Abstract
Pulmonary veno-occlusive disease (PVOD) is a rare but devastating cause of pulmonary hypertension (PH) characterized by preferential remodeling of the pulmonary venules.
Mitomycin-C (MMC) is an alkylating agent commonly used in chemotherapy with documented lung toxicity as well as PVOD adverse effect. The incidence of PVOD in patients with anal cancer is much higher than in those with idiopathic PVOD, especially following treatment with MMC. An accurate diagnosis of PVOD can be made based on noninvasive investigations utilizing oxygen parameters, low diffusing capacity for carbon monoxide and characteristic signs on high-resolution computed tomography of the chest. No evidence-based medical therapy exists for PVOD at present and lung transplant remains the preferred definitive therapy for eligible patients. We present a case of autopsy confirmed MMC induced PVOD in a patient with metastatic anal cancer.
Keywords: Pulmonary veno-occlusive disease, Pulmonary arterial hypertension, Pulmonary hypertension, Pulmonary vasodilators, Mitomycin-C, Respiratory failure, Anal cancer
1. Introduction
Pulmonary veno-occlusive disease (PVOD) is a rare but devastating cause of pulmonary hypertension (PH) characterized by preferential remodeling of pulmonary venules. In the current PH classification based on the 6th world symposium on PH, PVOD and pulmonary capillary hemangiomatosis (PCH) are considered to be a common entity and represent varied expressions of the same disease [1]. Incidence of PVOD in the general population is estimated at 0.5/million persons per year while the estimated incidence in patients with anal cancer based off a French registry was 3.9/1000 patients per year. This is significantly higher than in those with idiopathic PVOD, especially following treatment with Mitomycin-C (MMC) [2]. We present a case of MMC induced PVOD in a patient with metastatic anal cancer, confirmed on autopsy.
2. Case presentation
Patient was a 50-year-old man who initially presented to the emergency department (ED) with painful peri-anal mass and was later diagnosed with locally advanced invasive anal squamous cell cancer. He received 6 weeks of concomitant radiation and chemotherapy with 5-fluoro-uracil and MMC. His treatment course was complicated by poor wound healing in his right groin and recurrent hospital admissions for hypoxic respiratory failure thought to be from multifactorial etiologies including right heart failure, recurrent pleural effusions and pneumonia. Echo initially only showed left ventricular diastolic dysfunction and he improved with diuresis and antibiotics on different admissions. Chest computed tomography (CT) revealed smooth interlobular septal thickening, ground-glass opacities (GGOs), mediastinal lymphadenopathy (LAD), pleural effusions and enlarged central pulmonary arteries. Thoracentesis of the effusions and endoscopic bronchial ultrasound fine needle aspirates of mediastinal LAD were negative for malignancy.
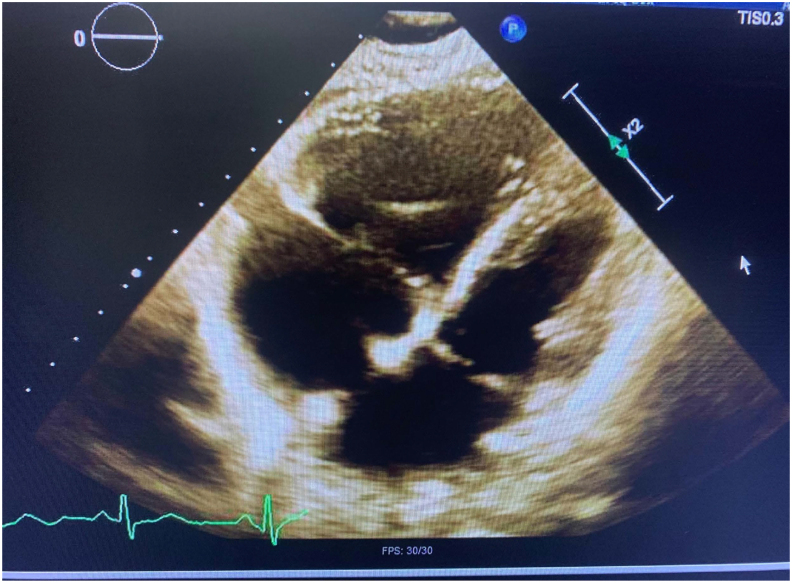
A year after his initial cancer diagnosis, he received systemic chemotherapy with carboplatin and paclitaxel as well as local radiation for recurrent metastatic disease but ended up on admission shortly after. He presented with worsening shortness of breath and increasing oxygen requirements and was found to be in cardiogenic shock requiring multiple pressors. Echo showed dilated right ventricle (RV) with systolic and diastolic septal flattening consistent with RV pressure and volume overload (Fig. 1).
Fig. 1.
Echo showing dilated RV with systolic and diastolic septal flattening consistent with RV pressure and volume overload.
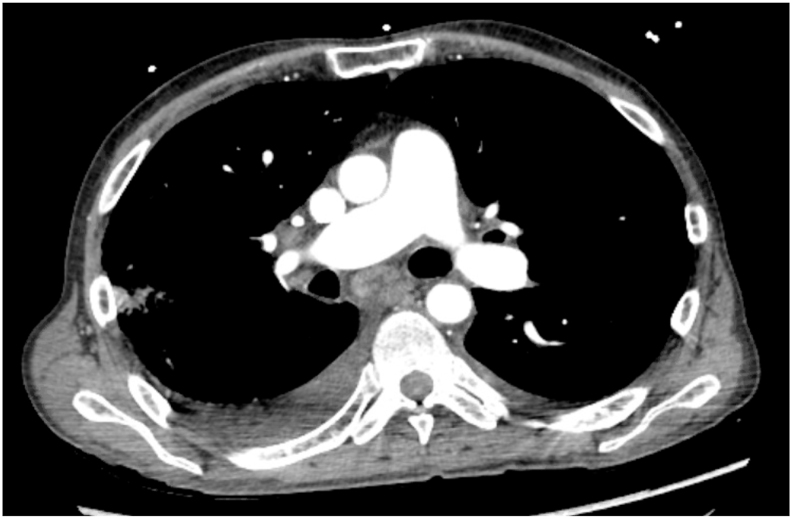
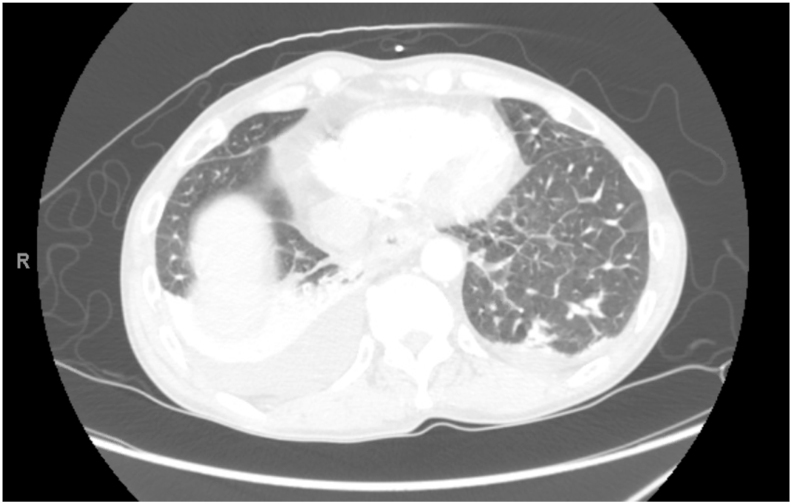
Chest computed tomography angiography (CTA) ruled out pulmonary embolism but again, had nonspecific findings of interlobular septal thickening, patchy GGOs, bilateral pleural effusions, mediastinal LAD and enlarged central pulmonary arteries (Fig. 2, Fig. 3, Fig. 4).
Fig. 2.
Mediastinal view of Chest CTA showing mediastinal LAD and enlarged central pulmonary artery.
Fig. 3.
Lung window of CTA showing interlobular septal thickening and pleural effusions.
Fig. 4.
Lung window of CTA showing patchy GGOs (albeit not in classic centrilobular fashion) and pleural effusions.
He was admitted to the intensive care unit (ICU) in cardiogenic shock requiring multiple pressors and had pulmonary artery catheter (PAC) placed which showed elevated pulmonary artery and wedge pressures, increased pulmonary vascular resistance (PVR) and decreased cardiac index (CI).
He was aggressively diuresed and put on inhaled epoprostenol but later had to be switched to intravenous epoprostenol. After volume optimization, PAC numbers showed hemodynamics suggestive of pre-capillary PH with normal wedge pressure (Table 1). These findings, along with his extreme hypoxia, radiographic imaging and timeline from exposure to MMC raised concerns for possible MMC induced PVOD. Lung biopsy was not an option given his markedly elevated pulmonary artery pressures and hypoxemia. Despite our high clinical suspicion for PVOD, an informed and shared decision was made to try pulmonary vasodilators as the only other option was comfort measures. He fortunately responded well. His shock resolved with reduced oxygen requirements and after closely monitoring him in the ICU for a few more days, he was discharged home on supplemental oxygen, sildenafil, furosemide and intravenous epoprostenol. He unfortunately got re-admitted a week later in cardiogenic shock and succumbed to his disease. Genetic studies were negative for eukaryotic translation initiation factor 2 alpha kinase 4 (EIF2AK4) and bone morphogenetic protein receptor type II (BMPR2).
Table 1.
Shows pulmonary artery catheter readings on admission and after volume optimization but while still on a small dose of norepinephrine.
| PAC Variables (Normal PAC Values) | Admission PAC readings | PAC numbers after volume optimization |
|---|---|---|
| Right atrial pressure (mmHg) | 10 | 0 |
| Pulmonary artery pressure (mmHg) | ||
| Systolic | 97 | 54 |
| Diastolic | 48 | 18 |
| Mean | 63 | 30 |
| Pulmonary capillary wedge pressure | 17 | 12 |
| Cardiac output | 2.65 | 3.69 |
| Cardiac index | 1.62 | 2.27 |
| Pulmonary vascular resistance-calculated (dyn/sec/cm−5) | 1389 | 390 |
| Systemic vascular resistance (dyn/sec/cm−5) | 1680 | 2081 |
| Mixed venous oxygen saturation | 20 | 57 |
Postmortem autopsy done showed slight cardiomegaly, dilated hypertrophy of right ventricle, left ventricular dilatation and pericardial effusion. Histopathologic findings of the lungs showed extensive interstitial fibrosis and organizing pneumonia involving both lungs as well as centrilobular emphysema, alveolar edema, pleural effusions, organizing bronchopneumonia, subpleural infarcts and thrombotic microangiopathy (small vessel thromboemboli). Based on the clinical history and autopsy findings his cardiac arrest was deemed to be secondary to right heart failure from pulmonary hypertension and PVOD secondary to MMC.
3. Discussion
PVOD is a rare form of pulmonary hypertension characterized by obliteration of small pulmonary veins by fibrous intimal thickening and patchy capillary proliferation [3]. It is often misclassified as pulmonary arterial hypertension (PAH) in about 10% of cases as they share similar clinical presentation with features of severe precapillary PH. It is however important to differentiate these two conditions as PVOD carries a much worse prognosis [1].
PVOD may be idiopathic, heritable, drug/toxin induced, or it may develop in association with connective tissue diseases among other causes. Eyries et al., in 2013, discovered biallelic mutations in the EIF2AK4 gene as the cause of heritable PVOD and PCH(4). Current evidence therefore supports the concept that PVOD and PCH are in fact varied expressions of the same disorder(1). Regardless of etiology, PVOD tends to have poor prognosis and poor response to typical PAH therapies. Certain et al., in a small retrospective study, looked at characteristics and long-term outcomes of PVOD induced by MMC and came to the conclusion that ‘like other forms of PVOD, prognosis remains dismal despite MMC withdrawal and PAH-specific therapy’. However, they noted that whereas sporadic PVOD is male predominant and may be associated with mutations in EIF2AK4, MMC-PVOD is female predominant and has no association with EIF2AK4 mutations [4,5].
Clinical findings suggestive of PVOD are similar to PAH and include insidious onset of fatigue and breathlessness progressing to symptoms of right heart failure in end-stage disease, out-of-proportion dyspnea, hypoxemia, and low diffusion capacity of carbon monoxide (DLCO) on pulmonary function tests [6]. Definitive diagnosis of PVOD, regardless of etiology requires lung biopsy which carries a high risk of life-threatening complications in these patients. A noninvasive diagnostic approach is therefore favored. Decreased diffusing capacity of the lung for carbon monoxide, severe hypoxemia, radiological abnormalities on high-resolution chest CT, and the occurrence of pulmonary edema after starting pulmonary vasodilator therapy strongly argue for PVOD. The presence of two or three radiological abnormalities including mediastinal lymph node enlargement, interlobular septal thickening (Kerley B lines), and centrilobular ground-glass opacities had a sensitivity of 75% and a specificity of 84.6% for the detection of PVOD in a retrospective study by Montani et al., 2008 [6]. Ogawa et al., in 2018, based off a small retrospective study, also published a novel scoring system designed to clinically help diagnose PVOD/PCH: male sex, smoking history, 6-minute walk distance <285 m, minimum SpO2 < 92% during the 6-minute walk test (6MWT), %DLCO < 34%, GGO plus thickening of the interlobular septa on high-resolution CT, defects in the perfusion lung scan, and pulmonary edema due to vasodilators. They predicted a score of ≥5 points had 95% sensitivity and 98% specificity to predict PVOD/PCH (area under the curve: 0.991; 95% CI: 0.976–1.000) [7]. By their criteria, our patient had a score of 5 even though we did not have some of these variables like DLCO and 6MWT due to the patient presenting with such acuity in shock.
Other suggestive CT findings include enlarged central pulmonary arteries and pleural effusions [8,9]. Bronchoalveolar lavage (BAL) may show increased hemosiderin-laden macrophages as occult alveolar hemorrhage tends to occur in these patients due to the presence of post-capillary block [10].
MMC is an alkylating agent commonly used in chemotherapy. Lung toxicity and PVOD are documented in patients taking MMC [[11], [12], [13]] and the incidence of PVOD in patients with anal cancer is higher than in those with idiopathic PVOD, especially following treatment with MMC [2]. To confirm a causal link between MMC and human PVOD, Perros et al., in 2015 found that in rats, intraperitoneal administration of MMC induced PVOD, as demonstrated by pulmonary hypertension at right-heart catheterization at days 21–35 and major remodeling of small pulmonary veins associated with foci of intense microvascular endothelial-cell proliferation of the capillary bed. They also evaluated 7 cases of PVOD induced by MMC therapy from the French Pulmonary Hypertension Registry. These patients had received 2 to 4 cycles of chemotherapy except for one who also received 8 cycles of 5-fluorouracil plus oxaliplatin [2]. The average interval between the end of chemotherapy with MMC and the diagnosis of PVOD was four months (range: 2–12 months). It was about 11 months in the case of our patient. They also demonstrated in a follow up publication that MMC exposed lungs displayed areas of exuberant microvascular endothelial cell proliferation which mimics pulmonary capillary hemangiomatosis, one of the pathologic hallmarks of human PVOD.
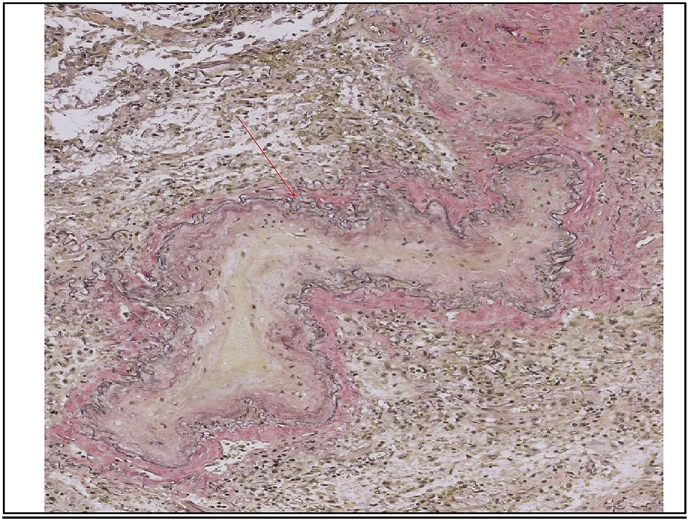
Histopathological features of PVOD include prominent alveolar septa, which are thickened secondary to capillary congestion. Evidence of old hemorrhage may be found in intra-alveolar hemosiderin-laden macrophages and interstitial hemosiderin. Interstitial fibrosis and focal interstitial lymphocytic infiltrates may be seen. These findings give the appearance of a chronic fibrosing interstitial process at low power. At higher power, examination of small and medium-sized veins shows fibrous intimal thickening by dense collagen or by edematous, loose connective tissue within the intima and media. Venous lumina are greatly narrowed or show total fibrous occlusion (Slide 1). Additional associated features include arterial hypertrophy, arterial thrombi, venous infarcts, and lymphatic dilation [14].
Slide 1.

Postmortem histopathology section from our patient showing a vein with its lumen significantly narrowed by fibrous intimal thickening.
For our patient, extensive interstitial fibrosis was reported in the lung microscopic sections on the autopsy report. Additionally, subpleural infarcts, thrombotic microangiopathy, and small vessel thromboemboli were noted. Fibrous intimal thickening of veins was shown with an elastin stain (Slide 2). Elastin stains are crucial in diagnosing PVOD as they highlight the single external elastic membranes of veins and the internal and external elastic membranes of arteries. This is important as completely obliterated veins can sometimes only be appreciated with these special stains. This helps to distinguish PVOD from usual interstitial pneumonia (UIP) and other hemorrhagic and fibrosing processes [14].
Slide 2.
Elastin stain outlining the external elastic laminae of this vein in black. Fibrous intimal thickening is present. Red arrow: external elastic laminae of vessel.
No evidence-based medical therapy exists for PVOD at present and lung transplant remains the preferred definitive therapy for eligible patients. The mean time from diagnosis to death or lung transplantation was 11.8 months, and the mean time from the first reported symptom to death or lung transplantation was 24.4 months in a study by Montani et al., 2008 [5]. Exposure to pulmonary vasodilators used to treat PAH may precipitate acute pulmonary edema in patients with PVOD but can be used with extreme caution and close monitoring [15]. The alveolar edema seen on our patient's autopsy could have been due to the pulmonary vasodilator he was on (edema can occur weeks or even months after treatment initiation) or more likely because he received a significant amount of fluid bolus for shock when he presented to the outside hospital the second time.
4. Conclusion
PVOD is an uncommon form of PH that is usually difficult to diagnose and is refractory to treatment with poor prognosis. It should be considered in cancer patients with PH, extreme hypoxemia, and suggestive CT findings in the setting of exposure to MMC. It is equally important to differentiate PVOD from other causes of PAH as standard therapy for PAH can be detrimental in PVOD if not used with caution. Lung transplant is the recommended treatment. Our case is particularly challenging because the patient presented to our center in such acuity and we could not get some tests that we would otherwise get when patients are more stable. There are very few reported cases of mitomycin induced PVOD (possibly under recognized) and we hope to increase the awareness with this report to facilitate diagnosis and expedite management because of the poor outcomes.
Declaration of competing interest
None.
Authors have no conflicts of interest to declare.
References
- 1.Montani D., Lau E., Dorfmüller P., Girerd B., Jaïs X., Savale L. Pulmonary veno-occlusive disease. Eur. Respir. J. 2016;47(5):1518–1534. doi: 10.1183/13993003.00026-2016. [DOI] [PubMed] [Google Scholar]
- 2.Perros F., Günther S., Ranchoux B., Godinas L., Antigny F., Chaumais M. Mitomycin-induced pulmonary veno-occlusive disease. Circulation. 2015;132(9):834–847. doi: 10.1161/circulationaha.115.014207. [DOI] [PubMed] [Google Scholar]
- 3.Mandel J., Mark E., Hales C. Pulmonary veno-occlusive disease. Am. J. Respir. Crit. Care Med. 2000;162(5):1964–1973. doi: 10.1164/ajrccm.162.5.9912045. [DOI] [PubMed] [Google Scholar]
- 4.Eyries M., Montani D., Girerd B., Perret C., Leroy A., Lonjou C. EIF2AK4 mutations cause pulmonary veno-occlusive disease, a recessive form of pulmonary hypertension. Nat. Genet. 2013;46(1):65–69. doi: 10.1038/ng.2844. [DOI] [PubMed] [Google Scholar]
- 5.Certain M., Chaumais M., Jaïs X., Savale L., Seferian A., Parent F. Characteristics and long-term outcomes of pulmonary veno-occlusive disease induced by mitomycin C. Chest. 2021;159(3):1197–1207. doi: 10.1016/j.chest.2020.09.238. [DOI] [PubMed] [Google Scholar]
- 6.Montani D., Achouh L., Dorfmüller P., Le Pavec J., Sztrymf B., Tchérakian C. Pulmonary veno-occlusive disease. Medicine. 2008;87(4):220–233. doi: 10.1097/md.0b013e31818193bb. [DOI] [PubMed] [Google Scholar]
- 7.Ogawa A., Takahashi Y., Matsubara H. Clinical prediction score for identifying patients with pulmonary veno-occlusive disease/pulmonary capillary hemangiomatosis. J. Cardiol. 2018;72(3):255–260. doi: 10.1016/j.jjcc.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 8.Pulmonary hypertension: CT of the chest in pulmonary veno-occlusive disease. AJR Am. J. Roentgenol. 2004;183:65–70. doi: 10.2214/ajr.183.1.1830065. [DOI] [PubMed] [Google Scholar]
- 9.Frazier A., Franks T., Mohammed T., Ozbudak I., Galvin J. Pulmonary veno-occlusive disease and pulmonary capillary hemangiomatosis. Radiographics. 2007;27(3):867–882. doi: 10.1148/rg.273065194. [DOI] [PubMed] [Google Scholar]
- 10.Rabiller A. Occult alveolar hemorrhage in pulmonary veno-occlusive disease. Eur. Respir. J. 2013;27:108–113. doi: 10.1183/09031936.06.00054105. 2006. [DOI] [PubMed] [Google Scholar]
- 11.Botros L., Van Nieuw Amerongen G., Noordegraaf A., Bogaard H. Recovery from mitomycin-induced pulmonary arterial hypertension. Annals of The American Thoracic Society. 2014;11(3):468–470. doi: 10.1513/annalsats.201312-426le. [DOI] [PubMed] [Google Scholar]
- 12.Khalid H., Damjanov I., Latham H. Mitomycin induced pulmonary veno-occlusive disease in a patient with carcinoma of the breast. Kansas Journal of Medicine. 2013;6(1):31–33. doi: 10.17161/kjm.v6i1.11433. [DOI] [Google Scholar]
- 13.Joselson R., Warnock M. Pulmonary veno-occlusive disease after chemotherapy. Hum. Pathol. 1983;14(1):88–91. doi: 10.1016/s0046-8177(83)80052-5. [DOI] [PubMed] [Google Scholar]
- 14.Hoda S., D'Alfonso T. Sternberg's diagnostic surgical Pathology. Adv. Anat. Pathol. 2015;22(3):225. doi: 10.1097/pap.0000000000000070. [DOI] [Google Scholar]
- 15.Montani D., O'Callaghan D. Pulmonary veno-occlusive disease: recent progress and current challenges. Respir. Med. 2010 doi: 10.1016/j.rmed.2010.03.014. [DOI] [PubMed] [Google Scholar]