Abstract
Background
Fetal docosahexaenoic acid (DHA) supply relies on preferential transplacental transfer, which is regulated by placental DHA lipid metabolism. Maternal hyperglycemia and obesity associate with higher birthweight and fetal DHA insufficiency but the role of placental DHA metabolism is unclear.
Methods
Explants from 17 term placenta were incubated with 13C-labeled DHA for 48 h, at 5 or 10 mmol/L glucose treatment, and the production of 17 individual newly synthesized 13C-DHA labeled lipids quantified by liquid chromatography mass spectrometry.
Results
Maternal BMI positively associated with 13C-DHA-labeled diacylglycerols, triacylglycerols, lysophospholipids, phosphatidylcholine and phosphatidylethanolamine plasmalogens, while maternal fasting glycemia positively associated with five 13C-DHA triacylglycerols. In turn, 13C-DHA-labeled phospholipids and triacylglycerols positively associated with birthweight centile. In-vitro glucose treatment increased most 13C-DHA-lipids, but decreased 13C-DHA phosphatidylethanolamine plasmalogens. However, with increasing maternal BMI, the magnitude of the glucose treatment induced increase in 13C-DHA phosphatidylcholine and 13C-DHA lysophospholipids was curtailed, with further decline in 13C-DHA phosphatidylethanolamine plasmalogens. Conversely, with increasing birthweight centile glucose treatment induced increases in 13C-DHA triacylglycerols were exaggerated, while glucose treatment induced decreases in 13C-DHA phosphatidylethanolamine plasmalogens were diminished.
Conclusions
Maternal BMI and glycemia increased the production of different placental DHA lipids implying impact on different metabolic pathways. Glucose-induced elevation in placental DHA metabolism is moderated with higher maternal BMI. In turn, findings of associations between many DHA lipids with birthweight suggest that BMI and glycemia promote fetal growth partly through changes in placental DHA metabolism.
Supplementary Information
The online version contains supplementary material available at 10.1186/s10020-021-00344-w.
Keywords: Pregnancy, Placenta, Lipid, Stable-isotope, Gestational diabetes, LCMS
Background
The placenta regulates fetal nutritional supply and communication across the maternal–placental–fetal axis (Haggarty 2010; Lewis et al. 2018). DHA-containing lipids are important for fetal growth and development (Knopp et al. 1998; Higa and Jawerbaum 2013; Khan 2007), and the fetus relies on placental transfer of maternal DHA since the fetal–placental unit has limited synthetic capability (Hanebutt et al. 2008). Transplacental DHA supply is regulated by placental lipid metabolism, but there is limited understanding of the specific pathways involved (Haggarty 2010; Lewis et al. 2018). Understanding these mechanisms could pave the way to optimizing in-utero DHA supply for fetal development and designing strategies to rectify possible fetal DHA insufficiency in pathological conditions such as gestational diabetes (GDM) and maternal obesity (Haggarty 2010; Harris and Baack 2015).
Excessive fetal growth and adiposity, which are more prevalent with GDM and maternal obesity, increases risk of birth trauma, neonatal morbidity and perinatal mortality, as well as long term cardiometabolic disease (Leng et al. 2015; Boney et al. 2005; Catalano 2003). Lipids serve as metabolic fuel for the fetus and variations in fat accretion contribute to birthweight differences (Catalano 2003). However, fetal development also depends on less abundant, but highly bioactive DHA-lipids, which impact processes such as placental nutrient uptake, storage and export (Larqué et al. 2014; Herrera and Ortega-Senovilla 2018a; Delhaes et al. 2018; Duttaroy 2016). DHA-lipids modulate lipid membrane properties, transport, and membrane-related-processes such as G-Protein-coupled and interleukin-2 receptor-signaling (Li et al. 2005; Mitchell et al. 2003) and lipid droplet formation (Lecchi et al. 2013; Digel et al. 2010; Onuki et al. 2006); processes which regulate placental transfer of nutrients (Kolahi et al. 2016; Saito 2001). DHA and related derivatives also act as signaling molecules (Larqué et al. 2014; Herrera and Ortega-Senovilla 2018a; Delhaes et al. 2018; Gallo et al. 2017) that regulate fetal growth, inflammatory processes and labor onset (Duttaroy 2016; Rogers et al. 2013; Farhat et al. 2020), and protect against oxidative stress (Yavin et al. 2002; Lessig and Fuchs 2009; Wang and Wang 2010).
DHA is particularly important for fetal brain growth and neurological development (Helland et al. 2008; Steenweg-de Graaff et al. 2015; Wainwright 2002) while circulating maternal DHA in late pregnancy is also associated with fetal insulin sensitivity and long-term future weight gain for the child (Zhao et al. 2014; Moon et al. 2013). However, factors contributing to differences in levels of fetal–placental DHA lipids and mechanisms by which they may affect growth and development are poorly understood.
Alterations in placental and cord DHA lipids (Meher et al. 2016; Foreman-van Drongelen et al. 1995; Felton et al. 1994; Hellmuth et al. 2017; Patel et al. 2017) have indeed been linked with birthweight, likely predominantly due to the signaling actions of DHA-lipids. The directionality of associations is lipid-dependent, with cord phospholipid and lyso-phospholipid DHA positively linked with birthweight (Foreman-van Drongelen et al. 1995; Felton et al. 1994; Patel et al. 2017), and un-esterified cord DHA negatively linked (Hellmuth et al. 2017). This is consistent with the idea that different DHA-derivatives, may signal different effects.
The placenta selectively and preferentially transfers DHA leading to higher DHA content in umbilical cord blood compared to the maternal circulation (Larqué et al. 2014; Haggarty 2002; Pagán et al. 2013). Transplacental DHA transfer is substantially regulated by placental lipid metabolism, with compartmentalisation between different lipid pools influencing the bio-availability of un-esterified DHA and related compounds for placental processes and fetal supply (Lewis et al. 2018; Kolahi et al. 2016,2018). Although not well understood, accessibility likely depends on both lipid location and lipid type. For example, DHA stored in placental lipid droplets is more accessible than DHA in lipid-membrane structures important for cell function (Kolahi et al. 2016,2015). Meanwhile, triacylglycerols (TGs) undergo turnover more quickly than phospholipids suggesting that TG metabolism could be an important regulator of the availability of free un-esterified fatty acids (Watkins et al. 2019a). Furthermore, placental DHA-phosphatidyl-ethanolamine (DHA-PE), rather than DHA-phosphatidylcholine (DHA-PC) may be a more important source of DHA for the fetus (Uhl et al. 2015). The actual DHA-lipid forms that are being transferred from the placenta to the fetus (as signaling agents or building blocks) may include non-esterified DHA, other forms such as lysophospholipids which can cross transmembrane channels like MFSD2A, or by exocytosis of vesicles. Hence, tracking the metabolism of individual DHA-containing lipid species is necessary for understanding transplacental DHA supply and placental DHA-lipid action.
Both pre-pregnancy BMI and GDM have been negatively associated with DHA-phospholipids in umbilical cord blood erythrocytes (Thomas et al. 2005; Wijendran et al. 2000), whilst GDM has also been associated with reduced DHA in cord plasma (Zornoza-Moreno et al. 2014; Ortega-Senovilla et al. 2009), but other studies reported no difference (Thomas et al. 2005; Wijendran et al. 2000). Studies on endogenous 12C-DHA, and in-vivo studies using 13C-DHA, have shown that transplacental DHA transfer and consequent enrichment of cord plasma with DHA-lipids is impaired in GDM pregnancies and with obesity, suggesting placental dysfunction as a possible contributor to fetal DHA deficiency (Pagán et al. 2013; Wijendran et al. 2000; Visiedo et al. 2015; Gázquez et al. 2020). While functional studies in isolated primary human trophoblasts demonstrated impaired placental DHA uptake with GDM (Araújo et al. 2013), descriptive studies of GDM placental biopsies found elevated DHA-lipids (Uhl et al. 2015; Bitsanis et al. 2006). Since isolated primary human trophoblasts exposed to GDM-like conditions in-vitro showed decreased DHA transfer across a trophoblast monolayer, increased TG content, and decreased expression of proteins which suppress lipid synthesis, it was hypothesized that reduced DHA transfer to the fetus in GDM could result from increased placental DHA-lipid synthesis and retention as well as impaired uptake (Mishra et al. 2020). Indeed, DHA-phospholipids were previously found to be elevated in placenta in GDM or obesity (Uhl et al. 2015; Bitsanis et al. 2006) but reduced in the fetal compartment (Thomas et al. 2005; Wijendran et al. 2000) suggestive of placental DHA retention, while placental TGs in general are increased with hyperglycemia (Visiedo et al. 2015; Visiedo et al. 2013; Mishra et al. 2020; Tewari et al. 2011). However, these studies have not directly measured DHA-lipid synthesis, nor fully characterized the specific DHA-containing lipids involved.
Our present in-vitro study measures the fresh production of specific 13C-DHA-labeled lipids by placental explants from 13C-labeled-DHA. 13C-DHA is not naturally present in placenta, but is metabolized identically to naturally occurring 12C-DHA. Measured 13C-DHA-lipids therefore reflect the capacity of the placenta to take up, synthesize and metabolize new individual DHA-lipids, details which were not possible to assess with older methods involving radiolabeled 14C-DHA that could, at best, only measure overall uptake into broad extractable fractions such as total phospholipids by quantifying beta radiation (Araújo et al. 2013). Furthermore, the effects of in-vivo confounding, such as maternal dietary intake, maternal and fetal tissue DHA metabolism, circulating lipid concentrations, and placental blood flow, are all reduced in an in-vitro explant model, thus findings of 13C-DHA lipids are reflective of placental tissue DHA uptake and metabolism in isolation.
Current evidence therefore suggests that placental lipid metabolism is important for preferential transplacental DHA transfer, and that in GDM or maternal obesity some DHA lipids may be reduced in the fetal compartment (Uhl et al. 2015; Bitsanis et al. 2006) but elevated in placenta, suggestive of placental DHA retention. Given the reported lipid-dependent links between placental and cord blood DHA lipids with birthweight (Helland et al. 2008; Steenweg-de Graaff et al. 2015; Wainwright 2002; Meher et al. 2016; Varastehpour et al. 2006), characterizing the specific alterations in individual placental DHA lipids could uncover specific lipidomic mechanisms by which increasing maternal glycemia and BMI may lead to dysregulated fetal growth commonly observed with these conditions.
Thus, we hypothesized that placental DHA metabolism is programed by maternal BMI and antenatal glycemia, leading to alterations in placental DHA-lipids, which could in turn promote fetal growth and birthweight. We aimed to establish the association between maternal BMI and glycemia with the production and metabolism of individual placental 13C-DHA-lipids. We determined the direct effects of glucose treatment in-vitro on placental 13C-DHA lipid metabolism, and how this response to glucose is modulated by prior programing from in-vivo exposure to higher maternal BMI and glycemia. Relationships between placental 13C-DHA-lipids and birthweight were then examined alongside interactions with maternal BMI and glycemia.
Methods
Recruitment and clinical characteristics of participants
Placenta (n = 17) were collected from women recruited at the National University Hospital, Singapore with informed written consent. Universal screening for gestational diabetes (GDM) was performed at mid-gestation by a three time-point 75 g oral glucose tolerance test (OGTT) using WHO 2013 criteria. Cases of known pre-existing type 1 and type 2 diabetes mellitus were excluded as well as cases of possible pre-existing diabetes defined by antenatal OGTT results of fasting glycemia ≥ 7 mmol/L or 2 h glycemia ≥ 11.1 mmol/L. Non-smoking mothers who delivered after 37 weeks’ gestation by elective Caesarean section, with newborns who were greater than the 10th percentile (by local sex- and gestational age-standardized references (Mikolajczyk et al. 2011; Ong et al. 2020); this would exclude cases of potential malplacentation and uteroplacental insufficiency) were eligible. Women with GDM (n = 9) and normoglycemia (non-GDM; n = 8) were recruited and matched for first trimester BMI (similar mean (SD) kg/m2: GDM: 24.1 (3.9); non-GDM: 26.6 (5.0)), hence neither fasting glycemia nor post-load glycemia (by OGTT) were significantly associated with BMI in this study population. This selection strategy ensured a balance of cases across the more physiological glycemic range and across a range of maternal BMIs for these mechanistic studies of placental DHA lipid metabolism. Researchers were blinded to the clinical characteristics of the women and neonates involved until the completion of all explant culture experiments and LCMS. Table 1 shows the characteristics of the study population (individual details in Additional file 1) as extracted from medical records.
Table 1.
Clinical characteristics of study population
| Clinical characteristics | Total (n = 17) | Non-GDM (n = 8) | GDM (n = 9) |
|---|---|---|---|
| Maternal age (years) | 33.4 (2.9) | 32.5 (2.6) | 34.1 (3.2) |
| Chinese: Indian ethnicity | 11 (65%): 6 (35%) | 6 (75%): 2 (25%) | 5 (56%): 4 (44%) |
| Maternal BMI in first trimester (kg/m2) | 25.4 (4.6) | 24.1 (3.9) | 26.6 (5) |
| Normal weight: Overweight: Obesea | 5 (29%): 6 (35%): 6 (35%) | 3 (38%): 2 (25%): 3 (38%) | 2 (22%): 4 (44%): 3 (33%) |
| Fasting glycemia (mmol/L)b | 4.5 (0.5) | 4.4 (0.2) | 4.6 (0.6) |
| 1-h glycemia (mmol/L)b | 9.0 (1.7) | 7.8 (1.4) | 10.1 (1.15) |
| 2-h glycemia (mmol/L)b | 7.6 (1.6) | 6.4 (0.9) | 8.8 (1.2) |
| Antenatal PUFA-containing supplementation | 6 (35%) | 3 (33%) | 3 (38%) |
| Gestational age at delivery (days) | 271.4 (5.9) | 273.3 (7.4) | 269.7 (3.7) |
| Female neonates | 9 (52%) | 4 (50%) | 5 (56%) |
| Birthweight (g) | 3276.0 (323) | 3298.1 (326) | 3255.6 (339) |
| Birthweight centile (%)c | 61 (30) | 59 (31) | 66 (31) |
Data presented as Mean (SD) or n (%)
aWHO recommended Asian-specific BMI cut-off that is associated with increased metabolic risk (WHO EC 2004): Normal 18.5–22.9 kg/m2, Overweight 23–27.4 kg/m2, Obese ≥ 27.5 kg/m2
bIn mid-gestation 75 g three time-point OGTT
cBy local sex and gestational age-standardized references
Placenta collection and placental explant culture
Placental explants were cultured as previously described. Briefly, five pieces of villous placental tissue were randomly biopsied from five different sites across the placenta. These biopsies were then cut into small explants (approximately 3 × 3 × 3 mm) and added to each experimental well, with one explant coming from each different placental biopsy.
Explants were cultured in 12 well plates (Thermo-scientific, NunclonTM Delta surface) in basal serum-free CMRL media (1.8 mL, contains a physiological glucose concentration and no detectable fatty acids; GIBCO 1066-L Glutamine, Thermofisher, New York, USA) with 1.5% BSA (to match the albumin: fatty-acid ratio of maternal blood in the last trimester (0.78) (Haggarty et al. 1997); HI Clone fraction V, Culture grade, pH 7.00 lyophilized powder, GE Life Sciences, South Logan, Utah) and either no DHA or 13C22-DHA (24 µmol/L, 99 atom % 13C, 99% CP, Cambridge isotope laboratories), and either no additional (5 mmol/L) or 10 mmol/L final concentration of glucose (Biowest, P5030, France).
The levels of local un-esterified DHA available for placental uptake at the maternal-facing apical membrane of the placental syncytiotrophoblast barrier in-vivo are unknown. Un-esterified DHA was found to be 5.8 (SD 1.6) µmol/L in the maternal vein, 19.3 (2.8) µmol/L in the placental intervillous space containing maternal blood, but the actual amount available at the apical membrane is likely higher since much of the DHA available for uptake is lipid bound and only hydrolysed at the apical membrane surface to release unesterified DHA, which is rapidly internalized (Benassayag et al. 1997). We therefore added 24 µmol/L of 13C-DHA in our experiments, which was high enough to enable quantification of a range of 13C-DHA placental lipids.
Explants were incubated in a Thermo-scientific Foma Direct heat CO2 Incubator, Hepa Class 100 Model 37 °C in a humidified atmosphere of 5% CO2/air. All placental processing was finished within 2 h of delivery. Treatments were applied in triplicate wells and explants harvested at 48 h with triplicates combined in a pre-weighed Omnitube. Unlike simple uptake of fatty acids, the conversion of newly taken up 13C-DHA into 13C-DHA lipids is a relatively slow process such that few 13C-DHA lipids could be quantified in experimental times shorter than 48 h (Watkins et al. 2019a). Based on time-course experiments 48 h was selected as optimal for accurate quantification of a wide range of newly synthesized 13C-DHA lipids and when structural integrity of placental explants, including the syncytial cell layer, is maintained (Watkins et al. 2019a).
Confirmation of explant viability in these culture conditions were previously assessed by HCG and LDH over the period of culture (Watkins et al. 2019a). Explants were washed with PBS (1 mL) then stored at − 80 ºC until lipid extraction. Lipids were extracted as described in Additional file 2.
LC–MS/MS methodology
The LCMS method was developed based on our previous method (Watkins et al. 2019a) and the lipidomics methods of the Baker Institute (Weir et al. 2013). A dMRM transition list was developed containing transitions for all lipids possibly containing DHA (22:6). Transitions were then calculated for a 13C-DHA (13C22-DHA) containing partner for each lipid and these candidate transitions added to the method and this method tested on placental lipid extracts from placenta incubated with 13C-DHA. Proposed 13C-DHA lipid transitions that gave peaks exactly co-eluting with their 12C-DHA counterpart were kept, whilst those that did not were removed from the method. Candidate 13C-DHA lipid peaks present in lysate from placenta not incubated with 13C-DHA were also removed from the list. Additional file 3 illustrates the structure of DHA containing PC 38:6, showing how the incorporation of either 12C-DHA or 13C-DHA can produce an unlabeled or 13C-labeled PC 38:6, respectively, which can be quantified separately by LCMS due to differences in mass, but with identical physical characteristics and elution times. Lipid extracts (5 μL) were injected into an Agilent 6490 triple quadrupole (QQQ) liquid chromatography mass spectrometry (LC–MS/MS) instrument alongside a range of standards and analysed using a targeted dynamic multiple reaction monitoring (dMRM) method as described in Additional file 4.
Data and statistical analysis
LC–MS/MS data was analysed using Mass Hunter QQQ Quantitative Analysis Version 8 and peak area quantified by integration. Peak areas were normalized against their corresponding lipid class internal standard to give concentration expressed as pmol/ml. Dried placental mass was used to calculate the lipid amount expressed as pmol/mg. Calculations used for analysis are defined in Additional file 4 and quantified lipid data in Additional file 5. Statistical analysis was run on Z-scored log2 lipid amount or enrichment. Linear regression was then run for each lipid (outcome) with each variable of interest (predictor): maternal BMI, fasting glycemia or post-load 2 h glycemia, maternal age, or gestational age at delivery. Sensitivity analyses were performed excluding outliers of fasting glycemia. Linear regression was then run for birthweight centile (outcome) with each lipid (one lipid per model; predictor). Where indicated, multiple linear regression was then performed with mutual adjustments for these variables.
The effects of in-vitro glucose treatment (10 mmol/L) were expressed as log2 fold-change relative to controls from the same placenta with no additional glucose (5 mmol/L). The fold-change was calculated as amount of labeled lipid in explants treated with 10 mmol/L glucose divided by amount of labeled lipid in control explants. Fold-change was then log-2-transformed to give a glucose response value for each lipid. Glucose response therefore represents the relative amount of 13C-DHA lipid in placental explants treated with glucose compared to control explants from the same placenta. A 13C-DHA lipid glucose response > 0 indicates an increase in 13C-DHA lipid compared to the control, while a glucose response < 0 indicates a decrease. Linear regression was used to determine the relationships between glucose response and maternal characteristics or birthweight. Repeated measures analysis of variance was used to test whether glucose treatment altered the relative amount of placental 13C-DHA lipid compared to the control and the effects of covariate adjustment with one lipid per model. Data was analyzed in R using packages described in Additional file 4. Additional sensitivity analyses were performed with exclusion of those taking PUFA-containing supplements. For all sets of statistical analyses, the Benjamini–Hochberg method was used to correct for multiple testing to minimize false discovery. Statistical significance was set at a two-sided alpha level of p < 0.05.
Results
Placental incorporation of 13C-DHA into lipids
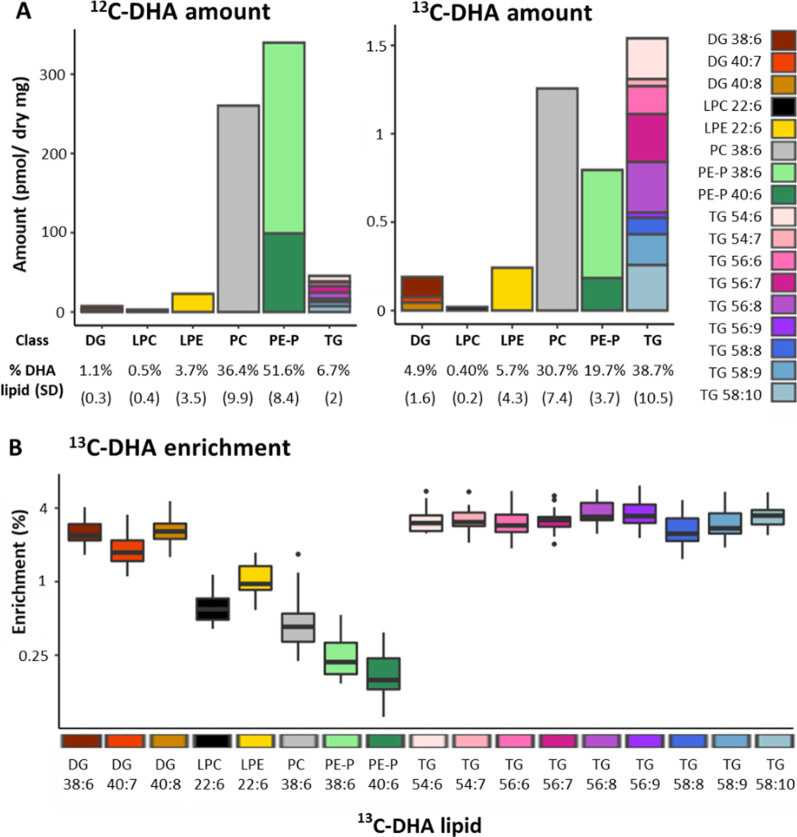
Placental explants incubated with stable-isotope 13C-DHA for 48 h produced stable-isotope labeled 13C-DHA-lipids of which seventeen could be reliably quantified using our LCMS method (Fig. 1). Quantifiable 13C-DHA phospholipids and their 12C-DHA counterparts included phosphatidyl-ethanolamine-plasmalogens (PE-P 48:6 [16:0_22:6], PE-P 40:6 [18:0_22:6]), phosphatidyl-choline (PC 38:6 [16:0_22:6]), lyso-phosphatidyl-choline (LPC 22:6) and lyso-phosphatidyl-ethanolamine (LPE 22:6). Quantifiable 13C-DHA glycerolipids and their 12C-DHA counterparts included diacylglycerides (DG 38:6 [16:0_22:6], 40:7 [18:1_22:6], 40:8 [18:2_22:6]) and nine triacylglycerides (TG; all contained 13C-DHA and two other fatty acids).
Fig. 1.
Placental lipids containing 12C-DHA and 13C-DHA in placental explants from 17 placenta incubated for 48 h with 24 µmol/L 13C-DHA at a physiological glucose concentration (5 mmol/L). A Amount of each lipid (pmol/mg) with percentages showing relative amount of each lipid class compared to total 12C-DHA or 13C-DHA lipids. Brackets show standard deviation. Colours represent individual lipids. B Boxplots showing the enrichment of each lipid with 13C-DHA. Enrichment is the percentage of each lipid containing 13C-DHA calculated by the formula 100 * 13C-lipid/(13C-lipid + 12C-lipid). DHA docosahexaenoic acid, DG diacylglycerol, LPC lyso-phosphatidylcholine, LPE lyso-phosphatidylethanolamine, PC phosphatidylcholine, PE-P phosphatidylethanolamine-plasmalogen, TG triacylglycerols. Labeled TGs contained 13C-DHA and two other fatty acids which cannot be identified with certainty. Since the total number of fatty acid carbons (x) and the total number of fatty acid double bonds (y) is known for each TG (format x:y), the combined carbon count and saturation of the two remaining fatty acids can be calculated, then predicted based on known abundance in humans. Two TGs contained 13C-DHA and two saturated lipids (TG 54:6 [predicted 16:0_16:0_22:6], TG 56:6 [predicted 16:0_18:0_22:6]), two contained DHA with one saturated fatty acid and one mono-unsaturated fatty acid (TG 54:7 [predicted 16:0_16:1_22:6], TG 56:7 [predicted 16:0_18:1_22:6 or 16:1_18:0_22:6]) and five TGs contained DHA with unknown combinations of various unsaturated lipids (TG 56:8, TG 58:8, TG 56:9, TG 58:9 and TG 58:10)
While the bulk of endogenous 12C-DHA lipids was found in PE-Ps (51.6% of total 12C-DHA lipids ± SD 8.4) followed by PCs (36.4% ± 9.9), then TGs (6.7% ± 2), for freshly produced 13C-DHA lipids, most were found in TGs (38.7% of total 13C-DHA lipids ± 10.5) and PCs (30.7% ± 7.4) followed by PE-Ps (19.7% ± 3.7) (Fig. 1A). This indicates that newly synthesized total 13C-DHA-TGs made up a much greater proportion of newly synthesised DHA lipids than any of the other individual lipid classes. However, the overall total of newly synthesized 13C-DHA-phospholipids from all its classes still amounted to more than total 13C-DHA-TGs, consistent with previous studies (Johnsen et al. 2009; Gil-Sánchez et al. 2010).
The difference between 12C-DHA lipids and their corresponding 13C-DHA lipids was especially obvious for PE-P, consistent with the idea that plasmalogens become enriched with PUFA mainly via the slow remodelling of existing phospholipids (Braverman and Moser 2012). Placental PEs (which includes PE-Ps) are known to be an important source of DHA for the fetus (Uhl et al. 2015), while PC 38:6 was previously found to be the most abundant PC with at least 6 double bonds (i.e. possibly DHA containing) in placenta (Uhl et al. 2015), and in placental lipid droplets (Gázquez et al. 2018).
DGs and LPCs made up the lowest proportion of both 12C-DHA and 13C-DHA labeled lipids, which is expected since these molecules are normally lowly abundant, transitory, and often act as signaling molecules (Wasserman et al. 2020).
Enrichment of each lipid with 13C-DHA (i.e. 100*13C-lipid/(13C-lipid + 12C-lipid)) gives an indication of lipid turnover (i.e. how quickly new lipids are produced and how quickly existing lipids are replaced by new lipids) and was found to be highest in TGs and DGs (Fig. 1B). This finding is consistent with the role of DGs as a precursor to most other lipids (Fig. 7) and suggests that TGs provide rapid storage for newly taken up 13C-DHA. Phospholipid enrichment in contrast was much lower, consistent with the idea that these structural lipids are produced and replaced more slowly. Similar enrichment patterns of these lipid types had previously been described for placental lipids incorporating 13C-PA and 13C-OA (Watkins et al. 2019b).
Fig. 7.
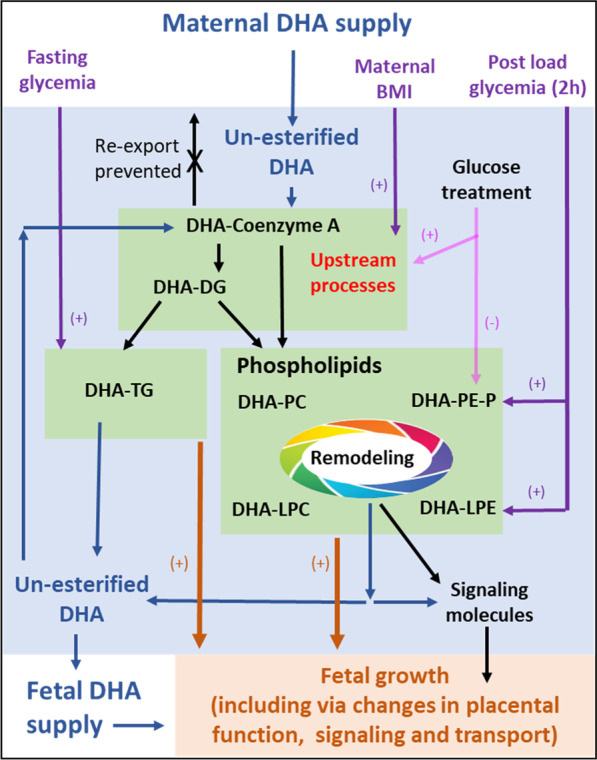
Placental DHA metabolism regulates the amount of DHA available for the synthesis of signaling molecules and for fetal supply. The conversion of DHA into DHA-COA and lipids such as diacylglycerols (DG), triacylglycerols (TG), or phospholipids such as phosphatidylcholine (PC) or phosphatidylethanolamine-plasmalogen (PE-P) traps DHA in the placenta and prevents re-export. DHA released from lipids (blue arrows), may be remade into lipids, used to form signaling molecules such as eicosanoids or exported to the fetus. In our experiments, increased BMI (purple) and in-vitro glucose treatment (pink) was associated with the increased synthesis of most 13C-DHA-lipids, suggesting that upstream processes such as activation into DHA-CoA or the early stages of lipid synthesis may be affected. Glucose treatment also decreased PE-Ps suggesting a separate PE-P specific process may also be affected. Fasting glycemia (purple) is only associated with TGs, suggesting a link with TG specific processes. DHA lipid metabolism and change in response to glucose treatment, was also associated with birthweight centile (shown by brown arrows) suggesting that placental DHA metabolism could influence birthweight
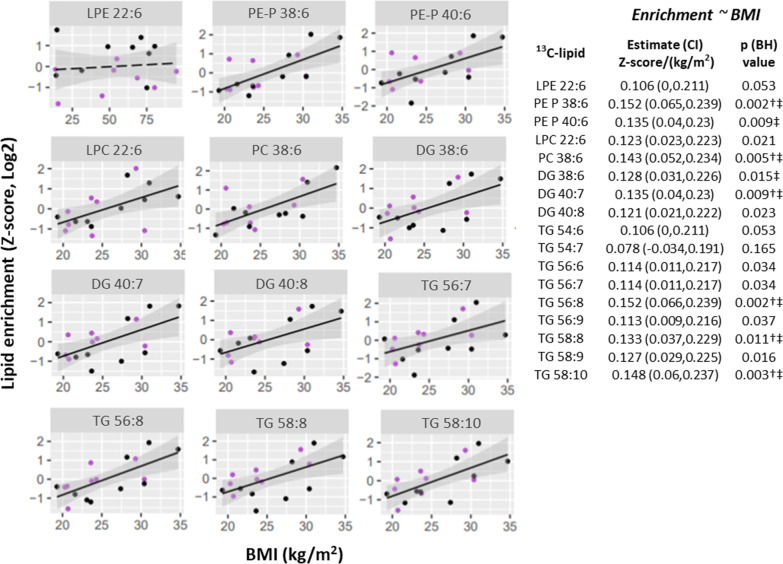
Association of maternal glycemia and BMI with placental 13C-DHA incorporation under culture in a physiological glucose condition (5 mmol/L)
Maternal BMI was positively associated with placental 13C-DHA-lipid enrichment for 14 out of the 17 lipids and associations remained similar after adjusting for maternal fasting or 2 h glycemia (Fig. 2). Of all quantified 13C-DHA-lipids, post-load 2 h glycemia was only associated with 13C-DHA-LPE enrichment [estimate (95% CI) 0.44 (0.20, 0.68) log2 z-score per mmol/L glucose, p = 0.002; Additional file 6] with associations remaining significant after adjusting for BMI. No association with 13C-DHA-lipid enrichment was observed for fasting glycemia, gestational age or maternal age.
Fig. 2.
Positive associations of BMI with the enrichment of placental 13C-DHA lipids (Z-scored, log2 transformed; n = 17 placenta) in culture at a physiological glucose condition (5 mmol/L). Linear regression was run with lipid as the outcome and BMI as the predictor. The Benjamini–Hochberg (BH) method was used to correct for multiple testing. Solid lines show significant associations while dashed lines show non-significant associations. Shaded areas show 95% confidence intervals. Key for dots—Purple: non-GDM, Black: GDM. †Significant after adjusting for fasting glycemia, ‡Significant after adjusting for 2 h glycemia. CI confidence interval, DG diacylglycerol, DHA docosahexaenoic acid, GDM gestational diabetes, LPC lyso-phosphatidylcholine, LPE lyso-phosphatidylethanolamine, PC phosphatidylcholine, PE-P phosphatidylethanolamine-plasmalogen, TG triacylglycerol
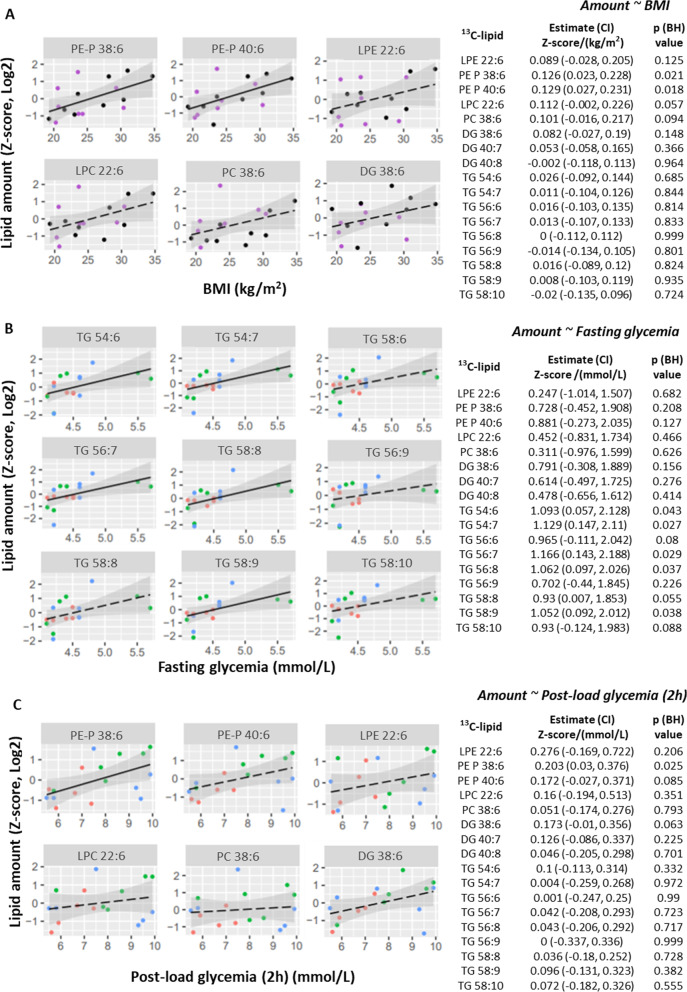
Maternal BMI was also positively associated with 13C-DHA phospholipid amount, with significant associations observed for PE-P 38:6 and PE-P 40:6 and similar trends seen for PC 36:6 and LPC 22:6 (Fig. 3A). Post-load 2 h glycemia was also positively associated with 13C-DHA phospholipid amount, with significant associations observed for 13C-DHA labeled PE-P 38:6 and similar trends observed for PE-P 40:6 and DG 38:6 (Fig. 3C).
Fig. 3.
Variations in patterns of association between fasting glycemia (A), post-load (2 h) glycemia (B) and maternal BMI (C) with the amount of placental 13C-DHA lipids (Z-score, log2; n = 17 placenta). Linear regression was run with lipid as the outcome and glycemia or BMI as the predictor. The Benjamini–Hochberg method was used to correct for multiple testing. Solid lines show statistically significant associations, while dashed lines show non-significant trends. Shaded areas show 95% confidence intervals. Key for dots in A and B—Red: Normal BMI (< 23 kg/m2), Green: Overweight (BMI 23.0 to < 27.5 kg/m2), Blue: Obese (BMI ≥ 27.5 kg/m2). Key for dots in C—Purple: non-GDM, Black: GDM. BH Benjamini–Hochberg, CI confidence interval, DG diacylglycerol, DHA docosahexaenoic acid, GDM gestational diabetes, LPC lyso-phosphatidylcholine, LPE lyso-phosphatidylethanolamine, PC phosphatidylcholine, PE-P phosphatidylethanolamine-plasmalogen, TG triacylglycerol
Even though BMI was associated with TG enrichment and therefore TG turnover, neither BMI nor post-load glycemia showed any significant associations with 13C-DHA TG amount. Instead, antenatal fasting glycemia was positively associated with the amount of 13C-DHA labeled TGs (significant for 5 out of the 9 with similar trends seen for the remainder), but not other lipids (Fig. 3B). In sensitivity analyses, which excluded the two outliers of higher fasting glycemia, results remained similar. Similar but non-significant trends in these associations were also observed after mutual adjustment for BMI and fasting glycemia (Additional file 7). Adjustment for maternal age or gestational age at delivery did not materially alter these results. No associations were observed between maternal age or gestational age with any 13C-DHA labeled lipid in univariate analyses.
Association between placental 13C-DHA lipid incorporation under culture in a physiological glucose condition (5 mmol/L) and birthweight centile
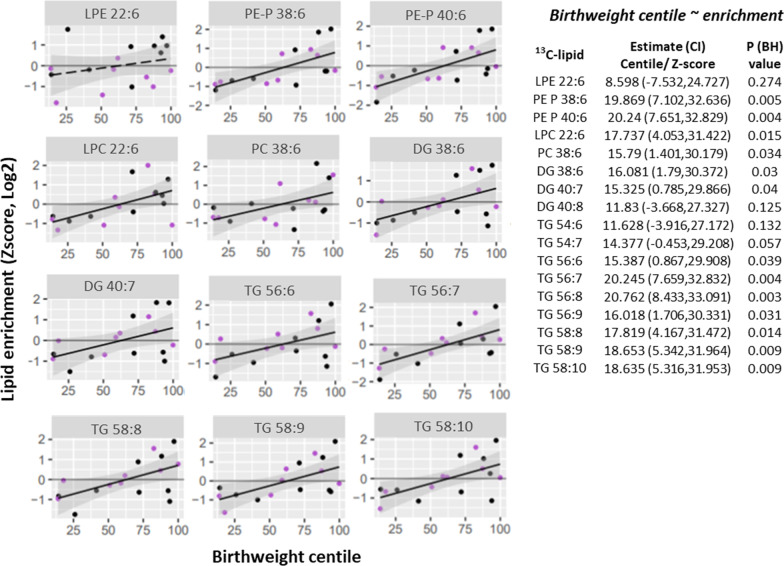
Birthweight centile was positively associated with increasing 13C-DHA enrichment of lipids in general (13 out of 17 13C-DHA lipids, Fig. 4) and increasing amounts of 13C-DHA phospholipids (LPC 22:6, PE-P 38:6, PE-P 40:6, Additional file 8).
Fig. 4.
Positive associations between placental 13C-DHA-lipid enrichment (Z-score, log2) and birthweight centile. Linear regression was run with birthweight centile as the outcome and either lipid amount or lipid enrichment as the predictor. The Benjamini–Hochberg method was used to correct for multiple testing. Solid lines show significant associations while dashed lines show non-significant results. Shaded areas show 95% confidence intervals. Key—Purple: non-GDM, Black: GDM. BH Benjamini–Hochberg, CI confidence interval, DG diacylglycerol, DHA docosahexaenoic acid, GDM gestational diabetes, LPC lyso-phosphatidylcholine, LPE lyso-phosphatidylethanolamine, PC phosphatidylcholine, PE-P phosphatidylethanolamine-plasmalogen, TG triacylglycerol
Since associations were observed between maternal BMI and birthweight centile [estimate (95% CI) 19.9 (7.2, 32.7) % per SD increase in log2-BMI, p = 0.004] and between BMI with DHA-lipids, we hypothesized that BMI may impact birthweight partly through affecting placental DHA metabolism. We thus adjusted for each DHA lipid in turn in our BMI (predictor)-birthweight centile (outcome) model (Additional file 9) to assess for any attenuation of the associations. BMI was no longer significantly associated with birthweight centile after adjusting for the enrichment of DG 38:6, DG 40:7, LPC 22:6, PC 38:6, PE-P 38:6, PE-P 40:6 and 8 out of 9 TGs, or for the amount of LPC 22:6, PE-P 38:6, PE-P 40:6. A reduction in the magnitude of birthweight centile change per unit BMI was also consistently observed across these DHA-lipid models. This suggests that placental DHA lipid metabolism may be one of the mechanistic pathways by which maternal BMI could increase birthweight.
Glucose treatment in-vitro and placental DHA-lipid metabolism
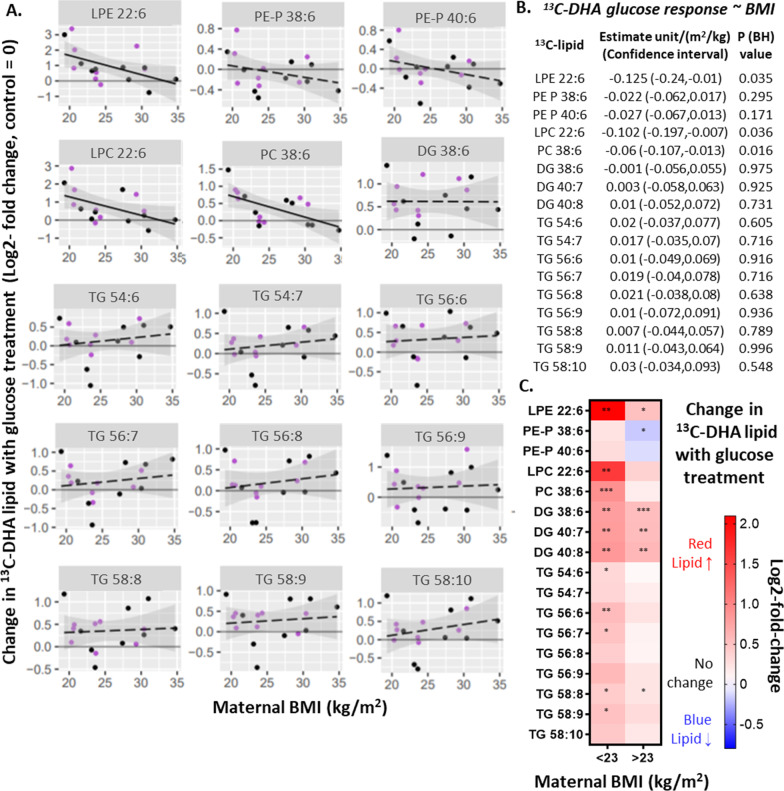
To determine if glucose can directly affect placental DHA-lipid metabolism, placental explants were cultured in media with added glucose. Overall, the amount of most 13C-DHA lipids in most placental explants was increased by glucose treatment (10 mmol/L) compared with the respective control (5 mmol/L glucose), as indicated by a log2-fold change greater than zero (i.e. dots above the x-axis in Fig. 5). The relative amount of 13C-DHA lipids between the glucose-treated and control, for each placenta, is henceforth termed glucose response. Since it has been postulated that how the placenta responds to glucose in-vitro could be dependent on prior exposure to maternal metabolic factors in-vivo, associations between maternal BMI or glycemia, with glucose response were investigated. Neither fasting glycemia nor 2 h glycemia, were associated with glucose response (Table 2). However, BMI was found to be negatively associated with 13C-DHA-phospholipid glucose response (Fig. 5).
Fig. 5.
Associations between maternal BMI and change in 13C-DHA lipids with glucose treatment (i.e. glucose response, log2-fold change, n = 17). Change in 13C-DHA lipid with glucose treatment represents the relative amount of 13C-DHA lipid in placental explants treated with glucose (10 mmol/L) compared to control explants (cultured in 5 mmol/L glucose) from the same placenta. Positive values indicate an increase in 13C-DHA lipids compared to the control, whilst negative values indicate a decrease. A, B Linear regression was run with glucose response as the outcome and BMI as the exposure variable. Benjamini–Hochberg (BH) method was used to correct for multiple testing. Solid lines show statistically significant associations, while dashed lines show non-significant results. Shaded areas show 95% confidence intervals. Key for dots—Purple: non-GDM, Black: GDM. C Analyses stratified by maternal BMI. Heat map illustrating glucose response in placental explants from normal weight women (BMI < 23 kg/m2) and from overweight/obese women (BMI ≥ 23 kg/m2). Colours indicate an increase (red; positive glucose response) or a decrease (blue; negative glucose response). Asterisks indicate significant differences between 10 mmol/L glucose treatment and the control (5 mmol/L) *p < 0.05, **p < 0.01, ***p < 0.001. Comparisons were made by repeated measures analysis of variance for each lipid followed by the Benjamini–Hochberg correction for multiplicity. BH Benjamini–Hochberg, CI confidence interval, DG diacylglycerol, DHA docosahexaenoic acid, GDM gestational diabetes, LPC lyso-phosphatidylcholine, LPE lyso-phosphatidylethanolamine, PC phosphatidylcholine, PE-P phosphatidylethanolamine-plasmalogen, TG triacylglycerol
Table 2.
Associations between maternal glycemia and change in 13C-DHA lipids with in-vitro glucose treatment (glucose response) in placental explants (n = 17)
| 13C-DHA lipid | Fasting glycemiaa | Post load glycemia (2 h)a | ||
|---|---|---|---|---|
| Estimate (lower CI, upper CI) Relative amountb/ (mmol/L) |
p value (BH) | Estimate (lower CI, upper CI) Relative amountb/ (mmol/L) |
p value (BH) | |
| DG 38:6 | 0.106 (− 0.45, 0.663) | 0.911 | − 0.091 (− 0.241, 0.059) | 0.216 |
| DG 40:7 | 0.065 (− 0.542, 0.673) | 0.822 | − 0.108 (− 0.27 ,0.053) | 0.172 |
| DG 40:8 | 0.116 (− 0.506, 0.739) | 0.919 | − 0.093 (− 0.262, 0.076) | 0.261 |
| LPC 22:6 | − 0.688 (− 1.732, 0.355) | 0.18 | − 0.228 (− 0.515, 0.059) | 0.111 |
| LPE 22:6 | − 0.82 (− 2.092, 0.451) | 0.189 | − 0.26 (− 0.613, 0.093) | 0.137 |
| PC 38:6 | − 0.474 (− 0.993, 0.046) | 0.071 | − 0.111 (− 0.264, 0.041) | 0.139 |
| PE-P 38:6 | − 0.034 (− 0.45, 0.382) | 0.912 | − 0.079 (− 0.189, 0.03) | 0.186 |
| PE-P 40:6 | − 0.196 (− 0.612, 0.219) | 0.329 | − 0.055 (− 0.172, 0.062) | 0.338 |
| TG 54:6 | − 0.164 (− 0.741, 0.414) | 0.555 | − 0.075 (− 0.235, 0.085) | 0.334 |
| TG 54:7 | − 0.123 (− 0.658, 0.413) | 0.632 | − 0.059 (− 0.208, 0.09) | 0.412 |
| TG 56:6 | − 0.243 (− 0.823, 0.337) | 0.387 | − 0.06 (− 0.225, 0.105) | 0.451 |
| TG 56:7 | − 0.128 (− 0.729, 0.474) | 0.658 | − 0.037 (− 0.207, 0.133) | 0.65 |
| TG 56:8 | − 0.042 (− 0.647, 0.562) | 0.883 | − 0.053 (− 0.221, 0.115) | 0.512 |
| TG 56:9 | − 0.256 (− 1.065, 0.553) | 0.511 | − 0.117 (− 0.34, 0.106) | 0.28 |
| TG 58:8 | 0.112 (− 0.398, 0.622) | 0.89 | − 0.01 (− 0.155, 0.135) | 0.881 |
| TG 58:9 | − 0.086 (− 0.628, 0.457) | 0.741 | − 0.076 (− 0.224, 0.072) | 0.289 |
| TG 58:10 | 0.009 (− 0.649, 0.668) | 0.976 | − 0.052 (− 0.236, 0.132) | 0.553 |
CI confidence interval, BH Benjamini–Hochberg corrected
aIn mid-gestation 75 g three time-point OGTT
bChange in 13C-DHA lipid with glucose treatment or glucose response (log2-fold-change) represents the relative amount of 13C-DHA lipid in placental explants treated with glucose (10 mmol/L) compared to control explants from the same placenta. Positive values indicate an increase in 13C-DHA lipids compared to the control, whilst negative values indicate a decrease
Since BMI was associated with phospholipid glucose response, further analyses were conducted with stratification by maternal BMI (normal BMI < 23 kg/m2 versus overweight/obese: BMI ≥ 23 kg/m2) using the WHO recommended Asian-specific BMI cut-off that is associated with increased metabolic risk (WHO EC 2004). Among cases of normal BMI, glucose treatment (compared with no added glucose control) increased 13C-DHA DGs (all three), LPC, LPE, PC and over half of 13C-DHA TGs (Fig. 5C). In contrast, in the overweight/obese group glucose treatment (compared with control) increased fewer lipids; there was only increased 13C-DHA DGs (all three), LPE and TG 58:8, whilst PE-P 38:6 was decreased in this group (Fig. 5C).
Glucose-treatment-induced changes in placental DHA metabolism and birthweight
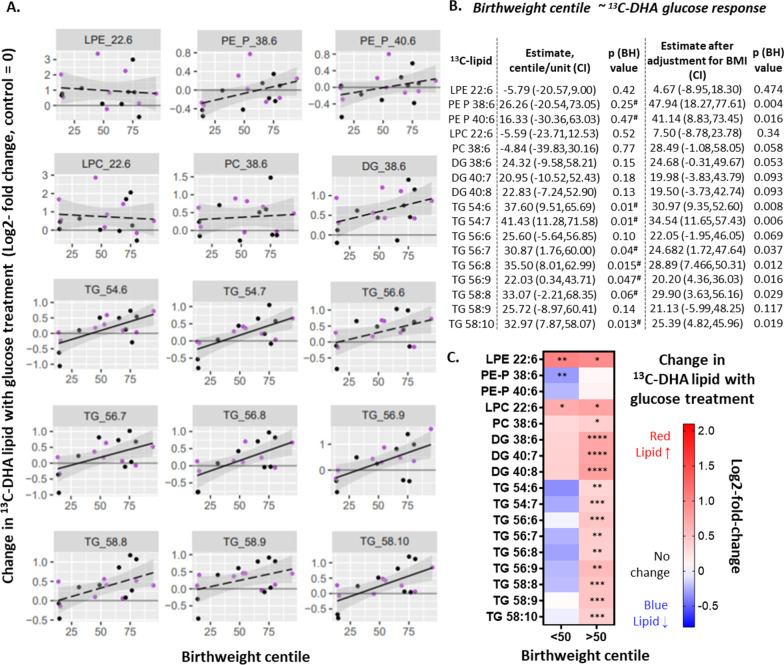
Next, we examined the association between glucose response and birthweight, since how the placenta responds to glucose during pregnancy might impact fetal growth. Positive associations were found between the glucose response of 13C-DHA-TGs and birthweight centile (six out of nine TGs; Fig. 6), and these associations remained similar after adjusting for maternal BMI (Fig. 6), suggesting that BMI does not substantially confound this relationship with DHA-TGs. Similar trends were seen between the glucose response of DGs and PE-Ps and birthweight centile. Moreover, the association of the glucose response of 13C-DHA PE-P 38:6 and PE-P 40:6 with birthweight centile was even strengthened and became statistically significant after adjusting for BMI [estimate (95% CI) 47.9 (18.3, 77.6) % per increase in log2-fold-change, p = 0.004; and 41.1 (8.8, 73.4), p = 0.016, respectively].
Fig. 6.
Associations between glucose-treatment-induced changes in 13C-DHA lipids (glucose response) and birthweight centile (n = 17). Change in 13C-DHA lipid with glucose treatment or glucose response (log2-fold-change) represents the relative amount of 13C-DHA lipid in placental explants treated with glucose (10 mmol/L) compared to control explants from the same placenta. Positive values indicate an increase in 13C-DHA lipids compared to the control, whilst negative values indicate a decrease. Linear regression was run with birthweight centile as the outcome and glucose response as the exposure variable, with or without adjusting for (Z-score, log-2) BMI. Solid lines show significant associations while dashed lines show non-significant associations. Shaded areas show 95% confidence intervals. Key—Purple: non GDM, Black: GDM. Significance retained after adjusting for (Z-score, log-2) BMI (#). The Benjamini–Hochberg method was used to correct for multiple testing. C Heat map illustrating glucose response in placental explants from births with birthweight centile < 50 and from births with birthweight centile > 50. Positive values (red) indicate an increase in 13C-DHA lipids compared to the control, while negative values (blue) indicate a decrease. Asterisks indicate significant differences between 10 mmol/L glucose treatment and the control (5 mmol/L) *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. Comparisons were made for each lipid by repeated measures analysis of variance followed by the Benjamini–Hochberg correction for multiple comparisons. BH Benjamini–Hochberg, CI confidence interval, DG diacylglycerol, DHA docosahexaenoic acid, GDM gestational diabetes, LPC lyso-phosphatidylcholine, LPE lyso-phosphatidylethanolamine, PC phosphatidylcholine, PE-P phosphatidylethanolamine-plasmalogen, TG triacylglycerol
Since glucose response was associated with birthweight centile, with decreases generally seen at lower birthweight centiles, and increases generally seen with higher birthweight centiles, cases were stratified by birthweight 50th centile for further analyses to better identify patterns of glucose-treatment-induced DHA-lipid changes. Among placentas of heavier neonates (birthweight centile > 50%) there were increases in almost all 13C-DHA lipids (except for the PE-Ps) with glucose treatment (compared with no added glucose control), but among placentas of lighter neonates (birthweight centile < 50%) there were fewer 13C-DHA-lipid alterations with increases only seen in lyso-phospholipids (13C-DHA-LPC and 13C-DHA-LPE), while PE-P 38:6 was decreased in this group (Fig. 6C).
With the exclusion of cases where antenatal PUFA-containing supplements were taken (leaving n = 11 for analysis), the magnitude and the direction of all reported associations were materially unchanged (data not shown) compared with results from the entire group of 17 cases.
Discussion
Findings here are consistent with our hypothesis that the activity of term placental DHA-lipid synthesis is increased by the programing effects of higher maternal BMI and higher glycemia, and that this may in turn result in increased birthweight. Higher maternal BMI was associated with greater placental enrichment in 13C-DHA DGs, TGs and phospholipids, and greater phospholipid amounts, while fasting glycemia was associated with increases in the amount of 13C-DHA-TGs, suggesting that these maternal characteristics affect different DHA metabolic pathways in placenta. Furthermore, positive associations between newly synthesized 13C-DHA-lipids and birthweight suggest that placental DHA-lipid metabolism during pregnancy could influence fetal growth. Glucose treatment in-vitro at 10 mmol/L, equivalent to excursions seen in diabetes, increased the amount of almost all newly synthesized 13C-DHA lipids, but the magnitude of glucose-treatment-induced 13C-DHA phospholipid increases were reduced with increasing maternal BMI. Taken together, increasing maternal BMI programs the placenta to increase DHA-lipid synthetic capacity, which is associated with increasing birthweight, but at the same time BMI may limit the stimulatory effect of glucose on placental DHA-phospholipid synthesis. We therefore postulate that the placental-DHA-lipid-mediated actions leading to an increase in birthweight with increasing maternal glycemia could be altered in the possible co-morbid condition of higher BMI.
Associations between maternal BMI or glycemia with DHA lipid metabolism
Being able to reliably measure the incorporation of 13C-DHA into 17 newly synthesized 13C-DHA lipids was an improvement over our previous DHA methods that were only able to measure three 13C-DHA TGs (Watkins et al. 2019b). Further, we confirmed our previous findings that programed differences in placental lipid metabolism by maternal BMI and glycemia are retained by placental explants in culture (Watkins et al. 2019a).
The association of maternal BMI and short term in-vitro glucose treatment with almost all 13C-DHA lipids, including DHA-DGs, a precursor of other DHA-lipids in placental explants, suggests that these factors influence common upstream processes (Fig. 7) which may include activation into DHA-COA (by placental acyl-CoA synthetase long-chain [ACSL] 2, 3 and 4) or the early stages of lipid synthesis, or placental DHA import and intracellular transport by enzymes such as fatty acid transport proteins (FATP and CD36) and fatty acid binding protein (FABP), which are known to be increased in GDM and obesity (Magnusson et al. 2004; Radaelli et al. 2009; Díaz et al. 2015; Hall et al. 2003; Segura et al. 2017). Whereas the more chronic exposure to antenatal fasting glycemia being only associated with TGs, while post-load glycemia associated mostly with PE-P and LPE, suggest differential glycemic impact (directly or indirectly) on specific downstream lipid metabolic pathways. That in-vitro glucose treatment also decreased 13C-DHA PE-Ps, a change in the opposite direction to all other lipids, suggests that glucose particularly influences PE-P specific downstream process. Overall glucose appears to influence DHA processing at multiple points of the various DHA-lipid metabolic pathways (Fig. 7).
At high BMI, there appeared to be limited capacity for further glucose-induced increases in DHA-lipid synthesis in an already heightened DHA-lipid state, but there was still capacity for glucose-induced decreases, for example, in 13C-DHA-PE-P. The less exaggerated glucose-induced increase in placental DHA-phospholipids was only observed with increasing maternal BMI, but not by prior in-vivo glycemia exposure, suggesting that BMI (and not maternal glycemia) specifically induces a lasting programing effect on the glucose-responsive aspects of placental DHA-metabolism. Possibly, the critical window period for such programming occurs early in gestation, when the placenta is exposed to pre-pregnancy factors like high BMI, predating the development of gestational hyperglycemia, which typically arises from mid-gestation onwards. Since our study did not include cases of pre-existing diabetes, the potential programming effect of more established hyperglycemia periconception and during very early gestation could not be determined.
In agreement with our current work, several studies had reported increased placental TGs and lipid droplets with maternal hyperglycemia and in-vitro glucose treatment (Visiedo et al. 2015; Visiedo et al. 2013; Mishra et al. 2020; Tewari et al. 2011), while others showed that obesity is linked with increases in many placental lipid classes (Calabuig-Navarro et al. 2017). However, these studies did not specifically measure the DHA content of lipids. Studies that measured naturally occurring DHA lipids found placental DHA-phospholipids to be elevated in GDM or obesity (Uhl et al. 2015; Bitsanis et al. 2006), consistent with our in-vitro 13C-DHA studies. However, these studies cannot separate the systemic effects of maternal and fetal DHA metabolism and availability, from local placental processing. Our study, in contrast, demonstrated that both maternal BMI and glycemia influence placental DHA lipid metabolism itself, and that different lipid pathways are affected by BMI and glycemia.
Placental DHA uptake, metabolism and export
Studies on the in-vivo processing of orally-administered 13C-DHA in an obese pregnant population reported reduced placental 13C-DHA phospholipids but not TGs with GDM, possibly as a consequence of altered maternal metabolism and placental uptake (Pagán et al. 2013). However, this study also reported that it was the cord/maternal plasma ratio, rather than the placenta/maternal 13C-DHA plasma ratio that was most decreased in GDM, suggesting that fetal DHA deficiency is a consequence of alterations in both placental DHA uptake and metabolism (Pagán et al. 2013). The phospholipids involved were not identified, but we observed a similar decrease in freshly produced 13C-DHA PE-Ps (a major class of DHA-containing phospholipids) with glucose treatment in the high BMI population.
Isolated primary human trophoblasts from GDM pregnancies were reported to take up less 14C-DHA compared to controls over a short incubation of 2 h and that uptake was not affected by in-vitro glucose treatment (Araújo et al. 2013). This may appear inconsistent with our findings of increased 13C-DHA-lipids with increasing glycemia and with in-vitro glucose treatment of placental explants. However, measurable incorporation of 13C-labeled fatty acids into lipids by placental explants takes more than 3 h (Watkins et al. 2019a), thus the published experiments likely measured (by total radioactivity) predominantly uptake of un-esterified DHA rather than incorporation into lipids like in our experiments.
If indeed DHA uptake is reduced in GDM, our finding of increased placental 13C-DHA-lipids (particularly DHA-TGs that are quickly synthesized and turned-over to prevent re-export) with glycemia and glucose treatment, suggests that the hyperglycemia-exposed placenta may compensate for reduced uptake by increasing DHA trapping in placenta and DHA-lipid synthesis. Although such compensation mechanisms may protect the placenta from DHA deficiency, they may not necessarily increase fetal DHA supply. Indeed, it has been suggested that increased placental DHA-lipid content associated with hyperglycemia or BMI represents increased placental retention and decreased export of un-esterified DHA to the fetus (Uhl et al. 2015; Bitsanis et al. 2006; Mishra et al. 2020). Unfortunately, our understanding remains limited as studies have not assessed fetal tissue DHA utilization as a determinant of cord blood DHA levels.
DHA placental metabolism and birthweight
Variations in placental DHA-lipid metabolism could influence fetal growth and development, through providing DHA-lipid substrates for growth, and by regulating the synthesis and release of DHA-lipid signals to affect local placental function and fetal nutritional supply, as well as maternal and fetal metabolism in distant tissues (Haggarty 2002; Lager and Powell 2012; Herrera and Ortega-Senovilla 2018b). Whether the positive association between fresh placental 13C-DHA lipids with birthweight in our study imply a causal relationship remains unclear. Nonetheless, our findings are consistent with studies describing increased placental DHA-neutral lipids (TGs and cholesterol esters) with higher neonatal body fat (Varastehpour et al. 2006), and more placental phospholipid fraction DHA from a normal birthweight group compared with a low birthweight group, possibly mediated by DHA-induced PPARγ-promotion of fetal growth (Meher et al. 2016). In general, lyso-phospholipids are known to regulate a wide array of metabolic processes, including insulin-induced release of glycogen and lipid synthesis, and mitochondrial function (Soga et al. 2005; Hollie et al. 2014; Labonté et al. 2010; Rivera and Chun 2006). Much of the cord blood lyso-phospholipids may originate from the placenta (Gázquez et al. 2018; Ferchaud-Roucher et al. 2019) and cord blood lyso-phospholipids, including DHA-LPC (Hellmuth et al. 2017) as well as cord DHA phospholipids in general (Foreman-van Drongelen et al. 1995; Felton et al. 1994), have been positively associated with birthweight. Increased placental DHA lyso-phospholipids (and their phospholipid precursors) observed with higher maternal BMI, post-load glycemia and in heavier neonates in our study, could possibly result in the transfer of more lyso-phospholipids to the fetus and increase fetal growth. Furthermore the activation of placental secretory phospho-lipase A2 (which produces lyso-phospholipids from placental phospholipids) is associated with higher neonatal adiposity (Varastehpour et al. 2006).
Maternal glycemia is known to increase birthweight largely mediated through transplacental glucose supply. Our findings of in-vitro glucose-induced increases in most 13C-DHA lipids in placentas from heavier neonates, but only increased 13C-DHA lyso-phospholipids in placentas from lighter neonates, suggests that variations in glucose-responsive placental DHA lipid metabolism in-vivo may also partly explain glycemia-promoted fetal growth. Our finding that the magnitude of association between BMI and birthweight centile was reduced following adjustment for individual 13C-DHA lipids raises the possibility that placental DHA metabolism could also partially mediate the birthweight-promoting effects of maternal BMI. However, much larger studies will be needed to confirm the mediation effects of DHA-lipids.
Our data showed positive associations between DHA-PE-Ps and birthweight centile, suggesting that PE-P may promote fetal growth, possibly by acting as a large DHA reservoir for fetal supply and synthesis of signaling molecules. However, in contrast to other lipids, in-vitro glucose treatment decreased placental 13C-DHA-PE-P, especially with maternal obesity and in cases of lighter neonates. Decreased PE-P may reflect decreased production (thus reducing DHA storage) and/or increased catabolism (which would release un-esterified DHA). We speculate that glucose-induced reduction of placental DHA-PE-P when maternal BMI is high may enable some neonates to compensate for glucose-induced growth acceleration, resulting in more moderated birthweights and lower risk of macrosomia, but possibly at the expense of fetal DHA supply, which is thought to be disrupted in GDM and maternal obesity. Regulation of placental DHA-PE-P metabolism appears pivotal to such a balancing act and should be a focus of future research in pregnancies complicated by diabetes and obesity.
Alterations in placental DHA metabolism could change the availability of un-esterified DHA, DHA-lipids and various DHA-based signaling molecules which could influence fetal growth in different and divergent ways (Hellmuth et al. 2017); the relative strength of influence between them remains undetermined. Our findings of increased DHA-lipids in placenta with maternal metabolic disorders that promote fetal growth, may represent placental retention of some DHA-lipids or increased bioavailability of specific DHA-lipids for fetal transfer and regulation of placental function. These concepts are in keeping with studies reporting an overall lower total DHA content in the fetal circulation with maternal metabolic disorders, since circulating DHA lipids reflect the combined impact of variations in placental metabolism and transplacental transfer, as well as fetal tissue consumption and metabolism, which are likely to increase with greater fetal size. Whether increased placental DHA lipids underlie part of the pathophysiology of excessive fetal growth associated with GDM and maternal obesity (which is more in keeping with our findings that adjustment for DHA-lipids attenuated associations between maternal BMI and birthweight) or could actually be an adaptive response to limit the impact of the maternal condition on the offspring through placental lipid retention remains inconclusive. We also do not exclude the possibility of reverse causation where increased birthweight may be driving increased placental DHA metabolism as part of fetal-tailoring of its own nutritional supply.
Limitations of the study
The modest sample size in this in-vitro study limits the ability to study interactions between different clinical factors, and other demographic factors such as ethnicity and sex, and we are therefore unable to definitively determine whether DHA-lipids mediate links between maternal BMI or glycemia with birthweight. Consideration of the potential effects of pre-pregnancy BMI and total gestational weight gain were not possible due to the lack of data while noting that a measured weight in early pregnancy provides a more precise assessment of pre-pregnancy weight than recalled weight (Inskip 2020). Since participants were Chinese and Indian, findings may not be generalizable to other ethnicities. A balance of GDM/non-GDM and high/normal BMI cases were selected to understand mechanisms across a spectrum without the extremes of hyperglycemia consistent with pre-existing type 1 and 2 diabetes, and of morbid obesity (BMI > 40 kg/m2), and therefore may not represent wider variation within the general pregnant population. Our method does not assume that the syncytiotrophoblast is the only cell type involved in the regulation of the transplacental transfer of DHA. Using mixed cell placental explants has the advantage of preserving microarchitecture and maintenance of cell–cell interactions, including lipid signaling, which may participate in the regulation of overall placental DHA metabolism. However, our method precludes the ability to pinpoint the actual cell types within which newly produced DHA-lipids are synthesized and stored. Placental explants were cultured under 20% O2 tension, which is higher than the 8% O2 tension within the placental bed and effects may be different in-vivo. The incorporation of 13C-DHA into other lipids not discussed here could not be reliably quantified using this methodology. Our method does not allow the quantification of transfer of DHA from the maternal to fetal compartments and exactly how placental metabolism may alter the availability of DHA, either as un-esterified fatty acids or in other lipid forms, for transfer to the fetus is still unclear.
Conclusions
Our novel evidence demonstrate that maternal BMI and glycemia could increase placental DHA-lipid synthesis itself, without mediation by other confounding factors such as maternal lipid supply and placental blood flow. Increases in placental DHA-lipid production could be one potential mechanism by which higher maternal BMI and glycemia could lead to increased birthweight. Maternal BMI, fasting and post-load glycemia differentially influenced the placental capacity to synthesize individual DHA-lipids. Furthermore, glucose-responsive placental DHA-lipid metabolism can be programed by maternal BMI to limit glucose-stimulation of excessive placental DHA-lipid synthesis and turnover, and may represent a compensatory mechanism to moderate glucose-driven acceleration in fetal growth, but possibly at the expense of transplacental fetal DHA supply. Future studies with larger sample sizes will be required to confirm these postulations and pave the way to development of different approaches to intervention that are tailored to maternal BMI and glycemia status to optimize fetal DHA supply and growth.
Supplementary Information
Additional file 1. Clinical characteristics.
Additional file 3. Structure of PC 38:6 illustrating the incorporation of 13C-DHA or 12C-DHA (A) and chromatogram illustrating the identical retention times of 13C and 12C PC 38:6 by LCMS (B).
Additional file 6. The association of post-load glycemia with the enrichment of placental 13C-DHA lipids (Z-score, log2 transformed) for 17 placenta.
Additional file 7. Associations (linear regression) between maternal metabolic factors and lipid amount (Z-score, log2) after mutual adjustment
Additional file 8. The association of 13C-DHA-lipid amount (Z-score, Log2) with birthweight centile for 17 placenta.
Additional file 9. Association between BMI (Z-score, log2) and birthweight centile adjusted for each DHA lipid.
Acknowledgements
The authors would like to thank the staff of the National University Hospital in coordinating the recruitment of women, and assisting with placental collection, as well as thank the women for generously donating their placenta for research.
Abbreviations
- BMI
Body mass index
- BSA
Bovine serum albumin
- CMRL
Connaught Medical Research Laboratories
- DG
Diacylglycerols
- DHA
Docosahexaenoic acid
- dMRM
Dynamic multiple reaction monitoring
- GDM
Gestational diabetes mellitus
- IS
Internal standard
- LC–MS/MS
Tandem liquid chromatography mass spectrometry
- LC-PUFA
Long-chain polyunsaturated fatty acid
- LPC
Lyso-phosphatidylcholine
- LPE
Lyso-phosphatidylethanolamine
- PC
Phosphatidylcholine
- PE
Phosphatidylethanolamine
- PE-P
Phosphatidylethanolamine plasmalogen
- PUFA
Polyunsaturated fatty acids
- TG
Triacylglycerol
Authors’ contributions
Project was conceptualized by SYC and OCW. SYC and MW were responsible for the acquisition of the financial support for the project leading to this publication. Experiments and analysis were performed by OCW, PS, VC, RP, HY and NS with supervision provided by SYC and AC-G. Manuscript was written by OCW and SYC with the assistance of AC-G, AB, KG, RL and MW. All authors have given consent for publication. All authors read and approved the final manuscript.
Funding
This research is supported by a Clinician Scientist Award awarded to SYC from the Singapore National Medical Research Council (NMRC/CSA-INV/0010/2016, MOH-CSAINV19nov-0002), by the National University of Singapore, National University Health System Singapore and the Singapore Institute for Clinical Sciences A*STAR. The Singapore Lipidomics Incubator receives funding from the Life Sciences Institute, the National University of Singapore Yong Loo Lin School of Medicine, the National Research Foundation (Grant number NRFI2015-05) and A*STAR (IAF-ICP I1901E0040). Funders played no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Availability of data and materials
The dataset supporting the conclusions of this article are included within the article and its additional files.
Declarations
Ethics approval and consent to participate
Ethical approval was obtained from the National Healthcare Group Domain Specific Review Board (2016/00183) and written informed consent was received from participants prior to inclusion in the study.
Consent for publication
Not applicable.
Competing interests
SYC and KMG are part of an academic consortium that has received research funding from Abbott Nutrition, Nestlé S.A., Danone and BenevolentAI Bio Ltd for work unrelated to this manuscript. KMG has received reimbursement for speaking at conferences sponsored by companies selling nutritional products. SYC has received reimbursement and honoraria into her research funds from Société Des Produits Nestlé S.A. for a half-day consultancy and for speaking at a conference. The other authors have no financial or personal conflict of interest to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Araújo JR, Correia-Branco A, Ramalho C, Keating E, Martel F. Gestational diabetes mellitus decreases placental uptake of long-chain polyunsaturated fatty acids: involvement of long-chain acyl-CoA synthetase. J Nutr Biochem. 2013;24:1741–1750. doi: 10.1016/j.jnutbio.2013.03.003. [DOI] [PubMed] [Google Scholar]
- Benassayag C, et al. High polyunsaturated fatty acid, thromboxane A2, and alpha-fetoprotein concentrations at the human feto-maternal interface. J Lipid Res. 1997;38:276–286. doi: 10.1016/S0022-2275(20)37440-X. [DOI] [PubMed] [Google Scholar]
- Bitsanis D, Ghebremeskel K, Moodley T, Crawford MA, Djahanbakhch O. Gestational diabetes mellitus enhances arachidonic and docosahexaenoic acids in placental phospholipids. Lipids. 2006;41:341–346. doi: 10.1007/s11745-006-5104-8. [DOI] [PubMed] [Google Scholar]
- Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics. 2005;115:290–296. doi: 10.1542/peds.2004-1808. [DOI] [PubMed] [Google Scholar]
- Braverman NE, Moser AB. Functions of plasmalogen lipids in health and disease. Biochim Biophys Acta (BBA) Mol Basis Dis. 2012;1822:1442–1452. doi: 10.1016/j.bbadis.2012.05.008. [DOI] [PubMed] [Google Scholar]
- Calabuig-Navarro V, et al. Effect of maternal obesity on placental lipid metabolism. Endocrinology. 2017;158:2543–2555. doi: 10.1210/en.2017-00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalano PM. Obesity and pregnancy—the propagation of a viscous cycle? J Clin Endocrinol Metab. 2003;88:3505–3506. doi: 10.1210/jc.2003-031046. [DOI] [PubMed] [Google Scholar]
- Delhaes F, et al. Altered maternal and placental lipid metabolism and fetal fat development in obesity: current knowledge and advances in non-invasive assessment. Placenta. 2018;69:118–124. doi: 10.1016/j.placenta.2018.05.011. [DOI] [PubMed] [Google Scholar]
- Díaz P, Harris J, Rosario FJ, Powell TL, Jansson T. Increased placental fatty acid transporter 6 and binding protein 3 expression and fetal liver lipid accumulation in a mouse model of obesity in pregnancy. Am J Physiol Regul Integr Comp Physiol. 2015;309:R1569–R1577. doi: 10.1152/ajpregu.00385.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Digel M, Ehehalt R, Füllekrug J. Lipid droplets lighting up: insights from live microscopy. FEBS Lett. 2010;584:2168–2175. doi: 10.1016/j.febslet.2010.03.035. [DOI] [PubMed] [Google Scholar]
- Duttaroy AK. Docosahexaenoic acid supports feto-placental growth and protects cardiovascular and cognitive function: a mini review. Eur J Lipid Sci Technol. 2016;118:1439–1449. doi: 10.1002/ejlt.201500496. [DOI] [Google Scholar]
- Farhat S, et al. Association of resolvin level in pregnant women with preeclampsia and metabolic syndrome. Taiwan J Obstet Gynecol. 2020;59:105–108. doi: 10.1016/j.tjog.2019.11.016. [DOI] [PubMed] [Google Scholar]
- Felton C, et al. Umbilical vessel wall fatty acids after normal and retarded fetal growth. Arch Dis Child Fetal Neonatal Ed. 1994;70:F36–F39. doi: 10.1136/fn.70.1.F36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferchaud-Roucher V, et al. A potential role for lysophosphatidylcholine in the delivery of long chain polyunsaturated fatty acids to the fetal circulation. Biochim Biophys Acta Mol Cell Biol Lipids. 2019;1864:394. doi: 10.1016/j.bbalip.2018.12.007. [DOI] [PubMed] [Google Scholar]
- Foreman-van Drongelen MM, et al. Long-chain polyunsaturated fatty acids in preterm infants: status at birth and its influence on postnatal levels. J Pediatr. 1995;126:611–618. doi: 10.1016/S0022-3476(95)70363-2. [DOI] [PubMed] [Google Scholar]
- Gallo LA, Barrett HL, Dekker NM. Review: Placental transport and metabolism of energy substrates in maternal obesity and diabetes. Placenta. 2017;54:59–67. doi: 10.1016/j.placenta.2016.12.006. [DOI] [PubMed] [Google Scholar]
- Gázquez A, et al. Placental lipid droplet composition: effect of a lifestyle intervention (UPBEAT) in obese pregnant women. Biochim Biophys Acta (BBA) Mol Cell Biol Lipids. 2018;1863:998–1005. doi: 10.1016/j.bbalip.2018.04.020. [DOI] [PubMed] [Google Scholar]
- Gázquez A, et al. Altered materno-fetal transfer of 13C-polyunsaturated fatty acids in obese pregnant women. Clin Nutr. 2020;39:1101–1107. doi: 10.1016/j.clnu.2019.04.014. [DOI] [PubMed] [Google Scholar]
- Gil-Sánchez A, et al. Maternal-fetal in vivo transfer of [13C]docosahexaenoic and other fatty acids across the human placenta 12 h after maternal oral intake. Am J Clin Nutr. 2010;92:115–122. doi: 10.3945/ajcn.2010.29589. [DOI] [PubMed] [Google Scholar]
- Haggarty P. Placental regulation of fatty acid delivery and its effect on fetal growth—a review. Placenta. 2002;23:S28–S38. doi: 10.1053/plac.2002.0791. [DOI] [PubMed] [Google Scholar]
- Haggarty P. Fatty acid supply to the human fetus. Annu Rev Nutr. 2010;30:237–255. doi: 10.1146/annurev.nutr.012809.104742. [DOI] [PubMed] [Google Scholar]
- Haggarty P, Page K, Abramovich DR, Ashton J, Brown D. Long-chain polyunsaturated fatty acid transport across the perfused human placenta. Placenta. 1997;18:635–642. doi: 10.1016/S0143-4004(97)90004-7. [DOI] [PubMed] [Google Scholar]
- Hall AM, Smith AJ, Bernlohr DA. Characterization of the Acyl-CoA synthetase activity of purified murine fatty acid transport protein 1. J Biol Chem. 2003;278:43008–43013. doi: 10.1074/jbc.M306575200. [DOI] [PubMed] [Google Scholar]
- Hanebutt FL, Demmelmair H, Schiessl B, Larqué E, Koletzko B. Long-chain polyunsaturated fatty acid (LC-PUFA) transfer across the placenta. Clin Nutr. 2008;27:685–693. doi: 10.1016/j.clnu.2008.05.010. [DOI] [PubMed] [Google Scholar]
- Harris W, Baack M. Beyond building better brains: bridging the docosahexaenoic acid (DHA) gap of prematurity. J Perinatol. 2015;35:1–7. doi: 10.1038/jp.2014.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helland IB, et al. Effect of supplementing pregnant and lactating mothers with omega-3 very-long-chain fatty acids on childrens IQ and body mass index at 7 years of age. Pediatrics. 2008;122:472. doi: 10.1542/peds.2007-2762. [DOI] [PubMed] [Google Scholar]
- Hellmuth C, et al. Cord blood metabolome is highly associated with birth weight, but less predictive for later weight development. Obes Facts. 2017;10:85–100. doi: 10.1159/000453001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera E, Ortega-Senovilla H. Implications of lipids in neonatal body weight and fat mass in gestational diabetic mothers and non-diabetic controls. Curr Diabetes Rep. 2018;18:7. doi: 10.1007/s11892-018-0978-4. [DOI] [PubMed] [Google Scholar]
- Herrera E, Ortega-Senovilla H. Implications of lipids in neonatal body weight and fat mass in gestational diabetic mothers and non-diabetic controls. Curr Diabetes Rep. 2018;18:7. doi: 10.1007/s11892-018-0978-4. [DOI] [PubMed] [Google Scholar]
- Higa R, Jawerbaum A. Intrauterine effects of impaired lipid homeostasis in pregnancy diseases. Curr Med Chem. 2013;20:2338–2350. doi: 10.2174/0929867311320180005. [DOI] [PubMed] [Google Scholar]
- Hollie NI, et al. Micromolar changes in lysophosphatidylcholine concentration cause minor effects on mitochondrial permeability but major alterations in function. Biochim Biophys Acta (BBA) Mol Cell Biol Lipids. 2014;1841:888–895. doi: 10.1016/j.bbalip.2013.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inskip H, et al. Measured weight in early pregnancy is a valid method for estimating pre-pregnancy weight. J Dev Orig Health Dis. 2020;12:561–569. doi: 10.1017/S2040174420000926. [DOI] [PubMed] [Google Scholar]
- Johnsen G, Weedon-Fekjaer M, Tobin K, Staff A, Duttaroy A. Long-chain polyunsaturated fatty acids stimulate cellular fatty acid uptake in human placental choriocarcinoma (BeWo) cells. Placenta. 2009;30:1037–1044. doi: 10.1016/j.placenta.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Khan NA. Role of lipids and fatty acids in macrosomic offspring of diabetic pregnancy. Cell Biochem Biophys. 2007;48:79–88. doi: 10.1007/s12013-007-0019-4. [DOI] [PubMed] [Google Scholar]
- Knopp RH, Bonet B, Zhu X. Lipid metabolism in pregnancy. In: Cowett RM, editor. Principles of perinatal—neonatal metabolism. Berlin: Springer; 1998. pp. 221–258. [Google Scholar]
- Kolahi K, Louey S, Varlamov O, Thornburg K. Real-time assessment of fatty acid kinetics in the human placenta. Placenta. 2015;36:A33. doi: 10.1016/j.placenta.2015.07.284. [DOI] [Google Scholar]
- Kolahi K, Louey S, Varlamov O, Thornburg K. Real-time tracking of BODIPY-C12 long-chain fatty acid in human term placenta reveals unique lipid dynamics in cytotrophoblast cells. PLoS ONE. 2016;11:e0153522. doi: 10.1371/journal.pone.0153522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolahi KS, Valent AM, Thornburg KL. Real-time microscopic assessment of fatty acid uptake kinetics in the human term placenta. Placenta. 2018;72:1–9. doi: 10.1016/j.placenta.2018.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labonté ED, et al. Postprandial lysophospholipid suppresses hepatic fatty acid oxidation: the molecular link between group 1B phospholipase A2 and diet-induced obesity. FASEB J. 2010;24:2516–2524. doi: 10.1096/fj.09-144436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lager S, Powell TL. Regulation of nutrient transport across the placenta. J Pregnancy. 2012;2012:1–14. doi: 10.1155/2012/179827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larqué E, et al. Placental fatty acid transfer: a key factor in fetal growth. Ann Nutr Metab. 2014;64:247–253. doi: 10.1159/000365028. [DOI] [PubMed] [Google Scholar]
- Lecchi C, et al. Effects of EPA and DHA on lipid droplet accumulation and mRNA abundance of PAT proteins in caprine monocytes. Res Vet Sci. 2013;94:246–251. doi: 10.1016/j.rvsc.2012.09.019. [DOI] [PubMed] [Google Scholar]
- Leng J, et al. GDM women’s pre-pregnancy overweight/obesity and gestational weight gain on offspring overweight status. PLoS ONE. 2015;10:e0129536. doi: 10.1371/journal.pone.0129536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessig J, Fuchs B. Plasmalogens in biological systems: their role in oxidative processes in biological membranes, their contribution to pathological processes and aging and plasmalogen analysis. Curr Med Chem. 2009;16:2021–2041. doi: 10.2174/092986709788682164. [DOI] [PubMed] [Google Scholar]
- Lewis RM, Wadsack C, Desoye G. Placental fatty acid transfer. Curr Opin Clin Nutr Metab Care. 2018;21:78–82. doi: 10.1097/MCO.0000000000000443. [DOI] [PubMed] [Google Scholar]
- Li Q, et al. Docosahexaenoic acid changes lipid composition and interleukin-2 receptor signaling in membrane rafts. J Lipid Res. 2005;46:1904–1913. doi: 10.1194/jlr.M500033-JLR200. [DOI] [PubMed] [Google Scholar]
- Magnusson A, Waterman I, Wennergren M, Jansson T, Powell T. Triglyceride hydrolase activities and expression of fatty acid binding proteins in the human placenta in pregnancies complicated by intrauterine growth restriction and diabetes. J Clin Endocrinol Metab. 2004;89:4607–4614. doi: 10.1210/jc.2003-032234. [DOI] [PubMed] [Google Scholar]
- Meher AP, et al. Placental DHA and mRNA levels of PPARγ and LXRα and their relationship to birth weight. J Clin Lipidol. 2016;10:767–774. doi: 10.1016/j.jacl.2016.02.004. [DOI] [PubMed] [Google Scholar]
- Mikolajczyk RT, et al. A global reference for fetal-weight and birthweight percentiles. Lancet. 2011;377:1855–1861. doi: 10.1016/S0140-6736(11)60364-4. [DOI] [PubMed] [Google Scholar]
- Mishra JS, Zhao H, Hattis S, Kumar S. Elevated glucose and insulin levels decrease DHA transfer across human trophoblasts via SIRT1-dependent mechanism. Nutrients. 2020;12:1271. doi: 10.3390/nu12051271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell DC, Niu S-L, Litman BJ. Enhancement of G protein-coupled signaling by DHA phospholipids. Lipids. 2003;38:437–443. doi: 10.1007/s11745-003-1081-1. [DOI] [PubMed] [Google Scholar]
- Moon RJ, et al. Maternal plasma polyunsaturated fatty acid status in late pregnancy is associated with offspring body composition in childhood. J Clin Endocrinol Metab. 2013;98:299–307. doi: 10.1210/jc.2012-2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong YY, et al. Mismatch between poor fetal growth and rapid postnatal weight gain in the first 2 years of life is associated with higher blood pressure and insulin resistance without increased adiposity in childhood: the GUSTO cohort study. Int J Epidemiol. 2020;49:1591–1603. doi: 10.1093/ije/dyaa143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onuki Y, Morishita M, Chiba Y, Tokiwa S, Takayama K. Docosahexaenoic acid and eicosapentaenoic acid induce changes in the physical properties of a lipid bilayer model membrane. Chem Pharm Bull. 2006;54:68–71. doi: 10.1248/cpb.54.68. [DOI] [PubMed] [Google Scholar]
- Ortega-Senovilla H, Alvino G, Taricco E, Cetin I, Herrera E. Gestational diabetes mellitus upsets the proportion of fatty acids in umbilical arterial but not venous plasma. Diabetes Care. 2009;32:120–122. doi: 10.2337/dc08-0679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagán A, et al. Materno-fetal transfer of docosahexaenoic acid is impaired by gestational diabetes mellitus. Am J Physiol Endocrinol Metab. 2013;305:E826–E833. doi: 10.1152/ajpendo.00291.2013. [DOI] [PubMed] [Google Scholar]
- Patel N, et al. Cord metabolic profiles in obese pregnant women: insights into offspring growth and body composition. J Clin Endocrinol Metab. 2017;103:346–355. doi: 10.1210/jc.2017-00876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radaelli T, et al. Differential regulation of genes for fetoplacental lipid pathways in pregnancy with gestational and type 1 diabetes mellitus. Am J Obstet Gynecol. 2009;201(209):e201–209.e210. doi: 10.1016/j.ajog.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera R, Chun J. Biological effects of lysophospholipids. In: Amara SG, Bamberg E, Fleischmann B, Gudermann T, Hebert SC, Jahn R, Lederer WJ, Lill R, Miyajima A, Offermanns S, Zechner R, editors. Reviews of physiology biochemistry and pharmacology. Berlin: Springer; 2006. pp. 25–46. [DOI] [PubMed] [Google Scholar]
- Rogers LK, Valentine CJ, Keim SA. DHA supplementation: current implications in pregnancy and childhood. Pharmacol Res. 2013;70:13–19. doi: 10.1016/j.phrs.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito S. Cytokine cross-talk between mother and the embryo/placenta. J Reprod Immunol. 2001;52:15–33. doi: 10.1016/S0165-0378(01)00112-7. [DOI] [PubMed] [Google Scholar]
- Segura MT, et al. Maternal BMI and gestational diabetes alter placental lipid transporters and fatty acid composition. Placenta. 2017;57:144–151. doi: 10.1016/j.placenta.2017.07.001. [DOI] [PubMed] [Google Scholar]
- Soga T, et al. Lysophosphatidylcholine enhances glucose-dependent insulin secretion via an orphan G-protein-coupled receptor. Biochem Biophys Res Commun. 2005;326:744–751. doi: 10.1016/j.bbrc.2004.11.120. [DOI] [PubMed] [Google Scholar]
- Steenweg-de Graaff JCJ, et al. Maternal LC-PUFA status during pregnancy and child problem behavior: the Generation R Study. Pediatr Res. 2015;77:489–497. doi: 10.1038/pr.2014.204. [DOI] [PubMed] [Google Scholar]
- Tewari V, Tewari A, Bhardwaj N. Histological and histochemical changes in placenta of diabetic pregnant females and its comparision with normal placenta. Asian Pac J Trop Dis. 2011;1:1–4. doi: 10.1016/S2222-1808(11)60001-7. [DOI] [Google Scholar]
- Thomas B, Ghebremeskel K, Lowy C, Offley-Shore B, Crawford MA. Plasma fatty acids of neonates born to mothers with and without gestational diabetes. Prostaglandins Leukot Essent Fatty Acids. 2005;72:335–341. doi: 10.1016/j.plefa.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Uhl O, et al. Effects of obesity and gestational diabetes mellitus on placental phospholipids. Diabetes Res Clin Pract. 2015;109:364–371. doi: 10.1016/j.diabres.2015.05.032. [DOI] [PubMed] [Google Scholar]
- Varastehpour A, et al. Activation of phospholipase A2 is associated with generation of placental lipid signals and fetal obesity. J Clin Endocrinol Metab. 2006;91:248–255. doi: 10.1210/jc.2005-0873. [DOI] [PubMed] [Google Scholar]
- Visiedo F, et al. High glucose levels reduce fatty acid oxidation and increase triglyceride accumulation in human placenta. Am J Physiol Endocrinol Metab. 2013;305:E205–E212. doi: 10.1152/ajpendo.00032.2013. [DOI] [PubMed] [Google Scholar]
- Visiedo F, et al. Glucose and fatty acid metabolism in placental explants from pregnancies complicated with gestational diabetes mellitus. Reprod Sci. 2015;22:798–801. doi: 10.1177/1933719114561558. [DOI] [PubMed] [Google Scholar]
- Wainwright PE. Dietary essential fatty acids and brain function: a developmental perspective on mechanisms. Proc Nutr Soc. 2002;61:61–69. doi: 10.1079/PNS2001130. [DOI] [PubMed] [Google Scholar]
- Wang G, Wang T. The role of plasmalogen in the oxidative stability of neutral lipids and phospholipids. J Agric Food Chem. 2010;58:2554–2561. doi: 10.1021/jf903906e. [DOI] [PubMed] [Google Scholar]
- Wasserman AH, Venkatesan M, Aguirre A. Bioactive lipid signaling in cardiovascular disease, development, and regeneration. Cells. 2020;9:1391. doi: 10.3390/cells9061391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins OC, et al. Metabolism of 13C-labeled fatty acids in term human placental explants by liquid chromatography-mass spectrometry. Endocrinology. 2019;160:1394–1408. doi: 10.1210/en.2018-01020. [DOI] [PubMed] [Google Scholar]
- Watkins OC, et al. Myo-inositol alters 13C-labeled fatty acid metabolism in human placental explants. J Endocrinol. 2019;243:73–84. doi: 10.1530/JOE-19-0267. [DOI] [PubMed] [Google Scholar]
- Weir JM, et al. Plasma lipid profiling in a large population-based cohort. J Lipid Res. 2013;54:2898–2908. doi: 10.1194/jlr.P035808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO EC Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet (lond, Engl) 2004;363:157. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- Wijendran V, et al. Fetal erythrocyte phospholipid polyunsaturated fatty acids are altered in pregnancy complicated with gestational diabetes mellitus. Lipids. 2000;35:927–931. doi: 10.1007/S11745-000-0602-2. [DOI] [PubMed] [Google Scholar]
- Yavin E, Brand A, Green P. Docosahexaenoic acid abundance in the brain: a biodevice to combat oxidative stress. Nutr Neurosci. 2002;5:149–157. doi: 10.1080/10284150290003159. [DOI] [PubMed] [Google Scholar]
- Zhao J-P, et al. Circulating docosahexaenoic acid levels are associated with fetal insulin sensitivity. PLoS ONE. 2014;9:e85054. doi: 10.1371/journal.pone.0085054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zornoza-Moreno M, et al. Is low docosahexaenoic acid associated with disturbed rhythms and neurodevelopment in offsprings of diabetic mothers? Eur J Clin Nutr. 2014;68:931–937. doi: 10.1038/ejcn.2014.104. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Clinical characteristics.
Additional file 3. Structure of PC 38:6 illustrating the incorporation of 13C-DHA or 12C-DHA (A) and chromatogram illustrating the identical retention times of 13C and 12C PC 38:6 by LCMS (B).
Additional file 6. The association of post-load glycemia with the enrichment of placental 13C-DHA lipids (Z-score, log2 transformed) for 17 placenta.
Additional file 7. Associations (linear regression) between maternal metabolic factors and lipid amount (Z-score, log2) after mutual adjustment
Additional file 8. The association of 13C-DHA-lipid amount (Z-score, Log2) with birthweight centile for 17 placenta.
Additional file 9. Association between BMI (Z-score, log2) and birthweight centile adjusted for each DHA lipid.
Data Availability Statement
The dataset supporting the conclusions of this article are included within the article and its additional files.