Abstract
Background
The development of functional neural circuits requires the precise formation of synaptic connections between diverse neuronal populations. The molecular pathways that allow GABAergic interneuron subtypes in the mammalian brain to initially recognize their postsynaptic partners remain largely unknown. The transmembrane glycoprotein Dystroglycan is localized to inhibitory synapses in pyramidal neurons, where it is required for the proper function of CCK+ interneurons. However, the precise temporal requirement for Dystroglycan during inhibitory synapse development has not been examined.
Methods
In this study, we use NEXCre or Camk2aCreERT2 to conditionally delete Dystroglycan from newly-born or adult pyramidal neurons, respectively. We then analyze forebrain development from postnatal day 3 through adulthood, with a particular focus on CCK+ interneurons.
Results
In the absence of postsynaptic Dystroglycan in developing pyramidal neurons, presynaptic CCK+ interneurons fail to elaborate their axons and largely disappear from the cortex, hippocampus, amygdala, and olfactory bulb during the first two postnatal weeks. Other interneuron subtypes are unaffected, indicating that CCK+ interneurons are unique in their requirement for postsynaptic Dystroglycan. Dystroglycan does not appear to be required in adult pyramidal neurons to maintain CCK+ interneurons. Bax deletion did not rescue CCK+ interneurons in Dystroglycan mutants during development, suggesting that they are not eliminated by canonical apoptosis. Rather, we observed increased innervation of the striatum, suggesting that the few remaining CCK+ interneurons re-directed their axons to neighboring areas where Dystroglycan expression remained intact.
Conclusion
Together these findings show that Dystroglycan functions as part of a synaptic partner recognition complex that is required early for CCK+ interneuron development in the forebrain.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13064-021-00153-1.
Keywords: Dystroglycan, Cannabinoid receptor, Cholecystokinin, Interneuron, Synapse, Apoptosis
Background
Proper function of neural circuits requires precise connections between specific populations of excitatory pyramidal and inhibitory neurons. GABAergic interneurons are a highly diverse group of neurons that control brain function by synchronizing and shaping the activity of populations of excitatory pyramidal neurons (PyNs) [1–5]. In mice and humans, the majority of interneurons in the cortex and hippocampus are produced in the medial and caudal ganglionic eminences (MGE and CGE) of the ventral forebrain, and migrate long distances to their final destinations [6–8]. The importance of interneurons for brain function is underscored by their involvement in a wide variety of neurodevelopmental and neurological disorders including autism, schizophrenia, seizures, and Alzheimer’s disease [9–12].
The proper integration of inhibitory interneurons into neural circuits during development relies on multiple processes such as proliferation, migration, axon guidance, cell death, synaptic target selection, synapse formation (synaptogenesis) and synaptic maintenance. Although much progress has been made in identifying candidate molecules that regulate inhibitory synaptogenesis, our understanding of how molecularly defined subtypes of inhibitory interneurons initially identify specific postsynaptic target cells is lacking [13, 14]. One prominent hypothesis for explaining how diverse interneuron subtypes recognize one another during synapse development is the “molecular code” hypothesis, whereby different cell types use unique pairs or complexes of cell adhesion molecules to select their target cells [15–18]. Cell adhesion molecules are ideally suited to regulate synaptic target recognition due to their large diversity and presence at pre- and postsynaptic membranes. Several recent studies support the idea that cell adhesion molecules are key players in regulating subcellular targeting and synaptic specificity [19–22]. Although many families of cell adhesion molecules have been implicated in controlling synapse development, they are often involved in multiple aspects of neural circuit development, making it difficult to determine their precise role in mediating synaptic specificity.
Dystroglycan is a cell adhesion molecule widely expressed throughout the body including the developing and adult brain. Dystroglycan is extensively glycosylated, and mutations in at least 19 genes that participate in synthesizing and elongating specific O-mannose sugar chains on Dystroglycan result in a form of congenital muscular dystrophy called dystroglycanopathy, characterized by muscle weakness and neurological defects of varying severity [23–25]. Dystroglycan (Dag1) is expressed by multiple cell types in the developing nervous system, including neuroepithelial cells, astrocytes, oligodendrocytes, and excitatory neurons [26, 27]. Loss of Dystroglycan function in the nervous system phenocopies the most severe forms of dystroglycanopathy, and causes multiple structural brain and retinal abnormalities due to its indirect role in regulating neuronal migration and axon guidance [28–33]. However, some individuals with milder forms of dystroglycanopathy exhibit cognitive impairments even in the absence of detectable brain malformations, suggesting a possible role for Dystroglycan at later stages of brain development including synaptogenesis [34, 35]. In PyNs, Dystroglycan is highly concentrated on the cell body and proximal dendrites where it is a major postsynaptic component of inhibitory synapses (Fig. 1A [27, 36–38]). However, because of its importance in early aspects of brain development, the role of Dystroglycan at synapses has remained obscure. Using a mouse genetic approach to selectively delete Dystroglycan from PyNs, a recent study showed that Dystroglycan is required for the formation and maintenance of CCK+ interneuron (CCK+ IN) synapses in adult animals, but its specific role in the early development of these interneurons has not been examined [39].
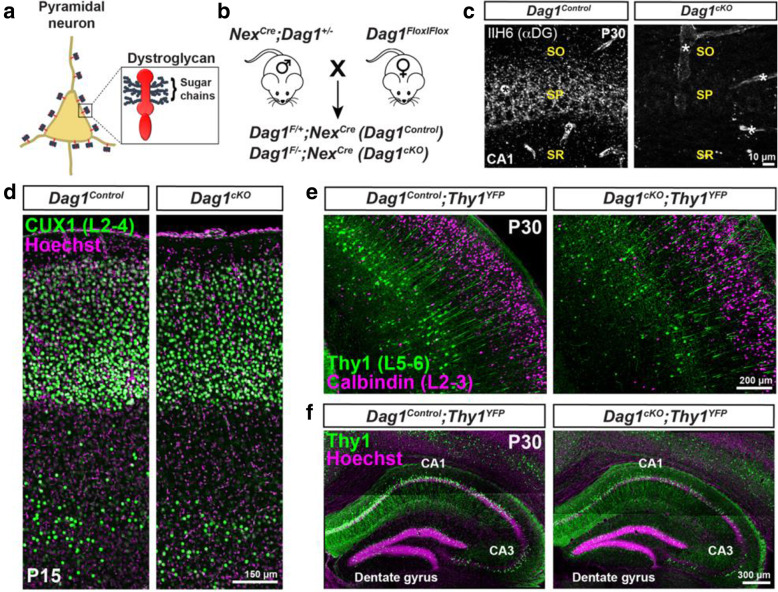
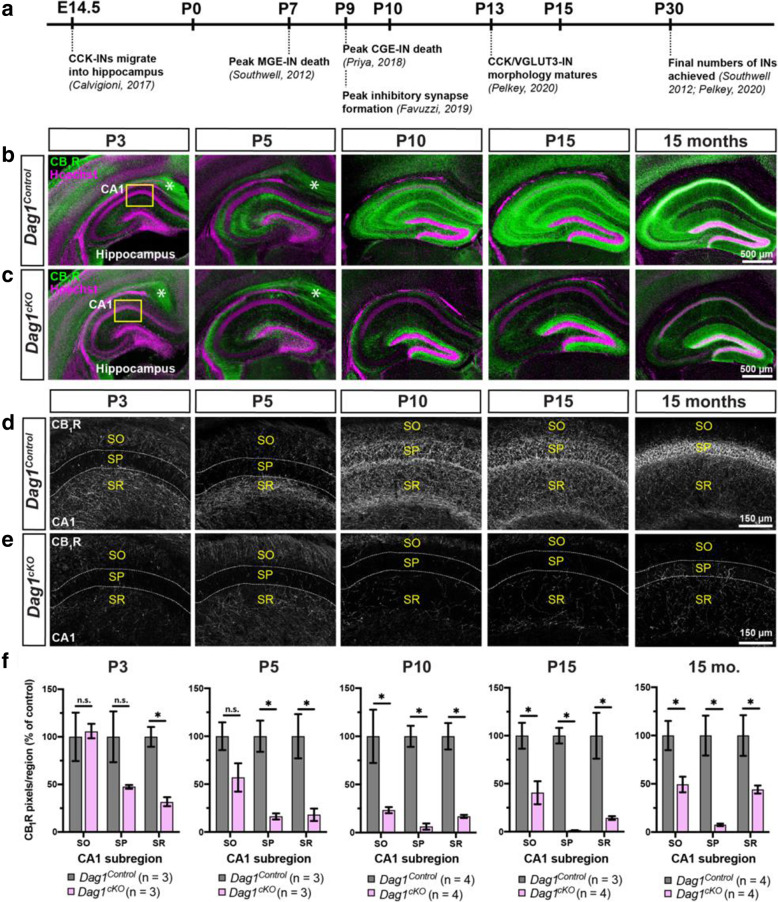
Fig. 1.
Neuronal Dystroglycan is not required for pyramidal neuron migration. (a) Schematic of Dystroglycan on pyramidal neurons. Inset shows the structure of Dystroglycan and sugar chain moieties present on the extracellular subunit. (b) Mouse breeding scheme for generating pyramidal neuron-specific Dag1 conditional knockout mice using NexCre driver mice. (c) Immunostaining for Dystroglycan in the hippocampal CA1 region of P30 Dag1Control mice (left panel) shows punctate Dystroglycan protein on the soma and proximal dendrites of pyramidal neurons, whereas Dag1cKO mice (right panel) lack perisomatic staining. Asterisks denote Dystroglycan staining on blood vessels which is retained in Dag1cKO mice. (d) Coronal sections from P15 Dag1Control and Dag1cKO cortex were immunostained for upper layer marker CUX1 (L2–4). (e) Coronal sections of the cortex from P30 Dag1Control and Dag1cKO mice crossed with a Thy1YFP reporter mouse to sparsely label layer 5–6 pyramidal neurons (green) and stained for Calbindin (magenta) to label layer 2–3 pyramidal neurons. (f) Coronal sections of the hippocampus from P30 Dag1Control and Dag1cKO mice crossed with a Thy1YFP reporter mouse to label excitatory neurons (green) in the CA regions and dentate gyrus
In this study, we show that postsynaptic Dystroglycan on PyNs is required for the proper development of presynaptic CCK+ INs throughout the forebrain. In mice lacking Dystroglycan in PyNs, CCK+ INs fail to elaborate their axons during the first postnatal week and are largely absent by P10. CCK+ INs were not rescued by genetic deletion of Bax suggesting that CCK+ INs may undergo Bax-independent cell death or fail to differentiate in the absence of Dystroglycan. Some remaining CCK+ INs retarget their axons into the striatum, where Dystroglycan expression is retained, suggesting that Dystroglycan functions to allow CCK+ INs to recognize their synaptic partners. Collectively, these results demonstrate that Dystroglycan is a critical regulator of CCK+ IN development.
Results
CCK+ interneurons are largely absent in mice lacking Dystroglycan from pyramidal neurons
To investigate the role of neuronal Dystroglycan in forebrain development, we used a conditional genetic approach to delete Dystroglycan selectively from pyramidal neurons (PyNs). We crossed Dystroglycan conditional mice (Dag1Flox/Flox) with NexCre driver mice to delete Dystroglycan in all postmitotic excitatory neurons of the forebrain except Cajal-Retzius cells, beginning at E12.5 [40–43]. Control (NexCre;Dag1F/+) and conditional knockout mice (NexCre;Dag1F/−) are hereafter referred to as Dag1Control and Dag1cKO mice, respectively (Fig. 1B). We verified the recombination specificity of the NexCre line by crossing it with a reporter mouse that expresses mCherry in the nuclei of Cre-recombined cells (R26LSL-H2B-mCherry [44]). mCherry+ nuclei were detected in excitatory neurons of the forebrain including the cortex, hippocampus, amygdala, and nucleus of the lateral olfactory tract (nLOT) (Fig. S1A). Importantly, mCherry+ nuclei did not overlap with markers for interneurons (CB1R, PV, Calbindin) or astrocytes (GFAP), confirming the specificity of the NexCre mouse (Fig. S1B, C). In Dag1Control mice, Dystroglycan staining was observed as puncta concentrated primarily on the cell bodies and proximal dendrites of PyNs, as well as blood vessels (Fig. 1C). In Dag1cKO mice, Dystroglycan staining was absent from PyNs but was still present on blood vessels, confirming the specificity of the conditional knockout.
Deletion of Dystroglycan from neuroepithelial cells results in disrupted neuronal migration, axon guidance, and dendrite development in the brain, spinal cord and retina [28–33]. In contrast, deletion of Dystroglycan from PyNs with NexCre did not affect overall brain architecture, consistent with previous results [32]. Cortical lamination in Dag1cKO mice was normal based on CUX1 immunostaining of layer 2–4 PyNs and labeling of layer 5–6 and hippocampal PyNs with a Thy1GFP-H transgenic line (Fig. 1D-F). Therefore, neuronal Dystroglycan is not required for PyN migration in the forebrain.
Forebrain interneurons (INs) are a remarkably diverse population, with multiple molecularly and morphologically distinct IN subtypes forming synapses onto different subcellular domains of PyNs [2, 45, 46]. Since Dystroglycan is localized to inhibitory synapses on the soma and dendrites of PyNs, we examined whether IN development is affected in Dag1cKO mice. We performed immunostaining with a panel of molecular markers that label IN subpopulations in the hippocampus of adult mice (Fig. 2). In Dag1Control mice, parvalbumin (PV) and somatostatin (SOM) positive INs, which label the majority of interneurons that originate from the medial ganglionic eminence (MGE), were abundant throughout the hippocampus. The distribution of PV+ and SOM+ cell bodies and their synaptic targeting patterns appeared the same in Dag1cKO mice, suggesting these populations are unaffected by the loss of Dystroglycan (Fig. 2A, B).
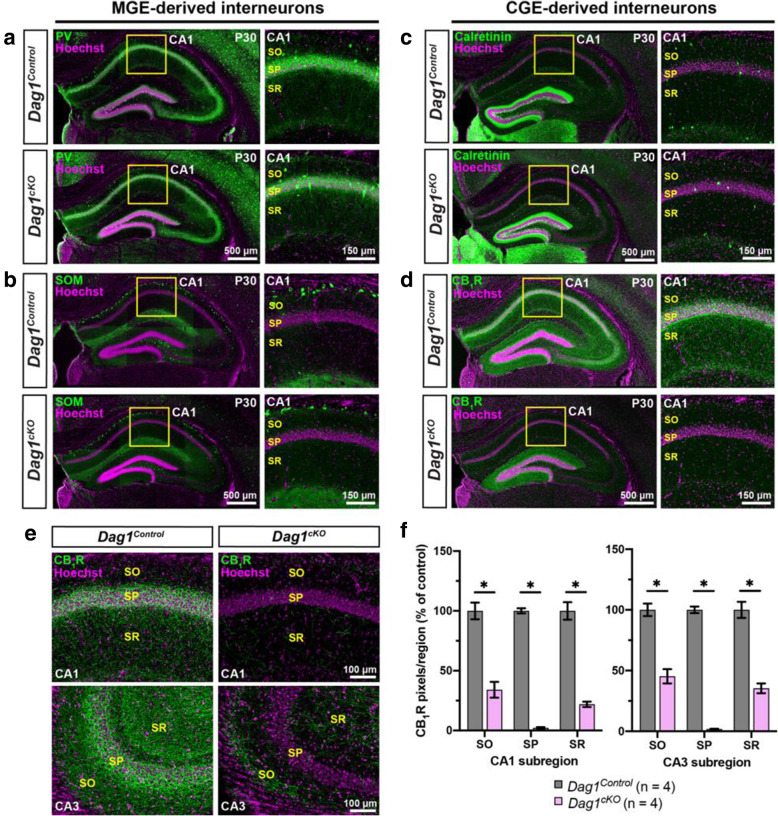
Fig. 2.
CCK+ interneurons are selectively reduced in mice lacking Dystroglycan from pyramidal neurons. (a-b) Immunostaining for medial ganglionic eminence (MGE)-derived interneuron markers (green) parvalbumin (PV) (a) and somatostatin (SOM) (b) show normal innervation of the hippocampus in P30 Dag1Control and Dag1cKO mice. Insets (yellow boxed regions) show enlarged images of the CA1. (c-d) Immunostaining for caudal ganglionic eminence (CGE)-derived interneuron markers (green) Calretinin (c), and CB1R (d) show normal innervation of Calretinin interneurons in Dag1Control and Dag1cKO mice, whereas CB1R is largely absent from the CA regions of Dag1cKO mice. Insets (yellow boxed regions) show enlarged images of the CA1. (e) Immunostaining for CB1R in hippocampal CA1 (top) and CA3 (bottom) of P30 Dag1Control and Dag1cKO mice. (f) Quantification of CB1R pixels for each CA layer of the CA1 and CA3 shows a significant reduction in CB1R staining in Dag1cKO mice (*P < 0.05, unpaired two-tailed Student’s t-test; n = 4 mice/genotype). Data are presented as mean values ± s.e.m. Data are normalized to Dag1Control signal in each CA layer. CA layers: SO, stratum oriens; SP, stratum pyramidale; SR, stratum radiatum
We next stained the hippocampus for IN subtypes that originate from the caudal ganglionic eminence (CGE). The distribution and synaptic targeting of Calretinin interneurons, which target other INs as well as PyN dendrites, appeared normal (Fig. 2C [47, 48]). In contrast, we found a dramatic reduction in cannabinoid receptor-1 (CB1R) staining in the hippocampus, which labels the axon terminals of cholecystokinin (CCK) + INs (Fig. 2D [49–51]). CB1R+ terminals were significantly reduced in all CA subregions (Fig. 2E, F). In both the CA1 and CA3, the magnitude of the reduction varied by layer. CB1R+ terminals were most strongly reduced (> 95%) in the stratum pyramidale (SP) where CCK+ INs form basket synapses onto PyN cell bodies, and more moderately reduced in the stratum radiatum (SR) and stratum oriens (SO) where CCK/CB1R+ INs synapse onto PyN dendrites (Fig. 2E, F). In contrast, CB1R+ terminals were abundant in the dentate gyrus of Dag1cKO mice (Fig. S2). This is likely because NexCre recombination is restricted to the outer third of granular layer neurons (Fig. S1C [41]).
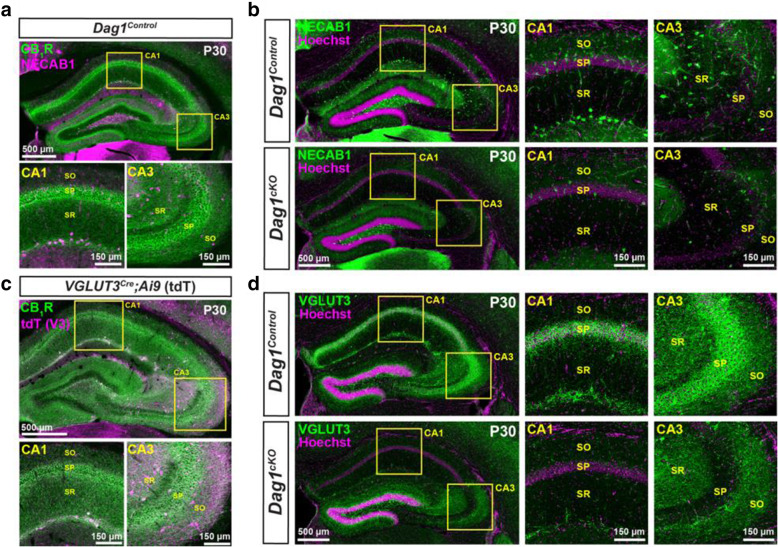
The loss of CB1R staining in the hippocampus of Dag1cKO mice could reflect either downregulation of CB1R expression or a loss of CCK+ INs. To distinguish between these possibilities, we examined whether other independent markers of CGE-derived CCK+ INs were similarly reduced. These include NECAB1, a calcium binding protein that specifically labels CCK+ IN cell bodies (Fig. 3A) [52], and VGLUT3, a vesicular glutamate transporter enriched at CCK+ IN synapses (Fig. 3C) [53–55]. Both NECAB1+ cell bodies and VGLUT3+ synaptic terminals were reduced in the hippocampus of Dag1cKO mice (Fig. 3B, D). Based on the loss of all three markers, we conclude that CCK+ INs are largely absent from the hippocampus of Dag1cKO mice.
Fig. 3.
Cell body and synaptic markers for CCK+ interneurons are reduced in Dag1cKO mice. (a) Immunostaining showing the co-localization of CB1R (green) and NECAB1 (magenta) in CCK+ interneurons. Insets (yellow boxed regions) show enlarged images of the CA1 and CA3. (b) Immunostaining for NECAB1 (green) shows a reduction of NECAB1+ interneurons in the hippocampus of P30 Dag1cKO mice. Insets (yellow boxed regions) show enlarged images of the CA1 and CA3. (c) Immunostaining of hippocampal sections from VGLUT3Cre mice crossed with a Lox-STOP-Lox-tdTomato (Ai9) reporter mouse showing the co-localization of CB1R (green) and VGLUT3 (magenta) in a subset of CCK+ interneurons. Insets (yellow boxed regions) show enlarged images of the CA1 and CA3. (d) Immunostaining for VGLUT3 (green) shows a reduction of CCK+ interneuron synaptic terminals in the hippocampus of P30 Dag1cKO mice. Insets (yellow boxed regions) show enlarged images of the CA1 and CA3. CA layers: SO, stratum oriens; SP, stratum pyramidale; SR, stratum radiatum
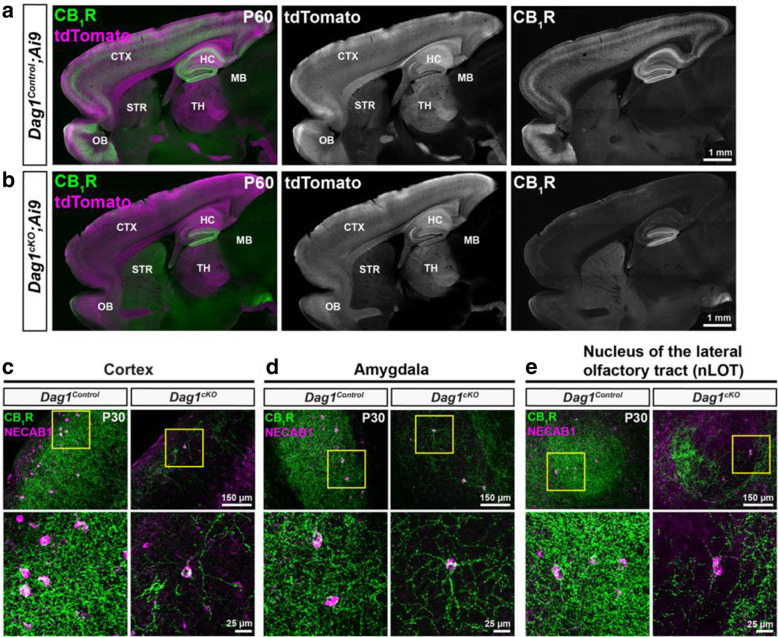
In addition to the hippocampus, Dystroglycan is present in PyNs of the cortex, amygdala, and nucleus of the lateral olfactory tract (nLOT) [27], which all receive extensive innervation from CCK+ INs [56–58]. Therefore, we assessed whether deletion of Dystroglycan from PyNs affects CCK+ INs and their terminals in these forebrain regions. We first performed immunostaining for CB1R on sagittal sections from Dag1Control and Dag1cKO mice. CB1R terminals were largely absent throughout the entire forebrain of Dag1cKO mice (Fig. 4A, B). Next, we stained P30 Dag1Control and Dag1cKO mice for NECAB1 and CB1R to label the cell bodies and terminals of CCK+ INs, respectively (Fig. 4C-E). In Dag1Control mice, NECAB1+ cell bodies were numerous and CB1R innervation was extensive in the cortex, amygdala, and nLOT. In contrast, NECAB1+ cell bodies were dramatically reduced, and CB1R staining was almost completely absent in all regions of Dag1cKO mice (Fig. 4C-E). In each region, a few NECAB1+ cell bodies remained in Dag1cKO mice, and these co-localized with CB1R. Therefore, Dystroglycan expressed in PyNs is required broadly in the developing forebrain for the proper integration of CCK+ INs.
Fig. 4.
CCK+ interneurons are reduced throughout the forebrain of mice lacking Dystroglycan from pyramidal neurons. (a-b) Sagittal sections from P60 Dag1Control;Ai9 (a) and Dag1cKO;Ai9 mice (b) immunostained for CB1R (green; right panels) and tdTomato/Ai9 (magenta; middle panels). In Dag1cKO;Ai9 mice, CB1R staining is lacking in all the forebrain regions where NexCre drives recombination in excitatory neurons (tdTomato expression, middle panels) including the cortex (CTX), hippocampus (HC), and olfactory bulb (OB). Note the absence of tdTomato signal in the striatum (STR) and midbrain (MB), which are not targeted by NexCre. (c-e) Immunostaining for CB1R (green) and NECAB1 (magenta) in the cortex (c), amygdala (d), and nucleus of the lateral olfactory tract (e) shows the reduction of CCK+ interneuron markers in the forebrain of P30 Dag1cKO mice (right panels). Enlarged images (yellow boxed regions) show individual NECAB1+ cell bodies (magenta) co-localized with CB1R (green)
Postnatal development of CCK+ interneurons is impaired in the forebrains of Dag1cKO mice
Our results showing that deletion of Dystroglycan from PyNs leads to a reduction in CCK+ IN innervation is consistent with previous work [39]. However, the temporal onset of this phenotype has not been determined. During embryonic development, CCK+ INs generated in the caudal ganglionic eminence (CGE) begin populating the hippocampus around E14.5 [59, 60] (Fig. 5A). At postnatal ages, CCK+ INs settle into their final positions within the hippocampus and initially extend axons throughout the hippocampal layers before refining their projections to form characteristic basket synapses onto PyN somas (Fig. 5B, D) [61–63]. We first examined the development of CB1R+ terminals in Dag1Control mice during the first two postnatal weeks (P3-P15), as CB1R staining is largely absent from CCK+ INs before birth [64–67]. At early postnatal ages (P3-P5), the majority of CB1R+ terminals were observed in the stratum radiatum (SR) layer of the hippocampus, where immature PyN dendrites are located (Fig. 5B, D). Between P5 and P10, CB1R+ terminals increased in the stratum pyramidale (SP) where PyN cell bodies are located. From P15 through adulthood (15 months), CB1R+ terminals became progressively concentrated in the SP.
Fig. 5.
Postnatal development of CCK+ interneurons is impaired in the hippocampus of Dag1cKO mice. (a) Timeline of interneuron developmental milestones including interneuron migration, cell death, and inhibitory synapse formation. (b-c) Immunostaining for CB1R (green) in the hippocampus of Dag1Control mice (b) shows a progressive increase in CCK+ interneuron axon terminals from P3-P15. In contrast, CB1R+ axon terminals are diminished at all ages in Dag1cKO mice (c). Asterisks (P3 and P5) denote the presence of CB1R immunoreactivity in pyramidal neuron axons at early postnatal ages. Yellow boxes (b, c) indicate approximate locations of high magnification images in (d-e). High magnification (20X), single channel images (gray) of CB1R+ axon terminals in the CA1 of Dag1Control (d) and Dag1cKO mice (e) from P3–15 months. Dotted white lines indicate the position of the pyramidal cell layer (SP). SO, stratum oriens; SP, stratum pyramidale; SR, stratum radiatum. f Quantification of CB1R pixels in hippocampal CA1 layers from Dag1Control (gray) and Dag1cKO (pink) mice shows significantly reduced CB1R staining at all ages examined (*P < 0.05, unpaired two-tailed Student’s t-test; n = 3–4 mice/genotype). Data are presented as mean values ± s.e.m. Data are normalized to Dag1Control signal in each CA layer
Next, we examined CB1R+ terminal development in Dag1cKO mice. At P3, the earliest age we were able to conclusively identify CCK+ INs, CB1R+ staining was already reduced in the hippocampus of Dag1cKO mice. This reduction persisted throughout the period of postnatal development and into adulthood, as late as 15 months (Fig. 5C, E, F). To further confirm this finding, we stained the hippocampus for VGLUT3, an independent synaptic marker for CCK+ IN terminals that is upregulated during early postnatal ages (Fig. S3A). In Dag1Control mice, VGLUT3+ terminals increased in the hippocampus during the first two postnatal weeks, and showed a similar pattern of innervation as CB1R+ staining (Fig. S3B). In contrast, VGLUT3+ terminals were reduced at all ages examined in Dag1cKO mice (Fig. S3B). PV staining, which increases between P10 and P15 [68], was unaltered in Dag1cKO mice compared with controls (Fig. S3C).
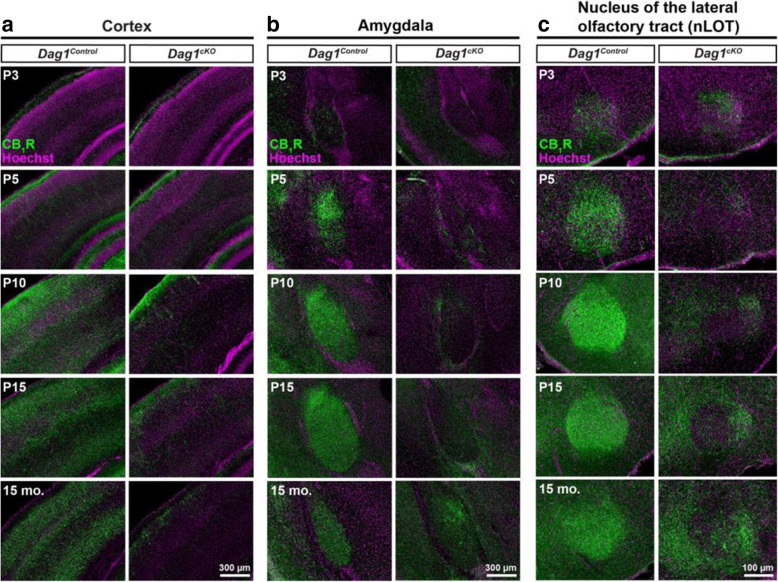
We next determined whether the reduction of CCK+ INs in the cortex, amygdala, and nLOT followed the same developmental time course as the hippocampus. In Dag1Control mice, CB1R+ terminals gradually increased in density in all regions between P3 and P15, and remained stable beyond this age into adulthood (15 months) (Fig. 6). In contrast, CB1R+ terminals in Dag1cKO mice failed to elaborate during the first two postnatal weeks, and remained sparse in adult animals. Collectively, these results demonstrate that Dystroglycan in PyNs is critical during the first two postnatal weeks for the development and integration of CCK+ INs throughout the forebrain.
Fig. 6.
Postnatal development of CCK+ interneurons is impaired in the forebrain of Dag1cKO mice. (a-c) Immunostaining for CB1R (green) and Hoechst (magenta) shows the progressive innervation of the cortex (a), amygdala (b), and nucleus of the lateral olfactory tract (c) of Dag1Control (left panels) mice by CCK+ interneurons from P3-P15. CB1R staining is decreased in all regions of Dag1cKO mice (right panels) at all ages examined from P3–15 months
Post-developmental maintenance of CCK+ interneurons does not require Dystroglycan
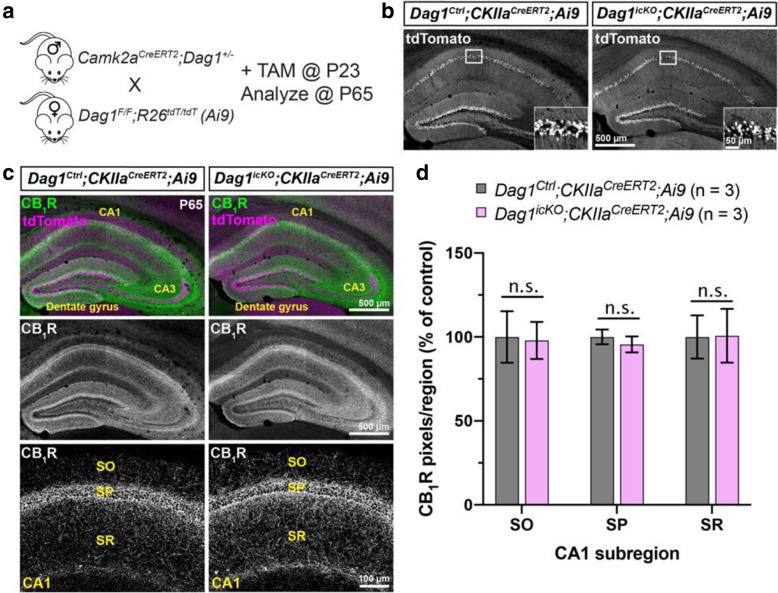
Inhibitory synaptogenesis increases between P5-P15, and is largely complete by P30 [20, 22, 54]. Therefore, we wanted to assess whether deletion of Dystroglycan after inhibitory synapse formation impairs the maintenance of CCK+ INs. To achieve temporal control of Dystroglycan deletion from PyNs, we generated mice expressing tamoxifen-inducible Cre recombinase under the control of an excitatory neuron-specific promoter Camk2a, (Calcium/calmodulin-dependent protein kinase II alpha [69]). Control (Camk2aCreERT2;DGF/+;Ai9) or inducible-cKO (Camk2aCreERT2;DGF/−;Ai9) mice were administered tamoxifen at P23 via oral gavage, which induced recombination in the majority of PyNs in the hippocampus (Fig. 7A, B). We then analyzed CB1R+ innervation 6 weeks later at P65. No differences were found between the Dag1 inducible-cKO and controls, suggesting that Dystroglycan is not required for the post-developmental maintenance of CCK+ INs (Fig. 7C, D).
Fig. 7.
Post-developmental maintenance of CCK+ interneurons does not require Dystroglycan. (a) Breeding scheme and experimental approach for generating tamoxifen-inducible Dystroglycan conditional knockout mice. Dag1Ctrl;Camk2aCreERT2;Ai9 and Dag1icKO;Camk2aCreERT2;Ai9 mice were treated with tamoxifen (5 mg/ml) at P23 and brains were collected for immunohistochemistry 6 weeks later at P65. (b) Single channel images of tdTomato staining in the hippocampus show the recombination pattern in PyNs. Insets show enlarged view of tdT+ pyramidal neurons in the CA1. (c) Immunostaining for CB1R+ terminals (green) and tdTomato signal (magenta) in the hippocampus of P65 Dag1Ctrl;Camk2aCreERT2;Ai9 (left panels) and Dag1icKO;Camk2aCreERT2;Ai9 mice (right panels) shows that the deletion of Dystroglycan in adult PyNs does not affect CB1R+ terminal maintenance. (d) Quantification of CB1R pixels in hippocampal CA1 of Dag1Ctrl;Camk2aCreERT2;Ai9 (gray) and Dag1icKO;Camk2aCreERT2;Ai9 (pink) mice (n.s. = not significant, unpaired two-tailed Student’s t-test; n = 3 mice/genotype). Data are presented as mean values ± s.e.m. Data are normalized to Dag1Control signal in each layer. SO, stratum oriens; SP, stratum pyramidale; SR, stratum radiatum
Blocking Bax-dependent cell death does not rescue CCK+ interneurons in Dag1cKO mice
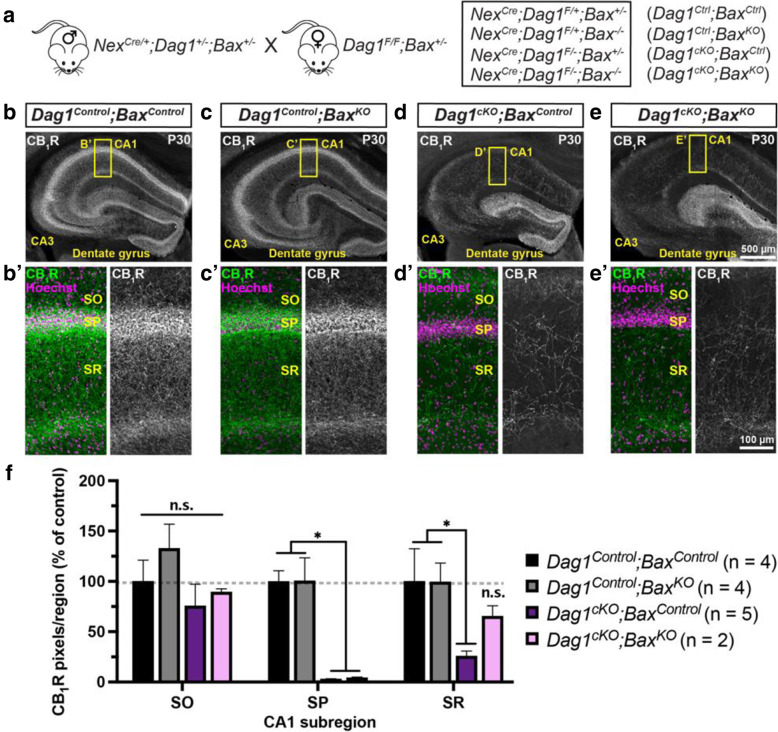
The number of PyNs and INs in the forebrain is tightly regulated during early postnatal development, with excess or inappropriately connected neurons pruned by Bax-dependent apoptosis [70–73]. PyN apoptosis is largely complete by P5, followed by IN apoptosis which peaks at P7-P9. We hypothesized that in the absence of PyN Dystroglycan, CCK+ INs are unable to recognize their postsynaptic targets and are therefore eliminated by apoptosis. We tested this hypothesis by generating Dag1Ctrl and Dag1cKO mice that lack either one (Dag1Ctrl;BaxCtrl and Dag1cKO;BaxCtrl) or both copies of Bax (Dag1Ctrl;BaxKO and Dag1cKO;BaxKO) to block apoptosis (Fig. 8A). Deletion of Bax from control mice (Dag1Ctrl;BaxKO) did not alter CB1R+ innervation in the CA1 subregion of the hippocampus (Fig. 8B-C, F). In line with our previous results, Dag1cKO;BaxCtrl mice lacking one copy of Bax had a similar reduction in CB1R+ terminals as Dag1cKO mice (Fig. 8D, F). Surprisingly, we found that complete deletion of Bax in Dag1cKO mice (Dag1cKO;BaxKO) was not sufficient to rescue CB1R+ innervation (Fig. 8E, F). Staining for an additional CCK+ IN synapse marker (VGLUT3) further confirmed this result (Fig. S4). Finally, we examined whether deletion of Bax could rescue CB1R+ innervation in the cortex, amygdala, and the nucleus of the lateral olfactory tract (nLOT) of Dag1cKO mice (Fig. S5). In all regions examined, CB1R+ terminals were reduced in mice lacking Dystroglycan (Dag1cKO;BaxCtrl). Similar to our observations in the hippocampus, deleting both copies of Bax (Dag1cKO;BaxKO) was not sufficient to rescue CB1R+ innervation in the cortex, amygdala, or nLOT (Fig. S5). Collectively, these results suggest that loss of CB1R+ innervation in the absence of PyN Dystroglycan is not due to CCK+ INs undergoing Bax-dependent apoptosis.
Fig. 8.
Constitutive deletion of Bax does not rescue CB1R+ terminals in the hippocampus. (a) Breeding scheme for deletion of Bax in Dag1Control and Dag1cKO mice; the four genotypes analyzed and their abbreviations are shown to the right. (b-e) Coronal sections of the hippocampus stained for CB1R (gray) from (b) Dag1Control;BaxControl, (c) Dag1Control;BaxKO, (d) Dag1cKO;BaxControl and (e) Dag1cKO;BaxKO mice. (B′-E’) Enlarged images of the CA1 (yellow boxed regions) stained for CB1R (green; Right, gray single channel images) and Hoechst (magenta). (f) Quantification of CB1R pixels in hippocampal CA1 layers from Dag1Control;BaxControl (black bars), Dag1Control;BaxKO (gray bars), Dag1cKO;BaxControl (purple bars), and Dag1cKO;BaxKO (pink bars) shows that deleting Bax fails to rescue the loss of CB1R in Dag1cKO mice (n.s. = not significant; *P < 0.05, unpaired two-tailed Student’s t-test; n = 2–5 mice/genotype). Data are presented as mean values ± s.e.m. Data are normalized to Dag1Control;BaxControl signal in each CA1 layer. SO, stratum oriens; SP, stratum pyramidale; SR, stratum radiatum
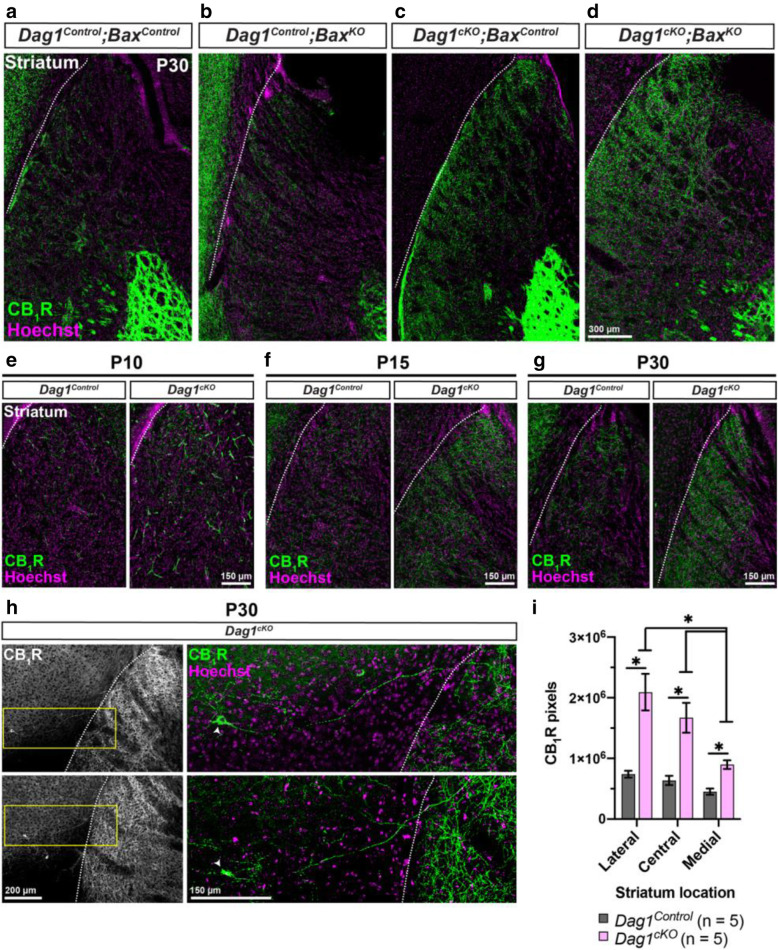
CCK+ interneurons inappropriately innervate the striatum of Dag1cKO mice
During embryonic development, CCK+ INs are produced in and migrate through the caudal ganglionic eminence (CGE), one of two ventral forebrain regions that ultimately develop into the striatum. Expression of Dystroglycan in striatal neurons is retained in Dag1cKO mice, as they are not targeted by NexCre (Fig. 4A-B [39, 41]). We therefore examined CB1R innervation of the striatum in Dag1Control and Dag1cKO mice. In Dag1Control mice, CB1R innervation in the striatum was present, but sparse compared with neighboring regions of the cortex (Fig. 9A) [74, 75]. In contrast, CB1R innervation in the striatum of Dag1cKO mice was noticeably increased (Fig. 9C, I). The lateral regions of the striatum closest to the cortex exhibited dense CB1R innervation, which decreased towards the medial striatum. Global deletion of Bax from Dag1Control or Dag1cKO mice did not alter the pattern of CB1R innervation in the striatum (Fig. 9B, D).
Fig. 9.
CCK+ interneurons inappropriately innervate the striatum of Dag1cKO mice. (a-d) Immunostaining for CB1R (green) and Hoechst (magenta) shows minimal CB1R innervation in the striatum of P30 (a) Dag1Control;BaxControl and (B) Dag1Control;BaxKO mice. Striatal innervation by CB1R+ axons is abnormally increased in (c) Dag1cKO;BaxControl and (d) Dag1cKO;BaxKO mice. (e-g) Immunostaining for CB1R (green) and Hoechst (magenta) in the striatum of Dag1Control and Dag1cKO mice at P10 (e), P15 (f), and P30 (g), showing that the inappropriate CB1R innervation in the striatum of Dag1cKO mice increases gradually between P10-P30. (h) Low magnification images (10X) of CB1R+ cell bodies and their axons (Left panels, gray) near the cortico-striatal boundary from two separate Dag1cKO mice at P30. Yellow boxed regions (right panels) show high magnification (20X) images of individual CB1R+ cell bodies (arrowheads, green) and their axons projecting from the cortex into the striatum. White dotted lines (a-h) indicate the approximate boundary between the cortex and striatum. (i) Quantification of CB1R pixels in the caudal striatum from P30 Dag1Control (black bars) and Dag1cKO (pink bars) mice shows increased CB1R staining in Dag1cKO (*P < 0.05, unpaired two-tailed Student’s t-test; n = 5 mice/genotype). Data are presented as mean values ± s.e.m.
Examination of the developmental timecourse of CB1R+ innervation in the striatum revealed sparse CB1R+ terminals at P10 in both Dag1Control and Dag1cKO mice (Fig. 9E), which increased in Dag1cKO mice compared with controls between P15 and P30 (Fig. 9F-G). This coincides with the period of CB1R+ innervation of forebrain targets in Dag1Control mice. Occasionally, CB1R+ cell bodies could be seen in the cortex near the striatal boundary, with their axon terminals projecting into the striatum (Fig. 9H). These results suggest that some CCK+ INs in the cortex of Dag1cKO mice may redirect their axons into the neighboring regions of the striatum that retain Dystroglycan.
Discussion
Dystroglycan plays a critical role in maintaining the integrity of the neuroepithelial scaffold during early stages of brain development, which has made it difficult to assess its function within neurons at subsequent stages. In the current study, we show that Dystroglycan in pyramidal neurons regulates the development of a subset of their pre-synaptic partners. When Dystroglycan is selectively deleted from PyNs, CCK+ INs throughout the entire forebrain fail to properly integrate, and largely disappear during the first postnatal week. Surprisingly, we found that deletion of Bax did not rescue CCK+ INs in Dag1cKO mice, suggesting their disappearance is not due to apoptotic cell death. The few remaining CCK+ INs redirect their axons into neighboring regions of the brain in which Dystroglycan is still present, suggesting that Dystroglycan functions as a part of a synaptic partner recognition complex.
What stage of CCK+ interneuron development requires Dystroglycan?
The localization of Dystroglycan to inhibitory synapses in forebrain pyramidal neurons has been described by multiple studies, while its function at these synapses has remained obscure [36–38, 76]. Recently, it was found that Dystroglycan is required for the formation and function of CCK+ inhibitory basket synapses, but not PV+ basket synapses onto the same PyNs [39]. This finding is significant, because very little is known about the molecules and mechanisms involved in orchestrating the formation of specific subtypes of inhibitory synapses [17]. However, since the earliest timepoint examined in this previous study was P21, it was unclear what stage of synapse development requires Dystroglycan.
During neural circuit development, neurons must first migrate and direct their axons to their appropriate targets, then recognize the appropriate synaptic partners from a myriad of potential choices, then finally form functional synapses [13]. Our data suggest that Dystroglycan is required for the least understood of these processes: synaptic partner recognition. This is supported by the observation that CCK+ INs are present at the earliest stages they can be conclusively identified in the forebrain of Dag1cKO mice (P3), but then fail to elaborate their axons and integrate into these circuits during the first postnatal week (Figs. 5, S3, Fig. 6). Interestingly, the few remaining CCK+ INs appear to project their axons into regions that continue to express Dystroglycan in Dag1cKO mice (Figs. 5, 9). Taken together, this data suggests that CCK+ INs in Dag1cKO mice fail to recognize their normal postsynaptic PyN targets in the hippocampus and cortex during early postnatal development, and instead re-route to neighboring Dag1+ neurons, discussed further below.
The process of synaptic partner recognition in mammals has been difficult to study due to our inability to precisely identify and genetically manipulate the specific neuronal populations during development. Determining whether loss of Dystroglycan impairs CCK+ IN development before birth is technically challenging due to the lack of immunohistochemical and genetic tools for detecting CCK+ INs prenatally [60]. The cannabinoid receptor-1 (Cnr1) and cholecystokinin (Cck) genes are both expressed in PyNs at prenatal timepoints, limiting their usefulness for detecting CCK+ INs. Transcription factors such as Prox1 are also of limited usefulness due to its broad expression in multiple CGE-derived IN subtypes [77]. VGLUT3, which labels a subset of CCK+ INs, does not increase in expression until the first postnatal week [54]. Other IN subtypes exhibit delayed expression of selective molecular markers as well. For instance, MGE-derived Parvalbumin INs do not begin to express Parvalbumin until P10, well after the period of initial synaptic partner recognition [68, 78].
What happens to CCK+ interneurons in the absence of Dystroglycan?
Our results show that deletion of Dystroglycan from PyNs resulted in a loss of all of the markers we used to identify CCK+ INs in the forebrain (Figs. 2, 3, 4). What happens to these neurons in the absence of PyN Dystroglycan? One possibility that we examined is that CCK+ INs undergo apoptosis. During the first 2 weeks of development (P5-P10), a significant number of excitatory and inhibitory neurons are pruned by Bax-dependent apoptotic cell death [70–73]. This ensures the proper number of neurons and removes neurons that fail to integrate into the developing circuit. Whereas Bax-dependent developmental cell death has been described for most MGE and CGE-derived interneuron subtypes, whether CCK+ INs normally undergo the same process has not been directly examined [72, 73]. We tested whether the loss of CCK+ INs in Dag1cKO mice could reflect premature or amplified developmental apoptosis, which peaks around P9 for other IN subtypes. However, constitutive deletion of Bax, which is sufficient to block developmental apoptosis in other neuronal populations, did not rescue CCK+ INs (Figs. 8, S4, S5). This suggests that canonical apoptosis is not responsible for the loss of CCK+ INs in Dag1cKO mice. It is possible that CCK+ INs are eliminated in a Bax-independent manner, similar to some populations of Cajal-Retzius cells in the cortex and astrocytes in the developing retina [79, 80].
CCK+ INs comprise a molecularly and morphologically diverse group of cells that include both cell body targeting (perisomatic) and multiple dendrite targeting subtypes [54, 81, 82]. In the hippocampus, CCK+ INs frequently express one of two non-overlapping markers, VGLUT3 (~ 45%) and VIP (~ 16%) [53]. In Dag1cKO mice, all synaptic and cell body markers selective for CCK+ INs (CB1R, VGLUT3, NECAB1) that we examined were reduced at the onset of their expression. While it is formally possible that Dystroglycan in PyNs is required for CCK+ INs to fully differentiate into their mature, molecularly defined subtype, we consider this unlikely. In this situation, Dystroglycan present on PyNs would be required to transmit a retrograde signal to CCK+ INs to direct their differentiation. We are unaware of any cell adhesion molecules that function in this manner. Rather, fate switching or failure to fully differentiate is usually observed upon cell-autonomous loss of specific transcription factors [83].
Our data also indicate that Dystroglycan is not required to maintain CCK+ INs after the period of synapse formation (Fig. 7). This is in contrast to a previous study that showed a gradual reduction in the number of Vglut3+ puncta when Dag1 was deleted in adult mice using AAV-Cre [39]. Aside from the different approaches used for adult deletion, this difference may arise from the level of analysis: in our study, we saw no difference in the cellular organization of CCK+ INs following adult deletion, whereas the previous study was focused specifically on presynaptic puncta. It is possible that in our inducible-cKO (Camk2aCreERT2;DGF/−;Ai9) mice, synaptic inputs from CCK+ INs are reduced without altering the survival of these neurons. Alternatively, there may still be some residual Dystroglycan protein remaining in Camk2aCreERT2;DGF/−;Ai9 mice, at levels sufficient to support CCK+ IN maintenance.
Although CCK+ INs and their terminals were dramatically reduced throughout the brains of Dag1cKO mice, some CCK+ IN terminals were still present, particularly along the cortico-striatal boundary and in the upper dendritic layers of the cortex (layer 1) and hippocampus. Importantly, striatal neurons and Cajal-Retzius cells, which are located in superficial cortical layers during postnatal development, are not targeted by NexCre [84]. This suggests that in the absence of Dystroglycan on their normal postsynaptic targets (PyNs), CCK+ INs may direct their axons to secondary synaptic targets that retain Dystroglycan expression.
A number of studies have indicated that synaptic partner recognition and targeting may be “stringent” or “flexible”, depending on the cell type involved. Studies in the Drosophila visual system have shown that synaptic cell adhesion molecules such as DIP/Dprs can promote either stringent or flexible outcomes among synaptic partners depending on the cellular context and the molecules involved. For instance, postsynaptic Dm8 neurons containing the receptor DIP-γ undergo cell death if not innervated by a matching R7 photoreceptor containing the cognate ligand Dpr11 [85]. In contrast, loss of DIP-β from L4 neurons does not impair synapse formation or cause cell death, but instead leads to ectopic synapses onto alternative synaptic partners [86]. Synaptic partner recognition “flexibility” and “stringency” has also been demonstrated in the mammalian nervous system. In the developing retina, On-alpha retinal ganglion cells will re-wire to increase inputs from neighboring bipolar cell types when their normal presynaptic inputs (Type 6 bipolar cells) are genetically ablated [87]. In contrast preGABA INs in the developing spinal cord retract their processes when their primary targets (proprioceptor axons) are not present, rather than forming synapses onto secondary targets [88]. Despite retracting their axons, preGABA INs do not undergo cell death, suggesting that loss of neurons is not a necessary consequence of losing synaptic partners. In Dag1cKO mice, CCK+ INs may stringently require Dystroglycan for their ability to recognize their primary synaptic targets and die in a Bax-independent manner in its absence. The observation that some CCK+ INs near the cortico-striatal boundary survive and innervate the striatum suggests that they may exhibit some degree of flexibility to make contacts onto secondary targets. Determining whether the remaining CCK+ INs in Dag1cKO mice exhibit normal morphological and physiological properties will require fate mapping these neurons, which is difficult with currently available genetic tools.
Why are CCK+ interneurons selectively affected in Dag1cKO mice?
CCK+ INs appear to be the only interneuron subtype affected by deletion of Dystroglycan from PyNs. Compared to other IN populations, CCK+ INs express high levels of CB1Rs, which can play important roles in neuronal proliferation, migration, and axon outgrowth [89]. In utero exposure to exogenous cannabinoids results in a specific loss of CCK+ INs through unknown mechanisms [90]. However, conditional deletion of the cannabinoid receptor-1 gene Cnr1 from CCK+ INs does not affect interneuron migration or neurochemical specification, but rather increases the number of perisomatic VGLUT3+ inhibitory synapses on cortical PyNs [67]. In addition, CB1R signaling is not necessary for the survival of CCK+ INs [91]. Therefore, it is unlikely that alterations in CB1R activity underlie the selective loss of CCK+ INs in Dag1cKO mice.
One possible explanation for this selective loss is that Dystroglycan interacts with specific molecules on presynaptic CCK+ INs compared with other IN subtypes. Dystroglycan is highly glycosylated, and unique matriglycan moieties present on its extracellular domain bind to proteins containing Laminin G (LG) domains [92]. Proteins that bind Dystroglycan through their LG domains include extracellular matrix proteins (Agrin, Laminins, Perlecan), axon guidance molecules (Slits, Celsr3), as well as synaptic proteins (Neurexin, Pikachurin) [29, 33, 93–98]. Several other putative synaptic proteins also contain LG domains (ie: Cntnap1–6), although their binding to Dystroglycan has not been examined.
Biochemical experiments have identified α-DG as a major interaction partner of α- and β-neurexins in whole brain lysates, and these interactions are dependent upon the lack of splice inserts in LNS2 and LNS6 of neurexin [98–101]. Conditional deletion of all three Neurexins from interneurons revealed distinct outcomes depending on the IN population examined [102]. Deletion of all Neurexin isoforms from PV+ INs results in a significant decrease in the number of PV+ synapses in the cortex, whereas it does not affect inhibitory synapse numbers when deleted from SST+ INs. While PV+ IN numbers were not affected by conditional deletion of Neurexins, this could reflect the timing of deletion, which is unlikely to occur before three weeks of age based on the onset of Cre expression [68, 78]. Nrxn1α and Neurexin 3α/β are expressed at significantly higher levels in CCK+ INs than in PV+ INs, and CCK+ INs predominantly express Neurexin isoforms lacking splice inserts in LNS6 [100]. Therefore, CCK+ INs may show a larger degree of Nrxn:Dystroglycan interaction than other IN subtypes. Mice harboring a mutation in Dystroglycan that exhibits reduced glycosylation, and thus Neurexin binding capacity (Dag1T190M), showed no impairments in CCK+ IN terminal development [39, 103]. However, these mice do not display the cortical migration phenotypes associated with a complete loss of Dystroglycan, suggesting that Dystroglycan retains some residual function, which may be sufficient for CCK+ IN terminal development. Whether Neurexins are required cell autonomously in CCK+ INs for their development has not been directly tested, in part due to a lack of genetic tools.
Limitations in studying CCK+ interneuron development
Our understanding of CCK+ IN development and function has lagged behind other interneuron subtypes (PV, SOM, VIP, etc) due in part to the lack of viral and mouse genetic tools available for selectively labeling and manipulating CCK+ INs. All major markers of CCK+ INs (CCK, CB1R, and VGLUT3) are also expressed at lower levels in PyNs, limiting the usefulness of single promoter/recombinase approaches for targeting CCK+ INs [104–106]. Specific targeting of CCK+ INs therefore requires dual recombinase-based intersectional approaches, including CCK-Cre;Dlx5/6-Flp double transgenic mice [107–111], dual CCK-dsRed;GAD67-GFP reporter mice [60], or VGLUT3Cre mice which label approximately half of CCK+ INs [54, 112]. Other reporter lines (5HT3AREGFP) target the entire CGE-derived interneuron population, of which CCK+ INs only comprise ~ 10% [113, 114]. A recently developed SncgFlpO mouse line appears to provide selective genetic access CCK+ basket cells by taking advantage of the fact that Sncg is specifically expressed in CCK+ INs [115]. However, it is not clear when the onset of recombination occurs in this line, and whether it will be useful for studying the early development of CCK+ INs. Indeed, many of the genes used for targeting IN subtypes are not significantly expressed until after the first postnatal week in mice, when much of the process of synaptic partner recognition and initial synapse formation has already occurred (Fig. S3 [54, 68, 78]).
Conclusion
In this study, we identified a critical role for excitatory neuron Dystroglycan in regulating the development of forebrain CCK+ interneurons during the first postnatal week. Given the emerging role for CCK+ INs and cannabinoid signaling in controlling neural circuit activity, Dag1cKO mice may be useful for studying the consequences of losing a major IN population.
Materials and methods
Animal husbandry
All animals were housed and cared for by the Department of Comparative Medicine (DCM) at Oregon Health and Science University (OHSU), an AAALAC-accredited institution. Animal procedures were approved by OHSU Institutional Animal Care and Use Committee (Protocol # IS00000539) and adhered to the NIH Guide for the care and use of laboratory animals. Animals older than postnatal day 6 (P6) were euthanized by administration of CO2, animals <P6 were euthanized by rapid decapitation. Animal facilities are regulated for temperature and humidity and maintained on a 12 h light-dark cycle and animals were provided food and water ad libitum.
Mouse strains and genotyping
The day of birth was designated postnatal day 0 (P0). Ages of mice used for each analysis are indicated in the figure and figure legends. Mice were maintained on a C57BL/6 background and have been previously described or obtained from JAX (Table 1): Dystroglycan conditional mice (Dag1Flox) [30, 117], NexCre [41, 42], VGLUT3Cre [118], Bax−/− [119, 120], Camk2aCreERT2 [69], Ai9LSL-tdTomato [69], and R26LSL-H2B-mCherry [44]. Loss of BAX protein in Bax−/− mouse brains was validated by western blot in a previous study [121]. Genomic DNA extracted from tissue samples (Quanta BioSciences) was used to genotype animals. The presence of the Cre allele in NexCre mice and Camk2aCreERT2 mice was detected using generic Cre primers (JAX).
Table 1.
Mouse strains
| Common name | Strain name | Reference | Stock # |
|---|---|---|---|
| Dag1−/− | B6.129-Dag1tm1Kcam/J | [116] | 006836 |
| Dag1Flox | B6.129(Cg)-Dag1tm2.1Kcam/J | [117] | 009652 |
| NexCre | NeuroD6tm1(cre)Kan | [41] | MGI:4429523 |
| Vglut3Cre | Tg(Slc17a8-icre)1Edw | [118] | 018147 |
| Ai9LSL-tdTomato | B6.Cg-Gt(ROSA)26Sortm9(CAG-tdTomato)Hze/J | [69] | 007909 |
| Camk2aCreERT2 | B6.Tg(Camk2a-cre/ERT2)1Aibs/J | [69] | 012362 |
| R26LSL-H2B-mCherry | B6.Gt(ROSA)26Sortm1.1Ksvo | [44] | 023139 |
| Bax−/− | B6.129X1-Baxtm1Sjk/J | [119] | 002994 |
Tamoxifen administration
Tamoxifen (Sigma; Cat# T5648-1G) was dissolved 1:10 in sunflower seed oil. Each mouse was orally gavaged with 200 μL of tamoxifen at a final concentration of 5 mg/ml tamoxifen.
Perfusions and tissue preparation
Brains from mice younger than P15 were dissected and fixed in 4% paraformaldehyde (PFA) in phosphate buffered saline (PBS) overnight for 18–24 h at 4 °C. Mice P15 and older were deeply anesthetized using CO2 and transcardially perfused with ice cold 0.1 M PBS for two minutes to clear blood from the brain, followed by 15 mL of ice cold 4% PFA in PBS. After perfusion, brains were dissected and post-fixed in 4% PFA for two hours. Brains were rinsed with PBS, embedded in 4% low-melt agarose (Fisher: Cat# 16520100), and 50 μm sections were cut on a vibratome (VT1200S, Leica Microsystems Inc., Buffalo Grove, IL).
Immunohistochemistry and antibodies
Single and multiple immunofluorescence detection of antigens was performed as follows: Free-floating vibratome sections (50 μm) were briefly rinsed with PBS, then blocked for 1 h in PBS containing 0.2% Triton-X (PBST) plus 10% normal donkey serum. Sections were incubated with primary antibodies (Table 2) diluted in PBST at 4 °C overnight (18–24 h) or for 3 days for Dystroglycan staining. The following day, sections were rinsed briefly with PBS, then washed with PBST three times for 20 min each. Sections were then incubated with a cocktail of secondary antibodies (1:1000, Alexa Fluor 488, 546, 647; Fisher) in PBST for 90 min at room temperature. Sections were washed with PBS three times for 20 min each and counterstained with Hoechst 33342 (Life Technologies, Cat# H3570) for 10 min to visualize nuclei. Finally, sections were mounted on slides using Fluoromount-G (Fisher; SouthernBiotech) and sealed using nail polish.
Table 2.
Primary antibodies used for immunohistochemistry
| Target | Host species | Dilution | Source | Catalog # | RRID |
|---|---|---|---|---|---|
| α-Dystroglycan (IIH6C4) | Mouse | 1:200 | Millipore | 05–593 | AB_309828 |
| Calbindin | Rabbit | 1:4000 | Swant | CB38 | AB_10000340 |
| Calretinin | Rabbit | 1:4000 | Swant | CG1 | AB_2619710 |
| CB1R | Guinea pig | 1:2000 | Synaptic Systems | 258–104 | AB_2661870 |
| Cux1 | Rabbit | 1:250 | Santa Cruz Biotech | sc-13,024 | AB_2261231 |
| GFAP | Mouse | 1:1000 | Millipore | MAB360 | AB_2109815 |
| NECAB1 | Rabbit | 1:2000 | Sigma | HPA023629 | AB_1848014 |
| Parvalbumin | Goat | 1:2000 | Swant | PVG-213 | AB_2650496 |
| Somatostatin | Rabbit | 1:2000 | Peninsula Labs | T-4103 | AB_518614 |
| tdTomato | Goat | 1:1000 | Biorbyt | orb182397 | AB_2687917 |
| VGlut3 | Rabbit | 1:2000 | Synaptic Systems | 135–203 | AB_887886 |
Microscopy
Imaging was performed on a Zeiss Axio Imager M2 fluorescence upright microscope equipped with an Apotome.2 module for structured illumination microscopy. The microscope uses a metal halide light source (HXP 200 C), Axiocam 506 mono camera, and 10X/0.3 NA EC Plan-Neofluar, 20X/0.8 NA Plan-Apochromat objectives. Z-stack images were acquired and processed as maximum projection images using Zeiss Zen Imaging software, and analyzed offline in ImageJ/FIJI [122]. Images used for quantification between genotypes were acquired using the same exposure times. Brightness and contrast were adjusted in FIJI to improve visibility of images for publication. Figures were composed in Adobe Illustrator CS6 (Adobe Systems).
Quantification
Quantification of CB1R terminals in the hippocampus (CA1, CA3, Dentate gyrus) and caudal striatum was performed on 5 μm z-stacks acquired using a 20X objective. Six to twelve sections per animal (technical replicates) from at least three animals per genotype (biological replicates) were used for analysis, except where noted in the text and figure legends. Sections were taken from equivalent rostro-caudal positions including the dorsal hippocampus (Bregma between − 1.48 to − 1.94 mm) using coordinates from the mouse brain atlas (Franklin and Paxinos, 1997). All images used for quantification were processed identically. Briefly, background subtraction (Rolling ball radius = 50) and mean filtering (Smooth function in FIJI) were applied to each image to enhance the detection of CB1R terminals by thresholding. To measure CB1R signal in specific regions of interest (ROIs), a threshold was manually set and applied equally across images to detect only CB1R signal. Separate regions of interest (ROIs) were used to quantify CB1R pixels in CA1 and CA3 layers: stratum oriens (SO), stratum pyramidale (SP) and stratum radiatum (SR). Three separate ROIs were used to analyze Dentate gyrus layers: Outer molecular layer (OML), Inner molecular layer (IML), and Granule cell layer (GCL). Hoechst signal in the SP (CA regions) and GCL (Dentate regions) were used to align the ROIs consistently for each image. Raw integrated density values from each ROI were averaged across all images for each animal and normalized to the mean intensity of the control group (set to 100% for each ROI).
Experimental design and statistical analysis
All phenotypic analyses were conducted using tissue collected from at least three mice per genotype from at least two independent litters unless otherwise noted. The number of mice used for each analysis (“n”) are indicated in the figures and figure legends. No specific power analyses were performed, but sample sizes were similar to our previous work and other published literature [28, 29, 33]. Male and female mice were analyzed together. In many cases, highly penetrant phenotypes revealed the genotypes of the mice and no blinding could be performed. Significance between groups was determined using unpaired two-tailed Student’s t-test. Data are presented as mean ± standard error of the mean (s.e.m) and statistical significance was set at alpha = 0.05 (P < 0.05). Graphical representations of data and statistical analyses were performed in GraphPad Prism 8 (San Diego, CA).
Supplementary Information
Additional file 1: Fig. S1. NexCre drives recombination in forebrain pyramidal neurons but not interneurons or glia. (A) Coronal sections from NexCre;R26LSL-H2B-mCherry reporter mice at P21 show mCherry+ nuclei (magenta) of pyramidal neurons in the hippocampus, cortex, amygdala, and nucleus of the lateral olfactory tract (nLOT). (B) Hippocampal sections from NexCre;R26LSL-H2B-mCherry reporter mice immunostained for interneuron markers (green) Calbindin (left panels), Parvalbumin (middle panel), and CB1R (right panel) show no overlap of interneuron cell bodies with mCherry+ nuclei. White arrowheads indicate CB1R+ cell bodies. SO, stratum oriens; SP, stratum pyramidale; SR, stratum radiatum. (C) The astrocyte marker GFAP (green) shows no overlap with mCherry+ nuclei in the hippocampal CA regions or dentate gyrus (left and middle panels). Inset (middle panel) shows a magnified view of astrocyte nuclei (blue). mCherry+ nuclei occupy the outer third of the dentate gyrus granule cell layer (right panel). ML, molecular layer; GCL, granule cell layer.
Additional file 2: Fig. S2. CCK+ interneuron innervation of the dentate gyrus is minimally altered in Dag1cKO mice. (A) Immunostaining of CB1R in the dentate gyrus from P30 Dag1Control (left panels) and Dag1cKO mice (right panels). Single channel images of CB1R (gray) are shown below. (B) Quantification of CB1R pixels for each dentate gyrus layer (*P < 0.05, unpaired two-tailed Student’s t-test; n = 4 mice/genotype). Data are presented as mean values ± s.e.m. Data are normalized to Dag1Control signal in each dentate gyrus layer. OML, outer molecular layer; IML, inner molecular layer; GCL, granule cell layer.
Additional file 3: Fig. S3. CCK+ interneuron markers are reduced postnatally in Dag1cKO mice. (A) Images of hippocampal CA1 from VGLUT3Cre;Ai9 mice from P3-P18. Immunostaining for tdTomato (green) shows progressive increase in VGLUT3 expression in the pyramidal cell layer (SP, magenta). (B) Immunostaining for VGLUT3 in the CA1 of Dag1Control (top panels) and Dag1cKO mice (bottom panels) from P3-P15. Note the lack of VGLUT3 expression at all ages in Dag1cKO mice. (C) Parvalbumin (PV) labeling is similar in the CA1 of Dag1Control (top panels) and Dag1cKO mice (bottom panels) from P5-P30.
Additional file 4: Fig. S4. Constitutive deletion of Bax in Dag1cKO mice does not rescue VGLUT3+ terminals. (A-D) Coronal sections of the hippocampus stained for VGLUT3 (gray) from P30 (A) Dag1Control;BaxControl, (B) Dag1Control;BaxKO, (C) Dag1cKO;BaxControl and (D) Dag1cKO;BaxKO mice. (A’-D′) Magnified images of the CA1 (yellow boxed regions) stained for VGLUT3 (green; Right, gray single channel images) and Hoechst (magenta) to stain the pyramidal cell layer (SP). SO, stratum oriens; SP, stratum pyramidale; SR, stratum radiatum.
Additional file 5: Fig. S5. Constitutive deletion of Bax in Dag1cKO mice does not rescue CB1R+ terminals in the forebrain. (A-C) Coronal sections immunostained for CB1R (green) and Hoechst (magenta) in the cortex (A), amygdala (B), and nucleus of the lateral olfactory tract (C) of P30 Dag1Control;BaxControl, Dag1Control;BaxKO, Dag1cKO;BaxControl and Dag1cKO;BaxKO mice.
Acknowledgments
This work was funded by NIH Grants R01-NS091027 (K.M.W.), CureCMD (K.M.W.), NINDS P30–NS061800 (OHSU ALM), NINDS F31 NS108522 (D.S.M), and a Tartar Trust Fellowship (D.S.M).
Abbreviations
- BAX
BCL2-associated X protein
- CAM
Cell adhesion molecule
- CB1R
Cannabinoid receptor 1
- CCK
Cholecystokinin
- CGE
Caudal ganglionic eminence
- cKO
Conditional knockout
- CNS
Central nervous system
- GABA
Gamma-aminobutyric acid
- IN
Interneuron
- MGE
Medial ganglionic eminence
- P
Postnatal day
- PV
Parvalbumin
- PyN
Pyramidal neuron
- SST
Somatostatin
- VGLUT3
Vesicular glutamate transporter 3
Authors’ contributions
DSM and KMW designed the experiments. DSM performed experiments and analyzed the data. DSM prepared the figures and wrote the original draft. DSM and KMW revised and edited the final draft. Both authors read and approved the final manuscript.
Funding
Funding bodies were not involved in the design of the study or involved in collection, analysis, or interpretation of data.
Availability of data and materials
The datasets used during the current study are available from the corresponding author upon request.
Declarations
Ethics approval and consent to participate
All experiments were carried out in the Vollum Institute at Oregon Health and Science University, an American Association of Laboratory Animal Care (AAALAC)-accredited institution. Animal procedures were approved by OHSU IACUC and adhered to the NIH Guide for the care and use of laboratory animals.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Harris KD, Hochgerner H, Skene NG, Magno L, Katona L, Bengtsson Gonzales C, et al. Classes and continua of hippocampal CA1 inhibitory neurons revealed by single-cell transcriptomics. PLoS Biol. 2018;16(6):e2006387. doi: 10.1371/journal.pbio.2006387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kepecs A, Fishell G. Interneuron cell types are fit to function. Nature. 2014;505(7483):318–326. doi: 10.1038/nature12983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lim L, Mi D, Llorca A, Marín O. Development and functional diversification of cortical interneurons. Neuron. 2018;100(2):294–313. doi: 10.1016/j.neuron.2018.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paul A, Crow M, Raudales R, He M, Gillis J, Huang ZJ. Transcriptional architecture of synaptic communication delineates GABAergic neuron identity. Cell. 2017;171(3):522–539.e20. doi: 10.1016/j.cell.2017.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pelkey KA, Chittajallu R, Craig MT, Tricoire L, Wester JC, McBain CJ. Hippocampal GABAergic inhibitory interneurons. Physiol Rev. 2017;97(4):1619–1747. doi: 10.1152/physrev.00007.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson SA, Eisenstat DD, Shi L, Rubenstein JL. Interneuron migration from basal forebrain to neocortex: dependence on dlx genes. Science. 1997;278(5337):474–476. doi: 10.1126/science.278.5337.474. [DOI] [PubMed] [Google Scholar]
- 7.Tamamaki N, Fujimori KE, Takauji R. Origin and route of tangentially migrating neurons in the developing neocortical intermediate zone. J Neurosci. 1997;17(21):8313–8323. doi: 10.1523/JNEUROSCI.17-21-08313.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zecevic N, Hu F, Jakovcevski I. Interneurons in the developing human neocortex. Dev Neurobiol. 2011;71(1):18–33. doi: 10.1002/dneu.20812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chao H-T, Chen H, Samaco RC, Xue M, Chahrour M, Yoo J, et al. Dysfunction in GABA signalling mediates autism-like stereotypies and Rett syndrome phenotypes. Nature. 2010;468(7321):263–269. doi: 10.1038/nature09582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lanerolle NC, Kim JH, Robbins RJ, Spencer DD. Hippocampal interneuron loss and plasticity in human temporal lobe epilepsy. Brain Res. 1989;495(2):387–395. doi: 10.1016/0006-8993(89)90234-5. [DOI] [PubMed] [Google Scholar]
- 11.Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6(4):312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- 12.Verret L, Mann EO, Hang GB, Barth AMI, Cobos I, Ho K, et al. Inhibitory interneuron deficit links altered network activity and cognitive dysfunction in Alzheimer model. Cell. 2012;149(3):708–721. doi: 10.1016/j.cell.2012.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanes JR, Zipursky SL. Synaptic specificity, recognition molecules, and assembly of neural circuits. Cell. 2020;181(6):1434–1435. doi: 10.1016/j.cell.2020.05.046. [DOI] [PubMed] [Google Scholar]
- 14.Südhof TC. Towards an understanding of synapse formation. Neuron. 2018;100(2):276–293. doi: 10.1016/j.neuron.2018.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wit J, Ghosh A. Specification of synaptic connectivity by cell surface interactions. Nat Rev Neurosci. 2016;17(1):22–35. doi: 10.1038/nrn.2015.1. [DOI] [PubMed] [Google Scholar]
- 16.Földy C, Darmanis S, Aoto J, Malenka RC, Quake SR, Südhof TC. Single-cell RNAseq reveals cell adhesion molecule profiles in electrophysiologically defined neurons. Proc Natl Acad Sci U S A. 2016;113(35):E5222–E5231. doi: 10.1073/pnas.1610155113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krueger-Burg D, Papadopoulos T, Brose N. Organizers of inhibitory synapses come of age. Curr Opin Neurobiol. 2017;45:66–77. doi: 10.1016/j.conb.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 18.Lu W, Bromley-Coolidge S, Li J. Regulation of GABAergic synapse development by postsynaptic membrane proteins. Brain Res Bull. 2017;129:30–42. doi: 10.1016/j.brainresbull.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson GR, Maxeiner S, Sando R, Tsetsenis T, Malenka RC, Südhof TC. Postsynaptic adhesion GPCR latrophilin-2 mediates target recognition in entorhinal-hippocampal synapse assembly. J Cell Biol. 2017;216(11):3831–3846. doi: 10.1083/jcb.201703042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Favuzzi E, Deogracias R, Marques-Smith A, Maeso P, Jezequel J, Exposito-Alonso D, et al. Distinct molecular programs regulate synapse specificity in cortical inhibitory circuits. Science. 2019;363(6425):413–417. doi: 10.1126/science.aau8977. [DOI] [PubMed] [Google Scholar]
- 21.Sando R, Jiang X, Südhof TC. Latrophilin GPCRs direct synapse specificity by coincident binding of FLRTs and teneurins. Science. 2019;363(6429):eaav7969. doi: 10.1126/science.aav7969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tai Y, Gallo NB, Wang M, Yu J-R, Van Aelst L. Axo-axonic innervation of neocortical pyramidal neurons by GABAergic chandelier cells requires AnkyrinG-associated L1CAM. Neuron. 2019;102(2):358–372.e9. doi: 10.1016/j.neuron.2019.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manya H, Endo T. Glycosylation with ribitol-phosphate in mammals: new insights into the O-mannosyl glycan. Biochim Biophys Acta Gen Subj. 2017;1861(10):2462–2472. doi: 10.1016/j.bbagen.2017.06.024. [DOI] [PubMed] [Google Scholar]
- 24.Taniguchi-Ikeda M, Morioka I, Iijima K, Toda T. Mechanistic aspects of the formation of alpha-dystroglycan and therapeutic research for the treatment of alpha-dystroglycanopathy: a review. Mol Asp Med. 2016;51:115–124. doi: 10.1016/j.mam.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 25.Barresi R, Campbell KP. Dystroglycan: from biosynthesis to pathogenesis of human disease. J Cell Sci. 2006;119(Pt 2):199–207. doi: 10.1242/jcs.02814. [DOI] [PubMed] [Google Scholar]
- 26.Nickolls AR, Bönnemann CG. The roles of dystroglycan in the nervous system: insights from animal models of muscular dystrophy. Dis Model Mech. 2018;11(12):dmm035931. doi: 10.1242/dmm.035931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zaccaria ML, Di Tommaso F, Brancaccio A, Paggi P, Petrucci TC. Dystroglycan distribution in adult mouse brain: a light and electron microscopy study. Neuroscience. 2001;104(2):311–324. doi: 10.1016/S0306-4522(01)00092-6. [DOI] [PubMed] [Google Scholar]
- 28.Clements R, Turk R, Campbell KP, Wright KM. Dystroglycan maintains inner limiting membrane integrity to coordinate retinal development. J Neurosci. 2017;37(35):8559–8574. doi: 10.1523/JNEUROSCI.0946-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lindenmaier LB, Parmentier N, Guo C, Tissir F, Wright KM. Dystroglycan is a scaffold for extracellular axon guidance decisions. Elife. 2019;8 10.7554/eLife.42143. [DOI] [PMC free article] [PubMed]
- 30.Moore SA, Saito F, Chen J, Michele DE, Henry MD, Messing A, et al. Deletion of brain dystroglycan recapitulates aspects of congenital muscular dystrophy. Nature. 2002;418(6896):422–425. doi: 10.1038/nature00838. [DOI] [PubMed] [Google Scholar]
- 31.Myshrall TD, Moore SA, Ostendorf AP, Satz JS, Kowalczyk T, Nguyen H, et al. Dystroglycan on radial glia end feet is required for pial basement membrane integrity and columnar organization of the developing cerebral cortex. J Neuropathol Exp Neurol. 2012;71(12):1047–1063. doi: 10.1097/NEN.0b013e318274a128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Satz JS, Ostendorf AP, Hou S, Turner A, Kusano H, Lee JC, et al. Distinct functions of glial and neuronal dystroglycan in the developing and adult mouse brain. J Neurosci. 2010;30(43):14560–14572. doi: 10.1523/JNEUROSCI.3247-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wright KM, Lyon KA, Leung H, Leahy DJ, Ma L, Ginty DD. Dystroglycan organizes axon guidance cue localization and axonal pathfinding. Neuron. 2012;76(5):931–944. doi: 10.1016/j.neuron.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Godfrey C, Clement E, Mein R, Brockington M, Smith J, Talim B, et al. Refining genotype phenotype correlations in muscular dystrophies with defective glycosylation of dystroglycan. Brain. 2007;130(Pt 10):2725–2735. doi: 10.1093/brain/awm212. [DOI] [PubMed] [Google Scholar]
- 35.Mercuri E, Messina S, Bruno C, Mora M, Pegoraro E, Comi GP, et al. Congenital muscular dystrophies with defective glycosylation of dystroglycan: a population study. Neurology. 2009;72(21):1802–1809. doi: 10.1212/01.wnl.0000346518.68110.60. [DOI] [PubMed] [Google Scholar]
- 36.Brünig I, Suter A, Knuesel I, Lüscher B, Fritschy J-M. GABAergic terminals are required for postsynaptic clustering of dystrophin but not of GABA(a) receptors and gephyrin. J Neurosci. 2002;22(12):4805–4813. doi: 10.1523/JNEUROSCI.22-12-04805.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lévi S, Grady RM, Henry MD, Campbell KP, Sanes JR, Craig AM. Dystroglycan is selectively associated with inhibitory GABAergic synapses but is dispensable for their differentiation. J Neurosci. 2002;22(11):4274–4285. doi: 10.1523/JNEUROSCI.22-11-04274.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Uezu A, Hisey E, Kobayashi Y, Gao Y, Bradshaw TW, Devlin P, et al. Essential role for InSyn1 in dystroglycan complex integrity and cognitive behaviors in mice. Elife. 2019;8 10.7554/eLife.50712. [DOI] [PMC free article] [PubMed]
- 39.Früh S, Romanos J, Panzanelli P, Bürgisser D, Tyagarajan SK, Campbell KP, et al. Neuronal dystroglycan is necessary for formation and maintenance of functional CCK-positive basket cell terminals on pyramidal cells. J Neurosci. 2016;36(40):10296–10313. doi: 10.1523/JNEUROSCI.1823-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Belvindrah R, Graus-Porta D, Goebbels S, Nave K-A, Müller U. Beta1 integrins in radial glia but not in migrating neurons are essential for the formation of cell layers in the cerebral cortex. J Neurosci. 2007;27(50):13854–13865. doi: 10.1523/JNEUROSCI.4494-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goebbels S, Bormuth I, Bode U, Hermanson O, Schwab MH, Nave K-A. Genetic targeting of principal neurons in neocortex and hippocampus of NEX-Cre mice. Genesis. 2006;44(12):611–621. doi: 10.1002/dvg.20256. [DOI] [PubMed] [Google Scholar]
- 42.Schwab MH, Druffel-Augustin S, Gass P, Jung M, Klugmann M, Bartholomae A, et al. Neuronal basic helix-loop-helix proteins (NEX, neuroD, NDRF): spatiotemporal expression and targeted disruption of the NEX gene in transgenic mice. J Neurosci. 1998;18(4):1408–1418. doi: 10.1523/JNEUROSCI.18-04-01408.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu S-X, Goebbels S, Nakamura K, Nakamura K, Kometani K, Minato N, et al. Pyramidal neurons of upper cortical layers generated by NEX-positive progenitor cells in the subventricular zone. Proc Natl Acad Sci U S A. 2005;102(47):17172–17177. doi: 10.1073/pnas.0508560102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peron SP, Freeman J, Iyer V, Guo C, Svoboda K. A cellular resolution map of barrel cortex activity during tactile behavior. Neuron. 2015;86(3):783–799. doi: 10.1016/j.neuron.2015.03.027. [DOI] [PubMed] [Google Scholar]
- 45.Huang ZJ, Di Cristo G, Ango F. Development of GABA innervation in the cerebral and cerebellar cortices. Nat Rev Neurosci. 2007;8(9):673–686. doi: 10.1038/nrn2188. [DOI] [PubMed] [Google Scholar]
- 46.Miyoshi G, Hjerling-Leffler J, Karayannis T, Sousa VH, Butt SJB, Battiste J, et al. Genetic fate mapping reveals that the caudal ganglionic eminence produces a large and diverse population of superficial cortical interneurons. J Neurosci. 2010;30(5):1582–1594. doi: 10.1523/JNEUROSCI.4515-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gulyás AI, Hájos N, Freund TF. Interneurons containing calretinin are specialized to control other interneurons in the rat hippocampus. J Neurosci. 1996;16(10):3397–3411. doi: 10.1523/JNEUROSCI.16-10-03397.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Urbán Z, Maglóczky Z, Freund TF. Calretinin-containing interneurons innervate both principal cells and interneurons in the CA1 region of the human hippocampus. Acta Biol Hung. 2002;53(1–2):205–220. doi: 10.1556/ABiol.53.2002.1-2.19. [DOI] [PubMed] [Google Scholar]
- 49.Katona I, Sperlágh B, Sík A, Käfalvi A, Vizi ES, Mackie K, et al. Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J Neurosci. 1999;19(11):4544–4558. doi: 10.1523/JNEUROSCI.19-11-04544.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marsicano G, Lutz B. Expression of the cannabinoid receptor CB1 in distinct neuronal subpopulations in the adult mouse forebrain: CB1 expression in murine forebrain. Eur J Neurosci. 1999;11(12):4213–4225. doi: 10.1046/j.1460-9568.1999.00847.x. [DOI] [PubMed] [Google Scholar]
- 51.Tsou K, Brown S, Sañudo-Peña MC, Mackie K, Walker JM. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 1998;83(2):393–411. doi: 10.1016/S0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]
- 52.Miczán V, Kelemen K, Glavinics JR, László ZI, Barti B, Kenesei K, et al. NECAB1 and NECAB2 are prevalent calcium-binding proteins of CB1/CCK-positive GABAergic interneurons. Cereb Cortex. 2021;31(3):1786–1806. doi: 10.1093/cercor/bhaa326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Del Pino I, Brotons-Mas JR, Marques-Smith A, Marighetto A, Frick A, Marin O, et al. Abnormal wiring of CCK(+) basket cells disrupts spatial information coding. Nat Neurosci. 2017;20(6):784–792. doi: 10.1038/nn.4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pelkey KA, Calvigioni D, Fang C, Vargish G, Ekins T, Auville K, et al. Paradoxical network excitation by glutamate release from VGluT3(+) GABAergic interneurons. Elife. 2020;9 10.7554/eLife.51996. [DOI] [PMC free article] [PubMed]
- 55.Somogyi J, Baude A, Omori Y, Shimizu H, El Mestikawy S, Fukaya M, et al. GABAergic basket cells expressing cholecystokinin contain vesicular glutamate transporter type 3 (VGLUT3) in their synaptic terminals in hippocampus and isocortex of the rat. Eur J Neurosci. 2004;19(3):552–569. doi: 10.1111/j.0953-816X.2003.03091.x. [DOI] [PubMed] [Google Scholar]
- 56.Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, de Costa BR, et al. Cannabinoid receptor localization in brain. Proc Natl Acad Sci U S A. 1990;87(5):1932–1936. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J Neurosci. 1991;11(2):563–583. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Katona I, Rancz EA, Acsady L, Ledent C, Mackie K, Hajos N, et al. Distribution of CB1 cannabinoid receptors in the amygdala and their role in the control of GABAergic transmission. J Neurosci. 2001;21(23):9506–9518. doi: 10.1523/JNEUROSCI.21-23-09506.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tricoire L, Pelkey KA, Erkkila BE, Jeffries BW, Yuan X, McBain CJ. A blueprint for the spatiotemporal origins of mouse hippocampal interneuron diversity. J Neurosci. 2011;31(30):10948–10970. doi: 10.1523/JNEUROSCI.0323-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Calvigioni D, Máté Z, Fuzik J, Girach F, Zhang M-D, Varro A, et al. Functional differentiation of cholecystokinin-containing interneurons destined for the cerebral cortex. Cereb Cortex. 2017;27(4):2453–2468. doi: 10.1093/cercor/bhw094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Morozov YM, Freund TF. Postnatal development and migration of cholecystokinin-immunoreactive interneurons in rat hippocampus. Neuroscience. 2003;120(4):923–939. doi: 10.1016/S0306-4522(03)00409-3. [DOI] [PubMed] [Google Scholar]
- 62.Morozov YM, Freund TF. Post-natal development of type 1 cannabinoid receptor immunoreactivity in the rat hippocampus. Eur J Neurosci. 2003;18(5):1213–1222. doi: 10.1046/j.1460-9568.2003.02852.x. [DOI] [PubMed] [Google Scholar]
- 63.Morozov YM, Torii M, Rakic P. Origin, early commitment, migratory routes, and destination of cannabinoid type 1 receptor-containing interneurons. Cereb Cortex. 2009;19(suppl_1):i78–i89. doi: 10.1093/cercor/bhp028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Eggan SM, Mizoguchi Y, Stoyak SR, Lewis DA. Development of cannabinoid 1 receptor protein and messenger RNA in monkey dorsolateral prefrontal cortex. Cereb Cortex. 2010;20(5):1164–1174. doi: 10.1093/cercor/bhp179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mulder J, Aguado T, Keimpema E, Barabás K, Ballester Rosado CJ, Nguyen L, et al. Endocannabinoid signaling controls pyramidal cell specification and long-range axon patterning. Proc Natl Acad Sci U S A. 2008;105(25):8760–8765. doi: 10.1073/pnas.0803545105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vitalis T, Lainé J, Simon A, Roland A, Leterrier C, Lenkei Z. The type 1 cannabinoid receptor is highly expressed in embryonic cortical projection neurons and negatively regulates neurite growth in vitro. Eur J Neurosci. 2008;28(9):1705–1718. doi: 10.1111/j.1460-9568.2008.06484.x. [DOI] [PubMed] [Google Scholar]
- 67.Berghuis P, Dobszay MB, Wang X, Spano S, Ledda F, Sousa KM, et al. Endocannabinoids regulate interneuron migration and morphogenesis by transactivating the TrkB receptor. Proc Natl Acad Sci U S A. 2005;102(52):19115–19120. doi: 10.1073/pnas.0509494102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.del Río JA, Lecea L, Ferrer I, Soriano E. The development of parvalbumin-immunoreactivity in the neocortex of the mouse. Brain Res Dev Brain Res. 1994;81(2):247–259. doi: 10.1016/0165-3806(94)90311-5. [DOI] [PubMed] [Google Scholar]
- 69.Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13(1):133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Carriere CH, Wang WX, Sing AD, Fekete A, Jones BE, Yee Y, et al. The γ-Protocadherins regulate the survival of GABAergic interneurons during developmental cell death. J Neurosci. 2020;40(45):8652–8668. doi: 10.1523/JNEUROSCI.1636-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mancia Leon WR, Spatazza J, Rakela B, Chatterjee A, Pande V, Maniatis T, et al. Clustered gamma-protocadherins regulate cortical interneuron programmed cell death. Elife. 2020;9 10.7554/eLife.55374. [DOI] [PMC free article] [PubMed]
- 72.Priya R, Paredes MF, Karayannis T, Yusuf N, Liu X, Jaglin X, et al. Activity regulates cell death within cortical interneurons through a calcineurin-dependent mechanism. Cell Rep. 2018;22(7):1695–1709. doi: 10.1016/j.celrep.2018.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Southwell DG, Paredes MF, Galvao RP, Jones DL, Froemke RC, Sebe JY, et al. Intrinsically determined cell death of developing cortical interneurons. Nature. 2012;491(7422):109–113. doi: 10.1038/nature11523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Davis MI, Crittenden JR, Feng AY, Kupferschmidt DA, Naydenov A, Stella N, et al. The cannabinoid-1 receptor is abundantly expressed in striatal striosomes and striosome-dendron bouquets of the substantia nigra. PLoS One. 2018;13(2):e0191436. doi: 10.1371/journal.pone.0191436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Van Waes V, Beverley JA, Siman H, Tseng KY, Steiner H. CB1 cannabinoid receptor expression in the striatum: association with corticostriatal circuits and developmental regulation. Front Pharmacol. 2012;3:21. doi: 10.3389/fphar.2012.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pribiag H, Peng H, Shah WA, Stellwagen D, Carbonetto S. Dystroglycan mediates homeostatic synaptic plasticity at GABAergic synapses. Proc Natl Acad Sci U S A. 2014;111(18):6810–6815. doi: 10.1073/pnas.1321774111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Miyoshi G, Young A, Petros T, Karayannis T, McKenzie Chang M, Lavado A, et al. Prox1 regulates the subtype-specific development of caudal ganglionic eminence-derived GABAergic cortical interneurons. J Neurosci. 2015;35(37):12869–12889. doi: 10.1523/JNEUROSCI.1164-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Carlén M, Meletis K, Siegle JH, Cardin JA, Futai K, Vierling-Claassen D, et al. A critical role for NMDA receptors in parvalbumin interneurons for gamma rhythm induction and behavior. Mol Psychiatry. 2012;17(5):537–548. doi: 10.1038/mp.2011.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ledonne F, Orduz D, Mercier J, Vigier L, Grove EA, Tissir F, et al. Targeted inactivation of Bax reveals a subtype-specific mechanism of Cajal-retzius neuron death in the postnatal cerebral cortex. Cell Rep. 2016;17(12):3133–3141. doi: 10.1016/j.celrep.2016.11.074. [DOI] [PubMed] [Google Scholar]
- 80.Puñal VM, Paisley CE, Brecha FS, Lee MA, Perelli RM, Wang J, et al. Large-scale death of retinal astrocytes during normal development is non-apoptotic and implemented by microglia. PLoS Biol. 2019;17(10):e3000492. doi: 10.1371/journal.pbio.3000492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cope DW, Maccaferri G, Márton LF, Roberts JDB, Cobden PM, Somogyi P. Cholecystokinin-immunopositive basket and Schaffer collateral-associated interneurones target different domains of pyramidal cells in the CA1 area of the rat hippocampus. Neuroscience. 2002;109(1):63–80. doi: 10.1016/S0306-4522(01)00440-7. [DOI] [PubMed] [Google Scholar]
- 82.Szabó GG, Papp OI, Máté Z, Szabó G, Hájos N. Anatomically heterogeneous populations of CB1 cannabinoid receptor-expressing interneurons in the CA3 region of the hippocampus show homogeneous input-output characteristics: CB1-expressing interneurons in CA3. Hippocampus. 2014;24(12):1506–1523. doi: 10.1002/hipo.22330. [DOI] [PubMed] [Google Scholar]
- 83.Guillemot F. Spatial and temporal specification of neural fates by transcription factor codes. Development. 2007;134(21):3771–3780. doi: 10.1242/dev.006379. [DOI] [PubMed] [Google Scholar]
- 84.del Rio JA, Martínez A, Fonseca M, Auladell C, Soriano E. Glutamate-like immunoreactivity and fate of Cajal-Retzius cells in the murine cortex as identified with calretinin antibody. Cereb Cortex. 1995;5(1):13–21. doi: 10.1093/cercor/5.1.13. [DOI] [PubMed] [Google Scholar]
- 85.Courgeon M, Desplan C. Coordination between stochastic and deterministic specification in the Drosophila visual system. Science. 2019;366(6463):eaay6727. doi: 10.1126/science.aay6727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Xu C, Theisen E, Maloney R, Peng J, Santiago I, Yapp C, et al. Control of synaptic specificity by establishing a relative preference for synaptic partners. Neuron. 2019;106(2):355. doi: 10.1016/j.neuron.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tien N-W, Soto F, Kerschensteiner D. Homeostatic plasticity shapes cell-type- specific wiring in the retina. Neuron. 2017;94(3):656–665.e4. doi: 10.1016/j.neuron.2017.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Betley JN, Wright CVE, Kawaguchi Y, Erdélyi F, Szabó G, Jessell TM, et al. Stringent specificity in the construction of a GABAergic presynaptic inhibitory circuit. Cell. 2009;139(1):161–174. doi: 10.1016/j.cell.2009.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gaffuri A-L, Ladarre D, Lenkei Z. Type-1 cannabinoid receptor signaling in neuronal development. Pharmacology. 2012;90(1–2):19–39. doi: 10.1159/000339075. [DOI] [PubMed] [Google Scholar]
- 90.Vargish GA, Pelkey KA, Yuan X, Chittajallu R, Collins D, Fang C, et al. Persistent inhibitory circuit defects and disrupted social behaviour following in utero exogenous cannabinoid exposure. Mol Psychiatry. 2017;22(1):56–67. doi: 10.1038/mp.2016.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Albayram Ö, Passlick S, Bilkei-Gorzo A, Zimmer A, Steinhäuser C. Physiological impact of CB1 receptor expression by hippocampal GABAergic interneurons. Pflugers Arch. 2016;468(4):727–737. doi: 10.1007/s00424-015-1782-5. [DOI] [PubMed] [Google Scholar]
- 92.Yoshida-Moriguchi T, Campbell KP. Matriglycan: a novel polysaccharide that links dystroglycan to the basement membrane. Glycobiology. 2015;25(7):702–713. doi: 10.1093/glycob/cwv021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Campanelli JT, Roberds SL, Campbell KP, Scheller RH. A role for dystrophin-associated glycoproteins and utrophin in agrin-induced AChR clustering. Cell. 1994;77(5):663–674. doi: 10.1016/0092-8674(94)90051-5. [DOI] [PubMed] [Google Scholar]
- 94.Gee SH, Montanaro F, Lindenbaum MH, Carbonetto S. Dystroglycan-alpha, a dystrophin-associated glycoprotein, is a functional agrin receptor. Cell. 1994;77(5):675–686. doi: 10.1016/0092-8674(94)90052-3. [DOI] [PubMed] [Google Scholar]
- 95.Ibraghimov-Beskrovnaya O, Ervasti JM, Leveille CJ, Slaughter CA, Sernett SW, Campbell KP. Primary structure of dystrophin-associated glycoproteins linking dystrophin to the extracellular matrix. Nature. 1992;355(6362):696–702. doi: 10.1038/355696a0. [DOI] [PubMed] [Google Scholar]
- 96.Peng HB, Ali AA, Daggett DF, Rauvala H, Hassell JR, Smalheiser NR. The relationship between perlecan and dystroglycan and its implication in the formation of the neuromuscular junction. Cell Adhes Commun. 1998;5(6):475–489. doi: 10.3109/15419069809005605. [DOI] [PubMed] [Google Scholar]
- 97.Sato S, Omori Y, Katoh K, Kondo M, Kanagawa M, Miyata K, et al. Pikachurin, a dystroglycan ligand, is essential for photoreceptor ribbon synapse formation. Nat Neurosci. 2008;11(8):923–931. doi: 10.1038/nn.2160. [DOI] [PubMed] [Google Scholar]
- 98.Sugita S, Saito F, Tang J, Satz J, Campbell K, Südhof TC. A stoichiometric complex of neurexins and dystroglycan in brain. J Cell Biol. 2001;154(2):435–445. doi: 10.1083/jcb.200105003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Boucard AA, Chubykin AA, Comoletti D, Taylor P, Südhof TC. A splice code for trans-synaptic cell adhesion mediated by binding of neuroligin 1 to alpha- and beta-neurexins. Neuron. 2005;48(2):229–236. doi: 10.1016/j.neuron.2005.08.026. [DOI] [PubMed] [Google Scholar]
- 100.Fuccillo MV, Földy C, Gökce Ö, Rothwell PE, Sun GL, Malenka RC, et al. Single-cell mRNA profiling reveals cell-type-specific expression of neurexin isoforms. Neuron. 2015;87(2):326–340. doi: 10.1016/j.neuron.2015.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Reissner C, Stahn J, Breuer D, Klose M, Pohlentz G, Mormann M, et al. Dystroglycan binding to alpha-neurexin competes with neurexophilin-1 and neuroligin in the brain. J Biol Chem. 2014;289(40):27585–27603. doi: 10.1074/jbc.M114.595413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chen LY, Jiang M, Zhang B, Gokce O, Südhof TC. Conditional deletion of all neurexins defines diversity of essential synaptic organizer functions for neurexins. Neuron. 2017;94(3):611–625.e4. doi: 10.1016/j.neuron.2017.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hara Y, Balci-Hayta B, Yoshida-Moriguchi T, Kanagawa M. Beltrán-Valero de Bernabé D, Gündeşli H, et al. a dystroglycan mutation associated with limb-girdle muscular dystrophy. N Engl J Med. 2011;364(10):939–946. doi: 10.1056/NEJMoa1006939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dimidschstein J, Chen Q, Tremblay R, Rogers SL, Saldi G-A, Guo L, et al. A viral strategy for targeting and manipulating interneurons across vertebrate species. Nat Neurosci. 2016;19(12):1743–1749. doi: 10.1038/nn.4430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tasic B, Menon V, Nguyen TN, Kim TK, Jarsky T, Yao Z, et al. Adult mouse cortical cell taxonomy revealed by single cell transcriptomics. Nat Neurosci. 2016;19(2):335–346. doi: 10.1038/nn.4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zeisel A, Muñoz-Manchado AB, Codeluppi S, Lönnerberg P, La Manno G, Juréus A, et al. Brain structure. Cell types in the mouse cortex and hippocampus revealed by single-cell RNA-seq. Science. 2015;347(6226):1138–1142. doi: 10.1126/science.aaa1934. [DOI] [PubMed] [Google Scholar]
- 107.Nguyen R, Venkatesan S, Binko M, Bang JY, Cajanding JD, Briggs C, et al. Cholecystokinin-expressing interneurons of the medial prefrontal cortex mediate working memory retrieval. J Neurosci. 2020;40(11):2314–2331. doi: 10.1523/JNEUROSCI.1919-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rovira-Esteban L, Gunduz-Cinar O, Bukalo O, Limoges A, Brockway E, Müller K, et al. Excitation of diverse classes of cholecystokinin interneurons in the basal amygdala facilitates fear extinction. eNeuro. 2019:6, ENEURO.0220–19.2019–6. [DOI] [PMC free article] [PubMed]
- 109.Taniguchi H, He M, Wu P, Kim S, Paik R, Sugino K, et al. A resource of Cre driver lines for genetic targeting of GABAergic neurons in cerebral cortex. Neuron. 2011;71(6):995–1013. doi: 10.1016/j.neuron.2011.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Whissell PD, Cajanding JD, Fogel N, Kim JC. Comparative density of CCK- and PV-GABA cells within the cortex and hippocampus. Front Neuroanat. 2015;9:124. doi: 10.3389/fnana.2015.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Whissell PD, Bang JY, Khan I, Xie Y-F, Parfitt GM, Grenon M, et al. Selective activation of cholecystokinin-expressing GABA (CCK-GABA) neurons enhances memory and cognition. eNeuro. 2019;6(1):ENEURO.0360–18.2019. doi: 10.1523/ENEURO.0360-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fasano C, Rocchetti J, Pietrajtis K, Zander J-F, Manseau F, Sakae DY, et al. Regulation of the hippocampal network by VGLUT3-positive CCK- GABAergic basket cells. Front Cell Neurosci. 2017;11:140. doi: 10.3389/fncel.2017.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chittajallu R, Craig MT, McFarland A, Yuan X, Gerfen S, Tricoire L, et al. Dual origins of functionally distinct O-LM interneurons revealed by differential 5-HT(3A)R expression. Nat Neurosci. 2013;16(11):1598–1607. doi: 10.1038/nn.3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lee S, Hjerling-Leffler J, Zagha E, Fishell G, Rudy B. The largest group of superficial neocortical GABAergic interneurons expresses ionotropic serotonin receptors. J Neurosci. 2010;30(50):16796–16808. doi: 10.1523/JNEUROSCI.1869-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dudok B, Klein PM, Hwaun E, Lee BR, Yao Z, Fong O, et al. Alternating sources of perisomatic inhibition during behavior. Neuron. 2021;109(6):997–1012.e9. doi: 10.1016/j.neuron.2021.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Williamson RA, Henry MD, Daniels KJ, Hrstka RF, Lee JC, Sunada Y, Ibraghimov-Beskrovnaya O, Campbell KP. Dystroglycan is essential for early embryonic development: disruption of Reichert's membrane in Dag1-null mice. Hum Mol Genet. 1997;6(6):831–841. doi: 10.1093/hmg/6.6.831. [DOI] [PubMed] [Google Scholar]
- 117.Cohn RD, Henry MD, Michele DE, Barresi R, Saito F, Moore SA, et al. Disruption of DAG1 in differentiated skeletal muscle reveals a role for dystroglycan in muscle regeneration. Cell. 2002;110(5):639–648. doi: 10.1016/S0092-8674(02)00907-8. [DOI] [PubMed] [Google Scholar]
- 118.Grimes WN, Seal RP, Oesch N, Edwards RH, Diamond JS. Genetic targeting and physiological features of VGLUT3+ amacrine cells. Vis Neurosci. 2011;28(5):381–392. doi: 10.1017/S0952523811000290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Knudson CM, Tung KS, Tourtellotte WG, Brown GA, Korsmeyer SJ. Bax-deficient mice with lymphoid hyperplasia and male germ cell death. Science. 1995;270(5233):96–99. doi: 10.1126/science.270.5233.96. [DOI] [PubMed] [Google Scholar]
- 120.White FA, Keller-Peck CR, Knudson CM, Korsmeyer SJ, Snider WD. Widespread elimination of naturally occurring neuronal death in Bax-deficient mice. J Neurosci. 1998;18(4):1428–1439. doi: 10.1523/JNEUROSCI.18-04-01428.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Slupe AM, Villasana L, Wright KM. GABAergic neurons are susceptible to BAX-dependent apoptosis following isoflurane exposure in the neonatal period. PLoS One. 2021;16(1):e0238799. doi: 10.1371/journal.pone.0238799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9(7):676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Fig. S1. NexCre drives recombination in forebrain pyramidal neurons but not interneurons or glia. (A) Coronal sections from NexCre;R26LSL-H2B-mCherry reporter mice at P21 show mCherry+ nuclei (magenta) of pyramidal neurons in the hippocampus, cortex, amygdala, and nucleus of the lateral olfactory tract (nLOT). (B) Hippocampal sections from NexCre;R26LSL-H2B-mCherry reporter mice immunostained for interneuron markers (green) Calbindin (left panels), Parvalbumin (middle panel), and CB1R (right panel) show no overlap of interneuron cell bodies with mCherry+ nuclei. White arrowheads indicate CB1R+ cell bodies. SO, stratum oriens; SP, stratum pyramidale; SR, stratum radiatum. (C) The astrocyte marker GFAP (green) shows no overlap with mCherry+ nuclei in the hippocampal CA regions or dentate gyrus (left and middle panels). Inset (middle panel) shows a magnified view of astrocyte nuclei (blue). mCherry+ nuclei occupy the outer third of the dentate gyrus granule cell layer (right panel). ML, molecular layer; GCL, granule cell layer.
Additional file 2: Fig. S2. CCK+ interneuron innervation of the dentate gyrus is minimally altered in Dag1cKO mice. (A) Immunostaining of CB1R in the dentate gyrus from P30 Dag1Control (left panels) and Dag1cKO mice (right panels). Single channel images of CB1R (gray) are shown below. (B) Quantification of CB1R pixels for each dentate gyrus layer (*P < 0.05, unpaired two-tailed Student’s t-test; n = 4 mice/genotype). Data are presented as mean values ± s.e.m. Data are normalized to Dag1Control signal in each dentate gyrus layer. OML, outer molecular layer; IML, inner molecular layer; GCL, granule cell layer.
Additional file 3: Fig. S3. CCK+ interneuron markers are reduced postnatally in Dag1cKO mice. (A) Images of hippocampal CA1 from VGLUT3Cre;Ai9 mice from P3-P18. Immunostaining for tdTomato (green) shows progressive increase in VGLUT3 expression in the pyramidal cell layer (SP, magenta). (B) Immunostaining for VGLUT3 in the CA1 of Dag1Control (top panels) and Dag1cKO mice (bottom panels) from P3-P15. Note the lack of VGLUT3 expression at all ages in Dag1cKO mice. (C) Parvalbumin (PV) labeling is similar in the CA1 of Dag1Control (top panels) and Dag1cKO mice (bottom panels) from P5-P30.
Additional file 4: Fig. S4. Constitutive deletion of Bax in Dag1cKO mice does not rescue VGLUT3+ terminals. (A-D) Coronal sections of the hippocampus stained for VGLUT3 (gray) from P30 (A) Dag1Control;BaxControl, (B) Dag1Control;BaxKO, (C) Dag1cKO;BaxControl and (D) Dag1cKO;BaxKO mice. (A’-D′) Magnified images of the CA1 (yellow boxed regions) stained for VGLUT3 (green; Right, gray single channel images) and Hoechst (magenta) to stain the pyramidal cell layer (SP). SO, stratum oriens; SP, stratum pyramidale; SR, stratum radiatum.
Additional file 5: Fig. S5. Constitutive deletion of Bax in Dag1cKO mice does not rescue CB1R+ terminals in the forebrain. (A-C) Coronal sections immunostained for CB1R (green) and Hoechst (magenta) in the cortex (A), amygdala (B), and nucleus of the lateral olfactory tract (C) of P30 Dag1Control;BaxControl, Dag1Control;BaxKO, Dag1cKO;BaxControl and Dag1cKO;BaxKO mice.
Data Availability Statement
The datasets used during the current study are available from the corresponding author upon request.