Abstract
Survivors of head and neck squamous cell cancers (HNSCC) frequently complain of taste dysfunction long after radiation therapy is completed, which contradicts findings from most sensory evaluation studies that predict dysfunction should resolve few months after treatment. Therefore, it remains unclear whether taste and smell function fully recovers in HNSCC survivors. We evaluated HNSCC survivors (n = 40; age 63 ± 12 years, mean ± standard deviation) who received radiation therapy between 6 months and 10 years before recruitment and compared their responses to those of a healthy control group (n = 20) equivalent in age, sex, race, smoking history, and body mass index. We assessed regional (tongue tip) and whole-mouth taste intensity perception using the general Labeled Magnitude Scale and smell function using the University of Pennsylvania Smell Identification Test (UPSIT). To determine possible differences between groups in retronasal smell perception, we used solutions of sucrose with strawberry extract, citric acid with lemon extract, sodium chloride in vegetable broth, and caffeine in coffee and asked participants to rate perceived smell and taste intensities with and without nose clips. We found groups had similar UPSIT and taste intensity scores when solutions were experienced in the whole mouth. However, HNSCC survivors were less likely to identify low concentrations of bitter, sweet, or salty stimuli in the tongue tip relative to healthy controls. Our findings suggest persistent and subtle localized damage to the chorda tympani or to the taste buds in the fungiform papillae of HNSCC survivors, which could explain their sensory complaints long after completion of radiotherapy.
Keywords: cancer, dysgeusia, hypogeusia, oncology, radiation, UPSIT
Introduction
Head and neck squamous cell cancers (HNSCC), which comprise primarily tumors of the oral cavity, pharynx, and larynx, are the seventh most prevalent form of cancer worldwide (World Cancer Research Fund and American Institute for Cancer Research 2018). Traditionally, these cancers were associated with alcohol and tobacco use, but currently a large proportion of these cancers, particularly in the oropharynx, are driven by exposure to the human papilloma virus (HPV) (Marur et al. 2010; Chaturvedi et al. 2013). The prognosis of HPV-related HNSCC following treatment is excellent, as these patients have notably higher survival rates than non-HPV-related cancers (O’Rorke et al. 2012; Tahtali et al. 2013). In addition, technological improvements in radiation therapies over the last decade, which use modulated intensity radiation and can more accurately target cancerous tissue and spare healthy tissue, have also increased survival rate (Chen et al. 2019). Therefore, the pool of long-term HNSCC survivors is increasing (Miller et al. 2019), and attention to long-term treatment side effects is critical.
There are different treatment plans for HNSCC, for example, some patients are treated with surgery or radiation alone, but often times, the disease requires a combination of therapies, such as chemoradiation. One of the most common side effects reported by patients after being treated for head and neck cancer (HNC) with these treatments is taste dysfunction (McLaughlin 2013, 2014). Although frequently underappreciated by medical practitioners (McLaughlin and Mahon 2014), taste dysfunction has a significant negative impact in patients’ quality of life (Epstein et al. 2001; Baharvand et al. 2013; Alvarez-Camacho et al. 2016), nutritional status and health (McQuestion et al. 2011; Ganzer et al. 2015; Jin et al. 2018). In recent qualitative studies that included a subgroup of the HNSCC survivors who participated in this current study, we found that 94% (29 of 31) reported having taste dysfunctions that were described as being “frustrating,” “troublesome,” and “aggravating” and stated they no longer enjoyed eating (Crowder et al. 2020). For many HNSCC survivors, eating was considered “boring” and an activity they did “to survive” because food was enjoyed “not near” like it used to be (Crowder et al. 2021).
While it is clear that radiation therapy for HNC induces taste dysfunction during treatment and for a few weeks after (Mossman and Henkin 1978; Just et al. 2005; Mirza et al. 2008), there is controversy over when and to what extent taste dysfunction recovers after treatment. More important, most studies suggest gustatory ability recovers within a few months of treatment (Tomita and Osaki 1990; Yamashita et al. 2006, see Supplementary Table 1), but this does not match reports from HNC survivors, who continue to experience taste dysfunction 6 or more months after treatment completion (McLaughlin and Mahon 2014).
Remarkably, most of the studies that assessed taste function in these patients did so by measuring taste thresholds or by using magnitude matching procedures (Supplementary Table 1). Unfortunately, results from these studies are limited, in part, because threshold sensitivity measures typically do not correlate with above-threshold sensory function (Bartoshuk 1978; Pepino et al. 2010). An extreme example of this is illustrated by sensory data from a 52-year-old woman who underwent radiation therapy for cancer of the neck (Bartoshuk 1978). Although data from her recognition thresholds suggested an initial acute impairment of taste perception that was followed by recovery at about 60 days (similar to findings from other studies: Maes et al. 2002; Yamashita et al. 2006; Kamprad et al. 2008), data from her above-threshold sensory function showed significant hypogeusia up to 173 days after treatment (Bartoshuk 1978). Notably, the patient’s description of her taste world matched well with her scaling data: she indicated that by 173 days after treatment foods still did not taste as strong as they used to (Bartoshuk 1978).
The second method, magnitude matching, is also limited in its ability to infer differences in taste perception due to an intervention, because magnitude estimates express individual perceptions of different stimuli in terms of ratios, not absolute perceived intensities (Bartoshuk 1978). It is possible that after treatment the relationship between ratios remains constant yet patients perceive all taste solutions as significantly less intense than before radiation treatment.
Another confounding factor is that studies measuring taste intensity perception did so using whole-mouth procedures. This approach may not identify regional impairments in taste perception, because reduced signal from the anterior tongue, via the chorda-tympani nerve, can release central inhibition of the glossopharyngeal nerve, resulting in a net recovery of overall taste function when the whole mouth is assessed (Bartoshuk et al. 2012). However, the regional application of taste at the tip of the tongue isolates taste perception that is carried by the chorda-tympani nerve division of the facial nerve and therefore allows detection of specific nerve damage. Thus, taste dysfunction in HNC patients may be more evident when using regional procedures than when using whole-mouth procedures.
Another potential explanation for the lack of agreement between patients’ self-reported taste function and function measured using sensory evaluation techniques is that patients may misidentify olfactory dysfunction as taste dysfunction (Rozin 1982; Stevenson et al. 1999; Spence 2015). Studies have shown that olfactory dysfunction also occur in HNC patients after radiotherapy (Alvarez-Camacho et al. 2017) (Supplementary Table 2). Although smell and taste are separate senses, they both integrate and interact in the brain to provide us with the perception of flavor (Rozin 1982). The intimate entwining of these 2 chemicals senses, combined with the erroneous use of “flavor” as a synonym of “taste,” may contribute to confusion between symptoms arising from olfactory dysfunction and those caused by taste dysfunction. However, few normative studies have assessed both taste and smell function in the same HNC patient (Sandow et al. 2006; Riva et al. 2015).
The primary goal of the present study was to test the hypothesis that radiation therapy for the treatment of HNSCC is associated with long-term (or chronic) alterations of taste and smell function. We evaluated both taste and smell function in HNSCC survivors who received radiation therapy between 6 months and 10 years before recruitment, as well as a healthy control (HC) group equivalent in age, sex race, smoking history, and weight who had no history of cancer. We used validated psychophysical tests that included regional and whole-mouth procedures for the assessment of taste perception (Coldwell et al. 2013), as well as measures of retronasal smell intensity and smell identification.
Materials and methods
Participants and study design
The HNSCC survivor group comprised cancer-free participants who were previously diagnosed with squamous cell carcinoma in the areas of the oral cavity, pharynx (oropharynx, nasopharynx, and hypopharynx), and larynx and completed their radiation therapy (alone or combined with surgery or chemotherapy) at least 6 months but less than 10 years before enrolling in the study. Survivors who received radiation therapy ≥10 years before the time of recruitment were excluded because of the profound changes in radiation treatments over the last decade (van der Laan et al. 2012; Christianen et al. 2015). In addition, we recruited a HC group equivalent to the HNSCC group in age, sex, race, smoking history, and body mass index. We identified HNSCC participants via the Carle Foundation Hospital Cancer Registry and HC participants via flyers and newsletter emails posted at the University of Illinois at Urbana-Champaign campus. All participants completed the same taste and smell psychophysical assessments in 1 study visit, which lasted approximately 2 h. The Human Research Protection Office at Carle Foundation Hospital and the University of Illinois at Urbana-Champaign Institutional Review Board approved the study protocol, and all included participants gave informed written consent before initiating study procedures, according to the Declaration of Helsinki.
Taste assessment procedure
We assessed both taste quality identification and taste intensity ratings via both regional and whole-mouth taste presentation methods, as recommended in the NIH Toolbox for Assessment of Neurological and Behavioral Function (Coldwell et al. 2013). For the regional presentation session, a cotton swab soaked with stimulus was applied in a semicircular motion around the tip of the tongue. For the whole-mouth presentation session, participants were asked to swish the taste sample in their mouth for approximately 5 s before expectorating it. The whole-mouth session was assessed twice: once without a nose clip and once with a nose clip, which precludes both orthonasal and retronasal smell stimulation. The order of presence/absence of the nose clip was randomized among participants. After tasting, participants identified the taste quality by selecting from a list containing the following options: sweet, salty, sour, bitter, umami, and no sensation.
Participants then rated the strength of the perceived taste intensity of the solution (as well as perceived smell intensity; see below) using a generalized Labeled Magnitude Scale (gLMS) (Green et al. 1996; Bartoshuk et al. 2004). The gLMS is a measure of perceived intensity with 7 anchor labels: Strongest of any kind, Very strong, Strong, Moderate, Weak, Barely detectable, No sensation). Prior to participants using the gLMS scale, we trained them on the use of the scale by providing different examples. To assess whether they understood how to use the scale, we asked them to rate remembered intensities of different sources of light: the intensity of light in a candle-lit restaurant, light in a well-lit room, and the strongest/brightest light they have ever seen.
During the testing sessions, participants were asked to complete their ratings in the gLMS immediately after sample expectoration. They were also instructed to rinse their mouth twice with deionized water and to wait 30 s before tasting the next sample. In total, each participant completed 3 tasting sessions (i.e., tip of the tongue, whole mouth wearing nose clip, and whole mouth without nose clip), each with 9 stimuli. All psychophysical data were collected using Academic Consortium, Compusense Cloud (Compusense Inc., Guelph, Canada).
Sensory stimuli
We used ~10 mL of liquid stimuli that contained both taste and smell components. We used 2 concentrations each of sucrose (Domino Sugar; 180 mM, 700 mM) with 0.78 g strawberry extract (Watson’s), sodium chloride (Morton Salt; 100 mM, 320 mM) in a vegetable broth (Kitchen Basics Unsalted Vegetable Broth), citric acid (Purisure; 3.2 mM, 32 mM) with 0.78 g lemon extract (Watson’s), caffeine in caffeinated instant coffee (Nescafe Taster’s Choice House Blend; 1 mM, prepared with 0.59 g instant coffee; and 10 mM, prepared with 5.97 g instant coffee), and deionized water as a blank. All 9 stimuli were placed in small clear medicine cups, with order of presentation randomized, except for the highest caffeine concentration, which was always presented at the end of each testing round. All taste stimuli were presented at room temperature.
Smell assessment
Participants completed the University of Pennsylvania Smell Identification Test (UPSIT) (Doty et al. 1984), which contains 40 “scratch and sniff” boxes with microencapsulated common odorants. Participants scratched the box with a pencil to release the odorant, sniffed the stimulus, and then indicated the text descriptor that best matched the perceived smell. Standardized UPSIT scores from normative data in published literature (Doty 1995) adjust the score for age and sex of the participant and define the participant’s corresponding percentile, which determines smell identification function. In addition, we assessed judgments of smell intensity during the tasting test (while participants were tasting cups with and without nose clips) using the gLMS.
Statistical analyses
For analyses of taste quality identification data, participants’ responses were stratified as “expected,” “unexpected,” or “no sensation”. Expected identification included selecting mainly “sweet” for solutions of sucrose, “sour/bitter” for solutions of citric acid, “salty/umami” for solutions of sodium chloride in vegetable broth, and “bitter/sour” for solutions of coffee. We accepted more than 1 taste quality as the expected response for some of the stimuli because people frequently confuse bitter with sour (Meiselman and Dzendolet 1967), and the vegetable broth used to prepare the salty solutions contained vegetables that have a weak umami taste.
To detect frequency differences in responses between the HNSCC and HC groups, we used omnibus chi-square analyses for 3 independent samples (expected, unexpected, no sensation) for each solution and testing condition (tip of the tongue, whole mouth with nose clip, whole mouth without nose clip), followed by Fisher’s Exact tests to determine where the difference occurred (Shan and Gerstenberger 2017). In cases where a chi-square test was not appropriate (no observations in a particular cell, or the number of observations in 20% of the cells was less than 5), we used the Freeman–Halton extension of the Fisher’s Exact test for a 2 × 3 contingency instead of the chi-square test (http://vassarstats.net/fisher2x3.html).
Taste intensity data in the tip of the tongue and smell intensity (when samples were tasted in the whole mouth without wearing nose clip) were analyzed by separate mixed 2-way analyses of variance (ANOVAs) for each taste stimulus, using group (HNSCC vs. HC) as the between-subjects factor and concentration (low vs. high) as the within-subject factor. We considered and selected the highest rated quality between sour and bitter for solutions containing caffeine or citric acid, salty, and umami for solutions containing sodium chloride and sweet for solutions containing sucrose. For taste intensity data in the whole mouth, we used separate mixed 3-way ANOVAs for each taste stimulus using group (HNSCC vs. HC) as the between-subjects factor and nose clip condition (with and without nose clip) and concentration (low vs. high) as the within-subject factors. Finally, a 1-way ANOVA was used to determine whether groups (HNSCC vs. HC) differed in their ability to identify odorants (UPSIT scores). All parametric analyses were performed in Statistica v.13.3 (TIBCO Software Inc.), and the criterion for significance in all analyses was set at α = 0.05.
Results
Participant characteristics
We evaluated 40 HNSCC survivors (mean age, 63 ± 12 years, 24 males) and 20 HC participants who were equivalent in age, sex, race, smoking history, and body mass index (58 ± 14 years, 11 males; Table 1). The mean time from treatment to testing for the HNSCC group was 3 ± 2 years (range: 0.5–8.4 years). This mean time from treatment was calculated from 35 of 40 HNSCC survivors because, although all survivors received treatment within the range used for inclusion in the study, we did not have the exact date of treatment for 5 of them. All HNSCC received radiation therapy and 60% received concurrent chemotherapy (Table 1).
Table 1.
Characteristics of participants by study group
| Characteristic | HC group (n = 20) | HNSCC group (n = 40) | P value |
|---|---|---|---|
| Age, years (mean [SD]) | 58 (14) | 63 (12) | 0.13 |
| Body mass index, kg/m2 (mean [SD]) | 27 (6) | 26 (5) | 0.37 |
| Sex (n [%]) | 0.71 | ||
| Male | 11 (55) | 24 (60) | |
| Female | 9 (45) | 16 (40) | |
| Race (n [%]) | 0.74 | ||
| White | 18 (90) | 37 (92.5) | |
| Other | 2 (10) | 3 (7.5) | |
| Smoking status (n [%]) | 0.85 | ||
| Current | 1 (5) | 2 (5) | |
| Former | 10 (50) | 23 (57.5) | |
| Never | 9 (45) | 15 (37.5) | |
| Chemotherapy (n [%]) | |||
| Yes | 24 (60) | ||
| No | 16 (40) | ||
| Primary tumor site (n [%]) | |||
| Oral cavity | 19 (47.5) | ||
| Pharynx | 18 (45) | ||
| Larynx | 3 (7.5) | ||
| Cancer stage (n [%]) | |||
| I | 2 (5) | ||
| II | 10 (25) | ||
| III | 3 (7.5) | ||
| IV | 25 (62.5) |
Taste quality identification
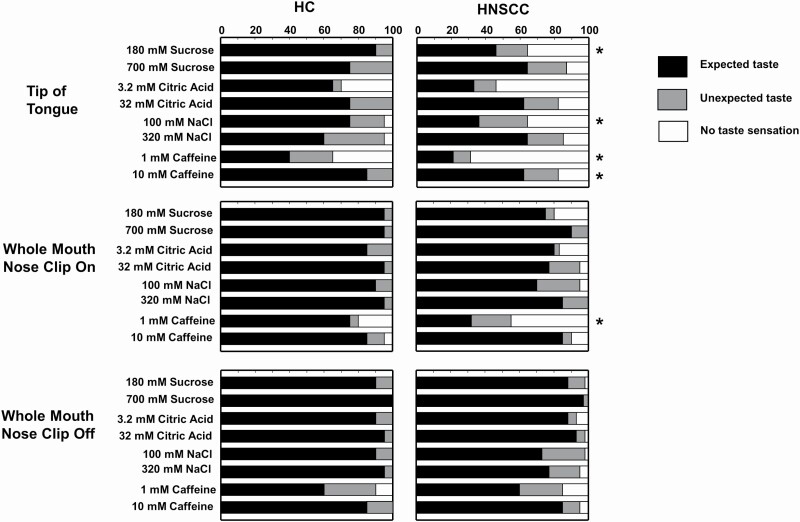
When taste perception was assessed at the tip of the tongue, compared with the HC group the HNSCC survivors were more likely to respond “no sensation” or to misidentify the taste quality for several stimuli (low sucrose and low sodium chloride: both: P < 0.01 low and high caffeine: both P < 0.05; Figure 1). However, when participants were evaluated using the whole-mouth procedure, only 1 difference was observed between the groups: HNSCC survivors were more likely to misidentify or perceive no taste sensation from the low caffeine solution when using the nose clip (i.e., deprived of retronasal smell; P < 0.01). When taste was assessed using the whole mouth without a nose clip, there were no significant differences between groups in the identification of taste qualities of any of the solutions (P > 0.05; Figure 1). Data on taste quality identification at the tip of the tongue for 1 participant with HNSCC were not available due to technical difficulties when performing the regional test, due to tongue anatomic consequences of the treatment.
Figure 1.
Effect of group on taste identification of different solutions by testing condition. The cumulative percentage of participants in the HC group and in the HNSCC group who reported perceiving no taste sensation (white bars), a taste quality that was different than the 1 expected for the stimulus presented (unexpected taste; gray bars), or the expected taste quality (black bars) when sampling each of the solutions on the tip of the tongue (top row), in the whole mouth with nose clip on (middle row), or in the whole mouth with nose clip off (bottom row). *Significantly different from HC by χ 2 or 2 × 3 Freeman–Halton extension of the Fisher’s Exact test; P < 0.05. NaCl, sodium chloride. Note: sucrose solutions contained 0.78 g strawberry extract, and citric acid solutions, 0.78 g lemon extract; NaCl solutions were prepared in a vegetable broth, and caffeine solutions were prepared from instant coffee.
Taste intensity ratings
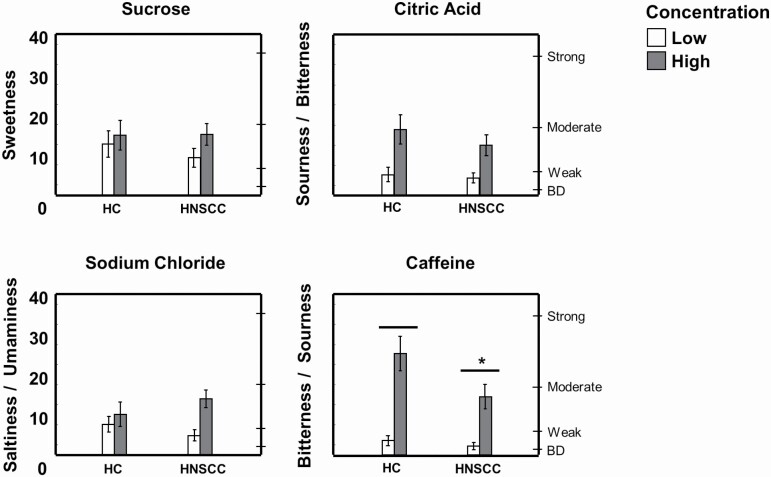
Compared with the HC group, the HNSCC group perceived less intensity from the caffeine solutions when assessed at the tip of the tongue (F(1,57) = 4.79; P = 0.03). However, there were no other statistically significant differences between groups in their intensity ratings for the other solutions, either regionally (Figure 2) or in the whole mouth (Supplementary Figure 1). Ratings of expected responses increased with increasing concentrations of sucrose, citric acid, and caffeine similarly in both groups (main effect of concentration for all stimuli, P < 0.02).
Figure 2.
Regional taste intensity perception in HNSCC and HC groups. Taste intensity was measured using the general Labeled Magnitude Scale after application of low (180 mM sucrose, 3.2 mM citric acid, 100 mM sodium chloride, or 1 mM caffeine; white bars) or high (700 mM sucrose, 32 mM citric acid, 320 mM sodium chloride, or 10 mM caffeine; gray bars) concentrations of tastants on the tip of the tongue. *Significantly different from HC by 2-way ANOVA, main effect of group; P < 0.05; there was also a main effect of concentration (low significantly different than high concentrations for all stimuli); P < 0.05. Data are mean values ± SEM. BD, barely detectable.
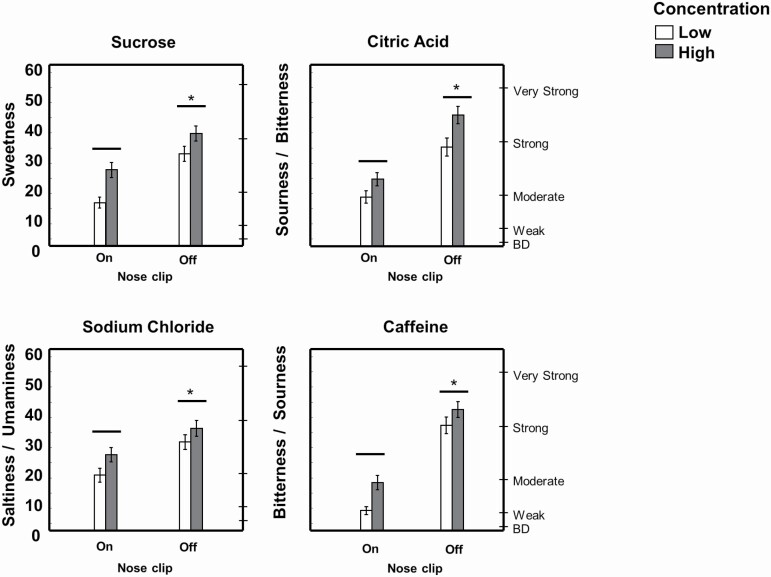
Perception of sweetness of sucrose solutions, sourness/bitterness of citric acid solutions, saltiness/umaminess of NaCl solutions, and bitterness/sourness of caffeine solutions was similarly enhanced in both groups when solutions were tasted without a nose clip (main effect of nose clip condition for all stimuli, P < 0.005; Figure 3). There were no significant group × concentration, group × nose clip, or group × concentration × nose clip interactions in taste intensity ratings.
Figure 3.
Retronasal enhancement of taste intensity (both groups combined). Taste intensity was measured using the general Labeled Magnitude Scale after participants savored samples in their whole mouth under 2 testing conditions: with nose clip on, blocking retronasal smell, or nose clip off. *Significantly different from nose clip on by 3-way ANOVA, main effect of nose clip condition; P < 0.05; there was also a main effect of concentration (low significantly different than high concentrations for all stimuli); P < 0.05. Data are mean values ± SEM. BD, barely detectable. Note: sucrose solutions contained 0.78 g strawberry extract, and citric acid solutions, 0.78 g lemon extract; NaCl solutions were prepared in a vegetable broth, and caffeine solutions were prepared from instant coffee.
Smell intensity
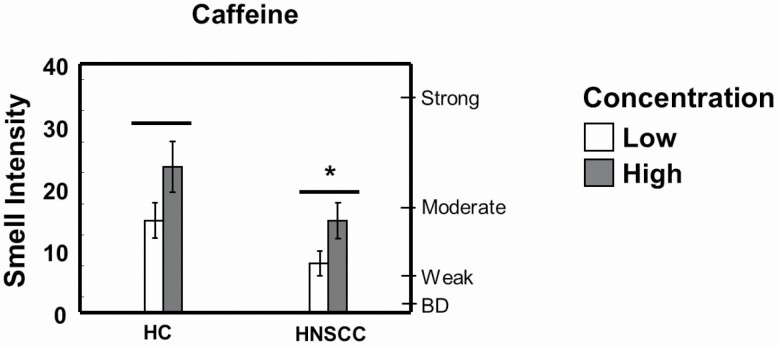
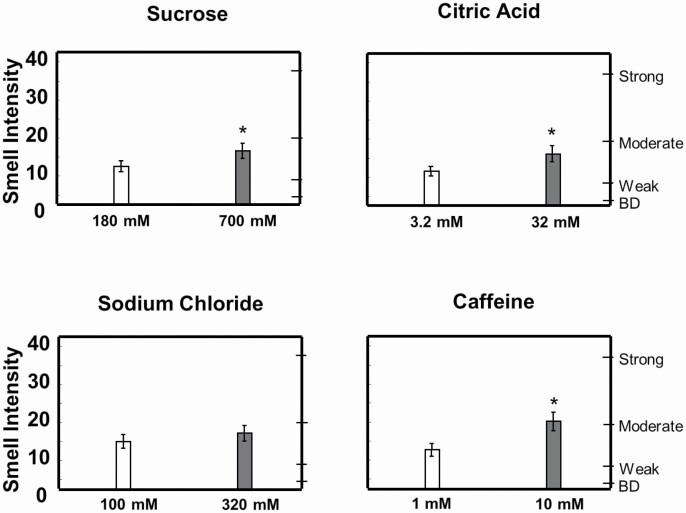
Ratings of smell intensity when tasting caffeine solutions were lower for the HNSCC group than for the HC group (F(1,58) = 4.02; P = 0.05; Figure 4). There were no other statistically significant differences between the groups in their ratings of smell intensity for the other solutions (data not shown). Ratings of smell intensity increased with increasing concentration for sucrose, citric acid, and caffeine solutions similarly in both groups (Figure 5).
Figure 4.
Reduced intensity of coffee smell in HNSCC survivors compared with HCs. Smell intensity was measured using the general Labeled Magnitude Scale. *Significantly different from HC by 2-way ANOVA, main effect of group; P < 0.05; there was also a main effect of concentration; P < 0.05. Data are mean values ± SEM. BD, barely detectable.
Figure 5.
Tastants enhance retronasal smell intensity. Smell intensity was measured using the general Labeled Magnitude Scale after participants (both groups combined) savored samples in their whole mouth with nose clips off. *Significantly different from low concentration by 2-way ANOVA, main effect of concentration; P < 0.05. Data are mean values ± SEM. BD, barely detectable. Note: both sucrose solutions contained 0.78 g strawberry extract; both citric acid solutions, 0.78 g lemon extract; NaCl solutions were prepared in the same vegetable broth, and caffeine solutions were prepared from 2 doses of the same instant coffee.
Smell identification
UPSIT mean scores did not differ significantly between HNSCC and HC groups (HNSCC = 32.5 ± 0.8, HC = 34.6 ± 1.17; F(1,58) = 2.001, P = 0.16). Additionally, when adjusting UPSIT scores for normative sex and age data, there was no significant difference in the percentage of participants within each group who were classified as normosmic (HNC = 62.5%, HC = 80%; P = 0.24).
Discussion
The results of our cross-sectional study suggest that these HNSCC survivors experienced subtle differences in taste perception in the long term compared with the HC group. Although results from the taste test that involved stimulation of the whole mouth suggested that taste function was overall normal and well preserved which is in agreement with previous studies that solely used whole-mouth procedures, results from the regional test uncovered some taste deficits in HNSCC survivors. Relative to a healthy peer group, which was equivalent in age, sex, race, body mass index, and smoking history, HNSCC survivors were less likely to perceive a taste from and less likely to identify low concentrations of bitter, sweet, and salty stimuli in the anterior tongue. Differences in the sense of smell between groups were almost negligible and limited to a weaker perception of the smell of coffee in HNSCC survivors.
Declines in taste function in HNSCC survivors can result from damage at any step of the gustatory pathway: the taste buds, the peripheral gustatory nerves, or the central nervous system (Bartoshuk et al. 2012; Barlow and Klein 2015; Yang et al. 2015; Chen et al. 2019). For example, the fast turnover of taste bud cells (~10 days), which allows rapid recovery from injury (if stem cells are preserved), makes taste buds vulnerable to effects of chemotherapy (Kumari et al. 2015; Castillo-Azofeifa et al. 2017; Mukherjee et al. 2017) and radiotherapy (Nguyen et al. 2012; Gaillard et al. 2019). These treatment modalities, via direct cytotoxic and antiproliferative effects, cause a loss of taste buds, which is associated with transient reduced taste (hypogeusia to ageusia) or altered taste (dysgeusia) (Comeau et al. 2001). Dysgeusia may also occur after HNC surgery, due to injury to the gustatory nerves (facial, glossopharyngeal, and/or vagal) that innervate taste buds (Bartoshuk et al. 2012). In addition, hyposalivation, another common side effect of radiotherapy, can contribute to impairments in taste perception, as taste stimuli need to be dissolved in saliva to interact with taste receptor cells (Kuten et al. 1986). Finally, mucositis-induced epithelial changes in the mouth can also affect taste perception, as high levels of inflammatory cytokines can induce taste cell death and inhibit taste bud cell renewal (Feng et al. 2014).
Our findings of a deficit in taste identification in the anterior tongue but normal taste function when stimulating the whole mouth suggest localized damage to the chorda tympani or to the taste buds in the fungiform papillae and underscore the importance of assessing taste function using both regional and whole-mouth taste presentation methods. It has been shown that reduced signal input from the anterior tongue can release central inhibition of the glossopharyngeal nerve, resulting in a net recovery of overall taste function when the whole mouth is assessed (Bartoshuk et al. 2012). However, these regional taste dysfunction, which would go unnoticed when evaluating taste function using whole-mouth procedures, can lead to oral phantoms (Logan et al. 2008), burning mouth syndrome (Coculescu et al. 2014), or the phenomenon of metallic taste (Reith and Spence 2020), which might underlie taste complaints reported by HNC survivors. We did not survey or assess participants to evaluate whether any of these taste phenomena were present, but future studies in this area are warranted.
Except for a reduced perception in the intensity of the smell of coffee solutions, smell function in HNSCC survivors was not different from the HC group either in UPSIT identification scores or in their judgment of smell intensity of aromas presented in solution. Previous studies that assessed smell function in HNC survivors found discrepant results (Supplementary Table 2). Depending on the characteristics of HNC survivors studied (e.g., with or without radiation to the nasopharynx) and the methods used to evaluate smell function (e.g., detection thresholds or smell identification), studies found none to some dysfunction. Our findings agree with those of a small study that also used UPSIT to determine smell function and found it to be unaffected (Sandow et al. 2006). However, most studies that used detection threshold methods found decreased smell sensitivity in HNC survivors (Supplementary Table 2), suggesting that some subtle effects on smell function might also persist.
An innovative aspect of our study is that, in addition to assessing taste and smell function separately, we explored whether sensory interactions between taste and retronasal odors differed between HNSCC survivors and the HC group. We found that HNSCC survivors experienced enhancement of a gustatory stimulus by the presence of aromatic extracts of similar magnitude as experienced by the HC group. Interestingly, in agreement with findings from previous studies in healthy participants (Green et al. 2012; Veldhuizen et al. 2018), we found that the concurrent presentation of smell and taste stimuli in the mouth resulted on mutual enhancements of perceived taste and smell intensity. That is, sucrose solutions were rated as sweeter, and citric acid solutions were rated as more sour/bitter, when not using the nose clip, allowing the aromatic extracts to be retronasally perceived, compared with when using nose clip, which blocks retronasal stimulation. Reciprocally, despite using the same amount of strawberry extract and lemon extract for the 2 concentrations of sucrose and citric acid, respectively, the smell of strawberry and lemon was perceived as stronger for the higher sucrose or citric acid concentration.
The findings of our study should be interpreted considering some strengths and limitations. Among its strengths, our study design included a HC group that was equivalent in many factors that can affect taste and smell function, such as sex, age, race, and smoking history. By matching study groups on these confounding variables, our findings place HNSCC survivors’ taste dysfunction into perspective relative to the function of their peers. Other strengths are the assessment of taste function using both regional and whole-mouth procedures, the assessment of taste perception in the presence and absence of retronasal stimulation (Feeney and Hayes 2014), and the inclusion of all basic tastes. Among its limitations, due to time constraints for the sensory study visit, we could include only 2 concentrations for each taste stimulus, and we did not assess smell detection thresholds. In addition, the HNSCC group consisted of a heterogeneous group who received several oncology treatments. Although all survivors received radiotherapy, we included a wide range of time since completion of the treatment and only some of them additionally received chemotherapy, which has specific effects on taste (Speck et al. 2013), smell (Yakirevitch et al. 2006; Riga et al. 2015), and cognition (Steinbach et al. 2011). Future studies with more homogeneous groups are needed to test specific oncology treatment effects.
In conclusion, we found that while smell function and taste function in the whole mouth seemed to be generally well preserved in HNSCC survivors, subtle taste dysfunction in the anterior tongue persisted for several months after patients completed their oncology treatment. Future studies should determine if the regional taste dysfunction is associated with other oral symptoms, such as the presence of a metallic taste, burning mouth syndrome, or taste phantoms, and how such alterations might impact HNC survivors’ eating behavior.
Supplementary material
Supplementary material can be found at Chemical Senses online.
Supplementary Table 1. Sensory studies of taste dysfunction in head and neck cancer (HNC) survivors.
Supplementary Table 2. Sensory studies of smell dysfunction in head and neck cancer (HNC) survivors.
Acknowledgments
The authors thank Alexander D. Nichol for helping with data collection, Patricia Watson for editing assistance, and the study subjects for their participation.
Funding
This work was supported by the United States Department of Agriculture, National Institute of Food and Agriculture Hatch projects [698-921 and 1011487]; the Academy of Nutrition and Dietetics Colgate-Palmolive Fellowship in Nutrition and Oral Health; and a Division of Nutritional Sciences Vision 20/20 Grant. S.C. was supported by NCI Cancer Prevention and Control Training Grant [5T32CA090314-17] and a Carle Illinois Cancer Scholars for Translational and Applied Research Fellowship. The funding sources had no role in the design of the study or the drafting and publication of the manuscript.
Conflict of interest
The authors report no conflict of interest.
References
- Alvarez-Camacho M, Gonella S, Campbell S, Scrimger RA, Wismer WV. 2017. A systematic review of smell alterations after radiotherapy for head and neck cancer. Cancer Treat Rev. 54:110–121. [DOI] [PubMed] [Google Scholar]
- Alvarez-Camacho M, Gonella S, Ghosh S, Kubrak C, Scrimger RA, Chu KP, Wismer WV. 2016. The impact of taste and smell alterations on quality of life in head and neck cancer patients. Qual Life Res. 25(6):1495–1504. [DOI] [PubMed] [Google Scholar]
- Baharvand M, ShoalehSaadi N, Barakian R, Moghaddam EJ. 2013. Taste alteration and impact on quality of life after head and neck radiotherapy. J Oral Pathol Med. 42(1):106–112. [DOI] [PubMed] [Google Scholar]
- Barlow LA, Klein OD. 2015. Developing and regenerating a sense of taste. Curr Top Dev Biol. 111:401–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoshuk LM. 1978. The psychophysics of taste. Am J Clin Nutr. 31(6):1068–1077. [DOI] [PubMed] [Google Scholar]
- Bartoshuk LM, Catalanotto F, Hoffman H, Logan H, Snyder DJ. 2012. Taste damage (otitis media, tonsillectomy and head and neck cancer), oral sensations and BMI. Physiol Behav. 107(4):516–526. [DOI] [PubMed] [Google Scholar]
- Bartoshuk LM, Duffy VB, Green BG, Hoffman HJ, Ko CW, Lucchina LA, Marks LE, Snyder DJ, Weiffenbach JM. 2004. Valid across-group comparisons with labeled scales: the gLMS versus magnitude matching. Physiol Behav. 82(1):109–114. [DOI] [PubMed] [Google Scholar]
- Castillo-Azofeifa D, Losacco JT, Salcedo E, Golden EJ, Finger TE, Barlow LA. 2017. Sonic hedgehog from both nerves and epithelium is a key trophic factor for taste bud maintenance. Development. 144(17):3054–3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi AK, Anderson WF, Lortet-Tieulent J, Curado MP, Ferlay J, Franceschi S, Rosenberg PS, Bray F, Gillison ML. 2013. Worldwide trends in incidence rates for oral cavity and oropharyngeal cancers. J Clin Oncol. 31(36):4550–4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WC, Tsai MS, Tsai YT, Lai CH, Lee CP, Chen MF. 2019. Long-term taste impairment after intensity-modulated radiotherapy to treat head-and-neck cancer: correlations with glossectomy and the mean radiation dose to the oral cavity. Chem Senses. 44(5):319–326. [DOI] [PubMed] [Google Scholar]
- Christianen ME, Verdonck-de Leeuw IM, Doornaert P, Chouvalova O, Steenbakkers RJ, Koken PW, Leemans CR, Oosting SF, Roodenburg JL, van der Laan BF, et al. 2015. Patterns of long-term swallowing dysfunction after definitive radiotherapy or chemoradiation. Radiother Oncol. 117(1):139–144. [DOI] [PubMed] [Google Scholar]
- Coculescu EC, Tovaru S, Coculescu BI. 2014. Epidemiological and etiological aspects of burning mouth syndrome. J Med Life. 7(3):305–309. [PMC free article] [PubMed] [Google Scholar]
- Coldwell SE, Mennella JA, Duffy VB, Pelchat ML, Griffith JW, Smutzer G, Cowart BJ, Breslin PA, Bartoshuk LM, Hastings L, et al. 2013. Gustation assessment using the NIH Toolbox. Neurology. 80:S20–S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comeau TB, Epstein JB, Migas C. 2001. Taste and smell dysfunction in patients receiving chemotherapy: a review of current knowledge. Support Care Cancer. 9(8):575–580. [DOI] [PubMed] [Google Scholar]
- Crowder SL, Najam N, Sarma KP, Fiese BH, Arthur AE. 2020. Head and neck cancer survivors’ experiences with chronic nutrition impact symptom burden after radiation: a qualitative study. J Acad Nutr Diet. 120(10):1643–1653. [DOI] [PubMed] [Google Scholar]
- Crowder SL, Najam N, Sarma KP, Fiese BH, Arthur AE. 2021. Quality of life, coping strategies, and supportive care needs in head and neck cancer survivors: a qualitative study. Support Care Cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doty RL. 1995. The Smell Identification Test™ administration manual. Haddon Heights (NJ): Sensonics Inc. [Google Scholar]
- Doty RL, Shaman P, Dann M. 1984. Development of the University of Pennsylvania Smell Identification Test: a standardized microencapsulated test of olfactory function. Physiol Behav. 32(3):489–502. [DOI] [PubMed] [Google Scholar]
- Epstein JB, Robertson M, Emerton S, Phillips N, Stevenson-Moore P. 2001. Quality of life and oral function in patients treated with radiation therapy for head and neck cancer. Head Neck. 23(5):389–398. [DOI] [PubMed] [Google Scholar]
- Feeney EL, Hayes JE. 2014. Regional differences in suprathreshold intensity for bitter and umami stimuli. Chemosens Percept. 7(3–4):147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng P, Huang L, Wang H. 2014. Taste bud homeostasis in health, disease, and aging. Chem Senses. 39(1):3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard D, Shechtman LA, Millar SE, Barlow LA. 2019. Fractionated head and neck irradiation impacts taste progenitors, differentiated taste cells, and Wnt/β-catenin signaling in adult mice. Sci Rep. 9(1):17934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganzer H, Touger-Decker R, Byham-Gray L, Murphy BA, Epstein JB. 2015. The eating experience after treatment for head and neck cancer: a review of the literature. Oral Oncol. 51(7):634–642. [DOI] [PubMed] [Google Scholar]
- Green BG, Dalton P, Cowart B, Shaffer G, Rankin K, Higgins J. 1996. Evaluating the ‘Labeled Magnitude Scale’ for measuring sensations of taste and smell. Chem Senses. 21(3):323–334. [DOI] [PubMed] [Google Scholar]
- Green BG, Nachtigal D, Hammond S, Lim J. 2012. Enhancement of retronasal odors by taste. Chem Senses. 37(1):77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin S, Lu Q, Jin S, Zhang L, Cui H, Li H. 2018. Relationship between subjective taste alteration and weight loss in head and neck cancer patients treated with radiotherapy: a longitudinal study. Eur J Oncol Nurs. 37:43–50. [DOI] [PubMed] [Google Scholar]
- Just T, Pau HW, Bombor I, Guthoff RF, Fietkau R, Hummel T. 2005. Confocal microscopy of the peripheral gustatory system: comparison between healthy subjects and patients suffering from taste disorders during radiochemotherapy. Laryngoscope. 115(12):2178–2182. [DOI] [PubMed] [Google Scholar]
- Kamprad F, Ranft D, Weber A, Hildebrandt G. 2008. Functional changes of the gustatory organ caused by local radiation exposure during radiotherapy of the head-and-neck region. Strahlenther Onkol. 184(3):157–162. [DOI] [PubMed] [Google Scholar]
- Kumari A, Ermilov AN, Allen BL, Bradley RM, Dlugosz AA, Mistretta CM. 2015. Hedgehog pathway blockade with the cancer drug LDE225 disrupts taste organs and taste sensation. J Neurophysiol. 113(3):1034–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuten A, Ben-Aryeh H, Berdicevsky I, Ore L, Szargel R, Gutman D, Robinson E. 1986. Oral side effects of head and neck irradiation: correlation between clinical manifestations and laboratory data. Int J Radiat Oncol Biol Phys. 12(3):401–405. [DOI] [PubMed] [Google Scholar]
- Logan HL, Bartoshuk LM, Fillingim RB, Tomar SL, Mendenhall WM. 2008. Metallic taste phantom predicts oral pain among 5-year survivors of head and neck cancer. Pain. 140(2):323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes A, Huygh I, Weltens C, Vandevelde G, Delaere P, Evers G, Van den Bogaert W. 2002. De Gustibus: time scale of loss and recovery of tastes caused by radiotherapy. Radiother Oncol. 63(2):195–201. [DOI] [PubMed] [Google Scholar]
- Marur S, D’Souza G, Westra WH, Forastiere AA. 2010. HPV-associated head and neck cancer: a virus-related cancer epidemic. Lancet Oncol. 11(8):781–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin L. 2013. Taste dysfunction in head and neck cancer survivors. Oncol Nurs Forum. 40(1):E4–E13. [DOI] [PubMed] [Google Scholar]
- McLaughlin L. 2014. Taste dysfunction and eating behaviors in survivors of head and neck cancer treatment. Medsurg Nurs. 23(3):165–170, 184. [PubMed] [Google Scholar]
- McLaughlin L, Mahon S. 2014. A meta-analysis of the relationship among impaired taste and treatment, treatment type, and tumor site in head and neck cancer treatment survivors. Oncol Nurs Forum. 41(3):E194–E202. [DOI] [PubMed] [Google Scholar]
- McQuestion M, Fitch M, Howell D. 2011. The changed meaning of food: physical, social and emotional loss for patients having received radiation treatment for head and neck cancer. Eur J Oncol Nurs. 15(2):145–151. [DOI] [PubMed] [Google Scholar]
- Meiselman HL, Dzendolet E. 1967. Variability in gustatory quality identification. Percept Psychophys. 2:496–498. [Google Scholar]
- Miller KD, Nogueira L, Mariotto AB, Rowland JH, Yabroff KR, Alfano CM, Jemal A, Kramer JL, Siegel RL. 2019. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin. 69(5):363–385. [DOI] [PubMed] [Google Scholar]
- Mirza N, Machtay M, Devine PA, Troxel A, Abboud SK, Doty RL. 2008. Gustatory impairment in patients undergoing head and neck irradiation. Laryngoscope. 118(1):24–31. [DOI] [PubMed] [Google Scholar]
- Mossman KL, Henkin RI. 1978. Radiation-induced changes in taste acuity in cancer patients. Int J Radiat Oncol Biol Phys. 4(7–8):663–670. [DOI] [PubMed] [Google Scholar]
- Mukherjee N, Pal Choudhuri S, Delay RJ, Delay ER. 2017. Cellular mechanisms of cyclophosphamide-induced taste loss in mice. PLoS One. 12(9):e0185473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen HM, Reyland ME, Barlow LA. 2012. Mechanisms of taste bud cell loss after head and neck irradiation. J Neurosci. 32(10):3474–3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Rorke MA, Ellison MV, Murray LJ, Moran M, James J, Anderson LA. 2012. Human papillomavirus related head and neck cancer survival: a systematic review and meta-analysis. Oral Oncol. 48(12):1191–1201. [DOI] [PubMed] [Google Scholar]
- Pepino MY, Finkbeiner S, Beauchamp GK, Mennella JA. 2010. Obese women have lower monosodium glutamate taste sensitivity and prefer higher concentrations than do normal-weight women. Obesity. 18(5):959–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reith AJM, Spence C. 2020. The mystery of “metal mouth” in chemotherapy. Chem Senses. 45(2):73–84. [DOI] [PubMed] [Google Scholar]
- Riga M, Chelis L, Papazi T, Danielides V, Katotomichelakis M, Kakolyris S. 2015. Hyposmia: an underestimated and frequent adverse effect of chemotherapy. Support Care Cancer. 23(10):3053–3058. [DOI] [PubMed] [Google Scholar]
- Riva G, Raimondo L, Ravera M, Moretto F, Boita M, Potenza I, Rampino M, Ricardi U, Garzaro M. 2015. Late sensorial alterations in different radiotherapy techniques for nasopharyngeal cancer. Chem Senses. 40(4):285–292. [DOI] [PubMed] [Google Scholar]
- Rozin P. 1982. “Taste-smell confusions” and the duality of the olfactory sense. Percept Psychophys. 31(4):397–401. [DOI] [PubMed] [Google Scholar]
- Sandow PL, Hejrat-Yazdi M, Heft MW. 2006. Taste loss and recovery following radiation therapy. J Dent Res. 85(7):608–611. [DOI] [PubMed] [Google Scholar]
- Shan G, Gerstenberger S. 2017. Fisher’s exact approach for post hoc analysis of a chi-squared test. PLoS One. 12(12):e0188709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speck RM, DeMichele A, Farrar JT, Hennessy S, Mao JJ, Stineman MG, Barg FK. 2013. Taste alteration in breast cancer patients treated with taxane chemotherapy: experience, effect, and coping strategies. Support Care Cancer. 21(2):549–555. [DOI] [PubMed] [Google Scholar]
- Spence C. 2015. Just how much of what we taste derives from the sense of smell? Flavour. 4:1–10. [Google Scholar]
- Steinbach S, Proft F, Schulze-Koops H, Hundt W, Heinrich P, Schulz S, Gruenke M. 2011. Gustatory and olfactory function in rheumatoid arthritis. Scand J Rheumatol. 40(3):169–177. [DOI] [PubMed] [Google Scholar]
- Stevenson RJ, Prescott J, Boakes RA. 1999. Confusing tastes and smells: how odours can influence the perception of sweet and sour tastes. Chem Senses. 24(6):627–635. [DOI] [PubMed] [Google Scholar]
- Tahtali A, Hey C, Geissler C, Filman N, Diensthuber M, Leinung M, Stöver T, Wagenblast J. 2013. HPV status and overall survival of patients with oropharyngeal squamous cell carcinoma—a retrospective study of a German head and neck cancer center. Anticancer Res. 33(8):3481–3485. [PubMed] [Google Scholar]
- Tomita Y, Osaki T. 1990. Gustatory impairment and salivary gland pathophysiology in relation to oral cancer treatment. Int J Oral Maxillofac Surg. 19(5):299–304. [DOI] [PubMed] [Google Scholar]
- van der Laan HP, Christianen ME, Bijl HP, Schilstra C, Langendijk JA. 2012. The potential benefit of swallowing sparing intensity modulated radiotherapy to reduce swallowing dysfunction: an in silico planning comparative study. Radiother Oncol. 103(1):76–81. [DOI] [PubMed] [Google Scholar]
- Veldhuizen MG, Siddique A, Rosenthal S, Marks LE. 2018. Interactions of lemon, sucrose and citric acid in enhancing citrus, sweet and sour flavors. Chem Senses. 43(1):17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Cancer Research Fund, American Institute for Cancer Research . 2018. Diet, nutrition, physical activity and cancers of the mouth, pharynx, and larynx. Continuous Update Project Expert Report 2018. 10–11. Available at dietandcancerreport.org. [Google Scholar]
- Yakirevitch A, Bercovici M, Migirov L, Adunsky A, Pfeffer MR, Kronenberg J, Talmi YP. 2006. Olfactory function in oncologic hospice patients. J Palliat Med. 9(1):57–60. [DOI] [PubMed] [Google Scholar]
- Yamashita H, Nakagawa K, Tago M, Nakamura N, Shiraishi K, Eda M, Nakata H, Nagamatsu N, Yokoyama R, Onimura M, et al. 2006. Taste dysfunction in patients receiving radiotherapy. Head Neck. 28(6):508–516. [DOI] [PubMed] [Google Scholar]
- Yang H, Cong W, Yoon J, Egan J. 2015. Vismodegib, an antagonist of hedgehog signaling, directly alters taste molecular signaling in taste buds. Cancer Med. 4(2):245–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.