Abstract
Cardiovascular disease (CVD) is the number one contributor to death in the United States and worldwide. A risk factor for CVD is high serum low-density lipoprotein cholesterol (LDL-C) concentrations; however, LDL particles exist in a variety of sizes that may differentially affect the progression of CVD. The small, dense LDL particles, compared to the large, buoyant LDL subclass, are considered to be more atherogenic. It has been suggested that replacing saturated fatty acids with monounsaturated and polyunsaturated fatty acids decreases the risk for CVD. However, certain studies are not in agreement with this recommendation, as saturated fatty acid intake did not increase the risk for CVD, cardiovascular events, and/or mortality. Furthermore, consumption of saturated fat has been demonstrated to increase large, buoyant LDL particles, which may explain, in part, for the differing outcomes regarding fat consumption on CVD risk. Therefore, the objective was to review intervention trials that explored the effects of fat consumption on LDL particle size in healthy individuals. PubMed and Web of Science were utilized during the search process for journal articles. The results of this review provided evidence that fat consumption increases large, buoyant LDL and/or decreases small, dense LDL particles, and therefore, influences CVD risk.
Keywords: Low-density lipoprotein size, Fat, Fatty acids, Cardiovascular disease, Human, Clinical trial
Introduction
Cardiovascular disease (CVD) (includes heart disease and stroke) is the number one cause of death in the United States [1] and worldwide [2]. A risk factor for CVD is high serum concentrations of low-density lipoprotein cholesterol (LDL-C) [3–6], which may be significantly increased by saturated fatty acids [7]; however, some individuals with heart disease have normal levels of LDL-C [8–10]. Furthermore, LDL particles consist of a variety of sizes which may differentially affect the development of CVD [9, 11, 12]. There are larger, more buoyant LDL particles and smaller, denser LDL particles. These smaller, denser LDL particles are considered to be more atherogenic, and therefore, a significant risk factor for CVD and cardiovascular events [12–23]. LDL subclasses may be characterized by densities and diameters [11, 24–27], as presented in Table 1.
Table 1.
The properties of LDL subclasses
| LDL subclass | Density (g/mL) | Diameter (nm) | Size |
|---|---|---|---|
| 1: LDL-I | 1.019–1.023 | 27.2–28.5 | Larger |
| 2: LDL-IIa | 1.023–1.028 | 26.5–27.2 | Larger |
| 3: LDL-IIb | 1.028–1.034 | 25.6–26.5 | Medium |
| 4: LDL-IIIa | 1.034–1.041 | 24.7–25.6 | Medium |
| 5: LDL-IIIb | 1.041–1.044 | 24.2–24.7 | Smaller |
| 6: LDL-IVa | 1.044–1.051 | 23.3–24.2 | Smaller |
| 7: LDL-IVb | 1.051–1.063 | 22.0–23.3 | Smaller |
Abbreviations: LDL low-density lipoprotein, g grams, mL milliliters, nm nanometers
The atherogenic properties of small, dense (sd) LDL particles include: increased transport into the arterial wall [15, 28], increased binding to arterial wall proteoglycans [29], increased susceptibility to oxidation, which are subsequently taken up by macrophages as part of the plaque formation process [28, 30], and decreased binding to the LDL receptor [15, 31, 32]. Individuals may be characterized as pattern A (predominance of larger, more buoyant LDL particles) or pattern B (predominance of smaller, denser LDL particles) [16]. The peak particle diameter for pattern A is greater than 25.5 nm, whereas pattern B is characterized by a peak of less than 25.5 nm [16, 33]. Pattern B appears to be more common among men compared to women, which may explain, in part, why males are more at risk for CVD [34–36].
Epidemiological studies have investigated the effects of LDL particle size on CVD risk. In the MESA study, it was discovered that individuals with higher concentrations of sdLDL-C displayed a higher risk for coronary heart disease (CHD) [37]. In addition, as reported in the prospective ARIC investigation, sdLDL-C was associated with an increased risk for CHD [38]. According to the Framingham Offspring Study [39], sdLDL-C was higher in females with CHD compared to controls. In agreement, the Cardiovascular Health Study concluded that small LDL particles were related to CHD in women [40]. These results coincide with the outcomes from the Quebec Cardiovascular Study [41, 42] in which it was demonstrated that sdLDL concentrations increased the risk for heart disease in men. In another study, stroke patients were characterized with having smaller LDL sizes compared to controls; furthermore, sdLDL was a significant predictor of stroke and stroke mortality [43].
Certain studies report that replacing a portion of saturated fat with unsaturated fat – particularly polyunsaturated fat – decreases the risk factors for CVD and total mortality [44–47]. A replacement of 5% energy (E) from saturated fatty acids (SFA) with equivalent E from polyunsaturated fatty acids (PUFA), monounsaturated fatty acids (MUFA), or carbohydrates from whole grains reduced the risk for CHD, according to the Nurses’ Health Study and the Health Professionals Follow Up Study [46–48]. The PREDIMED trial, in which a Mediterranean diet was supplemented with nuts, the participants displayed decreased concentrations of medium-small and very small LDL particles and increased large LDL particles [49]. In the cross-sectional ERA-JUMP study, it was found that serum linoleic acid (the essential omega-6 PUFA) concentrations were inversely associated with small LDL particles [50]. A meta-analysis of randomized controlled trials reported that replacing a portion of saturated fat with polyunsaturated fat decreased CHD events [51].
On the other hand, not all studies and authors are in agreement regarding the replacement of saturated fat with unsaturated fat on decreasing the risk factors for CVD, cardiovascular events, and/or mortality [52–55]. Re-analyses of the Minnesota Coronary Experiment [56] and the Sydney Diet Heart Study [57] found that while replacing saturated fat with linoleic acid decreased serum cholesterol [56], it did not reduce the risk of death from CVD [56, 57]. The PURE prospective cohort study illustrated that intakes of total, saturated, and unsaturated fats were not significantly associated with myocardial infarction risk or CVD mortality. Interestingly, saturated fat consumption lowered the risk of stroke. The types of carbohydrates, however, that were consumed in the baseline diet (whole versus refined grains), as well as the details on fatty acid intakes (trans-fatty acids and vegetable oils) were not identified [58]. A meta-analysis of prospective cohort studies concluded that intake of saturated fat was not associated with an increased CVD risk [59]. Moreover, there was an inverse association between saturated fat consumption and the risk of stroke in a recent meta-analysis of 14 prospective cohort studies [60].
Certain randomized controlled trials have supported the results from epidemiological studies. In a meta-analysis of randomized controlled trials, it was reported that replacing saturated fat with primarily polyunsaturated fat is “unlikely” to lower CVD events or mortality [61]. Replacing saturated fats with polyunsaturated fats was not associated with a lowered risk for secondary prevention of CHD in a meta-analysis of randomized controlled trials [62]. A recent review of 15 randomized controlled trials, Hooper et al. [63] concluded that there is “little or no effect of reducing saturated fat on all-cause mortality or cardiovascular mortality.” It was further stated that “cutting down on saturated fat led to a 17% reduction in the risk for cardiovascular disease (including heart disease and strokes), but had little effect on the risk of dying.”
Altering the diet composition of fat and carbohydrate, for example, low-fat diets, and in turn, high-carbohydrate diets, differentially impacts serum lipid levels. If fat is replaced with carbohydrate, LDL-C is reduced; however, high-density lipoprotein cholesterol (HDL-C) is also reduced and there is an increase in triglycerides (TG) or very-low-density lipoproteins (VLDL) [10, 64–67]. In a recent meta-analysis [68], it was reported that low-carbohydrate diets improve HDL and TG values; however, they also lead to increases in LDL and total cholesterol levels. Another meta-analysis of randomized controlled trials demonstrated that consumption of low-carbohydrate diets generated higher HDL-C and lower TG concentrations compared with low-fat diets [69]. Adhering to a low-carbohydrate, high-fat diet also results in decreased blood glucose and insulin concentrations [70].
As noted, there is controversy regarding whether dietary fatty acid compositions increase the risk for CVD and/or mortality – and if the mechanisms involve LDL modifications – as saturated fat has been demonstrated to increase large, buoyant LDL particles and/or decrease the sdLDL subclass [14, 71, 72]. Additionally, based on a search of the literature, a review article has not been published on this topic. Therefore, the objective is to review intervention trials that explored the effects of fat consumption on LDL particle size in healthy individuals.
Methods
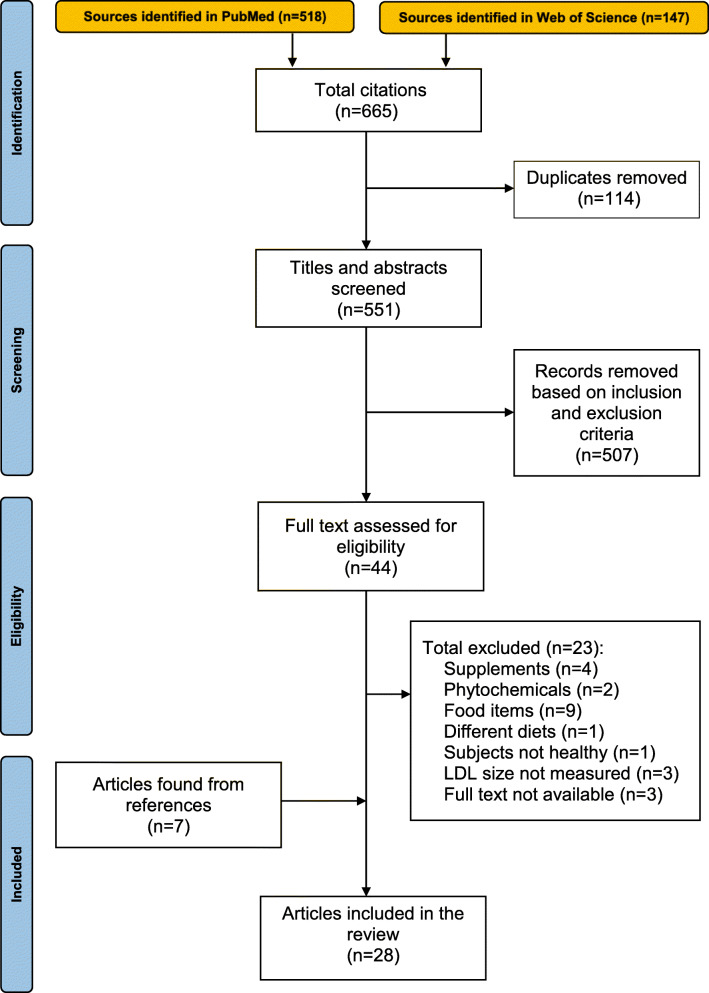
The procedure for this narrative review included searching PubMed and Web of Science for peer-reviewed journal articles. The accepted articles on intervention trials that investigated the effects of fat consumption on LDL particle size were published from 1995 to 2021. Overall, the articles used as references ranged from 1978 to 2021. The following search terms were used: low density lipoprotein, small dense LDL, dense LDL, small LDL, LDL subclasses, LDL subfractions, LDL size, LDL classes, LDL fractions, LDL diameter, healthy human clinical trial, healthy human intervention trial, healthy human randomized controlled trial, and combinations thereof. Inclusion criteria included the following: 1) specific fatty acids or saturated, monounsaturated, and polyunsaturated fatty acids consumed in grams (g), g/100 g, or % E were stated; 2) the authors indicated that the participants were “healthy”; 3) the participants had no history of CVD or other chronic diseases; 4) lipid-lowering drugs were not taken; and 5) details on the effects of varying amounts of fatty acids or fat on LDL particle size were reported. There were no restrictions on the length of the studies. The following topics that investigated whether they impacted LDL particle size were excluded: drugs, exercise, calorie-restriction, pregnancy/lactation, fibers, phytochemicals, particular food items, various diets with differing food items, supplements with multiple ingredients, plant sterols and stanols, nutrition education, children (< 18 years), genetic variants, and postprandial studies. The references of the journal articles found via PubMed and Web of Science were also analyzed for additional articles. Using this strategy, 28 journal articles were found (Fig. 1). The journal articles include studies that investigated the effects of individual fatty acids or saturated, monounsaturated, and polyunsaturated fatty acids. Therefore, the collection of journal articles includes studies on the effects of individual fatty acids, as well as saturated, monounsaturated, and polyunsaturated fatty acids, in general, on LDL particle size. As such, both types of studies are reviewed.
Fig. 1.
PRISMA flow diagram [73]
It should also be underscored that a variety of techniques may be used to determine LDL particle size [24, 74, 75], such as nondenaturing polyacrylamide gradient gel electrophoresis (PAGGE), analytic or density gradient ultracentrifugation (UC), nuclear magnetic resonance (NMR) spectroscopy [75], and ion mobility (IM) [76]. PAGGE is used to separate LDL particles by size and shape – in addition to determining peak particle diameter [71, 75]. UC typically detects the mass and density (g/mL) of up to four LDL subclasses (LDL-I to LDL-IV) [11, 71, 77]. NMR spectroscopy may be used to measure serum concentrations of LDL subclasses, particle sizes, and numbers [24, 78], while IM detects concentrations of LDL particles based on their size [76, 79]. Thus, the reviewed studies included these techniques, which are highlighted in Table 2. Additionally, the language used in the reviewed studies regarding LDL particle size is summarized in the results column of the table.
Table 2.
Intervention studies on the effects of fat intake on LDL particle size
| Author (Year) | Study/Method | Subjects | Age | Duration | Treatment | Results |
|---|---|---|---|---|---|---|
| Campos et al. (1995) [80] |
Randomized Crossover PAGGE UC |
43 males | Mean, 50 years (SD, ± 11) | 12 weeks |
Each diet was consumed for 6 wk. Low-fat diet: 24.2% E fat (6% E SFA, 11.6% E MUFA, 4.3% E PUFA), 58.8% E CHO, 16.8% E PRO. High-fat diet: 45.2% E fat (18.1% E SFA, 12.4% E MUFA, 11.8% E PUFA), 39.2% E CHO, 16.3% E PRO. Calories, cholesterol, fiber, and P:S were kept constant. |
↓LDL-C (Low-fat diet) ↑mean peak LDL diameter ↓LDL peak density ↑large, buoyant LDL particle mass (LDL I and LDL II) ↓sdLDL particle mass (LDL III and LDL IV) (High-fat diet) |
| Krauss et al. (1995) [77] |
Randomized Crossover PAGGE UC |
105 males |
28 to 79 years mean, 48.9 (SD, ± 11.1) |
12 weeks |
Each diet was consumed for 6 wk. Low-fat diet: 23.9% E fat (5.4% E SFA, 12.3% E MUFA, 4% E PUFA), 60% E CHO, 16.1% E PRO. High-fat diet: 46% E fat (18.3% E SFA, 12.4% E MUFA, 12.5% E PUFA), 38.6% E CHO, 16.2% E PRO. |
↓LDL-C (Low-fat diet) ↑large, buoyant LDL particle mass (LDL I and LDL II) ↓sdLDL particle mass (LDL III and LDL IV) (High-fat diet) Thirty-six subjects (about one-third) switched from pattern A (or intermediate pattern) to pattern B by following the low-fat diet. |
| Carmena et al. (1996) [81] |
Intervention PAGGE |
18 males |
30 to 69 years mean, 57.1 (SD, ± 17.2) |
6 weeks |
The SFO diet was consumed for 3 wk., followed by 3 wk. on the OO diet. Sunflower seed oil (SFO) diet: 31% E fat (6.8% E SFA, 10.9% E MUFA, 13.3% E PUFA), 48% E CHO, 11% E PRO. Olive oil (OO) diet: 30.5% E fat (6.9% E SFA, 21.6% E MUFA, 4.7% E PUFA), 48% E CHO, 11% E PRO. Vitamin E, beta-carotene, and vitamin C were significantly higher in the SFO diet. |
↓LDL-C ↑LDL size (SFO diet compared to OO diet) |
| Kasim-Karakas et al. (1997) [82] |
Intervention PAGGE |
14 females |
Mean, 61 years (SD, ± 11) |
4 months |
Consumption of a “habitual diet”, followed by the intakes of a 31% fat diet for 4 wk., followed by the 24% fat diet for 6 wk., then the 14% fat diet for 6 wk. Habitual diet: 33% E fat (10% E SFA, 13% E MUFA, 8% E PUFA), 51% E CHO, 71 g PRO. 31% fat diet: 31% E fat (10% E SFA, 12% E MUFA, 9% E PUFA), 53% E CHO, 17% E PRO. 24% fat diet: 23% E fat (6% E SFA, 10% E MUFA, 7% E PUFA), 60% E CHO, 18% E PRO. 14% fat diet: 14% E fat (3% E SFA, 7% E MUFA, 4% E PUFA), 67% E CHO, 19% E PRO. |
↓LDL-C (14% fat diet) ↔LDL particle size (all diets compared) |
| Clifton et al. (1998) [26] |
Randomized Double-blind PAGGE |
54 males 51 females |
Males: 30–66 years mean, 50 years (SD, ± 7.5) Females: 23–76 years mean, 51.1 years (SD, ± 9.5) |
8 weeks |
Consumption of a “self-selected” low fat baseline diet for 2 wk., followed by the addition of a fat-containing (high fat phase) or fat free (low fat phase) liquid supplement to the baseline diet for 3 wk. each. Low fat baseline diet: 26.2% E fat (9% E SFA, 9% E MUFA, 5.4% E PUFA), 51.1% E CHO, 19.7% E PRO. Low fat phase: 20.7% E fat (7.5% E SFA, 7.1% E MUFA, 4.3% E PUFA), 59.3% E CHO, 20% E PRO. High fat phase: 35.7% E fat (14.9% E SFA, 12.1% E MUFA, 3.7% E PUFA), 46.3% E CHO, 18% E PRO. The high fat phase had significantly higher cholesterol (748 mg) compared to the low fat phase (182 mg) |
↑LDL-C (high fat phase compared to low fat phase) ↑smaller LDL particles (men compared to women, in both low fat and high fat phases) ↔LDL particle size (low fat compared to high fat phase) |
| Dreon et al. (1998) [71] |
Randomized Crossover PAGGE UC |
103 males |
28–79 years mean, 48.9 years (SD, ± 11.1) |
12 weeks |
Consumption of each experimental diet for 6 wk. Low-fat diet: 24.2% E fat, 5.9% E SFA (0.1% E lauric acid, 0.3% E myristic acid, 3.7% E PA, 1.5% E SA), 11.8% E MUFA (11.7% E OA), 4.2% E PUFA (0.1% E AA, 0.3% E ALA, 3.9% E LA), 59% E CHO, 16.6% E PRO. High-fat diet: 45.5% E fat, 18.4% E SFA (0.5% E lauric acid, 2.3% E myristic acid, 4% E SA, 9% E PA), 12.5% E MUFA (11.7% E OA), 11.8% E PUFA (0.6% E ALA, 10.8% E LA), 38.8% E CHO, 16.3% E PRO. Significant differences in reported intakes of cholesterol, P:S, and fiber. |
↑LDL-C (High-fat diet) ↔plasma lipoproteins [SA, MUFA (and OA), PUFA (and LA)] ↑large LDL particle mass ↑LDL peak particle diameter (High-fat diet, high SFA, myristic and palmitic acids) ↓sdLDL mass (High-fat diet; total SFA; myristic acid) Dietary protein, carbohydrate, cholesterol, P:S, and fiber were not associated with plasma lipoproteins. |
| Lagrost et al. (1999) [83] |
Randomized PAGGE |
32 total 14 males 18 females |
20–60 years (mean, 41 years) | 23 weeks |
Three different diets were consumed for 6 wk. each, with 2 to 3 wk. washout periods. Lauric acid diet: 41.5% E fat, 22.2% E SFA (10.6% E lauric acid, 4.2% E myristic acid, 5.9% E PA), 11% E MUFA (10.3% E OA), 4.6% E PUFA (4.4% E LA), 43.9% E CHO, 14.3% E PRO. Palmitic acid diet: 41% E fat, 18.9% E SFA (13.2% E PA, 2.2% E myristic acid, 1.9% E lauric acid), 11.4% E MUFA (10.6% E OA), 4.6% E PUFA (4.4% E LA), 44.7% E CHO, 14.3% E PRO. Oleic acid diet: 41.8% E fat, 11.8% E SFA (5.7% E PA, 2.5% E lauric acid, 1.9% E myristic acid), 19.9% E MUFA (19% E OA), 5.4% E PUFA (5.2% E LA), 44.3% E CHO, 14% E PRO. Nutrient compositions were similar for each diet, except about 8.5% E was supplied by lauric (+ 2.2% E myristic acid), palmitic, or oleic acids. |
↓LDL-C (Oleic acid diet compared to lauric acid and palmitic acid diets). There were no significant differences between lauric acid and palmitic acid diets. ↔LDL particle mean size (all diets compared) |
|
Dreon et al. (1999) [84] |
Randomized Crossover PAGGE UC |
38 males |
32–71 years mean, 52.5 years (SD, ± 12.1) |
20 days |
Participants displayed phenotype A by following both a low- and high-fat diet for 4–6 wk. in a previous study. Consumption of their usual diet and very-low-fat diet for 10 d each. Usual diet: 31.8% E fat (10.8% E SFA, 11.8% E MUFA, 6.9% E PUFA), 52.1% E CHO, 14% E PRO. 10%-Fat diet: 10.4% E fat (2.7% E SFA, 3.7% E MUFA, 2.6% E PUFA), 75.7% E CHO, 14.5% E PRO. |
↓LDL-C ↓mass larger LDL-I ↑mass smaller LDL-III and LDL-IV subfractions ↓LDL particle size ↓LDL peak diameter (10%-Fat diet compared to usual diet) Twelve individuals (about one-third) converted to phenotype B, whereas 26 remained phenotype A. |
| Pedersen et al. (2000) [27] |
Randomized Double-blind Crossover UC |
18 males | 20–28 years (mean, 24 years) | Up to 33 weeks |
Three identical diets were consumed for 3 wk. each (5–12 wk. washout periods), except that 19% E was from either extra virgin olive oil, physically refined rapeseed oil, or chemically refined sunflower oil. Olive oil (OO) diet: 35% E fat (11% E SFA, 21% E MUFA, 3% E PUFA), 53% E CHO, 12% E PRO. Rapeseed oil (RO) diet: 35% E fat (9% E SFA, 18% E MUFA, 7% E PUFA), 52% E CHO, 13% E PRO. Sunflower oil (SO) diet: 35% E fat (10% E SFA, 9% E MUFA, 15% E PUFA), 53% E CHO, 12% E PRO. OO diet contained significantly more squalene and less campesterol and sitosterol compared to RO and SO diets. |
↓LDL-C (RO and SO diets compared to OO diet) ↔LDL subfraction average size (all diets compared) ↑number of larger and medium-sized LDL subfractions (LDL-1 to LDL-3) (OO diet compared to RO and SO diets) ↑number of medium-sized and sdLDL subfractions (LDL-4 to LDL-5) (OO diet compared to RO diet) ↔number of smallest, dense LDL particles (LDL-6) (all diets compared) |
| Kratz et al. (2002) [85] |
Randomized Parallel PAGGE |
56 total 30 males 26 females |
18 to 43 years (69 initial participants) mean, 25.8 years (SD, ± 5.5) |
6 weeks |
Baseline diet rich in SFA was consumed for 2 wk., followed by participants assigned to one of three treatment diets for 4 wk. Baseline diet: 38% E fat (19% E SFA, 11.2% E MUFA, 5.2% E n-6 PUFA, 0.4% E n-3 PUFA, 45.1% E CHO, 16.9% E PRO. Refined olive oil diet: 38.7% E fat (10.7% E SFA, 23.2% E MUFA, 3% E n-6 PUFA, 0.4% E n-3 PUFA, 47% E CHO, 14.3% E PRO. Rapeseed oil diet: 38.4% E fat (9.1% E SFA, 19.1% E MUFA, 6.5% E n-6 PUFA, 2.5% E n-3 PUFA, 47.3% E CHO, 14.3% E PRO. Sunflower oil diet: 38.3% E fat (10% E SFA, 8.7% E MUFA, 18.2% E n-6 PUFA, 0.3% E n-3 PUFA, 47.6% E CHO, 14.2% E PRO. The diets were identical, save for fatty acid composition. |
↓LDL size ↓LDL peak particle diameter (all 3 diets compared to baseline diet) ↔LDL size (all 3 treatment diets compared) |
| Sharman et al. (2002) [33] |
Intervention PAGE (nongradient) |
20 males |
Ketogenic diet: mean, 36.7 years (SD, ± 11.6) Control diet: mean, 35 years (SD, ± 13) |
6 weeks |
Twelve subjects switched from their usual dietary pattern to a ketogenic diet, whereas 8 subjects continued their usual dietary pattern (controls) for 6 wk. Ketogenic diet: 61% E fat (25% E SFA, 25% E MUFA, 11% E PUFA), 8% E CHO, 30% E PRO. Habitual diet: 25% E fat (12% E SFA, 9% E MUFA, 4% E PUFA), 59% E CHO, 15% E PRO. All nutrients were significantly different between diets, except for energy and alcohol consumption. |
↔LDL-C (both diets after 6 wk) ↑LDL peak particle diameter (ketogenic diet after 3 wk) ↑LDL-1 percentage (ketogenic diet) All 7 initial pattern A subjects remained pattern A after the ketogenic diet (no significant changes in percentages of any LDL subclasses, or the mean and peak LDL particle size). Most initial pattern B subjects (3 out of 5) changed to pattern A after the ketogenic diet. |
| Rivellese et al. (2003) [86] |
Randomized PAGGE UC |
162 total 86 males 76 females |
30–65 years SFA diet: mean, 48 years (SD, ± 8) (n-3 group) and mean, 49 years (SD, ± 7) (placebo) MUFA diet: mean, 49 years (SD, ± 7) (n-3 group and placebo) |
90 days |
Consumption of a diet high in SFA or MUFA, followed by a second random assignment to capsule supplements of fish oil (3.6 g n-3 FA, containing 2.4 g EPA and DHA) or placebo capsules (with same amount of olive oil). The test period was preceded by a 2 wk. “stabilisation period” on their “habitual” diets and placebo capsules. SFA diet: 37.1% E fat (17.6% E SFA, 13.1% E MUFA, 4.7% E PUFA), 44.1% E CHO, 15.2% E PRO. MUFA diet: 37.1% E fat (9.6% E SFA, 21.2% E MUFA, 4.6% E PUFA), 45.9% E CHO, 14.8% E PRO. |
↑LDL-C (SFA diet compared to MUFA diet) ↑LDL-C (n-3 supplementation in both diets) ↔LDL size (all diets compared) |
| Archer et al. (2003) [87] |
Randomized PAGGE |
65 males | Mean, 37.5 years (SD, ± 11.2) | 6–7 weeks |
Subjects consumed one of the diets for 6–7 wk. in an ad libitum manner. Low fat, high CHO diet: 25.8% E fat (6% E SFA, 13.3% E MUFA, 5.1% E PUFA. 58.3% E CHO, 15.9% E PRO. High MUFA diet: 40.1% E fat (8.2% E SFA, 22.5% E MUFA, 7.6% E PUFA, 44.7% E CHO, 15.2% E PRO. |
↓LDL-C (both diets; no significant difference between diets) ↓LDL peak particle diameter (High CHO diet; in subjects with large LDL peak particle diameters at baseline) ↑percentage of small LDL particles (High CHO diet; no significant difference between diets) |
| Smith et al. (2003) [88] |
Randomized Single-blind Parallel UC |
51 total 26 males 25 females |
18–28 years Moderate MUFA diet: Males: mean, 21 years (SD, ± 3) Females: mean, 20 (SD, ± 1) High MUFA diet: Males: mean, 20 years (SD, ± 2) Females: mean, 20 years (SD, ± 2) |
24 weeks |
Consumption of a SFA-rich reference diet for 8 wk., followed by either a moderate- or high-MUFA diet for 16 wk. SFA reference diet (one for each MUFA diet): 39.8% E/37.7% E fat (15.4% E/14.5% E SFA, 12.5% E/11.9% E MUFA, 7.3% E/6.7% E PUFA, 47.9% E/50% E CHO, 10.5% E/10.7% E PRO Moderate-MUFA diet: 39.7% E fat (12.1% E SFA, 15.1% E MUFA, 7.2% E PUFA), 47.7% E CHO, 11.2% E PRO High-MUFA diet: 37.1% E fat (9.7% E SFA, 16.6% E MUFA, 6.9% E PUFA, 50.3% E CHO, 11% E PRO MUFA intakes were not significantly different between the two MUFA diets. MUFA intakes were significantly higher and SFA intakes were significantly lower than the reference diets. |
↓LDL-C (moderate- and high-MUFA diets compared to baseline, after SFA reference diet) ↑LDL-1 percentage (moderate-MUFA diet compared to SFA reference diet) ↔proportions of LDL subfractions (between each diet) |
| Volek et al. (2003) [89] |
Randomized Crossover PAGE (nongradient) |
10 females | Mean, 26.3 years (SD, ± 6.1) | 12 weeks |
Each diet was consumed for 4 wk., with a 4 wk. break between diets. Very low CHO diet: 60% E, 118 g fat (41 g SFA, 35 g MUFA, 20 g PUFA), 10% E CHO (43 g), 29% E PRO (128 g). Low fat diet: 19% E, 34 g fat (10 g SFA, 9 g MUFA, 6 g PUFA), 62% E CHO (249 g), 17% E PRO (68 g). |
↑LDL-C (very low CHO diet compared to baseline and low fat diet) ↔relative percentages or concentrations of LDL subclasses (after consumption of each diet) Three of ten participants with pattern B displayed larger peak LDL size after following the very low CHO diet. |
| Goyens et al. (2005) [78] |
Randomized Double-blind Parallel NMR |
54 total 21 males 33 females 29 total (NMR analyses) 14 males 15 females |
Males: mean, 52.6 years (SD, ± 13.7) Females: mean, 47.7 years (SD, ± 11.1) |
10 weeks |
A 4 wk. period, followed by consumption of one of the following diets for 6 wk. Control diet: 33.5% E fat (11.6% E SFA, 12.8% E MUFA, 8% E PUFA, 7.3% E LA and 0.4% E ALA), 50.5% E CHO, 14.5% E PRO. Low-LA diet: 34% E fat (12.4% E SFA, 16.9% E MUFA, 3.7% E PUFA, 3% E LA, 0.4% E ALA, 49.8% E CHO, 14.9% E PRO. High-ALA diet: 32.6% E fat (10.4% E SFA, 12.6% E MUFA, 8.6% E PUFA, 7.1% E LA, 1.1% E ALA, 50.4% E CHO, 15.5% E PRO. |
↓LDL-C (High-ALA diet compared to control diet) ↔mean LDL particle size (all groups compared) |
| Thijssen et al. (2005) [90] |
Randomized Crossover NMR |
45 total 18 males 27 females 22 total (NMR analyses) 9 males 13 females |
28–66 years mean, 51 years (SD, ± 10) |
17 weeks |
Consumption of each diet for 5 wk., with a washout period of ≥1 wk. between diets. Stearic acid diet: 38.2% E fat, 18% E SFA (7.7% E SA), 12.9% E MUFA (6.8% E OA), 4.7% E PUFA (2.1% E LA, 0.2% E ALA), 45.8% E CHO, 14% E PRO. Oleic acid diet: 37.7% E fat, 11% E SFA (1.2% E SA), 19.1% E MUFA (13.1% E OA), 5% E PUFA (2.4% E LA, 0.2% E ALA), 46.3% E CHO, 14% E PRO. Linoleic acid diet: 38% E fat, 11.2% E SFA (1.2% E SA), 12.5% E MUFA (6.6% E OA), 11.8% E PUFA (9.3% E LA, 0.2% E ALA), 46.3% E CHO, 13.8% E PRO. The diets did not differ, save for the difference of 7% E from SA, OA, or LA. |
↔LDL-C ↔LDL particle size and subclass concentrations (all 3 diets compared) |
| Faghihnia et al. (2010) [91] |
Randomized Crossover PAGGE UC |
63 total 61 males 2 females |
At least 20 years mean, 47.9 years (SD, ± 11.2) |
8 weeks |
Each diet was consumed for 4 wk. High-fat low-carbohydrate diet: 40% E fat (13% E SFA, 11% E MUFA, 14% E PUFA), 45% E CHO, 15% E PRO. Low-fat high-carbohydrate diet: 20% E fat (5% E SFA, 10% E MUFA, 5% E PUFA), 65% E CHO, 15% E PRO. There were no differences in cholesterol and simple:complex CHO ratios. |
↓LDL-C ↓large and medium LDL particle concentrations ↑small and very small LDL particle concentrations ↓mean LDL peak particle diameter (Low-fat high-carbohydrate diet compared with the high-fat low-carbohydrate diet) |
| Egert et al. (2011) [92] |
Randomized Parallel PAGGE |
37 total 12 males 25 females |
18–34 years mean, 22.6 years (SD, ± 4.2) | 6 weeks |
Consumption of a 2 wk. wash-in SFA-rich diet followed by consumption of one of the treatment diets for 4 wk. Wash-in SFA-rich diet: 40.8% E fat (18.1% E SFA, 13.1% E MUFA, 6.6% E n-6 PUFA, 1.1% E n-3 PUFA), 42.6% E CHO, 15.7% E PRO. Low-fat diet (MUFA-rich): 28.7% E fat (7.2% E SFA, 13.9% E MUFA, 5.3% E n-6 PUFA, 0.9% E n-3 PUFA), 54.4% E CHO, 15.6% E PRO. High-fat diet (MUFA-rich): 40.2% E fat (9.9% E SFA, 19.8% E MUFA, 7% E n-6 PUFA, 1.6% E n-3 PUFA), 43.1% E CHO, 15.6% E PRO. Both diets were isocaloric, rich in MUFA, with similar FA, CHO, cholesterol, fiber, and antioxidant proportions. |
↓LDL-C ↓LDL size of the major fraction (both diets compared to the wash-in SFA-rich diet; no significant difference between treatment diets) |
| Mangravite et al. (2011) [79] |
Randomized Crossover IM |
40 males |
Mean, 45 years (SD, ± 15) |
13 weeks |
Consumption of a baseline diet for 3 wk., followed by intakes of two intervention diets for 3 wk. each. There were 2 wk. washout periods after the baseline diet and between intervention diets. Baseline diet: 38% E fat (15% E SFA, 15% E MUFA, 6% E PUFA), 50% E CHO, 13% E PRO (no beef protein). Lower carbohydrate, high-saturated fat (LCHSF) diet: 38% E fat (15% E SFA, 15% E MUFA, 5% E PUFA), 31% E CHO, 31% E PRO (10% E beef protein). Lower carbohydrate, low-saturated fat (LCLSF) diet: 38% E fat (8% E SFA, 21% E MUFA, 6% E PUFA), 31% E CHO, 32% E PRO (11% E beef protein). |
↓LDL-C ↓total LDL ↓medium LDL concentrations (LCLSF diet compared to LCHSF and baseline diets) ↓small LDL concentrations (LCLSF diet compared to LCHSF diet) ↔large LDL ↔very small LDL ↔LDL peak diameter ↔LDL subclass phenotype (all diets compared) |
| Faghihnia et al. (2012) [93] |
Randomized Crossover UC |
14 males |
24–67 years mean, 44.5 years (SD, ± 14.4) |
11 weeks |
Consumption of a baseline diet for 3 wk., followed by intakes of two experimental diets for 3 wk. each. There was a 2 wk. washout period between experimental diets. Baseline diet: 38% E fat (15% E SFA, 15% E MUFA, 6% E PUFA), 50% E CHO, 13% E PRO. Low CHO, high SFA diet: 38% E fat (15% E SFA, 15% E MUFA, 5% E PUFA), 31% E CHO, 31% E PRO (10% E beef protein). Low CHO, low SFA diet: 38% E fat (8% E SFA, 21% E MUFA, 6% E PUFA), 31% E CHO, 31% E PRO (11% E beef protein). |
↓LDL-C (low CHO, low SFA diet compared to low CHO, high SFA diet) ↑LDL total mass concentration ↑LDL subclass I (large), II (medium), and III (small) mass concentrations (low CHO, high SFA diet compared to low CHO, low SFA diet) ↔LDL subclass IV (very small) (compared to each diet) |
| Guay et al. (2012) [94] |
Randomized Double-blind Crossover PAGGE |
12 males |
18 to 50 years mean, 27.1 years (SD, ± 3.9) |
2 weeks plus 6 days |
Consumption of two experimental diets for 3 d each, separated by a 2 wk. washout period. Low fat diet: 25% E fat (6% E SFA, 12% E MUFA, 4.9% E PUFA), 61.8% E CHO, 15% E PRO. High fat diet: 37% E fat (15% E SFA, 12.7% E MUFA, 4.3% E PUFA), 49.8% E CHO, 15% E PRO. The experimental diets consisted of the same calories, proteins, fiber, MUFA, and PUFA. |
↑LDL-C ↑LDL particle size ↔LDL peak particle diameter ↑percentage of large (not significant) and medium LDL particles ↓percentage of small LDL particles (High fat diet compared with low fat diet) |
| Wang et al. (2015) [95] |
Randomized Crossover NMR |
45 total 27 males 18 females |
21–70 years mean, 45 years (SD, ± 13.3) |
14 weeks |
A 2 wk. intake of an average American diet, followed by dietary treatments for 5 wk. each. There was a 2 wk. “compliance break” between treatments. Average American diet (AAD): 34% E fat (13% E SFA, 12% E MUFA, 7% E PUFA), 51% E CHO, 16% E PRO. Lower-fat diet (LF): 24% E fat (7% E SFA, 11% E MUFA, 6% E PUFA), 59% E CHO, 16–17% E PRO. Moderate-fat diet (MF): 34% E fat (6% E SFA, 17% E MUFA, 9% E PUFA), 49% E CHO, 16–17% E PRO. Diets were designed to meet calorie needs. |
↓LDL-C ↓large LDL particle number ↓mean LDL particle size (LF and MF compared to AAD; no significant difference between LF and MF) ↓total LDL particle number (MF compared to LF; no significant difference compared to AAD) ↑small LDL particle number (LF and MF compared to AAD; there was also a significant increase with LF compared to MF) |
| Dias et al. (2017) [96] |
Randomized Parallel NMR |
26 total 11 males 15 females |
21–65 years (29 subjects recruited) SFA-rich diet: median, 32 years n-6 PUFA-rich diet: median, 28 years |
4 weeks plus 10 days |
Consumption of 4 × 1 g fish oil capsules (100 mg EPA and 500 mg DHA each) for 4 wk., followed by one of the treatment diets for 10 d while consuming the fish oil capsules. SFA + LC n-3 PUFA diet: 38.8% E fat (50.4 g SFA/100 g, 34.6 g MUFA/100 g, 13.5 g PUFA/100 g, 9.1 g LA/100 g, 4 g LC n-3 PUFA/100 g), 37.6% E CHO, 17.8% E PRO. n-6 PUFA + LC n-3 PUFA diet: 38.6% E fat (25.4 g SFA/100 g, 32.3 g MUFA/100 g, 39.1 g PUFA/100 g, 34.5 g LA/100 g, 4.6 g LC n-3 PUFA/100 g), 34% E CHO, 21% E PRO. |
↓LDL-C ↓total LDL particle concentration ↓very large, medium-large, and small LDL particle concentrations (n-6 PUFA + LC n-3 PUFA diet compared to SFA + LC n-3 PUFA diet) |
| Dias et al. (2017) [97] |
Randomized Parallel NMR |
26 total 6 males 20 females |
18–65 years | 6 weeks |
Diets were consumed for 6 wk. The diets contained 400 mg EPA and 2000 mg DHA. SFA-rich diet: 40.9% E fat (18.9% E SFA, 13.8% E MUFA, 4.4% E PUFA, 2.9% E LA, 1.13% E n-3 PUFA), 38.1% E CHO, 16.6% E PRO. n-6 PUFA-rich diet: 42.4% E fat (12.6% E SFA, 13.2% E MUFA, 14.4% E PUFA, 12.7% E LA, 1% E n-3 PUFA), 41.6% E CHO, 18.1% E PRO. |
↔LDL-C ↔LDL particle size concentrations (between diets) |
| Ulven et al. (2019) [98] |
Randomized Double-blind NMR |
99 total Control diet: 52 total 21 males 31 females Exp. diet: 47 total 20 males 27 females |
25–70 years Control diet: mean, 55.2 years (SD, ± 9.8) Exp. diet: mean, 53.6 years (SD, ± 9.7) |
10 weeks |
A 2 wk. duration which consisted of the control food items, followed by the consumption of 1 of 2 intervention diets for 8 wk. Control diet: 42.8% E fat (18% E SFA, 15.4% E MUFA, 5.6% E PUFA), 36.6% E CHO, 15% E PRO. Experimental diet: 42.9% E fat (11.5% E SFA, 15.7% E MUFA, 12% E PUFA), 34.2% E CHO, 16.5% E PRO. There was a 6.5% E lower SFA and a 6.4% E higher PUFA in the experimental diet. PRO, CHO, and fiber intakes were also significantly different. |
↓LDL-C ↓Large, medium and small LDL particle concentrations (Experimental diet compared to control diet) |
| Bergeron et al. (2019) [99] |
Randomized Parallel (high or low SFA arm) Crossover IM |
113 total High-SFA arm: 62 total 27 males 35 females Low-SFA arm: 51 total 17 males 34 females |
21–65 years High-SFA arm: mean, 45 years (SD, ± 12) Low-SFA arm: mean, 42 years (SD, ± 13) |
Up to 28 weeks |
A 2 wk. baseline diet, followed by random assignment to a low-SFA (~ 7% E) or high-SFA (~ 14% E) arm. Within each SFA arm, 3 experimental diets were consumed for 4 wk. each, with a 2–7 wk. washout period between experimental diets. High-SFA arm: Red meat diet: 35% E fat (13% E SFA, 12% E MUFA, 5% E PUFA), 41% E CHO, 24% E PRO (11.5% E red meat). White meat diet: 34% E fat (14% E SFA, 13% E MUFA, 5% E PUFA), 42% E CHO, 24% E PRO (11.5% E white meat). Nonmeat diet: 35% E fat (14% E SFA, 12% E MUFA, 6% E PUFA), 41% E CHO, 24% E PRO (15.4% E vegetable protein). Low-SFA arm: Red meat diet: 35% E fat (8% E SFA, 21% E MUFA, 5% E PUFA), 39% E CHO, 26% E PRO (12.5% E red meat). White meat diet: 31% E fat (7% E SFA, 18% E MUFA, 6% E PUFA), 46% E CHO, 23% E PRO (11% E white meat). Nonmeat diet: 34% E fat (7% E SFA, 20% E MUFA, 5% E PUFA), 41% E CHO, 25% E PRO (16% E vegetable protein). |
↑LDL-C ↑large LDL particle concentrations (High SFA compared with low SFA, independent of protein source) ↔small- and medium-sized LDL particle concentrations (High SFA intake compared with low SFA intake) |
| Buren et al. (2021) [100] |
Randomized Crossover PAGGE |
17 females |
19–27 years median, 23.8 years |
23 weeks |
Each diet was consumed for 4 wk., separated by a 15 wk. washout period. Ketogenic low-carbohydrate high-fat (LCHF) diet: 77% E fat (33% E SFA), 4% E CHO (not exceeding 25 g, excluding fiber), 19% E PRO. Control diet: 33% E fat, 44% E CHO, 19% E PRO. |
↑LDL-C ↑sdLDL-C ↑large,buoyant LDL-C (LCHF diet compared to control diet) |
Abbreviations: AA arachidonic acid, ALA alpha-linolenic acid, CHO carbohydrate, d days, DHA docosahexaenoic acid, E energy, EPA eicosapentaenoic acid, FA fatty acids, g grams, IM ion mobility, LA linoleic acid, LC long chain, LDL-C low-density lipoprotein cholesterol, MUFA monounsaturated fatty acids, NMR nuclear magnetic resonance, OA oleic acid, PA palmitic acid, PAGGE polyacrylamide gradient gel electrophoresis, PRO protein, P:S, ratio of polyunsaturated to saturated fatty acids, PUFA polyunsaturated fatty acids, SA stearic acid, SD standard deviation, sdLDL small, dense low-density lipoprotein, SFA saturated fatty acids, UC ultracentrifugation, wk. weeks, ↑, increase; ↓, decrease; ↔, no significant difference between groups
Results
A summary of the results for the reviewed journal articles is provided in Table 2. The results in the table focus on the effects of fatty acid or fat consumption on LDL particle size. The effects of the treatments are indicated above the respective treatments, which are stated in parentheses. Following the table, the results are organized according to the effects of dietary fatty acid or fat compositions on LDL particle size.
The effects of low-fat and high-fat diets on LDL particle size
In a study by Campos et al. [80], it was demonstrated that the consumption of a high-fat (45.2% E) diet increased large, buoyant LDL particles and reduced sdLDL particles compared to a low-fat (24.2% E) diet. These results are in agreement with similar studies in which the participants consumed a high-fat diet for 6 weeks (wk) [71, 77]. Interestingly, in the investigation by Krauss and Dreon [77], about one-third of pattern A (or intermediate pattern) individuals converted to pattern B by following the low-fat (23.9% E) diet. In the study by Dreon et al. [71], intakes of the high-fat (45.5% E) diet, high SFA (18.4% E), myristic acid (2.3% E), and palmitic acid (9% E) increased large LDL particle mass compared to the low-fat (24.2% E) diet. Additionally, the intake of the high-fat diet, SFA, and myristic acid decreased sdLDL. Interestingly, the consumption of stearic acid (a SFA), MUFA (including oleic acid), and PUFA (including linoleic acid) did not affect plasma lipoproteins.
In agreement with the aforementioned studies, consumption of a very-low-fat (10.4% E) diet for 10 days (d) decreased large LDL and increased smaller LDL fractions compared to a higher fat (31.8% E) diet. Furthermore, about one-third of participants switched to pattern B after following the very-low fat diet [84]. The consumption of a low-fat, high-carbohydrate (CHO) diet (20% E fat and 65% E CHO) for 4 wk. decreased large and medium LDL particle concentrations, while increasing small and very small LDL particle concentrations compared to the high-fat, low-carbohydrate diet (40% E fat and 45% E CHO) [91]. The percentage of small LDL particles decreased while the percentage of medium LDL particles increased after following a high-fat diet (37% E fat, 15% E SFA, and 49.8% E CHO) for 3 d versus a low-fat diet (25% E fat, 6% E SFA, and 61.8% E CHO) [94].
Contrary to these outcomes, an investigation in which post-menopausal females consumed a higher fat (31% E) diet, followed by intakes of lower fat (24% E and 14% E) diets in a step-wise manner, showed no significant differences in LDL size [82]. Another study found no changes in LDL particle size after male and female participants consumed a high-fat diet (35.7% E fat and 14.9% E SFA) for 3 wk. compared to a low-fat diet (20.7% E fat and 7.5% E SFA); however, male participants had higher concentrations of sdLDL particles compared to females [26]. Ad libitum consumption of a low-fat, high-carbohydrate diet (25.8% E fat, 6% E SFA, and 58.3% E CHO) for ~ 7 wk. significantly decreased LDL peak particle diameter (in subjects with large diameters at baseline) and increased the percentage of small LDL particles; however, there were no significant differences compared with the high MUFA diet (40.1% E fat, 8.2% E SFA, and 44.7% E CHO) [87].
The effects of higher or mixed fat diets on LDL particle size
A study by Lagrost et al. [83] demonstrated that consumption of higher fat (~ 41% E) diets rich in lauric acid (a SFA) (10.6% E), palmitic acid (13.2% E), or oleic acid (19% E) for 6 wk. each resulted in no significant differences in mean LDL size. Consistent with this study, the intakes of a low linoleic acid diet [34% E fat, 3% E linoleic acid, and 0.4% E alpha-linolenic acid (the essential omega-3 fatty acid)] and a high alpha-linolenic acid diet (32.6% E fat, 7.1% E linoleic acid, and 1.1% E alpha-linolenic acid) for 6 wk. did not affect mean LDL particle size compared to the control diet (33.5% E fat, 7.3% E linoleic acid and 0.4% E alpha-linolenic acid) [78]. These results coincide with the consumption of three diets (~ 38% E fat) rich in stearic acid (7.7% E), oleic acid (13.1% E), or linoleic acid (9.3% E) for 5 wk. each in which there were no significant differences in LDL particle size and concentrations [90]. The consumption of diets containing 37.1% E fat, one rich in SFA (17.6% E) and one rich in MUFA (21.2% E) [both supplemented with the omega-3 fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA)], also did not significantly differ regarding LDL size [86]. In agreement with this study, intakes of SFA- and n-6 PUFA-rich diets (~ 41% E fat) for 6 wk., both diets supplemented with EPA and DHA, displayed no significant differences regarding LDL particle size concentrations [97].
The effects of saturated fatty acids on LDL particle size
Small LDL concentrations decreased after following a lower-carbohydrate, low-saturated fat diet (38% E fat, 8% E SFA, 21% E MUFA, and 31% E CHO) for 3 wk. compared to a lower-carbohydrate, high-saturated fat diet (38% E fat, 15% E SFA, 15% E MUFA, and 31% E CHO). Intake of the lower-carbohydrate, low-saturated fat diet also decreased medium LDL concentrations in comparison to the lower-carbohydrate, high-saturated fat diet and the baseline diet (38% E fat, 15% E SFA, 15% E MUFA, and 50% E CHO). Both lower-carbohydrate diets were rich in beef protein, whereas the baseline diet was not. No significant differences were observed between diets regarding large and very small LDL concentrations [79]. In a similar study [93], the consumption of a low-carbohydrate, high-saturated fat diet (38% E fat, 15% E SFA, 15% E MUFA, and 31% E CHO) for 3 wk. increased large, medium, and small LDL mass concentrations compared to a low-carbohydrate, low-saturated fat diet (38% E fat, 8% E SFA, 21% E MUFA, and 31% E CHO). There were no significant changes for very small LDL concentrations, which coincide with the previous investigation. Another study reported that the inclusion of various protein sources (red meat, white meat, or vegetable) – along with a high SFA (~ 14% E) intake – increased large LDL particle concentrations compared to the low SFA (~ 7% E) diet after 4 wk.; however, no significant differences were found for small- and medium-sized LDL subclasses [99].
The effects of saturated and monounsaturated fatty acids on LDL particle size
The intake of a moderate-MUFA diet (39.7% E fat, 12.1% E SFA, and 15.1% E MUFA) for 16 wk. increased the percentage of large LDL particles compared to the SFA reference diet (39.8% E fat, 15.4% E SFA, and 12.5% E MUFA); however, there were no significant differences in the proportions of the LDL subfractions between the moderate-MUFA and high-MUFA (37.1% E, 9.7% E SFA, and 16.6% E MUFA) diets [82]. Following a low-fat diet (28.7% E fat and 7.2% E SFA) and a high-fat diet (40.2% E fat and 9.9% E SFA), both rich in MUFA (13.9% E and 19.8% E, respectively), decreased the size of the major LDL fraction compared to the wash-in SFA-rich diet (40.8% E fat, 18.1% E SFA, and 13.1% E MUFA) after 4 wk. No significant differences, however, were found between the MUFA-rich diets [92]. Interestingly, there were increases in small LDL particle numbers with the consumption of a lower-fat diet (24% E fat, 7% E SFA, 11% E MUFA, and 59% E CHO) or a moderate-fat diet (34% E fat, 6% E SFA, 17% E MUFA, and 49% E CHO) for 5 wk. each in comparison to an average American diet (34% E fat, 13% E SFA, 12% E MUFA, and 51% E CHO). There was also a significant increase in small LDL particle numbers with the lower-fat diet versus the moderate-fat diet. Moreover, intakes of the lower-fat and higher-fat diets reduced large LDL particle numbers compared with the average American diet, which was higher in saturated fat [95].
The effects of polyunsaturated fatty acids on LDL particle size
It was demonstrated by Dias et al. [96] that intake of an n-6 PUFA diet (38.6% E fat) decreased very large, medium-large, and small LDL particle concentrations compared with a SFA diet (38.8% E fat) after 10 d (both diets were supplemented with EPA and DHA). In agreement with these results, large, medium, and small LDL particle concentrations decreased by following an experimental diet which contained 6.4% E higher PUFA and 6.5% E lower SFA in comparison to the control diet after 8 wk. [98].
The effects of oils on LDL particles size
The consumption of a sunflower seed oil diet (13.3% E PUFA) increased LDL size compared to an olive oil diet (21.6% E MUFA) after 3 wk. on each diet [81]. In another study, it was reported that the consumption of an olive oil diet increased the number of larger and medium-sized LDL subfractions compared to the rapeseed oil and sunflower oil diets (all 35% E fat) after 3 wk. The olive oil diet also increased the number of medium-sized and sdLDL subfractions compared with the rapeseed oil diet. No significant differences were observed, however, regarding the number of the smallest, dense LDL particles [27]. In contrast to these findings, the intakes of refined olive oil, rapeseed oil, or sunflower oil diets (all ~ 38% E fat) for 4 wk. did not differ significantly regarding LDL size; however, these three diets significantly reduced LDL size compared to a baseline diet rich in SFA (19% E) [85].
The effects of very-low carbohydrate and ketogenic diets on LDL particle size
The intake of a ketogenic diet (61% E fat and 8% E CHO) increased LDL peak particle diameter (after 3 wk) and LDL-1 percentage (after 3 and 6 wk) compared to a habitual diet (25% E fat and 59% E CHO). Additionally, all initial pattern A subjects stayed pattern A following the ketogenic diet, whereas most initial pattern B participants changed to pattern A following the ketogenic diet [33]. In another investigation, the consumption of a ketogenic diet (77% E fat and 4% E CHO) for 4 wk. increased both sdLDL and large, buoyant LDL particles [100]. There were no significant differences in percentages or concentrations of LDL subclasses in participants following a very-low CHO diet (60% E fat and 10% E CHO) or a low-fat diet (19% E fat and 62% E CHO) for 4 wk. each; however, 3 of 10 individuals with pattern B exhibited larger peak LDL size after the very-low CHO diet [89].
Discussion
This review sought to summarize intervention studies that explored the effects of fat intake on LDL size in healthy individuals. A summary of the reviewed studies will be presented in this section. The mechanisms of LDL particle formation – along with factors that influence LDL particle production – will be briefly discussed. Additionally, recommendations to decrease the risk factors for CVD will be suggested. Lastly, the major findings and concluding remarks will be covered.
A summary of the reviewed studies on the effects of fat consumption on LDL particle size
The consumption of high-fat (~ 32 to 46% E) diets increased large, buoyant LDL particles and/or decreased sdLDL particles compared to low-fat (~ 10 to 25% E) diets [71, 77, 80, 84, 91, 94]. About one-third of pattern A subjects switched to pattern B by following low-fat diets [77, 84]. The intakes of higher fat (~ 33 to 41% E) diets with a variety of individual fatty acid compositions resulted in no significant differences in LDL particle size [78, 83, 86, 88, 90, 97]. In the context of a lower-carbohydrate (31% E) diet, the consumption of low-saturated fat (38% E fat and 8% E SFA) or high-saturated fat (38% E fat and 15% E SFA) diets produced mixed results on LDL subclasses [79, 93]. In other studies, high SFA (~ 13 to 19% E) intakes increased large and/or decreased small LDL particle size, numbers, mass, and/or concentrations compared to low SFA (~ 6 to 11% E) intakes, with total fat consumption of ~ 24 to 46% E in the respective diets [71, 85, 92, 95, 99]. The consumption of PUFA-rich diets decreased large, medium, and small LDL concentrations versus diets higher in SFA [96, 98]. The inclusion of diets containing olive oil, sunflower oil, or rapeseed oil produced inconsistent outcomes regarding LDL size, perhaps due to the differing ingredients, such as vitamins and phytochemicals [27, 81, 85]. There were inconsistent results in LDL size after adhering to ketogenic or very-low carbohydrate diets [33, 89, 100]. Lastly, males displayed higher concentrations of sdLDL particles compared to females [26].
Regarding individual fatty acids, myristic acid (2.3% E) and palmitic acid (9% E) increased large LDL particle mass, with myristic acid also decreasing sdLDL mass [71]. There were reductions in very large, medium-large, and small LDL particle concentrations after following a diet rich in linoleic acid compared to a diet high in SFA [96]. In contrast, there were no significant changes in LDL particle mean size after consuming diets rich in lauric acid (10.6% E), palmitic acid (13.2% E), or oleic acid (19% E) [83], or diets low in linoleic acid (3% E linoleic acid and 0.4% E alpha-linolenic acid), high in alpha-linolenic acid (7.1% E linoleic acid and 1.1% E alpha-linolenic acid), or the control provision (7.3% E linoleic acid and 0.4% E alpha-linolenic acid) [78]. Additionally, there were no significant differences in LDL particle size and subclass concentrations after intakes of diets rich in stearic acid (7.7% E), oleic acid (13.1% E), or linoleic acid (9.3% E) [90].
The mechanisms regarding the production of LDL particle size
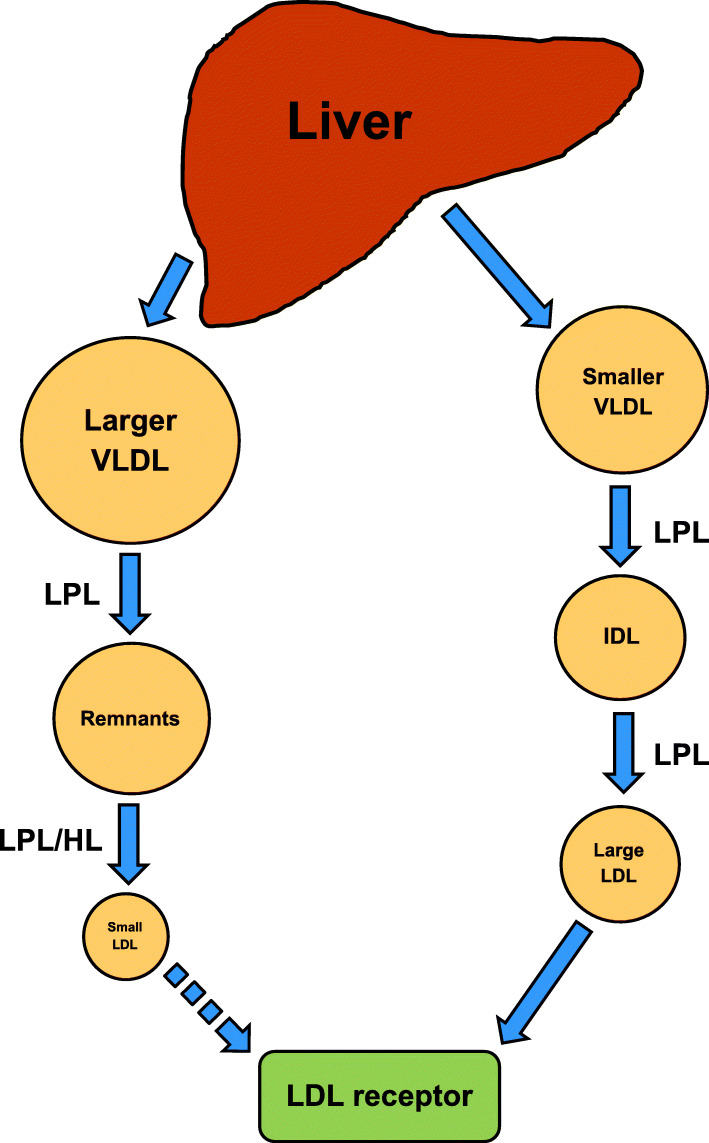
In a detailed review by Berneis and Krauss [101], it was illustrated that there is “metabolic channeling within the VLDL-IDL-LDL delipidation cascade” in which pathways produce different intermediate-density lipoprotein (IDL) and LDL particles from various precursor TG-rich lipoproteins, such as VLDL. For example, VLDL particles are produced in the liver and the TG in VLDL particles are hydrolyzed by lipoprotein lipase (LPL) in various tissues, thereby generating IDL. The IDL-TG particles are further hydrolyzed, which produce LDL particles. The cholesteryl ester transfer protein (CETP) exchanges the cholesteryl esters in LDL for TG in VLDL. This is followed by hydrolysis of TG in LDL particles by hepatic lipase (HL), which generates sdLDL particles [102]. These sdLDL particles are formed due to “prolonged residence” in the bloodstream – primarily from VLDL particles – and display reduced binding to the LDL receptor [28, 31, 32] (Fig. 2). The CETP, additionally, participates in a similar exchange of lipids between VLDL and HDL particles, which results in HDL that are low in cholesteryl esters [102, 103].
Fig. 2.
The production of LDL particles. HL, hepatic lipase; IDL, intermediate-density lipoprotein; LDL, low-density lipoprotein; LPL, lipoprotein lipase; VLDL, very-low-density lipoprotein
There is a “strong” relationship between plasma TG and sdLDL whereby increased TG concentrations are associated with smaller LDL size. Decreased HDL cholesterol concentrations are also correlated with smaller LDL particles [17]. Very large VLDL particles typically generate lower quantities of LDL as their “delipidation” likely stops in the VLDL or IDL density range which may be removed from plasma or remain in circulation. It has been suggested that there is a relationship between large VLDL particles and sdLDL, as certain individuals convert VLDL to IDL, and eventually sdLDL particles [25, 101]. Interestingly, the intake of a whole food, high-carbohydrate diet increases [TG] [67]. The metabolic reactions that generate sdLDL also generate “abnormalities” in VLDL and HDL subfractions that likely increase CVD risk [34].
The effects of fat consumption on LDL particle size
The consumption of very-low fat (< 8% E) diets, and consequently, high-carbohydrate diets, reduces lipase activity, such as LPL, whereas a high-fat diet and a higher saturated fat diet increase HL and LPL activities [80, 104]. HL activity has been indicated to be positively correlated with sdLDL particles, whereas LPL activity has been shown to be inversely associated with sdLDL concentrations [80]. However, these enzymes do not seem to be “primary determinants” in generating sdLDL particles. Additionally, HL activity was demonstrated to be inversely correlated with small VLDL and small IDL. These smaller VLDL particles are “more effective” ligands for LDL receptors compared to the larger varieties [101, 105]. Moreover, LPL activity was shown to be positively associated with small IDL and large, buoyant LDL particles. Increased LPL activity stimulated by intake of a high-fat diet may increase the production of large, buoyant LDL particles and small IDL, and increased HL activity may increase the catabolism and clearance of TG-rich lipoprotein remnants [80]. The involvement of CETP in the production of the sdLDL subfraction is controversial, and may depend upon underlying health conditions [101, 103].
According to the reviewed studies [71, 77, 80, 84, 91, 94], the consumption of the high-fat diets increased the large, buoyant LDL class and/or decreased sdLDL particles compared to the low-fat diets. Furthermore, there were increases in LDL particle size following higher SFA intakes compared to lower SFA consumption [71, 85, 92, 95, 99]. As such, the results of these reviewed investigations are in agreement with the proposed mechanisms of fat consumption on LDL particle size. However, additional research is needed regarding the effects of individual fatty acids on LDL particle size.
The effects of additional factors on LDL particle size
An increased number of sdLDL particles may result from a disorder in the metabolism of TG-rich lipoproteins [101]. Insulin resistance, for example, may cause decreased retention of fatty acids in adipocytes, and thus, increased plasma free fatty acids that are returned to the liver. Hence, this leads to increased synthesis of TG, and therefore, increased production and “residence time” of large, TG-rich VLDL particles during the postprandial period, thereby increasing CVD risk [15, 34, 102, 106].
The more dense LDL subclasses may also be influenced by age, gender, body weight, smoking, exercise, fiber, plant sterols, hormones, postmenopausal status, and oral contraceptive use [80, 107–111]. For example, increased fat deposition is associated with higher amounts of TG, and in turn, reduced LDL particle size [112]. In another example, women have larger LDL particles compared to men, with the sex differences regarding CVD risk narrowing with age [34, 113]. Premenopausal women also display reduced LDL-C and TG concentrations, as well as higher HDL-C levels [114]. There are also genes regulating this metabolic cascade, such as those involving LPL, HL, and CETP; however, there are other candidates – as well as the genetic variants – that may impact this cascade [9, 102].
LDL particle size as a risk marker for CVD risk
As suggested previously, sdLDL particles are associated with increased TG and apolipoprotein B levels (associated with LDL-C), and decreased HDL-C and apolipoprotein A1 levels (associated with HDL-C) [36]. Additionally, the sdLDL subclass is considered to be more atherogenic, and therefore, a significant risk factor for CVD [12–23]. On the other hand, sdLDL particles may not be an independent risk factor for CVD after other risk factors have been considered, such as total cholesterol, LDL particle number, and LDL-C, HDL-C, and TG concentrations, according to certain studies [24, 34, 115–119]. There are also additional lipid factors to consider, such as lipoprotein(a) [120], the total cholesterol:HDL-C ratio, apolipoproteins, and measuring LDL size, number, and concentrations [24, 99]. Hence, more research is needed to develop strong risk markers for CVD. Moreover, there are non-lipid factors that may increase CVD risk, such as hypertension, endothelial dysfunction, cardiac arrhythmia, oxidative stress, inflammation, thrombosis, the glucose-insulin axis, and the microbiome [10]. Additional CVD risk factors include smoking, obesity, a lack of exercise, a high alcohol consumption [121], and genetic factors [9, 17, 102, 122].
Dietary recommendations to decrease the risk factors for CVD
The American College of Cardiology/American Heart Association Task Force has recently provided suggestions to reduce CVD risk factors including: consumption of adequate amounts of fruits, vegetables, legumes, whole grains, nuts, lean vegetable or animal protein sources, and fish, as well as reducing the intakes of trans-fatty acids, red meats and processed red meats, refined carbohydrates, cholesterol, sodium, and sugar-sweetened beverages. It is also recommended to replace a % of E from saturated fatty acids with monounsaturated and polyunsaturated fatty acids [121].
The recently published Dietary Guidelines for Americans emphasize using vegetable oils in place of sources high in saturated fat, such as lard, coconut oil, palm kernel oil, palm oil, butter, shortening, high-fat meats, and full-fat dairy products. Additionally, it is suggested to limit the intake of saturated fats to less than 10% E by replacing them with unsaturated fats – especially polyunsaturated fats [123].
According to the World Health Organization, it is recommended that total fat intake should not exceed 30% E, with less than 10% E as saturated fat. Trans-fat consumption should be less than 1% E and replace saturated fats with unsaturated fats (fish, avocado, nuts, and safflower, sunflower, corn, soybean, olive, and canola oils) [124].
In a prospective cohort study, it was noted that a plant-based dietary pattern (fruits, vegetables, beans, and fish) was associated with a reduced risk for heart failure, whereas the intakes of fried food, processed meats, added fats, and sugar-sweetened beverages increased the risk [125]. The PREDIMED investigation reported that a Mediterranean diet supplemented with extra-virgin olive oil or nuts lowered the incidence of cardiovascular events compared to a reduced-fat diet [126]. Further, consumption of a low-fat diet (20% E fat) during a long-term randomized clinical trial, did not reduce the risk for CVD [127]. A report by Kris-Etherton et al. suggested that a moderate intake of fat – 25 to 35% E – should be consumed. It was additionally stated that “extremes in dietary fat should be avoided” [128].
It has been recently proposed that we should focus on whole foods and overall dietary patterns, rather than individual fatty acids [52, 129]. For example, certain populations that consume higher amounts of cholesterol and saturated fat do not display high CHD mortality rates, as they consume more plant foods, as well as monounsaturated and polyunsaturated fatty acids [53]. Moreover, some saturated fatty acid-rich food items have not been demonstrated to increase CVD risk. A possible explanation is due to the food matrix of these particular foods, such as the components of vitamins, minerals, proteins, phospholipids, probiotics, and phytochemicals. Therefore, guidelines should focus on food-based recommendations and overall dietary patterns rather than individual fatty acid recommendations [10, 52].
Review strengths and limitations
A strength of this narrative review is that it covers human intervention trials on this topic that were published over an extended period of time (1995 to 2021); the reviewed studies provided evidence that fat consumption impacts LDL particle size. Additionally, based on a search of the literature, a review article has not been published in this area. This review, however, has limitations, such as not analyzing risk of bias in the intervention studies and the heterogeneity of the investigations (ages, sex distributions, diets, study durations, and methods).
Conclusions and future perspectives
The objective of this review was to assess whether fat consumption affects LDL particle size in healthy individuals. Interestingly, it was found that consuming higher fat diets increased large, buoyant LDL and/or decreased sdLDL particles compared to lower fat diets. It was also discovered that higher SFA consumption increased large and/or decreased small LDL particles. In limited studies, intakes of PUFA-rich diets decreased both large and small LDL particles compared to SFA-rich diets. In contrast, the consumption of higher fat diets containing a variety of individual fatty acids did not differ with respect to LDL particle size. Therefore, it appears that the major finding from this review is that LDL particle size is primarily influenced by the consumption of high- and low-fat diets, as well as high and low SFA intakes (Fig. 3). These outcomes emphasize the recommendation to consume adequate amounts of fat to reduce the formation of sdLDL particles, thereby decreasing the risk for CVD. However, more research is needed regarding the effects of individual fatty acids on LDL particle size – in addition to the mechanisms. Overall dietary patterns should also be considered, as food components (macro- and micronutrients, fiber, and phytochemicals) may affect LDL size. It is suggested that further investigations address the effects of dietary patterns (whole versus refined carbohydrates, types and amounts of fat, and plant-based versus meat-based diets) on a variety of cardiovascular disease risk markers – including sdLDL particles. Moreover, it is recommended to ascertain the clinical relevance of the relative and absolute levels, size, number, and peak particle diameter of LDL particles involved with the primary and secondary prevention of CVD.
Fig. 3.
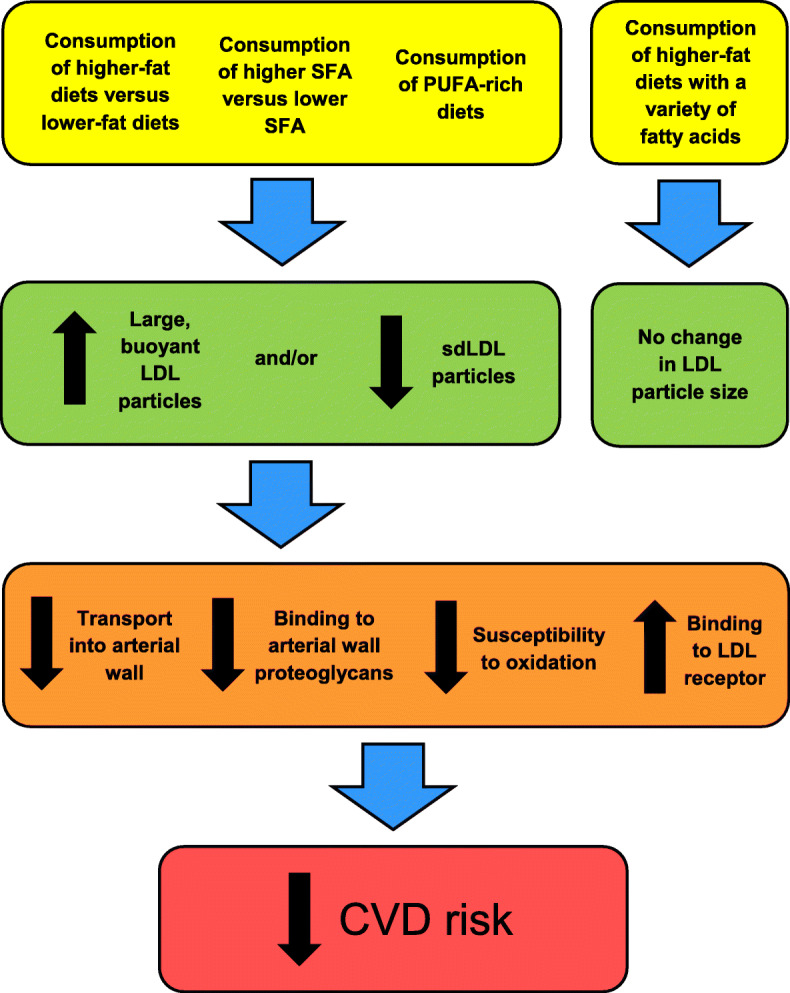
A summary of the major findings from this review. CVD, cardiovascular disease; LDL, low-density lipoprotein; PUFA, polyunsaturated fatty acid; sd, small dense; SFA, saturated fatty acids
Acknowledgements
Not applicable.
Abbreviations
- CETP
Cholesteryl ester transfer protein
- CHD
Coronary heart disease
- CVD
Cardiovascular disease
- CHO
Carbohydrate
- d
days
- DHA
Docosahexaenoic acid
- E
Energy
- EPA
Eicosapentaenoic acid
- g
grams
- HDL-C
High-density lipoprotein cholesterol
- HL
Hepatic lipase
- IDL
Intermediate-density lipoproteins
- IM
Ion mobility
- LDL-C
Low-density lipoprotein cholesterol
- LPL
Lipoprotein lipase
- MUFA
Monounsaturated fatty acids
- NMR
Nuclear magnetic resonance
- PAGGE
Polyacrylamide gradient gel electrophoresis
- PUFA
Polyunsaturated fatty acids
- sdLDL
small dense low-density lipoprotein
- SFA
Saturated fatty acids
- TG
Triglycerides
- UC
Ultracentrifugation
- VLDL
Very-low-density lipoproteins
- wk.
weeks
Author’s contributions
EF solely conceptualized, visualized, wrote, reviewed and edited the manuscript. EF read and approved the final manuscript.
Funding
Not applicable.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The author declares that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Deaths: Leading Causes for 2017. https://www.cdc.gov/nchs/nvss/leading-causes-of-death.htm. Accessed 29 Mar 2021.
- 2.World Health Organization. The top 10 causes of death. https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death. Accessed 29 Mar 2021.
- 3.Ference BA, Ginsberg HN, Graham I, Ray KK, Packard CJ, Bruckert E, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European atherosclerosis society consensus panel. Eur Heart J. 2017;38(32):2459–2472. doi: 10.1093/eurheartj/ehx144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ference BA, Majeed F, Penumetcha R, Flack JM, Brook RD. Effect of naturally random allocation to lower low-density lipoprotein cholesterol on the risk of coronary heart disease mediated by polymorphisms in NPC1L1, HMGCR, or both: a 2 x 2 factorial Mendelian randomization study. J Am Coll Cardiol. 2015;65(15):1552–1561. doi: 10.1016/j.jacc.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen JC, Boerwinkle E, Mosley TH, Jr, Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med. 2006;354(12):1264–1272. doi: 10.1056/NEJMoa054013. [DOI] [PubMed] [Google Scholar]
- 6.Jacobson TA, Maki KC, Orringer CE, Jones PH, Kris-Etherton P, Sikand G, et al. National Lipid Association Recommendations for patient-centered Management of Dyslipidemia: part 2. J Clin Lipidol. 2015;9(6 Suppl):S1–122 e1. doi: 10.1016/j.jacl.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 7.Denke MA. Dietary fats, fatty acids, and their effects on lipoproteins. Curr Atheroscler Rep. 2006;8(6):466–471. doi: 10.1007/s11883-006-0021-0. [DOI] [PubMed] [Google Scholar]
- 8.Genest J, Jr, McNamara JR, Ordovas JM, Jenner JL, Silberman SR, Anderson KM, et al. Lipoprotein cholesterol, apolipoprotein A-I and B and lipoprotein (a) abnormalities in men with premature coronary artery disease. J Am Coll Cardiol. 1992;19(4):792–802. doi: 10.1016/0735-1097(92)90520-W. [DOI] [PubMed] [Google Scholar]
- 9.Carr MC, Ayyobi AF, Murdoch SJ, Deeb SS, Brunzell JD. Contribution of hepatic lipase, lipoprotein lipase, and cholesteryl ester transfer protein to LDL and HDL heterogeneity in healthy women. Arterioscler Thromb Vasc Biol. 2002;22(4):667–673. doi: 10.1161/01.ATV.0000013284.47317.95. [DOI] [PubMed] [Google Scholar]
- 10.Forouhi NG, Krauss RM, Taubes G, Willett W. Dietary fat and cardiometabolic health: evidence, controversies, and consensus for guidance. BMJ. 2018;361:k2139. doi: 10.1136/bmj.k2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krauss RM, Burke DJ. Identification of multiple subclasses of plasma low density lipoproteins in normal humans. J Lipid Res. 1982;23(1):97–104. doi: 10.1016/S0022-2275(20)38178-5. [DOI] [PubMed] [Google Scholar]
- 12.Blake GJ, Otvos JD, Rifai N, Ridker PM. Low-density lipoprotein particle concentration and size as determined by nuclear magnetic resonance spectroscopy as predictors of cardiovascular disease in women. Circulation. 2002;106(15):1930–1937. doi: 10.1161/01.CIR.0000033222.75187.B9. [DOI] [PubMed] [Google Scholar]
- 13.Coresh J, Kwiterovich PO, Jr, Smith HH, Bachorik PS. Association of plasma triglyceride concentration and LDL particle diameter, density, and chemical composition with premature coronary artery disease in men and women. J Lipid Res. 1993;34(10):1687–1697. doi: 10.1016/S0022-2275(20)35731-X. [DOI] [PubMed] [Google Scholar]
- 14.DiNicolantonio JJ, O'Keefe JH. Effects of dietary fats on blood lipids: a review of direct comparison trials. Open Heart. 2018;5(2):e000871. doi: 10.1136/openhrt-2018-000871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Griffin BA. Lipoprotein atherogenicity: an overview of current mechanisms. Proc Nutr Soc. 1999;58(1):163–169. doi: 10.1079/PNS19990022. [DOI] [PubMed] [Google Scholar]
- 16.Austin MA, King MC, Vranizan KM, Krauss RM. Atherogenic lipoprotein phenotype. A proposed genetic marker for coronary heart disease risk. Circulation. 1990;82(2):495–506. doi: 10.1161/01.CIR.82.2.495. [DOI] [PubMed] [Google Scholar]
- 17.Austin MA. Triglyceride, small, dense low-density lipoprotein, and the atherogenic lipoprotein phenotype. Curr Atheroscler Rep. 2000;2(3):200–207. doi: 10.1007/s11883-000-0021-4. [DOI] [PubMed] [Google Scholar]
- 18.St-Pierre AC, Ruel IL, Cantin B, Dagenais GR, Bernard PM, Despres JP, et al. Comparison of various electrophoretic characteristics of LDL particles and their relationship to the risk of ischemic heart disease. Circulation. 2001;104(19):2295–2299. doi: 10.1161/hc4401.098490. [DOI] [PubMed] [Google Scholar]
- 19.Skoglund-Andersson C, Tang R, Bond MG, de Faire U, Hamsten A, Karpe F. LDL particle size distribution is associated with carotid intima-media thickness in healthy 50-year-old men. Arterioscler Thromb Vasc Biol. 1999;19(10):2422–2430. doi: 10.1161/01.ATV.19.10.2422. [DOI] [PubMed] [Google Scholar]
- 20.Mackey RH, Kuller LH, Sutton-Tyrrell K, Evans RW, Holubkov R, Matthews KA. Lipoprotein subclasses and coronary artery calcium in postmenopausal women from the healthy women study. Am J Cardiol. 2002;90(8A):71i–76i. doi: 10.1016/S0002-9149(02)02636-X. [DOI] [PubMed] [Google Scholar]
- 21.Williams PT, Superko HR, Haskell WL, Alderman EL, Blanche PJ, Holl LG, et al. Smallest LDL particles are most strongly related to coronary disease progression in men. Arterioscler Thromb Vasc Biol. 2003;23(2):314–321. doi: 10.1161/01.ATV.0000053385.64132.2D. [DOI] [PubMed] [Google Scholar]
- 22.Duran EK, Aday AW, Cook NR, Buring JE, Ridker PM, Pradhan AD. Triglyceride-rich lipoprotein cholesterol, small dense LDL cholesterol, and incident cardiovascular disease. J Am Coll Cardiol. 2020;75(17):2122–2135. doi: 10.1016/j.jacc.2020.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liou L, Kaptoge S. Association of small, dense LDL-cholesterol concentration and lipoprotein particle characteristics with coronary heart disease: a systematic review and meta-analysis. PLoS One. 2020;15(11):e0241993. doi: 10.1371/journal.pone.0241993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Superko HR, Gadesam RR. Is it LDL particle size or number that correlates with risk for cardiovascular disease? Curr Atheroscler Rep. 2008;10(5):377–385. doi: 10.1007/s11883-008-0059-2. [DOI] [PubMed] [Google Scholar]
- 25.Krauss RM. Lipids and lipoproteins in patients with type 2 diabetes. Diabetes Care. 2004;27(6):1496–1504. doi: 10.2337/diacare.27.6.1496. [DOI] [PubMed] [Google Scholar]
- 26.Clifton PM, Noakes M, Nestel PJ. LDL particle size and LDL and HDL cholesterol changes with dietary fat and cholesterol in healthy subjects. J Lipid Res. 1998;39(9):1799–1804. doi: 10.1016/S0022-2275(20)32167-2. [DOI] [PubMed] [Google Scholar]
- 27.Pedersen A, Baumstark MW, Marckmann P, Gylling H, Sandstrom B. An olive oil-rich diet results in higher concentrations of LDL cholesterol and a higher number of LDL subfraction particles than rapeseed oil and sunflower oil diets. J Lipid Res. 2000;41(12):1901–1911. doi: 10.1016/S0022-2275(20)32351-8. [DOI] [PubMed] [Google Scholar]
- 28.Taskinen MR. LDL-cholesterol, HDL-cholesterol or triglycerides--which is the culprit? Diabetes Res Clin Pract. 2003;61(Suppl 1):S19–S26. doi: 10.1016/S0168-8227(03)00126-8. [DOI] [PubMed] [Google Scholar]
- 29.Anber V, Millar JS, McConnell M, Shepherd J, Packard CJ. Interaction of very-low-density, intermediate-density, and low-density lipoproteins with human arterial wall proteoglycans. Arterioscler Thromb Vasc Biol. 1997;17(11):2507–2514. doi: 10.1161/01.ATV.17.11.2507. [DOI] [PubMed] [Google Scholar]
- 30.Dejager S, Bruckert E, Chapman MJ. Dense low density lipoprotein subspecies with diminished oxidative resistance predominate in combined hyperlipidemia. J Lipid Res. 1993;34(2):295–308. doi: 10.1016/S0022-2275(20)40756-4. [DOI] [PubMed] [Google Scholar]
- 31.Nigon F, Lesnik P, Rouis M, Chapman MJ. Discrete subspecies of human low density lipoproteins are heterogeneous in their interaction with the cellular LDL receptor. J Lipid Res. 1991;32(11):1741–1753. doi: 10.1016/S0022-2275(20)41629-3. [DOI] [PubMed] [Google Scholar]
- 32.Galeano NF, Milne R, Marcel YL, Walsh MT, Levy E, Ngu'yen TD, et al. Apoprotein B structure and receptor recognition of triglyceride-rich low density lipoprotein (LDL) is modified in small LDL but not in triglyceride-rich LDL of normal size. J Biol Chem. 1994;269(1):511–519. doi: 10.1016/S0021-9258(17)42379-9. [DOI] [PubMed] [Google Scholar]
- 33.Sharman MJ, Kraemer WJ, Love DM, Avery NG, Gomez AL, Scheett TP, et al. A ketogenic diet favorably affects serum biomarkers for cardiovascular disease in normal-weight men. J Nutr. 2002;132(7):1879–1885. doi: 10.1093/jn/132.7.1879. [DOI] [PubMed] [Google Scholar]
- 34.Freedman DS, Otvos JD, Jeyarajah EJ, Shalaurova I, Cupples LA, Parise H, et al. Sex and age differences in lipoprotein subclasses measured by nuclear magnetic resonance spectroscopy: the Framingham study. Clin Chem. 2004;50(7):1189–1200. doi: 10.1373/clinchem.2004.032763. [DOI] [PubMed] [Google Scholar]
- 35.McNamara JR, Campos H, Ordovas JM, Peterson J, Wilson PW, Schaefer EJ. Effect of gender, age, and lipid status on low density lipoprotein subfraction distribution. Results from the Framingham offspring study. Arteriosclerosis. 1987;7(5):483–490. doi: 10.1161/01.ATV.7.5.483. [DOI] [PubMed] [Google Scholar]
- 36.Campos H, Blijlevens E, McNamara JR, Ordovas JM, Posner BM, Wilson PW, et al. LDL particle size distribution. Results from the Framingham offspring study. Arterioscler Thromb. 1992;12(12):1410–1419. doi: 10.1161/01.ATV.12.12.1410. [DOI] [PubMed] [Google Scholar]
- 37.Tsai MY, Steffen BT, Guan W, McClelland RL, Warnick R, McConnell J, et al. New automated assay of small dense low-density lipoprotein cholesterol identifies risk of coronary heart disease: the multi-ethnic study of atherosclerosis. Arterioscler Thromb Vasc Biol. 2014;34(1):196–201. doi: 10.1161/ATVBAHA.113.302401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoogeveen RC, Gaubatz JW, Sun W, Dodge RC, Crosby JR, Jiang J, et al. Small dense low-density lipoprotein-cholesterol concentrations predict risk for coronary heart disease: the atherosclerosis risk in communities (ARIC) study. Arterioscler Thromb Vasc Biol. 2014;34(5):1069–1077. doi: 10.1161/ATVBAHA.114.303284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ai M, Otokozawa S, Asztalos BF, Ito Y, Nakajima K, White CC, et al. Small dense LDL cholesterol and coronary heart disease: results from the Framingham offspring study. Clin Chem. 2010;56(6):967–976. doi: 10.1373/clinchem.2009.137489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuller L, Arnold A, Tracy R, Otvos J, Burke G, Psaty B, et al. Nuclear magnetic resonance spectroscopy of lipoproteins and risk of coronary heart disease in the cardiovascular health study. Arterioscler Thromb Vasc Biol. 2002;22(7):1175–1180. doi: 10.1161/01.ATV.0000022015.97341.3A. [DOI] [PubMed] [Google Scholar]
- 41.Lamarche B, Tchernof A, Moorjani S, Cantin B, Dagenais GR, Lupien PJ, et al. Small, dense low-density lipoprotein particles as a predictor of the risk of ischemic heart disease in men. Prospective results from the Quebec cardiovascular study. Circulation. 1997;95(1):69–75. doi: 10.1161/01.CIR.95.1.69. [DOI] [PubMed] [Google Scholar]
- 42.St-Pierre AC, Cantin B, Dagenais GR, Mauriege P, Bernard PM, Despres JP, et al. Low-density lipoprotein subfractions and the long-term risk of ischemic heart disease in men: 13-year follow-up data from the Quebec cardiovascular study. Arterioscler Thromb Vasc Biol. 2005;25(3):553–559. doi: 10.1161/01.ATV.0000154144.73236.f4. [DOI] [PubMed] [Google Scholar]
- 43.Zeljkovic A, Vekic J, Spasojevic-Kalimanovska V, Jelic-Ivanovic Z, Bogavac-Stanojevic N, Gulan B, et al. LDL and HDL subclasses in acute ischemic stroke: prediction of risk and short-term mortality. Atherosclerosis. 2010;210(2):548–554. doi: 10.1016/j.atherosclerosis.2009.11.040. [DOI] [PubMed] [Google Scholar]
- 44.Sacks FM, Lichtenstein AH, Wu JHY, Appel LJ, Creager MA, Kris-Etherton PM, et al. Dietary fats and cardiovascular disease: a presidential advisory from the American Heart Association. Circulation. 2017;136(3):e1–e23. doi: 10.1161/CIR.0000000000000510. [DOI] [PubMed] [Google Scholar]
- 45.Clifton PM, Keogh JB. A systematic review of the effect of dietary saturated and polyunsaturated fat on heart disease. Nutr Metab Cardiovasc Dis. 2017;27(12):1060–1080. doi: 10.1016/j.numecd.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 46.Wang DD, Hu FB. Dietary fat and risk of cardiovascular disease: recent controversies and advances. Annu Rev Nutr. 2017;37(1):423–446. doi: 10.1146/annurev-nutr-071816-064614. [DOI] [PubMed] [Google Scholar]
- 47.Wang DD, Li Y, Chiuve SE, Stampfer MJ, Manson JE, Rimm EB, et al. Association of Specific Dietary Fats with Total and Cause-Specific Mortality. JAMA Intern Med. 2016;176(8):1134–1145. doi: 10.1001/jamainternmed.2016.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li Y, Hruby A, Bernstein AM, Ley SH, Wang DD, Chiuve SE, et al. Saturated fats compared with unsaturated fats and sources of carbohydrates in relation to risk of coronary heart disease: a prospective cohort study. J Am Coll Cardiol. 2015;66(14):1538–1548. doi: 10.1016/j.jacc.2015.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Damasceno NR, Sala-Vila A, Cofan M, Perez-Heras AM, Fito M, Ruiz-Gutierrez V, et al. Mediterranean diet supplemented with nuts reduces waist circumference and shifts lipoprotein subfractions to a less atherogenic pattern in subjects at high cardiovascular risk. Atherosclerosis. 2013;230(2):347–353. doi: 10.1016/j.atherosclerosis.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 50.Choo J, Ueshima H, Curb JD, Shin C, Evans RW, El-Saed A, et al. Serum n-6 fatty acids and lipoprotein subclasses in middle-aged men: the population-based cross-sectional ERA-JUMP study. Am J Clin Nutr. 2010;91(5):1195–1203. doi: 10.3945/ajcn.2009.28500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mozaffarian D, Micha R, Wallace S. Effects on coronary heart disease of increasing polyunsaturated fat in place of saturated fat: a systematic review and meta-analysis of randomized controlled trials. PLoS Med. 2010;7(3):e1000252. doi: 10.1371/journal.pmed.1000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Astrup A, Magkos F, Bier DM, Brenna JT, de Oliveira Otto MC, Hill JO, et al. Saturated fats and health: a reassessment and proposal for food-based recommendations: JACC state-of-the-art review. J Am Coll Cardiol. 2020;76(7):844–857. doi: 10.1016/j.jacc.2020.05.077. [DOI] [PubMed] [Google Scholar]
- 53.Artaud-Wild SM, Connor SL, Sexton G, Connor WE. Differences in coronary mortality can be explained by differences in cholesterol and saturated fat intakes in 40 countries but not in France and Finland. A Paradox. Circulation. 1993;88(6):2771–2779. doi: 10.1161/01.CIR.88.6.2771. [DOI] [PubMed] [Google Scholar]
- 54.de Oliveira Otto MC, Mozaffarian D, Kromhout D, Bertoni AG, Sibley CT, Jacobs DR, Jr, et al. Dietary intake of saturated fat by food source and incident cardiovascular disease: the multi-ethnic study of atherosclerosis. Am J Clin Nutr. 2012;96(2):397–404. doi: 10.3945/ajcn.112.037770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.de Souza RJ, Mente A, Maroleanu A, Cozma AI, Ha V, Kishibe T, et al. Intake of saturated and trans unsaturated fatty acids and risk of all cause mortality, cardiovascular disease, and type 2 diabetes: systematic review and meta-analysis of observational studies. BMJ. 2015;351:h3978. doi: 10.1136/bmj.h3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ramsden CE, Zamora D, Majchrzak-Hong S, Faurot KR, Broste SK, Frantz RP, et al. Re-evaluation of the traditional diet-heart hypothesis: analysis of recovered data from Minnesota coronary experiment (1968-73) BMJ. 2016;353:i1246. doi: 10.1136/bmj.i1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ramsden CE, Zamora D, Leelarthaepin B, Majchrzak-Hong SF, Faurot KR, Suchindran CM, et al. Use of dietary linoleic acid for secondary prevention of coronary heart disease and death: evaluation of recovered data from the Sydney diet heart study and updated meta-analysis. BMJ. 2013;346(feb04 3):e8707. doi: 10.1136/bmj.e8707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dehghan M, Mente A, Zhang X, Swaminathan S, Li W, Mohan V, et al. Associations of fats and carbohydrate intake with cardiovascular disease and mortality in 18 countries from five continents (PURE): a prospective cohort study. Lancet. 2017;390(10107):2050–2062. doi: 10.1016/S0140-6736(17)32252-3. [DOI] [PubMed] [Google Scholar]
- 59.Siri-Tarino PW, Sun Q, Hu FB, Krauss RM. Meta-analysis of prospective cohort studies evaluating the association of saturated fat with cardiovascular disease. Am J Clin Nutr. 2010;91(3):535–546. doi: 10.3945/ajcn.2009.27725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kang ZQ, Yang Y, Xiao B. Dietary saturated fat intake and risk of stroke: systematic review and dose-response meta-analysis of prospective cohort studies. Nutr Metab Cardiovasc Dis. 2020;30(2):179–189. doi: 10.1016/j.numecd.2019.09.028. [DOI] [PubMed] [Google Scholar]
- 61.Hamley S. The effect of replacing saturated fat with mostly n-6 polyunsaturated fat on coronary heart disease: a meta-analysis of randomised controlled trials. Nutr J. 2017;16(1):30. doi: 10.1186/s12937-017-0254-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schwingshackl L, Hoffmann G. Dietary fatty acids in the secondary prevention of coronary heart disease: a systematic review, meta-analysis and meta-regression. BMJ Open. 2014;4(4):e004487. doi: 10.1136/bmjopen-2013-004487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hooper L, Martin N, Jimoh OF, Kirk C, Foster E, Abdelhamid AS. Reduction in saturated fat intake for cardiovascular disease. Cochrane Database Syst Rev. 2020;8:CD011737. doi: 10.1002/14651858.CD011737.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mensink RP, Zock PL, Kester AD, Katan MB. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials. Am J Clin Nutr. 2003;77(5):1146–1155. doi: 10.1093/ajcn/77.5.1146. [DOI] [PubMed] [Google Scholar]
- 65.Krauss RM. Dietary and genetic probes of atherogenic dyslipidemia. Arterioscler Thromb Vasc Biol. 2005;25(11):2265–2272. doi: 10.1161/01.ATV.0000186365.73973.f0. [DOI] [PubMed] [Google Scholar]
- 66.Siri-Tarino PW, Sun Q, Hu FB, Krauss RM. Saturated fat, carbohydrate, and cardiovascular disease. Am J Clin Nutr. 2010;91(3):502–509. doi: 10.3945/ajcn.2008.26285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Parks EJ, Krauss RM, Christiansen MP, Neese RA, Hellerstein MK. Effects of a low-fat, high-carbohydrate diet on VLDL-triglyceride assembly, production, and clearance. J Clin Invest. 1999;104(8):1087–1096. doi: 10.1172/JCI6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chawla S, Tessarolo Silva F, Amaral Medeiros S, Mekary RA, Radenkovic D. The effect of low-fat and low-carbohydrate diets on weight loss and lipid levels: a systematic review and meta-analysis. Nutrients. 2020;12(12):3774. doi: 10.3390/nu12123774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hu T, Mills KT, Yao L, Demanelis K, Eloustaz M, Yancy WS, Jr, et al. Effects of low-carbohydrate diets versus low-fat diets on metabolic risk factors: a meta-analysis of randomized controlled clinical trials. Am J Epidemiol. 2012;176(Suppl 7):S44–S54. doi: 10.1093/aje/kws264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Noakes TD, Windt J. Evidence that supports the prescription of low-carbohydrate high-fat diets: a narrative review. Br J Sports Med. 2017;51(2):133–139. doi: 10.1136/bjsports-2016-096491. [DOI] [PubMed] [Google Scholar]
- 71.Dreon DM, Fernstrom HA, Campos H, Blanche P, Williams PT, Krauss RM. Change in dietary saturated fat intake is correlated with change in mass of large low-density-lipoprotein particles in men. Am J Clin Nutr. 1998;67(5):828–836. doi: 10.1093/ajcn/67.5.828. [DOI] [PubMed] [Google Scholar]
- 72.Krauss RM, Kris-Etherton PM. Public health guidelines should recommend reducing saturated fat consumption as much as possible: NO. Am J Clin Nutr. 2020;112(1):19–24. doi: 10.1093/ajcn/nqaa111. [DOI] [PubMed] [Google Scholar]
- 73.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sninsky JJ, Rowland CM, Baca AM, Caulfield MP, Superko HR. Classification of LDL phenotypes by 4 methods of determining lipoprotein particle size. J Investig Med. 2013;61(6):942–949. doi: 10.2310/JIM.0b013e31829d9d17. [DOI] [PubMed] [Google Scholar]
- 75.Desroches S, Lamarche B. Diet and low-density lipoprotein particle size. Curr Atheroscler Rep. 2004;6(6):453–460. doi: 10.1007/s11883-004-0086-6. [DOI] [PubMed] [Google Scholar]
- 76.Caulfield MP, Li S, Lee G, Blanche PJ, Salameh WA, Benner WH, et al. Direct determination of lipoprotein particle sizes and concentrations by ion mobility analysis. Clin Chem. 2008;54(8):1307–1316. doi: 10.1373/clinchem.2007.100586. [DOI] [PubMed] [Google Scholar]
- 77.Krauss RM, Dreon DM. Low-density-lipoprotein subclasses and response to a low-fat diet in healthy men. Am J Clin Nutr. 1995;62(2):478S–487S. doi: 10.1093/ajcn/62.2.478S. [DOI] [PubMed] [Google Scholar]
- 78.Goyens PL, Mensink RP. The dietary alpha-linolenic acid to linoleic acid ratio does not affect the serum lipoprotein profile in humans. J Nutr. 2005;135(12):2799–2804. doi: 10.1093/jn/135.12.2799. [DOI] [PubMed] [Google Scholar]
- 79.Mangravite LM, Chiu S, Wojnoonski K, Rawlings RS, Bergeron N, Krauss RM. Changes in atherogenic dyslipidemia induced by carbohydrate restriction in men are dependent on dietary protein source. J Nutr. 2011;141(12):2180–2185. doi: 10.3945/jn.111.139477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Campos H, Dreon DM, Krauss RM. Associations of hepatic and lipoprotein lipase activities with changes in dietary composition and low density lipoprotein subclasses. J Lipid Res. 1995;36(3):462–472. doi: 10.1016/S0022-2275(20)39880-1. [DOI] [PubMed] [Google Scholar]
- 81.Carmena R, Ascaso JF, Camejo G, Varela G, Hurt-Camejo E, Ordovas JM, et al. Effect of olive and sunflower oils on low density lipoprotein level, composition, size, oxidation and interaction with arterial proteoglycans. Atherosclerosis. 1996;125(2):243–255. doi: 10.1016/0021-9150(96)05882-0. [DOI] [PubMed] [Google Scholar]
- 82.Kasim-Karakas SE, Lane E, Almario R, Mueller W, Walzem R. Effects of dietary fat restriction on particle size of plasma lipoproteins in postmenopausal women. Metabolism. 1997;46(4):431–436. doi: 10.1016/S0026-0495(97)90061-5. [DOI] [PubMed] [Google Scholar]
- 83.Lagrost L, Mensink RP, Guyard-Dangremont V, Temme EH, Desrumaux C, Athias A, et al. Variations in serum cholesteryl ester transfer and phospholipid transfer activities in healthy women and men consuming diets enriched in lauric, palmitic or oleic acids. Atherosclerosis. 1999;142(2):395–402. doi: 10.1016/S0021-9150(98)00244-5. [DOI] [PubMed] [Google Scholar]
- 84.Dreon DM, Fernstrom HA, Williams PT, Krauss RM. A very low-fat diet is not associated with improved lipoprotein profiles in men with a predominance of large, low-density lipoproteins. Am J Clin Nutr. 1999;69(3):411–418. doi: 10.1093/ajcn/69.3.411. [DOI] [PubMed] [Google Scholar]
- 85.Kratz M, Gulbahce E, von Eckardstein A, Cullen P, Cignarella A, Assmann G, et al. Dietary mono- and polyunsaturated fatty acids similarly affect LDL size in healthy men and women. J Nutr. 2002;132(4):715–718. doi: 10.1093/jn/132.4.715. [DOI] [PubMed] [Google Scholar]
- 86.Rivellese AA, Maffettone A, Vessby B, Uusitupa M, Hermansen K, Berglund L, et al. Effects of dietary saturated, monounsaturated and n-3 fatty acids on fasting lipoproteins, LDL size and post-prandial lipid metabolism in healthy subjects. Atherosclerosis. 2003;167(1):149–158. doi: 10.1016/S0021-9150(02)00424-0. [DOI] [PubMed] [Google Scholar]
- 87.Archer WR, Lamarche B, St-Pierre AC, Mauger JF, Deriaz O, Landry N, et al. High carbohydrate and high monounsaturated fatty acid diets similarly affect LDL electrophoretic characteristics in men who are losing weight. J Nutr. 2003;133(10):3124–3129. doi: 10.1093/jn/133.10.3124. [DOI] [PubMed] [Google Scholar]
- 88.Smith RD, Kelly CN, Fielding BA, Hauton D, Silva KD, Nydahl MC, et al. Long-term monounsaturated fatty acid diets reduce platelet aggregation in healthy young subjects. Br J Nutr. 2003;90(3):597–606. doi: 10.1079/BJN2003953. [DOI] [PubMed] [Google Scholar]
- 89.Volek JS, Sharman MJ, Gomez AL, Scheett TP, Kraemer WJ. An isoenergetic very low carbohydrate diet improves serum HDL cholesterol and triacylglycerol concentrations, the total cholesterol to HDL cholesterol ratio and postprandial pipemic responses compared with a low fat diet in normal weight, normolipidemic women. J Nutr. 2003;133(9):2756–2761. doi: 10.1093/jn/133.9.2756. [DOI] [PubMed] [Google Scholar]
- 90.Thijssen MA, Mensink RP. Small differences in the effects of stearic acid, oleic acid, and linoleic acid on the serum lipoprotein profile of humans. Am J Clin Nutr. 2005;82(3):510–516. doi: 10.1093/ajcn/82.3.510. [DOI] [PubMed] [Google Scholar]
- 91.Faghihnia N, Tsimikas S, Miller ER, Witztum JL, Krauss RM. Changes in lipoprotein(a), oxidized phospholipids, and LDL subclasses with a low-fat high-carbohydrate diet. J Lipid Res. 2010;51(11):3324–3330. doi: 10.1194/jlr.M005769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Egert S, Kratz M, Kannenberg F, Fobker M, Wahrburg U. Effects of high-fat and low-fat diets rich in monounsaturated fatty acids on serum lipids, LDL size and indices of lipid peroxidation in healthy non-obese men and women when consumed under controlled conditions. Eur J Nutr. 2011;50(1):71–79. doi: 10.1007/s00394-010-0116-9. [DOI] [PubMed] [Google Scholar]
- 93.Faghihnia N, Mangravite LM, Chiu S, Bergeron N, Krauss RM. Effects of dietary saturated fat on LDL subclasses and apolipoprotein CIII in men. Eur J Clin Nutr. 2012;66(11):1229–1233. doi: 10.1038/ejcn.2012.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Guay V, Lamarche B, Charest A, Tremblay AJ, Couture P. Effect of short-term low- and high-fat diets on low-density lipoprotein particle size in normolipidemic subjects. Metabolism. 2012;61(1):76–83. doi: 10.1016/j.metabol.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 95.Wang L, Bordi PL, Fleming JA, Hill AM, Kris-Etherton PM. Effect of a moderate fat diet with and without avocados on lipoprotein particle number, size and subclasses in overweight and obese adults: a randomized, controlled trial. J Am Heart Assoc. 2015;4(1):e001355. doi: 10.1161/JAHA.114.001355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dias CB, Amigo N, Wood LG, Mallol R, Correig X, Garg ML. Improvement of the omega 3 index of healthy subjects does not alter the effects of dietary saturated fats or n-6PUFA on LDL profiles. Metabolism. 2017;68:11–19. doi: 10.1016/j.metabol.2016.11.014. [DOI] [PubMed] [Google Scholar]
- 97.Dias CB, Amigo N, Wood LG, Correig X, Garg ML. Effect of diets rich in either saturated fat or n-6 polyunsaturated fatty acids and supplemented with long-chain n-3 polyunsaturated fatty acids on plasma lipoprotein profiles. Eur J Clin Nutr. 2017;71(11):1297–1302. doi: 10.1038/ejcn.2017.56. [DOI] [PubMed] [Google Scholar]
- 98.Ulven SM, Christensen JJ, Nygard O, Svardal A, Leder L, Ottestad I, et al. Using metabolic profiling and gene expression analyses to explore molecular effects of replacing saturated fat with polyunsaturated fat-a randomized controlled dietary intervention study. Am J Clin Nutr. 2019;109(5):1239–1250. doi: 10.1093/ajcn/nqy356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bergeron N, Chiu S, Williams PT, Krauss RM. Effects of red meat, white meat, and nonmeat protein sources on atherogenic lipoprotein measures in the context of low compared with high saturated fat intake: a randomized controlled trial. Am J Clin Nutr. 2019;110(1):24–33. doi: 10.1093/ajcn/nqz035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Buren J, Ericsson M, Damasceno NRT, Sjodin A. A ketogenic low-carbohydrate high-fat diet increases LDL cholesterol in healthy, young, Normal-weight women: a randomized controlled feeding trial. Nutrients. 2021;13(3):814. doi: 10.3390/nu13030814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Berneis KK, Krauss RM. Metabolic origins and clinical significance of LDL heterogeneity. J Lipid Res. 2002;43(9):1363–1379. doi: 10.1194/jlr.R200004-JLR200. [DOI] [PubMed] [Google Scholar]
- 102.Kwiterovich PO., Jr Clinical relevance of the biochemical, metabolic, and genetic factors that influence low-density lipoprotein heterogeneity. Am J Cardiol. 2002;90(8A):30i–47i. doi: 10.1016/S0002-9149(02)02749-2. [DOI] [PubMed] [Google Scholar]
- 103.Hogue JC, Lamarche B, Gaudet D, Lariviere M, Tremblay AJ, Bergeron J, et al. Relationship between cholesteryl ester transfer protein and LDL heterogeneity in familial hypercholesterolemia. J Lipid Res. 2004;45(6):1077–1083. doi: 10.1194/jlr.M300420-JLR200. [DOI] [PubMed] [Google Scholar]
- 104.Wang CS, Weingand KW, Anthony MS. Effect of atherogenic diet on lipoprotein lipase activity in cynomolgus monkeys. Atherosclerosis. 1987;67(2–3):173–180. doi: 10.1016/0021-9150(87)90277-2. [DOI] [PubMed] [Google Scholar]
- 105.Chappell DA, Fry GL, Waknitz MA, Muhonen LE, Pladet MW. Low density lipoprotein receptors bind and mediate cellular catabolism of normal very low density lipoproteins in vitro. J Biol Chem. 1993;268(34):25487–25493. doi: 10.1016/S0021-9258(19)74418-4. [DOI] [PubMed] [Google Scholar]
- 106.Malmstrom R, Packard CJ, Caslake M, Bedford D, Stewart P, Yki-Jarvinen H, et al. Defective regulation of triglyceride metabolism by insulin in the liver in NIDDM. Diabetologia. 1997;40(4):454–462. doi: 10.1007/s001250050700. [DOI] [PubMed] [Google Scholar]
- 107.de Graaf J, Swinkels DW, de Haan AF, Demacker PN, Stalenhoef AF. Both inherited susceptibility and environmental exposure determine the low-density lipoprotein-subfraction pattern distribution in healthy Dutch families. Am J Hum Genet. 1992;51(6):1295–1310. [PMC free article] [PubMed] [Google Scholar]
- 108.Applebaum-Bowden D, McLean P, Steinmetz A, Fontana D, Matthys C, Warnick GR, et al. Lipoprotein, apolipoprotein, and lipolytic enzyme changes following estrogen administration in postmenopausal women. J Lipid Res. 1989;30(12):1895–1906. doi: 10.1016/S0022-2275(20)38202-X. [DOI] [PubMed] [Google Scholar]
- 109.Nikkila EA, Taskinen MR, Rehunen S, Harkonen M. Lipoprotein lipase activity in adipose tissue and skeletal muscle of runners: relation to serum lipoproteins. Metabolism. 1978;27(11):1661–1667. doi: 10.1016/0026-0495(78)90288-3. [DOI] [PubMed] [Google Scholar]
- 110.Shrestha S, Volek JS, Udani J, Wood RJ, Greene CM, Aggarwal D, et al. A combination therapy including psyllium and plant sterols lowers LDL cholesterol by modifying lipoprotein metabolism in hypercholesterolemic individuals. J Nutr. 2006;136(10):2492–2497. doi: 10.1093/jn/136.10.2492. [DOI] [PubMed] [Google Scholar]
- 111.Sarzynski MA, Burton J, Rankinen T, Blair SN, Church TS, Despres JP, et al. The effects of exercise on the lipoprotein subclass profile: a meta-analysis of 10 interventions. Atherosclerosis. 2015;243(2):364–372. doi: 10.1016/j.atherosclerosis.2015.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rainwater DL, Mitchell BD, Comuzzie AG, Haffner SM. Relationship of low-density lipoprotein particle size and measures of adiposity. Int J Obes Relat Metab Disord. 1999;23(2):180–189. doi: 10.1038/sj.ijo.0800813. [DOI] [PubMed] [Google Scholar]
- 113.Price JF, Fowkes FG. Risk factors and the sex differential in coronary artery disease. Epidemiology. 1997;8(5):584–591. doi: 10.1097/00001648-199709000-00018. [DOI] [PubMed] [Google Scholar]
- 114.Hazzard WR. Atherogenesis: why women live longer than men. Geriatrics. 1985;40(1):42–51 4. [PubMed] [Google Scholar]
- 115.Campos H, Genest JJ, Jr, Blijlevens E, McNamara JR, Jenner JL, Ordovas JM, et al. Low density lipoprotein particle size and coronary artery disease. Arterioscler Thromb. 1992;12(2):187–195. doi: 10.1161/01.ATV.12.2.187. [DOI] [PubMed] [Google Scholar]
- 116.El Harchaoui K, van der Steeg WA, Stroes ES, Kuivenhoven JA, Otvos JD, Wareham NJ, et al. Value of low-density lipoprotein particle number and size as predictors of coronary artery disease in apparently healthy men and women: the EPIC-Norfolk prospective population study. J Am Coll Cardiol. 2007;49(5):547–553. doi: 10.1016/j.jacc.2006.09.043. [DOI] [PubMed] [Google Scholar]
- 117.Austin MA, Rodriguez BL, McKnight B, McNeely MJ, Edwards KL, Curb JD, et al. Low-density lipoprotein particle size, triglycerides, and high-density lipoprotein cholesterol as risk factors for coronary heart disease in older Japanese-American men. Am J Cardiol. 2000;86(4):412–416. doi: 10.1016/S0002-9149(00)00956-5. [DOI] [PubMed] [Google Scholar]
- 118.Freedman DS, Otvos JD, Jeyarajah EJ, Barboriak JJ, Anderson AJ, Walker JA. Relation of lipoprotein subclasses as measured by proton nuclear magnetic resonance spectroscopy to coronary artery disease. Arterioscler Thromb Vasc Biol. 1998;18(7):1046–1053. doi: 10.1161/01.ATV.18.7.1046. [DOI] [PubMed] [Google Scholar]
- 119.Kamigaki AS, Siscovick DS, Schwartz SM, Psaty BM, Edwards KL, Raghunathan TE, et al. Low density lipoprotein particle size and risk of early-onset myocardial infarction in women. Am J Epidemiol. 2001;153(10):939–945. doi: 10.1093/aje/153.10.939. [DOI] [PubMed] [Google Scholar]
- 120.Fogacci F, Cicero AFG, D'Addato S, Giovannini M, Borghi C. Effect of spontaneous changes in dietary components and lipoprotein(a) levels: data from the Brisighella heart study. Atherosclerosis. 2017;262:202–204. doi: 10.1016/j.atherosclerosis.2017.03.036. [DOI] [PubMed] [Google Scholar]
- 121.Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J Am Coll Cardiol. 2019;74(10):e177–e232. doi: 10.1016/j.jacc.2019.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Krauss RM. Dietary and genetic effects on low-density lipoprotein heterogeneity. Annu Rev Nutr. 2001;21(1):283–295. doi: 10.1146/annurev.nutr.21.1.283. [DOI] [PubMed] [Google Scholar]
- 123.Dietary Guidelines for Americans 2020-2025. https://www.dietaryguidelines.gov/. Accessed 30 Mar 2021.
- 124.World Health Organization. Healthy diet. https://www.who.int/news-room/fact-sheets/detail/healthy-diet. Accessed 30 Mar 2021.
- 125.Lara KM, Levitan EB, Gutierrez OM, Shikany JM, Safford MM, Judd SE, et al. Dietary patterns and incident heart failure in U.S. adults without known coronary disease. J Am Coll Cardiol. 2019;73(16):2036–2045. doi: 10.1016/j.jacc.2019.01.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Estruch R, Ros E, Salas-Salvado J, Covas MI, Corella D, Aros F, et al. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. N Engl J Med. 2018;378(25):e34. doi: 10.1056/NEJMoa1800389. [DOI] [PubMed] [Google Scholar]
- 127.Howard BV, Van Horn L, Hsia J, Manson JE, Stefanick ML, Wassertheil-Smoller S, et al. Low-fat dietary pattern and risk of cardiovascular disease: the Women’s Health Initiative randomized controlled dietary modification trial. JAMA. 2006;295(6):655–666. doi: 10.1001/jama.295.6.655. [DOI] [PubMed] [Google Scholar]
- 128.Kris-Etherton PM, Kris-Etherton PM, Binkoski AE, Zhao G, Coval SM, Clemmer KF, et al. Dietary fat: assessing the evidence in support of a moderate-fat diet; the benchmark based on lipoprotein metabolism. Proc Nutr Soc. 2002;61(2):287–298. doi: 10.1079/PNS2002157. [DOI] [PubMed] [Google Scholar]
- 129.Liu AG, Ford NA, Hu FB, Zelman KM, Mozaffarian D, Kris-Etherton PM. A healthy approach to dietary fats: understanding the science and taking action to reduce consumer confusion. Nutr J. 2017;16(1):53. doi: 10.1186/s12937-017-0271-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.