Abstract
The gut mounts secretory immunoglobulin A (SIgA) responses to commensal bacteria through non-redundant T cell-dependent (TD) and T cell-independent (TI) pathways that promote the establishment of mutualistic host-microbiota interactions. SIgAs from the TD pathway target penetrant bacteria and their induction requires engagement of CD40 on B cells by CD40 ligand on T follicular helper cells. In contrast, SIgAs from the TI pathway bind a larger spectrum of bacteria, but the mechanism underpinning their production remains elusive. Here we show that the intestinal TI pathway required CD40-independent B cell-activating signals from TACI, a receptor for the innate CD40 ligand-like factors BAFF and APRIL. TACI-induced SIgA responses targeted a fraction of the gut microbiota without shaping its overall composition. Of note, TACI was dispensable for TD induction of IgA in gut-associated lymphoid organs. Thus, BAFF/APRIL signals acting on TACI orchestrate commensal bacteria-specific SIgA responses through an intestinal TI program.
Introduction
Gut homeostasis involves the continuous release of microbiota-reactive polymeric IgA antibodies by plasma cells (PCs) emerging from complementary T cell-dependent (TD) and T cell-independent (TI) B cell-activation pathways (1). Binding of IgA to an epithelial antibody transporter called polymeric Ig receptor mediates transcytosis of polymeric IgA, which is followed by intraluminal accumulation of secretory IgA (SIgA) (1). While some SIgAs remain bacteria-free, the remaining SIgAs target commensal bacteria to regulate their composition, topography, fitness, motility and/or immunometabolic properties (2–4).
The TD pathway involves microbiota-dependent activation of B cells from Peyer’s patches (PPs) and mesenteric lymph nodes (MLNs) by antigen-primed T follicular helper (TFH) cells, which express CD40 ligand (CD40L) (5–7). In the presence of interleukin-21 (IL-21) and transforming growth factor-β (TGF-β) from TFH cells, engagement of CD40 on antigen-specific B cells by CD40L initiates a germinal center (GC) reaction encompassing IgM-to-IgA class switch recombination and IgA somatic hypermutation (6–8). The resulting high-affinity SIgA binds to some members of the microbiota, thereby limiting their growth, motility and contact with gut epithelial cells (2, 9).
In addition to yielding PCs that release high-affinity IgA, intestinal TD inductive sites generate unswitched IgM+ and class-switched IgA+ memory B cells that iteratively enter mucosal GCs to continuously diversify gut IgA and IgM via somatic hypermutation (10–12). This process permits the rapid accommodation of gut antibodies to transient antigenic changes of the microbiota and, over time, may render memory B cells a dominant precursor of gut PCs (11, 12).
Though eliciting IgM-to-IgA class switching through myeloid, stromal and possibly epithelial cells (1, 13, 14), the intestinal TI pathway does not induce antibody affinity maturation via somatic hypermutation, but generates SIgA responses that specifically recognize commensal bacteria with polyreactive binding modalities (15, 16). This TI pathway could be involved in the early shaping of the postnatal intestinal naïve B cell repertoire by B cell receptor-editing signals emanating from the commensal microbiota (17).
While CD40L-CD40 interaction dominates the gut TD pathway, it is unclear whether an equivalent receptor-ligand pair regulates the gut TI pathway. Aside from microbial Toll-like receptor (TLR) ligands, TGF-β, IL-6, retinoic acid and nitric oxide, TI induction of gut IgA is likely to involve microbiota-induced production of structurally related innate CD40L-like molecules termed B cell-activating factor of the TNF family (BAFF) and a proliferation-inducing ligand (APRIL) (18–23). BAFF/APRIL elicit TI production of systemic IgM, IgG, IgA or IgE by engaging a CD40-related receptor on B cells known as transmembrane activator and CAML interactor (TACI) (24–26). Despite this evidence, the role of BAFF/APRIL in TI gut IgA responses and the contribution of TACI to these responses remain debated or unknown (27).
By using multiple TACI-deficient mouse models, we found that TACI orchestrated an intestinal TI pathway that elicited specific SIgA responses to gut microbes by B cells located outside the specialized environment of mucosal GCs. Possibly due to their low binding affinity, TACI-induced SIgA responses targeted a fraction of gut commensal bacteria, but had no major effect on the overall composition of the intestinal microbiota. Remarkably, TACI did not play any role in the induction of IgA by the CD40-controlled TD pathway in gut-associated lymphoid organs. These findings place TACI at the center of the regulatory signaling networks that control the gut TI pathway for IgA production. They also point to a dualism of TACI and CD40 in the specific control of intestinal IgA-inducing TI and TD programs, respectively.
Results
TACI triggers CD40-independent IgA production
TACI has been detected on B cells as well as a fraction of T cells and macrophages from systemic lymphoid tissues (28–31). However, little is known about TACI expression by immune cells from the intestinal compartment. Flow cytometry initially characterized TACI expression in gut-associated PPs and MLNs from wild type (WT) C57BL/6 mice. For comparison, TACI was also measured in spleen and peritoneum.
In all tissues analyzed, TACI expression was readily detected on B cells and PCs, but was negligible on T cells, dendritic cells, macrophages, neutrophils and eosinophils (Fig. S1 A–J). Consistent with recently published findings (32), innate-like splenic or gut-associated B-1a, B-1b and marginal zone B cells expressed more TACI compared to follicular B cells (Fig. S1 A–B, D–J), which belong to the conventional B-2 lineage together with marginal zone B cells. Of note, PCs had the highest TACI expression in splenic but not gut-associated compartments (Fig. S1 D–J). Considering that intestinal B-1b and B-2 cells promote TI production of IgA and that TACI drives CD40-independent IgM-to-IgA class switching (15, 33), these observations are consistent with the hypothesis that BAFF/APRIL signals from TACI are likely to be involved in the regulation of the intestinal TI pathway for IgA production.
We next determined the possible impact of TACI on TI induction of IgA by exposing splenic resting naive B cells from WT or TACI-deficient Tnfrsf13b−/− mice to APRIL. Of note, this TACI ligand is not recognized by BAFF receptor (BAFF-R), which is expressed by all peripheral B cells and drives powerful B cell-specific survival signals (34). Enzyme-linked immunosorbent assay (ELISA) showed that, compared to B cells from control WT mice, B cells from Tnfrsf13b−/− mice secreted less IgA following exposure to APRIL for 5 days in combination with the Gram-negative microbial product lipopolysaccharide (LPS) and the cytokine transforming growth factor-β1 (TGF-β1) (Fig. S1 K). These mucosa-associated molecules up-regulate TACI expression and promote IgM-to-IgA class switching, respectively (32, 35). Thus, APRIL signals from TACI can trigger IgA production via a CD40-independent program that may mimic the gut TI pathway.
TACI orchestrates TI induction of intestinal IgA+ PCs
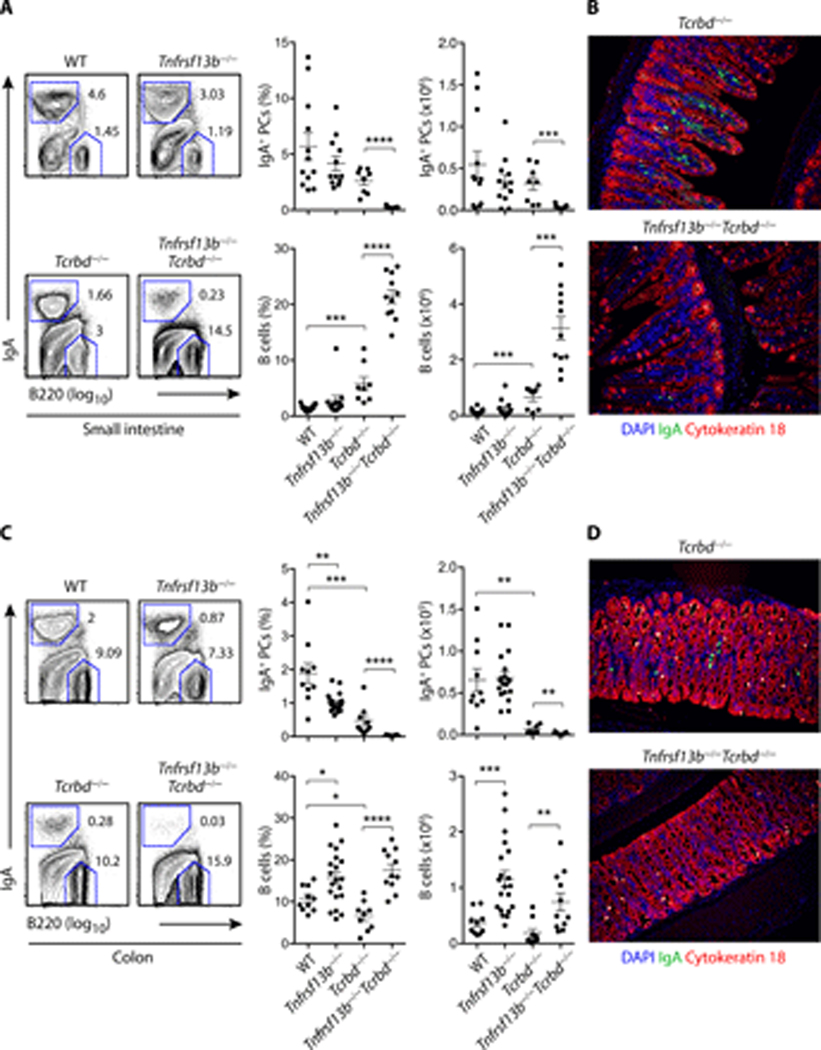
We next evaluated whether TACI promoted homeostatic induction of IgA class-switched PCs in the small intestine. WT and Tnfrsf13b−/− breeders were set up from Tnfrsf13b+/− parents to control for microbiota and genetic background variability between strains. Flow cytometry demonstrated that, compared to WT controls, Tnfrsf13b−/− mice showed a comparable frequency and absolute number of IgA+ PCs in the small intestinal lamina propria (Fig. 1 A). Given that TACI was not involved in the homeostatic induction of intestinal IgA+ PCs from T cell-sufficient Tnfrsf13b−/− mice, we next explored the role of TACI in the homeostatic induction of intestinal IgA+ PCs from T cell-deficient Tnfrsf13b−/− mice (15, 16). For this, we first generated T cell-deficient Tcrb−/−Tcrd−/− mice (hereafter referred to as Tcrbd−/− mice), which were subsequently crossed to TACI-deficient Tnfrsf13b−/− animals. Tcrbd−/− and Tnfrsf13b−/−Tcrbd−/− breeders were obtained from Tnfrsf13b+/−Tcrbd−/− parents. We found that, compared to Tcrbd−/− controls, Tnfrsf13b−/−Tcrbd−/− mice showed a severely reduced frequency and absolute number of IgA+ PCs in the small intestine (Fig. 1 A). Tissue immunofluorescence analysis visually confirmed that the intestinal lamina propria of Tnfrsf13b−/−Tcrbd−/− mice contained fewer IgA+ PCs compared to Tcrbd−/− controls (Fig. 1 B).
Figure 1.
TACI drives homeostatic induction of IgA class-switched PCs through intestinal TI but not TD pathways. (A) Flow cytometric analysis of frequency (% of live) and absolute number of B220−IgA+ PCs and B220+IgA− B cells from the small intestine lamina propria of control WT or Tnfrsf13b−/−, Tcrbd−/− and Tnfrsf13b−/−Tcrbd−/− mice. Left: representative flow cytometric contour plots. Numbers indicate cell frequency. Right: summary of multiple independent experiments. (B) IFA of small intestine from Tcrbd−/− and Tnfrsf13b−/−Tcrbd−/− mice stained for IgA (green), cytokeratin 18 (red) and counterstained with DNA-binding 4',6-diamidino-2-phenylindole (DAPI, blue), which labels nuclei. (C-D) Results from the colon lamina propria obtained through experimental settings identical to those depicted in (A) and (B). Flow cytometry plots show data representative of the mean (A, C, left panels) or pooled 2–4 independent experiments from 8 to 21 (A, C, right panels) 9–17-week-old mice or show one representative experiment of at least two yielding similar results (B, D). Error bars, s.e.m.; * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001 (two-tailed Student’s t test for normally distributed data and Mann-Whitney U test for other data).
We then determined the impact of TACI on the homeostatic induction of colonic IgA class-switched PCs, which derive from distal as well as local inductive sites (1, 12, 36). Compared to WT controls, Tnfrsf13b−/− mice showed a decreased frequency but a conserved absolute number of IgA+ PCs in the colonic lamina propria (Fig. 1 C). In addition, Tnfrsf13b−/− mice showed an increased frequency and absolute number of IgA− B cells in the colon (Fig. 1 C). Of note, Tnfrsf13b−/−Tcrbd−/− mice lacked the small pool of IgA+ PCs that consistently inhabited the colon of Tcrbd−/− controls (Fig. 1 C and 1 D).
The loss of IgA+ PCs was not linked to a B cell depletion, as both frequency and absolute number of B cells were increased in the small intestine and colon from Tnfrsf13b−/−Tcrbd−/− mice compared to Tcrbd−/− controls (Fig. 1 A and 1 C). Thus, BAFF/APRIL signals from TACI drive homeostatic TI induction of IgA class-switched PCs in the intestinal mucosa.
TACI induces intestinal IgA+ PCs independently of GCs
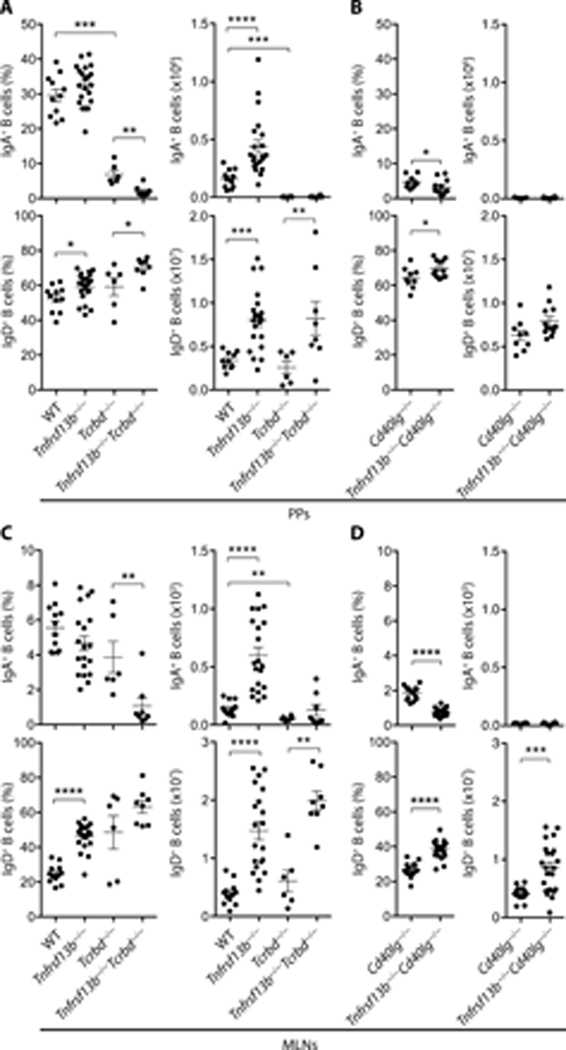
We then verified whether TACI could induce intestinal IgA+ PCs independently of the TD microenvironment of GCs (8). To this purpose, Tnfrsf13b−/− mice were crossed with CD40L-deficient Cd40lg−/− mice, which do not develop GCs (Fig. S2) but have conserved lymphoid follicles and T cells (37). Cd40lg−/− and Tnfrsf13b−/−Cd40lg−/− breeders were obtained from Tnfrsf13b+/−Cd40lg+/− parents. Compared to Cd40lg−/− controls, Tnfrsf13b−/−Cd40lg−/− mice showed a decreased frequency and a reduced absolute number of IgA+ PCs in both small intestine and colon, which was coupled with an increased frequency but a conserved absolute number of IgA− B cells (Fig. 2 A and 2 B). Thus, BAFF/APRIL signals from TACI drive homeostatic TI induction of intestinal IgA class-switched PCs through a GC-independent pathway that does not require CD40L-CD40-mediated T-B cell interaction.
Figure 2.
TACI drives homeostatic induction of IgA class-switched PCs through an intestinal GC-independent pathway. (A) Flow cytometric analysis of frequency (% of live) and absolute number of B220−IgA+ PCs and B220+IgA− B cells from the small intestine lamina propria of Cd40lg−/− and Tnfrsf13b−/−Cd40lg−/− mice. (B) IgA+ PCs and B cells from the colon lamina propria of Cd40lg−/− and Tnfrsf13b−/−Cd40lg−/− mice. Data shown pooled from 2 independent experiments from 9 to 12 mice that were 13–15-week-old. Error bars, s.e.m.; * p < 0.05, ** p < 0.01, *** p <0.001 (two-tailed Student’s t test for normally distributed data and Mann-Whitney U test for other data).
TACI does not regulate TD induction of intestinal IgA
Next, we evaluated the contribution of TACI to the TD induction of IgA in the small intestine. Indeed, TACI may activate T cells (38, 39) and modulate the size of both TFH cell and GC B cell compartments (30). Flow cytometry showed that, compared to WT controls, PPs from Tnfrsf13b−/− mice included a comparable frequency but an increased absolute number of class-switched IgA+ B cells (Fig. 3 A and Fig. S1 C).
Figure 3.
TACI does not regulate TD induction of IgA in gut-associated lymphoid tissue. (A-D) Flow cytometric analysis of frequency and absolute numbers of IgA class-switched IgD−B220+IgA+ B cells (% of IgD−B220+ cells) and unswitched IgD+B220+ B cells (% of live cells) from (A-B) PPs or (C-D) MLNs of WT, Tnfrsf13b−/−, Tcrbd−/−, Tnfrsf13b−/−Tcrbd−/−, Cd40lg−/− or Tnfrsf13b−/−Cd40l−/− mice. Data summarize 2–4 independent experiments from 6 to 22 mice that were 9–17-week-old. Error bars, s.e.m.; * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001 (two-tailed Student’s t test for normally distributed data and Mann-Whitney U test for other data).
In agreement with their prevailing emergence from the TD pathway, IgA+ B cells from PPs of Tcrbd−/− mice were virtually undetectable compared to WT controls (Fig. 3 A). Aside from the TD pathway (7), PPs may include an alternative TI pathway for IgA production (40). Thus, we determined whether this TI pathway required signals from TACI. Compared to Tcrbd−/− or Cd40lg−/− controls, Tnfrsf13b−/−Tcrbd−/− or Tnfrsf13b−/−Cd40lg−/− mice showed a decreased frequency, but a comparable absolute number of IgA+ B cells, which, in general, were barely detectable in PPs from either T cell-deficient or CD40L-deficient animals (Fig. 3 A and 3 B).
Subsequently, we verified whether the increase of class-switched IgA+ B cells from PPs of TACI-deficient mice was associated with a global expansion of B cells, including non-switched naive IgD+ B cells. Indeed, the frequency and absolute number of IgD+ B cells were increased in PPs from Tnfrsf13b−/− mice compared to WT controls. Similarly, the frequency and/or absolute number of IgD+ B cells were increased in PPs from Tnfrsf13b−/−Tcrbd−/− or Tnfrsf13b−/−Cd40lg−/− mice compared to Tcrbd−/− or Cd40lg−/− controls (Fig. 3 A and 3 B). In MLNs, class-switched IgA+ B cells from Tnfrsf13b−/− mice showed a comparable frequency, but an increased absolute number compared to WT controls (Fig. 3 C). In addition, the absolute number of IgA+ B cells from MLNs was reduced in Tcrbd−/− mice compared to WT controls (Fig. 3 C). Moreover, compared to Tcrbd−/− or Cd40lg−/− controls, Tnfrsf13b−/−Tcrbd−/− or Tnfrsf13b−/−Cd40lg−/− mice showed a decreased frequency, but a comparable though barely detectable absolute number of IgA+ B cells (Fig. 3 C and 3 D).
Finally, the frequency and/or absolute number of non-switched IgD+ B cells were increased in MLNs from Tnfrsf13b−/−, Tnfrsf13b−/−Tcrbd−/− or Tnfrsf13b−/−Cd40lg−/− mice compared to WT, Tcrbd−/− or Cd40lg−/− controls (Fig. 3 C and 3 D). Thus, BAFF/APRIL signals from TACI play little or no role in homeostatic IgA responses from the CD40-controlled TD pathway operating in GCs associated with gut lymphoid follicles. Moreover, this TD pathway does not contribute to the gut B cell hyperplasia that develops in TACI-deficient mice.
TACI modulates B cell numbers but not IgA affinity in gut GCs
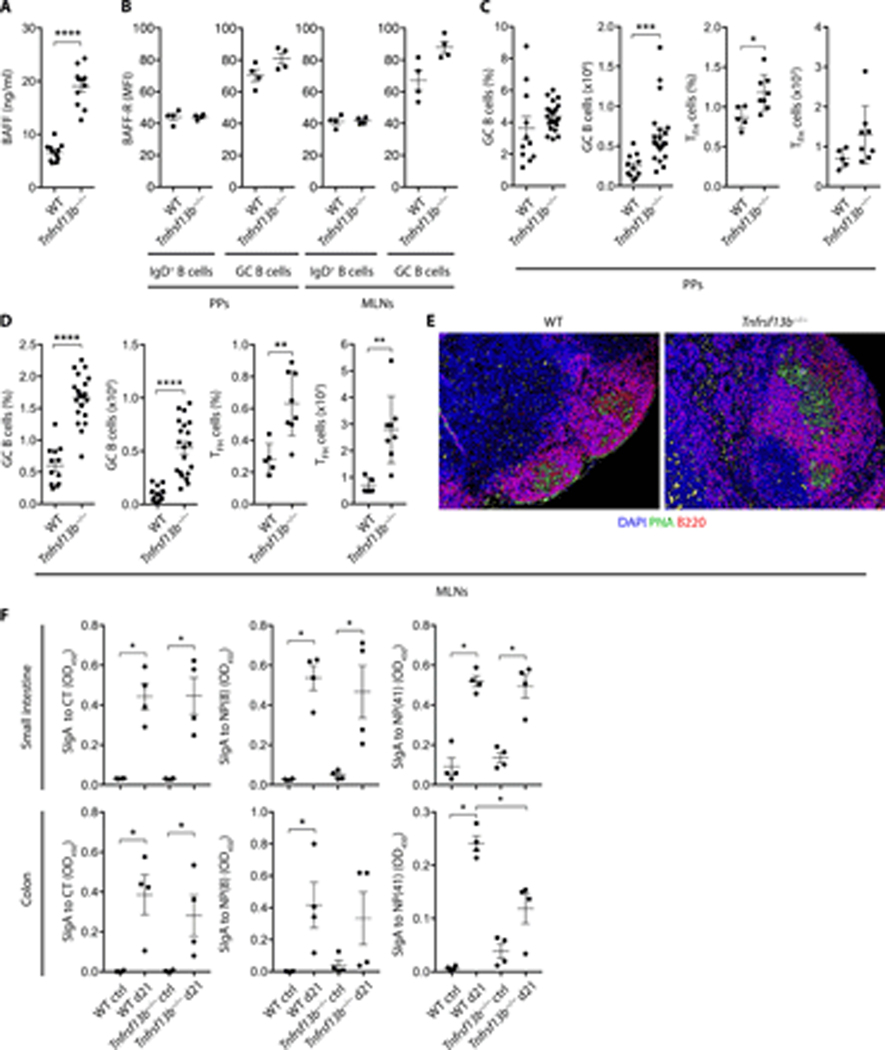
Having shown that T cells and CD40L played little or no role in the gut B cell expansion detected in TACI-deficient mice, we verified whether this phenomenon was linked to an increased availability of BAFF, an innate inducer of peripheral B cell survival (29, 41, 42). ELISAs confirmed that serum BAFF was increased in Tnfrsf13b−/− mice compared to WT controls (Fig. 4 A). In addition, flow cytometry indicated that unswitched and GC B cells from PPs or MLNs of WT and Tnfrsf13b−/− mice expressed comparable levels of surface BAFF-R (Fig. 4 B), a specific BAFF receptor inducing peripheral B cell survival (34).
Figure 4.
TACI modulates B cell and TFH cell numbers but not IgA affinity in gut GCs. (A) Plasma BAFF ELISA of WT or Tnfrsf13b−/− mice. (B-D) Flow cytometric analysis of PPs and MLNs of WT or Tnfrsf13b−/− mice of (B) BAFF-R on IgD+B220+ and IgD−B220+GL7+CD95+ GC B cells, MFI, median fluorescence intensity, (C-D) frequency (% of live) and absolute numbers of GC B cells and TCRβ+CD4+PD-1highCXCR5high TFH cells. (E) IFA of MLNs from WT or Tnfrsf13b−/− mice stained with peanut agglutinin (PNA, green), B220 (red) and DAPI (blue). (F) Cholera toxin (CT)- or NP-specific fecal SIgA ELISA from the small intestine or colon of WT or Tnfrsf13b−/− mice orally immunized with NP-OVA and CT. Bovine serum albumin (BSA) haptenated with 8 or 41 NP epitopes (NP(8)-BSA or NP(41)-BSA) used to measure high- and low-affinity SIgA to NP, respectively. Ctrl, PBS-immunized mice. OD, optical density. Data summarize 2–4 independent experiments from 5 to 21 9–15-week-old mice (A, C-D) or one representative experiment of at least two (B, E) or one experiment (n=4) (F). Error bars, s.e.m.; * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001 (two-tailed Student’s t test or Mann-Whitney U test).
Having shown that gut-associated lymphoid organs from Tnfrsf13b−/− mice show hyperplasia of follicular IgD+ B cells, we determined whether this hyperplasia further involved GC B cells. In agreement with published data (30), compared to WT controls, Tnfrsf13b−/− mice showed an increased frequency and absolute number of not only GC B cells, but also TFH cells in both PPs and MLNs (Fig. 4 C and 4 D, Fig. S1 C and Fig. S3). Of note, TFH cells constitute a BCL6-dependent CD4+ T cell subset specialized in the activation of GC B cells (6). Accordingly, tissue immunofluorescence showed that peanut agglutinin-positive GCs from MLNs were enlarged in Tnfrsf13b−/− mice compared to WT controls (Fig. 4 E).
Considering that B cell hyperplasia dampens the selection of high-affinity IgA-expressing B cells in gut-associated GCs (6), we evaluated the impact of TACI on the TD induction of high-affinity IgA by orally immunizing WT or Tnfrsf13b−/− mice with 4-hydroxy-3-nitrophenyl (NP)-haptenated ovalbumin (OVA) supplemented with the adjuvant cholera toxin (CT). ELISAs demonstrated that high-affinity SIgAs to NP-OVA or CT were comparable in the small intestine or colon from WT and Tnfrsf13b−/− mice (Fig. 4 F). However, low-affinity SIgA to NP-OVA, which could involve an NP-reactive TI pathway, was somewhat decreased in the colon from Tnfrsf13b−/− mice (Fig. 4 F). Thus, loss of BAFF/APRIL signals from TACI does not impair TD induction of high-affinity IgA despite causing GC B cell and TFH cell hyperplasia in gut-associated lymphoid follicles.
TACI does not influence the prevailing gut IgA gene repertoire
Consistent with our finding that TACI deficiency induces B cell expansion in gut-associated (GCs) centers, TACI has been recently shown to constrain the expansion of GC B cells in systemic lymphoid follicles (43). A corollary of this observation is that TACI deficiency could perturb the selection of IgA class-switched B cells in gut follicles, as found in mice lacking the immune inhibitory receptor programmed cell death-1 (PD-1) (6). To explore this possibility, we adopted a recently published strategy involving next generation sequencing of gut-derived heavy chain (H) variable (V), diversity (D) and joining (J) gene segments encoding the antigen-binding VH domain of IgA (11). In adults harboring a stable microbiota, the gut IgA gene repertoire is mainly shaped by the TD pathway, which continuously diversifies pre-existing memory IgA+ B cells by channeling them into constitutively active GCs from PPs (11). We found that the IgA VHDJH gene repertoire from both small intestine and colon of Tnfrsf13b−/− mice showed no major differences compared to WT controls (Fig. 5 A). Similarly, no major differences were detected in the frequency of total, non-silent (or replacement) and silent mutations targeting the framework region 1 (FR1), complementary determining region 1 (CDR1), FR2, CDR2, and FR3 of IgA VHDJH genes from the small intestine or colon of WT and Tnfrsf13b−/− mice (Fig. 5 B).
Figure 5.
TACI deficiency does not perturb the prevailing TD clonal architecture of IgA+ PCs from adult mice. (A) Next generation sequencing (NGS) of the IgA VHDJH gene repertoire from the small intestine (SI) or colon lamina propria (LP) of WT or Tnfrsf13b−/− mice. (B) NGS analysis of non-silent (replacement) and silent mutations targeting the FR1, CDR1, FR2, CDR2 and FR3 regions of IgA VHDJH genes from the SI or colon LP of WT or Tnfrsf13b−/− mice. (C-E) NGS analysis of (C) clonal IgA VHDJH gene networks in the colon, (D) clonal IgA VHDJH gene diversity, and (E) clonal IgA VHDJH gene relatedness between the SI and colon LP of WT or Tnfrsf13b−/− mice. (F) Fluorescent in situ hybridization analysis of the colon from WT or Tnfrsf13b−/− mice stained with a fluorochrome-labeled universal eubacterial probe (red) and counterstained with DAPI (blue). Data summarize one experiment including 2 to 4 mice (A-B, D-E) that were 10–14-week-old or show one representative mouse (C) or experiment of two yielding similar results (F). Error bars, s.d. (A-B); horizontal bar, mean (D, E).
In addition, the general architecture of different IgA clones from either expanded or small clonal networks was similar in the small intestine or colon of WT and Tnfrsf13b−/− mice (Fig. S4 and 5 C). In this analysis, CDR3 sequences showing at least 95% nucleotide identity were assigned to an identical clone and IgA gene networks were generated by depicting each IgA VHDJH gene sequence as a node and by drawing lines to connect nodes belonging to the same clone (11). Finally, as demonstrated by the calculation of Shannon and Morisita-Horn indexes (11), the IgA VHDJH gene repertoires from WT and Tnfrsf13b−/− mice showed comparable clonal diversity and clonal gene relatedness between the small intestine and colon, respectively (Fig. 5 D and 5 E).
Considering that SIgAs from the TD pathway may target penetrant commensals to constrain their motility and penetrance (2, 9, 15), we also performed fluorescence in situ hybridization to determine whether TACI deficiency influenced intestinal immune exclusion of bacteria. Unlike the small intestine, the colon has a visible bacteria-free mucus layer that can be readily identified after staining the local microbiota with a fluorochrome-labeled universal bacterial probe (44). Therefore, we analyzed immune exclusion in the colon and found that Tnfrsf13b−/− mice excluded commensals from the epithelium as efficiently as WT controls did (Fig. 5 F). Thus, consistent with the central role of the TD pathway in shaping the adult intestinal IgA repertoire (11, 12, 45), TI signals from TACI play no role in the composition, clonal architecture, diversity or function of this repertoire.
TACI orchestrates TI induction of SIgAs to gut commensals
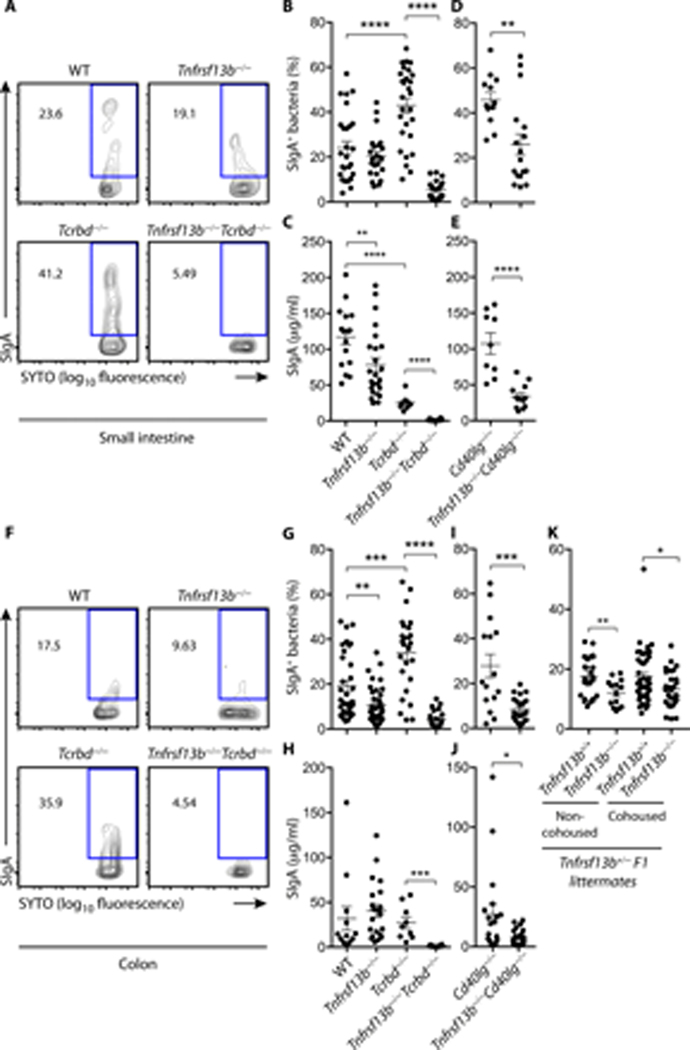
Given that SIgAs from the TI pathway bind a large spectrum of gut commensals (15, 16), we set up a flow cytometric strategy to determine the impact of TACI deficiency on SIgA binding to gut microbes. Compared to WT controls, Tnfrsf13b−/− mice had fewer SIgA-bound commensals in the small intestine (Fig. 6 A–B). This finding was somewhat in contrast with our earlier observation that IgA+ PCs were conserved in the small intestine from Tnfrsf13b−/− mice. One possible interpretation is that gut PCs from Tnfrsf13b−/− mice generate SIgA antibodies with lower specificity to gut bacteria compared to WT controls. However, this latter conclusion would also imply an increased detection of free (i.e., unbound) intestinal SIgA (6). Instead, we found smaller amounts of free SIgA in the small intestine from Tnfrsf13b−/− mice compared to WT controls (Fig. 6 C). Thus, homeostatic SIgA responses to the gut commensal microbiota involve a TI pathway orchestrated by BAFF/APRIL signals from TACI.
Figure 6.
TACI drives TI homeostatic SIgA responses to the intestinal microbiota. (A-B, D, F-G, I, K) Flow cytometry of SIgA+SYTO+ bacteria from the small intestine (A-B, D) or colon (F-G, I, K) of WT, Tnfrsf13b−/−, Tcrbd−/−, Tnfrsf13b−/−Tcrbd−/−, Cd40lg−/− or Tnfrsf13b−/−Cd40lg−/− mice. Representative flow cytometric contour plots from (A) small intestinal or (F) colonic contents. Numbers indicate % of SIgA-coated bacteria. (B-D, G-I) graphs summarize 3–5 independent experiments involving 12 to 33 mice that were 6–13 week-old. ELISA of free SIgA from (C, E) small intestine or (H, J) colon feces from WT, Tnfrsf13b−/−, Tcrbd−/−, Tnfrsf13b−/−Tcrbd−/−, Cd40lg−/− or Tnfrsf13b−/−Cd40lg−/− mice. (K) Flow cytometry of SIgA+SYTO+ bacteria from non-cohoused and cohoused Tnfrsf13b+/+ and Tnfrsf13b−/− littermates from Tnfrsf13b+/− breeders. Data pooled from 8–16-week-old mice from 2–5 independent experiments (n=14 to 47). Error bars, s.e.m. (B-E, G-K). * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001 (two-tailed Student’s t test for normally distributed data and Mann-Whitney U test for other data).
Despite showing increased bacteria-bound SIgA, the small intestine from Tcrbd−/− mice contained less free SIgA compared to WT controls (Fig. 6 B–C), suggesting that SIgA emanating from the TI pathway retains a significant avidity for commensals. Of note, TI binding of gut bacteria by SIgAs was specific, because SIgA-coated microbes were readily detectable in Tcrbd−/− mice but not Igha−/− controls (Fig. S5). These results echo a recent report showing that SIgA from the TI pathway specifically recognizes a broad set of gut bacteria (15).
In agreement with the key role played by TACI in the orchestration of the gut TI pathway, SIgA-bound bacteria were significantly reduced in the small intestine from Tnfrsf13b−/−Tcrbd−/− mice compared to Tcrbd−/− controls (Fig. 6 B). Remarkably, also free SIgA was reduced in the small intestine from Tnfrsf13b−/−Tcrbd−/− mice (Fig. 6 C). Thus, SIgA from the intestinal TI pathway specifically targets commensal bacteria through a mechanism that requires TACI.
Consistent with this possibility, we found that both bacteria-bound and free SIgAs were decreased in the small intestine from Tnfrsf13b−/−Cd40lg−/− mice compared to Cd40lg−/− controls (Fig. 6 D–E). Similar to the small intestine, the colon showed fewer SIgA-coated bacteria in Tnfrsf13b−/−, Tnfrsf13b−/−Tcrbd−/− or Tnfrsf13b−/−Cd40lg−/− mice compared to WT, Tcrbd−/− or Cd40lg−/− controls, respectively, and less free SIgA in Tnfrsf13b−/−Tcrbd−/− and Tnfrsf13b−/−Cd40lg−/− mice compared to control Tcrbd−/− and Cd40lg−/− mice, respectively (Fig. 6 F–J and Fig. S5 B).
To demonstrate that the depletion of SIgA-coated gut bacteria in Tnfrsf13b−/− mice was specifically linked to the loss of TI signals from TACI rather than non-specific microbiota-related differences accumulating overtime as a result of distinct breeding strategies, we went on to analyze SIgA-coated gut bacteria from littermates of Tnfrsf13b+/− breeders. We found decreased SIgA-coated bacteria in Tnfrsf13b−/− compared to Tnfrsf13b+/+ littermate controls from Tnfrsf13b+/− breeders (Fig. 6 K). In another breeding strategy, Tnfrsf13b−/− mice rederived to be Helicobacter-free also showed decreased SIgA-coated bacteria compared to WT controls, originating from Tnfrsf13b−/− and WT breeders from common Tnfrsf13b+/− parents (Fig. S5 B). This finding suggests that TACI deficiency causes loss of SIgA-coated bacteria independently of a specific microbiota environment.
In a third model, Tnfrsf13b−/− pups raised by a WT dam as of 1–2 days after birth and thereby colonized by WT microbiota showed decreased SIgA-coated bacteria compared to WT pups raised by a Tnfrsf13b−/− dam, indicating an intrinsic defect in SIgA bacteria binding mediated by TACI-deficiency (Fig. S5 C). Altogether, these data provide convincing evidence that depletion of SIgA-coated gut bacteria in Tnfrsf13b−/− mice is causally linked to the loss of SIgA emerging from the TACI-controlled TI pathway. Thus, BAFF/APRIL signals from TACI orchestrate homeostatic SIgA responses to gut commensal bacteria through a TI pathway that develops outside GCs. Of note, SIgA from this TACI-controlled TI pathway is sufficient to coat a set of gut microbes.
TACI-induced commensal-reactive SIgAs do not modify the composition of the gut microbiota
Considering that TACI orchestrates TI-induced SIgA responses to gut microbes and having found an impairment of these responses not only in T cell-deficient Tnfrsf13b−/−Tcrbd−/− mice but also in T cell-sufficient Tnfrsf13b−/− mice, we went on to compare the composition of the fecal microbiota in co-housed Tnfrsf13b+/+ and Tnfrsf13b−/− littermates. Next generation sequencing of bacterial 16S ribosomal DNA (rDNA) showed that, compared to Tnfrsf13b+/+ controls, Tnfrsf13b−/− littermates had a similar fecal microbiota composition at the phylum, genus and operational taxonomic unit (OTU) levels (Fig. 7 A and S6 A), with the expected predominance of Bacteroidetes and Firmicutes (6, 46). Given that the composition and regulation of the commensal microbiota from stool and gut mucosa could show unexpected differences, we also analyzed the microbiota associated with the colonic tissue. While no differences were detected between Tnfrsf13b−/− mice and Tnfrsf13b+/+ controls, the composition of the colon-associated microbiota was dramatically different from the composition of the fecal microbiota at the phylum, genus and OTU levels (Fig. 7 B and S6 A–B).
Figure 7.
SIgAs from the TACI-controlled intestinal TI pathway do not affect microbiota richness and diversity in the presence of an intact CD40-controlled TD pathway. (A-B) Relative abundance of microbial phyla from the stool (A) or colon tissue-associated microbiota (colon) (B) of co-housed Tnfrsf13b+/+ or Tnfrsf13b−/− mice from Tnfrsf13b+/− breeders. (C) Rarefaction plot comparing alpha-diversity of the microbiota from the stool or colon of Tnfrsf13b+/+ or Tnfrsf13b−/− mice. (D) Principal component analysis of beta-diversity of the microbiota from the stool and colon of Tnfrsf13b+/+ or Tnfrsf13b−/− mice. Data show one experiment including 8–9-week-old mice co-housed littermates from Tnfrsf13b+/− breeders (Tnfrsf13b+/+ n=27, Tnfrsf13b−/− n=20). Error bars, s.e.m.. * p < 0.05 (Wilcoxon rank sum test).
Moreover, alpha diversity expressed as the number of observed OTUs of the gut microbiota from Tnfrsf13b−/− mice was comparable to that of Tnfrsf13b+/+ controls (Fig. 7 C). Similarly, principal component analysis of beta diversity indicated that the gut microbiota from Tnfrsf13b−/− mice clustered together with the gut microbiota from Tnfrsf13b+/+ controls (Fig. 7 D). Of note, the colon-associated microbiota showed decreased number of observed OTUs and tended to cluster separately from the fecal microbiota. Thus, aside from showing that microbial communities from stool and gut mucosa have major differences in the relative abundance of the major gut phyla, these findings indicate that bacteria-reactive SIgA antibodies induced by the TACI-controlled TI pathway have no effect on the composition or diversity of the intestinal microbiota.
Discussion
Here we have shown that the intestinal SIgA-inducing TI pathway required TACI, a BAFF/APRIL receptor predominantly expressed by B cells. Signals from TACI generated IgA class-switched PCs through a CD40-independent program that was not involved in TD induction of IgA affinity maturation within gut-associated GCs. Yet, SIgAs from the TACI-controlled TI pathway bound to a fraction of gut bacteria.
The gut-associated lymphoid tissue includes a dominant CD40-regulated TD pathway that unfolds within GCs and generates hypermutated SIgAs that specifically target a discrete set of highly penetrant commensals with higher affinity (6, 7, 9, 15, 45). A complementary TI pathway develops outside intestinal GCs and generates unmutated SIgAs that bind a broader spectrum of bacteria with lower affinity and polyreactive modalities (15, 16, 47). This TI pathway requires poorly understood CD40-independent B cell-activating signals that, based on multiple ex vivo and in vivo lines of evidence, may include APRIL and, to a lesser extent, BAFF (20, 48, 49). Despite this evidence, the master regulator of the gut TI pathway remains elusive.
Our present findings demonstrate that this regulator is TACI, a B cell-activating receptor that binds both APRIL and BAFF (34). These CD40L-related TACI ligands trigger CD40-independent class switching both in vitro (19) and in vivo (24, 48). However, the role of these ligands in intestinal SIgA induction, including their specific contribution to the gut TI pathway, remains unclear. While a recent study questions the positive impact of APRIL and BAFF on gut IgA responses (27), more evidence proposes that these innate B cell activation ligands enhance intestinal PC survival in addition to IgM-to-IgA class switching (50). In general, it remains unknown whether BAFF/APRIL signals from TACI induce gut SIgA production by specifically controlling the TI pathway or, rather, by generically supporting the survival of PCs induced by TI or TD signals. Indeed, published evidence gathered from the analysis of B cells inhabiting extra-intestinal compartments indicates that TACI may enhance systemic IgM and IgG responses by merely sustaining the differentiation and/or survival of PCs induced by TI or TD antigens (26, 49, 51, 52). Our findings from the intestinal compartment show that innate-like BAFF/APRIL signals from TACI selectively orchestrated the TI pathway for SIgA induction, but were not relevant to the induction of the TD pathway. The following evidence supports this conclusion.
First, gut-associated TACI expression was mostly detected on B cells, but involved neither T cells nor dendritic cells nor macrophages. Thus, it is unlikely that TACI initiates TD intestinal IgA responses by influencing the function of T cells and/or antigen-presenting myeloid cells, as suggested by some studies (31, 43). Second, in the presence of LPS and TGF-β1, TACI ligation by APRIL elicits CD40-independent IgA production in B cells. Thus, TACI induces IgA upon engagement by APRIL, which is highly expressed by intestinal epithelial, dendritic and stromal cells (1, 20).
Third, T cell and TACI double-deficient Tnfrsf13b−/−Tcrbd−/− mice showed no TI induction of intestinal IgA+ PCs, which instead were conserved in T cell-deficient but TACI-sufficient Tcrbd−/− controls. Thus, TACI does not merely promote the survival of IgA+ PCs emerging irrespectively from TI or TD pathways, as suggested by earlier studies (26, 51, 52). Rather, TACI specifically elicits the generation of IgA+ PCs by the TI pathway. Consistent with this interpretation, intestinal SIgA responses to an oral TD protein antigen were conserved in Tnfrsf13b−/− mice. Fourth, the depletion of intestinal IgA+ PCs from Tnfrsf13b−/−Tcrbd−/− mice was combined with a profound hyperplasia of intestinal IgD+ B cells. Thus, TACI does not support TI induction of intestinal IgA+ PCs by merely enhancing the survival of their B cell progenitors (53). Fifth, Tnfrsf13b−/− mice developed conserved homeostatic or post-immune TD intestinal SIgA responses, displayed a normally hypermutated intestinal IgA repertoire and showed no depletion but, instead, an expansion of GC B cells and class-switched IgA+ B cells from major TD intestinal inductive sites, including PPs and MLNs (1). Thus, TACI plays no major role in shaping the adult intestinal IgA repertoire and does not induce intestinal IgA in PPs or MLNs, two functions that depend on the TD pathway.
Despite showing conserved TD induction of intestinal IgA+ PCs, T cell-sufficient Tnfrsf13b−/− mice developed a partial loss of microbiota-coating by SIgA. A possible interpretation of this result is that SIgAs from the TD pathway are quantitatively normal but qualitatively perturbed. In principle, this potential perturbation could stem from BAFF-mediated expansion of B cells in intestinal GCs, which may impair the stringency of B cell selection and the efficiency of IgA affinity maturation (6, 29). Indeed, earlier studies show accumulation of peripheral B cells in Tnfrsf13b−/− mice due to pathologically increased BAFF-R-mediated survival signals from BAFF (24, 29, 41, 42). Accordingly, we found increased serum BAFF and conserved BAFF-R expression by gut-associated B cells from Tnfrsf13b−/− mice.
In addition to B cells, TFH cells were expanded in gut-associated GCs. This expansion may result from the increased activation of TFH cells by signals from ICOSL (inducible co-stimulator ligand), a TACI-constrained molecule with increased expression on TACI-deficient GC B cells from Tnfrsf13b−/− mice (43). By reducing the stringency of IgA affinity maturation through the TD pathway, the hyperplasia of both GC B and T cells from Tnfrsf13b−/− mice could decrease the reactivity of SIgAs for commensals, as previously shown in Pdcd1−/− mice lacking the inhibitory PD-1 receptor (6). However, an alternative explanation involves impaired early priming of follicular B cells by certain commensals physiologically transported into PPs by phagocytes with the help of TACI-induced SIgAs (54–56). These bacteria-reactive SIgAs may include polyreactive specificities emanating from the TACI-controlled TI pathway (56).
Although plausible, impaired IgA affinity maturation in Tnfrsf13b−/− mice is at odds with two additional sets of observations. First, we found that free SIgA was decreased in the small intestine from adult Tnfrsf13b−/− mice, whereas published evidence shows that free SIgA is more (not less) abundant when gut B cell affinity maturation is impaired (6). Second, additional published studies show that systemic B cells from Tnfrsf13b−/− mice induce increased (not decreased) IgG somatic hypermutation in splenic GCs (30). Consistent with this evidence, Tnfrsf13b−/− mice mount more abundant and higher affinity IgG responses to an invasive intestinal pathogen, which thereby is cleared more effectively in Tnfrsf13b−/− mice compared to WT controls (30).
Thus, the GC B and T cell hyperplasia that we observed in the gut-associated lymphoid organs from Tnfrsf13b−/− mice is unlikely to have a negative impact on the quality of intestinal IgA responses, including their affinity for antigen. Accordingly, intestinal IgA showed no alteration of somatic hypermutation and conserved affinity upon oral immunization of Tnfrsf13b−/− mice with a canonical TD antigen. We are left with the possibility that, in these mice, the conserved and overwhelming induction of IgA+ PCs through the intestinal TD pathway masks the loss and/or dysfunction of a discrete pool of IgA+ PCs generated by the TACI-controlled TI pathway. In the T cell-sufficient context of Tnfrsf13b−/− mice, a selective perturbation of the TI pathway may become apparent only when highly specific and very sensitive parameters such as bacteria-bound SIgAs are measured.
By showing that TACI drives TI intestinal IgA responses to commensals independently of CD40-induced GCs, our findings indicate that signals from the BAFF/APRIL system account for the abundant induction of microbiota-coating SIgAs that was recently detected in GC-deficient Bcl6−/− mice (15). The reactivity of TACI-controlled SIgAs for bacteria is somewhat surprising, as the TD microenvironment of GCs is commonly thought to be required for the development of high-affinity SIgA responses to the gut microbiota (8). However, gut bacteria may also shape the reactivity of intestinal B cells by delivering BCR-editing signals at an early time after birth, possibly through a TI mechanism (17). In principle, these signals may generate TACI-responsive B cells with an intrinsically elevated reactivity to commensals independent of the induction of affinity maturation in mucosal GCs. An additional consideration relates to the fact that gut commensals express a mosaic of surface TD proteins and glycoproteins along with surface TI glycans. This implies that, in principle, TI-induced SIgA to bacterial glycans could sterically interfere with the binding of TD-induced SIgA to bacterial glycoproteins, at least under homeostatic conditions.
While being highly reactive to commensals, the specific function of SIgAs from the TACI-controlled TI pathway remains elusive. Indeed, Tnfrsf13b−/− mice show no major perturbations of the composition and diversity of the gut microbiota. This negative finding may be linked to the low binding affinity of TACI-controlled SIgAs, which are uncoupled from GC-induced antibody affinity maturation via somatic hypermutation. In general, higher affinity SIgA from the CD40-controlled TD pathway may be more effective in controlling the composition of the gut microbiota by regulating competitive mucosal niches via bacteria-derived metabolites (3, 57). Of note, the lack of TACI-induced SIgAs in Tnfrsf13b−/− mice did not hamper the confinement of commensals within the intestinal lumen, which indicates that also immune exclusion may be predominantly implemented by higher affinity SIgAs from the CD40-controlled TD pathway.
In the presence of an intact TD pathway, commensal-specific but low-affinity SIgAs from the TACI-controlled TI pathway may mediate rather subtle functions, which could involve the control of the overall immunogenicity of the gut microbiota. For example, broadly reactive SIgAs induced by TACI-activated B cells might enhance the pro-inflammatory properties of coated gut bacteria by engaging the Fcα/μ receptor (in mice) or the Fcα receptor I (in humans) (58, 59).
While the diminished binding of SIgA to the gut microbiota from Tnfrsf13b−/− or Tnfrsf13b−/−Tcrbd−/− mice supports the above interpretation, an alternative interpretation is that the gut microbiota from our mouse colony lacks responsiveness to both TI-induced and TD-induced SIgA. Indeed, published studies are often discordant on the overall impact that SIgA has on the gut microbiota, regardless of its origin from a TI or a TD pathway. This raises the question of what impact colony-specific host genetic differences have on the gut microbiota with respect to its responsiveness to SIgA (60, 61). To address this potential caveat, future studies will dissect the composition of the gut microbiota also in our TCR- or CD40L-deficient colonies, which selectively lack the TD pathway for IgA production. In principle, the detection of compositional differences in the gut microbiota from TCR- or CD40L-deficient mice would further validate our initial conclusion that intestinal microbial communities respond more profoundly to SIgA from the TD-induced pathways compared to SIgA from the TI-induced pathway.
An additional limitation of the present study relates to the distinct biology of TACI in humans compared to mice. Indeed, human but not mouse TACI is comprised of long and short isoforms that originate from alternative splicing of the mRNA encoding TNFRSF13B (62). While the long isoform does not influence PC induction, the short isoform effectively induces PC differentiation (62). However, this evidence derives from in vitro studies that do not ascertain the relative impact of long and short TACI isoforms on PC responses in the human intestine. The in vivo elucidation of such responses will require the generation of knock-in mice expressing long or short human TACI.
In summary, we identified the APRIL/BAFF receptor TACI as a dominant orchestrator of the intestinal TI pathway for SIgA production. We also found that SIgAs emanating from the TACI-controlled TI pathway specifically target a fraction of the gut microbiota independently of CD40-controlled GCs.
Materials and Methods
Study Design
This study was designed to identify the receptor on B cells deputed to the induction and maintenance of the intestinal TI pathway for in vivo IgA production. Our hypothesis was that this receptor was TACI, a BAFF/APRIL-binding member of the TNF receptor superfamily related to CD40, which instead governs the intestinal TD pathway for IgA production. A corollary hypothesis was that TACI additionally contributed to some aspects of the intestinal TD pathway, which relies on CD40-dependent GCs. First, we compared WT and Tnfrsf13b−/− mice to define the impact of TACI in the TD pathway. Next, we compared Tcrbd−/− and Tnfrsf13b−/−Tcrbd−/− mice to dissect the role of TACI in the TI pathway. To ascertain whether TACI required GCs to induce intestinal IgA responses, we compared Cd40lg−/− and Tnfrsf13b−/−Cd40lg−/− mice. Most experiments were performed 2–6 times, showed good reproducibility and were summarized by pooling the data from all the experiments. The following were the exceptions. BAFF-R MFI values were shown from one representative flow cytometry experiment out of three, which could not be pooled. Indeed, BAFF-R MFI showed inter-experimental variability, possibly due to unexpected changes in instrument settings. In addition, oral immunizations with NP-OVA and CT were performed once, as they incontrovertibly showed conserved IgA responses in Tnfrsf13b−/− mice compared to WT controls. Moreover, the 16S rDNA sequencing analysis of the microbiota from colon tissue and fecal pellets was performed only once, but this experiment involved a large number of mice to account for inter-cage variability. To obtain enough samples from age-matched co-housed mice, samples were collected and frozen at 7 distinct time points. Importantly, the initial flow cytometric analysis of fecal bacteria-bound SIgA from Tnfrsf13b−/− and WT mice was repeated in Tnfrsf13b−/− and Tnfrsf13b+/+ littermates, and in separate cohorts of mice. The latter experiments were performed to demonstrate that the depletion of SIgA-coated gut bacteria in Tnfrsf13b−/− mice was specifically linked to the loss of TI signals from TACI rather than to non-specific microbiota-related differences accumulating overtime or non-specific differences in the genetic background. Furthermore, the analysis of the gut IgA VHDJH gene repertoire was performed on samples from small intestine and colon that were originally collected from four Tnfrsf13b−/− mice and three WT controls. One colon sample from each strain did not yield enough sequences to be included in the analysis. Since the mutation frequencies of gut IgA showed no significant differences in Tnfrsf13b−/− mice compared to WT controls and considering that this finding was consistent with additional data showing no involvement of TACI in pre- or post-immune TD-induced IgA responses, we chose not to repeat this large gut IgA VHDJH gene sequencing experiment. Finally, samples in which most of the cells were dead were excluded from the analysis. This happened mostly with small intestine or colon samples, which are overly sensitive to changes in the ex vivo environment.
Mice
WT C57BL/6, Tcrb−/−, Tcrd−/− and Cd40lg−/− mice were obtained from commercially available sources (The Jackson Laboratory). Tnfrsf13b−/− mice were generated as described (24, 63) and backcrossed for ten generations to C57BL/6 mice. Tcrbd−/− were obtained by breeding Tcrb−/− mice with Tcrd−/− mice. Finally, Tnfrsf13b−/−Tcrbd−/− mice were generated by crossing Tnfrsf13b−/− with Tcrbd−/− mice, whereas Tnfrsf13b−/−Cd40lg−/− were obtained by breeding Tnfrsf13b−/− with Cd40lg−/− mice. WT and Tnfrsf13b−/− breeders were set up from Tnfrsf13b+/− parents, Tcrbd−/− and Tnfrsf13b−/−Tcrbd−/− breeders from Tnfrsf13b+/−Tcrbd−/− parents, Cd40lg−/− and Tnfrsf13b−/−Cd40lg−/− breeders from Tnfrsf13b+/−Cd40lg+/− parents to control for microbiota and genetic background variability between strains. Igha−/− mice were generated as described (64). All mouse strains were on C57BL/6 background. Mice were housed at Icahn School of Medicine at Mount Sinai and maintained in specific pathogen-free conditions. All experiments involving mice were performed in accordance with the guidelines of the Mount Sinai Animal Care and Use Committee.
Isolation of cells from gut lamina propria, PPs, MLNs and spleen
Cells were isolated as detailed in Supplementary Materials and Methods and subsequently pelleted before resuspension in IMDM with 2% FBS.
Flow cytometry
Cell suspensions were labeled as described in Supplementary Materials and Methods.
Isolation of B cells and in vitro IgA-inducing assay
Splenocyte suspensions were obtained as described in Supplementary Materials and Methods and naive B-2 cells were negatively selected using an EasySep Mouse B Cell isolation Kit (STEMCELL Technologies) according to the manufacturer’s instructions. PBS supplemented with 2% FBS and 1 mM EDTA was used as cell separation buffer. 200,000 B cells/well were seeded in 96-well plates in RPMI medium supplemented with 10% FBS, 1% antibiotics (Gibco), 1% MEM non-essential amino acids (Sigma), and 0.1% 2-mercaptoethanol (Gibco). Cells were stimulated for 5 days with 0.75 μg/ml mouse APRIL (R&D Systems), 10 μg/ml LPS, and/or 0.5 ng/ml mouse TGF-β1, which was added 24 h after the onset of the culture.
ELISA
Antibodies in feces and cell culture supernatants, and BAFF in sera, were measured by ELISA as detailed in Supplementary Materials and Methods.
Tissue IFA
Small intestine and colon tissues were processed as detailed in Supplementary Materials and Methods. Images were collected using fluorescence microscope Nikon Eclipse Ni-U and a DS- QiMc monochrome CCD camera. The acquisition software used was NIS-Elements BR. Images were processed in Photoshop software (Adobe Systems).
Next generation sequencing of IgA genes
The murine IgA VHDJH gene repertoire was analyzed as described (8, 11) and further detailed in Supplementary Materials and Methods (66–71).
IgA gene analysis
Immunoglobulin sequence analysis was performed as described with some modifications (8, 11). Details are provided in Supplementary Materials and Methods.
FISH analysis
Tissue fragments from colon were processed and hybridized with an Alexa594-conjugated eubacterial GCTGCCTCCCGTAGGAGT probe (Invitrogen) as detailed in Supplementary Materials and Methods. Images were collected using Nikon Eclipse Ni-U and a DS-QiMc monochrome CCD camera. The acquisition software used was NIS-Elements BR. Images were processed in Photoshop software (Adobe Systems).
Immunization with cholera toxin and NP-OVA
Mice were immunized by oral gavage with 10 μg CT from Vibrio cholerae (Sigma-Aldrich) supplemented with 1 mg NP(15)-OVA, batch N-5051–100 (Biosearch Technologies) at day 0, 7 and 14 (72). Immunized mice were sacrificed at day 21.
Staining of SIgA-coated bacteria
Intestinal contents were suspended in sterile PBS (100 mg feces/ml), homogenized by vortexing 10 min and centrifuged at 2000 rpm for 5 min twice to remove large particles. Fecal bacteria from the supernatants (100 μl/sample) were washed with 1 ml PBS staining buffer containing 1% BSA (w/v) and centrifuged at 3184 g for 6 min to remove free antibodies. After an additional wash, bacterial pellets were resuspended in 25 μl blocking buffer made of staining buffer supplemented with 20% normal rat serum (STEMCELL), incubated for 20 min and stained with PE-conjugated mA-6E1 mAb to IgA (1:100 in staining buffer) for 30 min on ice (eBioscience). Samples were washed 3 times with 1 ml staining buffer as reported previously (73). Samples were then resuspended in staining buffer with 5 nM SYTO BC Green Fluorescent Nucleic Acid stain (Life Technologies) to label bacteria, incubated for 1–30 min, and 0.5 ml staining buffer was added prior to flow cytometric analysis.
DNA Extraction, 16S rRNA gene amplification, and Multiplex Sequencing
Mice used for 16S DNA sequencing were 8–9-week-old co-housed Tnfrsf13b+/+ and Tnfrsf13b−/− littermates from Tnfrsf13b+/− breeders kept in specific pathogen-free conditions. DNA from colonic tissue and fecal pellets was extracted and the V4 region of 16S rRNA genes was amplified and multiplex sequenced as previously described (74–76) and detailed in Supplementary Materials and Methods.
16S rRNA gene analysis
The raw reads obtained from 150 bp paired-end MiSeq runs were pre-processed through the MacQIIME 1.9.1 pipeline (77). After demultiplexing and base quality-trimming (q20), a reference-based OTU picking protocol was applied and 97% OTUs were picked against the August 2013 Greengenes database (78) pre-filtered at 97% identity using uclust (68). Reads were assigned to OTUs based on their best match to a Greengenes sequence and reads that did not match a Greengenes sequence at 97% or greater sequence identity were discarded. The Greengenes taxonomy associated with the best match in Greengenes was assigned to each OTU. The resulting OTU table was normalized and quality filtered by discarding OTUs with less than 3 counts across all samples or present in less than 5 samples (79). Finally, filtered OTU counts were used to calculate Jackknifed Weighted UniFrac beta-diversity indices (80) and alpha-diversity at an even depth of 22,000 sequences/sample and taxonomic plots were generated. Wilcoxon rank sum test was used to test the differences of alpha diversity between groups.
To evaluate differences between groups, one-way ANOVA (CRAN/R v3.4.1) was performed on log2 transformed count data (all zeroes were set to 0.5 of the smallest non-zero entry) assuming equal variance. OTUs with q < 0.05 (false discovery rate/Benjamini & Hochberg) were subjected to Tukey HSD post hoc test (CRAN/R v3.4.1) and significant differences among groups were defined as q < 0.05, p < 0.05 and −2 > fold-change > 2. Abundance profiles were hierarchically clustered using Spearman correlation as the distance metric; heat map diagrams were generated using CRAN/R v3.4.1.
Statistical analysis
Two-tailed Student’s-t test was performed to analyze normally distributed data, whereas non-parametric two-tailed Mann-Whitney test was used to analyze data that were not normally distributed. Wilcoxon rank sum test was used to test the differences of alpha diversity between stool and colon. p values < 0.05 were considered significant.
Supplementary Material
Acknowledgments
Funding: Supported by US National Institutes of Health grant P01 AI61093, Innovator Award 2015-936 from The Kenneth Rainin Foundation, Ministerio de Ciencia, Innovación y Universidades grant RTI2018-093894-B-I00, and European Advanced Grant ERC-2011-ADG-20110310 to A. Cerutti; Sara Borrell post-doctoral fellowships to A. Chorny; and post-doctoral fellowship from the Swedish Research Council 2015-06486 and Swedish Society of Medicine to E.K. Grasset.
M.R is a full-time employee of Boehringer-Ingelheim. C.G. is currently a full-time employee at GlaxoSmithKline Vaccines. R.B. is listed as a co-inventor on patents related to the TACI gene and protein held by St. Jude Children’s Research Hospital.
Footnotes
Competing Interests: The other authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper or the Supplementary Materials.
References and Notes
- 1.Fagarasan S, Kawamoto S, Kanagawa O, Suzuki K, Adaptive immune regulation in the gut: T cell-dependent and T cell-independent IgA synthesis. Annu Rev Immunol 28, 243 (2010). [DOI] [PubMed] [Google Scholar]
- 2.Kubinak JL, Round JL, Do antibodies select a healthy microbiota? Nat Rev Immunol 16, 767 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakajima A, Vogelzang A, Maruya M, Miyajima M, Murata M, Son A, Kuwahara T, Tsuruyama T, Yamada S, Matsuura M, Nakase H, Peterson DA, Fagarasan S, Suzuki K, IgA regulates the composition and metabolic function of gut microbiota by promoting symbiosis between bacteria. J Exp Med 215, 2019 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Macpherson AJ, Yilmaz B, Limenitakis JP, Ganal-Vonarburg SC, IgA Function in Relation to the Intestinal Microbiota. Annu Rev Immunol 36, 359 (2018). [DOI] [PubMed] [Google Scholar]
- 5.Hapfelmeier S, Lawson MA, Slack E, Kirundi JK, Stoel M, Heikenwalder M, Cahenzli J, Velykoredko Y, Balmer ML, Endt K, Geuking MB, Curtiss R 3rd, McCoy KD, Macpherson AJ, Reversible microbial colonization of germ-free mice reveals the dynamics of IgA immune responses. Science 328, 1705 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kawamoto S, Tran TH, Maruya M, Suzuki K, Doi Y, Tsutsui Y, Kato LM, Fagarasan S, The inhibitory receptor PD-1 regulates IgA selection and bacterial composition in the gut. Science 336, 485 (2012). [DOI] [PubMed] [Google Scholar]
- 7.Reboldi A, Arnon TI, Rodda LB, Atakilit A, Sheppard D, Cyster JG, IgA production requires B cell interaction with subepithelial dendritic cells in Peyer's patches. Science 352, aaf4822 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lindner C, Wahl B, Fohse L, Suerbaum S, Macpherson AJ, Prinz I, Pabst O, Age, microbiota, and T cells shape diverse individual IgA repertoires in the intestine. J Exp Med 209, 365 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okai S, Usui F, Yokota S, Hori IY, Hasegawa M, Nakamura T, Kurosawa M, Okada S, Yamamoto K, Nishiyama E, Mori H, Yamada T, Kurokawa K, Matsumoto S, Nanno M, Naito T, Watanabe Y, Kato T, Miyauchi E, Ohno H, Shinkura R, High-affinity monoclonal IgA regulates gut microbiota and prevents colitis in mice. Nat Microbiol 1, (2016). [DOI] [PubMed] [Google Scholar]
- 10.Bergqvist P, Stensson A, Hazanov L, Holmberg A, Mattsson J, Mehr R, Bemark M, Lycke NY, Re-utilization of germinal centers in multiple Peyer's patches results in highly synchronized, oligoclonal, and affinity-matured gut IgA responses. Mucosal Immunol 6, 122 (2013). [DOI] [PubMed] [Google Scholar]
- 11.Lindner C, Thomsen I, Wahl B, Ugur M, Sethi MK, Friedrichsen M, Smoczek A, Ott S, Baumann U, Suerbaum S, Schreiber S, Bleich A, Gaboriau-Routhiau V, Cerf-Bensussan N, Hazanov H, Mehr R, Boysen P, Rosenstiel P, Pabst O, Diversification of memory B cells drives the continuous adaptation of secretory antibodies to gut microbiota. Nat Immunol 16, 880 (2015). [DOI] [PubMed] [Google Scholar]
- 12.Magri G, Comerma L, Pybus M, Sintes J, Llige D, Segura-Garzon D, Bascones S, Yeste A, Grasset EK, Gutzeit C, Uzzan M, Ramanujam M, van Zelm MC, Albero-Gonzalez R, Vazquez I, Iglesias M, Serrano S, Marquez L, Mercade E, Mehandru S, Cerutti A, Human Secretory IgM Emerges from Plasma Cells Clonally Related to Gut Memory B Cells and Targets Highly Diverse Commensals. Immunity 47, 118 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen K, Magri G, Grasset EK, Cerutti A, Rethinking mucosal antibody responses: IgM, IgG and IgD join IgA. Nat Rev Immunol, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pabst O, New concepts in the generation and functions of IgA. Nat Rev Immunol 12, 821 (2012). [DOI] [PubMed] [Google Scholar]
- 15.Bunker JJ, Flynn TM, Koval JC, Shaw DG, Meisel M, McDonald BD, Ishizuka IE, Dent AL, Wilson PC, Jabri B, Antonopoulos DA, Bendelac A, Innate and Adaptive Humoral Responses Coat Distinct Commensal Bacteria with Immunoglobulin A. Immunity 43, 541 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bunker JJ, Erickson SA, Flynn TM, Henry C, Koval JC, Meisel M, Jabri B, Antonopoulos DA, Wilson PC, Bendelac A, Natural polyreactive IgA antibodies coat the intestinal microbiota. Science, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wesemann DR, Portuguese AJ, Meyers RM, Gallagher MP, Cluff-Jones K, Magee JM, Panchakshari RA, Rodig SJ, Kepler TB, Alt FW, Microbial colonization influences early B-lineage development in the gut lamina propria. Nature 501, 112 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He B, Xu W, Santini PA, Polydorides AD, Chiu A, Estrella J, Shan M, Chadburn A, Villanacci V, Plebani A, Knowles DM, Rescigno M, Cerutti A, Intestinal bacteria trigger T cell-independent immunoglobulin A(2) class switching by inducing epithelial-cell secretion of the cytokine APRIL. Immunity 26, 812 (2007). [DOI] [PubMed] [Google Scholar]
- 19.Tezuka H, Abe Y, Iwata M, Takeuchi H, Ishikawa H, Matsushita M, Shiohara T, Akira S, Ohteki T, Regulation of IgA production by naturally occurring TNF/iNOS-producing dendritic cells. Nature 448, 929 (2007). [DOI] [PubMed] [Google Scholar]
- 20.Tsuji M, Suzuki K, Kitamura H, Maruya M, Kinoshita K, Ivanov II, Itoh K, Littman DR, Fagarasan S, Requirement for lymphoid tissue-inducer cells in isolated follicle formation and T cell-independent immunoglobulin A generation in the gut. Immunity 29, 261 (2008). [DOI] [PubMed] [Google Scholar]
- 21.Tezuka H, Abe Y, Asano J, Sato T, Liu J, Iwata M, Ohteki T, Prominent role for plasmacytoid dendritic cells in mucosal T cell-independent IgA induction. Immunity 34, 247 (2011). [DOI] [PubMed] [Google Scholar]
- 22.Kruglov AA, Grivennikov SI, Kuprash DV, Winsauer C, Prepens S, Seleznik GM, Eberl G, Littman DR, Heikenwalder M, Tumanov AV, Nedospasov SA, Nonredundant function of soluble LTalpha3 produced by innate lymphoid cells in intestinal homeostasis. Science 342, 1243 (2013). [DOI] [PubMed] [Google Scholar]
- 23.Wang Y, Liu L, Moore DJ, Shen X, Peek RM, Acra SA, Li H, Ren X, Polk DB, Yan F, An LGG-derived protein promotes IgA production through upregulation of APRIL expression in intestinal epithelial cells. Mucosal Immunol 10, 373 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.von Bulow GU, van Deursen JM, Bram RJ, Regulation of the T-independent humoral response by TACI. Immunity 14, 573 (2001). [DOI] [PubMed] [Google Scholar]
- 25.Castigli E, Wilson SA, Garibyan L, Rachid R, Bonilla F, Schneider L, Geha RS, TACI is mutant in common variable immunodeficiency and IgA deficiency. Nat Genet 37, 829 (2005). [DOI] [PubMed] [Google Scholar]
- 26.Mantchev GT, Cortesao CS, Rebrovich M, Cascalho M, Bram RJ, TACI is required for efficient plasma cell differentiation in response to T-independent type 2 antigens. J Immunol 179, 2282 (2007). [DOI] [PubMed] [Google Scholar]
- 27.Hashiguchi M, Kashiwakura Y, Kanno Y, Kojima H, Kobata T, Tumor necrosis factor superfamily member (TNFSF) 13 (APRIL) and TNFSF13B (BAFF) downregulate homeostatic immunoglobulin production in the intestines. Cell Immunol 323, 41 (2018). [DOI] [PubMed] [Google Scholar]
- 28.von Bulow GU, Bram RJ, NF-AT activation induced by a CAML-interacting member of the tumor necrosis factor receptor superfamily. Science 278, 138 (1997). [DOI] [PubMed] [Google Scholar]
- 29.Goenka R, Matthews AH, Zhang B, O'Neill PJ, Scholz JL, Migone TS, Leonard WJ, Stohl W, Hershberg U, Cancro MP, Local BLyS production by T follicular cells mediates retention of high affinity B cells during affinity maturation. J Exp Med 211, 45 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsuji S, Stein L, Kamada N, Nunez G, Bram R, Vallance BA, Sousa AE, Platt JL, Cascalho M, TACI deficiency enhances antibody avidity and clearance of an intestinal pathogen. J Clin Invest 124, 4857 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Allman WR, Dey R, Liu L, Siddiqui S, Coleman AS, Bhattacharya P, Yano M, Uslu K, Takeda K, Nakhasi HL, Akkoyunlu M, TACI deficiency leads to alternatively activated macrophage phenotype and susceptibility to Leishmania infection. Proc Natl Acad Sci U S A 112, E4094 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Figgett WA, Fairfax K, Vincent FB, Le Page MA, Katik I, Deliyanti D, Quah PS, Verma P, Grumont R, Gerondakis S, Hertzog P, O'Reilly LA, Strasser A, Mackay F, The TACI receptor regulates T-cell-independent marginal zone B cell responses through innate activation-induced cell death. Immunity 39, 573 (2013). [DOI] [PubMed] [Google Scholar]
- 33.Castigli E, Wilson SA, Scott S, Dedeoglu F, Xu S, Lam KP, Bram RJ, Jabara H, Geha RS, TACI and BAFF-R mediate isotype switching in B cells. J Exp Med 201, 35 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mackay F, Schneider P, Cracking the BAFF code. Nat Rev Immunol 9, 491 (2009). [DOI] [PubMed] [Google Scholar]
- 35.Cazac BB, Roes J, TGF-beta receptor controls B cell responsiveness and induction of IgA in vivo. Immunity 13, 443 (2000). [DOI] [PubMed] [Google Scholar]
- 36.Masahata K, Umemoto E, Kayama H, Kotani M, Nakamura S, Kurakawa T, Kikuta J, Gotoh K, Motooka D, Sato S, Higuchi T, Baba Y, Kurosaki T, Kinoshita M, Shimada Y, Kimura T, Okumura R, Takeda A, Tajima M, Yoshie O, Fukuzawa M, Kiyono H, Fagarasan S, Iida T, Ishii M, Takeda K, Generation of colonic IgA-secreting cells in the caecal patch. Nat Commun 5, 3704 (2014). [DOI] [PubMed] [Google Scholar]
- 37.Xu J, Foy TM, Laman JD, Elliott EA, Dunn JJ, Waldschmidt TJ, Elsemore J, Noelle RJ, Flavell RA, Mice deficient for the CD40 ligand. Immunity 1, 423 (1994). [DOI] [PubMed] [Google Scholar]
- 38.Wang H, Marsters SA, Baker T, Chan B, Lee WP, Fu L, Tumas D, Yan M, Dixit VM, Ashkenazi A, Grewal IS, TACI-ligand interactions are required for T cell activation and collagen-induced arthritis in mice. Nat Immunol 2, 632 (2001). [DOI] [PubMed] [Google Scholar]
- 39.Seyler TM, Park YW, Takemura S, Bram RJ, Kurtin PJ, Goronzy JJ, Weyand CM, BLyS and APRIL in rheumatoid arthritis. J Clin Invest 115, 3083 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bergqvist P, Stensson A, Lycke NY, Bemark M, T cell-independent IgA class switch recombination is restricted to the GALT and occurs prior to manifest germinal center formation. J Immunol 184, 3545 (2010). [DOI] [PubMed] [Google Scholar]
- 41.Mackay F, Woodcock SA, Lawton P, Ambrose C, Baetscher M, Schneider P, Tschopp J, Browning JL, Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J Exp Med 190, 1697 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seshasayee D, Valdez P, Yan M, Dixit VM, Tumas D, Grewal IS, Loss of TACI causes fatal lymphoproliferation and autoimmunity, establishing TACI as an inhibitory BLyS receptor. Immunity 18, 279 (2003). [DOI] [PubMed] [Google Scholar]
- 43.Ou X, Xu S, Lam KP, Deficiency in TNFRSF13B (TACI) expands T-follicular helper and germinal center B cells via increased ICOS-ligand expression but impairs plasma cell survival. Proc Natl Acad Sci U S A 109, 15401 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johansson ME, Larsson JM, Hansson GC, The two mucus layers of colon are organized by the MUC2 mucin, whereas the outer layer is a legislator of host-microbial interactions. Proc Natl Acad Sci U S A 108 Suppl 1, 4659 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kato LM, Kawamoto S, Maruya M, Fagarasan S, The role of the adaptive immune system in regulation of gut microbiota. Immunol Rev 260, 67 (2014). [DOI] [PubMed] [Google Scholar]
- 46.Belkaid Y, Hand TW, Role of the microbiota in immunity and inflammation. Cell 157, 121 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Macpherson AJ, Gatto D, Sainsbury E, Harriman GR, Hengartner H, Zinkernagel RM, A primitive T cell-independent mechanism of intestinal mucosal IgA responses to commensal bacteria. Science 288, 2222 (2000). [DOI] [PubMed] [Google Scholar]
- 48.Castigli E, Scott S, Dedeoglu F, Bryce P, Jabara H, Bhan AK, Mizoguchi E, Geha RS, Impaired IgA class switching in APRIL-deficient mice. Proc Natl Acad Sci U S A 101, 3903 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Belnoue E, Pihlgren M, McGaha TL, Tougne C, Rochat AF, Bossen C, Schneider P, Huard B, Lambert PH, Siegrist CA, APRIL is critical for plasmablast survival in the bone marrow and poorly expressed by early-life bone marrow stromal cells. Blood 111, 2755 (2008). [DOI] [PubMed] [Google Scholar]
- 50.Chu VT, Beller A, Rausch S, Strandmark J, Zanker M, Arbach O, Kruglov A, Berek C, Eosinophils promote generation and maintenance of immunoglobulin-A-expressing plasma cells and contribute to gut immune homeostasis. Immunity 40, 582 (2014). [DOI] [PubMed] [Google Scholar]
- 51.Castigli E, Wilson SA, Elkhal A, Ozcan E, Garibyan L, Geha RS, Transmembrane activator and calcium modulator and cyclophilin ligand interactor enhances CD40-driven plasma cell differentiation. J Allergy Clin Immunol 120, 885 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tsuji S, Cortesao C, Bram RJ, Platt JL, Cascalho M, TACI deficiency impairs sustained Blimp-1 expression in B cells decreasing long-lived plasma cells in the bone marrow. Blood 118, 5832 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McCarthy DD, Kujawa J, Wilson C, Papandile A, Poreci U, Porfilio EA, Ward L, Lawson MA, Macpherson AJ, McCoy KD, Pei Y, Novak L, Lee JY, Julian BA, Novak J, Ranger A, Gommerman JL, Browning JL, Mice overexpressing BAFF develop a commensal flora-dependent, IgA-associated nephropathy. J Clin Invest 121, 3991 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kadaoui KA, Corthesy B, Secretory IgA mediates bacterial translocation to dendritic cells in mouse Peyer's patches with restriction to mucosal compartment. J. Immunol 179, 7751 (2007). [DOI] [PubMed] [Google Scholar]
- 55.Obata T, Goto Y, Kunisawa J, Sato S, Sakamoto M, Setoyama H, Matsuki T, Nonaka K, Shibata N, Gohda M, Kagiyama Y, Nochi T, Yuki Y, Fukuyama Y, Mukai A, Shinzaki S, Fujihashi K, Sasakawa C, Iijima H, Goto M, Umesaki Y, Benno Y, Kiyono H, Indigenous opportunistic bacteria inhabit mammalian gut-associated lymphoid tissues and share a mucosal antibody-mediated symbiosis. Proc. Natl. Acad. Sci. USA 107, 7419 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fransen F, Zagato E, Mazzini E, Fosso B, Manzari C, El Aidy S, Chiavelli A, D'Erchia AM, Sethi MK, Pabst O, Marzano M, Moretti S, Romani L, Penna G, Pesole G, Rescigno M, BALB/c and C57BL/6 Mice Differ in Polyreactive IgA Abundance, which Impacts the Generation of Antigen-Specific IgA and Microbiota Diversity. Immunity 43, 527 (2015). [DOI] [PubMed] [Google Scholar]
- 57.Donaldson GP, Ladinsky MS, Yu KB, Sanders JG, Yoo BB, Chou WC, Conner ME, Earl AM, Knight R, Bjorkman PJ, Mazmanian SK, Gut microbiota utilize immunoglobulin A for mucosal colonization. Science 360, 795 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shibuya A, Honda SI, Shibuya K, A pro-inflammatory role of Fcalpha/muR on marginal zone B cells in sepsis. Int Immunol 29, 519 (2017). [DOI] [PubMed] [Google Scholar]
- 59.Breedveld A, van Egmond M, IgA and FcalphaRI: Pathological Roles and Therapeutic Opportunities. Front Immunol 10, 553 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Elinav E, Henao-Mejia J, Strowig T, Flavell R, NLRP6 and Dysbiosis: Avoiding the Luring Attraction of Over-Simplification. Immunity 48, 603 (2018). [DOI] [PubMed] [Google Scholar]
- 61.Wullaert A, Lamkanfi M, McCoy KD, Defining the Impact of Host Genotypes on Microbiota Composition Requires Meticulous Control of Experimental Variables. Immunity 48, 605 (2018). [DOI] [PubMed] [Google Scholar]
- 62.Garcia-Carmona Y, Cols M, Ting AT, Radigan L, Yuk FJ, Zhang L, Cerutti A, Cunningham-Rundles C, Differential induction of plasma cells by isoforms of human TACI. Blood 125, 1749 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.von Bulow GU, Russell H, Copeland NG, Gilbert DJ, Jenkins NA, Bram RJ, Molecular cloning and functional characterization of murine transmembrane activator and CAML interactor (TACI) with chromosomal localization in human and mouse. Mamm Genome 11, 628 (2000). [DOI] [PubMed] [Google Scholar]
- 64.Harriman GR, Bogue M, Rogers P, Finegold M, Pacheco S, Bradley A, Zhang Y, Mbawuike IN, Targeted deletion of the IgA constant region in mice leads to IgA deficiency with alterations in expression of other Ig isotypes. J Immunol 162, 2521 (1999). [PubMed] [Google Scholar]
- 65.Sanos SL, Diefenbach A, Isolation of NK cells and NK-like cells from the intestinal lamina propria. Methods Mol Biol 612, 505 (2010). [DOI] [PubMed] [Google Scholar]
- 66.Brochet X, Lefranc MP, Giudicelli V, IMGT/V-QUEST: the highly customized and integrated system for IG and TR standardized V-J and V-D-J sequence analysis. Nucleic Acids Res 36, W503 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lefranc MP, Giudicelli V, Ginestoux C, Jabado-Michaloud J, Folch G, Bellahcene F, Wu Y, Gemrot E, Brochet X, Lane J, Regnier L, Ehrenmann F, Lefranc G, Duroux P, IMGT, the international ImMunoGeneTics information system. Nucleic Acids Res 37, D1006 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Edgar RC, Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26, 2460 (2010). [DOI] [PubMed] [Google Scholar]
- 69.Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T, Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res 13, 2498 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cline MS, Smoot M, Cerami E, Kuchinsky A, Landys N, Workman C, Christmas R, Avila-Campilo I, Creech M, Gross B, Hanspers K, Isserlin R, Kelley R, Killcoyne S, Lotia S, Maere S, Morris J, Ono K, Pavlovic V, Pico AR, Vailaya A, Wang PL, Adler A, Conklin BR, Hood L, Kuiper M, Sander C, Schmulevich I, Schwikowski B, Warner GJ, Ideker T, Bader GD, Integration of biological networks and gene expression data using Cytoscape. Nat Protoc 2, 2366 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jost L, Entropy and diversity. Oikos 113, 363 (2006). [Google Scholar]
- 72.Yamamoto M, Rennert P, McGhee JR, Kweon MN, Yamamoto S, Dohi T, Otake S, Bluethmann H, Fujihashi K, Kiyono H, Alternate mucosal immune system: organized Peyer's patches are not required for IgA responses in the gastrointestinal tract. J Immunol 164, 5184 (2000). [DOI] [PubMed] [Google Scholar]
- 73.Palm NW, de Zoete MR, Cullen TW, Barry NA, Stefanowski J, Hao L, Degnan PH, Hu J, Peter I, Zhang W, Ruggiero E, Cho JH, Goodman AL, Flavell RA, Immunoglobulin A coating identifies colitogenic bacteria in inflammatory bowel disease. Cell 158, 1000 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Faith JJ, Guruge JL, Charbonneau M, Subramanian S, Seedorf H, Goodman AL, Clemente JC, Knight R, Heath AC, Leibel RL, Rosenbaum M, Gordon JI, The long-term stability of the human gut microbiota. Science 341, 1237439 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bongers G, Pacer ME, Geraldino TH, Chen L, He Z, Hashimoto D, Furtado GC, Ochando J, Kelley KA, Clemente JC, Merad M, van Bakel H, Lira SA, Interplay of host microbiota, genetic perturbations, and inflammation promotes local development of intestinal neoplasms in mice. J Exp Med 211, 457 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen L, He Z, Iuga AC, Martins Filho SN, Faith JJ, Clemente JC, Deshpande M, Jayaprakash A, Colombel JF, Lafaille JJ, Sachidanandam R, Furtado GC, Lira SA, Diet Modifies Colonic Microbiota and CD4(+) T-Cell Repertoire to Induce Flares of Colitis in Mice With Myeloid-Cell Expression of Interleukin 23. Gastroenterology 155, 1177 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Pena AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R, QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7, 335 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McDonald D, Price MN, Goodrich J, Nawrocki EP, DeSantis TZ, Probst A, Andersen GL, Knight R, Hugenholtz P, An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ISME J 6, 610 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bokulich NA, Subramanian S, Faith JJ, Gevers D, Gordon JI, Knight R, Mills DA, Caporaso JG, Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat Methods 10, 57 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lozupone C, Lladser ME, Knights D, Stombaugh J, Knight R, UniFrac: an effective distance metric for microbial community comparison. ISME J 5, 169 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.