Abstract
Vaccinations against influenza viruses are widely used all over the world. There are reports, however, of some associated adverse events, and there are some case reports of interstitial lung disease occurring after influenza vaccination. We experienced the case of exacerbation of connective tissue disease-associated interstitial lung disease (CTD-ILD) after influenza vaccination, and this is the first reported case, as far as we know. The patient responded quite well to corticosteroids administration. Influenza vaccination for patients with chronic lung disease including CTD-ILD is strongly recommended, but we should be aware of possible adverse events.
Keywords: Influenza vaccine, Connective tissue disease-associated interstitial lung disease, CTD-ILD, Interstitial pneumonia
1. Introduction
Influenza is one of the most prevalent contagious diseases in the world and spreads every year. Influenza symptoms are mostly mild, although occasionally can be fatal, especially for the elderly and those with underlying diseases. Influenza vaccinations reduce the number of hospitalizations and deaths [1,2]. The European League Against Rheumatism (EULAR) strongly recommends that influenza vaccination should be considered for patients with autoimmune inflammatory rheumatic disease (AIIRD) [3]. However, there are some case reports that indicate a relationship between influenza vaccination and interstitial lung disease. We hereby report a case where influenza vaccination seems to have caused exacerbation of connective tissue disease-associated interstitial lung disease (CTD-ILD).
1.1. Case presentation
A 71-year-old woman was referred to our hospital for evaluation of fever, exertional dyspnea and fatigue in November 2020. She had received the influenza vaccine (A/Guangdong-Maonan/SWL/2019 [H1N1], A/HongKong/2671/2019 [H3N2], B/Phuket/3073/2013, and B/Victoria/705/2018) nine days before her visit. She was in good shape on the day of vaccination and for weeks beforehand. She developed a slight fatigue and exertional dyspnea the night of administration and it gradually worsened. Three days later she developed a fever. There was no improvement in spite of levofloxacin use. Therefore, she was referred to our hospital for further evaluation of her symptoms. She had been diagnosed with rheumatoid arthritis, Sjögren's syndrome and connective tissue disease-associated interstitial lung disease (CTD-ILD) in 2009. These had been well controlled with tacrolimus and abatacept administration. Additionally, she was diagnosed as Burkitt lymphoma in February 2019, but she achieved complete remission in January 2020 after eight months of chemotherapy (R-THP-COP, R-hyper-CVAD, R-MTX-AraC) and peripheral blood stem cell transplantation (PBSCT). She experienced no deterioration of underlying interstitial lung disease after its diagnosis. She received influenza vaccination annually for several years till 2018 with no adverse events, but she didn't have vaccination in 2019 season due to her hospitalization. She started smoking at the age of 30 and quit at 60.
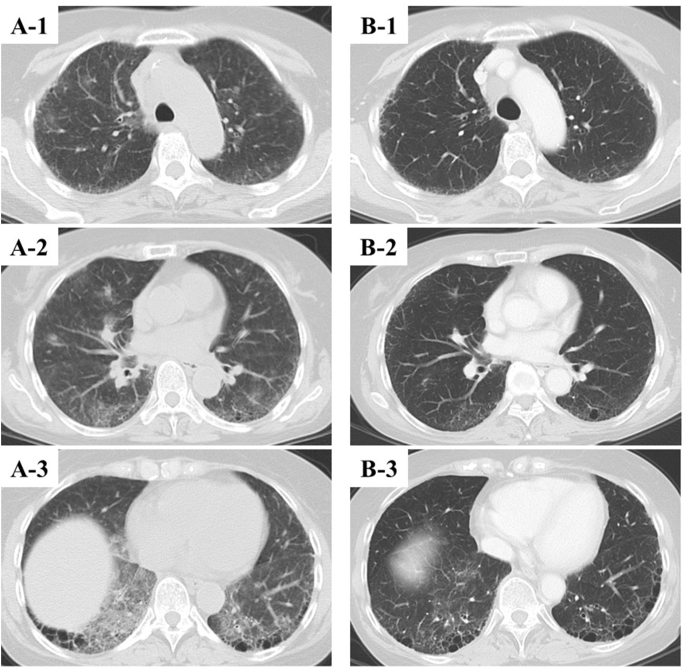
On examination her temperature was 38.7 °C, blood pressure was 120/70 mmHg, heart rate was 100/min, respiratory rate was 20/min and SpO2 was 94% (room air). She felt difficulty in breathing even when walking on level ground. A physical examination revealed fine crackles in both lung bases. Complete blood count revealed white blood cells at 6600 /μL (with neutrophils comprising 77.9%, lymphocytes 12.3%, eosinophils 2.8%, and basophils 0.2%), mild anemia with hemoglobin of 10.1 g/dL, and a platelet count of 122,000 /μL. Biochemistry assays showed C-reactive protein (CRP) to be 15.07 mg/dL, lactate dehydrogenase (LDH) 310 U/L, soluble interleukin-2 receptor (sIL-2R) 1448 U/mL (122-496), and Krebs von den Lungen-6 (KL-6) 355 U/mL (0-499). Serology tests for anti-cyclic citrullinated peptide antibody (ACPA) were positive (21.8 U/mL), but was negative for any autoimmune markers and for infectious diseases including an influenza antigen test and SARS-CoV-2 PCR test. Chest radiograph and computed tomography revealed ground-glass opacities and infiltrates in both lungs and no enlarged lymph nodes to indicate the recurrence of lymphoma (Fig. 1A).
Fig. 1.
A) Computed tomography (CT) on the day she visited our hospital shows bilateral ground-glass opacities and infiltrates. B) Follow up CT at 10 days after discharge shows almost complete vanishment of bilateral ground-glass opacities and infiltrates.
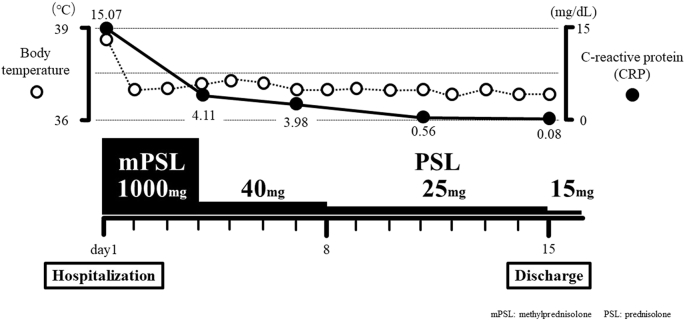
Based on the clinical course, influenza vaccination may have triggered the exacerbation of underlying interstitial lung disease and the progression of acute respiratory failure. Her fever came down to normal immediately after intravenous methylprednisolone administration at 1000 mg for 3 days. Thus, oral prednisolone administration at 40 mg was started and gradually tapered. KL-6 slightly rose to 566 U/mL, but other laboratory data improved (CRP 0.09 mg/dL, LDH 235 U/L, sIL-2R 476 U/mL). Her complaint of fatigue and exertional dyspnea gradually decreased day by day and chest radiograph showed improvement of bilateral ground-glass opacities. Hence, she left our hospital at the 15th hospital day (Fig. 2). The computed tomography performed 10 days after her discharge demonstrated almost complete vanishment of bilateral ground-glass opacities and infiltrates (Fig. 1B). No recurrence of interstitial pneumonia was observed. Oral prednisolone was withdrawn at four months.
Fig. 2.
Clinical course of our case.
2. Discussion
Influenza vaccine is one of the most prevalent inactivated vaccines and is administered all over the world. Based on the national law in Japan every elderly person over 65 years old and the persons over 60 years old with underlying diseases are designated to receive a vaccination annually. Consequently, over 50 million Japanese people get vaccinated every year. Its efficacy in decreasing hospitalization and the mortality rate of influenza has been reported by several researchers [ [1,2]]. It is said to be a relatively safe vaccination, but some adverse events including interstitial pneumonia are reported every year. According the Ministry of Health, Labor and Welfare of Japan, 278 cases (0.00049%) were reported as adverse events due to influenza vaccination in the 2019 season, and three were suspected of vaccination-related interstitial pneumonia [4].
A systematic search of PubMed found nine case reports including 11 cases of interstitial lung disease associated with the influenza vaccine (Table) [ [[5], [6], [7], [8], [9], [10], [11], [12], [13]]]. Nine patients of them survived due to early detection and corticosteroids administration, but the others died despite all efforts. Four patients had a medical history of chronic pulmonary disease (1 lung cancer, 1 extrinsic allergic alveolitis to parakeets, 2 idiopathic pulmonary fibrosis), but none had suffered from connective tissue disease-associated interstitial lung disease (CTD-ILD). As far as we know, this is the first reported case of CTD-ILD exacerbation caused by influenza vaccination. However, just like other cases, the patient was over 60 years old, the symptoms occurred within several days after administration, with a CT showing bilateral ground-glass opacities and infiltrates and she responded well to corticosteroid treatment.
Table 1.
Summary of the eleven reviewed cases and our case.
| Case | First author | Sex | Age | Onset of ILD (day) | Previous illness | Symptoms | Chest CT | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Johnston [5] | M | 58 | 10 | Pneumothoraxes, arthritis, AMI, nephrolithiasis | Fever, dyspnea, weight loss | Not available | Corticosteroids | Survival |
| 2 | Heinrichs [6] | F | 59 | 6 | Extrinsic allergic alveolitis to parakeets | Fever, dyspnea, Joint pain |
Bilateral GGO, patchy infiltrates | Corticosteroids | Survival |
| 3 | Kanemitsu [7] | M | 74 | 1 | GERD | Fever, dry cough | Bilateral patchy infiltrates | Corticosteroids | Survival |
| 4 | Bhurayanontachai [8] | F | 38 | 1 | None | Dyspnea, myalgia | Bilateral lung infiltrates | Aggressive organ support | Survival |
| 5 | Umeda [9] | M | 57 | 2 | Idiopathic pulmonary fibrosis | Fever, dyspnea | Bilateral GGO | Corticosteroids, immunosuppressants | Survival |
| 6 | Kumamoto [10] | M | 61 | 2 | Lung cancer | Fever, dyspnea, dry cough | Bilateral GGO | Corticosteroids | Survival |
| 7 | Watanabe [11] | F | 75 | 7 | Hypertension, diabetes mellitus, dialysis | Fever | Bilateral patchy infiltrates | Corticosteroids | Survival |
| 8 | Hirasawa [12] | M | 78 | 3 | Hypertension, hyperuricemia, GERD, allergic rhinitis |
Fever, cough | Bilateral lung infiltrates | Corticosteroids, immunosuppressants | Death |
| 9 | Hirasawa [12] | M | 68 | 3 | Idiopathic pulmonary fibrosis, hypertension, BPH, chronic renal failure | Dyspnea | Bilateral GGO | Corticosteroids | Death |
| 10 | Hibino [13] | F | 71 | 2 | Diabetes mellitus, dyslipidemia, renal tuberculosis | Dry cough, chest pain | Bilateral patchy consolidations, bilateral GGO | Corticosteroids | Survival |
| 11 | Hibino [13] | M | 67 | 6 | Unremarkable | Fever, dry cough, dyspnea, fatigue | Bilateral patchy consolidations, bilateral GGO | Corticosteroids | Survival |
| 12 | Present study | F | 71 | 1 | Rheumatoid arthritis, Sjögren's syndrome, CTD-ILD, Burkitt lymphoma |
Fever, fatigue, exertional dyspnea | Bilateral GGO, bilateral patchy infiltrates | Corticosteroids | Survival |
AMI: acute myocardial infarction GERD: gastro-esophageal reflux disease BPH: benign prostatic hyperplasia CTD-ILD: connective tissue disease-associated interstitial lung disease GGO: Ground-glass opacities.
Influenza vaccination is strongly recommended for patients with autoimmune inflammatory rheumatic disease (AIIRD) by The European League Against Rheumatism (EULAR) [3]. It is quite meaningful that in this case we experienced the possibility that an influenza vaccination might trigger the deterioration of underlying interstitial lung disease in spite of its other benefits.
It is unclear why this was her first exacerbation of CTD-ILD after diagnosis, as she was subsequently vaccinated annually. The chemotherapy for Burkitt lymphoma might have been affected in some way.
The reason why we selected high-dose (1000 mg) intravenous methylprednisolone treatment is that we assumed it could be severe and fatal exacerbation of interstitial pneumonia. There is a retrospective case-control study which indicates a mortality of 64% for initial acute exacerbation of rheumatoid arthritis-associated interstitial lung disease (RA-ILD) [14]. There is no established treatment for acute exacerbation of RA-ILD, thus treatments for acute exacerbation of idiopathic pulmonary fibrosis (IPF) are often selected. In particular, we generally perform high-dose (500-1000 mg/day) intravenous methylprednisolone administration for three days every other week for 1-4 iterations until the patient’s condition is ameliorated. In retrospect, however, high-dose corticosteroid administration might have been unnecessary as the patient responded to corticosteroids quite well.
We could not perform a bronchoscopy before corticosteroid administration. The Japan Society for Respiratory endoscopy recommended to not to perform unnecessary bronchoscopy exams during the COVID-19 pandemic [15]. In addition, it was risky to perform the bronchoscopy, because the acute respiratory failure of this patient progressed rapidly. Therefore, we prioritized treatment over diagnosis.
3. Conclusion
We experienced the case of the exacerbation of CTD-ILD due to influenza vaccination and early corticosteroids treatment achieved a favorable outcome. It is strongly recommended that the elderly and patients with chronic pulmonary disease including CTD-ILD should have influenza vaccination for the overall benefit, but physicians should also know that influenza vaccination can cause and exacerbate underlying interstitial lung disease. Early detection and corticosteroids administration could prevent pneumonia from worsening. Thus, it is very important to tell patients to see doctor as soon as possible when there is something wrong after vaccination.
Declaration of competing interest
The authors state that they have no conflict of interest (COI) about this case report.
References
- 1.Arriola C., Garg S., Anderson J.E. Influenza vaccination modifies disease severity among community-dwelling adults hospitalized with influenza. Clin. Infect. Dis. 2017;65:1289–1297. doi: 10.1093/cid/cix468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chan T.C., Hung F.N.I., Luk K.H.J., Chu L.W., Chan H.W.F. Effectiveness of influenza vaccination in institutionalized older adults: a systematic review. J. Am. Med. Dir. Assoc. 2014;15:226. doi: 10.1016/j.jamda.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 3.Furer V., Rondaan C., Haijstek M. Update of EULAR recommendations for vaccination in adult patients with autoimmune inflammatory rheumatic diseases. Ann. Rheum. Dis. 2019;79(2020):39–52. doi: 10.1136/annrheumdis-2019-215882. [DOI] [PubMed] [Google Scholar]
- 4.2020. Pharmaceuticals and medical devices safety information No. 376 october 2020. [Google Scholar]
- 5.Johnston S.D., Kempston A., Robinson T.J. Pneumonitis secondary to the influenza vaccine. Postgrad. Med. 1998;74:541–542. doi: 10.1136/pgmj.74.875.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heinrichs D., Sennekamp J., Kirsten A., Kirsten D. Allergic alveolitis after influenza vaccination. Pneumologie. 2009;63:508–511. doi: 10.1055/s-0029-1214909. (in German, Abstract in English) [DOI] [PubMed] [Google Scholar]
- 7.Kanemitsu Y., Kita H., Fuseya Y. Interstitial pneumonitis caused by seasonal influenza vaccine Nihon Kokyuki Gakkai Zasshi. J. Jpn. Respir. Soc. 2010;48:739–742. (in Japanese, Abstract in English) [PubMed] [Google Scholar]
- 8.Bhurayanontachai R. Possible life-threatening adverse reaction to monovalent H1N1 vaccine. Crit. Care. 2010;14:422. doi: 10.1186/cc9022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Umeda Y., Morikawa M., Anzai M. Acute exacerbation of idiopathic pulmonary fibrosis after pandemic influenza A (H1N1) vaccination. Intern. Med. 2010;49:2333–2336. doi: 10.2169/internalmedicine.49.3890. [DOI] [PubMed] [Google Scholar]
- 10.Kumamoto T., Mitsuyama H., Hamasaki T. Case report; Drug induced lung injury caused by 2009 pandemic H1N1 vaccine Nihon Naika Gakkai Zasshi. J. Jpn. Soc. Intern. Med. 2011;100:3034–3037. doi: 10.2169/naika.100.3034. (in Japanese) [DOI] [PubMed] [Google Scholar]
- 11.Watanabe S., Waseda Y., Takato H. Influenza vaccine-induced interstitial lung disease. Eur. Respir. J. 2013;41:474–477. doi: 10.1183/09031936.00146912. [DOI] [PubMed] [Google Scholar]
- 12.Hirasawa Y., Kono C., Yamada Y. Case Report; Influenza vaccination-associated acute lung injury: two cases report. Nihon Naika Gakkai Zasshi. 2015;104:1457–1459. doi: 10.2169/naika.104.1457. (in Japanese) [DOI] [PubMed] [Google Scholar]
- 13.Hibino M., Kondo T. Interstitial pneumonia assosiated with the influenza vaccine: a report of two cases. Intern. Med. 2017;56:197–201. doi: 10.2169/internalmedicine.56.7239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hozumi H, Nakamura Y, Johkoh T. Acute exacerbation in rheumatoid arthritis-associated interstitial lung disease: a retrospective case control study. BMJ Open. 2013;3(9) doi: 10.1136/bmjopen-2013-003132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The Japan Society for Respiratory Endoscopy. Urgent Recommendations on Corona Virus Disease on 2019 (COVID-19) [Online]. Available at: http://www.jsre.org/info/200217_corona_teigen.pdf (in Japanese) (Accessed: June 2021).