Abstract
Objective
This study aims to evaluate the isokinetic performance for the peak torque and average power of the spinal flexor and extensor muscles in adolescents aged 12 to 18 years. The study also analyses the differences between the trunk muscle peak torque and average power with body mass index (BMI).
Method
The peak torque and average power of the trunk flexor and extensor muscles were measured in 180 adolescents (aged 12–18 years). The participants were classified into four groups according to BMI. The Biodex isokinetic dynamometer in concentric mode at speeds of 60° and 120°/sec was used for assessment.
Results
One-way multivariate analysis of variance MANOVA results demonstrated a significant difference in trunk muscle peak torque and average power with different BMI (F = 14.692, p = 0.0005). A Pearson's correlation analysis demonstrated a significantly negative correlation between weight and peak torque of trunk flexors and extensors (r = - 0.43, p = 0.0001; r = −0.31, p = 0.007, respectively). Finally, the results showed a negative correlation between weight and average power of trunk flexors and extensors (r = −0.54, p = 0.0001; r = −0.31, p = 0.007).
Conclusion
In this study, overweight and obese adolescents are found to be correlated with decreased trunk muscle torque and power. Thus, therapeutic interventions for overweight and obese adolescents, along with exercise training programmes, may help improve muscle performance including peak torque and power. Finally, these measures may enhance the quality of life of such adolescents.
Keywords: Adolescents, BMI, Isokinetic evaluation, Peak torque, Trunk flexors and extensors
الملخص
أهداف البحث
تقييم ذروة عزم الدوران ومتوسط قوة العضلات القابضة والباسطة للعمود الفقري لدى المراهقين الذين تتراوح أعمارهم بين ١٢ ١٨ عاما ومعرفة ما إذا كان هناك اختلاف في أداء عضلات الجذع باختلاف نوع مؤشر كتلة الجسم.
طرق البحث
تم قياس ذروة عزم الدوران ومتوسط قوة العضلات القابضة والباسطة للعمود الفقري لدى ١٨٠ شخصا (تتراوح أعمارهم من ١٢ إلى ١٨ عاما) تم تصنيفهم الى أربع مجموعات وفقا لمؤشر كتلة الجسم. كما تم إجراء التقييم باستخدام جهاز الايزوكينيتك حيث تم تقييم ذروة عزم الدوران ومتوسط قوة العضلات القابضة والباسطة للعمود الفقري بسرعات ٦٠ درجة و١٢٠ درجة / ثانية. وتم تحليل البيانات باستخدام تحليل التباين متعدد الاتجاهات لتحديد ما إذا كانت هناك أي فروق ذات دلالة إحصائية بين المجموعات الأربع.
النتائج
أشارت نتائج تحليل التباين متعدد الاتجاهات إلى انخفاض في قوة عضلات الجذع مع زيادة مؤشر كتلة الجسم. وكذلك أوضحت النتائج ارتفاعا ملحوظا في عزم الدوران ومتوسط قوة عضلات الجذع في مجموعة الأشخاص ذوي الوزن الطبيعي مقارنة بالأشخاص الذين يعانون من نقص الوزن وزيادة الوزن والسمنة. وأيضا أظهرت نتائج معامل ارتباط بيرسون وجود ارتباطا معنويا سلبيا بين الوزن وعزم دوران الذروة للعضلات القابضة والباسطة للعمود الفقري. وكان هناك ارتباط سلبي ملحوظ بين الوزن ومتوسط قوة هذه العضلات.
الاستنتاجات
خلصت هذه الدراسة إلى وجود علاقة سلبية بين مؤشر كتلة الجسم وعزم الدوران الأقصى ومتوسط قوة عضلات الجذع والعضلات القابضة والباسطة للظهر. وبالتالي يجب أن تتضمن برامج إعادة التأهيل للمراهقين الذين يعانون من زيادة الوزن والسمنة برنامج لتقوية عضلات الظهر بهدف تحسين فاعلية تلك التدخلات العلاجية وبالتالي تحسين نوعية حياة هؤلاء الأشخاص.
الكلمات المفتاحية: التقييم باستخدام جهاز الايزوكينيتك, قوة عضلات الظهر القابضة, قوة عضلات الظهر الباسطة, عزم الدوران الأقصى, مؤشر كتلة الجسم, المراهقين.
Introduction
Recently, increased body mass index (BMI) at young age has emerged as a prevalent health concern.1 Additionally, in Saudi children and adolescents, overweight and obesity have demonstrated alarming pervasiveness of 13.4% and 18.2%, respectively.2
Being overweight or obese can have many negative effects on the well-being of children and adolescents and lead to various health problems, including diabetes, cancer, musculoskeletal problems and respiratory and cardiac issues.3,4 A recent study on 343 participants with different BMI found that the prevalence of low back pain among obese adolescents (age 12–15 years) was 67%.5
Researchers have examined the impact of obesity on maximum isotonic,6 isometric,7 and isokinetic8, 9, 10 strength in different age groups from adolescents to the elderly. They concluded that muscle strength is lower when normalised to total body mass. Additionally, relative muscle strength of knee extension, trunk flexion, and handgrip in relation to body mass was about 10% lower in obese persons.11
Pajoutan et al.12 conducted a study to investigate the effect of body composition on trunk extensor muscles’ maximum capacity across all levels of BMI-defined categories and over a wider age range. They found that absolute back muscle strength was comparable between BMI categories, while relative strength to body mass in obese participants was about 38% lower than that in people with normal weight and approximately 26% less than the overweight group.
Early detection and treatment of childhood musculoskeletal abnormalities is a vital intervention that should be undertaken by healthcare professionals.13,14 Efficient prevention programmes for children and adolescents can be established based on early detection of musculoskeletal problems, including spinal conditions.13
Measuring the strength and performance of trunk muscles is a challenging issue.15,16 However, this examination is crucial for research and clinical practice to attain certain goals, including providing guidance to rehabilitation and prevention programmes.17,18
Many objective tools can measure and train the strength of back muscles. Isokinetic testing ranks top among such methods.15,16 The assessment of muscle strength of trunk musculature at various positions, velocities, and modes shows a high level of safety,19 reliability,20 and sensitivity that is sufficient for the detection of muscle weakness21 and achieving the goals of rehabilitation.22 Thus, isokinetic dynamometry is an effective and reliable tool that objectively assesses the strength of specific muscle groups.23 In a systematic review, Estrázulas et al24 concluded that the isokinetic testing of trunk muscles showed a high level of reliability; thus, they recommended the use of isokinetic measurement of trunk muscle performance in clinical settings.
Although muscle strength is considered an excellent indicator of general health when based on reliable measurements, most studies involving isokinetic measurements of trunk muscle performance recruit adult participants.25, 26, 27, 28, 29, 30, 31 However, few have measured trunk muscle strength isokinetically in normal healthy children and adolescents.32 To the best of our knowledge, there is a lack of published information on the values of isokinetic peak torque and the power of trunk muscles in Saudi children and adolescents and the relationship between BMI and these parameters.
Given the well-documented importance of the good performance of trunk muscles for optimal physical function, along with our limited knowledge of the effects of body weight on trunk muscle peak torque and power in young people, we conduct the present study to achieve the following objectives: (1) evaluate the isokinetic peak torque and mean power of trunk flexors and extensors in Saudi adolescents aged 12 to 18 years, (2) compare the isokinetic peak torque and mean power of trunk flexors and extensors in these adolescents with different BMI, and (3) determine the effects of weight on trunk muscle peak torque and power. We hope that our results provide strength-test administrators and teams working in rehabilitation with the normal values of peak torque and mean power of trunk flexors and extensors, as well as knowledge of variation in these parameters depending on weight. We hypothesise that trunk muscle peak torque and power is associated with body mass and that increasing mass reduces trunk muscle peak torque and power in adolescents.
Materials and Methods
Participants
The sample size of the present school-based cross-sectional study was 180 healthy male students who were recreationally active but non-participants in physical exercise. They were recruited from intermediate and secondary schools from Almadinah Almunawwarah city, KSA. The participants were selected using a multistage stratified sampling method. No participant reported practising sports or exercising for over half an hour. Adolescents were included in the study if they were registered in public schools, were in the classroom on the day of measurements, and were aged 12 to 18 years. Participants with postural abnormalities recognised by observation, a history of musculoskeletal trauma to spine or hip areas, a history of surgery in the abdominal region and spine, lower back pain, trauma, or deformity of the knees were excluded from the study.
Procedures
Weight (kg) and height (m) were measured to the nearest 0.5 kg and 0.5 cm, respectively, using Detecto Physician's Scale Model 2392, USA. BMI was calculated by dividing weight in kilograms with height in square metres. Participants were considered obese if the BMI specific for their age and sex was at the 95th percentile or more of the growth charts of the Centres for Disease Control and Prevention. If the BMI was between the 85th and 95th percentiles, the participant was classified as overweight. If the BMI was between the 85th percentile and the 5th percentiles, the participant was classified as having normal weight. Participants were classified as underweight when their BMI was lower than the 5th percentile.33
Testing protocol description
Before commencing with testing, a warm–up activity was performed, such as static cycling for 5 min at level two resistance.34 Back flexor/extensor strength and power were measured on a Biodex isokinetic dynamometer 4 Pro (Biodex Corporation, Shirley, NY, USA). Participants were positioned on the back attachment designed to measure trunk extension–flexion. The spine was set upright and the hips and knees are positioned in 90° flexion position with the thighs aligned parallel to the ground. The mechanical axis of the dynamometer was aligned with the anatomical axis of the trunk, which is represented by a line joining the anterior superior iliac spines. This position is regarded as the anatomic reference position (Figure 1). Adjustable pads are positioned posterior to the head, the upper trunk, the pelvis, and on the anterior shaft of the tibia to fix the participant to the back attachment. The upper trunk, the pelvis, and the thighs are stabilised using straps. The range of movement of the back was set at 50°, which is composed of a flexion component of 30° (−30°) and an extension component of 20° (+20°), in relation to the previously mentioned anatomic reference angle (0°) (Figure 1). A trunk range of motion that does not exceed 50° helps in isolating the lumbar spine motion with decreasing flexion and extension of the hip joint.35 Furthermore, the previously described alignment of mechanical and anatomical axes, the presence of the pad support behind the pelvis, and the stabilising belt on the pelvis limit unwanted motion at the hip joint during the measurements.
Figure 1.
flexion–extension trunk motion on the isokinetic machine through a 50° ROM.
The measurement protocol began with the person in a flexed position, with their arms and hands crossed over the thorax, and included three sets of five maximum sequential concentric lumbar flexions and extensions with 60°/s and 120°/s angular velocities. In this study, each participant was verbally encouraged to exert maximum effort throughout the whole protocol.
Data analysis
Statistical analysis was performed using SPSS 21.0 (Statistical Package for Social Sciences, IBM, New York). Normality of the data was verified using the Shapiro–Wilk test. Thus, parametric tests were applied to analyse quantitative data. One-way multiple analysis of variance (one-way MANOVA) with Tukey's post-hoc tests was used to observe differences in peak torque and average power of trunk flexors and extensors at 60 and 120 °/s in underweight, normal-weight, overweight, and obese conditions. Pearson's correlation was used to investigate the correlation between weight and peak torque and the average power of trunk flexors and extensors. Statistical significance was set at p < 0.05.
Results
Demographic data of the participants is presented in Table 1. Our sample includes 180 Saudi male participants (45 participants for each subgroup). The mean ± SD age, weight, and height of the entire sample were 15.374 ± 2.015 years, 61.733 ± 27.560 kg, and 157.233 ± 16.921 cm, respectively
Table 1.
Demographic data of participants.
| Variable | Mean ± SD |
F | p | |||
|---|---|---|---|---|---|---|
| Underweight (N = 45) | Normal weight (N = 45) | Overweight (N = 45) | Obese (N = 45) | |||
| Age (years) | 15.184 ± 2.606 | 15.768 ± 2.029 | 15.079 ± 2.261 | 15.755 ± 2.165 | 1.166 | 0.3241 |
| Weight (kg) | 35.066 ± 8.2204 | 53.311 ± 16.641 | 70.488 ± 8.04779 | 88.066 ± 33.066 | 61.975 | 0.000 |
| Height (cm) | 146.822 ± 10.149 | 148.511 ± 8.381 | 147.177 ± 8.7549 | 147.422 ± 11.081 | 0.256 | 0.8570 |
The one-way MANOVA showed a statistically significant difference in peak torque and average power of trunk extensors and flexors at the two selected speeds based on the participants' BMI (F (3, 176) = 14.692, p = 0.0005; Wilks’ Lambda = 0.450, partial η2 = 0.408) (see Table 2).
Table 2.
Descriptive statistics of peak torque and average power of trunk flexors and extensors based on BMI type.
| Variable | Mean ± SD |
F | p | |||
|---|---|---|---|---|---|---|
| Underweight (N = 45) | Normal weight (N = 45) | Overweight (N = 45) | Obese (N = 45) | |||
| PTTE at 60°/s | 89.573 ± 23.726 | 109.113 ± 28.161 | 79.188 ± 32.444 | 76.740 ± 37.135 | 10.312 | 0.000∗ |
| PTTF at 60°/s | 82.055 ± 24.884 | 136.286 ± 40.248 | 78.793 ± 26.8054 | 77.637 ± 29.247 | 38.228 | 0.000∗ |
| APTE at 60°/s | 36.104 ± 21.032 | 46.222 ± 19.363 | 35.6867 ± 25.39903 | 32.942 ± 19.001 | 3.352 | 0.020∗ |
| APTF at 60°/s | 28.600 ± 12.418 | 32.635 ± 11.834 | 24.0622 ± 19.64635 | 20.940 ± 17.683 | 4.767 | 0.003∗ |
| PTTE at 120°/s | 62.075 ± 23.234 | 102.688 ± 40.1513 | 66.404 ± 36.565 | 53.280 ± 23.090 | 21.165 | 0.000∗ |
| PTTF 120°/s | 72.757 ± 23.311 | 83.137 ± 24.04366 | 66.6733 ± 28.41589 | 59.9867 ± 34.449 | 5.5824 | 0.001∗ |
| APTE at 120°/s | 24.686 ± 12.827 | 36.8933 ± 8.998 | 28.5067 ± 11.7839 | 27.49267 ± 9.448 | 10.4903 | 0.000∗ |
| APTF at 120°/s | 17.271 ± 6.080 | 27.0133 ± 9.392 | 19.4822 ± 5.283 | 18.1822 ± 4.790 | 11.221 | 0.000∗ |
PTTE at 60°/s: Peak torque of trunk extensors at 60 degrees/second, PTTF at 60°/s: Peak torque of trunk flexors at 60 degrees/second, PTTE at 120°/s:Peak torque of trunk extensors at 120 degrees/second, APTF at 120°/s: Average power of trunk flexors at 120 degrees/second, APTF at 120°/s: Average power of trunk flexors at 120 degrees/second.APTE°/s at 120°/s: Average power of trunk extensors at 120 degrees/second.
∗ = Significant difference (p < 0.05).
Additionally, BMI was found to have a statistically significant effect on the peak torque of trunk extensors at 60 degrees/second (F (3, 176) = 9.759; p < .0005; partial η2 = 0.192) and the peak torque of trunk flexors at 60 degrees/second (F (3, 176) = 7.410; p = .0001; partial η2 = 0.112).
Furthermore, the peak torque of trunk extensors at 60 degrees/second for the underweight condition was significantly lower than that for the normal-weight condition (mean difference = 19.54). Moreover, the normal-weight condition is significantly higher than the overweight condition (mean difference = 29.931). Furthermore, the normal-weight condition significantly differs from the obese condition (mean difference = 32.373). Therefore, these results suggest that overweight and obesity impact the peak torque of trunk extensors at 60 degrees/second was higher in normal weight condition. Specifically, our results suggest that the peak torque decreases with increase in body weight (Figure 2).
Figure 2.
Peak torque of trunk extensors at 60 degrees/second in four groups.
Moreover, the peak torque of trunk flexors at 60 degrees/second for the underweight condition differed significantly from that for the obese condition (mean difference = 4.418). Additionally, the normal-weight condition significantly differed from the overweight condition (mean difference = 57.493) and from the obese (mean difference = 58.649) in this regard. Thus, these results suggest that overweight and obesity influence the peak torque of trunk flexors at 60 degrees/second. Specifically, when body weight increases, the peak torque decreases.
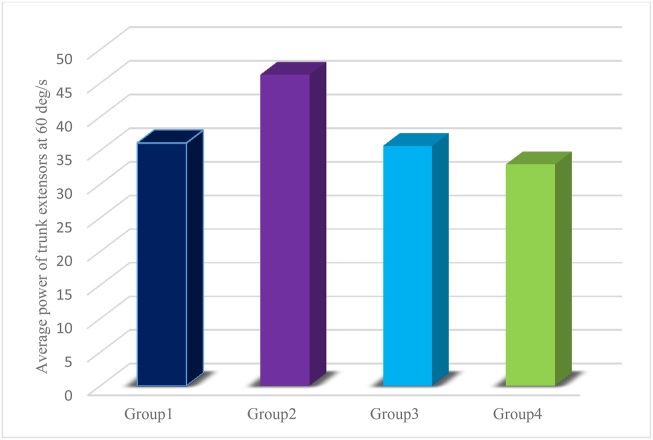
BMI also had a statistically significant effect on the average power of trunk extensors at 60 degrees/second (F (3, 176) = 3.352; p = 0.02; partial η2 = 0.37) and average power of trunk flexors at 60 degrees/second (F (2, 57) = 14.30; p < .0005; partial η2 = .054). Additionally, the results revealed that the average power of trunk extensors at 60 degrees/second for the normal-weight condition differed significantly from that for the obese condition (mean difference = 13.28) (Figure 3). Furthermore, the average power of trunk flexors at 60 degrees/second for the normal-weight condition differed significantly from that for the obese condition (mean difference = 11.695).
Figure 3.
Average power of trunk extensors at 60 degrees/second.
Similarly, a statistically significant effect of BMI on the peak torque of trunk extensors at 120 degrees/second (F (3, 176) = 21.165; p = .000; partial η2 = 0.265) and peak torque of trunk flexors at 120 degrees/second (F (3, 176) = 43.518; p = .000; partial η2 = 0 .426) was observed. The peak torque of trunk extensors at 120 degrees/second for the normal-weight condition differed significantly from that for the underweight condition (mean difference = 40.613) Furthermore, normal weight differs significantly from overweight (mean difference = 36.284), and from the obese (mean difference = 49.408). Similarly, the peak torque of trunk flexors at 120 degrees/second was higher in normal-weight condition was significantly different from that for the overweight condition (mean difference = 16.464) and the obese condition (mean difference = 23.151) (Figure 4).
Figure 4.
Peak torque of trunk flexors at 120 degrees/second.
Additionally, our results showed that BMI had a statistically significant effect on the average power of trunk extensors at 120 degrees/second (F (3, 156) = 6.851; p = 0 .0001; partial η2 = 0.31) and average power of trunk flexors at 120 degrees/second (F (3, 176) = 2.452; p = 0.038; partial η2 = 0.057). The average power of trunk extensors at 120 degrees/second for the underweight condition differed significantly from that for the normal-weight condition (mean difference = 12.207). The normal-weight condition differed significantly from the overweight condition (mean difference = 8.387) and from the obese condition (mean difference = 9.401) in this regard as well. The average power of trunk flexors at 120 degrees/second for the underweight condition differed significantly from that for the normal-weight condition (mean difference = 9.742). The normal-weight condition differed significantly from the overweight condition (mean difference = 7.531) and from the obese condition (mean difference = 8.831) (Figure 5) in this regard as well.
Figure 5.
Average power of trunk flexors at 120 degrees/second.
Correlation between weight and peak torque and average power of trunk flexors and extensors
Pearson's correlation analysis demonstrated a significant negative correlation between weight and the peak torque of trunk flexors and extensors at 60 degrees/second (r = - 0.43, p = 0.0001; r = −0.31, p = 0.007, respectively). Additionally, there was a notable negative correlation between weight and average power of trunk flexors and extensors at 60 degrees/second (r = −0.54, p = 0.0001; r = −0.31, p = 0.007). Similarly, a significant negative correlation between weight and the peak torque of trunk flexors and extensors at 120 degrees/second (r = −0.51, p = 0.0001; r = −0.53, p = 0.0001, respectively). Furthermore, a significant negative correlation was found between weight and the average power of trunk flexors and extensors at 120 degrees/second (r = −0.21, p = 0.04; r = −0.24, p = 0.007).
Discussion
The current study aimed to compare the respective strength and average power of trunk flexors and extensors in adolescents aged 12–8 years with different BMI using an isokinetic dynamometer.
In general, our findings show that increased BMI has a significant negative relationship with the performance of spinal flexors and extensors, including peak torque and average power. Normal weight adolescents’ values of muscle performance are higher than those of the overweight and obese adolescents, with significant differences.
In the present study, the isokinetic measurement of trunk flexors and extensors was performed in a seated position as this position seems to be minimally influenced by the contraction of the muscles of the hip, leading to reduction of stress on the lumbar region.36 Therefore, this position is more suitable for the proper assessment of the strength of trunk flexors and extensors.
The results show that torque increases when moving towards lower velocities. This can be explained by the existence of an inverse relationship between muscle force and velocity of concentric muscle contraction. The slower the contraction of the muscle, the larger the tension it produces, and vice versa. Termed as the force–velocity relationship, this is considered as a basic principle of the physiology of skeletal muscle.37
Our results are in line with those of Pajoutan et al.12 who examined the effect of obesity on the muscle performance of back extensor musculatures. They recruited obese, overweight, and normalweight groups and had them undertake four sets of maximum volitional isometric contractions of back extensors, repeated thrice. They concluded that obesity negatively affects muscle performance in activities demanding stronger contractions. Generally, obesity at different ages results in reduced peak strength when expressed in relation to body mass.38,39
Moreover, these findings are consistent with a previous study of Al Abdulwahab et al.40 that concluded that excessive body weight has a significant negative effect on the control of the muscles around the abdomen and back in the adult population.
These results are also consistent with Mayer et al.,41 who state that obesity, as measured by BMI, is correlated with decreased trunk muscular endurance. Duncan et al.42 also support these findings by reporting that overweight and obesity in British children is significantly associated with poor body function compared to children with normal weight.
Several explanations may clarify the decreased trunk muscular strength in overweight and obese children and adolescents. First, the possible variations between normal-weight adolescents and overweight and obese adolescents in the morphology and geometry of the trunk muscles could be due to excessive accumulation of adipose tissue inside and over the back muscles. This is in agreement with the hypothesis of Ryan et al.43 who state that overweight and obese children may accumulate adipose tissue in the abdominal region, where the greatest fatty deposition occurs in the rectus abdominal muscle, followed by the lateral abdominal muscle and the erector spinae. This accumulation of adipose tissue inside the muscle may interfere with its functional capability, as Hicks et al.44 have reported. The reduction of the functional capability of the core musculature, especially in terms of strength and endurance, may alter the induction of these muscles into the spinal stability.45
Second, the possible variations between normal-weight adolescents and overweight and obese ones are in their general fitness levels. Most overweight and obese adolescents have lower fitness levels than normal-weight adolescents; although their extremities are strong, their trunks are weak. The trunk muscles fail to stabilise the body to enable the extremities to generate sufficient force to produce efficient movement patterns. This is in line with the results of Tse et al.46 who find that core stability is essential to enable the trunk to control the movement of the distal extremities during participation in sports.
Third, weight is also an important factor of performance on measuring strength.47,48 On physical assessments demanding thrusting or elevating body mass, young obese persons show poor performance compared to non-obese young persons because of the effect of overload imposed by excess fat in addition to the original physical load.
Fourth, according to Cavuoto49 and Maffiuletti et al.,50 muscle fatigue in overweight and obese children negatively affects the strength and endurance periods during exercises. Moreover, overweight and obese children are not familiar with physical fitness and experience greater harassment during physical activities than normal-weight children.51
The strengths of the current study are that it included a large sample of adolescents and that it assessed the relationship between trunk flexors and extensors and BMI in paediatrics, which has not been extensively covered in previous studies.
The current study has some limitations that should be taken into consideration. First, the BMI measured in this study is the most popular index of adiposity, but it does not differentiate between fat and lean body masses. Second, only males participated in this study as access to femle schools was not available.
Conclusion
This study clearly indicates that obesity is correlated with decreased trunk muscle torque and power in adolescents. Therapeutic interventions for overweight and obese adolescents should be supported by exercise training programmes to improve muscle performance variables including peak torque and power. These interventions and programmes would consequently enhance the quality of life of overweight and obese adolescents.
Recommendations
Further studies are required that examine the differences between males and females in trunk muscle performance across different age groups. Future studies should also examine the predictors of the trunk flexor and extensor torque using sociodemographic and clinical (BMI) variables.
Source of funding
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Conflict of interest
The authors do not have any conflict of interest to declare.
Ethical approval
The Ethics Committee of College of Medical Rehabilitation Sciences, Taibah University, approved this study on 05/03/2020 (Approval No. CMR-PT-2020-03).
Consent
During the research activities, each student was informed about the study objectives, emphasising the confidentiality of collected data, and verbal consent was provided by the subject prior to the study.
Authors’ contributions
Design and conceptualisation of the study, in addition to data analysis, were carried out by AMA and HAE. Data interpretation was done by all the authors. AMA and HAE conducted data collection and organisation procedures and provided the manuscript in its initial form. Critical review, modifications, and approval of the manuscript's final form were done by OAK and FSA. All authors conducted a similarity check using iThenticate software to approve the final form of the manuscript.
Acknowledgment
The authors would like to thank all participants for their efforts and patience during data collection.
Footnotes
Peer review under responsibility of Taibah University.
References
- 1.Cecily L., Betz Special issue on childhood overweight and obesity. J Pediatr Nurs. 2014;29:491–492. doi: 10.1016/j.pedn.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 2.Al-Hussaini A., Bashir M.S., Khormi M., AlTuraiki M., Alkhamis W., Alrajhi M. Overweight and obesity among Saudi children and adolescents: where do we stand today? Saudi J Gastroenterol. 2019;25(4):229. doi: 10.4103/sjg.SJG_617_18. official journal of the Saudi Gastroenterology Association. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reilly J.J., Kelly J. Long-term impact of overweight and obesity in childhood and adolescence on morbidity and premature mortality in adulthood: systematic review. Int J Obes. 2011;35:891–898. doi: 10.1038/ijo.2010.222. [DOI] [PubMed] [Google Scholar]
- 4.Wilson Louise F., Green Adele C., Jordan Susan J., Neale Rachel E., Webb Penelope M., Whiteman David C. The proportion of cancers attributable to social deprivation: a population-based analysis of Australian health data. Cancer Epidemiology. 2020;67:101742. doi: 10.1016/j.canep.2020.101742. [DOI] [PubMed] [Google Scholar]
- 5.Silva M.R., Badaró A.F., Dall'Agnol M.M. Low back pain in adolescent and associated factors: a cross sectional study with schoolchildren. Braz J Phys Ther. 2014;18(5):402–409. doi: 10.1590/bjpt-rbf.2014.0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lafortuna C.L., Maffiuletti N.A., Agosti F., Sartorio A. Gender variations of body composition, muscle strength and power output in morbid obesity. Int J Obes. 2005;29(7):833–841. doi: 10.1038/sj.ijo.0802955. [DOI] [PubMed] [Google Scholar]
- 7.Tomlinson D.J., Erskine R.M., Morse C.I., Winwood K., Onambélé-Pearson G.L. Combined effects of body composition and ageing on joint torque, muscle activation and co-contraction in sedentary women. Age. 2014;36(3):9652. doi: 10.1007/s11357-014-9652-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maffiuletti N.A., Ratel S., Sartorio A., Martin V. The impact of obesity on in vivo human skeletal muscle function. Curr Obes Rep. 2013;2:251–260. [Google Scholar]
- 9.Delmonico M.J., Harris T.B., Visser M., Park S.W., Conroy M.B., Velasquez-Mieyer P. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am J Clin Nutr. 2009;90(6):1579–1585. doi: 10.3945/ajcn.2009.28047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hilton Tiffany N. Excessive adipose tissue infiltration in skeletal muscle in individuals with obesity, diabetes mellitus, and peripheral neuropathy: association with performance and function. Phys Ther. 2008;88(11):1336–1344. doi: 10.2522/ptj.20080079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hulens M., Vansant G., Lysens R., Claessens A.L., Muls E., Brumagne S. Study of differences in peripheral muscle strength of lean versus obese women: an allometric approach. Int J Obes Relat Metab Disord. 2001;25:676–681. doi: 10.1038/sj.ijo.0801560. [DOI] [PubMed] [Google Scholar]
- 12.Pajoutan Mojdeh, Mehta Ranjana K., Cavuoto Lora A. Proceedings of the human factors and ergonomics society annual meeting. SAGE Publications; Los Angeles, CA: 2016. Obesity effect on isometric strength of the trunk extensors. 60 ( 1). Sage CA. [Google Scholar]
- 13.Harreby M., Neergaard K., Hesselsoe G., Kjer J. Are radiologic changes in the thoracic and lumbar spine of adolescents risk factors for low back pain in adults? A 25-year prospective cohort study of 640 schoolchildren. Spine. 1995;20:2298–2302. doi: 10.1097/00007632-199511000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Leboeuf-Yde C., Kyvik K.O. At what age does low back pain become a common problem? A study of 29,424 individuals aged 12–41 years. Spine. 1998;23:228–234. doi: 10.1097/00007632-199801150-00015. [DOI] [PubMed] [Google Scholar]
- 15.Grabiner M.D., Jeziorowski J.J., Divekar A.D. Isokinetic measurements of trunk extension and flexion performance collected with the biodex clinical data station. J Orthop Sports Phys Ther. 1990;11(12):590–598. doi: 10.2519/jospt.1990.11.12.590. [DOI] [PubMed] [Google Scholar]
- 16.Newton M., Thow M., Somerville D., Henderson I., Waddell G. Trunk strength testing with iso-machines. Part 2: experimental evaluation of the Cybex II Back Testing System in normal subjects and patients with chronic low back pain. Spine. 1993;18(7):812–824. [PubMed] [Google Scholar]
- 17.Iwai K., Nakazato K., Irie K., Fujimoto H., Nakajima H. Trunk muscle strength and disability level of low back pain in collegiate wrestlers. Med Sci Sports Exerc. 2004;36(8):1296–1300. doi: 10.1249/01.mss.0000135791.27929.c1. [DOI] [PubMed] [Google Scholar]
- 18.Yahia A., Jribi S., Ghroubi S., Elleuch M., Baklouti S., Habib Elleuch M. Evaluation of the posture and muscular strength of the trunk and inferior members of patients with chronic lumbar pain. Joint Bone Spine. 2011;78(3):291–297. doi: 10.1016/j.jbspin.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 19.Den Hartog D., Eker H.H., Tuinebreijer W.E., Kleinrensink G.J., Stam H.J., Lange J.F. Isokinetic strength of the trunk flexor muscles after surgical repair for incisional hernia. Hernia. 2010;14(3):243–247. doi: 10.1007/s10029-010-0627-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hupli M., Sainio P., Hurri H., Alaranta H. Comparison of trunk strength measurements between two different isokinetic devices used at clinical settings. J Spinal Disord. 1997;10(5):391–397. [PubMed] [Google Scholar]
- 21.Langrana N.A., Lee C.K., Alexander H., Mayott C.W. Quantitative assessment of back strength using isokinetic testing. Spine. 1984;9(3):287–290. doi: 10.1097/00007632-198404000-00011. [DOI] [PubMed] [Google Scholar]
- 22.Brady S., Mayer T., Gatchel R.J. Physical progress and residual impairment quantification after functional restoration. Part II: isokinetic trunk strength. Spine. 1994;19(4):395–400. [PubMed] [Google Scholar]
- 23.Granacher U., Gollhofer A., Hortobagyi T., Kressig R.W., Muehlbauer T. The importance of trunk muscle strength for balance, functional performance, and fall prevention in seniors: a systematic review. Sports Med. 2013;43(7):627–641. doi: 10.1007/s40279-013-0041-1. [DOI] [PubMed] [Google Scholar]
- 24.Estrázulas J.A., de Jesus K., da Silva R.A., Libardoni Dos Santos J.O. Evaluation isometric and isokinetic of trunk flexor and extensor muscles with isokinetic dynamometer: a systematic review. Phys Ther Sport. 2020;45:93–102. doi: 10.1016/j.ptsp.2020.06.008. [DOI] [PubMed] [Google Scholar]
- 25.Grabiner M.D., Jeziorowski J.J. Isokinetic trunk extension discriminates uninjured subjects from subjects with previous low back pain. Clin BioMech. 1992;7(4):195–200. doi: 10.1016/S0268-0033(92)90001-K. [DOI] [PubMed] [Google Scholar]
- 26.García-Vaquero M.P., Barbado D., Juan-Recio C., Lopez-Valenciano A., Vera- Garcia F.J. Isokinetic trunk flexion extension protocol to assess trunk muscle strength and endurance: reliability, learning effect, and sex differences. J Sport Health Sci. 2016;328:1–10. doi: 10.1016/j.jshs.2016.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Juan-Recio C., L_opez-Plaza D., Barbado Murillo D., García-Vaquero M.P., Vera- García F.J. Reliability assessment and correlation analysis of 3 protocols to measure trunk muscle strength and endurance. J Sports Sci. 2018;36(4):357–364. doi: 10.1080/02640414.2017.1307439. [DOI] [PubMed] [Google Scholar]
- 28.Verbrugghe J., Agten A., Eijnde B.O., Vandenabeele F., De Baets L., Huybrechts X. Reliability and agreement of isometric functional trunk and isolated lumbar strength assessment in healthy persons and persons with chronic nonspecific low back pain. Phys Ther Sport. 2019;38:1–7. doi: 10.1016/j.ptsp.2019.03.009. [DOI] [PubMed] [Google Scholar]
- 29.Roth R., Donath L., Kurz E., Zahner L., Faude O. Absolute and relative reliability of isokinetic and isometric trunk strength testing using the IsoMed- 2000 dynamometer. Phys Ther Sport. 2017;24:26–31. doi: 10.1016/j.ptsp.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 30.Harding A.T., Weeks B.K., Horan S.A., Little A., Watson S.L., Beck B.R. Validity and testeretest reliability of a novel simple back extensor muscle strength test. SAGE open medicine. 2017;5:1–9. doi: 10.1177/2050312116688842. (2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guilhem G., Giroux C., Couturier A., Maffiuletti N.A. Validity of trunk extensor and flexor torque measurements using isokinetic dynamometry. J Electromyogr Kinesiol. 2014;24(6):986–993. doi: 10.1016/j.jelekin.2014.07.006. (2014) [DOI] [PubMed] [Google Scholar]
- 32.Balague F., Nordin M., Skovron M.L., Dutoit G., Yee A., Waldburger M. Non-specific low-back pain among schoolchildren: a field survey with analysis of some associated factors. J Spinal Disord. 1994;7(5):374–379. [PubMed] [Google Scholar]
- 33.Ogden C.L., Flegal K.M. Changes in terminology for childhood overweight and obesity. Natl Health Stat Rep. 2010;25:1–5. [PubMed] [Google Scholar]
- 34.Volek J.S., Kraemer W.J., Bush J.A., Boetes M., Incledon T., Clark K.L. Creatine supplementation enhances muscular performance during high-intensity resistance exercise. J Am Dietetic Assoc. 1997;97(7):765–770. doi: 10.1016/S0002-8223(97)00189-2. [DOI] [PubMed] [Google Scholar]
- 35.Grabiner M., Jeziorowski J. Isokinetic trunk extension and flexion strength-endurance relationships. Clin Biomech. 1991;6:118–122. doi: 10.1016/0268-0033(91)90009-F. [DOI] [PubMed] [Google Scholar]
- 36.Morini S., Ciccarelli A., Cerulli C., Giombini A., Di Cesare A., Ripani M. Functional anatomy of trunk flexion-extension in isokinetic exercise: muscle activity in standing and seated positions. J Sports Med Phys Fit. 2008;48:17–23. [PubMed] [Google Scholar]
- 37.Alcazar Julian, Csapo Robert, Ara Ignacio, Alegre Luis M. On the shape of the force-velocity relationship in skeletal muscles: the linear, the hyperbolic, and the double-hyperbolic. Front Physiol. 2019;10:769. doi: 10.3389/fphys.2019.00769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maffiuletti N.A., Jubeau M., Munzinger U., Bizzini M., Agosti F., De Col A. Differences in quadriceps muscle strength and fatigue between lean and obese subjects. Eur J Appl Physiol. 2007;101:51–59. doi: 10.1007/s00421-007-0471-2. [DOI] [PubMed] [Google Scholar]
- 39.Abdelmoula A., Martin V., Bouchant A., Walrand S., Lavet C., Taillardat M. Knee extension strength in obese and nonobese male adolescents. Appl Physiol Nutr Metabol. 2012;37:269–275. doi: 10.1139/h2012-010. [DOI] [PubMed] [Google Scholar]
- 40.Alabdulwahab Sami S., Kachanathu Shaji John. Effects of body mass index on foot posture alignment and core stability in a healthy adult population. J Exercise Rehabil. 2016;12(3):182. doi: 10.12965/jer.1632600.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mayer John M., Nuzzo James L., Chen Ren, Quillen William S., Verna Joe L., Miro Rebecca. The impact of obesity on back and core muscular endurance in firefighters. J Obesity. 2012:1–7. doi: 10.1155/2012/729283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Duncan M.J., Stanley M., Wright S.L. The association between functional movement and overweight and obesity in British primary school children. Sports Med Arthrosc Rehabil Ther Technol. 2013;5(1):11. doi: 10.1186/2052-1847-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ryan A.S., Harduarsingh-Permaul A.S. Effects of weight loss and exercise on trunk muscle composition in older women. Clin Interv Aging. 2014;9:395–402. doi: 10.2147/CIA.S56662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dedering A., Németh G., Harms-Ringdahl K. Correlation between electromyographic spectral changes and subjective assessment of lumbar muscle fatigue in subjects without pain from the lower back. Clin BioMech. 1999;14(2):103–111. doi: 10.1016/s0268-0033(98)00053-9. [DOI] [PubMed] [Google Scholar]
- 45.Hibbs A.E., Thompson K.G., French D., Wrigley A., Spears I. Optimizing performance by improving core stability and core strength. Sports Med. 2008;38(12):995–1008. doi: 10.2165/00007256-200838120-00004. [DOI] [PubMed] [Google Scholar]
- 46.Tse M.A., McManus A.M., Masters R.S.W. Development and validation of a core endurance intervention program:implications for performance in college-age rowers. J Strength Condit Res. 2005;19(3):547–552. doi: 10.1519/15424.1. [DOI] [PubMed] [Google Scholar]
- 47.Dumith S.C., Ramires V.V., Souza M.A., Moraes D.S., Petry F.G., Oliveira E.S. Overweight/obesity and physical fitness among children and adolescents. J Phys Activ Health. 2010;7(5):641–648. doi: 10.1123/jpah.7.5.641. [PubMed: 20864760] [DOI] [PubMed] [Google Scholar]
- 48.Deforche B., Lefevre J., De Bourdeaudhuij I., Hills A.P., Duquet W., Bouckaert J. Physical fitness and physical activity in obese and nonobese Flemish youth. Obes Res. 2003;11(3):434–441. doi: 10.1038/oby.2003.59. [PubMed: 12634442] [DOI] [PubMed] [Google Scholar]
- 49.Cavuoto Lora A., Maury A., Nussbaum Differences in functional performance of the shoulder musculature with obesity and aging. Int J Ind Ergon. 2013;43(5):393–399. [Google Scholar]
- 50.Maffiuletti N.A., Jubeau M., Munzinger U., Bizzini M., Agosti F., De Col A. Differences in quadriceps muscle strength and fatigue between lean and obese subjects. Eur J Appl Physiol. 2007;101(1):51–59. doi: 10.1007/s00421-007-0471-2. [DOI] [PubMed] [Google Scholar]
- 51.Sun Q., Van Dam R.M., Spiegelman D., Heymsfield S.B., Willett W.C., Hu F.B. Comparison of dual-energy X-ray absorptiometric and anthropometric measures of adiposity in relation to adiposity-related biologic factors. Am J Epidemiol. 2010;172(2):1442–1454. doi: 10.1093/aje/kwq306. [DOI] [PMC free article] [PubMed] [Google Scholar]