Abstract
Introduction
Esophagectomy is a key component in the curative treatment of esophageal cancer. Little is understood about the impact of smoking status on perioperative morbidity and mortality and the long-term outcome of patients following esophagectomy.
Objective
This study aimed to evaluate morbidity and mortality according to smoking status in patients undergoing esophagectomy for esophageal cancer.
Methods
Consecutive patients undergoing two-stage transthoracic esophagectomy (TTE) for esophageal cancers (adenocarcinoma or squamous cell carcinoma) between January 1997 and December 2016 at the Northern Oesophagogastric Unit were included from a prospectively maintained database. The main explanatory variable was smoking status, defined as current smoker, ex-smoker, and non-smoker. The primary outcome was overall survival (OS), while secondary outcomes included perioperative complications (overall, anastomotic leaks, and pulmonary complications) and survival (cancer-specific survival [CSS], recurrence-free survival [RFS]).
Results
During the study period, 1168 patients underwent esophagectomy for cancer. Of these, 24% (n = 282) were current smokers and only 30% (n = 356) had never smoked. The median OS of current smokers was significantly shorter than ex-smokers and non-smokers (median 36 vs. 42 vs. 48 months; p = 0.015). However, on adjusted analysis, there was no significant difference in long-term OS between smoking status in the entire cohort. The overall complication rates were significantly higher with current smokers compared with ex-smokers or non-smokers (73% vs. 66% vs. 62%; p = 0.018), and there were no significant differences in anastomotic leaks and pulmonary complications between the groups. On subgroup analysis by receipt of neoadjuvant therapy and tumor histology, smoking status did not impact long-term survival in adjusted multivariable analyses.
Conclusion
Although smoking is associated with higher rates of short-term perioperative morbidity, it does not affect long-term OS, CSS, and RFS following esophagectomy for esophageal cancer. Therefore, implementation of perioperative pathways to optimize patients may help reduce the risk of complications.
Supplementary Information
The online version contains supplementary material available at 10.1245/s10434-021-09720-6.
Esophagectomy remains a key part of the treatment for patients with potentially curable esophageal cancer; however, esophagectomy is a technically demanding procedure and is associated with a high incidence of postoperative morbidity and mortality.1–5 Anastomotic leaks and pulmonary complications remain a major cause of postoperative mortality.6–9 Thus, identifying those at risk of pulmonary morbidities and optimizing their perioperative management has an important role for patients being considered for esophagectomy.
Because smoking is recognized as a risk factor for morbidity following esophagectomy, particularly with respect to pulmonary complications,7,9–12 smoking cessation has been demonstrated to reduce postoperative pulmonary morbidity.13,14 However, the impact of smoking status on long-term survival is unclear and is limited to small case series from eastern cohorts focused on esophageal squamous cell carcinoma (SCC).5,15 A recent analysis of 5354 esophagectomies from a Japanese nationwide web-based database demonstrated that smoking within 1 year of undergoing esophagectomy is an independent risk factor for 30-day mortality.5
Despite this, its impact on long-term survival has not been fully established. The aim of this study was to investigate the impact of smoking status on long-term survival in patients undergoing esophagectomy. These findings will be useful for counseling patients and targeting prehabilitation in patients undergoing thoracoabdominal surgery.
Methods
Study Population
Consecutive patients undergoing two-stage transthoracic esophagectomy (TTE) for esophageal cancer (adenocarcinoma or SCC) were included in this study. Patients were identified from the Northern Oesophagogastric Unit (NOGU) in Newcastle-upon-Tyne between January 1997 and December 2016 through a contemporaneously maintained database. Patients with metastases, non-resectable tumors during exploratory surgery, or macroscopically incomplete resections (R2) were excluded.
Staging
Patients were staged using esophagogastroduodenoscopy (OGD) with biopsy, endoscopic ultrasonography, and thoracoabdominal computerized tomography (CT). Positron emission tomography (PET) CT scan became routine for patients during the study period. Laparoscopy with peritoneal washings was used for junctional tumors. All cases were discussed by the multidisciplinary team and treatment recommendations were discussed with patients. All patients in this study were staged according to the American Joint Committee on Cancer (AJCC) 8th edition.16
Neoadjuvant Therapy and Operative Approach
Multiple neoadjuvant regimens were employed in the present study, determined by the standard of care as practiced in the UK, and recruiting trials at the time of each patient’s treatment. The most commonly used regimen was a combination of epirubicin, cisplatin, and capecitabine (ECX), over the course of this study.17,18 TTE with two- or three-field lymphadenectomy was carried out 4–6 weeks after completion of neoadjuvant therapy using a conventional approach as previously reported.19 As per protocol, after surgery, each lymph node station was dissected from the specimen by the operating surgeon and placed into individual pots for analysis by the pathologist.20
Pathology
Histopathological reporting was carried out by specialist gastrointestinal pathologists using a standardized proforma in line with guidelines produced by the Royal College of Pathologists;21 this included tumor type and differentiation, depth of tumor infiltration, and degree of tumor regression according to the Mandard criteria. The total number of lymph nodes and number of nodal metastases from each location was also recorded.
Follow-Up and Definition of Recurrence
Smoking status was defined as follows: (1) current smokers were defined as ongoing smokers at the time of diagnosis, or had stopped within 6 weeks of surgery; (2) ex-smokers were those who stopped >6 weeks prior to surgery; and (3) non-smokers were defined as those who have never smoked, consistent with previously published multicenter studies.22–24 Patients were reviewed in the outpatient clinic at 3- to 6-month intervals during the first 2 years and every 6 months or annually for 5 years, then yearly until 10 years post initial surgery. Recurrence of disease suspected on clinical grounds was confirmed with either CT or endoscopy.
Statistical Analysis
Categorical variables were compared using the Kruskal–Wallis test, and non-normally distributed data were analyzed using the Mann–Whitney U test. Survival was estimated using Kaplan–Meier survival curves and compared using the log-rank test. A p-value <0.05 was considered statistically significant. Data analysis was performed using R Foundation Statistical software (R 3.2.2) with TableOne, ggplot2, Hmisc, and survival packages (The R Foundation for Statistical Computing, Vienna, Austria) as previously described.19,25,26
Results
Baseline Characteristics
During the study period, 1168 patients underwent esophagectomy for cancer, of whom 282 were current (24%) smokers and 530 (45%) were ex-smokers. Current smokers were more likely to be younger (median 62 vs. 67 vs. 67 years; p < 0.001), have an SCC (27% vs. 19% vs. 28%; p = 0.005), be from more deprived groups (Index of Multiple Deprivation [IMD] 1/2: 40% vs. 39% vs. 42%; p < 0.001), and have higher American Society of Anesthesiologists (ASA) grade 3/4 (34% vs. 26% vs. 21%; p = 0.003) than ex-smokers or never smokers. There were no significant differences in overall pathological stage, tumor grades, lymphadenectomy, and margin-positive rates between groups. Clinicopathologic variables are presented in Table 1. Trends in the rates of two-stage TTE and neoadjuvant therapy are presented in electronic supplementary Fig. 1. The two-stage TTE rates over the past 2 decades ranged from 92% to 100%.
Table 1.
Baseline demographics and postoperative outcomes of all patients undergoing transthoracic esophagectomy for esophageal cancer, stratified by smoking status
| Current [n = 282] | Ex-smoker [n = 530] | Never [n = 356] | Total [n = 1168] | p value | ||
|---|---|---|---|---|---|---|
| Age at diagnosis, years | Median (IQR) | 62.0 (14.0) | 66.5 (11.0) | 67.0 (13.2) | 65.0 (13.0) | < 0.001 |
| Sex | Male | 204 (72.3) | 430 (81.1) | 228 (64.0) | 862 (73.8) | < 0.001 |
| Female | 78 (27.7) | 100 (18.9) | 128 (36.0) | 306 (26.2) | ||
| Histology | Adenocarcinoma | 205 (72.7) | 428 (80.8) | 258 (72.5) | 891 (76.3) | 0.005 |
| SCC | 77 (27.3) | 102 (19.2) | 98 (27.5) | 277 (23.7) | ||
| Body mass index, kg/m2 | Median (IQR) | 24.2 (6.9) | 26.8 (5.6) | 26.0 (5.6) | 26.0 (6.0) | < 0.001 |
| IMD decile | 1 | 86 (30.5) | 122 (23.0) | 51 (14.3) | 259 (22.2) | < 0.001 |
| 2 | 26 (9.2) | 84 (15.8) | 98 (27.5) | 208 (17.8) | ||
| 3 | 77 (27.3) | 113 (21.3) | 75 (21.1) | 265 (22.7) | ||
| 4 | 45 (16.0) | 110 (20.8) | 65 (18.3) | 220 (18.8) | ||
| 5 | 35 (12.4) | 80 (15.1) | 59 (16.6) | 174 (14.9) | ||
| Unknown | 13 (4.6) | 21 (4.0) | 8 (2.2) | 42 (3.6) | ||
| ASA grade | 1 | 33 (11.7) | 70 (13.2) | 72 (20.2) | 175 (15.0) | 0.003 |
| 2 | 125 (44.3) | 276 (52.1) | 180 (50.6) | 581 (49.7) | ||
| 3 | 92 (32.6) | 134 (25.3) | 70 (19.7) | 296 (25.3) | ||
| 4 | 3 (1.1) | 3 (0.6) | 2 (0.6) | 8 (0.7) | ||
| Unknown | 29 (10.3) | 47 (8.9) | 32 (9.0) | 108 (9.2) | ||
| Overall treatment | NAC + surgery | 145 (51.4) | 282 (53.2) | 174 (48.9) | 601 (51.5) | 0.449 |
| Surgery only | 137 (48.6) | 248 (46.8) | 182 (51.1) | 567 (48.5) | ||
| AJCC pathological stage classification | 0 | 16 (5.7) | 34 (6.4) | 23 (6.5) | 73 (6.2) | 0.304 |
| I | 53 (18.8) | 114 (21.5) | 81 (22.8) | 248 (21.2) | ||
| II | 49 (17.4) | 118 (22.3) | 86 (24.2) | 253 (21.7) | ||
| III | 134 (47.5) | 219 (41.3) | 139 (39.0) | 492 (42.1) | ||
| IVA | 30 (10.6) | 45 (8.5) | 27 (7.6) | 102 (8.7) | ||
| Tumor grade | Well | 26 (9.2) | 39 (7.4) | 36 (10.1) | 101 (8.6) | 0.216 |
| Moderate | 127 (45.0) | 264 (49.8) | 165 (46.3) | 556 (47.6) | ||
| Poor | 111 (39.4) | 187 (35.3) | 118 (33.1) | 416 (35.6) | ||
| Unknown | 18 (6.4) | 40 (7.5) | 37 (10.4) | 95 (8.1) | ||
| Lymph nodes examined | Median (IQR) | 31.0 (17.0) | 29.0 (15.5) | 30.0 (13.0) | 30.0 (15.2) | 0.260 |
| Margin status | R0 | 272 (96.5) | 524 (98.9) | 347 (97.5) | 1143 (97.9) | 0.064 |
| R1 | 10 (3.5) | 6 (1.1) | 9 (2.5) | 25 (2.1) | ||
| Lymphatic involvement | No | 149 (52.8) | 289 (54.5) | 203 (57.0) | 641 (54.9) | 0.559 |
| Yes | 133 (47.2) | 241 (45.5) | 153 (43.0) | 527 (45.1) | ||
| Venous involvement | No | 181 (64.2) | 337 (63.6) | 246 (69.1) | 764 (65.4) | 0.211 |
| Yes | 101 (35.8) | 193 (36.4) | 110 (30.9) | 404 (34.6) | ||
| Perineural involvement | No | 144 (51.1) | 296 (55.8) | 212 (59.6) | 652 (55.8) | 0.100 |
| Yes | 138 (48.9) | 234 (44.2) | 144 (40.4) | 516 (44.2) | ||
| Extracapsular spread | No | 242 (85.8) | 445 (84.0) | 296 (83.1) | 983 (84.2) | 0.647 |
| Yes | 40 (14.2) | 85 (16.0) | 60 (16.9) | 185 (15.8) | ||
| Critical care stay | Median (IQR) | 3.0 (7.0) | 2.0 (4.0) | 2.0 (3.0) | 2.0 (4.0) | < 0.001 |
| Length of stay | Median (IQR) | 15.0 (11.0) | 15.0 (10.8) | 15.0 (9.0) | 15.0 (10.0) | 0.515 |
| Overall complications | No | 76 (27.0) | 181 (34.2) | 136 (38.2) | 393 (33.6) | 0.011 |
| Yes | 206 (73.0) | 349 (65.8) | 220 (61.8) | 775 (66.4) | ||
| Surgical site infection | No | 251 (89.0) | 477 (90.0) | 325 (91.3) | 1053 (90.2) | 0.621 |
| Yes | 31 (11.0) | 53 (10.0) | 31 (8.7) | 115 (9.8) | ||
| Pulmonary complications | No | 244 (86.5) | 471 (88.9) | 321 (90.2) | 1036 (88.7) | 0.348 |
| Yes | 38 (13.5) | 59 (11.1) | 35 (9.8) | 132 (11.3) | ||
| Cardiac complications | No | 269 (95.4) | 494 (93.2) | 328 (92.1) | 1091 (93.4) | 0.250 |
| Yes | 13 (4.6) | 36 (6.8) | 28 (7.9) | 77 (6.6) | ||
| Anastomotic leaks | No | 253 (89.7) | 483 (91.1) | 334 (93.8) | 1070 (91.6) | 0.154 |
| Yes | 29 (10.3) | 47 (8.9) | 22 (6.2) | 98 (8.4) | ||
| In-hospital mortality | No | 267 (94.7) | 516 (97.4) | 342 (96.1) | 1125 (96.3) | 0.149 |
| Yes | 15 (5.3) | 14 (2.6) | 14 (3.9) | 43 (3.7) | ||
| 30-day mortality | No | 273 (96.8) | 520 (98.1) | 344 (96.6) | 1137 (97.3) | 0.328 |
| Yes | 9 (3.2) | 10 (1.9) | 12 (3.4) | 31 (2.7) |
Data are expressed as n (%) unless otherwise specified
IMD Index of Multiple Deprivation, ASA American Society of Anesthesiologists, AJCC American Joint Committee on Cancer, IQR interquartile range, SCC squamous cell carcinoma, NAC neoadjuvant chemotherapy
Postoperative Outcomes and Survival
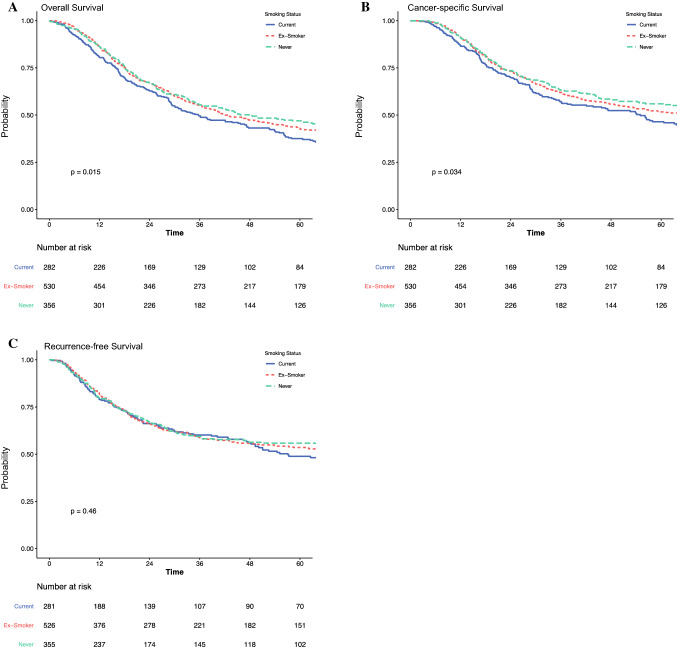
Trends in the rates of overall complications and anastomotic leaks are presented in electronic supplementary Fig. 1. Current smokers had significantly higher rates of overall complications compared with ex-smokers and never smokers (73% vs. 66% vs. 62%, p = 0.011); however, there were no significant differences in the rates of surgical site infections, pulmonary complications, cardiac complications, or anastomotic leaks between the groups. The median overall survival (OS; median 36 vs. 42 vs. 48 months, p = 0.015) (Fig. 1a) and cancer-specific survival (CSS; median 55 vs. 68 vs. 119 months; p = 0.034) (Fig. 1b) of current smokers was significantly shorter than ex-smokers and non-smokers. There were no statistical differences on recurrence-free survival (RFS) between smoking status groups (Fig. 1c). On adjusted Cox regression, smoking statuses were not independent prognostic factors for OS, CSS, and RFS (Table 2, electronic supplementary Tables 1–3).
Fig. 1.
Impact of smoking on (a) overall survival, (b) cancer-specific survival, and (c) recurrence-free survival, in patients undergoing transthoracic esophagectomy for esophageal cancer
Table 2.
Impact of smoking on long-term overall, cancer-specific, and recurrence-free survival following transthoracic esophagectomy for esophageal cancers
| No. of patients | Median survival, months | Univariable HR (95% CI) | Multivariable HR (95% CI) | |
|---|---|---|---|---|
| Overall survival | ||||
| Current | 282 | 35.6 (29.3–47.3) | REF | REF |
| Ex-smoker | 530 | 42.0 (37.0–53.3) | 0.85 (0.71–1.01), p = 0.060 | 0.94 (0.77–1.14), p = 0.499 |
| Never | 356 | 48.3 (36.9–76.9) | 0.76 (0.62–0.92), p = 0.004 | 0.93 (0.75–1.16), p = 0.529 |
| Cancer-specific survival | ||||
| Current | 282 | 55.0 (37.6–68.8) | REF | REF |
| Ex-smoker | 530 | 68.2 (52.3–170.5) | 0.83 (0.67–1.01), p = 0.068 | 0.94 (0.75–1.19), p = 0.615 |
| Never | 356 | 118.6 (62.2–NR) | 0.74 (0.59–0.93), p = 0.011 | 0.90 (0.70–1.16), p = 0.420 |
| Recurrence-free survival | ||||
| Current | 281 | 57.1 (47.7–NR) | REF | REF |
| Ex-smoker | 526 | NR (54.2–NR) | 0.90 (0.72–1.13), p = 0.377 | 1.00 (0.78–1.29), p = 0.980 |
| Never | 355 | NR (76.0–NR) | 0.84 (0.65–1.08), p = 0.173 | 1.03 (0.78–1.36), p = 0.842 |
HR hazard ratio, CI confidence interval, NR not reached
Interaction Between Smoking Status and Neoadjuvant Therapy
Of the entire cohort, 601 patients received neoadjuvant therapy. Interaction analyses were performed to further understand the impact of smoking status in patients receiving neoadjuvant therapy. Baseline demographics and postoperative outcomes of patients with and without neoadjuvant therapy are presented in electronic supplementary Tables 4 and 5, respectively. As above, similar trends were observed for age, IMD decile, and ASA grade between the different smoking status groups in patients with neoadjuvant therapy. The overall complication rates were significantly higher in current smokers compared with ex-smokers and never smokers in patients receiving one therapy (72% vs. 64% vs. 56%; p = 0.012) [electronic supplementary Table 4), but this was not the case for patients without neoadjuvant therapy (74% vs. 69% vs. 67%, p = 0.4).
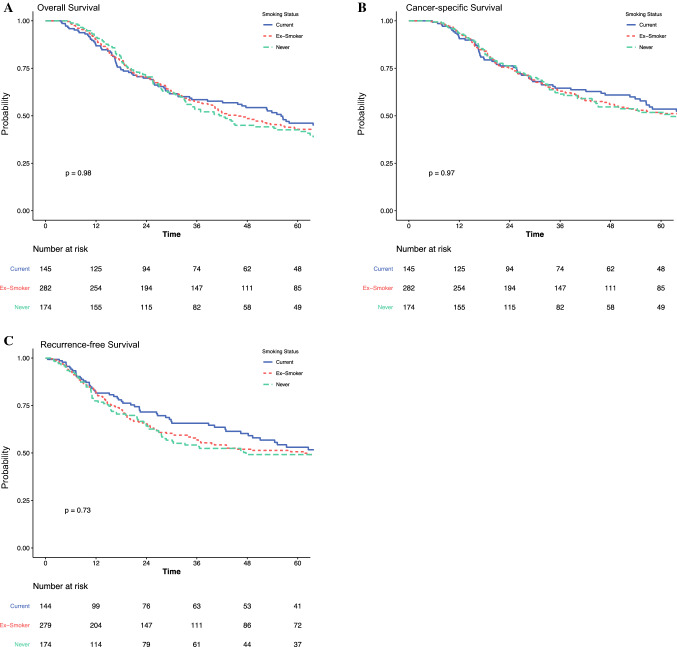
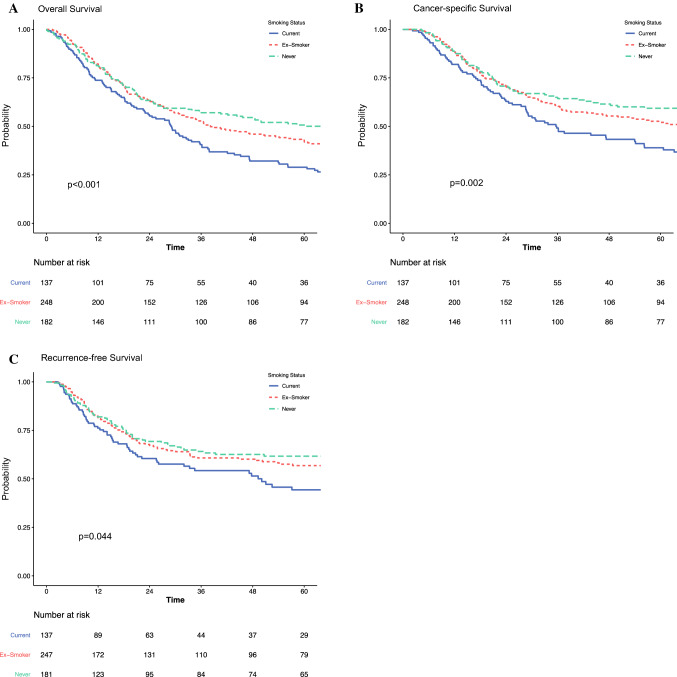
In patients with neoadjuvant therapy, there were no significant differences in OS for current smokers compared with ex-smokers and never smokers (median 56 vs. 46 vs. 42; p = 1.0) (Table 3, Fig. 2a). There were also no significant differences for CSS (Table 3, Fig. 2b) and RFS (Table 3, Fig. 2c). These results were consistent on multivariable analysis, as presented in Table 3. In contrast, current smokers undergoing unimodality surgery only had significantly shorter survival than ex-smokers or never smokers, for OS (median 29 vs. 38 vs. 70 months; p < 0.001) (Table 3, Fig. 3a), CSS (median 36 vs. 78 vs. 200 months; p = 0.002) (Table 3, Fig. 3b), and RFS (median 50 vs. not reached vs. not reached; p = 0.044) (Table 3, Fig. 3c). These results were consistent on multivariable analysis, as presented in Table 3. In multivariable analyses modeling the interaction between receipt of neoadjuvant therapy and smoking status, there were no survival differences in ex-smokers or never smokers, by neoadjuvant therapy status, for OS, CSS, and RFS.
Table 3.
Subset analyses, by neoadjuvant therapy, on the impact of smoking on long-term overall, cancer-specific, and recurrence-free survival following transthoracic esophagectomy for esophageal cancers
| No. of patients | Median survival, months | Univariable HR (95% CI) | Multivariable HR (95% CI) | |
|---|---|---|---|---|
| Neoadjuvant and surgery | ||||
| Overall survival | ||||
| Current | 145 | 56.0 (38.5–67.9) | REF | REF |
| Ex-smoker | 282 | 46.0 (39.8–59.2) | 1.02 (0.79–1.32), p = 0.877 | 1.10 (0.82–1.48), p = 0.513 |
| Never | 174 | 42.2 (33.4–62.2) | 1.00 (0.75–1.33), p = 0.999 | 1.09 (0.80–1.51), p = 0.582 |
| Cancer-specific survival | ||||
| Current | 145 | 69.7 (55.0–NR) | REF | REF |
| Ex-smoker | 282 | 67.5 (48.9–NR) | 1.03 (0.77–1.39), p = 0.822 | 1.17 (0.83–1.65), p = 0.372 |
| Never | 174 | 62.2 (44.2–NR) | 1.01 (0.73–1.40), p = 0.957 | 1.07 (0.74–1.55), p = 0.720 |
| Recurrence-free survival | ||||
| Current | 144 | 81.4 (49.3–NR) | REF | REF |
| Ex-smoker | 279 | 61.5 (36.4–NR) | 1.13 (0.82–1.55), p = 0.451 | 1.19 (0.83–1.69), p = 0.346 |
| Never | 174 | 47.7 (28.2–NR) | 1.12 (0.79–1.59), p = 0.524 | 1.20 (0.82–1.76), p = 0.351 |
| Unimodality surgery only | ||||
| Overall survival | ||||
| Current | 137 | 29.1 (22.8–35.8) | REF | REF |
| Ex-smoker | 248 | 38.2 (31.5–59.4) | 0.72 (0.57–0.91), p = 0.006 | 0.86 (0.66–1.13), p = 0.278 |
| Never | 182 | 69.5 (40.5–111.3) | 0.61 (0.47–0.78), p < 0.001 | 0.86 (0.63–1.18), p = 0.359 |
| Cancer-specific survival | ||||
| Current | 137 | 35.8 (28.7–56.2) | REF | REF |
| Ex-smoker | 248 | 78.2 (46.5–186.0) | 0.68 (0.51–0.91), p = 0.009 | 0.81 (0.58–1.14), p = 0.229 |
| Never | 182 | 199.8 (88.1–NR) | 0.57 (0.42–0.79), p = 0.001 | 0.79 (0.53–1.17), p = 0.236 |
| Recurrence-free survival | ||||
| Current | 137 | 50.1 (26.1–NR) | REF | REF |
| Ex-smoker | 247 | NR (NR–NR) | 0.72 (0.52–1.00), p = 0.052 | 0.89 (0.61–1.31), p = 0.560 |
| Never | 181 | NR (NR–NR) | 0.65 (0.45–0.93), p = 0.019 | 1.02 (0.66–1.59), p = 0.918 |
HR hazard ratio, CI confidence interval, NR not reached
Fig. 2.
Impact of smoking on (a) overall survival, (b) cancer-specific survival, and (c) recurrence-free survival, in patients undergoing neoadjuvant therapy and transthoracic esophagectomy for esophageal cancer
Fig. 3.
Impact of smoking on (a) overall survival, (b) cancer-specific survival, and (c) recurrence-free survival, in patients undergoing transthoracic esophagectomy only for esophageal cancer
Subgroup Analysis by Tumor Histology
Interaction analyses were performed to further understand the impact of smoking status by tumor histology (adenocarcinoma and SCC). Baseline demographics and postoperative outcomes for esophageal adenocarcinoma and SCC are presented in electronic supplementary Tables 6 and 7, respectively. As above, similar trends were observed for age and IMD decile between the different smoking status groups in patients with adenocarcinoma and SCC.
In patients with esophageal adenocarcinoma, there were no significant differences in OS for current smokers compared with ex-smokers and never smokers (median 38 vs. 42 vs. 40; p = 0.4) (Table 4, electronic supplementary Fig. 2a). There were also no significant differences for CSS (Table 4, electronic supplementary Fig. 2b) and RFS (Table 4, electronic supplementary Fig. 2c). These results were consistent with adjusted Cox multivariable regression analysis, as presented in Table 4. In contrast, for esophageal SCC, current smokers had significantly shorter survival than ex-smokers or never smokers, for OS (median 34 vs. 52 vs. 96 months; p = 0.001) (Table 4, electronic supplementary Fig. 3a) and CSS (median 47 vs. 68 vs. 154 months; p = 0.040) (Table 4, electronic supplementary Fig. 3b), but not RFS (Table 3, electronic supplementary Fig. 3c). On adjusted Cox regression analyses, there was no significant impact of smoking on OS, CSS, and RFS, as presented in Table 4. In multivariable analyses modeling the interaction between receipt of neoadjuvant therapy and smoking status, there were no survival differences in ex-smokers or never smokers, by neoadjuvant therapy status, for OS, CSS, and RFS.
Table 4.
Subset analyses, by tumor histology, on the impact of smoking on long-term overall, cancer-specific, and recurrence-free survival following transthoracic esophagectomy for esophageal cancers
| No. of patients | Median survival, months | Univariable HR (95% CI) | Multivariable HR (95% CI) | |
|---|---|---|---|---|
| Adenocarcinoma | ||||
| Overall survival | ||||
| Current | 205 | 37.9 (28.9–56.0) | REF | REF |
| Ex-smoker | 428 | 42.0 (36.9–52.6) | 0.89 (0.73–1.09), p = 0.260 | 0.94 (0.75–1.18), p = 0.585 |
| Never | 258 | 40.1 (33–56.2) | 0.87 (0.70–1.09), p = 0.232 | 1.00 (0.78–1.29), p = 1.000 |
| Cancer-specific survival | ||||
| Current | 205 | 55.0 (38.5–81.5) | REF | REF |
| Ex-smoker | 428 | 68.5 (49.7–170.5) | 0.83 (0.65–1.05), p = 0.115 | 0.86 (0.66–1.12), p = 0.272 |
| Never | 258 | 86.8 (45.1–NR) | 0.81 (0.62–1.05), p = 0.110 | 0.86 (0.64–1.16), p = 0.315 |
| Recurrence-free survival | ||||
| Current | 204 | 51.1 (42.7–NR) | REF | REF |
| Ex-smoker | 424 | NR (49.0–NR) | 0.89 (0.69–1.15), p = 0.373 | 0.91 (0.69–1.22), p = 0.540 |
| Never | 258 | NR (36.6–NR) | 0.88 (0.66–1.18), p = 0.401 | 0.97 (0.70–1.33), p = 0.844 |
| Squamous cell carcinoma | ||||
| Overall survival | ||||
| Current | 77 | 33.8 (28.6–47.2) | REF | REF |
| Ex-smoker | 102 | 51.6 (28.8–85.7) | 0.73 (0.51–1.04), p = 0.082 | 0.91 (0.60–1.38), p = 0.648 |
| Never | 98 | 96.2 (58.3–NR) | 0.48 (0.32–0.70), p < 0.001 | 0.77 (0.48–1.24), p = 0.278 |
| Cancer-specific survival | ||||
| Current | 77 | 46.7 (33.8–NR) | REF | REF |
| Ex-smoker | 102 | 68.2 (40.9–NR) | 0.81 (0.53–1.25), p = 0.342 | 1.10 (0.65–1.86), p = 0.711 |
| Never | 98 | 153.6 (96.2–NR) | 0.55 (0.35–0.88), p = 0.013 | 1.09 (0.60–1.98), p = 0.780 |
| Recurrence-free survival | ||||
| Current | 77 | 97.2 (48.2–NR) | REF | REF |
| Ex-smoker | 102 | NR (37.0–NR) | 0.91 (0.56–1.49), p = 0.716 | 1.04 (0.59–1.86), p = 0.882 |
| Never | 97 | NR (NR–NR) | 0.73 (0.43–1.22), p = 0.224 | 1.42 (0.75–2.68), p = 0.280 |
HR hazard ratio, CI confidence interval, NR not reached
Discussion
This single-center study analysis from a high-volume unit in patients undergoing TTE demonstrates that ongoing smoking significantly reduces long-term survival in patients with an SCC and also in those who receive unimodality surgery. However, a higher number of complications were seen in patients who received neoadjuvant treatment, although this does not translate to higher rates of anastomotic leaks or pulmonary complications, which might be expected, and likely reflects the overall deconditioning associated with neoadjuvant therapy.27 These findings are relevant in counseling patients preoperatively on their expected operative and oncological outcomes following surgery. Furthermore, interventions to reduce smoking are needed and prehabilitation pathways for these high-risk groups may improve outcomes in smokers and ex-smokers, as both groups have similar operative and oncological profiles compared with non-smokers.28
Although smoking is a well-established risk factor for esophageal SCC and adenocarcinoma,29 little is known about the impact of smoking on long-term survival. Until recently, most studies have focused on the relationship between smoking and cancer incidence.30–32 A nationwide case-control study in Sweden first reported that ex-smokers had a worse outcome for esophageal SCC, but a current smoker status was not statistically significant (hazard ratio 1.4, 95% confidence interval 0.7–2.8).33 In contrast, Sun et al.15 (n = 488 patients) demonstrated that smokers were associated with poor long-term survival, but not disease-free survival, following esophagectomy for esophageal SCC. In the present study, the survival of current smokers was significantly shorter than ex-smokers and non-smokers (median 35 vs. 43 vs. 48 months; p = 0.016); however, on adjusted analysis, there was no significant difference in long-term survival between smoking status in the entire cohort.
It is difficult to fully explain why patients with an SCC appeared to be more compromised by being current smokers than those with an adenocarcinoma. Underlying mechanisms on the impact of smoking on long-term survival is not well understood. Several mechanisms have been investigated to explain these findings. Recent data from the field of epigenetics show that active smoking is associated with an aberrant DNA methylation pattern, which is linked to carcinogenesis and poor oncological outcomes.34–36 Shui et al. demonstrated that aberrant DNA methylation in key gene promoters associated with active smoking can lead to tumor recurrence in patients with prostate cancer.34 Their study also suggested that this process might be reversible following smoking cessation.34 The most severe impact of smoking was found in patients treated with chemotherapy, which might be explained by the reduced chemosensitivity of esophageal cancer cells exposed to nicotine, previously described in in vitro research reports.37,38 However, in our study, there were no significant differences in survival between smokers and never smokers receiving neoadjuvant therapy even when stratified by tumor response, highlighting variability in the genetic landscape of these tumors.
This study has a number of limitations to address. First, the data on pack-year history could have been more comprehensive. It may be that a certain threshold exists where patients are significantly affected. Furthermore, it would be good to establish whether a specific time frame exists for ex-smokers where a benefit develops. Second, it was impossible to capture whether all patients had any advice or intervention for smoking cessation and the compliance rates during neoadjuvant therapy and/or surgery. Third, this study was not able to incorporate better definitions of smoking, such as the Brinkman index, to allow refined analysis. Finally, we were unable to capture whether recurrent smoking in patients who stopped smoking >6 weeks prior to surgery had an impact on long-term survival. Nevertheless, this study represents a large study evaluating the long-term impact of smoking on OS.
Conclusion
This study demonstrates that for patients with adenocarcinoma undergoing unimodality surgery, and for all SCC patients, current smokers have significantly poorer long-term survival compared with ex-smokers or non-smokers. However, there is no survival difference in patients who receive neoadjuvant therapy for adenocarcinoma, which is contrary to in vitro reports. This warrants additional investigation to further delineate the genetic landscape of esophageal cancers to identify high-risk groups that may warrant further multimodality therapy.
Supplementary Information
Below is the link to the electronic supplementary material.
Disclosure
None declared.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Grotenhuis BA, van Hagen P, Reitsma JB, et al. Validation of a nomogram predicting complications after esophagectomy for cancer. Ann Thorac Surg. 2010;90(3):920–925. doi: 10.1016/j.athoracsur.2010.06.024. [DOI] [PubMed] [Google Scholar]
- 2.Goense L, Meziani J, Ruurda JP, van Hillegersberg R. Impact of postoperative complications on outcomes after oesophagectomy for cancer. Br J Surg. 2019;106(1):111–119. doi: 10.1002/bjs.11000. [DOI] [PubMed] [Google Scholar]
- 3.Markar S, Gronnier C, Duhamel A, et al. The impact of severe anastomotic leak on long-term survival and cancer recurrence after surgical resection for esophageal malignancy. Ann Surg. 2015;262(6):972–980. doi: 10.1097/SLA.0000000000001011. [DOI] [PubMed] [Google Scholar]
- 4.Mantziari S, Hubner M, Demartines N, Schafer M. Impact of preoperative risk factors on morbidity after esophagectomy: is there room for improvement? World J Surg. 2014;38(11):2882–2890. doi: 10.1007/s00268-014-2686-9. [DOI] [PubMed] [Google Scholar]
- 5.Takeuchi H, Miyata H, Gotoh M, et al. A risk model for esophagectomy using data of 5354 patients included in a Japanese nationwide web-based database. Ann Surg. 2014;260(2):259–266. doi: 10.1097/SLA.0000000000000644. [DOI] [PubMed] [Google Scholar]
- 6.Schieman C, Wigle DA, Deschamps C, et al. Patterns of operative mortality following esophagectomy. Dis Esophagus. 2012;25(7):645–651. doi: 10.1111/j.1442-2050.2011.01304.x. [DOI] [PubMed] [Google Scholar]
- 7.Ferguson MK, Celauro AD, Prachand V. Prediction of major pulmonary complications after esophagectomy. Ann Thorac Surg. 2011;91(5):1494-1500; discussion 1500-1. doi:10.1016/j.athoracsur.2010.12.036 [DOI] [PubMed]
- 8.Griffin SM, Shaw IH, Dresner SM. Early complications after Ivor Lewis subtotal esophagectomy with two-field lymphadenectomy: risk factors and management. J Am Coll Surg. 2002;194(3):285–297. doi: 10.1016/S1072-7515(01)01177-2. [DOI] [PubMed] [Google Scholar]
- 9.Zingg U, Smithers BM, Gotley DC, et al. Factors associated with postoperative pulmonary morbidity after esophagectomy for cancer. Ann Surg Oncol. 2011;18(5):1460–1468. doi: 10.1245/s10434-010-1474-5. [DOI] [PubMed] [Google Scholar]
- 10.Dhungel B, Diggs BS, Hunter JG, Sheppard BC, Vetto JT, Dolan JP. Patient and peri-operative predictors of morbidity and mortality after esophagectomy: American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP), 2005–2008. J Gastrointest Surg. 2010;14(10):1492–1501. doi: 10.1007/s11605-010-1328-2. [DOI] [PubMed] [Google Scholar]
- 11.Yoshida N, Watanabe M, Baba Y, et al. Risk factors for pulmonary complications after esophagectomy for esophageal cancer. Surg Today. 2014;44(3):526–532. doi: 10.1007/s00595-013-0577-6. [DOI] [PubMed] [Google Scholar]
- 12.Kamarajah SK, Lin A, Tharmaraja T, et al. Risk factors and outcomes associated with anastomotic leaks following esophagectomy: a systematic review and meta-analysis. Dis Esophagus. 2020 doi: 10.1093/dote/doz089. [DOI] [PubMed] [Google Scholar]
- 13.Yoshida N, Baba Y, Hiyoshi Y, et al. Duration of smoking cessation and postoperative morbidity after esophagectomy for esophageal cancer: how long should patients stop smoking before surgery? World J Surg. 2016;40(1):142–147. doi: 10.1007/s00268-015-3236-9. [DOI] [PubMed] [Google Scholar]
- 14.Yoshida N, Nakamura K, Kuroda D, et al. Preoperative smoking cessation is integral to the prevention of postoperative morbidities in minimally invasive esophagectomy. World J Surg. 2018;42(9):2902–2909. doi: 10.1007/s00268-018-4572-3. [DOI] [PubMed] [Google Scholar]
- 15.Sun P, Chen C, Zhang F, et al. Combined heavy smoking and drinking predicts overall but not disease-free survival after curative resection of locoregional esophageal squamous cell carcinoma. Onco Targets Ther. 2016;9:4257–4264. doi: 10.2147/OTT.S104182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amin MB, Greene FL, Edge SB, et al. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin. 2017;67:7. doi: 10.3322/caac.21388. [DOI] [PubMed] [Google Scholar]
- 17.Allum WH, Stenning SP, Bancewicz J, Clark PI, Langley RE. Long-term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J Clin Oncol. 2009;27(30):5062–5067. doi: 10.1200/JCO.2009.22.2083. [DOI] [PubMed] [Google Scholar]
- 18.Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355(1):11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 19.Kamarajah SK, Navidi M, Wahed S, et al. Anastomotic leak does not impact on long-term outcomes in esophageal cancer patients. Ann Surg Oncol. 2020 doi: 10.1245/s10434-020-08199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Phillips AW, Hardy K, Navidi M, et al. Impact of lymphadenectomy on survival after unimodality transthoracic esophagectomy for adenocarcinoma of esophagus. Ann Surg Oncol. 2020;27(3):692–700. doi: 10.1245/s10434-019-07905-8. [DOI] [PubMed] [Google Scholar]
- 21.Mapstone N. Dataset for the histopahtological reporting of oesophageal carcinoma. 2007. Royal College of Pathologists (London).
- 22.STARSurg Collaborative. Impact of postoperative non-steroidal anti-inflammatory drugs on adverse events after gastrointestinal surgery. Br J Surg. 2014;101(11):1413-23. doi:10.1002/bjs.9614 [DOI] [PubMed]
- 23.STARSurg Collaborative. Multicentre prospective cohort study of body mass index and postoperative complications following gastrointestinal surgery. Br J Surg. 2016;103(9):1157-72. doi:10.1002/bjs.10203 [DOI] [PMC free article] [PubMed]
- 24.STARSurg Collaborative. Outcomes After Kidney injury in Surgery (OAKS): protocol for a multicentre, observational cohort study of acute kidney injury following major gastrointestinal and liver surgery. BMJ Open. 2016;6(1):e009812. doi:10.1136/bmjopen-2015-009812 [DOI] [PMC free article] [PubMed]
- 25.Kamarajah SK, Sonnenday CJ, Cho CS, et al. Association of adjuvant radiotherapy with survival after margin-negative resection of pancreatic ductal adenocarcinoma: a propensity-matched National Cancer Database (NCDB) Analysis. Ann Surg. 2021;273(3):587–594. doi: 10.1097/SLA.0000000000003242. [DOI] [PubMed] [Google Scholar]
- 26.Kamarajah SK, Newton N, Navidi M, et al. Long-term outcomes of clinical and pathological-staged T3 N3 esophageal cancer. Dis Esophagus. 2020;33(8):doz109. doi: 10.1093/dote/doz109. [DOI] [PubMed] [Google Scholar]
- 27.Navidi M, Phillips AW, Griffin SM, et al. Cardiopulmonary fitness before and after neoadjuvant chemotherapy in patients with oesophagogastric cancer. Br J Surg. 2018;105(7):900–906. doi: 10.1002/bjs.10802. [DOI] [PubMed] [Google Scholar]
- 28.Kamarajah SK, Bundred J, Weblin J, Tan BHL. Critical appraisal on the impact of preoperative rehabilitation and outcomes after major abdominal and cardiothoracic surgery: a systematic review and meta-analysis. Surgery. 2020;167(3):540–549. doi: 10.1016/j.surg.2019.07.032. [DOI] [PubMed] [Google Scholar]
- 29.Lagergren J, Smyth E, Cunningham D, Lagergren P. Oesophageal cancer. Lancet. 2017;390(10110):2383–2396. doi: 10.1016/S0140-6736(17)31462-9. [DOI] [PubMed] [Google Scholar]
- 30.Ramus JR, Gatenby PA, Caygill CP, Watson A, Winslet MC. The relationship between smoking and severe dysplastic disease in patients with Barrett's columnar-lined oesophagus. Eur J Cancer Prev. 2012;21(6):507–510. doi: 10.1097/CEJ.0b013e328350b06f. [DOI] [PubMed] [Google Scholar]
- 31.Lee CH, Lee KW, Fang FM, et al. The neoplastic impact of tobacco-free betel-quid on the histological type and the anatomical site of aerodigestive tract cancers. Int J Cancer. 2012;131(5):E733–E743. doi: 10.1002/ijc.27401. [DOI] [PubMed] [Google Scholar]
- 32.Coleman HG, Bhat S, Johnston BT, McManus D, Gavin AT, Murray LJ. Tobacco smoking increases the risk of high-grade dysplasia and cancer among patients with Barrett's esophagus. Gastroenterology. 2012;142(2):233–240. doi: 10.1053/j.gastro.2011.10.034. [DOI] [PubMed] [Google Scholar]
- 33.Sundelof M, Lagergren J, Ye W. Patient demographics and lifestyle factors influencing long-term survival of oesophageal cancer and gastric cardia cancer in a nationwide study in Sweden. Eur J Cancer. 2008;44(11):1566–1571. doi: 10.1016/j.ejca.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 34.Shui IM, Wong CJ, Zhao S, et al. Prostate tumor DNA methylation is associated with cigarette smoking and adverse prostate cancer outcomes. Cancer. 2016;122(14):2168–2177. doi: 10.1002/cncr.30045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee KW, Pausova Z. Cigarette smoking and DNA methylation. Front Genet. 2013;4:132. doi: 10.3389/fgene.2013.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaz AM, Grady WM, Stachler MD, Bass AJ. Genetic and epigenetic alterations in Barrett's Esophagus and Esophageal adenocarcinoma. Gastroenterol Clin North Am. 2015;44(2):473–489. doi: 10.1016/j.gtc.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hamano R, Miyata H, Yamasaki M, et al. Overexpression of miR-200c induces chemoresistance in esophageal cancers mediated through activation of the Akt signaling pathway. Clin Cancer Res. 2011;17(9):3029–3038. doi: 10.1158/1078-0432.CCR-10-2532. [DOI] [PubMed] [Google Scholar]
- 38.Yoshioka A, Miyata H, Doki Y, et al. The activation of Akt during preoperative chemotherapy for esophageal cancer correlates with poor prognosis. Oncol Rep. 2008;19(5):1099–1107. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.