Abstract
Objectives
Urinary tract infections (UTIs) in children are rapidly increasing worldwide and are commonly caused by extensively drug-resistant bacteria. This study determines the prevalence of UTIs in paediatric patients and evaluates the pattern of extensively drug-resistance in Escherichia coli and Klebsiellapneumoniae strains isolated from paediatric UTI patients.
Methods
Uropathogenic bacterial strains were isolated from paediatric patients with UTIs admitted to the Institute of Child Health, Lahore, Pakistan. Strains of both E. coli and K. pneumoniae were identified using biochemical characterisation and subjected to antibiotic susceptibility assays for 21 common antimicrobial drugs in order to determine their extensively drug-resistant profile.
Results
We isolated 63 E. coli and 37 K. pneumoniae strains from 130 paediatric patients with UTIs over a period of six months. The antibiotic susceptibility assays showed that both the E. coli and K. pneumoniae strains exhibited a high degree of resistance against co-amoxiclav, cefuroxime, cefixime, cefotaxime, ceftazidime, ceftriaxone, ciprofloxacin, nalidixic acid, norfloxacin, pepedemic acid, and co-trimoxazole. However, several of the antimicrobial agents, including polymyxin B, colistin sulphate, chloramphenicol, nitrofurantoin, and fosfomycin, were found to retain their antimicrobial activities against both pathogens. The five highest antibiotic resistant strains were identified as E. coli strains ZK9, ZK40, and ZK60 and K. pneumoniae ZK32 and ZK89 using 16S rRNA gene sequencing.
Conclusion
Our study demonstrates that E. coli and K. pneumonia are the dominant extensively drug-resistant uropathogenic bacteria in community-acquired UTIs in our cohort. These uropathogens were found to be resistant to the majority of the routinely-used classes of β-lactams, pyridopyrimidines, quinolones, and fluoroquinolone antibiotics, and these findings may be useful for clinicians in their treatment of paediatric UTIs.
Keywords: 16S rRNA gene sequencing, Klebsiella pneumoniae, Multiple drug-resistance, Paediatric infections, Urinary tract infections
الملخص
أهداف البحث
تتزايد التهابات المسالك البولية في الأطفال بسرعة في جميع أنحاء العالم، وعادة ما تكون ناجمة عن البكتيريا المقاومة للأدوية. هذه الدراسة تحدد انتشار التهاب المسالك البولية في الأطفال، وتقيم نمط البكتيريا المقاومة للأدوية من قبل سلالات معزولة من الإشريكية والكلبسيلة عن التهابات المسالك البولية في مرضى الأطفال.
طرق البحث
تم عزل السلالات البكتيرية الممرضة للجهاز البولي من المرضى الأطفال بالتهابات المسالك البولية التي تم إدخالها في معهد صحة الطفل بلاهور، باكستان. تم تحديد السلالات من جنس الإشريكية والكلبسيلة، من خلال التوصيف الكيميائي الحيوي، وتعرضت لمقاييس الحساسية للمضادات الحيوية لتحديد البكتيريا المقاومة للأدوية ضد ٢١ من الأدوية المضادة للميكروبات.
النتائج
خلال ستة أشهر، تم عزل ما مجموعه ٦٣ من الإشريكية و٣٧ من سلالات الكلبسيلة من ١٣٠ طفلا مصابين بالتهابات المسالك البولية. وأظهرت مقاييس حساسية المضادات الحيوية أن الإشريكية والكلبسيلة لها مقاومة عالية ضد كوأموكسيكلاف، وسيفروكسيم، وسيفيكسيم، وسيفوتاكسيم، وسيفتازيديم، وسيفترياكسون، وسيبروفلوكساسين، وحمض ناليديكسيك، ونورفلوكساسين، وحمض بيبيديمك، وكوتريموكسيزول. ومع ذلك، فقد وجد أن للعقاقير المضادة للميكروبات بما في ذلك بوليميكسين-ب، وكبريتات الكوليستين، والكلورامفينيكول، ونيتروفوارانتون، وفوسفومايسين فاعلية كبرى للتحكم بالسلالات الممرضة للجهاز البولي من القولونية الإشريكيشية والكلبسيلة الرئوية. وتم تحديد السلالات الخمس التي تعاني من أعلى مقاومة ضد المضادات الحيوية المختبرة على أنها سلالات من الإشريكية القولونية والكلبسيلة الرئوية.
الاستنتاجات
أظهرت دراستنا أن الإشريكية القولونية والكلبسيلة الرئوية كانت البكتيريا المهيمنة لمقاومة للأدوية من التهابات المسالك البولية المكتسبة من المجتمع في مجموعتنا. وتم الوصول إلى أن هذه البكتيريا الممرضة للجهاز البولي غير مستجيبة للعقارات المستخدمة بشكل روتيني من المضادات الحيوية مثل بيتا اللاكتام، والبيريدوبيريميدين، والكينولون، والفلوروكينولون. قد تكون هذه النتائج مفيدة للأطباء لتعزيز العلاج التجريبي لالتهابات المسالك البولية في الأطفال.
الكلمات المفتاحية: متعددة مقاومة للأدوية, طب الأطفال, عدوى المسالك البولية, تسلسل الجينات, الكلبسيلة الرئوية
Introduction
Urinary tract infections (UTIs) are among the most prevalent and serious infections in children.1 They frequently affect the lower urinary tract and are commonly referred to as bladder infections. UTIs may also affect the kidneys and are commonly referred to as pyelonephritis UTIs. Failure to effectively treat these infections may result in renal scarring, hypertension, and end-stage renal failure.2,3 Owing to the high number of asymptomatic UTIs, its diagnosis and treatment remain challenging in this population. The risk of UTIs increases in hospitalised children with complicated severe acute malnutrition,4 and UTIs are often diagnosed in children who exhibit poor toilet and hygiene habits, which may result in increased incidence of genetic transfer between pathogens and/or blockage in the normal urine flow between the ureters and kidney. UTIs are most common in girls as their urethra is closer to the anus.5 The incidence of UTIs in boys increases within the first three months of starting school, but then decreases over time.6 These infections are also commonly associated with the incorrect use of prescribed medications. This means that the rapid diagnosis and treatment of UTIs in small children is critical in preventing the long-term morbidities associated with renal scarring, such as hypertension, toxaemia in pregnancy, chronic kidney disease, and ultimately the need for renal transplantation.2,3
UTIs are caused by Gram-negative and Gram-positive bacterial pathogens commonly found in the gastrointestinal tract. Gram-negative bacteria, including Citrobacter spp., Enterococcus spp., Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, Pseudomonas aeruginosa, and Serratia spp., and Gram-positive bacteria, including Staphylococcus epidermidis, Staphylococcus saprophyticus, and Streptococcus spp., are the most commonly identified uropathogenic bacteria in these UTIs.7,8 Such uropathogenic bacteria demonstrate extensive resistance to third-generation cephalosporins, classifying most of these infections as multiple drug-resistant (MDR) or extensive drug resistant (XDR).9, 10, 11, 12 MDR infections demonstrate resistance to more than one antimicrobial agent, while XDR have resistance to almost all antimicrobial agents.12 Recent developments in paediatric urology have highlighted growing concerns around the emergence of extensive antibiotic resistance in urinary pathogens as a result of improper use and overuse of antibiotics.13 Patients with sepsis in the intensive care unit also demonstrate high levels of MDR bacteria and produce morbid urine.14,15 The antimicrobial susceptibility patterns in most pathogens demonstrate some geographical variation, and these changes are critical to establishing effective antimicrobial policies in local healthcare units. The use of antibiotics has resulted in a 95% reduction in infectious diseases with high mortality rates.16 Given their effectiveness in mitigating health risks, antibiotic consumption has continued to increase in modern society.
UTI-causing uropathogenic bacteria experience extensive antimicrobial resistance with most strains exhibiting some resistance to nalidixic acid (81%), trimethoprim/sulfamethoxazole (83%), amoxicillin (67%), co-trimoxazole (61%), cephalexin (43%), gentamicin (49%), and ciprofloxacin (46%).17 The discovery of novel antibiotics could help promote efforts to combat MDR and XDR in bacteria; however, where resistance is mediated by changes in cell wall permeability, novel antibiotics may be insufficient to combat widespread resistance.18 Reduced permeability causes a reduction in the intracellular concentration of antibiotics and may be the result of efflux pumps, which actively transport the antibiotics out of the cell.19,20 Bacteria also produce a variety of antibiotic metabolising enzymes, including β-lactamases, aminoglycoside-modifying enzymes, and chloramphenicol acetyltransferases that inactivate the antibiotics before they can exert their effects. Variations in the target site might also occur, in which case the antibiotic will lose its efficacy.21 Most of these genes are encoded on plasmids, which may be transferred between bacterial populations, resulting in the emergence of the ever-growing numbers of MDR strains. These gene transfers have been studied extensively, and remain a critical concern in antimicrobial resistance.22 This study determines the prevalence of UTIs and the frequency of the most commonly-found MDR and XDR uropathogenic E.coli and Klebsiella spp. in paediatric patients from Lahore, Pakistan. These uropathogenic bacteria are characterised using biochemical assays and screened for antibiotic resistance before final identification using 16S rRNA gene sequencing.
Materials and Methods
Urine collection
This study was conducted using a cohort of paediatric patients being treated in the outpatient department (OPD) and inpatient departments (nephrology, neonatal unit, general medical, urology, and neurology wards) of The Children's Hospital and the Institute of Child Health in Lahore, Pakistan, between June 2018 and February 2019. Freshly voided midstream urine specimens were collected from both male and female patients aged 0–13 years suffering from high fever, abdominal pain, and vomiting. Urine samples were collected using a clean catch method in dry, sterile, wide neck, leak-proof containers. Informed consent from each participant or their guardians was recorded on a form that included explicit consent for the collection, storage, and testing of the samples. More than 130 specimens were aseptically collected and transported to the Laboratory of Microbiology at the Institute for Molecular Biology and Biotechnology at the University of Lahore in Pakistan. The children were diagnosed with symptomatic UTI and presented with a variety of symptoms, including fever, chills, nausea, vomiting, dysuria, frequency, urgency, incontinence, and flank pain.23 The 5 mL urine samples were centrifuged at 10,000×g for 5 min and the supernatant was stained and processed to estimate white blood cell counts (pus cells) using a light microscope at low (10x) and high (40x) power as described by Cappuccino and Sherman.24 Urine samples with a white blood cell count [WBC] of ≥5 cells/high power field (HPF) were considered positive for UTI and were then used to isolate bacterial pathogens.
Isolation of uropathogenic bacteria
Uropathogenic bacteria were isolated from urine samples using cysteine lactose electrolyte-deficient (CLED) agar.25 Urine was poured over autoclaved CLED agar media at different dilutions and incubated at 37 °C for 72 h. Colonies with a yellow, opaque, or slightly deeper colour were tentatively identified as Escherichia spp., while large mucoid yellow or yellow-white colonies were considered to belong to the Klebsiella spp. Selected colonies were then picked, purified, and preserved at −20 °C until further experiments.
Biochemical characterization
The isolated strains were screened for Gram staining using the Duguid et al. method.26 To differentiate between lactose fermenting and non-fermenting bacterial strains, freshly grown strains were streaked on MacConkey agar and incubated at 37 °C for 24 h. After incubation, bacterial strains were evaluated for pigmentation and any colony appearing as pink-red was classified as a lactose fermenting organism. To check the haemolysis of each bacterial strain, freshly grown strains were streaked on blood agar plates and incubated at 37 °C for 24 h. A clear zone around the colony confirmed the haemolytic capacity of each of the screened strains and enzymatic activities, such as oxidase, catalase, and urease activities, were then evaluated. Strains were also evaluated for citrate utilisation, motility, and indole production using biochemical tests, as described by Cappuccino and Sherman.24
Antibiotic resistance test
The disk diffusion method, M100, from the Clinical and Laboratory Standard Institute (CLSI)27 was used to evaluate the antimicrobial susceptibility of each strain against 21 different antibiotics, including co-amoxiclav, amikacin, cefuroxime, cefixime, cefotaxime, ceftazidime, ceftriaxone, ciprofloxacin, nalidixic acid, nitrofurantoin, norfloxacin, pipemidic acid, sulbactam/cefoperazone, colistin sulphate, fosfomycin, meropenem, imipenem, chloramphenicol, piperacillin/tazobactam, co-trimoxazole, and polymyxin B. Uropathogenic strains were freshly grown overnight and heavily streaked on Mueller-Hinton (MH) plates.28 Then, commercially available 4 mm antibiotic paper discs (Abtek Biologicals Ltd, UK) were placed on the MH plates and incubated at 37 °C for 24 h. Subsequently, the zone of inhibition in the bacterial lawn was then measured and recorded. E. coli ATCC 23509 and E. coli ATCC 25922 were also tested for antibiotic susceptibility and used as the control for our observations. The diameters of the zones of inhibition were then categorised as sensitive or resistant using the standard diameters provided by the CLSI.27 The non-susceptible strains were classified as MDR, XDR, and pan drug-resistance (PDR) based on the criteria reported in Mogiorakos et al.12 Non-susceptible strains presenting with resistance to ≥1 agent were non-susceptible; strains resistant to ≥3 antimicrobial categories were considered MDR, while those with susceptibility to ≥1 agent in all but ≤2 antimicrobial categories were considered to be XDR. Those bacterial strains that were resistant to all the listed antimicrobial agents were considered PDR.
Identification of uropathogenic bacterial strains using 16S rRNA gene sequencing
The genomic DNA from selected antibiotic-resistant uropathogenic bacterial strains (ZK9, ZK32, ZK40, ZK60, and ZK89) was isolated using proteinase K treatment and the polymerase chain reaction was performed as described by Mumtaz et al.29 The PCR product was then sequenced using a commercial service provided by Macrogen (Seoul, Korea) and the sequences were identified using the BLAST algorithm from the NCBI web browser. The phylogenetic tree of closely related species was constructed using MEGA 7.0.26. DNA sequences were aligned using ClustalW, and phylogeny was determined using the maximum parsimony method.
Results
Uropathogenic Escherichia and Klebsiella spp. were isolated from urine samples collected from 130 paediatric UTI patients in various wards, including OPD (29), nephrology (23), neonatal unit (18), general medical (12), urology (11), and neurology (7), of the Children's Hospital and Institute of Child Health in Lahore, Pakistan (Table 1). The demographic data of these paediatric patients are summarised in Table 2. Some urine samples (30) exhibited no bacterial growth on the CLED agar and were thus excluded from the study. Of the remaining 100 paediatric patients, 23 were less than one year old and 77 were in the range of 1–14 years of age. UTI prevalence between the two sexes revealed that 51% of the UTIs were found in male children and 49% in female paediatric patients. Uropathogens were isolated from both symptomatic UTIs, presenting with vomiting (64%), fever (87%), and non-symptomatic UTIs, without vomiting (36%) or fever (13%). Health complications, including immunosuppression (11%), urethral malformation (34%), and neurological disorders (7%), were also identified in several UTI patients. A total of 26% of the paediatric patients had a history of antibiotic use and the highest bacterial count, 105 CFU mL−1, was observed in 72% of urine samples, while 28% of the samples had a reduced bacterial cell count of 103 CFU mL−1 (Table 2).
Table 1.
Distribution of selected paediatric patients across different hospital wards.
| Wards | n (%) |
|---|---|
| Out Patient Department | 29 |
| Nephrology | 23 |
| Neonatal unit | 18 |
| General medical | 12 |
| Urology | 11 |
| Neurology | 7 |
Table 2.
Baseline demographic and clinical characterization of paediatric UTI patients.
| Variable | n (%) |
|---|---|
| Age | |
| Less than 1 year | 23 |
| 1 year to 14 years | 77 |
| Gender | |
| Male | 51 |
| Female | 49 |
| Vomiting | |
| Yes | 64 |
| No | 36 |
| Fever | |
| Yes | 87 |
| No | 13 |
| Complications | |
| Immunosuppression | 11 |
| Urethra malformation | 34 |
| Neurological disorders | 7 |
| UTI history | |
| Yes | 26 |
| No | 74 |
| Bacterial count | |
| ˃ 103 CFU mL−1 | 28 |
| ˃ 105 CFU mL−1 | 72 |
Characterization of the uropathogenic strains
A total of 100 strains were isolated and identified as Escherichia and Klebsiella spp. using morphological and biochemical characterisation. These isolates were coded as ZK1, ZK2, ZK3, … to ZK100 and divided into groups A and B based on the results of the biochemical characterisation (Table 3). Biochemical characterisation of selected strains revealed that all the strains in both groups were Gram-negative, positive for catalase activity, and negative for oxidase activities. All the strains presented with a yellow acidic slant and butt with gas production in the TSI test. Group A strains were negative for urease activity and citrate utilisation tests but positive for indole production and motility. The group A strains were negative for urease activity and citrate utilisation, however, group B strains were positive to these assays (Table 3). Based on the biochemical characterisation, group A strains were identified as belonging to the Escherichia spp. and group B strains were identified as Klebsiella spp. (Table 3 and Figure 1a). Among the E. coli strains, 38% were isolated from male patients and 25% were from female patients. In the Klebsiella strains, 21% of the strains were from boy and 16% were from girl patients (Figure 1b).
Table 3.
Characterization of the uropathogenic bacterial strains identified in paediatric UTI patients.
| Characterization | Group A (n = 63)∗ | Group B (n = 37)∗∗ |
|---|---|---|
| Gram-staining | Gram-negative | Gram-negative |
| Catalase activity | Positive | Positive |
| Oxidase activity | Negative | Negative |
| Urease activity | Negative | Positive |
| Citrate utilization test | Negative | Positive |
∗ Group A was identified as Escherichia spp. through biochemical characterization. ∗∗ Group B was identified as Klebsiella spp. through biochemical characterization.
Figure 1.
Prevalence of uropathogenic Escherichia and Klebsiella strains (A) and their distribution in male and female paediatric UTIs (B).
Antibiotic susceptibility
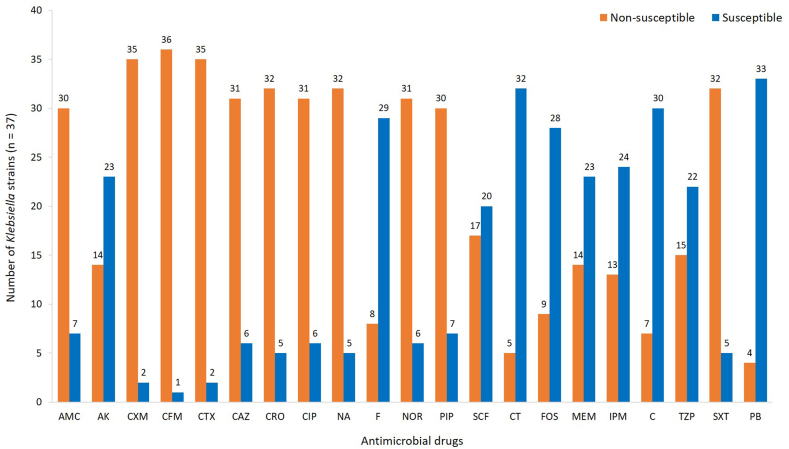
The susceptibility patterns of the Gram-negative uropathogens is summarized in Figure 2, Figure 3. Overall, ≥90% of the uropathogenic strains were not susceptible to cefuroxime, cefixime, and cefotaxime. More than 80% of uropathogenic strains were also found to be resistant to co-amoxiclav, ceftazidime, ceftriaxone, ciprofloxacin, nalidixic acid, norfloxacin, pipemidic acid, and co-trimoxazole. The antimicrobial susceptibility patterns of the Escherichia and Klebsiella spp. are shown in Figure 2, Figure 3. All of the E. coli strains (group A) were screened against all 21 antimicrobial drugs, and the results are depicted in Figure 2. The highest degree of resistance (93%) was reported for cefixime, followed by cefuroxime (90%). More than 80% of the E. coli strains were not susceptible to cefotaxime, ceftazidime, ceftriaxone, ciprofloxacin, nalidixic acid, norfloxacin, pepedemic acid, and co-trimoxazole. However, E. coli strains were susceptible to colistin sulphate (85%), polymyxin B (85%), chloramphenicol (84%), nitrofurantoin (79%), fosfomycin (76%), and meropenem (71%). The Klebsiella strains (group B) demonstrated a similar pattern of resistance and susceptibility (Figure 3). The highest resistance (95%) was observed against cefixime, followed by cefuroxime (92%) and cefotaxime (92%). More than 75% of the Klebsiella strains were not susceptible to co-amoxiclav, ceftazidime, ceftriaxone, ciprofloxacin, nalidixic acid, norfloxacin, pepedemic acid, and co-trimoxazole. Antimicrobial agents polymyxin B (89%), colistin sulphate (86%), chloramphenicol (81%), nitrofurantoin (78%), and fosfomycin (76%) were more effective against the Klebsiella strains (Figure 3). The estimated MDR and XDR patterns are reported in Table 4 and reveal that 14% of the strains can be classified as MDR, and 86% of the uropathogenic strains identified in this study could be classified as XDR. None of the strains were found to be PDR. Among the MDR strains, five were identified as Escherichia spp. and nine were Klebsiella spp. In contrast, 58 of the Escherichia spp. and 28 of the Klebsiella spp. were found to be XDR. The XDR strains ZK9, ZK32, ZK40, ZK60, and ZK89 were shown to demonstrate the highest degree of resistance to the antibiotics used in this study and reported resistance to 17 of the 21 antimicrobials evaluated here. These strains were then selected for molecular identification.
Figure 2.
Antimicrobial susceptibility of uropathogenic Escherichia strains.
Figure 3.
Antimicrobial susceptibility of uropathogenic Klebsiella strains.
Table 4.
Multiple drug-resistant and extensively drug-resistant uropathogenic E. coli and K. pneumonia from paediatric UTIs in Lahore, Pakistan.
| Antimicrobial resistance | Uropathogen identifier |
|---|---|
| Multiple drug-resistant (MDR) |
Escherichia spp. strains (n = 5) ZK52, ZK53, ZK63, ZK64, ZK98, |
|
Klebsiella spp. strains (n = 9) ZK19, ZK20, ZK42, ZK46, ZK47, ZK54, ZK55, ZK97, ZK99 | |
| Extensively drug resistant (XDR) |
Escherichia spp. strains (n = 58) ZK1, ZK3, ZK4, ZK6, ZK7, ZK9a, ZK10, ZK11, ZK12, ZK13, ZK16, ZK21, ZK22, ZK23, ZK24, ZK26, ZK27, ZK29, ZK31, ZK33, ZK34, ZK38, ZK39, ZK40a, ZK44, ZK49, ZK56, ZK57, ZK58, ZK59, ZK60a, ZK61, ZK65, ZK66, ZK67, ZK68, ZK69, ZK70, ZK71, ZK73, ZK76, ZK78, ZK79, ZK80, ZK81, ZK82, ZK83, ZK84, ZK85, ZK87, ZK88, ZK90, ZK91, ZK92, ZK93, ZK95, ZK96, ZK100 |
|
Klebsiella spp. strains (n = 28) ZK2, ZK5, ZK8, ZK14, ZK15, ZK17, ZK18, ZK25, ZK28, ZK30, ZK32a, ZK35, ZK36, ZK37, ZK41, ZK43, ZK45, ZK48, ZK50, ZK51, ZK62, ZK72, ZK74, ZK75, ZK77, ZK86, ZK89a, ZK94, |
Indicated XDR strains were selected for molecular identification using 16S rRNA gene sequencing.
Identification of selected antibiotic-resistant uropathogens
The five most representative MDR strains, ZK9, ZK32, ZK40, ZK60, and ZK89, were selected for molecular identification using 16S rRNA partial gene sequencing. These strains were identified as Escherichia coli strain ZK9, Klebsiella pneumoniae strain ZK32, Escherichia coli strain ZK40, Escherichia coli strain ZK60, and Klebsiella pneumoniae strain ZK89. A phylogenetic tree of these strains was constructed using the neighbour-joining method and broadened by selecting closely matched Enterobacteriaceae strains, as shown in Figure 4. The resulting sequences were deposited in NCBI GenBank with accession numbers MT764342 for ZK9, MT764343 for ZK32, MT764344 for ZK40, MT764345 for ZK60, and MT764346 for ZK89 (Figure 4).
Figure 4.
Phylogenetic tree describing the 16S rRNA gene sequences of E. coli strains ZK9, ZK40, ZK60 (accession number MT764342, MT764344, and MT764345, respectively) and K. pneumoniae strains ZK32 and ZK89 (accession number MT764343 and MT764346, respectively) and the other closely related bacterial strains found in the GenBank database.
Discussion
Antimicrobial drug resistance is a life-threatening issue, especially in paediatric patients, when treating infections caused by Escherichia and Klebsiella spp. Antimicrobial drug-resistance is increasing every day and the normal microbial flora in the urinary tract is becoming a reservoir for resistance genes.30 In this study, hundreds of uropathogenic Escherichia and Klebsiella strains were isolated from paediatric patients with UTIs and their antimicrobial susceptibility was evaluated. The E. coli and K. pneumoniae strains isolated from the paediatric patients in this study showed the highest antimicrobial resistance (≥60%) against tested antibiotics. Among these strains, ≥80% were found to be not susceptible to cefixime, cefuroxime, cefotaxime, nalidixic acid, ceftazidime, co-trimoxazole, pepedemic acid, ceftriaxone, ciprofloxacin, and norfloxacin, and 86% of these strains, 58 Escherichia strains and 28 Klebsiella strains, were found to be XDR. Similar findings have also been reported in various clinical settings across Nepal and Ethiopia.9,31,32 However, the prevalence of MDR Escherichia and Klebsiella strains has not yet been reported for Lahore, Pakistan. This study addresses this lack of information and provides insight into the increasing incidence of MDR in paediatric UTIs in Pakistan.
In this study, uropathogenic bacterial strains were identified as E. coli and K. pneumoniae based on their biochemical characterisation. These uropathogens are Gram-negative and are known to be the dominant pathogens in UTIs across the world due to their unique structure, which facilitates their attachment to the host cells.33 These structural features help the bacteria avoid exclusion by urinary lavage and support bacterial reproduction and expansion in these tissues, supporting invasive infection and pyelonephritis.34 The Escherichia strains in this study were found to be negative for citrate utilisation and positive for indole production, while the Klebsiella strains showed the opposite result. Indole and citrate-utilization tests are commonly used to identify the Enterobacteriaceae including Escherichia, Enterobacter, and Klebsiella spp.35 In this study the Escherichia strains were indole positive, which is likely related to their ability to produce tryptophanase enzymes that can convert tryptophan into indole. When indole reacts with para-dimethyl-amino benzaldehyde, a pink-coloured complex is produced that differentiates Klebsiella from Enterobacter spp. In this study, all Escherichia and Klebsiella strains were oxidase negative, which may be due to the inactivation of cytochrome C-oxidase and an inability to utilise oxygen for energy production via the electron transfer chain, which confirms their link to the Enterobacteriaceae family.36 In our study, the production of a yellow slant and butt with gas and no H2S on TSI media indicates the carbohydrate fermentation ability of these strains.37 However, the molecular identification method is more reliable and provides information up to the species level. In this study, the evaluation of the 16s rRNA gene revealed that most MDR strains were Escherichia coli strain ZK9, Klebsiella pneumoniae strain ZK32, Escherichia coli ZK40, Escherichia coli strain ZK60, and Klebsiella pneumoniae strain ZK89. Strains ZK9, ZK40, and ZK60 were shown to be closely related to Escherichia sp. strain UA32, which is an MDR strain isolated from female patients during pregnancy.38 The XDR Klebsiella pneumoniae strains ZK32 and ZK89 were shown to be closely related to MDR Klebsiella sp. strain UA47 from pregnant females.38
In this study, the highest degree of resistance (≥90%) in the E. coli strains was observed for cefixime and cefuroxime, while the antimicrobial agents cefotaxime, ceftazidime, ceftriaxone, ciprofloxacin, nalidixic acid, norfloxacin, pepedemic acid, and co-trimoxazole reported a ≥80% non-susceptibility rate in these strains. Recently, Vazouras et al.39 reported their findings from Greece, wherein 79% of the strains in their study were E. coli, making them the most common UTI-causing organism in paediatric patients. This study also reported the resistance rates for ampicillin (42%), trimethoprim/sulfamethoxazole (up to 26.5%), amoxicillin/clavulanic acid (up to 12.2%), cephalosporins (up to 1.7%), nitrofurantoin (up to 2.3%), ciprofloxacin (up to 1.4%), and amikacin (up to 0.9%), which were much lower than those found in our study. This difference may be the result of geographical variations resulting from local differences in the frequency of antibiotic use and ease of antibiotic availability.40 Kayastha et al.9 reported a rising prevalence in resistance, up to 62%, of extended-spectrum β-lactamase-producing Gram-negative bacteria in paediatric UTIs and Shrestha et al.10 reported MDR uropathogenic E. coli (53%), E. faecalis (22%), K. pneumoniae (7%), and S. aureus (7%). Hsueh et al.11 evaluated urogenital pathogens and found that 23% of their urine samples were positive for the most common resistance factors in bacterial uropathogens. Mirzarazi et al.41 reported that their E. coli isolates were resistant to nalidixic acid and trimethoprim-sulfamethoxazole. In this study, we found that the Klebsiella strains (≥92%) were not susceptible to cefixime, cefuroxime, or cefotaxime. More than 75% of these strains were also resistant to co-amoxiclav, ceftazidime, ceftriaxone, ciprofloxacin, nalidixic acid, norfloxacin, pepedemic acid, and co-trimoxazole. Similarly, Hayat et al.42 reported that the prevalence of paediatric UTIs was up to 52% in Pakistan, with the majority of infections occurring due to extended-spectrum β-lactamase-producing K. pneumoniae, which demonstrated a high degree of resistance (96%) to ceftazidime. Klebsiella spp. also showed resistance to trimethoprim-sulfamethoxazole, ciprofloxacin, and nalidixic acid.41
Across the world, most patients carry a variety of MDR genes that have developed as a result of the misuse and improper administration of antibiotics. This has resulted in the widespread sharing of β-lactamase enzymes in the commensal bacteria, promoting the development of novel MDR strains.43 In our study, we observed the highest MDR patterns for the aminoglycosides and β-lactam antibiotics. This is of significant clinical concern, especially in children, as their immune system is still developing.44 This is particularly problematic when uropathogenic organisms present with MDR characteristics transferred from other organisms via genetic exchange.45 Longer hospital stays also result in infections with extended-spectrum β-lactamase-producing organisms.46 MDR could also be due to an unavoidable genetic reaction to the solid discriminating strength forced by antibiotic therapy which plays its dynamic role in the development of MDR in uropathogens and their plasmid having MDR genes.25
Conclusion
Our data shows that the majority of the uropathogenic Escherichia and Klebsiella strains isolated in this study are resistant to cefixime, cefuroxime, nalidixic acid, and cefotaxime, suggesting that such antibiotic treatment should be suspended following extensive long-term evaluations. Self-medication and improper diagnosis should be avoided to prevent increasing antibiotic resistance in both of these clinically-important bacterial genera.
Recommendations
Uropathogenic E. coli and K. pneumoniae were found to responsible for the majority of the MDR UTIs identified in paediatric patients in Lahore, Pakistan. Efforts must be made to control MDR infections in paediatric patients by executing evidence-based monitoring of UTI treatment and creating awareness of the judicious use of antibiotics.
Source of funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors have no conflict of interest to declare.
Ethical approval
Ethical approval for this study protocol was granted by the Institute of Molecular Biology and Biotechnology at The University of Lahore, Pakistan, with the reference number IMBB-744 and dated 10-11-2018.
Authors' contributions
ZI conducted the research, provided the research materials, and collected and organised the data. MZM conceptualised and supervised the study, analysed and interpreted the data, and wrote the initial and final drafts of the manuscript. AM provided the research materials and logistical support for this project. All authors critically reviewed and approved the final draft of this manuscript and are responsible for the content and similarity index of the manuscript.
Acknowledgments
The authors would like to acknowledge the research facility provided by the Institute of Molecular Biology and Biotechnology (IMBB) at the University of Lahore (UOL), Lahore, Pakistan.
Footnotes
Peer review under responsibility of Taibah University.
References
- 1.Mahony M., McMullan B., Brown J., Kennedy S.E. Multidrug-resistant organisms in urinary tract infections in children. Pediatr Nephrol. 2020;35(9):1–11. doi: 10.1007/s00467-019-04316-5. [DOI] [PubMed] [Google Scholar]
- 2.Lane D.R., Takhar S.S. Diagnosis and management of urinary tract infection and pyelonephritis. Emerg Med Clin. 2011;29(3):539–552. doi: 10.1016/j.emc.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Whiting P., Westwood M., Watt I., Cooper J., Kleijnen J. Rapid tests and urine sampling techniques for the diagnosis of urinary tract infection (UTI) in children under five years: a systematic review. BMC Pediatr. 2005;5(1):1–13. doi: 10.1186/1471-2431-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Uwaezuoke S.N., Ndu I.K., Eze I.C. The prevalence and risk of urinary tract infection in malnourished children: a systematic review and meta-analysis. BMC Pediatr. 2019;19(1):1–20. doi: 10.1186/s12887-019-1628-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stauffer C.M., Weg B., Donadini R., Ramelli G.P., Marchand S., Bianchetti M.G. Family history and behavioral abnormalities in girls with recurrent urinary tract infections: a controlled study. J Urol. 2004;171(4):1663–1665. doi: 10.1097/01.ju.0000117701.81118.f0. [DOI] [PubMed] [Google Scholar]
- 6.Rabasa A.I., Shattima D. Urinary tract infection in severely malnourished children at the University of Maiduguri Teaching Hospital. J Trop Pediatr. 2002;48(6):359–361. doi: 10.1093/tropej/48.6.359. [DOI] [PubMed] [Google Scholar]
- 7.Mickymaray S., Al Aboody M.S. In vitro antioxidant and bactericidal efficacy of 15 common spices: novel therapeutics for urinary tract infections? Medicina. 2019;55(6):1–18. doi: 10.3390/medicina55060289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Behzadi P., Urbán E., Matuz M., Benkő R., Gajdács M. Advances in experimental medicine and biology. Springer; New York: 2020. The role of Gram-negative bacteria in urinary tract infections: current concepts and therapeutic options. [DOI] [PubMed] [Google Scholar]
- 9.Kayastha K., Dhungel B., Karki S., Adhikari B., Banjara M.R., Rijal K.R. Extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella species in pediatric patients visiting international friendship children's hospital, Kathmandu, Nepal. Infect Dis Res Treat. 2020;13:1–7. doi: 10.1177/1178633720909798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shrestha L.B., Baral R., Poudel P., Khanal B. Clinical, etiological and antimicrobial susceptibility profile of pediatric urinary tract infections in a tertiary care hospital of Nepal. BMC Pediatr. 2019;19(1):36. doi: 10.1186/s12887-019-1410-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsueh P.R., Hoban D.J., Carmeli Y., Chen S.Y., Desikan S., Alejandria M., Binh T.Q. Consensus review of the epidemiology and appropriate antimicrobial therapy of complicated urinary tract infections in Asia-Pacific region. J Infect. 2011;63(2):114–123. doi: 10.1016/j.jinf.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 12.Magiorakos A.P., Srinivasan A., Carey R.T., Carmeli Y., Falagas M.T., Giske C.T. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 13.Mojtahedzadeh M., Panahi Y., Fazeli M.R., Najafi A., Pazouki M., Navehsi B.M., Beiraghdar F. Intensive care unit-acquired urinary tract infections in patients admitted with sepsis: etiology, risk factors, and patterns of antimicrobial resistance. Int J Infect Dis. 2008;12(3):312–318. doi: 10.1016/j.ijid.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization (WHO). 2005. Urinary tract infections in infants and children in developing countries in the context of IMCI (No. WHO/FCH/CAH/05.11) [Google Scholar]
- 15.Shepherd A.K., Pottinger P.S. Management of urinary tract infections in the era of increasing antimicrobial resistance. Med Clin. 2013;97(4):737–757. doi: 10.1016/j.mcna.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 16.Afsharpaiman S., Bairaghdar F., Torkaman M., Kavehmanesh Z., Amirsalari S., Moradi M., Safavimirmahalleh M. Bacterial pathogens and resistance patterns in children with community-acquired urinary tract infection: a cross sectional study. J Compr Pediatr. 2012;3(1):16–20. [Google Scholar]
- 17.Ray J., Paul R., Haldar A., Mondol S. A study on antibiotic resistance pattern of Escherichia coli isolated from urine specimens in Eastern India. Int J Med Sci Publ Health. 2015;4(12):1670–1674. [Google Scholar]
- 18.Ghai I., Ghai S. Understanding antibiotic resistance via outer membrane permeability. Infect Drug Resist. 2018;11:523–530. doi: 10.2147/IDR.S156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Masi M., Réfregiers M., Pos K.M., Pagès J.M. Mechanisms of envelope permeability and antibiotic influx and efflux in Gram-negative bacteria. Nature Microb. 2017;2(3):1–7. doi: 10.1038/nmicrobiol.2017.1. [DOI] [PubMed] [Google Scholar]
- 20.Džidic S., Šuškovic J., Kos B. Antibiotic resistance mechanisms in bacteria: biochemical and genetic aspects. Food Technol Biotechnol. 2008;46:11–21. [Google Scholar]
- 21.Dockrell H.M., Goering R.V., Roitt I., Wakelin D., Zuckerman M. In: Attacking the enemy: antimicrobial agents and chemotherapy. Medical Microbiology. Mims C., Dockrell H.M., Goering R.V., Roitt I., Wakelin D., Zuckerman M., editors. Elsevier; Netherlands: 2004. pp. 473–507. [Google Scholar]
- 22.Mills S., McAuliffe O.E., Coffey A., Fitzgerald G.F., Ross R.P. Plasmids of lactococci-genetic accessories or genetic necessities? FEMS Microbiol Rev. 2006;30:243–273. doi: 10.1111/j.1574-6976.2005.00011.x. [DOI] [PubMed] [Google Scholar]
- 23.Woldemariam H.K., Geleta D.A., Tulu K.D., Aber N.A., Legese M.H., Fenta G.M. Common uropathogens and their antibiotic susceptibility pattern among diabetic patients. BMC Infect Dis. 2019;19:1–10. doi: 10.1186/s12879-018-3669-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cappuccino J.C., Sherman N. 3rd ed. A Laboratory Manual; 1992. Microbiology; pp. 125–179. [Google Scholar]
- 25.Chakraborty S.P., Mahapatra S.K., Roy S. Biochemical characters and antibiotic susceptibility of Staphylococcus aureus isolates. Asian Pac J Trop Biomed. 2011;1(3):212–216. doi: 10.1016/S2221-1691(11)60029-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duguid J.P. Staining methods. In: Collee J.G., Fraser A.G., Marmion B.P., Simmons A., editors. Mackie and McCartney practical medical microbiology. Churchill Livingstone; Edinburgh: 1996. [Google Scholar]
- 27.CLSI . 30th ed. Clinical and Laboratory Standards Institute (CLSI); Weinstein, MP: 2020. Performance standards for antimicrobial susceptibility testing (M100) [Google Scholar]
- 28.Murray P.R., Zeitinger J.R. Evaluation of Mueller-Hinton agar for disk diffusion susceptibility tests. J Clin Microbiol. 1983;18(5):1269–1271. doi: 10.1128/jcm.18.5.1269-1271.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mumtaz M.Z., Ahmad M., Jamil M., Hussain T. Zinc solubilizing Bacillus spp. potential candidates for biofortification in maize. Microbiol Res. 2017;202:51–60. doi: 10.1016/j.micres.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 30.Okeke I.N., Fayinka S.T., Lamikanra A. Antibiotic resistance in Escherichia coli from Nigerian students. Emerg Infect Dis. 2000;6(4):393–396. doi: 10.3201/eid0604.009913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shakya P., Shrestha D., Maharjan E., Sharma V.K., Paudyal R. ESBL production among E. coli and Klebsiella spp. causing urinary tract infection: a hospital-based study. Open Microbiol J. 2017;11:23–30. doi: 10.2174/1874285801711010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seifu W.D., Gebissa A.D. Prevalence and antibiotic susceptibility of uropathogens from cases of urinary tract infections (UTI) in Shashemene referral hospital, Ethiopia. BMC Infect Dis. 2018;18(1):1–9. doi: 10.1186/s12879-017-2911-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vaca D.J., Thibau A., Schütz M., Kraiczy P., Happonen L., Malmström J. Interaction with the host: the role of fibronectin and extracellular matrix proteins in the adhesion of Gram-negative bacteria. Med Microbiol Immunol. 2020;209(3):277–299. doi: 10.1007/s00430-019-00644-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ayelign B., Abebe B., Shibeshi A., Meshesha S., Shibabaw T., Addis Z. Bacterial isolates and their antimicrobial susceptibility patterns among pediatric patients with urinary tract infections. Turk J Urol. 2018;44(1):62–69. doi: 10.5152/tud.2017.33678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bahjat S.A., Al-Taee M.F., Al-Hassani A.M., Al-Hassani O.M. Molecular and bacteriological method for identification of lactose fermenting Salmonella in Mosul province. Indian J Public Health Res Dev. 2019;10(12):1428–1434. [Google Scholar]
- 36.Prescott L.M., Harley J.P., Klein D.A. McGraw’s Hill Companies; Boston: 1999. Isolation of pure bacterial cultures from specimens: Microbiology. [Google Scholar]
- 37.Buxton A., Fraser G. Blackwell Scientific Publications; 1977. Animal microbiology, vol. 1: immunology, bacteriology, mycology, diseases of fish and laboratory methods. [Google Scholar]
- 38.Asmat U., Mumtaz M.Z., Malik A. Rising prevalence of multidrug-resistant uropathogenic bacteria from urinary tract infections in pregnant women. J Taibah Univ Medical Sci. 2020;16(1):102–111. doi: 10.1016/j.jtumed.2020.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vazouras K., Velali K., Tassiou I., Anastasiou-Katsiardani A., Athanasopoulou K., Barbouni A. Antibiotic treatment and antimicrobial resistance in children with urinary tract infections. J Glob Antimicrob Resist. 2020;20:4–10. doi: 10.1016/j.jgar.2019.06.016. [DOI] [PubMed] [Google Scholar]
- 40.Zorc J.J., Kiddoo D.A., Shaw K.N. Diagnosis and management of pediatric urinary tract infections. Clin Microbiol Rev. 2005;18(2):417–422. doi: 10.1128/CMR.18.2.417-422.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mirzarazi M., Rezatofighi S.E., Pourmahdi M., Mohajeri M.R. Antibiotic resistance of isolated Gram-negative bacteria from urinary tract infections (UTIs) in Isfahan. Jundishapur J Microbiol. 2013;6(8):1–5. [Google Scholar]
- 42.Hayat S., Siddique M.H., Aslam B., Nadeem H., Ashraf A., Saqalein M. Extended-spectrum-β-lactamase producing multidrug resistant Klebsiella pneumoniae isolates from pediatrics. Pakistan J Zool. 2019;51(4):1251–1257. [Google Scholar]
- 43.Tiwari B., Drogui P., Tyagi R.D. Multidrug-resistant genes and pathogenic bacteria in hospital wastewater. In: Tyagi R.D., Sellamuthu B., Pandey A., editors. Current developments in biotechnology and bioengineering: environmental and health impact of hospital wastewater. 2020. pp. 177–202. [Google Scholar]
- 44.Isturiz R. Global resistance trends and the potential impact on empirical therapy. Int J Antimicrob Agents. 2008;32:S201–S206. doi: 10.1016/S0924-8579(09)70003-2. [DOI] [PubMed] [Google Scholar]
- 45.Ju F., Beck K., Yin X., Maccagnan A., McArdell C.S., Singer H.P. Wastewater treatment plant resistomes are shaped by bacterial composition, genetic exchange, and upregulated expression in the effluent microbiomes. ISME J. 2019;13(2):346–360. doi: 10.1038/s41396-018-0277-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dayan N., Dabbah H., Weissman I., Aga I., Even L., Glikman D. Urinary tract infections caused by community-acquired extended-spectrum β-lactamase-producing and nonproducing bacteria: a comparative study. J Pediatr. 2013;163(5):1417–1421. doi: 10.1016/j.jpeds.2013.06.078. [DOI] [PubMed] [Google Scholar]