Abstract
Children have often been treated as small adults in relation to drug formulation, but research has now shown this not to be the case. Therefore, there is a push from regulatory bodies to provide drug formulations specifically tailored towards the needs of this fragmented population. Orally dissolving films (ODFs) have been identified as an emerging opportunity, to bridge this gap. Therefore, the aim of this study was to prepare ODFs containing topiramate, an antiepileptic drug, using solvent casting method as a potential alternative to oral tablets/powders for paediatrics. For this purpose, a Design of Experiment (DoE) was employed to optimise formulation parameters. 24 formulations were prepared by changing the polymer type (HPMC, Guar-Gum or PEO), concentration (0.4%-1.2%w/v); plasticizer type (glycerol\sorbitol) and concentration (0.1–0.3%w/v). Disintegration time, content-uniformity, film quality and thickness uniformity were the responses. Surface and molecular profiling were conducted on the optimal formulation (N4). TGA and XRD results demonstrated the stability of materials upon production into films, while the SEM images showed smooth films that proved to be resilient due to good mechanical properties. HPMC-glycerine based ODFs are presented as an effective dosage form to enhance the ease of administration and patient compliance of topiramate, specifically for paediatric patients.
Keywords: Orally dissolving films, Drug load, Pediatric formulations, Topiramate, Solvent casting, Design of experiment (DoE)
1. Introduction
The European Medicines Agency (EMA) has recognised the need for legal guidance for the development of medicines for paediatric use, since medications intended for paediatric patients have a number of challenges associated with them. These challenges are pertinent to dose flexibility, high load efficiency of above 30%, dose accuracy, overcoming dysphagia and developing user friendly dosage forms; which are capable of meeting the needs of a population with diverse physiological characteristics and levels of maturity; and to ensure patient adherence (such as with taste masking) (Ivanovska et al., 2014).
Children differ from adults in relation to pharmacotherapy, including capabilities for drug administration, drugs toxicity, and taste preferences. A focus on novel preparations is required particularly upon the changes in regulations in 2007. Where it became important that paediatric drugs be formulated to best suit a child’s age, size, physiological condition, and treatment requirements. To ensure suitable treatment of all children, different routes of administration, dosage forms, and strengths may be required. Many formulations are not suitable for children, therefore, the selection of an all encompassing paediatric dosage form is challenging (Hanning et al., 2016, Lopez et al., 2015). One of the challenges stem from the use of inactive ingredients (excipients) which although generally regarded as safe (GRAS) in adults, have not been validated for pediatric use. Some excipients commonly used in adult medicines showed elevated toxicity and safety issues in children, especially neonates (Cuzzolin, 2018, Ivanovska et al., 2014, Turner et al., 2014). Therefore paediatric drug formulation development is associated with numerous challenges, including methodologic and ethical requirements for pediatric trials, high developmental costs, and a small and fragmented market. As a result of these challenges, there have only been limited research efforts to adapt medicines according to paediatric needs (Ivanovska et al., 2014).
Orally dissolving films (ODFs) are a type of oral drug delivery system that was developed based on transdermal patch technology. This delivery system consists of a thin film, which is simply placed on the patient’s tongue or mucosal tissue, instantly gets wetted by saliva, then rapidly disintegrates and dissolves to release the medication for oral mucosal absorption. This fast dissolving action is primarily due to the large surface area of the film, which wets quickly when exposed to oral moisture. ODFs, extensively reviewed in literature, improve the efficacy of the active pharmaceutical ingredient (API) by dissolving in short duration in the oral cavity after contact with less amount of saliva as compared to dissolving tablets (Abay and Ugurlu, 2015, Ouda et al., 2020, Saab and Mehanna, 2019, Thakur et al., 2013). ODFs offer several advantages such as ease of management for pediatrics, they do not require water for swallowing – a very convenient feature for patients who travel. They possess a good mouth feel and therefore help to change the perception of the medicine as a bitter medicine, especially for paediatric patients. Some drugs may be absorbed from the mouth, pharynx and oesophagus as the saliva passes down into the stomach, which enhances bioavailability of drugs. This pre-gastric absorption can lead to improved bioavailability resulting in dose reduction. The risk of suffocation or choking during oral administration of conventional solid formulations due to physical obstruction is avoided, thus providing improved safety. ODFs are useful in cases where a rapid onset of action is required. ODFs demonstrate stability for longer periods of time, since the drug remains in solid state until it is consumed. Therefore, ODFs combine the advantages of solid dosage forms in terms of stability and liquid dosage forms in terms of bioavailability (Ouda et al., 2020, Patil and Shrivastava, 2014, Saab and Mehanna, 2019).
Epilepsy is a relatively common condition in children, where its prevalence is approximately 3.2–5.5 per 1000 children in the developed world. It has been estimated that 12–39% of children with epilepsy also have some form of attention deficit hyperactivity disorder (ADHD) (Curatolo et al., 2017). Topiramate (TPM) was approved for treatment of epilepsy in the US in 1995. It is a sulfamate substituted monosaccharide with a novel combination of pharmacological properties. Pharmacokinetically, TPM shows many desirable characteristics such as rapid absorption, minimal binding to plasma proteins, no enzymatic auto induction and linear kinetics. However, its metabolism is induced by phenytoin and carbamazepine. Hence, concomitant administration of these antiepileptic drugs accelerates TPM elimination rate and decreases its plasma concentration by 50% (Pellock and Watemberg, 1997). Topiramate use in pediatrics has been established, where it is effective and well tolerated in children under the age of 12 years in many epileptic conditions, including refractory partial epilepsy and symptomatic and myoclonic generalized epilepsy (Mikaeloff et al., 2003). Topiramate is a crystalline solid with melting point of 125–126 °C, water solubility reaching 9.8 mg/mL, with bitter taste. The saturated solution has a pH of 6.3 (PubChem, 2019). According to the biopharmaceutical classification system (BCS), topiramate is classified as Class I drug (high solubility and high permeability at the maximum dose) (EMA, 2013, Talati et al., 2012). It has a recommended therapeutic dose of 25 mg once or twice daily for children 6–17 years old, and can go higher according to the classification and severity of the condition (Mikaeloff et al., 2003). The currently available paediatric dosage form is based on capsules that are opened and sprinkled on soft food then swallowed (BNF, 2017). Therefore, the provision of topiramate as ODFs may provide a more convenient dosage form for patients.
Based on the foregoing, the aim of this work was to develop and characterise paediatric-centric orally disintegrating film formulations containing 25 mg TPM per film, as a novel, safe and acceptable dosage forms for treatment of epilepsy in children using design of experiments (DoE) mathematical approach. Different polymers (Hydroxypropyl methyl cellulose, guar gum, polyethylene oxide) and plasticizers (glycerine or sorbitol) were used over a range of concentrations.
2. Materials and methods
2.1. Materials
Topiramate, hydroxypropyl methyl cellulose K100 (HPMC), guar gum (GG), polyethylene oxide (molecular weight 100,000 Da) (PEO) glycerine, sorbitol, potassium dihydrogen phosphate, and ethanol (absolute HPLC grade) were purchased from Sigma Aldrich (Pool, UK). Disodium hydrogen phosphate, sodium chloride and phosphoric acid were purchased from AZ Chemicals, Inc. (ON, Canada). Distilled water was used in all experiments. All chemicals were used as received.
2.2. Methods
2.2.1. Preparation of topiramate loaded orally dissolving films
The topiramate loaded ODFs were prepared using solvent casting method according to the method described by (Ouda et al., 2020). Initially, the accurately weighed film-forming polymer (HPMC, PEO or GG) was dissolved in 50 ml distilled water at temperature of 35–40 °C using a hotplate-magnetic stirrer (Dragonlab- MS-H-S, China), stirred at 1250 rpm, and then allowed to stand for 2 h for swelling and complete hydration. Then, the plasticizer (glycerine or sorbitol) was added to the polymer solution and stirred at 1250 rpm and 40 °C to obtain a homogenous solution. The polymeric solution was kept for 45–60 min in an ultrasonic bath [(Sonorex Digitec (BANDELIN electronics, GmbH)] set at medium speed to enable the removal of bubbles. Next, an accurately weight of topiramate powder was added to 40 ml of distilled water and stirred on magnetic stirrer at 1000 rpm till complete dissolution. The topiramate solution was then added slowly to the polymeric solution and stirred to form a homogenous solution. The final volume was adjusted to 100 ml with distilled water, then was degassed using the ultrasonic bath. After degassing, the solution was casted on plastic Petri dishes (25 ml per dish) and dried at room temperature for 24 hrs, to avoid quick drying and film deformation, then in an oven at 40 °C for 10 hrs. The films were carefully removed from the surface and cut into the rectangles of 3 × 2 cm2 per film. The films were stored in a dry place for further analysis. Results of our previous work (Ouda et al., 2020) employing different polymers and plasticizers showed successful films with polymer concentration around 0.6–1% and plasticizer concentration of 0.1–0.2%. This study aimed at optimizing the polymer and plasticizer types and concentrations, therefore, the employed polymer concentrations were 0.4%, 0.8% or 1.2%w/v. The plasticizer concentrations were 0.1%, 0.2% and 0.3% w/v. Table 1 summarizes the concentrations used in this study.
Table 1.
Summary of the Composition of Topiramate loaded ODFs Highlighting Polymer Type (HPMC, PEO or GG) and Concentration; Plasticizer type (Sorbitol or Glycerine) and concentrations.
| Range | Concentration [%w/v] per Batch |
Amount (mg) per ODF 2*3 (6 cm2)] Based on Dry Weight |
Film Weight (mg)) | ||||
|---|---|---|---|---|---|---|---|
| Polymer | Plasticizer | Topiramate | Polymer | Plasticizer | Topiramate | ||
| −1 | 0.4 | 0.1 | 0.9 | 11.1 | 2.8 | 25.0 | 38.9 |
| 0 | 0.8 | 0.2 | 0.9 | 22.2 | 5.6 | 25.0 | 52.8 |
| 1 | 1.2 | 0.3 | 0.9 | 33.33 | 8.33 | 25.0 | 66.7 |
2.2.2. Design of experiment (DoE)
The statistical analytical technique was performed using MODDE software version 12.1 (Umetrics Inc., Sweden). D-optimal design for the DoE with quadratic model was selected, which was further fitted using partial least squares (PLS) method. Whereas response surface modelling (RSM) was employed to investigate and optimize the non-linear multidimensional relationship between factors and responses. A total of 24 experiments were produced, that include triplicate runs to evaluate the repeatability and error estimation. To fit the quadratic model. The experiments were conducted depending on proposed run order which was given by the software; so, the randomness of the process could be assessed. Table 2 specifies the factors and responses which were used in the DoE, respectively.
Table 2.
List of factors and responses with their details that were employed in the DoE study.
| Factor | Abbr. | Units | Type | Settings | Precision |
|---|---|---|---|---|---|
| Polymer type | Pol | Qualitative | HPMC, PEO, GG | ||
| Polymer concentration* | PC | % | Quantitative | −1 to 1 | 0.05 |
| Plasticizer type | Pla | Qualitative | Glycerine, Sorbitol | ||
| Plasticizer concentration** | PLC | % | Quantitative | −1 to 1 | 0.05 |
| Responses | Abbr. | Units | Min | Target | Max |
| Disintegration time | Dis | Sec | 5 | 20 | 60 |
| Film Quality | Fil | % | 0 | 80 | 100 |
| Content uniformity | Con | % | None | 80 | 100 |
| Thickness Uniformity RSD | Thi | % | None | 1 | 2 |
*Polymer concentration %w/v: −1 = 0.4%, 0 = 0.8%, +1 = 1.2%.
**Plasticizer concentration %w/v: −1 = 0.1%, 0 = 0.2%, +1 = 0.3%.
In the design, none of the factors or responses underwent transformation of values and hence the type was regular. However, various proportions of the polymer concentration and plasticizer concentration were encoded in the D-optimal design as −1, 0 or 1 that stand for the lowest value, intermediate value, and the highest value respectively. Table 3 summarizes the D-optimal design worksheet with the proportions of factors, the total number of runs as well as the run order.
Table 3.
The D-optimal design worksheet with factors, responses the total number of runs as well as the run order (Inc/Ecl: inclusion and exclusions; Gly: glycerine; Sor: Sorbitol), *are the used responses in the DoE study.
| Exp No | Exp Name | Run Order | Incl/Excl | Polymer type | Polymer Concentration (%w/v) | Plasticizer type | Plasticizer Concentration (%w/v) | Disintegration time (s)* | Film Quality |
Content uniformity (%)* | Thickness(mm) | Thickness Uniformity RSD (%)* | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Film flexibility (%) | Spreadability (%) | Stickiness(%) | Easy to peel off (%) | Smooth(%) | Total Film Quality (%)* | ||||||||||||
| 1 | N1 | 6 | Incl | HPMC | −1 | Gly | 0 | 10 | 0 | 10 | 10 | 10 | 10 | 40 | 90.3 | 0.061 | 5.8 |
| 2 | N2 | 11 | Incl | HPMC | 1 | Gly | 0 | 75 | 20 | 20 | 20 | 20 | 20 | 100 | 94.3 | 0.074 | 1.03 |
| 3 | N3 | 16 | Incl | HPMC | 0 | Gly | −1 | 28 | 10 | 20 | 20 | 10 | 20 | 80 | 95.6 | 0.068 | 2.2 |
| 4 | N4 | 15 | Incl | HPMC | 0 | Gly | 1 | 30 | 20 | 20 | 20 | 20 | 20 | 100 | 94.7 | 0.066 | 1.95 |
| 5 | N5 | 13 | Incl | PEO | 1 | Gly | −1 | 90 | 10 | 20 | 20 | 10 | 20 | 80 | 93.6 | 0.078 | 3.6 |
| 6 | N6 | 7 | Incl | PEO | 1 | Gly | 1 | 86 | 20 | 20 | 20 | 20 | 20 | 100 | 94.6 | 0.079 | 2.5 |
| 7 | N7 | 17 | Incl | PEO | −1 | Gly | 0 | 35 | 10 | 20 | 10 | 10 | 10 | 60 | 93.9 | 0.071 | 5.67 |
| 8 | N8 | 12 | Incl | GG | −1 | Gly | −1 | 27 | 0 | 10 | 0 | 0 | 0 | 10 | 82.6 | 0.070 | 6.8 |
| 9 | N9 | 24 | Incl | GG | −1 | Gly | 1 | 25 | 0 | 10 | 0 | 0 | 0 | 10 | 82.7 | 0.073 | 8.7 |
| 10 | N10 | 20 | Incl | GG | 1 | Gly | 0 | 60 | 0 | 20 | 10 | 0 | 10 | 40 | 87.5 | 0.076 | 5.9 |
| 11 | N11 | 10 | Incl | HPMC | −1 | Sor | −1 | 21 | 10 | 10 | 0 | 10 | 10 | 40 | 98.4 | 0.061 | 3.1 |
| 12 | N12 | 21 | Incl | HPMC | 1 | Sor | −1 | 80 | 10 | 20 | 20 | 20 | 10 | 80 | 95.9 | 0.070 | 1.76 |
| 13 | N13 | 19 | Incl | HPMC | −1 | Sor | 1 | 20 | 10 | 20 | 10 | 0 | 0 | 40 | 98.6 | 0.063 | 2.87 |
| 14 | N14 | 23 | Incl | HPMC | 1 | Sor | 1 | 77 | 20 | 20 | 10 | 10 | 0 | 60 | 97.6 | 0.075 | 1.96 |
| 15 | N15 | 9 | Incl | HPMC | 0 | Sor | 0 | 44 | 20 | 20 | 20 | 10 | 10 | 80 | 105 | 0.069 | 2.3 |
| 16 | N16 | 4 | Incl | PEO | −1 | Sor | −1 | 26 | 0 | 10 | 10 | 0 | 0 | 20 | 94.2 | 0.065 | 3.87 |
| 17 | N17 | 2 | Incl | PEO | −1 | Sor | 1 | 18 | 10 | 10 | 10 | 10 | 0 | 40 | 96.7 | 0.069 | 4.65 |
| 18 | N18 | 22 | Incl | PEO | 1 | Sor | 0 | 63 | 10 | 20 | 10 | 10 | 10 | 60 | 89.9 | 0.080 | 5.65 |
| 19 | N19 | 1 | Incl | GG | 1 | Sor | −1 | 50 | 10 | 20 | 10 | 0 | 0 | 40 | 84.3 | 0.078 | 9.45 |
| 20 | N20 | 8 | Incl | GG | 1 | Sor | 1 | 47 | 10 | 20 | 10 | 0 | 0 | 40 | 85.9 | 0.080 | 6.65 |
| 21 | N21 | 18 | Incl | GG | −1 | Sor | 0 | 30 | 0 | 10 | 0 | 0 | 0 | 10 | 106.1 | 0.066 | 8.76 |
| 22 | N22 | 14 | Incl | GG | 0 | Sor | 0 | 35 | 0 | 20 | 0 | 0 | 0 | 20 | 104.3 | 0.069 | 8.97 |
| 23 | N23 | 3 | Incl | GG | 0 | Sor | 0 | 35 | 0 | 20 | 0 | 0 | 0 | 20 | 105.7 | 0.068 | 9.21 |
| 24 | N24 | 5 | Incl | GG | 0 | Sor | 0 | 32 | 0 | 20 | 0 | 0 | 0 | 20 | 101.5 | 0.068 | 7.45 |
2.2.3. UV–VIS spectrophotometric assay method for topiramate
The amount of topiramate loaded in, or released from, the ODFs was quantified using a UV–VIS spectrophotometer method. Maximum wavelength of topiramate was determined using Thermo Fisher Scientific Double Beam UV–VIS Spectrophotometer with maximum detected at 264 nm. 50 mg of topiramate was accurately weighed, transferred into a 100 ml volumetric flask. The final volume was adjusted to 100 ml with ethanol (for content uniformity studies) or water (for the dissolution study). Serial dilutions were made from the solution using ethanol or water to obtain the following concentrations: 500 μg/ml, 250 μg/ml, 125 μg/ml, 62.5 μg/ml, and 31.25 μg/ml, 15.625 μg/ml. Samples were analyzed using UV– VIS spectrophotometer at 264 nm using ethanol as blank. Experiments were performed in triplicate and repeated three times on consecutive days. The data were plotted as absorbance verses concentration and regression coefficient was calculated from the straight-line equation. The method was validated according to the International Conference on Harmonization (ICH) guidelines (Validation of Analytical Procedures Q2(R1)) in terms of specificity, accuracy, precision, linearity and limit of detection/quantification (ICH, 2005). Linearity was over a range of 0–125 µg/ml (for water and ethanol), LOD and LOQ were 3.00 µg/ml and 9.09 µg/ml respectively for ethanol based data and 3.76 µg/ml and 11.02 µg/ml for water based data respectively. None of the excipients interfered with the absorbance at the used higher concentrations.
2.2.4. Preparation of artificial saliva
The artificial saliva solution was prepared according to the method in (Ouda et al., 2020). To prepare a litre of artificial saliva 2.382 g of di-sodium hydrogen phosphate was dissolved in 500 ml distilled water. Then 0.190 g of potassium dihydrogen phosphate (0.019%) and 8 g of sodium chloride (0.8%) were added to form a homogenous saliva solution. The final volume was adjusted to 1 L using distilled water. The pH of the solution was further adjusted to 6.75 with phosphoric acid and was used as test medium for disintegration.
2.2.5. Characterization of the ODFs
The prepared ODFs for all the produced formulations N1-N24 were characterized using various techniques including disintegration time, content uniformity, film quality, and thickness uniformity. Selected formulations that demonstrated good properties were further analysed using in-vitro dissolution profile, SEM, mechanical properties, XRD, and TGA.
2.2.5.1. Film quality
The quality of the produced films was assessed based on 5 criteria. Each criterion was given 20% of the total value. The criteria include 1) film flexibility (endurance >30 times), 2) good spreadability upon pouring onto the casting tray, 3) not sticky film, 4) easy to peel off casting tray, and 5) smooth appearance. Table 4 summarise the scoring criteria.
Table 4.
Scoring Criteria for ODF highlighting the distribution of scores.
| Elements | Scoring Scheme |
||
|---|---|---|---|
| Score (%) |
|||
| Poo (0%) | Average (10%) | Good (20%) | |
| Film flexibility (endurance >30 times) | <20 times | 20–30 | >30 times |
| Spreadability upon pouring onto the casting tray | Does not spread | Spread with aid of spatula | Spread easily without aid |
| Not sticky film | Very sticky that could not be handled easily | Some stickiness but could be handled | Not sticky |
| Easy to peel off the casting tray | Could not be peeled off | Peeled off but takes time | Peeled off easily |
| Smooth appearance | Surface not smooth with irregularity or patches | Smooth surface with some irregularities | Very smooth surface |
2.2.5.2. Disintegration time
Disintegration test was performed based on our previously reported method (Ouda et al., 2020). Since there is no specific time limit for rapidly dissolving ODFs in compendial references, the orally dissolving time frame was employed to assess ODFs (Ph.Eur, 2010, Saab and Mehanna, 2019). The 180 s limit identified by European Pharmacopeia was employed with a set target of 60 s. The film was placed in a beaker containing 10 ml of artificial salvia (pH 6.75). To simulate in vivo conditions the test was performed in 10 ml of artificial saliva. The ODF was placed horizontally in a 30 ml beaker with 10 ml of artificial salvia media. The beaker was placed in a larger beaker containing 100 ml distilled water, and the temperature was maintained at 37 °C using hot plate. Magnetic stirrer was used set at 10 rpm. All studies were performed in triplicate for each formulation. The disintegration time was determined when the film dissolved or fragmented into small pieces.
2.2.5.3. Content uniformity
One ODF was dissolved in ethanol and volume completed to 50 ml ethanol. The solution was sonicated without heat for 10 min to ensure complete dissolution of the active ingredient. From this solution, 1 ml was diluted with 1 ml of ethanol, then the sample was filtered using syringe filter (0.45 µm), then the absorbance was measured using a UV spectrophotometer at 264 nm for topiramate using ethanol as blank. Experiments were performed in triplicate for each formulation and the average values were recorded.
2.2.5.4. Thickness uniformity
The uniformity of thickness for the ODFs was carried out using 3 films per formulation. Each film (3 × 2 cm) was taken randomly, and the thickness was measured from five locations (4 corners and the centre) using Kendo digital calliper (Shanghai, China). The mean and relative standard deviation (RSD) were calculated, and RSD reported.
2.2.5.5. Thermogravimetric analysis (TGA)
The TG curves were obtained using a Thermogravimetric Analyser Pyris 1 TGA instrument, (Perkin Elmer, USA). Pyris manager software was used to analyse the results. A sample mass 5 to 10 mg was used in the test. Samples were held for 2 min at 25 °C then measurements were obtained from 25 to 800 °C under nitrogen atmosphere. The heating rate was set at 10 °C/min.
2.2.5.6. Scanning electron microscope (SEM)
SEM images were obtained using a JSM-IT300 (Manufacture JEOL, JAPAN). A 2x2 mm piece of the film was mounted on a double adhesive carbon tape placed on an aluminium tub. Samples were analysed at low vacuum without further coating.
2.2.5.7. X-ray diffraction (XRD)
X-ray diffractions were obtained for targeted ODF sample using X-ray diffractometer (D8 Phaser, Bruker AXS GmbH, Germany). The X-ray generator was operated at 30 kV and 10 mA employing Co tube at λ 1.79026 Å as a radiation source and using LYNXEYE detector. The angular range (2θ) varied from 4 to 50° at a scanning rate of 0.02° 2θ s−1 and measured at 0.24 s/step. The diffraction patterns were produced as counts per step which were analysed using Topas software (Bruker, AXS).
2.2.5.8. Mechanical properties
The mechanical properties of the ODFs were evaluated using Instron universal testing machine (Instron, USA), with load cell 50 N. Films with size 3x2 cm2 were attached on two clamps at the distance at 30 mm. These films were pulled by two clamps at rate of 50 mm/min. The parameters of mechanical properties including tensile strength and % elongation were assessed. Three samples per batch were used and results are reported as mean ± SD. Tensile strength was measured using the Eq. (1) (Ouda et al., 2020):
| (1) |
Whereas % elongation was determined by the following equation (Eq. (2)):
| (2) |
2.2.5.9. In vitro dissolution studies
The release profile of a selected ODF formulation was conducted using the USP type II (paddle) dissolution apparatus with distilled water that was maintained at 37 ± 0.5 °C. The dissolution medium was stirred at 100 rpm. 5 ml samples were taken at 5 min interval for 30 min. Distilled water was added to replace the withdrawn samples. Amount of topiramate was determined by UV spectrophotometer after filtration using syringe filters (0.45 µm). The percent drug released was plotted against time. Results are reported in triplicates with mean and standard deviation.
2.2.6. Statistical analysis
All data generated in replicates were analysed and presented as mean and standard deviation (SD) or relative standard deviation (RSD) using Minitab v. 18. Level of significance was quoted as p < 0.05, with a confidence interval of 95%.
3. Results and discussion
3.1. Design of experiment (DoE)
The aim of this work was to optimize formulation parameters for the development of orally dissolving films (ODFs) containing topiramate. Theoretically, a successful film should establish excellent content uniformity, exhibit adequate mechanical strength and integrity, and provide fast disintegration upon hydration. Good mechanical strength and film integrity can be controlled by increasing the polymer concentration, which will increase the film thickness, and optimizing the plasticizer concentration, whereas the disintegration time is affected by the film thickness and the ratio of polymer concentration to other components. Therefore, critical responses selected as dependent variables (responses) were disintegration time, film thickness uniformity, content uniformity and film quality.
HPMC, GG, and PEO were selected as film forming polymers which should give shape and provide mechanical strength to the ODF. Moreover, the polymers are hydrophilic which will enable quick hydration upon contact with saliva and hence dissolution / disintegration of the thin films (Ouda et al., 2020). The use of plasticizer would enhance the elasticity and flexibility of the films (Ding et al., 2015, Irfan et al., 2016). Our previous studies (Ouda et al., 2020) showed that HPMC within a concentration range of 0.5–1% is capable of producing good film and that GG with insoluble drug produced films with poor coalescence, poor flexibility and difficult to peel off the casting tray. Accordingly, the influence of varying the polymer and plasticizer type (independent variables - factors) at three concentration levels on the dependent variables (responses) was investigated using the RSM model.
D-optimal model was selected based on the software preference as the most effective and efficacious that would enable the prediction of best model. The model was comprised of 24 experiments in total that entailed 3 replicated centre points. The formulations were developed according to the software proposed run order to avoid bias. The films were prepared and evaluated for disintegration time, content uniformity, film quality and thickness uniformity and are results depicted in Table 3.
The results in Table 3 revealed that the disintegration time of the ODFs varied from 10 to 90 s, the film quality from 10% to 100%, content uniformity from 82% to 108.1%, and thickness uniformity from 1.03% to 9.45%. Further, it was noted that all films prepared from GG resulted in poor quality in terms of roughness, not easy to peel off casting surface and elasticity. This results are in line with our previously reported data (Ouda et al., 2020). Even with the increase in plasticizer concentration, the films were marked as poor. Furthermore, in general, HPMC showed better results in terms of disintegration time while maintaining better film quality. Low concentration of PEO produced films with favourably low disintegration time, but film quality was poor, particularly in terms of peeling off the film from the tray. Films were fragile and tore off upon peeling.
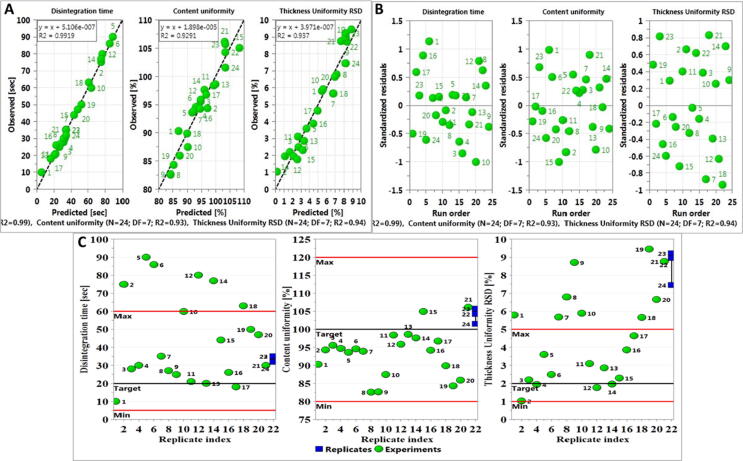
Data was fitted into the model and results are depicted in Fig. 1A. Film quality showed low validity. This might be explained as the influence of all the factors within film properties in various formulations is expected to show variations and the assessment of response might include some subjectivity of the criteria. Also, the qualitative nature of film quality that depends on individual evaluation and hence subjectivity can result in error that jeopardized the validity of the model. Therefore, this response was excluded from the model and the model was refitted with three responses (disintegration time, content uniformity and thickness uniformity). For the three responses, model fitting as expressed in R2 that exceeded 90% mark for all the three responses, whereas, the prediction power (Q2) showed good prediction ability (0.89, 0.72 and 0.64 for disintegration time, content uniformity and thickness uniformity respectively) as they exceeded the threshold of 0.25 (Dahmash et al., 2018). Further, the model was valid for the three responses, the highest was for thickness uniformity 0.80 and the lowest was for disintegration time 0.59. The three responses showed excellent reproducibility that exceeded 88% for all of them. The wide variation in the response’s values for different formulations and the high degree of fitting of the model (Fig. 1B) suggest that the responses (except for film quality) are strongly dependent on the selected independent factors.
Fig. 1.
Summary of fit for all responses highlighting the degree of fit (R2), prediction power (Q2) model validity and reproducibility. Total number of runs 24. (A) The model included film quality which demonstrated poor validity, (B) Summary of Fit upon the exclusion of film quality as a response and revision of all model terms.
Further examination of the model validity once the response, film quality, was excluded, was done through several plots as can be seen in Fig. 2. Fig. 2A showed that the predicted versus observed response showed strong correlation with R2 exceeding 0.9 (0.99 for disintegration time, 0.93 for content uniformity and 0.94 for thickness uniformity).
Fig. 2.
(A) Observed versus predicted for all responses highlightimg the significance of the model. (B) Residual versus run order of all responses. (C) Replicate index for all responses, highlighting the closeness of the centre points (replicates).
Similarly, residual versus run order (Fig. 2B) demonstrated the ideal random order indicating absence of trend and pattern according to run order (i.e., error was not built up with run order). Plotting Replicate index (Fig. 2C) showed that all replicated runs were close to each other (i.e., less than 5% variations between them) and hence high reproducibility of the model.
Statistical analysis for testing the model validity is summarized in Table 5. A P value of less than 0.05 for the regression model suggests a statistically significant model for each response. The correlation coefficient (R2) for the three responses indicates good fit of the data (observed data) in the revised model. From the ANOVA analysis also, the P value due to lack of fit of more than 0.05 for all responses also, indicate valid model and that the errors are not due to lack of fit. The results indicate that the model is statistically significant.
Table 5.
Summary of the ANOVA results for the three responses to test model validity, where P is the probability and R2 is the regression coefficient. (valid model is when the P value is less than 0.05).
| P | R2 | |
|---|---|---|
| Disintegration time | ||
| Regression | 0.000 | 0.990 |
| Lack of Fit | 0.195 | |
| Content Uniformity | ||
| Regression | 0.000 | 0.926 |
| Lack of Fit | 0.411 | |
| Thickness Uniformity | ||
| Regression | 0.000 | 0.916 |
| Lack of Fit | 0.452 | |
3.1.1. Regression model equations for all responses
Once the overall significance of the model factors was determined; the regression coefficients of the model terms were examined to identify the significant model terms for each response using the regression coefficients plots (Fig. 3). The first seven coefficients represent the linear terms and reveal the effect of each individual factor. The other coefficients (bars) represent the interaction terms, which show the interactions (if any) among the factors. The length of the coefficient bar indicates the magnitude of the effect, while the confidence interval represents the noise (Dahmash et al., 2018). For example, the most significant factor affecting disintegration time was the polymer concentration. Increasing the polymer concentration resulted in an increase in the disintegration time. Increasing the polymer concentration resulted in thicker films, producing a more viscous outer layer upon hydration and hence took longer to disintegrate or dissolve (Irfan et al., 2016, Ouda et al., 2020, Saab and Mehanna, 2019). Polymer concentration and the polymer HPMC were the most significant interaction model terms that increased disintegration time. The quadratic term of polymer concentration also had high significance.
Fig. 3.

Regression coefficient plot of model terms for: (A) disintegration time in seconds (B) content uniformity % and (C) thickness uniformity %. The length of the bar indicates the magnitude of the effect of each model term, whereas the direction represents negative or positive effect. Significant effect is determined when the confidence intervals do not cross zero.
The regression model of the three responses that included significant terms was built, where for each response, the quadratic model comprised of 21 terms. The model terms that showed significance (P value less than 0.05) were included into the regression equations (Eqs. (3)–(5), Table 6). The value of each coefficient and the sign represented the impact and effect (negative or positive) respectively. For example, in thickness uniformity, the impact of HPMC polymer (X11) was the highest (2.18) and the negative sign indicates the reduction in RSD, which is favourable. The results also indicate that content uniformity of the films was significantly influenced by the linear models of HPMC polymer (X11), guar gum (X13) and polymer concentration (X2).
Table 6.
Regression equations for the responses (dependent variable) highlighting the effect of all significant model terms on each response.
| Y1 = 39.01 + 2.44*X11 − 2.59*X13 + 19.57*X2 + 1.73*X31 − 1.73*X32 + 5.48*X2*X2 + 6.59*X11*X2 − 7.12*X13*X2 −6.14 X11*X31 + 6.14*X11*X32 + 6.27 *X12*X31 − 6.27*X12*X32 + 3.28*X2*X31 − 3.28*X2*X32(3) | |||||||
| Y2 = 102.06 + 2.01 X11 − 3.33* X31 + 3.33 X32 − 5.15* X2*X2 − 2.61 * X4*X4 + 1.81 X11*X2 + 2.19 X12*X31 −2.19 * X12*X32 + 3.17 *X2*X31 −3.17* X2*X32(4) | |||||||
| Y3 = 5.47–2.18 *X11 + 1.95*X13 – 0.63 *X2 − 0.91 X2*X31 + 0.91* X2*X32(5) | |||||||
| Y1 | Disintegration time | ||||||
| Y2 | Content uniformity | ||||||
| Y3 | Thickness uniformity | ||||||
| X1 | Polymer type | X11 | HPMC | X12 | PEO | X13 | GG |
| X2 | Polymer concentration | ||||||
| X3 | Plasticizer type | X31 | Glycerine | X32 | Sorbitol | ||
| X4 | Plasticizer concentration | ||||||
3.1.2. The effect of independent variables (factors) on dependent variables (responses)
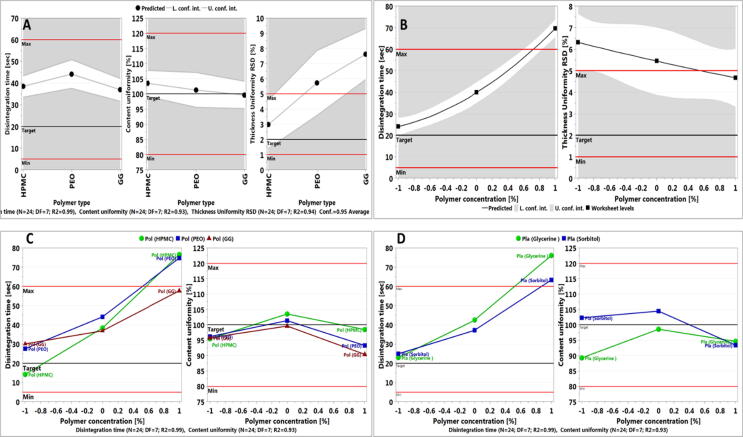
The effect of the linear model terms on film properties (Fig. 4A) showed that the HPMC polymer produced films with lower disintegration time, excellent content uniformity and the lowest RSD in thickness uniformity. Although PEO produced films with excellent content uniformity, disintegration time was higher than that of HPMC and thickness uniformity was above the maximum allowed limit of 5%. Such results could be attributed to the use of high grade of PEO and which produced more viscous environment upon hydration when compared to HPMC and hence the increase in disintegration time. Although, guar gum containing films produced low disintegration time, it resulted in poor film properties (difficult to peel off the casting surface) and hence produced inconsistency in film thickness as can be seen from the high RSD values.
Fig. 4.
Main effect plots of (A) the polymer type on the three responses, when other factors maintained at their middle values. Note the D-optimal design has no centre points and hence were not displayed. (B) The polymer concentration on disintegration time and film thickness uniformity when other factors maintained at their middle values. Note the D-optimal design has no centre points and hence were not displayed. (C) Interaction effect plot of polymer type with increasing polymer concentration on disintegration time and content uniformity while the other factors were maintained at their middle values. (D) Interaction effect plot of polymer concentration with plasticizer type on disintegration time and content uniformity while the other factors were maintained at their middle values.
Polymer concentration had a detrimental effect on disintegration time of the films but better film thickness uniformity (Fig. 4B). Higher polymer concentration as discussed earlier produced more viscous solutions upon hydration and hence delayed dissolution and disintegration time particularly, in the absence of disintegrating agent. However, slight increase in solution viscosity resulted in easy spread of the solution on the casting surface and hence better film thickness uniformity. However, the results revealed that the polymer concentration did not affect content uniformity where the drug content was optimal. This could be attributed to high drug load of the film and the small range of polymer concentration used, as one of the aims of this study was polymer concentration optimisation. Our previous work (Ouda et al., 2020) with very high polymer concentration resulted in detrimental content uniformity due to difficulty in pouring the solution on the casting tray and huge variations in film thickness.
The interaction between polymer type and concentration was the most significant on disintegration time and content uniformity as evident from the regression model (Eq. (3) and (4)) with coefficient factor of + 6.59 for HPMC and − 7.12 for GG on disintegration time and + 1.81 for HPMC on content uniformity. From Fig. 4C, low polymer concentration produced films with low disintegration time for the three polymers, yet, the lowest was for HPMC. Overall, HPMC demonstrated the best film forming characteristic and good disintegration time. Similar results were reported by (Ouda et al., 2020). However, when the polymer concentration was further increased, films prepared from HPMC and PEO showed similar high disintegration profile while GG produced poor films that were hard to peel but lacked mechanical strength and hence disintegrated rapidly. However, the interaction effect on content uniformity was minimal with the most prevailing effect observed when HPMC was used. But, despite the change with polymer concentration it was within the targeted range.
Eq. (3) and (4) also showed significant interaction terms; polymer concentration (X2) and plasticizer type (X3) on disintegration time and content uniformity. The results in Fig. 4D suggest that at low polymer concentration, the plasticizer type has no effect. While, upon the increase in the polymer concentration, glycerine containing films demonstrate higher disintegration time when compared to sorbitol. Unfortunately, when high polymer concentration was used sorbitol produced a lot of bubbles that could not be removed upon sonication, which resulted in presences of air bubbles that resulted in faster disintegration time. Such films obtained low scores in terms of film quality. The effect of plasticizer on content uniformity was acceptable for both plasticizers. Despite a slight difference at low polymer concentration, the effect was trivial as the results were in the targeted range for both plasticizers.
From Eq. (3) also, the disintegration time was reduced when HPMC was used as a polymer with glycerine, while it was increased when sorbitol was used. On the other hand, for PEO, the addition of plasticizer resulted in an increase in disintegration time.
The addition of plasticizers to films is necessary to decrease polymer intermolecular forces, enhance the mobility of the polymer chains, and the mechanical properties of the ODF. Both glycerine and sorbitol are hydrophilic, though, glycerine demonstrated better plasticity of produced films (Müller et al., 2008). However, in this study when the polymer concentration was high for HPMC and when PEO was used sorbitol produced high level of bubble and films produced demonstrated lower disintegration time upon hydration due to the presence of bubbles. (i.e., air voids and hence more porous films). The presence of bubbles produced films with bad appearance (not smooth and air bubbles could be seen after drying).
Response surface plots that simulate the influence of independent factors on each response were generated by the software. The plots for disintegration time, content uniformity and thickness uniformity are presented in Fig. 5. The plots provide uninterrupted visual assessment of the change in response surface upon the variation of independent variables from low to high values individually and concurrently (AlHusban et al., 2010). From the plots, it was noted that the main effect of varying the polymer concentration when HPMC was used and the plasticizer concentration when glycerine was selected is on disintegration time. Lower polymer concentration was required to obtain low disintegration time (20–30 s), while plasticizer effect was trivial. However, at higher polymer concentration, higher plasticizer concertation was preferred for lower disintegration time. Such results can be explained by the effect of plasticizer on polymer interaction and higher hydrophilicity of the film. Varying polymer and plasticizer concentration did not produce films outside the targeted range for content uniformity and /or thickness uniformity.
Fig. 5.
Response Surface Model (RSM) plot showing the effect of varying the polymer concentration (HPMC) and plasticizer concentration (Glycerine) on the three responses (disintegration time, content uniformity and thickness uniformity).
3.1.3. Setting optimal formulation parameters
Based on the results obtained from the response surface plot, optimal formulation parameters within the independent variables that reveal the ranges that could result in optimal responses (low disintegration time of less than 60 s, content uniformity within ± 5% and RSD of lower than 2%) are illustrated in Fig. 6. From the graph, the green area represents the design space with independent factors (polymer concentration and plasticizer concentration) that could result in desired responses for both disintegration time, content uniformity and thickness uniformity. The zones marked with light blue and marine blue represent areas where the factors meet the criteria for disintegration and content uniformity or disintegration alone respectively. Whereas the white zone represents the factors when none of the criteria is met. For example, within the operable space optimal film properties could be obtained at point A and point B. At point A, the polymer concentration was set at −0.08 (equivalent to 0.78% of HPMC) while plasticizer concentration set at −0.899017 (equivalent to 0.11% glycerine) which is expected to produce disintegration time of 36.2 s, content uniformity of 95.0934% and RSD 1.98%. Another operable zone was obtained (point B) when polymer was 0.0366077 (equivalent to 0.82% HPMC) and plasticizer 0.946721 (equivalent to 0.29% glycerine) to produce films with disintegration time is 29.49 s, content uniformity 95.04% and thickness uniformity of 1.996%. Overall, optimal ODFs can be obtained with polymer HPMC concentration ranging from 0.83% to 1.1% and glycerine plasticizer concentration ranging from 0.11% to 0.3%.
Fig. 6.
Sweet spot for optimal ranges of the independent variables (polymer concentration and plasticizer concentration) for the desired profile of ODFs (disintegration time, content uniformity and thickness uniformity) while HPMC and Glycerine were selected as polymer and plasticizer respectively.
3.2. Characterization of optimal topiramate ODF formulation
Optimal formulation was selected based on the results of the DoE particularly N4 with medium polymer concentration (0.8%) and high glycerine concentration (0.3%). Lower plasticizer concentration resulted in films that were difficult to peel off the casting surface and it was not homogeneous. Resulting films were good in term of homogeneity and ease of removal from the casting surface. The formulation produced films with good physical properties particulaly in terms of consistency, transparency and easy peeling off the casting tray. Fig. 7 shows the obtained film from formulation N4, where it clearly shows that the film as trasparent and homogeneous. This formulation was taken for further investigations.
Fig. 7.
Topiramate loaded ODF (N4) made from HPMC, topiramate and glycerine.
Dissolution profile was assessed and as can be seen from Fig. 8, more than 98% of topiramate was released within the first 10 min. Such results suggest possible increase in onset time and hence enhancement of pharmacokinetic profile of topiramate.
Fig. 8.
Percentage released over time of topiramate loaded ODF (N4) made from HPMC, topiramate and glycerine size 2x3 cm2 (mean ± SD, n = 3).
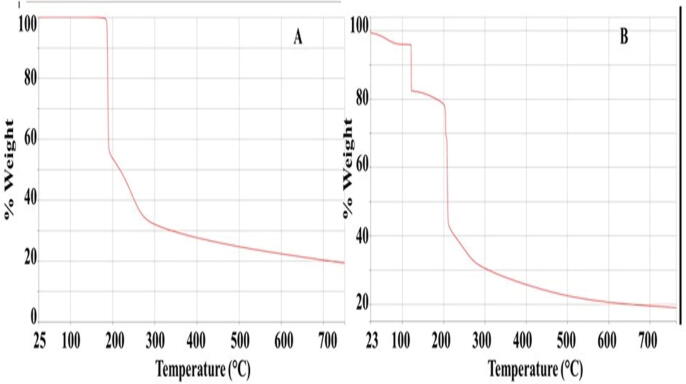
Thermal stability of the topiramate loaded ODF (N4) was assessed using TGA. The TGA analysis is employed to determine the amount and rate of change in the weight of a sample as a function of temperature. The results are used to determine moisture content, as well as thermal and oxidative stability of materials. Thermal stability of the films comprising 25 mg topiramate, HPMC, and glycerine was investigated and results as depicted in Fig. 9 (A) showed the topiramate powder TGA profile with a mass loss of around 45% at around 180–200 °C, followed by a further loss of mass in excess of 20% in the range of 200–300 °C. This loss of mass continued till 750 °C, where only around 20% of the material was left. Similar results were reported by Sena et al. (2008). Fig. 9 (B) revealed that the film contained about 5% residual water that evaporated around 100 °C; this could be attributed to the presence of glycerine that has the ability to retain water. Such water retention property of glycerine is responsible to its property as a plasticizer (Liu et al., 2001). The second decomposition and loss of mass at around 100–120 °C could be attributed to glycerine (Crnkovic et al., 2012). The next loss of material / decomposition was observed at about 200 °C represents the decomposition of topiramate. Both topiramate and HPMC demonstrated thermal decomposition at around 350 °C (Ding et al., 2015). In summary the topiramate ODF demonstrated good thermal stability.
Fig. 9.
TGA profile of (A) topiramate powder, (B) ODF (N4) composed of 25 mg topiramate, HPMC and glycerine, all obtained at a heating rate of 10 °C/min under N2 atmosphere.
The next set of experiments were focused on investigating the change in crystallinity of the formulation and the effect of production processes on material properties. Fig. 10 (A) showed the XRD patterns of topiramate powder that demonstrated numerous characteristic sharp peaks, suggesting that the drug was present in a crystalline state with major characteristic diffraction peaks appearing at a diffraction angle of 2θ value of 11.93°,15.15°, 17.1°, 20°, which is in line with published literature (Yang et al., 2014) . HPMC is a known amorphous material that showed a broad “halo” pattern with few maxima. HPMC had two peaks at 10° and 22° 2θ (Storey and Ymén, 2011). Using topiramate with HPMC resulted in XRD patterns for the film that revealed many characteristic high intensity peaks and relative intensities of topiramate that are presented in XRD confirming that the process of ODF production did not affect the crystallinity of the drug Fig. 10 (B).
Fig. 10.
Typical XRD patterns of (A) topiramate pure powder, (B) ODF N4 topiramate loaded 25 mg collected on D8 Phaser diffractometer.
SEM images of the obtained topiramate containing film (N4) are depicted in Fig. 11. It is clear from the SEM images that the surface is smooth and coalescent (A and B) and that HPMC particles coalesced into a smooth film. The crystalline active pharmaceutical ingredient (topiramate) particles were distributed throughout the film with few clusters of topiramate are seen at the centre.
Fig. 11.
SEM micrographs of (A) topiramate containing ODF- N4 at 50× magnification showing the surface of the film, (B) N4 at 100× magnification, (C) N4 at 500× magnification and (D) N4 at 1000× magnification. The while patches in C and D represents clusters of Crystalline topiramate.
Determining the mechanical properties of the ODFs is critical to its use. Tensile strength and elongation were measured. The tensile strength is calculated by the applied forces of break divided by the cross-sectional area of the film (Irfan et al., 2016). Good ODFs should have moderate tensile strength and high elongation. The elongation is a measure of plasticity, with results demonstrating more than 3% capability and good tensile strength that exceeded 22 MPa. Nevertheless, no specific limits are defined to ensure the appropriate mechanical properties of films (Irfan et al., 2016). Table 7 lists the mechanical properties of the selected formulation (N4).
Table 7.
Mechanical Properties of topiramate loaded ODF (N4) 3 × 2 cm2 (Mean ± SD, n = 3).
| Property | Mean | SD |
|---|---|---|
| Tensile strength (MPa) | 22.53 | 4.54 |
| % Elongation | 1.81 | 0.34 |
| Energy to break (J) | 0.0073 | 0.0006 |
| Thickness (mm) | 0.066 | 0.0013 |
| Width (mm) | 19.87 | 0.63 |
Overall, this study revealed that topiramate could successfully be loaded onto ODFs using HPMC as film forming polymer and glycerine as plasticizer, to produce doses of 25 mg/ film.
4. Conclusions
This work has successfully developed and validated orally dissolving films containing 25 mg topiramate as a potential alternative dosage form for treatment of indicated epileptic disorders in children. The D-optimal multifactorial design of experiment applied in this study enabled the understanding of the effect of independent variables (polymer type, polymer concentration, plasticizer type and plasticizer concentration) on four responses. The analysis of variance revealed that all the independent variables had significant effect on three responses (disintegration time, content uniformity and thickness uniformity). The revised model showed high degree of reliability and enabled the identification of design space that produced ODF formulations with optimal properties. The optimized films contained hydroxypropyl methyl cellulose (HPMC) as hydrophilic film forming agent and glycerine as plasticizer. The developed film released 98% of topiramate within 10 mins and maintained the physicochemical stability of the drug as analysed using TGA, XRD and SEM. This study identified the type of polymer used as having the highest influence on the characteristics of the film produced.
CRediT authorship contribution statement
Eman Zmaily Dahmash: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Resources, Data curation, Writing - original draft, Writing - review & editing, Visualization, Supervision, Project administration, Funding acquisition. Affiong Iyire: Methodology, Writing - original draft, Writing - review & editing. Hamad S. Alyami: Methodology, Formal analysis, Resources, Writing - original draft, Visualization, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors would like to acknowledge the funding from Isra University (Jordan) for funding E.D. Najran University are thanked for their financial support in funding H.A work in this research and Aston University for supporting A.I.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Eman Zmaily Dahmash, Email: eman.zmaily@iu.edu.jo.
Affiong Iyire, Email: a.iyire@aston.ac.uk.
Hamad S. Alyami, Email: hsalmukalas@nu.edu.sa.
References
- Abay F., Ugurlu T. Orally disintegrating tablets: A short review. J. Pharm. Drug Dev. 2015;3:1–8. doi: 10.15744/2348-9782.3.303. [DOI] [Google Scholar]
- AlHusban F., ElShaer A.M., Kansara J.H., Smith A.M., Grover L.M., Perrie Y., Mohammed A. Investigation of formulation and process of lyophilised orally disintegrating tablet (ODT) using novel amino acid combination. Pharmaceutics. 2010;2:1–17. doi: 10.3390/pharmaceutics2010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BNF, 2017. BNF for children 2017-2018: September 2017-18. London: BMJ Group; Pharmaceutical Press.
- Crnkovic P.M., Koch C., Ávila I., Mortari D.A., Cordoba A.M., Moreira dos Santos A. Determination of the activation energies of beef tallow and crude glycerin combustion using thermogravimetry. Biomass Bioenergy. 2012;44:8–16. doi: 10.1016/j.biombioe.2012.04.013. [DOI] [Google Scholar]
- Curatolo P., Panzarino G., Rizzo R., Verrotti A., Moavero R., Di Paolantonio C. The Challenge of Pharmacotherapy in Children and Adolescents with Epilepsy-ADHD Comorbidity. Clin. Drug Investig. 2017;38:1–8. doi: 10.1007/s40261-017-0585-1. [DOI] [PubMed] [Google Scholar]
- Cuzzolin, L., 2018. Neonates exposed to excipients: concern about safety. J. Pediatr. Neonatal Individ. Med. 7, e070112–e070112.
- Dahmash E.Z., Al-Khattawi A., Iyire A., Al-Yami H., Dennison T.J., Mohammed A.R. Quality by Design (QbD) based process optimisation to develop functionalised particles with modified release properties using novel dry particle coating technique. PLoS One. 2018;13 doi: 10.1371/journal.pone.0206651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding C., Zhang M., Li G. Preparation and characterization of collagen/hydroxypropyl methylcellulose (HPMC) blend film. Carbohydr. Polym. 2015;119:194–201. doi: 10.1016/j.carbpol.2014.11.057. [DOI] [PubMed] [Google Scholar]
- EMA, 2013. Assessment Report: International non-proprietary name: Phentermine/ Topiramate. London.
- Hanning S.M., Lopez F.L., Wong I.C.K., Ernest T.B., Tuleu C., Orlu Gul M. Patient centric formulations for paediatrics and geriatrics: Similarities and differences. Int. J. Pharm. 2016;512:355–359. doi: 10.1016/j.ijpharm.2016.03.017. [DOI] [PubMed] [Google Scholar]
- ICH, 2005. Validation of analytical procedures: Text and methodology Q2(R1). Int. Conf. Harmon. Tech. Requir. Regist. Pharm. Hum. Use 4.
- Irfan M., Rabel S., Bukhtar Q., Imran M., Jabeen F., Khan A. Orally disintegrating films : A modern expansion in drug delivery system. Saudi Pharm. J. 2016;24:537–546. doi: 10.1016/j.jsps.2015.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanovska V., Rademaker C.M.A., van Dijk L., Mantel-Teeuwisse A.K. Pediatric Drug Formulations: A Review of Challenges and Progress. Pediatrics. 2014;134:361–372. doi: 10.1542/peds.2013-3225. [DOI] [PubMed] [Google Scholar]
- Liu Z.Q., Yi X.-S., Feng Y. Effects of glycerin and glycerol monostearate on performance of thermoplastic starch. J. Mater. Sci. 2001;36:1809–1815. [Google Scholar]
- Lopez F.L., Ernest T.B., Tuleu C., Gul M.O. Formulation approaches to pediatric oral drug delivery: benefits and limitations of current platforms. Expert Opin. Drug Deliv. 2015;12:1727–1740. doi: 10.1517/17425247.2015.1060218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikaeloff Y., de Saint-Martin A., Mancini J., Peudenier S., Pedespan J.-M., Vallée L., Motte J., Bourgeois M., Arzimanoglou A., Dulac O., Chiron C. Topiramate: efficacy and tolerability in children according to epilepsy syndromes. Epilepsy Res. 2003;53:225–232. doi: 10.1016/S0920-1211(03)00028-7. [DOI] [PubMed] [Google Scholar]
- Müller C.M.O., Yamashita F., Laurindo J.B. Evaluation of the effects of glycerol and sorbitol concentration and water activity on the water barrier properties of cassava starch films through a solubility approach. Carbohydr. Polym. 2008;72:82–87. [Google Scholar]
- Ouda G.I., Dahmash E.Z., Alyami H., Iyire A. A Novel Technique to Improve Drug Loading Capacity of Fast/Extended Release Orally Dissolving Films with Potential for Paediatric and Geriatric Drug Delivery. AAPS PharmSciTech. 2020;21:126. doi: 10.1208/s12249-020-01665-5. [DOI] [PubMed] [Google Scholar]
- Patil P., Shrivastava S.K. Fast Dissolving Oral Films : a Novel Drug Delivery System. Int. J. Sci. Res. 2014;3:2088–2093. [Google Scholar]
- Pellock J.M., Watemberg N. New antiepileptic drugs in children: Present and future. Semin. Pediatr. Neurol. 1997;4:9–18. doi: 10.1016/S1071-9091(97)80004-6. [DOI] [PubMed] [Google Scholar]
- Ph.Eur, 2010. European pharmacopoeia. Council of Europe.
- PubChem, 2019. National Center for Biotechnology Information. PubChem Database. Topiramate, CID=5284627, [WWW Document].
- Saab M., Mehanna M.M. Disintegration time of orally dissolving films: various methodologies and in-vitro/in-vivo correlation. Die Pharm. Int. J. Pharm. Sci. 2019;74:227–230. doi: 10.1691/ph.2019.8231. [DOI] [PubMed] [Google Scholar]
- Sena Jr, D.M., Freire, P.T.C., M Filho, J., Melo, F.E.A., Pontes, F.M., Longo, E., Ferreira, O.P., Alves, O.L., 2008. Vibrational and thermal properties of crystalline topiramate. J. Braz. Chem. Soc. 19, 1607–1613.
- Storey, R.A., Ymén, I., 2011. Solid state characterization of pharmaceuticals. John Wiley & Sons.
- Talati, R., Scholle, J.M., Phung, O.J., Baker, W.L., Baker, E.L., Ashaye, A., Kluger, J., Quercia, R., Mather, J., Giovenale, S., 2012. Effectiveness and safety of antiepileptic medications in patients with epilepsy. [PubMed]
- Thakur, N., Bansal, M., Sharma, N., 2013. Overview “ A Novel Approach of Fast Dissolving Films and Their Patients ” 7, 50–58. doi:10.5829/idosi.abr.2013.7.2.72134
- Turner M.A., Catapano M., Hirschfeld S., Giaquinto C. Paediatric drug development: the impact of evolving regulations. Adv. Drug Deliv. Rev. 2014;73:2–13. doi: 10.1016/j.addr.2014.02.003. [DOI] [PubMed] [Google Scholar]
- Yang M., Xie S., Li Q., Wang Y., Chang X., Shan L., Sun L., Huang X., Gao C. Effects of polyvinylpyrrolidone both as a binder and pore-former on the release of sparingly water-soluble topiramate from ethylcellulose coated pellets. Int. J. Pharm. 2014;465:187–196. doi: 10.1016/j.ijpharm.2014.02.021. [DOI] [PubMed] [Google Scholar]