Abstract
Background
While several studies have observed that solid organ transplant recipients experience diminished antibody responses to SARS-CoV-2 mRNA vaccination, data specific to heart and lung transplant (HT/LT) recipients remains sparse.
Methods
US adult HT and LT recipients completed their vaccine series between January 7 and April 10, 2021. Reactogencity and SARS-CoV-2 anti-spike antibody were assessed after a priming dose (D1) and booster dose (D2). Modified Poisson regression with robust variance estimator was used to evaluate associations between participant characteristics and antibody development.
Results
Of 134 heart recipients, there were 38% non-responders (D1-/D2-), 48% booster responders (D1-/D2+), and 14% priming dose responders (D1+/D2+). Of 103 lung recipients, 64% were non-responders, 27% were booster responders, and 9% were priming dose responders. Lung recipients were less likely to develop antibodies (p < .001). Priming dose antibody response was associated with younger recipient age (p = .04), transplant-to-vaccination time ≥6 years (p < .01), and lack of anti-metabolite maintenance immunosuppression (p < .001). Pain at injection site was the most commonly reported reaction (85% after D1, 76% after D2). Serious reactions were rare, the most common being fatigue (2% after D1 and 3% after D2). No serious adverse events were reported.
Conclusions
HT and LT recipients experienced diminished antibody response following vaccination; reactogenicity was comparable to that of the general population. LT recipients may exhibit a more impaired antibody response than HT recipients. While current recommendations are to vaccinate eligible candidates and recipients, further studies characterizing the cell-mediated immune response and clinical efficacy of these vaccines in this population are needed.
KEYWORDS: SARS-CoV-2, COVID-19, mRNA vaccination, heart transplant, lung transplant
Abbreviations: aIRR, Adjusted incident rate ratio; BMI, Body mass index; CI, Confidence interval; COVID-19, Coronavirus disease 2019; D1/D2, Dose 1/Dose 2; FDA, Food and Drug Administration; HT, Heart transplant; IgG, Immunoglobulin G; IQR, Interquartile range; LT, Lung transplant; mRNA, messenger ribonucleic acid; PCR, Polymerase chain reaction; RBD, Receptor binding domain; REDCap, Research Electronic Data Capture; SARS-CoV-2, Severe acute respiratory disease coronavirus 2; SOTR, Solid organ transplant recipients
Heart and lung transplant (HT/LT) recipients experience an elevated rate of severe illness due to SARS-CoV-2 infection.1, 2, 3, 4, 5, 6, 7, 8 While two SARS-CoV-2 messenger RNA (mRNA) vaccines are currently available, solid organ transplant recipients (SOTR) were excluded from Phase 1 to 3 trials.9 , 10 International Society for Heart and Lung Transplantation (ISHLT) guidelines strongly recommend SARS-CoV-2 vaccination in transplant candidates and recipients.11 , 12 While evidence concerning vaccine-based immune response among SOTRs is emerging, data specific to HT and LT recipients remain sparse with further study necessary to inform patient and provider decision-making.
SOTRs are capable of generating a robust humoral response to natural SARS-CoV-2 infection with 78% testing positive for antibodies more than three months after diagnosis.13 However, a national study of 658 SOTRs who received two doses of the BNT162b2 (Pfizer/BioNTech) or mRNA-1273 (Moderna) vaccine reported dramatically reduced humeral response with just 15% developing anti-spike antibodies after a dose 1 (D1), 46% developing no antibodies, and 39% developing antibodies only after dose 2 (D2).14 Antibody development was associated with lower recipient age, lack of anti-metabolite maintenance immunosuppression, longer time since transplantation, and receipt of the mRNA-1273 vaccine. A safety analysis of 187 SOTRs demonstrated predominantly mild perivaccine reactogenicity and no major safety events such as acute rejection, new neurological illness, or anaphylaxis.15 Several other studies among kidney transplant recipients have corroborated these findings.16, 17, 18 More recently, a report of 77 HT recipients who received the BNT162b2 vaccine reported anti-spike antibody development in only 18% of patients at 3 weeks after the second dose.19 A report of 48 LT recipients who received the same vaccine was unable to demonstrate any antibody response up to 6 weeks after the second dose.20
Safety and humoral response following SARS-CoV-2 mRNA vaccination in HT and LT recipients must be understood in order to inform clinical decision-making and vaccination guidelines. To investigate this, we studied SARS-CoV-2 antibody development in HT and LT recipients who completed a two-dose vaccine series. Demographic and clinical correlates were explored.
Methods
Study population
Participants were recruited through social media or their transplant centers and completed a two-dose vaccine course between January 7, 2021 and April 10, 2021. English-speaking SOTRs at least 18 years old were eligible to participate. Age, sex, race, body mass index (BMI), prior COVID-19 diagnosis and hospitalization, transplant type and date, medications, other immune conditions, and allergies were collected using Research Electronic Data Capture (REDCap) hosted at Johns Hopkins.21 REDCap is a secure, web-based software platform designed to support data capture for research studies, providing 1) an intuitive interface for validated data capture; 2) audit trails for tracking data manipulation and export procedures; 3) automated export procedures for seamless data downloads to common statistical packages; and 4) procedures for data integration and interoperability with external sources. The study was approved by the Institutional Review Board at the Johns Hopkins School of Medicine and is compliant with the ISHLT statement on transplant ethics. Participants provided informed consent.
Blood specimen collection and testing
Samples were collected shortly before receipt of D2 and as close to 28 days after D2 as possible. As described previously, participants were provided the option of blood sampling by standard venipuncture in a local lab or at home with the TAPII blood collection device (Seventh Sense Biosystems; investigational use only) and tested for antibodies against the SARS-CoV-2 spike protein.22 Venipuncture samples were tested using an immunoassay (Roche Elecsys) for antibodies against the receptor-binding domain (RBD). TAPII samples were tested using an immunoassay (EUROIMMUN) for antibodies to the S1 domain. Both tests are semiquantitative, correspond to mRNA vaccine antigens and are correlated with neutralizing immunity.23, 24, 25 The sensitivity and specificity of the enzyme immunoassays are excellent for detection of the humoral response to SARS-CoV-2 infection (sensitivity 87.1%, specificity 98.9% for EUROIMMUN;24 sensitivity 84.0%, specificity 100% for Roche Elecsys25). Both are comparable to anti-spike antibody assays used during immunogenicity assessments in mRNA vaccine clinical trials.
Anti-RBD immunoassay (Roche Elecsys) results were reported as a concentration of immunoglobulin G (IgG) against the target protein with a measurement range of 0.4 to 250 U/mL; results ≥250 U/mL were reported as 250 U/mL. Anti-S1 immunoassay (EUROIMMUN) results were reported as a sample-to-control ratio of optical density. Antibody-positive cut-offs (determined by the manufacturer) were ≥0.80 U/mL for the former and ≥1.1 arbitrary units for the latter.
Antibody response after SARS-CoV-2 mRNA vaccination
Participants were divided into three categories according to immunoassay results. Those who developed positive results after both doses 1 and 2 (D1, D2) were classified as priming dose responders. Those who failed to develop antibodies after both doses were classified as non-responders. If an individual failed to develop antibodies after D1 but subsequently did so after D2, they were classified as a booster responder.
Reactogenicity and adverse events after SARS-CoV-2 mRNA vaccination
Questionnaires were distributed to participants 7 days after D1 and D2. After each dose, participants were asked if they had received new diagnoses of COVID-19, other infections, acute rejection, or neurological illness and, if so, whether they required hospitalization, intensive care unit management, or mechanical ventilation. Local symptoms, including pain, redness, and swelling, as well as systemic adverse reactions, including fever, fatigue, headaches, chills, vomiting, diarrhea, and myalgias, were solicited using an ordinal scale of mild, moderate, or severe. Mild symptoms were defined as symptoms that did not interfere with daily activities. Moderate symptoms were defined as those that caused some interference with daily activity. Severe symptoms were defined as those that prevented daily activity.
Statistical analysis
The proportion of patients who developed a positive antibody response was assessed with exact binomial 95% CIs. Associations between demographic and clinical characteristics, vaccine manufacturer, and positive antibody response were assessed using modified Poisson regression with a robust variance estimator and reported as adjusted incident rate ratios (aIRR). The risk factors for antibody development included age, sex, time since transplant (<6 years vs ≥6 years), anti-metabolite maintenance immunosuppression (including mycophenolate mofetil, mycophenolic acid, and azathioprine), and vaccine manufacturer. All tests were 2-sided with an α level of 0.05. Analyses were performed using Stata 16.0/IC for Windows (College Station, TX).
Results
Study population demographics and clinical characteristics
A total of 237 participants, including 134 (57%) heart and 103 (43%) lung transplant recipients, were included in the study (Table 1 ). None had a prior polymerase chain reaction (PCR)–confirmed diagnosis of COVID-19. Among HT recipients, the median age was 60 years (interquartile range [IQR], 44-69 years), 51% were women, 10% were non-white, and 4% were Hispanic. The median BMI was 25.5 (IQR, 22.3-29.9). The median time since transplant was 5.5 years (IQR, 2.6-12.4 years). The most common maintenance immunosuppression regimen was a combination of tacrolimus and mycophenolate (43%). With regard to vaccines, 70/134 (52%) received the BNT162b2 vaccine and 64/134 (48%) received the mRNA-1273 vaccine.
Table 1.
Demographic and Clinical Characteristics of Heart and Lung Transplant Recipients Who Completed a Two-Dose SARS-CoV-2 mRNA Vaccine Series
| Participant characteristics | Total | HT recipients | LT recipients |
|---|---|---|---|
| n = 237 | n = 134 | n = 103 | |
| Age, median (IQR) years | 62 (46-69) | 60 (44-69) | 63 (48-70) |
| Female sex, n (%)a | 127 (55) | 67 (51) | 60 (59) |
| Non-white, n (%)b | 19 (8) | 13 (10) | 6 (6) |
| Hispanic ethnicity, n (%)c | 7 (3) | 5 (4) | 2 (2) |
| BMI, median kg/m2 (IQR) | 25.6 (21.9-29.7) | 25.5 (22.3-29.9) | 25.7 (21.9-29.4) |
| Years since transplant, median (IQR) | 5.1 (2.5-11.0) | 5.5 (2.6-12.4) | 5.0 (2.0-9.4) |
| Maintenance immunosuppression, n (%)d | |||
| Tacrolimus | 203 (86) | 113 (84) | 90 (87) |
| Mycophenolate | 148 (62) | 84 (63) | 64 (62) |
| Corticosteroids | 134 (57) | 35 (26) | 99 (96) |
| Sirolimus | 32 (14) | 22(16) | 10 (10) |
| Cyclosporine | 19 (8) | 13 (10) | 6 (6) |
| Azathioprine | 19 (8) | 4 (3) | 15 (15) |
| Everolimus | 17 (7) | 12 (9) | 5 (5) |
| Belatacept | 2 (1) | 0 (0) | 2 (2) |
| Maintenance immunosuppression combination, n (%) | |||
| Tacrolimus, mycophenolate, prednisone | 66 (28) | 12 (9) | 54 (52) |
| Cyclosporine, mycophenolate, prednisone | 5 (2) | 2 (1) | 3 (3) |
| Tacrolimus, mycophenolate | 58 (24) | 57 (43) | 1 (1) |
| Cyclosporine, mycophenolate | 6 (3) | 6 (4) | 0 (0) |
| Tacrolimus, everolimus, prednisone | 8 (3) | 5 (4) | 3 (3) |
| Everolimus, cyclosporine | 2 (1) | 2 (1) | 0 (0) |
| Everolimus, mycophenolate | 1 (<1) | 1 (1) | 0 (0) |
| Cyclosporine, prednisone | 9 (4) | 3 (2) | 6 (6) |
| Tacrolimus, prednisone | 44 (19) | 13 (10) | 31 (30) |
| Tacrolimus, everolimus, mycophenolate, prednisone | 1 (<1) | 0 (0) | 1 (1) |
| Vaccine manufacturer, n (%) | |||
| BNT162b2 (Pfizer-BioNTech) | 126 (53) | 70 (52) | 56 (54) |
| mRNA-1273 (Moderna) | 111 (47) | 64 (48) | 47 (46) |
BMI, body mass index; HT, Heart transplant; IQR, interquartile range; LT, Lung transplant.
Missing data for 3 participants.
Missing data for 3 participants.
Missing data for 4 participants.
Not mutually exclusive.
Among LT recipients, the median age was 63 years (IQR, 48-70 years), 59% were women, 6% were non-white, and 2% were Hispanic. The median BMI was 25.7 (IQR, 21.9-29.4). The median time since transplant was 5.0 years (IQR, 2.0-9.4 years). The most common maintenance immunosuppression regimen was a combination of tacrolimus, mycophenolate, and prednisone (52%). With regard to vaccines, 56/103 (54%) received the BNT162b2 vaccine and 47/103 (46%) received the mRNA-1273 vaccine.
Immunogenicity after each SARS-CoV-2 mRNA vaccine dose
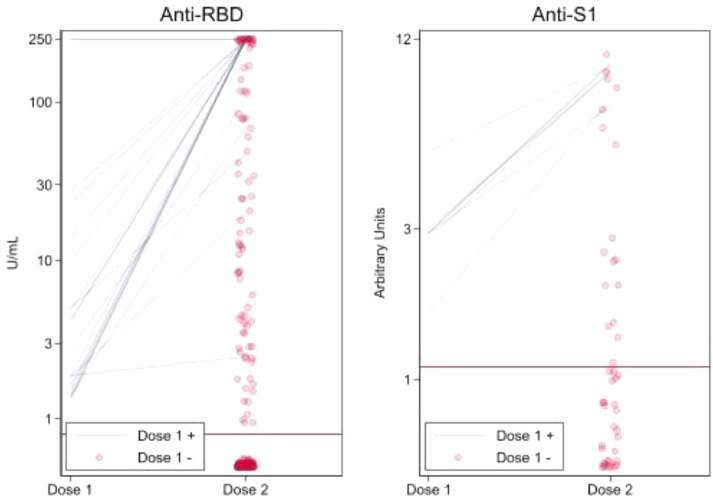
At a median of 21 days (IQR, 19-26 days) after D1, anti-spike antibody (anti-S1 or anti–RBD) was detectable in 28/237 participants (12%; 95%CI, 8%-17%) (Figure 1 ). At a median of 29 days (IQR, 28-32 days) after D2, anti-spike antibody was detectable in 120/237 participants (51%, 95%CI, 44%-57%). Only 28 (12%) participants demonstrated a priming dose response to the vaccine series while 92 (39%) were booster responders and 117 (49%) were non-responders. Of 134 heart recipients, 14% were priming dose responders, 48% were booster responders, and 38% were non-responders (Table 2a ). Of 103 lung recipients, 9% were priming dose responders, 27% were booster responders, and 64% were non-responders (Table 2b ).
Figure 1.
Semiquantitative SARS-CoV-2 anti-spike antibody immunoassay results of heart and lung transplant recipients by assay type. Individual priming dose responders (Dose 1+) are represented by lines connecting immunoassay results following Dose 1 and Dose 2. Individual booster responders and non-responders (Dose 1-) are represented by points indicating immunoassay results following Dose 2. Antibody-positive cut-offs (determined by the manufacturer and indicated here by thick, horizontal lines) were ≥0.80 U/mL for the anti-RBD immunoassay (Roche Elecsys) and ≥1.1 arbitrary units for the anti-S1 immunoassay (EUROIMMUN). RBD, receptor binding domain. n = 237.
Table 2a.
Demographic and Clinical Characteristics of Heart and Lung Transplant Recipients With Stratification by Antibody Response to a Two-Dose Course of SARS-CoV-2 Messenger RNA Vaccine
| HT recipient vaccine response, No. (%) |
|||
|---|---|---|---|
| Priming dose responders | Booster responders | Non-responders | |
| n = 19 | n = 64 | n = 51 | |
| Age group, ya | |||
| 18-39 | 7 (24) | 9 (31) | 13 (45) |
| 40-59 | 7 (18) | 20 (53) | 11 (29) |
| ≥60 | 5 (8) | 34 (52) | 27 (41) |
| Sexb | |||
| Male | 9 (14) | 34 (52) | 22 (34) |
| Female | 9 (13) | 30 (45) | 28 (42) |
| Racec | |||
| White | 17 (14) | 59 (49) | 44 (37) |
| Non-white | 2 (16) | 5 (38) | 6 (46) |
| Time since transplant, y | |||
| <3 | 4 (11) | 10 (26) | 24 (63) |
| 3-6 | 6 (15) | 19 (46) | 16 (39) |
| 7-11 | 3 (15) | 13 (65) | 4 (20) |
| ≥12 | 6 (17) | 22 (63) | 7 (20) |
| Type of regimen | |||
| Includes anti-metabolite maintenance immunosuppressiond | 7 (8) | 41 (47) | 40 (45) |
| Does not include anti-metabolite maintenance immunosuppression | 12 (26) | 23 (50) | 11 (24) |
| Vaccine | |||
| mRNA-1273 (Moderna) | 12 (19) | 29 (45) | 23 (36) |
| BNT16b2 (Pfizer-BioNTech) | 7 (10) | 35 (50) | 28 (40) |
HT, Heart transplant.
Missing data for 1 participant.
Missing data for 2 participants.
Missing data for 1 participant.
Antimetabolite maintenance immunosuppressive regimens included mycophenolate mofetil, mycophenolic acid, and azathioprine.
Table 2b.
Demographic and Clinical Characteristics of Heart and Lung Transplant Recipients With Stratification by Antibody Response to a Two-Dose Course of SARS-CoV-2 Messenger RNA vaccine
| LT recipient vaccine response, No. (%) |
|||
|---|---|---|---|
| Priming dose responders | Booster responders | Non-responders | |
| n = 9 | n = 28 | n = 66 | |
| Age group, y | |||
| 18–39 | 3 (19) | 2 (12) | 11 (69) |
| 40–59 | 1 (4) | 11 (41) | 15 (55) |
| ≥60 | 5 (8) | 15 (25) | 40 (67) |
| Sexa | |||
| Male | 4 (10) | 14 (34) | 23 (56) |
| Female | 5 (8) | 13 (22) | 42 (70) |
| Raceb | |||
| White | 9 (10) | 24 (25) | 62 (65) |
| Non-white | 0 (0) | 3 (50) | 3 (50) |
| Time since transplant, y | |||
| <3 | 1 (3) | 10 (28) | 25 (69) |
| 3-6 | 4 (12) | 7 (21) | 22 (67) |
| 7-11 | 2 (11) | 5 (28) | 11 (61) |
| ≥12 | 2 (13) | 6 (37) | 8 (50) |
| Type of regimen | |||
| Includes anti-metabolite maintenance immunosuppressionc | 6 (8) | 19 (25) | 52 (68) |
| Does not include anti-metabolite maintenance immunosuppression | 3 (12) | 9 (34) | 14 (54) |
| Vaccine | |||
| mRNA-1273 (Moderna) | 5 (10) | 13 (28) | 29 (62) |
| BNT16b2 (Pfizer-BioNTech) | 4 (7) | 15 (27) | 37 (66) |
LT, Lung transplant.
Missing data for 2 participants.
Missing data for 2 participants.
Antimetabolite maintenance immunosuppressive regimens included mycophenolate mofetil, mycophenolic acid, and azathioprine.
The median D2 anti-spike RBD assay results were 250 U/mL (IQR, 174-250 U/mL) for priming dose responders, 23.8 U/mL (IQR, 3.9-244.2 U/mL) for booster responders, and 0 U/mL (IQR, 0-0 U/mL) for non-responders (Table 3 ). The median D2 anti-S1 assay results were 9.1 (IQR, 7.2-9.4) for priming dose responders, 5.5 (IQR, 2.1-8.0) for booster responders, and 0.2 (IQR, 0.1-0.5) for non-responders. Crossover between immunoassays was minimal (1/237). Following D2, 54 (23%) specimens were collected by TAPII device and 183 (77%) were collected by venipuncture; immunoassay type was not associated with antibody response (p = 1.0 for D1, p = .4 for D2).
Table 3.
Median Semiquantitative SARS-CoV-2 Anti-Spike Antibody Immunoassay Results of Heart and Lung Transplant Recipients by Antibody Response
| D1 |
D2 |
|||
|---|---|---|---|---|
| Roche Elecsys | EUROIMMUN | Roche Elecsys | EUROIMMUN | |
| U/mL (IQR) | (IQR) | U/mL (IQR) | (IQR) | |
| n = 183 | n = 54 | n = 183 | N = 54 | |
| Priming dose responders | 2.9 (1.1, 11.5) | 2.4 (2.3, 2.5) | 250 (174, 250) | 9.1 (7.2, 9.4) |
| Booster responders | 0 (0, 0) | 0.2 (0.03, 0.5) | 23.8 (3.9, 244.2) | 5.5 (2.1, 8.0) |
| Non-responders | 0 (0, 0) | 0.1 (0.03, 0.2) | 0 (0, 0) | 0.2 (0.1, 0.5) |
Anti-RBD immunoassay (Roche Elecsys) results are reported as a concentration of immunoglobulin G (IgG) against the target protein with a measurement range of 0.4 to 250 U/mL; results ≥250 U/mL are reported as 250 U/mL. Anti-S1 immunoassay (EUROIMMUN) results are reported as a sample-to-control ratio of optical density. Antibody-positive cut-offs (determined by the manufacturer) were ≥0.80 U/mL for the former and ≥1.1 arbitrary units for the latter. Priming dose responders developed positive results after both D1 and D2. Booster responders developed positive results only after D2. Non-responders maintained negative results after D1 and D2. D1/D2; Dose1/2. RBD; Receptor binding domain.
Heart transplant recipients were more likely to develop an antibody response to D2 (aIRR, 1.55 [95%CI, 1.18-2.03], p = .001) and were more likely to be priming dose responders than LT recipients (p < .001) (Table 4 ). Participants receiving anti–metabolite maintenance immunosuppression therapy were less likely to develop an antibody response to D1 (aIRR, 0.43 [95%CI, 0.22-0.85], p = .02) and D2 (aIRR, 0.71 [95%CI, 0.58-0.88], p < .01) and were less likely to be priming dose responders (p < .001). Younger participants were more likely to develop an antibody response to D1 (aIRR, 0.61 [95%CI, 0.41-0.92], p = .02) and were more likely to be priming dose responders (p = .04). Those who were 6 or more years out from transplantation were more likely to develop an antibody response to D2 (aIRR, 1.22 [95%CI, 1.10-1.35], p < .001) and more likely to be priming dose responders (p < .01) compared to those who underwent transplant in the last 6 years. No significant interactions were observed between vaccine type and additional participant characteristics. No association was observed between immunoassay type and antibody response (p = .7). No participants reported a PCR- or antigen-confirmed diagnosis of COVID-19 following vaccination by end of follow-up on April 10, 2021.
Table 4.
Association Between Characteristics of Heart and Lung Transplant Recipients and Development of an Antibody Response to Each Dose of SARS-CoV-2 Messenger RNA Vaccine
| Dose 1 |
Dose 2 |
|||||
|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis |
Univariate analysis | Multivariate analysis |
|||
| Recipient characteristics | p value | aIRRa | p value | p value | aIRRa | p value |
| (95% CI) | (95% CI) | |||||
| Ageb | .054 | 0.61 (0.41-0.92) | .02 | .2 | 0.93 (0.81-1.08) | .4 |
| Female sex | .7 | 0.74 (0.38-1.43) | .4 | .054 | 0.80 (0.64-1.00) | .051 |
| Type of organ transplantc | .3 | 1.19 (0.59-2.42) | .6 | <.001 | 1.55 (1.18-2.03) | .001 |
| Time since transplantd | .2 | 1.28 (0.95-1.72) | .11 | <.001 | 1.22 (1.10-1.35) | <.001 |
| Anti-metabolite maintenance immunosuppressione | <.01 | 0.43 (0.22-0.85) | .02 | <.001 | 0.71 (0.58-0.88) | <.01 |
| Vaccine typef | .09 | 1.66 (0.83-3.30) | .15 | .4 | 1.10 (0.89-1.37) | .2 |
aIRR, adjusted incident rate ratio. n = 237.
Model adjusted for age, sex, transplant type, time since transplant, anti-metabolite maintenance immunosuppression, and vaccine type. Comparison of mRNA-1273 and BNT16b2 was further adjusted for number of days between vaccination and antibody testing.
Age treated as continuous.
Comparison of heart (reference) vs lung transplant recipients.
Comparison of 6 or more years since transplant vs less than 6 years since transplant.
Antimetabolite maintenance immunosuppressive regimens included mycophenolate mofetil, mycophenolic acid, and azathioprine.
Comparison of mRNA-1273 (reference) BNT162b2 vaccine.
Reactogenicity after each SARS-CoV-2 mRNA vaccine dose
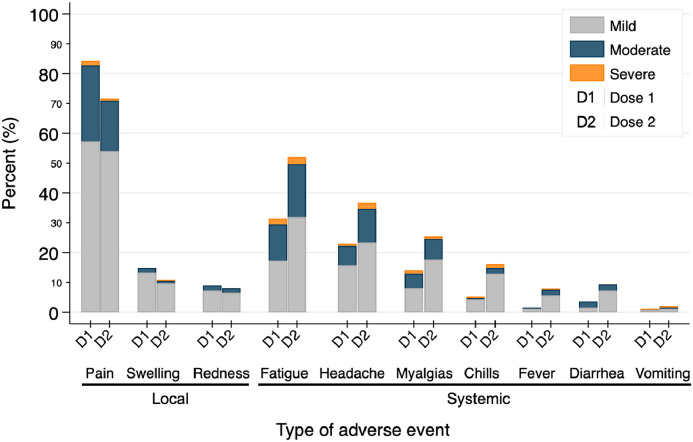
Pain at the injection site was the most commonly reported local reaction (85% after D1, 76% after D2), while the most common systemic symptoms were fatigue (32% after D1, 56% after D2) and headache (24% after D1, 39% after D2) (Figure 2 ). Severe symptoms were rare, the most common being fatigue (2% after D1 and 3% after D2). No major safety events, such as acute rejection, new neurological illness, or anaphylaxis, were reported with 98% and 96% of participants completing surveys after D1 and D2.
Figure 2.
Development of local and systemic adverse events after each SARS-CoV-2 messenger RNA vaccine dose among heart and lung transplant recipients. Percent of participants reporting adverse events within 7 day of receipt of doses 1 and 2 by symptom severity.
Discussion
In this national study of 134 HT and 103 LT recipients who completed two doses of SARS-CoV-2 mRNA vaccines we found dramatically diminished antibody responses compared to clinical trials in the general population. Only 12% of participants attained a priming dose antibody response; 39% developed antibodies only after a second dose (i.e., booster responders) and 49% failed to develop antibodies at all (i.e., non-responders); LT recipients were less likely to develop an antibody response than HT recipients. In addition to transplant organ type, priming dose antibody response was associated with lack of anti-metabolite maintenance immunosuppression, lower recipient age, and transplantation 6 or more years prior to vaccination. Adverse symptoms were predominantly mild and consistent with reactogenicity reported among the general population; no major safety events were reported. These results suggest that existing SARS-CoV-2 mRNA vaccine regimens are safe for use in HT and LT recipients; however, recipients (particularly LT recipients) experience an attenuated humeral response to vaccination relative to the general population.
These findings are consistent with published reports of diminished antibody response in SOTRs following receipt of SARS-CoV-2 mRNA vaccines. Most notably, a US national cohort of 658 SOTRs (including 90 HT and 62 LT recipients) who received two doses of the BNT162b2 or mRNA-1273 vaccine reported priming and booster dose response rates of 15% and 39%.14 Antibody development was associated with lower recipient age, lack of anti-metabolite maintenance immunosuppression, longer time since transplantation, and receipt of the mRNA-1273 vaccine. With regard to thoracic transplant recipients, a study of 77 HT recipients who received two doses of the BNT162b2 vaccine reported development of anti-spike IgG in 18% of participants 3 weeks after course completion.19 Among those with detectable antibody responses, only 57% developed neutralizing antibodies. Another study of 48 LT recipients was unable to demonstrate any antibody response 4-6 weeks after two doses of the same vaccine.20 This is in stark contrast to the robust antibody responses reported in the original clinical trials in the general population.26 , 27 The present study confirms previous findings of diminished antibody response among vaccinated HT and LT recipients and extends them, suggesting that the latter may be at significantly greater risk of immune paresis. As noted elsewhere, variability in antibody response rates between this and other studies may be due to differences in immunoassay sensitivity and timing of serologic assessment.28
Our findings also parallel reported reactogenicity in SOTRs and the general population. A safety analysis of 187 SOTRs who received D1 of the BNT162b2 or mRNA-1273 vaccine demonstrated mild adverse symptoms15 Injection site pain, fatigue, and headache were the most common reported symptoms, which were identical to those reported in the original clinical trials among the general population.26 , 27 No major safety events, including acute rejection, new neurological illness, or anaphylaxis, were reported. Clinical trials in the general population have reported similar symptom profiles. While anaphylaxis was not documented in these trials, it has been reported following widespread distribution.29 , 30 Our study identified the same symptoms as most common among HT and LT recipients with no reports of anaphylaxis, acute rejection, or incident neurological conditions.
Importantly, our study extends previous reports of vaccine outcomes among transplant recipients with the addition of antibody response and reactogenicity data following a second vaccine dose. Of the 28 participants who developed anti-spike antibodies following D1, all maintained positive results after D2 (i.e., priming dose responders). Of the remaining 209, 92 developed antibodies after D2 but with lower semiquantitative titers than priming dose responders (i.e., booster responders). The remaining 117 failed to generate an antibody response at a median of 29 days after D2 (i.e., non-responders). This differs dramatically from the 100% antibody response documented in clinical trials in the general population.31 This suggests that, for a majority of HT and LT recipients, a diminished antibody response persists despite completion of the vaccine course. Augmented vaccination schedules, alternative vaccine platforms, temporary adjustment of immunosuppressive regimens, or other viral prophylaxis strategies may be required in this population and should be the topic of future studies.
The magnitude of immunoassay results among priming dose responders reported here also compares favorably with those previously reported in healthy vaccinated individuals. A study of 34 healthy individuals who received a two-dose series of the BNT162b2 vaccine reported median results of 57.7 U/mL (range 5.35-2049 U/mL) at 3 weeks and 3284 U/mL (106-2501 U/mL) at 4 weeks after D2 using an anti-RBD immunoassay (Roche Elecsys).32 Results were fully quantitative reflecting the total concentration of anti-spike RBD IgG. Priming dose responders in the present study assessed with the same assay but in semiquantitative fashion had a median of at least 250 U/mL 28 days after D2, making their antibody response at least as robust as healthy individuals 3 weeks from series completion. Another study of 27 healthy individuals who received one or two doses of SARS-CoV-2 mRNA vaccine reported median results of 3.3 at 19 days post-D1 and 6.1 at 3 days post-D2 using an anti-S1 immunoassay (EUROIMMUN).33 Priming dose responders in the present study assessed with the same assay had median results of 2.4 at 20 days and 9.3 at 28 days following D1 and D2, respectively. Booster responders, by comparison, fell short of these results when assessed with either assay.
This study benefits from a national sample of heart and lung transplant recipients with early and novel data following completion of two-dose regimens of the BNT162b2 and mRNA-1273 vaccines. However, this study must also be understood in the context of its limitations. Our use of non-consecutive convenience sampling contributed to sociodemographic homogeneity and reduced generalizability. The lack of a non-SOTR control group makes true comparisons difficult but the robust 100% antibody response rates seen in clinical trials among the general population are reasonable benchmarks. The use of two immunoassays made direct quantitative comparisons between their results impossible; however, their similar distribution of seropositivity and seronegativity in vaccinated transplant recipients and clear manufacturer standards allowed for their reliable and equivalent use in the detection of anti-spike antibodies.14 , 34 Neutralization assays were not directly assessed, however both of the assays used correlate favorably with neutralizing immunity.24 Our study also relies on participant self-report which limited available clinical data and which may be subject to response bias. Our ability to investigate associations between specific immunosuppressive protocols and antibody response is limited by the lack of medication dosing, adherence, and serum trough levels. Crucially, long-term follow-up and characterization of participant cell-mediated responses to vaccination are needed before the durability of antibody response and its implications for vaccine effectiveness can be fully assessed. Future investigations into the incidence of COVID-19-related infections, hospitalizations, and deaths among priming dose responders, booster responders, and non-responders as well as further immunophenotyping, including B-cell and T-cell responses, are needed.
In conclusion, HT and LT recipients experience diminished antibody response with reactogenicity comparable to the general population following completion of a two-dose series of SARS-CoV-2 mRNA vaccine. LT recipients are at greater risk of a diminished response than HT recipients. Younger recipients, those who are 6 or more years post-transplant, and those not on anti-metabolite maintenance immunosuppression, are most likely to develop a robust antibody response. Longer term follow-up and deeper immunophenotyping, including characterization of B-cell and T-cell primary and secondary responses, will be important in determining vaccination strategies in this population. Further studies are needed to determine whether the diminished humoral response following SARS-CoV-2 mRNA vaccination observed here is correlated with lower clinical efficacy. Until then, clinicians and thoracic transplant recipients should continue to follow ISHLT guidelines promoting vaccination among transplant recipients as well as continued masking and social distancing following vaccination.
Author contribution
Substantial contributions to the conception or design of the work: AMH, RSG, BJB, PDS, MTO, ATT, MRK, JIL, WAW, RKA, SB, AAT, ABM, RSDH, JMGW, DLS, ELB, Data analysis, acquisition, and interpretation; AMH, RSG, BJB, MTO, ATT, MRK, JIL, ABM, Drafting the work or revising it critically for important intellectual content; AMH, RSG, BJB, MTO, Critical manuscript revisions for important intellectual content; PDS, WAW, RKA, SB, AAT, ABM, RSDH, JMGW, DLS, ELB, Project supervision; ABM, DLS, ELB, Funding; AMH, BJB, JMGW, DLS, WAW, Final approval of the version to be published; AMH, RSG, BJB, PDS, MTO, ATT, MRK, JIL, WAW, RKA, SB, AAT, ABM, RSDH, JMGW, DLS, ELB, Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. AMH, RSG, BJB, PDS, MTO, ATT, MRK, JIL, WAW, RKA, SB, AAT, ABM, RSDH, JMGW, DLS, ELB.
Disclosure statements
The named authors have no conflicts of interest.
Dorry L. Segev, MD PhD has the following financial disclosures: consulting and speaking honoraria from Sanofi, Novartis, CSL Behring, Jazz Pharmaceuticals, Veloxis, Mallincrodt, Thermo Fisher Scientific. Robin Avery MD has the following financial disclosures: study/grant support from Aicuris, Astellas, Chimerix, Merck, Oxford Immunotec, Qiagen, and Takeda/Shire. The other authors of this manuscript have no financial disclosures to make.
This research was made possible with generous support of the Ben-Dov family. This work was supported by grant numbers T32DK007732 (Hallett), F32DK124941 (Boyarsky), and K23DK115908 (Garonzik‐Wang) from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), K24AI144954 (Segev) from National Institute of Allergy and Infectious Diseases (NIAID), and by a grant from the Transplantation and Immunology Research Network of the American Society of Transplantation (Werbel). The analyses described here are the responsibility of the authors alone and do not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products or organizations imply endorsement by the US Government.
References
- 1.Granger C, Guedeney P, Arnaud C, et al. Clinical manifestations and outcomes of coronavirus disease-19 in heart transplant recipients: a multicentre case series with a systematic review and meta-analysis. Transpl Int Off J Eur Soc Organ Transplant. 2021;34:721–731. doi: 10.1111/tri.13837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rivinius R, Kaya Z, Schramm R, et al. COVID-19 among heart transplant recipients in Germany: a multicenter survey. Clin Res Cardiol. 2020;109:1531–1539. doi: 10.1007/s00392-020-01722-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marcondes-Braga FG, Murad CM, Belfort DSP, et al. Characteristics and outcomes of heart transplant recipients with Coronavirus-19 disease in a high-volume transplant center. Transplantation. Published online March 22, 2021 doi: 10.1097/TP.0000000000003770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iacovoni A, Boffini M, Pidello S, et al. A case series of novel coronavirus infection in heart transplantation from 2 centers in the pandemic area in the North of Italy. J Hear Lung Transplant. 2020;39:1081–1088. doi: 10.1016/j.healun.2020.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bottio T, Bagozzi L, Fiocco A, et al. COVID-19 in heart transplant recipients: a multicenter analysis of the Northern Italian outbreak. JACC Hear Fail. 2021;9:52–61. doi: 10.1016/j.jchf.2020.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Myers CN, Scott JH, Criner GJ, et al. COVID-19 in lung transplant recipients. Transpl Infect Dis. 2020;22:e13364. doi: 10.1111/tid.13364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morlacchi LC, Rossetti V, Gigli L, et al. COVID-19 in lung transplant recipients: a case series from Milan, Italy. Transpl Infect Dis. 2020;22:e13356. doi: 10.1111/tid.13356. [DOI] [PubMed] [Google Scholar]
- 8.Gergen AK, Madsen HJ, Tilva KR, Smith JB, Weyant MJ. Coronavirus disease 2019 in lung transplant recipients. Ann Thorac Surg. 2021;111:e343–e345. doi: 10.1016/j.athoracsur.2020.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Study to Describe the Safety . Tolerability, Immunogenicity, and Efficacy of RNA Vaccine Candidates Against COVID-19 in Healthy Individuals - Full Text View - ClinicalTrials.gov. Accessed April 13, 2021. https://clinicaltrials.gov/ct2/show/NCT04368728 [Google Scholar]
- 10.A Study to Evaluate Efficacy . Safety, and Immunogenicity of mRNA-1273 Vaccine in Adults Aged 18 Years and Older to Prevent COVID-19 - Full Text View - ClinicalTrials.gov. Accessed April 13, 2021. https://clinicaltrials.gov/ct2/show/NCT04470427 [Google Scholar]
- 11.Surgery C. Guidance from the International Society of Heart and Lung Transplantation regarding the SARS CoV-2 pandemic. ISHLT. 2020:2–16. Published online. [Google Scholar]
- 12.SARS-CoV-2 Vaccination in Heart and Lung Transplantation: Recommendations from the ISHLT COVID-19 Task Force. Accessed April 15, 2021. https://ishlt.org/ishlt/media/Documents/COVID19_Vaccine-Recommendations_3-15-2021.pdf
- 13.Boyarsky BJ, Ou MT, Werbel WA, et al. Early development and durability of SARS-CoV-2 antibodies among solid organ transplant recipients: a pilot study. Transplantation. 9000; Online Fir. Accessed August 24, 2021. https://journals.lww.com/transplantjournal/Fulltext/9000/Early_Development_and_Durability_of_SARS_CoV_2.95419.aspx [DOI] [PMC free article] [PubMed]
- 14.Boyarsky BJ, Werbel WA, Avery RK, et al. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021;325:2204–2206. doi: 10.1001/jama.2021.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boyarsky BJ, Ou MT, Greenberg RS, et al. Safety of the first oose of SARS-CoV-2 vaccination in solid organ transplant recipients. Transplantation. 9000; Online Fir. Accessed August 24, 2021. https://journals.lww.com/transplantjournal/Fulltext/9000/Safety_of_the_First_Dose_of_SARS_CoV_2_Vaccination.95390.aspx [DOI] [PMC free article] [PubMed]
- 16.Yi SG, Knight RJ, Graviss EA, et al. Kidney transplant recipients rarely show an early antibody response following the first COVID-19 vaccine administration. Transplantation. 2021;105:e72–e73. doi: 10.1097/TP.0000000000003764. [DOI] [PubMed] [Google Scholar]
- 17.Sattler A, Schrezenmeier E, Weber U, et al. Impaired humoral and cellular immunity after SARS-CoV2 BNT162b2 (Tozinameran) prime-boost vaccination in kidney transplant recipients. medRxiv. Published online January 1, 2021 doi: 10.1101/2021.04.06.21254963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grupper A, Rabinowich L, Schwartz D, et al. Reduced humoral response to mRNA SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients without prior exposure to the virus. Am J Transplant. 2021;21:2719–2726. doi: 10.1111/ajt.16615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peled Y, Ram E, Lavee J, et al. BNT162b2 vaccination in heart transplant recipients: clinical experience and antibody response. J Heart Lung Transplant. April 2021;40:759–762. doi: 10.1016/j.healun.2021.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Havlin J, Svorcova MDE. Immunogenicity of BNT162b2 mRNA COVID-19 Vaccine and SARS-CoV-2 infection in lung transplant recipients. J Hear Lung Transpl. 2021 doi: 10.1016/j.healun.2021.05.004. In Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boyarsky BJ, Werbel WA, Avery RK, et al. Immunogenicity of a single dose of SARS-CoV-2 messenger RNA vaccine in solid organ transplant recipients. JAMA. 2021;325:1784–1786. doi: 10.1001/jama.2021.4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klein SL, Pekosz A, Park H-S, et al. Sex, age, and hospitalization drive antibody responses in a COVID-19 convalescent plasma donor population. J Clin Invest. 2020;130:6141–6150. doi: 10.1172/JCI142004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patel EU, Bloch EM, Clarke W, et al. Comparative performance of five commercially available serologic assays to detect antibodies to SARS-CoV-2 and identify individuals with high Neutralizing Titers. J Clin Microbiol. 2021;59 doi: 10.1128/JCM.02257-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Higgins V, Fabros A, Kulasingam V. Quantitative measurement of anti-SARS-CoV-2 antibodies: analytical and clinical evaluation. J Clin Microbiol. 2021;59 doi: 10.1128/JCM.03149-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aslam S, Danziger-Isakov L, Mehra MR. COVID-19 vaccination immune paresis in heart and lung transplantation. J Heart Lung Transplant. 2021;40:763–766. doi: 10.1016/j.healun.2021.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shimabukuro T. Allergic reactions including anaphylaxis after receipt of the first dose of Moderna COVID-19 vaccine - United States, December 21, 2020-January 10, 2021. Am J Transplant. 2021;21:1326–1331. doi: 10.1111/ajt.16517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine - United States, December 14-23, 2020. MMWR Morb Mortal Wkly Rep. 2021;70:46–51. doi: 10.15585/mmwr.mm7002e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walsh EE, Frenck RW, Falsey AR, et al. Safety and immunogenicity of two RNA-Based Covid-19 vaccine candidates. N Engl J Med. 2020;383:2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mueller T. Antibodies against severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) in individuals with and without COVID-19 vaccination: a method comparison of two different commercially available serological assays from the same manufacturer. Clin Chim Acta. 2021;518:9–16. doi: 10.1016/j.cca.2021.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dörschug A, Frickmann H, Schwanbeck J, et al. Comparative assessment of Sera from individuals after S-Gene RNA-based SARS-CoV-2 vaccination with spike-protein-based and nucleocapsid-based serological assays. Diagnostics. 2021;11:426. doi: 10.3390/diagnostics11030426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rubio-Acero R, Castelletti N, Fingerle V, et al. In search of the SARS-CoV-2 protection correlate: head-to-head comparison of two quantitative S1 assays in pre-characterized Oligo-/Asymptomatic Patients. Infect Dis Ther. 2021;10:1505–1518. doi: 10.1007/s40121-021-00475-x. [DOI] [PMC free article] [PubMed] [Google Scholar]