Abstract
The increase in malaria transmission in the Amazon region motivated vector control units of the Ministry of Health of Ecuador and Peru to investigate Anopheles (Diptera: Culicidae) species present in transmission hotspots. Mosquitoes were collected using prokopack aspirators and CDC light traps (Ecuador) and human landing catch in Peru. In Ecuador, 84 Anopheles were captured from Pastaza, Morona Santiago, and Orellana provinces and identified morphologically [An. (An.) apicimacula Dyar and Knab, An. (Nys.) near benarrochi, An. (Nys.) near oswaldoi, An. (Nys.) near strodei, An. (An.) nimbus (Theobald, 1902), and An. (Nyssorhynchus) sp.]. In Peru, 1,150 Anopheles were collected in Andoas District. A subsample of 166 specimens was stored under silica and identified as An. near oswaldoi, An. darlingi, and An. (An.) mattogrossensis Lutz and Neiva. COI barcode region sequences were obtained for 137 adults (107 from Peru, 30 from Ecuador) identified by ITS2 PCR-RFLP as An. benarrochi Gabaldon, Cova Garcia, and Lopez and retained in the final analysis. Haplotypes from the present study plus An. benarrochi B GenBank sequences grouped separately from Brazilian An. benarrochi GenBank sequences by 44 mutation steps, indicating that the present study specimens were An. benarrochi B. Our findings confirm the presence of An. benarrochi B in Ecuador and reported here for the first time from the Amazonian provinces of Orellana and Morona Santiago. Furthermore, we confirm that the species collected in Andoas District in the Datem del Maranon Province, Peru, is An. benarrochi B, and we observed that it is highly anthropophilic. Overall, the known distribution of An. benarrochi B has been extended and includes southern Colombia, much of Peru and eastern Ecuador.
Keywords: malaria hotspot, Ecuador, Peru, anopheline identification
Malaria, caused by protozoan parasites of the genus Plasmodium and transmitted by female mosquitoes of the genus Anopheles (WHO 2018), is reemerging globally, including some parts of Latin America (PAHO 2018). In Ecuador, the ~99% decrease in malaria cases from 106,641 in 2001 to 558 in 2012 moved Ecuador into the pre-elimination phase (Krisher et al. 2016), and the World Health Organization recognized this progress by including Ecuador in the E-2020 initiative with 20 other countries expected to reach the goal of zero autochthonous or indigenous cases by 2020 (WHO 2018). However, since 2015, case numbers have increased to 2,081 in 2019 (Ministerio de Salud Publica 2020) and the provinces with the highest number of confirmed malaria cases (as of epidemiological week 41) are Pastaza (582), Morona Santiago (717), and Orellana (276; PAHO/WHO 2019). The most common species of malaria parasite reported in Ecuador is Plasmodium vivax (87%), with the remaining 13% attributed to Plasmodium falciparum (Ministerio de Salud Publica 2019).
Malaria in Peru remains an important public health problem (WHO 2018), and an estimated 90% of malaria transmission occurs in the Department of Loreto in the Amazon region, mainly in riverine villages linked to occupations of agriculture, fishing, and timber extraction (Parker et al. 2013, Rosas-Aguirre et al. 2016). After the success of the global PAMAFRO (2006–2011) initiative wherein incidence dropped to a low of 11,504 cases in 2011 (Soto-Calle et al. 2017), malaria was considered to be under control. Subsequently, cases began to increase and rose steadily through 2018 (Recht et al. 2017) to an estimated 45,443 (Ministerio de Salud del Perú 2018). By early 2019, numbers had been halved to 22,070 cases (Ministerio de Salud del Perú 2019, Rosas-Aguirre et al. 2020), at least partly from adoption of the ambitious Plan Malaria Cero that recommended an initial focus on control of high-risk malaria villages (Ministerio de Salud del Perú 2017). In Peru throughout 2018, P. vivax predominated (79.2%), with P. falciparum responsible for the remaining 20.8% (Rosas-Aguirre et al. 2020).
There are approximately 465 Anopheles species worldwide and 41 that are considered dominant vectors (Sinka et al. 2012, Harbach 2013), effectively transmitting to humans one or more of the five species of Plasmodium. Currently, Anopheles is subdivided into eight subgenera: Anopheles is cosmopolitan; Cellia is present in the Afrotropical, Australasian, and Oriental regions; Kerteszia, Lophopodomyia, Stethomyia, and Nyssorhynchus are restricted to the Neotropics (Foster et al. 2017). The latter subgenus, provisionally elevated to genus status (Foster et al. 2013, 2017), includes most of the species involved in Plasmodium transmission in the Neotropics. Despite their importance as vectors, many Nyssorhynchus species remain relatively poorly characterized, especially in more remote areas of the Amazon Basin (Lounibos and Conn 2000, Bourke et al. 2018).
In Ecuador, 30 Anophelinae species have been reported in the Amazonian, Coastal, and Andean regions and malaria transmission has been attributed to six of these: An. albimanus, An. aquasalis, An. neivai, An. pseudopunctipennis, An. punctimula, and An. oswaldoi (Pinault and Hunter 2011, 2012; Linton et al. 2013; Ramón et al. 2019). In contrast, in Peru, 43 species of Anopheles have been identified throughout the country with four recognized as primary malaria vectors: An. albimanus and An. pseudopunctipennis along the Pacific coast (Sinka et al. 2012) and An. darlingi and An. benarrochi in the Amazon region (Calderón et al. 1995, Aramburú Guarda et al. 1999, Flores-Mendoza et al. 2004). The focus of the present study, Anopheles benarrochi, was originally described from Trujillo state, Venezuela, and its distribution was thought to include Colombia, Venezuela, Brazil, and Peru (Faran and Linthicum 1981, Rubio-Palis 2000). Ruiz et al. (2005) first described a new species from southern Colombia that differed from An. benarrochi in morphology, genetics (ITS2 rDNA region sequences from link-reared progeny) and behavior (highly anthropophilic). They named it An. benarrochi B and concluded that An. benarrochi is a species complex.
In practice, identification of anopheline females by morphological keys is the most commonly used strategy for entomological field studies. Limitations of such keys, including for major characters such as leg banding and wing coloration, extensive overlapping intra- and interspecific variation, and the existence of species complexes, are well known and have been described elsewhere (Faran and Linthicum 1981, Obando and Gironza 2009). The outcome is that unless complementary molecular methods are used to confirm initial identification, some specimens (even potential vectors) remain misidentified, confounding efforts to curtail human–mosquito interactions that lead to transmission. Molecular methods have helped to identify several previously unrecognized species, some of which are putative Plasmodium vectors (Matson et al. 2008, Linton et al. 2013, Arregui et al. 2015). Furthermore, the use of COI barcode sequences is a valuable tool in molecular taxonomy for confirming species identification (Ruiz-Lopez et al. 2013) and in the creation of networks of haplotypes to provide a deeper understanding of intraspecific haplotype relationships (Prussing et al. 2018).
In 2018, an increase in the number of malaria cases in the provinces of Pastaza, Morona Santiago, and Orellana in Ecuador, as well as in the neighboring district of Andoas, Peru, motivated the Ministerio de Salud Publica (MSP) of Ecuador and the Health Unit of Loreto Department in Peru, respectively, to collect and identify the Anopheles species present in transmission hotspots in these provinces. The objectives of this study were 1) to test the hypothesis that the distribution of Anopheles benarrochi B includes Amazonian Ecuador and Andoas District, Peru, and 2) to investigate the relationship between An. benarrochi B and other reported An. benarrochi using a combination of barcode sequences from the present study, Barcode of Life Database (BOLD), and GenBank.
Materials and Methods
Study Areas
According to the epidemiological reports of the Ecuadorian Ministry of Health and the Ministry of Health of Peru, the historical and active areas of malaria transmission are concentrated in provinces located in the Amazon region (Aramburú Guarda et al. 1999, Arregui et al. 2015). These communities are remote, accessible by water, on foot via community trails, or occasionally by light aircraft. Human populations are scattered along river margins and most housing is made of local material, with wooden walls and palm-thatch roofs (Saavedra et al. 2019). Generally, people here live in conditions of extreme poverty. Contact is limited mainly to government workers or other personnel involved in providing healthcare that requires considerable economic and logistical resources. Therefore, surveillance of malaria vectors by national institutions is challenging; this has made it difficult to identify local factors responsible for transmission that could be targeted to reduce human–vector interactions.
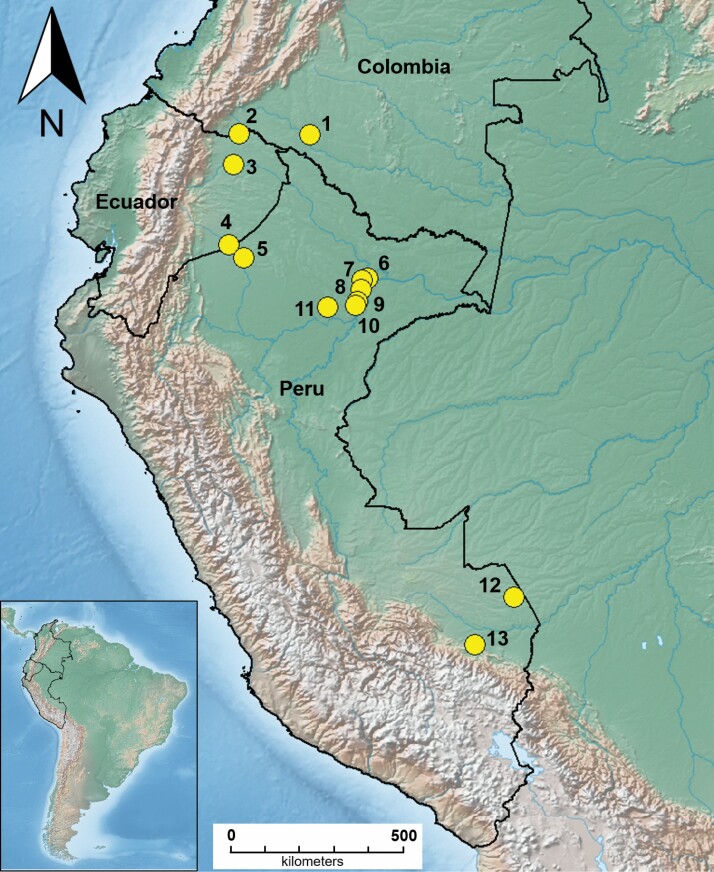
Wachirpas and Lunchi are Amazonian communities in the provinces of Morona Santiago and Orellana, respectively, in Ecuador (Fig. 1; map sites 3, 4; Supp Table 1 [online only]). Wachirpas has 162 inhabitants and is located within the Evergreen Forest lowland ecosystem of the Pastaza river (Ministerio del Ambiente 2013), whereas Orellana has 3,038 inhabitants and is situated in the Evergreen lowland forest of the Napo-Curaray river (Ministerio del Ambiente 2013). The Ecuadorian field collection was carried out in April 2018 for a week as part of entomological surveillance toward strengthening activities of the National Network of Entomology Laboratories, directed by the National Reference Center for Vectors (CRNV) in Quito, Ecuador.
Fig. 1.
Localities where Anopheles benarrochi B has been molecularly identified with COI (see Supp Table S1 [online only] for location details). Map made with DIVAGIS v7.5, base layer from Natural Earth (https://www.naturalearthdata.com/).
Santa Maria Manchari is a community in Andoas District in the Datem del Maranon Province of Loreto Department. Andoas is located approximately 362 km northwest of Iquitos on the Pastaza river, near the Ecuadorian border (Fig. 1; map site 5; Supp Table 1 [online only]). Local vegetation surrounding the village is characteristic of tropical rainforest (Need et al. 1993). Entomological surveys were carried out between 5 and 7 July 2018 as part of the surveillance activities by the Dirección Regional de Salud, Iquitos, Loreto, to evaluate Anopheles susceptibility to insecticides in Andoas District.
Mosquito Collections
In Ecuador, adult female mosquitoes were collected for four nights outside six houses in Wachirpas within a distance of ~20 m and in Lunchi in two houses within ~250 m using a prokopack motor vacuum aspirator (John W. Hock, Model 1419). Inside each house, a prokopack was used with two collectors who rotated every hour to account for possible bias in capture capability and attractiveness to mosquitoes, from 18:00 to 06:00. In addition, CDC light traps were hung inside each house at 1.5 m above the ground from 18:00 to 06:00 h and were checked and emptied each morning. In Peru, mosquitoes were collected using human landing catch (HLC) from 18:00 to 22:00 h and from 04:00 to 06:00 h, during peak anopheline activity reported in the area (Need et al. 1993). Mosquito sampling was conducted by six collectors in three houses, two per house, one person inside (indoors), and the other 1–2 m from the house (outdoors).
All mosquitoes collected were individually stored in 1.5-ml Eppendorf tubes with the cap punctured to prevent the accumulation of water vapor and resultant fungal infection and DNA degradation, then stored in sealed sleeves for transport to the laboratories in Quito (Ecuador) and Iquitos (Peru). Samples were separated by date, collection method, and location.
Anopheles Species Identification
Mosquitoes were identified with one of three morphological keys: Faran and Linthicum (1981), Consoli and Lourenco-de-Oliveira (1994), or Obando and Gironza (2009), and entered into the CRNV database in Ecuador. Several mosquito samples with complete morphological structures were deposited in the Reference Collection of the National Vector Reference Center, Quito, and others were stored in 70% ethanol. Genomic DNA extraction of unfed females was conducted using DNeasy Blood & Tissue Kit (Qiagen, Hilden, Germany). All samples were stored at −20°C until use.
For all samples from Ecuador and Peru, we followed methods for species identification as in Conn et al. (2013) and Prussing et al. (2019), starting with a PCR-RFLP assay of the ribosomal internal transcribed spacer 2 (ITS2; Matson et al. 2008), followed by a barcode cytochrome oxidase c subunit I (COI) PCR (Folmer et al. 1994, Hebert et al. 2004) and Sanger sequencing in the forward direction at the Wadsworth Center Applied Genomic Technologies Core (New York State Department of Health). Sequences were cleaned, edited, and checked for pseudogenes and stop codons with Geneious v9.1.4 (http://www.geneious.com; Kearse et al. 2012). COI sequences were queried against the BOLD Identification System (Ratnasingham and Hebert 2007) or GenBank (https://www.ncbi.nlm.nih.gov/genbank/) for species identification. Anopheles benarrochi B COI sequences from this study were aligned with MUSCLE (Edgar 2004) using default settings in MEGA X (Kumar et al. 2018), with published NCBI GenBank sequences for An. benarrochi B (Conn et al. 2013, Orjuela et al. 2013, Prussing et al. 2019) and An. benarrochi (Foster et al. 2013). Sequences were trimmed to the length of the shortest sequence, leaving a 598-bp region for comparison, and PopART v1.7 (Leigh and Bryant 2015), with epsilon set to 0, was utilized to create a median-joining haplotype (Bandelt et al. 1999).
A bootstrap consensus neighbor-joining (NJ) tree was constructed using the Kimura 2-parameter model (NJ K-2P), with 1,000 bootstrap replicates, to examine the phylogenetic relationships among the An. benarrochi complex haplotypes (n = 39) in MEGA X (Kumar et al. 2018). Sequences of Anopheles rangeli (GenBank ID JF923725) and Anopheles cruzii (GenBank ID JF923692) were used as outgroups (Foster et al. 2013) to root the tree. Using these species haplotype clusters, mean between group genetic distance measurements (and SE) were calculated using the K-2P model and 1,000 bootstrap replicates (Kimura 1980, Kumar et al. 2018).
Ethics Statement
No permit or authorization is required for personnel from the Ecuador National Reference Center to collect mosquito samples in Ecuador. Nevertheless, the samples analyzed herein were collected under a project called ‘Strengthening the National Network of Entomology Laboratories’, funded by the Pan American Health Organization (PAHO) in 2018. Likewise, the mosquito samples from Andoas, Peru, were collected by members of the Unidad de Entomología, Laboratorio Referencial Regional de Salud Pública de Loreto, Dirección Regional de Salud (DIRESA), Loreto, Peru; thus, no permit was required.
Results
In Ecuador, a total of 84 Anopheles mosquitoes were collected, of which 58 corresponded to the locality of Wachirpas and 26 to Lunchi. These specimens were identified morphologically as An. apicimacula, An. near benarrochi, An. near oswaldoi, An. near strodei, An. nimbus, An. (Nyssorhynchus) sp., and An. sp., and further molecular identifications were conducted to identify An. benarrochi (Table 1). In Peru, a total of 1,150 Anopheles were collected during night nights in the locality of Santa Maria in Andoas District. A subsample of 166 specimens was stored under dry conditions for subsequent morphological identification. Three species were identified: An. near oswaldoi, An. darling, and An. mattogrossensis. Fourteen specimens were selected randomly from the An. near oswaldoi group to be sequenced.
Table 1.
Identities of anopheline specimens from Ecuador and Peru, 2018
| Country | Locality | No. | Trap | Morph. Id. | COI Id. |
|---|---|---|---|---|---|
| Ecuador | Wachi rpas | 10 | CDC BA | An. apicimacula | — |
| 5 | CDC BA | An. near benarrochi | An. benarrochi B | ||
| 1 | CDC BA | An. near oswaldoi | An. benarrochi B | ||
| 1 | CDC BA | An. near strodei | An. benarrochi B | ||
| 2 | CDC | An. nimbus | — | ||
| 25 | CDC | Anopheles sp. | — | ||
| 14 | CDC BA | Nyssorhynchus sp. | — | ||
| Lunchi | 23 | CDC BA | An. near benarrochi | An. benarrochi B | |
| 2 | CDC BA | An. near oswaldoi | — | ||
| 1 | CDC BA | Nyssorhynchus sp. | — | ||
| Peru | Santa Maria | 107 | HLC | An. near oswaldoi | An. benarrochi B |
| 40 | HLC | An. darlingi | |||
| 19 | HLC | An. mattogrossensis |
No., number of specimens; CDC, CDC light trap; CDC BA (prokopack Aspirator); HLC, human landing catch; Morph. Id., morphological identification; COI Id, mtDNA COI sequence identification.
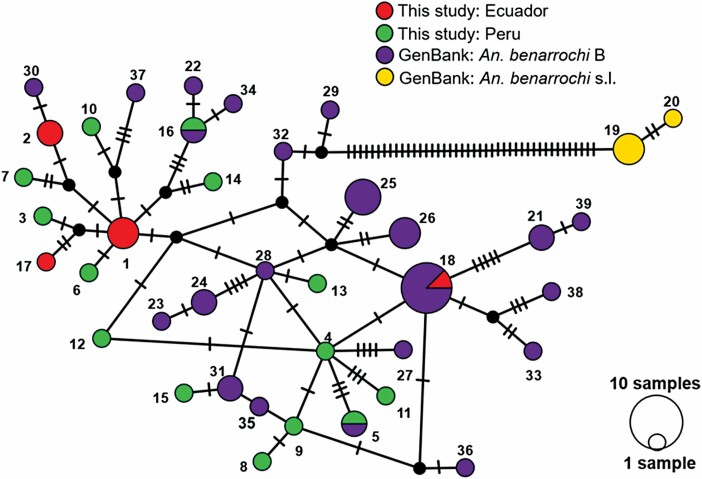
COI barcode region sequences were obtained for 137 adults identified by ITS2 PCR-RFLP as An. benarrochi retained in the final analysis (n = 30 adults collected in Ecuador, 2018; n = 107 adults collected in Peru, 2018). The median-joining haplotype network (Fig. 2) detected 39 unique haplotypes: 15 included only sequences from this study, 3 included sequences both from this study and GenBank sequences identified as An. benarrochi B, 19 included only GenBank sequences of An. benarrochi B, and 2 included only GenBank sequences identified as An. benarrochi (Supp Table 2 [online only]). The haplotypes comprising samples from the present study plus An. benarrochi B GenBank sequences grouped separately from the An. benarrochi GenBank sequences (Fig. 2; Supp Table 2 [online only]) by 44 mutation steps, indicating that the samples from the present study were all An. benarrochi B. Unique sequences of An. benarrochi B from this study were deposited in GenBank: MT556439–MT556442, MK604185–MK604187, and MT503203–MT503216 (Supp Table 2 [online only]).
Fig. 2.
Median-joining COI (598-bp) haplotype network (epsilon = 0) for members of the Anopheles benarrochi complex from the current study and GenBank sequences (Supp Table 2 [online only]). Circles represent unique haplotypes and are colored based on species or sample origin (sequences from this study or GenBank). The size of each circle is proportional to the number of individual sequences sharing the haplotype. Black nodes indicate theoretical missing haplotypes, and hash marks represent mutation steps between haplotypes.
A NJ K-2P tree and mean genetic distances corresponding to the NJ K-2P tree clusters (Supp Fig. 1 [online only]; Supp Table 3 [online only]) further supported the identification of sequences from this study with previously reported An. benarrochi B, with a mean 8.81% genetic distance between the An. benarrochi complex haplotype clusters, yet an approximate 6% genetic distance between An. rangeli and the An. benarrochi complex members.
Discussion
One of the advantages of networks is that they can suggest divisions between species and are thus especially valuable for members of species complexes that typically present little morphological differentiation (Motoki et al. 2020). This study provides support for a high number of mutational differences between the COI sequences of An. benarrochi (Suppl. Data [online only]; source is Acre state, Brazil; Foster et al. 2013) and those of An. benarrochi B from GenBank plus sequenced specimens from Ecuador and Peru (the current study). We do not know whether the Brazilian GenBank An. benarrochi sequences are An. benarrochi s.s. (Foster et al. 2013), but Bourke et al. (2018) found clade-level differences between An. benarrochi from Acre and Rondonia states in Brazil and An. benarrochi B using COI sequences and concluded that there are other species in this complex.
Our findings support the hypothesis that the distribution of An. benarrochi B includes Ecuador, reported here for the first time from the Amazonian provinces of Orellana and Morona Santiago. However, it should be emphasized that only An. benarrochi B was identified from this region; the distribution of An. benarrochi s.s. is hypothesized to be restricted to Venezuela and Brazil (Rubio-Palis 2000). Furthermore, we confirm that the species collected with HLC in Andoas, Napo district, Alto Amazonas Province, Peru, is An. benarrochi B, and we observed that it is highly anthropophilic. Overall, the known distribution of An. benarrochi B has been extended and includes southern Colombia (Ruiz et al. 2005), much of Peru (Conn et al. (2013), plus the present study) and eastern (Amazonian) Ecuador.
As originally described, An. benarrochi is mainly zoophilic, crepuscular, and present in low densities (Rubio-Palis 2000, Ruiz et al. 2005). Furthermore, in susceptibility trials, laboratory-reared F1An. benarrochi from Costa Marques, Rondônia state, western Brazil, which fed on P. vivax-infected human volunteers, developed oocysts but not sporozoites (Klein et al. 1991). The conclusion was that An. benarrochi in western Amazonian Brazil did not contribute to local malaria transmission.
Despite these earlier conclusions, Anopheles benarrochi was incriminated in the transmission of P. vivax near Iquitos, Loreto department (Fernández et al. 1996) and of P. vivax and P. falciparum in Loreto and Ucayali departments, eastern Peru (Flores-Mendoza et al. 2004). Of considerable relevance is the extensive anopheline survey conducted by Schoeler et al. (2003) along multiple river systems (including the Pastaza River along which the community of Andoas is located), in the Peruvian departments of Loreto and Ucayali. From a total of 93 putative collection sites (82 of which yielded anophelines), the most abundant species was An. benarrochi, which was present in about 1/3 of the sites (n = 32) and represented 70.7% of the total number (60,585 specimens) of anophelines collected. Based on our detection herein of abundant, anthropophilic An. benarrochi B in Andoas, Peru, we hypothesize that this species is broadly distributed in Peru. In Putumayo, Colombia, and in Madre de Dios, Peru, molecularly confirmed specimens of An. benarrochi B were detected positive for P. vivax (Orjuela et al. 2013, Conn et al. 2015).
A study in the Yasuni Biosphere Reserve and National Park updated the list of mosquitoes of Ecuador to 179 species, of which 25 belong to the genus Anopheles, including a new record of An. nr. konderi belonging to the Oswaldoi Group (Linton et al. 2013). Since then (2013), there have been no extensive collections in the Ecuadorian Amazon. In 2018, the MSP through CRNV conducted several interventions in Amazonian locations where malaria cases were recorded (Ministerio de Salud Publica 2019), identifying An. benarrochi with morphological keys in the communities of Wachirpas and Lunchi. The confirmation of a subsample of these specimens as An. benarrochi B further supports our hypothesis of the involvement of this species in malaria transmission in Amazonian Ecuador.
Supplementary Data
Supplementary data are available at Journal of Medical Entomology online.
Supplementary Fig. 1. Neighbor-Joining Kimura 2-parameter model (NJ K-2P) bootstrap consensus tree, with 1,000 bootstrap replicates, including Anopheles benarrochi complex 39 haplotypes (as in Fig. 2) and outgroup sequences of Anopheles rangeli and Anopheles cruzii. Only bootstrap values ≥ 70% are shown.
Acknowledgments
The authors thank the National Network of Entomology Laboratories for their hours collecting mosquitoes. Thanks to PAHO for the support with the entomology laboratories strengthening project with which the Ecuadorian collections were financed. Peruvian authors acknowledge the entomology technicians from Laboratorio Referencial Regional de Salud Pública de Loreto and the personnel at the health facility in the Datem del Marañon Province, Peru, for all the assistance during the field collections. This project was funded in part by the United States National Institutes of Health (grant no. U19AI089681) as the Amazonian International Center of Excellence in Malaria Research to J.M.V. Sample DNA was sequenced at the Wadsworth Center Applied Genomics Technology Core, Albany, NY.
References Cited
- Aramburú Guarda, J., Ramal Asayag C., and Witzig R.. 1999. Malaria reemergence in the Peruvian Amazon region. Emerg. Infect. Dis. 5: 209–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arregui, G., Enriquez S., Benítez-Ortiz W., and Navarro J.-C.. 2015. Molecular taxonomy of Anopheles from Ecuador using mitochondrial DNA (cytochrome c oxidase I) and optimization by parsimony maximum. Bol. Malariol. Salud Ambient. 55: 132–154. [Google Scholar]
- Bandelt, H. J., Forster P., and Röhl A.. 1999. Median-joining networks for inferring intraspecific phylogenies. Mol. Biol. Evol. 16: 37–48. [DOI] [PubMed] [Google Scholar]
- Bourke, B. P., Conn J. E., de Oliveira T. M. P., Chaves L. S. M., Bergo E. S., Laporta G. Z., and Sallum M. A. M.. 2018. Exploring malaria vector diversity on the Amazon Frontier. Malar. J. 17: 342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderón, G., Fernández R., and Valle J.. 1995. Especies de la fauna anofelina, su distribucion y algunas consideraciones sobre su abundancia e infectividad en el Peru. Rev. Peru Epidemiol. 8: 5–23. [Google Scholar]
- Conn, J. E., Moreno M., Saavedra M., Bickersmith S. A., Knoll E., Fernandez R., Vera H., Burrus R. G., Lescano A. G., Sanchez J. F., et al. 2013. Molecular taxonomy of Anopheles (Nyssorhynchus) benarrochi (Diptera: Culicidae) and malaria epidemiology in southern Amazonian Peru. Am. J. Trop. Med. Hyg. 88: 319–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn, J. E., Norris D. E., Donnelly M. J., Beebe N. W., Burkot T. R., Coulibaly M. B., Chery L., Eapen A., Keven J. B., Kilama M., et al. 2015. Entomological monitoring and evaluation: diverse transmission settings of ICEMR projects will require local and regional Malaria elimination strategies. Am. J. Trop. Med. Hyg. 93: 28–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consoli, R. A., and Lourenco-de-Oliveira R.. 1994. Principais mosquitos de importância sanitária no Brasil. Editora Fiocruz, Fundação Oswaldo Cruz, Rio de Janeiro. [Google Scholar]
- Edgar, R. C. 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32: 1792–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faran, M. E., and Linthicum K. J.. 1981. A handbook of the Amazonian species of Anopheles (Nyssorhynchus) (Diptera: Culicidae). Mosq. Syst. 13: 1–81. [Google Scholar]
- Fernández, R., Carbajal F., Quintana J., Chauca H., and Watts D. M.. 1996. Presencia del A. (N) darlingi (Diptera: Culicidae), en alrededores de la ciudad de Iquitos, Loreto-Peru. Bol. Soc. Peru. Enferm. Infecc. Trop. 5: 10–20. [Google Scholar]
- Flores-Mendoza, C., Fernández R., Escobedo-Vargas K. S., Vela-Perez Q., and Schoeler G. B.. 2004. Natural Plasmodium infections in Anopheles darlingi and Anopheles benarrochi (Diptera: Culicidae) from eastern Peru. J. Med. Entomol. 41: 489–494. [DOI] [PubMed] [Google Scholar]
- Folmer, O., Black M., Hoeh W., Lutz R., and Vrijenhoek R.. 1994. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 3: 294–299. [PubMed] [Google Scholar]
- Foster, P. G., Bergo E. S., Bourke B. P., Oliveira T. M., Nagaki S. S., Sant’Ana D. C., and Sallum M. A.. 2013. Phylogenetic analysis and DNA-based species confirmation in Anopheles (Nyssorhynchus). PLoS One 8: e54063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster, P. G., de Oliveira T. M. P., Bergo E. S., Conn J. E., Sant’Ana D. C., Nagaki S. S., Nihei S., Lamas C. E., González C., Moreira C. C., et al. 2017. Phylogeny of Anophelinae using mitochondrial protein coding genes. R. Soc. Open Sci. 4: 170758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbach, R. E. 2013. The phylogeny and classification of Anopheles, pp. 3–55. InManguin S. (ed.), Anopheles mosquitoes – new insights into malaria vectors. InTech, Rijeka, Croatia. [Google Scholar]
- Hebert, P. D., Penton E. H., Burns J. M., Janzen D. H., and Hallwachs W.. 2004. Ten species in one: DNA barcoding reveals cryptic species in the neotropical skipper butterfly Astraptes fulgerator. Proc. Natl. Acad. Sci. USA 101: 14812–14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearse, M., Moir R., Wilson A., Stones-Havas S., Cheung M., Sturrock S., Buxton S., Cooper A., Markowitz S., Duran C., et al. 2012. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28: 1647–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura, M. 1980. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 16: 111–120. [DOI] [PubMed] [Google Scholar]
- Klein, T. A., Lima J. B., and Tada M. S.. 1991. Comparative susceptibility of anopheline mosquitoes to Plasmodium falciparum in Rondonia, Brazil. Am. J. Trop. Med. Hyg. 44: 598–603. [DOI] [PubMed] [Google Scholar]
- Krisher, L. K., Krisher J., Ambuludi M., Arichabala A., Beltrán-Ayala E., Navarrete P., Ordoñez T., Polhemus M. E., Quintana F., Rochford R., et al. 2016. Successful malaria elimination in the Ecuador-Peru border region: epidemiology and lessons learned. Malar. J. 15: 573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, S., Stecher G., Li M., Knyaz C., and Tamura K.. 2018. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 35: 1547–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh, J. W., and Bryant D.. 2015. popart: full-feature software for haplotype network construction. Methods Ecol. Evol. 6: 1110–1116. [Google Scholar]
- Linton, Y. M., Pecor J. E., Porter C. H., Mitchell L. B., Garzón-Moreno A., Foley D. H., Pecor D. B., and Wilkerson R. C.. 2013. Mosquitoes of eastern Amazonian Ecuador: biodiversity, bionomics and barcodes. Mem. Inst. Oswaldo Cruz 108(Suppl 1): 100–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lounibos, L. P., and Conn J. E.. 2000. Malaria vector heterogeneity in South America. Am. Entomol. 46: 238–249. [Google Scholar]
- Matson, R., Rios C. T., Chavez C. B., Gilman R. H., Florin D., Sifuentes V. L., Greffa R. C., Yori P. P., Fernandez R., Portocarrero D. V., et al. 2008. Improved molecular technique for the differentiation of neotropical anopheline species. Am. J. Trop. Med. Hyg. 78: 492–498. [PMC free article] [PubMed] [Google Scholar]
- Ministerio de Salud del Perú . 2017. Plan Malaria Cero Periodo 2017–2021: Resolucion Ministerial No 244–2017. Ministerio de Salud del Perú, Lima, Peru. [Google Scholar]
- Ministerio de Salud del Perú . 2018. Boletín Epidemiologico del Peru – SE 52–2018 (del 23 al 28 de diciembre del 2018), pp. 1218–1302. Centro Nacional de Epidemiología, Prevención y Control de Enfermedades, Ministerio de Salud del Perú, Lima, Peru. [Google Scholar]
- Ministerio de Salud del Perú . 2019. Boletín Epidemiologico del Peru – SE 52–2019 (del 22 al 28 de diciembre del 2019), pp. 1346–1351. Centro Nacional de Epidemiología, Prevención y Control de Enfermedades, Ministerio de Salud del Perú, Lima, Preu. [Google Scholar]
- Ministerio de Salud Publica. 2019. Subsistema de vigilancia SIVE-ALERTA, Enfermedades Transmitidas por Vectores Ecuador SE 1-52, 2019. InDirrecion Nacional de Vigilancia Epidemiologica (ed.). Ministerio de Salud Publica, Quito, Ecuador. [Google Scholar]
- Ministerio de Salud Publica. 2020. Subsistema de vigilancia SIVE-ALERTA, Enfermedades Transmitidas por Vectores Ecuador SE 01-17, 2020. InDirección Nacional de Vigilancia Epidemiológica (ed.). Ministerio de Salud Publica, Quito, Ecuador. [Google Scholar]
- Ministerio del Ambiente . 2013. Sistema de Clasificación de Ecosistemas de Ecuador Continental. Subsecretaría de Patrimonio Natural, Quito, Ecuador. [Google Scholar]
- Motoki, M. T., Linton Y.-M., Conn J. E., Ruiz-Lopez F., and Wilkerson R. C.. 2020. Phylogenetic network of mitochondrial COI gene sequences distinguishes 10 taxa within the Neotropical Albitarsis Group (Diptera: Culicidae), confirming the separate species status of Anopheles albitarsis H (Diptera: Culicidae) and revealing a novel lineage, Anopheles albitarsis J. J. Med. Entomol. XX: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Need, J. T., Rogers E. J., Phillips I. A., Falcon R., Fernández R., Carbajal F., and Quintana J.. 1993. Mosquitoes (Diptera: Culicidae) captured in the Iquitos area of Peru. J. Med. Entomol. 30: 634–638. [DOI] [PubMed] [Google Scholar]
- Obando, R. G., and Gironza N. S. C.. 2009. Introducción al estudio taxonómico de Anopheles de Colombia Claves y notas de distribución, 2nd ed. Universidad del Valle, Cali, Colombia. [Google Scholar]
- Orjuela, L. I., Herrera M., Erazo H., and Quiñones M. L.. 2013. Especies de Anopheles presentes en el departamento del Putumayo y su infección natural con Plasmodium. Biomédica 33: 42–52. [DOI] [PubMed] [Google Scholar]
- PAHO . 2018. Actualización Epidemiológica Aumento de malaria en las Américas. Pan American Health Organization, Washington, DC. [Google Scholar]
- PAHO/WHO . 2019. Epidemiological update: malaria in the Americas. Pan American Health Organization/World Health Organization, Washington, DC. [Google Scholar]
- Parker, B. S., Paredes Olortegui M., Peñataro Yori P., Escobedo K., Florin D., Rengifo Pinedo S., Cardenas Greffa R., Capcha Vega L., Rodriguez Ferrucci H., Pan W. K., et al. 2013. Hyperendemic malaria transmission in areas of occupation-related travel in the Peruvian Amazon. Malar. J. 12: 178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinault, L. L., and Hunter F. F.. 2011. New highland distribution records of multiple Anopheles species in the Ecuadorian Andes. Malar. J. 10: 236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinault, L. L., and Hunter F. F.. 2012. Malaria in highlands of Ecuador since 1900. Emerg. Infect. Dis. 18: 615–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prussing, C., Bickersmith S. A., Moreno M., Saavedra M. P., Alava F., Sallum M. A. M., Gamboa D., Vinetz J. M., and Conn J. E.. 2018. Nyssorhynchus dunhami: bionomics and natural infection by Plasmodium falciparum and P. vivax in the Peruvian Amazon. Mem. Inst. Oswaldo Cruz 113: e180380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prussing, C., Saavedra M. P., Bickersmith S. A., Alava F., Guzmán M., Manrique E., Carrasco-Escobar G., Moreno M., Gamboa D., Vinetz J. M., et al. 2019. Malaria vector species in Amazonian Peru co-occur in larval habitats but have distinct larval microbial communities. PLoS Negl. Trop. Dis. 13: e0007412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramón, G. M., Pérez R., and Jarrín P.. 2019. Francisco Campos-Rivadeneira and Roberto Levi-Castillo: their lives and contributions to the study of mosquitoes (Diptera: Culicidae) in Ecuador. Biomedica 39: 172–198. [DOI] [PubMed] [Google Scholar]
- Ratnasingham, S., and Hebert P. D.. 2007. bold: the Barcode of Life Data System. Mol. Ecol. Notes 7: 355–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recht, J., Siqueira A. M., Monteiro W. M., Herrera S. M., Herrera S., and Lacerda M. V. G.. 2017. Malaria in Brazil, Colombia, Peru and Venezuela: current challenges in malaria control and elimination. Malar. J. 16: 273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas-Aguirre, A., Gamboa D., Manrique P., Conn J. E., Moreno M., Lescano A. G., Sanchez J. F., Rodriguez H., Silva H., Llanos-Cuentas A., et al. 2016. Epidemiology of Plasmodium vivax Malaria in Peru. Am. J. Trop. Med. Hyg. 95: 133–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas-Aguirre, A., Guzman-Guzman M., Chuquiyauri R., Moreno M., Manrique P., Ramirez R., Carrasco-Escobar G., Rodriguez H., Speybroeck N., Conn J. E., et al. 2020. Temporal and micro-spatial heterogeneity in transmission dynamics of co-endemic Plasmodium vivax and Plasmodium falciparum in two rural cohort populations in the Peruvian Amazon. J. Infect. Dis. doi: 10.1093/infdis/jiaa526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio-Palis, Y. 2000. Anopheles (Nyssorhynchus) de Venezuela. Taxonomía, bionomía, ecología e importancia médica., pp. 120. Publicaciones de la Dirrecion de Malariologia, Maracay, Venezuela. [Google Scholar]
- Ruiz, F., Quiñones M. L., Erazo H. F., Calle D. A., Alzate J. F., and Linton Y. M.. 2005. Molecular differentiation of Anopheles (Nyssorhynchus) benarrochi and An. (N.) oswaldoi from southern Colombia. Mem. Inst. Oswaldo Cruz 100: 155–160. [DOI] [PubMed] [Google Scholar]
- Ruiz-Lopez, F., Wilkerson R. C., Ponsonby D. J., Herrera M., Sallum M. A., Velez I. D., Quiñones M. L., Flores-Mendoza C., Chadee D. D., Alarcon J., et al. 2013. Systematics of the oswaldoi complex (Anopheles, Nyssorhynchus) in South America. Parasit. Vectors 6: 324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saavedra, M. P., Conn J. E., Alava F., Carrasco-Escobar G., Prussing C., Bickersmith S. A., Sangama J. L., Fernandez-Miñope C., Guzman M., Tong C., et al. 2019. Higher risk of malaria transmission outdoors than indoors by Nyssorhynchus darlingi in riverine communities in the Peruvian Amazon. Parasit. Vectors 12: 374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoeler, G. B., Flores-Mendoza C., Fernández R., Davila J. R., and Zyzak M.. 2003. Geographical distribution of Anopheles darlingi in the Amazon Basin region of Peru. J. Am. Mosq. Control Assoc. 19: 286–296. [PubMed] [Google Scholar]
- Sinka, M. E., Bangs M. J., Manguin S., Rubio-Palis Y., Chareonviriyaphap T., Coetzee M., Mbogo C. M., Hemingway J., Patil A. P., Temperley W. H., et al. 2012. A global map of dominant malaria vectors. Parasit. Vectors 5: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto-Calle, V., Rosas-Aguirre A., Llanos-Cuentas A., Abatih E., DeDeken R., Rodriguez H., Rosanas-Urgell A., Gamboa D., D Alessandro U., Erhart A., et al. 2017. Spatio-temporal analysis of malaria incidence in the Peruvian Amazon Region between 2002 and 2013. Sci. Rep. 7: 40350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . 2018. World malaria report 2018, pp. 210. WHO, Geneva, Switzerland. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.