Abstract
Serratia species are gram-negative bacteria, which could be isolated from soil, water, plants, animals and air. They are responsible for a heterogeneous spectrum of diseases, affecting the central nervous system, the urinary tract, the respiratory tract and the bloodstream.
Pulmonary involvement is rare and typically occurs in immunocompromised patients; radiological appearances include haemorrhagic bronchopneumonia, even with the development of pulmonary abscesses and cavitated parenchymal lesions, or diffuse alveolar damage. Concerning pulmonary cavities, the differential diagnosis should include metastatic lung nodules, rheumatoid arthritis, Langerhans cell histiocytosis, mycotic infections and septic emboli. The knowledge of these radiological features, in association with clinical history and laboratory findings, is mandatory to make the correct diagnosis, suggesting the right treatment and the adequate follow-up. We described a challenging case of a Serratia marcescens’ pulmonary infection, which occurred in a patient with breast cancer: clinical features and main imaging findings have been discussed – in order to help clinicians and radiologists in the management of the disease.
Keywords: X-ray, Computed tomography, Serratia marcescens, Infection, Cavitated nodule, Breast cancer
Highlights
-
•
Breast cancer may involve lung parenchyma with metastatic cavitated nodules.
-
•
Serratia can cause cavitated pulmonary nodules.
-
•
Differential diagnosis of cavitated pulmonary nodules includes infectious and non-infectious diseases.
-
•
Serratia commonly causes haemorrhagic bronchopneumonia or diffuse alveolar damage.
1. Introduction
Serratia species are gram-negative, motile, bacilli, which belong to the family Enterobacteriaceae; these pathogens can cause a heterogeneous spectrum of diseases in humans and animals. They are responsible for both opportunistic and nosocomial infections, the latter due to hospitalization, placement of intravenous catheters, intraperitoneal catheters, urinary catheters and prior use of respiratory tract instrumentation [1].
The type species Serratia marcescens represents the most frequently associated with human infections, affecting the central nervous system, the urinary tract, the respiratory tract and the bloodstream; it may cause meningitis, pneumonia and endocarditis [2]. The pathogenic mechanism consists of cytotoxic effects on human epithelial cells, characterized by vacuolization with subsequent cell lysis [3].
It could be isolated from soil, water, plants, animals and air [4]; the survival and growth potential of these microorganisms are much better under semi-anaerobic and strictly anaerobic conditions [5]. An important characteristic of Serratia marcescens is the presence of R-factor – which confers resistance to different types of antimicrobial agents [6].
2. Case report
We report a case of a 46-year-old caucasian female, who was admitted to our hospital for erythematous and painful right breast, having a “peau d'orange” appearance and increased consistency. Breast ultrasound examination showed an altered echo-texture, with ill-defined hypoechoic mass and axillary pathological lymph nodes. Tru-cut biopsy of the breast and fine-needle aspiration of the axillary nodes were performed – revealing the presence of ductal invasive carcinoma (G4). CT staging revealed no metastasis in the lung and in the abdomen; no signs of bone involvement was found. The patient underwent mastectomy, followed by radiotherapy and chemotherapy with docetaxel and doxorubicin; then, she was scheduled for clinical and radiological follow-up evaluations.
A CT examination, acquired 12 months after the end of treatment, has shown the onset of multiple liver metastasis; no other organs were involved by disease dissemination (Fig. 1). A multidisciplinary evaluation was done and a second line of treatment was performed, adding to the previous treatment a biological therapy based on trastuzumab and pertuzumab; CT scan – performed three months later – revealed complete resolution of disease with disappearance of hepatic lesions (Fig. 2). During the second line of treatment, the patient did not report acute episodes of drug toxicity.
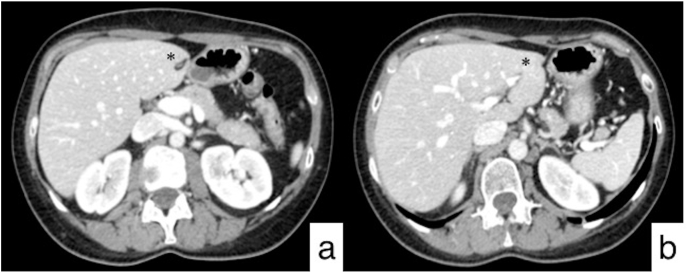
Fig. 1.
Follow-up CT acquired one year after mastectomy and adjuvant chemo-radiotherapy. Abdominal axial CT images show multiple hypodense liver lesions (black arrows) with ring enhancement, due to metastatic disease, well depicted at third (a) and second (b) hepatic segments. Axial (c) and coronal (d) HRCT images do not demonstrate any pulmonary involvement.
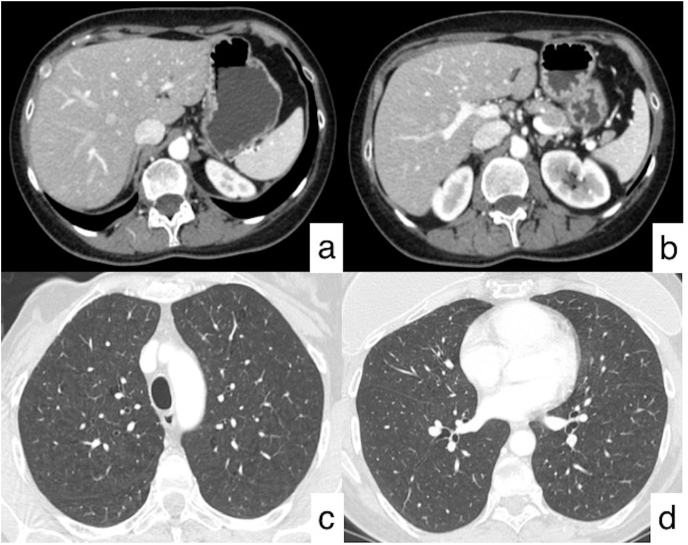
Fig. 2.
Follow-up CT performed after second line treatment. Axial CT images (a, b) show the complete disappearance of the lesions in the left hepatic lobe (black asterisk).
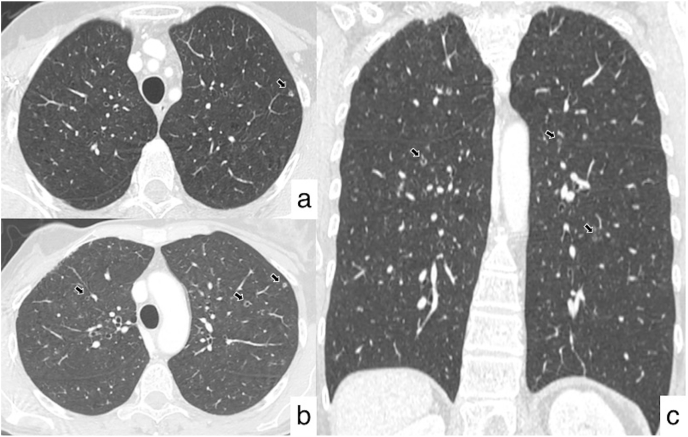
The following CT examinations have demonstrated the patient's stability over a few years, with no evidence of metastasis (Fig. 3). After five years from the diagnosis of the oncological disease, a new CT follow-up revealed multiple pulmonary nodular lesions, showing predominant centrilobular and peribronchial distribution, with no gradient between upper and lower regions. The majority of these tiny nodules showed cavitated appearance – the biggest lesion reporting a size of 6 mm. In some cases, they were ill-defined with a perilesional area of ground-glass attenuation (Fig. 4). No other pulmonary findings were observed on HRCT images – except for the presence of moderate thickened bronchial walls. According to the patient's clinical history, no signs of lymphatic disease were observed. The bronchocentric distribution of the cavitated nodules suggested the possibility of a small airway disease – due to inflammation and/or infection: however, these CT appearances were not specific, and a differential diagnosis of relapsing disease was considered.
Fig. 3.
Follow-up CT. Axial CT images show the radiological stability of the patient, with no evidence of metastasis in the liver (a, b) and in the lungs (c, d).
Fig. 4.
Axial (a, b) and coronal (c) HRCT images reveal the presence of multiple bilateral cavitated nodular lesions, having a predominant centrilobular and peribronchial distribution, with no gradient between upper and lower regions.
After a multidisciplinary discussion, further investigations were proposed and the patient was scheduled for bronchoscopy and Broncho-Alveolar Lavage (BAL) evaluation. The radiological hypothesis of a small airway disease was fully taken into consideration – even if no clinical signs of pulmonary infection were present. Laboratory tests were not significant; clinical evaluation did not reveal findings of active infections.
BAL showed growth of Serratia marcescens, confirming the hypothesis of small airway disease related to pulmonary infection.
3. Discussion
Serratia represents the seventh most common cause of pneumonia, with an incidence of 4.1% in the United States and 3.2% in Europe; it is also the tenth most common cause of blood flow infection, reporting an incidence of 2.0% among hospitalized patients. There is no significant difference in the prevalence between males and females [7].
Serratia marcescens gives rise to a wide spectrum of clinical manifestations; pulmonary involvement is rare and typically occurs in immunocompromised patients [8]. There is a correlation between different serotypes of Serratia marcescens and neoplastic lesions: namely, the serotype 014:H12 has been isolated from the upper respiratory tract (sputum), whereas the serotype 013:H17 has been frequently recovered from urine [9].
Serratia marcescens pneumonia has been described in 1982 by Goldstein et Al; they have investigated the histological appearance of 16 patients having pneumonia related to Serratia. In 9 patients, the pathogens caused haemorrhagic bronchopneumonia, with micro-abscesses and cavitated lesions; vessels larger than 75 μm were also observed, due to the presence of vasculitis. In the remaining patients – represented by a population of 7 neutropenic cases – more extensive and diffuse pneumonia was found, sometimes resembling a diffuse alveolar damage lung pattern, with intra-alveolar fibrinous exudates and haemorrhage [10].
In our clinical case, the radiological appearance was similar to the morphological condition of a Serratia infection in non-neutropenic patients: no signs of diffuse alveolar damage were observed on CT images. Several pulmonary diseases have been considered in the differential diagnosis.
The possibility of disease progression was accurately evaluated, because many cancers may involve lung parenchyma with metastatic nodules having cavitated appearance. Previous reports published in literature have emphasized the possibility of cavitated metastasis coming from breast, bladder, kidney, synovial sarcoma and gallbladder. However, as reported in 2002 by Mermershtain et al. cavitated pulmonary nodules can be related also to immunosuppressive therapy. They described the clinical case of a 40-year old female with breast cancer treated with adjuvant chemotherapy (5-fluorouracil, doxorubicin, cyclophosphamide, given on day 1 each of six 21-day cycles). The CT revealed multiple lesions in the pulmonary parenchyma, caused by the dissemination of breast cancer. Docetaxel was then used for 2 months; the therapy was stopped for disease progression and the patient started weekly paclitaxel. The new CT performed after the new treatment demonstrated disappearance of lung nodules, which were partially cavitated. This pattern – found on CT images – was considered as a partial response to treatment [11].
In our case, after the second line treatment, the CT revealed the disappearance of multiple hepatic lesions, whereas no pulmonary lesions were demonstrated at the beginning and during the treatment. Cavitated nodules were found five years after the onset of breast cancer, without any clinical findings of immunocompromising status. Therefore, in the differential diagnoses of cavitated nodules, several diseases should be considered: metastases, rheumatoid arthritis, Langerhans cell histiocytosis, mycotic infections, and septic emboli [12] (Table 1).
Table 1.
Differential diagnosis of cavitary lung lesions.
| Acute infections |
Lung abscess Necrotizing pneumonia Septic emboli Fungal infection Nocardia |
| Chronic infections |
TB NTB Fungal Parasitic Viral |
| Malignancy |
Primary lung cancer Metastasis from squamous cell carcinoma (SCC) of the lung, head and neck, adenocarcinoma of the gastrointestinal tract or bladder, breast cancer, sarcoma |
| Autoimmunity |
Rheumatoid arthritis Granulomatosis with polyangiitis |
| Other | Langerhans cell histiocytosis |
Metastases represent the main diagnostic hypothesis to be considered in the differential diagnosis of cavitated pulmonary nodules. The most common causes include squamous cell carcinoma (SCC) of the lung, head and neck, and adenocarcinoma of the gastrointestinal tract or bladder. Rarely, also in breast cancer and sarcomas cavitated pulmonary nodules may be depicted. Generally, a cavitated lung nodules show thick and irregular walls; however, thin-walled cavities can be found in cases of metastases from adenocarcinomas and sarcomas [13].
Rheumatoid arthritis may manifest with interstitial lung disease in a percentage of about 5–58% of cases with lung involvement; other patterns include pleural disease, airway disease – sometimes resembling a mosaic attenuation – and bronchocentric nodules (0–37%) [14]. The latter may cavitate in rare cases, as reported by Kanitez et al. [15].
Langerhans cell histiocytosis is characterized by multiple nodules, which may cavitate in the acute phase of the disease; however, these nodules typically spare costo-phrenic angles and lower regions of the lung [16]. In our patient, nodules were homogeneously distributed through the lung, so that this cause of disease was excluded. Finally, mycotic infections and septic emboli have been accurately evaluated among differential diagnosis; however, no clinical signs of systemic infection were observed. In addition, there was no evidence of mycotic infection or typical consolidation in the pulmonary parenchyma.
The sudden appearance of the lung nodules oriented the radiologists towards two possible hypotheses: relapsing disease or airway disease related to infection. The BAL excluded neoplastic cells and revealed the growth of Serratia marcescens: the clinical history was more in depth analysed – trying to identify behaviours at risk for this pathogen.
Our patient referred a previous holiday in a spa hotel, having several baths in the hot tub; as reported in literature by Kiyohara et al. among possible pathogenic mechanisms of Serratia infection, it's possible to include inadvertent swallowing of swimming pool water contaminated [17].
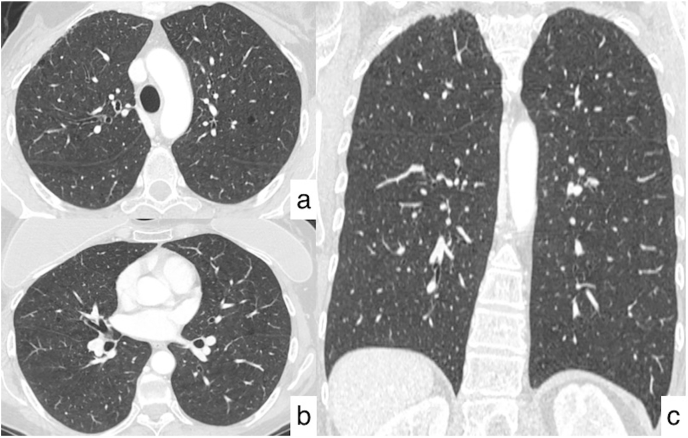
Considering this clinical history, a medical treatment with levofloxacin was performed for 14 days; CT acquired one month later demonstrated complete resolution of nodular opacities, so that patient has been scheduled for a new radiological evaluation (Fig. 5).
Fig. 5.
Resolution of pulmonary infection after treatment. Axial (a, b) and coronal (c) HRCT images demonstrate the disappearance of lung nodules, excluding the malignant nature of the lesions.
In conclusion, the knowledge of radiological features, in association with clinical history and laboratory findings, is important to achieve a right diagnosis, suggesting the optimal management. Cavitated nodules – even if rarely reported in literature – may be associated with Serratia infections due to contamination of swimming pool water.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Giulia Fazio, Email: giuliafazio8@gmail.com.
Federica Galioto, Email: federicagalioto91@gmail.com.
Agata Ferlito, Email: agataferlito91@gmail.com.
Maria Coronella, Email: mcoronella@sirm.org.
Stefano Palmucci, Email: spalmucci@unict.it.
Antonio Basile, Email: basile.antonello73@gmail.com.
References
- 1.Khanna A., Khanna M., Aggarwal A. Serratia marcescens - a rare opportunistic nosocomial pathogen and measures to limit its spread in hospitalized patients. J. Clin. Diagn. Res. 2013;7(2):243–246. doi: 10.7860/JCDR/2013/5010.2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hejazi A., Falkiner F.R. Serratia marcescens. J. Med. Microbiol. 1997;46:903–912. doi: 10.1099/00222615-46-11-903. [DOI] [PubMed] [Google Scholar]
- 3.Carbonell G.V., Amorim C.R.N., Furumura M.T., Darini A.L.C., Fonseca B.A.L., Yano T. Biological activity of Serratia marcescens cytotoxin. Braz. J. Med. Biol. Res. 2003;36(3):351–359. doi: 10.1590/s0100-879x2003000300010. [DOI] [PubMed] [Google Scholar]
- 4.Grimont P.A.D., Grimont F. Genus VIII. Sermtia. In: Krieg N.R., Holt J.G., editors. vol. 1. Williams and Wilkins; Baltimore: 1984. pp. 477–484. (Bergey's Manual of Systematic Bacteriology). [Google Scholar]
- 5.Szewzyk U., Szewzyk R., Stenstrom T.-A. Growth and survival of Serratia marcescens under aerobic and anaerobic conditions in the presence of materials from blood bags. J Clin Micmbiol. 1993;31:1826–1830. doi: 10.1128/jcm.31.7.1826-1830.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Medeiros A.A., O'Brien T. Contribution of R factors to the antibiotic resistance of hospital isolates of Serratia. Antimicrob. Agents Chemother. 1969:30–35. doi: 10.1128/AAC.8.1.30. 1968. [DOI] [PubMed] [Google Scholar]
- 7.Herra C, Falkiner FR. Serratia marcescens. In: Antimicrobe Microbes. Available at: http://www.antimicrobe.org/b26.asp. Accessed July 27, 2017.
- 8.Edmund Cope Thomas, Cope Wei, Martin Beaumont David. A case of necrotising fasciitis caused by Serratia marcescens: extreme age as functional immunosuppression? Age Ageing. March 2013;42(Issue 2):266–268. doi: 10.1093/ageing/afs198. [DOI] [PubMed] [Google Scholar]
- 9.Minck R., Le Minor S., Pigache F., Dammron A., Reeb E., Kohen M., Le Faou A. Intérêt de la sérotypie dans l'étude d'infections à Serratia marcescens observées dans les hôpitaux de Strasbourg [Utility of serotyping in the study of Serratia marcescens infections in Strasbourg Hospitals (author's transl)] Pathol. Biol. 1980 Oct;28(8):501–507. French. PMID: 7001322. [PubMed] [Google Scholar]
- 10.Goldstein J.D., Godleski J.J., Balikian J.P., Herman P.G. Pathologic patterns of Serratia marcescens pneumonia. Hum. Pathol. 1982 May;13(5):479–484. doi: 10.1016/s0046-8177(82)80031-2. [DOI] [PubMed] [Google Scholar]
- 11.Mermershtain W., Schulman H., Hertzanu Y., Cohen Y. Massive cavitation of solid pulmonary metastatic lesions in a breast cancer patient: a case report. Ann. Oncol. 2002;13(Issue 1):173–174. doi: 10.1093/annonc/mdf001. ISSN 0923-7534. [DOI] [PubMed] [Google Scholar]
- 12.Parkar A.P., Kandiah P. Differential diagnosis of cavitary lung lesions. J Belg Soc Radiol. 2016 Nov 19;100(1):100. doi: 10.5334/jbr-btr.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seo J.B., Im J.G., Goo J.M., Chung M.J., Kim M.Y. Atypical pulmonary metastases: spectrum of radiologic findings. Radiographics. 2001 Mar-Apr;21(2):403–417. doi: 10.1148/radiographics.21.2.g01mr17403. [DOI] [PubMed] [Google Scholar]
- 14.De Lauretis A., Veeraraghavan S., Renzoni E. Review series: aspects of interstitial lung disease: connective tissue disease-associated interstitial lung disease: how does it differ from IPF? How should the clinical approach differ? Chron. Respir. Dis. 2011;8(1):53–82. doi: 10.1177/1479972310393758. [DOI] [PubMed] [Google Scholar]
- 15.Kanıtez N.A., Çelik S., Öner S.Y., Ürer H.N., Bes C., Çetinkaya E. Cavitary pulmonary nodules in rheumatoid arthritis; case reports and review of the literature. Eur J Rheumatol. 2018 Mar;5(1):65–68. doi: 10.5152/eurjrheum.2017.16106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jawad H., Walker C.M., Wu C.C., Chung J.H. Cystic interstitial lung diseases: recognizing the common and uncommon entities. Curr. Probl. Diagn. Radiol. 2014;43:115–127. doi: 10.1067/j.cpradiol.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 17.Kiyohara Nobuhiko, Kobayakawa Yuri, Lyman Harvard, Osafune Tetsuaki. Identification of bacterial flora in the water of swimming pools throughout the year. Taiikugaku Kenkyu. 2006;51:1–9. doi: 10.5432/jjpehss.51.1. [DOI] [Google Scholar]