Summary
Glia, the non-neuronal cells of the nervous system, were long considered secondary cells only necessary for supporting the functions of their more important neuronal neighbors. Work by many groups over the past two decades has completely overturned this notion, revealing the myriad and vital functions of glia in nervous system development, plasticity, and health. The largest population of glia outside the brain is in the enteric nervous system, a division of the autonomic nervous system that constitutes a key node of the gut-brain axis. Here, we review the latest in the understanding of these enteric glia in mammals with a focus on their putative roles in human health and disease.
Subject area: Neuroscience, Microbiome, Gastroenterology
Graphical abstract
Neuroscience; Microbiome; Gastroenterology
Introduction
The mammalian enteric nervous system (ENS) is a complex network of neurons and glial cells organized into two interconnected plexuses that span the entire length of the alimentary tract in some capacity (Figure 1). ENS circuits operate both autonomously and in collaboration with the central nervous system (CNS) to regulate virtually every aspect of gut function including motility, epithelial turnover, fluid secretion, and mucosal immunity (Furness, 2012; Furness et al., 2014; Rao and Gershon, 2016; Sharkey et al., 2018; Spencer and Hu, 2020). In humans, the ENS is composed of more than 100 million neurons in the small intestine alone, which is at least as many as are in the spinal cord and much more than in all other peripheral ganglia combined (Furness, 2006). Glial cells outnumber neurons in the ENS by approximately 4-fold in mice and 6-fold in humans (Gabella and Trigg, 1984; Hoff et al., 2008), suggesting a major role in gut physiology. Despite the abundance of these cells in mammals, enteric glia have been far less studied than their counterparts in the CNS, and much remains to be discovered about their underlying biology.
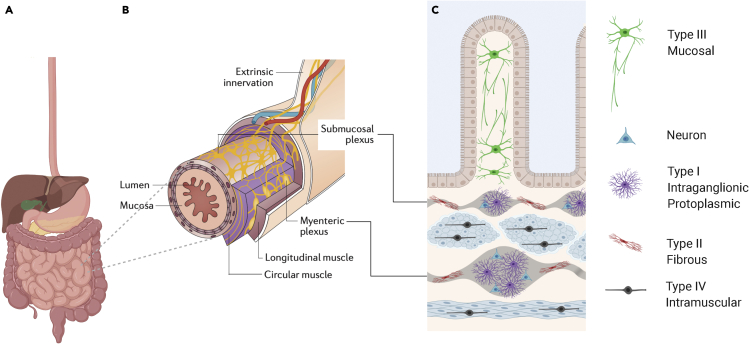
Figure 1.
The enteric nervous system and its glial components
(A) The human alimentary tract extending from the esophagus to the rectum contains an intrinsic network of neuro-glial circuits termed the enteric nervous system (ENS).
(B) The bowel is visualized in cross-section (here the small intestine) to illustrate the location of the two major enteric plexuses in relation to the laminar structure of the bowel. The larger plexus, the myenteric plexus, is located in between the outer longitudinal and inner circular layers of the muscularis propria. The smaller submucosal plexus is situated in the submucosa, in between the mucosa and the muscularis propria.
(C) Schematic of higher magnification view of small bowel cross-section illustrating that while neuronal cell bodies are largely restricted to the enteric plexuses (gray), enteric glial cells (EGCs) are distributed more widely across all the lamina. Mucosal EGCs (Type III), located closest to the intestinal lumen, are multipolar with long branches. Intraganglionic EGCs (Type I), located within both the submucosal and myenteric plexuses, are extensively branched. EGCs within nerve fibers that connect ganglia of the same type are elongated and less extensively branched (Type II), and intramuscular EGCs located along small nerve fibers in the muscle layers are bipolar in morphology (Type IV). Glia have been detected in all layers of the human bowel wall, and while the morphological subtypes shown here have been established in rodent models, emerging evidence supports similar morphologies in humans.
The CNS contains 3 main types of glia: astrocytes and oligodendrocytes, both of which derive from neuroectoderm, and microglia, macrophage-like cells of distinct developmental origin that express the chemokine receptor CX3CR1. Similarly, in the gut, there is a large population of enteric glia derived from neural crest as well as developmentally distinct populations of tissue-resident macrophages, many of which are CX3CR1+. The roles of these enteric macrophages have been well reviewed elsewhere (Muller et al., 2020; Moreira Lopes et al., 2020), and therefore, here, we focus our attention exclusively on enteric glia, which most closely associate with enteric neurons. We describe the development and diversification of enteric glia, their homeostatic functions, potential interactions with gut microbiota, and contributions to the progression of disease states along the gut-brain axis. Aiming to understand the aspects of enteric glial biology most directly relevant to human health, we focus this review on studies in human tissue and mammalian model organisms. Some nonmammalian vertebrates, such as zebrafish, do not have a dual plexus ENS structure and have relatively fewer enteric glia (Mccallum et al., 2020; Kuil et al., 2020). Thus, here, we discuss key advances in human enteric glial biology, placed in context of what has been shown in murine counterparts, and highlight the many knowledge gaps that make a compelling case for further study of these fascinating cells.
Enteric glial morphology and ultrastructure – evidence for distinct subtypes?
From their first description by Russian anatomist Alexandre Dogiel in 1899 (Dogiel, 1899), glia in the gut were considered to be analogous to Schwann cells, the dominant population of glia in the extraenteric peripheral nervous system (PNS). Many Schwann cells myelinate nerve fibers and, like enteric glia, are neural crest-derived. Detailed studies of ENS ultrastructure by electron microscopy (EM), however, showed that while glia in both rodent and human intestines partially ensheathed axons, no evidence of myelin-like structures could be found (Cook and Burnstock, 1976; Dvorak et al., 1980; Gabella, 1981; Ferri et al., 1982). Intestinal glia also did not structurally resemble Remak Schwann cells, the nonmyelinating glia in peripheral nerves that support small-diameter, unmyelinated axons (Harty and Monk, 2017). Gabella was the first to label glia in the gut “enteric glial cells” (EGCs), in recognition that they were phenotypically distinct from Schwann cells (Gabella, 1981). He and others showed that many EGCs had small central perikarya and stellate processes, more similar in ultrastructure to astrocytes than Schwann cells (Gabella, 1971; Komuro et al., 1982). These morphological studies combined with the discovery that enteric glia expressed S100B and glial fibrillary acidic protein (GFAP) (Ferri et al., 1982; Jessen and Mirsky, 1983), molecular markers characteristic of astrocytes, led to the idea that enteric glia were the “astroglia” of the gut. This paradigm defined the expected functions of enteric glia for many decades, and S100B and GFAP were long used interchangeably to identify glial cells in the intestine.
Recent work has challenged the notion that all EGCs are analogous to astrocytes, showing that GFAP protein expression is not universal among enteric glia, reflecting phenotypic plasticity and/or functional heterogeneity (Boesmans et al., 2015; Rao et al., 2015). Similarly, Aldh1L1, a widely utilized marker of astrocytes, is restricted to a subset of mouse EGCs and is more widely expressed by nonglial cell types in the gut (Boesmans et al., 2014). As a group, the transcriptional profile of enteric glia is actually more similar to that of myelinating glia than astrocytes (Rao et al., 2015), further indication that they differ from astrocytes in meaningful ways. Consistent with this, SOX10 and PLP1, two proteins important for the development and function of myelinating glia, including Schwann cells and oligodendrocytes, are widely expressed by enteric glia and are now recognized, along with S100B, as among the best markers of EGCs in the murine intestine (Young et al., 2003; Hoff et al., 2008; Rao et al., 2015). Recent single-cell sequencing studies suggest that human enteric glia also express a similar mix of astrocyte and oligodendrocyte molecular markers at the transcript level (Kinchen et al., 2018; Smillie et al., 2019; Drokhlyansky et al., 2020), evidence that EGC biology is unique in the nervous system and likely conserved across species. Further work rigorously comparing EGCs to other populations of nonmyelinating glia such as olfactory ensheathing cells, supporting cells of the inner ear, perisynaptic Schwann cells, and satellite glia, as was recently done for splenic glia (Lucas et al., 2021), would be illuminating.
Enteric glia are widely distributed throughout the gut, and their vastly different local microenvironments and morphologies hint that heterogeneity in molecular marker expression reflects functional diversity (Figure 2). The ENS is organized into two main networks of ganglia, clusters of neurons and glia, found in distinct locations along the radial (serosa-to-lumen) axis of the bowel wall (Figure 1B). Submucosal ganglia are located closest to the gut lumen, just beneath the mucosa, and are found throughout the small and large intestines. Submucosal ganglia are rare in the esophagus and relatively sparse in the stomach (Furness, 2006). Myenteric ganglia, in contrast, are found in between the circular and longitudinal smooth muscle layers throughout the digestive tract. Nerve fiber tracts connect individual ganglia within each layer, forming the two plexuses. The submucosal and myenteric plexuses are extensively interconnected with each other as well as with extrinsic nerves. Isolated neuronal soma and very small ganglia have also been reported within the gut mucosa of rats, pigs and humans (Newson et al., 1979; Balemba et al., 1998; Wedel et al., 1999). This more diminutive network of mucosal ganglia has not been as well characterized. Unlike enteric neurons, glia are found both within and outside ENS ganglia (Figure 1C, Figure 2). Intraganglionic glia are present within both myenteric and submucosal ganglia in close association with neuronal soma and are presumed to have similar functions in both locations. Extraganglionic glia are found both along nerve fibers in the smooth muscle layers (intramuscular glia) and distributed throughout the mucosa (mucosal glia), and they have been much less studied. Each of these spatial niches presents very different stimuli and interaction partners, suggesting that each of these EGC groups might have very different homeostatic functions.
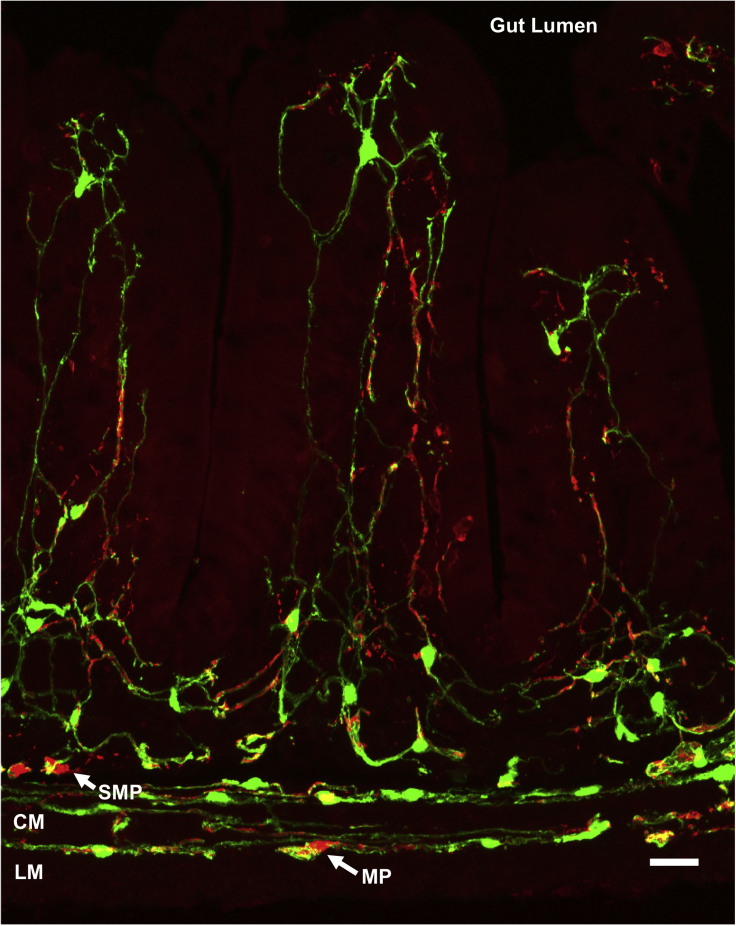
Figure 2.
Enteric glial diversity and distribution in the mouse small intestine
Immunohistochemical staining of a cross section of small intestine from an adult PLP1-eGFP mouse in which green fluorescent protein (GFP) expression marks all enteric glia illustrates the extensive glial network in the gut. Neurons are immunoreactive for PGP9.5 (red), and their somas are visible in the myenteric (MP) and submucosal (SMP) plexuses. The muscular propria consists of the longitudinal (LM) and circular (CM) muscle layers. Scale bar = 25μm.
In concert with their varying microenvironments, enteric glia also vary dramatically in cellular morphology. A study that used horseradish peroxidase injections into individual cells of the guinea pig small intestine to visualize morphology was the first to show that EGCs, even just within the myenteric plexus, were morphologically diverse. The investigators described extensively branched “protoplasmic” EGCs within myenteric ganglia and less branched, more elongated “fibrous” EGCs in nerve fiber tracts between ganglia (Hanani and Reichenbach, 1994). These were classified as Type I and Type II EGC types, respectively. This morphological classification was later broadened to 4 types including multipolar glia with a limited number of branches that are found mostly in the mucosa (Type III) and glia with small central soma that extend long, thin, bipolar projections, mostly found along small nerve fibers in the muscle layers (Type IV) (Gulbransen and Sharkey, 2012). More recent genetic labeling studies in mice have elegantly illustrated this morphological diversity (Boesmans et al., 2015) and suggested that a combinatorial code of molecular markers may define at least one of these morphological types (Type IV intramuscular glia) (Rao et al., 2015).
Fewer studies have examined glial morphologies and molecular marker expression patterns in the esophagus, which differs from the stomach and intestines in that it has a squamous epithelial lining, incorporates both striated and smooth muscle, lacks a submucosal plexus, and is more extensively innervated by the vagus nerve. S100B- and GFAP-immunoreactive glia are present in the esophageal myenteric plexus of rodents and humans (Chadi et al., 2004; Raab and Neuhuber, 2004; Gao et al., 2006; Nascimento et al., 2010). A recent study used genetic reporters and immunohistochemistry to characterize expression of PLP1, GFAP, and myelin basic protein in the mouse esophagus in detail. It found that all four morphological types of glia were detectable in the esophagus and that they shared similarities with their counterparts in the intestines, such as presence of GFAP expression in myenteric glia and absence of GFAP in Type IV intramuscular glia (Kapitza et al., 2021). Many esophageal glia expressed both PLP1 and GFAP, but overall, PLP1 expression was much more widespread. An important exception was a perivascular population of GFAP+ cells with elongated soma and variable PLP1 expression that the authors proposed could be a fifth morphological type of esophageal glia.
The majority of studies to date characterizing glial morphology and ultrastructure have focused on rodent models. The accurate depiction of EGC morphology in the human gut is an ongoing endeavor. Recent advances in tissue preparation and imaging that have enabled 3-dimensional (3D) visualization of glial networks in whole gut segments have delivered fresh insights. As in mice, the calcium-binding protein S100B is relatively specific to glia in the healthy human intestine (Gonzalez-Martinez et al., 2003), and its immunoreactivity is useful for visualizing glial morphology. Immunohistochemical labeling of S100B in human colon resection specimens followed by optical clearing revealed that mucosal EGCs closely associate with lymphatic channels at the bases of colonic glands and with blood vessels at the apex (Liu et al., 2013). Mucosal EGC processes appear to orient differently depending on the cell's location, traversing longitudinally near the crypt base and axially closer to the lumen (Liu et al., 2013). Another study using similar approaches visualized myenteric and intramuscular glia in human colons and showed that these cells resembled their counterparts in rodent tissues (Graham et al., 2020).
In contrast to morphological studies of human EGCs that have preferentially surveyed the colon, EM studies have focused on the small intestine. These studies have largely described EGCs incidentally as a consequence of their association with axons, the primary focus of these reports. Moreover, virtually all the ultrastructural analyses of rodent and human EGCs have focused on the myenteric plexus. A single study described putative EGCs in the mucosa of the mouse small intestine coming into close contact with enteroendocrine cells, specialized sensory epithelial cells that release hormones and neurotransmitters (Bohorquez et al., 2014). The putative EGCs that were visualized, however, were not immunolabeled and there was limited evidence confirming that they were glia. Mucosal glia are situated as individuals amidst a dense collection of many other cell types in the lamina propria, and genetic labeling approaches in combination with immuno-EM will be helpful to identify them more definitively. Overall, the ultrastructural features of submucosal, mucosal, and intramuscular glia and their interactions with neighboring cells are almost completely unknown in mice and humans. Evidence to date suggests that the morphological features of EGCs are conserved among mammals; however, the relationships between glial structure and function are yet to be fully defined.
Development and diversification of enteric glia
Enteric glia derive from neural crest cells that originate in the vagal and sacral levels of the neural tube and migrate into the developing bowel to give rise to the ENS (Rao and Gershon, 2018; Kang et al., 2021). These crest-derived progenitors are bipotential, giving rise to both neurons and glia, first in myenteric ganglia and then submucosal ganglia in an “outside-in” fashion (Pham et al., 1991; Wallace and Burns, 2005). This developmental program results in a topological organization of clonally related cells in columns along the radial axis (Lasrado et al., 2017). In mice, the first S100B-expressing cells are detected in the ENS as early as embryonic day 14.5 (E14.5) (Rothman et al., 1986; Young et al., 2003; Lake and Heuckeroth, 2013). GFAP expression is detectable shortly afterward at E16 (Rothman et al., 1986). Human EGCs are also detectable in enteric ganglia during prenatal development. A survey of human fetal tissue recognized neural crest-derived cells at locations characteristic of the submucosal and myenteric plexuses by 8–11 postconceptual weeks (PCWs) in multiple segments along the gastrointestinal (GI) tract (Wallace and Burns, 2005). At least some of these precursors seem to have committed to either neurogenic or gliogenic programs by this time point because distinct glial and neuronal precursor transcriptional profiles are detectable (Fawkner-Corbett et al., 2021). S100B-expressing cells are present in every segment of the human GI tract in the myenteric and submucosal ganglia by 12 and 15 PCWs, respectively. In contrast to mice, human EGCs do not express GFAP in any gut segment until after birth (Grundmann et al., 2019). Recent transcriptional profiling studies show that the majority of colonic EGCs in the healthy, adult human bowel express S100B and PLP1 but little to no GFAP (Kinchen et al., 2018; Drokhlyansky et al., 2020), suggesting that GFAP may not be a robust marker for human EGCs at any age. GFAP levels fluctuate in other glial types with stress, and it is possible that environmental conditions and low baseline transcript levels account for these observed differences, rather than species-specific biology. Spatial proteomic approaches to studying ENS development could offer additional insights.
Bipotential ENS progenitors that give rise to glia must downregulate neurogenic programs, including expression of RET receptor tyrosine kinase, while maintaining expression of the transcription factor SOX10, known to be important for glial differentiation in the CNS and extraenteric PNS (Paratore et al., 2001; Bondurand and Sham, 2013). A number of other molecules, including transcription factor TBX3 (Lopez et al., 2018), the orphan nuclear receptor NR2F1 (Charrier and Pilon, 2017), and the Notch and Hedgehog signaling pathways (Taylor et al., 2007; Liu and Ngan, 2014) have all been implicated in mouse EGC development and differentiation. It is unclear to what degree these pathways are conserved in the human ENS or if they play a role in glial diversification. The majority of human EGCs across all layers in the ileum and colon are immunoreactive for SOX10 (Hoff et al., 2008), suggesting that at least this transcription factor is important for human EGC development as well. Mucosal EGCs derive from the same progenitors as EGCs in other locations along the bowel wall (Kabouridis et al., 2015), but their development has some unique features. These features are reviewed in detail in the subsequent section.
While enteric neuronal diversity has been studied in great detail, much less is known about the molecular mechanisms that drive glial diversification and to what extent there are functionally distinct types of EGCs in the mature ENS. Recent single-cell sequencing studies have started to address these gaps. A study of the juvenile nervous system at postnatal day 21 (P21) profiled glia in the small intestinal myenteric plexus of transgenic reporter mice and reported seven transcriptionally distinct enteric glial subtypes, one of which had a proliferative signature (Zeisel et al., 2018). Drokhlyansky et al developed new methods to isolate intact cell nuclei from gut tissues of mice and humans and utilized them to transcriptionally profile the adult colonic ENS (Drokhlyansky et al., 2020). In mice, they identified 3 transcriptionally distinct populations of colonic EGCs that could be distinguished based on expression of the membrane receptor Gfra2, the monoamine transporter Slc18a2 (also identified as a cluster marker by Zeisel et al., 2018), and the neurotensin receptor Ntsr1. In humans, they found that glia derived from colonic muscularis propria isolated from cancer-adjacent resection specimens segregated into 3 main subsets. There were 3 additional cell clusters that were patient-specific, perhaps reflecting disease-specific phenotypes. All of these muscularis EGC populations were found to be transcriptionally distinct from mucosal EGCs, profiled in a previous study by the same group (Smillie et al., 2019). Surprisingly, all had relatively low S100B expression. The genes enriched in a cluster-specific manner were not similar between murine and human EGCs in this study, hinting at divergence, but no formal comparative analysis was done analogous to that done in neurons (Drokhlyansky et al., 2020; May-Zhang et al., 2021). Furthermore, it remains unclear how these transcriptional groups relate to the known diversity in EGC spatial niches and morphological types.
Another study using spatial transcriptomics to analyze human fetal tissue described five transcriptionally distinct glial populations in the small and large intestines that emerged over the developmental period examined, defined by shared expression of both SOX10 and S100B (Fawkner-Corbett et al., 2021). This study is an exciting resource for exploring developmental diversification of glia, but the labels assigned to the EGC populations in this study need to be interpreted with some caution. At 8–10 PCWs, a cluster of “glial progenitors” dominated and was distinguished by low levels of mature glial gene expression, early appearance during development, and expression of PHOX2B. While mature glia are known to have reduced PHOX2B expression in comparison to neurons (Corpening et al., 2008), they still express robust levels, and no clear threshold of PHOX2B expression has been defined to distinguish glia from neurons. Thus, some cells assigned to this group may be mature glia rather than progenitors. The next two clusters to emerge were classified as “submucosal” and were groups of cells expressing TGFB1 and genes associated with regulating epithelial cell proliferation. These groups presumably represented mucosal glia in the lamina propria and not glia in the submucosal plexus. One of these two groups showed relatively lower expression of “submucosal” EGC genes and simultaneous expression of HAND2, a transcription factor important for ENS development, and was thus designated as containing “submucosal precursors.” The two clusters that emerged latest in development were those characterized as “intraganglionic” and “lymphoid-associated.” EGCs marked by ENTPD2 expression were categorized as “intraganglionic”; however, earlier studies have shown that while intramuscular EGCs do not express ENTPD2, many other extraganglionic EGCs, including mucosal EGCs, express robust levels, so ENTPD2 expression is not specific to intraganglionic EGCs (Rao et al., 2015; Grubisic et al., 2019). “Lymphoid-associated” EGCs expressed high levels of genes involved in immune cell function and TGF-β receptors (TGFBR3), and they were the most transcriptionally distinct from the other groups. Supporting their designation, these cells expressed several genes previously shown to be important for development of Peyer's patches, small aggregates of lymphoid tissue in the small intestine (Veiga-Fernandes et al., 2007).
There is growing evidence in mammalian and nonmammalian systems that the ENS is populated not just by neural crest-derived progenitors, but also by Schwann cell precursors (SCPs) associated with extrinsic peripheral nerves that innervate the bowel (Uesaka et al., 2015; Soret et al., 2020; El-Nachef and Bronner, 2020). These SCPs give rise to a relatively small population of submucosal neurons in the small intestine but contribute nearly 20% of all neurons in the mouse colon (Uesaka et al., 2015). Neurogenic SCPs do not seem to pass through a bipotential state (Uesaka et al., 2015), and therefore, it is less clear to what extent they contribute to enteric glial populations at steady state. Regardless, SCPs express both SOX10 and PLP1, and thus, at least some proportion of cells identified as EGCs based on these two markers are probably neurogenic SCPs. SCPs express many molecular markers in common with EGCs, and discriminating these two cell populations in order to interrogate their relative proportions and contributions will be challenging. Recent single-cell transcriptional profiling data suggest that expression of Dhh, Mal, and Mpz genes may distinguish SCPs from EGCs, at least in the muscular layers of the embryonic and perinatal mouse intestine (Zeisel et al., 2018; Morarach et al., 2021).
In summary, enteric glia in mice and humans arise from neural crest and mature into phenotypes with distinct morphologies, spatial niches, and transcriptional signatures. Much work remains to determine the molecular mechanisms that generate this diversity, how differences in gene expression relate to physical features or locations of EGCs, and to what extent any of this diversity reflects the existence of distinct subtypes with different homeostatic functions. There have been substantial advances in describing human EGCs at the gene expression level, and these data suggest some fundamental differences between human and rodent EGCs. The evaluation of these differences is currently limited by our understanding of enteric glial biology and the ways in which these gene products might support EGC function.
Enteric glia and the gut microbiome
Enteric glia, particularly those in the mucosa, are auspiciously located for effective, if not direct, interaction with microbes in the gut lumen. The first evidence of functional interactions between EGCs and microbes was the observation that glia in germ-free (GF) mice fail to fully populate the mucosa of the small intestine, despite normal appearance of glia in the myenteric and submucosal plexuses (Kabouridis et al., 2015). The authors of this study found that mucosal EGCs were largely undetectable during normal fetal development and seemed to populate the mucosa in the postnatal period in tandem with the maturation of the intestinal microbiome. These observations indicated that mucosal EGCs are developmentally distinct from other EGCs and require the presence of microbes to establish a normal population. Lineage tracing experiments showed that the small intestinal mucosa in adult mice is continuously repopulated by glia that arise from Sox10- and Gfap-expressing progenitors located in the myenteric plexus and migrate into the mucosa (Kabouridis et al., 2015). Administration of broad-spectrum antibiotics to mice raised in standard conditions caused diminished mucosal EGCs in the small intestine with no effect on myenteric EGCs, similar to what was seen in GF mice. These findings raised the exciting possibility that gut microbes are necessary not just to establish a normal mucosal EGC population but also to continuously maintain it. Consistent with this possibility, restoring gut microbiota to GF mice completely rescued the deficit in mucosal EGCs. This study did not probe the effects of microbial depletion in the colon, where the microbial burden is orders of magnitude higher and might have even greater influence.
Subsequent studies have both challenged and supported these initial observations on EGC-microbe interactions. De Vadder et al. found that microbial recolonization of GF mice increased S100B immunoreactivity in the myenteric plexus of the colon but not the number of SOX10+ cells, suggesting that there may be microbial effects on myenteric glial phenotypes but not number (De Vadder et al., 2018). A recent preprint manuscript reports on the results of broad-spectrum antibiotic treatment of PLP1-eGFP reporter mice and suggests that microbial depletion has minimal effects on EGCs in the small and large intestines, with no defects observed in mucosal glia at all (Vicentini et al., 2020). Instead, they report a small deficit in glial number only in the ileal myenteric plexus. The relevance of microbes to human EGC development and migration is even less clear. A spatial transcriptomics study detected SOX10+/S100B+ cells located outside the muscularis propria in presumptive mucosa in human fetal tissue (Fawkner-Corbett et al., 2021), suggesting that human EGCs migrate out of the myenteric plexus early in fetal development. In accordance, S100B-immunoreactive glia are present in the human gut mucosa prior to birth (Inlender et al., 2021). Interestingly, these investigators found that while native S100B+ mucosal EGCs in mice were vulnerable to broad-spectrum antibiotic treatment, S100B+ mucosal EGCs in human fetal gut explants transplanted into the same mice were not vulnerable (Inlender et al., 2021). There may be important species- and/or microbe-specific differences leading to these differing observations. Study of human mucosal EGCs in situ, potentially in tissues isolated from patients treated with antibiotics compared to those from untreated controls, would help provide clarity.
While the role of microbes in EGC development remains to be clarified, there is strong evidence that mucosal EGCs are well poised to respond to microbes both directly and indirectly. Mucosal EGCs in mice closely approach blood vessel endothelial cells (Savidge et al., 2007b), lymphatic endothelial cells (Liu et al., 2013), immune effector cells (Grubisic et al., 2020), and enteroendocrine cells (Bohorquez et al., 2014), all of which can be affected by the presence or absence of microbiota. Furthermore, EGCs in mice and humans express toll-like receptors (TLRs), proteins capable of detecting microbe-associated molecular patterns (Turco et al., 2014; Esposito et al., 2014). Human EGCs in the mucosa and ganglia also express major histocompatibility complex class II (MHC-II), a class of molecules critical in presentation of foreign protein to immune effector cells in inflammatory disease states including infection (Hirata et al., 1986; Koretz et al., 1987; Geboes et al., 1992; Da Silveira et al., 2011). These data combine to suggest roles for EGCs in microbial detection, antigen presentation, and immune modulation that remain to be defined.
One molecular mechanism that EGCs might use to modulate gut function in response to microbial cues is the secretion of neurotrophic factors, such as those belonging to the glial-derived neurotrophic factor (GDNF) family of ligands (GFLs). GFLs are secreted proteins that signal through the RET receptor tyrosine kinase to support the growth and survival of neurons in the context of development, homeostasis, and injury. In the gut, GFL signaling is critical for normal development of the ENS and Peyer's patches (Schuchardt et al., 1994; Veiga-Fernandes et al., 2007). The roles of this signaling in gut homeostasis are still being determined. The GFLs include GDNF, neurturin, artemin (ARTN), and persephin (Airaksinen and Saarma, 2002). At least two of these ligands have been detected in EGCs: GDNF expression localizes to EGCs in the mature ENS (Bar et al., 1997) as well as gut smooth muscle (Brun et al., 2015; Han et al., 2015); ARTN is detectable in EGCs, at least at the transcript level, in human fetal tissues (Fawkner-Corbett et al., 2021). Conversely, RET is expressed by myriad cell types in the mature gut including Type 3 innate lymphoid cells (ILCs) in which RET signaling modulates expression of IL-22, a secreted factor important for epithelial reactivity and repair (Ibiza et al., 2016). Interfering with either RET expression in ILC3s or the immune adaptor protein MYD88 in Gfap-expressing cells impairs ILC3-derived IL-22 release and increases susceptibility to gut inflammation. These observations indicate that EGCs might sense microbial molecules and release GFLs to modulate ILC3 functions via RET signaling. Type 2 ILCs also express RET, but its role in these cells is yet undetermined. Enteric glial MYD88 has also been shown in a recent preprint manuscript to modulate the response to a high-fat diet (Liu et al., 2020), a condition known to alter the gut microbiome (Hildebrandt et al., 2009). EGCs of mice fed a high-fat diet assumed a reactive phenotype and increased production of S100B and GFLs, including GDNF and ARTN. This response was attenuated in mice lacking MYD88 in GFAP+ cells. Diet may alter the intestinal microbiome, in turn eliciting an immune response that is partly integrated by EGCs and their signaling mechanisms. A potential limitation of both of these studies is the use of the Gfap promoter to drive gene deletion in enteric glia as this promoter may be active in nonglial cells that also can modulate immune functions (Rao et al., 2017).
A smaller number of studies have investigated the behavior of human EGCs in response to microbes or microbial products. Primary cultures of human myenteric plexus EGCs exposed to bacterial lipopolysaccharide upregulate a variety of transcripts for proinflammatory cytokines (Linan-Rico et al., 2016). Similar cultures exposed to enteroinvasive Escherichia coli increased their expression of MHC-II, S100B, and TLRs (Turco et al., 2014). These cultured cells also released nitric oxide (NO), a proinflammatory antimicrobial molecule, in response to enteroinvasive E. coli. In contrast, exposure to the probiotic Lactobacillus paracasei did not increase production of either S100B or NO, implying selectivity of response. Gut inflammation might prime this response because mucosal EGCs isolated from patients with ulcerative colitis, a type of inflammatory bowel disease (IBD), have higher levels of S100B and TLR4 compared to mucosal EGCs isolated from healthy controls (Esposito et al., 2014). Similar to proposed mechanisms in mice, human EGCs may respond to changes in gut microbial composition, such as the arrival of pathogens, through specialized recognition proteins and secretion of proinflammatory molecules.
Enteric glial functions in gastrointestinal homeostasis and disease
EGCs are considered to enable a number of physiological functions of the gut, and one of the first functions to be attributed to them was maintenance of the intestinal epithelial barrier (IEB). In IBD and other digestive disorders, dysregulation of this barrier is thought to play a major part in disease progression (Odenwald and Turner, 2017). In an animal model enabling inducible ablation of Gfap-expressing cells upon ganciclovir administration (GfapHSV−Tk), loss of enteric glia was associated with severe inflammation in the distal small intestine (Bush et al., 1998), suggesting that enteric glia were necessary to prevent gut inflammation. Subsequent studies of this model and others that employed the Gfap promoter to target glia showed that depleting EGCs was associated with increased IEB permeability, impaired epithelial cell proliferation, and immune cell invasion into enteric ganglia (Cornet et al., 2001; Aube et al., 2006; Neunlist et al., 2007; Savidge et al., 2007a). These and other in vitro studies in mice supported the idea that EGCs influence the integrity of the IEB and that EGC dysfunction contributes to inflammatory GI disease (Sharkey, 2015; Pochard et al., 2018).
A number of recent studies have questioned this dogma on glial-epithelial interactions. When a strictly cell-autonomous approach was used to target Plp1-expressing cells for genetic ablation, for example, there was no resulting intestinal inflammation in the small or large intestines, even when mice were challenged with the inflammatory agent dextran sodium sulfate (DSS) (Rao et al., 2017). IEB function remained intact, both in terms of permeability to macromolecules and microbes as well as its ultrastructure. Rare epithelial cells in the distal small intestine express GFAP, and the ganciclovir-derived antimetabolite generated within HSV-Tk-expressing cells can affect neighboring cells, two features of the GfapHSV−Tk mouse model that could both contribute to off-target effects (Rao et al., 2017). Subsequent work using intermittent ganciclovir dosing to deplete glia with fewer off-target effects in the GfapHSV−Tk model or using the inducible diphtheria toxin receptor system as an alternative means to target Gfap- or Plp1-expressing cells added further evidence that glial loss does not cause or exacerbate intestinal inflammation (Yuan et al., 2020). Similarly, neither disruption of EGC function by ATP depletion (Nasser et al., 2006) nor by ablation of connexins important for EGC communication (Grubisic and Gulbransen, 2017) provokes inflammation. In summary, there is clear evidence that EGCs secrete signaling molecules that can alter IEB permeability and epithelial proliferation in vitro, but in vivo, glia seem to be dispensable for these epithelial functions, and their depletion is not sufficient to cause intestinal inflammation in mice.
Given the challenges in identifying the role of EGCs in the pathogenesis of inflammation in mice, it is not surprising that their role in human IBD, such as ulcerative colitis and Crohn's disease, is even less clear. There is some evidence that EGCs in patients with IBD may be increased in number and phenotypically distinct, at least at the transcriptional level. Cell circuit analysis of single-cell transcriptional profiling data from human colonic tissue isolated from ulcerative colitis patients compared to controls implies that EGCs might serve to repress expansion of CD8+IL-17+ T-cells, a major source of the potent inflammatory cytokine IL-17 in patients with ulcerative colitis (Smillie et al., 2019). GFAP elevation is a hallmark of reactive astrocytes in the CNS, and intestinal biopsies from patients with IBD have higher levels of GFAP protein relative to controls (Cornet et al., 2001; von Boyen et al., 2011). This difference could also reflect the increased number of EGCs identified in the submucosa of both the small and large intestines of patients with IBD (Villanacci et al., 2008; Bassotti et al., 2009; Li et al., 2018b). If there are “reactive” EGCs associated with IBD, it is unclear if they contribute to disease pathogenesis or repair, or if their reactivity is simply a reflection of the disease.
Just as CNS glia can have both proinflammatory and anti-inflammatory effects in neurological disorders, there is growing evidence that EGC activities can be helpful as well as harmful in inflammatory GI conditions. For example, the EGC-derived factor S-nitrosoglutathione restored epithelial defects caused by Shigella infection in human colonic mucosa in vitro (Flamant et al., 2011). Similarly, colonic biopsies from Crohn's disease patients secreted less of the polyunsaturated fatty acid metabolite 15-hydroxyeicosatetraenoic acid (15-HETE) relative to those from controls, and application of 15-HETE to immortalized cell lines bolstered cell membrane integrity by reducing permeability across the epithelium (Pochard et al., 2016). These findings indicate that human EGCs can secrete molecules that promote intestinal barrier function and repair in vitro. Regardless of the extent to which glia and these factors are necessary for maintaining IEB function in vivo, stimulating EGC secretion of molecules that drive epithelial repair could represent a promising therapeutic strategy for IBD.
EGCs not only secrete key signals but also receive them from neighboring cells. One important mode of communication from enteric neurons to EGCs is via pannexin-mediated release of purines (Gulbransen et al., 2012; Boesmans et al., 2019). In mice with inflammatory colitis, this purinergic signaling activates EGCs to increase NO and ATP release, driving neuroinflammation and cell death (Gulbransen et al., 2012; Brown et al., 2016; Delvalle et al., 2018a). While the extent of neuronal loss in human IBD is still being established, the role of glia in this process clearly merits further investigation.
Whatever the myriad roles of EGCs are in neuronal injury during inflammation, there is exciting new work suggesting that they may participate in restoring homeostasis by contributing to neurogenesis. In zebrafish, EGCs readily function as neuronal progenitors in vivo (Mccallum et al., 2020). Mouse EGCs differentiate into neurons in vitro but seem less likely to do so in vivo, except in rare cases when chemical injury drives neuronal loss and presumably stimulates EGCs to replace them (Laranjeira et al., 2011; Joseph et al., 2011). In a mouse model of inflammatory colitis, however, lineage tracing revealed that neuronal loss in enteric ganglia was associated with generation of new neurons from Plp1-and Sox2-expressing cells (Belkind-Gerson et al., 2017). Consistent with this finding, in colonic tissue isolated from patients with Clostridium difficile-associated colitis or ulcerative colitis, enteric neurons positive for SOX2, a marker of EGCs, were markedly increased (Belkind-Gerson et al., 2017). Another study, however, found that while Gfap-expressing cells gave rise to new glia, especially when stimulated by injury, there was no evidence that they gave rise to neurons in a slew of physiological or pathophysiological conditions (Joseph et al., 2011). It may be that the enteric neuronal progenitors most relevant to homeostasis are Schwann cell precursors (Uesaka et al., 2015; Soret et al., 2020) or Nestin-positive nonglial cells (Azan et al., 2011; Kulkarni et al., 2017), rather than EGCs. Overall, it is evident that adult neurogenesis occurs in the ENS in at least some contexts, and mammalian EGCs, among other candidates, may be one potential source of new neurons (Jonscher and Belkind-Gerson, 2019). Glia seem capable of stimulating both neuronal death and generation in the ENS, suggesting that their net effects in inflammatory disease may be context-dependent or potentially subtype-specific.
While the understanding of EGCs in promoting and resolving inflammation is still evolving, one aspect of intestinal health in which they have a clear role is GI motility. Virtually, every mouse model of EGC ablation or disruption exhibits GI dysmotility (Aube et al., 2006; Nasser et al., 2006; Mcclain et al., 2014). Interestingly, one model showed a highly sex-dependent defect in colonic transit upon glial loss (Rao et al., 2017), suggesting that EGC dysfunction might have links to the prominent sex differences in functional GI disorders. A series of studies in mice using conditional gene deletion and chemogenetics has revealed that calcium signaling in EGCs is critical for their function in intestinal motility (Broadhead et al., 2012; Mcclain et al., 2014; Mcclain et al., 2015). EGC activity may influence motility through the release of signals, such as the neurotransmitter GABA (Fried et al., 2017) or by influencing neuronal activity in response to detection of neurotransmitters such as acetylcholine, ATP, and 5-HT (Boesmans et al., 2013; Delvalle et al., 2018b). Astrocytes in the brain refine synapses between neurons, facilitating synapse growth and elimination. Rodent EGCs cocultured with enteric neurons also promote synapse formation (Le Berre-Scoul et al., 2017), suggesting yet another mechanism by which EGC activity could influence enteric circuits important for motility.
EGC changes in human tissues, particularly alterations in cell number, have been observed in multiple GI motility disorders (Figure 3). Hirschsprung disease is a developmental disorder characterized by congenital absence of enteric ganglia (aganglionosis) in a segment of bowel, typically the distal colon. Patients present with severe constipation, and even functional bowel obstruction, caused by lack of peristalsis in their affected bowel segments. Standard treatment is resection of the affected segment, but many patients experience lingering postoperative symptoms including dysmotility (Menezes et al., 2006). Patients with Hirschsprung disease exhibit more EGC precursors in ganglia of nonaffected portions of the GI tract than controls (Tani et al., 2017), raising the possibility that altered ENS composition in ganglionic bowel is linked to the chronic GI dysmotility in patients following surgery. The most life-threatening complication of Hirschsprung disease is severe enterocolitis, an inflammatory condition with a risk that is undiminished by surgery and that remains poorly understood (Demehri et al., 2013). Intriguingly, the major susceptibility gene for Hirschsprung disease is RET (Angrist et al., 1996; Amiel et al., 2008), discussed above as playing a potential role in EGC signaling in gut inflammation. The possible contributions of EGCs to the inflammatory and motility-related complications of Hirschsprung disease clearly deserve further exploration.
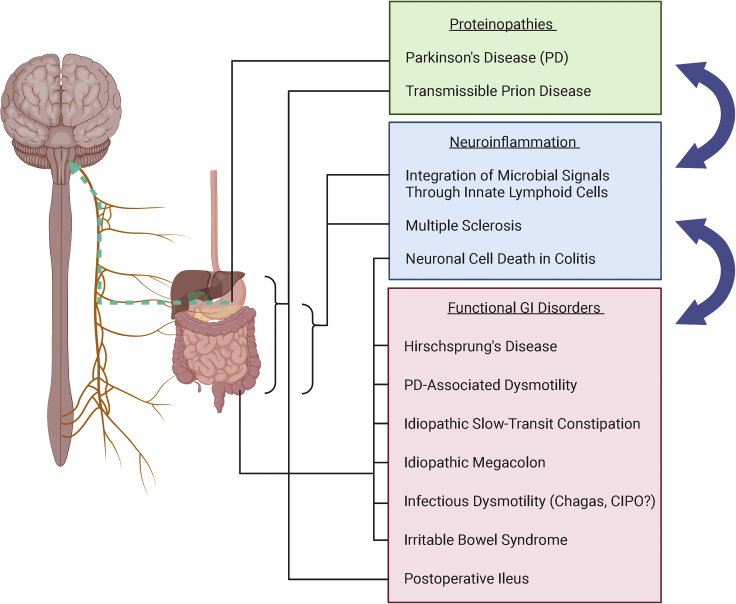
Figure 3.
Enteric glia-associated human diseases along the gut-brain axis
Proteinopathies (green box) involve misfolded proteins that may travel from the gastrointestinal (GI) system to the brain, and these include Parkinson's disease and transmissible prion disease. Enteric glial cells (EGCs) may be involved in transmission of these proteins. Neuroinflammatory conditions of the enteric nervous system (blue box) can be induced by dysregulation of the gut microbiome or infection, and these states may be mediated in part by EGCs. A host of functional GI disorders (red box) may be associated with enteric glial dysfunction, largely affecting the colon. Groups of EGC-associated conditions may exacerbate or influence each other (blue arrows): neuroinflammatory disorders may cause dysmotility and bowel dysfunction, and dysmotility may promote neuroinflammation; neuroinflammation may allow easier transmission of misfolded proteins, and aggregates of misfolded protein may in turn promote neuroinflammation.
EGC deficits are also linked to idiopathic slow-transit constipation (STC). Small intestinal and colonic ganglia of patients with STC contain fewer EGCs than those of control patients (Bassotti et al., 2006a, 2006b). Additional conditions that involve constipation such as obstructed defecation, Chagasic megacolon, and idiopathic megacolon all are similarly linked to fewer EGCs in colonic ganglia (Bassotti et al., 2007; Iantorno et al., 2007). Chronic intestinal pseudo-obstruction (CIPO) is a rare condition characterized by severe dysmotility leading to signs of bowel obstruction without any identifiable occlusion (Downes et al., 2018). Not only did patients with CIPO possess fewer EGCs in enteric ganglia, but a case series identified the presence of JC virus, known to cause fatal infections in the immunosuppressed, within EGCs in a majority of patients with CIPO (Selgrad et al., 2009).
A common acquired cause of GI dysmotility is postoperative ileus (POI), impaired intestinal transit that can follow both abdominal and extra-abdominal surgery. A model of POI elicited by intestinal manipulation in mice demonstrated that the immune adaptor protein MYD88 as well as the inflammatory signaling molecule IL-1R1 is important in the progression of POI (Stoffels et al., 2014). Intramuscular EGCs express IL-1R1, signifying that EGCs may be involved in triggering POI through this inflammatory pathway. Subsequent work has found ATP release to be involved in eliciting a reactive EGC phenotype in the same mouse model (Schneider et al., 2021). In this study, human intestinal tissue retrieved from surgery and mechanically manipulated also exhibited reactive EGC phenotypes, and human EGCs in culture released the inflammatory signaling molecule IL-6 upon stimulation with ATP. All together, these findings indicate that ATP-mediated EGC responses may contribute to POI in both rodents and humans.
As detailed above, animal models have revealed a clear role for enteric glia in the regulation of gut transit, and changes in EGC number as well as phenotypes are linked to a variety of human disorders of GI motility. EGC functions in immune communication may influence motility when infectious organisms are involved, such as in Chagas disease (Trypanosoma cruzi) and CIPO (JC virus). Similar to the relationship between EGC changes and inflammatory GI disease, the EGC deficits reported in motility disorders could also result because they are targets or innocent bystanders of the disease process rather than the cause. Further basic and translational studies combining animal models and human tissue will help to tease apart these causalities and uncover the roles of enteric glia in regulating GI motility in both health and disease.
Glial functions in both motility and inflammation may play a role in irritable bowel syndrome (IBS), a functional GI disorder characterized by abdominal pain and altered stool patterns that affects 5–10% of the population worldwide (Black and Ford, 2020). IBS lacks the overt inflammatory histological findings of IBD but often manifests after GI infections, and its pathophysiology is poorly understood. EGC changes, such as reduced S100B immunoreactivity, have been observed in colonic mucosal biopsies of patients with IBS (Lilli et al., 2018). A subset of patients with IBS exhibit visceral hypersensitivity, defined by diminished thresholds for pain in response to colorectal distention (Ritchie, 1973; Zhou and Verne, 2011), and EGCs may participate in this aspect of IBS pathophysiology. Nerve growth factor (NGF) has been implicated in promoting visceral hypersensitivity in patients with IBS (Dothel et al., 2015) and is upregulated in the colon in a rat model of IBS (Xu et al., 2013). In this rat model, NGF colocalized with GFAP, suggesting that EGCs may promote visceral hypersensitivity via NGF secretion (Long et al., 2018). In mice, EGCs are involved in mediating communication between enteric neurons and the spinal afferent nerve endings that signal visceral pain (Delvalle et al., 2018a). Disruption of connexin-43 signaling in Sox10-expressing cells, which include EGCs, abolishes the visceral hypersensitivity caused by chronic inflammatory colitis (Grubisic et al., 2020). EGCs, along with satellite glia and Schwann cells that associate with visceral afferent neurons outside the bowel, are thus likely to play a role in disorders of visceral hypersensitivity such as IBS.
In addition to inflammatory and functional GI disorders, the other group of digestive disorders in which EGCs have been implicated is cancer. Patients with multiple endocrine neoplasia type 1 (MEN1) are prone to developing gastrin-secreting neuroendocrine tumors, gastrinomas, in the duodenum (Anlauf et al., 2005). In mouse models of MEN1, gastrin-expressing EGCs accumulate in the duodenum, and gastrinomas from patients with MEN1 express glial markers including GFAP and S100B (Sundaresan et al., 2017). These data highlight the possibility that gastrinomas in patients with MEN1 originate from duodenal EGCs. A more common tumor entity associated with EGCs is colorectal carcinoma (CRC). Both human EGCs and EGC cell lines promote expansion of tumor number and size from a CRC stem cell line (Vales et al., 2019). This effect requires bidirectional communication between EGCs and tumor cells, mediated by prostaglandin E2 and epidermal growth factor signaling. In vivo, mice injected with tumor stem cells and EGCs had higher tumor burdens than those injected with tumor stem cells alone, further supporting a tumorigenic role for EGCs (Vales et al., 2019). Consistent with this possibility, genetic depletion of EGCs in vivo in mice was associated with a lower tumor burden in the azoxymethane-DSS model of inflammatory CRC (Yuan et al., 2020). The data on EGCs in human CRC pathology, however, are sparse and conflicting. EGCs are intimately associated with colorectal tumors in human tissue, suggesting that they are a component of the tumor microenvironment (Vales et al., 2019). Arguing against a protumorigenic role, however, GFAP content is diminished in higher grade, more proliferative tumors, and elevated in lower grade tumors (Tartea et al., 2017). This observation could reflect a tumor effect on EGC expression of GFAP and/or fewer tumor-associated EGCs in patients with more aggressive tumors. Nevertheless, the studies to date suggest that EGCs are part of the tumor microenvironment, and their role in CRC pathogenesis needs to be unraveled.
Roles for enteric glia in neurological disease
The ENS participates in bidirectional communication with the CNS, and when hijacked, this axis may serve as a route for the progression of disease from the gut to the brain (Figure 3). One such disease is Parkinson's disease (PD), where the ENS has been proposed to be a conduit of PD pathology to the CNS. Lewy bodies, hallmark aggregates of misfolded α-synuclein typically identified within CNS neurons of patients with PD, were first noticed in the ENS in postmortem esophageal tissue (Qualman et al., 1984) and subsequently shown throughout the GI tract (Kupsky et al., 1987; Wakabayashi et al., 1988). Braak et al. showed that deposits of α-synuclein were distributed in the stomach both in submucosal and myenteric ganglia of human patients with PD at various stages of disease progression (Braak et al., 2006). They proposed that a putative pathogenic insult to the stomach could induce misfolding of α-synuclein within enteric neurons, which then transmit protein aggregates to the CNS through synaptic connections with vagal nerve fibers. A number of studies in rodents have since established the proof-of-concept that administration of pathogenic proteins into the gut wall is sufficient to cause ascending pathology that propagates from the GI tract to the brain (Holmqvist et al., 2014; Kim et al., 2019; Challis et al., 2020).
EGCs are altered in patients with PD, but it is unclear to what extent this reflects a role in disease pathogenesis. Colonic biopsies from patients with PD contain higher levels of GFAP transcripts and protein relative to biopsies from controls (Devos et al., 2013; Clairembault et al., 2014). These increased GFAP levels correlate with expression of a variety of proinflammatory cytokines including IL-1β, IL-6, IFN-γ, and TNF-α (Devos et al., 2013). Reactive EGCs in patients with PD may contribute to a proinflammatory environment that facilitates misfolding of α-synuclein, igniting a fuse that drives pathology from the gut to the brain.
GI motility symptoms are common in patients with PD (Gallagher et al., 2010; Pfeiffer, 2011), and in many patients, they precede the somatic motor symptoms characteristic of the disease (Abbott et al., 2001). Given the evident role of EGCs in regulating GI motility, their activity may influence the development of motility symptoms in patients with PD regardless of a role in facilitating α-synuclein aggregation. Postmortem assessment of the ENS in patients with PD did not reveal neuronal loss anywhere along the GI tract from stomach to rectum, suggesting that dysmotility is not secondary to EGC effects on neuronal survival, at least in the myenteric plexus (Annerino et al., 2012). Further studies are needed to determine whether there might be deficits in synapse formation or disruptions in glial interactions with non-neuronal cells important for motility, such as muscularis macrophages, that could contribute to dysmotility in PD. Lewy body aggregation affects several autonomic ganglia separate from the ENS, including those that innervate the GI tract (Beach et al., 2010). Alterations at these sites could also act in concert with EGC dysfunction to disrupt motility.
As detailed previously, mucosal EGCs interact at least indirectly with the gut microbiome, integrating signals to and from the immune system. These interactions may promote PD pathology, especially if microbial changes are involved in the pathogenesis of PD. Numerous studies have documented altered composition of the GI microbiome in patients with PD, although without concordance in specific taxonomies (Lubomski et al., 2019). It is unclear whether this dysbiosis is the cause or consequence of PD progression. Although there is evidence that microbiome changes precede PD onset (Heintz-Buschart et al., 2018), GI dysmotility is a symptom that often precedes diagnosis and can alter gut microbiota. The ways human EGCs might integrate microbial signals to influence PD or PD-associated dysmotility are far from understood, but animal models have started to provide insights. In the rotenone model, administration of the pesticide rotenone to rodents reproduces abnormal brain and behavioral findings of PD, mimicking chronic pesticide exposure as a risk factor for the disease (Betarbet et al., 2000). Mice treated with rotenone exhibited GI dysbiosis before the onset of motor symptoms (Yang et al., 2017), and mice lacking TLR4 demonstrated attenuation of motor symptoms in this model (Perez-Pardo et al., 2019). TLR4 expression is by no means restricted to EGCs, but given the links between microbes and mucosal glia, TLR4 signaling in EGCs could link GI dysbiosis to neuronal pathology and dysmotility in PD.
Analogous to PD, prion diseases are also associated with misfolded proteins that accumulate in the brain. Prion diseases are characterized by rapidly progressive and fatal neurological decline, and there are no effective treatments. Most cases of prion disease are either genetic or sporadic (of unclear etiology), but some are of transmissible origin, beginning with ingestion of abnormally folded protein. In these cases, transmissible prions are likely to travel from enteric neurons to vagal neurons that innervate the gut and the brain (Mcbride and Beekes, 1999; Sigurdson et al., 2001). Intriguingly, a number of sporadic and genetic cases also exhibit prion deposition in the vagus nerve (Kresl et al., 2019), raising the possibility that the ENS plays a role in nontransmissible as well as transmissible prion disease. Propagation of prions requires expression of normally folded protein PrPc by the host, which misfolds upon coming into contact with the infectious isoform PrPsc. In addition to the brain, a variety of mammalian tissue types express PrPc, including the heart, lung, intestine, and kidney (Oesch et al., 1985; Peralta and Eyestone, 2009). PrPc is expressed in the human and rodent ENS, by both EGCs and neurons in both plexuses of the small and large intestines (Shmakov et al., 2000; Albanese et al., 2008). Thus, PrPc reservoirs in EGCs could participate in the transmission of misfolded prions from the gut to the brain.
Enteric glia may be both protagonists and victims of neurological disease. In multiple sclerosis (MS), an autoimmune disorder characterized by demyelinating lesions in the CNS (Levinthal et al., 2013), many patients experience GI symptoms. New evidence suggests that EGCs may be peripheral targets in MS. There is increased GFAP immunoreactivity in the myenteric ganglia of patients with MS, suggestive of gliosis (Wunsch et al., 2017). The experimental autoimmune encephalomyelitis (EAE) model of MS in which mice are immunized with myelin antigens, however, has shown conflicting results. One group reported increased GFAP in the small intestinal myenteric plexus, while another reported a decrease in the colon (Wunsch et al., 2017; Spear et al., 2018). Nevertheless, both groups found clear evidence of GI dysmotility in EAE mice. Circulating autoantibodies to enteric neurons and glia were identified in EAE mice, and a small subset of patients with MS was found to have antibodies to one of the identified antigens, apolipoprotein A-I (Wunsch et al., 2017). Consistent with these observations, antibodies from the serum of patients with MS colocalized with S100B and HuD in the guinea pig myenteric plexus (Spear et al., 2018), indicating that both enteric glia and neurons could be targets of the autoimmune process in MS.
Conclusions
The brain-gut axis is a two-way highway by which the CNS modulates many gut functions from motility to fluid secretion, and the gut, in turn, transmits a great deal of information about the host's environment, nutrient state, and gut microbial composition to the brain. The ENS and its major constituents, EGCs, are key components of this axis. It is now evident that while EGCs share features of glia in other parts of the nervous system, they are highly unique and without direct analogy. Presumably, at least one reason for this unique biology is because they are embedded in the distinct and complex microenvironment of the GI tract where they are in close contact with myriad cell types including blood vessel endothelial cells, lymphatic endothelial cells, immune effector cells, enteric neurons, extrinsic nerve fibers, and intestinal epithelial cells. This makes EGCs well positioned to receive, integrate, and transmit information between the nervous, immune, and endocrine systems to regulate GI homeostasis. Less advantageously, this may perpetuate miscommunication or maladaptive responses in the context of disease (Figure 3).
There have been major advances in the understanding of EGCs over the last several decades, but in almost every aspect of their biology, fundamental questions remain. A pressing question is to what degree there are functionally distinct subtypes of enteric glia. Recent single-cell genomics studies have added to the understanding of EGC diversity, and these will undoubtedly provide an important foundation for determining the functional significance of different EGC populations in both homeostasis and disease. The majority of studies to date have focused on glia in the myenteric plexus, and much more work is needed to understand the biology of submucosal, mucosal, and intramuscular EGCs. Similarly, glial biology in the upper GI tract, particularly the stomach and esophagus, is largely unexplored.
Studies in animal models have been and will continue to be essential for defining the fundamental biology of EGCs, especially because these cells are at the nexus of multiple systems that interact to determine host physiology. Nevertheless, to determine the relevance of these observations to human disease it is also necessary to understand which EGC attributes are conserved across species. New approaches such as human tissue explants into rodent hosts (Nagy et al., 2018), intestinal organoids that incorporate ENS cells (Schlieve et al., 2017), and transplantation of neural crest stem cells derived from human-induced pluripotent stem cells into animal models in vivo or human intestinal explants in vitro (Workman et al., 2017; Li et al., 2018a) are all promising ways to examine human EGC behavior in physiologically relevant environments.
Studies in animal models and human tissues have already revealed a role for EGCs in intestinal inflammation, colonic dysmotility, and colorectal cancer. The understanding of EGC roles in neurodegenerative disorders is still in its infancy, and potential roles in neurodevelopmental disorders are almost completely unexplored. Human tissue studies that are limited to analysis of glia in paraffin tissue sections or mucosal biopsies ultimately sample a vanishingly small number of cells, which may be one reason why they sometimes lead to conflicting findings. The advent of 3D approaches to visualizing glial networks throughout the gut wall will enable more effective sampling and detection of ENS pathology in human disorders. Conversely, dedicated ultrastructural analyses of human EGCs and the nature of their intercellular interactions will also reveal new insights on human EGC biology.
Regardless of their roles in the pathogenesis of various disorders, the ability of EGCs to secrete factors that influence neurons, immune cells, and the intestinal epithelium means that they could be highly valuable targets for treating human disease. The accumulating evidence that systemic inflammation has a role in CNS pathology means that enteric glia-based therapies could be considered not just for digestive disorders but also for neurological disorders such as PD. Further exploration of both the fundamental biology and human pathology of enteric glia will be vital for all of these efforts.
Acknowledgments
We thank A. Muppirala for helpful comments. M.R. is supported by funding from the National Institutes of Health (K08 DK110532, R03 DK125636) and the Richard and Susan Smith Family Foundation (Odyssey Award). Schematics in Figures were created using Biorender.com.
Author contributions
Both authors participated in conceptualization of the manuscript, writing the initial draft, and editing. M.R. acquired funding.
Declaration of interests
M.R. is a consultant for Boston Pharmaceuticals and 89bio. M.R.’s spouse is an employee of Takeda Pharmaceuticals.
References
- Abbott R.D., Petrovitch H., White L.R., Masaki K.H., Tanner C.M., Curb J.D., Grandinetti A., Blanchette P.L., Popper J.S., Ross G.W. Frequency of bowel movements and the future risk of Parkinson's disease. Neurology. 2001;57:456–462. doi: 10.1212/wnl.57.3.456. [DOI] [PubMed] [Google Scholar]
- Airaksinen M.S., Saarma M. The GDNF family: signalling, biological functions and therapeutic value. Nat. Rev. Neurosci. 2002;3:383–394. doi: 10.1038/nrn812. [DOI] [PubMed] [Google Scholar]
- Albanese V., Lawson V.A., Hill A.F., Cappai R., Di Guardo G., Staikopoulos V., Thacker M., Furness J.B., Chiocchetti R. Evidence for prion protein expression in enteroglial cells of the myenteric plexus of mouse intestine. Auton. Neurosci. 2008;140:17–23. doi: 10.1016/j.autneu.2008.01.008. [DOI] [PubMed] [Google Scholar]
- Amiel J., Sproat-Emison E., Garcia-Barcelo M., Lantieri F., Burzynski G., Borrego S., Pelet A., Arnold S., Miao X., Griseri P. Hirschsprung disease, associated syndromes and genetics: a review. J. Med. Genet. 2008;45:1–14. doi: 10.1136/jmg.2007.053959. [DOI] [PubMed] [Google Scholar]
- Angrist M., Bolk S., Halushka M., Lapchak P.A., Chakravarti A. Germline mutations in glial cell line-derived neurotrophic factor (GDNF) and RET in a Hirschsprung disease patient. Nat. Genet. 1996;14:341–344. doi: 10.1038/ng1196-341. [DOI] [PubMed] [Google Scholar]
- Anlauf M., Perren A., Meyer C.L., Schmid S., Saremaslani P., Kruse M.L., Weihe E., Komminoth P., Heitz P.U., Kloppel G. Precursor lesions in patients with multiple endocrine neoplasia type 1-associated duodenal gastrinomas. Gastroenterology. 2005;128:1187–1198. doi: 10.1053/j.gastro.2005.01.058. [DOI] [PubMed] [Google Scholar]
- Annerino D.M., Arshad S., Taylor G.M., Adler C.H., Beach T.G., Greene J.G. Parkinson's disease is not associated with gastrointestinal myenteric ganglion neuron loss. Acta Neuropathol. 2012;124:665–680. doi: 10.1007/s00401-012-1040-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aube A.C., Cabarrocas J., Bauer J., Philippe D., Aubert P., Doulay F., Liblau R., Galmiche J.P., Neunlist M. Changes in enteric neurone phenotype and intestinal functions in a transgenic mouse model of enteric glia disruption. Gut. 2006;55:630–637. doi: 10.1136/gut.2005.067595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azan G., Low W.C., Wendelschafer-Crabb G., Ikramuddin S., Kennedy W.R. Evidence for neural progenitor cells in the human adult enteric nervous system. Cell Tissue Res. 2011;344:217–225. doi: 10.1007/s00441-011-1130-9. [DOI] [PubMed] [Google Scholar]
- Balemba O.B., Grondahl M.L., Mbassa G.K., Semuguruka W.D., Hay-Smith A., Skadhauge E., Dantzer V. The organisation of the enteric nervous system in the submucous and mucous layers of the small intestine of the pig studied by VIP and neurofilament protein immunohistochemistry. J. Anat. 1998;192(Pt 2):257–267. doi: 10.1046/j.1469-7580.1998.19220257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar K.J., Facer P., Williams N.S., Tam P.K., Anand P. Glial-derived neurotrophic factor in human adult and fetal intestine and in Hirschsprung's disease. Gastroenterology. 1997;112:1381–1385. doi: 10.1016/s0016-5085(97)70154-9. [DOI] [PubMed] [Google Scholar]
- Bassotti G., Villanacci V., Cathomas G., Maurer C.A., Fisogni S., Cadei M., Baron L., Morelli A., Valloncini E., Salerni B. Enteric neuropathology of the terminal ileum in patients with intractable slow-transit constipation. Hum. Pathol. 2006;37:1252–1258. doi: 10.1016/j.humpath.2006.04.027. [DOI] [PubMed] [Google Scholar]
- Bassotti G., Villanacci V., Maurer C.A., Fisogni S., Di Fabio F., Cadei M., Morelli A., Panagiotis T., Cathomas G., Salerni B. The role of glial cells and apoptosis of enteric neurones in the neuropathology of intractable slow transit constipation. Gut. 2006;55:41–46. doi: 10.1136/gut.2005.073197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassotti G., Villanacci V., Antonelli E., Morelli A., Salerni B. Enteric glial cells: new players in gastrointestinal motility? Lab Invest. 2007;87:628–632. doi: 10.1038/labinvest.3700564. [DOI] [PubMed] [Google Scholar]
- Bassotti G., Villanacci V., Nascimbeni R., Cadei M., Fisogni S., Antonelli E., Corazzi N., Salerni B. Enteric neuroglial apoptosis in inflammatory bowel diseases. J. Crohns Colitis. 2009;3:264–270. doi: 10.1016/j.crohns.2009.06.004. [DOI] [PubMed] [Google Scholar]
- Beach T.G., Adler C.H., Sue L.I., Vedders L., Lue L., White Iii C.L., Akiyama H., Caviness J.N., Shill H.A. Multi-organ distribution of phosphorylated alpha-synuclein histopathology in subjects with Lewy body disorders. Acta Neuropathol. 2010;119:689–702. doi: 10.1007/s00401-010-0664-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkind-Gerson J., Graham H.K., Reynolds J., Hotta R., Nagy N., Cheng L., Kamionek M., Shi H.N., Aherne C.M., Goldstein A.M. Colitis promotes neuronal differentiation of Sox2+ and PLP1+ enteric cells. Sci. Rep. 2017;7:2525. doi: 10.1038/s41598-017-02890-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Berre-Scoul C., Chevalier J., Oleynikova E., Cossais F., Talon S., Neunlist M., Boudin H. A novel enteric neuron-glia coculture system reveals the role of glia in neuronal development. J. Physiol. 2017;595:583–598. doi: 10.1113/JP271989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betarbet R., Sherer T.B., Mackenzie G., Garcia-Osuna M., Panov A.V., Greenamyre J.T. Chronic systemic pesticide exposure reproduces features of Parkinson's disease. Nat. Neurosci. 2000;3:1301–1306. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- Black C.J., Ford A.C. Global burden of irritable bowel syndrome: trends, predictions and risk factors. Nat. Rev. Gastroenterol. Hepatol. 2020;17:473–486. doi: 10.1038/s41575-020-0286-8. [DOI] [PubMed] [Google Scholar]
- Boesmans W., Cirillo C., Van Den Abbeel V., Van Den Haute C., Depoortere I., Tack J., Vanden Berghe P. Neurotransmitters involved in fast excitatory neurotransmission directly activate enteric glial cells. Neurogastroenterol. Motil. 2013;25:e151–e160. doi: 10.1111/nmo.12065. [DOI] [PubMed] [Google Scholar]
- Boesmans W., Rocha N.P., Reis H.J., Holt M., Vanden Berghe P. The astrocyte marker Aldh1L1 does not reliably label enteric glial cells. Neurosci. Lett. 2014;566:102–105. doi: 10.1016/j.neulet.2014.02.042. [DOI] [PubMed] [Google Scholar]
- Boesmans W., Lasrado R., Vanden Berghe P., Pachnis V. Heterogeneity and phenotypic plasticity of glial cells in the mammalian enteric nervous system. Glia. 2015;63:229–241. doi: 10.1002/glia.22746. [DOI] [PubMed] [Google Scholar]
- Boesmans W., Hao M.M., Fung C., Li Z., Van Den Haute C., Tack J., Pachnis V., Vanden Berghe P. Structurally defined signaling in neuro-glia units in the enteric nervous system. Glia. 2019;67:1167–1178. doi: 10.1002/glia.23596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohorquez D.V., Samsa L.A., Roholt A., Medicetty S., Chandra R., Liddle R.A. An enteroendocrine cell-enteric glia connection revealed by 3D electron microscopy. PLoS One. 2014;9:e89881. doi: 10.1371/journal.pone.0089881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondurand N., Sham M.H. The role of SOX10 during enteric nervous system development. Dev. Biol. 2013;382:330–343. doi: 10.1016/j.ydbio.2013.04.024. [DOI] [PubMed] [Google Scholar]
- von Boyen G.B., Schulte N., Pfluger C., Spaniol U., Hartmann C., Steinkamp M. Distribution of enteric glia and GDNF during gut inflammation. BMC Gastroenterol. 2011;11:3. doi: 10.1186/1471-230X-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H., De Vos R.A., Bohl J., Del Tredici K. Gastric alpha-synuclein immunoreactive inclusions in Meissner's and Auerbach's plexuses in cases staged for Parkinson's disease-related brain pathology. Neurosci. Lett. 2006;396:67–72. doi: 10.1016/j.neulet.2005.11.012. [DOI] [PubMed] [Google Scholar]
- Broadhead M.J., Bayguinov P.O., Okamoto T., Heredia D.J., Smith T.K. Ca2+ transients in myenteric glial cells during the colonic migrating motor complex in the isolated murine large intestine. J. Physiol. 2012;590:335–350. doi: 10.1113/jphysiol.2011.219519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown I.A., Mcclain J.L., Watson R.E., Patel B.A., Gulbransen B.D. Enteric glia mediate neuron death in colitis through purinergic pathways that require connexin-43 and nitric oxide. Cell Mol. Gastroenterol. Hepatol. 2016;2:77–91. doi: 10.1016/j.jcmgh.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brun P., Gobbo S., Caputi V., Spagnol L., Schirato G., Pasqualin M., Levorato E., Palu G., Giron M.C., Castagliuolo I. Toll like receptor-2 regulates production of glial-derived neurotrophic factors in murine intestinal smooth muscle cells. Mol. Cell Neurosci. 2015;68:24–35. doi: 10.1016/j.mcn.2015.03.018. [DOI] [PubMed] [Google Scholar]
- Bush T.G., Savidge T.C., Freeman T.C., Cox H.J., Campbell E.A., Mucke L., Johnson M.H., Sofroniew M.V. Fulminant jejuno-ileitis following ablation of enteric glia in adult transgenic mice. Cell. 1998;93:189–201. doi: 10.1016/s0092-8674(00)81571-8. [DOI] [PubMed] [Google Scholar]
- Chadi G., Gomide V.C., Rodrigues De Souza R., Scabello R.T., Mauricio Da Silva C. Basic fibroblast growth factor, neurofilament, and glial fibrillary acidic protein immunoreactivities in the myenteric plexus of the rat esophagus and colon. J. Morphol. 2004;261:323–333. doi: 10.1002/jmor.10252. [DOI] [PubMed] [Google Scholar]
- Challis C., Hori A., Sampson T.R., Yoo B.B., Challis R.C., Hamilton A.M., Mazmanian S.K., Volpicelli-Daley L.A., Gradinaru V. Gut-seeded alpha-synuclein fibrils promote gut dysfunction and brain pathology specifically in aged mice. Nat. Neurosci. 2020;23:327–336. doi: 10.1038/s41593-020-0589-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charrier B., Pilon N. Toward a better understanding of enteric gliogenesis. Neurogenesis (Austin) 2017;4:e1293958. doi: 10.1080/23262133.2017.1293958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clairembault T., Kamphuis W., Leclair-Visonneau L., Rolli-Derkinderen M., Coron E., Neunlist M., Hol E.M., Derkinderen P. Enteric GFAP expression and phosphorylation in Parkinson's disease. J. Neurochem. 2014;130:805–815. doi: 10.1111/jnc.12742. [DOI] [PubMed] [Google Scholar]
- Cook R.D., Burnstock G. The ultrastructure of Auerbach's plexus in the Guinea-pig. II. Non-neuronal elements. J. Neurocytol. 1976;5:195–206. doi: 10.1007/BF01181656. [DOI] [PubMed] [Google Scholar]
- Cornet A., Savidge T.C., Cabarrocas J., Deng W.L., Colombel J.F., Lassmann H., Desreumaux P., Liblau R.S. Enterocolitis induced by autoimmune targeting of enteric glial cells: a possible mechanism in Crohn's disease? Proc. Natl. Acad. Sci. U S A. 2001;98:13306–13311. doi: 10.1073/pnas.231474098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corpening J.C., Cantrell V.A., Deal K.K., Southard-Smith E.M. A Histone2BCerulean BAC transgene identifies differential expression of Phox2b in migrating enteric neural crest derivatives and enteric glia. Dev. Dyn. 2008;237:1119–1132. doi: 10.1002/dvdy.21498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delvalle N.M., Dharshika C., Morales-Soto W., Fried D.E., Gaudette L., Gulbransen B.D. Communication between enteric neurons, glia, and nociceptors underlies the effects of Tachykinins on neuroinflammation. Cell Mol. Gastroenterol. Hepatol. 2018;6:321–344. doi: 10.1016/j.jcmgh.2018.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delvalle N.M., Fried D.E., Rivera-Lopez G., Gaudette L., Gulbransen B.D. Cholinergic activation of enteric glia is a physiological mechanism that contributes to the regulation of gastrointestinal motility. Am. J. Physiol. Gastrointest. Liver Physiol. 2018;315:G473–G483. doi: 10.1152/ajpgi.00155.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demehri F.R., Halaweish I.F., Coran A.G., Teitelbaum D.H. Hirschsprung-associated enterocolitis: pathogenesis, treatment and prevention. Pediatr. Surg. Int. 2013;29:873–881. doi: 10.1007/s00383-013-3353-1. [DOI] [PubMed] [Google Scholar]
- Devos D., Lebouvier T., Lardeux B., Biraud M., Rouaud T., Pouclet H., Coron E., Bruley Des Varannes S., Naveilhan P., Nguyen J.M. Colonic inflammation in Parkinson's disease. Neurobiol. Dis. 2013;50:42–48. doi: 10.1016/j.nbd.2012.09.007. [DOI] [PubMed] [Google Scholar]
- Dogiel A.S. Uber den Bau der Ganglien in den Geflechten des Darmes und der Gallenblase des Menschen und des Saugetiere. Zeit. Naturforsch. B. 1899;5:130–158. [Google Scholar]
- Dothel G., Barbaro M.R., Boudin H., Vasina V., Cremon C., Gargano L., Bellacosa L., De Giorgio R., Le Berre-Scoul C., Aubert P. Nerve fiber outgrowth is increased in the intestinal mucosa of patients with irritable bowel syndrome. Gastroenterology. 2015;148:1002–1011.e4. doi: 10.1053/j.gastro.2015.01.042. [DOI] [PubMed] [Google Scholar]
- Downes T.J., Cheruvu M.S., Karunaratne T.B., De Giorgio R., Farmer A.D. Pathophysiology, diagnosis, and management of chronic intestinal pseudo-obstruction. J. Clin. Gastroenterol. 2018;52:477–489. doi: 10.1097/MCG.0000000000001047. [DOI] [PubMed] [Google Scholar]
- Drokhlyansky E., Smillie C.S., Van Wittenberghe N., Ericsson M., Griffin G.K., Eraslan G., Dionne D., Cuoco M.S., Goder-Reiser M.N., Sharova T. The human and mouse enteric nervous system at single-cell resolution. Cell. 2020;182:1606–1622.e23. doi: 10.1016/j.cell.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorak A.M., Monahan R.A., Osage J.E., Dickersin G.R. Crohn's disease: transmission electron microscopic studies. II. Immunologic inflammatory response. Alterations of mast cells, basophils, eosinophils, and the microvasculature. Hum. Pathol. 1980;11:606–619. doi: 10.1016/s0046-8177(80)80072-4. [DOI] [PubMed] [Google Scholar]
- El-Nachef W.N., Bronner M.E. De novo enteric neurogenesis in post-embryonic zebrafish from Schwann cell precursors rather than resident cell types. Development. 2020;147 doi: 10.1242/dev.186619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito G., Capoccia E., Turco F., Palumbo I., Lu J., Steardo A., Cuomo R., Sarnelli G., Steardo L. Palmitoylethanolamide improves colon inflammation through an enteric glia/toll like receptor 4-dependent PPAR-alpha activation. Gut. 2014;63:1300–1312. doi: 10.1136/gutjnl-2013-305005. [DOI] [PubMed] [Google Scholar]
- Fawkner-Corbett D., Antanaviciute A., Parikh K., Jagielowicz M., Geros A.S., Gupta T., Ashley N., Khamis D., Fowler D., Morrissey E. Spatiotemporal analysis of human intestinal development at single-cell resolution. Cell. 2021;184:810–826.e23. doi: 10.1016/j.cell.2020.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferri G.L., Probert L., Cocchia D., Michetti F., Marangos P.J., Polak J.M. Evidence for the presence of S-100 protein in the glial component of the human enteric nervous system. Nature. 1982;297:409–410. doi: 10.1038/297409a0. [DOI] [PubMed] [Google Scholar]
- Flamant M., Aubert P., Rolli-Derkinderen M., Bourreille A., Neunlist M.R., Mahe M.M., Meurette G., Marteyn B., Savidge T., Galmiche J.P. Enteric glia protect against Shigella flexneri invasion in intestinal epithelial cells: a role for S-nitrosoglutathione. Gut. 2011;60:473–484. doi: 10.1136/gut.2010.229237. [DOI] [PubMed] [Google Scholar]
- Fried D.E., Watson R.E., Robson S.C., Gulbransen B.D. Ammonia modifies enteric neuromuscular transmission through glial gamma-aminobutyric acid signaling. Am. J. Physiol. Gastrointest. Liver Physiol. 2017;313:G570–G580. doi: 10.1152/ajpgi.00154.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness J.B. Blackwell Pub; 2006. The Enteric Nervous System. [Google Scholar]
- Furness J.B. The enteric nervous system and neurogastroenterology. Nat. Rev. Gastroenterol. Hepatol. 2012;9:286–294. doi: 10.1038/nrgastro.2012.32. [DOI] [PubMed] [Google Scholar]
- Furness J.B., Callaghan B.P., Rivera L.R., Cho H.J. The enteric nervous system and gastrointestinal innervation: integrated local and central control. Adv. Exp. Med. Biol. 2014;817:39–71. doi: 10.1007/978-1-4939-0897-4_3. [DOI] [PubMed] [Google Scholar]
- Gabella G. Glial cells in the myenteric plexus. Z. Naturforsch B. 1971;26:244–245. doi: 10.1515/znb-1971-0313. [DOI] [PubMed] [Google Scholar]
- Gabella G. Ultrastructure of the nerve plexuses of the mammalian intestine: the enteric glial cells. Neuroscience. 1981;6:425–436. doi: 10.1016/0306-4522(81)90135-4. [DOI] [PubMed] [Google Scholar]
- Gabella G., Trigg P. Size of neurons and glial cells in the enteric ganglia of mice, Guinea-pigs, rabbits and sheep. J. Neurocytol. 1984;13:49–71. doi: 10.1007/BF01148318. [DOI] [PubMed] [Google Scholar]
- Gallagher D.A., Lees A.J., Schrag A. What are the most important nonmotor symptoms in patients with Parkinson's disease and are we missing them? Mov. Disord. 2010;25:2493–2500. doi: 10.1002/mds.23394. [DOI] [PubMed] [Google Scholar]
- Gao H., He C., Fang X., Hou X., Feng X., Yang H., Zhao X., Ma T. Localization of aquaporin-1 water channel in glial cells of the human peripheral nervous system. Glia. 2006;53:783–787. doi: 10.1002/glia.20336. [DOI] [PubMed] [Google Scholar]
- Geboes K., Rutgeerts P., Ectors N., Mebis J., Penninckx F., Vantrappen G., Desmet V.J. Major histocompatibility class II expression on the small intestinal nervous system in Crohn's disease. Gastroenterology. 1992;103:439–447. doi: 10.1016/0016-5085(92)90832-j. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Martinez T., Perez-Pinera P., Diaz-Esnal B., Vega J.A. S-100 proteins in the human peripheral nervous system. Microsc. Res. Tech. 2003;60:633–638. doi: 10.1002/jemt.10304. [DOI] [PubMed] [Google Scholar]
- Graham K.D., Lopez S.H., Sengupta R., Shenoy A., Schneider S., Wright C.M., Feldman M., Furth E., Valdivieso F., Lemke A. Robust, 3-dimensional visualization of human colon enteric nervous system without tissue sectioning. Gastroenterology. 2020;158:2221–2235.e5. doi: 10.1053/j.gastro.2020.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubisic V., Gulbransen B.D. Enteric glial activity regulates secretomotor function in the mouse colon but does not acutely affect gut permeability. J. Physiol. 2017 doi: 10.1113/JP273492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubisic V., Perez-Medina A.L., Fried D.E., Sevigny J., Robson S.C., Galligan J.J., Gulbransen B.D. NTPDase1 and -2 are expressed by distinct cellular compartments in the mouse colon and differentially impact colonic physiology and function after DSS colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2019;317:G314–G332. doi: 10.1152/ajpgi.00104.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubisic V., Mcclain J.L., Fried D.E., Grants I., Rajasekhar P., Csizmadia E., Ajijola O.A., Watson R.E., Poole D.P., Robson S.C. Enteric glia modulate macrophage phenotype and visceral sensitivity following inflammation. Cell Rep. 2020;32:108100,. doi: 10.1016/j.celrep.2020.108100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundmann D., Loris E., Maas-Omlor S., Huang W., Scheller A., Kirchhoff F., Schafer K.H. Enteric glia: S100, GFAP, and beyond. Anat. Rec. (Hoboken) 2019;302:1333–1344. doi: 10.1002/ar.24128. [DOI] [PubMed] [Google Scholar]
- Gulbransen B.D., Sharkey K.A. Novel functional roles for enteric glia in the gastrointestinal tract. Nat. Rev. Gastroenterol. Hepatol. 2012;9:625–632. doi: 10.1038/nrgastro.2012.138. [DOI] [PubMed] [Google Scholar]
- Gulbransen B.D., Bashashati M., Hirota S.A., Gui X., Roberts J.A., Macdonald J.A., Muruve D.A., Mckay D.M., Beck P.L., Mawe G.M. Activation of neuronal P2X7 receptor-pannexin-1 mediates death of enteric neurons during colitis. Nat. Med. 2012;18:600–604. doi: 10.1038/nm.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han T.Y., Lourenssen S., Miller K.G., Blennerhassett M.G. Intestinal smooth muscle phenotype determines enteric neuronal survival via GDNF expression. Neuroscience. 2015;290:357–368. doi: 10.1016/j.neuroscience.2015.01.056. [DOI] [PubMed] [Google Scholar]
- Hanani M., Reichenbach A. Morphology of horseradish peroxidase (HRP)-injected glial cells in the myenteric plexus of the Guinea-pig. Cell Tissue Res. 1994;278:153–160. doi: 10.1007/BF00305787. [DOI] [PubMed] [Google Scholar]
- Harty B.L., Monk K.R. Unwrapping the unappreciated: recent progress in Remak Schwann cell biology. Curr. Opin. Neurobiol. 2017;47:131–137. doi: 10.1016/j.conb.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heintz-Buschart A., Pandey U., Wicke T., Sixel-Doring F., Janzen A., Sittig-Wiegand E., Trenkwalder C., Oertel W.H., Mollenhauer B., Wilmes P. The nasal and gut microbiome in Parkinson's disease and idiopathic rapid eye movement sleep behavior disorder. Mov Disord. 2018;33:88–98. doi: 10.1002/mds.27105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt M.A., Hoffmann C., Sherrill-Mix S.A., Keilbaugh S.A., Hamady M., Chen Y.Y., Knight R., Ahima R.S., Bushman F., Wu G.D. High-fat diet determines the composition of the murine gut microbiome independently of obesity. Gastroenterology. 2009;137:1716–1724.e1-2. doi: 10.1053/j.gastro.2009.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata I., Austin L.L., Blackwell W.H., Weber J.R., Dobbins W.O., 3rd Immunoelectron microscopic localization of HLA-DR antigen in control small intestine and colon and in inflammatory bowel disease. Dig. Dis. Sci. 1986;31:1317–1330. doi: 10.1007/BF01299810. [DOI] [PubMed] [Google Scholar]
- Hoff S., Zeller F., Von Weyhern C.W., Wegner M., Schemann M., Michel K., Ruhl A. Quantitative assessment of glial cells in the human and Guinea pig enteric nervous system with an anti-Sox8/9/10 antibody. J. Comp. Neurol. 2008;509:356–371. doi: 10.1002/cne.21769. [DOI] [PubMed] [Google Scholar]
- Holmqvist S., Chutna O., Bousset L., Aldrin-Kirk P., Li W., Bjorklund T., Wang Z.Y., Roybon L., Melki R., Li J.Y. Direct evidence of Parkinson pathology spread from the gastrointestinal tract to the brain in rats. Acta Neuropathol. 2014;128:805–820. doi: 10.1007/s00401-014-1343-6. [DOI] [PubMed] [Google Scholar]
- Iantorno G., Bassotti G., Kogan Z., Lumi C.M., Cabanne A.M., Fisogni S., Varrica L.M., Bilder C.R., Munoz J.P., Liserre B. The enteric nervous system in chagasic and idiopathic megacolon. Am. J. Surg. Pathol. 2007;31:460–468. doi: 10.1097/01.pas.0000213371.79300.a8. [DOI] [PubMed] [Google Scholar]
- Ibiza S., Garcia-Cassani B., Ribeiro H., Carvalho T., Almeida L., Marques R., Misic A.M., Bartow-Mckenney C., Larson D.M., Pavan W.J. Glial-cell-derived neuroregulators control type 3 innate lymphoid cells and gut defence. Nature. 2016;535:440–443. doi: 10.1038/nature18644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inlender T., Nissim-Eliraz E., Stavely R., Hotta R., Goldstein A.M., Yagel S., Gutnick M.J., Shpigel N.Y. Homeostasis of mucosal glial cells in human gut is independent of microbiota. Sci. Rep. 2021;11:12796. doi: 10.1038/s41598-021-92384-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen K.R., Mirsky R. Astrocyte-like glia in the peripheral nervous system: an immunohistochemical study of enteric glia. J. Neurosci. 1983;3:2206–2218. doi: 10.1523/JNEUROSCI.03-11-02206.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonscher R., Belkind-Gerson J. Concise review: cellular and molecular mechanisms of postnatal injury-induced enteric neurogenesis. Stem Cells. 2019;37:1136–1143. doi: 10.1002/stem.3045. [DOI] [PubMed] [Google Scholar]
- Joseph N.M., He S., Quintana E., Kim Y.G., Nunez G., Morrison S.J. Enteric glia are multipotent in culture but primarily form glia in the adult rodent gut. J. Clin. Invest. 2011;121:3398–3411. doi: 10.1172/jci58186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabouridis P.S., Lasrado R., Mccallum S., Chng S.H., Snippert H.J., Clevers H., Pettersson S., Pachnis V. Microbiota controls the homeostasis of glial cells in the gut lamina propria. Neuron. 2015;85:289–295. doi: 10.1016/j.neuron.2014.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y.N., Fung C., Vanden Berghe P. Gut innervation and enteric nervous system development: a spatial, temporal and molecular tour de force. Development. 2021;148 doi: 10.1242/dev.182543. [DOI] [PubMed] [Google Scholar]
- Kapitza C., Chunder R., Scheller A., Given K.S., Macklin W.B., Enders M., Kuerten S., Neuhuber W.L., Worl J. Murine esophagus expresses glial-derived central nervous system Antigens. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms22063233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Kwon S.H., Kam T.I., Panicker N., Karuppagounder S.S., Lee S., Lee J.H., Kim W.R., Kook M., Foss C.A. Transneuronal propagation of pathologic alpha-synuclein from the gut to the brain models Parkinson's disease. Neuron. 2019;103:627–641.e7. doi: 10.1016/j.neuron.2019.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinchen J., Chen H.H., Parikh K., Antanaviciute A., Jagielowicz M., Fawkner-Corbett D., Ashley N., Cubitt L., Mellado-Gomez E., Attar M. Structural remodeling of the human colonic mesenchyme in inflammatory bowel disease. Cell. 2018;175:372–386.e17. doi: 10.1016/j.cell.2018.08.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komuro T., Baluk P., Burnstock G. An ultrastructural study of neurons and non-neuronal cells in the myenteric plexus of the rabbit colon. Neuroscience. 1982;7:1797–1806. doi: 10.1016/0306-4522(82)90037-9. [DOI] [PubMed] [Google Scholar]
- Koretz K., Momburg F., Otto H.F., Moller P. Sequential induction of MHC antigens on autochthonous cells of ileum affected by Crohn's disease. Am. J. Pathol. 1987;129:493–502. [PMC free article] [PubMed] [Google Scholar]
- Kresl P., Rahimi J., Gelpi E., Aldecoa I., Ricken G., Danics K., Keller E., Kovacs G.G. Accumulation of prion protein in the vagus nerve in creutzfeldt-jakob disease. Ann. Neurol. 2019;85:782–787. doi: 10.1002/ana.25451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuil L.E., Chauhan R.K., Cheng W.W., Hofstra R.M.W., Alves M.M. Zebrafish: a model organism for studying enteric nervous system development and disease. Front. Cell Dev. Biol. 2020;8:629073. doi: 10.3389/fcell.2020.629073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni S., Micci M.A., Leser J., Shin C., Tang S.C., Fu Y.Y., Liu L., Li Q., Saha M., Li C. Adult enteric nervous system in health is maintained by a dynamic balance between neuronal apoptosis and neurogenesis. Proc. Natl. Acad. Sci. U S A. 2017;114:E3709–E3718. doi: 10.1073/pnas.1619406114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupsky W.J., Grimes M.M., Sweeting J., Bertsch R., Cote L.J. Parkinson's disease and megacolon: concentric hyaline inclusions (Lewy bodies) in enteric ganglion cells. Neurology. 1987;37:1253–1255. doi: 10.1212/wnl.37.7.1253. [DOI] [PubMed] [Google Scholar]
- Lake J.I., Heuckeroth R.O. Enteric nervous system development: migration, differentiation, and disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2013;305:G1–G24. doi: 10.1152/ajpgi.00452.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laranjeira C., Sandgren K., Kessaris N., Richardson W., Potocnik A., Vanden Berghe P., Pachnis V. Glial cells in the mouse enteric nervous system can undergo neurogenesis in response to injury. J. Clin. Invest. 2011;121:3412–3424. doi: 10.1172/jci58200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasrado R., Boesmans W., Kleinjung J., Pin C., Bell D., Bhaw L., Mccallum S., Zong H., Luo L., Clevers H. Lineage-dependent spatial and functional organization of the mammalian enteric nervous system. Science. 2017;356:722–726. doi: 10.1126/science.aam7511. [DOI] [PubMed] [Google Scholar]
- Levinthal D.J., Rahman A., Nusrat S., O'leary M., Heyman R., Bielefeldt K. Adding to the burden: gastrointestinal symptoms and syndromes in multiple sclerosis. Mult. Scler. Int. 2013;2013:319201. doi: 10.1155/2013/319201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Huang L., Zeng J., Lin W., Li K., Sun J., Huang W., Chen J., Wang G., Ke Q. Characterization and transplantation of enteric neural crest cells from human induced pluripotent stem cells. Mol. Psychiatry. 2018;23:499–508. doi: 10.1038/mp.2016.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Ge Y., Zhu W., Gong J., Cao L., Guo Z., Gu L., Li J. Increased enteric glial cells in proximal margin of resection is associated with postoperative recurrence of Crohn's disease. J. Gastroenterol. Hepatol. 2018;33:638–644. doi: 10.1111/jgh.13973. [DOI] [PubMed] [Google Scholar]
- Lilli N.L., Queneherve L., Haddara S., Brochard C., Aubert P., Rolli-Derkinderen M., Durand T., Naveilhan P., Hardouin J.B., De Giorgio R. Glioplasticity in irritable bowel syndrome. Neurogastroenterol Motil. 2018;30:e13232. doi: 10.1111/nmo.13232. [DOI] [PubMed] [Google Scholar]
- Linan-Rico A., Turco F., Ochoa-Cortes F., Harzman A., Needleman B.J., Arsenescu R., Abdel-Rasoul M., Fadda P., Grants I., Whitaker E. Molecular signaling and dysfunction of the human reactive enteric glial cell phenotype: implications for GI infection, IBD, POI, neurological, motility, and GI disorders. Inflamm. Bowel Dis. 2016;22:1812–1834. doi: 10.1097/MIB.0000000000000854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J.A., Ngan E.S. Hedgehog and Notch signaling in enteric nervous system development. Neurosignals. 2014;22:1–13. doi: 10.1159/000356305. [DOI] [PubMed] [Google Scholar]
- Liu Y.A., Chung Y.C., Pan S.T., Shen M.Y., Hou Y.C., Peng S.J., Pasricha P.J., Tang S.C. 3-D imaging, illustration, and quantitation of enteric glial network in transparent human colon mucosa. Neurogastroenterol. Motil. 2013;25:e324–e338. doi: 10.1111/nmo.12115. [DOI] [PubMed] [Google Scholar]
- Liu Z., Sun H., Liang M., Gao J., Meng L., Zheng X., Wei Y., Yanbo K. Enteric glial cells respond to a dietary change in the lamina propria in a MyD88-dependent manner. bioRxiv. 2020 doi: 10.1101/2020.11.23.394601. [DOI] [Google Scholar]
- Long X., Li M., Li L.X., Sun Y.Y., Zhang W.X., Zhao D.Y., Li Y.Q. Butyrate promotes visceral hypersensitivity in an IBS-like model via enteric glial cell-derived nerve growth factor. Neurogastroenterol. Motil. 2018;30:e13227. doi: 10.1111/nmo.13227. [DOI] [PubMed] [Google Scholar]
- Lopez S.H., Avetisyan M., Wright C.M., Mesbah K., Kelly R.G., Moon A.M., Heuckeroth R.O. Loss of Tbx3 in murine neural crest reduces enteric glia and causes cleft palate, but does not influence heart development or bowel transit. Dev. Biol. 2018;444(Suppl 1):S337–S351. doi: 10.1016/j.ydbio.2018.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubomski M., Davis R.L., Sue C.M. The gut microbiota: a novel therapeutic target in Parkinson's disease? Parkinsonism Relat. Disord. 2019;66:265–266. doi: 10.1016/j.parkreldis.2019.08.010. [DOI] [PubMed] [Google Scholar]
- Lucas T.A., Zhu L., Buckwalter M.S. Spleen glia are a transcriptionally unique glial subtype interposed between immune cells and sympathetic axons. Glia. 2021;69:1799–1815. doi: 10.1002/glia.23993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May-Zhang A.A., Tycksen E., Southard-Smith A.N., Deal K.K., Benthal J.T., Buehler D.P., Adam M., Simmons A.J., Monaghan J.R., Matlock B.K. Combinatorial transcriptional profiling of mouse and human enteric neurons identifies shared and disparate subtypes in Situ. Gastroenterol. 2021;160:755–770.e26. doi: 10.1053/j.gastro.2020.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcbride P.A., Beekes M. Pathological PrP is abundant in sympathetic and sensory ganglia of hamsters fed with scrapie. Neurosci. Lett. 1999;265:135–138. doi: 10.1016/s0304-3940(99)00223-2. [DOI] [PubMed] [Google Scholar]
- Mccallum S., Obata Y., Fourli E., Boeing S., Peddie C.J., Xu Q., Horswell S., Kelsh R.N., Collinson L., Wilkinson D. Enteric glia as a source of neural progenitors in adult zebrafish. Elife. 2020;9 doi: 10.7554/eLife.56086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcclain J.L., Grubisic V., Fried D., Gomez-Suarez R.A., Leinninger G.M., Sevigny J., Parpura V., Gulbransen B.D. Ca2+ responses in enteric glia are mediated by connexin-43 hemichannels and modulate colonic transit in mice. Gastroenterology. 2014;146:497–507.e1. doi: 10.1053/j.gastro.2013.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcclain J.L., Fried D.E., Gulbransen B.D. Agonist-evoked Ca2+ signaling in enteric glia drives neural programs that regulate intestinal motility in mice. Cell Mol. Gastroenterol. Hepatol. 2015;1:631–645. doi: 10.1016/j.jcmgh.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menezes M., Corbally M., Puri P. Long-term results of bowel function after treatment for Hirschsprung's disease: a 29-year review. Pediatr. Surg. Int. 2006;22:987–990. doi: 10.1007/s00383-006-1783-8. [DOI] [PubMed] [Google Scholar]
- Morarach K., Mikhailova A., Knoflach V., Memic F., Kumar R., Li W., Ernfors P., Marklund U. Diversification of molecularly defined myenteric neuron classes revealed by single-cell RNA sequencing. Nat. Neurosci. 2021;24:34–46. doi: 10.1038/s41593-020-00736-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira Lopes T.C., Mosser D.M., Goncalves R. Macrophage polarization in intestinal inflammation and gut homeostasis. Inflamm. Res. 2020;69:1163–1172. doi: 10.1007/s00011-020-01398-y. [DOI] [PubMed] [Google Scholar]
- Muller P.A., Matheis F., Mucida D. Gut macrophages: key players in intestinal immunity and tissue physiology. Curr. Opin. Immunol. 2020;62:54–61. doi: 10.1016/j.coi.2019.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy N., Marsiano N., Bruckner R.S., Scharl M., Gutnick M.J., Yagel S., Arciero E., Goldstein A.M., Shpigel N.Y. Xenotransplantation of human intestine into mouse abdomen or subcutaneous tissue: novel platforms for the study of the human enteric nervous system. Neurogastroenterol. Motil. 2018;30 doi: 10.1111/nmo.13212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascimento R.D., De Souza Lisboa A., Fujiwara R.T., De Freitas M.A., Adad S.J., Oliveira R.C., D'avila Reis D., Da Silveira A.B. Characterization of enteroglial cells and denervation process in chagasic patients with and without megaesophagus. Hum. Pathol. 2010;41:528–534. doi: 10.1016/j.humpath.2009.05.018. [DOI] [PubMed] [Google Scholar]
- Nasser Y., Fernandez E., Keenan C.M., Ho W., Oland L.D., Tibbles L.A., Schemann M., Macnaughton W.K., Ruhl A., Sharkey K.A. Role of enteric glia in intestinal physiology: effects of the gliotoxin fluorocitrate on motor and secretory function. Am. J. Physiol. Gastrointest. Liver Physiol. 2006;291:G912–G927. doi: 10.1152/ajpgi.00067.2006. [DOI] [PubMed] [Google Scholar]
- Neunlist M., Aubert P., Bonnaud S., Van Landeghem L., Coron E., Wedel T., Naveilhan P., Ruhl A., Lardeux B., Savidge T. Enteric glia inhibit intestinal epithelial cell proliferation partly through a TGF-beta1-dependent pathway. American journal of physiology. Gastrointest. Liver Physiol. 2007;292:G231–G241. doi: 10.1152/ajpgi.00276.2005. [DOI] [PubMed] [Google Scholar]
- Newson B., Ahlman H., Dahlstrom A., Das Gupta T.K., Nyhus L.M. Are there sensory neurons in the mucosa of the mammalian gut? Acta Physiol. Scand. 1979;105:521–523. doi: 10.1111/j.1748-1716.1979.tb00118.x. [DOI] [PubMed] [Google Scholar]
- Odenwald M.A., Turner J.R. The intestinal epithelial barrier: a therapeutic target? Nat. Rev. Gastroenterol. Hepatol. 2017;14:9–21. doi: 10.1038/nrgastro.2016.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesch B., Westaway D., Walchli M., Mckinley M.P., Kent S.B., Aebersold R., Barry R.A., Tempst P., Teplow D.B., Hood L.E. A cellular gene encodes scrapie PrP 27-30 protein. Cell. 1985;40:735–746. doi: 10.1016/0092-8674(85)90333-2. [DOI] [PubMed] [Google Scholar]
- Paratore C., Goerich D.E., Suter U., Wegner M., Sommer L. Survival and glial fate acquisition of neural crest cells are regulated by an interplay between the transcription factor Sox10 and extrinsic combinatorial signaling. Development. 2001;128:3949–3961. doi: 10.1242/dev.128.20.3949. [DOI] [PubMed] [Google Scholar]
- Peralta O.A., Eyestone W.H. Quantitative and qualitative analysis of cellular prion protein (PrP(C)) expression in bovine somatic tissues. Prion. 2009;3:161–170. doi: 10.4161/pri.3.3.9772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Pardo P., Dodiya H.B., Engen P.A., Forsyth C.B., Huschens A.M., Shaikh M., Voigt R.M., Naqib A., Green S.J., Kordower J.H. Role of TLR4 in the gut-brain axis in Parkinson's disease: a translational study from men to mice. Gut. 2019;68:829–843. doi: 10.1136/gutjnl-2018-316844. [DOI] [PubMed] [Google Scholar]
- Pfeiffer R.F. Gastrointestinal dysfunction in Parkinson's disease. Parkinsonism. Relat. Disord. 2011;17:10–15. doi: 10.1016/j.parkreldis.2010.08.003. [DOI] [PubMed] [Google Scholar]
- Pham T.D., Gershon M.D., Rothman T.P. Time of origin of neurons in the murine enteric nervous system: sequence in relation to phenotype. J. Comp. Neurol. 1991;314:789–798. doi: 10.1002/cne.903140411. [DOI] [PubMed] [Google Scholar]
- Pochard C., Coquenlorge S., Jaulin J., Cenac N., Vergnolle N., Meurette G., Freyssinet M., Neunlist M., Rolli-Derkinderen M. Defects in 15-HETE production and control of epithelial permeability by human enteric glial cells from patients with Crohn's disease. Gastroenterology. 2016;150:168–180. doi: 10.1053/j.gastro.2015.09.038. [DOI] [PubMed] [Google Scholar]
- Pochard C., Coquenlorge S., Freyssinet M., Naveilhan P., Bourreille A., Neunlist M., Rolli-Derkinderen M. The multiple faces of inflammatory enteric glial cells: is Crohn's disease a gliopathy? Am. J. Physiol. Gastrointest. Liver Physiol. 2018;315:G1–G11. doi: 10.1152/ajpgi.00016.2018. [DOI] [PubMed] [Google Scholar]
- Qualman S.J., Haupt H.M., Yang P., Hamilton S.R. Esophageal Lewy bodies associated with ganglion cell loss in achalasia. Similarity to Parkinson's disease. Gastroenterology. 1984;87:848–856. [PubMed] [Google Scholar]
- Raab M., Neuhuber W.L. Intraganglionic laminar endings and their relationships with neuronal and glial structures of myenteric ganglia in the esophagus of rat and mouse. Histochem. Cell Biol. 2004;122:445–459. doi: 10.1007/s00418-004-0703-z. [DOI] [PubMed] [Google Scholar]
- Rao M., Gershon M.D. The bowel and beyond: the enteric nervous system in neurological disorders. Nat. Rev. Gastroenterol. Hepatol. 2016;13:517–528. doi: 10.1038/nrgastro.2016.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao M., Gershon M.D. Enteric nervous system development: what could possibly go wrong? Nat. Rev. Neurosci. 2018;19:552–565. doi: 10.1038/s41583-018-0041-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao M., Nelms B.D., Dong L., Salinas-Rios V., Rutlin M., Gershon M.D., Corfas G. Enteric glia express proteolipid protein 1 and are a transcriptionally unique population of glia in the mammalian nervous system. Glia. 2015;63:2040–2057. doi: 10.1002/glia.22876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao M., Rastelli D., Dong L., Chiu S., Setlik W., Gershon M.D., Corfas G. Enteric glia regulate gastrointestinal motility but are not required for maintenance of the epithelium in mice. Gastroenterology. 2017;153:1068–1081.e7. doi: 10.1053/j.gastro.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie J. Pain from distension of the pelvic colon by inflating a balloon in the irritable colon syndrome. Gut. 1973;14:125–132. doi: 10.1136/gut.14.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman T.P., Tennyson V.M., Gershon M.D. Colonization of the bowel by the precursors of enteric glia: studies of normal and congenitally aganglionic mutant mice. J. Comp. Neurol. 1986;252:493–506. doi: 10.1002/cne.902520406. [DOI] [PubMed] [Google Scholar]
- Savidge T.C., Newman P., Pothoulakis C., Ruhl A., Neunlist M., Bourreille A., Hurst R., Sofroniew M.V. Enteric glia regulate intestinal barrier function and inflammation via release of S-nitrosoglutathione. Gastroenterology. 2007;132:1344–1358. doi: 10.1053/j.gastro.2007.01.051. [DOI] [PubMed] [Google Scholar]
- Savidge T.C., Sofroniew M.V., Neunlist M. Starring roles for astroglia in barrier pathologies of gut and brain. Lab. Invest. 2007;87:731–736. doi: 10.1038/labinvest.3700600. [DOI] [PubMed] [Google Scholar]
- Schlieve C.R., Fowler K.L., Thornton M., Huang S., Hajjali I., Hou X., Grubbs B., Spence J.R., Grikscheit T.C. Neural crest cell implantation restores enteric nervous system function and alters the gastrointestinal transcriptome in human tissue-engineered small intestine. Stem Cell Rep. 2017;9:883–896. doi: 10.1016/j.stemcr.2017.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider R., Leven P., Glowka T., Kuzmanov I., Lysson M., Schneiker B., Miesen A., Baqi Y., Spanier C., Grants I. A novel P2X2-dependent purinergic mechanism of enteric gliosis in intestinal inflammation. EMBO Mol. Med. 2021;13:e12724. doi: 10.15252/emmm.202012724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuchardt A., D'agati V., Larsson-Blomberg L., Costantini F., Pachnis V. Defects in the kidney and enteric nervous system of mice lacking the tyrosine kinase receptor Ret. Nature. 1994;367:380–383. doi: 10.1038/367380a0. [DOI] [PubMed] [Google Scholar]
- Selgrad M., De Giorgio R., Fini L., Cogliandro R.F., Williams S., Stanghellini V., Barbara G., Tonini M., Corinaldesi R., Genta R.M. JC virus infects the enteric glia of patients with chronic idiopathic intestinal pseudo-obstruction. Gut. 2009;58:25–32. doi: 10.1136/gut.2008.152512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey K.A. Emerging roles for enteric glia in gastrointestinal disorders. J. Clin. Invest. 2015;125:918–925. doi: 10.1172/JCI76303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey K.A., Beck P.L., Mckay D.M. Neuroimmunophysiology of the gut: advances and emerging concepts focusing on the epithelium. Nat. Rev. Gastroenterol. Hepatol. 2018;15:765–784. doi: 10.1038/s41575-018-0051-4. [DOI] [PubMed] [Google Scholar]
- Shmakov A.N., Mclennan N.F., Mcbride P., Farquhar C.F., Bode J., Rennison K.A., Ghosh S. Cellular prion protein is expressed in the human enteric nervous system. Nat. Med. 2000;6:840–841. doi: 10.1038/78558. [DOI] [PubMed] [Google Scholar]
- Sigurdson C.J., Spraker T.R., Miller M.W., Oesch B., Hoover E.A. PrP(CWD) in the myenteric plexus, vagosympathetic trunk and endocrine glands of deer with chronic wasting disease. J. Gen. Virol. 2001;82:2327–2334. doi: 10.1099/0022-1317-82-10-2327. [DOI] [PubMed] [Google Scholar]
- Da Silveira A.B., De Oliveira E.C., Neto S.G., Luquetti A.O., Fujiwara R.T., Oliveira R.C., Brehmer A. Enteroglial cells act as antigen-presenting cells in chagasic megacolon. Hum. Pathol. 2011;42:522–532. doi: 10.1016/j.humpath.2010.06.016. [DOI] [PubMed] [Google Scholar]
- Smillie C.S., Biton M., Ordovas-Montanes J., Sullivan K.M., Burgin G., Graham D.B., Herbst R.H., Rogel N., Slyper M., Waldman J. Intra- and inter-cellular rewiring of the human colon during ulcerative colitis. Cell. 2019;178:714–730.e22. doi: 10.1016/j.cell.2019.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soret R., Schneider S., Bernas G., Christophers B., Souchkova O., Charrier B., Righini-Grunder F., Aspirot A., Landry M., Kembel S.W. Glial cell derived neurotrophic factor induces enteric neurogenesis and improves colon structure and function in mouse models of Hirschsprung disease. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.07.018. [DOI] [PubMed] [Google Scholar]
- Spear E.T., Holt E.A., Joyce E.J., Haag M.M., Mawe S.M., Hennig G.W., Lavoie B., Applebee A.M., Teuscher C., Mawe G.M. Altered gastrointestinal motility involving autoantibodies in the experimental autoimmune encephalomyelitis model of multiple sclerosis. Neurogastroenterol. Motil. 2018;30:e13349. doi: 10.1111/nmo.13349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer N.J., Hu H. Enteric nervous system: sensory transduction, neural circuits and gastrointestinal motility. Nat. Rev. Gastroenterol. Hepatol. 2020;17:338–351. doi: 10.1038/s41575-020-0271-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoffels B., Hupa K.J., Snoek S.A., Van Bree S., Stein K., Schwandt T., Vilz T.O., Lysson M., Veer C.V., Kummer M.P. Postoperative ileus involves interleukin-1 receptor signaling in enteric glia. Gastroenterology. 2014;146:176–187.e1. doi: 10.1053/j.gastro.2013.09.030. [DOI] [PubMed] [Google Scholar]
- Sundaresan S., Meininger C.A., Kang A.J., Photenhauer A.L., Hayes M.M., Sahoo N., Grembecka J., Cierpicki T., Ding L., Giordano T.J. Gastrin induces nuclear export and proteasome degradation of menin in enteric glial cells. Gastroenterology. 2017;153:1555–1567.e15. doi: 10.1053/j.gastro.2017.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tani G., Tomuschat C., O'donnell A.M., Coyle D., Puri P. Increased population of immature enteric glial cells in the resected proximal ganglionic bowel of Hirschsprung's disease patients. J. Surg. Res. 2017;218:150–155. doi: 10.1016/j.jss.2017.05.062. [DOI] [PubMed] [Google Scholar]
- Tartea E.A., Florescu C., Donoiu I., Pirici D., Mihailovici A.R., Albu V.C., Balseanu T.A., Iancau M., Badea C.D., Vere C.C. Implications of inflammation and remodeling of the enteric glial cells in colorectal adenocarcinoma. Rom. J. Morphol. Embryol. 2017;58:473–480. [PubMed] [Google Scholar]
- Taylor M.K., Yeager K., Morrison S.J. Physiological Notch signaling promotes gliogenesis in the developing peripheral and central nervous systems. Development. 2007;134:2435–2447. doi: 10.1242/dev.005520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turco F., Sarnelli G., Cirillo C., Palumbo I., De Giorgi F., D'alessandro A., Cammarota M., Giuliano M., Cuomo R. Enteroglial-derived S100B protein integrates bacteria-induced Toll-like receptor signalling in human enteric glial cells. Gut. 2014;63:105–115. doi: 10.1136/gutjnl-2012-302090. [DOI] [PubMed] [Google Scholar]
- Uesaka T., Nagashimada M., Enomoto H. Neuronal differentiation in Schwann cell lineage underlies postnatal neurogenesis in the enteric nervous system. J. Neurosci. 2015;35:9879–9888. doi: 10.1523/JNEUROSCI.1239-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vadder F., Grasset E., Manneras Holm L., Karsenty G., Macpherson A.J., Olofsson L.E., Backhed F. Gut microbiota regulates maturation of the adult enteric nervous system via enteric serotonin networks. Proc. Natl. Acad. Sci. U S A. 2018;115:6458–6463. doi: 10.1073/pnas.1720017115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vales S., Bacola G., Biraud M., Touvron M., Bessard A., Geraldo F., Dougherty K.A., Lashani S., Bossard C., Flamant M. Tumor cells hijack enteric glia to activate colon cancer stem cells and stimulate tumorigenesis. EBioMedicine. 2019;49:172–188. doi: 10.1016/j.ebiom.2019.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veiga-Fernandes H., Coles M.C., Foster K.E., Patel A., Williams A., Natarajan D., Barlow A., Pachnis V., Kioussis D. Tyrosine kinase receptor RET is a key regulator of Peyer's patch organogenesis. Nature. 2007;446:547–551. doi: 10.1038/nature05597. [DOI] [PubMed] [Google Scholar]
- Vicentini F., Keenan C., Wallace L., Woods C., Cavin J.-B., Flockton A., Macklin W., Belkind-Gerson J., Hirota S., Sharkey K. 2020. Intestinal Microbiota Shapes Gut Physiology and Regulates Enteric Neurons and Glia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villanacci V., Bassotti G., Nascimbeni R., Antonelli E., Cadei M., Fisogni S., Salerni B., Geboes K. Enteric nervous system abnormalities in inflammatory bowel diseases. Neurogastroenterol. Motil. 2008;20:1009–1016. doi: 10.1111/j.1365-2982.2008.01146.x. [DOI] [PubMed] [Google Scholar]
- Wakabayashi K., Takahashi H., Takeda S., Ohama E., Ikuta F. Parkinson's disease: the presence of Lewy bodies in Auerbach's and Meissner's plexuses. Acta Neuropathol. 1988;76:217–221. doi: 10.1007/BF00687767. [DOI] [PubMed] [Google Scholar]
- Wallace A.S., Burns A.J. Development of the enteric nervous system, smooth muscle and interstitial cells of Cajal in the human gastrointestinal tract. Cell Tissue Res. 2005;319:367–382. doi: 10.1007/s00441-004-1023-2. [DOI] [PubMed] [Google Scholar]
- Wedel T., Roblick U., Gleiss J., Schiedeck T., Bruch H.P., Kuhnel W., Krammer H.J. Organization of the enteric nervous system in the human colon demonstrated by wholemount immunohistochemistry with special reference to the submucous plexus. Ann. Anat. 1999;181:327–337. doi: 10.1016/S0940-9602(99)80122-8. [DOI] [PubMed] [Google Scholar]
- Workman M.J., Mahe M.M., Trisno S., Poling H.M., Watson C.L., Sundaram N., Chang C.F., Schiesser J., Aubert P., Stanley E.G. Engineered human pluripotent-stem-cell-derived intestinal tissues with a functional enteric nervous system. Nat. Med. 2017;23:49–59. doi: 10.1038/nm.4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wunsch M., Jabari S., Voussen B., Enders M., Srinivasan S., Cossais F., Wedel T., Boettner M., Schwarz A., Weyer L. The enteric nervous system is a potential autoimmune target in multiple sclerosis. Acta Neuropathol. 2017;134:281–295. doi: 10.1007/s00401-017-1742-6. [DOI] [PubMed] [Google Scholar]
- Xu D., Wu X., Grabauskas G., Owyang C. Butyrate-induced colonic hypersensitivity is mediated by mitogen-activated protein kinase activation in rat dorsal root ganglia. Gut. 2013;62:1466–1474. doi: 10.1136/gutjnl-2012-302260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Qian Y., Xu S., Song Y., Xiao Q. Longitudinal analysis of fecal microbiome and pathologic processes in a rotenone induced mice model of Parkinson's disease. Front. Aging Neurosci. 2017;9:441. doi: 10.3389/fnagi.2017.00441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young H.M., Bergner A.J., Muller T. Acquisition of neuronal and glial markers by neural crest-derived cells in the mouse intestine. J. Comp. Neurol. 2003;456:1–11. doi: 10.1002/cne.10448. [DOI] [PubMed] [Google Scholar]
- Yuan R., Bhattacharya N., Kenkel J.A., Shen J., Dimaio M.A., Bagchi S., Prestwood T.R., Habtezion A., Engleman E.G. Enteric glia play a critical role in promoting the development of colorectal cancer. Front. Oncol. 2020;10:595892. doi: 10.3389/fonc.2020.595892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisel A., Hochgerner H., Lonnerberg P., Johnsson A., Memic F., Van Der Zwan J., Haring M., Braun E., Borm L.E., La Manno G. Molecular architecture of the mouse nervous system. Cell. 2018;174:999–1014.e22. doi: 10.1016/j.cell.2018.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q., Verne G.N. New insights into visceral hypersensitivity–clinical implications in IBS. Nat. Rev. Gastroenterol. Hepatol. 2011;8:349–355. doi: 10.1038/nrgastro.2011.83. [DOI] [PMC free article] [PubMed] [Google Scholar]