Abstract
Plant-based expression system can be used to produce biopharmaceuticals with many potential advantages, but plants cannot be directly used to express functional human glycoproteins because of their differences in glycosylation abilities from mammals. To exploit a plant-based expression for producing recombinant human erythropoietin (rhuEPO), we glycoengineered tobacco plants by stably introducing seven to eight mammalian genes including a target human EPO into tobacco genome in order to generate capacities for β1,4-galactosylation, bisecting N-acetylglucosamine (GlcNAc) and sialylation. Wild type human β1,4-galactosyltransferase gene (GalT) or a chimeric GalT gene (ST/GalT) with GalT cytoplasmic-transmembrane-stem region (CTS) replaced by the CTS from the rat 2,6-sialyltransferase (ST) was co-expressed to produce rhuEPO bearing β1,4-galactose-extended N-glycan chains as well as to compare their β1,4-galactosylation efficiencies. Mammalian UDP-N-acetylglucosamine 2-epimerase/N-acetylmannosamine kinase gene (GNE), N-acetylneuraminic acid phosphate synthase gene (NANS), CMP-N-acetylneuraminic acid synthetase gene (CMAS), CMP-sialic acid transporter gene (CST) and α−2,6-sialyltransferase gene (ST) were co-expressed to build sialylation capacity in plants. In addition, the human MGAT3 encoding N-acetylglucosaminyltransferase III (GnTIII) was also co-expressed to produce N-glycan chains with bisecting GlcNAc. Our PCR and RT-PCR results demonstrated that the above transgenes were not only incorporated into tobacco genome but also properly transcribed. Both GalT and ST/GalT were found to add β1,4-galactose residues to the N-glycan chains, but the latter was more efficient. Furthermore, co-expressing MGAT3 could generate bisected GlcNAc. However, our current efforts did result in generating sialylation capacity. Created transgenic plants expressing EPO and ST/GalT could be used to produce rhuEPO with high proportion of β1,4-galactose-extended N-glycan chains for tissue protective purposes.
Keywords: RhuEPO, N-glycosylation, β1, 4-galactosylation, bisecting GlcNAc, glycoengineering, tobacco
1. Introduction
Recombinant human erythropoietin (rhuEPO) is a heavily glycosylated glycoprotein with three N-linked and one O-linked glycan chains (Takeuchi and Kobata, 1991; Jelkmann 1992). Its N-linked chains are essential for its stability and in vivo biological activity (Wasley et al. 1991). Furthermore, different N-glycan terminal sugar residues not only affect the function of rhuEPO but also determine its potential application. Mammalian cell-produced rhuEPO (rhuEPOM) with terminal sialic acids possesses a long plasma half-life and exhibits both hematopoietic (Tsuda et al., 1990; Fukuda et al., 1989) and tissue-protective activities (Ghezzi and Brines, 2004), and is widely used to treat anemia caused by various disease conditions (Jelkmann 1992; Smith et al. 2003). However, rhuEPOM cannot be directly used for tissue protection because of its hematopoietic activity related adverse effects (Erbayraktar et al. 2003; Wiessner et al. 2001). On the other hand, desialylated rhuEPO (asialo-rhuEPO) prepared by enzymatic removal of its terminal sialic acid residues with a very short plasma half-life displays only cytoprotective activity (Erbayraktar et al. 2003). Asialo-rhuEPO exhibits remarkable tissue protection against damage triggered by ischemia/reperfusion, hypoxia or cytotoxic agents in the brain, the heart, the kidneys and the liver (Brines et al., 2000; Calvillo et al., 2003; Vesey et al., 2004; Grief et al., 2010). Therefore, finding alternative ways to produce rhuEPO with different N-glycan profiles is essential to meet demand for its various applications.
RhuEPO is presently produced in mammalian cells (CHO cells) because of their capacities to make human-like glycosylation, including sialylation which essential for its hematopoietic activity. Other expression systems such as bacteria, yeasts, insect cells and plants, either lack or have different glycosylation capacities (Ghaderi et al., 2012). The major disadvantages of using mammalian cell system to manufacture rhuEPO are high production cost, potential risk of pathogen contamination and limited expandability. Despite many efforts to reduce its production cost, rhuEPO remains to be a very expensive (Weise et al. 2007), which imposes an economic burden on patients. The other drawback of CHO cell-produced rhuEPO is a slight difference in N-glycan structures from those produced in humans. Glycoproteins produced in mammalian cells carry non-human N-glycolylneuraminic acids (Neu5Gc) instead of N-acetylneuraminic acids (Neu5Ac), which could potentially cause an immune response and affect its efficacy (Jacobs and Callewaert, 2009; Padler-karavani and Varki 2011; Ghaderi et al. 2012). Producing asialo-rhuEPO for tissue-protective purpose is even more challenging because there is no mammalian cell line that can be used to express asialo-rhuEPO directly. Preparation of asialo-rhuEPO by enzymatic removal of sialic acid residues from commercial rhuEPOM is not economically viable because of high cost and limited production of rhuEPOM (Weise et al. 2007). In addition, high doses of asialo-rhuEPO are needed for the tissue-protection purpose (Brines and Cerami, 2008). Therefore, there is a need for an alternative expression system that can produce rhuEPO with different glycan profiles inexpensively.
Recent progress in plant glycoengineering (Strasser et al., 2014; Montero-Morales and Steinkellner, 2018) have enabled plant-based expression system to be used an alternative system to produce rhuEPO with different N-glycan profiles. The N-glycan core is conserved in both plants and animals, but plants lack activities for β1,4-galactosylation and sialylation (Lerouge et al. 1998; Bakker et al. 2001; Ma et al. 2003). Based on verified ability of human β1,4-galactosyltransferase (GalT) expressed in plants to extend N-glycan chains of antibodies with β1,4-galactose residues (Palacpac et al. 1999; Bakker et al. 2001, 2006), we (Kittuet al. 2013) and Parson et al. (2012) stably co-expressed human EPO and wild-type GalT genes in tobacco plants and moss, respectively to produce asialo-rhuEPO with β1,4-galactose-extended glycan chains. However, the proportion of N-glycan chains bearing β1,4-galactose in rhuEPO was low (Kittur et al. 2013). Since the terminal β1,4-galactose residues can have an impact on rhuEPO tissue-protective activity, and are also pre-requisite for sialylation, further improving β1,4-galactosylation efficiency is necessary. Using a chimeric gene ST/GalT in which GalT cytoplasmictransmembrane-stem region (CTS) replaced with CTS from the rat 2,6-sialyltransferase (ST) has been found to be more efficient than wild-type GalT in generating homogeneously β1,4-galactosylated Nglycan chains on antibodies (Strasser et al., 2009). For sialylation, in a proof-of-concept study, Castilho and co-workers (2010) recently showed that transient co-expression of six mammalian genes, including GalT in plants produced sialylated antibodies. They also genetically modified plants and proved their capacities for producing sialylated (Castilho et al., 2013; Jez et al., 2013; Kallolimath et al., 2016), bisected and multiantennary complex N-glycans (Castilho et al., 2011, 2013) by transient expression of target proteins.
Recently, human EPO alone or EPO fused with a sequence encoding the IgG-Fc domain (EPO-Fc) has been transiently expressed in the above glycoengineered plants to produce β1,4-galactosylated and sialylated rhuEPO with our without bisected GlcNAc (Castilho et al. 2011, 2013; Jez et al. 2013). A potential disadvantage of using a transient expression system for producing glycoproteins is potential batch-to-batch variation in the glycosylation since it is a challenge to have all multiple genes expressed simultaneously and efficiently (Kallolimath et al., 2016). Variation in glycosylation is known to affect biological activities of glycoproteins (Raju, 2003), and it could potentially cause delay or deny the approval by a regulatory agency. Conversely, the stable expression approach with the selected line(s) that is thoroughly characterized would be expected to have least batch-to-batch variation thereby preserving biological activity and easing the regulatory process. The transgenic seeds could be seed-banked and used indefinitely.
In the present study, we set out to generate stable tobacco transgenic lines expressing human EPO together with genes for β1,4-galactosylation, bisecting GlcNAc and sialylation (Fig. 1), which are absent in plants (Stanley and Campbell, 1984; Edmund et al, 1998; Gomord and Faye, 204; Castilho et al., 2010). Nicotiana tabacum variety W38 plants were chosen for this study because of its high biomass suitable for large scale production of therapeutic glycoproteins. We introduced a chimeric ST/GalT along with sialylation pathway genes into N. tabacum to determine the β1,4-galactosylation efficiency and sialylation capacity. In addition, we expressed wild-type GalT, mammalian MGAT3 encoding GnTIII along with sialylation pathway genes to validate whether expressed rhuEPO would have bisecting GlcNAc with sialic acids in its N-glycan chains. N-glycan analysis of purified rhuEPO from transgenic line expressing ST/GalT and sialylation pathway genes revealed as high as 68% β1,4-galactosylation while another line expressing wild-type GalT, MGAT3 and sialylation pathway genes had moderate β1,4-galactosylation and demonstrated the presence of N-glycans with bisected GlcNAc. Intriguingly, in transgenic lines expressing chimeric ST/GalT and sialylation pathway genes, about 16–18% of N-glycans bore pentoses and hexoses at the non-reducing terminal instead of Neu5Ac residues. The transgenic plants generated in the present study with capacities to add high levels of terminal β1,4-galactose residues to the N-glycan chains could potentially be used to produce asialo-rhuEPO for special therapeutic purposes.
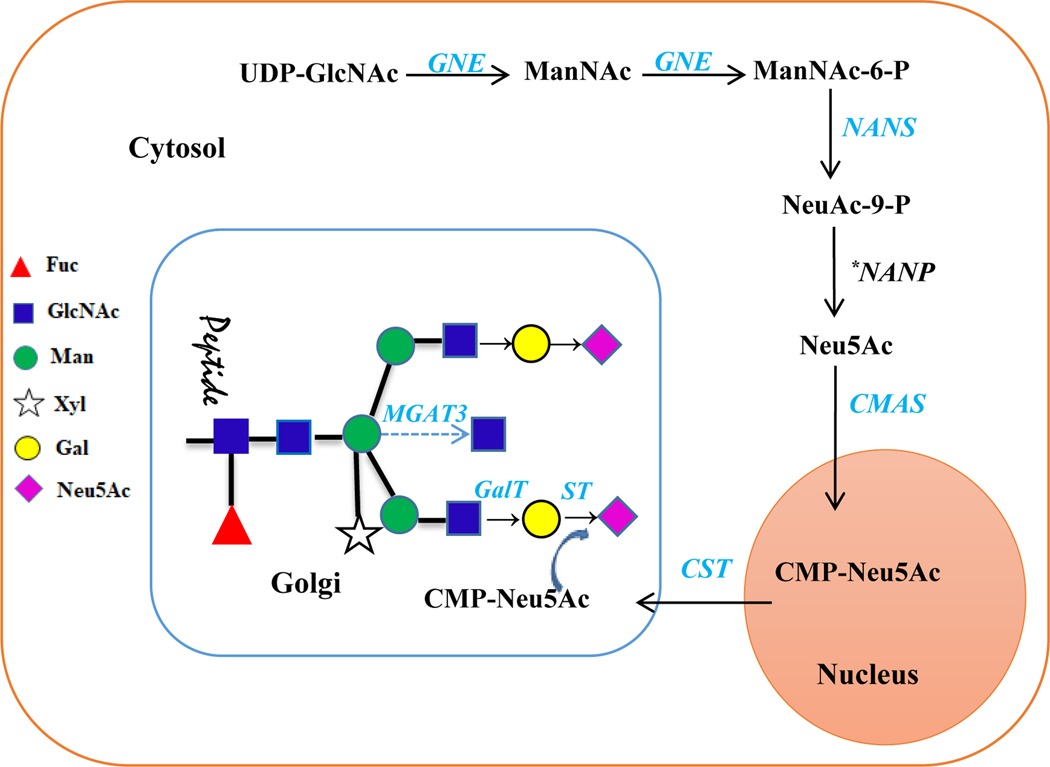
Fig. 1. Schematic representation of N-glycosylation pathway modified from Castilho et al., 2010.
Seven mammalian genes (GNE, NANS, CMAS, CST, GalT, MGAT3 and ST) were used for generating β1,4-galactosylation, bisecting GlcNAc and sialylation capacities in glycoengineered tobacco plants. *: Plants have a endogenous NANP.
2. Materials and methods
2.1. Plant materials
Sterilized seedlings of tobacco (N. tabacum L., cultivar W38) prepared as described previously (Musa et al., 2009) were used in the current study to generate stable transgenic plants expressing mammalian N-glycosylation pathway genes. Transgenic plants grown under greenhouse conditions were used for transgene analysis as well as protein isolation and characterization.
2.2. Construction of expression cassettes for modulating plant N-glycosylation pathway
To generate human-like N-glycosylation pathway in transgenic tobacco plants, five mammalian genes GNE, NANS, CMAS, CST and ST involved in Neu5Ac acid synthesis, transport and transfer were cloned into binary vector pBI121 (Fig. 2, cassette A). For generating β1,4-galactosylation and bisecting GlcNAc capacities, GalT or chimeric ST/GalT with and without MGAT3 were cloned along with target glycoprotein gene EPO into another binary vector MpBI121 to create two separate genetic cassettes (Fig. 2, cassettes B1 and B2). MpBI121 was modified from pBI121 by replacing selection gene nptII with bar. Information of all nine genes is listed in Supplementary Table 1. Coding region sequences of GNE, NANS, CMAS, CST and ST were synthesized by the Life Technologies (www.lifetechnologies.com). The MGAT3 cDNA was purchased from the GeneCopoeia (Product ID: Z1511, www.genecopoeia.com). Previously used coding regions of GalT and EPO without any tags (Kittur et al., 2012) were used for subcloning. Chimeric ST/GalT was synthesized based on the report by Strasser et al. (2009) by replacing cytoplasmic-transmembrane-stem region (CTS) of GalT with 156 bp fragment encoding 52 amino acids from the N-terminal CTS of ST. Coding regions of all genes were sequenced to verify their accuracy before sub-cloning. Different combination of promoters (P), including tobacco glyceraldehyde-3-phosphate dehydrogenase C gene promoter (GapC-P), nopaline synthase gene promoter (nos-P), UbiU4 promoter (UbiU4-P), CaMV35S promoter (35S-P), or double CaMV35S promoter (35S2-P), and GapC terminator (GapC-T) or nos terminator (nos-T), were used to drive the expression of each gene. The combinations of the promoter and terminator used to regulate each gene expression are shown in Fig. 2.
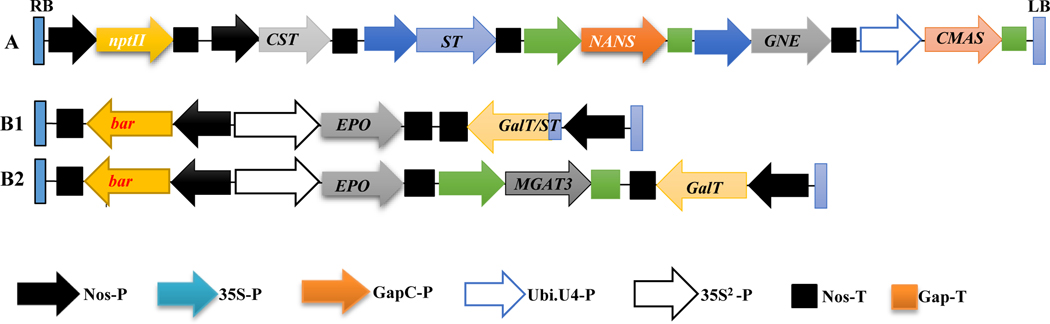
Fig. 2. Schematic representation of three genetic cassettes created for transformation.
Cassette A: Five mammalian genes were cloned into pBI121 expression vector. Resultant cassette contains nos-P::CST::nos-T, 35S-P::ST::nos-T, GapC-P::NANS::GapC-T, 35S-P::GNE::nos-T, and Ubi.U4-P::CMAS::GapC-T with nptII as a selective marker. Cassette B1: Human EPO and chimeric GalT genes were cloned into modified expression vector MpBI121. Resultant cassette contains 35S2-P::EPO::nos-T and nos-P::ST/GalT::nos-T with bar as a selection gene. B2: MGAT3, wild-type GalT and human EPO genes were cloned into modified expression vector MpBI121. Resultant cassette B3 contains 35S2P::EPO::nos-T, GapC-P::MGAT3::GapC-T and nos-P::GalT::nos-T with bar as a selection gene.
2.3. Confirmation of the presence of each gene in different genetic cassettes before and after Agrobacterium transformation
To confirm the presence of each gene in three genetic cassettes, including nptII and bar selection genes, PCR amplification was performed with a pair of gene specific primers (Supplementary Table 2). After confirming the presence of each gene in three different genetic cassettes by PCR, they were introduced into A. tumefaciens strain LBA4404 using the freeze-thaw method (Holsters et al., 1978). The presence of each gene in each genetic cassette was further confirmed by PCR analysis in transformed Agrobacterium cells to check their stabilities inside of Agrobacterium cells.
2.4. Agrobacterium-mediated tobacco plant transformation
The three generated genetic cassettes were used in two combinations (A+B1 and A+B2) to transform tobacco plants. Our previous Agrobacterium-mediated transformation method (Musa et al., 2009) was used with some modifications. Briefly, Agrobacterium strain carrying cassette A was mixed with the strain carrying B1 or B2 separately to infect leaf discs. After 3-day dark co-culture, infected explants were transferred onto selection medium with both 5 mg/L glufosinate ammonium (herbicide) and 300 mg/L kanamycin. Regenerated shoots resistant to both glufosinate and kanamycin were rooted. A total of 95 and 120 glufosinate and kanamycin resistant lines were obtained for genetic cassettes A+B1 and A+B2 combination, respectively.
2.6. Extraction of total soluble protein from transgenic tobacco lines
All 215 tobacco transgenic lines transformed with cassettes A+B1 and A+B2 were pre-screened for rhuEPO expression levels while they were growing in baby jars with 5–6 leaves. Total soluble proteins (TSP) from each line were extracted by grinding 1 g of leaf tissues with 3 ml of ice-cold 0.1 M Tris-HCl (pH 8) containing 0.1 M KCl, 2% PVPP and protease inhibitor cocktail. The extracts were centrifuged at 15,000 x g for 15 min at 4°C to remove plant debris. The clear supernatants were transferred to another microcentrifuge tube and spun again at 15,000 x g to remove particulates. The clear extracts were then transferred to microcentrifuge tubes, and stored at −20°C until analyses for total protein by Bradford assay and soluble rhuEPO by sandwich ELISA assay, respectively. After pre-screen, about twenty lines with higher expression levels from each genetic cassette combination were selected and transplanted in greenhouse. When they reached 15–16 leaf-stages, the third leaf from the top of each line was harvested to measure rhuEPO levels again with the same TSP extraction procedure described above.
2.7. Protein determination and quantification of soluble rhuEPO levels in transgenic lines
In order to prepare samples for sandwich ELISA, the protein concentration was first determined by Bradford method (Bradford, 1976). The extracts were thawed, spun at 15,000 x g for 10 min, and the clear supernatants were diluted 3 times with PBS. Protein concentration was determined using Bradford reagent with BSA as standard (0–1.0 mg/mL concentration range) in a microassay format as described by the manufacturer (Bio-Rad, Hercules, CA, USA). To detect total soluble rhuEPO in the above extracts, sandwich ELISA was performed as described previously (Kittur et al., 2012). Approximately 25 μg of TSP was taken into 400 μL of ELISA blocking buffer (EBB, PBS containing 2.7% BSA and 0.05% Tween-20), and ~6.25 μg of proteins was applied in each well. Assay was performed in triplicates.
2.8. PCR analysis to confirm the presence of transgenes in transgenic lines
Six transgenic lines from each genetic cassette combination were selected for transgene analysis. To confirm the presence of all transgenes, including selection genes nptII and bar, total genomic DNA was isolated by Plant DNeasy kit (Qiagen) and PCR was performed under same reaction conditions for each gene as mentioned in section 2.3 except for using genomic DNA as template. Plasmid DNA was used as a positive control.
2.9. Reverse transcriptase PCR (RT-PCR) analysis to confirm the expression of transgenes in transgenic lines
The same transgenic lines were used to detect transcripts of each gene by RT-PCR. RNA isolation and first strand cDNA synthesis were carried out as described previously (Musa et al. 2009). Primers and PCR amplification conditions were the same as described for genomic DNA PCR except that cDNA was used as template. The primer pair from QuantumRNA™ 18S Internal Standards kit (Ambion, Austin, TX, USA) targeting the 18S rRNA was used as an internal control. Plasmid DNA was used as a positive control.
2.10. Western blot analysis to determine the size of plant-produced rhuEPO
For Western blot analysis, same transgenic lines were used to determine the size of plant-produced rhuEPO. Soluble protein extracts containing ~40–45 μg of proteins from were boiled with Laemmli sample buffer and separated on a 12.5% SDS/PAGE. Ten nanograms of rhuEPOM and about 21 μg of soluble proteins from transgenic tobacco line A56–5 expressing asialoerythropoietin (asialorhuEPO) (Kittur et al., 2013) were used as a size control. Following separation, proteins were transferred onto PVDF membrane using 10 mM CAPS (pH 11) as transfer buffer. The membrane was blocked with 5% BSA in PBST for 1 h at room temperature, followed by incubation with mouse anti-EPO monoclonal antibody (0.5 μg/mL) at room temperature for 1 h with gentle shaking. A secondary HRP-conjugated goat anti-mouse IgG antibody (dilution of 1:5,000) (Jackson Immuno Research, West Grove, PA, USA) was used to reveal the protein bands. The luminescent signal was generated after 30 sec incubations with Super Signal ®West Pico Chemiluminescent substrate (Thermo Scientific, Rockford, IL, USA) and captured by Kodak Biomax light film (PerkinElmer, Waltham, MA, USA).
2.11. Purification of rhuEPO from transgenic tobacco lines
For N-glycan analysis, rhuEPO was purified from transgenic lines B1–1 (A+B1) and B2–5 (A+B2) using immunoaffinity chromatography. Briefly, about 1 kg of leaf tissues was grounded into fine powder in liquid nitrogen and transferred to a beaker containing 3 L of chilled 0.1 M Tris-HCl buffer (pH 8.0) with protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO, USA). The grounded tissue was allowed to soak in the buffer for 1 h, followed by brief homogenization using a blender. The extracts were filtered through a mira cloth to remove leaf debris. After filtration, the extract was centrifuged at 8,000 x g for 20 min to remove particulates. The pH of the extract was adjusted to 7.5 with HCl, and a second centrifugation was performed at 8,000 x g for 20 min. The clear extract was filtered again through a mira cloth to remove any particulates. Anti-EPO antibody coupled resin (10 ml) (Kittur et al., 2013) was then added to the extract and stirred overnight to allow binding of rhuEPO to the resin. The antibody resin was then packed in a column and washed with 10 CV of 0.1 M Tris-HCl buffer to remove unbound proteins. Bound rhuEPO was eluted with 0.1 M Glycine-HCl buffer (pH 2.5), and immediately neutralized with 1 M Tris-HCl, pH 8.0. About fifteen 10 ml fractions were collected, pooled and concentrated using Centricon-70 Plus centrifugal device (Millipore, Burlington, MA, USA). The amount of rhuEPO recovered was determined by sandwich ELISA as described above. Purification was performed at 4 °C.
2.12. Glycoproteomics analysis and site mapping
Purified rhuEPO proteins from transgenic lines B1–1 and B2–5 were subjected to glycoproteomics analysis. Proteins were resolved on a 10% SDS-PAGE and stained with Coomassie. The protein bands corresponding to the molecular weights of rhuEPO glycoforms were excised, cut into small pieces of about 1 mm2 and destained with 1:1 (v/v) digestion buffer (50 mM aq. NH4HCO3)-acetonitrile followed by 100 % acetonitrile. The proteins in the gels were then reduced by adding 50 μL of DTT solution (25 mM), carbamidomethylated by adding 50 μL of iodoacetamide solution (25 mM), and finally washed with 200 μL acetonitrile. Furthermore, 50 μL of digestion buffer was added to the gel pieces and proteins in the gels were digested by adding 5 μL of sequencing-grade trypsin (0.5 μg/μL) (Promega, Madison, WI, USA) and incubated at 37 °C for 12 h. The gel pieces were subsequently treated with 5 μL of sequencing-grade GluC (0.5 μg/μL) (Promega) and incubated again at 37 °C for 12 h. The digested peptides were extracted with 5% formic acid in 1:2 (v/v) water-acetonitrile. The digests were then dried, and peptides and glycopeptides were subsequently re-dissolved in solvent A (0.1% formic acid in water) and stored at −30 °C until analysis by nano-LC-MS/MS.
Data acquisition of protein digested samples was performed using nano-LC-MS/MS. Briefly, desalted digests of glycoproteins were analyzed on an Orbitrap Fusion Tribrid mass spectrometer equipped with a nanospray ion source and connected to a nano-LC system. Pre-packed nano-LC columns of 15 cm length with 75 μm internal diameter (id) filled with 3 μm C18 material (reverse phase) were used for chromatographic separation of digests. The precursor ion scan was acquired at 120,000 resolutions in the Orbitrap analyzer and precursors at a time frame of 3 sec were selected for subsequent fragmentation using an HCD product triggered CID program. The threshold for triggering an MS/MS event was set to 1,000 counts. Charge state screening was enabled, and precursors with unknown charge state or a charge state of +1 were excluded (positive ion mode). Dynamic exclusion was also enabled (exclusion duration of 30 sec). The fragment ions were analyzed on Orbitrap for HCD and CID at 30,000 resolutions.
The LC-MS/MS spectra of trypsin and endoproteinase GluC digest of B1–1 and B2–5 samples were analyzed manually for the glycopeptides with the support of Xcalibur 3.0 software. The HCD and CID MS2 spectra of glycopeptides were evaluated for the glycan neutral loss pattern, oxonium ions and the glycopeptide fragmentations to assign the glycan composition and the presence of N-glycans in the glycopeptides. The LC-MS/MS spectra of the B1–1 and B2–5 samples were further searched against. fasta sequence of human erythropoietin using Byonic™ V 2.3.5 software with trypsin and GluC as digestion enzyme (amino acids cleavage sites such as R, K, E, and D) with semi-specific cleavage option enabled. Carbamidomethylation of cysteine, oxidation of methionine and the possible N-glycans based on manual glycan analysis were used as variable modification search parameters.
3. Results
3.1. Creation of three genetic cassettes and transgenic tobacco plants
For glycoengineering tobacco plants to add β1,4-galactose and bisected GlcNAc on rhuEPO N-glycan chains, EPO with chimeric ST/GalT or with wild-type GalT and MGAT3 were cloned in expression vector MpBI121 to create two genetic cassettes B1 and B2, respectively with bar as a selection gene (Fig. 2). Genetic cassette B1 containing 35S2-P::EPO::nos-T and nos-P::ST/GalT::nos-T was used to investigate whether chimeric ST/GalT provides improved β1,4-galactosylation efficiency. Genetic cassette B2 contained 35S2-P::EPO::nos-T, GapC-P::MGAT3::GapC-T and nos-P::GalT::nos-T, and was used to introduce both bisecting GlcNAc and β1,4-galactose residues. To generate sialylation capacity in plants and extend the β1,4-galactose residues on rhuEPO with sialic acid residues, five mammalian genes GNE, NANS, CMAS, CST and ST regulated by its own promoter and terminator were cloned into expression vector pBI121 to create genetic cassette A, which contained 35S-P::GNE::nos-T, GapC-P::NANS::GapC-T, Ubi.U4-P::CMAS::GapC-T, nos-P::CST::nos-T and 35S-P::ST::nos-T with nptII as a selection gene (Fig. 2). Each gene (including selection genes) in genetic cassettes and transformed Agrobacterium strain LBA4404 was confirmed by PCR analysis with gene specific primers.
In order to produce stable transgenic lines for the production of rhuEPO with different glycan profiles, two genetic cassette combinations A+B1 and A+B2 were used to co-transform tobacco cultivar W38. Using a selection system with two antibiotics, a total of 95 individual transgenic lines resistant to both glufosinate and kanamycin from genetic cassettes A+B1 and 120 from genetic cassettes A+B2 were successfully regenerated and rooted. They were used for downstream N-glycan and other molecular analyses.
3.2. Quantification of expression levels of rhuEPO in young and mature transgenic tobacco lines
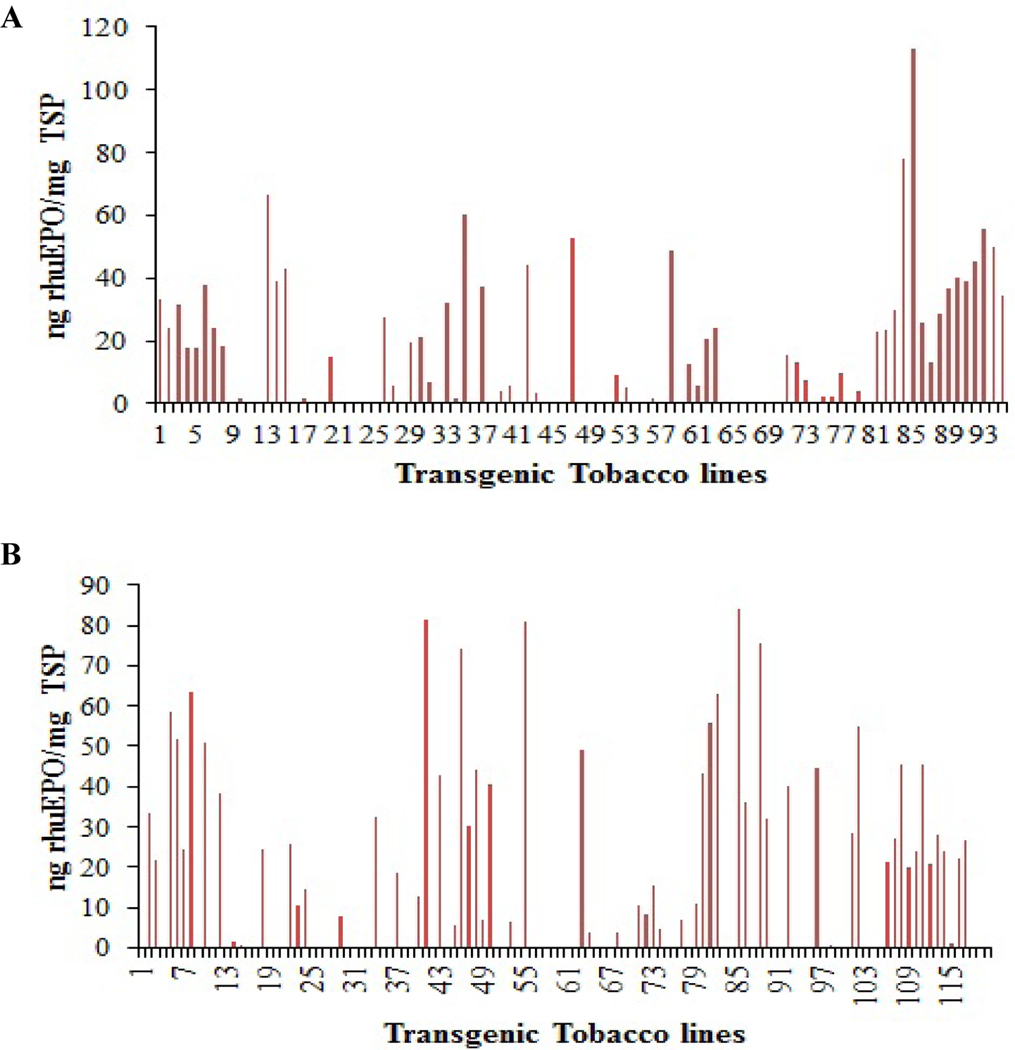
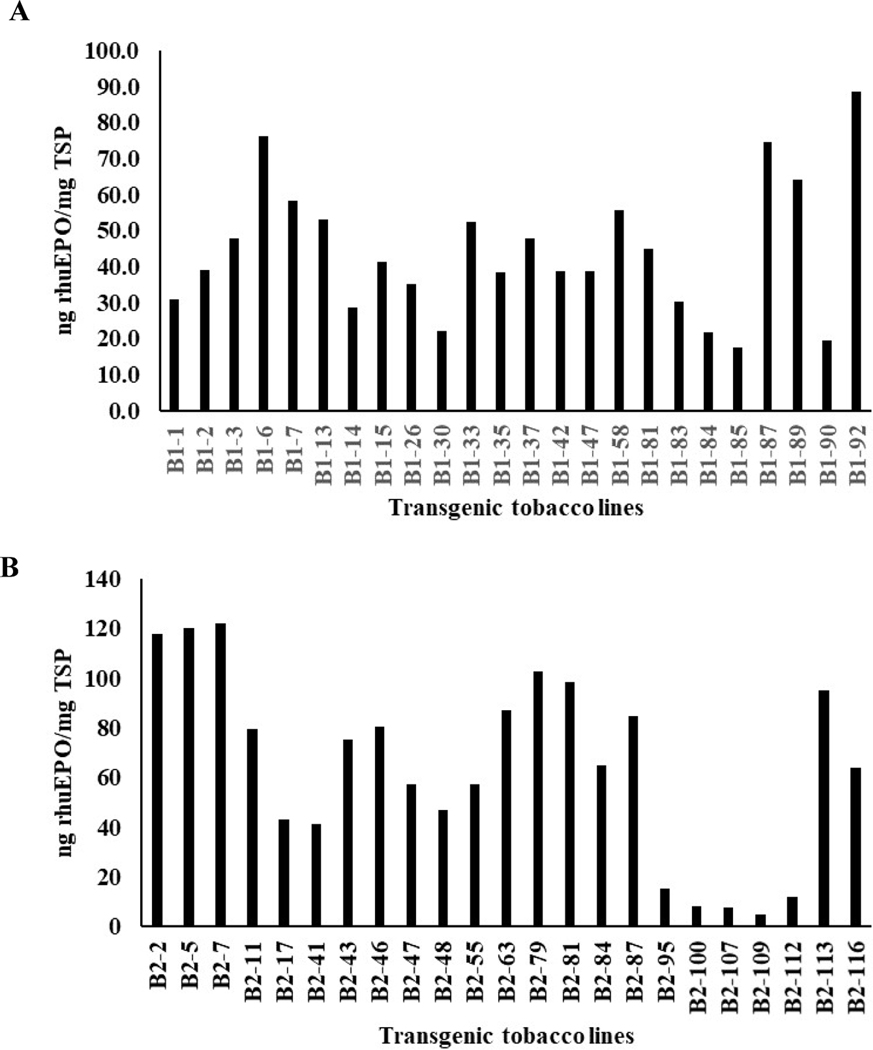
A total of 215 (95 for cassettes A+B1 and 120 for A+B2) baby jar grown putative transgenic lines resistant to both glufosinate and kanamycin with 5–6 leaves were prescreened to check the expression levels of soluble rhuEPO using sandwich ELISA. In the case of genetic cassette combination of A+B1, 59 (62%) out of 95 had detectable levels of rhuEPO with expression levels around 0.5–113.2 ng/mg TSP (Fig. 3A) while 60 (50%) out of 120 transgenic lines from genetic cassette combination of A+B2 had expression levels around 1.3–84 ng/mg TSP (Fig. 3B). These results indicated that EPO was not only transcribed but also translated with detectable levels of rhuEPO in these transgenic lines. After prescreening, about 20 transgenic lines with higher expression levels of rhuEPO from each genetic cassette combination were selected and transferred into greenhouse. Most of them grew well with few exceptions. When they reached 14–15 leaf-stages, the expression levels of total soluble rhuEPO were measured again by aforementioned sandwich ELISA method. Results showed that all transgenic lines from genetic cassette combinations A+B1 and A+B2 had rhuEPO levels of 17.5–88.6 ng/mg TSP and 7.9–122.3 ng/mg TSP, respectively (Fig. 4A and B). When their expression levels between 5–6 leaf-stages and 14–15 leaf-stages (Supplementary Table 3) were compared, their correlation coefficients were −0.32 and 0.28 for A+B1 and A+ B2 transgenic lines, respectively. These results indicate the prescreening EPO expression levels from baby jar grown transgenic plantlets works cannot well represent expression levels of greenhouse grown transgenic plants. The greenhouse grown transgenic plants were used for molecular analyses.
Fig. 3. Expression levels of rhuEPO in baby jar grown transgenic tobacco lines.
Expression levels of 215 transgenic tobacco lines co-transformed co-transformedwith six or seven mammalian genes responsible for β1,4-galactosylationand sialylation with a target gene human EPO were measured. A) RhuEPO levels in 95 transgenic lines obtained from co-transformation of genetic cassettes A and B1; B) rhuEPO levels in 120 transgenic lines obtained from co-transformation of genetic cassettes A and B2.
Fig. 4. Expression levels of rhuEPO in soil grown transgenic lines.
Expression levels of rhuEPO in 23–24 selected transgenic lines from each genetic cassette combination were measured when they reached 15–16 leaf-stages. A) Expression levels of rhuEPO in transgenic lines from genetic cassettes A and B1. B) expression levels of rhuEPO in transgenic lines from genetic cassettes A and B2.
3.3. Confirmation of the presence of transgenes and their transcripts in transgenic lines by PCR and RT-PCR analysis
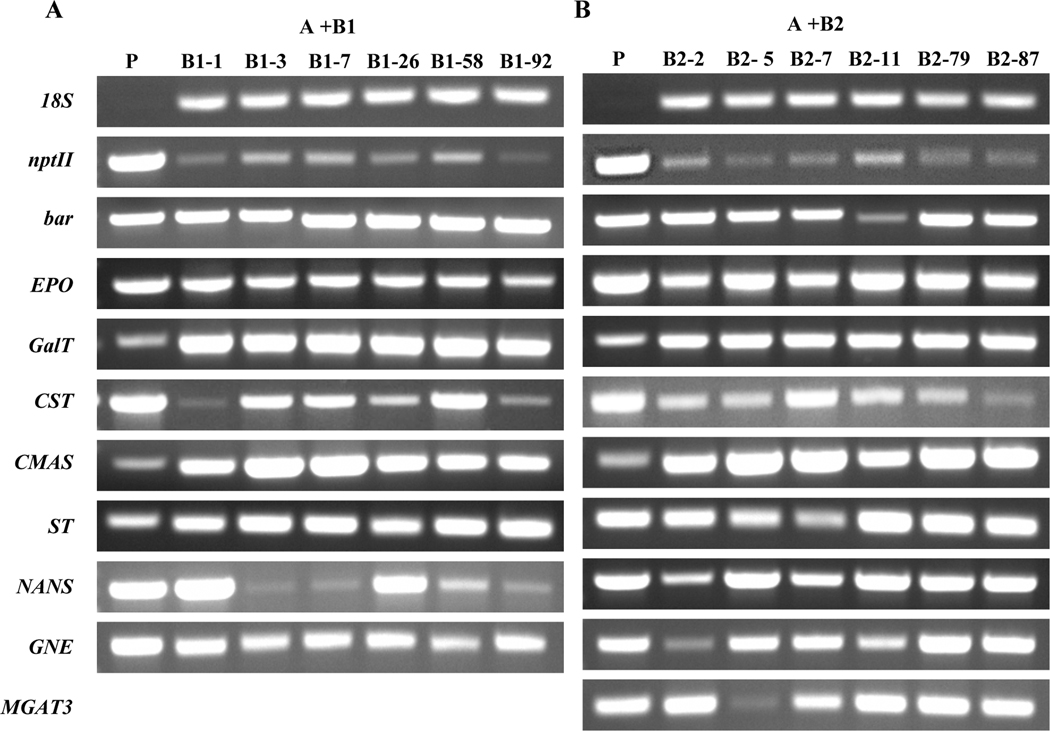
To confirm the integration of transgenes including selection genes, six transgenic lines from each genetic cassette combination were used. PCR analysis with genomic DNA confirmed that all transgenes and selection genes could be detected in each transgenic line with expected sizes of PCR products (Fig. 5), indicating that all transgenes including selection genes were integrated in tobacco genome in these transgenic plants. The same set of six transgenic plants from each genetic cassette combination was used to monitor whether all integrated transgenes could be properly transcribed or not by RT-PCR analysis. The results showed that all transgenes including selection genes had positive amplification results with expected sizes of PCR products (Fig. 6). These results indicate that all transgenes were transcribed in each transgenic line.
Fig. 5. PCR analysis to determine the presence of transgenes including two selection genes in six transgenic lines from each genetic cassette combination.
A) Six transgenic lines from genetic cassettes A and B1 were used for confirming the presence of nptII, bar, EPO, ST/GalT, CST, CMAS, ST, NANS, GNE. B) six transgenic lines from genetic cassettes A and B2 used for confirming the presence of nptII, bar, EPO, GalT, CST, CMAS, ST, NANS, GNE and MGAT3. Genomic DNA obtained from leaves of each transgenic line was used for PCR amplification with gene specific primers. For ST/GalT, only GalT part was amplified. P: plasmid DNA containing respective genes.
Fig. 6. RT-PCR to detect transcripts of all transgenes including two selection genes in six transgenic lines from each genetic cassette combination.
A) Six transgenic lines from genetic cassettes A+B1 were used to detect transcripts of nptII, bar, EPO, ST/GalT, CST, CMAS, ST, NANS and GNE. B) Six transgenic lines from genetic cassettes A+B2 were used to detect transcripts of nptII, bar, EPO, GalT, CST, CMAS, ST, NANS, GNE and MGAT3. cDNAs made from total RNAs isolated from leaf tissue of transgenic lines were used for RT-PCR amplification. The standard primers from the QuantumRNATM 18S internal standard kit was used to target the 18S rRNA gene to ensure equal loading. For ST/GalT, only GalT part was detected.
3.4. Confirmation and determination of sizes of rhuEPO expressed in glycoengineered transgenic lines by Western blot analysis
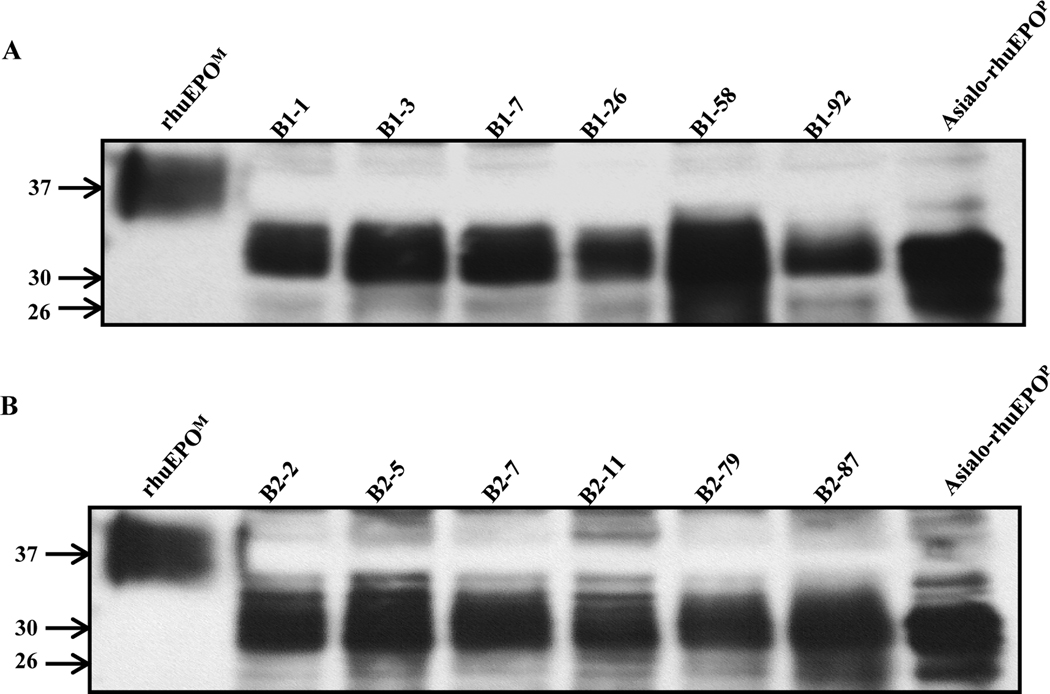
To further confirm rhuEPO expression in glycoengineered plants and determine its sizes, Western blot analysis was performed. Both mammalian cell-produced rhuEPO (rhuEPOM) and TSPs from our previous transgenic tobacco line A56–5 expressing asialo-rhuEPO (Kittur et al., 2013) were used as controls for size comparison. As can be seen in Fig. 7, all transgenic lines transformed with cassette combinations A+B1 and A+B2 had immuno-reactive bands whose sizes were larger than that of asialorhuEPO, suggesting that that rhuEPO expressed in the above transgenic lines bear extended N-glycan chains. However, the sizes of plant-produced rhuEPO were smaller than that of rhuEPOM because plant-produced glycoproteins bear diantennary N-glycans in contrast to multiantennary in mammalian cell-produced glycoproteins.
Fig. 7. Western blots of crude protein extracts made from six transgenic tobacco lines from each genetic cassette combination.
A) Transgenic lines from genetic cassettes A+B1; B) transgenic lines from genetic cassettes A+B2. RhuEPOM is a fully sialylated rhuEPO containing tri- and tetraantennary N-glycans, whereas asialo-rhuEPOP is a plant-produced diantennary rhuEPO lacking sialic acid residues. Both of them were used as controls
3.5. Detailed N-glycan patterns of rhuEPO expressed in glycoengineered transgenic lines
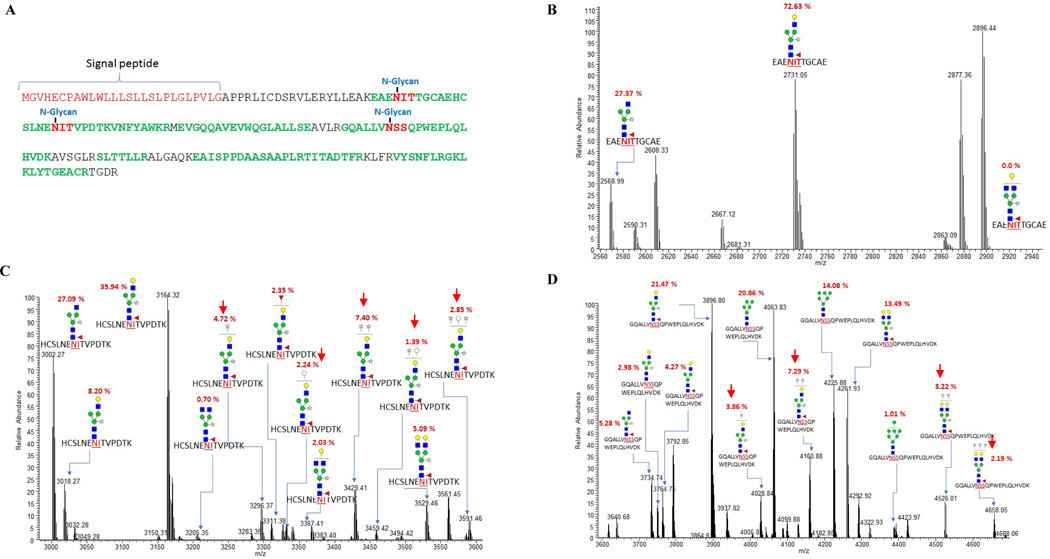
Glycoproteomics approach was applied to determine the N-glycan profiles of rhuEPO expressed in the glycoengineered tobacco plants. Purified rhuEPO from transgenic lines B1–1 (cassettes A+B1) and B2–5 (cassettes A+B2) were selected for N-glycan analysis. For protein digestion, GluC was used in addition to trypsin because in-gel digestion with trypsin alone was not sufficient to release the di-N-glycosylated peptide (EAENITTGCAEHCSLNENITVPDTK) (Fig. 8A) from plant-produced rhuEPO as well as rhuEPOM.
Fig. 8. Amino acid sequence of human EPO showing N-glycosylation sites and LC-MS/MS spectra of trypsin+GluC glycopeptides of rhuEPO purified from transgenic line B1–1 co-expressing chimeric GalT and sialylation pathway genes.
A) Amino acid sequence of human EPO showing signal peptide with three Nglycoylation sites (shown in red). Amino acid sequence highlighted in green indicate regions with good peptide scores in mass spectra; B) N-glycans identified at site I; C) N-glycans at site II; D) N-glycans at site III.
LC-MS/MS analysis of rhuEPO purified from B1–1 line expressing chimeric ST/GalT along with sialylation pathway genes revealed higher proportion of N-glycans bearing β1,4-galactose residues (Fig. 8B–D) compared to transgenic line expressing wild-type GalT (Fig. 9A–C), indicating that the former is superior than the latter in generating β1,4-galactosylation capacity in plants. All three N-glycosylation sites in rhuEPO purified from B1–1 transgenic line were occupied by N-glycan chains. GalGlcNAc(Xyl)Man3(Fuc)GlcNAc2 was the most predominant N-glycan chain, followed by GlcNAc(Xyl)Man3(Fuc)GlcNAc2 at sites I and II (Fig. 8B and C, Table 1) while site III was dominated by GalGlcNAc(Xyl)Man3(Fuc)GlcNAc2 and high mannose type Man5–6GlcNAc2 (Fig. 8D, Table 1). The percentage of mono-and di-galactosylated N-glycans at site I, II and III were 73, 72 and 59%, respectively. Surprisingly, no sialic acid was detected. Intriguingly, unusual N-glycans bearing 2–3 extra pentoses (Pen2–3GalGlcNAc(Xyl)Man3(Fuc)GlcNAc2) in addition to core β1,2-xylose, and in some an extra hexose (Pen2–3HexGalGlcNAc(Xyl)Man3(Fuc)GlcNAc2) were observed in the N-glycan chains attached to sites II and III (16–18%, indicated with red arrowheads in Fig. 8C and D, Table 1). The presence of multiple pentoses on the N-glycans were confirmed by evaluating the neutral losses of pentoses on the HCD MS2 fragmentation spectra of N-glycopeptides (Supplemantary Fig. 1). The identity of both pentose and hexose, and their position and linkage within the N-glycan chain still remains to be determined.
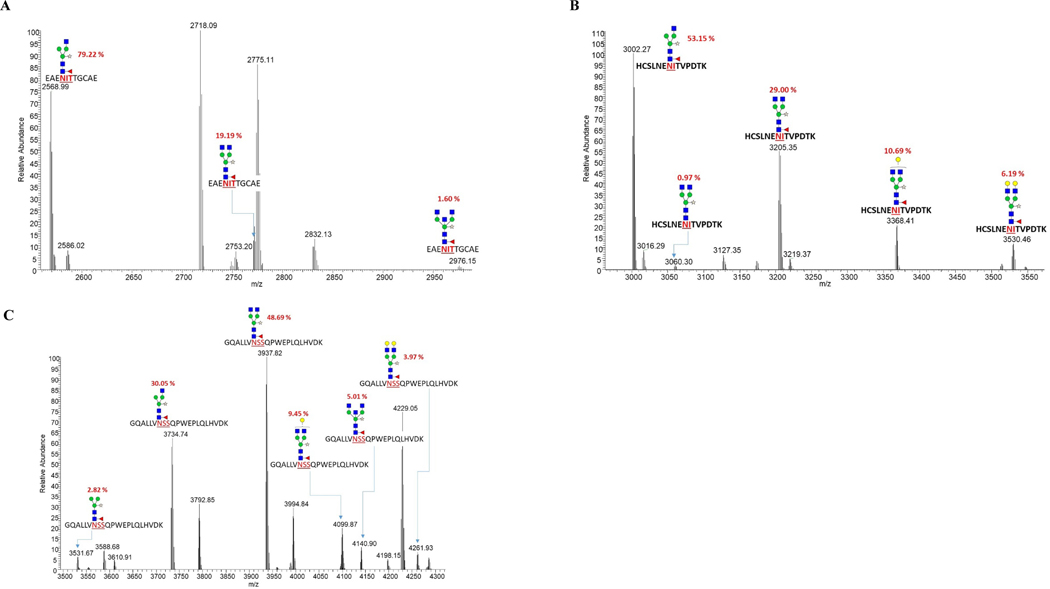
Fig. 9. LC-MS/MS spectra of trypsin+GluC glycopeptides of rhuEPO purified from transgenic line B2–5 co-expressing wild-type GalT and sialylation pathway genes.
A) N-glycans at site I; B) N-glycans at site II; C) N-glycans at site III.
Table 1.
N-glycan chains identified in rhuEPO purified from transgenic tobacco lines B1–1 and B2–5
| B1–1 | B2–5 | |||||
|---|---|---|---|---|---|---|
| N-glycans | *S1 | S2 | S3 | S1 | S2 | S3 |
| GlcNAc(Xyl)Man3(Fuc)GlcNAc2 | 27.4 | 27.1 | 5.3 | 79.2 | 53.1 | 30.0 |
| GlcNAc2(Xyl)Man3(Fuc)GlcNAc2 | 0.7 | 19.2 | 30.0 | 48.7 | ||
| GalGlcNAc(Xyl)Man3(Fuc)GlcNAc2 | 72.6 | 35.9 | 21.5 | |||
| Gal2GlcNAc2(Xyl)Man3(Fuc)GlcNAc2 | 5.1 | 13.5 | 6.2 | 4.0 | ||
| GalGlcNAc(Xyl)Man3GlcNAc2 | 8.2 | 3.0 | ||||
| GalGlcNAcMan3(Fuc)GlcNAc2 | 4.3 | |||||
| GalGlcNAc2(Xyl)Man3(Fuc)GlcNAc2 | 2.0 | 10.7 | 9.5 | |||
| †GlcNAc3(Xyl)Man3(Fuc)GlcNAc2 | 1.6 | 5.0 | ||||
| ‡(Fuc)GalGlcNAc(Xyl)Man3(Fuc)GlcNAc2 | 2.4 | |||||
| ¶PentGalGlcNAc(Xyl)Man3(Fuc)GlcNAc2 | 4.7 | 3.8 | ||||
| Pent2GalGlcNAc(Xyl)Man3(Fuc)GlcNAc2 | 7.4 | 7.3 | ||||
| Pent2Gal2GlcNAc2(Xyl)Man3(Fuc)GlcNAc2 | 3.2 | |||||
| Pent3Gal2GlcNAc2(Xyl)Man3(Fuc)GlcNAc2 | 2.2 | |||||
| §HexGalGlcNAc(Xyl)Man3(Fuc)GlcNAc2 | 2.2 | |||||
| HexPentGalGlcNAc(Xyl)Man3(Fuc)GlcNAc2 | 1.4 | |||||
| Hex1Pent2GalGlcNAc(Xyl)Man3(Fuc)GlcNAc2 | 2.9 | |||||
| (Xyl)Man3(Fuc)GlcNAc2 | 2.8 | |||||
| GlcNAc2(Xyl)Man3GlcNAc2 | 1.0 | |||||
| Man5GlcNAc2 | 21.8 | |||||
| Man6GlcNAc2 | 14.1 | |||||
| Man7GlcNAc2 | 1.0 | |||||
| Total (%) | 100 | 100 | 100 | 100 | 100 | 100 |
N-glycosylation site
N-glycan containing bisected GlcNAc
most probably Lewis A
N-glycan containing additional pentose
N-glycan containing additional hexose.
Transgenic line B2–5 expressing MAGT3, wild-type GalT and sialylation pathway genes (cassettes A+B2) showed a small proportion of N-glycans with bisected GlcNAc (Fig. 9A and C, Table 1), indicating that GnTIII is functional but not very efficient. The N-glycan chains were also decorated with β1,4-galactose residues but their proportion was smaller compared to transgenic line expressing chimeric ST/GalT. All three N-glycosylation sites were occupied by N-glycan chains. GlcNAc(Xyl)Man3(Fuc)GlcNAc2 was the most abundant N-glycan (79.2%) at site I, followed by GlcNAc2(Xyl)Man3(Fuc)GlcNAc2 (19.2%) and a minor amount (1.6%) of N-glycan contained a bisected GlcNAc (Fig. 9A, Table 1). Site II also carried the same types of N-glycan chains as at site I with high abundances (53.1% and 30%, respectively) (Fig. 9B, Table 1), but also carried mono-(10.7%) and di-β1,4-galactosylated (6.2%) N-glycans (Fig. 9B, Table 1). At site III however, GlcNAc2(Xyl)Man3(Fuc)GlcNAc2 was the most predominant N-glycan (48.7%), followed by GlcNAc(Xyl)Man3(Fuc)GlcNAc2 (30%) (Fig. 9C, Table 1). About 13.4% mono-and di-β1,4-galactosylated N-glycans were also present at site III along with a N-glycan containing a bisected GlcNAc (5%) (Fig. 9C, Table 1). The overall β1,4-galactosylation was moderate in B2–5 transgenic expressing wild-type GalT. Surprisingly, no Neu5Ac was detected in this transgenic line either.
4. Discussion
Plant-based expression system is now emerging as an attractive alternative because it offers many potential advantages, such as low cost, ease of scaling up in production, and no risk of transmitting pathogens (Lerouge et al., 1998; Ma et al., 2003). Recent progress in plant glycoengineering also shows that plants are flexible to glycosylation engineering to express pharmaceutically important glycoproteins (Strasser, 2014; Montero-Morales and Steinkellner, 2018). In spite of plants having capacity to synthesize complex N-glycans, there exist significant differences in the glycosylation capacities between plant-based and mammalian cell-based expression systems (Denecke et al., 1990; Ma et al., 2003). Plants lack enzyme activities responsible for adding β1,4-galactose, terminal sialic acid residues and bisected GlcNAc (Stanley and Campbell, 1984; Edmund et al, 1998; Gomord and Faye, 2004; Castilho et al., 2010). The presence of β1,4-galactose residues is a prerequisite for adding terminal sialic acid residues to the N-glycan chains, and in antibodies the exposed β1,4-galactose residues play an important role in mediating effector functions (Houde et al., 2010). In certain special cases, a desialylated glycoprotein with β1,4-galactose-extended N-glycan chains, such as asialo-rhuEPO, has a special function and application (Erbayraktar et al., 2003; Kittur et al., 2013, 2019; Arthur et al., 2017). Therefore, glycoengineering tobacco plants to have an efficient β1,4-galactosylation capacity is important. Our glycan analysis results revealed that both chimeric ST/GalT (Fig. 8C–D) and wild type GalT (Fig. 9A–B) can generate β1,4-galactosylation capacity in plants, but the former is more efficient for adding β1,4-galactose residues to the growing N-glycan chains. These results are consistent with previous reports (Strasser et al., 2009; Castilho et al., 2011). The high β1,4-galactosylation efficiency of chimeric ST/GalT is thought due to proper sub-Golgi targeting (Castilho et al., 2011) and use of rat ST-CTS region to target GalT to a late stage of the pathway could be an efficient way to generate β1,4-galactosylated diantennary N-glycans as demonstrated by Strasser et al. (2009).
About 10% of N-glycan chains in human IgG contain bisecting GlcNAc (Raju et al., 2000) that has been reported to play an important role in mediating antibody effector function via modulation of antibody-dependent cellular cytotoxicity (Hodonickzy et al., 2005). Although no bisected GlcNAc has been observed in human urinary EPO N-glycan chains (considered as a natural EPO molecule) (Skibeli et al., 2001), but rhuEPO produced in human lympholastoid cell line RPMI 1788 has been reported to have 80% N-glycans chains with bisecting GlcNAc with no effect on its hematopoietic activity (Cointe et al., 2000). However, what effect bisected GlcNAc exerts on tissue-protective property of rhuEPO still needs to be determined because a different receptor is thought to be involved in mediating the tissue-protective function of EPO (Brines et al., 2004). Previous studies have shown that the expression of GnTIII in wildtype plants resulted in the attachment of a bisecting GlcNAc to the N-glycans of antibodies (Rouwendal et al., 2007; Frey et al., 2009; Karg et al., 2010). Transiently co-expression of EPO-Fc and GnTIII also generated bisecting GlcNAc on EPO-Fc N-glycan chains (Castilho et al., 2011). In our stable transgenic plant B2–5 expressing GnTIII, we also observed rhuEPO with bisected diantennary N-glycans (Fig. 9C), but at lower proportion. Low efficiency of N-glycans with bisected GlcNAc could be due to improper sub-Golgi targeting of GnTIII for appropriate N-glycan modification in plants. In order to enhance bisected oligosaccharide levels, targeting GnTIII to a medial or late sub-Golgi compartment is essential, which can be achieved by replacing CTS region of GnTIII by that of β1,2-xylosyltransferase (XT) or ST (Castilho et al., 2011).
As for sialylation in transgenic plants, all five genes GNE, NANS, CMAS, CST and ST that have been previously proved to build an entire sialylation pathway into plant genome for producing terminal sialic acid residues (Castilho et al., 2013; Jez et al., 2013; Kallolimath et al., 2016) were confirmed to be not only stably incorporated into tobacco genome (Fig. 5) but also properly transcribed (Fig. 6). However, to our surprise, no sialic acid residues were detected in purified rhuEPO from either type of transgenic plants. This could have resulted from one or more genes not properly translated or localized. Future studies to measure CMP-sialic acid in transgenic leaf tissues will assist to narrow down affected steps in the created stable transgenic lines. Unexpectedly, we observed about 16–18% of N-glycans bearing pentoses at the non-reducing terminal instead of Neu5Ac residues in transgenic line expressing chimeric ST/GalT and sialylation pathway genes. Whether these additional sugar residues present at the nonreducing end of the N-glycans is caused by improper targeting of chimeric ST/GalT or sialylation pathway genes is not clear yet. However, these abnormal sugar residues were not observed in rhuEPO purified from transgenic line expressing wild-type GalT. Nevertheless, further studies to check protein expression levels, activities and localization may allow us to understand why sialylation failed and to explain presence of unusual pentoses on the N-glycan chains of rhuEPO.
In conclusion, our current efforts have allowed us to produce rhuEPO with high β1,4-galactose-extended and certain levels of bisecting GlcNAc containing N-glycan chains. Our previous studies have shown that rhuEPO produced in transgenic tobacco plants co-expressing human EPO and wild-type GalT with certain β1,4-galactose-extended N-glycan chains displays broad cytoprotective functions in neuronal cells, cardiomyocytes, and pancreatic β-cells (Kittur et a., 2013; 2019; Arthur et al., 2017). This newly created transgenic plants co-expressing EPO and ST/GalT will provide asialo-rhuEPO with high proportion of β1,4-galactose-extended N-glycan chains for cytoprotective purposes. Further improving bisecting GlcNAc efficiency will allow us to have yet another version of rhuEPO with a novel N-glycan profile, and would be interesting to see what impact bisected GlcNAc will have on its cytoprotective function.
Supplementary Material
Supplementary Table 1. Information of eight mammalian genes used for plant transformation
Supplementary Table 2. Sequences of primers used for PCR and RT-PCR amplification
Supplementary Table 3. Expression levels of each transgenic line measured from baby jar grown and greenhouse grown (ng rhuEPO/mg TSP)
Acknowledgments
Sources of funding
Research conducted in these studies was supported by North Carolina Biotechnology Center Grant (2013-BRG-1207) and National Institute of General Medical Sciences (SC1GM111178-01A1) to J.H. Xie, a scholarship from China Scholarship Council (201306305041) to C.S. Zhu.
References
- Takeuchi M, Kobata A, Structural and functional roles of the sugar chains of human erythropoietins, Glycobiol. 1 (1991) 337–346. [DOI] [PubMed] [Google Scholar]
- Jelkmann W, 1992. Erythropoietin: structure, control of production, and function, Physiol. Rev 72 (1992) 449–489. [DOI] [PubMed] [Google Scholar]
- Wasley LC, Timony G, Murtha P, Stoudemire J, Dorner AJ et al. The importance of N-an O-linked oligosaccharides for the biosynthesis and in vitro and in vivo biologic activities, Blood. 77 (1991) 2624–2632. [PubMed] [Google Scholar]
- Tsuda E, Kawanishi G, Ueda M, Masuda S, Sasaki R, The role of carbohydrates in recombinant human erythropoietin, Eur. J. Biochem 18 (1990) 405–411. [DOI] [PubMed] [Google Scholar]
- Fukuda MN, Sasaki H, Lopez L, Fukuda M, Survival of recombinant erythropoietin in the circulation: the role of carbohydrates, Blood 73 (1989) 84–89. [PubMed] [Google Scholar]
- Ghezzi P, Brines M, Erythropoietin as an antiapoptotic, tissue-protective cytokine, Cell Death Differ. 11 (2004) S37–S44. [DOI] [PubMed] [Google Scholar]
- Smith KJ, Bleyer AJ, Little WC, Sane DC The cardiovascular effects of erythropoietin, Cardiovas. Res 59 (2003) 538–548. [DOI] [PubMed] [Google Scholar]
- Erbayraktar S, Grasso G, Sfacteria A, Xie QW, Coleman T. et al. , Asialoerythropoietin is a non-erythropoietic cytokine with broad neuroprotective activity in vivo, Proc. Natl. Acad. Sci. USA 100 (2003) 6741–6746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissner C, Allergini PR, Ekatodramis D, Jewel UR, Stallmach T. et al. Increased cerebral infarct volumes in polyglobulic mice overexpressing erythropoietin, J. Cereb. Blood Flow Metab 21 (2001) 857–864. [DOI] [PubMed] [Google Scholar]
- Brines M, Ghezzi P, Keenan S, Agnello D, de Lanerolle NC et al. Erythropoietin crosses the blood-brain barrier to protect against experimental brain injury, Proc. Natl. Acad. Sci. USA 97 (2000) 10526–10531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvillo L, Latini R, Kajstura J, Leri A, Anversa P. et al. Recombinant human erythropoietin protects the myocardium from ischemia-reperfusion injury and promotes beneficial remodeling, Proc. Natl. Acad. Sci. USA 100 (2003) 4801–4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesey DA, Cheung C, Pat B, Endre Z, Gobe G. et al. Erythropoietin protects ischemic acute renal injury, Nephrol. Dial. Transplant 19 (2004) 348–355. [DOI] [PubMed] [Google Scholar]
- Greif F, Ari ZB, Taya R, Pappo O, Kurtzwad E. et al. , Dual effect of erythropoietin on liver protection and regeneration after subtotal hepatectomy in rats, Liver Transplantation. 16 (2010) 631–638. [DOI] [PubMed] [Google Scholar]
- Ghaderi D, Zhang M, Hurtado Z, Varki A, Production platforms for biotherapeutic glycoproteins. Occurrence, impact, and challenges of non-human sialylation, Biotechnol. Genet. Eng. Rev 28 (2012)147–175. [DOI] [PubMed] [Google Scholar]
- Weise A, Altmann F, Rodriguez-Franco M, Sjoberg ER, Baumer W, et al. , High level expression of secreted complex glycosylated recombinant human erythropoietin in the Physcomitrella Δ-fuct-Δ-xyl-t mutant, Plant Biotechnol. J 5 (2007) 389–401. [DOI] [PubMed] [Google Scholar]
- Jacobs PP, Callewaert N, N-glycosylation engineering of biopharmaceutical expression systems, Curr. Mol. Med 9 (2009) 774–800. [DOI] [PubMed] [Google Scholar]
- Padler-Karavani V, Varki A, Potential impact of the non-human sialic acid N-glyconeuraminic acid on transplant rejection, Xenotransplantation. 18 (2011) 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brines M, Cerami A, Erythropoietin-mediated tissue protection: reducing collateral damage from the primary injury response, J. Intern. Med 264 (2008) 405–432. [DOI] [PubMed] [Google Scholar]
- Strasser R, Biological significance of complex N-glycans in plants and their impact on plant physiology, Front. Plant Sci 5 (2014) 363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero-Morales L, Steinkellner H, Advanced plant-based glycan engineering, Front. Bioeng. Biotechnol 6 (2018) 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerouge P, Cabanes-Macheteau M, Rayon C, Fischette-Laine AC, Gomord V, Faye L, 1998. N-glycoprotein biosynthesis in plants: recent developments and future trends, Plant Mol. Biol 38 (1998) 31–48. [PubMed] [Google Scholar]
- Bakker H, Bardor M, Molthoff JW, Gomord V, Elbers I, Stevens LH, Jordi W, Lommen A, Faye L, Lerouge P, Bosch D, Galactose-extended glycans of antibodies produced by transgenic plants, Proc. Natl. Acad. Sci. USA 98 (2001) 2899–2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma JK, Drake PM, Christou P, The production of recombinant pharmaceutical proteins in plants, Nat. Rev. Genet 4 (2003) 794–805. [DOI] [PubMed] [Google Scholar]
- Palacpac NQ, Yoshida S, Sakai H, Kimura Y, Fijiyama K, Yoshida T, Seki T, Stable expression of human beta 1,4-galactosyltransferase in plant cells modifies N-linked glycosylation patterns, Proc. Natl. Acad. Sci. USA 96 (1999) 4692–4697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker H, Rouwendal GJ, Karnoup AS, Florack DE, Stoopen GM, Helsper JP, van Ree R, van Die I, Bosch D, An antibody produced in tobacco expressing a hybrid beta-1,4-galactosyl-transferase is essentially devoid of plant carbohydrate epitopes, Proc. Natl. Acad. Sci. USA 103 (2006) 7577–7582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittur FS, Bah M, Archer-Hartmann S, Hung CY, Azadi P, Ishihara M, Sane DC, Xie J, Cytoprotective effect of recombinant human erythropoietin produced in transgenic tobacco plants. PLoS One 8 (2013) e76468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parson J, Altmann F, Arrenberg CK, Koprivova A, Beike AK et al. Moss-based production of asialoerythropoietin devoid of Lewis A and other plant-typical carbohydrate determinants, Plant Biotechnol. J 10 (2012) 851–861. [DOI] [PubMed] [Google Scholar]
- Strasser R, Castilho A, Stadlmann J, Kunert R, Quendler H, Gattinger P, Jez J, Rademacher T, Altmann F, Mach L, Steinkellner H, 2009. Improved virus neutralization by plant-produced anti-HIV antibodies with a homogeneous beta1,4-galactosylated N-glycan profile, J. Biol. Chem 284 (2009) 20479–20485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castilho A, Strasser R, Stadlmann J, Grass J, Jez J, Gattinger P, Kunert R, Quendler H, Pabst M, Leonard R, Altmann F, Steinkellner H, 2010. In planta protein sialylation through overexpression of the respective mammalian pathway, J. Biol. Chem 285 (2010)15923–15930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castilho A, Neumann L, Gattinger P, Strasser R, Vorauer-Uhl K. et al. , Generation of biologically active multi-sialylated recombinant human EPOFc in plants, PloS One. 8 (2013) c54836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jez J, Castilho A, Grass J, Vorauer-Uhl K, Sterovsky T, Altmann F, Steinkellner H, Expression of functionally active sialylated human erythropoietin in plants, Biotechnol. J 8 (2013) 371–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollolimath S, Castilho A, Strasser R, Grunwald-Gruber C, Altmann F. et al. , Engineering of complex protein sialylation in plants, Proc. Natl. Acad. Sci. USA 113 (2016) 9498–9503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castilho A, Gattinger P, Grass J, Jez J, Pabst M. et al. , N-glycosylation engineering of plants for the biosynthesis of glycoproteins with bisected and branched complex N-glycans, Glycobiol. 21 (2011) 813–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raju TS, Glycosylation variations with expressions systems and their impact on biological activity of therapeutic immunoglobulins, BioProcess Intl. 1 (2003) 44–54. [Google Scholar]
- Stanley P, Campbell CA, A dominant mutation to ricin resistance in Chinese hamster ovary cells induces UDP-GlcNAc:glycopeptide β1,4-N-acetylglucosaminyl-transferase III activity, J. Biol. Chem 259 (1984) 13370–13378. [PubMed] [Google Scholar]
- Edmund GT, Wee D, Sherrier J, Prime TA, Dupree P, 1998. Targeting of active sialyltransferase to the plant Golgi apparatus, Plant Cell 10 (1998) 1759–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomord V, Faye L, Posttranslational modification of therapeutic proteins in plants, Curr. Opin. Plant Biol 7 (2004) 171–181. [DOI] [PubMed] [Google Scholar]
- Musa TA, Hung CY, Darlington DE, Sane DC, Xie J, Overexpression of human erythropoietin in tobacco does not affect plant fertility or morphology, Plant Biotechnol. Rep 3 (2009) 157–165. [Google Scholar]
- Kittur FS, Hung CY, Darlington DE, Sane DC, Xie J, N-glycosylation engineering of tobacco plants to produce asialoerythropoietin, Plant Cell Rep. 31 (2012) 1233–1243. [DOI] [PubMed] [Google Scholar]
- Holsters M, de Waele D, Depicker A, Messens E, Van Montagu M, Schell J, Transfection and transformation of Agrobacterium tumefaciens, Mol. Gen. Genet 163 (1978) 181–187. [DOI] [PubMed] [Google Scholar]
- Bradford MM, A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding, Anal. Biochem 72 (1976) 248–254. [DOI] [PubMed] [Google Scholar]
- Denecke J, Botterman J, Deblaere R, 1990. Protein secretion in plant cells can occur via a default pathway, Plant Cell 2 (1990) 51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houde D, Peng Y, Berowitz SA, Engen JR Post-translational modification differentially affects IgG1 conformation and receptor binding, Mol. Cellular Proteomics 9 (2010) 1716–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittur FS, Lin Y, Arthur E, Hung CY, Li PA, Sane DC, Xie J, Recombinant asialoerythropoietin protects HL-1 cardiomyocytes from injury via suppression of Mst1 activation, Biochem. Biophys. Rep 17 (2019) 157–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur E, Kittur FS, Lin Y, Hung CY, Sane DC, Xie J. Plant-produced asialoerythropoietin restore pancreatic β cell function by suppressing mammalian sterilie-20-like kinase (Mst1) and caspase-3 activation, Front. Pharmacol 8 (2017) 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raju TS, Briggs JB, Borge SM, Jones AJS, Species specific variation in glycosylation of IgG: Evidence for the species-specific sialylation and branch-specific galactosylation and importance for engineering recombinant glycoprotein therapeutics, Glycobiol. 10 (2000) 477–486. [DOI] [PubMed] [Google Scholar]
- Hodonickzy J, Zhen YZ, James DC, Control of recombinant antibody effector functions by Fc N-glycan remodeling in vitro, Biotechnol. Prog 21 (2005) 1644–1652. [DOI] [PubMed] [Google Scholar]
- Skibeli V, Nissen-Lie G, Torjesen P, Sugar profiling proves that human serum erythropoietin differs from recombinant human erythropoietin, Blood. 98 (2001) 3626–3634. [DOI] [PubMed] [Google Scholar]
- Cointe D, Beliard R, Jorieux S, Leroy Y, Glacet A, Verbert A, Bourel D, Chirat F. Unusual N-glycosylation of a recombinant human erythropoietin expressed in a human lymphoblastoid cell line does not alter its biological properties. Glycobiol, 10 (2000) 511–519. [DOI] [PubMed] [Google Scholar]
- Brines M, Grasso G, Fiordaliso F, Sfacteria A, Ghezzi P, Erythropoietin mediates tissue protection through an erythropoietin and common beta-subunit hetero-receptor, Proc. Natl. Acad. Sci. USA 101 (2004) 14907–14912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouwendal GJA, Deedler AM, Bakker H, Stoopen GM, Die IV, Helsper JPFG, Hokke CH, Bosch D, Efficient introduction of a bisecting GlcNAc residue in tobacco N-glycans by expressing of the gene encoding human N-acetylglucosaminyltransferase III, Glycobiol. 17 (2007) 334–344. [DOI] [PubMed] [Google Scholar]
- Frey AD, Karg SR, Kallio PT, Expression of rat β(1,4)-N-acetylglucosaminyltransferase III in Nicotiana tabacum remodels the plant-specific N-glycosylation, Plant Biotechnol. J 7 (2009) 33–48. [DOI] [PubMed] [Google Scholar]
- Karg SR, Frey AD, Kallio PT, Reduction of N-linked xylose and fucose by expression of rat β1,4-N-acetylglucosaminyltransferase III in tobacco BY-2 cells depends on Golgi enzyme localization domain and genetic elements used for expression, J. Biotechnol 146 (2010) 54–65. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Information of eight mammalian genes used for plant transformation
Supplementary Table 2. Sequences of primers used for PCR and RT-PCR amplification
Supplementary Table 3. Expression levels of each transgenic line measured from baby jar grown and greenhouse grown (ng rhuEPO/mg TSP)