ABSTRACT
Background
Although fructose as a source of excess calories increases uric acid, the effect of the food matrix is unclear.
Objectives
To assess the effects of fructose-containing sugars by food source at different levels of energy control on uric acid, we conducted a systematic review and meta-analysis of controlled trials.
Methods
MEDLINE, Embase, and the Cochrane Library were searched (through 11 January 2021) for trials ≥ 7 days. We prespecified 4 trial designs by energy control: substitution (energy-matched replacement of sugars in diets); addition (excess energy from sugars added to diets); subtraction (energy from sugars subtracted from diets); and ad libitum (energy from sugars freely replaced in diets) designs. Independent reviewers (≥2) extracted data and assessed the risk of bias. Grading of Recommendations, Assessment, Development, and Evaluation was used to assess the certainty of evidence.
Results
We included 47 trials (85 comparisons; N = 2763) assessing 9 food sources [sugar-sweetened beverages (SSBs), sweetened dairy, fruit drinks, 100% fruit juice, fruit, dried fruit, sweets and desserts, added nutritive sweetener, and mixed sources] across 4 energy control levels in predominantly healthy, mixed-weight adults. Total fructose-containing sugars increased uric acid levels in substitution trials (mean difference, 0.16 mg/dL; 95% CI: 0.06–0.27 mg/dL; P = 0.003), with no effect across the other energy control levels. There was evidence of an interaction by food source: SSBs and sweets and desserts increased uric acid levels in the substitution design, while SSBs increased and 100% fruit juice decreased uric acid levels in addition trials. The certainty of evidence was high for the increasing effect of SSBs in substitution and addition trials and the decreasing effect of 100% fruit juice in addition trials and was moderate to very low for all other comparisons.
Conclusions
Food source more than energy control appears to mediate the effects of fructose-containing sugars on uric acid. The available evidence provides reliable indications that SSBs increase and 100% fruit juice decreases uric acid levels. More high-quality trials of different food sources are needed. This trial was registered at clinicaltrials.gov as NCT02716870.
Keywords: food sources of fructose-containing sugars, sugar-sweetened beverages, fruit, fruit juice, gout, uric acid, systematic review, meta-analysis
Introduction
High blood uric acid is a risk factor for gout, cardiovascular disease, and type 2 diabetes mellitus (1), and can be influenced by diet (2). Fructose has a direct effect on uric acid metabolism, with oral or intravenous administration of excess fructose increasing blood uric acid levels in humans (3–5). Fructose intake has also been associated with gout in individual large, prospective cohort studies and systematic reviews and meta-analyses of prospective cohort studies (6–9). Total dietary energy intake, however, may be an important mediating factor. Systematic reviews and meta-analyses of controlled feeding trials have shown that fructose increases uric acid when it provides excess calories to diets, but not when given in isocaloric substitution for other carbohydrates (mainly in the form of refined starches or glucose) (10).
Dietary guidelines for chronic disease prevention are moving away from a focus on single nutrients towards a focus on foods and dietary patterns, in recognition of the fact that a focus on single nutrients may miss important interactions related to the food matrix in which the nutrients are contained (11). Much of the evidence linking fructose to the increased risk of cardiometabolic diseases is derived from studies of sugar-sweetened beverages (SSBs) (12–15). Whether the evidence for SSBs holds for other commonly consumed food sources of fructose, including fruit, 100% fruit juices, sweetened cereal grains, and sweetened dairy and dairy alternatives, or as a component of crystalline sugars and other sources at different energy control levels is unclear. To address this gap, we conducted a systematic review and meta-analysis of controlled trials to examine the effects of different food sources of fructose-containing sugars at different energy control levels on blood uric acid levels and assess the certainty of evidence using the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach.
Methods
We followed the Cochrane Handbook for Systematic Reviews of Interventions (16) for the conduct of our systematic review and meta-analysis and reported our results following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines (17). The study protocol is registered at clinicaltrials.gov as NCT02716870.
Data sources and search strategy
We conducted a systematic search in MEDLINE, Embase, and the Cochrane Central Register of Controlled Studies through 11 January 2021. Supplemental Tables 1 and 2 show the search strategy based on the population, intervention, comparator, outcome, time, and study design framework without language restrictions. Validated filters from the McMaster University Health Information Research Unit were applied to limit the database search to controlled studies only (18). Manual searches of the reference lists of included studies complemented the systematic search.
Study selection
We included randomized and nonrandomized controlled feeding trials in humans of all health backgrounds and ages that had intervention periods ≥ 7 days investigating the role of orally consumed fructose-containing sugars from various food sources compared with control diets free of or lower in fructose-containing sugars on serum uric acid or plasma urate levels. We excluded studies of liquid meal replacement interventions and studies of interventions or comparators of rare sugars that contain fructose (e.g., isomaltulose or melzitose) or were low-calorie epimers of fructose (e.g., allulose, tagatose, sorbose). Four study designs based on energy control were prespecified: 1) “substitution” or isocaloric trials, in which energy from the food sources of fructose-containing sugars was substituted for other nonfructose-containing macronutrients under energy-matched conditions; 2) “addition” trials, in which excess energy from the food sources of fructose-containing sugars was added to the background diet compared to the same diet alone without the excess energy (with or without the use of nonnutritive/low-calorie sweeteners to match sweetness); 3) “subtraction” trials, in which energy from the food sources of fructose-containing sugars was subtracted from background diets compared with the original background diets through displacement by water or low-calorie sweeteners or elimination altogether; and 4) “ad libitum” trials, in which energy from the food sources of fructose-containing sugars was freely replaced (that is, the participants could eat as much or as little as they liked within reasonable limits: e.g., intake required to be between 75% and 125% of predicted daily energy requirements) with other nonfructose-containing macronutrients without any strict control of either the study foods or the background diets, allowing for free replacement of energy. In reports containing more than 1 eligible trial comparison, we included all available trial comparisons.
Data extraction
At least 2 independent reviewers (SA-C, QL, and/or LC) extracted relevant data from eligible studies. Relevant information included the food sources of fructose-containing sugars, number of participants, setting, participant health status, study design, level of feeding control, randomization, comparator, fructose-containing sugar types, macronutrient profiles of the diets, follow-up duration, energy balance, relevant medication use, funding source, and outcome data. Different food sources of fructose-containing sugars are coded based on definitions shown in Supplemental Table 3. Authors were contacted for missing outcome data when it was indicated that a fasting blood uric acid or plasma urate level was measured but data were not reported. In the absence of numerical values for fasting blood uric acid and an inability to obtain the original data from authors, values were extracted from figures using Plot Digitizer where available (19).
Quality assessment
Included studies were assessed for risk of bias independently and in duplicate by ≥2 independent reviewers (SA-C, QL, and/or LC) with the Cochrane Collaboration Risk of Bias Tool (20). An assessment was done across 6 domains of bias (sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting, and other). The risk of bias for each domain was assessed as either “low” (proper methods taken to reduce bias), “high” (improper methods creating bias), or “unclear” (insufficient information provided). A rating of “high” on the other risk of bias domain was given to crossover trials which had no washout between interventions; otherwise, the trial was rated as “low” for this domain. Reviewer discrepancies were resolved by consensus or arbitration by the senior author (JLS).
Outcomes
The primary outcome was the fasting serum uric acid or plasma urate level, which is the ionized form of uric acid. For this analysis, fasting serum uric acid and plasma urate were considered equivalent and are collectively referred to as uric acid. Mean differences (MDs) between the intervention and control arm and respective SEs were extracted for each trial. If these were not provided, they were derived from available data using published formulas (21). Mean pairwise differences in change-from-baseline values were preferred over end values. When median data were provided, they were converted to mean data with corresponding variances using methods developed by Luo et al. (22) and Wan et al. (23). When no variance data were available, the SD of the MDs was borrowed from a trial similar in size, participant health status, and the nature of the intervention.
Data synthesis and analysis
We used STATA software, version 16.1 (StataCorp) for all analyses. As our primary research question sought to assess the effects of different food sources of fructose-containing sugars at different energy control levels, we performed separate pairwise meta-analyses for each of the 4 prespecified designs by energy control level (substitution, addition, subtraction, and ad libitum trials) and assessed the interaction between food sources within each energy control level using the Cochrane Handbook's recommended standard Q-test for subgroup differences (significance at P < 0.10) (24–26). According to the Cochrane Handbook for Systematic Reviews of Interventions, we have defined the term interaction as measuring the varying intervention effect with different populations or intervention characteristics or testing the differences across a subgroup analysis within each energy control level (16).
The principal effect measures were the mean pairwise differences in changes from baseline (or alternatively, end differences) between the food sources of the fructose-containing sugars arm and the comparator arm (significance at P < 0.05). Results are reported as MDs with 95% CIs. Data were analyzed using the generic inverse variance method with a DerSimonian and Laird random-effects model (27). A fixed-effects model was used when the number of trials was <5 (28). Paired analyses were applied to all crossover trials with the use of a within-individual correlation coefficient between treatments of 0.5, as described by Elbourne et al. (29–31). To mitigate a unit-of-analysis error, when arms of trials with multiple intervention or control arms were used more than once, the corresponding sample size was divided by the number of times it was used for a calculation of the standard error (16). Each pairwise trial comparison was considered a separate trial for the purpose of this analysis.
Heterogeneity was assessed using the Cochrane Q statistic and quantified using the I2 statistic (32). We considered an I2 ≥ 50% and PQ < 0.10 as evidence of substantial heterogeneity (21). Sources of heterogeneity were explored by sensitivity and subgroup analyses. We conducted sensitivity analyses by an influence analysis, in which each trial is systematically removed from the meta-analysis with recalculation of the summary effect estimate. A trial whose removal explained the heterogeneity or changed the significance, direction, or magnitude [by more than the minimally important difference for uric acid; ±0.113 mg/dL (33)] of the effect was considered an influential trial. To determine whether the overall results were robust to the use of different correlation coefficients in crossover trials, we also conducted sensitivity analyses using correlation coefficients of 0.25 and 0.75. If ≥10 trials were available (24, 34), we conducted subgroup analyses to explore sources of heterogeneity using meta-regression (significance at P < 0.05). An a priori subgroup analysis was conducted by participant health status, age, baseline blood uric acid levels, fructose sugar type, type of comparator, study design, follow-up, feeding control, fructose-containing sugar dose, randomization, energy balance, funding source, and domains of risk of bias. Post hoc subgroup analyses were conducted by medication use, sugar regulatory designation, and type of mean difference. Meta-regression analyses were used to assess the significance of each subgroup categorically and, when possible, continuously.
Dose-response analyses were performed using meta-regression to assess linear and nonlinear (restricted cubic splines) dose-response gradients (significance at P < 0.05) if there were ≥6 trials (35). We also assessed nonlinear dose-threshold effects with 3 prespecified spline knots at the public health thresholds of 5% (36, 37), 10% (37, 38) and 25% (39) of energy.
If ≥10 trials were available (40), we assessed publication bias by inspecting contour-enhanced funnel plots and conducting formal tests with the Egger's and Begg's tests (significance at P < 0.10) (41–43). If there was evidence of publication bias, we adjusted for funnel plot asymmetry by imputing the missing trial data using the Duval and Tweedie trim-and-fill method and assessed for small study effects (44).
Certainty of the evidence
The certainty of the evidence was assessed using the GRADE approach (45) and software (GRADEpro GDT, McMaster University and Evidence Prime Inc.) (46). Evidence was rated as being of high, moderate, low, or very low certainty. The included controlled trials were initially rated as high certainty by default and then downgraded or upgraded based on prespecified criteria. Reasons for downgrading the evidence included risk of bias [assessed via the Cochrane Risk of Bias tool (21)], inconsistency (substantial unexplained interstudy heterogeneity; I2 ≥ 50%; P < 0.100), indirectness (absence or presence of factors that limit the generalizability of the results), imprecision [95% CI for pooled effect estimates cross the minimally important difference for harm or benefit for uric acid; ±0.113 mg/dL (33)], and publication bias (significant evidence of small study effects). The reason for upgrading the evidence was presence of a significant dose-response gradient (47–54).
Results
Search results
Figure 1 shows the flow of the literature. We identified 1733 reports from databases and manual searches, 1518 of which were excluded based on the title and abstract. Of the 215 reports reviewed in full, 47 reports of controlled feeding trials (85 trial comparisons) in 2763 participants met the eligibility criteria (55–101). These trials included 9 different food sources of fructose-containing sugars [SSBs, sweetened dairy, fruit drink (lemonade), 100% fruit juice, fruit, dried fruit (raisins), sweets and desserts, added nutritive (caloric) sweetener, and mixed sources] across 4 energy control levels: substitution (49 trial comparisons), addition (30 trial comparisons), subtraction (4 trial comparisons), and ad libitum (2 trial comparisons) trials. The mixed-sources food category includes those trials in which the intervention included more than 1 of the food sources (e.g., SSBs and fruits). For the present meta-analysis, all trials categorized as mixed sources included SSBs. Out of the authors who were contacted for missing blood uric acid data, 4 responded with unpublished data (60, 76, 97, 99).
FIGURE 1.
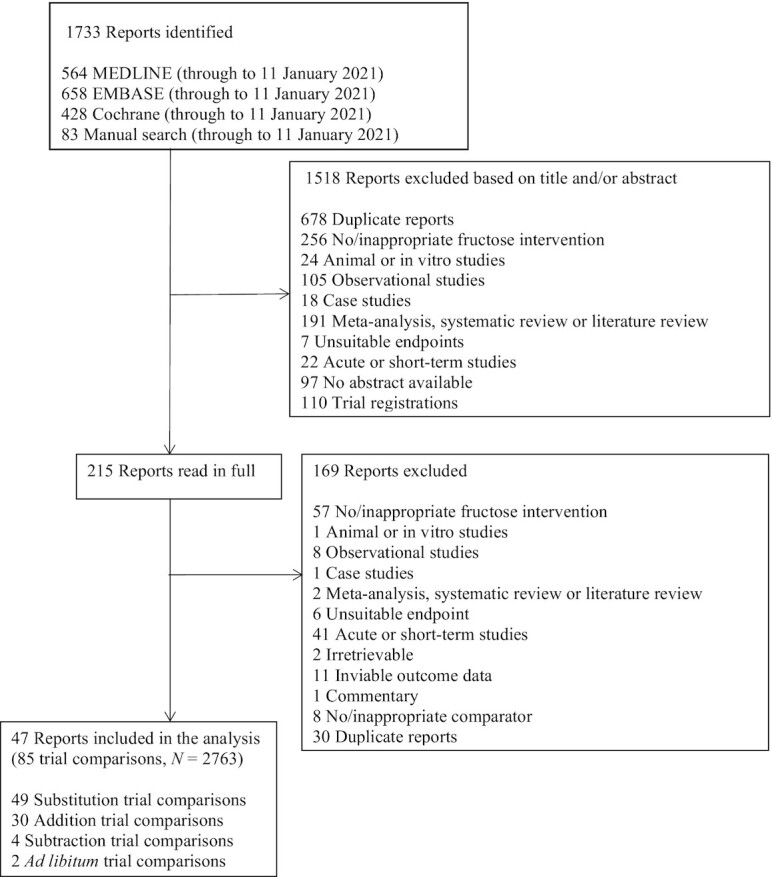
Flow of the literature for the effect of different food sources of fructose-containing sugars on blood uric acid levels.
Trial characteristics
Table 1 and Supplemental Table 4 show the trial characteristics for each energy control level. Mixed sources were the most common food source in substitution (29%) and ad libitum (100%) trials, while SSBs were the most common in addition (43%) and subtraction trials (100%). In substitution trials, most comparators were starch (33%), mixed comparators (33%), or glucose (29%). Most comparators were diet alone (47%) in addition trials, nonnutritive sweeteners (100%) in subtraction trials, and sugar alcohols in ad libitum trials (100%). Trial sizes ranged from a median of 14 participants (range, 8–142 participants) in substitution trials to 82 participants (range, 81–83 participants) in ad libitum trials. Most participants in substitution and addition trials were healthy and had mixed weights (mix of participants with normal weight, overweight, or obese BMIs; 47% and 70%, respectively). Half (50%) of the subtraction trials were conducted with overweight or obese individuals and half (50%) with healthy, mixed-weight individuals, and all ad libitum trials were conducted in healthy and mixed-weight individuals. Participants tended to be middle-aged, with ages ranging from a median of 28 years (range, 26–19 years) in subtraction trials to 40 years (range, 15–63 years) in substitution trials, with approximately equal ratios of both sexes. Most trials were performed in an outpatient setting, with over half of all substitution (57%) trials conducted in the United States, nearly half of all addition (47%) trials conducted in Europe, and half of all subtraction trials conducted in the United States. All ad libitum trials were conducted in Europe. Most trials were randomized (76% of substitution trials, 60% of addition trials, and 100% of subtraction trials), except for ad libitum trials, which were partially randomized (where half of participants were allocated based on their preferences). The mean fructose-containing-sugar dose ranged from a median of 14% of total energy in ad libitum trials (range, 14%–15% of total energy) to 21% in addition trials (range, 2%–35% of total energy). The follow-up durations ranged from a median of 2 weeks in addition trials (range, 1–24 weeks) to 72 weeks in the 1 ad libitum trial. Most of the addition trials were agency funded (government, not-for-profit health agency, or university sources; 73%), half of subtraction trials were agency or agency and industry funded, and most substitution trials were both agency and industry funded (33%). None of the ad libitum trials reported funding sources.
TABLE 1.
Summary of trial characteristics of included trial comparisons for the effects of different food-sources of fructose-containing sugars on blood uric acid levels
| Trial characteristics | Substitution trials | Addition trials | Subtraction trials | Ad libitum trials |
|---|---|---|---|---|
| Trials, n | 49 | 30 | 4 | 2 |
| Participants, n1 | 14 (8–142) | 18 (8–112) | 52.5 (12–96) | 82 (81–83) |
| Underlying disease status, n trials | Healthy, mixed weight = 23, OW/OB = 6, T2D = 9, T1D = 2, HI = 2, CKD = 2, NAFLD = 1, IGT = 2, mixed health status = 2 | Healthy, mixed weight = 21, OW/OB = 1, T2D = 1, HIV = 3, COPD = 1, SF = 1, mixed health status = 2 | Healthy, mixed weight = 2, OW/OB = 2 | Healthy, mixed weight = 2 |
| Sex ratio, %men: women2 | 51: 374 | 55: 455 | NR | NR |
| Age, years1,2 | 40 (15–63)6 | 30 (24–62)7 | 28 (26–29) | NR |
| Age category ratio, %adults: children: mixed | 90: 2: 8 | 100: 0: 0 | 100: 0: 0 | 0: 0: 100 |
| Medication use ratio, %no: unclear: mixed: NR2 | 65: 4: 22: 8 | 93: 0: 3: 3 | 100: 0: 0: 0 | 100: 0: 0: 0 |
| Country, n trials | Europe = 19, USA = 28, Mexico = 2 | Europe = 14, USA = 10, China = 1, Indonesia = 1, Iran = 1, Malaysia = 3 | USA = 2, Switzerland = 2 | Finland = 2 |
| Setting ratio, %inpatients: outpatients: inpatients + outpatient | 12: 53: 35 | 13: 53: 33 | 0: 100: 0 | 0: 100: 0 |
| Baseline fasting UA, mg/dL1,2 | 5.5 (3.4–7.1)8 | 4.8 (4.2–6.9)9 | 5.3 (4.6–6.4) | 4.6 |
| Study design ratio, %crossover: parallel | 59: 41 | 43: 57 | 0: 100 | 0: 100 |
| Feeding control ratio, %met: sup: DA: met + sup | 45: 39: 10: 6 | 30: 47: 0: 23 | 0: 100: 0: 0 | 0: 100: 0: 0 |
| Randomization ratio, %yes: no: partial3 | 76: 24: 0 | 60: 40: 0 | 100: 0: 0 | 0: 0: 100 |
| Fructose-containing sugars dose, % of total energy intake1 | 18 (5–47) | 21 (2–35) | 15.1 (15–15.3) | 14 (14–15) |
| Follow-up duration, weeks1 | 5 (1–52) | 2 (1–24) | 32 (12–52) | 72 |
| Funding sources ratio, %A: I: A + I: NR | 29: 8: 33: 31 | 73: 3: 23: 0 | 50: 0: 50: 0 | 0: 0: 0: 100 |
| Fructose-containing sugars type, n trials | Fructose = 23, sucrose = 12, honey = 1, fruit = 5, HFCS = 4, mixed type = 4 | Fructose = 7, sucrose = 3, honey = 3, fruit = 12, HFCS = 5 | Sucrose = 2, HFCS = 2 | Fructose = 1, Sucrose = 1 |
| Sugar regulatory designation, n trials | Naturally occurring = 5, added = 36, mixed designations = 8 | Naturally occurring = 13, added = 14, mixed designations = 3 | Added = 4 | Mixed designations = 2 |
| Comparator, n trials | Starch = 16, glucose = 14, lactose = 2, nuts = 1, mixed comparators = 16 | NNS = 9, water = 6, diet alone = 14, glucose = 1 | NNS = 3, water = 1 | Sugar alcohol = 2 |
| Food sources of fructose-containing sugars, n trials | SSB = 9; sweetened dairy = 3, fruit = 2, dried fruit = 3, sweets and desserts = 10, added nutritive (caloric) sweetener = 8, mixed sources = 14 | SSB = 13,100%FJ = 8, fruit drink = 1, fruit = 4, sweets and desserts = 1, added nutritive (caloric) sweetener = 3 | SSB = 4 | Mixed sources = 2 |
All values were rounded up, except for baseline UA values. Abbreviations: A, agency funding; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; DA, dietary advice; FJ, fruit juice; HFCS, high-fructose corn syrup; HI, hyperinsulinemic; I, industry funding; IGT, impaired glucose tolerance; met, metabolic diet; NAFLD, nonalcoholic fatty liver disease; NNS, nonnutritive sweetener; NR, not reported; OW/OB, overweight or obese; SF, stone formers; SSB, sugar-sweetened beverages; sup, supplemented diet; T1D, type 1 diabetes mellitus; T2D, type 2 diabetes mellitus; UA, uric acid.
Data presented as median (range).
Based on trials which reported data.
Half of the participants were assigned to groups according to personal preference, while the other half of the participants were randomly allocated (68).
n = 10 trials missing information on sex ratio.
n = 6 trials missing information on sex ratio.
n = 6 trials missing information on age.
n = 1 trial missing information on age.
n = 9 trials missing information on baseline uric acid levels.
n = 7 trials missing information on baseline uric acid levels.
Trial quality
Supplemental Figures 1–4 show the risk of bias assessments by the Cochrane Risk of Bias Tool. Due to poor reporting, most trials were assessed as having an unclear risk of bias for most domains. Exceptions include blinding of the outcome assessment, where most studies were assessed as having a low risk of bias for substitution (69%), addition (73%), and ad libitum (100%) trials. Few trials were assessed as having a high risk of bias for each domain (24% for sequence generation, 24% for allocation concealment, 5% for blinding, 0% for incomplete outcome data and selective outcome reporting, and 26% for other). Overall, there was no serious summary risk of bias across the available trials.
Outcomes
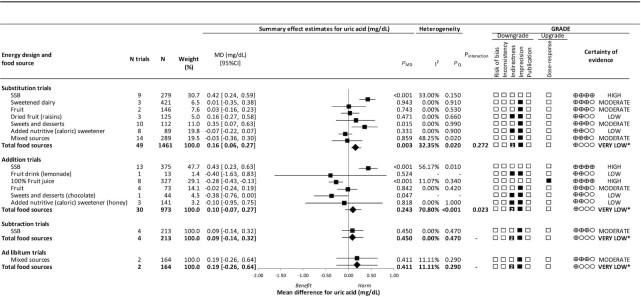
Figure 2 and Supplemental Figures 5–8 show the effects of different food sources of fructose-containing sugars on blood uric acid levels at 4 energy control levels. The total of fructose-containing sugars, independent of the food source, increased uric acid in substitution [49 trials; MD, 0.16 mg/dL; 95% CI: 0.06–0.27 mg/dL; PMD = 0.003; no substantial heterogeneity, I2 = 32.35% (PQ = 0.020)] trials but not in addition [30 trials; MD, 0.10 mg/dL; 95% CI: −0.07 to 0.27 mg/dL; PMD = 0.243; substantial heterogeneity, I2 = 70.80% (PQ < 0.001)], subtraction [4 trials; MD, 0.09 mg/dL; 95% CI: −0.14 to −0.32 mg/dL; PMD = 0.450; no substantial heterogeneity, I2 = 0.00% (PQ = 0.470)] or ad libitum [2 trials; MD, 0.19 mg/dL; 95% CI: −0.26 to 0.64 mg/dL; PMD = 0.411; no substantial heterogeneity, I2 = 11.11% (PQ = 0.290)] trials.
FIGURE 2.
Summary plot for the effects of different food sources of fructose-containing sugars on fasting blood uric acid. Data are weighted mean differences (95% CIs) for summary effects of individual food sources and total food sources on fasting blood uric acid. Analyses were conducted by generic, inverse variance random-effects models (at least 5 trials available) or fixed-effects models (fewer than 5 trials available). Between-study heterogeneity was assessed by the Cochrane Q statistic, where PQ < 0.100 is considered statistically significant, and quantified by the I2 statistic, where I2 ≥ 50% is considered evidence of substantial heterogeneity. The GRADEs of randomized controlled trials are rated as “high” certainty of evidence and can be downgraded by 5 domains and upgraded by 1 domain. The filled black squares indicate a single downgrade and/or upgrade for each outcome, whereas the black squares with a white “2” indicate a double downgrade for each outcome. *Where there was a significant interaction by food source in substitution and addition trials and SSBs and mixed sources were the sole food sources in subtraction and ad libitum trials, we performed the GRADE analysis for each individual food source. To convert uric acid to mmol/L, multiply by 0.0595. Abbreviations: GRADE, Grading of Recommendations, Assessment, Development, and Evaluation; MD, mean difference; PMD, P value for the overall effect; PQ, Cochrane's Q statistic; SSB, sugar-sweetened beverage.
Although there was no statistical evidence for an interaction by food source in substitution trials (P = 0.272), the significant effect of harm seen for total fructose−containing sugars was mainly driven by the significant harm from SSBs, which contributed 30.7% of the weight in the overall analysis. Further, a sensitivity analysis where we removed SSBs changed the effect of the overall analysis so that it was no longer significant (MD, 0.02 mg/dL; 95% CI: −0.07 to 0.1 mg/dL; PMD = 0.656). SSBs [9 trials; MD, 0.42 mg/dL; 95% CI: 0.24–0.59 mg/dL; PMD < 0.001; no substantial heterogeneity, I2 = 33.00% (PQ = 0.150)] and sweets and desserts [10 trials; MD, 0.35 mg/dL; 95% CI: 0.07–0.63 mg/dL; PMD = 0.015; no substantial heterogeneity, I2 = 0.00% (PQ = 0.990)] increased fasting uric acid, while all other food sources showed null effects on fasting uric acid (PMD ranged from 0.331 to 0.943) in substitution trials. There was an interaction by food source in addition trials (P = 0.023) that explained the heterogeneity in the pooled estimate for total fructose-containing sugars, reducing the heterogeneity from I2 = 70.80% (PQ < 0.001) to 39.50% (PQ = 0.023). In addition trials, SSBs increased uric acid levels [13 trials; MD, 0.43 mg/dL; 95% CI: 0.23–0.63 mg/dL; PMD < 0.001; substantial heterogeneity, I2 = 56.17% (PQ = 0.010)], while 100% fruit juice [8 trials; MD, −0.28 mg/dL; 95% CI: −0.43 to −0.13 mg/dL; PMD < 0.001; no substantial heterogeneity, I2 = 11.07% (PQ = 0.340)] and sweets and desserts (chocolate; 1 trial; MD, −0.38 mg/dL; 95% CI: −0.76–0.00 mg/dL; PMD = 0.047) decreased uric acid levels. All other food sources showed null effects on uric acid (PMD ranged from 0.524 to 0.842) in addition trials. We could not assess interactions by food source at the other energy control levels, as SSBs and mixed sources were the sole food sources in subtraction and ad libitum trials.
Sensitivity and subgroup analyses
Supplemental Table 5 and Supplemental Figures 9–23 show sensitivity analyses for the use of different correlation coefficients and an influence analysis for the overall analyses by energy control level and for the analysis by food source. The sensitivity analyses did not alter the significance, direction, or magnitude of any effects, nor did they reduce the evidence of heterogeneity across the 4 energy control levels. Where there was a significant interaction by food source in substitution and addition trials and SSBs and mixed sources were the sole food sources in subtraction and ad libitum trials, sensitivity analyses similarly did not alter the results for most of the individual food sources in these analyses, except for mixed sources of fructose-containing sugars in substitution trials, for which removal of Forster and Heller (sucrose) (66) changed the MD direction and magnitude, without altering its significance [original MD, −0.03 mg/dL (95% CI: −0.36 to 0.30 mg/dL; PMD = 0.859) compared with adjusted MD, 0.11 mg/dL (95% CI: −0.10 to 0.32 mg/dL; PMD = 0.314)], and SSBs in addition trials, for which removal of Büsing et al. (SSB) (96) reduced the heterogeneity from I2 = 56% (PQ = 0.007) to 24% (PQ = 0.208), without changing the magnitude, direction or significance of the effect. Supplemental Figures 24–38 show the subgroup analyses. In substitution trials, there was evidence of subgroup differences by risk of bias in the 2 domains of blinding (P = 0.011, increasing effect of high risk of bias) and other (carry-over effects; P = 0.011, increasing effect of low risk of bias). In addition trials, there was evidence of subgroup differences by fructose type (P = 0.004, increasing effect of fructose and decreasing effect of fruit), comparator [P = 0.007, increasing effect of nonnutritive sweetener (NNS)], feeding control (P = 0.002, increasing effect of metabolic feeding control), sugar regulatory designation (P = 0.006, increasing effect of added sugars and decreasing effects of mixed and naturally occurring sugar designations), and by risk of bias in the domain of sequence generation (P = 0.046, decreasing effect of low risk of bias). Where there was a significant interaction by food source in substitution and addition trials, the only food source showing significant subgroup differences was SSBs in addition trials. Here, there were subgroup differences by fructose type (P = 0.033, increasing effects of fructose and sucrose), comparator (P = 0.019, increasing effects of NNS and water), design (P = 0.014, increasing effect of parallel design), follow-up duration (P = 0.011, increasing effect of trials ≤8 weeks and >8 weeks in duration), and the risk of bias in the 2 domains of blinding (P = 0.002, increasing effects of high and low risk of bias) and other (carry-over effects; P < 0.001, increasing effect of low risk of bias). There were also significant subgroup differences by the continuous subgroup of follow-up duration (P = 0.017; for each additional week, uric acid increased by 0.05 mg/dL; 95% CI: 0.01–0.09 mg/dL). Subgroup analyses were not performed in subtraction or ad libitum trials, as there were <10 trials.
Dose response analyses
Supplemental Figures 39–50 show the linear and nonlinear dose response analyses. We observed a significant nonlinear (P = 0.006) dose response and evidence of a dose threshold at 25% energy for total fructose-containing sugars (P = 0.003), where increases in uric acid were only seen in up to 25% energy in substitution trials. No dose-response gradient or threshold was found in addition trials. As there was a significant interaction by food source in substitution and addition trials, we conducted linear and nonlinear dose response analyses for each of the food sources in these analyses. We observed a significant linear (P = 0.009) dose response for mixed sources of fructose-containing sugars in substitution trials, and a significant linear (P = 0.028) dose response for 100% fruit juice in addition trials. No dose response gradient or threshold was found in any other food source in substitution and addition trials. Dose-response analyses were not performed for subtraction and ad libitum trials, as there were <6 unique doses.
Publication bias
Supplemental Figures 51–55 show the funnel plots. There was no evidence of publication bias in any overall analysis by energy control level. Where there was a significant interaction by food source in substitution and addition trials, we did not detect evidence of a publication bias for any of the other individual food sources. Publication bias analyses were not performed in subtraction or ad libitum trials, as there were <10 trials.
GRADE assessment
Figure 2 and Supplemental Tables 6–7 show certainty of the evidence assessments by GRADE. The evidence for the effects of total fructose-containing sugars on blood uric acid levels was graded as very low in substitution, addition, subtraction, and ad libitum trials (double downgrades for indirectness and single downgrades for imprecision in each case). As there was a significant interaction by food source in substitution and addition trials and because SSBs were the sole food source in subtraction trials and mixed sources were the sole food source in the ad libitum trials, we assessed the certainty of evidence for each of the food sources in these analyses. The evidence was graded as high for the increasing effects of SSBs in substitution and addition trials (no downgrades or upgrades), high for the decreasing effect of 100% fruit juice in addition trials (upgrades for dose-response), moderate for the increasing effect of sweets and desserts in substitution trials (downgrades for imprecision in each case), and low for the decreasing effect of sweets and desserts (chocolate) in addition trials (downgrades for indirectness and imprecision in each case). The evidence of the null effects of other food sources ranged from high to low (owing to downgrades for indirectness and/or imprecision).
Discussion
We conducted a systematic review and meta-analysis of 47 controlled feeding trials involving 85 trial comparisons in 2763 predominantly healthy, mixed-weight adult participants, assessing the effects of 9 different food sources of fructose-containing sugars with median doses ranging from 14% to 21% of total energy across 4 different energy control levels over a median follow-up of 2–72 weeks. We showed that the effects of fructose-containing sugars on uric acid were more dependent on the food source than on energy control. Whereas total fructose-containing sugars increased uric acid levels in substitution trials, no effects were seen in addition, subtraction, or ad libitum trials and there was evidence of an interaction by food source in both substitution and addition trials. SSBs (median dose, 25% energy) and sweets and desserts (median dose, 18% energy) at high doses increased uric acid levels in substitution trials. When consumed as excess calories, SSBs at high doses (median dose, 24% excess energy) increased uric acid, while 100% fruit juice at high doses (median dose, 21% excess energy) decreased uric acid in addition trials. Sweets and desserts (chocolate) providing excess calories also decreased uric acid in an addition trial, but this was based on 1 trial.
Findings in relation to the literature
We failed to replicate the overall results from our previous systematic review and meta-analysis, which showed a harmful effect of fructose on blood uric acid in addition trials, which we did not find in our overall analysis. However, Wang et al. (10) included only 3 trials, all of which were of SSBs, which is consistent with our results for SSBs in the analysis.
These results appear to translate to gout risk. A recent systematic review and meta-analysis of prospective cohort studies investigating the roles of different food sources of fructose-containing sugars found significant, positive associations of SSBs and fruit juice with the risk of gout, but no effect of fruit (102). We found similar associations in our meta-analysis. However, unlike the previous work, which included both fruit drinks and 100% fruit juice as a single group, we were able to separately assess the effects of 100% fruit juice in the addition trials, which may explain our discrepant results.
Several mechanisms can explain the observed effects when food sources of fructose-containing sugars, specifically SSBs, are added to the diet. Excess intake of fructose can increase uric acid through an unregulated fructokinase pathway that uses substantial amounts of ATP (103) to convert fructose into fructose-1-phosphate in the liver (104). Net ATP degradation leads to the accumulation of AMP, which is subsequently degraded to uric acid. Additionally, fructose can increase de novo purine synthesis, which further produces uric acid (105). SSBs do not offer many nutrients besides sugars, whereas other foods, like fruits, present sugars in a complex food matrix in which components like antioxidants, polyphenols, and so forth may counteract the negative effects of fructose (11, 106–114). Interestingly, the food sources which increased uric acid all have a high glycemic index (GI), while food sources showing a reduction in uric acid have a lower GI, which may counteract the effect of fructose by itself on uric acid (115).
The lack of a reduction in uric acid with the displacement of excess calories from SSBs in the subtraction trials was not expected. Systematic reviews and meta-analyses of randomized controlled trials have shown that displacing calories from SSBs using low-calorie sweetened beverages [which was the main method of displacement in the 4 included subtraction trials (83)] leads to weight loss (116), an outcome which, combined with the reduction in fructose exposure, would be expected to lead to a reduction in uric acid. The lack of an effect may have reflected the lack of weight loss in the trial and unexpected reductions in intrahepatocellular lipid (IHCL) on SSBs in the trial comparison in those with high baseline IHCL levels (84). When mixed sources of fructose-containing sugars were freely exchanged in the diet, we observed no effect, perhaps because the diet in the 2 included ad libitum trials (68) included a range of different foods, including SSBs, fruits, and fruit juices, which may have opposing effects on uric acid levels.
Strengths and limitations
Our systematic review and meta-analysis has several strengths. First, we employed a comprehensive and reproducible search and selection process of the literature. Second, we collated and synthesized available evidence from a large body (47 studies; N = 2763) of controlled feeding trials, a design which provides the greatest protection against bias. Third, none of our analyses had substantial unexplained heterogeneity. Fourth, we comprehensively explored possible sources of heterogeneity. Fifth, we evaluated the shape and strength of the dose-response relationships. Finally, we used the GRADE assessment approach to assess the certainty of evidence.
Our analysis also presented limitations. First, there was evidence of serious indirectness in all overall pooled analyses (substitution, addition, subtraction, and ad libitum trials). There were significant interactions by food source in the substitution and addition trials, and so we double downgraded the overall evidence for indirectness for total fructose-containing sugars at these energy control levels. Despite the inclusion of 85 trial comparisons, there were also a limited number of food sources. For example, few or no trials reported on 2 of the important sources of added fructose-containing sugars outside of SSBs; no trials reported on sweetened breakfast cereals; only 3 studies included controlled trials, both from the same trial that reported on sweetened dairy (117); and SSBs were the sole food source in subtraction trials and mixed sources were the sole food source in ad libitum trials, leading to double downgrades for indirectness for total fructose-containing sugars at these energy control levels. Other sources of indirectness that resulted in downgrades included reduced generalizability due to limited health status representations in several analyses [e.g., n = 3 trials with substitution of dried fruit (raisins), all in individuals with nonalcoholic fatty liver disease or type 2 diabetes]. Further, most included trials were conducted in healthy, mixed-weight adults and did not include children, who have been shown to be at risk for developing high blood pressure as a result of high uric acid levels (118). We did not downgrade for this source of indirectness, but instead elected to make our conclusions specific to healthy, mixed-weight adults. Supporting this, our categorical subgroup analysis by age and health status did not support differences between these categories. Second, there was potential inconsistency in some analyses. The pooled estimates for both total fructose-containing sugars and SSBs in addition trials were complicated by evidence of substantial heterogeneity (I2 ≥ 50%; P < 0.1). We did not downgrade for inconsistency, as heterogeneity was explained in addition trials by the interaction by food source for total fructose-containing sugars and, in addition trials of SSBs, the removal of a single trial [Büsing et al. (96)] explained heterogeneity without altering the conclusion of the overall estimate. Further, when looking at the forest plot, we see that most addition SSB trials (12 out of 13) show harm, indicating consistency in the results across trials. Finally, there was evidence of serious imprecision in all overall analyses by energy control level and in most analyses by food source, except for SSBs in substitution and addition trials and 100% fruit juice in addition trials, as CIs crossed the minimally important difference for harm and/or benefit for uric acid.
Weighing the strengths and limitations, the overall certainty of evidence was graded as high for the increasing effects of SSBs in substitution and addition trials and for the decreasing effect of 100% fruit juice in addition trials, moderate for the increasing effect of sweets and desserts in substitution trials, and generally moderate to low for all other comparisons.
Implications
As dietary guidelines have shifted towards a food and dietary pattern–based approach to nutrition and health (11), our findings have implications for guiding recommendations on different food sources of fructose-containing sugars in the prevention and management of hyperuricemia and gout and their downstream cardiometabolic complications. Our results support previous evidence that SSB consumption within or above caloric needs increases uric acid; thus, public health strategies to reduce consumption of SSBs could be useful. Our demonstration of the benefit of 100% fruit juice may offer a potential avenue of SSB replacement, although future research is warranted.
Conclusion
In conclusion, the effects of fructose-containing sugars on blood uric acid appear more dependent on the food source than on energy control. SSBs increase uric acid at high doses and sweets and desserts likely increase uric acid at high doses, whereas 100% fruit juice in excess calories decreases uric acid and other food sources show null effects in predominantly healthy, mixed-weight adults. The anticipated benefit of displacing excess calories from SSBs was not observed. Our confidence in the estimates is generally strongest for the increasing effects of SSBs at high doses and/or when providing excess calories and for the decreasing effect of 100% fruit juice on uric acid when providing excess calories, owing to no downgrades and/or an upgrade for the dose response. This evidence is of high certainty, suggesting that the available evidence provides a reliable indication that SSBs increase and 100% fruit juice decreases uric acid. We are less confident in the evidence for the other food sources at the different energy control levels, which were graded as having generally moderate to low certainty. Owing to serious imprecision in the evidence, there remains a need for more large, high-quality randomized trials to improve our estimates. Owing to serious indirectness in the evidence, trials which broaden the variety of food sources of fructose-containing sugars would be particularly useful to understand whether certain food sources with an apparent benefit might even have advantages for uric acid under free-living conditions over the longer term (6 months or longer). While awaiting these data, policy and guideline makers should consider the influences of energy control and food sources in recommendations.
Supplementary Material
Acknowledgments
JLS attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
The authors’ responsibilities were as follows – JLS, DJAJ, LC, VLC, SBM: designed the research (project conception, development of overall research plan, and study oversight); SA-C, LC, QL, FA-Y, AC, AA, DL: conducted the research (hands-on conduct of the experiments and data collection); SA-C, LC, TAK, AZ, FA-Y: analyzed the data and performed the statistical analysis; SA-C: wrote the paper; SA-C, LC, JLS: had primary responsibility for the final content and take responsibility for the integrity of the data and the accuracy of the data analysis; JLS, DJAJ: supervised the study; and all authors: contributed to the critical revision of the manuscript for important intellectual content and read and approved the final manuscript.
Notes
Aspects of this work were presented at The Diabetes and Nutrition Study Group (DNSG)’s 37th International Symposium on Diabetes and Nutrition, Kerkrade, Netherlands (12–15 June 2019); The St. Michael's Hospital 2018 Research Training Centre Research Day, Toronto, Canada (5 November 2018); and The Canadian Nutrition Society's Thematic 2021 Online Conference (22–23 January 2021).
This work was supported by Diabetes Canada (CS-5–15–4771-JS), the Canadian Institutes of Health Research (CIHR) (funding reference number, 129,920). The Diet, Digestive Tract, and Disease (3-D) Centre, funded through the Canada Foundation for Innovation (CFI) and the Ministry of Research and Innovation's Ontario Research Fund (ORF), provided the infrastructure for the conduct of this project. SA-C was funded by a CIHR Canada Graduate Scholarships Master's Award, the Loblaw Food as Medicine Graduate Award and the Ontario Graduate Scholarship (OGS). LC was funded by a Mitacs-Elevate Postdoctoral Fellowship Award. TAK was funded by a Toronto 3D Foundation Postdoctoral Fellowship Award. AZ was funded by a Banting and Best Diabetes Centre Postdoctoral Fellowship. AC and AA were funded by Toronto 3D Foundation MSc Scholarship Awards. DL was funded by a St. Michael's Hospital Research Training Centre Scholarship. DJAJ was funded by the Government of Canada through the Canada Research Chair Endowment. JLS was funded by a PSI Graham Farquharson Knowledge Translation Fellowship, Diabetes Canada Clinician Scientist award, CIHR INMD/CNS (Institute of Nutrition, Metabolism, and Diabetes/Canadian Nutrition Society) New Investigator Partnership Prize, and Banting & Best Diabetes Centre Sun Life Financial New Investigator Award.
Author disclosures: All authors declare support from Diabetes Canada and the Diet, Digestive Tract, and Disease Centre through the Canada Foundation for Innovation and the Ministry of Research and Innovation's Ontario Research Fund for the submitted work. SA-C has received funding from CIHR Canadian Graduate Scholarship-Master's, the Loblaw Food as Medicine Award, and the Ontario Graduate Scholarship. LC is a Mitacs-Elevate postdoctoral fellow jointly funded by the Government of Canada and the Canadian Sugar Institute and was previously employed as a casual clinical coordinator at INQUIS Clinical Research, Ltd. (formerly Glycemic Index Laboratories, Inc.), a contract research organization. TAK has received research support from CIHR, the International Life Science Institute (ILSI), and the National Honey Board; has been an invited speaker at the Calorie Control Council Annual meeting, for which he received an honorarium; and was funded by a Toronto 3D Postdoctoral Fellowship Award. AZ is a part-time Research Associate at INQUIS Clinical Research, Ltd., a contract research organization, and has received funding from a Banting and Best Diabetes Centre (BBDC) Postdoctoral Fellowship. FA-Y is a part-time Research Assistant at INQUIS Clinical Research, Ltd., a contract research organization. AC had received funding from a Toronto3D MSc Scholarship award, and is now a part-time private practice Dietitian at Axis Healthcare and an employee of the Ministry of Health and Long Term Care for Ontario Health, Home and Community Care Support Services. AA has received funding from a Toronto 3D MSc Scholarship award. DL received a stipend from the University of Toronto Department of Nutritional Sciences Graduate Student Fellowship, University of Toronto Fellowship in Nutritional Sciences, University of Toronto Supervisor's Research Grant–Early Researcher Awards, and Dairy Farmers of Canada Graduate Student Fellowships; and a scholarship from St. Michael's Hospital Research Training Centre. RJdS has served as an external resource person to the WHO’s Nutrition Guidelines Advisory Group on trans fats, saturated fats, and polyunsaturated fats. The WHO paid for his travel and accommodation to attend meetings from 2012–2017 to present and discuss this work. He has also done contract research for the CIHR’s Institute of Nutrition, Metabolism, and Diabetes, Health Canada, and the WHO for which he received remuneration. He has received speaker’s fees from the University of Toronto, and McMaster Children’s Hospital. He has held grants from the CIHR, Canadian Foundation for Dietetic Research, Population Health Research Institute, and Hamilton Health Sciences Corporation as a principal investigator, and is a co-investigator on several funded team grants from the CIHR. He serves as a member of the Nutrition Science Advisory Committee to Health Canada (Government of Canada), and as an independent director of the Helderleigh Foundation (Canada). TMSW is a part owner and president of Glycemic Index Laboratories, Toronto, Canada; has authored several diet books on the glycemic index, for which he received royalties from Phillipa Sandall Publishing Services and CABI Publishers; and has received consultant fees, honorariums, travel funding, or research support from or served on the scientific advisory board for CIHR, Canadian Diabetes Association, Dairy Farmers of Canada, McCain Foods, Temasek Polytechnic, Northwestern University, Royal Society of London, Glycemic Index Symbol programme, CreaNutrition AG, McMaster University, Canadian Society for Nutritional Sciences, National Sports and Conditioning Association, Faculty of Public Health and Nutrition–Autonomous University of Nuevo Leon, and Diabetes and Nutrition Study Group of the European Association for the Study of Diabetes. CWCK has received grants or research support from the Advanced Food Materials Network, Agriculture and Agri-Foods Canada, Almond Board of California, American Pistachio Growers, Barilla, Calorie Control Council, CIHR, Canola Council of Canada, International Nut and Dried Fruit Council, International Tree Nut Council Research and Education Foundation, Loblaw Brands, Pulse Canada, Saskatchewan Pulse Growers, The Peanut Institute and Unilever; has received in-kind research support from the Almond Board of California, The Peanut Institute, Barilla, California Walnut Commission, Kellogg Canada, Loblaw Companies, Quaker (PepsiCo), Primo, Unico, Unilever, and WhiteWave Foods; has received travel support or honorariums from The Peanut Institute, American Pistachio Growers, Barilla, California Walnut Commission, Canola Council of Canada, General Mills, International Nut and Dried Fruit Council, International Pasta Organization, Loblaw Brands Ltd, Nutrition Foundation of Italy, Oldways Preservation Trust, Paramount Farms, Peanut Institute, Pulse Canada, Sabra Dipping, Saskatchewan Pulse Growers, Sun-Maid, Tate & Lyle, Unilever, and White Wave Foods; has served on the scientific advisory board for the International Tree Nut Council, International Pasta Organization, Lantmannen, McCormick Science Institute, Oldways Preservation Trust, Paramount Farms, and Pulse Canada; is a member of the International Carbohydrate Quality Consortium; is an executive board member of the Diabetes and Nutrition Study Group of the European Association for the Study of Diabetes; is on the Clinical Practice Guidelines Expert Committee for Nutrition Therapy of the European Association for the Study of Diabetes; and is a director of the Toronto 3D Knowledge Synthesis and Clinical Trials Foundation. DJAJ has received research grants from Saskatchewan & Alberta Pulse Growers Associations, the Agricultural Bioproducts Innovation Program through the Pulse Research Network, the Advanced Foods and Material Network, Loblaw Companies Ltd., Unilever Canada and Netherlands, Barilla, Almond Board of California, Agriculture and Agri-food Canada, Pulse Canada, Kellogg's Company, Canada, Quaker Oats, Canada, Procter & Gamble Technical Centre Ltd., Bayer Consumer Care, Springfield, NJ, Pepsi/Quaker, International Nut and Dried Fruit council (INC), Soy Foods Association of North America, Coca-Cola Company (investigator initiated, unrestricted grant), Solae, Haine Celestial, the Sanitarium Company, Orafti, the International Tree Nut Council Nutrition Research and Education Foundation, the Peanut Institute, Soy Nutrition Institute (SNI), the Canola and Flax Councils of Canada, the Calorie Control Council, the CIHR, the Canada Foundation for Innovation (CFI) and the Ontario Research Fund (ORF). He has received in-kind supplies for trials as a research support from the Almond Board of California, Walnut Council of California, The Peanut Institute, Barilla, Unilever, Unico, Primo, Loblaw Companies, Quaker (PepsiCo), Pristine Gourmet, Bunge Limited, Kellogg Canada, WhiteWave Foods. He has been on the speaker's panel, served on the scientific advisory board and/or received travel support and/or honoraria from 2020 China Glycemic Index (GI) International Conference, Atlantic Pain Conference, Academy of Life Long Learning, the Almond Board of California, Canadian Agriculture Policy Institute, Loblaw Companies Ltd., the Griffin Hospital (for the development of the NuVal scoring system), the Coca-Cola Company, EPICURE, Danone, Diet Quality Photo Navigation (DQPN), Better Therapeutics (FareWell), Verywell, True Health Initiative (THI), Institute of Food Technologists (IFT), Soy Nutrition Institute (SNI), Herbalife Nutrition Institute (HNI), Saskatchewan & Alberta Pulse Growers Associations, Sanitarium Company, Orafti, the American Peanut Council, the International Tree Nut Council Nutrition Research and Education Foundation, the Peanut Institute, Herbalife International, Pacific Health Laboratories, Nutritional Fundamentals for Health (NFH), Barilla, Metagenics, Bayer Consumer Care, Unilever Canada and Netherlands, Solae, Kellogg, Quaker Oats, Procter & Gamble, Abbott Laboratories, Dean Foods, the California Strawberry Commission, Haine Celestial, PepsiCo, the Alpro Foundation, Pioneer Hi-Bred International, DuPont Nutrition and Health, Spherix Consulting and WhiteWave Foods, the Advanced Foods and Material Network, the Canola and Flax Councils of Canada, Agri-Culture and Agri-Food Canada, the Canadian Agri-Food Policy Institute, Pulse Canada, the Soy Foods Association of North America, the Nutrition Foundation of Italy (NFI), Nutra-Source Diagnostics, the McDougall Programme, the Toronto Knowledge Translation Group (St. Michael's Hospital), the Canadian College of Naturopathic Medicine, The Hospital for Sick Children, the Canadian Nutrition Society (CNS), the American Society of Nutrition (ASN), Arizona State University, Paolo Sorbini Foundation, and the Institute of Nutrition, Metabolism, and Diabetes. He received an honorarium from the United States Department of Agriculture to present the 2013 W.O. Atwater Memorial Lecture. He received the 2013 Award for Excellence in Research from the International Nut and Dried Fruit Council. He received funding and travel support from the Canadian Society of Endocrinology and Metabolism to produce mini cases for the Canadian Diabetes Association (CDA). He is a member of the International Carbohydrate Quality Consortium (ICQC). His wife, Alexandra L Jenkins, is a director and partner of INQUIS Clinical Research for the Food Industry, his 2 daughters, Wendy Jenkins and Amy Jenkins, have published a vegetarian book that promotes the use of the foods described here, The Portfolio Diet for Cardiovascular Risk Reduction (Academic Press/Elsevier 2020 ISBN:978-0-12-810510-8) and his sister, Caroline Brydson, received funding through a grant from the St. Michael's Hospital Foundation to develop a cookbook for one of his studies. JLS has received research support from the Canadian Foundation for Innovation, Ontario Research Fund, Province of Ontario Ministry of Research and Innovation and Science, CIHR, Diabetes Canada, PSI Foundation, Banting and Best Diabetes Centre (BBDC), American Society for Nutrition (ASN), INC International Nut and Dried Fruit Council Foundation, National Dried Fruit Trade Association, National Honey Board (the honey “Checkoff” program overseen by the Agricultural Marketing Service of the U.S. Department of Agriculture [USDA]), International Life Sciences Institute (ILSI), Pulse Canada, Quaker, The United Soybean Board (the soy “Checkoff” program overseen by the Agricultural Marketing Service of the USDA), The Tate and Lyle Nutritional Research Fund at the University of Toronto, The Glycemic Control and Cardiovascular Disease in Type 2 Diabetes Fund at the University of Toronto (a fund established by the Alberta Pulse Growers), and the Nutrition Trialists Fund at the University of Toronto (a fund established by an inaugural donation from the Calorie Control Council). He has received in-kind food donations to support a randomized controlled trial from the Almond Board of California, California Walnut Commission, The Peanut Institute, Barilla, Unilever/Upfield, Unico/Primo, Loblaw Companies, Quaker, Kellogg Canada, WhiteWave Foods/Danone, and Nutrartis. He has received travel support, speaker fees, and/or honoraria from Diabetes Canada, Dairy Farmers of Canada, FoodMinds LLC, International Sweeteners Association, Nestlé, Pulse Canada, Canadian Society for Endocrinology and Metabolism (CSEM), GI Foundation, Abbott, General Mills, Biofortis, ASN, Northern Ontario School of Medicine, INC Nutrition Research & Education Foundation, European Food Safety Authority (EFSA), Comité Européen des Fabricants de Sucre (CEFS), Nutrition Communications, International Food Information Council (IFIC), Calorie Control Council and Physicians Committee for Responsible Medicine. He has or has had ad hoc consulting arrangements with Perkins Coie LLP, Tate & Lyle, Wirtschaftliche Vereinigung Zucker e.V., Danone, and INQUIS Clinical Research. He is a member of the European Fruit Juice Association Scientific Expert Panel and Soy Nutrition Institute (SNI) Scientific Advisory Committee. He is on the Clinical Practice Guidelines Expert Committees of Diabetes Canada, European Association for the Study of Diabetes (EASD), Canadian Cardiovascular Society (CCS), and Obesity Canada/Canadian Association of Bariatric Physicians and Surgeons. He serves or has served as an unpaid scientific advisor for the Food, Nutrition, and Safety Program (FNSP) and the Technical Committee on Carbohydrates of ILSI North America. He is a member of the International Carbohydrate Quality Consortium (ICQC), Executive Board Member of the Diabetes and Nutrition Study Group (DNSG) of the EASD, and is Director of the Toronto 3D Knowledge Synthesis and Clinical Trials foundation. JLS’ wife is an employee of AB InBev. All other authors report no conflicts of interest.
Supplemental Tables 1–7 and Supplemental Figures 1–55 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn.
Abbreviations used: GI, glycemic index; GRADE, Grading of Recommendations, Assessment, Development, and Evaluation; IHCL, intrahepatocellular lipid; MD, mean difference; NNS, nonnutritive sweetener; SSB, sugar-sweetened beverages.
Contributor Information
Sabrina Ayoub-Charette, Department of Nutritional Sciences, Temerty Faculty of Medicine, University of Toronto, Toronto, Ontario, Canada; Toronto 3D Knowledge Synthesis and Clinical Trials Unit, Clinical Nutrition and Risk Factor Modification Centre, St. Michael's Hospital, Toronto, Ontario, Canada.
Laura Chiavaroli, Department of Nutritional Sciences, Temerty Faculty of Medicine, University of Toronto, Toronto, Ontario, Canada; Toronto 3D Knowledge Synthesis and Clinical Trials Unit, Clinical Nutrition and Risk Factor Modification Centre, St. Michael's Hospital, Toronto, Ontario, Canada.
Qi Liu, Department of Nutritional Sciences, Temerty Faculty of Medicine, University of Toronto, Toronto, Ontario, Canada; Toronto 3D Knowledge Synthesis and Clinical Trials Unit, Clinical Nutrition and Risk Factor Modification Centre, St. Michael's Hospital, Toronto, Ontario, Canada.
Tauseef Ahmad Khan, Department of Nutritional Sciences, Temerty Faculty of Medicine, University of Toronto, Toronto, Ontario, Canada; Toronto 3D Knowledge Synthesis and Clinical Trials Unit, Clinical Nutrition and Risk Factor Modification Centre, St. Michael's Hospital, Toronto, Ontario, Canada.
Andreea Zurbau, Department of Nutritional Sciences, Temerty Faculty of Medicine, University of Toronto, Toronto, Ontario, Canada; Toronto 3D Knowledge Synthesis and Clinical Trials Unit, Clinical Nutrition and Risk Factor Modification Centre, St. Michael's Hospital, Toronto, Ontario, Canada; INQUIS Clinical Research Ltd. (formerly Glycemic Index Laboratories, Inc.), Toronto, Ontario, Canada.
Fei Au-Yeung, Department of Nutritional Sciences, Temerty Faculty of Medicine, University of Toronto, Toronto, Ontario, Canada; Toronto 3D Knowledge Synthesis and Clinical Trials Unit, Clinical Nutrition and Risk Factor Modification Centre, St. Michael's Hospital, Toronto, Ontario, Canada; INQUIS Clinical Research Ltd. (formerly Glycemic Index Laboratories, Inc.), Toronto, Ontario, Canada.
Annette Cheung, Department of Nutritional Sciences, Temerty Faculty of Medicine, University of Toronto, Toronto, Ontario, Canada; Toronto 3D Knowledge Synthesis and Clinical Trials Unit, Clinical Nutrition and Risk Factor Modification Centre, St. Michael's Hospital, Toronto, Ontario, Canada.
Amna Ahmed, Department of Nutritional Sciences, Temerty Faculty of Medicine, University of Toronto, Toronto, Ontario, Canada; Toronto 3D Knowledge Synthesis and Clinical Trials Unit, Clinical Nutrition and Risk Factor Modification Centre, St. Michael's Hospital, Toronto, Ontario, Canada.
Danielle Lee, Department of Nutritional Sciences, Temerty Faculty of Medicine, University of Toronto, Toronto, Ontario, Canada; Toronto 3D Knowledge Synthesis and Clinical Trials Unit, Clinical Nutrition and Risk Factor Modification Centre, St. Michael's Hospital, Toronto, Ontario, Canada.
Vivian L Choo, Department of Nutritional Sciences, Temerty Faculty of Medicine, University of Toronto, Toronto, Ontario, Canada; Toronto 3D Knowledge Synthesis and Clinical Trials Unit, Clinical Nutrition and Risk Factor Modification Centre, St. Michael's Hospital, Toronto, Ontario, Canada; Department of Family and Community Medicine, University of Toronto, Toronto, Ontario, Canada.
Sonia Blanco Mejia, Department of Nutritional Sciences, Temerty Faculty of Medicine, University of Toronto, Toronto, Ontario, Canada; Toronto 3D Knowledge Synthesis and Clinical Trials Unit, Clinical Nutrition and Risk Factor Modification Centre, St. Michael's Hospital, Toronto, Ontario, Canada.
Russell J de Souza, Department of Nutritional Sciences, Temerty Faculty of Medicine, University of Toronto, Toronto, Ontario, Canada; Toronto 3D Knowledge Synthesis and Clinical Trials Unit, Clinical Nutrition and Risk Factor Modification Centre, St. Michael's Hospital, Toronto, Ontario, Canada; Department of Health Research Methods, Evidence, and Impact, Faculty of Health Sciences, McMaster University, Hamilton, Ontario, Canada; Population Health Research Institute, Hamilton Health Sciences Corporation, Hamilton, Ontario, Canada.
Thomas Ms Wolever, Department of Nutritional Sciences, Temerty Faculty of Medicine, University of Toronto, Toronto, Ontario, Canada; Toronto 3D Knowledge Synthesis and Clinical Trials Unit, Clinical Nutrition and Risk Factor Modification Centre, St. Michael's Hospital, Toronto, Ontario, Canada; INQUIS Clinical Research Ltd. (formerly Glycemic Index Laboratories, Inc.), Toronto, Ontario, Canada; Division of Endocrinology and Metabolism, Department of Medicine, St. Michael's Hospital, Toronto, Ontario, Canada; Department of Medicine, Temerty Faculty of Medicine, University of Toronto, Toronto, Ontario, Canada.
Lawrence A Leiter, Department of Nutritional Sciences, Temerty Faculty of Medicine, University of Toronto, Toronto, Ontario, Canada; Toronto 3D Knowledge Synthesis and Clinical Trials Unit, Clinical Nutrition and Risk Factor Modification Centre, St. Michael's Hospital, Toronto, Ontario, Canada; Division of Endocrinology and Metabolism, Department of Medicine, St. Michael's Hospital, Toronto, Ontario, Canada; Department of Medicine, Temerty Faculty of Medicine, University of Toronto, Toronto, Ontario, Canada; Li Ka Shing Knowledge Institute, St. Michael's Hospital, Toronto, Ontario, Canada.
Cyril Wc Kendall, Department of Nutritional Sciences, Temerty Faculty of Medicine, University of Toronto, Toronto, Ontario, Canada; Toronto 3D Knowledge Synthesis and Clinical Trials Unit, Clinical Nutrition and Risk Factor Modification Centre, St. Michael's Hospital, Toronto, Ontario, Canada; College of Pharmacy and Nutrition, University of Saskatchewan, Saskatoon, Saskatchewan, Canada.
David Ja Jenkins, Department of Nutritional Sciences, Temerty Faculty of Medicine, University of Toronto, Toronto, Ontario, Canada; Toronto 3D Knowledge Synthesis and Clinical Trials Unit, Clinical Nutrition and Risk Factor Modification Centre, St. Michael's Hospital, Toronto, Ontario, Canada; Division of Endocrinology and Metabolism, Department of Medicine, St. Michael's Hospital, Toronto, Ontario, Canada; Department of Medicine, Temerty Faculty of Medicine, University of Toronto, Toronto, Ontario, Canada; Li Ka Shing Knowledge Institute, St. Michael's Hospital, Toronto, Ontario, Canada.
John L Sievenpiper, Department of Nutritional Sciences, Temerty Faculty of Medicine, University of Toronto, Toronto, Ontario, Canada; Toronto 3D Knowledge Synthesis and Clinical Trials Unit, Clinical Nutrition and Risk Factor Modification Centre, St. Michael's Hospital, Toronto, Ontario, Canada; Division of Endocrinology and Metabolism, Department of Medicine, St. Michael's Hospital, Toronto, Ontario, Canada; Department of Medicine, Temerty Faculty of Medicine, University of Toronto, Toronto, Ontario, Canada; Li Ka Shing Knowledge Institute, St. Michael's Hospital, Toronto, Ontario, Canada.
References
- 1. Campion EW, Glynn RJ, DeLabry LO.. Asymptomatic hyperuricemia. Risks and consequences in the Normative Aging Study. Am J Med. 1987;82(3):421–6. [DOI] [PubMed] [Google Scholar]
- 2. Singh JA, Reddy SG, Kundukulam J.. Risk factors for gout and prevention: A systematic review of the literature. Curr Opin Rheumatol. 2011;23(2):192–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Emmerson BT. Effect of oral fructose on urate production. Ann Rheum Dis. 1974;33(3):276–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fox IH, Kelley WN.. Studies on the mechanism of fructose-induced hyperuricemia in man. Metabolism. 1972;21(8):713–21. [DOI] [PubMed] [Google Scholar]
- 5. Johnson RJ, Perez-Pozo SE, Sautin YY, Manitius J, Sanchez-Lozada LG, Feig DI, Shafiu M, Segal M, Glassock RJ, Shimada Met al. . Hypothesis: Could excessive fructose intake and uric acid cause type 2 diabetes?. Endocr Rev. 2009;30(1):96–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Choi HK, Curhan G.. Soft drinks, fructose consumption, and the risk of gout in men: Prospective cohort study. BMJ. 2008;336(7639):309–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Choi HK, Willett W, Curhan G.. Fructose-rich beverages and risk of gout in women. JAMA. 2010;304(20):2270–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jamnik J, Rehman S, Blanco Mejia S, de Souza RJ, Khan TA, Leiter LA, Wolever TM, Kendall CW, Jenkins DJ, Sievenpiper JL. Fructose intake and risk of gout and hyperuricemia: A systematic review and meta-analysis of prospective cohort studies. BMJ Open. 2016;6(10):e013191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Williams PT. Effects of diet, physical activity and performance, and body weight on incident gout in ostensibly healthy, vigorously active men. Am J Clin Nutr. 2008;87(5):1480–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang DD, Sievenpiper JL, de Souza RJ, Chiavaroli L, Ha V, Cozma AI, Mirrahimi A, Yu ME, Carleton AJ, Di Buono Met al. . The effects of fructose intake on serum uric acid vary among controlled dietary trials. J Nutr. 2012;142(5):916–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sievenpiper JL, Dworatzek PD.. Food and dietary pattern-based recommendations: An emerging approach to clinical practice guidelines for nutrition therapy in diabetes. Can J Diabetes. 2013;37(1):51–7. [DOI] [PubMed] [Google Scholar]
- 12. Hauk L. DGAC makes food-based recommendations in the 2015–2020 Dietary Guidelines for Americans. Am Fam Physician. 2016;93(6):525. [PubMed] [Google Scholar]
- 13. Te Morenga L, Mallard S, Mann J. Dietary sugars and body weight: Systematic review and meta-analyses of randomised controlled trials and cohort studies. BMJ. 2013;346:e7492. [DOI] [PubMed] [Google Scholar]
- 14. Ivers NM, Jiang M, Alloo J, Singer A, Ngui D, Casey CG, Catherine HY.. Diabetes Canada 2018 clinical practice guidelines: Key messages for family physicians caring for patients living with type 2 diabetes. Can Fam Physician. 2019;65(1):14–24. [PMC free article] [PubMed] [Google Scholar]
- 15. Johnson RK, Appel LJ, Brands M, Howard BV, Lefevre M, Lustig RH, Sacks F, Steffen LM, Wylie-Rosett J.. Dietary sugars intake and cardiovascular health: A scientific statement from the American Heart Association. Circulation. 2009;120(11):1011–20. [DOI] [PubMed] [Google Scholar]
- 16. Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, Welch V (editors).. Cochrane handbook for systematic reviews of interventions, version 6.0. (updated July 2019). Cochrane, 2019. [Internet]. Available from: www.training.cochrane.org/handbook. [Google Scholar]
- 17. Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–12. [DOI] [PubMed] [Google Scholar]
- 18. Wilczynski NL, Morgan D, Haynes RB, Team H.. An overview of the design and methods for retrieving high-quality studies for clinical care. BMC Med Inform Decis Mak. 2005;5(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. SourceForge . Plot digitizer. 2001. Updated 24 Oct 2015 [Internet]. Available from: http://plotdigitizer.sourceforge.net/. [Google Scholar]
- 20. Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JAet al. . The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Higgins JPT, Green S (editors).. Cochrane handbook for systematic reviews of interventions version 5.1 (updated March 2011). Cochrane, 2011. [Internet]. Available from: www.handbook.cochrane.org. [Google Scholar]
- 22. Luo D, Wan X, Liu J, Tong T.. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. 2018;27(6):1785–805. [DOI] [PubMed] [Google Scholar]
- 23. Wan X, Wang W, Liu J, Tong T.. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Method. 2014;14(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Borenstein M, Hedges LV, Higgins JP, Rothstein HR.. Introduction to meta-analysis. Chichester, UK: John Wiley & Sons; 2011. [Google Scholar]
- 25. Borenstein M, Higgins JP.. Meta-analysis and subgroups. Prev Sci. 2013;14(2):134–43. [DOI] [PubMed] [Google Scholar]
- 26. Deeks JJ, Higgins JP, Altman DG. Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane; 2019. Available fromwww.training.cochrane.org/handbook. [Google Scholar]
- 27. DerSimonian R, Laird N.. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. [DOI] [PubMed] [Google Scholar]
- 28. Tufanaru C, Munn Z, Stephenson M, Aromataris E.. Fixed or random effects meta-analysis? Common methodological issues in systematic reviews of effectiveness. Int J Evid Based Healthc. 2015;13(3):196–207. [DOI] [PubMed] [Google Scholar]
- 29. Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A.. Meta-analyses involving cross-over trials: Methodological issues. Int J Epidemiol. 2002;31(1):140–9. [DOI] [PubMed] [Google Scholar]
- 30. Follmann D, Elliott P, Suh I, Cutler J.. Variance imputation for overviews of clinical trials with continuous response. J Clin Epidemiol. 1992;45(7):769–73. [DOI] [PubMed] [Google Scholar]
- 31. Balk EM, Earley A, Patel K, Trikalinos TA, Dahabreh IJ.. Empirical assessment of within-arm correlation imputation in trials of continuous outcomes [Internet]. Rockville, MD: Agency for Healthcare Research and Quality (US);2012. Available from:https://www.ncbi.nlm.nih.gov/sites/books/NBK115797/. [PubMed] [Google Scholar]
- 32. Higgins JP, Thompson SG, Deeks JJ, Altman DG.. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shiozawa A, Szabo SM, Bolzani A, Cheung A, Choi HK.. Serum uric acid and the risk of incident and recurrent gout: A systematic review. J Rheumatol. 2017;44(3):388–96. [DOI] [PubMed] [Google Scholar]
- 34. Thompson SG, Higgins JP.. How should meta-regression analyses be undertaken and interpreted?. Stat Med. 2002;21(11):1559–73. [DOI] [PubMed] [Google Scholar]
- 35. Fu R, Gartlehner G, Grant M, Shamliyan T, Sedrakyan A, Wilt TJ, Griffith L, Oremus M, Raina P, Ismaila A.. Conducting quantitative synthesis when comparing medical interventions: AHRQ and the Effective Health Care Program. J Clin Epidemiol. 2011;64(11):1187–97. [DOI] [PubMed] [Google Scholar]
- 36. World Health Organization . Guideline: Sugars intake for adults and children. [Internet]. Geneva, Switzerland: WHO; 2015. Available from: http://apps.who.int/iris/bitstream/handle/10665/149782/9789241549028_eng.pdf;jsessionid=F9FAD19E165BB45830BA1A484FC6FD93?sequence=1. [PubMed] [Google Scholar]
- 37. Scientific Advisory Committee on Nutrition. Carbohydrates and health [Internet]. Norwich, UK: The Stationery Office; 2015. Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/445503/SACN_Carbohydrates_and_Health.pdf. [Google Scholar]
- 38. US Department of Health and Human Services and US Department of Agriculture. 2015–2020 Dietary Guidelines for Americans. 8th Edition. 2015; [Internet]. Available from: https://health.gov/dietaryguidelines/2015/guidelines/. [Google Scholar]
- 39. Panel on Macronutrients, Panel on the Definition of Dietary Fiber, Subcommittee on Upper Reference Levels of Nutrients, Subcommittee on Interpretation and Uses of Dietary Reference Intakes, and the Standing Committee on the Scientific Evaluation of Dietary Reference Intakes, Food and Nutrition Board, Institute of Medicine of the National Academies . Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids. Washington, DC: National Academies Press; 2005. [Google Scholar]
- 40. Sterne JA, Gavaghan D, Egger M.. Publication and related bias in meta-analysis: Power of statistical tests and prevalence in the literature. J Clin Epidemiol. 2000;53(11):1119–29. [DOI] [PubMed] [Google Scholar]
- 41. Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L.. Contour-enhanced meta-analysis funnel plots help distinguish publication bias from other causes of asymmetry. J Clin Epidemiol. 2008;61(10):991–6. [DOI] [PubMed] [Google Scholar]
- 42. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Begg CB, Mazumdar M.. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–101. [PubMed] [Google Scholar]
- 44. Duval S, Tweedie R.. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–63. [DOI] [PubMed] [Google Scholar]
- 45. Schünemann H, Jan Brożek J, Guyatt G, Oxman A. (editors). GRADE handbook 2013 [Internet]. Updated October 2013. Available from: https://gdt.gradepro.org/app/handbook/handbook.html. [Google Scholar]
- 46. GRADEpro GDT . GRADEpro guideline development tool [Software]. McMaster University. Evidence Prime, Inc; 2020; [Internet]. Available from: https://gradepro.org/. [Google Scholar]
- 47. Guyatt GH, Thorlund K, Oxman AD, Walter SD, Patrick D, Furukawa TA, Johnston BC, Karanicolas P, Akl EA, Vist Get al. . GRADE guidelines: 13. Preparing summary of findings tables and evidence profiles–Continuous outcomes. J Clin Epidemiol. 2013;66(2):173–83. [DOI] [PubMed] [Google Scholar]
- 48. Guyatt GH, Oxman AD, Schunemann HJ.. GRADE guidelines–An introduction to the 10th–13th articles in the series. J Clin Epidemiol. 2013;66(2):121–3. [DOI] [PubMed] [Google Scholar]
- 49. Brunetti M, Shemilt I, Pregno S, Vale L, Oxman AD, Lord J, Sisk J, Ruiz F, Hill S, Guyatt GHet al. . GRADE guidelines: 10. Considering resource use and rating the quality of economic evidence. J Clin Epidemiol. 2013;66(2):140–50. [DOI] [PubMed] [Google Scholar]
- 50. Guyatt GH, Oxman AD, Santesso N, Helfand M, Vist G, Kunz R, Brozek J, Norris S, Meerpohl J, Djulbegovic Bet al. . GRADE guidelines: 12. Preparing summary of findings tables–Binary outcomes. J Clin Epidemiol. 2013;66(2):158–72. [DOI] [PubMed] [Google Scholar]
- 51. Guyatt G, Oxman AD, Sultan S, Brozek J, Glasziou P, Alonso-Coello P, Atkins D, Kunz R, Montori V, Jaeschke Ret al. . GRADE guidelines: 11. Making an overall rating of confidence in effect estimates for a single outcome and for all outcomes. J Clin Epidemiol. 2013;66(2):151–7. [DOI] [PubMed] [Google Scholar]
- 52. Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, Norris S, Falck-Ytter Y, Glasziou P, Debeer H.. GRADE guidelines: 1. Introduction–GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383–94. [DOI] [PubMed] [Google Scholar]
- 53. Andrews J, Guyatt G, Oxman AD, Alderson P, Dahm P, Falck-Ytter Y, Nasser M, Meerpohl J, Post PN, Kunz Ret al. . GRADE guidelines: 14. Going from evidence to recommendations: The significance and presentation of recommendations. J Clin Epidemiol. 2013;66(7):719–25. [DOI] [PubMed] [Google Scholar]
- 54. Santesso N, Glenton C, Dahm P, Garner P, Akl EA, Alper B, Brignardello-Petersen R, Carrasco-Labra A, De Beer H, Hultcrantz M.. GRADE guidelines 26: Informative statements to communicate the findings of systematic reviews of interventions. J Clin Epidemiol. 2020;119:126–35. [DOI] [PubMed] [Google Scholar]
- 55. Kanellos P, Kaliora A, Tentolouris N, Argiana V, Perrea D, Kalogeropoulos N, Kountouri A, Karathanos V.. A pilot, randomized controlled trial to examine the health outcomes of raisin consumption in patients with diabetes. Nutrition. 2014;30(3):358–64. [DOI] [PubMed] [Google Scholar]
- 56. McAnulty SR, Mcanulty LS, Nieman DC, Dumke CL, Morrow JD, Utter AC, Henson DA, Proulx WR, George GL.. Consumption of blueberry polyphenols reduces exercise-induced oxidative stress compared to vitamin C. Nutr Res. 2004;24(3):209–21. [Google Scholar]
- 57. Bruun JM, Maersk M, Belza A, Astrup A, Richelsen B.. Consumption of sucrose-sweetened soft drinks increases plasma levels of uric acid in overweight and obese subjects: A 6-month randomised controlled trial. Eur J Clin Nutr. 2015;69(8):949–53. [DOI] [PubMed] [Google Scholar]
- 58. Cox CL, Stanhope KL, Schwarz JM, Graham JL, Hatcher B, Griffen SC, Bremer AA, Berglund L, McGahan JP, Keim NLet al. . Consumption of fructose- but not glucose-sweetened beverages for 10 weeks increases circulating concentrations of uric acid, retinol binding protein-4, and gamma-glutamyl transferase activity in overweight/obese humans. Nutr Metab. 2012;9(68):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Johnston RD, Stephenson MC, Crossland H, Cordon SM, Palcidi E, Cox EF, Taylor MA, Aithal GP, Macdonald IA. No difference between high-fructose and high-glucose diets on liver triacylglycerol or biochemistry in healthy overweight men. Gastroenterology. 2013;145(5):1016–25.e2. [DOI] [PubMed] [Google Scholar]
- 60. Sock ETN, Lê K-A, Ith M, Kreis R, Boesch C, Tappy L.. Effects of a short-term overfeeding with fructose or glucose in healthy young males. Br J Nutr. 2010;103(7):939–43. [DOI] [PubMed] [Google Scholar]
- 61. Silbernagel G, Machann J, Unmuth S, Schick F, Stefan N, Haring HU, Fritsche A.. Effects of 4-week very-high-fructose/glucose diets on insulin sensitivity, visceral fat and intrahepatic lipids: An exploratory trial. Br J Nutr. 2011;106(1):79–86. [DOI] [PubMed] [Google Scholar]
- 62. Angelopoulos TJ, Lowndes J, Sinnett S, Rippe JM.. Fructose containing sugars do not raise blood pressure or uric acid at normal levels of human consumption. J Clin Hypert (Greenwich). 2015;17(2):87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hallfrisch J, Ellwood K, Michaelis OE 4th, Reiser S, Prather ES. Plasma fructose, uric acid, and inorganic phosphorus responses of hyperinsulinemic men fed fructose. J Am Coll Nutr. 1986;5(1):61–8. [DOI] [PubMed] [Google Scholar]
- 64. Israel KD, Michaelis OE 4th, Reiser S, Keeney M. Serum uric acid, inorganic phosphorus, and glutamic-oxalacetic transaminase and blood pressure in carbohydrate-sensitive adults consuming three different levels of sucrose. Ann Nutr Metab. 1983;27(5):425–35. [DOI] [PubMed] [Google Scholar]
- 65. Despland C, Walther B, Kast C, Campos V, Rey V, Stefanoni N, Tappy L.. A randomized-controlled clinical trial of high fructose diets from either Robinia honey or free fructose and glucose in healthy normal weight males. Clin Nutri ESPEN. 2017;19:16–22. [Google Scholar]
- 66. Forster HH, Heller G. Studies on the significance of carbohydrates in a fully synthetic fat-free diet. Dtsch Med Wochenschr. 1973;98(23):1156–63. [DOI] [PubMed] [Google Scholar]
- 67. Grigoresco C, Rizkalla SW, Halfon P, Bornet F, Fontvieille AM, Bros M, Dauchy F, Tchobroutsky G, Slama G.. Lack of detectable deleterious effects on metabolic control of daily fructose ingestion for 2 mo in NIDDM patients. Diabetes Care. 1988;11(7):546–50. [DOI] [PubMed] [Google Scholar]
- 68. Huttunen JK, Mäkinen KK, Scheinin A.. Turku sugar studies XI. Effects of sucrose, fructose and xylitol diets on glucose, lipid and urate metabolism. Acta Odontol Scand. 1976;34(6):345–51. [DOI] [PubMed] [Google Scholar]
- 69. Koh E, Ard N, Mendoza F.. Effects of fructose feeding on blood parameters and blood pressure in impaired glucose-tolerant subjects. J Am Diet Assoc. 1988;88(8):932–8. [PubMed] [Google Scholar]
- 70. Madero M, Arriaga JC, Jalal D, Rivard C, McFann K, Pérez-Méndez O, Vázquez A, Ruiz A, Lanaspa MA, Jimenez CRet al. . The effect of two energy-restricted diets, a low-fructose diet versus a moderate natural fructose diet, on weight loss and metabolic syndrome parameters: A randomized controlled trial. Metabolism. 2011;60(11):1551–9. [DOI] [PubMed] [Google Scholar]
- 71. Madero M, Rodríguez Castellanos FE, Jalal D, Villalobos-Martín M, Salazar J, Vazquez-Rangel A, Johnson RJ, Sanchez-Lozada LG. A pilot study on the impact of a low fructose diet and allopurinol on clinic blood pressure among overweight and prehypertensive subjects: A randomized placebo controlled trial. J Am Soc Hypertens. 2015;9(11):837–44. [DOI] [PubMed] [Google Scholar]
- 72. Porta M, Pigione M, Minonne A, Morisio Guidetti L. Moderate amounts of sucrose with mixed meals do not impair metabolic control in patients with type II (non-insulin dependent) diabetes. Diabetes Nutr Metab Clin Exp. 1989;2(2):133–7. [Google Scholar]
- 73. Reiser S, Powell AS, Scholfield DJ, Panda P, Ellwood KC, Canary JJ.. Blood lipids, lipoproteins, apoproteins, and uric acid in men fed diets containing fructose or high-amylose cornstarch. Am J Clin Nutr. 1989;49(5):832–9. [DOI] [PubMed] [Google Scholar]
- 74. Solyst JT, Michaelis OE 4th, Reiser S, Ellwood KC, Prather ES. Effect of dietary sucrose in humans on blood uric acid, phosphorus, fructose, and lactic acid responses to a sucrose load. Nutr Metab. 1980;24(3):182–8. [DOI] [PubMed] [Google Scholar]
- 75. Gettman MT, Ogan K, Brinkley LJ, Adams-Huet B, Pak CY, Pearle MS. Effect of cranberry juice consumption on urinary stone risk factors. J Urol. 2005;174(2):590–4. [DOI] [PubMed] [Google Scholar]
- 76. Lê K-A, Ith M, Kreis R, Faeh D, Bortolotti M, Tran C, Boesch C, Tappy L.. Fructose overconsumption causes dyslipidemia and ectopic lipid deposition in healthy subjects with and without a family history of type 2 diabetes. Am J Clin Nutr. 2009;89(6):1760–5. [DOI] [PubMed] [Google Scholar]
- 77. Odvina CV. Comparative value of orange juice versus lemonade in reducing stone-forming risk. Clin J Am Soc Nephrol. 2006;1(6):1269–74. [DOI] [PubMed] [Google Scholar]
- 78. Stanhope KL, Medici V, Bremer AA, Lee V, Lam HD, Nunez MV, Chen GX, Keim NL, Havel PJ.. A dose-response study of consuming high-fructose corn syrup-sweetened beverages on lipid/lipoprotein risk factors for cardiovascular disease in young adults. Am J Clin Nutr. 2015;101(6):1144–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Prymont-Przyminska A, Bialasiewicz P, Zwolinska A, Sarniak A, Wlodarczyk A, Markowski J, Rutkowski KP, Nowak D.. Addition of strawberries to the usual diet increases postprandial but not fasting non-urate plasma antioxidant activity in healthy subjects. J Clin Biochem Nutr. 2016;59(3):191–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Amagase H, Sun B, Nance DM.. Immunomodulatory effects of a standardized Lycium barbarum fruit juice in Chinese older healthy human subjects. J Med Food. 2009;12(5):1159–65. [DOI] [PubMed] [Google Scholar]
- 81. Osei K, Falko J, Bossetti BM, Holland GC.. Metabolic effects of fructose as a natural sweetener in the physiologic meals of ambulatory obese patients with type II diabetes. Am J Med. 1987;83(2):249–55. [DOI] [PubMed] [Google Scholar]
- 82. Osei K, Bossetti B.. Dietary fructose as a natural sweetener in poorly controlled type 2 diabetes: A 12-month crossover study of effects on glucose, lipoprotein and apolipoprotein metabolism. Diabet Med. 1989;6(6):506–11. [DOI] [PubMed] [Google Scholar]
- 83. Campos V, Despland C, Brandejsky V, Kreis R, Schneiter P, Chiolero A, Boesch C, Tappy L.. Sugar- and artificially sweetened beverages and intrahepatic fat: A randomized controlled trial. Obesity. 2015;23(12):2335–9. [DOI] [PubMed] [Google Scholar]
- 84. Brymora A, Flisiński M, Johnson RJ, Goszka G, Stefańska A, Manitius J.. Low-fructose diet lowers blood pressure and inflammation in patients with chronic kidney disease. Nephrol Dial Transplant. 2012;27(2):608–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Bantle JP, Laine DC, Thomas JW.. Metabolic effects of dietary fructose and sucrose in types I and II diabetic subjects. JAMA. 1986;256(23):3241–6. [PubMed] [Google Scholar]
- 86. Anderson JW, Story LJ, Zettwoch NC, Gustafson NJ, Jefferson BS.. Metabolic effects of fructose supplementation in diabetic individuals. Diabetes Care. 1989;12(5):337–44. [DOI] [PubMed] [Google Scholar]
- 87. Blayo A, Fontvieille AM, Acosta M, Bruzzo F, Tchobroutsky G, Slama G. Metabolic effects of a one year daily intake of granulated sucrose or fructose by diabetic patients. Diabetologia. 1988;31(7):A472. [Google Scholar]
- 88. Alatas H, Sja'bani M, Mustofa M, Mukti AG, Bawazier LA, Irijanto F, Zulaela Z, Tomino Y.. The effects of soursop supplementation on blood pressure, serum uric acid, and kidney function in a prehypertensive population in accordance with the 2017 ACC/AHA guideline. J Hum Hypertens. 2020;34(3):223–32. [DOI] [PubMed] [Google Scholar]
- 89. Crujeiras AB, Parra MD, Rodríguez MC, de Morentin BEM, Martínez JA.. A role for fruit content in energy-restricted diets in improving antioxidant status in obese women during weight loss. Nutrition. 2006;22(6):593–9. [DOI] [PubMed] [Google Scholar]
- 90. Cerdá B, Soto C, Albaladejo M, Martinez P, Sanchez-Gascon F, Tomás-Barberán F, Espin J.. Pomegranate juice supplementation in chronic obstructive pulmonary disease: A 5-week randomized, double-blind, placebo-controlled trial. Eur J Clin Nutr. 2006;60(2):245–53. [DOI] [PubMed] [Google Scholar]
- 91. Kanellos PT, Kaliora AC, Protogerou AD, Tentolouris N, Perrea DN, Karathanos VT.. The effect of raisins on biomarkers of endothelial function and oxidant damage; An open-label and randomized controlled intervention. Food Res Int. 2017;102:674–80. [DOI] [PubMed] [Google Scholar]
- 92. Alvarez-Suarez JM, Giampieri F, Tulipani S, Casoli T, Di Stefano G, González-Paramás AM, Santos-Buelga C, Busco F, Quiles JL, Cordero MD.. One-month strawberry-rich anthocyanin supplementation ameliorates cardiovascular risk, oxidative stress markers and platelet activation in humans. J Nutr Biochem. 2014;25(3):289–94. [DOI] [PubMed] [Google Scholar]
- 93. Kaliora AC, Kokkinos A, Diolintzi A, Stoupaki M, Gioxari A, Kanellos PT, Dedoussis GV, Vlachogiannakos J, Revenas C, Ladas SD.. The effect of minimal dietary changes with raisins in NAFLD patients with non-significant fibrosis: A randomized controlled intervention. Food Funct. 2016;7(11):4533–44. [DOI] [PubMed] [Google Scholar]
- 94. Karlsen A, Paur I, Bøhn SK, Sakhi AK, Borge GI, Serafini M, Erlund I, Laake P, Tonstad S, Blomhoff R.. Bilberry juice modulates plasma concentration of NF-κB related inflammatory markers in subjects at increased risk of CVD. Eur J Nutr. 2010;49(6):345–55. [DOI] [PubMed] [Google Scholar]
- 95. Chiu S, Siri-Tarino P, Bergeron N, Suh JH, Krauss RM.. A randomized study of the effect of replacing sugar-sweetened soda by reduced fat milk on cardiometabolic health in male adolescent soda drinkers. Nutrients. 2020;12(2):405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Büsing F, Hägele FA, Nas A, Döbert L-V, Fricker A, Dörner E, Podlesny D, Aschoff J, Pöhnl T, Schweiggert R.. High intake of orange juice and cola differently affects metabolic risk in healthy subjects. Clin Nutr. 2019;38(2):812–19. [DOI] [PubMed] [Google Scholar]
- 97. Hieronimus B, Medici V, Bremer AA, Lee V, Nunez MV, Sigala DM, Keim NL, Havel PJ, Stanhope KL.. Synergistic effects of fructose and glucose on lipoprotein risk factors for cardiovascular disease in young adults. Metabolism. 2020;112:154356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Ebbeling CB, Feldman HA, Steltz SK, Quinn NL, Robinson LM, Ludwig DS.. Effects of sugar-sweetened, artificially sweetened, and unsweetened beverages on cardiometabolic risk factors, body composition, and sweet taste preference: A randomized controlled trial. J Am Heart Assoc. 2020;9(15):e015668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Palacios OM, Maki KC, Xiao D, Wilcox ML, Dicklin MR, Kramer M, Trivedi R, Burton-Freeman B, Edirisinghe I.. Effects of consuming almonds on insulin sensitivity and other cardiometabolic health markers in adults with prediabetes. J Am Coll Nutr. 2020;39(5):397–406. [DOI] [PubMed] [Google Scholar]
- 100. Jafarirad S, Ayoobi N, Karandish M, Jalali M-T, Haghighizadeh MH, Jahanshahi A.. Dark chocolate effect on serum adiponectin, biochemical and inflammatory parameters in diabetic patients: A randomized clinical trial. Int J Prev Med. 2018;9:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Tang SP, Wan Yusuf WN, Abd Aziz CB, Mustafa M, Mohamed M. Effects of six-month tualang honey supplementation on physiological and biochemical profiles in asymptomatic, treatment-naïve HIV-infected patients. Trop J Nat Prod Res. 2020;4(12):1116–23. [Google Scholar]
- 102. Ayoub-Charette S, Liu Q, Khan TA, Au-Yeung F, Blanco-Mejia S, De Souza Rj, Wolever Tms, Leiter LA, Kendall C, Sievenpiper JL. Important food sources of fructose-containing sugars and incident gout: A systematic review and meta-analysis of prospective cohort studies. BMJ Open. 2019;5:e024171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Kanbay M, Jensen T, Solak Y, Le M, Roncal-Jimenez C, Rivard C, Lanaspa MA, Nakagawa T, Johnson RJ.. Uric acid in metabolic syndrome: From an innocent bystander to a central player. Eur J Intern Med. 2016;29:3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Garrett R, Grisham C, Andreopoulos S, Willmore W, Gallouzi I. Biochemistry. 1st Canadian ed. Toronto, ON: Nelson Education; 2013. [Google Scholar]
- 105. Choi HK, Mount DB, Reginato AM. Pathogenesis of gout. Ann Intern Med. 2005;143(7):499–516. [DOI] [PubMed] [Google Scholar]
- 106. Yahia EM, García-Solís P, Celis MEM.. Contribution of fruits and vegetables to human nutrition and health. In: Postharvest physiology and biochemistry of fruits and vegetables. Cambridge, UK: Woodhead Publishing; 2019. p. 19–45. [Google Scholar]
- 107. Cui J, Lian Y, Zhao C, Du H, Han Y, Gao W, Xiao H, Zheng J.. Dietary fibers from fruits and vegetables and their health benefits via modulation of gut microbiota. Compr Rev Food Sci Food Saf. 2019;18(5):1514–32. [DOI] [PubMed] [Google Scholar]
- 108. Jia X, Zhong L, Song Y, Hu Y, Wang G, Sun S.. Consumption of citrus and cruciferous vegetables with incident type 2 diabetes mellitus based on a meta-analysis of prospective study. Prim Care Diabetes. 2016;10(4):272–80. [DOI] [PubMed] [Google Scholar]
- 109. Du H, Li L, Bennett D, Guo Y, Key TJ, Bian Z, Sherliker P, Gao H, Chen Y, Yang L.. Fresh fruit consumption and major cardiovascular disease in China. N Engl J Med. 2016;374(14):1332–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Li B, Li F, Wang L, Zhang D.. Fruit and vegetables consumption and risk of hypertension: A meta-analysis. J Clin Hypertens (Greenwich). 2016;18(5):468–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Aune D, Giovannucci E, Boffetta P, Fadnes LT, Keum N, Norat T, Greenwood DC, Riboli E, Vatten LJ, Tonstad S.. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality–A systematic review and dose-response meta-analysis of prospective studies. Int J Epidemiol. 2017;46(3):1029–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Li M, Fan Y, Zhang X, Hou W, Tang Z.. Fruit and vegetable intake and risk of type 2 diabetes mellitus: Meta-analysis of prospective cohort studies. BMJ Open. 2014;4(11):e005497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Wang X, Ouyang Y, Liu J, Zhu M, Zhao G, Bao W, Hu FB.. Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: Systematic review and dose-response meta-analysis of prospective cohort studies. BMJ. 2014;349:g4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Wu L, Sun D, He Y.. Fruit and vegetables consumption and incident hypertension: Dose-response meta-analysis of prospective cohort studies. J Hum Hypertens. 2016;30(10):573–80. [DOI] [PubMed] [Google Scholar]
- 115. Juraschek SP, McAdams-Demarco M, Gelber AC, Sacks FM, Appel LJ, White KJ, Miller ER III. Effects of lowering glycemic index of dietary carbohydrate on plasma uric acid levels: The OmniCarb randomized clinical trial. Arthritis Rheumatol. 2016;68(5):1281–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Rogers P, Hogenkamp P, De Graaf C, Higgs S, Lluch A, Ness A, Penfold C, Perry R, Putz P, Yeomans M.. Does low-energy sweetener consumption affect energy intake and body weight? A systematic review, including meta-analyses, of the evidence from human and animal studies. Int J Obes. 2016;40(3):381–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Marriott BP, Cole N, Lee E.. National estimates of dietary fructose intake increased from 1977 to 2004 in the United States. J Nutr. 2009;139(6):1228S–35S. [DOI] [PubMed] [Google Scholar]
- 118. Park B, Lee HA, Lee SH, Park BM, Park EA, Kim HS, Cho SJ, Park H.. Association between serum levels of uric acid and blood pressure tracking in childhood. Am J Hypertens. 2017;30(7):713–18. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.