ABSTRACT
Background
Overweight or obesity among pregnant women may compromise maternal and neonatal iron status by upregulating hepcidin.
Objectives
This study determined the association of 1) maternal and neonatal iron status with maternal and neonatal hepcidin concentrations, and 2) maternal prepregnancy weight status with maternal and neonatal hepcidin concentrations.
Methods
We examined hematologic data from 405 pregnant women and their infants from the placebo treatment group of a pregnancy iron supplementation trial in rural China. We measured hepcidin, serum ferritin (SF), soluble transferrin receptor (sTfR), and high-sensitivity C-reactive protein in maternal blood samples at mid-pregnancy and in cord blood at delivery. We used regression analysis to examine the association of maternal prepregnancy overweight or obese status with maternal hepcidin concentration in mid-pregnancy and cord hepcidin concentrations. We also used path analysis to examine mediation of the association of maternal prepregnancy overweight or obese status with maternal iron status by maternal hepcidin, as well as with neonatal hepcidin by neonatal iron status.
Results
Maternal iron status was positively correlated with maternal hepcidin at mid-pregnancy (SF: r = 0.63, P < 0.001; sTfR: r = −0.37, P < 0.001). Neonatal iron status was also positively correlated with cord hepcidin (SF: r = 0.61, P < 0.001; sTfR: r = −0.39, P < 0.001). In multiple linear regression models, maternal prepregnancy overweight or obese status was not associated with maternal hepcidin at mid-pregnancy but was associated with lower cord hepcidin (coefficient = −0.21, P = 0.004). Using path analysis, we observed a significant indirect effect of maternal prepregnancy overweight or obese status on cord hepcidin, mediated by neonatal iron status.
Conclusions
In both pregnant women and neonates, hepcidin was responsive to iron status. Maternal prepregnancy overweight status, with or without including obese women, was associated with lower cord blood hepcidin, likely driven by lower iron status among the neonates of these mothers.
Keywords: iron deficiency, hepcidin, obesity, China, pregnancy
See corresponding editorial on page 2087.
Introduction
Iron is essential for an infant's short- and long-term neurocognitive and socioemotional development (1). Infants generally meet their iron requirements in the first 6 mo of life from iron stores accumulated during gestation (2). If infants are iron deficient at birth, they will prioritize iron for hemoglobin (Hb) synthesis over the iron requirements of the brain and other tissues, putting them at risk of deficits in early brain development (1). Despite changes in maternal iron physiology that maximize iron absorption and placental iron transfer to the growing fetus, maternal iron deficiency can compromise neonatal iron stores (3, 4). Thus, the primary limiting factor in the amount of iron available to the fetus during gestation is maternal iron status.
Iron deficiency and iron deficiency anemia among women, particularly during pregnancy, are persistent global problems (5, 6). Regions of the world where the prevalence of undernutrition and micronutrient deficiencies are highest are also now facing a rising prevalence of overweight and obesity (7). In China, amidst rapid economic growth and changing dietary patterns (8), the prevalence of adult obesity tripled between 2004 and 2014 (9). The co-occurrence of nutrient deficiencies and obesity, termed the “double burden” of malnutrition, occurs not only at the population level, but also at the household and even the individual level, wherein a person can both be obese and have micronutrient deficiencies (10, 11). Several studies have observed lower iron status among overweight or obese nonpregnant (12, 13) and pregnant (14–18) women, although nearly all of the studies among pregnant women (15–17) compared only obese with lean subjects, excluding nonobese overweight women. Although the mechanism is still under investigation, evidence suggests that reduced iron absorption (19), rather than lower dietary iron intake (20), contributes to obesity-associated hypoferremia.
Hepcidin is the primary iron-regulatory hormone in the body. In response to high iron stores and low iron demand, hepcidin lowers dietary iron absorption and suppresses iron efflux from storage cells and tissues by inducing internalization of the iron export protein, ferroportin (21). Hepcidin synthesis is also upregulated by IL-6 and other inflammatory signals (22, 23). Overweight and obesity have been associated with elevated hepcidin in nonpregnant populations, hypothesized to be due to the adiposity-induced release of inflammatory cytokines (24–25). It is thought that this inflammation-induced upregulation of hepcidin may in part explain reduced iron absorption among obese individuals (26).
Over the course of a healthy pregnancy, maternal hepcidin concentrations decrease (27, 28), which likely serves to increase iron absorption and iron availability to the placenta and growing fetus (29) as well as the woman. It follows that overweight and obesity during pregnancy could dysregulate iron homeostasis by increasing hepcidin, reducing placental iron transfer and compromising neonatal iron status. In support of this hypothesis, we previously found that higher prepregnancy BMI among pregnant Chinese women was associated with lower neonatal iron status (18). Studies examining the influence of maternal overweight or obesity (14, 30) or maternal obesity alone (15–17, 31) on maternal hepcidin concentrations during pregnancy, however, have reported mixed results. All but 2 studies compared obese with lean subjects, excluding those who were overweight but not obese. Neonates also begin to produce hepcidin early in gestation (32), and it is unclear whether maternal overweight or obesity influences hepcidin production in the neonate. Most studies have reported no effect of maternal overweight (14, 30) or obesity (14, 30, 31, 33) on neonatal hepcidin, whereas 1 recent study reported lower neonatal hepcidin associated with maternal obesity (34). Only 1 set of these studies was conducted in a low- or middle-income country (15, 17), and none were conducted in African or Asian regions. Few studies have examined variation in the hepcidin regulatory pathway across ethnic groups, although several recent findings have suggested such variation is plausible. Genetic variation in the HFEgene, a regulator of nonheme iron absorption and homeostasis, was observed between European and Asian populations (35). Higher frequency of single-nucleotide polymorphisms of the gene encoding protein matripase-2, a likely regulator of hepcidin, was observed among non-Hispanic black populations (36, 37) and possibly contributed to higher concentrations of maternal hepcidin found among non-Hispanic black pregnant women (38, 39). Given the mixed results emerging from the few recent studies that have examined associations of maternal overweight and obesity with maternal and neonatal hepcidin concentrations, there is a need to further define and corroborate these associations in diverse contexts, especially among previously unstudied populations. Furthermore, there is a need to examine how nonobese overweight status during pregnancy, a far more common condition among pregnant women than obesity (40), may be associated with maternal and neonatal hepcidin concentrations given our limited understanding of the BMI ranges at which iron homeostasis dysregulation may occur during pregnancy in response to overweight or obesity.
The objectives of this study were to determine the association of 1) maternal (mid-pregnancy) and neonatal iron status with maternal and neonatal hepcidin concentrations, respectively, and 2) maternal prepregnancy overweight or obese status with maternal and neonatal hepcidin concentrations. We further examined potential mediation of the association of maternal prepregnancy overweight or obese status with maternal iron status by maternal hepcidin. We also examined potential mediation of the association of maternal prepregnancy overweight or obese status with neonatal hepcidin by neonatal iron status. We hypothesized that maternal iron status during mid-pregnancy would be positively associated with maternal hepcidin concentrations and that neonatal iron status (proxied by cord blood iron status) would be positively associated with neonatal hepcidin concentrations. We further hypothesized that maternal prepregnancy overweight or obese status would be positively associated with both maternal and neonatal hepcidin concentrations.
Methods
Study design
We examined data from a double-randomized controlled trial of pregnancy and infancy iron supplementation in northeastern China. The design of the trial has been reported previously (41). Briefly, a total of 2367 women with uncomplicated singleton pregnancies who attended their first prenatal visit at 20 weeks of gestation or earlier were recruited and enrolled between June 2009 and December 2011 from 3 local hospitals in Hebei Province. Women with diabetes and other chronic health problems were excluded at enrollment. Participants were randomly assigned to receive 0.4 mg folic acid from enrollment to delivery and either a daily supplement of 300 mg iron sulfate (60 mg elemental iron) or placebo. After delivery, the infants of the women who participated in the trial were randomly assigned to receive either a daily supplement of ∼1 mg Fe/kg as oral iron protein succinylate or placebo from 6 wk to 9 mo. Hematologic data were collected at enrollment and in the late third trimester for 1613 women. Because our limited grant funding allowed for hepcidin analysis in only some of the women and neonates, we prioritized certain subsets for these analyses. We included the 385 women who were randomly assigned to the placebo treatment group of the pregnancy trial at enrollment and whose infants were similarly randomly assigned to the placebo treatment group of the infancy trial. We focused on this group because the variability in maternal iron status was greater than among iron-supplemented women. Examining only women who were not supplemented with iron during pregnancy also allowed us to eliminate the effect of iron supplementation that may have biased estimates of the associations of maternal prepregnancy weight status with maternal and neonatal hepcidin concentrations. To ensure the full range of neonatal iron status was represented, we also included infants who were excluded from the infancy trial because of very low cord serum ferritin (SF) concentrations (i.e., < 35 μg/L) (n = 34) and their mothers. All of these infants had been in the placebo arm of the pregnancy trial. To maximize variation in weight status among women in the study subsample, we further included overweight or obese women from the placebo arm of the pregnancy trial whose infants were assigned to the iron supplementation arm of the infancy trial (n = 61). We excluded participants in the targeted subsets who had missing data for maternal height (n = 3), prepregnant weight (n = 17), maternal iron status at enrollment (n = 17), or cord blood iron status (n = 38). Exclusion of the few women who developed pregnancy complications postenrollment (i.e., 1 pre-eclampsia, 1 severe eclampsia, and 3 gestational hypertension) did not alter our main findings and we therefore included these women in our sample. The total analytic sample was 405 women and their infants.
Data collection
Women were interviewed at enrollment to collect household sociodemographic data and information about current and previous pregnancies. Maternal height and weight were measured at enrollment. Maternal prepregnancy weight was assessed based on maternal recall. Maternal prepregnancy BMI (in kg/m2) was calculated. We also calculated maternal BMI based on the weight measurement taken at enrollment and found no differences in the reported associations when using this BMI measure or the prepregnancy BMI measure (mean ± SD maternal prepregnancy weight: 58.8 ± 10.5 kg; maternal weight at enrollment: 61.4 ± 10.7 kg; Pearson's r: 0.93; P < 0.0001). Given variability in the week of gestation at which weight at enrollment data were collected, we present data only using the prepregnancy BMI measure. Underweight, normal weight, overweight, and obese were defined as BMI <18.5, ≥18.5 and <25, ≥25 and <30, and ≥30, respectively. Adequate, low, and excessive gestational weight gain, defined according to Institute of Medicine recommendations (42), were calculated based on the difference in prepregnancy weight and weight measured at term (i.e., within 1 wk of birth). Gestational age was calculated from date of last menstrual period.
Blood sampling and hematologic assessment
Maternal blood samples (5–10 mL) were obtained by venipuncture at enrollment (i.e., mid-pregnancy) and at or near term. Cord blood samples were obtained immediately after cord clamping by sterile needle puncture. Cord clamping typically occurred within 60 s of delivery. Sampling procedures and protocols have been described previously (41). We assayed SF and soluble transferrin receptor (sTfR) in both maternal and cord blood using the Beckman Coulter Access 2 Immunoassay System with the chemiluminescent immunoassay method (Beckman Coulter Inc.). We assessed Hb using a Sysmex KX-21N Auto Hematology Analyzer (SYSMEX Corporation), and high-sensitivity C-reactive protein (hsCRP) concentrations by rate nephelometry using a Hitachi 7600 modular chemistry analyzer (Hitachi Co.). Hepcidin-25 was assessed in cryopreserved serum (stored at −80°C) using a solid-phase ELISA (DRG Instruments GmbH). We defined maternal anemia as Hb concentration <110 g/L (5) and anemia among neonates as cord blood Hb <130 g/L (43, 44). Low storage iron among pregnant women was defined as SF < 15 μg/L (45), and low SF among neonates was defined as cord SF < 75 μg/L (46).
Statistical analysis
Statistical analyses were carried out in Stata version 15.1 (StataCorp LLC, College Station, TX). Nonnormally distributed variables were log transformed before analysis (i.e., SF, sTfR, hsCRP, hepcidin). In sensitivity analyses, we also adjusted maternal SF and sTfR concentrations at mid-pregnancy and term for individuals with hsCRP >5.0 mg/L using the correction factor method developed by the Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia project (47). Regression results using these corrected values did not differ from the uncorrected values. We report uncorrected values for ease of interpretation and comparability. Means and proportions of maternal and neonatal characteristics were calculated by maternal BMI categories. We used ANOVA and chi-square test statistics to assess differences in means and proportions across categories. Pearson product-moment rs were calculated to assess the association of biomarkers of iron status in maternal and cord blood with maternal and cord hepcidin concentrations.
In separate models, we examined the association of maternal prepregnancy overweight or obese status with maternal hepcidin concentration in mid-pregnancy and cord hepcidin concentrations. These associations were assessed in both unadjusted bivariate linear regression models and multiple linear regression models that adjusted for maternal age, previous births, and child sex. In models assessing maternal hepcidin concentrations in mid-pregnancy, we further adjusted for maternal iron status in mid-pregnancy (i.e., log SF and log sTfR concentrations in separate models). In models assessing cord hepcidin concentrations, we adjusted for maternal iron status at term (i.e., log SF and log sTfR concentrations in separate models), gestational weight gain, gestational age at birth, and cord iron status (i.e., log SF and log sTfR concentrations in separate models). We did not adjust for maternal or cord hsCRP concentrations given that we hypothesized hsCRP to be on the causal pathway between maternal overweight or obesity status and hepcidin concentrations. We further examined these same models comparing women who were overweight prepregnancy (excluding obese women) with all other women.
Using path analysis, we examined mediation of the association of maternal prepregnancy overweight or obese status with maternal iron status by maternal hepcidin, as well as mediation of the association of maternal prepregnancy overweight or obese status with neonatal hepcidin by neonatal iron status. Standardized path coefficients were calculated through maximum-likelihood estimation of the direct, indirect (via the posited mediating variable), and total effects of maternal prepregnancy overweight or obese status on the outcome variable of the model. All models used the Huber–White sandwich estimator to calculate robust SEs.
Ethical approval
The study was approved by the Institutional Review Boards of the University of Michigan and Peking University First Hospital.
Results
Most women in the sample were normal weight before pregnancy (60.7%; n = 246). However, nearly one-quarter (23.2%) were overweight, but not obese (n = 94) and 4.0% (n = 16) were obese (range of obese BMIs: 30.3–35.2) (Table 1). Overweight and obese women were more likely than normal-weight women to gain excessive weight during pregnancy. The birth weight of their children was also higher (i.e., 128 g and 238 g higher for overweight and obese women, respectively). Overweight and obese women had higher Hb concentrations than underweight or normal-weight women but poorer iron status as reflected in higher sTfR concentrations (Table 1).
TABLE 1.
Sample characteristics and iron status measures by maternal prepregnancy BMI1
| Maternal prepregnancy BMI, kg/m2 | |||||
|---|---|---|---|---|---|
| Underweight (<18.5) (n = 49) (12.1%) | Normal weight (≥18.5 and <25) (n = 246) (60.7%) | Overweight (≥25 and <30) (n = 94) (23.2%) | Obese (≥30) (n = 16) (4.0%) | F or χ2 value | |
| Maternal characteristics at enrollment (mid-pregnancy) | |||||
| Age, y | 23.7 ± 3.4 | 24.3 ± 3.4 | 25.2 ± 4.2 | 23.9 ± 2.4 | 2.6* |
| Gave birth previously, % | 20.4 | 23.5 | 27.5 | 25.0 | 0.99 |
| Weeks of gestation | 16.2 ± 2.3 | 15.9 ± 1.9 | 16.1 ± 2.2 | 15.2 ± 1.9 | 1.1 |
| BMI, kg/m2 | 17.6 ± 0.76 | 21.2 ± 1.8 | 26.9 ± 1.3 | 31.7 ± 1.5 | 637*** |
| Pregnancy and birth outcomes | |||||
| Weight gain, kg | 18.1 ± 5.3 | 18.1 ± 5.6 | 16.2 ± 6.1 | 18.0 ± 7.3 | 2.4* |
| Excessive weight gain,2 % | 40.8 | 67.9 | 80.9 | 100 | 32.6*** |
| Gestational age at birth, wk | 39.8 ± 1.3 | 39.6 ± 1.1 | 39.6 ± 1.1 | 39.3 ± 1.0 | 0.77 |
| Birth weight, g | 3301 ± 382 | 3346 ± 381 | 3474 ± 369 | 3584 ± 395 | 4.9*** |
| Maternal mid-pregnancy iron and anemia status | |||||
| Hb, g/L | 117.9 ± 10.2 | 121.8 ± 9.6 | 124.7 ± 8.4 | 128.8 ± 7.4 | 8.5*** |
| Anemia (Hb <110 g/L), % | 18.4 | 12.6 | 4.3 | 6.3 | 8.0** |
| SF, μg/L | 42.2 ± 35.4 | 42.3 ± 36.6 | 39.5 ± 26.6 | 46.8 ± 40.8 | 0.28 |
| Iron depletion (SF <15 μg/L), % | 14.3 | 16.3 | 19.2 | 12.5 | 0.85 |
| sTfR, nmol/L | 15.4 ± 5.0 | 15.3 ± 5.0 | 17.0 ± 6.6 | 17.6 ± 8.9 | 2.7** |
| hsCRP, mg/L | 3.6 ± 6.6 | 3.1 ± 3.4 | 6.0 ± 5.7 | 4.8 ± 3.4 | 9.5*** |
| Hepcidin, μg/L | 9.4 ± 7.1 | 10.3 ± 8.3 | 8.9 ± 5.6 | 9.0 ± 4.6 | 0.88 |
| Neonatal iron and anemia status (cord blood) | |||||
| Hb, g/L | 147.2 ± 15.2 | 150.5 ± 15.4 | 151.8 ± 14.5 | 151.4 ± 13.6 | 1.0 |
| Anemia (Hb <130 g/L), % | 12.2 | 8.1 | 5.3 | 0 | 3.6 |
| Ferritin, μg/L | 143.2 ± 79.2 | 121.0 ± 71.0 | 109.1 ± 73.9 | 122.0 ± 66.7 | 2.4* |
| Low ferritin (<75 μg/L), % | 12.2 | 27.2 | 34.0 | 37.5 | 8.6** |
| sTfR, nmol/L | 32.6 ± 10.8 | 33.5 ± 14.1 | 34.7 ± 13.9 | 37.6 ± 26.2 | 0.66 |
| hsCRP, mg/L | 0.04 ± 0.20 | 0.02 ± 0.03 | 0.14 ± 0.97 | 0.01 ± 0.01 | 1.5 |
| Hepcidin, μg/L | 18.2 ± 12.8 | 14.9 ± 8.9 | 12.7 ± 8.0 | 14.0 ± 7.1 | 3.8** |
Values are means ± SDs or percentages. Differences in means and proportions were assessed using ANOVA (F values reported) and chi-square tests (χ2 statistic reported), respectively. *,**,***Significant difference: *P < 0.1, **P < 0.05, ***P < 0.01. Hb, hemoglobin; hsCRP, high-sensitivity C-reactive protein; SF, serum ferritin; sTfR, soluble transferrin receptor.
Excessive gestational weight gain was defined according to Institute of Medicine recommendations based on the difference in weight prepregnancy and at term (33).
Association of maternal and neonatal iron status with maternal and neonatal hepcidin concentrations
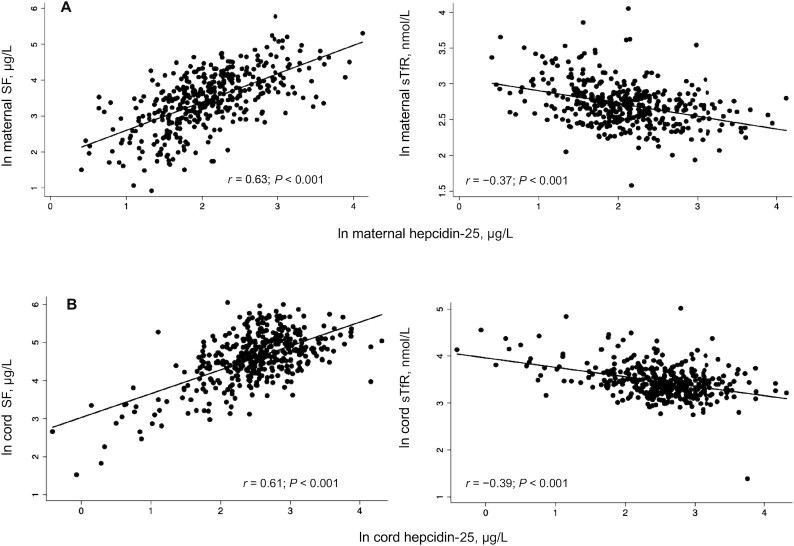
Maternal iron status in mid-pregnancy showed a strong positive correlation with maternal hepcidin concentrations in mid-pregnancy (SF: r = 0.63, P < 0.001; sTfR: r = −0.37, P < 0.001) (Figure 1A). Lower sTfR concentrations reflect higher iron status, because synthesis of sTfR is upregulated during conditions of tissue iron deficiency (48, 49). Cord iron status was similarly positively correlated with cord hepcidin concentrations (cord SF: r = 0.61, P < 0.001; cord sTfR: r = −0.39, P < 0.001) (Figure 1B). Neither maternal nor cord hsCRP concentrations were associated with maternal and cord hepcidin concentrations, respectively (Supplemental Figure 1).
FIGURE 1.
Association of maternal and cord blood iron status with maternal and cord blood hepcidin concentrations, respectively. (A) Associations of maternal SF and sTfR with maternal hepcidin concentrations in mid-pregnancy. (B) Associations of cord ferritin and sTfR with cord hepcidin concentrations. n = 405 for all associations. P values are shown for Pearson product-moment rs. SF, serum ferritin; sTfR, soluble transferrin receptor.
Association of maternal overweight or obese status with maternal and neonatal hepcidin concentrations
In adjusted and unadjusted analyses, maternal overweight or obese status prepregnancy was not associated with maternal hepcidin concentrations in mid-pregnancy (Table 2). However, in unadjusted analyses, maternal overweight or obese status prepregnancy was associated with lower cord hepcidin concentrations (coefficient = −0.22, P = 0.003) (Table 2). This association remained after adjusting for several potentially confounding covariates, including maternal iron status at term, gestational weight gain, gestational age at birth, and cord iron status (coefficient = −0.21, P = 0.004). Similar patterns of associations were observed when examining women who were overweight prepregnancy (excluding obese women) (Supplemental Table 1).
TABLE 2.
Regression analyses of the association of maternal prepregnancy overweight or obese status with maternal mid-pregnancy hepcidin concentration and neonatal hepcidin concentration1
| Maternal hepcidin (mid-pregnancy), μg/L | Cord hepcidin, μg/L | |||||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 | |
| Maternal overweight or obese status prepregnancy | ||||||
| Under- or normal weight | Reference | Reference | Reference | Reference | Reference | Reference |
| Overweight or obese (BMI ≥ 25) | −0.07 ± 0.07 | −0.06 ± 0.06 | 0.006 ± 0.07 | −0.22 ± 0.07*** | −0.11 ± 0.06* | −0.21 ± 0.07*** |
| Maternal age, y | — | 0.007 ± 0.008 | 0.01 ± 0.10 | — | −0.01 ± 0.01 | −0.01 ± 0.01 |
| Previous birth | ||||||
| No | Reference | Reference | Reference | Reference | Reference | Reference |
| Yes | — | −0.05 ± 0.07 | −0.11 ± 0.08 | — | 0.01 ± 0.08 | 0.07 ± 0.09 |
| Gestational age at birth, wk | — | — | — | — | −0.004 ± 0.02 | 0.05 ± 0.03 |
| Gestational weight gain2 | ||||||
| Adequate | Reference | Reference | Reference | Reference | Reference | Reference |
| Low | — | — | — | — | 0.08 ± 0.11 | 0.13 ± 0.13 |
| Excessive | — | — | — | — | −0.02 ± 0.07 | −0.02 ± 0.08 |
| Child sex | ||||||
| Male | Reference | Reference | Reference | Reference | Reference | Reference |
| Female | — | −0.002 ± 0.05 | −0.05 ± 0.06 | — | 0.04 ± 0.06 | 0.01 ± 0.06 |
| Maternal log SF concentration (mid-pregnancy), μg/L | 0.50 ± 0.03*** | — | — | — | — | |
| Maternal log sTfR concentration (mid-pregnancy), nmol/L | — | — | −0.82 ± 0.10*** | — | — | — |
| Maternal log SF concentration (at term), μg/L | — | — | — | — | 0.06 ± 0.05 | — |
| Maternal log sTfR concentration (at term), nmol/L | — | — | — | — | — | −0.24 ± 0.10** |
| Cord log SF concentration, μg/L | — | — | — | — | 0.58 ± 0.04*** | — |
| Cord log sTfR concentration, nmol/L | — | — | — | — | — | −0.74 ± 0.10*** |
| Maternal hepcidin (mid-pregnancy), μg/L | — | — | — | — | 0.02 ± 0.04 | 0.01 ± 0.05 |
Values are coefficients ± SEs. Sample sizes of mother–neonate pairs: Model 1: n = 405; Model 2: n = 394; Model 3: n = 394; Model 4: n = 405; Model 5: n = 379; Model 6: n = 379. *,**,***Significant association: *P < 0.1, **P < 0.05, ***P < 0.01. Models 1 and 4 adjusted for no covariates; Models 2 and 5 adjusted for all covariates shown and included SF as an iron status indicator; Models 3 and 6 adjusted for all covariates shown and included sTfR as an iron status indicator. SF, serum ferritin; sTfR, soluble transferrin receptor.
Excessive gestational weight gain was defined according to Institute of Medicine recommendations based on the difference in weight prepregnancy and at term (42).
Path analyses
In path analyses, the standardized path coefficients for the indirect effect of maternal prepregnancy overweight or obese status on maternal (mid-pregnancy) iron status mediated by maternal hepcidin were not statistically significant (Table 3). In models testing mediation of maternal prepregnancy overweight or obese status on cord hepcidin by cord SF status, the total effect of maternal prepregnancy overweight or obese status on cord hepcidin was statistically significantly smaller than the direct (unmediated) association of these 2 factors and the indirect effect was statistically significant (Table 4, Figure 2).
TABLE 3.
Association of maternal prepregnancy overweight or obese status with maternal (mid-pregnancy) iron status from path analysis testing for mediation by maternal (mid-pregnancy) hepcidin concentration1
| Indicator of maternal (mid-pregnancy) iron status | ||||
|---|---|---|---|---|
| Maternal log serum ferritin concentration (mid-pregnancy) | Maternal log soluble transferrin receptor concentration (mid-pregnancy) | |||
| Mediating variable: maternal hepcidin (mid-pregnancy) | Coefficient | SE | Coefficient | SE |
| Direct effect | 0.04 | 0.07 | 0.08** | 0.03 |
| Indirect effect | −0.05 | 0.05 | 0.01 | 0.01 |
| Total effect | −0.01 | 0.09 | 0.09*** | 0.03 |
n = 405. Coefficients shown are standardized path coefficients. For the direct effect, the coefficient is the partial regression coefficient of maternal prepregnancy overweight or obese status on maternal (mid-pregnancy) iron status, adjusting for maternal hepcidin. The indirect effect is calculated as the product of the path coefficient between maternal prepregnancy overweight or obese status and maternal hepcidin, and the path coefficient between maternal hepcidin and maternal iron status (i.e., the indirect effect of maternal prepregnancy overweight or obese status on maternal iron status as mediated by maternal hepcidin). The total effect is calculated as the sum of the direct and indirect effects. *,**,***Statistically significant point estimate: **P < 0.05, ***P < 0.01.
TABLE 4.
Association of maternal prepregnancy overweight or obese status with neonatal hepcidin from path analysis testing for mediation by neonatal iron status1
| Indicator of cord iron status used as mediator | ||||
|---|---|---|---|---|
| Cord log serum ferritin concentration | Cord log soluble transferrin receptor concentration | |||
| Mediating variable: cord iron status | Coefficient | SE | Coefficient | SE |
| Direct effect | −0.13** | 0.06 | −0.19*** | 0.07 |
| Indirect effect | −0.09* | 0.05 | −0.03 | 0.03 |
| Total effect | −0.22*** | 0.08 | −0.22*** | 0.08 |
n = 405. Coefficients shown are standardized path coefficients. For the direct effect, the coefficient is the partial regression coefficient of maternal prepregnancy overweight or obese status on cord hepcidin, adjusting for cord iron status. The indirect effect is calculated as the product of the path coefficient between maternal prepregnancy overweight or obese status and cord iron status, and the path coefficient between cord iron status and cord hepcidin (i.e., the indirect effect of maternal prepregnancy overweight or obese status on cord hepcidin as mediated by iron status). The total effect is calculated as the sum of the direct and indirect effects. *,**,***Statistically significant point estimate: *P < 0.1, **P < 0.05, ***P < 0.01.
FIGURE 2.
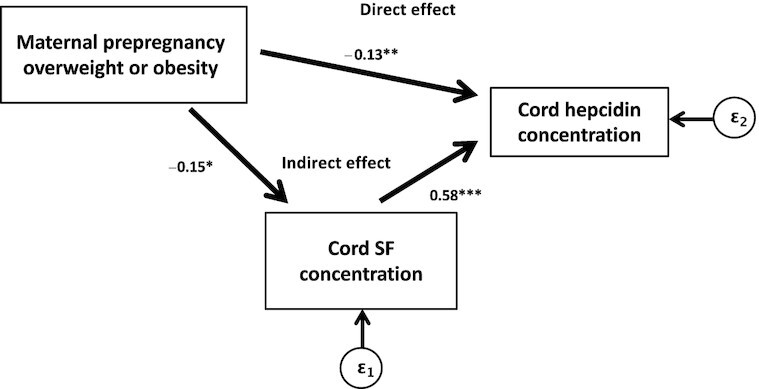
Path diagram with path coefficients showing the associations of maternal prepregnancy overweight or obese status with neonatal (cord) hepcidin mediated by neonatal (cord SF) iron status. *,**,***Statistically significant standardized path coefficient: *P < 0.1, **P < 0.05, ***P < 0.01. SF, serum ferritin.
Discussion
In this cohort of nondiabetic pregnant Chinese women, we found that maternal prepregnancy overweight or obesity status was not associated with maternal hepcidin at mid-gestation. However, neonates born to women who were overweight or obese before pregnancy had lower cord hepcidin concentrations than those born to women with a prepregnancy BMI <25. Findings were similar for analyses that included nonobese overweight women. Maternal and neonatal hepcidin concentrations were strongly positively correlated with maternal and neonatal iron status, respectively. Consistent with previous work, the correlations observed with SF were stronger than those observed with sTfR (50). This likely reflects hepcidin's primary response to changes in iron storage during pregnancy (29), whereas sTfR concentrations during pregnancy increase only in iron-deficient states. Neither maternal nor neonatal hepcidin concentrations were correlated with hsCRP, a biomarker of inflammation. Using path analysis, we found that maternal overweight or obese status had a significant indirect effect on cord blood hepcidin mediated by neonatal iron status.
We previously found that prepregnancy obesity was negatively associated with maternal iron status at mid-gestation and that obese pregnant women had higher inflammation than normal-weight women (27). We thus hypothesized that the chronic low-grade inflammation associated with obesity may elevate hepcidin among pregnant women with higher BMIs, leading to lower circulating iron concentrations across gestation. Our data from the current study, however, do not support this hypothesis. Prior studies examining the association between maternal overweight or obesity and hepcidin concentrations have reported mixed results, depending on the stage of gestation at which hepcidin was measured and the BMI range of the comparison groups. Studies have observed higher hepcidin concentrations among obese than among normal-weight pregnant women in mid-pregnancy (31), in mid-pregnancy but not later gestation (15), at delivery but not earlier in gestation (14), and throughout gestation (17). In contrast, Koenig et al. (38) found no differences in serum hepcidin among women during their third trimester of pregnancy when comparing those who were obese prepregnancy with those who were nonobese prepregnancy. The lack of association observed in our study between maternal overweight or obesity and hepcidin may be due to the mild degree of obesity in this sample (mean ± SD BMI in the obese group: 31.7 ± 1.5) relative to the aforementioned studies. Some studies have found elevated hepcidin concentrations only among pregnant women whose BMI was >35 (16, 29). These findings suggest that obesity-induced upregulation of hepcidin during pregnancy may occur only at very high BMIs. In our previous work examining BMI and iron status among pregnant women, we observed an inflection point in the range of BMI = 30.5–32.5 at which iron status biomarkers sharply contrasted with lower BMIs and trended toward lower iron status (27). Accordingly, there may be a threshold for BMI-induced upregulation of hepcidin during pregnancy.
Chronic low-grade inflammation has been proposed as the mechanism by which hepcidin is upregulated in obese pregnant women. Although overweight and obese pregnant women had higher inflammation in this study, we did not find an association between hepcidin and hsCRP. Several studies have likewise found no association between hepcidin and inflammatory biomarkers in obese and normal-weight pregnant women (16, 17, 25, 29). Instead, hepcidin appears to maintain its fundamental iron-regulatory role during pregnancy, responding normally to erythropoietic and fetal iron demands among pregnant women who are overweight or obese (17, 27, 28, 51, 52). Our findings support this prior evidence. Furthermore, recent work from Flores-Quijano et al. (17) suggests that hepcidin concentrations decrease across gestation in obese pregnant women as they do in normal-weight women. Thus, the physiological mechanism for how obesity induces upregulation of hepcidin during pregnancy and whether hepcidin upregulation substantially alters maternal iron status require further investigation.
Fetal iron status depends on the availability of iron in the maternal circulation and the regulation of iron transport across the placenta (53). In our previous work, we confirmed that neonatal iron status was lower among mothers with lower iron status in late pregnancy (27). We also found that maternal prepregnancy BMI was negatively associated with neonatal iron status, independently of maternal iron status. Some studies have similarly reported a negative association between maternal obesity and neonatal iron status (31, 33, 34, 54), whereas others have found no association (14, 16, 29). In this study, we examined whether fetal-derived hepcidin plays a role in the association between maternal overweight or obesity and neonatal iron status. Fetal hepcidin may regulate ferroportin expression at the basolateral (fetal) side of placental syncytiotrophoblasts to allow export of iron from the placenta into the fetal circulation (44, 53). Alternatively, some evidence suggests that ferroportin expression may be regulated by the cellular iron demand of the placenta, not systemic iron needs of the fetus, thus a reduction in fetal hepcidin may be unable to increase placental iron transfer (53, 55). In our sample, controlling for maternal iron status, cord iron status, and maternal hepcidin, neonates born to overweight and obese mothers had lower cord blood hepcidin concentrations than those born to mothers with a prepregnancy BMI <25. This same association was also observed among nonobese overweight mothers. With a single exception (31), previous studies have found no associations between maternal BMI and cord blood hepcidin (14, 28–30). Rather, existing evidence suggests that fetal hepcidin is primarily regulated by fetal iron status, independently of maternal signals (34, 56) or neonatal biomarkers of inflammation (56). Findings from our study support this evidence. We found 1) a strong association between cord blood hepcidin and neonatal SF and sTfR concentrations, independently of maternal prepregnancy BMI; 2) no correlation between neonatal hepcidin and neonatal hsCRP; and 3) significant mediation of the association between maternal prepregnancy overweight or obese status and cord hepcidin by cord SF concentration. We thus posit that the lower cord blood hepcidin concentrations observed among neonates of overweight and obese mothers in our sample were likely driven by lower iron status among these neonates. This lower iron status is likely a result of inadequate delivery of iron from maternal circulation to the placenta, whether due to poor maternal iron status or to dysregulation of iron transport across the apical side of the placenta. Fetal hepcidin is likely able to regulate iron uptake from the placenta based on tissue iron demand and iron stores. Thus, hepcidin, although a plausible target, maintains its responsiveness to iron signaling in both maternal and fetal iron metabolism and does not appear to be the primary mechanism that explains how obesity in pregnancy alters neonatal iron status. Our observation that neonatal hepcidin concentrations were lower even among nonobese overweight mothers warrants replication in future studies, especially because overweight in pregnancy is much more common than obesity. Further research is needed to investigate the extent to which the dysregulation of iron homeostasis observed among obese pregnant women may also be present among nonobese overweight pregnant women.
Our study had several limitations. Firstly, the study cohort included a small number of obese women, and the degree of obesity observed was relatively mild (generally class 1), possibly below the threshold of obesity-induced alterations to hepcidin concentrations. Secondly, limited funding allowed us to measure maternal hepcidin at only 1 time point during gestation. Thus, we were unable to observe how differences in hepcidin and iron and inflammatory biomarkers between obese and normal-weight women may change across gestation. Given that we were unable to assess hepcidin concentrations at various time points during gestation, we are also unable to definitively establish cord iron status as antecedent to cord hepcidin in path analyses. An alternative path analysis (data not shown), modeling cord hepcidin as mediating the association of maternal overweight or obese status with cord iron status, showed a similar mediating effect as that observed for mediation by cord iron of the association of maternal overweight or obese status with cord hepcidin. We prioritize the model shown in Figure 2, however, given that it aligns with the aforementioned emerging mechanistic evidence and the complementary findings from our regression models. Thirdly, although we measured hsCRP, which proxies inflammation known to upregulate hepcidin, we did not measure IL-6, which influences hepcidin transcription through the Janus kinase–signal transducers and activators of transcription (JAK-STAT) signaling pathway (22), or other proinflammatory cytokines released from adipose tissue, such as leptin. However, other studies have found no association of IL-6 or leptin with hepcidin among obese pregnant women (16, 17), possibly indicating that other mechanisms influencing hepcidin concentrations could be at play during pregnancy [e.g., erythroferrone (34, 57)]. Fourthly, we did not control for genetic variations in iron regulation (36), which may have confounded our results. Finally, we did not collect data on dietary iron intake among the pregnant mothers of the study, and therefore we were unable to include this information in our models.
The rising prevalence of overweight and obesity in low- and middle-income countries presents a serious public health challenge, especially given that overweight and obesity can exacerbate poor iron status. This is particularly relevant during pregnancy, when a woman's iron status may influence her infant's iron stores for the first 6 mo of life. Our findings suggest that neonates born to women entering pregnancy overweight or obese may have lower hepcidin concentrations, although this seems to be primarily driven by lower neonatal iron status. Further research is needed to determine whether higher adiposity in pregnant women alters placental iron transfer and via which mechanism. In addition, the mechanism by which obesity during pregnancy elevates hepcidin, and under what conditions of obesity and gestation, remain unclear. Understanding the multiple signals that may alter iron regulation during pregnancy, especially among overweight and obese populations, could provide further evidence to support the benefits of diet and lifestyle interventions aimed at weight management during pregnancy (58), and potentially identify novel interventions to improve neonatal iron status.
Supplementary Material
Acknowledgments
We are grateful to Blair Richards for his assistance with data management and data quality control. We also acknowledge the contributions of Gengli Zhao and Zhixiang Zhang to the conception and design of the parent study and of Guobin Xu to laboratory analyses. The authors’ responsibilities were as follows—ADJ: developed the research question and designed the statistical analyses; ADJ and NJL: drafted the manuscript; ZS: conducted the hepcidin assays and was guided by mentors JW, MB, and ML; YJ: conducted the assays for other iron status measures; BL and ML: contributed to the conception and design of the parent study; ADJ and BL: had primary responsibility for the final content of the manuscript; and all authors: critically revised the manuscript and read and approved the final manuscript.
Notes
The study of iron supplementation in pregnancy was supported by a grant from Vifor Pharma Ltd., Gengli Zhao, Principal Investigator (to ADJ). The laboratory measures of iron status were supported by US NIH grant R01 HD052069 (to BL), which included funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the Office of Dietary Supplements. Laboratory measures of hepcidin and travel to China were supported by grants from the Center for Human Growth and Development at the University of Michigan (Rauner Family Foundation) (to ADJ and MB, respectively). ZS was supported in part by Michigan Medicine–Peking University Health Sciences Center Joint Institute for Clinical and Translational Research grant BMU20140480 (to MB and ML). The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding sources or other donors. The authors had full control of the primary data and did not have an agreement with the funders that limited their ability to complete the research as planned.
Author disclosures: the authors report no conflicts of interest.
ADJ is a member of The Journal of Nutrition’s Editorial Board.
Supplemental Figure 1 and Supplemental Table 1 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
ADJ and ZS are co-first authors.
Abbreviations used: Hb, hemoglobin; hsCRP, high-sensitivity C-reactive protein; SF, serum ferritin; sTfR, soluble transferrin receptor.
Contributor Information
Andrew D Jones, Department of Nutritional Sciences, School of Public Health, University of Michigan, Ann Arbor, MI, USA.
Zhen Shi, Peking University First Hospital, Beijing, China; Guangzhou Women and Children's Medical Center, Guangzhou, China.
Nathalie J Lambrecht, Department of Nutritional Sciences, School of Public Health, University of Michigan, Ann Arbor, MI, USA.
Yaping Jiang, Peking University First Hospital, Beijing, China.
Jingmin Wang, Peking University First Hospital, Beijing, China.
Margit Burmeister, Department of Computational Medicine & Bioinformatics, Michigan Neuroscience Institute, University of Michigan, Ann Arbor, MI, USA; Department of Psychiatry, University of Michigan, Ann Arbor, MI, USA; Department of Human Genetics, University of Michigan, Ann Arbor, MI, USA.
Ming Li, Peking University First Hospital, Beijing, China.
Betsy Lozoff, Department of Pediatrics, Medical School, University of Michigan, Ann Arbor, MI, USA.
References
- 1. Lozoff B, Georgieff MK. Iron deficiency and brain development. Semin Pediatr Neurol. 2006;13(3):158–65. [DOI] [PubMed] [Google Scholar]
- 2. Radlowski EC, Johnson RW. Perinatal iron deficiency and neurocognitive development. Front Hum Neurosci. 2013;7:585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rao R, Georgieff MK. Iron in fetal and neonatal nutrition. Semin Fetal Neonatal Med. 2007;12(1):54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cao C, O'Brien KO. Pregnancy and iron homeostasis: an update. Nutr Rev. 2013;71(1):35–51. [DOI] [PubMed] [Google Scholar]
- 5. Stevens GA, Finucane MM, De-Regil LM, Paciorek CJ, Flaxman SR, Branca F, Peña-Rosas JP, Bhutta ZA, Ezzati M, Nutrition Impact Model Study Group (Anaemia) . Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non-pregnant women for 1995–2011: a systematic analysis of population-representative data. Lancet Glob Health. 2013;1(1):e16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kassebaum NJ, Jasrasaria R, Naghavi M, Wulf SK, Johns N, Lozano R, Regan M, Weatherall D, Chou DP, Eisele TPet al. A systematic analysis of global anemia burden from 1990 to 2010. Blood. 2014;123(5):615–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Prentice AM. The double burden of malnutrition in countries passing through the economic transition. Ann Nutr Metab. 2018;72(Suppl 3):47–54. [DOI] [PubMed] [Google Scholar]
- 8. Popkin BM, Adair LS, Ng SW. Global nutrition transition and the pandemic of obesity in developing countries. Nutr Rev. 2012;70(1):3–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang X, Zhang M, Zhao Z, Huang Z, Deng Q, Li Y, Pan A, Li C, Chen Z, Zhou Met al. Geographic variation in prevalence of adult obesity in China: results from the 2013–2014 National Chronic Disease and Risk Factor Surveillance. Ann Intern Med. 2020;172(4):291–3. [DOI] [PubMed] [Google Scholar]
- 10. García OP, Long KZ, Rosado JL. Impact of micronutrient deficiencies on obesity. Nutr Rev. 2009;67(10):559–72. [DOI] [PubMed] [Google Scholar]
- 11. Doak CM, Adair LS, Bentley M, Monteiro C, Popkin BM. The dual burden household and the nutrition transition paradox. Int J Obes. 2005;29(1):129–36. [DOI] [PubMed] [Google Scholar]
- 12. Micozzi MS, Albanes D, Stevens RG. Relation of body size and composition to clinical biochemical and hematologic indices in US men and women. Am J Clin Nutr. 1989;50(6):1276–81. [DOI] [PubMed] [Google Scholar]
- 13. Ausk KJ, Ioannou GN. Is obesity associated with anemia of chronic disease? A population-based study. Obesity. 2008;16(10):2356–61. [DOI] [PubMed] [Google Scholar]
- 14. Garcia-Valdes L, Campoy C, Hayes H, Florido J, Rusanova I, Miranda MT, McArdle HJ. The impact of maternal obesity on iron status, placental transferrin receptor expression and hepcidin expression in human pregnancy. Int J Obes. 2015;39(4):571–8. [DOI] [PubMed] [Google Scholar]
- 15. Flores-Quijano ME, Montalvo-Velarde I, Vital-Reyes VS, Rodríguez-Cruz M, Rendón-Macías ME, López-Alarcón M. Longitudinal analysis of the interaction between obesity and pregnancy on iron homeostasis: role of hepcidin. Arch Med Res. 2016;47(7):550–6. [DOI] [PubMed] [Google Scholar]
- 16. Flynn AC, Begum S, White SL, Dalrymple K, Gill C, Alwan NA, Kiely M, Latunde-Dada G, Bell R, Briley ALet al. Relationships between maternal obesity and maternal and neonatal iron status. Nutrients. 2018;10(8):1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Flores-Quijano ME, Vega-Sánchez R, Tolentino-Dolores MC, López-Alarcón MG, Flores-Urrutia MC, López-Olvera AD, Talavera JO. Obesity is associated with changes in iron nutrition status and its homeostatic regulation in pregnancy. Nutrients. 2019;11(3):693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jones AD, Zhao G, Jiang Y-p, Zhou M, Xu G, Kaciroti N, Zhang Z, Lozoff B. Maternal obesity during pregnancy is negatively associated with maternal and neonatal iron status. Eur J Clin Nutr. 2016;70(8):918–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zimmermann MB, Zeder C, Muthayya S, Winichagoon P, Chaouki N, Aeberli I, Hurrell RF. Adiposity in women and children from transition countries predicts decreased iron absorption, iron deficiency and a reduced response to iron fortification. Int J Obes. 2008;32(7):1098–104. [DOI] [PubMed] [Google Scholar]
- 20. Menzie CM, Yanoff LB, Denkinger BI, McHugh T, Sebring NG, Calis KA, Yanovski JA. Obesity-related hypoferremia is not explained by differences in reported intake of heme and nonheme iron or intake of dietary factors that can affect iron absorption. J Am Diet Assoc. 2008;108(1):145–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ganz T, Nemeth E. Hepcidin and iron homeostasis. Biochim Biophys Acta. 2012;1823(9):1434–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, Pedersen BK, Ganz T. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004;113(9):1271–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ganz T, Nemeth E. Iron homeostasis in host defence and inflammation. Nat Rev Immunol. 2015;15(8):500–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tussing-Humphreys L, Pusatcioglu C, Nemeth E, Braunschweig C. Rethinking iron regulation and assessment in iron deficiency, anemia of chronic disease, and obesity: introducing hepcidin. J Acad Nutr Diet. 2012;112(3):391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cepeda-Lopez AC, Melse-Boonstra A, Zimmermann MB, Herter-Aeberli I. In overweight and obese women, dietary iron absorption is reduced and the enhancement of iron absorption by ascorbic acid is one-half that in normal-weight women. Am J Clin Nutr. 2015;102(6):1389–97. [DOI] [PubMed] [Google Scholar]
- 26. Dao MC, Meydani SN. Iron biology, immunology, aging, and obesity: four fields connected by the small peptide hormone hepcidin. Adv Nutr. 2013;4(6):602–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bah A, Pasricha SR, Jallow MW, Sise EA, Wegmuller R, Armitage AE, Drakesmith H, Moore SE, Prentice AM. Serum hepcidin concentrations decline during pregnancy and may identify iron deficiency: analysis of a longitudinal pregnancy cohort in The Gambia. J Nutr. 2017;147(6):1131–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. van Santen S, Kroot JJC, Zijderveld G, Wiegerinck ET, Spaanderman MEA, Swinkels DW. The iron regulatory hormone hepcidin is decreased in pregnancy: a prospective longitudinal study. Clin Chem Lab Med. 2013;51(7):1395–401. [DOI] [PubMed] [Google Scholar]
- 29. Fisher AL, Nemeth E. Iron homeostasis during pregnancy. Am J Clin Nutr. 2017;106(Supplement 6):1567S–74S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cao C, Pressman EK, Cooper EM, Guillet R, Westerman M, O'Brien KO. Prepregnancy body mass index and gestational weight gain have no negative impact on maternal or neonatal iron status. Reprod Sci. 2016;23(5):613–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dao MC, Sen S, Iyer C, Klebenov D, Meydani SN. Obesity during pregnancy and fetal iron status: is hepcidin the link?. J Perinatol. 2013;33(3):177–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Evans P, Cindrova-Davies T, Muttukrishna S, Burton GJ, Porter J, Jauniaux E. Hepcidin and iron species distribution inside the first-trimester human gestational sac. Mol Hum Reprod. 2011;17(4):227–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dosch NC, Guslits EF, Weber MB, Murray SE, Ha B, Coe CL, Auger AP, Kling PJ. Maternal obesity affects inflammatory and iron indices in umbilical cord blood. J Pediatr. 2016;172:20–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Korlesky C, Kling PJ, Pham DQD, Ovasapyan AA, Leyns CEG, Weber MB, Coe CL. Cord blood erythropoietin and hepcidin reflect lower newborn iron stores due to maternal obesity during pregnancy. Am J Perinatol. 2019;36(5):511–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ye K, Cao C, Lin X, O'Brien KO, Gu Z. Natural selection on HFE in Asian populations contributes to enhanced non-heme iron absorption. BMC Genet. 2015;16(1):61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gichohi-Wainaina WN, Towers GW, Swinkels DW, Zimmermann MB, Feskens EJ, Melse-Boonstra A. Erratum to: Inter-ethnic differences in genetic variants within the transmembrane protease, serine 6 (TMPRSS6) gene associated with iron status indicators: a systematic review with meta-analyses. Genes Nutr. 2015;10(3):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. McLaren CE, McLachlan S, Garner CP, Vulpe CD, Gordeuk VR, Eckfeldt JH, Adams PC, Acton RT, Murray JA, Leiendecker-Foster Cet al. Associations between single nucleotide polymorphisms in iron-related genes and iron status in multiethnic populations. PLoS One. 2012;7(6):e38339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Koenig M, Klikuszowian E, O'Brien K, Pauls H, Steffen A, DeMartelly V, Ruchob R, Welke L, Hemphill N, LaBomascus Bet al. Prepregnancy obesity is not associated with iron utilization during the third trimester. J Nutr. 2020;150(6):1397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cao C, Fleming MD. The placenta: the forgotten essential organ of iron transport. Nutr Rev. 2016;74(7):421–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chen C, Xu X, Yan Y. Estimated global overweight and obesity burden in pregnant women based on panel data model. PLoS One. 2018;13(8):e0202183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhao G, Xu G, Zhou M, Jiang Y, Richards B, Clark KM, Kaciroti N, Georgieff MK, Zhang Z, Tardif Tet al. Prenatal iron supplementation reduces maternal anemia, iron deficiency, and iron deficiency anemia in a randomized clinical trial in rural China, but iron deficiency remains widespread in mothers and neonates. J Nutr. 2015;145(8):1916–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Institute of Medicine, National Research Council . Weight gain during pregnancy: reexamining the guidelines. Washington (DC): National Academies Press; 2009. [PubMed] [Google Scholar]
- 43. Diagne I, Archambeaud MP, Diallo D, d'Oiron R, Yvart J, Tchernia G. [Erythrocyte indices and iron stores in cord blood]. Arch Pediatr. 1995;2(3):208–14. [DOI] [PubMed] [Google Scholar]
- 44. Paterakis GS, Lykopoulou L, Papassotiriou J, Stamulakatou A, Kattamis C, Loukopoulos D. Flow-cytometric analysis of reticulocytes in normal cord blood. Acta Haematol. 1993;90(4):182–5. [DOI] [PubMed] [Google Scholar]
- 45. Centers for Disease Control and Prevention . Recommendations to prevent and control iron deficiency in the United States. MMWR Recomm Rep. 1998;47(RR-3):1–36. [PubMed] [Google Scholar]
- 46. Shao J, Lou J, Rao R, Georgieff MK, Kaciroti N, Felt BT, Zhao Z-Y, Lozoff B. Maternal serum ferritin concentration is positively associated with newborn iron stores in women with low ferritin status in late pregnancy. J Nutr. 2012;142(11):2004–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Namaste SM, Aaron GJ, Varadhan R, Peerson JM, Suchdev PS; BRINDA Working Group . Methodologic approach for the Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project. Am J Clin Nutr. 2017;106(Suppl 1):333S–47S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Carriaga MT, Skikne BS, Finley B, Cutler B, Cook JD. Serum transferrin receptor for the detection of iron deficiency in pregnancy. Am J Clin Nutr. 1991;54(6):1077–81. [DOI] [PubMed] [Google Scholar]
- 49. Zimmermann MB, Molinari L, Staubli-Asobayire F, Hess SY, Chaouki N, Adou P, Hurrell RF. Serum transferrin receptor and zinc protoporphyrin as indicators of iron status in African children. Am J Clin Nutr. 2005;81(3):615–23. [DOI] [PubMed] [Google Scholar]
- 50. Koenig MD, Tussing-Humphreys L, Day J, Cadwell B, Nemeth E. Hepcidin and iron homeostasis during pregnancy. Nutrients. 2014;6(8):3062–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Schulze KJ, Christian P, Ruczinski I, Ray AL, Nath A, Wu LS-F, Semba RD. Hepcidin and iron status among pregnant women in Bangladesh. Asia Pac J Clin Nutr. 2008;17(3):451–6. [PMC free article] [PubMed] [Google Scholar]
- 52. Rehu M, Punnonen K, Ostland V, Heinonen S, Westerman M, Pulkki K, Sankilampi U. Maternal serum hepcidin is low at term and independent of cord blood iron status. Eur J Haematol. 2010;85(4):345–52. [DOI] [PubMed] [Google Scholar]
- 53. Sangkhae V, Nemeth E. Placental iron transport: the mechanism and regulatory circuits. Free Radic Biol Med. 2019;133:254–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. McCarthy EK, Kenny LC, Hourihane JOB, Irvine AD, Murray DM, Kiely ME. Impact of maternal, antenatal and birth-associated factors on iron stores at birth: data from a prospective maternal–infant birth cohort. Eur J Clin Nutr. 2017;71(6):782–7. [DOI] [PubMed] [Google Scholar]
- 55. Sangkhae V, Fisher AL, Wong S, Koenig MD, Tussing-Humphreys L, Chu A, Lelic M, Ganz T, Nemeth E. Effects of maternal iron status on placental and fetal iron homeostasis. J Clin Invest. 2019;130(2):625–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ru Y, Pressman EK, Guillet R, Katzman PJ, Vermeylen F, O'Brien KO. Umbilical cord hepcidin concentrations are positively associated with the variance in iron status among multiple birth neonates. J Nutr. 2018;148(11):1716–22. [DOI] [PubMed] [Google Scholar]
- 57. Pasricha SR, McHugh K, Drakesmith H. Regulation of hepcidin by erythropoiesis: the story so far. Annu Rev Nutr. 2016;36:417–34. [DOI] [PubMed] [Google Scholar]
- 58. Thangaratinam S, Rogozińska E, Jolly K, Glinkowski S, Duda W, Borowiack E, Roseboom T, Tomlinson J, Walczak J, Kunz Ret al. Interventions to reduce or prevent obesity in pregnant women: a systematic review. Health Technol Assess. 2012;16(31):iii–iv, 1–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.