Abstract
Background
D-limonene and its derivatives have demonstrated potential chemopreventive and anticancer activity in preclinical and clinical studies. The aim of this scoping review was to assess and critically appraise current literature on the effect of these bioactive citrus peel compounds on breast cancer in human trials and to identify knowledge gaps for exploration in future studies.
Methods
This study followed a scoping review framework. Peer-reviewed journal articles were included if they reported the effect of d-limonene or its derivatives on breast cancer in human subjects. Articles were retrieved from academic databases – PubMed, EMBASE, CINAHL, Web of Science, and Cochrane reviews – and iteratively through review of bibliographies of relevant manuscripts. Titles and abstracts were appraised against the aforementioned inclusion criteria in a first round of screening. Through consensus meetings and full article review by authors, a final set of studies were selected. Results were reported according to the PRISMA extension for scoping reviews.
Results
Our search strategy yielded 367 records. Following screening and adjudication, five articles reporting on phase 1(n = 2), phase 2 (n = 2) and both trial phases (n = 1) were included as the final dataset for this review. Trials evaluating the effect of d-limonene (n = 2) showed it was well tolerated in subjects. One study (n = 43 participants) showed d-limonene concentrated in breast tissue (mean 41.3 μg/g tissue) and reduction in tumor cyclin D1 expression, which is associated with tumor proliferation arrest. This study did not show meaningful change in serum biomarkers associated with breast cancer, except for a statistically significant increase in insulin-like growth factor-1 (IGF-I) levels. While elevation of IGF-I is associated with increased cancer risk, the clinical implication of this study remains uncertain given its short duration. Trials with perillyl alcohol (n = 3) showed low tolerance and no effect on breast cancer.
Conclusion
This review demonstrated a dearth of clinical studies exploring the effect of d-limonene and its derivatives on breast cancer. Limited literature suggests d-limonene is safe and tolerable in human subjects compared to its derivative, perillyl alcohol. Our review demonstrates the need for additional well-powered placebo-controlled trials that assess d-limonene’s efficacy on breast cancer compared to other therapies.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12885-021-08639-1.
Keywords: Citrus peel, D-limonene, Perillyl alcohol, Breast cancer, Chemopreventive, Scoping review
Background
Breast cancer is the second most common cancer among women in the United States, accounting for approximately 30% of all cancers diagnosed in this population [1]. In 2021, an estimated 284,200 new cases and 44,130 deaths attributable to breast cancer are projected [1]. Depending on a confluence of factors – ranging from age, cancer type, and cancer stage – clinical treatment options for breast cancer include surgical interventions, chemotherapy, hormonal, biological, and radiation therapies. These currently available therapies are invasive, associated with severe side effects, and/or are expensive to administer [2–4]. There is therefore a need for cheaper and more tolerable alternative treatments.
Citrus fruits, including lemons, limes, oranges, tangerines, and grapefruits, are widely available at low cost. These fruits contain bioactive compounds – including phenols, flavonoids and terpenes – which have demonstrated chemotherapeutic properties in relation to breast cancer [5–8]. While other compounds in the citrus peel have been evaluated for anticancer activity, limonene, the simplest monocyclic monoterpene, shows substantial chemotherapeutic promise because: (1) it constitutes 3.8 wt% of dry orange peel, and about 90–95% of citrus oil [9]; (2) it is fat-soluble, allowing for absorption into fat tissue, thereby permitting for the monoterpene to accumulate in the body [10]; and (3) it is rapidly metabolized in humans in a fashion similar to animal models [11].
Limonene (Fig. 1) has demonstrated clinical and therapeutic applications [12, 13]. Among humans, limonene has been shown to be effective in dissolving gallstones [14], and in relieving Gastroesophageal Reflux Disease (GERD) symptoms [12]. Exploration into hydroxylated analogs of d-limonene – including perillyl alcohol, carveol, uroterpenol, and sobrerol – have been investigated, owing to their efficacy in causing tumor regression in animal models at lower concentrations than d-limonene [15, 16]. Their use, therefore, have been postulated as resulting in more favorable therapeutic ratios [15]. Perillyl alcohol (Fig. 1), a dietary monoterpene is found naturally in peppermint, lavender, and other plants.
Fig. 1.
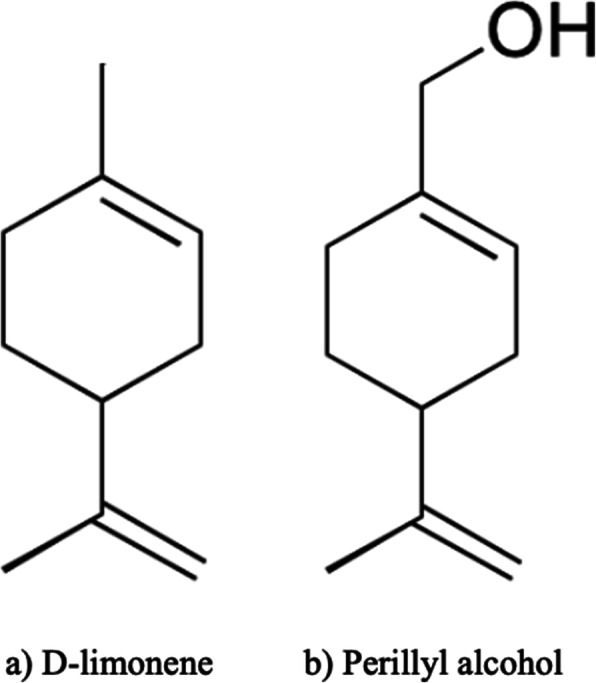
Chemical structure of d-limonene and perillyl alcohol
Given the positive results of in vivo and in vitro studies, chemotherapeutic evaluation of d-limonene and perillyl alcohol have progressed to human studies. To our knowledge, findings from these clinical studies have not been consolidated into a single review, which would allow for a comprehensive mapping of literature on the subject, as well as a repository of this evidence. The objectives of this review, therefore, are to explore the depth of knowledge currently available on the effect of d-limonene and its derivatives on breast cancer in humans. Specifically, this scoping review aims to: (1) systematically review and summarize evidence on citrus peel extracts and breast cancer in humans, including populations that have been studied, tolerable dosage, observed toxicity, pharmacokinetic profiles and anticancer effects; and (2) discuss studied underlying mechanisms leading to anticancer activity.
Methods
Inclusion criteria and search strategy
The design of this review was informed by Arksey and O’Malley’s methodological framework for scoping reviews [17]. Peer-reviewed journal articles were included in the present study if they: (1) reported on the effect of d-limonene or any of its derivatives; (2) measured breast cancer as a key outcome; (3) were conducted among human subjects; 4) employed an experimental design, with or without a comparison; and (5) were published at any time before June 20, 2020. Studies were excluded if they: (1) were not peer-reviewed; and (2) did not present original data.
A medical librarian collaborated on this review and contributed to the development of the search strategy. Academic databases – Legacy PubMed, Embase, EBSCOhost Cumulative Index of Nursing and Allied Health Literature (CINAHL), Web of Science (Core Databases), and Cochrane Reviews – were searched to retrieve academic peer-reviewed journal articles. To capture articles relevant to the review questions, a search strategy incorporating key words and controlled vocabulary pertinent to our exposure (d-limonene OR citrus oil OR orange oil OR Lemon oil OR Mandarin oil OR Lime oil OR Grapefruit oil OR citrus peel OR carveol OR uroterpenol OR sobrerol OR “limonene”[MeSH] OR “citrus”[MeSH] OR “citrus paradise”[MeSH]) and outcome (breast cancer OR breast carcinoma OR mammary cancer OR cancer of the breast OR “breast neoplasms”[MeSH]) was used (supplemental Table 1). In an iterative process, involving the review of bibliographies of relevant manuscripts, additional articles were included to ensure an exhaustive search. Articles retrieved from the academic databases and bibliography review were combined, and duplicates removed to arrive at a consolidated dataset to determine eligibility for inclusion in the review.
Data management and extraction
All article citations were managed using the Mendeley reference manager. To allow for collaboration and transparency through the screening process, Rayyan QCRI, a web- and mobile-based systematic review application was used [18]. In an initial round of screening, study authors reviewed the titles and abstracts in the consolidated dataset for relevance based on the abovementioned inclusion/exclusion criteria. Following this first review, authors convened to discuss the articles resulting from the first screening and came to a consensus about the articles to be excluded. In a secondary screening, articles were reviewed in their entirety and included in the present review if they met the eligibility criteria. Queries on the eligibility for inclusion were resolved through consensus of authors. A final set of articles fitting the scope of the present review were analyzed and summarized.
Data analysis and synthesis
To assess methodological quality, the Joanna Briggs Institute (JBI) Checklist for Quasi-Experimental Studies (non-randomized experimental studies) was applied to the final set of articles (supplemental Table 2) [19]. Additionally, the quality of each included article was assessed using the Risk Of Bias In Non-randomized Studies of Interventions (ROBINS-I) Tool [20]. Risk of bias was assessed under the following 7 domains of bias: confounding, selection of participants, classification of interventions, deviation from protocol, missing data, measurement of outcomes and selection of the reported result [20]. For each domain, articles were assigned a gradation of risk of bias – from no information, low, moderate, serious and critical risk.
A summary of each article – consisting of the author and publication details, compound under investigation, research aim and major findings – were performed. Findings were synthesized and categorized based on (1) study participant characteristics; (2) tolerance to monoterpene under evaluation; (3) toxicity to monoterpene under evaluation and corresponding dosing; and (4) effect of monoterpene on breast cancer. Data was reported using PRISMA extension for scoping reviews (PRISMA-ScR) (supplemental Table 3) [21].
Results
Included studies
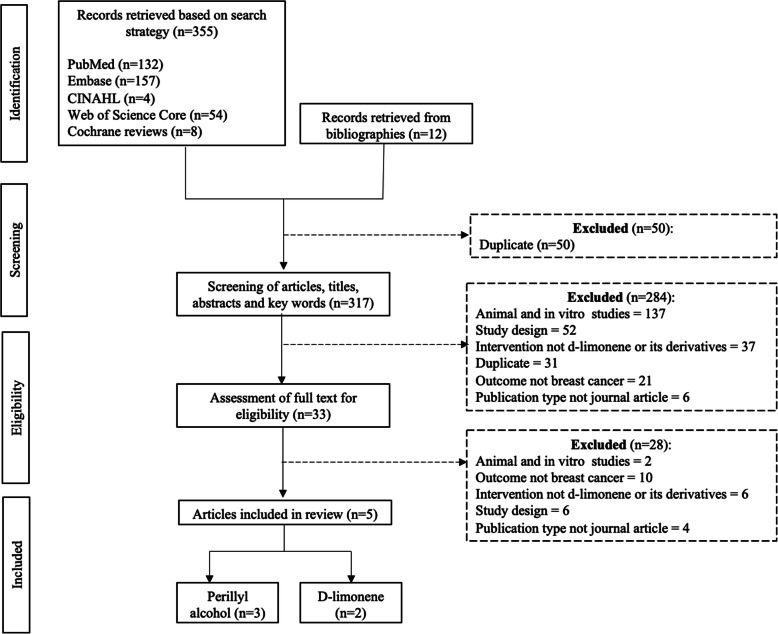
An initial search yielded 367 records from academic databases (n = 355) and review of bibliographies (n = 12). Following deduplication, initial screening of titles and abstracts, full text review and researcher adjudication, five articles met the eligibility criteria for inclusion in this present review (Fig. 2). These studies, conducted between 1998 and 2013, included clinical evaluation of d-limonene (n = 2) and perillyl alcohol (n = 3) on breast cancer-specific outcomes. Included studies reported findings from phase 1 safety and dose escalation (n = 3) and phase 2 efficacy (n = 3) clinical studies (note: one article included results from both a phase 1 and 2 study). As such, these clinical trials were relatively small (10–43 participants), non-randomized, and did not include a comparison group. Four of the five articles were found to have low overall risk of bias, with one found to be at serious risk of bias due to deviation from the study protocol (Table 1).
Fig. 2.
Flow chart illustrating the process of screening and identifying articles included in present review
Table 1.
Evaluation of bias in articles included in present review using the Risk Of Bias In Non-randomized Studies of Interventions (ROBINS-I) tool
| Bias domain | Vigushin et al. 1998 [22] | Ripple et al. 1998 [23] | Ripple et al. 2000 [24] | Bailey et al. 2008 [25] | Miller et al. 2013 [26] |
|---|---|---|---|---|---|
| Pre-intervention | |||||
| Confounding | low | low | low | low | low |
| Selection of participants | low | low | low | low | low |
| At intervention | |||||
| Classification of intervention | low | low | low | low | low |
| Post intervention | |||||
| Deviation from intended intervention | low | low | low | Serious | low |
| Missing data | low | low | low | low | low |
| Measurement of outcomes | low | low | low | low | low |
| Selection of reported results | low | low | low | low | low |
| Overall | low | low | low | Serious | low |
Participant characteristics
One hundred and thirty-three participants were included across the two d-limonene (n = 85 participants) and three perillyl alcohol (n = 48 participants) studies meeting eligibility for this review (Table 2). However, while 136 total participants were enrolled in the studies, 128 (94%) were evaluable for study outcomes. The 8 participants not evaluated in the studies were removed owing to death, severe adverse effects, non-compliance and disease progression. Three of the studies focused solely on the effect of the monoterpenes d-limonene and perillyl alcohol on breast cancer [22, 25, 26]. The remaining 2 studies included participants with other cancers, in addition to breast cancer [23, 24]. Majority of the participants included in this review were women (73%) and ranged between the ages of 23 and 90. Overall, 83% of the total number of participants in the studies included in this review were diagnosed with breast cancer.
Table 2.
Summary of collated participant characteristics of participants included in present review
| Vigushin et al. 1998 [22] | Ripple et al. 1998 [23] (n = 18) | Ripple et al. 2000 [24] (n = 16) | Bailey et al. 2008 [25] (n = 14) | Miller et al. 2013 [26] (n = 43) | Total (N = 133) |
||
|---|---|---|---|---|---|---|---|
| Phase 1 (n = 32) | Phase 2 (n = 10) |
||||||
| Enrolled participants; n | 32 | 10 | 18 | 19 | 14 | 43 | 136 |
| Evaluable participants n (%) | 32 (100) | 10 (100) | 16 (88.9) | 16 (84.2) | 14 (100) | 40 (93.0) | 128 (94.1) |
| Age; years | |||||||
| Median | 57 | 57 | 58.6a | 50 | 58 | 58.5 | |
| Range | 35–78 | 40–82 | 23–82 | 24–79 | 40–90 | – | 23–90 |
| Sex; n (%) | |||||||
| Male | 15 (46.9) | 0 (0) | 8 (44.4) | 13 (81.3) | 0 (0) | 0 (0) | 36 (27.1) |
| Female | 17 (53.1) | 10 (100) | 10 (55.6) | 6 (37.5) | 14 (100) | 40 (100) | 97 (72.9) |
| Breast cancer; n (%) | 16 (50.0) | 10 (100) | 2 (11.1) | 1(6.3) | 14 (100) | 40 (100) | 83 (62.4) |
| Prior therapies | |||||||
| Surgery | 29 (90.6) | 9 (90.0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 38 (28.6) |
| Chemotherapy | 28 (87.5) | 7 (70.0) | 14 (77.8) | 14 (87.5) | 11 (78.6) | 0 (0) | 74 (55.6) |
| Radiation therapy | 23 (71.9) | 7 (70.0) | 9 (50.0) | 9 (56.3) | 0 (0) | 0 (0) | 48 (36.1) |
| Hormone therapy | 20 (62.5) | 10 (100) | 6 (33.3) | 4 (25.0) | 13 (92.9) | 6 (14.0) | 59 (44.4) |
| Supportive therapy | 2 (6.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (1.5) |
| Biologic response modifiers | 0 (0) | 0 (0) | 4 (22.2) | 1 (6.3) | 0 (0) | 0 (0) | 5 (3.8) |
| Endocrine agents | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 7 (50.0) | 0 (0) | 7 (5.3) |
aOnly mean age provided in article
- not provided
Majority of the included studies enrolled participants with advanced or metastatic disease [22–25], that were unresponsive to conventional therapies [22–24]. Only one study by Miller et al. enrolled participants with early-stage malignancies [26]. Exclusion criteria included pregnancy/breastfeeding [22, 25, 26], brain metastasis [23, 25], HIV diagnosis [22], and receipt of treatment (hormonal, immunological, chemo or radiation therapies) in weeks preceding participation in study [24–26]. All studies excluded participants using cholesterol lowering drugs, vitamin supplements, and participants with poor kidney or bone marrow function. About half of the participants included in this review (56%) had undergone one or more cycles of chemotherapy prior to enrollment in respective studies. Furthermore, participants had undergone hormone therapy (44%), radiation therapy (36%) and surgery (29%).
Intervention and supplement formulation
In all studies included in this review, either d-limonene or perillyl alcohol was administered orally as an intervention (Table 3). In phase 1 dose escalation studies, perillyl alcohol was formulated as soft gelatin capsules consisting 250 mg perillyl alcohol and 250 mg soybean oil [23–25]. Dosing was escalated from 800 (level 1), to 1200 (level 2) and 1600 (level 3) mg/m2/dose administered 3 times a day in the Ripple et al. (1998) study [23], and 800 (level 1), to 1600 (level 2) and 2400 (level 3) mg/m2/dose administered 4 times a day in the Ripple et al. (2000) study [24]. In a more recent phase 2 trial by Bailey et al. [25], 1200 (level 1) and 1500 (level 2) mg/m2/dose were administered 4 times a day. Only 1 d-limonene trial by Vigushin et al. [22] included a dose escalation study, where the schedule ranged from 0.5–12 g/m2/day. In the second limonene study by Miller et al. [26], 2 g of commercially available d-limonene was administered.
Table 3.
Summary of clinical studies included in review of d-limonene and its derivatives on breast cancer
| Author(s) | Trial Phase | Compound | Cancer type | Dose | Toxicity | Maximum tolerated dose | Effect on breast cancer |
|---|---|---|---|---|---|---|---|
| d-limonene | |||||||
| Vigushin et al. 1998 [22] | Phase 1: Human subjects (n = 32) | Orally administered d-limonene | Phase 1: refractory solid tumorsa | 0.5 to 12 g/m2 per day in 21-day cycles | Gastrointestinal toxicities leading to nausea, vomiting and diarrhea were dose limiting | 8 g/m2 per day | Phase 1: Partial responseb observed in one breast cancer patient. Effect was sustained for 11 months. |
| Phase 2: Human subjects (n = 10) | Phase 2: locally advanced breast cancer | 8 g/m2 per day for 15 cycles | Not indicated | Phase 2: no response | |||
| Miller et al. 2013 [26] | Phase 2: Human subjects (n = 43) | Orally administered d-limonene | Newly diagnosed, operable cancers breast cancer | 2 g d-limonene for 2–6 weeks | Well tolerated | Not indicated | D-limonene concentrated in breast tissue (mean 41.3 μg/g tissue); Small but statistically significant increase in insulin-like growth factor levels; Reduction in tumor cyclin D1 expression |
| Perillyl alcohol | |||||||
| Ripple et al. 1998 [23] | Phase 1: Human subjects (n = 18) | Orally administered perillyl Alcohol | Advanced malignanciesc | Dose escalation: 800, 1200 and 1600 mg/m2/dose administered 3 time a day | Dose- related gastrointestinal toxicities leading to nausea and vomiting; 2 participants experienced severe drug related myelosuppression | Not indicated | No objective tumor response observed in any patients |
| Ripple et al. 2000 [24] | Phase 1: Human subjects (n = 16) | Orally administered perillyl Alcohol | Advanced refractory malignanciesd | Dose escalation: 800, 1600 and 2400 mg/m2/dose administered 4 time a day | Gastrointestinal toxicities, including nausea, vomiting, satiety, and eructation, that were dose limiting | 1200 mg/m2/dose | No anticancer activity observed in breast cancer patient. Tumor regression observed in one patient with metastatic colorectal cancer |
| Bailey et al. 2008 [25] | Phase 2: Women (N = 14) | Orally administered perillyl Alcohol | Advanced treatment-refractory breast cancer | Dose escalation: 1200–1500 mg/m2/dose administered 4 time a day | Poor toleration due to gastrointestinal and fatigue- related toxicities | Not indicated | No partial or complete regression observed in any participant. |
aIncluding breast cancer colorectal carcinoma, metastatic adenocarcinoma, esophagus, pancreas, bronchus, ovary, and soft tissue sarcoma
bPartial response defined as ≥ 50% reduction in tumor size assessed by two measurements conducted ≥ 4 weeks apart
cIncluding prostrate (n = 4), ovarian (n = 3), sarcoma, renal cell (n = 3), breast (n = 2), hepatocellular (n = 2), chronic myelogenous leukemia (n = 1), chronic lymphocytic leukemia (n = 1),adenocarcinoma (n = 1)
dincluding: prostrate (n = 4), ovarian (n = 3), adenocarcinoma (n = 2), colorectal (n = 1), chronic myelogenous leukemia (n = 1), melanoma (n = 1), non-Hodgkin’s lymphoma (n-1), pancreas (n = 1), salivary gland (n = 1), and sarcoma (n = 1)
Maximum tolerated dosing, toxicity and tolerance
Adverse events in all studies included in this review were classified according to the National Cancer Institute criteria and ranged from grade 0 (no events) to grade 4 (life threatening events). Overall, d-limonene was tolerable in both patients with advanced and early-stage malignancies, receiving single or multiple daily dosing [22, 26]. In the Vigushin et al. trial [22], 2 of the 32 participants receiving 6 g/m2/day discontinued the study as a result of limonene-linked gastrointestinal (GI) toxicity not exceeding grade 2 (nausea and diarrhea). A Maximum Tolerable Dose (MTD) of 8 g/m2/day was established in this study. However, dose escalation was limited by withdrawal from the studies due to disease progression. In the trial by Miller et al. [26], 3 of the 43 women terminated participation in the study early due to adverse events (heartburn, nausea and vomiting). In both the d-limonene trials, there were no grade 4 or serious organ toxicities observed.
Gastrointestinal toxicities were dose limiting in all three perillyl alcohol trials [23–25]. Dose-related adverse events including nausea, vomiting, diarrhea and fatigue were observed in these trials [23–25]. Two participants in the Ripple et al. (1998) trial [23] enrolled to level 3 dosing (2400 mg/m2/dose) required dose reduction to level 2 (1600 mg/m2/dose) due to these adverse events. Additionally, 3 participants experienced severe drug related myelosuppression – however, the three were ovarian (n = 2) and renal cell carcinoma (n = 1) cancer patients [23]. In the Ripple et al. (2000) trial [24], three participants experienced toxicities greater than grade 1 at 1200 and 1600 mg/m2/dose. This study established the MTD to be 1200 mg/m2/dose. This same MTD was arrived at by Bailey et al. trial [25] where 29 cycles of perillyl alcohol at 1200 mg/m2/dose were completed by all participants. Participants experienced majority of grade 3 and 4 toxicities in the first cycle. These included grade 3 nausea, vomiting, elevated alkaline phosphatase, and elevated aspartate transaminase; as well as grade 4 dyspnea and elevated lactate dehydrogenase [25]. Three participants whose dosing was escalated to 1500 mg/m2/dose, discontinued the study due to intolerability [25].
Pharmacokinetic profile
Transformation of d-limonene into bioactive monoterpenes was observed in the trial by Vigushin et al. [22]. Metabolites, including perillic acid, dihydroperillic acid, limonene-1,2-diol, and uroterpenol, were observed, with peak concentration levels achieved on day 21 [22]. D-limonene was also found to accumulate in breast tissue (mean 41.3 μg/g tissue) and induce a statistically significant, albeit small, increase in IGF-I levels among study participants in the Miller et al. trial [26]. Furthermore, a statistically significant reduction (22%) in the tumor cyclin D1 expression was observed in this trial [26].
Perillyl alcohol metabolites including perillic acid dihydroperillic acid were detected in participant plasma, with peak levels occurring between 2 and 3 and 3–5 h after ingestion respectively [23]. Furthermore, about 9% of perillyl alcohol was excreted within the first 24 h through urine [23, 24].
Anticancer activity
Overall, no objective complete tumor response – defined as absence of detectable clinical disease for more than 4 weeks – was observed in the d-limonene (n = 2) or perillyl alcohol (n = 3) trials. In the Vigushin et al. phase 1 d-limonene trial however, a partial response – defined as ≥ 50% reduction in tumor size assessed by two measurements conducted ≥ 4 weeks apart – was observed in one breast cancer patient [22]. This effect was sustained for 11 months and prompted a phase 2 trial exclusively among breast cancer patients. In this phase 2 trial (n = 10 participants), there was no response observed [22].
In the perillyl alcohol trials, no clinical benefit was observed among breast cancer patients [23–25]. In the Ripple et al. (2000) trial [24], however, chemotherapeutic activity was observed in 1 colorectal cancer patient, who experienced near-complete response – resolution of all but 1 lesion – for more than 2 years. Additionally, 2 prostate cancer patients treated at level 1 (800 mg/m2/dose) experienced disease stabilization for 13 and 10 months [24]. In the same study, a adenoidcystic carcinoma patient treated at level 2 (1200 mg/m2/dose) experienced stable disease for 8 months before progression [24]. Similarly, 2 metastatic breast patients in the Bailey et al. trial demonstrated disease stabilization [25]. However, there was no freedom from progression 1 year from initiation, with a median rate to disease progression of 35 days and a median survival of 389 days [25].
Discussion
This scoping review aimed to explore the breadth and depth of existing evidence on the effect of d-limonene and its derivatives on breast cancer on human subjects, with the goal of highlighting gaps in knowledge. Our review yielded five eligible studies with a total of 133 participants, evaluating the chemotherapeutic properties of d-limonene (n = 2 trials; 85 participants) and perillyl alcohol (n = 3 trials; 48 participants). The number of articles resulting from our search was noticeably small, demonstrating the dearth of evidence available on the effect of d-limonene on breast cancer in human subjects. All studies included in this review were early-phase (1&2) clinical trials evaluating the safety and efficacy of the monoterpenes d-limonene and Perillyl alcohol. Perillyl alcohol dose escalation studies ranged from 800 to 2400 mg/m2/dose with a MTD of 1200 mg/m2/dose. This hydroxylated monoterpene derivative was generally poorly tolerated with participants experiencing dose-limiting gastrointestinal toxicities. Conversely, d-limonene was well tolerated in participants with early- and advance-stage malignancies who received doses ranging between 0.5 and 12 g/m2. Neither perillyl alcohol nor d-limonene demonstrated significant chemotherapeutic properties. We postulate that this null result may have been as a result of the study small sample sizes, and/or the advance stage malignancies of the participants.
In rodent models with chemically induced carcinogenesis, d-limonene and its derivatives have demonstrated a statistically significant decrease in mammary tumors incidence (Table 4) [16, 27, 28]. In one study, a 72% decrease in tumor incidence was observed among rats fed d-limonene (10,000 ppm) compared with controls not given the monoterpene and followed for 18 weeks [27]. Additionally, rodents fed d-limonene in the initiation phase showed an increase in the duration between introduction of the carcinogen and development of the first tumors [16, 29]. This increased latency period observed a dose response relationship, with longer latency at higher d-limonene dosage [27].
Table 4.
Summary of findings from in vivo and in vitro pre-clinical studies evaluating the effect of d-limonene and its derivatives on breast cancer
| Author(s) | Model | Compound and dosage under investigation | Cancer type | Effect |
|---|---|---|---|---|
| Elegbede et al. 1984 [27] | Female Sprague-Dawley Rats | 1000 or 10,000 ppm d-limonene | DMBAa-induced rat mammary tumor | Inhibited mammary carcinogenesis due to increased latency; significant differences in incidence (72% reduction in tumors at 18 weeks among d-limonene fed animal); and regression of mammary tumors. |
| Elegbede et al.1986 [28] | Female (W/Fu X F344)F2 | 10% d-limonene | DMBAa-induced rat mammary tumor | Significant regression of chemically induced tumors in rats fed d-limonene (p-value 0.016). d-limonene inhibited formation of subsequent tumors (p-value < 0.025) |
| Elson et al. 1988 [29] | Female Sprague-Dawley Rats | 5% d-limonene | DMBAa-induced rat mammary tumor | Reduction of average number of rat mammary carcinomas when fed d-limonene during the initiation or during the promotion/progression stage of carcinogenesis (p < 0.05); time to appearance of first tumor extended only when d-limonene fed during initiation stage (p < 0.005). |
| Maltzman et al 1989 [30]. | Female Wistar-Furth rats | 5% d-limonene and 5% orange oil | NMUb-induced mammary tumors | Orange oil (p < 0.001) and d-limonene (p < 0.001) prevent rat NMU- induced mammary carcinomas when introduced in the promotion/progression phase. No statistical difference in effect of orange oil and limonene. |
| Crowell et al. 1992 [16] | Female Wistar-Furth rats | 1% d-limonene and 1% hydroxylated derivativesc | aDMBA-induced rat mammary tumor | No significant effect on tumor latency or multiplicity in rats receiving 1% d-limonene. Rats receiving 1% of uroterpenol and sobreol had significant increase in latency (p < 0.005 and p = 0.0001 respectively); significant decrease in tumor multiplicity in rats fed carveol (p < 0.05), uroterpenol (p < 0.025), and sobreol (p < 0.0001). |
| Haag et al. 1992 [31] | Female Wistar-Furth rats | 0, 2.5, 5, 7.5 and 10% d-limonene | DMBAa and NMUb -induced rat mammary tumor | Statically significant complete regression rate observed starting at 5% limonene dietary levels. At 10% dietary limonene level, there was a 68% (p < 0.001) and 96% (p < 0.001) complete tumor regression rate in DBMA and NMU induced rats respectively. Established minimum dose of 7.5% dietary limonene required for a significant increase in complete tumor regression. |
| Jirtle et al. 1993 [32] | Female Fischer 344 rats | 10% d-limonene | Advanced DMBAa-induced rat mammary tumor | Significant (p < 0.0001) regression in limonene fed rats (87%) compared to the control rodents (7%). Observed increased Growth Factor β1 and Mannose 6-Phosphate/Insulin-like Growth Factor II Receptor in limonene treated tumors, suggesting this as a possible mode of action. |
| Haag and Gould 1994 [15] | Female Wistar-Furth rats | 2% Perillyl alcohol | DMBAa-induced rat mammary tumor | Statistically significant difference (p < 0.01) in complete regression of primary carcinomas (≥ 3 mm in diameter) of perillyl alcohol fed (2.5% w/w) rats (81% and the controls 31%). Treatment group also had lower rates of secondary tumors. |
| Chander et al. 1994 [33] | Female Ludwig/Wistar/Olac rats | 10% limonene; 5% d-limonene; 12.5 mg/kg HADd; and combination 5% d-limonene and 12.5 mg/kg HADd | NMUb-induced mammary tumors | Significant rates of regression (p < 0.05) observed with 10% limonene; and 5% limonene, 4-HAD (12.5 mg/kg). Highest rate of tumor regression recorded in rats treated with the combination of 5% limonene and 4-HAD |
| Asamoto et al. 2002 [34] | Female Hras128e rats | 5% d-limonene | bNMU-induced mammary tumors | Significant reduction in multiplicity and tumor size (diameter p < 0.002, and volume p < 008) observed in rats treated with 5% d-limonene |
| Yuri et al. 2004 [35] |
In vitro: ERf+ and ERf- human breast cancer cell lines In vivo: female BALB/c mice |
500 μ M Perillyl alcohol for in vivo experiments; and 75 mg/Kg for in vitro studies | Human breast cancer cell inoculated mice | In vivo experiments: cell growth and proliferation inhibited by perillyl alcohol; In vitro study: treatment with perillyl alcohol resulted in significantly smaller tumors (p < 0.05) in terms of volume and weight |
aDMBA – 7,12-Dimethylbenz[a]anthracene
bNMU- N -methyl- N -nitrosourea
c Carveol, uroterpenol and sobrerol
d4-hydroxyandrostenedione
eHuman c-Ha-ras proto-oncogene
fEstrogen receptor
When limonene derivatives were introduced in the promotion/progression stage in preclinical models, a statistically significant reduction in tumor multiplicity was observed [16]. Limonene, too, demonstrated a two-fold increase in protection against the development of secondary tumors, and induced a 63% regression in the secondary tumors formed [31]. Perillyl alcohol also demonstrated the prevention of development of secondary tumors [15] and suppressed growth in estrogen receptor human breast cancer cells [35]. Statistically significant reduction in tumor size (diameter p < 0.002, and volume p < 008) was observed in rats treated with 5% d-limonene [34].
Limonene-induced regression greater than 80% has been observed in 7,12-Dimethylbenz [a] anthracene (DBMA)-induced tumors, during the initiation and promotion/progression carcinogenesis stages [27–29, 31, 32]. Furthermore, studies examining perillyl alcohol demonstrated this limonene derivative induced regression in both early- and advance-stage carcinomas in DBMA-induced rodent models. In one study, a statistically significant difference (p < 0.01) in complete regression of primary carcinomas ≥ 3 mm in diameter of perillyl alcohol fed rats (81%) and the controls (31%) was observed [15]. However, in models where N -methyl- N -nitrosourea (NMU) was used as the carcinogen, tumor regression was only observed in the promotion/progression stage [30].
In DBMA and NMU cancer-induced rodent models, statistically significant levels of regression were observed beginning at 5% limonene dietary levels [31]. A dose of 7.5% was determined as the minimum dose required to observe significant increase in complete tumor regression in one study [31]. Given limited suppressive activity of limonene in the initiation phase of NMU-induced mammary carcinogenesis, Chander et al. assessed if supplementation with a aromatase inhibitor (4- hydroxyandrostenedione) could enhance tumor inhibition [33]. The researchers found that suboptimal doses of limonene and 4-HAD (5%) resulted in an 83% overall tumor regression (p < 0.001, 35]. Limited toxicity has been observed among rodent models treated with d-limonene and its derivatives [15, 31, 35].
Pre-clinical trials suggest serum levels of Transforming Growth Factor Beta 1 (TGF- β1) are linked to anticancer activity of monoterpenes through cell cycle regulation [32, 36]. Pharmacokinetic findings in this review demonstrated metabolic elements that are supported by these in vivo and in vitro findings. Miller et al. [26] reported a reduction in cyclin D1 expression, which has been shown to play a role in cell cycle progression [37–39]. The same study showed an increase in IGF-I among patients. While elevation of IGF-I is associated with increased cancer risk, the clinical implication in this study was unknown given the short duration of the study. A parallel study evaluating the plasma metabolic profiles of the same participants showed d-limonene as changing metabolic pathways in the participants [42]. Additionally, Pre-clinical studies have suggested that d-limonene induces cytostasis through selective inhibition of isopreylation of small G proteins [31].
None of the studies included in this review reported complete tumor response in patients with breast cancer. This null finding should be interpreted with caution for two reasons. First, most of the trials (4 of 5) were conducted among patients with advanced disease, who were heavily pretreated prior to enrolling in the respective studies [22–25]. Additionally, cancer subtype information was not reported in the studies included in this review. Preclinical findings may be an indication that perillyl alcohol may be chemopreventive, and not act during the progression stages of disease. Metabolic and pharmacokinetic analyses in the Miller et al. trial among newly diagnosed patients with operable breast cancer indicated more promising chemotherapeutic properties during this early-stage carcinogenesis [26]. Therefore, additional investigation of the effect of d-limonene and its derivatives in patients in the initiation stage of disease may be warranted. Furthermore, future studies should disaggregate findings by breast cancer subtype. Second, all studies recruited few patients, ranging from 10 to 43, which may have limited their power to detect clinically significant difference. Of note, the Bailey et al. [25] study was designed to enroll 40 participants to achieve adequate power. However, only 14 participants were enrolled [25]. Furthermore, the percentage of breast cancer patients enrolled in some studies was low. Notably, breast cancer participants constituted 11 and 6% of enrolled participants in the Ripple et al. (1998 and 2000) studies respectively [23, 24].
Of the two monoterpenes, d-limonene showed more chemotherapeutic promise, with partial tumor response observed in one patient [22]. Furthermore, d-limonene was found to be fat soluble, accumulating in breast tissue and retained in the body for a longer period of time [26]. This finding has been supported by other research, suggesting that d-limonene may be a candidate for further human trials for efficacy [10, 40]. Conversely, perillyl alcohol was expelled from the body at higher rates and did not show evidence of accumulation [23, 24]. This suggests that perillyl alcohol may need to be used in concert with other active ingredients, such as aromatase inhibitors, to bolster efficacy.
The present review was the first to systematically collate research on the effect of d-limonene and its derivatives on breast cancer in human subjects. However, our study was limited by the heterogeneity of the studies included in the review. Different indicators were used in the included trials, restricting our ability to compare chemotherapeutic activity across the studies.
Conclusion
Citrus peel and citrus oils contain bioactive compounds, which laboratory and animal models have shown as promising to address breast cancer. This review of early clinical trials of d-limonene and its derivative, perillyl alcohol, demonstrated significant gaps in knowledge on the subject as evidenced by the few studies currently available. In the five trials included in the review, d-limonene (n = 2) was better tolerated and exhibited more promising chemopreventive properties compared to its derivative (n = 3). Well-powered clinical trials of d-limonene among patients with early-stage carcinogenesis may offer greater insight into the effect of d-limonene on breast cancer.
Supplementary Information
Additional file 1: Table S1. Databases and search terms used for scoping review
Additional file 2: Table S2. JBI Critical appraisal checklist for non-randomized experimental studies*.
Additional file 3: Table S3. PRISMA-ScR checklist*.
Acknowledgements
None.
Abbreviations
- ACA
American Cancer Association
- CINAHL
Cumulative Index of Nursing and Allied Health Literature
- DMBA
7,12-Dimethylbenz [a]anthracene
- GERD
Gastroesophageal Reflux Disease
- GI
Gastrointestinal
- JBI
Joanna Briggs Institute
- MTD
Maximum Tolerable Dose
- NMU
N -methyl- N -nitrosourea
- PRISMA-ScR
PRISMA Extension for Scoping Reviews
- ROBINS-I
Risk Of Bias In Non-randomized Studies of Interventions
- TGF- β1
Transforming Growth Factor Beta 1
Authors’ contributions
Conceptualization: IH, JE. Methodology and design: IH, JE, JJC, DT, DJM. Data abstraction: JJC, IH, JE. Original draft: JJC. Editing and review: JJC, JE, DT, IH, DJM. All authors have read and approved the manuscript.
Funding
This review was supported by the “Food as Medicine Initiative” of the Mel and Enid Zuckerman College of Public Health, University of Arizona. The funding body played no role in the study conception, design, data collection, data analysis, data interpretation or writing of the report.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
This study did not involve the collection of primary data and therefore did not undergo ethical review. All studies included in this review, however, received ethical clearance and participants provided informed consent as a prerequisite for participation.
Consent for publication
Not applicable.
Competing interests
Authors declare they have no competing interest
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 2.Hirbe A, Morgan EA, Uluçkan Ö, Weilbaecher K. Guise, Coleman, et al. skeletal complications of breast cancer therapies. Clin Cancer Res. 2006;12:6309–6315. doi: 10.1158/1078-0432.CCR-06-0652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moslehi JJ. Cardiovascular toxic effects of targeted cancer therapies. N Engl J Med. 2016;375(15):1457–1467. doi: 10.1056/NEJMra1100265. [DOI] [PubMed] [Google Scholar]
- 4.Shapiro CL, Recht A. Side effects of adjuvant treatment of breast cancer. N Engl J Med. 2001;344(26):1997–2008. doi: 10.1056/NEJM200106283442607. [DOI] [PubMed] [Google Scholar]
- 5.Sergeev IN, Ho CT, Li S, Colby J, Dushenkov S. Apoptosis-inducing activity of hydroxylated polymethoxyf lavones and polymethoxyflavones from orange peel in human breast cancer cells. Mol Nutr Food Res. 2007;51(12):1478–1484. doi: 10.1002/mnfr.200700136. [DOI] [PubMed] [Google Scholar]
- 6.Lakshmi A, Subramanian S. Chemotherapeutic effect of tangeretin, a polymethoxylated flavone studied in 7, 12-dimethylbenz(a) anthracene induced mammary carcinoma in experimental rats. Biochimie. 2014;99:96–109. doi: 10.1016/j.biochi.2013.11.017. [DOI] [PubMed] [Google Scholar]
- 7.Park JY, Shin MS, Kim SN, Kim HY, Kim KH, Shin KS, et al. Polysaccharides from Korean Citrus hallabong peels inhibit angiogenesis and breast cancer cell migration. Int J Biol Macromol. 2016;85:522–529. doi: 10.1016/j.ijbiomac.2016.01.015. [DOI] [PubMed] [Google Scholar]
- 8.Nipin SP, Kang DY, Joung YH, Park JH, Kim WS, Lee HK, et al. Nobiletin inhibits angiogenesis by regulating Src/FAK/STAT3-mediated signaling through PXN in ER+ breast cancer cells. Int J Mol Sci. 2017;18:935. doi: 10.3390/ijms18050935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ciriminna R, Lomeli-Rodriguez M, Demma Carà P, Lopez-Sanchez JA, Pagliaro M. Limonene: A versatile chemical of the bioeconomy. Chem Commun 2014;50:15288–15296. Royal Society of Chemistry. Available from: 10.1039/C4CC06147K [DOI] [PubMed]
- 10.Miller JA, Hakim IA, Chew W, Thompson P, Thomson CA, Chow HHS. Adipose tissue accumulation of d-limonene with the consumption of a lemonade preparation rich in d-limonene content. Nutr Cancer. 2010;62(6):783–788. doi: 10.1080/01635581003693066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crowell PL, Elson CE, Bailey HH, Elegbede A, Haag JD, Gould MN. Human metabolism of the experimental cancer therapeutic agent d-limonene. Cancer Chemother Pharmacol. 1994;35(1):31–37. doi: 10.1007/BF00686281. [DOI] [PubMed] [Google Scholar]
- 12.Sun J. D-Limonene. D-Limonene. 2007;12:259–264. [PubMed] [Google Scholar]
- 13.Miller JA, Thompson PA, Hakim IA, Chow HHS, Thomson CA. D-limonene: a bioactive food component from citrus and evidence for a potential role in breast cancer prevention and treatment. Oncol Rev. 2011;5(1):31–42. doi: 10.1007/s12156-010-0066-8. [DOI] [Google Scholar]
- 14.Igimi H, Hisatsugu T, Nishimura M. The use of d-limonene preparation as a dissolving agent of gallstones. Am J Dig Dis. 1976;21(11):926–939. doi: 10.1007/BF01071903. [DOI] [PubMed] [Google Scholar]
- 15.Haag JD, Gould MN. Mammary carcinoma regression induced by perillyl alcohol, a hydroxylated analog of limonene. Cancer Chemother Pharmacol. 1994;34(6):477–483. doi: 10.1007/BF00685658. [DOI] [PubMed] [Google Scholar]
- 16.Crowell PL, Kennan WS, Haag JD, Ahmad S, Vedejs E, Gould MN. Chemoprevention of mammary carcinogenesis by hydroxylated derivatives of d-limonene. Carcinogenesis. 1992;13(7):1261–1264. doi: 10.1093/carcin/13.7.1261. [DOI] [PubMed] [Google Scholar]
- 17.Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol Theory Pract. 2005;8(1):19–32. doi: 10.1080/1364557032000119616. [DOI] [Google Scholar]
- 18.Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev; 2016;5:1–10. Available from: 10.1186/s13643-016-0384-4, 1 [DOI] [PMC free article] [PubMed]
- 19.Tufanaru C, Munn Z, Aromataris E, Campbell J, Hopp L. In: JBI Manual for Evidence Synthesis. Aromataris E, Munn Z, editors. JBI Man. Evid. Synth. JBI; 2020. [Google Scholar]
- 20.Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:4–10. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467–473. doi: 10.7326/M18-0850. [DOI] [PubMed] [Google Scholar]
- 22.Vigushin DM, Poon GK, Boddy A, English J, Jarman M, Coombes CR. Phase I and pharmacokinetic study of lapatinib and docetaxel in patients with advanced cancer. Cancer Chemother Pharmacol. 1998;42(2):111–117. doi: 10.1007/s002800050793. [DOI] [PubMed] [Google Scholar]
- 23.Ripple GH, Gould MN, Stewart JA, Tutsch KD, Arzoomanian RZ, Alberti D, Feierabend C, Pomplun M, Wilding G, Bailey HH. Phase I clinical of Perillyl alcohol administered daily. Clin Cancer Res. 1998;4(5):1159–1164. [PubMed] [Google Scholar]
- 24.Ripple GH, Gould MN, Arzoomanian RZ, Alberti D, Feierabend C, Simon K, Binger K, Tutsch KD, Pomplun M, Wahamaki A, Marnocha R, Wilding G, Bailey HH. Phase I clinical and pharmacokinetic study of perillyl alcohol administered four times a day. Clin Cancer Res. 2000;6(2):390–396. [PubMed] [Google Scholar]
- 25.Bailey HH, Attia S, Love RR, Fass T, Chappell R, Tutsch K, Harris L, Jumonville A, Hansen R, Shapiro GR, Stewart JA. Phase II trial of daily oral perillyl alcohol (NSC 641066) in treatment-refractory metastatic breast cancer. Cancer Chemother Pharmacol. 2008;62(1):149–157. doi: 10.1007/s00280-007-0585-6. [DOI] [PubMed] [Google Scholar]
- 26.Miller JA, Lang JE, Ley M, Nagle R, Hsu CH, Thompson PA, Cordova C, Waer A, Chow HHS. Human breast tissue disposition and bioactivity of limonene in women with early-stage breast cancer. Cancer Prev Res. 2013;6(6):577–584. doi: 10.1158/1940-6207.CAPR-12-0452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elegbede JA, Elson CE, Qureshi A, Tanner MA, Gould MN. Inhibition of DMBA-induced mammary cancer by the monoterpene d-limonene. Carcinogenesis. 1984;5(5):661–664. doi: 10.1093/carcin/5.5.661. [DOI] [PubMed] [Google Scholar]
- 28.Elegbede JA, Elson CE, Tanner MA, Qureshi A, Gould MN. Regression of rat primary mammary tumors following dietary d-limonene. J Natl Cancer Inst. 1986;76(2):323–325. [PubMed] [Google Scholar]
- 29.Elson CE, Maltzman TH, Boston JL, Tanner MA, Gould MN. Anti-carcinogenic activity of d-limonene during the initiation and promotion/progression stages of dmba-induced rat mammary carcinogenesis. Carcinogenesis. 1988;9(2):331–332. doi: 10.1093/carcin/9.2.331. [DOI] [PubMed] [Google Scholar]
- 30.Maltzman TH, Hurt LM, Elson CE, Tanner MA, Gould MN. The prevention of nitrosomethylurea-induced mammary tumors by d-limonene and orange oil. Carcinogenesis. 1989;10(4):781–783. doi: 10.1093/carcin/10.4.781. [DOI] [PubMed] [Google Scholar]
- 31.Haag JD, Lindstrom MJ, Gould MN. Limonene-induced regression of mammary carcinomas. Cancer Res. 1992;52(14):4021–4026. [PubMed] [Google Scholar]
- 32.Jirtle RL, Haag JD, Ariazi EA, Gould MN. Increased mannose 6-phosphate/insulin-like growth factor II receptor and transforming growth factor B1 levels during monoterpene-induced regression of mammary tumors. Cancer Res. 1993;53(17):3849–3852. [PubMed] [Google Scholar]
- 33.Chander SK, Lansdown AGB, Luqmani YA, Gomm JJ, Coope RC, Gould N, Coombes RC. Effectiveness of combined limonene and 4-hydroxyandrostenedione in the treatment of NMU-induced rat mammary tumours. Br J Cancer. 1994;69(5):879–882. doi: 10.1038/bjc.1994.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Asamoto M, Ota T, Toriyama-Baha H, Hokaiwado N, Naito A, Tsuda H. Mammary carcinomas induced in human c-ha-ras proto-oncogene transgenic rats are estrogen-independent, but responsive to d-limonene treatment. Japan J Cancer Res. 2002;93(1):32–35. doi: 10.1111/j.1349-7006.2002.tb01197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yuri T, Danbara N, Tsujita-Kyutoku M, Kiyozuka Y, Senzaki H, Shikata N, Kanzaki H, Tsubura A. Perillyl alcohol inhibits human breast cancer cell growth in vitro and in vivo. Breast Cancer Res Treat. 2004;84(3):251–260. doi: 10.1023/B:BREA.0000019966.97011.4d. [DOI] [PubMed] [Google Scholar]
- 36.Ariazi EA, Satomi Y, Ellis MJ, Haag JD, Shi W, Sattler CA, Gould MN. Activation of the transforming growth factor β signaling pathway and induction of cytostasis and apoptosis in mammary carcinomas treated with the anticancer agent perillyl alcohol. Cancer Res. 1999;59(8):1917–1928. [PubMed] [Google Scholar]
- 37.Baldin V, Lukas J, Marcote MJ, Pagano M, Draetta G. Cyclin D1 is a nuclear protein required for cell cycle progression in G1. Genes Dev. 1993;7(5):812–821. doi: 10.1101/gad.7.5.812. [DOI] [PubMed] [Google Scholar]
- 38.Bardon S, Foussard V, Fournel S, Loubat A. Monoterpenes inhibit proliferation of human colon cancer cells by modulating cell cycle-related protein expression. Cancer Lett. 2002;181(2):187–194. doi: 10.1016/S0304-3835(02)00047-2. [DOI] [PubMed] [Google Scholar]
- 39.Watts CKW, Sweeney KJE, Warlters A, Musgrove EA, Sutherland RL. Antiestrogen regulation of cell cycle progression and cyclin D1 gene expression in MCF-7 human breast cancer cells. Breast Cancer Res Treat. 1994;31(1):95–105. doi: 10.1007/BF00689680. [DOI] [PubMed] [Google Scholar]
- 40.Miller JA, Thompson PA, Hakim IA, Lopez AM, Cynthia A, Chew W, et al. Massage oil to the breast. J Cancer Ther. 2013;3:1–12. doi: 10.4236/jct.2012.325094. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Databases and search terms used for scoping review
Additional file 2: Table S2. JBI Critical appraisal checklist for non-randomized experimental studies*.
Additional file 3: Table S3. PRISMA-ScR checklist*.
Data Availability Statement
Not applicable.