Abstract
Six lignols (1–6), including two new compounds (+)-(7R,8R)-palmitoyl alatusol D (1) and (+)-(7R,8R)-linoleyl alatusol D (2), along with four phenolics (7–10), a neolignan (11), three alkyl aryl ether-type lignans (12–14), two furofuran-type lignans (15–16), three benzofuran-type lignans (17–19), a tetrahydrofuran-type lignan (20), and a dibenzylbutane-type lignan (21) were isolated from the ethyl acetate-soluble fraction of the methanol extract of Platycodon grandiflorum (Jacq.) A. DC. root. The chemical structures of the obtained compounds were elucidated via high-resolution mass spectrometry and nuclear magnetic resonance (NMR) spectroscopy analyses. The obtained spectroscopic data agreed well with literature. Among the isolated compounds, eighteen (1–7 and 11–21) were isolated from P. grandiflorum and the Campanulaceae family for the first time. This is the first report on lignol and lignan components of P. grandiflorum. The anti-inflammatory effects of the isolated compounds were examined in terms of their ability to inhibit the production of pro-inflammatory cytokines IL-6, IL-12 p40, and TNF-α in lipopolysaccharide-stimulated murine RAW264.7 macrophage cells. Nine compounds (4–6, 12, and 15–19) exhibited inhibitory effects on IL-12 p40 production, eleven compounds (1–6, 12, 15–17, and 19) exhibited inhibitory activity on IL-6 production, and eleven compounds (1–6 and 15–19) exhibited inhibitory effects against TNF-α. These results warrant further investigation into the potential anti-inflammatory activity and general benefits of the phenolic constituents of P. grandiflorum root.
Keywords: Platycodon grandiflorum, lignol, phenolic, lignan, anti-inflammatory
1. Introduction
Platycodon grandiflorum (Jacq.) A. DC. is a perennial herb belonging to the Campanulaceae family that is mainly found in Northeast Asia, including countries such as China, Korea, and Japan. The root of P. grandiflorum (called Doraji in Korea, Jiegeng or Lingdanghua in China, and Kikyo in Japan) has been used as a food item in East Asia for thousands of years. In addition, P. grandiflorum has been used in traditional oriental medicine for treating lung and respiratory diseases such as cough, cold, bronchitis, asthma, and sore throat [1,2].
Results from previously published studies indicate that P. grandiflorum can have a relieving effect on cough and asthma; it has also been shown to exhibit extensive pharmacological effects, including anti-tumor, antioxidation, anti-inflammatory, and antibacterial activities; furthermore, it has been observed to afford protection against hypoglycemia and liver disease [3,4,5,6,7,8]. In the past few decades, P. grandiflorum has been reported to contain various chemical constituents such as triterpenoid saponins, flavonoids, phenolic acids, polyacetylenes, phytosterols, and polysaccharides. Platycodins, an oleanane-type pentacyclic triterpenoid saponin, is abundant in roots of P. grandiflorum, and they are the major bioactive constituents of this plant [1,2,9]. In addition, according to previous studies, platycodins identified in more than 70 from P. grandiflorum have a greatly diverse structure, and a wide range of biological and pharmacological activities, such as anti-tussive, anti-inflammatory, anti-cancer, anti-obesity, anti-fibrosis, and immune system-enhancing effects, have been reported [10,11,12,13]. Of these, platycodin D (PD) and platycodin D3 (PD3), the major platycosides of P. grandiflorum, exhibit significant anti-inflammatory activity. Moreover, previous studies have demonstrated that PD and PD3 regulate the production of pro-inflammatory cytokines, nitric oxide (NO), and secretion of tumor necrosis factor-alpha (TNF-α) in lipopolysaccharide (LPS)-stimulated murine RAW 264.7 macrophage cells [14,15].
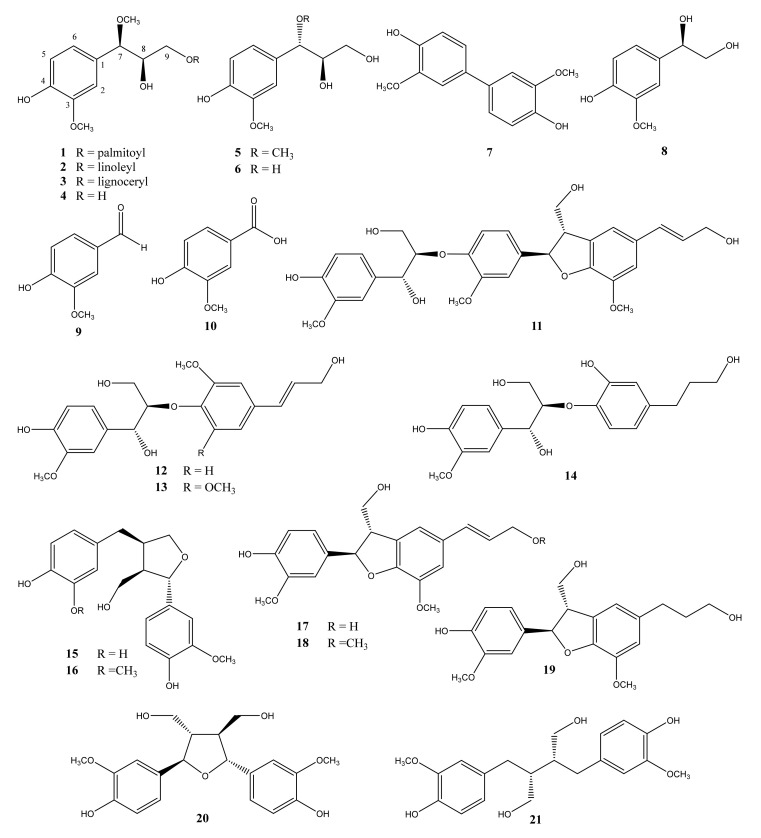
In practice, most pharmacological activity studies have been conducted on triterpenoid saponins, whereas research on the phenolic constituents of P. grandiflorum is lacking. Herein, we successfully isolated and elucidated the structures of 21 phenolic constituents present in methanol extracts of the P. grandiflorum root. In particular, the isolated compounds comprised two new and four known lignols, four phenols, a neolignan, nine lignans including two alkyl aryl ether type, two furofuran type, three benzofuran type, a tetrahydrofuran-type, and a dibenzylbutane-type (Figure 1). In addition, we report the results of experiments aimed at the inhibition of the pro-inflammatory cytokines IL-6, IL-12 p40, and TNF-α by the isolated phenol constituents of P. grandiflorum in lipopolysaccharide (LPS)-stimulated murine RAW264.7 macrophage cells.
Figure 1.
Structures of compounds 1–21 from the root of P. grandiflorum.
2. Results and Discussion
2.1. Isolation and Structural Elucidation of Compounds 1–21
A series of twenty-one compounds, including six lignols (1–6), four phenolic compounds (7–10), a neolignan (11), three alkyl aryl ether-type lignans (12–14), two furofuran-type lignans (15–16), three benzofuran-type lignans (17–19), a tetrahydrofuran-type lignans (20), and a dibenzylbutane-type lignan (21), were isolated from the ethyl acetate-soluble fraction of the methanol extract of the root of P. grandiflorum. Their structures were identified as (+)-(7R,8R)-palmitoyl alatusol D (1), (+)-(7R,8R)-linoleyl alatusol D (2), (+)-(7R,8R)-lignoceryl alatusol D (3) [16], (+)-(7R,8R)-alatusol D (4) [17], (−)-(7S,8R)-alatusol D (5) [18], (+)-(7S,8R)-guaiacylglycerol (6) [19], 3,3′-dimethoxy [1,1′-biphenyl]-4,4′-diol (7) [20], (+)-4-hydroxy-3-methoxyphenylglycol (8) [21], vanillin (9), vanillic acid (10), 2-[4-[2,3-dihydro-3-(hydroxymethyl)-5-(3-hydroxy-1-propen-1-yl)-7-methoxy-2-benzofuranyl]-2-methoxyphenoxy]-1-(4-hydroxy-3-methoxyphenyl)-1,3-propanediol (11) [22], threo-(7R,8R)-1-(4-hydroxy-3-methoxyphenyl)-2-[4-[(E)-3-hydroxy-1-propenyl]-2-methoxyphenoxy]-1,3-propanediol (12) [23], wikstroemol (13) [24], threo-4,7,9,9′-tetrahydroxy-3,3′-dimethoxy-8-O-4′-neolignan (14) [25], (+)-lariciresinol (15) [26], 3′-demethyl-(+)-lariciresinol (16) [27], (−)-dehydrodiconiferyl alcohol (17) [28], hawthornnin G (18) [29], dihydrodehydrodiconiferyl alcohol (19) [30], (+)-neoolivil (20) [31], and (−)-secoisolariciresinol (21) [32], based on the consistency of their analytical data with those from available literature reports (Figure 1). Notably, compounds 1 and 2 are new compounds; in addition, eighteen compounds (1–7 and 11–21) were isolated from P. grandiflorum and from the Campanulaceae family in general for the first time.
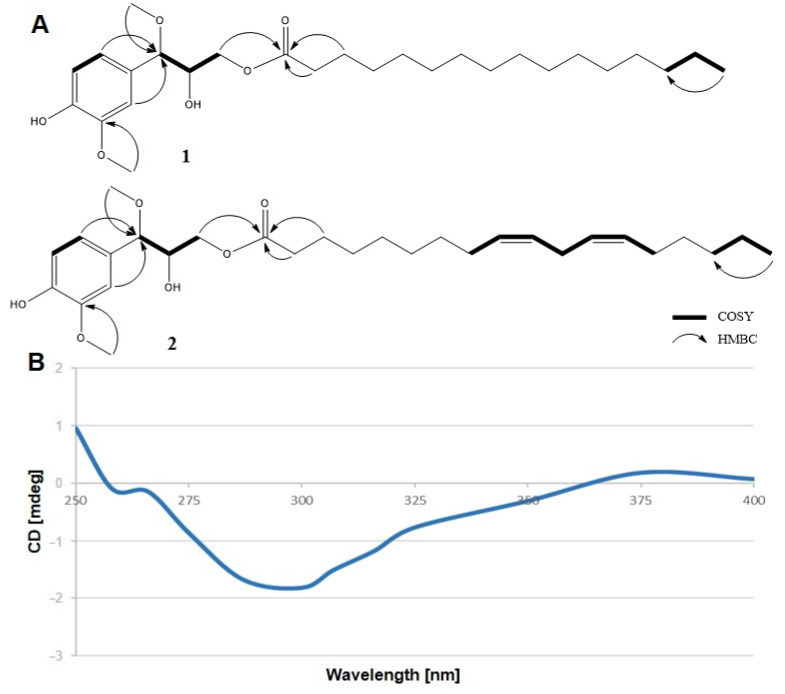
Focusing on the two new compounds, compound 1 was isolated as a white amorphous powder. Its molecular formula was determined based on the presence of a pseudomolecular ion peak at an m/z value of 489.3190 [M + Na]+ (calcd. 489.3187) in the high-resolution electrospray ionization time-of-flight mass (HR-ESI-TOF-MS) spectrum. The proton nuclear magnetic resonance (1H-NMR) spectrum of compound 1 (Table 1) was characterized using three aromatic signals due to an ABX system [δH 6.70 (dd, J = 8.0, 1.9 Hz, H-6), 6.72 (d, J = 1.9 Hz, H-2), and 6.82 (d, J = 8.0 Hz, H-5)], two resonance signals due to oxymethine moieties [δH 3.80 (ddd, J = 6.1, 5.8, 3.4 Hz, H-8) and 3.97 (d, J = 6.1 Hz, H-7)], two resonance signals due to an oxymethylene group [δH 3.77 (dd, J = 11.0, 3.4 Hz, H-9a) and 3.99 (dd, J = 11.0, 5.7 Hz, H-9b)], and two resonance signals due to two different methoxy groups [δH 3.18 (s, 7-OMe) and 3.82 (s, 3-OMe)] that belong to a lignol moiety, which is the same as that present in compound 4. The other resonance signals [δH 0.81 (t, J = 7.5 Hz, H-16′), 1.54 (m, H-3′), 1.17–1.30 (m, H-4′-15′), and 2.24 (t, J = 7.5 Hz, H-2′)] suggested the presence of a fatty acid moiety. Correspondingly, the 13C-NMR spectrum (Table 1) contained six aromatic signals corresponding to an ABX system of the aromatic rings [δC 109.1 (C-2), 114.4 (C-5), 120.9 (C-6), 129.2 (C-1), 145.9 (C-4), and 146.9 (C-3)], three lignol carbon signals [δC 64.5 (C-9), 73.6 (C-8), and 84.2 (C-7)], and a signal due to a carboxyl group [δC 173.7 (C-1′)], which proved that compound 1 is a lignol fatty acid ester [17]. The key heteronuclear multiple-bond correlation spectroscopy (HMBC) correlations between H-9 (δH 3.77 and 3.99)/C-1′ (δC 173.7), 7-OMe (δH 3.18)/C-7 (δC 84.2), and 3-OMe (δH 3.82)/C-3 (δC 146.9) are indicative of the connectivity between the lignol and fatty acid moieties, as well as the location of the methoxy groups.
Table 1.
1H and 13C NMR spectroscopic data for compounds 1 and 2 in chloroform-d.
| 1 | 2 | |||
|---|---|---|---|---|
| Position | δH a (J/Hz) | δC b | δH a (J/Hz) | δC b |
| 1 | 129.2 | 129.2 | ||
| 2 | 6.72 d (1.9) | 109.1 | 6.72 d (1.9) | 109.1 |
| 3 | 146.9 | 146.9 | ||
| 4 | 145.9 | 145.9 | ||
| 5 | 6.82 d (8.0) | 114.4 | 6.84 d (8.0) | 114.4 |
| 6 | 6.70 dd (8.0, 1.9) | 120.9 | 6.70 dd (8.0, 1.9) | 120.9 |
| 7 | 3.97 d (6.1) | 84.2 | 3.98 d (6.1) | 84.2 |
| 8 | 3.80 ddd (6.1, 5.8, 3.4) | 73.6 | 3.80 ddd (6.1, 5.8, 3.4) | 73.6 |
| 9 | 3.99 dd (11.0, 3.4) 3.77 dd (11.0, 5.7) |
64.5 | 4.00 dd (11.0, 3.4) 3.77 dd (11.0, 5.7) |
64.5 |
| 1′ | 173.7 | 173.7 | ||
| 2′ | 2.24 t (7.5) | 34.1 | 2.25 t (7.5) | 34.1 |
| 3′ | 1.54 m c | 24.9 | 1.53 m | 24.9 |
| 4′ | 1.17–1.30 m | 29.1–29.7 | 1.18–1.27 m | 29.1–29.8 |
| 5′ | 1.17–1.30 m | 29.1–29.7 | 1.18–1.27 m | 29.1–29.8 |
| 6′ | 1.17–1.30 m | 29.1–29.7 | 1.18–1.27 m | 29.1–29.8 |
| 7′ | 1.17–1.30 m | 29.1–29.7 | 1.18–1.27 m | 29.1–29.8 |
| 8′ | 1.17–1.30 m | 29.1–29.7 | 1.94 m | 27.2 |
| 9′ | 1.17–1.30 m | 29.1–29.7 | 5.27 m | 129.7 |
| 10′ | 1.17–1.30 m | 29.1–29.7 | 5.27 m | 129.7 |
| 11′ | 1.17–1.30 m | 29.1–29.7 | 1.52 m | 24.9 |
| 12′ | 1.17–1.30 m | 29.1–29.7 | 5.27 m | 130.2 |
| 13′ | 1.17–1.30 m | 29.1–29.7 | 5.27 m | 130.2 |
| 14′ | 1.17–1.30 m | 31.9 | 1.95 m | 27.2 |
| 15′ | 1.17–1.30 m | 22.7 | 1.18–1.27 m | 29.6 |
| 16′ | 0.81 t (7.5) | 14.1 | 1.18–1.27 m | 31.9 |
| 17′ | 1.18–1.27 m | 22.6 | ||
| 18′ | 0.81 t (7.5) | 14.1 | ||
| 3-OMe | 3.82 s | 56.0 | 3.84 s | 56.0 |
| 7-OMe | 3.18 s | 56.7 | 3.19 s | 56.7 |
Assignments were achieved by analyzing the HMQC and HMBC experiments; J values (Hz) are given in parentheses. a 600 MHz. b 150 MHz. c Overlapped.
To determine the absolute configuration of compound 1, the alkaline hydrolysis of 1 was conducted with 0.5% NaOH; consequently, palmitic acid and compound 1a were produced. Notably, 1a had the same structure as compound 4 did ((+)-(7R,8R)-alatusol D). The absolute configuration at C-8 of 1a was determined by implementing Snatzke’s method [17]. The ICD spectrum of compound 1a was characterized by a negative band at 300 nm (−1.8) (Figure 2B) [32], which is indicative of an 8R configuration at C-8. Based on the value of the coupling constant between H7 and H8 (J = 6.1 Hz), the absolute configuration at C-7 in compound 1 is 7R [17]. Based on these data, compound 1 was identified as (+)-(7R,8R)-palmitoyl alatusol D.
Figure 2.
Key COSY and HMBC correlations of 1 and 2 (A) and the ICD spectrum of 1a (B).
Compound 2 was isolated as a white amorphous powder. The molecular formula was established to be C29H46O6 based on the HR-ESI-TOF-MS peak observed at an m/z value of 513.3192 [M + Na]+ (calcd. 513.3187). The 1H-NMR and 13C-NMR spectra of this compound indicated that the lignol moiety in 2 was the same as that in 1. The difference between compounds 2 and 1 contained the fatty acid moiety. The 1H-NMR spectrum of 2 (Table 1) comprised four overlapped olefinic proton signals at δH 5.27 (H-9′, 10′, 12′, and 13′). Correspondingly, the 13C-NMR spectrum of compound 2 (Table 1) comprised the signals due to four olefinic carbons at δC 129.7 and 130.2 (C-9′, 10′, 12′, and 13′). NMR evidence and GC-MS data thus suggest that 2 includes a linoleic acid moiety. Analogous to the case of compound 1, the linoleic acid residue was attached to C-9, as indicated by the analysis results of the 1H–1H COSY and HMBC spectra (Figure 2). Therefore, compound 2 was determined to be (+)-(7R,8R)-linoleyl alatusol D.
2.2. Bioassay
Among the compounds present in the ethyl acetate-soluble fraction of the methanol extract of the root of P. grandiflorum, we identified those that exhibited anti-inflammatory activity by depressing the production of IL-12 p40, IL-6, and TNF-α in LPS-stimulated RAW264.7 cells. Preliminarily, the effect of compounds 1–21 (at a concentration of 100 μM) on the viability of RAW264.7 cells was evaluated by implementing the colorimetric MTT assay (Sigma, St. Louis, MO, USA.). According to the results of this assay, the tested compounds exhibited no cytotoxicity at the mentioned concentration (data not shown). Then, the effects of compounds 1–21 at various concentrations (1, 5, 25, 50, and 100 μM) on the production of IL-12 p40, IL-6, and TNF-α in LPS-stimulated RAW264.7 cells were investigated. Eight compounds (4–6 and 15–19) were observed to inhibit IL-12 p40, IL-6, and TNF-α production, with IC50 values determined to range from 5.0 to 60.6 μM; three compounds (1–3) were observed to inhibit the production of IL-6 and TNF-α, with IC50 values ranging from 6.5 to 20.2 μM; and compound 12 exhibited a weak inhibition of the production of IL-12 p40a and IL-6, with IC50 values of 56.2 and 62.7 μM, respectively (Table 2). The other nine compounds (7–11, 13, 14, 20, and 21) did not display any effect on the production of the mentioned cytokines at the indicated concentrations (IC50 > 100 μM). In these experiments, SB203580, a known inhibitor of p38 kinase, was used as a positive control; this compound inhibited IL-12 p40, IL-6, and TNF-α production with IC50 values of 5.3, 3.2, and 8.1 μM, respectively. Upon examination of the structure–activity relationship of the isolated compounds, we found that lignols (4–6) exhibited strong inhibitory activity on the three pro-inflammatory cytokines evaluated, whereas lignol fatty acid esters (1–3) only inhibited the production of IL-6 and TNF-α. This evidence can be preliminarily interpreted as indicating that the hydroxyl group on C-9 has a closely related effect on IL-12 p40; however, this hypothesis requires further research. A previous study revealed that alatusol E inhibited NO production in LPS-activated BV-2 cells, proving that lignol has a certain anti-inflammatory potential [33]. In contrast, among the 11 lignans (11–21), only furofuran-type (15–16) and benzofuran-type (17–19) lignans weakly inhibited the three pro-inflammatory cytokines considered. Previous reports have shown that various furofuran-type lignans significantly inhibit NO production [34]. However, there are no systematic reports on other inflammatory factors of furofuran-type lignans. Benzofuran-type lignans showed a strong inhibition of PGE2 synthesis [35], and some of them inhibited the pro-inflammation cytokines [36]. The present results also prove that benzofuran-type lignans can participate in the treatment of inflammation. These results not only reveal the anti-inflammatory activity of lignans in P. grandiflorum but also provide a side basis for research on the anti-inflammatory activity of lignans.
Table 2.
Anti-inflammatory effects of compounds 1–21 isolated from P. grandiflorum root on LPS-stimulated RAW264.7 cells.
| IC50 (µM) a | IC50 (µM) a | ||||||
|---|---|---|---|---|---|---|---|
| IL-12 p40 | IL-6 | TNF-α | IL-12 p40 | IL-6 | TNF-α | ||
| 1 | >100 | 8.1 ± 0.2 | 19.6 ± 0.5 | 12 | 56.2 ± 1.3 | 62.7 ± 3.1 | >100 |
| 2 | >100 | 6.5 ± 0.8 | 17.8 ± 1.1 | 13 | >100 | >100 | >100 |
| 3 | >100 | 9.2 ± 1.1 | 20.2 ± 0.9 | 14 | >100 | >100 | >100 |
| 4 | 29.7 ± 3.2 | 5.0 ± 0.1 | 16.5 ± 3.0 | 15 | 29.7 ± 1.5 | 42.6 ± 0.7 | 20.6 ± 0.2 |
| 5 | 35.2 ± 0.7 | 5.1 ± 0.2 | 17.1 ± 2.1 | 16 | 21.3 ± 0.2 | 19.8 ± 0.8 | 18.9 ± 1.0 |
| 6 | 40.0 ± 0.1 | 7.7 ± 0.1 | 14.2 ± 0.2 | 17 | 10.1 ± 0.6 | 19.2 ± 1.8 | 18.1 ± 0.4 |
| 7 | >100 | >100 | >100 | 18 | 60.6 ± 2.1 | 58.7 ± 2.9 | 49.3 ± 0.3 |
| 8 | >100 | >100 | >100 | 19 | 22.3 ± 0.9 | 30.7 ± 2.0 | 46.9 ± 1.3 |
| 9 | >100 | >100 | >100 | 20 | >100 | >100 | >100 |
| 10 | >100 | >100 | >100 | 21 | >100 | >100 | >100 |
| 11 | >100 | >100 | >100 | SB203580 b | 5.3 ± 0.1 | 3.2 ± 0.2 | 8.1 ± 0.1 |
a IC50 values for selected compounds are presented in columns IL-12 p40, IL-6, and TNF-α. Compounds exhibiting IC50 values > 100 µM were considered to be inactive. b Positive control.
3. Materials and Methods
3.1. General Information
A Jasco DIP-370 automatic polarimeter was used to determine the optical rotation. The UV spectra were measured on a UNICO UV-2102PCS spectrophotometer (Unico, Dayton, NJ, USA). The NMR spectra were determined using a Bruker AM-600 spectrometer (Bruck Biospin, Fallanden, Switzerland). The LCQ advantage trap mass spectrometer (Thermo Finnigan, San Jose, CA, USA) was equipped with an electrospray ionization (ESI) source, and high-resolution electrospray ionization mass spectra (HR-ESI-MS) were obtained using an Agilent 6530 Accurate-Mass Q-TOF LC/MS system. Column chromatography was performed using silica gel (Kieselgel 60, 70–230, and 230–400 mesh, Merck, Darmstadt, Germany) and YMC RP-18 resins, and thin layer chromatography (TLC) was performed using pre-coated silica-gel 60 F254 and RP-18 F254S plates (both 0.25 mm, Merck, Darmstadt, Germany). GC-MS data were obtained with an Clarus 600 GC equipped with a 600T mass selective detector and a 30 m (0.25 mm i.d., 0.25 μm film) HP-5 ms capillary column (Agilent, Wilmington, Germany). All isolation solvents were purchased from Daejung (Si Heung, Korea).
3.2. Plant Material
Dried roots of P. grandiflorum were purchased in the Yangyeongsi herbal market, Daegu, Korea, in January 2016. The sample was botanically identified by the author (W., Li). A voucher specimen (AA-031) was deposited at the Korean Medicine (KM) Application Center, Korea Institute of Oriental Medicine, Daegu, Korea.
3.3. Extraction and Isolation
Dried roots of P. grandiflorum (2.0 kg) were extracted with methanol (MeOH) (10 L × 3) under reflux. The MeOH extract (300.0 g) was suspended in water and partitioned with n-hexane and ethyl acetate (EtOAc). The EtOAc fraction (21.0 g) was subjected to silica gel (4.0 × 30 cm) column chromatography with hexane:EtOAc, 10:1–5:1; hexane:EtOAc:MeOH, 1.5:1:0.15; chloroform (CHCl3):acetone:MeOH, 2:1:0.1; CHCl3-MeOH-H2O, 4:1:0.1; and MeOH, 100% to give 8 fractions (Fr. 1–8). The fraction 2 (3.2 g) was subjected to silica gel (1.5 × 80 cm) column chromatography with hexane-acetone (acetone 1–20%) elution solvent to give 9 sub-fractions. The fraction 2C was subjected to YMC (1 × 80 cm) column chromatography with MeOH-H2O (MeOH 70–95%) elution solvent to give compounds 1 (11.2 mg), 2 (9.6 mg), and 3 (11.8 mg). The fraction 2F was subjected to YMC (1 × 80 cm) column chromatography with MeOH-H2O (MeOH 60–95%) elution solvent to give compounds 4 (22.7 mg), 5 (7.4 mg), and 6 (7.0 mg). The fraction 2G was subjected to YMC (1 × 80 cm) column chromatography with acetone-H2O (acetone 60–90%) elution solvent to give compounds 8 (3.5 mg), 9 (70.6 mg), and 10 (28.7 mg). The fraction 4 (4.0 g) was subjected to silica gel (2.0 × 80 cm) column chromatography with CHCl3-acetone (acetone 6–35%) elution solvent to give 18 sub-fractions. The fraction 4A was subjected to YMC (1 × 80 cm) column chromatography with acetone-H2O (acetone 25–70%) elution solvent to give compounds 7 (7.8 mg) and 21 (14.2 mg). The fraction 4C was subjected to YMC (1 × 80 cm) column chromatography with acetone-H2O (acetone 20–70%) elution solvent to give compounds 12 (7.7 mg), 13 (11.6 mg), and 14 (7.8 mg). The fraction 4D was subjected to YMC (1 × 80 cm) column chromatography with MeOH-H2O (MeOH 30–80%) elution solvent to give compounds 15 (5.1 mg), 16 (20.7 mg), 17 (3.2 mg), 18 (20.6 mg), and 19 (8.4 mg). The fraction 4L was subjected to YMC (1 × 80 cm) column chromatography with MeOH-H2O (MeOH 10–70%) elution solvent to give compounds 11 (4.7 mg), 20 (8.2 mg), and 21 (22.2 mg).
(+)-(7R,8R)-palmitoyl alatusol D (1): white amorphous powder; : –65.8 (c 0.05, MeOH); 1H NMR (methanol-d4, 600 MHz) and 13C NMR data (methanol-d4, 150 MHz), see Table 1; HR-ESI-MS: m/z 489.3190 [M+Na]+ (calcd. for 489.3187).
(+)-(7R,8R)-linoleyl alatusol D (2): white amorphous powder; : –59.6 (c 0.05, MeOH); 1H NMR (methanol-d4, 600 MHz) and 13C NMR data (methanol-d4, 150 MHz), see Table 1; HR-ESI-MS: m/z 513.3192 [M+Na]+ (calcd. for 513.3187).
3.4. Alkaline Hydrolysis
Compounds 1 and 2 (2.0 mg each) were treated with NaOH 1 N (2 mL) at 60 °C for 40 min, before the mixtures thus obtained were neutralized with HCl 1 N and extracted with CHCl3. The CHCl3 layers were then analyzed via silica gel thin-layer chromatography (TLC) using hexane–ethyl acetate (5:1 v/v) as the mobile phase; spots were visualized by spraying the TLC plate with 90% ethanol–H2SO4 (9:1, v/v) and then heating it at 180 °C for 2 min. The fatty acid moieties of compounds 1 and 2, including palmitic acid (compound 1) and linoleic acid (compound 2), were analyzed via GC-MS, and palmitic acid (tR = 27.87 min) and linoleic acid (tR = 31.06 min) were confirmed through comparison with authentic samples.
3.5. Absolute Configuration
A 0.6–0.7-mg/mL stock solution of commercial Mo2(AcO)4 in commercial dimethyl sulfoxide (DMSO) was used. One part of the solution was evenly added into pure DMSO, and its circular dichroism spectrum was measured to be used as the baseline; the other part was added into DMSO alongside Mo2(AcO)4, and the molecular ratio of ligand to metal was about 1.0. After 30 min, the ICD spectrum of this solution was recorded [24]. Three absorption bands could be noticed in the ICD spectrum of Mo2(AcO)4 in the DMSO solution, the most obvious of which was observed at 305 nm, which, based on literature data, was attributed to a metal-to-ligand charge-transfer transition.
3.6. Cell Culture
The RAW 264.7 cells were obtained from the Korean Cell Line Bank (KCLB, Chongno-gu, Seoul, Korea) and maintained at 37 °C in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS) and 100 U/mL of penicillin in a humidified incubator containing 5% CO2. The cells were cultured in RPMI 1640 medium containing 10% FBS to 2 × 105 cells/mL in 96-well tissue culture plates for 18 h; subsequently, they were pretreated with 0.4, 2, and 10 μM of the compounds to be tested 1 h prior to undergoing stimulation with LPS (1 μg/mL) for 24 h in an incubator.
3.7. Cell Viability
Cell viability was evaluated via the MTT method. Briefly, MTT was added to the cell culture medium. The supernatant was then removed, and the formazan crystals were dissolved in DMSO. The absorbance was measured at 570 nm. The percentage of dead cells was determined relative to the control group.
3.8. Measurement of Proinflammatory Cytokine Production
RAW 264.7 cells were incubated in 48-well plates containing 1 × 105 cells per well; they were then treated with the isolated compounds 1–21 at the indicated concentration for 1 h before being subjected to stimulation with 10 ng/mL of LPS from Salmonella minnesota (Alexis, Famingdale, NY, USA). Supernatants were harvested 18 h after stimulation. The concentrations of murine TNF-α, IL-6, and IL-12 p40 in the culture supernatants were determined using ELISA (BD PharMingen, San Jose, CA, USA) according to the manufacturer’s instructions. The data were presented as means ± standard deviation of at least three independent experiments performed in triplicate.
3.9. Statistical Analysis
All data represent means ± SD of at least three independent experiments performed in triplicate. Statistical significance is indicated as determined via one-way ANOVA, followed by Dunnett’s multiple comparison test (p < 0.05) utilizing GraphPad Prism 6.0 (GraphPad Software Inc., San Diego, CA, USA).
4. Conclusions
The results of the present study afforded a comprehensive chemical assessment of the phenolic constituents of P. grandiflorum root. Notably, (+)-(7R,8R)-palmitoyl alatusol D (1) and (+)-(7R,8R)-linoleyl alatusol D (2) are newly discovered compounds, and eighteen other compounds were isolated from P. grandiflorum and the Campanulaceae family for the first time. To the best of our knowledge, this is the first comprehensive report on the phenolic components of P. grandiflorum and their anti-inflammatory activity. Results from this study may provide a scientific basis for the complement of anti-inflammatory components in P. grandiflorum.
Author Contributions
The list of authors who contributed to this work is as follows: W.L. performed the isolation, structure elucidation of the constituents, and preparation of the manuscript. H.J.Y. conducted the isolation and bioassay experiments. The whole research was performed based on the planning of W.L. Both authors have read and agreed to the published version of the manuscript.
Funding
The present study was supported by the Basic Science Research Program through the National Research Foundation (NRF) funded by the Ministry of Education (Grant number NRF2020R1C1C1006749), Republic of Korea.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds 1–21 are available from the authors.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kim K.S., Ezaki O., Ikemoto S., Itakura H. Effects of Platycodon grandiflorum feeding on serum and liver lipid concentrations in rats with diet-induced hyperlipidemia. J. Nutr. Sci. Vitaminol. 1995;41:485–491. doi: 10.3177/jnsv.41.485. [DOI] [PubMed] [Google Scholar]
- 2.Zhang L., Wang Y.L., Yang D.W., Zhang C.H., Zhang N., Li M.H., Liu Y.Z. Platycodon grandifloras—An ethnopharmacological, phytochemical and pharmacological review. J. Ethnopharmacol. 2015;164:147–161. doi: 10.1016/j.jep.2015.01.052. [DOI] [PubMed] [Google Scholar]
- 3.Ji M.Y., Bo A., Yang M., Xu J.F., Jiang L.L., Zhou B.C., Li M.H. The pharmacological effects and health benefits of Platycodon grandifloras—A medicine food homology species. Foods. 2020;9:142. doi: 10.3390/foods9020142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi J.H., Hwang Y.P., Lee H.S., Jeong H.G. Inhibitory effect of Platycodi Radix on ovalbumin-induced airway inflammation in a murine model of asthma. Food. Chem. Toxicol. 2009;47:1272–1279. doi: 10.1016/j.fct.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 5.Lee K.J., Kim J.Y., Choi J.H., Kim H.G., Chung Y.C., Roh S.H., Jeong H.G. Inhibition of tumor invasion and metastasis by aqueous extract of the radix of Platycodon grandiflorum. Food. Chem. Toxicol. 2006;44:1890–1896. doi: 10.1016/j.fct.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 6.Lee J.H., Choi Y.H., Kang H.S., Choi B.T. An aqueous extract of Platycodi radix inhibits LPS-induced NF-kappaB nuclear translocation in human cultured airway epithelial cells. Int. J. Mol. Med. 2004;13:843–847. doi: 10.3892/ijmm.13.6.843. [DOI] [PubMed] [Google Scholar]
- 7.Zheng J., He J., Ji B., Li Y., Zhang X. Antihyperglycemic effects of Platycodon grandiflorum (Jacq.) A. DC. extract on streptozotocin-induced diabetic mice. Plant Foods Hum. Nutr. 2007;62:7–11. doi: 10.1007/s11130-006-0034-4. [DOI] [PubMed] [Google Scholar]
- 8.Khanal T., Choi J.H., Hwang Y.P., Chung Y.C., Jeong H.G. Saponins isolated from the root of Platycodon grandiflorum protect against acute ethanol-induced hepatotoxicity in mice. Food Chem. Toxicol. 2009;47:530–535. doi: 10.1016/j.fct.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 9.Yoo D.S., Choi Y.H., Cha M.R., Lee B.H., Kim S.J., Yon G.H., Hong K.S., Jang Y.S., Lee H.S., Kim Y.S., et al. HPLC-ELSD analysis of 18 platycosides from balloon flower roots (Platycodi Radix) sourced from various regions in Korea and geographical clustering of the cultivation areas. Food Chem. 2011;129:645–651. doi: 10.1016/j.foodchem.2011.04.106. [DOI] [PubMed] [Google Scholar]
- 10.Zhang L.L., Huang M.Y., Yang Y., Huang M.Q., Shi J.J., Zou L., Lu J.J. Bioactive platycodins from Platycodonis Radix: Phytochemistry, pharmacological activities, toxicology and pharmacokinetics. Food Chem. 2020;327:127029. doi: 10.1016/j.foodchem.2020.127029. [DOI] [PubMed] [Google Scholar]
- 11.Han L.K., Xu B.J., Kimura Y., Zheng Y.N., Okuda H. Platycodi radix affects lipid metabolism in mice with high fat diet–induced obesity. J. Nutr. 2000;130:2760–2764. doi: 10.1093/jn/130.11.2760. [DOI] [PubMed] [Google Scholar]
- 12.Lee K.J., Choi C.Y., Chung Y.C., Kim Y.S., Ryu S.Y., Roh S.H., Jeong H.G. Protective effect of saponins derived from roots of Platycodon grandiflorum on tert-butyl hydroperoxide-induced oxidative hepatotoxicity. Toxicol. Lett. 2004;147:271–282. doi: 10.1016/j.toxlet.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 13.Kim Y.S., Kim J.S., Choi S.U., Kim J.S., Lee H.S., Roh S.H., Jeong Y.C., Kim Y.K., Ryu S.Y. Isolation of a new saponin and cytotoxic effect of saponins from the root of Platycodon grandiflorum on human tumor cell lines. Planta Med. 2005;71:566–568. doi: 10.1055/s-2005-864161. [DOI] [PubMed] [Google Scholar]
- 14.Ahn K.S., Noh E.J., Zhao H.L., Jung S.H., Kang S.S., Kim Y.S. Inhibition of inducible nitric oxide synthase and cyclooxygenase II by Platycodon grandiflorum saponins via suppression of nuclear factor-κB activation in RAW 264.7 cells. Life Sci. 2005;76:2315–2328. doi: 10.1016/j.lfs.2004.10.042. [DOI] [PubMed] [Google Scholar]
- 15.Wang C., Schuller Levis G.B., Lee E.B., Levis W.R., Lee D.W., Kim B.S., Park S.Y., Park E. Platycodin D and D3 isolated from the root of Platycodon grandiflorum modulate the production of nitric oxide and secretion of TNF-α in activated RAW 264.7 cells. Int. Immunopharmacol. 2004;4:1039–1049. doi: 10.1016/j.intimp.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 16.Chin Y.W., Jung Y.H., Chae H.S., Yoon K.D., Kim J. Anti-inflammatory constituents from the roots of Saposhnikovia divaricate. Bull. Korean Chem. Soc. 2011;32:2132–2134. doi: 10.5012/bkcs.2011.32.6.2132. [DOI] [Google Scholar]
- 17.Shi X.L., Yan J.K., Li W.K., Donkor P.O., Gao X.M., Ding L.Q., Qiu F. Two pairs of phenylpropanoid enantiomers from the leaves of Eucommia ulmoides. J. Asian Nat. Prod. Res. 2018;20:1045–1054. doi: 10.1080/10286020.2018.1483347. [DOI] [PubMed] [Google Scholar]
- 18.Woo K.W., Suh W.S., Subedi L., Kim S.Y., Choi S.U., Kim K.H., Lee K.R. Phenolic derivatives from the stems of Lagerstroemia indica and their biological activity. Heterocycles. 2015;91:2355–2366. doi: 10.3987/COM-15-13328. [DOI] [Google Scholar]
- 19.Vichi S., Santini C., Natali N., Riponi C., Lopez-Tamames E., Buxaderas S. Volatile and semi-volatile components of oak wood chips analysed by Accelerated Solvent Extraction (ASE) coupled to gas chromatography-mass spectrometry (GC-MS) Food Chem. 2007;102:1260–1269. doi: 10.1016/j.foodchem.2006.07.023. [DOI] [Google Scholar]
- 20.Hirose K., Akizawa T., Asada K., Tanaka Y., Negoro Y., Yoshioka M. Syntheses of antigens conjugated with 3-methoxy-4-hydroxyphenyl-glycol by Mannich reaction for enzyme immunoassay. Anal. Chim. Acta. 1998;365:137–145. doi: 10.1016/S0003-2670(97)00617-X. [DOI] [Google Scholar]
- 21.Landucci L.L., Ralph S.A., Hammel K.E. 13C-NMR characterization of guaiacyl, guaiacyl/syringyl, and syringyl dehydrogenation polymers. Holzforschung. 1998;52:160–170. doi: 10.1515/hfsg.1998.52.2.160. [DOI] [Google Scholar]
- 22.Rao L., You Y.X., Su Y., Fan Y., Liu Y., He Q., Chen Y., Meng J., Hu L., Li Y., et al. Lignans and neolignans with antioxidant and human cancer cell proliferation inhibitory activities from Cinnamomum bejolghota confirm its functional food property. J. Agric. Food Chem. 2020;68:8825–8835. doi: 10.1021/acs.jafc.0c02885. [DOI] [PubMed] [Google Scholar]
- 23.Wu B., Wang J. Phenolic Compounds from Selaginella moellendorfii. Chem. Biodivers. 2011;8:1735–1747. doi: 10.1002/cbdv.201000340. [DOI] [PubMed] [Google Scholar]
- 24.Kim K.H., Moon E., Choi S.U., Kim S.Y., Lee K.R. Biological evaluation of phenolic constituents from the trunk of Berberis koreana. Bioorg. Med. Chem. Lett. 2011;21:2270–2273. doi: 10.1016/j.bmcl.2011.02.104. [DOI] [PubMed] [Google Scholar]
- 25.Li W., Yang H.J. Isolation and identification of lignans and other phenolic constituents from the stem bark of Albizia julibrissin Durazz and evaluation of their nitric oxide inhibitory activity. Molecules. 2020;25:2065. doi: 10.3390/molecules25092065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xie L.H., Akao T., Hamasaki K., Deyama T., Hattori M. Biotransformation of pinoresinol diglucoside to mammalian lignans by human intestinal microflora, and isolation of Enterococcus faecalis strain PDG-1 responsible for the transformation of (+)-pinoresinol to (+)-lariciresinol. Chem. Pharm. Bull. 2003;51:508–515. doi: 10.1248/cpb.51.508. [DOI] [PubMed] [Google Scholar]
- 27.Liang W., Sun L.Q., Qian F., Tian X.H. Chemical constituents from the whole plant of Liparis japonica. Biochem. Syst. Ecol. 2020;92:104126. doi: 10.1016/j.bse.2020.104126. [DOI] [Google Scholar]
- 28.Huang X.X., Bai M., Zhou L., Lou L.L., Liu Q.B., Zhang Y., Li L.Z., Song S.J. Food byproducts as a new and cheap source of bioactive compounds: Lignans with antioxidant and anti-inflammatory properties from Crataegus pinnatifida seeds. J. Agric. Food Chem. 2015;63:7252–7260. doi: 10.1021/acs.jafc.5b02835. [DOI] [PubMed] [Google Scholar]
- 29.Shataer D., Li J., Duan X.M., Liu L., Xin X.L., Aisa H.A. Chemical composition of the hazelnut kernel (Corylus avellana L.) and its anti-inflammatory, antimicrobial, and antioxidant activities. J. Agric. Food Chem. 2021;69:4111–4119. doi: 10.1021/acs.jafc.1c00297. [DOI] [PubMed] [Google Scholar]
- 30.Nishiwaki H., Nakayama K., Shuto Y., Yamauchi S. Synthesis of all stereoisomers of 3,3′-dimethoxy-7,7′-epoxylignane-4,4′-diol and their plant growth inhibitory activity. J. Agric. Food Chem. 2014;62:651–659. doi: 10.1021/jf4046396. [DOI] [PubMed] [Google Scholar]
- 31.Wang L.Q., Meselhy M.R., Li Y., Qin G.W., Hattori M. Human intestinal bacteria capable of transforming secoisolariciresinol diglucoside to mammalian lignans, enterodiol and enterolactone. Chem. Pharm. Bull. 2000;48:1606–1610. doi: 10.1248/cpb.48.1606. [DOI] [PubMed] [Google Scholar]
- 32.Shi C., Xu M.J., Bayer M., Deng Z.W., Kubbutat M.H.G., Waejen W., Proksch P., Lin W.H. Phenolic compounds and their anti-oxidative properties and protein kinase inhibition from Chinese mangrove plant Laguncularia racemose. Phytochemistry. 2010;71:435–442. doi: 10.1016/j.phytochem.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 33.Kim K.H., Ha S.K., Choi S.U., Kim S.Y., Lee K.R. Phenolic constituents from the twigs of Euonymus alatus and their cytotoxic and anti-inflammatory activity. Planta Med. 2013;79:361–364. doi: 10.1055/s-0032-1328286. [DOI] [PubMed] [Google Scholar]
- 34.Xu W.H., Zhao P., Wang M., Liang Q. Naturally occurring furofuran lignans: Structural diversity and biological activities. Nat. Prod. Res. 2018:1357–1373. doi: 10.1080/14786419.2018.1474467. [DOI] [PubMed] [Google Scholar]
- 35.Ragab F.A., Eid N.M., Hassan G.S., Nissan Y.M. Synthesis and anti-inflammatory activity of some benzofuran and benzopyran-4-one derivatives. Chem. Pharm. Bull. 2012;60:110–120. doi: 10.1248/cpb.60.110. [DOI] [PubMed] [Google Scholar]
- 36.Miao Y., Hu Y., Yang J., Liu T., Sun J., Wang X. Natural source, bioactivity and synthesis of benzofuran derivatives. RSC Adv. 2019;9:27510–27540. doi: 10.1039/C9RA04917G. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.