Abstract
Alzheimer’s disease (AD) is a progressive neurodegenerative disease and accounts for most cases of dementia. The prevalence of AD has increased in the current rapidly aging society and contributes to a heavy burden on families and society. Despite the profound impact of AD, current treatments are unable to achieve satisfactory therapeutic effects or stop the progression of the disease. Finding novel treatments for AD has become urgent. In this paper, we reviewed novel therapeutic approaches in five categories: anti-amyloid therapy, anti-tau therapy, anti-neuroinflammatory therapy, neuroprotective agents including N-methyl-D-aspartate (NMDA) receptor modulators, and brain stimulation. The trend of therapeutic development is shifting from a single pathological target to a more complex mechanism, such as the neuroinflammatory and neurodegenerative processes. While drug repositioning may accelerate pharmacological development, non-pharmacological interventions, especially repetitive transcranial magnetic stimulation (rTMS) and transcranial direct current stimulation (tDCS), also have the potential for clinical application. In the future, it is possible for physicians to choose appropriate interventions individually on the basis of precision medicine.
Keywords: Alzheimer’s disease, amyloid, tau, NMDA, neuroinflammation, neuroprotection, brain stimulation, rTMS, tDCS, precision medicine
1. Introduction
Alzheimer’s disease (AD) is a progressive neurodegenerative disease and the leading cause of dementia in the elderly [1]. Worldwide, around 50 million people have dementia, and 50–70% of cases are attributed to AD [2,3]. Both the prevalence and incidence of AD increase with age. Globally, the population aged 65 years or older is expected to increase from 9.3% in 2020 to around 16.0% in 2050 [4]. In the United States, the prevalence of AD is approximately 3% in people aged 65–74, 17% in people aged 75–84, and 32% in people aged 85 or older [5]. The incidence of AD doubles every 10 years in those aged older than 60 [6]. Currently, about 5.8 million American adults suffer from AD, and the number is predicted to reach nearly 14 million by 2050 [7].
AD causes functional disability in the elderly. Typical characteristics of AD are progressive memory loss and functional impairment. AD not only impacts the individual but also their families and society. In 2016, the Global Burden of Disease classification system listed AD as the fourth highest disease for premature death and the sixth most burdensome disease [8]. Patients with AD develop behavioral and psychological symptoms of dementia (BPSD), including delusions, misperceptions, mood disorders, and behavioral disturbances [9]. The presentation of BPSD increases the burden on caregivers [10]. Patients with AD or other dementia types require about 170 h of informal care per month, which is a twofold increase compared to those without dementia [11]. The heavy care burden of AD leads to physical, psychological, and financial impacts on both families and society.
Despite the profound and chronic effects of AD, current treatments are unable to achieve satisfactory therapeutic effects or stop disease progression. Today, only five drugs have been approved by the FDA for AD treatment: donepezil, rivastigmine, galantamine, tacrine, and memantine. The first four drugs are acetylcholinesterase inhibitors (AChEIs), while the last one is an N-methyl-D-aspartate receptor (NMDAR) antagonist [12]. American and European guidelines list AChEIs as first-line pharmacotherapies for mild to moderate AD. However, AChEIs only show modest efficacy on cognitive deficits and non-significant efficacy on functional capacity in mild to moderate AD [13]. Memantine shows very limited efficacy on cognitive symptoms without functional improvement [14]. Finding novel treatments for AD has become urgent.
Understanding AD pathogenesis may guide the development of novel treatments. Traditionally, the pathological hallmarks of AD include two misfolded proteins: β-amyloid (Aβ) and tau. Aβ deposition links to tau accumulation [15]. Tau accumulation is associated with glucose hypometabolism, brain atrophy, and neurodegeneration [16,17]. Some biological mechanisms also drive protein aggregation, including carriage of the apolipoprotein E type 4 allele (APOE4), neuroinflammation, sleep disturbance, and autophagy dysfunction [18,19].
This article aims to review novel therapeutic approaches to AD, including pharmacological interventions (Table 1) and non-pharmacological interventions (Table 2).
Table 1.
Summary of pharmacological interventions against AD.
| Class of Drugs | Compounds | Mechanism | Subjects | Status | Summary | [Ref] |
|---|---|---|---|---|---|---|
| 1. Anti-amyloid therapy | ||||||
| Secretase inhibitor | Verubecestat | BACE1 inhibitor | Prodromal to moderate AD | Phase II/III | Lack of efficacy | [20,21] |
| Atabecestat | BACE1 inhibitor | Prodromal AD | Phase II/III | Cognitive worsening, psychiatric disorder | [22] | |
| Lanabecestat | BACE1 inhibitor | MCI to mild AD | Phase III | Cognitive worsening, weight loss, psychiatric disorder | [23] | |
| LY3202626 | BACE1 inhibitor | Mild AD | Phase III | Lack of efficacy | [24] | |
| Umibecestat | BACE1 inhibitor | Cognitively healthy APOE4 carriers | Phase II/III | Completed, failed analysis due to small number of events | [25] | |
| Elenbecestat | BACE1 inhibitor | MCI to moderate AD | Phase III | Lack of efficacy, nightmare | [26,27] | |
| Semagacestat | γ-secretase inhibitor | Mild to moderate AD | Phase III | Lack of efficacy, skin cancer, weight loss, hematologic disorder, infection | [28] | |
| Avagacestat | γ-secretase inhibitor | MCI | Phase II | Lack of efficacy, non-melanoma cancer, gastrointestinal symptoms | [29] | |
| Tarenflurbil | γ-secretase modulator | Mild AD | Phase II | Lack of efficacy, anemia, infection | [30] | |
| Aβ aggregation inhibitor |
PBT1 | MPAC | MCI to moderate AD | Phase II | Rescue of cognitive decline in severely affected patients (ADAS-cog ≥25), visual impairment | [31] |
| PBT2 | MPAC | Mild to moderate AD | Phase II | Lack of efficacy, large individual variance | [32,33] | |
| Aβ immunotherapy | ACI-24 | Aβ vaccine | Adults with Down syndrome | Phase II | Lack of immunogenicity | [34] |
| CAD106 | Aβ vaccine | Mild AD | Phase II | Lack of efficacy | [34] | |
| UB-311 | Aβ vaccine | Mild AD | Phase II | No published data | [34] | |
| ABVac40 | Aβ vaccine | MCI to mild AD | Phase II | Ongoing | [34] | |
| BAN2401 | Monoclonal antibody | MCI to mild AD | Phase III | Modest efficacy among APOE4 carriers | [35] | |
| Gantenerumab | Monoclonal antibody | Prodromal to mild AD | Phase III | Lack of efficacy | [36] | |
| Aducanumab | Monoclonal antibody | Monoclonal antibody | Phase III | Termination, little change in efficacy FDA approval for now |
[37,38] | |
| 2. Anti-tau therapy | ||||||
| Phosphatase modifier | Selenate | PP2A activator | Mild to moderate AD | Phase II | Lack of efficacy | [39,40] |
| Kinase inhibitor | Roscovitine | CDK5 inhibitor | 5XFAD mice | In vivo | Prevention of tau phosphorylation | [41,42] |
| Flavopiridol | CDK5 inhibitor | CD1 mice | In vivo | Rescue of cognitive decline | [41,42] | |
| Tideglusib | GSK3β inhibitor | Mild to moderate AD | Phase II | Lack of efficacy, transaminase increase | [43] | |
| Lithium | GSK3β inhibitor | MCI | Phase II | Rescue of cognitive decline | [44,45,46] | |
| Tau aggregation inhibitor | MB | Disrupts polymerization | Mild to moderate AD | Phase II | Cognitive improvement | [47] |
| LMTX | Disrupts polymerization | Mild to moderate AD | Phase III | Lack of efficacy | [48] | |
| Curcumin | Decreases β-sheet formation in tau | Cognitively healthy elderly | Phase II | Improvement in working memory (short-term course) |
[49,50] | |
| Microtubule stabilizer | EpoD | Enhances microtubule bundling | Mild AD | Phase I | Discontinuation, frequent adverse effects without published data | [51] |
| NAP | Protects microtubules from katanin disruption | MCI | Phase II | Cognitive and functional improvement | [52,53] | |
| TPI-287 | Stabilizes microtubules | Mild to moderate AD | Phase I | Rescue of cognitive decline, anaphylactoid reactions | [54] | |
| Tau immunotherapy | AADvac1 | Tau vaccine | Mild AD | Phase II | Completed, no published data | [55] |
| ACI-35 | Tau vaccine | Mild to moderate AD | Phase I | Safe and tolerated | [56] | |
| Aβ 3–10-KLH | Tau vaccine | 3×Tg-AD mice | In vivo | Cognitive improvement | [57] | |
| BIIB092 | Monoclonal antibody | Early AD | Phase II | Ongoing | [58] | |
| ABBV-8E12 | Monoclonal antibody | Early AD | Phase II | Ongoing | [59,60] | |
| RO7105705 | Monoclonal antibody | Prodromal to moderate AD | Phase II | Ongoing | [61,62] | |
| BIIB076 | Monoclonal antibody | Healthy volunteers, MCI | Phase I | Safe and tolerated | [63] | |
| LY3303560 | Monoclonal antibody | Early AD | Phase II | Completed, no available data | [64] | |
| JNJ-63733657 | Monoclonal antibody | Early AD | Phase II | Ongoing | [65] | |
| UCB0107 | Monoclonal antibody | Healthy volunteers | Phase I | Ongoing | [66,67] | |
| 3. Anti-neuroinflammatory therapy | ||||||
| Microglia modulator | Thymoquinone | TLR4 inhibitor | AD mice induced by AlCl3 | In vivo | Rescue of cognitive impairment | [68] |
| Ethyl pyruvate | TLR4 inhibitor | AD mice induced by AlCl3 | In vivo | Rescue of cognitive impairment | [68] | |
| TAK-242 | TLR4 inhibitor | APP/PS1 mice | In vivo | Cognitive improvement | [68] | |
| GW2580 | CSF1R inhibitor | APP/PS1 mice | In vivo | Recovery of short-term memory and behavioral deficit | [69] | |
| JN-J527 | CSF1R inhibitor | P301S mice | In vivo | Functional improvement | [70] | |
| PLX3397 | CSF1R inhibitor | 5XFAD mice | In vivo | Recovery of spatial and emotional memory deficit | [71] | |
| Astrocyte modulator | Stattic | STAT3 inhibitor | 5XFAD mice | In vivo | Rescue of learning and memory impairment | [72,73] |
| FK506 | Calcineurin/NFAT inhibitor | MCI to AD | Phase II | Not yet recruiting | [74] | |
| SB202190 | P38 MAPK inhibitor | Wip1-deficient mice | In vivo | Rescue of learning and memory impairment | [75] | |
| PD169316 | P38 MAPK inhibitor | Aβ-injected mice | In vivo | Rescue of spatial memory and learning impairment | [75] | |
| MW108 | P38 MAPK inhibitor | hTau mice | In vivo | Rescue of cognitive impairment | [76] | |
| NJK14047 | P38 MAPK inhibitor | 5XFAD mice | In vivo | Cognitive improvement | [77] | |
| MRS2179 | P2Y1R inhibitor | APPPS1 mice | In vivo | Spatial learning improvement | [78] | |
| BPTU | P2Y1R inhibitor | APPPS1 mice | In vivo | Spatial learning improvement | [78] | |
| Insulin resistance management |
Intranasal insulin therapy | Intranasal supplement | MCI to moderate AD | Phase II | Cognitive improvement, modulation by APOE4 genotype | [79,80] |
| MCI to AD | Phase II/III | Lack of efficacy | [81] | |||
| Liraglutide | Incretin receptor agonist | Mild AD | Phase II | Delay of cognitive impairment | [82,83] | |
| Metformin | Biguanide | MCI | Phase II | Reduction in recall memory decline | [84] | |
| MCI to early AD | Phase II | Executive functional improvement | [85] | |||
| Gemfibrozil | PPAR-α agonist | MCI | Phase I | Completed, no published data | [86] | |
| Pioglitazone | PPAR-γ agonist | Mild AD | Phase II | Cognitive improvement | [87] | |
| MCI | Phase III | Lack of efficacy | [88,89] | |||
| T3D-959 | Hybrid PPAR-δ/γ agonist | STZ-induced AD mice | In vivo | Reduction in neuroinflammation | [90] | |
| Microbiome therapy | Sodium oligomannate | Dysbiosis of gut microbiota | Mild to moderate AD | Phase III | Cognitive improvement | [91,92] |
| 4. Neuroprotective agents | ||||||
| Antiepileptic drug | Levetiracetam | SV2A receptor | MCI | Phase III | Ongoing | [93] |
| Gabapentin | VGCCs inhibitor | Moderate to severe AD | Phase IV | Ongoing | [94] | |
| NMDAR modification | Sodium benzoate | DAAO inhibitor | MCI to mild AD | Phase II | Cognitive improvement | [95] |
| MCI | Phase II | Cognitive and functional improvement | [96] | |||
| Moderate to severe AD with BPSD | Phase II | Cognitive benefit in female gender | [97] | |||
| Riluzole | Glutamate modulator | Mild AD | Phase II | Completed, no published data | [98] | |
| Troriruzole | Glutamate modulator | Mild to moderate AD | Phase II | Ongoing | [99] | |
| Omega 3 polyunsaturated fatty acid supplements | DHA | Anti-oxidative effect | Mild to moderate AD | Phase III | Lack of efficacy | [100] |
| Cognitively healthy elderly | Phase II | Ongoing | [101] | |||
| Icosapent ethyl | Anti-oxidative effect | Cognitively healthy elderly | Phase III | Ongoing | [102] | |
BACE1—β-secretase1, APOE4—apolipoprotein E type 4, PBT1—clioquinol, PBT2—second-generation clioquinol, MPAC—metal protein attenuating compound, ADAS-cog—Alzheimer’s Disease Assessment Scale–Cognitive Subscale, MB—methylene blue, EpoD—Epothilone D, NAP—davunetide, TPI-287—abeotaxane, DHA—docosahexaenoic acid.
Table 2.
Summary of non-pharmacological interventions against AD.
| Methods | Targeted Region | Protocol | Subjects | Status | Summary | [Ref] |
|---|---|---|---|---|---|---|
| 1. Deep-brain stimulation | ||||||
| DBS | Fornix | Forneceal DBS | Mild AD | Phase II | Slight cognitive benefit in the elderly | [103,104] |
| Mild AD | Phase III | Ongoing | [105] | |||
| NBM | NBM-DBS | Mild to moderate AD | Phase I | Cognitive stabilization and improvement, response rate 67% | [106] | |
| 2. Vagus nerve stimulation | ||||||
| VNS | Tenth cranial nerve | Invasive VNS | Probable AD | Phase I | Cognitive stabilization and improvement, response rate 70% | [107,108] |
| Tenth cranial nerve | Non-invasive VNS | MCI | Not Applicable | Ongoing | [109] | |
| 3. Transcranial magnetic stimulation | ||||||
| High-frequency rTMS | Left DLPFC | 10 Hz/120% MT/3000 pulses per session/10 sessions/2 weeks * | MCI | Phase IV | Executive functional improvement | [110] |
| 10 Hz/120% MT/2000 pulses per session/20 sessions/4 weeks * | MCI | Not Applicable | Ongoing | [111] | ||
| 20 Hz/100% MT/2000 pulses per session/20 sessions/4 weeks * | Moderate AD | Not Applicable | Improved language performance | [112] | ||
| 20 Hz/80% MT/1200 pulses per session/20 sessions/4 weeks * | AD patients with BPSD | Not Applicable | Cognitive and functional improvement | [113] | ||
| 20 Hz/100% MT/2000 pulses per session/20 sessions/4 weeks * | Mild to moderate AD | Not Applicable | Improvement in trained associative memory, add-on effect | [114] | ||
| 20 Hz/80–100% MT/1000 pulses per session/20 sessions/4 weeks * | Mild to moderate AD | Not Applicable | Cognitive and functional improvement, add-on effect | [115] | ||
| 5 Hz/100% MT/1500 pulses per session/15 sessions/3 weeks * | Probable AD | Not Applicable | Cognitive and functional improvement | [116] | ||
| Bilateral DLPFCs | 20 Hz/90% MT/2000 pulses per session/5 sessions/5 days * | Mild to severe AD | Not Applicable | Cognitive and functional improvement in mild to moderate AD | [117] | |
| 20 Hz/90–100% MT/2000 pulses per session/13 sessions/4 weeks * | Mild to moderate AD | Not Applicable | Cognitive improvement | [118] | ||
| Right IFG | 10 Hz/90% MT/2250 pulses per session/single session * | MCI | Not Applicable | Improvement in attention and psychomotor speed | [119] | |
| 10 Hz/90% MT/2250 pulses per session/single session * | MCI to moderate AD | Not Applicable | Cognitive improvement | [120] | ||
| Right STG | 10 Hz/90% MT/2250 pulses per session/single session * | MCI to moderate AD | Not Applicable | Cognitive improvement | [120] | |
| Left parietal lobe | 20 Hz/100% MT/1600 pulses per session/10 sessions/1 week * | Early AD | Not Applicable | Improvement in episodic memory | [121] | |
| Bilateral parietal lobes | 20 Hz/Unavailable MT/1 h per session/30 sessions/6 weeks * | Mild to moderate AD | Not Applicable | Better performance in memory and language in mild AD | [122] | |
| Low-frequency rTMS | Bilateral DLPFCs | 1 Hz/90% MT/600 pulses per session/2 sessions/1 day * | Healthy individuals-MCI | Not Applicable | Improvement in recognition memory | [123] |
| 1 Hz/100% MT/2000 pulses per session/5 sessions/5 days * | Mild to severe AD | Not Applicable | Less cognitive efficacy than high-frequency rTMS | [117] | ||
| 4. Transcranial electrical stimulation | ||||||
| Transcranial direct current stimulation | Left DLPFC | 2 mA/30 min per session/single session ** | Mild to moderate AD | Not Applicable | Improved recognition memory | [124] |
| 2 mA/25 min per session/10 sessions/2 weeks ** | Mild to moderate AD | Not Applicable | Cognitive improvement | [125] | ||
| 2 mA/20 min per session/6 sessions/2 weeks ** | Moderate AD | Phase II | No cognitive or behavioral improvement, no change in apathy symptoms Requirement of more than 6 sessions |
[126] | ||
| 2 mA/30 min per session/daily session/6 months ** | Early AD | Not Applicable | Cognitive and functional improvement, rescue of executive function | [127] | ||
| 1.5 mA/15 min per session/single session ** | MCI | Not Applicable | Enhanced free recall and recognition of memory | [128] | ||
| 2 mA/30 min per session/1-5 sessions ** | MCI | Not Applicable | Improvement in selective attention, processing speed, and planning ability tasks Optimal frequency of 3 sessions/week |
[129] | ||
| Left parietal lobe | 2 mA/30 min per session/single session ** | Mild to moderate AD | Not Applicable | Improvement in recognition memory | [124] | |
| 2 mA/30 min per session/6 sessions/10 days ** | Mild to moderate AD | Not Applicable | No improved verbal memory function | [130] | ||
| Bilateral temporoparietal lobe | 1.5 mA/15 min per minute/2 sessions (anodal and cathodal) ** | Mild AD | Not Applicable | Improved word recognition in anodal group, cognitive worsening in cathodal group | [131] | |
| 2 mA/30 min per session/5 sessions/1 week ** | Mild to moderate AD | Not Applicable | Improvement in visual recognition memory | [132] | ||
| 2 mA/20 min per session/10 sessions/2 weeks ** | Early AD | Not Applicable | Improved cognitive performance | [133] | ||
| Left temporoparietal lobe | 2 mA/20 min per session/10 sessions/2 weeks ** | Advanced AD | Not Applicable | Stabilized neuropsychological performance, long-term protective effect | [134] | |
| Transcranial alternating current stimulation | Left DLPFC | 40 Hz/1.5 mA/30 min per session/40 sessions/4 weeks *** | MCI to moderate AD | Not Applicable | Improved cognitive performance | [135] |
| Superior parietal cortex | 40 Hz/3 mA/30 min per session/single session *** | AD patients | Not Applicable | Completed, no published data | [136] | |
| Left angular gyrus | 40 Hz/unavailable intensity/20 min per session/3 sessions *** | Healthy individuals to mild AD | Not Applicable | Ongoing | [137] | |
NBM—nucleus basalis of Meynert, DLPFC—dorsolateral prefrontal cortex, MT—motor threshold. * Protocol of rTMS: Frequency/Intensity/Number of pulses per session/Total number of sessions/Duration. ** Protocol of tDCS: Current intensity/Stimulation duration/Total number of sessions/Duration. *** Protocol of tACS: Frequency/Intensity/Stimulation duration/Total number of sessions/Duration.
2. Novel Therapeutic Approach
2.1. Anti-Amyloid Therapy
Amyloid plaques are composed of Aβ peptides in the extracellular space. Aβ is derived from the amyloid precursor protein (APP), a transmembrane protein. β-secretase and γ-secretase cleave the APP and generate pathological Aβ [138]. Accumulation of Aβ results in neurotoxicity [139,140]. Reducing the accumulation of Aβ has become a therapeutic purpose of AD [141]. Anti-amyloid therapy consists of three strategies: secretase inhibitors, Aβ aggregation inhibitors, and Aβ immunotherapy.
2.1.1. Secretase Inhibitors
Secretase inhibitors target the catalytic activities of β-secretase and γ-secretase, which is the rate-limiting step in Aβ production. These strategies have been studied for the past two decades. Inhibitors of β-secretase (BACE)1 decreased the Aβ levels in AD patients’ cerebrospinal fluid (CSF) [20,142]. Several BACE1 inhibitors have reached phase III clinical trials, such as verubecestat [21], atabecestat [22], lanabecestat [23], LY3202626 [24], and umibecestat [25], but these drugs have failed due to a lack of efficacy or worse cognitive function in patients with mild cognitive impairment (MCI), and mild to moderate AD [143]. The last BACE inhibitor, elenbecestat, was discontinued in phase III trials because it showed an unfavorable risk/benefit ratio in early AD [26,27]. The inhibition and modulation of γ-secretase were both therapeutic straggles. Double-blind randomized controlled trials (RCTs) of the γ-secretase inhibitors, including semagacestat [28] and avagacestat [29], were discontinued. Cognitive deterioration was noted in patients with MCI and mild to moderate AD. The γ-secretase modulator tarenflurbil also worsened cognition in patients with mild AD [30]. Therefore, the role of secretase inhibitors remains under debate [144,145].
2.1.2. Aβ aggregation Inhibitors
Several natural compounds have Aβ aggregation inhibitory properties [146]. However, multiple obstacles blocked these compounds from entering into clinical use. First, some compounds have poor permeability through the blood–brain barrier (BBB). Second, these compounds are small molecules and produce insufficient steric effects to disrupt Aβ aggregation. Third, the protein–protein binding regions are relatively featureless to small molecules without specific pockets or grooves [147,148].
One strategy is to target the chaperones in the brain, such as metals. Disrupting the interaction between Aβ peptides and metals provides a barrier against Aβ oligomerization. Metal protein attenuating compounds (MPACs) chelate copper and zinc ions and inhibit Aβ aggregation [149,150,151]. One such example is clioquinol (PBT1), a hydroxyquinoline ionophore. In patients with MCI to moderate AD, clioquinol showed no significant improvement in cognition or clinical global impression between the active treatment and placebo groups. Subgroup analysis showed that clioquinol treatment rescued cognitive decline in the more severely affected patients (The Alzheimer’s Disease Assessment Scale–Cognitive Subscale (ADAS-cog) ≥ 25). The adverse effect of visual impairment was reported in the treatment group [31]. Second-generation clioquinol (PBT2) had appeared to be safe and well tolerated in people with mild AD. However, the double-blind RCTs of PBT2 demonstrated no overall significant effect on cognition or function in treating MCI and mild to moderate AD [32,33].
Recently, advanced biophysical and structural biology experimental approaches have been used to investigate chemical features and identify potential compounds. Some compounds with binding epitopes or planar hydrophobic structures increase Aβ aggregation inhibitory activities, including tanshinone and uncarinic acid C. A few compounds, namely, epigallocatechin gallate (EGCG) and resveratrol, interact with the toxicity determinants of Aβ, the N-terminus and β1-turn regions. Epigallocatechin gallate (EGCG), oleuropein aglicone (OleA), and quercetin are potential therapeutic compounds for AD [147].
2.1.3. Aβ Immunotherapy
Aβ immunotherapy actively or passively decreases the Aβ burden. The active anti-Aβ vaccine AN1792 was first tested in humans, but the trial was discontinued because meningoencephalitis was developed in 6% of immunized AD patients [152]. Furthermore, although four second-generation active Aβ vaccines reached phase II trials: ACI-24, CAD106, UB-311, and ABVac40, none of them were proven to be clinically beneficial in treating AD at that time [34]. Passive immunotherapy promotes Aβ clearance by targeting neurotoxic Aβ oligomers [153]. Three humanized monoclonal antibodies underwent phase III trials, including BAN2401, gantenerumab, and aducanumab. Studies involving these agents were confirmed to engage amyloid oligomers and decrease downstream tau levels. BAN2401 has demonstrated modest cognitive and functional efficacy in APOE4 carriers with MCI to mild AD [35]. Gantenerumab has shown no clinical efficacy thus far in phase III trials in prodromal to mild AD [36]. Two double-blind phase III RCTs of aducanumab were terminated halfway through in March 2019. The futility determination was based on the low chance of therapeutic efficacy for AD. However, the company gathered additional data and announced that the final result of aducanumab showed positive treatment effects [37,38]. In June 2021, the U.S. Food and Drug Administration (FDA) approved aducanumab to treat AD patients [154]. The argument about this approval seems to be continued. Next-generation oral-form small-molecule agents targeting Aβ oligomers, such as CT1812, PQ912, and ALZ-801, are also in development [35].
2.2. Anti-tau Therapy
The tau protein is associated with microtubules and stabilizes microtubules in axons and dendrites. Tau undergoes the process of post-translational modifications, especially hyper-phosphorylation [155,156]. Hyper-phosphorylated tau proteins accumulate and form intracellular neurofibrillary tangles (NFTs). NFTs induce inflammatory responses and cause neurotoxicity. Unlike Aβ, the development of tau pathology correlates with the severity of cognitive deficit in AD [157]. The strategies for anti-tau therapy include phosphatase modifiers, kinase inhibitors, tau aggregation inhibitors, microtubule stabilizers, and tau immunotherapy.
2.2.1. Phosphatase Modifiers
Phosphatase modifiers decrease phosphorylation by activating phosphatases, such as protein phosphatase 2A (PP2A) [158]. Sodium selenate is a PP2A activator and an essential molecule in neurological functions [159]. Sodium selenite deficiency was related to oxidative damage and cognitive impairment [160]. A phase II trial on sodium selenate did not find any change in cognitive performance in mild to moderate AD [39]. A supranutritional supplement of sodium selenate increased selenium uptake in the CNS, but the clinical efficacy was minor for AD [40].
2.2.2. Kinase Inhibitors
Kinase inhibitors decrease post-translational modifications and limit the hyper-phosphorylation of tau. The degree of phosphorylation is related to the activity of protein kinases: cyclin-dependent-like kinase 5 (CDK5) [161] and glycogen synthase kinase-3β (GSK3β) [162]. Selective inhibitors of CDK5 have been reported in cancer therapy, including roscovitine [163] and flavopiridol [164]. In animal models of AD, roscovitine prevented tau phosphorylation, while flavopiridol reduced memory decline [41,42]. None of these agents have reached clinical trials in AD. Two types of GSK3β inhibitors, tideglusib and lithium, have been researched in AD. Tideglusib showed no clinical benefit in phase II trials of mild to moderate AD, and its short-term administration resulted in an adverse effect of a reversible transaminase increase [43]. Lithium, a mood stabilizer, was identified as a GSK3β inhibitor. One double-blind RCT revealed that a microdose of lithium prevented cognitive decline in AD patients [44]. The participants received a 15-month lithium treatment, with a daily dose of 300 mg. Meta-analyses concluded that lithium inhibited the progression of cognitive decline in AD patients, with a moderate effect size [45,46]. Whether lithium is effective in treating AD needs further verification.
2.2.3. Tau Aggregation Inhibitors
Methylene blue (MB) is a synthetic phenothiazine dye and the earliest tau aggregation inhibitor. MB blocks interactions between tau molecules and disrupts polymerization in vitro [165,166]. In a phase II double-blind RCT, 50-week administration of MB in which the participants received 138 mg of MB treatment showed a cognitive benefit in mild to moderate AD [47]. Methylthioninium chloride (LMTX), the MB derivative, failed to improve cognitive or functional performance in a phase III double-blind RCT in mild to moderate AD [48]. An advanced study demonstrated that MB inhibited tau fibril formation but accelerated the formation of neurotoxic tau oligomers [167]. Therefore, the role of MB remains ambivalent in AD therapy.
Curcumin is a coloring agent and food additive. Curcumin inhibits tau aggregation by decreasing β-sheet formation in tau and disintegrating tau oligomers in vitro [168]. Several phase II double-blind RCTs of curcumin displayed no clinical or biomarker improvement after a 6-month treatment in AD patients [169,170]. The failure of previous studies of curcumin was attributed to its low bioavailability [171]. In the cognitively healthy elderly, a bioavailability-improved formulation of curcumin administration improved working memory in an acute and short-term course (<4 weeks) [49,50]. However, a long-term course of curcumin treatment did not delay cognitive decline [172]. A recent systematic meta-analysis indicated that curcumin treatment worsened cognitive performance in AD patients [173].
2.2.4. Microtubule Stabilizers
Epothilone D (EpoD) is an anti-fungal agent and a microtubule stabilizer. Epothilone D induces tubulin’s polymerization into microtubules and enhances microtubule bundling in vitro [174]. In animal studies, EpoD rescued working and spatial memory deficits in aged tau transgenic mice [175,176,177], but the phase I trial of EpoD failed due to intolerable adverse effects [51].
NAP (davunetide), an activity-dependent neuroprotective protein (ADNP) derivative, protects microtubules from katanin disruption in vitro [178,179]. In a phase II double-blind RCT, NAP showed cognitive and functional improvement in MCI, when MCI patients received a 12-week intranasal NAP administration [52,53]. The clinical effect of NAP has not been researched in AD patients yet [180].
TPI-287 (abeotaxane) is a synthetic taxane derivative for central nerve system (CNS) malignancy or metastasis treatment [181,182]. A phase I double-blind RCT of TPI-287 showed less decline in Mini-Mental State Examination (MMSE) scores in the treated group compared to placebo in mild to moderate AD. Three serious adverse events (15%) with anaphylactoid reactions were reported [54]. In addition to EpoD, NAP, and TPI-287, the development of a peptide with the taxol-binding pocket of β-tubulin has become another innovative strategy [183].
2.2.5. Tau Immunotherapy
Active tau vaccines have been developed to trigger antibodies against tau proteins. Two tau vaccines have reached clinical trials: AADvac1 and ACI-35 [184]. The antibodies from AADvac1 target the microtubule-binding region of tau, decrease tau aggregation, and promote tau clearance [185]. In a phase I double-blind RCT in mild to moderate AD, almost all the patients receiving the AADvac1 injection (29/30) showed an IgG immune response within 12 weeks [186]. No case of meningoencephalitis or vasogenic edema was reported at a 72-week follow-up assessment [187]. One phase II double-blind RCT of AADvac1 was performed to evaluate its clinical efficacy in patients with mild AD [55]. ACI-35 is a liposome-based vaccine against phosphorylated tau. In animal studies, ACI-35 induced a rapid immune response and decreased phosphorylated tau in tau transgenic mice within 12 weeks [188]. One phase I double-blind RCT is underway in patients with mild to moderate AD to assess the tolerability and safety of the ACI-35 vaccine [56]. One novel tau vaccine, Aβ 3–10-keyhole limpet hemocyanin (KLH), reduced the phosphorylated tau level and improved cognitive functions in animal studies [57].
Passive immunotherapy is being developed for tau pathology. Several agents have achieved clinical trials for AD: BIIB092, ABBV-8E12, RO7105705, BIIB076, LY3303560, UCB0107, and JNJ-63733657 [189]. Three such agents are humanized lgG4 monoclonal antibodies BIIB092, ABBV-8E12, and RO7105705. Gosuranemab (BIIB092) was safe and well tolerated in healthy participants [190]. One large phase II double-blind RCT of BIIB092 is ongoing in patients with early AD [58]. ABBV-8E12 showed an acceptable safety profile in a phase I study [191]. Phase II double-blind RCTs of ABBV-8E12 are being continued with regard to the efficacy of treating patients with early AD [59,60]. Semorinemab (RO7105705) showed a fair safety profile in healthy individuals. Two phase II double-blind RCTs of RO7105705 are ongoing in prodromal to mild AD [61] and moderate AD [62].
Two agents belong to humanized lgG1 monoclonal antibodies: BIIB076 and LY3303560. BIIB076 was both safe and tolerable in healthy participants and MCI patients [63]. An advanced clinical trial of BIIB076 is not yet available. LY3303560 appeared to be tolerable in healthy individuals and AD patients [192,193]. One phase II triple-blind RCT of LY3303560 has ended in early AD, but the efficacy is currently not available [64]. JNJ-63733657, a monoclonal antibody, completed two phase I trials in healthy participants and patients with AD [194,195]. In early AD, the phase II double-blind RCT of the efficacy of JNJ-63733657 is still being studied [65]. UCB0107, a humanized version of antibody D, is undergoing a phase I investigator-blind RCT in a healthy population [66,67].
2.3. Anti-neuroinflammatory Therapy
Neuroinflammation contributes to the progression of AD and correlates with the severity of the disease [196]. Anti-neuroinflammatory strategies include microglia modulators, astrocyte modulators, insulin resistance management, and microbiome therapy.
2.3.1. Microglia Modulators
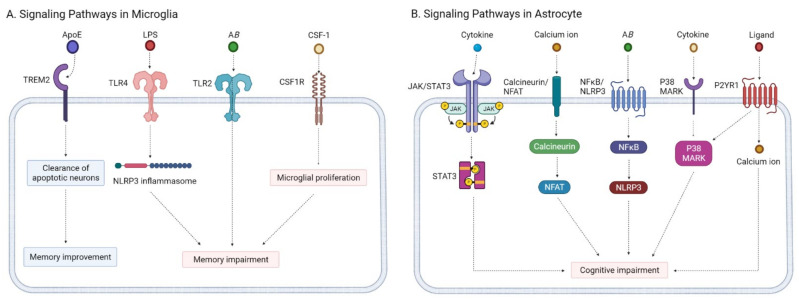
Microglial activation is recognized as a hallmark of neuroinflammation. Microglia interact with Aβ and the tau protein in the pathogenesis of AD [197,198]. Glial activation is associated with the signaling pathways of apolipoprotein E (ApoE), thus triggering the receptor expressed on myeloid cells 2 (TREM2), Toll-like receptor (TLR), and colony-stimulating factor-1 receptor (CSF1R) (Figure 1, panel A) [199].
Figure 1.
Signaling pathways of microglia modulators and astrocyte modulators. (A) Signaling pathways in microglia; (B) signaling pathways in astrocytes. Created with BioRender.com. * TREM2—triggering the receptor expressed on myeloid cells 2, TLR—Toll-like receptor, CSF1R—colony-stimulating factor-1 receptor, JAK—Janus kinase, STAT3—signal transducer and activator of transcription 3, NFAT—nuclear factor of activated T cells, NFκB—nuclear factor-kB, NLRP3—nod-like receptor family pyrin domain containing 3, MAPK—mitogen-activated protein kinase, P2Y1R—P2Y1 purinoreceptor.
Mutations of ApoE and TREM2 are considered strong risk factors of AD. The ApoE-TREM2 pathway shares similar mechanisms in regulating Aβ pathology in AD [200]. APOE is a primary cholesterol carrier and identified as a ligand for human TREM2 in microglia. The interaction increases TREM2-mediated phagocytosis of apoptotic neurons [201,202]. In an AD mouse model, increased TREM2 expression led to improved memory performance in 5xFAD mice [203]. The deficiency of TREM2 decreased plaque deposition during the early stage of AD but enhanced amyloid-β pathology in the advanced stage [204]. No agent targeting ApoE or TREM2 has reached clinical trials for AD treatment.
Multiple TLR pathways respond to the accumulation of Aβ and induce neuronal injuries in AD pathogenesis, especially TLR4 and TLR2. The TLR4 pathway interacts with NLRP3 inflammasomes and sustains neuroinflammation [205]. Furthermore, the TLR4 pathway is activated by lipopolysaccharide (LPS) and induces memory impairment in animal models of AD [206]. Several TLR4 inhibitors improved cognitive deficits in AD animal models, including thymoquinone, ethyl pyruvate, and TAK-242 [68]. TLR2 binds to Aβ and mediates the Aβ phagocytosis by microglia [207]. Dysregulation of the TLR2 pathway accelerated memory impairment in AD mice, either through inhibition [208,209,210] or activation [207]. None of these agents have reached clinical trials for AD therapy.
The CSF1R pathway drives microglial proliferation in animal models of AD. Selective CSF1R inhibitors were applied in transgenic AD mice, such as GW2580, JN-J527, and PLX3397. The efficacy of GW2580 blocked microglial proliferation and recovered the short-term memory and behavioral deficit in APP/PS1 mice [69]. Administration of JN-J527 improved tau-mediated neurodegeneration and functional impairment in P301S mice [70]. Long-term treatment of PLX3397 reversed spatial and emotional memory deficits in 5XFAD mice [71].
2.3.2. Astrocyte Modulators
The astrocyte reaction impairs the clearance of Aβ at the BBB. The astrocyte reaction in AD involves several signaling pathways: the Janus kinase/signal transducer and activator of transcription 3 (JAK/STAT3), the calcineurin/nuclear factor of activated T cells (calcineurin/NFAT), the nuclear factor-kB/nod-like receptor family pyrin domain containing 3(NFκB/NLRP3), the mitogen-activated protein kinase (MAPK), and the P2Y1 purinoreceptor (P2Y1R) pathways (Figure 1, panel B) [211].
The JAK/STAT3 pathway has been activated in reactive astrocyte transgenic mouse models of AD [212]. Stattic is a selective STAT3 inhibitor, and its intraperitoneal injection rescued learning and memory impairment in 5XFAD mice [72,73]. The calcineurin/NFAT pathway promotes the production of proinflammatory cytokines [213]. FK506 (Tacrolimus) inhibited the calcineurin/NFAT pathway and improved cognitive deficit in APP/PS1 mice [214,215]. An open-label phase II study of FK506 is underway to investigate the efficacy in MCI and AD. No results have yet been published [74]. The NFκB/NLRP3 pathway is activated by Aβ and promotes the production of proinflammatory cytokines [216]. Eliminating NLRP3 reduced brain Aβ levels in AD animal models [217,218]. Inhibition of the NFκB/NLRP3 pathway is a potential treatment, but no agents have yet entered clinical trials of AD [219].
P38 MAPK, a class of MAPKs, responds to inflammatory cytokines, mediates Aβ-induced neurotoxicity, and is correlated with tau phosphorylation [220]. Several p38 MAPK inhibitors were investigated in vivo, including SB202190 and PD169316 [75]. Two highly selective p38 MAPK inhibitors were investigated in animal studies of AD: MW181 and NJK14047. MW181 blocked tau phosphorylation and rescued cognitive impairment in aged hTau mice [76]. Meanwhile, NJK14047 decreased Aβ deposits, decreased neuron death, and improved cognitive functions in 5XFAD mice [77]. The P2Y1R pathway increases the frequency of spontaneous astroglial calcium events. The process promotes downstream p38 activity and glutamate-induced neuronal death in AD mice [221,222]. Several P2Y1R inhibitors have been involved in AD studies: MRS2179 and BPTU. Treatment of P2Y1R inhibitors normalized astrocyte activity and improved cognitive deficits in APPPS1 mice [78].
2.3.3. Insulin Resistance Management
AD features deficits in cerebral glucose utilization with progressive cognitive impairment [223]. The deficits in cerebral glucose utilization in human AD include insulin deficiency, insulin-like growth factor 1 (IGF-1) deficiency, and insulin resistance. Insulin resistance promotes oxidative stress, triggers inflammation, and increases tau phosphorylation and toxic Aβ levels [224].
Insulin therapy is applied when treating AD with an intranasal device. A double-blind RCT reported that intranasal insulin administration improved memory impairment in MCI and AD. The participants received intranasal regular insulin at 40 IU daily for 4 months [79]. A systematic review of RCTs indicated that patients with MCI and AD displayed improved verbal memory after insulin therapy. The patients without an APOE4 gene had more consistent cognitive benefits than the APOE4 carriers [80]. A recent RCT of intranasal insulin therapy also failed in treating MCI and AD. After 12 months of treatment, the treated group demonstrated no significant difference in cognition and function compared to the placebo group [81]. Intranasal insulin therapy is a relatively safe option of treatment without serious adverse events in the treated group [80].
Incretins are gut-derived hormones that stimulate insulin secretion, including glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP). Several incretin receptor agonists showed a potential therapeutic effect in animal models of AD and Parkinson’s disease: liraglutide, lixisenatide, exendin-4, semaglutide, peptide 17, peptide 18, peptide 20, DA-JC4, and DA-CIB [82]. A double-blind RCT of liraglutide was examined in AD treatment. The 12-month liraglutide treatment delayed cognitive impairment in the treated group compared to the placebo group [83]. Another phase II double-blind RCT of liraglutide is ongoing in patients with mild AD [225].
Metformin is the first-line therapy for diabetes mellitus. In diabetic patients, metformin demonstrated a neuroprotective effect and reduced the risk of developing dementia [226,227]. One study of metformin involved non-diabetic, overweight (BMI over 25) populations with MCI. The treated group received 500–2000 mg of metformin daily. After a 12-month intervention, the treated group showed a reduction in recall memory decline compared to the placebo group [84]. One pilot crossover RCT of metformin was tested in non-diabetic and non-overweight adults with MCI and early AD. The participants were randomized to receive an 8-month metformin or a placebo intervention. The daily dose of metformin was as high as 2000 mg. The results showed that the metformin administration improved executive functions in the treated group compared to placebo [85].
Peroxisome proliferator activator receptors (PPARs) mediate the anti-inflammatory process and metabolic pathways [228]. Three isotypes of PPARs have been identified: PPAR-α, PPAR-β/δ, and PPAR-γ. Four PPAR-α agonists showed therapeutic potential in animal models of AD: WY-14643, GW7647, fenofibrate, and gemfibrozil. A phase I trial of gemfibrozil in MCI patients has been completed, and advanced clinical studies are pending [86]. Pioglitazone is a PPAR-γ agonist for treating diabetes. An open-label phase II RCT of pioglitazone showed cognitive benefits in diabetic patients with mild AD. The participants received 15–30 mg of pioglitazone daily for 6 months [87]. Two phase III quadruple-blind trials of pioglitazone in MCI patients were terminated due to a lack of efficacy without safety concerns [88,89]. The PPAR-δ agonists have been evaluated in AD mouse models [229]. A hybrid PPAR-δ and PPAR-γ agonist, T3D-959, resolved neuroinflammation in an intracerebral streptozotocin (STZ) animal model of AD [90].
2.3.4. Microbiome Therapy
The composition of the gut microbiota affects the gut–brain communication and brain function by synthesizing various neurotransmitters and neuromodulators [230]. Dysbiosis of the gut microbiota leads to an overproduction of LPS in the gut, which increases permeability to the BBB [231]. Sodium oligomannate (GV-971), a marine-derived oligosaccharide, suppresses gut microbiota dysbiosis, regulates neuroinflammation, and destabilizes Aβ aggregates [232]. Phase III double-blind RCTs of sodium oligomannate showed a cognitive benefit in patients with mild to moderate AD [91]. The participants received a dose of 900 mg of sodium oligomannate for 36 weeks. The treated group showed significant improvement in ADAS-cog performance compared to the placebo group [92]. Sodium oligomannate was approved in November 2019 in China for treating mild to moderate AD [233].
2.4. Neuroprotective Agents
Neurodegenerative mechanisms are involved in the pathogenesis of AD. Therefore, applying neuroprotective strategies aims to delay both the AD onset and AD progression [234]. Three neuroprotective candidates are generally discussed: antiepileptic drugs, NMDAR modification, and omega 3 polyunsaturated fatty acid supplements.
2.4.1. Antiepileptic Drugs
Antiepileptic drugs are considered CNS depressants and have been found to deteriorate cognitive functions. In recent studies, some agents had the potential to enhance cognitive performance in epileptic patients, including levetiracetam and gabapentin [235].
Levetiracetam exerts a therapeutic effect by targeting the synaptic vesicle 2A (SV2A) protein [236]. Levetiracetam displayed neuroprotective properties in traumatic brain injury in both animal models and clinical trials [237]. In a mouse model of AD, administration of levetiracetam decreased the Aβ load and rescued the cognitive deficit in APP/PS1 mice after a 4-week treatment [238]. In the healthy elderly, the double-blind crossover RCT of levetiracetam showed potential with regard to enhancing cognitive functions. The volunteers received a dose of 1000 mg of levetiracetam during the 5-week treatment phase. The volunteers in the levetiracetam-treated phase showed cognitive improvement but with a tendency of irritability and fatigue [239]. In patients with MCI, a multicenter double-blind phase III RCT of AGB101 (levetiracetam) is currently ongoing to evaluate its potential to slow cognitive and functional decline [93].
Gabapentin is a voltage-gated calcium channel (VGCC) inhibitor that indirectly affects the glutamate system. In cerebral ischemia-reperfusion mice, gabapentin treatment showed a neuroprotective effect and reduced neural injury in a dose-dependent manner [240]. In healthy populations, administration of a single dose of 50–400 mg of gabapentin promoted a subtle cognitive improvement [241]. In dementia patients with BPSD, preliminary evidence indicated that gabapentin treatment had possible benefits in treating AD. The gabapentin treatment with a daily dose of 200–3600 mg decreased agitation and improved cognition. The result was based on low-grade evidence [242]. A double-blind phase IV RCT of gabapentin enacarbil is continuing to investigate the therapeutic efficacy of nighttime agitation and restless leg syndrome in patients with moderate to severe AD [94].
2.4.2. NMDAR Modification
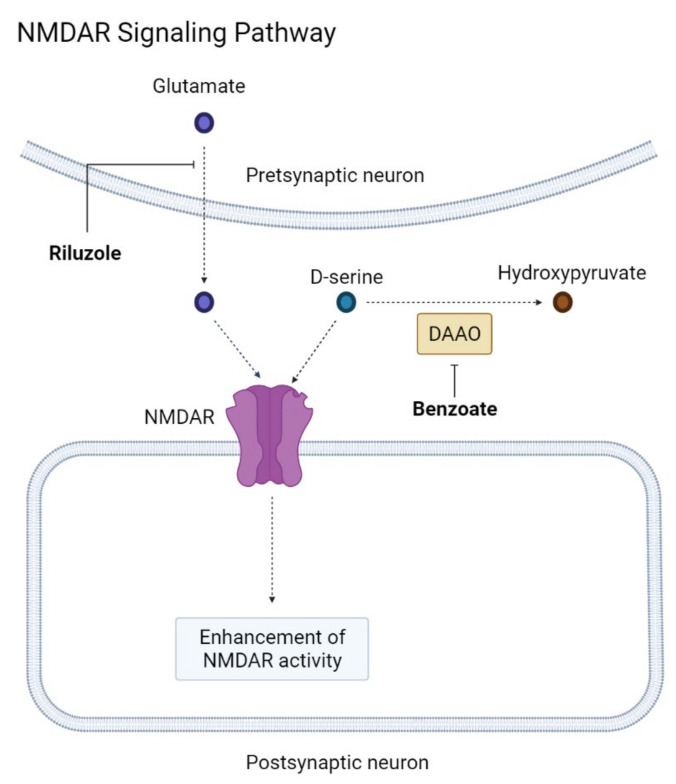
Glutamate is one of the major excitatory neurotransmitters in the CNS. The N-methyl-D-aspartate receptor (NMDAR) is a subtype of the ionotropic glutamate receptor and plays a critical role in regulating synaptic plasticity, neuronal survival, learning, and memory [243]. Individuals with AD had decreased glutamate levels in CSF and fewer NMDARs in the hippocampus and frontal cortex [244]. Enhancement or modulation of NMDAR activity demonstrated therapeutic potential in early AD.
The preservative sodium benzoate enhances NMDAR activity by inhibiting D-amino acid oxidase (DAAO). D-serine, the main co-agonist of NMDARs, is metabolized by DAAO into hydroxypyruvate. Inhibition of DAAO increases the level of downstream D-serine (Figure 2) [245]. In studies of schizophrenia, sodium benzoate inhibited reactive oxygen species and had a potent neuroprotective effect [246,247,248]. Sodium benzoate was tolerated in patients with MCI and mild AD. The participants received a 24-week benzoate treatment with a dose of 250 to 750 mg per day. The treated group showed greater improvement in ADAS-Cog than the placebo group [95]. In a phase II double-blind RCT, 24-week administration of sodium benzoate in which the participants received 250–1500 mg benzoate treatments showed both altered brain activity and cognitive benefit in MCI [96]. In patients with BPSD, a 6-week benzoate treatment demonstrated a benefit in specific individuals: those with a young age, those of the female gender, those with a higher BMI, those with a significant DAAO decrease, and those with antipsychotic use [247]. In a multicenter, double-blind RCT, benzoate treatment showed cognitive benefits in women with moderate to severe AD. The treated group received 250–1500 mg of benzoate daily for 6 weeks and showed improved ADAS-Cog performance compared to the placebo group [97].
Figure 2.
NMDAR signaling pathway. Created with BioRender.com. NMDAR—N-methyl-D-aspartate receptor, DAAO—D-amino acid oxidase. The sharo arrow means activation of the chemical reaction. The blunt head arrow means inhibition of the chemical reaction.
Riluzole is classified as a glutamate modulator and is used in amyotrophic lateral sclerosis therapy. Riluzole inhibits the presynaptic glutamate release indirectly and modulates the postsynaptic NMDAR activity. In animal models of early AD, the riluzole-treated group had better enhanced cognition and a reduced Aβ load compared to the placebo group in transgenic mice [249,250]. A phase II double-blind RCT of riluzole was completed to assess the cerebral metabolism and cognitive effect in mild AD. No results have yet been published [98]. Troriluzole (BHV-4157) is a riluzole derivative, and one phase II double-blind RCT of troriluzole is continuing to evaluate the cognitive change in patients with mild to moderate AD [99].
2.4.3. Omega 3 Polyunsaturated Fatty Acid Supplements
Omega 3 polyunsaturated fatty acids include three subtypes: α-linolenic acid (ALA), eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA). The latter two were derived from fish oil and demonstrated an anti-inflammatory effect against cardiovascular diseases [251]. In AD mouse models, a supplement of either EPA or DHA showed a neuroprotective property and improved memory and learning [252]. In MCI patients, several controlled studies have indicated that omega 3 fatty acid supplements from 3 to 12 months significantly improved cognitive performance over the placebo [253,254]. In APOE4 carriers, phospholipid DHA dietary supplements had the potential to prevent the development of AD [255]. A phase II double-blind RCT has been ongoing to evaluate the effect of the APOE4 genotype and the cognitive efficacy of DHA supplements [101]. In mild to moderate AD, a phase III RCT of a DHA supplement was evaluated. The 18-month 2 mg DHA supplements administered daily did not rescue the cognitive and functional decline in the treated group when compared to the placebo group [100]. A recent systematic review and meta-analysis study suggested that only combined DHA and EPA supplements improved certain aspects of cognitive performance in AD patients. No consistent evidence has supported the therapeutic efficacy in short- or medium-term treatment [256]. A phase III RCT of icosapent ethyl, an ethyl ester of EPA, is ongoing to evaluate the cognitive and cerebrovascular effect in cognitively healthy adults at increased risk for AD [102].
2.5. Brain Stimulation
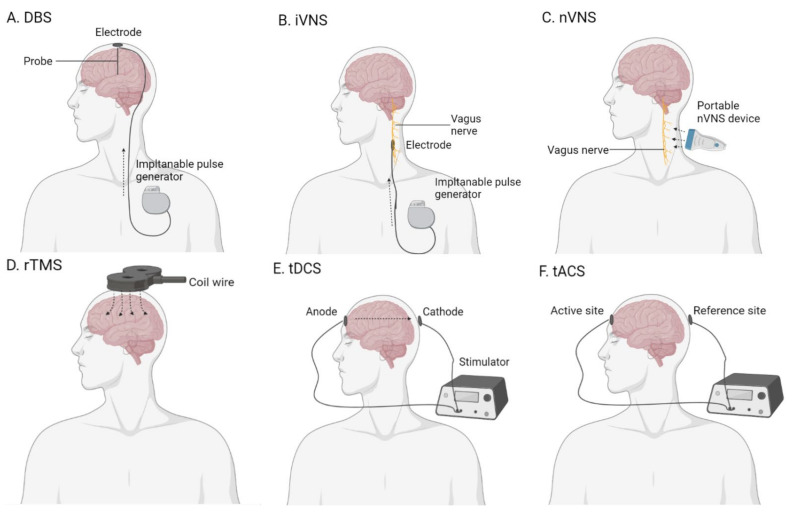
Brain stimulation is proposed as a promising non-pharmacological therapeutic option for AD [257]. In the field of AD therapy, several brain stimulation methods have been researched: deep-brain stimulation (DBS), vagus nerve stimulation (VNS), transcranial magnetic stimulation (TMS), and transcranial electrical stimulation (Figure 3) [258].
Figure 3.
The diagram of the brain stimulation devices. (A) Deep-brain stimulation (DBS); (B) invasive vagus nerve stimulation (iVNS); (C) non-invasive vagus nerve stimulation (nVNS); (D) repetitive transcranial magnetic stimulation (rTMS); (E) transcranial direct current stimulation (tDCS); (F) transcranial alternating current stimulation (tACS). Created with BioRender.com. The sharp arrow means the direction of energy flow.
2.5.1. Deep-Brain Stimulation
Deep-brain stimulation is an invasive brain stimulation technique. The surgeon implants electrodes at a targeted region of the brain and promotes electrical stimulation. The electrical stimulation is provided by an implantable pulse generator. DBS treatment is considered an advanced treatment for tremors in patients with Parkinson’s disease [259].
In 2010, a small-size phase I trial of DBS was investigated in six patients with mild AD. The DBS was placed in the fornix within the hypothalamus. After 12 months of continuous stimulation, the patients showed reduced cognitive decline and improved glucose metabolism at the temporoparietal lobe [260]. A phase II double-blind RCT of forniceal DBS was performed in mild AD patients. No significant difference in cognition or metabolism was observed between the treated and control groups. Subgroup analysis revealed the patients aged 65 or older had slight cognitive improvement, whereas younger patients demonstrated worsening of cognition after 12-month forniceal DBS treatment [103]. A two-year follow-up of the study reported the same conclusion. The forniceal DBS treatment had possible benefits in patients aged 65 and older [104]. A larger phase III multicenter RCT of forniceal DBS is currently underway to evaluate the effectiveness of cognition in the elderly with mild AD [105].
In addition to the fornix, the nucleus basalis of Meynert (NBM) is also considered a targeted region of DBS. A small-size, double-blind, sham-controlled phase I trial of NBM-DBS was assessed in patients with mild to moderate AD. At a 12-month follow-up assessment, two thirds of the patients had improved or stabilized cognitive performance [106]. The patients who responded to NBM-DBS had the characteristics of a higher baseline cognitive function and less advanced cortical atrophy [261,262].
2.5.2. Vagus Nerve Stimulation
Vagus nerve stimulation is categorized into invasive and non-invasive methods. With invasive VNS (iVNS), the surgeon places the electrode at the left side of the tenth cranial nerve. The electrical stimulation is generated by a connected implanted pulse generator. Invasive VNS is approved for treating epilepsy and treatment-resistant depression. In 2002, a small-size pilot study of iVNS was researched in AD patients for 6 months. After a 3-month treatment, an estimated 90% of patients showed an improved MMSE performance, and 70% of the patients had better performance on ADAS-Cog. After 6 months of treatment, the response rate was maintained at 70% in AD patients [107]. The same researchers recruited more AD patients and followed them up for 12 months. After 12-month iVNS treatment, an estimated 70% of patients showed stabilized or improved cognitive performance [108].
Non-invasive VNS (nVNS) is a non-invasive intervention. The portable nVNS device provides electrical stimulation transcutaneously at the ear or neck and indirectly stimulates the auricular branch of the vagus nerve. Non-invasive VNS has been proven to be effective in the treatment of cluster headaches and migraines [263]. In the cognitively healthy elderly, a single session of nVNS improved associative memory in the treated group compared to the placebo. No serious or long-term adverse effects were reported [264]. The potential of nVNS in AD treatment has been suggested, but the clinical evidence is still lacking [265]. One double-blind sham-controlled crossover study is underway to evaluate the therapeutic effect of nVNS in patients with MCI [109].
2.5.3. Transcranial Magnetic Stimulation
Transcranial magnetic stimulation (TMS) is a non-invasive brain stimulation technique. The TMS device produces an electric current through a coil wire, which is encased in plastic and placed above the patient’s scalp. The process produces a magnetic field across the cranial tissue and results in electrical stimulation at targeted sites of the brain [266]. The pulse of TMS can be single or repeated. Compared to single-pulse TMS, repetitive TMS (rTMS) modulates the cortical activity and promotes after effects beyond the stimulation period. Different rTMS protocols lead to variant after effects in the brain, with an inhibitory effect at low-frequency stimulation (≤1 Hz), and excitatory effects at high-frequency stimulation (≥5 Hz) [267]. A high-frequency rTMS protocol at the left dorsolateral prefrontal cortex (DLPFC) has been approved for treatment-resistant depression therapy in the United States [268].
High-frequency rTMS at the left DLPFC was also investigated in AD treatment. Three double-blind RCTs of 10 Hz rTMS were evaluated in MCI patients. Two studies of 2-week 10 Hz rTMS treatment showed a significant improvement in executive function in the treated group compared to the sham group [110,269]. One study of 4-week 10 Hz rTMS for evaluation in MCI patients is still recruiting and continuing [111].
The 20 Hz rTMS at the left DLPFC method was researched in studies for AD treatment. In AD patients, 2-week 20 Hz rTMS treatment led to improved language performance, and 4-week intervention brought an even greater change and longer-lasting effect [112]. The 20 Hz rTMS protocol was tested in AD patients with BPSD. Compared to the control group, which received low-dose antipsychotic medications alone, combined 20 Hz rTMS and medication resulted in significant improvement in both cognitive functions and BPSD symptoms after a 4-week treatment [113]. One double-blind RCT of 20 Hz rTMS was tested in patients with mild to moderate AD patients. The study aimed to evaluate the add-on effect of rTMS. All the participants received face–name associative memory cognitive training. After 4 weeks of treatment, the treated group showed better performance in trained associative memory than the sham group. Combined rTMS and cognitive training showed a greater benefit than cognitive training alone. The additional improvement was greater in participants with higher educational levels and cognitive baseline [114]. Another trial on the add-on effect of high-frequency rTMS was also investigated in AD treatment. Mild to moderate AD patients undergoing cognitive training received real or sham rTMS treatment for 4 weeks. Compared to the sham group, the treated group showed better performance in general cognitive and behavioral functions [115]. Stimulation at the left DLPFC seems to be the most popular and promising protocol in AD treatment.
Some studies of high-frequency rTMS targeted bilateral DLPFCs. The first study applied 20 Hz rTMS in patients with different degrees of AD. Following 5-day stimulation, patients receiving high-frequency rTMS showed cognitive and functional improvement in mild to moderate AD when compared to the sham group [117]. Another pilot crossover study of 4-week 20 Hz rTMS was evaluated in mild to moderate AD patients. The results revealed a stronger improvement in general cognition during the treatment phase than the sham phase [118]. Few studies have focused on the 5 Hz rTMS protocol in AD therapy. One clinical trial of 5 Hz rTMS compared the efficacy of different protocols in AD patients: simple (stimulation at left DLPFC) versus complex (stimulation at six other cortical sites). The results suggested that a 3-week intervention promoted both cognitive and functional improvement in both groups, and that there was no difference between the simple and complex protocols [116].
High-frequency rTMS is also performed at different targeted sites in the brain, such as the inferior frontal gyrus (IFG), superior temporal gyrus (STG), and parietal and posterior temporal lobes. One crossover RCT evaluated the clinical benefit of 10 Hz rTMS at the right IFG in MCI patients. All the patients received two sessions of stimulation in a random order: right IFG (active site), and right vertex (control site). The results indicated that high-frequency stimulation at the right IFG enhanced the improvement in attention and psychomotor speed, while the stimulation of the vertex showed a significant cognitive change [119]. Another crossover study tested the efficacy of high-frequency rTMS in patients with MCI and mild to moderate AD. Dementia patients had a pattern of gray matter atrophy, especially at the bilateral IFG, putamen, and cerebellum. The stimulation lasted a total of three sessions at 10 Hz over three regions in a random order: right IFG (active site), right STG (active site), and right vertex (control site). The stimulation of the right IFG and right STG revealed cognitive benefits, especially in attention and psychomotor speed performance. Patients with a greater gray matter volume reduction gained more benefit from the rTMS intervention [120]. The efficacy of high-frequency rTMS of the left parietal lobe (precuneus) was tested in MCI patients. The 20 Hz rTMS protocol was conducted for 2 weeks. The stimulation of the left parietal lobe revealed greater clinical benefits in episodic memory in the treated group than in the sham group, but the effect was not noted in other cognitive domains. Analysis of rTMS combined with electroencephalography uncovered the phenomenon of modulation of brain connectivity [121]. One double-blind RCT of 20 Hz rTMS targeted the region of bilateral posterior temporal regions of the brains of AD individuals. The study included mild to moderate AD patients and performed 6-week 20 Hz stimulation in the treated group and sham stimulation in the control group. The results showed that rTMS had advantages in the treatment of mild AD, with better performance in memory and language in the treated group than the sham group. However, the cognitive benefit was minimal or insignificant in moderate AD patients [122].
The low-frequency rTMS protocol has been less researched than the high-frequency protocol with regard to AD treatment. One randomized sham-controlled trial of 1 Hz rTMS at DLPFCs was applied in healthy individuals and MCI patients. The participants received two sessions on the same day, either at the right or left DLPFC. The results indicated that low-frequency rTMS enhanced recognition memory in both the healthy and MCI groups. Inhibition of the right DLPFC may modulate the excitability of the contralateral hemisphere [123]. In AD, one clinical study compared the efficacy of high-frequency (20 Hz) and low-frequency (1 Hz) rTMS targeting bilateral DLPFCs. The therapeutic efficacy of low-frequency rTMS was demonstrated to be less effective than high-frequency rTMS after a 5-day intervention [117].
A recent meta-analysis concluded that both high-frequency and low-frequency rTMS resulted in cognitive improvement in AD patients, with medium to large effect sizes [270]. The after effect of five or more sessions of rTMS could last from a few weeks to 4 months [114,115,116,117,118,270,271].
2.5.4. Transcranial Electrical Stimulation
Transcranial electrical stimulation involves passing a weak electrical current (1–2 mA) among two or more electrodes on the subject’s scalp. The electrode positioning could be based on the international 10–20 electrode placement system, the neuronavigation system, or physiology-based placement. Transcranial electrical stimulation comes in two major forms: transcranial direct current stimulation (tDCS) and transcranial alternating current stimulation (tACS). In tDCS, the electrodes are divided into anodal or cathodal sites, while in tACS, the electrodes are active or reference sites. The applied electrical current is direct in tDCS but sinusoidal in tACS [272,273].
Transcranial direct current stimulation is the most common choice of transcranial electrical stimulation in treating AD. Studies into tDCS in AD have focused on several targeted regions: left DLPFC, left temporal lobe, and temporoparietal lobe [274]. In 2009, a study of anodal tDCS at the left DLPFC was first performed in patients with mild to moderate AD. The participants received true stimulation with an intensity of 2 mA for 30 min, and sham stimulation was conventionally set as 30 s. The results indicated that a single session of left DLPFC stimulation led to improved recognition memory [124]. The 2 mA tDCS protocol over the left DLPFC was tested in AD patients with repeated sessions. One double-blind RCT of tDCS included mild to moderate AD patients. The participants were classified into anodal tDCS, cathodal tDCS, and sham tDCS groups. The true stimulation was 25 min long. After 10 sessions, the active treatment group showed a higher MMSE score than the sham group, and the cognitive benefit was similar in the anodal and cathodal tDCS groups [125]. One double-blind phase II RCT of the 2 mA anodal tDCS protocol was tested in moderate AD patients with apathy. Each true session persisted for 20 min, and the total session number was six times. However, the intervention of tDCS showed no significant effect regarding cognitive, behavioral, or apathy symptoms in moderate AD. The study suggested that more than six sessions may promote clinical change in patients with moderate AD [126]. One recent study of at-home tDCS was applied in patients with early AD. The 2 mA tDCS protocol was applied daily for 6 months with 30-min sessions. Compared to the sham group, the treated group showed improved or stabilized cognition, with improvement in global and language functions and a decreased reduction in executive function [127].
In MCI patients, the anodal tDCS protocol showed clinical benefits in cognition. One double-blind RCT of 1.5 mA anodal tDCS was evaluated in MCI treatment. Each true session was 15 min, while sham stimulation was 10 s each session. This study indicated that a single session of tDCS enhanced the free recall and recognition of memory in the treated group compared to the sham group [128]. One pilot study compared the efficacy between 2 mA anodal tDCS and cognitive stimulation in MCI patients. The study also aimed to determine the optimal frequency of tDCS in MCI treatment. Each true session lasted for 30 min, with a variance of one to five sessions in the treatment phase. The results revealed that tDCS treatment resulted in a significant but mild improvement in some cognitive aspects, especially in selective attention, processing speed, and planning ability tasks. The optimal frequency of tDCS was three sessions per week. The conclusion should be warranted due to the session’s variability [129].
Several studies of tDCS targeted the region of the left parietal lobe. In 2009, the anodal tDCS at the left parietal region was tested in patients with mild to moderate AD. Single-session 30-min 2 mA anodal tDCS promoted superior improved recognition memory in the treated group compared to the sham group [124]. One study of 2 mA anodal tDCS over the left temporal area was assessed in patients with mild to moderate AD. The participants received six 30-min sessions. Active tDCS stimulation did not result in a significant change in verbal memory function [130].
The other targeted site of tDCS was the bilateral temporoparietal lobe. In 2008, the 1.5 mA tDCS protocol of the bilateral temporoparietal region was first tested in patients with mild AD. All participants received one sham stimulation and two true stimulations (anodal and cantonal) in a random order. Each true session was performed for 15 min, while the sham stimulation lasted only 10 s. The subgroup analysis showed that the anodal tDCS group gained improved word recognition, but the cathodal tDCS group experienced cognitive worsening instead [131]. One trial of 2 mA anodal tDCS at the bilateral temporal areas was evaluated in patients with mild to moderate AD. The participants received five 30-min sessions. The anodal stimulation enhanced the visual recognition memory in the treated group compared to the sham group [132]. Another trial of 2 mA anodal tDCS over the bilateral temporal areas was investigated with regard to cognitive and biological changes in patients with early AD. The patients received ten 20-min sessions. Significantly improved cognitive performance and increased total serum Aβ levels were observed in the treated group, but there was no change in tau or lipid peroxidase [133]. One recent study assessed the short-term and long-term effects of 2 mA tDCS over the left temporoparietal region in the treatment of advanced AD. The true stimulation was administrated for 20 min daily, for a total of 10 times. The sham stimulation was 10 s each time. At one month, the tDCS intervention stabilized the neuropsychological performance in the treated group, while the sham group showed a significant decline. The treated group continued with the frequency of tDCS for five sessions per month for 8 months. The protective effect of tDCS was maintained in long-term follow-up [134]. The after effect of tDCS generally lasted for at least 4 weeks [128,132,134].
tACS is a choice among transcranial brain stimulations. Some small trials have indicated that tACS may enhance specific cognitive functions in cognitively healthy populations [275,276]. The evidence of the therapeutic effect of tACS in AD patients is limited. In 2020, one pilot study of tACS over the left DLPFC was conducted in patients with MCI and mild to moderate AD. The study aimed to investigate the additional cognitive effect of combined tDCS in patients undergoing brain exercises. The stimulation was scheduled as sinusoidal waveforms at the frequency of 40 Hz with an intensity of 1.5 mA, from −0.75 to +0.75 mA. The treated group received two 30-min sessions per day, with 40 sessions in total. At a 4-week follow-up assessment, the tACS group showed a slight improvement in cognitive performance, while the non-tACS group demonstrated slight cognitive decline instead. The study showed that tACS had potential in AD treatment, and that the after effect may be maintained for 4 weeks [135]. One crossover RCT of 40 Hz tACS at 3 mA in AD treatment has been completed, in which the targeted region of tACS was the superior parietal cortex. The study was completed, but the results have not been published [136]. Several clinical trials of 40 Hz tACS are underway in AD treatment [137,277].
3. Discussion
In this review, we described the development of AD therapy during the past two decades, identifying five mainstream categories: anti-Aβ therapy, anti-tau therapy, anti-neuroinflammatory therapy, neuroprotective agents, and brain stimulation. Initially, the pathological markers Aβ and tau were the main targets of therapy. Immunotherapies became the most popular method among the two fields. The target of intervention gradually shifted from specific pathological markers to complex mechanisms, such as neuroinflammatory and neurodegenerative processes. Compared to the pharmacological field, non-pharmacological interventions went even further. Among brain stimulation approaches, non-invasive methods have been more tolerable than invasive techniques, and the most commonly studied methods were rTMS and tDCS. rTMS and tDCS showed convincing outcomes in cognitive enhancement and maintenance. Non-invasive brain stimulations have the potential to be the next trend in AD treatment.
Drug repositioning is another potential method for accelerating pharmacological development. Drug repositioning has many advantages. First, repositioning existing drugs to new therapeutic uses is less expensive than developing a new drug. Second, both the safety and tolerance of existing drugs have already been investigated. It is easier for these drugs to achieve advanced clinical stages to evaluate the therapeutic effect in AD. Some potential drugs were proposed in this way, such as lithium, metformin, levetiracetam, and sodium benzoate. The challenges lie in the choice of existing drugs, which depends on our understanding of AD pathogenesis.
Precision medicine is another issue. Some patients gained more clinical benefits from a specific intervention. For example, sodium benzoate showed more cognitive improvement in female patients than in males. Phospholipid DHA supplements showed a preventive effect on AD in APOE4 carriers. Most of the AD patients who responded to rTMS and tDCS were in the early stage of AD. The etiology of AD is considered multifactorial, which may be distinct in each individual. Choosing the appropriate interventions according to the characteristics of AD patients can help to achieve a therapeutic effect as soon as possible and minimize the harm and adverse effects of treatment. Designing personalized interventions is one of the most critical milestones of further AD treatment.
4. Future Research Direction
In pharmacological interventions, researching the potential agents is still a challenge. AD is a multifactorial disorder and involves several pathogenic mechanisms: misfolded protein aggregation, neuroinflammatory process, neurodegeneration, and insulin dysregulation. Drug repositioning is a possible effective method. The potential candidates include anti-inflammatory agents, neuroprotective agents, and antidiabetic agents. As biophysical and structural biology experimental approaches progress, the pathophysiological mechanisms of Aβ and tau are being uncovered. The knowledge of AD pathogenesis helps us to find potential compounds or to design further immunotherapies.
In non-pharmacological interventions, standardizing the settings of the protocol is the current challenge. For example, to determine the protocol of rTMS, the parameters include the targeted sites, frequency, duration of each session, and schedule. The same rTMS protocol may show inconsistent efficacy in patients at different stages of AD. Designing several standardized protocols according to the disease severity is a possible strategy in the future.
Selecting responsive subgroups is important in both pharmacological and non-pharmacological interventions. The characteristics of patients are involved in designing the treatment, including age, gender, genetic factors, medical diseases, environmental factors, and lifestyles. Advances in machine learning allow us to deal with complex factors and build models which predict the optimal therapeutic regimens for AD patients. Further research is required to uncover the relationship between patients’ characteristics and response to a specific treatment. With further research efforts, the practice of precision medicine is possible and anticipated.
Acknowledgments
All the figures are created with BioRender.com. Publication license from BioRender are proof of our publication rights.
Author Contributions
H.-Y.L. and C.-H.L. developed the concept. T.-W.Y. drafted the original manuscript. H.-Y.L. provided expert opinions and edited the manuscript. C.-H.L. critically reviewed and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by National Health Research Institutes, Taiwan (No. NHRI-EX110-10816NC)(C.-H.L.), Ministry of Science and Technology, Taiwan (MOST 109-2628-B-182A-002)(C.-H.L.), Kaohsiung Chang Gung Memorial Hospital (No. CMRPG8K1461)(C.-H.L.), and China Medical University Hospital, Taiwan (No. DMR-HHC-110-9)(H.-Y.L).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
All authors declare that they have no conflict of interest concerning this article.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Scheltens P., Blennow K. Alzheimer’s disease. Lancet. 2016;388:505–517. doi: 10.1016/S0140-6736(15)01124-1. [DOI] [PubMed] [Google Scholar]
- 2.GBD 2016 Dementia Collaborators Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:88–106. doi: 10.1016/S1474-4422(18)30403-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Niu H., Álvarez-Álvarez I. Prevalence and incidence of Alzheimer’s disease in Europe: A meta-analysis. Neurologia. 2017;32:523–532. doi: 10.1016/j.nrl.2016.02.016. [DOI] [PubMed] [Google Scholar]
- 4.United Nations Department of Economic and Social Affairs, Population Division World Population Ageing 2020 Highlights: Living Arrangements of Older Persons. [(accessed on 4 April 2021)];2020 (ST/ESA/SER.A/451) Available online: https://www.un.org/development/desa/pd/sites/www.un.org.development.desa.pd/files/undesa_pd-2020_world_population_ageing_highlights.pdf.
- 5.Hebert L.E., Weuve J. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology. 2013;80:1778–1783. doi: 10.1212/WNL.0b013e31828726f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eratne D., Loi S.M. Alzheimer’s disease: Clinical update on epidemiology, pathophysiology and diagnosis. Australas Psychiatry. 2018;26:347–357. doi: 10.1177/1039856218762308. [DOI] [PubMed] [Google Scholar]
- 7.2020 Alzheimer’s Disease Facts and Figures. [(accessed on 4 April 2021)];Alzheimers Dementia. 2020 Available online: https://alz-journals.onlinelibrary.wiley.com/doi/full/10.1002/alz.12068.
- 8.Mokdad A.H., Ballestros K. The State of US Health, 1990–2016: Burden of Diseases, Injuries, and Risk Factors among US States. JAMA. 2018;319:1444–1472. doi: 10.1001/jama.2018.0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin C.H., Lane H.Y. The Role of N-Methyl-D-Aspartate Receptor Neurotransmission and Precision Medicine in Behavioral and Psychological Symptoms of Dementia. Front. Pharmacol. 2019;10:540. doi: 10.3389/fphar.2019.00540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baharudin A.D., Din N.C. The associations between behavioral-psychological symptoms of dementia (BPSD) and coping strategy, burden of care and personality style among low-income caregivers of patients with dementia. BMC Public Health. 2019;19(Suppl. 4):447. doi: 10.1186/s12889-019-6868-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friedman E.M., Shih R.A. US Prevalence and Predictors of Informal Caregiving for Dementia. Health Aff. 2015;34:1637–1641. doi: 10.1377/hlthaff.2015.0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kevadiya B.D., Ottemann B.M. Neurotheranostics as personalized medicines. Adv. Drug Deliv. Rev. 2019;148:252–289. doi: 10.1016/j.addr.2018.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blanco-Silvente L., Castells X. Discontinuation, Efficacy, and Safety of Cholinesterase Inhibitors for Alzheimer’s Disease: A Meta-Analysis and Meta-Regression of 43 Randomized Clinical Trials Enrolling 16 106 Patients. Int. J. Neuropsychopharmacol. 2017;20:519–528. doi: 10.1093/ijnp/pyx012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blanco-Silvente L., Capellà D. Predictors of discontinuation, efficacy, and safety of memantine treatment for Alzheimer’s disease: Meta-analysis and meta-regression of 18 randomized clinical trials involving 5004 patients. BMC Geriatr. 2018;18:168. doi: 10.1186/s12877-018-0857-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leal S.L., Lockhart S.N. Subthreshold Amyloid Predicts Tau Deposition in Aging. J. Neurosci. 2018;38:4482–4489. doi: 10.1523/JNEUROSCI.0485-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adams J.N., Lockhart S.N. Relationships between Tau and Glucose Metabolism Reflect Alzheimer’s Disease Pathology in Cognitively Normal Older Adults. Cereb. Cortex. 2019;29:1997–2009. doi: 10.1093/cercor/bhy078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang L., Benzinger T.L. Evaluation of Tau Imaging in Staging Alzheimer Disease and Revealing Interactions between β-Amyloid and Tauopathy. JAMA Neurol. 2016;73:1070–1077. doi: 10.1001/jamaneurol.2016.2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jagust W. Imaging the evolution and pathophysiology of Alzheimer disease. Nat. Rev. Neurosci. 2018;19:687–700. doi: 10.1038/s41583-018-0067-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hossain M.F., Wang N. Exploring the multifunctional role of melatonin in regulating autophagy and sleep to mitigate Alzheimer’s disease neuropathology. Ageing Res. Rev. 2021;67:101304. doi: 10.1016/j.arr.2021.101304. [DOI] [PubMed] [Google Scholar]
- 20.Egan M.F., Kost J. Randomized Trial of Verubecestat for Mild-to-Moderate Alzheimer’s Disease. N. Engl. J. Med. 2018;378:1691–1703. doi: 10.1056/NEJMoa1706441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Egan M.F., Kost J. Randomized Trial of Verubecestat for Prodromal Alzheimer’s Disease. N. Engl. J. Med. 2019;380:1408–1420. doi: 10.1056/NEJMoa1812840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henley D., Raghavan N. Preliminary Results of a Trial of Atabecestat in Preclinical Alzheimer’s Disease. N. Engl. J. Med. 2019;380:1483–1485. doi: 10.1056/NEJMc1813435. [DOI] [PubMed] [Google Scholar]
- 23.Wessels A.M., Tariot P.N. Efficacy and Safety of Lanabecestat for Treatment of Early and Mild Alzheimer Disease: The AMARANTH and DAYBREAK-ALZ Randomized Clinical Trials. JAMA Neurol. 2020;77:199–209. doi: 10.1001/jamaneurol.2019.3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lo A.C., Evans C.D. Phase II (NAVIGATE-AD study) Results of LY3202626 Effects on Patients with Mild Alzheimer’s Disease Dementia. J. Alzheimers Dis. Rep. 2021;5:321–336. doi: 10.3233/ADR-210296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.ClinicalTrials.gov A Study of CNP520 Versus Placebo in Participants at Risk for the Onset of Clinical Symptoms of Alzheimer’s Disease. NCT03131453. [(accessed on 4 April 2021)]; Available online: https://ClinicalTrials.gov/show/NCT03131453.
- 26.Imbimbo B.P., Watling M. Investigational BACE inhibitors for the treatment of Alzheimer’s disease. Expert Opin. Investig. Drugs. 2019;28:967–975. doi: 10.1080/13543784.2019.1683160. [DOI] [PubMed] [Google Scholar]
- 27.Iraji A., Khoshneviszadeh M. Novel small molecule therapeutic agents for Alzheimer disease: Focusing on BACE1 and multi-target directed ligands. Bioorg. Chem. 2020;97:103649. doi: 10.1016/j.bioorg.2020.103649. [DOI] [PubMed] [Google Scholar]
- 28.Doody R.S., Raman R. A phase 3 trial of semagacestat for treatment of Alzheimer’s disease. N. Engl. J. Med. 2013;369:341–350. doi: 10.1056/NEJMoa1210951. [DOI] [PubMed] [Google Scholar]
- 29.Coric V., Salloway S. Targeting Prodromal Alzheimer Disease with Avagacestat: A Randomized Clinical Trial. JAMA Neurol. 2015;72:1324–1333. doi: 10.1001/jamaneurol.2015.0607. [DOI] [PubMed] [Google Scholar]
- 30.Green R.C., Schneider L.S. Effect of tarenflurbil on cognitive decline and activities of daily living in patients with mild Alzheimer disease: A randomized controlled trial. JAMA. 2009;302:2557–2564. doi: 10.1001/jama.2009.1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ritchie C.W., Bush A.I. Metal-protein attenuation with iodochlorhydroxyquin (clioquinol) targeting Abeta amyloid deposition and toxicity in Alzheimer disease: A pilot phase 2 clinical trial. Arch. Neurol. 2003;60:1685–1691. doi: 10.1001/archneur.60.12.1685. [DOI] [PubMed] [Google Scholar]
- 32.Lannfelt L., Blennow K. Safety, efficacy, and biomarker findings of PBT2 in targeting Abeta as a modifying therapy for Alzheimer’s disease: A phase IIa, double-blind, randomised, placebo-controlled trial. Lancet Neurol. 2008;7:779–786. doi: 10.1016/S1474-4422(08)70167-4. [DOI] [PubMed] [Google Scholar]
- 33.Villemagne V.L., Rowe C.C. A randomized, exploratory molecular imaging study targeting amyloid β with a novel 8-OH quinoline in Alzheimer’s disease: The PBT2-204 IMAGINE study. Alzheimers Dement. 2017;3:622–635. doi: 10.1016/j.trci.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mantile F., Prisco A. Vaccination against β-Amyloid as a Strategy for the Prevention of Alzheimer’s Disease. Biology. 2020;9:425. doi: 10.3390/biology9120425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tolar M., Abushakra S. Aducanumab, gantenerumab, BAN2401, and ALZ-801-the first wave of amyloid-targeting drugs for Alzheimer’s disease with potential for near term approval. Alzheimers Res. Ther. 2020;12:95. doi: 10.1186/s13195-020-00663-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ostrowitzki S., Lasser R.A. A phase III randomized trial of gantenerumab in prodromal Alzheimer’s disease. Alzheimers Res. Ther. 2017;9:95. doi: 10.1186/s13195-017-0318-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alexander G.C., Emerson S. Evaluation of Aducanumab for Alzheimer Disease: Scientific Evidence and Regulatory Review Involving Efficacy, Safety, and Futility. JAMA. 2021;325:1717–1718. doi: 10.1001/jama.2021.3854. [DOI] [PubMed] [Google Scholar]
- 38.Howard R., Liu K.Y. Questions EMERGE as Biogen claims aducanumab turnaround. Nat. Rev. Neurol. 2020;16:63–64. doi: 10.1038/s41582-019-0295-9. [DOI] [PubMed] [Google Scholar]
- 39.Malpas C.B., Vivash L. A Phase IIa Randomized Control Trial of VEL015 (Sodium Selenate) in Mild-Moderate Alzheimer’s Disease. J. Alzheimers Dis. 2016;54:223–232. doi: 10.3233/JAD-160544. [DOI] [PubMed] [Google Scholar]
- 40.Cardoso B.R., Roberts B.R. Supranutritional Sodium Selenate Supplementation Delivers Selenium to the Central Nervous System: Results from a Randomized Controlled Pilot Trial in Alzheimer’s Disease. Neurotherapeutics. 2019;16:192–202. doi: 10.1007/s13311-018-0662-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khalil H.S., Mitev V. Discovery and development of Seliciclib. How systems biology approaches can lead to better drug performance. J. Biotechnol. 2015;202:40–49. doi: 10.1016/j.jbiotec.2015.02.032. [DOI] [PubMed] [Google Scholar]
- 42.Leggio G.M., Catania M.V. The antineoplastic drug flavopiridol reverses memory impairment induced by Amyloid-ß1-42 oligomers in mice. Pharmacol. Res. 2016;106:10–20. doi: 10.1016/j.phrs.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 43.Lovestone S., Boada M. A phase II trial of tideglusib in Alzheimer’s disease. J. Alzheimers Dis. 2015;45:75–88. doi: 10.3233/JAD-141959. [DOI] [PubMed] [Google Scholar]
- 44.Nunes M.A., Viel T.A. Microdose lithium treatment stabilized cognitive impairment in patients with Alzheimer’s disease. Curr. Alzheimer Res. 2013;10:104–107. doi: 10.2174/1567205011310010014. [DOI] [PubMed] [Google Scholar]
- 45.Matsunaga S., Kishi T. Lithium as a Treatment for Alzheimer’s Disease: A Systematic Review and Meta-Analysis. J. Alzheimers Dis. 2015;48:403–410. doi: 10.3233/JAD-150437. [DOI] [PubMed] [Google Scholar]
- 46.Matsunaga S., Fujishiro H. Efficacy and Safety of Glycogen Synthase Kinase 3 Inhibitors for Alzheimer’s Disease: A Systematic Review and Meta-Analysis. J. Alzheimers Dis. 2019;69:1031–1039. doi: 10.3233/JAD-190256. [DOI] [PubMed] [Google Scholar]
- 47.Wischik C.M., Staff R.T. Tau aggregation inhibitor therapy: An exploratory phase 2 study in mild or moderate Alzheimer’s disease. J. Alzheimers Dis. 2015;44:705–720. doi: 10.3233/JAD-142874. [DOI] [PubMed] [Google Scholar]
- 48.Gauthier S., Feldman H.H. Efficacy and safety of tau-aggregation inhibitor therapy in patients with mild or moderate Alzheimer’s disease: A randomised, controlled, double-blind, parallel-arm, phase 3 trial. Lancet. 2016;388:2873–2884. doi: 10.1016/S0140-6736(16)31275-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee M.S., Wahlqvist M.L. Turmeric improves post-prandial working memory in pre-diabetes independent of insulin. Asia Pac. J. Clin. Nutr. 2014;23:581–591. doi: 10.6133/apjcn.2014.23.4.24. [DOI] [PubMed] [Google Scholar]
- 50.Cox K.H., Pipingas A. Investigation of the effects of solid lipid curcumin on cognition and mood in a healthy older population. J. Psychopharmacol. 2015;29:642–651. doi: 10.1177/0269881114552744. [DOI] [PubMed] [Google Scholar]
- 51.ClinicalTrials.gov Study to Evaluate the Safety, Tolerability and the Effect of BMS-241027 on Cerebrospinal Fluid Biomarkers in Subjects with Mild Alzheimer’s Disease. NCT01492374. [(accessed on 4 April 2021)]; Available online: https://ClinicalTrials.gov/show/NCT01492374.
- 52.Morimoto B.H., Schmechel D. A double-blind, placebo-controlled, ascending-dose, randomized study to evaluate the safety, tolerability and effects on cognition of AL-108 after 12 weeks of intranasal administration in subjects with mild cognitive impairment. Dement. Geriatr. Cogn. Disord. 2013;35:325–336. doi: 10.1159/000348347. [DOI] [PubMed] [Google Scholar]
- 53.Gozes I., Stewart A. Addressing Alzheimer’s disease tangles: From NAP to AL-108. Curr. Alzheimer Res. 2009;6:455–460. doi: 10.2174/156720509789207895. [DOI] [PubMed] [Google Scholar]
- 54.Tsai R.M., Miller Z. Reactions to Multiple Ascending Doses of the Microtubule Stabilizer TPI-287 in Patients with Alzheimer Disease, Progressive Supranuclear Palsy, and Corticobasal Syndrome: A Randomized Clinical Trial. JAMA Neurol. 2020;77:215–224. doi: 10.1001/jamaneurol.2019.3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.ClinicalTrials.gov 24 Months Safety and Efficacy Study of AADvac1 in Patients with Mild Alzheimer’s Disease. NCT02579252. [(accessed on 4 April 2021)]; Available online: https://ClinicalTrials.gov/show/NCT02579252.
- 56.Congdon E.E., Sigurdsson E.M. Tau-targeting therapies for Alzheimer disease. Nat. Rev. Neurol. 2018;14:399–415. doi: 10.1038/s41582-018-0013-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang J.C., Zhu K. Early active immunization with Aβ(3-10)-KLH vaccine reduces tau phosphorylation in the hippocampus and protects cognition of mice. Neural Regen. Res. 2020;15:519–527. doi: 10.4103/1673-5374.266061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.ClinicalTrials.gov Phase 2 Study of BIIB092 in Participants with Early Alzheimer’s Disease. NCT03352557. [(accessed on 4 April 2021)]; Available online: https://ClinicalTrials.gov/show/NCT03352557.
- 59.ClinicalTrials.gov A Study to Evaluate the Efficacy and Safety of ABBV-8E12 in Subjects with Early Alzheimer’s Disease. NCT02880956. [(accessed on 4 April 2021)]; Available online: https://ClinicalTrials.gov/show/NCT02880956.
- 60.ClinicalTrials.gov An Extension Study of ABBV-8E12 in Early Alzheimer’s Disease (AD). NCT03712787. [(accessed on 4 April 2021)]; Available online: https://ClinicalTrials.gov/show/NCT03712787.
- 61.ClinicalTrials.gov A Study to Evaluate the Efficacy and Safety of Semorinemab in Patients with Prodromal to Mild Alzheimer’s Disease. NCT03289143. [(accessed on 4 April 2021)]; Available online: https://ClinicalTrials.gov/show/NCT03289143.
- 62.ClinicalTrials.gov A Study of Semorinemab in Patients with Moderate Alzheimer’s Disease. NCT03828747. [(accessed on 4 April 2021)]; Available online: https://ClinicalTrials.gov/show/NCT03828747.
- 63.ClinicalTrials.gov Single-Ascending-Dose Study of BIIB076 in Healthy Volunteers and Participants with Alzheimer’s Disease. NCT03056729. [(accessed on 4 April 2021)]; Available online: https://ClinicalTrials.gov/show/NCT03056729.
- 64.ClinicalTrials.gov A Study of LY3303560 in Participants with Early Symptomatic Alzheimer’s Disease. NCT03518073. [(accessed on 4 April 2021)]; Available online: https://ClinicalTrials.gov/show/NCT03518073.
- 65.ClinicalTrials.gov A Study of JNJ-63733657 in Participants with Early Alzheimer’s Disease. NCT04619420. [(accessed on 4 April 2021)]; Available online: https://ClinicalTrials.gov/show/NCT04619420.
- 66.ClinicalTrials.gov A Study to Test the Safety and Tolerability and Pharmacokinetics of Single Doses of UCB0107 in Healthy Japanese Subjects. NCT03605082. [(accessed on 4 April 2021)]; Available online: https://ClinicalTrials.gov/show/NCT03605082.
- 67.Courade J.P., Angers R. Epitope determines efficacy of therapeutic anti-Tau antibodies in a functional assay with human Alzheimer Tau. Acta Neuropathol. 2018;136:729–745. doi: 10.1007/s00401-018-1911-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhou Y., Chen Y. TLR4 Targeting as a Promising Therapeutic Strategy for Alzheimer Disease Treatment. Front. Neurosci. 2020;14:602508. doi: 10.3389/fnins.2020.602508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Olmos-Alonso A., Schetters S.T. Pharmacological targeting of CSF1R inhibits microglial proliferation and prevents the progression of Alzheimer’s-like pathology. Pt 3Brain. 2016;139:891–907. doi: 10.1093/brain/awv379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mancuso R., Fryatt G. CSF1R inhibitor JNJ-40346527 attenuates microglial proliferation and neurodegeneration in P301S mice. Brain. 2019;142:3243–3264. doi: 10.1093/brain/awz241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sosna J., Philipp S. Early long-term administration of the CSF1R inhibitor PLX3397 ablates microglia and reduces accumulation of intraneuronal amyloid, neuritic plaque deposition and pre-fibrillar oligomers in 5XFAD mouse model of Alzheimer’s disease. Mol. Neurodegener. 2018;13:11. doi: 10.1186/s13024-018-0244-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Choi M., Kim H. Inhibition of STAT3 phosphorylation attenuates impairments in learning and memory in 5XFAD mice, an animal model of Alzheimer’s disease. J. Pharmacol. Sci. 2020;143:290–299. doi: 10.1016/j.jphs.2020.05.009. [DOI] [PubMed] [Google Scholar]
- 73.Millot P., San C. STAT3 inhibition protects against neuroinflammation and BACE1 upregulation induced by systemic inflammation. Immunol. Lett. 2020;228:129–134. doi: 10.1016/j.imlet.2020.10.004. [DOI] [PubMed] [Google Scholar]
- 74.ClinicalTrials.gov A Pilot Open Labeled Study of Tacrolimus in Alzheimer’s Disease. NCT04263519. [(accessed on 18 April 2021)]; Available online: https://ClinicalTrials.gov/show/NCT04263519.
- 75.Kheiri G., Dolatshahi M. Role of p38/MAPKs in Alzheimer’s disease: Implications for amyloid beta toxicity targeted therapy. Rev. Neurosci. 2018;30:9–30. doi: 10.1515/revneuro-2018-0008. [DOI] [PubMed] [Google Scholar]
- 76.Maphis N., Jiang S. Selective suppression of the α isoform of p38 MAPK rescues late-stage tau pathology. Alzheimers Res. Ther. 2016;8:54. doi: 10.1186/s13195-016-0221-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gee M.S., Son S.H. A selective p38α/β MAPK inhibitor alleviates neuropathology and cognitive impairment, and modulates microglia function in 5XFAD mouse. Alzheimers Res. Ther. 2020;12:45. doi: 10.1186/s13195-020-00617-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Reichenbach N., Delekate A. P2Y1 receptor blockade normalizes network dysfunction and cognition in an Alzheimer’s disease model. J. Exp. Med. 2018;215:1649–1663. doi: 10.1084/jem.20171487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Craft S., Claxton A. Effects of Regular and Long-Acting Insulin on Cognition and Alzheimer’s Disease Biomarkers: A Pilot Clinical Trial. J. Alzheimers Dis. 2017;57:1325–1334. doi: 10.3233/JAD-161256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Avgerinos K.I., Kalaitzidis G. Intranasal insulin in Alzheimer’s dementia or mild cognitive impairment: A systematic review. J. Neurol. 2018;265:1497–1510. doi: 10.1007/s00415-018-8768-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Craft S., Raman R. Safety, Efficacy, and Feasibility of Intranasal Insulin for the Treatment of Mild Cognitive Impairment and Alzheimer Disease Dementia: A Randomized Clinical Trial. JAMA Neurol. 2020;77:1099–1109. doi: 10.1001/jamaneurol.2020.1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Salameh T.S., Rhea E.M. Brain uptake pharmacokinetics of incretin receptor agonists showing promise as Alzheimer’s and Parkinson’s disease therapeutics. Biochem. Pharmacol. 2020;180:114187. doi: 10.1016/j.bcp.2020.114187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gejl M., Gjedde A. In Alzheimer’s Disease, 6-Month Treatment with GLP-1 Analog Prevents Decline of Brain Glucose Metabolism: Randomized, Placebo-Controlled, Double-Blind Clinical Trial. Front. Aging Neurosci. 2016;8:108. doi: 10.3389/fnagi.2016.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Luchsinger J.A., Perez T. Metformin in Amnestic Mild Cognitive Impairment: Results of a Pilot Randomized Placebo Controlled Clinical Trial. J. Alzheimers Dis. 2016;51:501–514. doi: 10.3233/JAD-150493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Koenig A.M., Mechanic-Hamilton D. Effects of the Insulin Sensitizer Metformin in Alzheimer Disease: Pilot Data From a Randomized Placebo-controlled Crossover Study. Alzheimer Dis. Assoc. Disord. 2017;31:107–113. doi: 10.1097/WAD.0000000000000202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wójtowicz S., Strosznajder A.K. The Novel Role of PPAR Alpha in the Brain: Promising Target in Therapy of Alzheimer’s Disease and Other Neurodegenerative Disorders. Neurochem. Res. 2020;45:972–988. doi: 10.1007/s11064-020-02993-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sato T., Hanyu H. Efficacy of PPAR-γ agonist pioglitazone in mild Alzheimer disease. Neurobiol. Aging. 2011;32:1626–1633. doi: 10.1016/j.neurobiolaging.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 88.ClinicalTrials.gov AD-4833/TOMM40_303 Extension Study of the Safety and Efficacy of Pioglitazone to Slow Cognitive Decline in Participants with Mild Cognitive Impairment Due to Alzheimer Disease. NCT02284906. [(accessed on 22 April 2021)]; Available online: https://ClinicalTrials.gov/show/NCT02284906.
- 89.ClinicalTrials.gov Biomarker Qualification for Risk of Mild Cognitive Impairment (MCI) Due to Alzheimer’s Disease (AD) and Safety and Efficacy Evaluation of Pioglitazone in Delaying Its Onset. NCT01931566. [(accessed on 22 April 2021)]; Available online: https://ClinicalTrials.gov/show/NCT01931566.
- 90.Reich D., Gallucci G. Therapeutic Advantages of Dual Targeting of PPAR-δ and PPAR-γ in an Experimental Model of Sporadic Alzheimer’s Disease. J. Parkinsons Dis. Alzheimers Dis. 2018;5 doi: 10.13188/2376-922x.1000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang T., Kuang W. A phase II randomized trial of sodium oligomannate in Alzheimer’s dementia. Alzheimers Res. Ther. 2020;12:110. doi: 10.1186/s13195-020-00678-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xiao S., Chan P. A 36-week multicenter, randomized, double-blind, placebo-controlled, parallel-group, phase 3 clinical trial of sodium oligomannate for mild-to-moderate Alzheimer’s dementia. Alzheimers Res. Ther. 2021;13:62. doi: 10.1186/s13195-021-00795-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.ClinicalTrials.gov Study of AGB101 in Mild Cognitive Impairment Due to Alzheimer’s Disease. NCT03486938. [(accessed on 4 April 2021)]; Available online: https://ClinicalTrials.gov/show/NCT03486938.
- 94.ClinicalTrials.gov Nighttime Agitation and Restless Legs Syndrome in People with Alzheimer’s Disease. NCT03082755. [(accessed on 1 May 2021)]; Available online: https://ClinicalTrials.gov/show/NCT03082755.
- 95.Lin C.H., Chen P.K. Benzoate, a D-amino acid oxidase inhibitor, for the treatment of early-phase Alzheimer disease: A randomized, double-blind, placebo-controlled trial. Biol. Psychiatry. 2014;75:678–685. doi: 10.1016/j.biopsych.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 96.Lane H.Y., Tu C.H. Brain Activity of Benzoate, a D-Amino Acid Oxidase Inhibitor, in Patients with Mild Cognitive Impairment in a Randomized, Double-Blind, Placebo Controlled Clinical Trial. Int. J. Neuropsychopharmacol. 2021;24:392–399. doi: 10.1093/ijnp/pyab001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lin C.H., Chen P.K. Effect of Sodium Benzoate on Cognitive Function among Patients with Behavioral and Psychological Symptoms of Dementia: Secondary Analysis of a Randomized Clinical Trial. JAMA Netw. Open. 2021;4:e216156. doi: 10.1001/jamanetworkopen.2021.6156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.ClinicalTrials.gov Riluzole in Mild Alzheimer’s Disease. NCT01703117. [(accessed on 28 April 2021)]; Available online: https://ClinicalTrials.gov/show/NCT01703117.
- 99.ClinicalTrials.gov Study of BHV-4157 in Alzheimer’s Disease. NCT03605667. [(accessed on 28 April 2021)]; Available online: https://ClinicalTrials.gov/show/NCT03605667.
- 100.Quinn J.F., Raman R. Docosahexaenoic acid supplementation and cognitive decline in Alzheimer disease: A randomized trial. JAMA. 2010;304:1903–1911. doi: 10.1001/jama.2010.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.ClinicalTrials.gov DHA Brain Delivery Trial (PreventE4). NCT03613844. [(accessed on 19 July 2021)]; Available online: https://ClinicalTrials.gov/show/NCT03613844.
- 102.Bhatt D.L., Hull M.A. Beyond cardiovascular medicine: Potential future uses of icosapent ethyl. Eur. Heart J. Suppl. 2020;22(Suppl. J):J54–J64. doi: 10.1093/eurheartj/suaa119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lozano A.M., Fosdick L. A Phase II Study of Fornix Deep Brain Stimulation in Mild Alzheimer’s Disease. J. Alzheimers Dis. 2016;54:777–787. doi: 10.3233/JAD-160017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Leoutsakos J.S., Yan H. Deep Brain Stimulation Targeting the Fornix for Mild Alzheimer Dementia (the ADvance Trial): A Two Year Follow-up Including Results of Delayed Activation. J. Alzheimers Dis. 2018;64:597–606. doi: 10.3233/JAD-180121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lee D.J., Lozano A.M. Current Status of Deep Brain Stimulation for Alzheimer’s Disease: From Chance Observation to Clinical Trials. Cold Spring Harb. Symp. Quant. Biol. 2018;83:201–205. doi: 10.1101/sqb.2018.83.037440. [DOI] [PubMed] [Google Scholar]
- 106.Kuhn J., Hardenacke K. Deep brain stimulation of the nucleus basalis of Meynert in Alzheimer’s dementia. Mol. Psychiatry. 2015;20:353–360. doi: 10.1038/mp.2014.32. [DOI] [PubMed] [Google Scholar]
- 107.Sjögren M.J., Hellström P.T. Cognition-enhancing effect of vagus nerve stimulation in patients with Alzheimer’s disease: A pilot study. J. Clin. Psychiatry. 2002;63:972–980. doi: 10.4088/JCP.v63n1103. [DOI] [PubMed] [Google Scholar]
- 108.Merrill C.A., Jonsson M.A. Vagus nerve stimulation in patients with Alzheimer’s disease: Additional follow-up results of a pilot study through 1 year. J. Clin. Psychiatry. 2006;67:1171–1178. doi: 10.4088/JCP.v67n0801. [DOI] [PubMed] [Google Scholar]
- 109.ClinicalTrials.gov Treatment of Mild Cognitive Impairment with Transcutaneous Vagal Nerve Stimulation. NCT03359902. [(accessed on 3 May 2021)]; Available online: https://ClinicalTrials.gov/show/NCT03359902.
- 110.Padala P.R., Padala K.P. Repetitive transcranial magnetic stimulation for apathy in mild cognitive impairment: A double-blind, randomized, sham-controlled, cross-over pilot study. Psychiatry Res. 2018;261:312–318. doi: 10.1016/j.psychres.2017.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Taylor J.L., Hambro B.C. The effects of repetitive transcranial magnetic stimulation in older adults with mild cognitive impairment: A protocol for a randomized, controlled three-arm trial. BMC Neurol. 2019;19:326. doi: 10.1186/s12883-019-1552-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cotelli M., Calabria M. Improved language performance in Alzheimer disease following brain stimulation. J. Neurol. Neurosurg. Psychiatry. 2011;82:794–797. doi: 10.1136/jnnp.2009.197848. [DOI] [PubMed] [Google Scholar]
- 113.Wu Y., Xu W. Adjunctive treatment with high frequency repetitive transcranial magnetic stimulation for the behavioral and psychological symptoms of patients with Alzheimer’s disease: A randomized, double-blind, sham-controlled study. Shanghai Arch. Psychiatry. 2015;27:280–288. doi: 10.11919/j.issn.1002-0829.215107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bagattini C., Zanni M. Enhancing cognitive training effects in Alzheimer’s disease: rTMS as an add-on treatment. Brain Stimul. 2020;13:1655–1664. doi: 10.1016/j.brs.2020.09.010. [DOI] [PubMed] [Google Scholar]
- 115.Zhang F., Qin Y. High-frequency repetitive transcranial magnetic stimulation combined with cognitive training improves cognitive function and cortical metabolic ratios in Alzheimer’s disease. J. Neural Transm. (Vienna) 2019;126:1081–1094. doi: 10.1007/s00702-019-02022-y. [DOI] [PubMed] [Google Scholar]
- 116.Alcalá-Lozano R., Morelos-Santana E. Similar clinical improvement and maintenance after rTMS at 5 Hz using a simple vs. complex protocol in Alzheimer’s disease. Brain Stimul. 2018;11:625–627. doi: 10.1016/j.brs.2017.12.011. [DOI] [PubMed] [Google Scholar]
- 117.Ahmed M.A., Darwish E.S. Effects of low versus high frequencies of repetitive transcranial magnetic stimulation on cognitive function and cortical excitability in Alzheimer’s dementia. J. Neurol. 2012;259:83–92. doi: 10.1007/s00415-011-6128-4. [DOI] [PubMed] [Google Scholar]
- 118.Rutherford G., Lithgow B. Short and Long-term Effects of rTMS Treatment on Alzheimer’s Disease at Different Stages: A Pilot Study. J. Exp. Neurosci. 2015;9:43–51. doi: 10.4137/JEN.S24004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Eliasova I., Anderkova L. Non-invasive brain stimulation of the right inferior frontal gyrus may improve attention in early Alzheimer’s disease: A pilot study. J. Neurol. Sci. 2014;346:318–322. doi: 10.1016/j.jns.2014.08.036. [DOI] [PubMed] [Google Scholar]
- 120.Anderkova L., Eliasova I. Distinct Pattern of Gray Matter Atrophy in Mild Alzheimer’s Disease Impacts on Cognitive Outcomes of Noninvasive Brain Stimulation. J. Alzheimers Dis. 2015;48:251–260. doi: 10.3233/JAD-150067. [DOI] [PubMed] [Google Scholar]
- 121.Koch G., Bonnì S. Transcranial magnetic stimulation of the precuneus enhances memory and neural activity in prodromal Alzheimer’s disease. Neuroimage. 2018;169:302–311. doi: 10.1016/j.neuroimage.2017.12.048. [DOI] [PubMed] [Google Scholar]
- 122.Zhao J., Li Z. Repetitive transcranial magnetic stimulation improves cognitive function of Alzheimer’s disease patients. Oncotarget. 2017;8:33864–33871. doi: 10.18632/oncotarget.13060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Turriziani P., Smirni D. Enhancing memory performance with rTMS in healthy subjects and individuals with Mild Cognitive Impairment: The role of the right dorsolateral prefrontal cortex. Front. Hum. Neurosci. 2012;6:62. doi: 10.3389/fnhum.2012.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Boggio P.S., Khoury L.P. Temporal cortex direct current stimulation enhances performance on a visual recognition memory task in Alzheimer disease. J. Neurol. Neurosurg. Psychiatry. 2009;80:444–447. doi: 10.1136/jnnp.2007.141853. [DOI] [PubMed] [Google Scholar]
- 125.Khedr E.M., Gamal N.F. A double-blind randomized clinical trial on the efficacy of cortical direct current stimulation for the treatment of Alzheimer’s disease. Front. Aging Neurosci. 2014;6:275. doi: 10.3389/fnagi.2014.00275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Suemoto C.K., Apolinario D. Effects of a non-focal plasticity protocol on apathy in moderate Alzheimer’s disease: A randomized, double-blind, sham-controlled trial. Brain Stimul. 2014;7:308–313. doi: 10.1016/j.brs.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 127.Im J.J., Jeong H. Effects of 6-month at-home transcranial direct current stimulation on cognition and cerebral glucose metabolism in Alzheimer’s disease. Brain Stimul. 2019;12:1222–1228. doi: 10.1016/j.brs.2019.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Manenti R., Sandrini M. Effects of Transcranial Direct Current Stimulation on Episodic Memory in Amnestic Mild Cognitive Impairment: A Pilot Study. J. Gerontol. B Psychol. Sci. Soc. Sci. 2020;75:1403–1413. doi: 10.1093/geronb/gby134. [DOI] [PubMed] [Google Scholar]
- 129.Cruz Gonzalez P., Fong K.N.K. The Effects of Transcranial Direct Current Stimulation on the Cognitive Functions in Older Adults with Mild Cognitive Impairment: A Pilot Study. Behav. Neurol. 2018;2018:5971385. doi: 10.1155/2018/5971385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bystad M., Grønli O. Transcranial direct current stimulation as a memory enhancer in patients with Alzheimer’s disease: A randomized, placebo-controlled trial. Alzheimers Res. Ther. 2016;8:13. doi: 10.1186/s13195-016-0180-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ferrucci R., Mameli F. Transcranial direct current stimulation improves recognition memory in Alzheimer disease. Neurology. 2008;71:493–498. doi: 10.1212/01.wnl.0000317060.43722.a3. [DOI] [PubMed] [Google Scholar]
- 132.Boggio P.S., Ferrucci R. Prolonged visual memory enhancement after direct current stimulation in Alzheimer’s disease. Brain Stimul. 2012;5:223–230. doi: 10.1016/j.brs.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 133.Khedr E.M., Salama R.H. Therapeutic Role of Transcranial Direct Current Stimulation in Alzheimer Disease Patients: Double-Blind, Placebo-Controlled Clinical Trial. Neurorehabil. Neural Repair. 2019;33:384–394. doi: 10.1177/1545968319840285. [DOI] [PubMed] [Google Scholar]
- 134.Gangemi A., Colombo B. Effects of short- and long-term neurostimulation (tDCS) on Alzheimer’s disease patients: Two randomized studies. Aging Clin. Exp. Res. 2021;33:383–390. doi: 10.1007/s40520-020-01546-8. [DOI] [PubMed] [Google Scholar]
- 135.Kehler L., Francisco C.O. The effect of transcranial alternating current stimulation (tACS) on cognitive function in older adults with dementia; Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC); Montreal, QC, Canada. 20–24 July 2020; pp. 3649–3653. [DOI] [PubMed] [Google Scholar]
- 136.ClinicalTrials.gov Gamma tACS in Alzheimer’s Disease. NCT04515433. [(accessed on 7 May 2021)]; Available online: https://ClinicalTrials.gov/show/NCT04515433.
- 137.ClinicalTrials.gov Memory Functions in Mild Alzheimer’s Disease. NCT04785053. [(accessed on 7 May 2021)]; Available online: https://ClinicalTrials.gov/show/NCT04785053.
- 138.Ono K. Alzheimer’s disease as oligomeropathy. Neurochem. Int. 2018;119:57–70. doi: 10.1016/j.neuint.2017.08.010. [DOI] [PubMed] [Google Scholar]
- 139.Holtzman D.M., Morris J.C. Alzheimer’s disease: The challenge of the second century. Sci. Transl. Med. 2011;3:77sr1. doi: 10.1126/scitranslmed.3002369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hamelin L., Lagarde J. Early and protective microglial activation in Alzheimer’s disease: A prospective study using 18F-DPA-714 PET imaging. Pt 4Brain. 2016;139:1252–1264. doi: 10.1093/brain/aww017. [DOI] [PubMed] [Google Scholar]
- 141.Aisen P.S. The development of anti-amyloid therapy for Alzheimer’s disease: From secretase modulators to polymerisation inhibitors. CNS Drugs. 2005;19:989–996. doi: 10.2165/00023210-200519120-00002. [DOI] [PubMed] [Google Scholar]
- 142.Lynch S.Y., Kaplow J. Elenbecestat, e2609, a bace inhibitor: Results from a phase-2 study in subjects with mild cognitive impairment and mild-to-moderate dementia due to alzheimer’s disease. Alzheimer’s Dement. 2018;14(Suppl. 7):P1623. doi: 10.1016/j.jalz.2018.07.213. [DOI] [Google Scholar]
- 143.Huang L.K., Chao S.P. Clinical trials of new drugs for Alzheimer disease. J. Biomed. Sci. 2020;27:18. doi: 10.1186/s12929-019-0609-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Panza F., Lozupone M. Do BACE inhibitor failures in Alzheimer patients challenge the amyloid hypothesis of the disease? Expert Rev. Neurother. 2019;19:599–602. doi: 10.1080/14737175.2019.1621751. [DOI] [PubMed] [Google Scholar]
- 145.Miranda A., Montiel E. Selective Secretase Targeting for Alzheimer’s Disease Therapy. J. Alzheimers Dis. 2021 doi: 10.3233/JAD-201027. [DOI] [PubMed] [Google Scholar]
- 146.Uddin M.S., Hossain M.F. Exploring the multimodal role of phytochemicals in the modulation of cellular signaling pathways to combat age-related neurodegeneration. Sci. Total Environ. 2020;725:138313. doi: 10.1016/j.scitotenv.2020.138313. [DOI] [PubMed] [Google Scholar]
- 147.Pagano K., Tomaselli S. Natural Compounds as Inhibitors of Aβ Peptide Aggregation: Chemical Requirements and Molecular Mechanisms. Front. Neurosci. 2020;14:619667. doi: 10.3389/fnins.2020.619667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Nie Q., Du X.G. Small molecule inhibitors of amyloid β peptide aggregation as a potential therapeutic strategy for Alzheimer’s disease. Acta Pharmacol. Sin. 2011;32:545–551. doi: 10.1038/aps.2011.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sampson E.L., Jenagaratnam L. Metal protein attenuating compounds for the treatment of Alzheimer’s dementia. Cochrane Database Syst. Rev. 2014;2:Cd005380. doi: 10.1002/14651858.CD005380.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Makin S. The amyloid hypothesis on trial. Nature. 2018;559:S4–S7. doi: 10.1038/d41586-018-05719-4. [DOI] [PubMed] [Google Scholar]
- 151.Cheignon C., Tomas M. Oxidative stress and the amyloid beta peptide in Alzheimer’s disease. Redox. Biol. 2018;14:450–464. doi: 10.1016/j.redox.2017.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Schenk D. Amyloid-beta immunotherapy for Alzheimer’s disease: The end of the beginning. Nat. Rev. Neurosci. 2002;3:824–828. doi: 10.1038/nrn938. [DOI] [PubMed] [Google Scholar]
- 153.Loureiro J.C., Pais M.V. Passive antiamyloid immunotherapy for Alzheimer’s disease. Curr. Opin. Psychiatry. 2020;33:284–291. doi: 10.1097/YCO.0000000000000587. [DOI] [PubMed] [Google Scholar]
- 154.Mahase E. Three FDA advisory panel members resign over approval of Alzheimer’s drug. BMJ. 2021;373:n1503. doi: 10.1136/bmj.n1503. [DOI] [PubMed] [Google Scholar]
- 155.Naseri N.N., Wang H. The complexity of tau in Alzheimer’s disease. Neurosci. Lett. 2019;705:183–194. doi: 10.1016/j.neulet.2019.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Iqbal K., Liu F. Tau and neurodegenerative disease: The story so far. Nat. Rev. Neurol. 2016;12:15–27. doi: 10.1038/nrneurol.2015.225. [DOI] [PubMed] [Google Scholar]
- 157.Nelson P.T., Alafuzoff I. Correlation of Alzheimer disease neuropathologic changes with cognitive status: A review of the literature. J. Neuropathol. Exp. Neurol. 2012;71:362–381. doi: 10.1097/NEN.0b013e31825018f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Soeda Y., Takashima A. New Insights Into Drug Discovery Targeting Tau Protein. Front. Mol. Neurosci. 2020;13:590896. doi: 10.3389/fnmol.2020.590896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Pillai R., Uyehara-Lock J.H. Selenium and selenoprotein function in brain disorders. IUBMB Life. 2014;66:229–239. doi: 10.1002/iub.1262. [DOI] [PubMed] [Google Scholar]
- 160.Shultz S.R., Wright D.K. Sodium selenate reduces hyperphosphorylated tau and improves outcomes after traumatic brain injury. Pt 5Brain. 2015;138:1297–1313. doi: 10.1093/brain/awv053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Liu S.L., Wang C. The Role of Cdk5 in Alzheimer’s Disease. Mol. Neurobiol. 2016;53:4328–4342. doi: 10.1007/s12035-015-9369-x. [DOI] [PubMed] [Google Scholar]
- 162.Hernandez F., Lucas J.J. GSK3 and tau: Two convergence points in Alzheimer’s disease. J. Alzheimers Dis. 2013;33(Suppl. 1):S141–S144. doi: 10.3233/JAD-2012-129025. [DOI] [PubMed] [Google Scholar]
- 163.Cicenas J., Kalyan K. Roscovitine in cancer and other diseases. Ann. Transl. Med. 2015;3:135. doi: 10.3978/j.issn.2305-5839.2015.03.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zeidner J.F., Karp J.E. Clinical activity of alvocidib (flavopiridol) in acute myeloid leukemia. Leuk. Res. 2015;39:1312–1318. doi: 10.1016/j.leukres.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 165.Wischik C.M., Edwards P.C. Selective inhibition of Alzheimer disease-like tau aggregation by phenothiazines. Proc. Natl. Acad. Sci. USA. 1996;93:11213–11218. doi: 10.1073/pnas.93.20.11213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Taniguchi S., Suzuki N. Inhibition of heparin-induced tau filament formation by phenothiazines, polyphenols, and porphyrins. J. Biol. Chem. 2005;280:7614–7623. doi: 10.1074/jbc.M408714200. [DOI] [PubMed] [Google Scholar]
- 167.Soeda Y., Saito M. Methylene Blue Inhibits Formation of Tau Fibrils but not of Granular Tau Oligomers: A Plausible Key to Understanding Failure of a Clinical Trial for Alzheimer’s Disease. J. Alzheimers Dis. 2019;68:1677–1686. doi: 10.3233/JAD-181001. [DOI] [PubMed] [Google Scholar]
- 168.Rane J.S., Bhaumik P. Curcumin Inhibits Tau Aggregation and Disintegrates Preformed Tau Filaments in vitro. J. Alzheimers Dis. 2017;60:999–1014. doi: 10.3233/JAD-170351. [DOI] [PubMed] [Google Scholar]
- 169.Baum L., Lam C.W. Six-month randomized, placebo-controlled, double-blind, pilot clinical trial of curcumin in patients with Alzheimer disease. J. Clin. Psychopharmacol. 2008;28:110–113. doi: 10.1097/jcp.0b013e318160862c. [DOI] [PubMed] [Google Scholar]
- 170.Ringman J.M., Frautschy S.A. Oral curcumin for Alzheimer’s disease: Tolerability and efficacy in a 24-week randomized, double blind, placebo-controlled study. Alzheimers Res. Ther. 2012;4:43. doi: 10.1186/alzrt146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Tang M., Taghibiglou C. The Mechanisms of Action of Curcumin in Alzheimer’s Disease. J. Alzheimers Dis. 2017;58:1003–1016. doi: 10.3233/JAD-170188. [DOI] [PubMed] [Google Scholar]
- 172.Rainey-Smith S.R., Brown B.M. Curcumin and cognition: A randomised, placebo-controlled, double-blind study of community-dwelling older adults. Br. J. Nutr. 2016;115:2106–2113. doi: 10.1017/S0007114516001203. [DOI] [PubMed] [Google Scholar]
- 173.Zhu L.N., Mei X. Curcumin intervention for cognitive function in different types of people: A systematic review and meta-analysis. Phytother. Res. 2019;33:524–533. doi: 10.1002/ptr.6257. [DOI] [PubMed] [Google Scholar]
- 174.Bollag D.M., McQueney P.A. Epothilones, a new class of microtubule-stabilizing agents with a taxol-like mechanism of action. Cancer Res. 1995;55:2325–2333. [PubMed] [Google Scholar]
- 175.Zhang B., Carroll J. The microtubule-stabilizing agent, epothilone D, reduces axonal dysfunction, neurotoxicity, cognitive deficits, and Alzheimer-like pathology in an interventional study with aged tau transgenic mice. J. Neurosci. 2012;32:3601–3611. doi: 10.1523/JNEUROSCI.4922-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Barten D.M., Fanara P. Hyperdynamic microtubules, cognitive deficits, and pathology are improved in tau transgenic mice with low doses of the microtubule-stabilizing agent BMS-241027. J. Neurosci. 2012;32:7137–7145. doi: 10.1523/JNEUROSCI.0188-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Fernandez-Valenzuela J.J., Sanchez-Varo R. Enhancing microtubule stabilization rescues cognitive deficits and ameliorates pathological phenotype in an amyloidogenic Alzheimer’s disease model. Sci. Rep. 2020;10:14776. doi: 10.1038/s41598-020-71767-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Gozes I. Microtubules (tau) as an emerging therapeutic target: NAP (davunetide) Curr. Pharm. Des. 2011;17:3413–3417. doi: 10.2174/138161211798072553. [DOI] [PubMed] [Google Scholar]
- 179.Gozes I. NAP (davunetide) provides functional and structural neuroprotection. Curr. Pharm. Des. 2011;17:1040–1044. doi: 10.2174/138161211795589373. [DOI] [PubMed] [Google Scholar]
- 180.Morimoto B.H., Fox A.W. Davunetide: A review of safety and efficacy data with a focus on neurodegenerative diseases. Expert Rev. Clin. Pharmacol. 2013;6:483–502. doi: 10.1586/17512433.2013.827403. [DOI] [PubMed] [Google Scholar]
- 181.Fitzgerald D.P., Emerson D.L. TPI-287, a new taxane family member, reduces the brain metastatic colonization of breast cancer cells. Mol Cancer Ther. 2012;11:1959–1967. doi: 10.1158/1535-7163.MCT-12-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zumbar C.T., Usubalieva A. The CNS penetrating taxane TPI 287 and the AURKA inhibitor alisertib induce synergistic apoptosis in glioblastoma cells. J. Neurooncol. 2018;137:481–492. doi: 10.1007/s11060-018-2755-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Mondal P., Das G. Crafting of Neuroprotective Octapeptide from Taxol-Binding Pocket of β-Tubulin. ACS Chem. Neurosci. 2018;9:615–625. doi: 10.1021/acschemneuro.7b00457. [DOI] [PubMed] [Google Scholar]
- 184.Panza F., Solfrizzi V. Tau-based therapeutics for Alzheimer’s disease: Active and passive immunotherapy. Immunotherapy. 2016;8:1119–1134. doi: 10.2217/imt-2016-0019. [DOI] [PubMed] [Google Scholar]
- 185.Novak P., Zilka N. AADvac1, an Active Immunotherapy for Alzheimer’s Disease and Non Alzheimer Tauopathies: An Overview of Preclinical and Clinical Development. J. Prev. Alzheimers Dis. 2019;6:63–69. doi: 10.14283/jpad.2018.45. [DOI] [PubMed] [Google Scholar]
- 186.Novak P., Schmidt R. Safety and immunogenicity of the tau vaccine AADvac1 in patients with Alzheimer’s disease: A randomised, double-blind, placebo-controlled, phase 1 trial. Lancet Neurol. 2017;16:123–134. doi: 10.1016/S1474-4422(16)30331-3. [DOI] [PubMed] [Google Scholar]
- 187.Novak P., Schmidt R. Fundamant: An interventional 72-week phase 1 follow-up study of AADvac1, an active immunotherapy against tau protein pathology in Alzheimer’s disease. Alzheimers Res. Ther. 2018;10:108. doi: 10.1186/s13195-018-0436-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Theunis C., Crespo-Biel N. Efficacy and safety of a liposome-based vaccine against protein Tau, assessed in tau.P301L mice that model tauopathy. PLoS ONE. 2013;8:e72301. doi: 10.1371/journal.pone.0072301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Hoskin J.L., Sabbagh M.N. Tau immunotherapies for Alzheimer’s disease. Expert Opin. Investig. Drugs. 2019;28:545–554. doi: 10.1080/13543784.2019.1619694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Qureshi I.A., Tirucherai G. A randomized, single ascending dose study of intravenous BIIB092 in healthy participants. Alzheimers Dement. 2018;4:746–755. doi: 10.1016/j.trci.2018.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.ClinicalTrials.gov Safety, Tolerability, and Pharmacokinetics of C2N-8E12 in Subjects with Progressive Supranuclear Palsy. NCT02494024. [(accessed on 4 April 2021)]; Available online: https://ClinicalTrials.gov/show/NCT02494024.
- 192.ClinicalTrials.gov A Study of LY3303560 in Healthy Participants and Participants with Alzheimer’s Disease (AD). NCT02754830. [(accessed on 4 April 2021)]; Available online: https://ClinicalTrials.gov/show/NCT02754830.
- 193.ClinicalTrials.gov A Study of LY3303560 in Participants with Mild Cognitive Impairment or Alzheimer’s Disease. NCT03019536. [(accessed on 4 April 2021)]; Available online: https://ClinicalTrials.gov/show/NCT03019536.
- 194.ClinicalTrials.gov A Study of JNJ-63733657 in Healthy Japanese Participants. NCT03689153. [(accessed on 4 April 2021)]; Available online: https://ClinicalTrials.gov/show/NCT03689153.
- 195.ClinicalTrials.gov A Study to Investigate Safety and Tolerability, Pharmacokinetics and Pharmacodynamics of JNJ-63733657 in Healthy Subjects and Subjects with Alzheimer’s Disease. NCT03375697. [(accessed on 4 April 2021)]; Available online: https://ClinicalTrials.gov/show/NCT03375697.
- 196.Kreisl W.C., Lyoo C.H. In vivo radioligand binding to translocator protein correlates with severity of Alzheimer’s disease. Pt 7Brain. 2013;136:2228–2238. doi: 10.1093/brain/awt145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Kaur D., Sharma V. Activation of microglia and astrocytes: A roadway to neuroinflammation and Alzheimer’s disease. Inflammopharmacology. 2019;27:663–677. doi: 10.1007/s10787-019-00580-x. [DOI] [PubMed] [Google Scholar]
- 198.Regen F., Hellmann-Regen J. Neuroinflammation and Alzheimer’s Disease: Implications for Microglial Activation. Curr. Alzheimer Res. 2017;14:1140–1148. doi: 10.2174/1567205014666170203141717. [DOI] [PubMed] [Google Scholar]
- 199.Kinney J.W., Bemiller S.M. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimers Dement. 2018;4:575–590. doi: 10.1016/j.trci.2018.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Shi Y., Holtzman D.M. Interplay between innate immunity and Alzheimer disease: APOE and TREM2 in the spotlight. Nat. Rev. Immunol. 2018;18:759–772. doi: 10.1038/s41577-018-0051-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Atagi Y., Liu C.C. Apolipoprotein E Is a Ligand for Triggering Receptor Expressed on Myeloid Cells 2 (TREM2) J. Biol. Chem. 2015;290:26043–26050. doi: 10.1074/jbc.M115.679043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Wolfe C.M., Fitz N.F. The Role of APOE and TREM2 in Alzheimer’s Disease-Current Understanding and Perspectives. Int. J. Mol. Sci. 2018;20:81. doi: 10.3390/ijms20010081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Lee C.Y.D., Daggett A. Elevated TREM2 Gene Dosage Reprograms Microglia Responsivity and Ameliorates Pathological Phenotypes in Alzheimer’s Disease Models. Neuron. 2018;97:1032–1048.e5. doi: 10.1016/j.neuron.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Jay T.R., Hirsch A.M. Disease Progression-Dependent Effects of TREM2 Deficiency in a Mouse Model of Alzheimer’s Disease. J. Neurosci. 2017;37:637–647. doi: 10.1523/JNEUROSCI.2110-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Yang J., Wise L. TLR4 Cross-Talk with NLRP3 Inflammasome and Complement Signaling Pathways in Alzheimer’s Disease. Front. Immunol. 2020;11:724. doi: 10.3389/fimmu.2020.00724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Zakaria R., Wan Yaacob W.M. Lipopolysaccharide-induced memory impairment in rats: A model of Alzheimer’s disease. Physiol. Res. 2017;66:553–565. doi: 10.33549/physiolres.933480. [DOI] [PubMed] [Google Scholar]
- 207.Lax N., Fainstein N. Systemic microbial TLR2 agonists induce neurodegeneration in Alzheimer’s disease mice. J. Neuroinflamm. 2020;17:55. doi: 10.1186/s12974-020-01738-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Richard K.L., Filali M. Toll-like receptor 2 acts as a natural innate immune receptor to clear amyloid beta 1-42 and delay the cognitive decline in a mouse model of Alzheimer’s disease. J. Neurosci. 2008;28:5784–5793. doi: 10.1523/JNEUROSCI.1146-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.McDonald C.L., Hennessy E. Inhibiting TLR2 activation attenuates amyloid accumulation and glial activation in a mouse model of Alzheimer’s disease. Brain Behav. Immun. 2016;58:191–200. doi: 10.1016/j.bbi.2016.07.143. [DOI] [PubMed] [Google Scholar]
- 210.Zhou C., Sun X. Genomic deletion of TLR2 induces aggravated white matter damage and deteriorated neurobehavioral functions in mouse models of Alzheimer’s disease. Aging (Albany NY) 2019;11:7257–7273. doi: 10.18632/aging.102260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Perez-Nievas B.G., Serrano-Pozo A. Deciphering the Astrocyte Reaction in Alzheimer’s Disease. Front. Aging Neurosci. 2018;10:114. doi: 10.3389/fnagi.2018.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Ben Haim L., Ceyzériat K. The JAK/STAT3 pathway is a common inducer of astrocyte reactivity in Alzheimer’s and Huntington’s diseases. J. Neurosci. 2015;35:2817–2829. doi: 10.1523/JNEUROSCI.3516-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Nagamoto-Combs K., Combs C.K. Microglial phenotype is regulated by activity of the transcription factor, NFAT (nuclear factor of activated T cells) J. Neurosci. 2010;30:9641–9646. doi: 10.1523/JNEUROSCI.0828-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Hudry E., Wu H.Y. Inhibition of the NFAT pathway alleviates amyloid β neurotoxicity in a mouse model of Alzheimer’s disease. J. Neurosci. 2012;32:3176–3192. doi: 10.1523/JNEUROSCI.6439-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Rojanathammanee L., Floden A.M. Attenuation of microglial activation in a mouse model of Alzheimer’s disease via NFAT inhibition. J. Neuroinflamm. 2015;12:42. doi: 10.1186/s12974-015-0255-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Dugan L.L., Ali S.S. IL-6 mediated degeneration of forebrain GABAergic interneurons and cognitive impairment in aged mice through activation of neuronal NADPH oxidase. PLoS ONE. 2009;4:e5518. doi: 10.1371/journal.pone.0005518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.François A., Rioux Bilan A. Longitudinal follow-up of autophagy and inflammation in brain of APPswePS1dE9 transgenic mice. J. Neuroinflamm. 2014;11:139. doi: 10.1186/s12974-014-0139-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Heneka M.T., Kummer M.P. NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature. 2013;493:674–678. doi: 10.1038/nature11729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Feng Y.S., Tan Z.X. The involvement of NLRP3 inflammasome in the treatment of Alzheimer’s disease. Ageing Res. Rev. 2020;64:101192. doi: 10.1016/j.arr.2020.101192. [DOI] [PubMed] [Google Scholar]
- 220.Lee J.K., Kim N.J. Recent Advances in the Inhibition of p38 MAPK as a Potential Strategy for the Treatment of Alzheimer’s Disease. Molecules. 2017;22:1287. doi: 10.3390/molecules22081287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Chen Y., Li G. p38 MAPK mediates glial P2 × 7R-neuronal P2Y1R inhibitory control of P2 × 3R expression in dorsal root ganglion neurons. Mol. Pain. 2015;11:68. doi: 10.1186/s12990-015-0073-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Simões A.P., Silva C.G. Glutamate-induced and NMDA receptor-mediated neurodegeneration entails P2Y1 receptor activation. Cell Death Dis. 2018;9:297. doi: 10.1038/s41419-018-0351-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Kandimalla R., Thirumala V. Is Alzheimer’s disease a Type 3 Diabetes? A critical appraisal. Biochim. Biophys. Acta Mol. Basis Dis. 2017;1863:1078–1089. doi: 10.1016/j.bbadis.2016.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.de la Monte S.M. Insulin Resistance and Neurodegeneration: Progress Towards the Development of New Therapeutics for Alzheimer’s Disease. Drugs. 2017;77:47–65. doi: 10.1007/s40265-016-0674-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Femminella G.D., Frangou E. Evaluating the effects of the novel GLP-1 analogue liraglutide in Alzheimer’s disease: Study protocol for a randomised controlled trial (ELAD study) Trials. 2019;20:191. doi: 10.1186/s13063-019-3259-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Campbell J.M., Stephenson M.D. Metformin Use Associated with Reduced Risk of Dementia in Patients with Diabetes: A Systematic Review and Meta-Analysis. J. Alzheimers Dis. 2018;65:1225–1236. doi: 10.3233/JAD-180263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Samaras K., Makkar S. Metformin Use Is Associated With Slowed Cognitive Decline and Reduced Incident Dementia in Older Adults With Type 2 Diabetes: The Sydney Memory and Ageing Study. Diabetes Care. 2020;43:2691–2701. doi: 10.2337/dc20-0892. [DOI] [PubMed] [Google Scholar]
- 228.Iglesias J., Morales L. Metabolic and Inflammatory Adaptation of Reactive Astrocytes: Role of PPARs. Mol. Neurobiol. 2017;54:2518–2538. doi: 10.1007/s12035-016-9833-2. [DOI] [PubMed] [Google Scholar]
- 229.Tufano M., Pinna G. Is There a Future for PPARs in the Treatment of Neuropsychiatric Disorders? Molecules. 2020;25:1062. doi: 10.3390/molecules25051062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Megur A., Baltriukienė D. The Microbiota-Gut-Brain Axis and Alzheimer’s Disease: Neuroinflammation Is to Blame? Nutrients. 2020;13:37. doi: 10.3390/nu13010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Sochocka M., Donskow-Łysoniewska K. The Gut Microbiome Alterations and Inflammation-Driven Pathogenesis of Alzheimer’s Disease-a Critical Review. Mol. Neurobiol. 2019;56:1841–1851. doi: 10.1007/s12035-018-1188-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Wang X., Sun G. Sodium oligomannate therapeutically remodels gut microbiota and suppresses gut bacterial amino acids-shaped neuroinflammation to inhibit Alzheimer’s disease progression. Cell Res. 2019;29:787–803. doi: 10.1038/s41422-019-0216-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Syed Y.Y. Sodium Oligomannate: First Approval. Drugs. 2020;80:441–444. doi: 10.1007/s40265-020-01268-1. [DOI] [PubMed] [Google Scholar]
- 234.Longo F.M., Massa S.M. Neuroprotective strategies in Alzheimer’s disease. NeuroRx. 2004;1:117–127. doi: 10.1602/neurorx.1.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Eddy C.M., Rickards H.E. The cognitive impact of antiepileptic drugs. Ther. Adv. Neurol. Disord. 2011;4:385–407. doi: 10.1177/1756285611417920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Daniels V., Wood M. Modulation of the conformational state of the SV2A protein by an allosteric mechanism as evidenced by ligand binding assays. Br. J. Pharmacol. 2013;169:1091–1101. doi: 10.1111/bph.12192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Cortes-Altamirano J.L., Olmos-Hernández A. Levetiracetam as an antiepileptic, neuroprotective, and hyperalgesic drug. Neurol. India. 2016;64:1266–1275. doi: 10.4103/0028-3886.193801. [DOI] [PubMed] [Google Scholar]
- 238.Sola I., Aso E. Novel Levetiracetam Derivatives That Are Effective against the Alzheimer-like Phenotype in Mice: Synthesis, in Vitro, ex Vivo, and in Vivo Efficacy Studies. J. Med. Chem. 2015;58:6018–6032. doi: 10.1021/acs.jmedchem.5b00624. [DOI] [PubMed] [Google Scholar]
- 239.Schoenberg M.R., Rum R.S. A randomized, double-blind, placebo-controlled crossover study of the effects of levetiracetam on cognition, mood, and balance in healthy older adults. Epilepsia. 2017;58:1566–1574. doi: 10.1111/epi.13849. [DOI] [PubMed] [Google Scholar]
- 240.Yan B.C., Wang J. Neuroprotective Effects of Gabapentin Against Cerebral Ischemia Reperfusion-Induced Neuronal Autophagic Injury via Regulation of the PI3K/Akt/mTOR Signaling Pathways. J. Neuropathol. Exp. Neurol. 2019;78:157–171. doi: 10.1093/jnen/nly119. [DOI] [PubMed] [Google Scholar]
- 241.Ortinski P., Meador K.J. Cognitive side effects of antiepileptic drugs. Epilepsy Behav. 2004;5(Suppl. 1):S60–S65. doi: 10.1016/j.yebeh.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 242.Supasitthumrong T., Bolea-Alamanac B.M. Gabapentin and pregabalin to treat aggressivity in dementia: A systematic review and illustrative case report. Br. J. Clin. Pharmacol. 2019;85:690–703. doi: 10.1111/bcp.13844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Liu J., Chang L. The Role of NMDA Receptors in Alzheimer’s Disease. Front. Neurosci. 2019;13:43. doi: 10.3389/fnins.2019.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Huang Y.J., Lin C.H. NMDA Neurotransmission Dysfunction in Behavioral and Psychological Symptoms of Alzheimer’s Disease. Curr. Neuropharmacol. 2012;10:272–285. doi: 10.2174/157015912803217288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Lin C.H., Huang Y.J. NMDA neurotransmission dysfunction in mild cognitive impairment and Alzheimer’s disease. Curr. Pharm. Des. 2014;20:5169–5179. doi: 10.2174/1381612819666140110115603. [DOI] [PubMed] [Google Scholar]
- 246.Lin C.H., Chen Y.M. Novel Treatment for the Most Resistant Schizophrenia: Dual Activation of NMDA Receptor and Antioxidant. Curr. Drug Targets. 2020;21:610–615. doi: 10.2174/1389450120666191011163539. [DOI] [PubMed] [Google Scholar]
- 247.Lin C.H., Yang H.T. Precision Medicine of Sodium Benzoate for the Treatment of Behavioral and Psychological Symptoms of Dementia (BPSD) Neuropsychiatr. Dis. Treat. 2020;16:509–518. doi: 10.2147/NDT.S234371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Lin C.H., Lin C.H. Sodium Benzoate, a D-Amino Acid Oxidase Inhibitor, Added to Clozapine for the Treatment of Schizophrenia: A Randomized, Double-Blind, Placebo-Controlled Trial. Biol. Psychiatry. 2018;84:422–432. doi: 10.1016/j.biopsych.2017.12.006. [DOI] [PubMed] [Google Scholar]
- 249.Okamoto M., Gray J.D. Riluzole reduces amyloid beta pathology, improves memory, and restores gene expression changes in a transgenic mouse model of early-onset Alzheimer’s disease. Transl. Psychiatry. 2018;8:153. doi: 10.1038/s41398-018-0201-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Gulyaeva N.V. Hippocampal hyperglutamatergic signaling matters: Early targeting glutamate neurotransmission as a preventive strategy in Alzheimer’s disease: An Editorial Highlight for “Riluzole attenuates glutamatergic tone and cognitive decline in AβPP/PS1 mice” on page 513. J. Neurochem. 2021;156:399–402. doi: 10.1111/jnc.15238. [DOI] [PubMed] [Google Scholar]
- 251.Swanson D., Block R. Omega-3 fatty acids EPA and DHA: Health benefits throughout life. Adv. Nutr. 2012;3:1–7. doi: 10.3945/an.111.000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Dyall S.C. Long-chain omega-3 fatty acids and the brain: A review of the independent and shared effects of EPA, DPA and DHA. Front. Aging Neurosci. 2015;7:52. doi: 10.3389/fnagi.2015.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Thomas J., Thomas C.J. Omega-3 Fatty Acids in Early Prevention of Inflammatory Neurodegenerative Disease: A Focus on Alzheimer’s Disease. Biomed. Res. Int. 2015;2015:172801. doi: 10.1155/2015/172801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Mazereeuw G., Lanctôt K.L. Effects of ω-3 fatty acids on cognitive performance: A meta-analysis. Neurobiol. Aging. 2012;33:1482.e17–1482.e29. doi: 10.1016/j.neurobiolaging.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 255.Patrick R.P. Role of phosphatidylcholine-DHA in preventing APOE4-associated Alzheimer’s disease. FASEB J. 2019;33:1554–1564. doi: 10.1096/fj.201801412R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Araya-Quintanilla F., Gutiérrez-Espinoza H. Effectiveness of omega-3 fatty acid supplementation in patients with Alzheimer disease: A systematic review and meta-analysis. Neurologia. 2020;35:105–114. doi: 10.1016/j.nrl.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 257.Hansen N. Brain stimulation for combating Alzheimer’s disease. Front. Neurol. 2014;5:80. doi: 10.3389/fneur.2014.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Chang C.H., Lane H.Y. Brain Stimulation in Alzheimer’s Disease. Front. Psychiatry. 2018;9:201. doi: 10.3389/fpsyt.2018.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.McKinnon C., Gros P. Deep brain stimulation: Potential for neuroprotection. Ann. Clin. Transl. Neurol. 2019;6:174–185. doi: 10.1002/acn3.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Laxton A.W., Tang-Wai D.F. A phase I trial of deep brain stimulation of memory circuits in Alzheimer’s disease. Ann. Neurol. 2010;68:521–534. doi: 10.1002/ana.22089. [DOI] [PubMed] [Google Scholar]
- 261.Hardenacke K., Hashemiyoon R. Deep Brain Stimulation of the Nucleus Basalis of Meynert in Alzheimer’s Dementia: Potential Predictors of Cognitive Change and Results of a Long-Term Follow-Up in Eight Patients. Brain Stimul. 2016;9:799–800. doi: 10.1016/j.brs.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 262.Baldermann J.C., Hardenacke K. Neuroanatomical Characteristics Associated with Response to Deep Brain Stimulation of the Nucleus Basalis of Meynert for Alzheimer’s Disease. Neuromodulation. 2018;21:184–190. doi: 10.1111/ner.12626. [DOI] [PubMed] [Google Scholar]
- 263.Silberstein S.D., Yuan H. Non-invasive vagus nerve stimulation for primary headache: A clinical update. Cephalalgia. 2020;40:1370–1384. doi: 10.1177/0333102420941864. [DOI] [PubMed] [Google Scholar]
- 264.Jacobs H.I., Riphagen J.M. Transcutaneous vagus nerve stimulation boosts associative memory in older individuals. Neurobiol. Aging. 2015;36:1860–1867. doi: 10.1016/j.neurobiolaging.2015.02.023. [DOI] [PubMed] [Google Scholar]
- 265.Farmer A.D., Strzelczyk A. International Consensus Based Review and Recommendations for Minimum Reporting Standards in Research on Transcutaneous Vagus Nerve Stimulation (Version 2020) Front. Hum. Neurosci. 2020;14:568051. doi: 10.3389/fnhum.2020.568051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Luber B., McClintock S.M. Applications of transcranial magnetic stimulation and magnetic seizure therapy in the study and treatment of disorders related to cerebral aging. Dialogues Clin. Neurosci. 2013;15:87–98. doi: 10.31887/DCNS.2013.15.1/bluber. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Klomjai W., Katz R. Basic principles of transcranial magnetic stimulation (TMS) and repetitive TMS (rTMS) Ann. Phys. Rehabil. Med. 2015;58:208–213. doi: 10.1016/j.rehab.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 268.Somani A., Kar S.K. Efficacy of repetitive transcranial magnetic stimulation in treatment-resistant depression: The evidence thus far. Gen. Psychiatr. 2019;32:e100074. doi: 10.1136/gpsych-2019-100074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Drumond Marra H.L., Myczkowski M.L. Transcranial Magnetic Stimulation to Address Mild Cognitive Impairment in the Elderly: A Randomized Controlled Study. Behav. Neurol. 2015;2015:287843. doi: 10.1155/2015/287843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Chou Y.H., Ton That V. A systematic review and meta-analysis of rTMS effects on cognitive enhancement in mild cognitive impairment and Alzheimer’s disease. Neurobiol. Aging. 2020;86:1–10. doi: 10.1016/j.neurobiolaging.2019.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Rabey J.M., Dobronevsky E. Repetitive transcranial magnetic stimulation combined with cognitive training is a safe and effective modality for the treatment of Alzheimer’s disease: A randomized, double-blind study. J. Neural Transm. (Vienna) 2013;120:813–819. doi: 10.1007/s00702-012-0902-z. [DOI] [PubMed] [Google Scholar]
- 272.Woods A.J., Antal A. A technical guide to tDCS, and related non-invasive brain stimulation tools. Clin. Neurophysiol. 2016;127:1031–1048. doi: 10.1016/j.clinph.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Buss S.S., Fried P.J. Therapeutic noninvasive brain stimulation in Alzheimer’s disease and related dementias. Curr. Opin. Neurol. 2019;32:292–304. doi: 10.1097/WCO.0000000000000669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Lefaucheur J.P. A comprehensive database of published tDCS clinical trials (2005–2016) Neurophysiol. Clin. 2016;46:319–398. doi: 10.1016/j.neucli.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 275.Antonenko D., Faxel M. Effects of Transcranial Alternating Current Stimulation on Cognitive Functions in Healthy Young and Older Adults. Neural Plast. 2016;2016:4274127. doi: 10.1155/2016/4274127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Fröhlich F., Sellers K.K. Targeting the neurophysiology of cognitive systems with transcranial alternating current stimulation. Expert Rev. Neurother. 2015;15:145–167. doi: 10.1586/14737175.2015.992782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.ClinicalTrials.gov Transcranial Alternating Current Stimulation for Patients with Mild Alzheimer’s Disease (TRANSFORM-AD). NCT03920826. [(accessed on 7 May 2021)]; Available online: https://ClinicalTrials.gov/show/NCT03920826.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is contained within the article.