Abstract
Aging is an unavoidable part of life. The more aged we become, the more susceptible we become to various complications and damages to the vital organs, including the kidneys. The existing drugs for kidney diseases are mostly of synthetic origins; thus, natural compounds with minimal side-effects have attracted growing interest from the scientific community and pharmaceutical companies. A literature search was carried out to collect published research information on the effects of resveratrol on kidney aging. Recently, resveratrol has emerged as a potential anti-aging agent. This versatile polyphenol exerts its anti-aging effects by intervening in various pathologies and multi-signaling systems, including sirtuin type 1, AMP-activated protein kinase, and nuclear factor-κB. Researchers are trying to figure out the detailed mechanisms and possible resveratrol-mediated interventions in divergent pathways at the molecular level. This review highlights (i) the causative factors implicated in kidney aging and the therapeutic aspects of resveratrol, and (ii) the effectiveness of resveratrol in delaying the aging process of the kidney while minimizing all possible side effects.
Keywords: antioxidant, anti-aging, AKI, CKD, kidney aging, resveratrol
1. Introduction
The aged population is expanding exponentially worldwide [1]. Recently, healthy aging has been regarded as a critical issue due to the increase of the elderly population. The risk of kidney disease among the elderly has been increasing. Kidney aging is associated with age-related comorbidities. While the detailed molecular mechanism underlying kidney aging is not yet known, acute kidney injury (AKI) and chronic kidney disease (CKD) share many phenotypic similarities with aging, including cellular senescence, inflammation, fibrosis, vascular rarefaction, loss of glomeruli, and tubular dysfunction [2,3]. Interruption of the cell cycle leads to the accumulation of senescent tissues in multiple organs, including the kidney, with advancing age [4]. The stable transformation of the kidney structure and its functions in the elderly exhibits a significant reduction in the number of glomeruli (responsible for nephrosclerosis), cortical volume, glomerular filtration rate (GFR), and manifold kidney cysts [3,5,6]. Premature aging and a high mortality rate are exacerbated by inflammation leading to multiorgan dysfunction or diseases [7]. Thus, the mechanisms associated with kidney diseases may help in understanding the molecular pathways involved in kidney aging. At the same time, a promising therapeutic regimen is urgently needed to normalize age-associated issues, including kidney aging [3].
Resveratrol (3,4′,5-trihydroxy-trans-stilbene), a versatile phenolic compound found in various fruits, especially red grapes and vegetables, possesses anti-aging effects and thereby can promote life span through modulation of the important hallmarks of aging, for example, inflammation, oxidative stress, and fibrosis, angiotensin II (Ang II), cell senescence, telomere attrition, mitochondrial dysfunction, angiogenesis, and platelet aggregation [8,9,10,11]. Resveratrol tends to restrain cells from aging and inhibits senescence-associated secretory phenotype development [12]. Calorie restriction (CR) and resveratrol possess similar anti-aging properties by extending the lifespan [13]. Additionally, in a cellular senescence model of IMR-90 cells, 10 μM of resveratrol more effectively decreased cellular senescence and apoptosis than CR [13]. These findings indicate that resveratrol could be a potential alternate anti-aging therapy.
Furthermore, more than 244 clinical trials, along with 27 ongoing experiments, have investigated the safety and efficacy of resveratrol along with its pleiotropic functions. It is reported as safe at up to 5 g/day doses and shows therapeutic potential against various cancers, diabetes, obesity, hypertension, cardiovascular diseases, kidney diseases, inflammatory diseases, Alzheimer’s disease, and so on [14,15,16]. Nihei and colleagues reported that oral resveratrol treatment improves clinical parameters of nephrotic syndrome, including proteinuria and hypoalbuminemia, and normalized dyslipidemia in rats [17]. Resveratrol is shown to attenuate AKI by regulating antioxidant and anti-inflammatory mechanisms in rats [18,19]. Recently, health benefits of resveratrol for treating various kidney diseases have been reviewed [20,21,22]. Considering its pharmacological potential across the body systems and diverse conditions, here we mainly discuss the prospects of resveratrol in the therapeutic management of kidney aging and its associated abnormalities.
2. Methods
A search of the literature was carried out to collect published research information on the effects of resveratrol on kidney aging from available online databases, such as PubMed, Google Scholar, and Scopus, using the keywords ‘resveratrol on kidney diseases’ and ‘resveratrol on the aging process in the kidney such as telomere shortening, DNA damage, cellular senescence mitochondrial damage, endoplasmic reticulum stress (ER stress), autophagy dysfunction oxidative stress, inflammation, fibrosis, lifespan extension, calorie restriction, and epigenetic modulation’. All figures were generated using BioRender.com (accessed on 31 July 2021).
3. Pharmacological Effects of Resveratrol on Kidney Diseases
Patients who have a history of AKI may develop progressive CKD [23]. CKD is characterized by mitochondrial damage, ER stress, autophagy dysfunction, oxidative stress, inflammation, and fibrosis. The protective effects of resveratrol on various kidney diseases, such as AKI and CKD, in vitro and in vivo have been reported [20] and now are summarized below.
3.1. Acute Kidney Injury
AKI is a common kidney disease generally associated with an increased fatality rate [24,25]. Reduced glomerular function and urine output are the primary signs of AKI [26]. The severity of this disease can be estimated through a study demonstrating that an alarmingly high percentage of people (16.9% to 31.0% in Western countries) are suffering from this condition [27].
Many studies have shown that compounds, such as resveratrol, can provide us with a wide range of therapeutic options to act against various factors that are linked to different diseases [28,29]. In humans, resveratrol has proven its potency against AKI by inhibiting the formation of reactive oxygen species (ROS) [30]. Resveratrol-loaded nanoparticles could prevent ischemia/reperfusion (I/R)-induced kidney injury in a rat model [31]. Another study on rabbits showed that resveratrol reduced kidney hypoxia, mitochondrial dysfunction, and kidney tubular cell apoptosis [32]. Resveratrol has also been found to be an effective agent to downregulate tumor necrosis factor-α (TNF-α), interleukin (IL)-1β, and kidney injury molecule (KIM)-1 expression in AKI [33]. Resveratrol could decrease the mortality rate of septic rats and alleviates AKI by relieving ER stress, inhibiting NF-κB pathway activation, and mitigating the inflammatory response [34]. All of these studies indicate that resveratrol can be a potential agent to fight against AKI. An in vitro study showed that resveratrol was shown to increase cell viability while reducing phosphorylation of nuclear factor-κB (NF-κB) and the production of inflammatory factors in response to lipopolysaccharide (LPS), and reduced damage to tunicamycin-induced human kidney 2 (HK-2) cells through inhibiting inositol-requiring enzyme 1 (IRE1) activation [35]. While resveratrol reduced cadmium-induced mitochondrial ROS and apoptosis, it increased mitochondrial biogenesis as well as cell viability in TCMK-1 renal epithelial cells [36]. In addition, resveratrol decreased ochratoxin A (a nephrotoxin)-induced intracellular ROS production and cellular damage in HEK293 cells [37]. However, a detailed investigation is required to elucidate a precise mechanism of resveratrol action in AKI.
3.2. Chronic Kidney Disease
CKD is known to alter the regular function and structure of the kidney, usually irreversibly [38]. Several conditions, such as diabetes, hypertension, proteinuria, reduced cytokine clearance, and chronic infections, are the most common risk factors for CKD [39,40,41]. Resveratrol has been shown to increase the expression of muscle ring-finger 1 (MuRF1) and inhibit the phosphorylation of NF-κB in an in vivo model of CKD [42]. There is a link between mitochondrial dysfunction and CKD pathogenesis. Resveratrol was shown to play a significant role in the recovery of CKD by improving mitochondrial function through a mechanism that involved the preservation of mitochondrial membrane potential loss, enhancing the ATP level, reducing ROS generation, and facilitating oxidative phosphorylation in nephrectomized rats [43]. Resveratrol nanoparticles could be a better candidate for preventing CKD through attenuation of the NLR family pyrin domain containing 3 (NLRP3) inflammasome [44].
Individuals suffering from CKD may experience a reduced function of antioxidant defense due to reduced consumption of antioxidant vitamins and minerals, such as vitamin C and selenium. Due to its antioxidant capacity, resveratrol has the ability to fight CKD [45,46]. Consumption of white wine and olive oil together reduced CKD plasma biomarkers, suggesting a possible anti-inflammatory effect on CKD patients [47]. One study reported that resveratrol effectively reduced the degree of kidney tubular damage in unilateral ureteral obstruction-induced fibrotic rats [48]. Pterostilbene, an analog of resveratrol, prevents renal fibrosis and epithelial to mesenchymal transition (EMT) in mice [49]. All of this evidence suggests that resveratrol can act as a potential agent against CKD.
In HK-2 cells, resveratrol reduced high glucose-induced oxidative stress (such as MDA and ROS levels) by increasing superoxide dismutase (SOD) and catalase [50]. Additionally, resveratrol reduced the transforming growth factor-β (TGF-β)-induced EMT in HK-2 cells in a dose-dependent manner [51]. Resveratrol decreased the cisplatin-induced cellular injury and apoptosis in mouse proximal tubular cells [52]. Resveratrol inhibited oxalate-induced inflammatory cytokines, colonization, and hyaluronan protein level while it increased several antioxidant enzyme activities in human renal epithelial cells [53].
Platelet-derived growth factor (PDGF) is a potent stimulus for mesangial cell proliferation and involved in the pathogenesis of glomerulonephritis [54]. Treatment of mesangial cells with resveratrol inhibited PDGF-induced cell proliferation by regulating PI3K, Akt, ERK1/2, and c-Src [54]. In podocytes, resveratrol reduced high glucose-induced mitochondrial stress, mitochondrial ROS production, mitochondrial dysfunctions, and apoptosis [55]. In HEK293 cells, resveratrol reduced high glucose-induced aging markers, such as β-galactosidase, while it increased SIRT1 and thioredoxin [56].
However, long-term (72 h) exposure to high doses (40–80 μM) of resveratrol increased mitochondrial ROS, fibrotic, and apoptotic protein levels, while it reduced anti-apoptotic proteins and mitochondrial function in HK-2 cells [51]. Since higher ROS levels cause a ROS burst leading to mitochondrial damage [57], high-dose resveratrol-induced higher ROS levels in mitochondria may increase mitochondrial damage in the cells. Additionally, resveratrol at high doses (50–75 µM) increased NF-κB-mediated inflammatory effects in IL-1β or TNF-α-treated mesangial cells and kidney proximal tubular LLCPK1 cells [58]. However, considering the critical doses of resveratrol to induce either protective or cytotoxic effects, substantial research is needed to rule out the controversial effects caused by resveratrol treatment and to recommend for clinical use.
4. Pharmacological Effects of Resveratrol on Aging Biomarkers in the Kidney
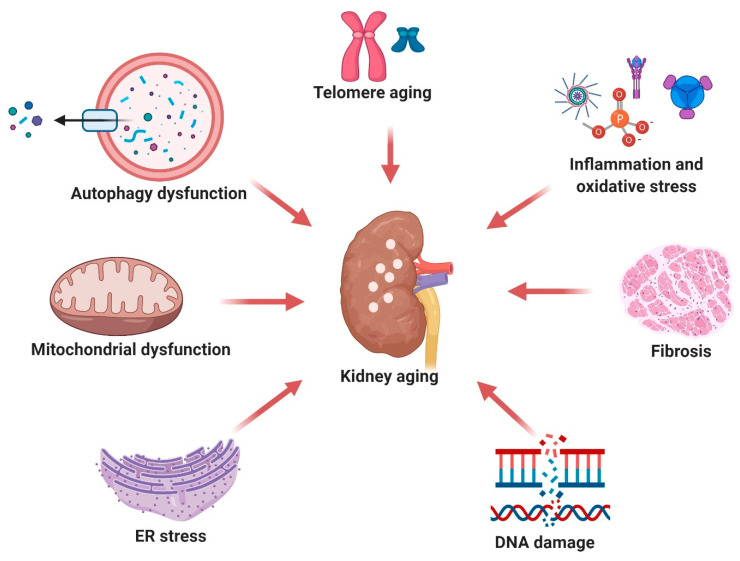
Resveratrol possesses a vast range of benefits in various organs, including the brain, liver, heart, lung, and pancreas, conferring anti-aging landmarks [14,15,16] (shown in Table 1). It also possesses various anti-aging landmarks in the kidney [20,21,22] (shown in Table 2). The anti-aging properties of resveratrol have been reviewed [59]. A growing body of evidence suggests that resveratrol exerts its protective effects against kidney aging by regulating the renin-angiotensin system (RAS) and alleviating inflammation, oxidative stress, and cellular senescence [10,11]. Health benefits of resveratrol on kidney epithelial cells, kidney corpuscle, kidney fibroblasts, and kidney cancer cells, even at the molecular level, both in vivo and in vitro, have been reported [20]. The pharmacological potential of resveratrol against some plausible factors (such as telomere shortening, cellular senescence through DNA damage, mitochondrial dysfunction, ER stress, and autophagy dysfunction oxidative stress, inflammation, and fibrosis, as shown in Figure 1) responsible for kidney aging are delineated in this section.
Table 1.
Pharmacological effects of resveratrol on different mechanisms involved in the aging process in various organs or cells or others.
| Biological Sample Types | Experimental Models | Resveratrol Doses | Mechanisms Involved | Ref. |
|---|---|---|---|---|
| Blood plasma | Older adult humans | daily dose 1–2 g for 4 weeks | ↑ Insulin sensitivity ↑ Plasma glucose in subjects with IGT |
[60] |
| Blood plasma | Patients with peritoneal dialysis | 150 or 450 mg/d | ↑ Angiogenesis ↓ Ang-II |
[61] |
| Brain | Ischemic brain in rats | 40 μM/kg | ↓ Lactate dehydrogenase ↓ Superoxide anion ↑ Mitophagy ↑ AMPK/autophagy ↓ Excess Ca2+ |
[62] |
| Brain | Traumatic brain injury in adult male rats | 100 mg/kg | ↓ IL-1β and IL-18 (inflammatory initiating cytokines) ↓ NLRP3 and caspase-1 pathways ↓ Inflammation and ROS ↑ SIRT1 activation |
[63] |
| Heart | Type 2 diabetes and kidney hypertension in rats | 5, 10 or 20 mg/kg/day for 4 weeks | ↓ Serum MDA ↓ Systolic pressure, blood glucose, heart rate ↑ Serum SOD, glutathione reductase |
[64] |
| Intestine and liver | Obesity in men | 1000 mg/day for 1 week followed by 2000 mg/day for 2 weeks | ↓ Intestinal as well as hepatic lipoprotein ↓ Apolipoprotein B (apoB-48) by 22% |
[65] |
| Liver | HFD-induced fatty liver in mice | 30 mg/kg/day | ↓ TNF-a, IL-6, and IL-1b ↓ NF-κB pathway ↑ AMPKa ↑ SIRT1 |
[66] |
| Lung | Lung fibrosis in mice | 50 and 100 mg/kg for 5 months | ↓ NLRP3 ↓ Cytotoxicity ↓ IL-1β production ↓ inflammation and fibrosis |
[67] |
| Lung | Nicotine-induced lung injury in rats | 20 mg/kg/b.w. for 4 weeks | ↓ IL-2, IL-6, TNF alpha, alpha-fetoprotein, plasma 8-hydroxydeoxyguanosine, Myeloperoxidase, XO, NO, lipid peroxidation ↑ Catalase, SOD, G6PD, and GSH-Px |
[68] |
| Muscle | Barrows/male pigs | 300 mg or 600 mg/kg of feed (dietary) for 49 days | ↓ Muscle lactate, glucose ↓ MDA ↑ Crude protein and myoglobin content, ↑ T-AOC ↑ GSH-Px |
[69] |
| Pancreas | Aged SAMP8 mice | 5 mg/kg/day | ↑ SIRT1 mRNA expression, ↓ NF-κB expression |
[70] |
| Skin | UV-skin change in mice | 10 µM /mouse | ↓ Cellular proliferation ↓ Established markers of tumor progression (epidermal cyclooxygenase-2 and ornithine decarboxylase) ↓ Survivin expression |
[71] |
| Mouse primary hepatocytes | NEFA-treated hepatocytes | 100 µM | ↓ TNF-a, IL-6, and IL-1b ↓ NF-κB pathway ↑ AMPKa ↑ SIRT1 |
[66] |
| Human epidermal keratinocytes | Foreskin biopsies-treated keratinoctyes | 20 µM up to 100 µM | ↑ Cellular glutathione content ↑ Nrf2 ↑ Cutaneous endogenous antioxidant status |
[72] |
| Human nucleus pulposus cells | H2O2-treated pulposus cells | 50 μM | ↓ Mitochondrial dysfunction ↑ Autophagy |
[73] |
| Human lung cancer cells (A549) | Non-small-cell lung cancer in cells | 200 μM | ↑ Beclin1 and LC3 II/I ↑ SIRT1 expression ↓ p62 expression ↑ Apoptosis ↑ Autophagy ↓ Akt/ mTOR pathway ↑ p-38-MAPK pathway |
[74] |
| L6 rat skeletal muscle cells | 2-deoxy-d-glucose-treated muscle cells | 100 µM | ↑ Glucose uptake in muscle ↑ Insulin action ↑ SIRT1, AMPK, GLUT4 |
[75] |
| Peritoneal mesothelial cells | High glucose in peritoneal dialysis solutions-treated cells | 50 μM | ↓ NLRP3 ↑ Autophagy ↑ AMPK-mediated autophagy |
[76] |
AMP-activated protein kinase, AMPK; angiotension II, ang-II; sirtuin type 1, glucose-6-phosphate dehydrogenases, G6PD; SIRT1; superoxide dismutase, SOD; glucose transporter 4, GLUT4; glutathione peroxidase, GSH-Px; malondialdehyde, microtubule-associated protein 1A/1B-light chain 3, LC3; MDA; mammalian target of rapamycin, mTOR; interleukin, IL; tumor necrosis factor alpha, TNF-α; nitric oxide, NO; non-alcoholic fatty liver disease, malondialdehyde, MDA; nuclear factor-κB, NF-κB; NLR family pyrin domain containing 3, NLRP3; impaired glucose tolerance, IGT; total antioxidative capacity, T-AOC; tumor necrosis factor-α, TNF-α, reactive oxygen species, ROS; non-esterified fatty acids, NEFA; ubiquitin-binding protein, p62; protein kinase B, Akt; and xanthine oxidase, XO; ↓: Decreased, and ↑: Increased.
Table 2.
Pharmacological effects of resveratrol on the aging process in kidney tissues or cells.
| Organs/Cells/ Tissues |
Experimental Models | Resveratrol Doses | Mechanisms Involved | Ref. |
|---|---|---|---|---|
| Kidney | AKI in mice | 100 μL of 100 mg/kg | ↓ TLR4 activation, iNOS, ↓ Apoptotic factors (Bax, Bcl-xL) |
[77] |
| Kidney | AKI in rats | 30 mg/kg | ↓ Serum creatinine and urea nitrogen levels, ↓ GRP78, Bip, pIRE1 and p65, TNF-α, IL-1β and IL-6 ↑ IL-10 |
[34] |
| Kidney | AKI in rats | 30 mg/kg | ↓ TNF-α, IL-1β and IL-6 ↓ pIRE1 and pNF-κB |
[35] |
| Kidney | AKI in rats | 100 mg/kg | ↓ MDA and TNF-α ↑ GSH levels and SOD |
[78] |
| Kidney | Cisplatin-induced kidney injury in mice | 10 mg/kg | ↑ SIRT1 and acetylation of p53 ↑ GFR |
[52] |
| Kidney | db/db mice | 40 mg/kg daily | ↓ Serum creatinine, albumin, NOX4, αSMA, and fibronection; ↑ AMPK, and ACC |
[79] |
| Kidney | Diabetic nephropathy in rats | (50 mg/kg/day) | ↓ ER stress related factors (p-PERK, GRP78, ATF4, and CHOP) | [80] |
| Kidney | Diabetic nephropathy in mice | 100 mg/kg /day for 12 weeks | ↑ LC3-II/LC3-I and synaptopodin ↓ Cleaved caspase 3 |
[81] |
| Kidney | Diabetic nephropathy in rats | 5 mg/kg/day | ↑ SIRT1-mediated autophagy | [82] |
| Kidney | Hypertensive rats | 50 mg for 9 weeks | ↓ Kidney inflammation and injury ↓ Oxidative stress, ↑ Nrf2 and GST activity |
[83] |
| Kidney | Progressive IgA nephropathy in mice | 100 mg/kg | ↓ NLRP3 inflammasome ↓ IL-1β, F4/80, CD3 ↓ Glomerular proliferation, glomerular sclerosis, and glomerular inflammation ↓ Superoxide anion levels |
[84] |
| Kidney | Polycystic kidney in rats | 200 mg/kg/day | ↓ NF-κB (p50/p65) ↓ MCP-1, TNF-α, and CFB |
[85] |
| Kidney | STZ-induced diabetes in rats | 30 mg/kg/day | ↓ Proteinuria, MDA, apoptosis ↑ Mn-SOD, SIRT1, PGC-1α |
[55] |
| Kidney | STZ-induced diabetes in rats | 30 mg/kg/day | ↓ Renal function glomerulosclerosis, MDA, and acetylated-FOXO3a; ↑ SIRT1 deacetylase activity; |
[50] |
| Kidney | UUO in mice |
20 mg/kg | ↓ Kidney injury & kidney fibrosis. ↓ MMP7, EMT |
[86] |
| Kidney | UUO in mice | 200 µg/g food | ↓ NF-κB, IL-8, and TNF-α ↑ IL-10 and SIRT1 |
[51] |
| Kidney | UUO in rats | 20 mg/kg/day | ↓ MAPK, PI3K/Akt ↓ TGF-β1-induced FMD ↓ Myofibroblastic phenotype |
[87] |
| Kidney | 5/6 nephrectomy in rats | 20 mg/kg | ↑ Mitochondrial membrane potential and ATP ↑ SIRT1 and PGC-1α deacetylation |
[43] |
| HK-2 cells | LPS-treated cells | 20 μM | ↓ TNF-α, IL-1β and IL-6 ↓ pIRE1 and pNF-κB |
[35] |
| HK-2 cells | High glucose-treated cells | 25 μM | ↓ MDA, and acetylated-FOXO3a ↑ SIRT1 deacetylase activity; |
[50] |
| Mouse podocytes | High glucose-treated cells | 10 μM | ↓ Mitochondrial ROS ↑ SOD, SIRT1, PGC-1α ↓ Apoptosis |
[55] |
| NRK-49F cells | High glucose-treated cells | 20 μM | ↓ ROS, NOX4, αSMA, and fibronection; ↑ AMPK, and ACC |
[79] |
| NRK-49F cells | Iohexol-treated cells | 10 μM | ↑ SIRT1, PGC-1α, SOD ↓ FoxO1, MDA |
[88] |
Acute kidney injury, AKI; AMP-activated protein kinase, AMPK; chronic kidney disease, CKD; glomerular filtration rate, GFR; sirtuin type 1, SIRT1; superoxide dismutase, SOD; glutathione peroxidase, GSH-Px; malondialdehyde, MDA; interleukin, IL; tumor necrosis factor alpha, TNF-α; nitric oxide, NO; nuclear factor- erythroid 2-related factor-2 (Nrf2); fibroblast growth factor 23 (FGF-23); glutathione-S-transferase, GST; NLR family pyrin domain containing 3, NLRP3; lipopolysaccharide, LPS; signal transducer and activator of transcription 3 (STAT3); forkhead box, FOXO; peroxisome proliferator-activated receptor-γ coactivator-1α, PGC-1α; caloric restriction, CR; monocyte chemoattractant protein 1, (MCP-1), Streptozotocin, STZ; tumor necrosis factor-α, TNF-α; and complement factor B, CFB, fibroblast–myofibroblast differentiation, FMD. ↓: Decreased, and ↑: Increased.
Figure 1.
Pathological features of the kidney aging process. Kidney aging mainly occurs through several events in a sequential manner. These include (i) kidney fibrosis along with a reduction in cortical mass, increased glomerulosclerosis, and promptness of RAS; (ii) cellular senescence occurs through a persisting DNA damage response and an increase in interferon-gamma (IFN-γ); (iii) mitochondrial damage induces an increased level of ROS through, which causes dysfunction with mitochondria; (iv) inflammation and oxidative stress are mediated through an increase in lipid peroxidation, NF-κB activation, and glutathione depletion; (v) ER stress; (vi) autophagy dysfunction; and (vii) telomere shortening occurs by limiting transcription by DNA polymerase, leading to GFR diminution, decreased urinary concentration, reduced urinary acidification, impaired potassium clearance, increased vascular resistance, a contracted kidney mass, and blood flow.
4.1. Telomere Shortening
The 6-bp recurred sequence, TTAGGG, which is known as the telomere, constructs the end of each mammal’s chromosome. Mitotic cell division is a fundamental process, and during each cycle of division, almost 50–200 bp telomeric sequences tend to be eroded due to the ″end replication problem″, causing telomere shortening [89]. The mechanism of limiting transcription by DNA polymerase is associated with replicative senescence, apoptosis, cancer, and CKD [90,91]. Thus, the telomeric length is considered to be a probable biomarker of kidney aging [92]. The most consolidated telomerase function having unlimited replicative dynamics is demonstrated in human cancers [91,93]. Telomeric shortening is ultimately responsible for significant changes in the kidney, such as decreases in GFR, urinary concentration, urinary acidification, kidney mass, and blood flow [94,95]. A critically short telomere evokes cell-cycle inhibitor p21 and CDKIs, which play a crucial role in arresting the cell cycle progression, resulting in apoptosis of kidney cells [96,97]. An inhibitory protein named p16INK4a is responsible for inhibiting the activity of CDK4 and CDK6 [96]. The manifestations of p21 and p16INK4a become exacerbated by activation of p53, with serious impacts on vital organs, such as the kidneys, in the elderly population [98]. The propensity to shortening of the telomeres is commensurate to kidney aging, along with aging in most other organs, tissues, and cells, including the lung, pancreas, liver, muscle, hepatocytes, intestinal epithelial cells, peripheral blood cells, lymphocytes, and vascular endothelial cells [99,100].
Telomerase is a reverse transcriptase enzyme (TERT) that adds repetitive sequences to telomeres in dividing cells to prevent the telomeres from shortening [101]. In CKD patients, the lowest levels of telomerase activity (TLMA) and TERT expression were detected, while they had the highest IL-6 and C-reactive protein (CRP) levels [102]. Resveratrol activates telomerase activity in epithelial [103] and endothelial progenitor cells [104]. Direct modulation of p53 by resveratrol has also been observed [52]. Resveratrol treatment promoted p53 deacetylation and thereby attenuated cisplatin-induced kidney apoptosis and improved the GFR [52].
However, in cancer, most cancer cells with limitless proliferative capacity protect their telomeres by expressing high TLMA. Resveratrol at higher concentrations (>2.5 µg/mL) induced a substantial and concentration-dependent downregulation of TLMA in carcinoma cell lines, with 100% inhibition at 40 µg/mL [105]. Besides, resveratrol was also shown to be effective in downregulating the expression of h-TERT protein in human A431 epidermoid carcinoma cells [106].
4.2. Cellular Senescence and DNA Damage
Aberrant accumulation of chronic senescent cells in response to prolonged signaling promotes kidney disease is also linked to age-related declines in kidney function [107]. Cellular senescence is a state where cellular differentiation and proliferation are inhibited. DNA damage is a ubiquitous mediator for both replicative superannuation that is brought about by premature cellular senescence and telomere shortening induced by various pathogenic factors, such as oxidative stress, mutations, and the failure of DNA repair mechanisms [108]. In the kidney, both G1- and G2-arrested senescent cells accumulate with advancing age and kidney disease [4]. A persistent DNA damage response (DDR) signal is provided by senescent cells to trigger the targeted arrest. The activation of DDR affects the chromatin of damaged DNA and the whole genome as well [109]. Moreover, the pathogenesis of atherosclerosis becomes exacerbated by interferon-gamma (IFN-γ), which entails the promotion of rapid cellular senescence following triggering of a p53-dominated DNA damage pathway [110]. Oxidative stress acts as a catalyst in the cellular senescence process, and this situation leads to kidney damage, which eventually turns into kidney aging.
Sepsis is a state of disrupted inflammatory homeostasis causing multiorgan failure, where resveratrol treatment is intended to alleviate oxidative DNA damage [78,111]. Resveratrol treatment attenuates the dysregulation of regulators involved in the cell cycle and senescence pathways [112]. Additionally, resveratrol treatment impedes high-glucose-induced cell senescence (β-galactosidase) in the kidneys [113]. Resveratrol administration resulted in dysregulation of regulators involved in cell cycle and senescence pathways, such as cyclin-dependent kinase (CDK4 and 6), cyclin D1, p21, and p16, leading to senescence instead of apoptosis in gastric cancer in vivo and in vitro [112].
4.3. Mitochondrial Damage
Mitochondria are considered highly dynamic organelles because they have numerous functions within a cell [114]. Mitochondrial damage has a significant impact on kidney function. Although mitochondrial ROS plays a protective role up to a certain level, it can cause mitochondrial dysfunction and cellular damage after exceeding the limit [115]. The mutation rate of mitochondrial DNA (mtDNA) is 10 to 1000 times higher than that of chromosomal DNA. Both exogenous (UV, base analog, ROS, and others) and endogenous stimuli (single or double strand breakdown, mismatch in base pair) may cause mtDNA damage [116]. Thus, a potential therapeutic option that can improve mitochondrial fitness is necessary to treat kidney diseases [117].
Resveratrol preserves mitochondrial integrity and enhances autophagy through inhibition of mitochondria damage signals like NLRP3 inflammasome-derived IL-1β production and pyroptosis in macrophages [84]. Resveratrol supports the escape of mtDNA from ROS by reducing cellular H2O2 and NO levels [118,119]. Resveratrol increases the expression of proteins involved in the electron transport chain, the mitochondrial content, and mitochondrial biogenesis markers [118]. In addition, resveratrol was shown to activate autophagy to attenuate mitochondrial dysfunction and apoptosis [73]. Administration of resveratrol improves mitochondrial function in kidneys through upregulation of sirtuin type 1 (SIRT1) and PGC-1α deacetylation, which constitute one of the most significant kidney protective mechanisms of resveratrol [43].
4.4. ER Stress
A role of ER stress is common to several kidney disease conditions, including kidney fibrosis, glomerulopathies, primary glomerulonephritis, diabetic nephropathy, and so on. ER stress induces apoptosis of kidney cells, leading to kidney damage [120,121,122]. Glycation stress is a contributing factor that involves an imbalance of protein homeostasis and post-translational modification of proteins in the kidney that leads to ER stress [123]. Even slightly diminished autophagy or micro-autophagy accelerates inflammation and ER stress in adipose tissue with aging [124]. The mechanism involved in ER stress in the kidney is related to a complicated chain/pool of stress signaling networks, where several parameters, such as oxidative stress, the Akt pathway, and lipid and epigenetic alterations, make the kidney susceptible to hypoxia stress [125]. Moreover, redox signaling mechanisms involved in ROS cascades become exacerbated by ER stress, which not only causes kidney injury but also results in multiple disorders in humans [126].
Looking for a solution, due to limited mechanism-based therapies targeting kidney diseases/aging, modulation of ER stress using pharmacological regimes could be a promising option/approach [121,127]. Apart from this, knockdown of ER stress-expressing proteins, for instance, reticulon 1 (RTN1), could contribute to halting the stress along with minimizing kidney risks [128]. Moreover, resveratrol, as a natural treatment, demonstrated a beneficial effect in alleviating ER stress and thereby improving kidney function and tubular cell injury [35]. Resveratrol has been proven to reduce the levels of ER stress-related factors, which in turn attenuate urinary protein, blood glucose, kidney damage, and AKI along with the mortality rate [34]. Resveratrol even protects against cadmium (Cd)-induced ER stress and nephrotoxicity [129]. Furthermore, H2O2-induced ER stress is also alleviated by treatment with resveratrol by improving the redox balance in bovine mammary epithelial cells [130].
4.5. Autophagy Dysfunction
Autophagy is crucial for protein homeostasis [121]. Though oxidative stress and autophagy dysfunction are intimately involved in kidney diseases, very little is known about the signaling processes that link them [131]. Autophagy is a degradation pathway of lysosomal proteins in a highly regulated manner, where ablation of protein aggregates and damaged organelles occurs to maintain intracellular homeostasis and cellular integrity, and therefore, an aberration of this pathway leads to the pathogenesis of a variety of kidney diseases and aging [132,133]. For instance, impairment/disturbances in autophagic flux may lead to pathogenesis of kidney lipotoxicity, kidney injury, lysosomal dysfunction, AKI, diabetic nephropathy, focal segmental glomerulosclerosis, polycystic kidney disease, and kidney aging [134,135,136]. However, the induction of autophagy during CKD in mice is responsible for the impairment of mitochondria and ATP production [137].
Activation of autophagy plays an important role against kidney diseases and the aging process, while sirtuins, mammalian target of rapamycin (mTOR), and AMP-activated protein kinase (AMPK) are the key regulators of autophagy [134,138]. Resveratrol inhibits the NLRP3 inflammasome pathway through induction of AMPK and SIRT1-mediated autophagy [139]. The effects of resveratrol on autophagy were shown against diabetic nephropathy, where this compound enhanced the LC3-II/LC3-I protein ratio and downregulated cleaved caspase-3 expression [81]. Long-term use of resveratrol is reported to reduce type 2 diabetic nephropathy since it promotes SIRT1-mediated autophagy induction [82]. Additionally, in vitro and in vivo studies showed that resveratrol reduced oxalate-induced kidney inflammation and oxidative stress through autophagy activation [140].
Pterostilbene, an analog of resveratrol, has several health benefits through activation of autophagy. Pterostilbene prevents kidney fibrosis via activation of autophagy and attenuation of the NLRP3 inflammasome and EMT [49]. Additionally, resveratrol nanoparticles induce autophagy and inhibit CKD through inhibition of the NLRP3 inflammasome and IL-1β production [44].
4.6. Oxidative Stress and Inflammation
Oxidative stress and inflammation are two common pathogenic factors that account for functional changes in the kidney during senescence. Oxygen-free radicals and pro-oxidants serve as an exaggerating player in both CKD and AKI [141,142]. CKD/aging, exacerbated by both oxidative stress and inflammation, involves a significant decline in SOD and glutathione peroxidase (GSH-Px) and a sudden increment of several pro-inflammatory cytokines, for instance, IL-1, IL-6, and TNF-α, along with CRP and MDA [143,144]. Excessive production of NO is triggered by inflammation-incited NO synthase, which exaggerates highly reactive superoxide radical generation. Furthermore, the reaction between excess NO synthase and SOD results in peroxynitrite formation. Besides, increased ROS and RNS elicit more age-provoking factors, for example, angiotensin II, chemokines, and so on [145]. The adverse effects exerted by reactive oxygen and nitrogen species (RONS) are obstructed by antioxidant defenses, and any inconsistency between them results in oxidative stress, and eventually tissue damage with further age-related complicacy [146]. The premature kidney aging phenotype incorporates muscle wasting, vascular calcification, depression, osteoporosis, and frailty, which are accelerated by systemic inflammation. On the other hand, uremic inflammation is engaged to alter the functional mechanism of the mitochondria, telomeres, and nutrient sensing [7]. The effects of oxidative stress and inflammation in the kidney provoke higher lipid peroxidation, NF-κB activation, and glutathione depletion, leading to Nrf2 deactivation and impaired antioxidant defenses [147].
As a forthright antioxidant, resveratrol serves as a shield for cellular biomolecules against oxidative damage by scavenging diverse RONS and secondary radicals [148]. Resveratrol was found to lower oxidative stress by activating the Nrf2 pathway [83,149], alleviating kidney inflammation and injury, recuperating the antioxidant capacity by promoting glutathione-S-transferase (GST) activity, mitigating hypertension, reducing apoptosis, and suppressing the NF-κB pathway along with caspase cascades [70,83,150]. Resveratrol treatment also exhibited its kidney protective effects by inhibiting inflammatory cytokines [151]. Resveratrol was proven to impede the manifestation of TNF-α and IL-1β in the hippocampus [152]. SIRT1 mRNA expression flourished, and age-related pro-inflammatory and pro-oxidant status was reduced in response to resveratrol treatment [70]. Additionally, a straightforward suppression of pro-inflammatory cytokines and, at the same time, the promotion of anti-inflammatory cytokine (IL-10) release by resveratrol were conspicuously exhibited [153]. The simultaneous prevention of inflammation and the disruption of endothelial cell permeability of kidney tissues by resveratrol significantly improved kidney function [77]. The blockage of NF-κB reduces pro-inflammatory factors (MCP-1, TNF-α, and CFB), and resveratrol alleviated inflammation in polycystic kidney disease [85]. Resveratrol can exert amazing renoprotection effects by inhibiting inflammatory responses and lowering oxidative stress via the Nrf2/TLR4/NF-κB pathway [150]. Additionally, resveratrol stimulates the induction of SOD expression and increases the GSH level while downregulating MDA and TNF-α [78]. Resveratrol treatment impedes high glucose-induced kidney oxidative stress by activating SIRT1 [113].
4.7. Fibrosis
Kidney aging is considerably associated with a reduced cortical mass and GFR, and with glomerulosclerosis, tubular atrophy, interstitial fibrosis, and arteriosclerosis [154,155]. EMT is the main mechanism of kidney fibrosis, while the TGFβ-1-SMAD pathway and hypoxia are known as the main modulator of EMT [156,157]. Kidney tubular EMT is a major contributing factor to age-related kidney fibrosis. Additionally, the activation of RAS causes kidney fibrosis. In particular, Ang II and its corresponding receptors are responsible for mediating kidney injury, while angiotensin 1-7 has a protective role by counteracting the effects of Ang II [10].
Resveratrol is an effective therapeutic agent against diabetic kidney fibrosis [79]. Additionally, resveratrol restrains Ang II expression and exaggerates Ang 1-7 with improved kidney histologic findings both in vivo and in vitro [10,158]. Anti-fibrotic or pro-fibrotic kidney effects with improved kidney function were demonstrated upon resveratrol administration in a dose-dependent manner [51]. As matrix metalloproteinase K (MMP) is a promotional player in aging, identifying an agent that can block its expression is worthwhile. Resveratrol may attenuate kidney injury and fibrosis through inhibition of EMT [51]. Additionally, pterostilbene prevents renal fibrosis and EMT in high adenine diet-induced CKD mice [49]. By targeting both EMT and fibroblast–myofibroblast differentiation (FMD), resveratrol may successfully impede fibrosis formation and the myofibroblastic phenotype by suppressing the activity of the proliferation-related signaling pathways, such as MAPK, PI3K/Akt, and SMAD2/3 [87]. Resveratrol also alleviates age-related EMT in aging kidneys [159].
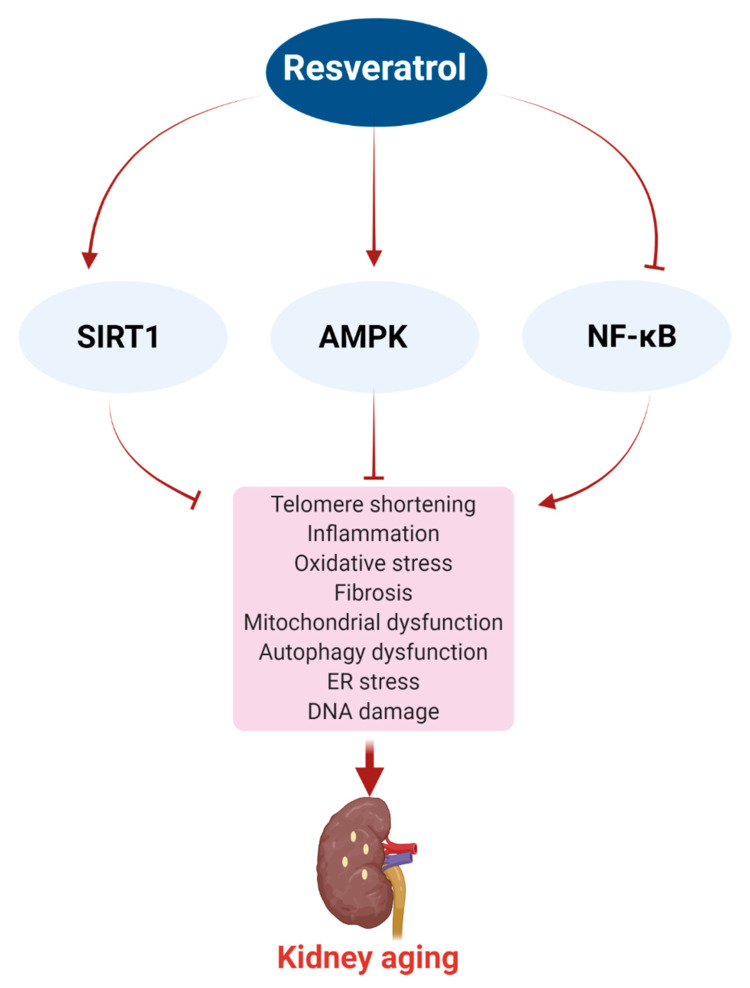
5. Effect of Resveratrol on Age-Related Mechanisms
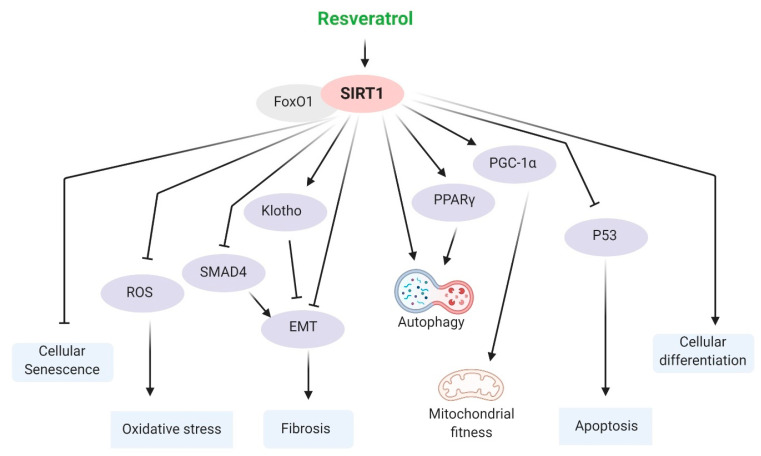
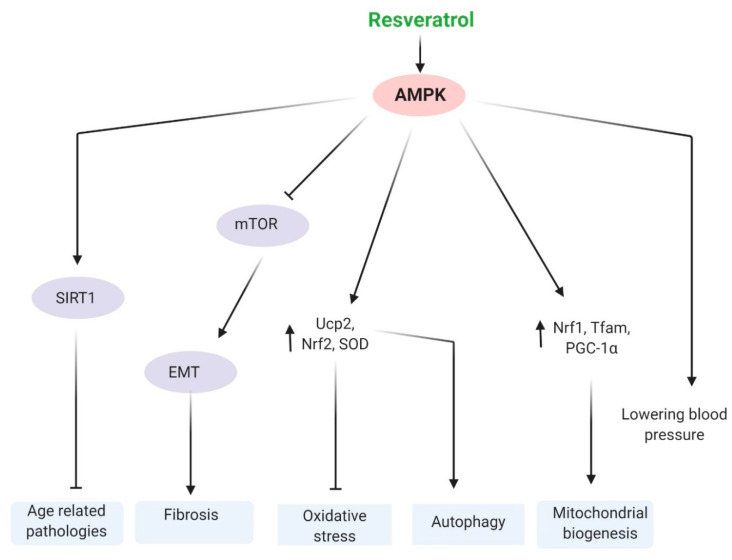
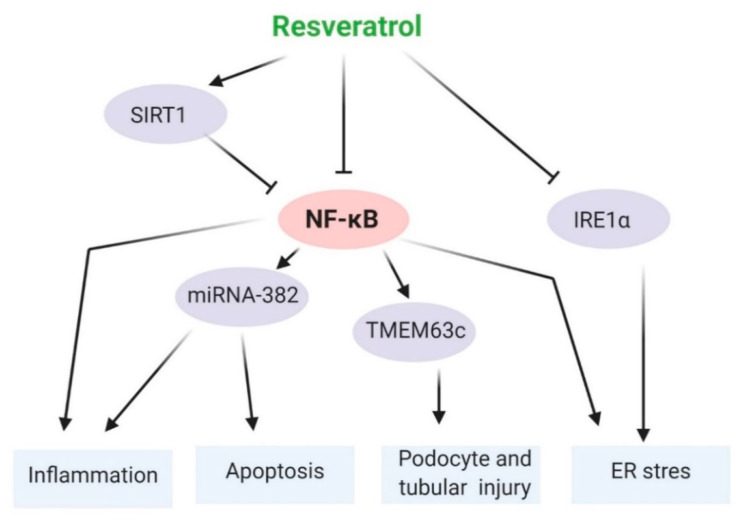
There are mainly three mechanisms involved in the anti-aging effects of resveratrol in the kidney, including SIRT1, AMPK, and the NF-κB pathways. These are discussed below and summarized in Figure 2, Figure 3 and Figure 4.
Figure 2.
Resveratrol-induced SIRT1 is associated with a modulation of various mechanisms of age-related pathologies. Resveratrol stimulates binding between FoxO1 and SIRT1, and it may reduce kidney damage, myocyte hypertrophy, cellular senescence, oxidative stress, tubular apoptosis, and interstitial fibrosis. It also stimulates cellular differentiation and autophagy through SIRT1 activation. All of these pathways are involved in the higher potency of resveratrol against the aging process. Arrow means activation, and ‘T’ arrow means inhibition.
Figure 3.
Resveratrol-induced AMPK is associated with the modulation of various mechanisms of age-related pathologies. Resveratrol-induced AMPK activates SIRT1, leading to the inhibition of many age-related pathologies. AMPK suppresses EMT and fibrosis through inhibition of mTOR. Nrf2, and SOD expression, which is associated with the activation of autophagy and the suppression of oxidative stress. Additionally, AMPK lowers blood pressure and increases mitochondrial biogenesis-related genes, such as Nrf1, Tfam, and PGC-1α. All of these pathways mediated by resveratrol-induced AMPK are associated with the anti-aging process. AMP-activated protein kinase, AMPK; epithelial–mesenchymal transition, EMT; sirtuin type 1, SIRT1; mammalian target of rapamycin, mTOR; mitochondrial uncoupling protein2 (UCP2); nuclear factor erythroid 2–related factor 2 (Nrf2); peroxisome proliferator-activated receptor-γ coactivator-1α, PGC-1α; and mitochondrial transcription factor A, Tfam. Arrow means activation, and ‘T’ arrow means inhibition.
Figure 4.
Resveratrol-inhibited NF-κB is associated with the inhibition of various mechanisms of age-related pathologies. Resveratrol inhibits NF-κB; thus, it may reduce kidney inflammation, tubular apoptosis and injury, podocyte apoptosis and damage, and ER stress. It also suppresses ER stress through inhibition of the IRE1α pathway. All of these pathways are involved in the higher potency of resveratrol against the NF-κB-associated aging process in kidneys. Membrane protein 63c, TMEM63c. Arrow means activation, and ‘T’ arrow means inhibition.
5.1. SIRT1
SIRT2 overexpression may extend longevity in the yeast Saccharomyces cerevisiae by 30%, while suppression of the SIRT2 gene causes a reduction of its lifespan by almost half [160]. In mammals, CR induces SIRT1 activation, which regulates various physiological processes, such as mitochondrial fitness, metabolism, and the aging process, leading to an extension of lifespan [161,162,163]. In zebrafish, resveratrol mediates the AMPKα-SIRT1-PPARγ pathway and lipid metabolism [164]. In diet-induced obese mice, resveratrol improves metabolic phenotypes related to the aging process by downregulating cAMP and phosphodiesterases [165]. Thus, both resveratrol and CR have a beneficial effect in terms of metabolic regulation [166].
Several studies have suggested beneficial effects of resveratrol on kidney aging. A recent study showed that resveratrol protects against glomerulosclerosis in aged mice, improving kidney oxidative stress via SIRT1-mediated klotho expression [167]. Another study showed that resveratrol increased the SIRT1 expression level, which ultimately improves EMT and Yin yang 1 (YY1) acetylation induced by high glucose [168]. Resveratrol also reduces cadmium (Cd)-induced nephrotoxicity and mitochondria dysfunction through upregulation of VDAC1, Cyt C, SIRT3, SIRT1, PGC-1α, Nrf1, and TFAM [169]. The resveratrol-activated SIRT1 pathway plays a protective role by autophagy induction in I/R-induced AKI [170,171]. Resveratrol stimulates binding between forkhead box protein O (FoxO) 1 and SIRT1; thus, it may reduce kidney damage, myocyte hypertrophy, and interstitial fibrosis in nephrectomized mice [172]. The deacetylating activity of the resveratrol-activated SIRT1 pathway results in alterations in various downstream regulators, such as PGC-1α [173]. Elevated levels of NAD induced SIRT1, which leads to enhanced PGC-1α transcriptional activity [174]. Evidence from both in vitro and in vivo experiments suggests that this polyphenol can act as a safeguard for the kidney through maintenance of the SIRT1-PGC1α-FoxO pathway [88]. A recent study suggested that PGC-1α might be a potential therapeutic target against kidney aging [175]. Additionally, activation of SIRT1 by resveratrol treatment caused p53 deacetylation and thereby attenuated cisplatin-induced kidney injury and tubular apoptosis [52]. Resveratrol protects against Cd-induced nephrotoxicity by inhibiting IRE-1α and activating SIRT1 [129]. Kidney injury and fibrosis are attenuated by the resveratrol-induced SIRT1 signaling pathway through inhibition of EMT, where deacetylation of SMAD4 plays a vital role in inhibiting TGF-β and MMP7 [51,86]. Resveratrol attenuates ROS-induced oxidative stress through activation of the SIRT1 pathway [119]. Additionally, resveratrol treatment impedes high-glucose-induced cell senescence in the kidney by activating SIRT1 [113]. SRT1720 (an analog of resveratrol), an activator of SIRT1, reduces renal fibrosis by attenuating TGF-β1 and oxidative stress in UUO mice [176]. All of this evidence supports the fact that resveratrol may become a potential therapeutic against kidney aging through activation of SIRT1 and its target pathways (Figure 2).
5.2. AMPK
A potential role of AMPK signaling in kidney diseases, including diabetic nephropathy, polycystic kidney disease, subtotal nephrectomy, lupus nephritis, and kidney fibrosis, has been reported [177]. AMPK induces autophagy through upregulation of several antioxidants, such as SOD, uncoupling protein 2 (UCP2), and Nrf2, while it downregulates nicotinamide adenine dinucleotide phosphate oxidase (NOX, a primary source of ROS), suggesting a role of AMPK in the inhibition of oxidative stress in kidney disease [178,179].
In the process of aging, AMPK is the main nutrient sensor. Pro-longevity interventions, such as dietary restriction, induce AMPK activation to regulate cellular homeostasis [180,181,182]. The anti-aging effect of resveratrol is strongly linked to the activation of AMPK. Activation of AMPK is involved in lowering blood pressure in hypertensive mice [183]. In vascular smooth muscle, resveratrol promotes cellular differentiation through activation of the AMPK-SIRT1 pathway [184]. In primary human keratinocytes, resveratrol reduces oxidative stress-induced senescence by activating AMPK-FOXO3 [185]. AICAR, an activator of AMPK, increases the endogenous Sirt1 in mouse embryonic fibroblasts [186]. Resveratrol is an effective therapeutic agent against diabetic kidney fibrosis via AMPK/NOX4/ROS signaling [79]. Additionally, resveratrol alleviates age-related EMT in aging kidneys via AMPK-mTOR signaling [159]. All of this evidence suggests that resveratrol has significant effects on AMPK, which ultimately may help to fight against kidney aging (Figure 3).
5.3. NF-κB
Increased activity of NF-κB has been implicated in the pathogenesis of AKI [187]. Additionally, NF-κB promotes inflammation and regulates apoptosis; these two factors are associated with the progression of CKD [188]. In an in vivo study with mice, the upregulation of microRNA-382 in kidney epithelial cells was mediated by the activation of NF-κB signaling, which elevates pro-inflammatory cytokines [189]. Ang II and NF-κB play a vital role in podocyte injury via membrane protein (Tmem) 63c [190]. Furthermore, the pathogenic role of NF-κB in mediating chronic inflammation in tubular epithelial cells, podocytes, mesangial cells, and macrophages during CKD has been reviewed [191].
In a rat model of AKI, resveratrol increases the survival rate by promoting NF-κB-p65 deacetylation by upregulating SIRT1 and it inhibits inflammatory responses [192]. Resveratrol attenuates ER stress through suppression of IRE1 and NF-κB in kidney tubular cell injury [35]. Skeletal muscle atrophy is an important clinical characteristic of CKD. Resveratrol reduces skeletal muscle atrophy through suppression of NF-κB activation in in vivo models [42]. In addition, SRT1720 reduces vascular endothelial dysfunction by inhibiting aortic NF-κB activation and TNF-α levels in old mice [193]. In humans, resveratrol inhibits the signaling pathway of NF-κB, thereby inhibiting inflammation [194]. These studies suggest that through modulation of NF-κB pathways, resveratrol may act as a protective agent against kidney aging (Figure 4).
6. Resveratrol as Epigenetic Modulator
Modulation of epigenetics is a major mechanism in aging [195]. Resveratrol mediates modifications to epigenetic enzymes, such as DNA methyltransferases (DNMTs), the histones acetyltransferases family (HATs), and the histone deacetylases family (HDACs), which ultimately impact our overall health and longevity [196]. While resveratrol increases AMPK, leading to activation of the SIRT1 pathway [164], SIRT1 catalyzes the deacetylation of histones and several transcription factors [197]. Thus, the beneficial effects of resveratrol are mediated through epigenetic modification by upregulation of the AMPK/SIRT1 pathway. SRT1720, a specific SIRT1 activator, mediates deacetylation and activation of PGC-1α, which restores tubular mitochondrial fitness, resulting in a decrease in I/R injury [198]. SIRT1 is suggested to deacetylate and inactivate the p65 subunit of NF-κB and STAT3, which reduces podocyte dysfunction in mice [199]. In addition, SIRT1 is found to deacetylate FoxO4 and it inhibits pro-apoptotic genes, such as Bcl2L11, which leads to a reduction in podocyte apoptosis [199]. SIRT1 increases the deacetylation of SMAD7, leading to inhibition of apoptosis in mesangial cells [200]. Resveratrol attenuates diabetic nephropathy through the activation of SIRT1 in rats. SIRT1 deficiency inhibits these effects [201]. Resveratrol protects the kidney by maintaining the SIRT1-PGC1α-FoxO pathway [88]. Histone H3.1 is a protein encoded by the HIST1H3E gene. In primary renal epithelial cells, epigenetic regulation of HIST1H3E has an overall effect on aging-related genes in humans. Additionally, resveratrol decreased HIST1H3E expression and increased SIRT5 in muscle cells [202].
A recent review suggests that resveratrol mediates neuroprotective effects against Alzheimer’s disease pathology through epigenetic changes, including anti-aging effects in the brain [203]. Furthermore, another study has reviewed the epigenetic regulation of resveratrol against ocular diseases [204].
7. Resveratrol as a Calorie Restriction Mimetic
CR is an effective way of delaying the aging process and preventing chronic diseases, such as abdominal obesity, diabetes, hypertension, and cardiovascular diseases [205]. Glomerulosclerosis and kidney interstitial fibrosis occur in the aging kidney. It has been shown that long-term CR reduces aging-related kidney fibrosis by downregulating microRNA21 [206]. Short-term CR has potential in treating AKI [207]. Heat shock protein 47 (Hsp47) promotes kidney fibrosis and glomerulosclerosis in a rat model of CKD, while CR has been found to downregulate this protein, thus slowing the aging process of the kidney in mice [208,209]. Resveratrol mediates several mechanisms, such as activation of SIRT1, development of insulin sensitivity, and utilization of energy, which are closely related to the effects of CR. Resveratrol supplementation has exhibited beneficial effects by altering metabolic activities by improving insulin and glucose tolerance in old mice [166]. A randomized controlled trial revealed that among individuals with diabetes who take resveratrol, it helped them to decrease their level of fasting glucose, reduce their insulin resistance, and reduce their glycated hemoglobin levels [210]. A double-blind crossover study showed that resveratrol supplementation for a month in 50-year-old men with obesity mediated CR-like effects by inducing some changes in metabolism by regulating the AMPK–SIRT1–PGC-1α axis [211]. Several studies have proposed that resveratrol and CR have the same impact on various targets, such as adiponectin, AMPK, Akt, MnSOD, and NF-kB, in the cardiovascular system in mammals [212]. Apart from these, resveratrol and CR mediate similar effects against the aging of neuromuscular junctions and muscle fibers in old mice [213]. Accordingly, CR significantly decreases urea nitrogen, creatinine, and urine protein in CKD rodents [214]. Resveratrol-induced CR-like effects might alleviate age-related EMT in aging kidneys via AMPK-mTOR signaling [159]. It has been observed in genetically obese animals that decreased food intake prevents or partially delays some specific degenerative lesions, more specifically glomerulonephritis associated with obesity and diabetes [215]. In individuals suffering from type 2 diabetes with abdominal obesity, CR has shown improved glomerular hyperfiltration, and a similar effect has also been reported with the supplementation of resveratrol, suggesting that both CR and resveratrol can act as a protective agent against kidney aging [79,216]. Additionally, resveratrol has been shown to repress microRNA21 and NF-κB expression, leading to a decrease in pro-inflammatory cytokines, such as TNF-α, IL-1β, IL-6, and IL–8, while it downregulates MAPK, JNK, and AP-1 [217,218,219,220,221,222]. This evidence indicates that resveratrol may become a potential CR mimetic for improving kidney disease and aging.
8. Resveratrol in Lifespan Expansion
Resveratrol is reported to increase the lifespan of many organisms. In Saccharomyces cerevisiae, it almost doubled its lifespan by stimulating SIRT2 activity [223]. Resveratrol also increases the lifespan of Caenorhabditis elegans (C. elegans) through SIRT2 activation without altering fertility [224]. Additionally, ad libitum addition of resveratrol to the diet may increase the lifespan of Apis mellifera [225]. Resveratrol extends the lifespan of N. furzeri along with a late decline in age-related brain action, particularly for motor and cognitive function [226]. In mammals, due to a lack of evidence, it cannot be said that there is a clear effect of resveratrol on lifespan extension. According to three different studies, resveratrol increases survival and insulin sensitivity, increases the lifespan, and improves locomotor activity; thus, it might be a potential treatment option against neurodegenerative diseases, as well as lengthening the lifespan, and it improves the function of motor neuron SOD1(G93A) in mice [227,228,229]. Resveratrol has shown protective effects against age-related kidney diseases by activation of SIRT1, an NAD(+)-dependent deacetylase, which may be a useful supplemental treatment for preventing age-related kidney injury [230]. Thus, these data suggest that resveratrol may also extend lifespan in the kidney.
However, a few studies support the fact that resveratrol treatment only slightly extends the lifespan of C. elegans and D. melanogaster and it had absolutely no effect on the crustacean model Daphnia [231,232]. A study on D. melanogaster also suggested that lifespan expansion solely depends on sex and diet [233]. Additionally, experiments with animal models showed that resveratrol administration for up to 1 year could not extend lifespan [234,235,236]. In aged mice, treatment with resveratrol delays age-related deterioration, including inflammation and oxidative stress, in vasculature and skeletal muscle [234,237]. A study found that resveratrol was associated with decreased survival rates in severe combined immunodeficiency in mice with prostate cancer xenografts [238].
9. Role of Resveratrol on Gut Dysbiosis and Associated CKD Pathobiology
Gut microbiota plays a crucial role in immunity and inflammation [239]. Evidence for the existence of a gut–kidney axis suggests an intimate correlation between the abnormal gut microbiota and the development of CKD [240,241]. Dysbiosis in gut microbiota disrupts gut integrity and produces toxic metabolites, including urea and trimethylamine-N-oxide (TMAO), which lead to the aberrant activation of immune cells, excess production of inflammatory factors, and infiltration of inflammatory cells that can potentially contribute to CKD pathobiology [242].
Evidence shows that various polyphenols, including resveratrol, can promote gut microbiota by inhibiting various bacterial pathogens, namely E. coli and Salmonella, and thereby can improve inflammation and mitigate kidney damage [243]. Oral administration of resveratrol ameliorates gut dysbiosis in db/db mice by increasing the intestinal bacterial population, such as Bacteroides, Alistipes, Rikenella, Odoribacter, Parabacteroides, and Alloprevotella. Moreover, transplantation of fecal microbiota derived from healthy resveratrol-treated db/m mice was found to attenuate the renal dysfunction, rebalance the gut microbiome, and improve intestinal permeability and inflammation in recipient db/db mice [244]. In a study by Hu and colleagues, resveratrol was also shown to promote Lactococcus lactis but inhibit Enterococcus faecalis [245].
Resveratrol also can help improve other CKD-related risk factors, such as obesity, dyslipidemia, atherosclerosis, and CVD [246]. For example, resveratrol can modify the relative Bacteroidetes:Firmicutes ratio and reverse the gut microbial dysbiosis caused by a high-fat diet, and thereby promote energy metabolism to produce anti-obesity effects in rodents [247]. High concentrations of TMAO, a gut microbe-dependent metabolite of dietary L-carnitine and choline, are indicative of the development of CVD and CKD [248]. Dietary supplementation with resveratrol increased the abundance of Lactobacillus, reduced the levels of TMAO, and abrogated the atherosclerosis phenotype of ApoE-/- mice fed a high-choline diet [249]. Together, these findings show that resveratrol modulates gut microbiota and thereby plays a pivotal role in maintaining gut homeostasis and the prevention of CKD.
10. Prospects, Limitations, and Conclusions
Kidney function decreases with age, and aging-associated kidney complications proportionately increase. Existing drugs for treating kidney diseases are limited by their side effects, and therefore natural compounds with fewer side effects are being evaluated. The literature highlighted in this review clearly suggests that resveratrol may modulate several pathological factors that are implicated in kidney aging, including inflammation, oxidative stress, fibrosis, mitochondrial dysfunction, cellular senescence, telomere shortening, ER stress, and autophagy dysfunction, and thus it may delay the aging process in the kidney (Figure 5). Aging biomarkers, such as SIRT1, AMPK, and NF-κB, and their associated signaling pathways are primarily targeted in resveratrol-mediated kidney protection. Moreover, resveratrol may increase the lifespan of model organisms and generate calorie restriction-mediated health effects, such as activation of SIRT1, development of insulin sensitivity, and utilization of energy.
Figure 5.
Potential anti-aging effects of resveratrol in the kidney. Resveratrol may mediate anti-aging effects in the kidney through the regulation of three main signaling pathways, SIRT1, AMPK, and NF-κB, which ultimately suppress pathological conditions, such as telomere shortening, inflammation, oxidative stress, fibrosis, mitochondrial dysfunction, autophagy dysfunction, ER stress, and DNA damage, in the kidney. Arrow means activation, and ‘T’ arrow means inhibition.
While the pharmacological benefits of resveratrol in kidney aging have been revealed, these findings were mostly based on preclinical studies. The clinical applications of resveratrol are limited by its poor bioavailability and limited durability during delivery. Additionally, several studies have recognized resveratrol as a pro-oxidizing and cell-damaging agent [250,251]. A range of formulation techniques have been employed to overcome these difficulties [252]. Several nanoparticle-loaded resveratrol and kidney biomarkers are currently being investigated for efficient and stable drug delivery with substantial efficacy, as mentioned earlier [31,253]. Intensified clinical trials must be conducted to further evaluate its efficacy following by suitable strategies to achieve facile delivery in the human body.
We anticipate that the points discussed in this review will direct future research to better understand how pharmacological interventions through natural products could modulate kidney aging and help develop resveratrol as a potential anti-aging agent to manage aging-associated kidney abnormalities.
Acknowledgments
This work acknowledges the National Research Foundation (No. 2015H1D3A1062189), Republic of Korea.
Author Contributions
Conceptualization, M.J.U. and A.M.; M.F., A.M., K.S.H. and M.J.U. drafted the manuscript. M.F. and K.S.H. wrote the initial draft of the manuscript. M.J.U., A.M., M.A.H. and H.H. reviewed the scientific content described in the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Research Foundation (No. 2020R1I1A1A01072879) and the Brain Pool program funded by the Ministry of Science and ICT through the National Research Foundation (No. 2020H1D3A2A02110924), Republic of Korea.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
There are no conflicts of interest of the authors regarding the publication of this manuscript.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.European Commission The 2015 ageing report. Eur. Econ. Rev. 2015;3:424. [Google Scholar]
- 2.Kooman J.P., Kotanko P., Schols A.M.W.J., Shiels P.G., Stenvinkel P. Chronic kidney disease and premature ageing. Nat. Rev. Nephrol. 2014;10:732–742. doi: 10.1038/nrneph.2014.185. [DOI] [PubMed] [Google Scholar]
- 3.Schmitt R., Melk A. Molecular mechanisms of renal aging. Kidney Int. 2017;92:569–579. doi: 10.1016/j.kint.2017.02.036. [DOI] [PubMed] [Google Scholar]
- 4.Valentijn F.A., Falke L.L., Nguyen T.Q., Goldschmeding R. Cellular senescence in the aging and diseased kidney. J. Cell Commun. Signal. 2018;12:69–82. doi: 10.1007/s12079-017-0434-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Denic A., Glassock R.J., Rule A.D. Structural and functional changes with the aging kidney. Adv. Chronic Kidney Dis. 2016;23:19–28. doi: 10.1053/j.ackd.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glassock R.J., Rule A.D. Aging and the kidneys: Anatomy, physiology and consequences for defining chronic kidney disease. Nephron. 2016;134:25–29. doi: 10.1159/000445450. [DOI] [PubMed] [Google Scholar]
- 7.Kooman J.P., Dekker M.J., Usvyat L.A., Kotanko P., van der Sande F.M., Schalkwijk C.G., Shiels P.G., Stenvinkel P. Inflammation and premature aging in advanced chronic kidney disease. Am. J. Physiol. Ren. Physiol. 2017;313:F938–F950. doi: 10.1152/ajprenal.00256.2017. [DOI] [PubMed] [Google Scholar]
- 8.Abu-Amero K.K., Kondkar A.A., Chalam K.V. Resveratrol and ophthalmic diseases. Nutrients. 2016;8:200. doi: 10.3390/nu8040200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Limmongkon A., Janhom P., Amthong A., Kawpanuk M., Nopprang P., Poohadsuan J., Somboon T., Saijeen S., Surangkul D., Srikummool M., et al. Antioxidant activity, total phenolic, and resveratrol content in five cultivars of peanut sprouts. Asian Pac. J. Trop. Biomed. 2017;7:332–338. doi: 10.1016/j.apjtb.2017.01.002. [DOI] [Google Scholar]
- 10.Jang I.A., Kim E.N., Lim J.H., Kim M.Y., Ban T.H., Yoon H.E., Park C.W., Chang Y.S., Choi B.S. Effects of resveratrol on the renin-angiotensin system in the aging kidney. Nutrients. 2018;10:1741. doi: 10.3390/nu10111741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Y.R., Li S., Lin C.C. Effect of resveratrol and pterostilbene on aging and longevity. BioFactors. 2018;44:69–82. doi: 10.1002/biof.1400. [DOI] [PubMed] [Google Scholar]
- 12.Liu S., Zheng Z., Ji S., Liu T., Hou Y., Li S., Li G. Resveratrol reduces senescence-associated secretory phenotype by SIRT1/NF-ΚB pathway in gut of the annual fish Nothobranchius guentheri. Fish Shellfish Immunol. 2018;80:473–479. doi: 10.1016/j.fsi.2018.06.027. [DOI] [PubMed] [Google Scholar]
- 13.Li J., Zhang C.X., Liu Y.M., Chen K.L., Chen G. A Comparative study of anti-aging properties and mechanism: Resveratrol and caloric restriction. Oncotarget. 2017;8:65717–65729. doi: 10.18632/oncotarget.20084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh A.P., Singh R., Verma S.S., Rai V., Kaschula C.H., Maiti P., Gupta S.C. Health benefits of resveratrol: Evidence from clinical studies. Med. Res. Rev. 2019;39:1851–1891. doi: 10.1002/med.21565. [DOI] [PubMed] [Google Scholar]
- 15.Savouret J.F., Quesne M. Resveratrol and cancer: A review. Biomed. Pharmacother. 2002;56:84–87. doi: 10.1016/S0753-3322(01)00158-5. [DOI] [PubMed] [Google Scholar]
- 16.Bhat K.P.L., Kosmeder J.W., Pezzuto J.M. Biological effects of resveratrol. Antioxid. Redox Signal. 2001;3:1041–1064. doi: 10.1089/152308601317203567. [DOI] [PubMed] [Google Scholar]
- 17.Nihei T., Miura Y., Yagasaki K. Inhibitory effect of resveratrol on proteinuria, Hypoalbuminemia and hyperlipidemia in Nephritic Rats. Life Sci. 2001;68 doi: 10.1016/S0024-3205(01)01061-X. [DOI] [PubMed] [Google Scholar]
- 18.Bertelli Alberto A.E., Migliori M., Panichi V., Longoni B., Origlia N., Ferreti A., Cuttano M., Giovannini L. Oxidative stress and inflammatory reaction modulation by white wine. Ann. N. Y. Acad. Sci. 2002;957:295–301. doi: 10.1111/j.1749-6632.2002.tb02929.x. [DOI] [PubMed] [Google Scholar]
- 19.Bertelli A.A.E., Migliori M., Panichi V., Origlia N., Filippi C., Das D.K., Giovannini L. Resveratrol, a component of wine and grapes, in the prevention of kidney disease. Ann. N. Y. Acad. Sci. 2002;957:230–238. doi: 10.1111/j.1749-6632.2002.tb02919.x. [DOI] [PubMed] [Google Scholar]
- 20.Den Hartogh D.J., Tsiani E. Health benefits of resveratrol in kidney disease: Evidence from in vitro and in vivo studies. Nutrients. 2019;11:1624. doi: 10.3390/nu11071624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caimi G., Carollo C., Presti L. Chronic renal failure: Oxidative stress, endothelial dysfunction and wine. Clin. Nephrol. 2004;62:331–335. doi: 10.5414/CNP62331. [DOI] [PubMed] [Google Scholar]
- 22.Saldanha J.F., de O.Leal V., Stenvinkel P., Carraro-Eduardo J.C., Mafra D. Resveratrol: Why is it a promising therapy for chronic kidney disease patients? Oxidative Med. Cell. Longev. 2013;2013 doi: 10.1155/2013/963217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coca S.G., Singanamala S., Parikh C.R. Chronic kidney disease after acute Kidney injury: A systematic review and meta-analysis. Kidney Int. 2012;81:442–448. doi: 10.1038/ki.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosner M.H., Okusa M.D. Acute Kidney Injury Associated with Cardiac Surgery. Clin. J. Am. Soc. Nephrol. 2006;1:19–32. doi: 10.2215/CJN.00240605. [DOI] [PubMed] [Google Scholar]
- 25.Chertow G.M., Burdick E., Honour M., Bonventre J.V., Bates D.W. Acute kidney injury, mortality, length of stay and costs in hospitalized patients. J. Am. Soc. Nephrol. 2005;16:3365–3370. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- 26.Mehta R.L., Kellum J.A., Shah S.V., Molitoris B.A., Ronco C., Warnock D.G., Levin A., Bagga A., Bakkaloglu A., Bonventre J.V., et al. Acute kidney injury network: Report of an initiative to improve outcomes in acute kidney injury. Crit. Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bouchard J., Mehta R.L. Acute kidney injury in western countries. Kidney Dis. 2016;2:103–110. doi: 10.1159/000445091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rauf A., Imran M., Sulera H.A.R., Ahmad B., Peters D.G., Mubarak M.S. A comprehensive review of the health perspectives of resveratrol. Food Funct. 2017;8:4284–4305. doi: 10.1039/C7FO01300K. [DOI] [PubMed] [Google Scholar]
- 29.Moni A., Iqbal A., Uddin M. Resveratrol attenuates inflammation through tristetraprolin expression in human hepatocytes. J. Adv. Biotechnol. Exp. Ther. 2018;1:78–82. doi: 10.5455/jabet.2018.d14. [DOI] [Google Scholar]
- 30.Huang Y.T., Chen Y.Y., Lai Y.H., Cheng C.C., Lin T.C., Su Y.S., Liu C.H., Lai P.C. Resveratrol alleviates the cytotoxicity induced by the radiocontrast agent, ioxitalamate, by reducing the production of reactive oxygen species in HK-2 human renal proximal tubule epithelial cells in vitro. Int. J. Mol. Med. 2016;37:83–91. doi: 10.3892/ijmm.2015.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li H.L., Yan Z., Ke Z.P., Tian X.F., Zhong L.L., Lin Y.T., Xu Y., Zheng D.H. IGFBP2 is a potential biomarker in acute kidney injury (AKI) and resveratrol-loaded nanoparticles prevent AKI. Oncotarget. 2018;9:36551–36560. doi: 10.18632/oncotarget.25663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Y., Wang B., Qi X., Zhang X., Ren K. Resveratrol protects against post-contrast acute kidney injury in rabbits with diabetic nephropathy. Front. Pharmacol. 2019;10:833. doi: 10.3389/fphar.2019.00833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Y., Feng F., Liu M., Xue J., Huang H. Resveratrol ameliorates sepsis-induced acute kidney injury in a pediatric Rat model via NRF2 signaling pathway. Exp. Ther. Med. 2018;16:3233–3240. doi: 10.3892/etm.2018.6533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luo C.-J., Luo F., Bu Q.-D., Jiang W., Zhang W., Liu X.-M., Che L., Luan H., Zhang H., Ma R.-X., et al. Protective effects of resveratrol on acute kidney injury in rats with sepsis. Biomed. Pap. 2019;164:49–56. doi: 10.5507/bp.2019.006. [DOI] [PubMed] [Google Scholar]
- 35.Wang N., Mao L., Yang L., Zou J., Liu K., Liu M., Zhang H., Xiao X., Wang K. Resveratrol protects against early polymicrobial sepsis-induced acute kidney injury through inhibiting endoplasmic reticulum stress-activated nf-κb pathway. Oncotarget. 2017;8:36449–36461. doi: 10.18632/oncotarget.16860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fu B., Zhao J., Peng W., Wu H., Zhang Y. Resveratrol rescues cadmium-induced mitochondrial injury by enhancing transcriptional regulation of PGC-1α and SOD2 via the Sirt3/FoxO3a pathway in TCMK-1 cells. Biochem. Biophys. Res. Commun. 2017;486 doi: 10.1016/j.bbrc.2017.03.027. [DOI] [PubMed] [Google Scholar]
- 37.Raghubeer S., Nagiah S., Phulukdaree A., Chuturgoon A. The phytoalexin resveratrol ameliorates ochratoxin a toxicity in human embryonic kidney (HEK293) cells. J. Cell. Biochem. 2015;116 doi: 10.1002/jcb.25242. [DOI] [PubMed] [Google Scholar]
- 38.Webster A.C., Nagler E.V., Morton R.L., Masson P. Chronic kidney disease. Lancet. 2017:1238–1252. doi: 10.1016/S0140-6736(16)32064-5. [DOI] [PubMed] [Google Scholar]
- 39.Xie Y., Bowe B., Mokdad A.H., Xian H., Yan Y., Li T., Maddukuri G., Tsai C.Y., Floyd T., Al-Aly Z. Analysis of the global burden of disease study highlights the global, regional, and national trends of chronic kidney disease epidemiology from 1990 to 2016. Kidney Int. 2018;94:567–581. doi: 10.1016/j.kint.2018.04.011. [DOI] [PubMed] [Google Scholar]
- 40.Carrero J.J., Stenvinkel P. Inflammation in end-stage renal disease—What have we learned in 10 years? Semin. Dial. 2010;23:498–509. doi: 10.1111/j.1525-139X.2010.00784.x. [DOI] [PubMed] [Google Scholar]
- 41.Meuwese C.L., Stenvinkel P., Dekker F.W., Carrero J.J. Monitoring of inflammation in patients on dialysis: Forewarned is forearmed. Nat. Rev. Nephrol. 2011;7:166–176. doi: 10.1038/nrneph.2011.2. [DOI] [PubMed] [Google Scholar]
- 42.Sun L.J., Sun Y.N., Chen S.J., Liu S., Jiang G.R. Resveratrol attenuates skeletal muscle atrophy induced by chronic kidney disease via MuRF1 signaling pathway. Biochem. Biophys. Res. Commun. 2017;487:83–89. doi: 10.1016/j.bbrc.2017.04.022. [DOI] [PubMed] [Google Scholar]
- 43.Hui Y., Lu M., Han Y., Zhou H., Liu W., Li L., Jin R. Resveratrol improves mitochondrial function in the remnant kidney from 5/6 nephrectomized rats. Acta Histochem. 2017;119:392–399. doi: 10.1016/j.acthis.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 44.Lin Y.F., Lee Y.H., Hsu Y.H., Chen Y.J., Lin Y.F., Cheng F.Y., Chiu H.W. Resveratrol-loaded nanoparticles conjugated with kidney injury molecule-1 as a drug delivery system for potential use in chronic kidney disease. Nanomedicine. 2017;12:2741–2756. doi: 10.2217/nnm-2017-0256. [DOI] [PubMed] [Google Scholar]
- 45.Vaziri N.D. Roles of oxidative stress and antioxidant therapy in chronic kidney disease and hypertension. Curr. Opin. Nephrol. Hypertens. 2004;13:93–99. doi: 10.1097/00041552-200401000-00013. [DOI] [PubMed] [Google Scholar]
- 46.Singh C.K., Liu X., Ahmad N. Resveratrol, in its natural combination in whole grape, for health promotion and disease management. Ann. N. Y. Acad. Sci. 2015;1348:150–160. doi: 10.1111/nyas.12798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Migliori M., Panichi V., de la Torre R., Fitó M., Covas M., Bertelli A., Muñoz-Aguayo D., Scatena A., Paoletti S., Ronco C. Anti-Inflammatory effect of white wine in CKD patients and healthy volunteers. Blood Purif. 2015;39:218–223. doi: 10.1159/000371570. [DOI] [PubMed] [Google Scholar]
- 48.Zhang C., Zhou Y., Zhou Y., Lu Y., Wang D. Regulation of EIF2α expression and renal interstitial fibrosis by resveratrol in rat renal tissue after unilateral ureteral obstruction. Ren. Fail. 2016;38:622–628. doi: 10.3109/0886022X.2016.1149774. [DOI] [PubMed] [Google Scholar]
- 49.Wang Y.-J., Chen Y.-Y., Hsiao C.-M., Pan M.-H., Wang B.-J., Chen Y.-C., Ho C.-T., Huang K.-C., Chen R.-J. Induction of autophagy by pterostilbene contributes to the prevention of renal fibrosis via attenuating NLRP3 inflammasome activation and epithelial-mesenchymal transition. Front. Cell Dev. Biol. 2020;8:436. doi: 10.3389/fcell.2020.00436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang X., Meng L., Zhao L., Wang Z., Liu H., Liu G., Guan G. Resveratrol ameliorates hyperglycemia-induced renal tubular oxidative stress damage via modulating the SIRT1/FOXO3a pathway. Diabetes Res. Clin. Pract. 2017;126 doi: 10.1016/j.diabres.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 51.Liu S., Zhao M., Zhou Y., Wang C., Yuan Y., Li L., Bresette W., Chen Y., Cheng J., Lu Y., et al. Resveratrol exerts dose-dependent anti-fibrotic or pro-fibrotic effects in kidneys: A potential risk to individuals with impaired kidney function. Phytomedicine. 2019;57:223–235. doi: 10.1016/j.phymed.2018.12.024. [DOI] [PubMed] [Google Scholar]
- 52.Kim D.H., Jung Y.J., Lee J.E., Lee A.S., Kang K.P., Lee S., Park S.K., Han M.K., Lee S.Y., Ramkumar K.M., et al. SIRT1 activation by resveratrol ameliorates cisplatin-induced renal injury through deacetylation of P53. Am. J. Physiol. Ren. Physiol. 2011;301:F427–F435. doi: 10.1152/ajprenal.00258.2010. [DOI] [PubMed] [Google Scholar]
- 53.Hong S.H., Lee H.-J., Sohn E.J., Ko H.-S., Shim B.S., Ahn K.S., Kim S.-H. Anti-Nephrolithic potential of resveratrol via inhibition of ROS, MCP-1, hyaluronan and osteopontin in vitro and in vivo. Pharmacol. Rep. 2013;65:970–979. doi: 10.1016/S1734-1140(13)71078-8. [DOI] [PubMed] [Google Scholar]
- 54.Venkatesan B., Ghosh-Choudhury N., Das F., Mahimainathan L., Kamat A., Kasinath B.S., Abboud H.E., Choudhury G.G. Resveratrol inhibits PDGF receptor mitogenic signaling in mesangial cells: Role of PTP1B. FASEB J. 2008;22:3469–3482. doi: 10.1096/fj.08-109488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang T., Chi Y., Kang Y., Lu H., Niu H., Liu W., Li Y. Resveratrol ameliorates podocyte damage in diabetic mice via SIRT1/PGC-1α mediated attenuation of mitochondrial oxidative stress. J. Cell. Physiol. 2019;234:5033–5043. doi: 10.1002/jcp.27306. [DOI] [PubMed] [Google Scholar]
- 56.Abharzanjani F., Afshar M., Hemmati M., Moossavi M. Short-Term High dose of quercetin and resveratrol alters aging markers in human kidney cells. Int. J. Prev. Med. 2017;8 doi: 10.4103/ijpvm.IJPVM_139_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zorov D.B., Juhaszova M., Sollott S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014;94:909–950. doi: 10.1152/physrev.00026.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Uchida Y., Yamazaki H., Watanabe S., Hayakawa K., Meng Y., Hiramatsu N., Kasai A., Yamauchi K., Yao J., Kitamura M. Enhancement of NF-kB activity by resveratrol in cytokine-exposed mesangial cells. Clin. Exp. Immunol. 2005;142:76–83. doi: 10.1111/j.1365-2249.2005.02895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baxter R.A. Anti-Aging properties of resveratrol: Review and report of a potent new antioxidant skin care formulation. J. Cosmet. Dermatol. 2008;7:2–7. doi: 10.1111/j.1473-2165.2008.00354.x. [DOI] [PubMed] [Google Scholar]
- 60.Crandall J.P., Oram V., Trandafirescu G., Reid M., Kishore P., Hawkins M., Cohen H.W., Barzilai N. Pilot study of resveratrol in older adults with impaired glucose tolerance. J. Gerontol. A Biol. Sci. Med. Sci. 2012;67:1307–1312. doi: 10.1093/gerona/glr235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lin C.T., Sun X.Y., Lin A.X. Supplementation with high-dose trans-resveratrol improves ultrafiltration in peritoneal dialysis patients: A prospective, randomized, double-blind study. Ren. Fail. 2016;38:214–221. doi: 10.3109/0886022X.2015.1128236. [DOI] [PubMed] [Google Scholar]
- 62.Pineda-Ramírez N., Alquisiras-Burgos I., Ortiz-Plata A., Ruiz-Tachiquín M.E., Espinoza-Rojo M., Aguilera P. Resveratrol activates neuronal autophagy through AMPK in the ischemic brain. Mol. Neurobiol. 2020;57:1055–1069. doi: 10.1007/s12035-019-01803-6. [DOI] [PubMed] [Google Scholar]
- 63.Zou P., Liu X., Li G., Wang Y. Resveratrol Pretreatment attenuates traumatic brain injury in rats by suppressing NLRP3 inflammasome activation via SIRT1. Mol. Med. Rep. 2018;17:3212–3217. doi: 10.3892/mmr.2017.8241. [DOI] [PubMed] [Google Scholar]
- 64.Mozafari M., Nekooeian A.A., Mashghoolozekr E., Panjeshahin M.R. The Cardioprotective effects of resveratrol in rats with simultaneous type 2 diabetes and renal hypertension. Nat. Prod. Commun. 2015;10:152–160. doi: 10.1177/1934578X1501000232. [DOI] [PubMed] [Google Scholar]
- 65.Dash S., Xiao C., Morgantini C., Szeto L., Lewis G.F. High-Dose resveratrol treatment for 2 weeks inhibits intestinal and hepatic lipoprotein production in overweight/obese men. Arterioscler. Thromb. Vasc. Biol. 2013;33:2895–2901. doi: 10.1161/ATVBAHA.113.302342. [DOI] [PubMed] [Google Scholar]
- 66.Tian Y., Ma J., Wang W., Zhang L., Xu J., Wang K., Li D. Resveratrol supplement inhibited the nf-κb inflammation pathway through activating AMPKα-SIRT1 pathway in mice with fatty liver. Mol. Cell. Biochem. 2016;422:75–84. doi: 10.1007/s11010-016-2807-x. [DOI] [PubMed] [Google Scholar]
- 67.Ding S., Wang H., Wang M., Bai L., Yu P., Wu W. Resveratrol alleviates chronic “real-world” ambient particulate matter-induced lung inflammation and fibrosis by inhibiting NLRP3 inflammasome activation in mice. Ecotoxicol. Environ. Saf. 2019;182 doi: 10.1016/j.ecoenv.2019.109425. [DOI] [PubMed] [Google Scholar]
- 68.Hamza R.Z., El-Shenawy N.S. Anti-Inflammatory and antioxidant role of resveratrol on nicotine-induced lung changes in male rats. Toxicol. Rep. 2017;4:399–407. doi: 10.1016/j.toxrep.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang C., Luo J., Yu B., Zheng P., Huang Z., Mao X., He J., Yu J., Chen J., Chen D. Dietary resveratrol supplementation improves meat quality of finishing pigs through changing muscle fiber characteristics and antioxidative status. Meat Sci. 2015;102:15–21. doi: 10.1016/j.meatsci.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 70.Ginés C., Cuesta S., Kireev R., García C., Rancan L., Paredes S.D., Vara E., Tresguerres J.A.F. Protective effect of resveratrol against inflammation, oxidative stress and apoptosis in pancreas of aged SAMP8 mice. Exp. Gerontol. 2017;90:61–70. doi: 10.1016/j.exger.2017.01.021. [DOI] [PubMed] [Google Scholar]
- 71.Aziz M.H., Afaq F., Ahmad N. Prevention of ultraviolet-B radiation damage by resveratrol in mouse skin is mediated via modulation in survivin. Photochem. Photobiol. 2005;81:25–31. doi: 10.1562/2004-08-13-RA-274.1. [DOI] [PubMed] [Google Scholar]
- 72.Soeur J., Eilstein J., Léreaux G., Jones C., Marrot L. Skin resistance to oxidative stress induced by resveratrol: From Nrf2 activation to GSH biosynthesis. Free Radic. Biol. Med. 2015;78:213–223. doi: 10.1016/j.freeradbiomed.2014.10.510. [DOI] [PubMed] [Google Scholar]
- 73.Zhang B., Xu L., Zhuo N., Shen J. Resveratrol protects against mitochondrial dysfunction through autophagy activation in human nucleus pulposus cells. Biochem. Biophys. Res. Commun. 2017;49:373–381. doi: 10.1016/j.bbrc.2017.09.015. [DOI] [PubMed] [Google Scholar]
- 74.Wang J., Li J., Cao N., Li Z., Han J., Li L. Resveratrol, an activator of SIRT1, induces protective autophagy in non-small-cell lung cancer via inhibiting Akt/MTOR and activating P38-MAPK. Onco Targets Ther. 2018;11:7777–7786. doi: 10.2147/OTT.S159095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Breen D.M., Sanli T., Giacca A., Tsiani E. Stimulation of muscle cell glucose uptake by resveratrol through sirtuins and AMPK. Biochem. Biophys. Res. Commun. 2008;374:117–122. doi: 10.1016/j.bbrc.2008.06.104. [DOI] [PubMed] [Google Scholar]
- 76.Wu J., Li X., Zhu G., Zhang Y., He M., Zhang J. The role of resveratrol-induced mitophagy/autophagy in peritoneal mesothelial cells inflammatory injury via NLRP3 inflammasome activation triggered by mitochondrial ROS. Exp. Cell Res. 2016;341:42–53. doi: 10.1016/j.yexcr.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 77.Chen L., Yang S., Zumbrun E.E., Guan H., Nagarkatti P.S., Nagarkatti M. Resveratrol attenuates lipopolysaccharide-induced acute kidney injury by suppressing inflammation driven by macrophages. Mol. Nutr. Food Res. 2015;59:853–864. doi: 10.1002/mnfr.201400819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Aydın S., Şahin T.T., Bacanlı M., Taner G., Başaran A.A., Aydın M., Başaran N. Resveratrol protects sepsis-induced oxidative DNA damage in liver and kidney of rats. Balk. Med. J. 2016;33:594–601. doi: 10.5152/balkanmedj.2016.15516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.He T., Xiong J., Nie L., Yu Y., Guan X., Xu X., Xiao T., Yang K., Liu L., Zhang D., et al. Resveratrol inhibits renal interstitial fibrosis in diabetic nephropathy by regulating AMPK/NOX4/ROS pathway. J. Mol. Med. 2016;94:1359–1371. doi: 10.1007/s00109-016-1451-y. [DOI] [PubMed] [Google Scholar]
- 80.Yuan D., Liu X.-M., Fang Z., Du L.-L., Chang J., Lin S.-H. Protective effect of resveratrol on kidney in rats with diabetic nephropathy and its effect on endoplasmic reticulum stress. Eur. Rev. Med. Pharmacol. Sci. 2018;22:1485–1493. doi: 10.26355/eurrev_201803_14497. [DOI] [PubMed] [Google Scholar]
- 81.Xu X.H., Ding D.F., Yong H.J., Dong C.L., You N., Ye X.L., Pan M.L., Ma J.H., You Q., Lu Y.B. Resveratrol transcriptionally regulates mirna-18a-5p expression ameliorating diabetic nephropathy via increasing autophagy. Eur. Rev. Med. Pharmacol. Sci. 2017;21:4952–4965. [PubMed] [Google Scholar]
- 82.Ma L., Fu R., Duan Z., Lu J., Gao J., Tian L., Lv Z., Chen Z., Han J., Jia L., et al. Sirt1 Is essential for resveratrol enhancement of hypoxia-induced autophagy in the type 2 diabetic nephropathy rat. Pathol. Res. Pract. 2016;212:310–318. doi: 10.1016/j.prp.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 83.Javkhedkar A.A., Quiroz Y., Rodriguez-Iturbe B., Vaziri N.D., Lokhandwala M.F., Banday A.A. Resveratrol restored Nrf2 Function, reduced renal inflammation, and mitigated hypertension in spontaneously hypertensive rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015;308:R840–R846. doi: 10.1152/ajpregu.00308.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chang Y.P., Ka S.M., Hsu W.H., Chen A., Chao L.K., Lin C.C., Hsieh C.C., Chen M.C., Chiu H.W., Ho C.L., et al. Resveratrol inhibits NLRP3 inflammasome activation by preserving mitochondrial integrity and augmenting autophagy. J. Cell. Physiol. 2015;230:1567–1579. doi: 10.1002/jcp.24903. [DOI] [PubMed] [Google Scholar]
- 85.Wu M., Gu J., Mei S., Xu D., Jing Y., Yao Q., Chen M., Yang M., Chen S., Yang B., et al. Resveratrol delays polycystic kidney disease progression through attenuation of nuclear factor κB-induced inflammation. Nephrol. Dial. Transplant. 2016;31:1826–1834. doi: 10.1093/ndt/gfw058. [DOI] [PubMed] [Google Scholar]
- 86.Xiao Z., Chen C., Meng T., Zhang W., Zhou Q. Resveratrol attenuates renal injury and fibrosis by inhibiting transforming growth factor-β pathway on matrix metalloproteinase 7. Exp. Biol. Med. 2016;241:140–146. doi: 10.1177/1535370215598401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang X., Lu H., Xie S., Wu C., Guo Y., Xiao Y., Zheng S., Zhu H., Zhang Y., Bai Y. Resveratrol suppresses the myofibroblastic phenotype and fibrosis formation in kidneys via proliferation-related signaling pathways. Br. J. Pharmacol. 2019;176:4745–4759. doi: 10.1111/bph.14842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hong Y.A., Bae S.Y., Ahn S.Y., Kim J., Kwon Y.J., Jung W.Y., Ko G.J. Resveratrol ameliorates contrast induced nephropathy through the activation of SIRT1-PGC-1α-Foxo1 signaling in mice. Kidney Blood Press. Res. 2017;42:641–653. doi: 10.1159/000481804. [DOI] [PubMed] [Google Scholar]
- 89.Muraki K., Nyhan K., Han L., Murnane J.P. Mechanisms of telomere loss and their consequences for chromosome instability. Front. Oncol. 2012;2 doi: 10.3389/fonc.2012.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jiang H., Ju Z., Rudolph K.L. Telomere shortening and ageing. Zeitschrift fur Gerontologie und Geriatrie. 2007;40:314–324. doi: 10.1007/s00391-007-0480-0. [DOI] [PubMed] [Google Scholar]
- 91.Gomez D.E., Armando R.G., Farina H.G., Menna P.L., Cerrudo C.S., Ghiringhelli P.D., Alonso D.F. Telomere structure and telomerase in health and disease (review) Int. J. Oncol. 2012;41:1561–1569. doi: 10.3892/ijo.2012.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wills L.P., Schnellmann R.G. Telomeres and telomerase in renal health. J. Am. Soc. Nephrol. 2011;22:39–41. doi: 10.1681/ASN.2010060662. [DOI] [PubMed] [Google Scholar]
- 93.Orlando C., Gelmini S., Selli C., Pazzagli M. Telomerase in urological malignancy. J. Urol. 2001;166:666–673. doi: 10.1016/S0022-5347(05)66040-5. [DOI] [PubMed] [Google Scholar]
- 94.Zhou X.J., Saxena R., Liu Z., Vaziri N.D., Silva F.G. Renal senescence in 2008: Progress and challenges. Int. Urol. Nephrol. 2008;40:823–839. doi: 10.1007/s11255-008-9405-0. [DOI] [PubMed] [Google Scholar]
- 95.Weinstein J.R., Anderson S. The aging kidney: Physiological changes. Adv. Chronic Kidney Dis. 2010;17:302–307. doi: 10.1053/j.ackd.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hara E., Smith R., Parry D., Tahara H., Stone S., Peters G. Regulation of P16CDKN2 expression and its implications for cell immortalization and senescence. Mol. Cell. Biol. 1996;16:859–867. doi: 10.1128/MCB.16.3.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Westhoff J.H., Schildhorn C., Jacobi C., Hömme M., Hartner A., Braun H., Kryzer C., Wang C., von Zglinicki T., Kränzlin B., et al. Telomere shortening reduces regenerative capacity after acute kidney injury. J. Am. Soc. Nephrol. 2010;21:327–336. doi: 10.1681/ASN.2009010072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.De Almeida A.J.P.O., Ribeiro T.P., de Medeiros I.A. Aging: Molecular pathways and implications on the cardiovascular system. Oxidative Med. Cell. Longev. 2017;2017:7941563. doi: 10.1155/2017/7941563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Djojosubroto M.W., Choi Y.S., Lee H.W., Rudolph K.L. Telomeres and telomerase in aging, regeneration and cancer. Mol. Cells. 2003;15:164–175. [PubMed] [Google Scholar]
- 100.Cherif H., Tarry J.L., Ozanne S.E., Hales C.N. Ageing and telomeres: A study into organ- and gender-specific telomere shortening. Nucleic Acids Res. 2003;31:1576–1583. doi: 10.1093/nar/gkg208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Blasco M.A. Telomeres and human disease: Ageing, cancer and beyond. Nat. Rev. Genet. 2005;6:611–622. doi: 10.1038/nrg1656. [DOI] [PubMed] [Google Scholar]
- 102.Kordinas V., Tsirpanlis G., Nicolaou C., Zoga M., Ioannidis A., Ioannidou V., Bersimis S., Petrihou C., Savva L., Legakis N.J., et al. Is there a connection between inflammation, telomerase activity and the transcriptional status of telomerase reverse transcriptase in renal failure? Cell. Mol. Biol. Lett. 2015;20:222–236. doi: 10.1515/cmble-2015-0016. [DOI] [PubMed] [Google Scholar]
- 103.Pearce V.P., Sherrell J., Lou Z., Kopelovich L., Wright W.E., Shay J.W. Immortalization of epithelial progenitor cells mediated by resveratrol. Oncogene. 2008;27 doi: 10.1038/sj.onc.1210886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Xia L., Wang X.X., Hu X.S., Guo X.G., Shang Y.P., Chen H.J., Zeng C.L., Zhang F.R., Chen J.Z. Resveratrol reduces endothelial progenitor cells senescence through augmentation of telomerase activity by Akt-dependent mechanisms. Br. J. Pharmacol. 2008;155 doi: 10.1038/bjp.2008.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fuggetta M.P., Lanzilli G., Tricarico M., Cottarelli A., Falchetti R., Ravagnan G., Bonmassar E. Effect of resveratrol on proliferation and telomerase activity of human colon cancer cells in vitro. J. Exp. Clin. Cancer Res. 2006;25:189–193. [PubMed] [Google Scholar]
- 106.Zhai X.X., Ding J.C., Tang Z.M., Li J.G., Li Y.C., Yan Y.H., Sun J.C., Zhang C.X. Effects of resveratrol on the proliferation, apoptosis and telomerase ability of human A431 epidermoid carcinoma cells. Oncol. Lett. 2016;11:3015–3018. doi: 10.3892/ol.2016.4351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sturmlechner I., Durik M., Sieben C.J., Baker D.J., Van Deursen J.M. Cellular senescence in renal ageing and disease. Nat. Rev. Nephrol. 2017;13:77–89. doi: 10.1038/nrneph.2016.183. [DOI] [PubMed] [Google Scholar]
- 108.Chen J.H., Hales C.N., Ozanne S.E. DNA damage, cellular senescence and organismal ageing: Causal or correlative? Nucleic Acids Res. 2007;35:7417–7428. doi: 10.1093/nar/gkm681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sulli G., Di Micco R., Di Fagagna F.D.A. Crosstalk between chromatin state and DNA damage response in cellular senescence and cancer. Nat. Rev. Cancer. 2012;12:709–720. doi: 10.1038/nrc3344. [DOI] [PubMed] [Google Scholar]
- 110.Kim K.S., Kang K.W., Seu Y.B., Baek S.H., Kim J.R. Interferon-γ induces cellular senescence through P53-dependent DNA damage signaling in human endothelial cells. Mech. Ageing Dev. 2009;130:179–188. doi: 10.1016/j.mad.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 111.Aydin S., Bacanli M., Taner G., Şahin T., Başaran A.A., Başaran N. Protective effects of resveratrol on sepsis-induced DNA damage in the lymphocytes of rats. Hum. Exp. Toxicol. 2013;32:1048–1057. doi: 10.1177/0960327112467047. [DOI] [PubMed] [Google Scholar]
- 112.Yang Q., Wang B., Zang W., Wang X., Liu Z., Li W., Jia J. Resveratrol inhibits the growth of gastric cancer by inducing G1 phase arrest and senescence in a Sirt1-dependent manner. PLoS ONE. 2013;8:e70627. doi: 10.1371/journal.pone.0070627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhang N., Li Z., Xu K., Wang Y., Wang Z. Resveratrol protects against high-fat diet induced renal pathological damage and cell senescence by activating SIRT1. Biol. Pharm. Bull. 2016;39:1448–1454. doi: 10.1248/bpb.b16-00085. [DOI] [PubMed] [Google Scholar]
- 114.Duann P., Lin P.H. Advances in Experimental Medicine and Biology. Volume 982. Springer; Berlin/Heidelberg, Germany: 2017. Mitochondria damage and kidney disease; pp. 529–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Galvan D.L., Green N.H., Danesh F.R. The hallmarks of mitochondrial dysfunction in chronic kidney disease. Kidney Int. 2017;92:1051–1057. doi: 10.1016/j.kint.2017.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Che R., Yuan Y., Huang S., Zhang A. Mitochondrial dysfunction in the pathophysiology of renal diseases. Am. J. Physiol. Ren. Physiol. 2014;306:F367–F378. doi: 10.1152/ajprenal.00571.2013. [DOI] [PubMed] [Google Scholar]
- 117.Pak E.S., Uddin M.J., Ha H. Inhibition of Src family kinases ameliorates LPS-induced acute kidney injury and mitochondrial dysfunction in mice. Int. J. Mol. Sci. 2020;21:8246. doi: 10.3390/ijms21218246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Csiszar A., Labinskyy N., Pinto J.T., Ballabh P., Zhang H., Losonczy G., Pearson K., De Cabo R., Pacher P., Zhang C., et al. Resveratrol induces mitochondrial biogenesis in endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2009;297:H13–H20. doi: 10.1152/ajpheart.00368.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ungvari Z., Labinskyy N., Mukhopadhyay P., Pinto J.T., Bagi Z., Ballabh P., Zhang C., Pacher P., Csiszar A. Resveratrol attenuates mitochondrial oxidative stress in coronary arterial endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2009;297:H1876–H1881. doi: 10.1152/ajpheart.00375.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Taniguchi M., Yoshida H. Endoplasmic reticulum stress in kidney function and disease. Curr. Opin. Nephrol. Hypertens. 2015:345–350. doi: 10.1097/MNH.0000000000000141. [DOI] [PubMed] [Google Scholar]
- 121.Cybulsky A.V. Endoplasmic reticulum stress, the unfolded protein response and autophagy in kidney diseases. Nat. Rev. Nephrol. 2017;13:681–696. doi: 10.1038/nrneph.2017.129. [DOI] [PubMed] [Google Scholar]
- 122.Uddin M.J., Jeong J., Pak E.S., Ha H. CO-Releasing Molecule-2 Prevents Acute Kidney Injury through Suppression of ROS-Fyn-ER Stress Signaling in Mouse Model. Oxidative Med. Cell. Longev. 2021;2021:9947772. doi: 10.1155/2021/9947772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Inagi R. RAGE and glyoxalase in kidney disease. Glycoconj. J. 2016;33:619–626. doi: 10.1007/s10719-016-9689-8. [DOI] [PubMed] [Google Scholar]
- 124.Ghosh A.K., Mau T., O’Brien M., Garg S., Yung R. Impaired autophagy activity is linked to elevated ER-stress and inflammation in aging adipose tissue. Aging. 2016;8:2525–2537. doi: 10.18632/aging.101083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Maekawa H., Inagi R. Stress signal network between hypoxia and ER stress in chronic kidney disease. Front. Physiol. 2017;8:74. doi: 10.3389/fphys.2017.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zeeshan H.M.A., Lee G.H., Kim H.R., Chae H.J. Endoplasmic reticulum stress and associated ROS. Int. J. Mol. Sci. 2016;17:327. doi: 10.3390/ijms17030327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Uddin M.J., Pak E.S., Ha H. Carbon monoxide releasing molecule-2 protects mice against acute kidney injury through inhibition of ER stress. Korean J. Physiol. Pharmacol. 2018;22 doi: 10.4196/kjpp.2018.22.5.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Fan Y., Xiao W., Li Z., Li X., Chuang P.Y., Jim B., Zhang W., Wei C., Wang N., Jia W., et al. RTN1 mediates progression of kidney disease by inducing ER stress. Nat. Commun. 2015;6:8710. doi: 10.1038/ncomms9710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chou X., Ding F., Zhang X., Ding X., Gao H., Wu Q. Sirtuin-1 ameliorates cadmium-induced endoplasmic reticulum stress and pyroptosis through XBP-1s deacetylation in human renal tubular epithelial cells. Arch. Toxicol. 2019;93:965–986. doi: 10.1007/s00204-019-02415-8. [DOI] [PubMed] [Google Scholar]
- 130.Jin X., Wang K., Liu H., Hu F., Zhao F., Liu J. Protection of bovine mammary epithelial cells from hydrogen peroxide-induced oxidative cell damage by resveratrol. Oxidative Med. Cell. Longev. 2016;2016:2572175. doi: 10.1155/2016/2572175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sureshbabu A., Ryter S.W., Choi M.E. Oxidative stress and autophagy: Crucial modulators of kidney injury. Redox Biol. 2015;4:208–214. doi: 10.1016/j.redox.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lin T.-A., Wu V.C.-C., Wang C.-Y. Autophagy in chronic kidney diseases. Cells. 2019;8:61. doi: 10.3390/cells8010061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lenoir O., Tharaux P.L., Huber T.B. Autophagy in kidney disease and aging: Lessons from rodent models. Kidney Int. 2016;90:950–964. doi: 10.1016/j.kint.2016.04.014. [DOI] [PubMed] [Google Scholar]
- 134.Tang C., Livingston M.J., Liu Z., Dong Z. Autophagy in kidney homeostasis and disease. Nat. Rev. Nephrol. 2020;16:489–508. doi: 10.1038/s41581-020-0309-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yamamoto T., Takabatake Y., Takahashi A., Kimura T., Namba T., Matsuda J., Minami S., Kaimori J.Y., Matsui I., Matsusaka T., et al. High-Fat Diet-induced lysosomal dysfunction and impaired autophagic flux contribute to lipotoxicity in the kidney. J. Am. Soc. Nephrol. 2017;28:1534–1551. doi: 10.1681/ASN.2016070731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yang D., Livingston M.J., Liu Z., Dong G., Zhang M., Chen J.K., Dong Z. Autophagy in diabetic kidney disease: Regulation, pathological role and therapeutic potential. Cell. Mol. Life Sci. 2018:669–688. doi: 10.1007/s00018-017-2639-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Su Z., Klein J.D., Du J., Franch H.A., Zhang L., Hassounah F., Hudson M.B., Wang X.H. Chronic kidney disease induces autophagy leading to dysfunction of mitochondria in skeletal muscle. Am. J. Physiol. Ren. Physiol. 2017;312:F1128–F1140. doi: 10.1152/ajprenal.00600.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sohn M., Kim K., Uddin M.J., Lee G., Hwang I., Kang H., Kim H., Lee J.H., Ha H. Delayed treatment with fenofibrate protects against high-fat diet-induced kidney injury in mice: The possible role of AMPK autophagy. Am. J. Physiol. Ren. Physiol. 2017;312 doi: 10.1152/ajprenal.00596.2015. [DOI] [PubMed] [Google Scholar]
- 139.He Q., Li Z., Wang Y., Hou Y., Li L., Zhao J. Resveratrol alleviates cerebral ischemia/reperfusion injury in rats by inhibiting NLRP3 inflammasome activation through Sirt1-dependent autophagy induction. Int. Immunopharmacol. 2017;50:208–215. doi: 10.1016/j.intimp.2017.06.029. [DOI] [PubMed] [Google Scholar]
- 140.Wu Y., Xun Y., Zhang J., Hu H., Qin B., Wang T., Wang S., Li C., Lu Y. Resveratrol attenuates oxalate-induced renal oxidative injury and calcium oxalate crystal deposition by regulating TFEB-induced autophagy pathway. Front. Cell Dev. Biol. 2021;9 doi: 10.3389/fcell.2021.638759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gyurászová M., Gurecká R., Bábíčková J., Tóthová Ľ. Oxidative stress in the pathophysiology of kidney disease: Implications for noninvasive monitoring and identification of biomarkers. Oxidative Med. Cell. Longev. 2020;2020:5478708. doi: 10.1155/2020/5478708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Uddin M.J., Kim E.H., Hannan M.A., Ha H. Pharmacotherapy against oxidative stress in chronic kidney disease: Promising small molecule natural products targeting nrf2-ho-1 signaling. Antioxidants. 2021;10:258. doi: 10.3390/antiox10020258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Xu G., Luo K., Liu H., Huang T., Fang X., Tu W. The progress of inflammation and oxidative stress in patients with chronic kidney disease. Ren. Fail. 2015;37:45–49. doi: 10.3109/0886022X.2014.964141. [DOI] [PubMed] [Google Scholar]
- 144.Michaud M., Balardy L., Moulis G., Gaudin C., Peyrot C., Vellas B., Cesari M., Nourhashemi F. Proinflammatory cytokines, aging, and age-related diseases. J. Am. Med. Dir. Assoc. 2013;14:877–882. doi: 10.1016/j.jamda.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 145.Ratliff B.B., Abdulmahdi W., Pawar R., Wolin M.S. Oxidant mechanisms in renal injury and disease. Antioxid. Redox Signal. 2016;25:119–146. doi: 10.1089/ars.2016.6665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Liguori I., Russo G., Curcio F., Bulli G., Aran L., Della-Morte D., Gargiulo G., Testa G., Cacciatore F., Bonaduce D., et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging. 2018;13:757–772. doi: 10.2147/CIA.S158513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kim H.J., Vaziri N.D. Contribution of impaired Nrf2-Keap1 pathway to oxidative stress and inflammation in chronic renal failure. Am. J. Physiol. Ren. Physiol. 2010;298:F662–F671. doi: 10.1152/ajprenal.00421.2009. [DOI] [PubMed] [Google Scholar]
- 148.Truong V.L., Jun M., Jeong W.S. Role of resveratrol in regulation of cellular defense systems against oxidative stress. BioFactors. 2018;44:36–49. doi: 10.1002/biof.1399. [DOI] [PubMed] [Google Scholar]
- 149.Jia R., Li Y., Cao L., Du J., Zheng T., Qian H., Gu Z., Jeney G., Xu P., Yin G. Antioxidative, anti-inflammatory and hepatoprotective effects of resveratrol on oxidative stress-induced liver damage in tilapia (Oreochromis niloticus) Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2019;215:56–66. doi: 10.1016/j.cbpc.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 150.Li J., Li L., Wang S., Zhang C., Zheng L., Jia Y., Xu M., Zhu T., Zhang Y., Rong R. Resveratrol alleviates inflammatory responses and oxidative stress in rat kidney ischemia-reperfusion injury and H2O2-induced NRK-52E cells via the Nrf2/TLR4/NF-kB pathway. Cell. Physiol. Biochem. 2018;45:1677–1689. doi: 10.1159/000487735. [DOI] [PubMed] [Google Scholar]
- 151.Cianciulli A., Dragone T., Calvello R., Porro C., Trotta T., Lofrumento D.D., Panaro M.A. IL-10 plays a pivotal role in anti-inflammatory effects of resveratrol in activated microglia cells. Int. Immunopharmacol. 2015;24:369–376. doi: 10.1016/j.intimp.2014.12.035. [DOI] [PubMed] [Google Scholar]
- 152.Tian X., Liu Y., Ren G., Yin L., Liang X., Geng T., Dang H., An R. Resveratrol limits diabetes-associated cognitive decline in rats by preventing oxidative stress and inflammation and modulating hippocampal structural synaptic plasticity. Brain Res. 2016;1650:1–9. doi: 10.1016/j.brainres.2016.08.032. [DOI] [PubMed] [Google Scholar]
- 153.Tao L., Ding Q., Gao C., Sun X. Resveratrol attenuates neuropathic pain through balancing pro-inflammatory and anti-inflammatory cytokines release in mice. Int. Immunopharmacol. 2016;34:165–172. doi: 10.1016/j.intimp.2016.02.033. [DOI] [PubMed] [Google Scholar]
- 154.Zhou X.J., Rakheja D., Yu X., Saxena R., Vaziri N.D., Silva F.G. The aging kidney. Kidney Int. 2008;74:710–720. doi: 10.1038/ki.2008.319. [DOI] [PubMed] [Google Scholar]
- 155.Yang H.C., Fogo A.B. Fibrosis and renal aging. Kidney Int. Suppl. 2014;4:75–78. doi: 10.1038/kisup.2014.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Schnaper H.W. Renal fibrosis. Methods Mol. Med. 2005;117:45–68. doi: 10.1385/1-59259-940-0:045. [DOI] [PubMed] [Google Scholar]
- 157.Hwang I., Uddin M.J., Lee G., Jiang S., Pak E.S., Ha H. Peroxiredoxin 3 deficiency accelerates chronic kidney injury in mice through interactions between macrophages and tubular epithelial cells. Free Radic. Biol. Med. 2019;131:162–172. doi: 10.1016/j.freeradbiomed.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 158.Kim E.N., Kim M.Y., Lim J.H., Kim Y., Shin S.J., Park C.W., Kim Y.S., Chang Y.S., Yoon H.E., Choi B.S. The protective effect of resveratrol on vascular aging by modulation of the renin—Angiotensin system. Atherosclerosis. 2018;270:123–131. doi: 10.1016/j.atherosclerosis.2018.01.043. [DOI] [PubMed] [Google Scholar]
- 159.Dong D., Cai G.Y., Ning Y.C., Wang J.C., Lv Y., Hong Q., Cui S.Y., Fu B., Guo Y.N., Chen X.M. Alleviation of senescence and epithelial-mesenchymal transition in aging kidney by short-term caloric restriction and caloric restriction mimetics via modulation of AMPK/MTOR signaling. Oncotarget. 2017;8:16109–16121. doi: 10.18632/oncotarget.14884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kaeberlein M., McVey M., Guarente L. The SIR2/3/4 Complex and SIR2 alone promote longevity in saccharomyces cerevisiae by two different mechanisms. Genes Dev. 1999;13:2570–2580. doi: 10.1101/gad.13.19.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bordone L., Guarente L. Calorie Restriction, SIRT1 and metabolism: Understanding Longevity. Nat. Rev. Mol. Cell Biol. 2005;6:298–305. doi: 10.1038/nrm1616. [DOI] [PubMed] [Google Scholar]
- 162.Dali-Youcef N., Lagouge M., Froelich S., Koehl C., Schoonjans K., Auwerx J. Sirtuins: The “magnificent seven”, function, metabolism and longevity. Ann. Med. 2007;39:335–345. doi: 10.1080/07853890701408194. [DOI] [PubMed] [Google Scholar]
- 163.Al-Regaiey K.A. The effects of calorie restriction on aging: A brief review. Eur. Rev. Med. Pharmacol. Sci. 2016;20:2468–2473. [PubMed] [Google Scholar]
- 164.Ran G., Ying L., Li L., Yan Q., Yi W., Ying C., Wu H., Ye X. Resveratrol ameliorates diet-induced dysregulation of lipid metabolism in Zebrafish (Danio rerio) PLoS ONE. 2017;12:e0180865. doi: 10.1371/journal.pone.0180865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Park S.J., Ahmad F., Philp A., Baar K., Williams T., Luo H., Ke H., Rehmann H., Taussig R., Brown A.L., et al. Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell. 2012;148:421–433. doi: 10.1016/j.cell.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Marchal J., Blanc S., Epelbaum J., Aujard F., Pifferi F. Effects of chronic calorie restriction or dietary resveratrol supplementation on insulin sensitivity markers in a primate, Microcebus murinus. PLoS ONE. 2012;7:e34289. doi: 10.1371/journal.pone.0034289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Chen C.C., Chang Z.Y., Tsai F.J., Chen S.Y. Resveratrol Pretreatment ameliorates concanavalin a-induced advanced renal glomerulosclerosis in aged mice through upregulation of Sirtuin 1-mediated klotho expression. Int. J. Mol. Sci. 2020;21:6766. doi: 10.3390/ijms21186766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Du L., Qian X., Li Y., Li X.Z., He L.L., Xu L., Liu Y.Q., Li C.C., Ma P., Shu F.L., et al. Sirt1 inhibits renal tubular cell epithelial—Mesenchymal transition through YY1 deacetylation in diabetic nephropathy. Acta Pharmacol. Sin. 2020 doi: 10.1038/s41401-020-0450-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zhang Q., Zhang C., Ge J., Lv M.-W., Talukder M., Guo K., Li Y.-H., Li J.-L. Ameliorative effects of resveratrol against cadmium-induced nephrotoxicity via modulating nuclear xenobiotic receptor response and PINK1/Parkin-mediated mitophagy. Food Funct. 2020;11:1856–1868. doi: 10.1039/C9FO02287B. [DOI] [PubMed] [Google Scholar]
- 170.Gong L., He J., Sun X., Li L., Zhang X., Gan H. Activation of sirtuin1 protects against ischemia/reperfusion-induced acute kidney injury. Biomed. Pharmacother. 2020;125:110021. doi: 10.1016/j.biopha.2020.110021. [DOI] [PubMed] [Google Scholar]
- 171.Mirhadi E., Roufogalis B.D., Banach M., Barati M., Sahebkar A. Resveratrol: Mechanistic and Therapeutic Perspectives in Pulmonary Arterial Hypertension. Pharmacol Res. 2020;163:105287. doi: 10.1016/j.phrs.2020.105287. [DOI] [PubMed] [Google Scholar]
- 172.Li P., Song X., Zhang D., Guo N., Wu C., Chen K., Liu Y., Yuan L., Chen X., Huang X. Resveratrol improves left ventricular remodeling in chronic kidney disease via Sirt1-mediated regulation of FoxO1 activity and MnSOD expression. BioFactors. 2020;46:168–179. doi: 10.1002/biof.1584. [DOI] [PubMed] [Google Scholar]
- 173.Pannu N., Bhatnagar A. Resveratrol: From enhanced biosynthesis and bioavailability to multitargeting chronic diseases. Biomed. Pharmacother. 2019;109:2237–2251. doi: 10.1016/j.biopha.2018.11.075. [DOI] [PubMed] [Google Scholar]
- 174.Rodgers J.T., Lerin C., Haas W., Gygi S.P., Spiegelman B.M., Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1α and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- 175.Lee G., Uddin M.J., Kim Y., Ko M., Yu I., Ha H. PGC-1α, a potential therapeutic target against kidney aging. Aging Cell. 2019;18:e12994. doi: 10.1111/acel.12994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ren Y., Du C., Shi Y., Wei J., Wu H., Cui H. The Sirt1 Activator, SRT1720, attenuates renal fibrosis by inhibiting CTGF and oxidative stress. Int. J. Mol. Med. 2017;39:1317–1324. doi: 10.3892/ijmm.2017.2931. [DOI] [PubMed] [Google Scholar]
- 177.Rajani R., Pastor-Soler N.M., Hallows K.R. Role of AMP-activated protein kinase in kidney tubular transport, metabolism, and disease. Curr. Opin. Nephrol. Hypertens. 2017;26:375–383. doi: 10.1097/MNH.0000000000000349. [DOI] [PubMed] [Google Scholar]
- 178.Trewin A.J., Berry B.J., Wojtovich A.P. Exercise and mitochondrial dynamics: Keeping in Shape with ROS and AMPK. Antioxidants. 2018;7:7. doi: 10.3390/antiox7010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Song P., Zou M.H. Regulation of NAD(P)H oxidases by AMPK in cardiovascular systems. Free Radic. Biol. Med. 2012;52:1607–1619. doi: 10.1016/j.freeradbiomed.2012.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Salminen A., Kaarniranta K., Kauppinen A. Age-Related changes in AMPK activation: Role for AMPK phosphatases and inhibitory phosphorylation by upstream signaling pathways. Ageing Res. Rev. 2016;28:15–26. doi: 10.1016/j.arr.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 181.Burkewitz K., Weir H.J.M., Mair W.B. Experimentia Supplementum. Volume 107. Springer; Berlin/Heidelberg, Germany: 2016. AMPK as a pro-longevity target; pp. 227–256. [DOI] [PubMed] [Google Scholar]
- 182.Ruiz R., María Pérez-Villegas E., Manuel Carrión Á. AMPK function in aging process. Curr. Drug Targets. 2016;17:932–941. doi: 10.2174/1389450116666151102095825. [DOI] [PubMed] [Google Scholar]
- 183.Sun G.Q., Li Y.B., Du B., Meng Y. Resveratrol via activation of AMPK lowers blood pressure in DOCA-salt hypertensive mice. Clin. Exp. Hypertens. 2015;37:616–621. doi: 10.3109/10641963.2015.1036060. [DOI] [PubMed] [Google Scholar]
- 184.Thompson A.M., Martin K.A., Rzucidlo E.M. Resveratrol induces vascular smooth muscle cell differentiation through stimulation of SirT1 and AMPK. PLoS ONE. 2014;9:e85495. doi: 10.1371/journal.pone.0085495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Ido Y., Duranton A., Lan F., Weikel K.A., Breton L., Ruderman N.B. Resveratrol prevents oxidative stress-induced senescence and proliferative dysfunction by activating the AMPK-FOXO3 cascade in cultured primary human keratinocytes. PLoS ONE. 2015;10:e0115341. doi: 10.1371/journal.pone.0115341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Chang C., Su H., Zhang D., Wang Y., Shen Q., Liu B., Huang R., Zhou T., Peng C., Wong C.C.L., et al. AMPK-Dependent phosphorylation of GAPDH Triggers Sirt1 activation and is necessary for autophagy upon glucose starvation. Mol. Cell. 2015;60 doi: 10.1016/j.molcel.2015.10.037. [DOI] [PubMed] [Google Scholar]
- 187.Markó L., Vigolo E., Hinze C., Park J.-K., Roël G., Balogh A., Choi M., Wübken A., Cording J., Blasig I.E., et al. Tubular epithelial NF-kB activity regulates ischemic AKI. J. Am. Soc. Nephrol. 2016;27:2658–2669. doi: 10.1681/ASN.2015070748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Chade A.R., Williams M.L., Engel J.E., Williams E., Bidwell G.L. Molecular targeting of renal inflammation using drug delivery technology to inhibit NF-kB improves renal recovery in chronic kidney disease. Am. J. Physiol. Ren. Physiol. 2020;319:F139–F148. doi: 10.1152/ajprenal.00155.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Wang X., Xue N., Zhao S., Shi Y., Ding X., Fang Y. Upregulation of MiR-382 contributes to renal fibrosis secondary to aristolochic acid-induced kidney injury via PTEN signaling pathway. Cell Death Dis. 2020;11:620. doi: 10.1038/s41419-020-02876-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Eisenreich A., Orphal M., Böhme K., Kreutz R. Tmem63c is a potential pro-survival factor in angiotensin II-treated human podocytes. Life Sci. 2020;258:118175. doi: 10.1016/j.lfs.2020.118175. [DOI] [PubMed] [Google Scholar]
- 191.Rangan G. NF-kappaB signaling in chronic kidney disease. Front. Biosci. 2009;14:3496–3522. doi: 10.2741/3467. [DOI] [PubMed] [Google Scholar]
- 192.Gan Y., Tao S., Cao D., Xie H., Zeng Q. Protection of resveratrol on acute kidney injury in septic rats. Hum. Exp. Toxicol. 2017;36:1015–1022. doi: 10.1177/0960327116678298. [DOI] [PubMed] [Google Scholar]
- 193.Gano L.B., Donato A.J., Pasha H.M., Hearon C.M., Sindler A.L., Seals D.R. The SIRT1 activator SRT1720 reverses vascular endothelial dysfunction, excessive superoxide production, and inflammation with aging in mice. Am. J. Physiol. Heart Circ. Physiol. 2014;307 doi: 10.1152/ajpheart.00377.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Yi H., Zhang W., Cui Z.-M., Cui S.-Y., Fan J.-B., Zhu X.-H., Liu W. Resveratrol alleviates the interleukin-1β-induced chondrocytes injury through the NF-kB signaling pathway. J. Orthop. Surg. Res. 2020;15:424. doi: 10.1186/s13018-020-01944-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.López-Otín C., Blasco M.A., Partridge L., Serrano M., Kroemer G. The hallmarks of aging. Cell. 2013;153 doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.de Lencastre A., Pincus Z., Zhou K., Kato M., Lee S.S., Slack F.J. MicroRNAs both promote and antagonize longevity in C. elegans. Curr. Biol. 2010;20:2159–2168. doi: 10.1016/j.cub.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Herskovits A.Z., Guarente L. SIRT1 in neurodevelopment and brain senescence. Neuron. 2014;81:471–483. doi: 10.1016/j.neuron.2014.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Funk J.A., Schnellmann R.G. Accelerated recovery of renal mitochondrial and tubule homeostasis with SIRT1/PGC-1α activation following ischemia—Reperfusion injury. Toxicol. Appl. Pharmacol. 2013;273:345–354. doi: 10.1016/j.taap.2013.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Liu R., Zhong Y., Li X., Chen H., Jim B., Zhou M.-M., Chuang P.Y., He J.C. Role of transcription factor acetylation in diabetic kidney disease. Diabetes. 2014;63:2440–2453. doi: 10.2337/db13-1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Kume S., Haneda M., Kanasaki K., Sugimoto T., Araki S., Isshiki K., Isono M., Uzu T., Guarente L., Kashiwagi A., et al. SIRT1 inhibits transforming growth factor β-induced apoptosis in glomerular mesangial cells via Smad7 deacetylation. J. Biol. Chem. 2007;282:151–158. doi: 10.1074/jbc.M605904200. [DOI] [PubMed] [Google Scholar]
- 201.Wen D., Huang X., Zhang M., Zhang L., Chen J., Gu Y., Hao C.-M. Resveratrol attenuates diabetic nephropathy via modulating angiogenesis. PLoS ONE. 2013;8:e82336. doi: 10.1371/journal.pone.0082336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Crossland H., Atherton P.J., Strömberg A., Gustafsson T., Timmons J.A. A reverse genetics cell-based evaluation of genes linked to healthy human tissue age. FASEB J. 2017;31:96–108. doi: 10.1096/fj.201600296rrr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Griñán-Ferré C., Bellver-Sanchis A., Izquierdo V., Corpas R., Roig-Soriano J., Chillón M., Andres-Lacueva C., Somogyvári M., Sőti C., Sanfeliu C., et al. The pleiotropic neuroprotective effects of resveratrol in cognitive decline and Alzheimer’s Disease pathology: From antioxidant to epigenetic therapy. Ageing Res. Rev. 2021;67:101271. doi: 10.1016/j.arr.2021.101271. [DOI] [PubMed] [Google Scholar]
- 204.Delmas D., Cornebise C., Courtaut F., Xiao J., Aires V. New highlights of resveratrol: A review of properties against ocular diseases. Int. J. Mol. Sci. 2021;22:1295. doi: 10.3390/ijms22031295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Omodei D., Fontana L. Calorie restriction and prevention of age-associated chronic disease. FEBS Lett. 2011;585:1537–1542. doi: 10.1016/j.febslet.2011.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Liu J.R., Cai G.Y., Ning Y.C., Wang J.C., Lv Y., Guo Y.N., Fu B., Hong Q., Sun X.F., Chen X.M. Caloric restriction alleviates aging-related fibrosis of kidney through downregulation of MiR-21 in extracellular vesicles. Aging. 2020;12:18052–18072. doi: 10.18632/aging.103591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Grundmann F., Müller R.U., Reppenhorst A., Hülswitt L., Späth M.R., Kubacki T., Scherner M., Faust M., Becker I., Wahlers T., et al. Preoperative short-term calorie restriction for prevention of acute kidney injury after cardiac surgery: A randomized, controlled, open-label, pilot trial. J. Am. Heart Assoc. 2018;7:e008181. doi: 10.1161/JAHA.117.008181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Razzaque M.S., Shimokawa I., Nazneen A., Higami Y., Taguchi T. Age-Related nephropathy in the Fischer 344 Rat is associated with overexpression of collagens and collagen-binding heat shock protein 47. Cell Tissue Res. 1998;293:471–478. doi: 10.1007/s004410051139. [DOI] [PubMed] [Google Scholar]
- 209.Razzaque M.S., Shimokawa I., Nazneen A., Liu D., Naito T., Higami Y., Taguchi T. Life-Long dietary restriction modulates the expression of collagens and collagen-binding heat shock protein 47 in aged Fischer 344 rat kidney. Histochem. J. 1999;31:123–132. doi: 10.1023/A:1003578928487. [DOI] [PubMed] [Google Scholar]
- 210.Guo X.-F., Li J.-M., Tang J., Li D. Effects of resveratrol supplementation on risk factors of non-communicable diseases: A meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2018;58:3016–3029. doi: 10.1080/10408398.2017.1349076. [DOI] [PubMed] [Google Scholar]
- 211.Timmers S., Konings E., Bilet L., Houtkooper R.H., Van De Weijer T., Goossens G.H., Hoeks J., Van Der Krieken S., Ryu D., Kersten S., et al. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011;14:612–622. doi: 10.1016/j.cmet.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Dolinsky V.W., Dyck J.R.B. Calorie restriction and resveratrol in cardiovascular health and disease. Biochim. Biophys. Acta Mol. Basis Dis. 2011;1812:1477–1489. doi: 10.1016/j.bbadis.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 213.Stockinger J., Maxwell N., Shapiro D., DeCabo R., Valdez G. Caloric restriction mimetics slow aging of neuromuscular synapses and muscle fibers. J. Gerontol. A Biol. Sci. Med. Sci. 2017;73:21–28. doi: 10.1093/gerona/glx023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Xu X.M., Cai G.Y., Bu R., Wang W.J., Bai X.Y., Sun X.F., Chen X.M. Beneficial effects of caloric restriction on chronic kidney disease in rodent models: A Meta-Analysis and systematic review. PLoS ONE. 2015;10:e0144442. doi: 10.1371/journal.pone.0144442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Stern J.S., Gades M.D., Wheeldon C.M., Borchers A.T. Calorie restriction in obesity: Prevention of kidney disease in rodents. J. Nutr. 2001;131:913S–917S. doi: 10.1093/jn/131.3.913S. [DOI] [PubMed] [Google Scholar]
- 216.Ruggenenti P., Abbate M., Ruggiero B., Rota S., Trillini M., Aparicio C., Parvanova A., Iliev I.P., Pisanu G., Perna A., et al. Renal and systemic effects of calorie restriction in patients with type 2 diabetes with abdominal obesity: A randomized controlled trial. Diabetes. 2017;66:75–86. doi: 10.2337/db16-0607. [DOI] [PubMed] [Google Scholar]
- 217.Li H., Jia Z., Li A., Jenkins G., Yang X., Hu J., Guo W. Resveratrol repressed viability of U251 cells by MiR-21 inhibiting of NF-kB pathway. Mol. Cell. Biochem. 2013;382:137–143. doi: 10.1007/s11010-013-1728-1. [DOI] [PubMed] [Google Scholar]
- 218.Latruffe N., Lançon A., Frazzi R., Aires V., Delmas D., Michaille J.J., Djouadi F., Bastin J., Cherkaoui-Malki M. Exploring new ways of regulation by resveratrol involving MiRNAs, with emphasis on inflammation. Ann. N. Y. Acad. Sci. 2015;1348:97–106. doi: 10.1111/nyas.12819. [DOI] [PubMed] [Google Scholar]
- 219.Tomé-Carneiro J., Larrosa M., Yáñez-Gascón M.J., Dávalos A., Gil-Zamorano J., Gonzálvez M., García-Almagro F.J., Ruiz Ros J.A., Tomás-Barberán F.A., Espín J.C., et al. One-Year supplementation with a grape extract containing resveratrol modulates inflammatory-related MicroRNAs and cytokines expression in peripheral blood mononuclear cells of type 2 diabetes and hypertensive patients with coronary artery disease. Pharmacol. Res. 2013;72:69–82. doi: 10.1016/j.phrs.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 220.Mostafa W.Z., Hegazy R.A. Vitamin D and the skin: Focus on a complex relationship: A review. J. Adv. Res. 2015;6:793–804. doi: 10.1016/j.jare.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Sheane B., Smyth P., Scott K., Aziz R., Buckley M., Lodge E., Kiely N., Kingston M., McGovern E., Healy M., et al. An association between MicroRNA-21 expression and vitamin D deficiency in coronary artery disease. MicroRNA. 2015;4:57–63. doi: 10.2174/2211536604666150414203919. [DOI] [PubMed] [Google Scholar]
- 222.Barrea L., Savanelli M.C., Di Somma C., Napolitano M., Megna M., Colao A., Savastano S. Vitamin D and its role in psoriasis: An overview of the dermatologist and nutritionist. Rev. Endocr. Metab. Disord. 2017;18:195–205. doi: 10.1007/s11154-017-9411-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Howitz K.T., Bitterman K.J., Cohen H.Y., Lamming D.W., Lavu S., Wood J.G., Zipkin R.E., Chung P., Kisielewski A., Zhang L.L., et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- 224.Collins J.J., Evason K., Kornfeld K. Pharmacology of delayed aging and extended lifespan of Caenorhabditis elegans. Exp. Gerontol. 2006;41:1032–1039. doi: 10.1016/j.exger.2006.06.038. [DOI] [PubMed] [Google Scholar]
- 225.Rascón B., Hubbard B.P., Sinclair D.A., Amdam G.V. The lifespan extension effects of resveratrol are conserved in the honey bee and may be driven by a mechanism related to caloric restriction. Aging. 2012;4:499–508. doi: 10.18632/aging.100474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Valenzano D.R., Terzibasi E., Genade T., Cattaneo A., Domenici L., Cellerino A. Resveratrol prolongs lifespan and retards the onset of age-related markers in a short-lived vertebrate. Curr. Biol. 2006;16:296–300. doi: 10.1016/j.cub.2005.12.038. [DOI] [PubMed] [Google Scholar]
- 227.Baur J.A., Pearson K.J., Price N.L., Jamieson H.A., Lerin C., Kalra A., Prabhu V.V., Allard J.S., Lopez-Lluch G., Lewis K., et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Gerhardt E., Gräber S., Szego É.M., Moisoi N., Martins L.M., Outeiro T.F., Kermer P. Idebenone and resveratrol extend lifespan and improve motor function of HtrA2 knockout mice. PLoS ONE. 2011;6:e28855. doi: 10.1371/journal.pone.0028855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Mancuso R., del Valle J., Modol L., Martinez A., Granado-Serrano A.B., Ramirez-Núñez O., Pallás M., Portero-Otin M., Osta R., Navarro X. Resveratrol improves motoneuron function and extends survival in SOD1G93A ALS mice. Neurotherapeutics. 2014;11:419–432. doi: 10.1007/s13311-013-0253-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Kitada M., Koya D. Renal protective effects of resveratrol. Oxidative Med. Cell. Longev. 2013;2013:568093. doi: 10.1155/2013/568093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Bass T.M., Weinkove D., Houthoofd K., Gems D., Partridge L. Effects of resveratrol on lifespan in Drosophila melanogaster and Caenorhabditis elegans. Mech. Ageing Dev. 2007;128:546–552. doi: 10.1016/j.mad.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 232.Kim E., Ansell C.M., Dudycha J.L. Resveratrol and food effects on lifespan and reproduction in the model crustacean Daphnia. J. Exp. Zool. A Ecol. Genet. Physiol. 2014;321:48–56. doi: 10.1002/jez.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Wang C., Wheeler C.T., Alberico T., Sun X., Seeberger J., Laslo M., Spangler E., Kern B., De Cabo R., Zou S. The Effect of resveratrol on lifespan depends on both gender and dietary nutrient composition in Drosophila melanogaster. Age. 2013;35:69–81. doi: 10.1007/s11357-011-9332-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Pearson K.J., Baur J.A., Lewis K.N., Peshkin L., Price N.L., Labinskyy N., Swindell W.R., Kamara D., Minor R.K., Perez E., et al. Resveratrol delays age-related deterioration and mimics transcriptional sspects of dietary restriction without extending life span. Cell Metab. 2008;8:157–168. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Miller R.A., Harrison D.E., Astle C.M., Baur J.A., Boyd A.R., De Cabo R., Fernandez E., Flurkey K., Javors M.A., Nelson J.F., et al. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J. Gerontol. A Biol. Sci. Med. Sci. 2011;66:191–201. doi: 10.1093/gerona/glq178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Strong R., Miller R.A., Astle C.M., Baur J.A., De Cabo R., Fernandez E., Guo W., Javors M., Kirkland J.L., Nelson J.F., et al. Evaluation of resveratrol, green tea extract, curcumin, oxaloacetic acid, and medium-chain triglyceride oil on life span of genetically heterogeneous mice. J. Gerontol. A Biol. Sci. Med. Sci. 2013;68:6–16. doi: 10.1093/gerona/gls070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Ryan M.J., Jackson J.R., Hao Y., Williamson C.L., Dabkowski E.R., Hollander J.M., Alway S.E. Suppression of oxidative stress by resveratrol after isometric contractions in gastrocnemius muscles of aged mice. J. Gerontol. A Biol. Sci. Med. Sci. 2010;65 doi: 10.1093/gerona/glq080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Klink J.C., Tewari A.K., Masko E.M., Antonelli J., Febbo P.G., Cohen P., Dewhirst M.W., Pizzo S.V., Freedland S.J. Resveratrol worsens survival in SCID mice with prostate cancer xenografts in a cell-line specific manner, through paradoxical effects on oncogenic pathways. Prostate. 2013;73:754–762. doi: 10.1002/pros.22619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Wu H.-J., Wu E. The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes. 2012;3 doi: 10.4161/gmic.19320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Ren Z., Fan Y., Li A., Shen Q., Wu J., Ren L., Lu H., Ding S., Ren H., Liu C., et al. Alterations of the human gut microbiome in chronic kidney disease. Adv. Sci. 2020;7:2001936. doi: 10.1002/advs.202001936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Rossi M., Johnson D.W., Campbell K.L. The kidney—Gut axis: Implications for nutrition care. J. Ren. Nutr. 2015;25:399–403. doi: 10.1053/j.jrn.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 242.Chi M., Ma K., Wang J., Ding Z., Li Y., Zhu S., Liang X., Zhang Q., Song L., Liu C. The immunomodulatory effect of the gut Microbiota in kidney disease. J. Immunol. Res. 2021;2021 doi: 10.1155/2021/5516035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Bao N., Chen F., Dai D. The Regulation of host intestinal Microbiota by polyphenols in the development and prevention of chronic kidney disease. Front. Immunol. 2020;10:2981. doi: 10.3389/fimmu.2019.02981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Cai T.-T., Ye X.-L., Li R.-R., Chen H., Wang Y.-Y., Yong H.-J., Pan M.-L., Lu W., Tang Y., Miao H., et al. Resveratrol modulates the gut Microbiota and inflammation to protect against diabetic nephropathy in mice. Front. Pharmacol. 2020;11:1249. doi: 10.3389/fphar.2020.01249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Hu Y., Chen D., Zheng P., Yu J., He J., Mao X., Yu B. The bidirectional interactions between resveratrol and gut Microbiota: An insight into oxidative stress and inflammatory bowel disease therapy. BioMed. Res. Int. 2019;2019 doi: 10.1155/2019/5403761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Arias N., Arboleya S., Allison J., Kaliszewska A., Higarza S.G., Gueimonde M., Arias J.L. The relationship between choline bioavailability from diet, intestinal Microbiota Composition and its modulation of human diseases. Nutrients. 2020;12:2340. doi: 10.3390/nu12082340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Bird J.K., Raederstorff D., Weber P., Steinert R.E. Cardiovascular and antiobesity effects of resveratrol mediated through the gut Microbiota. Adv. Nutr. Int. Rev. J. 2017;8:839–849. doi: 10.3945/an.117.016568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Song J.-Y., Shen T.-C., Hou Y.-C., Chang J.-F., Lu C.-L., Liu W.-C., Chen P.-J., Chen B.-H., Zheng C.-M., Lu K.-C. Influence of resveratrol on the cardiovascular health effects of chronic kidney disease. Int. J. Mol. Sci. 2020;21:6294. doi: 10.3390/ijms21176294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Chen M., Yi L., Zhang Y., Zhou X., Ran L., Yang J., Zhu J., Zhang Q., Mi M. Resveratrol Attenuates trimethylamine-N-Oxide (TMAO)-induced atherosclerosis by regulating TMAO synthesis and bile acid metabolism via remodeling of the gut Microbiota. mBio. 2016;7:e02210-15. doi: 10.1128/mBio.02210-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Salehi B., Mishra A., Nigam M., Sener B., Kilic M., Sharifi-Rad M., Fokou P., Martins N., Sharifi-Rad J. Resveratrol: A double-edged sword in health benefits. Biomedicines. 2018;6:91. doi: 10.3390/biomedicines6030091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.De la Lastra C.A., Villegas I. Resveratrol as an antioxidant and pro-oxidant agent: Mechanisms and clinical implications. Biochem. Soc. Trans. 2007;35:1156–1160. doi: 10.1042/BST0351156. [DOI] [PubMed] [Google Scholar]
- 252.Amri A., Chaumeil J.C., Sfar S., Charrueau C. Administration of resveratrol: What formulation solutions to bioavailability limitations? J. Control. Release. 2012;158:182–193. doi: 10.1016/j.jconrel.2011.09.083. [DOI] [PubMed] [Google Scholar]
- 253.Wan S., Zhang L., Quan Y., Wei K. Resveratrol-Loaded PLGA nanoparticles: Enhanced stability, solubility and bioactivity of resveratrol for non-alcoholic fatty liver disease therapy. R. Soc. Open Sci. 2018;5:181457. doi: 10.1098/rsos.181457. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.