Introduction
Coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has spread worldwide since December 2019. Despite optimized medical treatment, 6% to 10% of patients with end-stage COVID-19 pneumonia progress to acute respiratory distress syndrome (ARDS) requiring mechanical ventilation in 5% to 12% and Extra Corporeal Membrane Oxygenation (ECMO) support in less than 0,5% [1,2].
The place of lung transplantation (LT) as a therapeutic option has rarely been reported in ARDS and even less for COVID-19. Only a few cases have been reported in this setting [3-6]. The candidate selection and the timing of the LT procedure remains very questionable.
We report the first French case of lung transplantation for a patient affected by COVID-19 pneumonia with refractory ARDS and irreversible lung destruction.
Case report
On September 15th 2020, a 59-year-old man was admitted for dyspnea with a positive SARS-Cov2 nasopharyngeal swab in real time Real Time Polymerase Chain Reaction (RT-PCR). The patient was a non-smoker with an uneventful medical history, except for a body mass index (BMI) of 30 kg/m2. On first examination respiratory frequency was 30/min, oxygen arterial saturation was 85% with a partial arterial pressure of oxygen of 52 mmhg with ambient air. He was immediately transferred to the Intensive Care Unit (ICU) and treated with non-invasive ventilation. He received Dexamethasone (6 mg /24 h from day 2 to 10). The patient was intubated at day 5. Despite prone positioning, the patient was referred to the regional centre to benefit from a veno-venous femoro-jugular ECMO (25 French and 19 French, Quadrox I Adult Getingeࣨ, blood flow 5l/min and sweep gas flow 6l/min FiO2 100%).
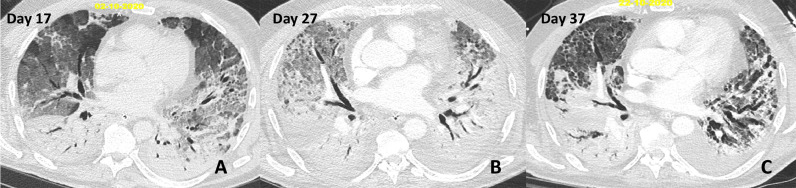
On day 17, lung CT scan showed persistent bilateral, symmetrical pulmonary opacification with anteroposterior gradient, including dense consolidation in the dependant areas and ground glass opacities in the non-dependant lung consistent with early ARDS (Fig. 1). Following negativisation of SARS-Cov2 RT-PCR and morphological CT Scan evolution with worsening ground glass opacities associated with reticulations in the non-dependant regions a treatment with Methylprednisolone bolus of 1 g for 3 days was started on day 27. On day 37, he remained fully dependent on the Veno-Venous (VV) ECMO support. Clinically, lung compliance was poor (10ml/cmH20) and imagery revealed partial resolution of the ground glass opacities, coarse reticular pattern in the anterior aerated lung with traction bronchiectasis suggesting underlying fibrosis, multiple small and coalescent pulmonary cysts probably related to prolonged ventilation and no sign of pulmonary embolism (Fig. 1 ).
Fig. 1.
Chest CT scans, axial images with parenchymal window at day 17 (A), day 27 (B) and day 37 (C).
(A) Bilateral, symmetrical pulmonary opacification with anteroposterior gradient, including dense consolidation in the dependant areas and ground glass opacities in the non-dependant lung consistent with early ARDS.
(B) Worsening ground glass opacities associated with a few reticulations in the non-dependant regions. No evidence of underlying fixed fibrosis (normal appearance of the bronchial tree).
(C) Partial resolution of the ground glass opacities. Coarse reticular pattern in the anterior, aerated lung with traction bronchiectasis suggesting underlying fibrosis. Multiple small and coalescent pulmonary cysts are probably related to prolonged ventilation.
Considering the onset of such irreversible morphological patterns despite maximal treatment, LT was considered as an option and discussed with our team. At that time, there was no extra-respiratory failure. Echocardiography showed normal heart function with a left ventricular ejection fraction of 70% and no pulmonary hypertension. Kidney, liver, and haematological functions were also normal. After a temporary sedation diminution, the patient was responding to simple orders. Nevertheless, a severe ICU acquired tetra-paresis was noted. A brain CT scan showed no evidence of intracerebral haemorrhage or ischaemic events. After this short evaluation, sedation had to be continued because of the severe respiratory failure. During this period, the patient developed three ventilator-associated pneumonias with bacteraemia involving Staphylococcus aureus, Enterobacter aerogenes and Citrobacter koseri successfully treated by antibiotics.
A videoconference between the patient's family and the lung transplant team was held to explain risks and benefits. The patient was transferred to Foch hospital for complete assessment at day 42. A PET CT scan (18F fdg) and a coronary angiography were performed and did not find any particularity. The final team decision to perform a LT was based on the five considerations: irreversible lung destruction under maximal support, single organ failure, patient without underlying disease, no more SARS-COV2 replication (Two successive BAL specimen negative RT-PCR), the patient's family signed informed consent for LT. At day 43, the patient was listed on the high emergency lung transplant (HELT) program, according to French regulations to prioritize graft allocation to patients with short-term severe prognoses [7].
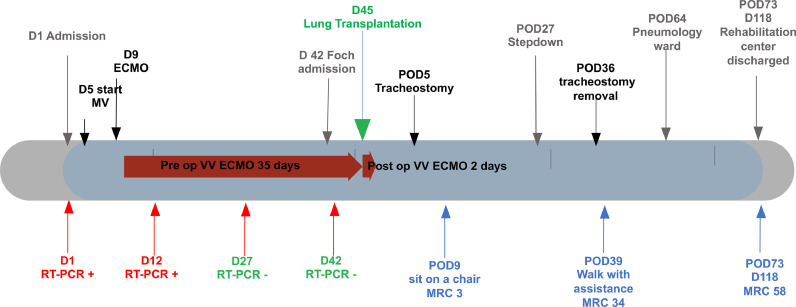
A sequential bilateral lung transplantation was performed at day 45. The surgical approach was a double anterolateral thoracotomy. VV ECMO was switched to a VVA ECMO which is our routine clinical practice. Central cannulation in the ascending aorta was performed through right thoracotomy. Femoral and jugular cannulas were used for venous drainage. We used the standardized anaesthetic management previously described [8]. Haemodynamic monitoring was done by continuous transoesophageal echography. Explantation was challenging due to the fragility of the tissues and bleeding. Ten blood packs, 2 platelet units and 8 fresh frozen plasma packs were transfused. At the end of the procedure, despite a chest Xray (Fig. 2 ) showing moderate infiltrates, lack of oedema during bronchoscopy and good heart function the patient remained hypoxic. For this reason, Central VVA ECMO was switched to peripheral femoro-jugular VV ECMO just in backup. The VV ECMO was weaned on Post-operative Day (POD) 2 and primary graft dysfunction grading at 72 h was 0. An early percutaneous tracheostomy was performed at POD 5 to help ventilation weaning. The first neuromuscular evaluation was done on POD 11 and showed severe neuromyopathy. The neuromuscular Medical Research Council strength score (MRC) sum score was quoted as 3/60 [9]. Then an intensive physiotherapy program was started. He was sitting on POD 9 and started walking on POD 39 (MRC sum 34).
Fig. 2.
A: Chest x-rays the day of inscription on transplant waiting list (on day 43). B: Immediate Chest x-ray after lung transplantation. C: Discharge Chest x-ray POD 73 (on day 118).
No specific alloimmunization was identified. Basiliximab induction and triple immunosuppression was initiated, including tacrolimus, mycophenolate mofetil, and steroids. Systematic lung biopsy following our protocol [10] revealed mild cellular acute rejection at POD 38 treated by steroid booster.
The total post-operative ventilation duration was 40 days. He left the ICU at POD 27 and stayed in the stepdown unit until POD 54. He was discharged to the rehabilitation centre on POD 73 with 58 MRC score (Fig. 3 ) and lung function evaluation showing: Forced expiratory volume for one second (FEV1): 3.18l (96%) and vital capacity (VC) 3,64l (83 %).
Fig. 3.
Clinical Time line (D: Day, MV: Mechanical Ventilation, ECMO: ExtraCorporeal Membrane Oxygenation, VV: Veno-Venous, RT-PCR: Sars cov 2 Real Time Polymerase Chain Reaction, POD: Post Operative Day, MRC: Medical Research Council sum neuromuscular score
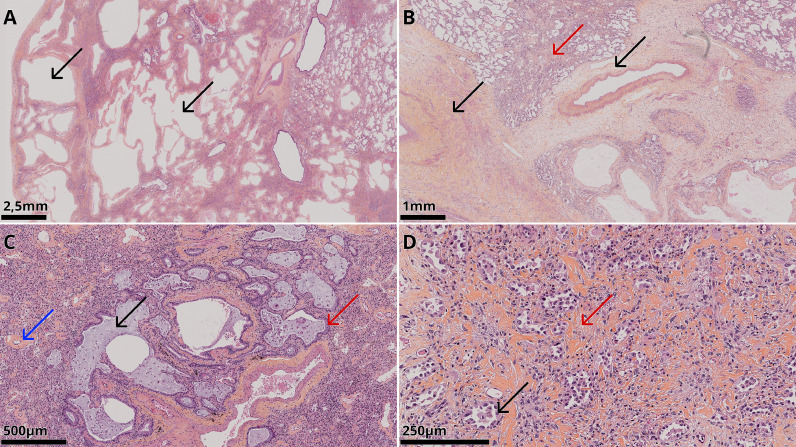
Of note, native lung specimens were pathologically examined (Fig. 4 ). The lung architecture was globally preserved with lesions that were temporally and geographically homogenous and suggestive of a fibrotic histological pattern without necrosis or thrombosis.
Fig. 4.
Microscopic images of haematoxylin and eosin staining of the explanted lungs.
A: chronic scarring with architectural destruction in the form of a microcystic appearance (black arrows) of the pulmonary parenchyma due to dilated alveolar spaces.
B: significant vascular changes with a significant amount of new vessel growth and fibrous and oedematous thickening of the media (black arrows) with very few thrombi. Interstitial fibrosis (red arrow).
C: traction bronchiectasis (black arrow) with peribronchiolar metaplasia (red arrow) and vascular changes with fibrosis thickening of small vessel walls (blue arrow).
D: interstitial fibrosis (red arrow) with mild to moderate interstitial inflammation composed of lymphocytes and plasma cells. Type II hyperplasia of the pneumocytes (black arrow).
Discussion
Here we describe the first French case of LT due to irreversible ARDS associated with COVID-19. To date, a few cases have already been published in China [5,3], USA [11] and Austria [4].
Acute respiratory insufficiency is not a classical indication for pulmonary transplantation. However, ARDS management has considerably changed in the last decade thanks to the possibilities of respiratory assistance by extracorporeal life support (ECLS). This strategy allows support for lung dysfunction and gives time for the lung to heal. Several patients have been weaned after extended periods [12]. However, prolonged ECMO is an invasive therapy that exposes the patients to many risks or complications which can be life-threatening.
Despite this maximal therapy, some patients develop irreversible lung injury and cannot be weaned from ECLS. This poor evolution could lead to death after therapy withdrawal. As it restores lung function, LT has been considered for a selected group of patients with refractory ARDS. However, only a few cases of LT for ARDS have already been reported [[13], [14], [15], [16], [17]]. Data, from the UNOS registry was recently published and suggested interesting results with a 63% survival rate three years after LT [18]. The current COVID-19 pandemic is responsible for a great increase in patients with ARDS and the mortality of the sickest patients needing ECLS is around 40 % at 90 days [19,20].
Ethical considerations are a major concern in this situation of ARDS because the possibilities of spontaneous lung healing are very unpredictable and fatal complications after 30 days of ECMO assistance are frequent. However, some patients are stabilized on ECMO with irreversible lung damage and without other organ dysfunction. For them LT should be a salvage therapy but challenging, with outcomes poorly known and a dilemma considering the shortage of suitable donors and standard recipients on the waiting list whose results could be better [21]. These considerations highlight two major points: first, to recognize the irreversible nature of the lung injury; second, to find the compromise between the moment when the lung lesions are irreversible and the moment when prolonged ECMO and ICU complication preclude the feasibility of LT.
In our case, radiologically, the initial ground glass opacities and condensation moved on to a fibrosis pattern despite high dose steroid treatment. This made us consider progression towards a non-reversible nature of the lung damage. This was confirmed by histological examination with air space enlargement, fibrosis without thrombosis. We note that this patterns were very different from the Austrian case where they observed necrosis and thrombosis [4].
Neurological evaluation was challenging in this specific disease. In this report, a short awakening testing and brain CT scan were possible. In case of neurologic doubt, functional imagery could be interesting but VV ECMO does not allow this strategy and brain CT probably remains the best solution. Moreover, the assessment of the muscles (strength, mass) is crucial before LT. As noted by Cypel et al. physical rehabilitation on an ECMO bridge improves outcomes in such patients [22]. But here, it appeared to be non-feasible according to the deepness of hypoxaemia.
The severe ICU-acquired paralysis and malnutrition were a considerable challenge for post-operative rehabilitation and mechanical ventilation weaning [23]. Good early graft function allowed an aggressive physiotherapeutic and nutritional program, and we observed a rapid improvement of MRC score. At discharge he had remarkable lung function and he was fully autonomous for walking.
According to ISHLT 2006, potential candidates should be well informed and demonstrate adequate healthy behavior and a willingness to adhere to guidelines from health care professionals [24]. Due to the patient's clinical status, it was not possible for him to sign informed consent. According to French law in case of emergency, his wife, after being well informed about the risks and benefits, gave consent for him to be treated.
During the surgery, the dissection showed significant tissue fragility and bleeding related to coagulopathy of prolonged ECMO as previously described [4]. We usually advocate femoral VA ECMO support because it has several advantages in our practice, including an ability to provide optimal respiratory and hemodynamic support. In this way, ECMO allows a partial bypass of the pulmonary circulation and then decreases mechanical shear stress, pulmonary reperfusion and pressure which are recognized as PGD risk factors. In this case, with destruction of the lung, we chose a central cannulation for the artery to avoid Harlequin syndrome.
Our case indicates that lung transplantation may be a dedicated option for strictly selected patients with refractory ARDS secondary to SARS-COV2 disease. Also, this pandemic condition creates a momentum to consider a high selective indication of LT in acute lung disease.
Disclosure
None
Funding
None
Data availability statement
None
Acknowledgments
The authors acknowledged the Foch Lung Transplant Group for its help in patients’ care and Polly Gobin for her linguistic help.
References
- 1.Guan W-J, Ni Z-Y, Hu Y, Liang W-H, Ou C-Q, He J-X, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA. 2020;323(20):2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen J-Y, Qiao K, Liu F, Wu B, Xu X, Jiao G-Q, et al. Lung transplantation as therapeutic option in acute respiratory distress syndrome for coronavirus disease 2019-related pulmonary fibrosis. Chin Med J. 2020;133(12):1390–1396. doi: 10.1097/CM9.0000000000000839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lang C, Jaksch P, Hoda MA, Lang G, Staudinger T, Tschernko E, et al. Lung transplantation for COVID-19-associated acute respiratory distress syndrome in a PCR-positive patient. Lancet Respiratory. 2020;8(10):1057–1060. doi: 10.1016/S2213-2600(20)30361-1. Oct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Han W, Zhu M, Chen J, Zhang J, Zhu S, Li T, et al. Lung transplantation for elderly patients with end-stage COVID-19 pneumonia. Ann Surg. 2020;272(1):e33–e34. doi: 10.1097/SLA.0000000000003955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bharat A, Machuca TN, Querrey M, Kurihara C, Garza-Castillon RJ, Kim S, et al. Early outcomes after lung transplantation for severe COVID-19: a series of the first consecutive cases from four countries. Lancet Respiratory. 2021;9(5):487–497. doi: 10.1016/S2213-2600(21)00077-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roux A, Beaumont-Azuar L, Hamid AM, De Miranda S, Grenet D, Briend G, et al. High emergency lung transplantation: dramatic decrease of waiting list death rate without relevant higher post-transplant mortality. Transpl Int. 2015;28(9):1092–1101. doi: 10.1111/tri.12604. [DOI] [PubMed] [Google Scholar]
- 8.Fessler J, Godement M, Pirracchio R, Marandon J-Y, Thes J, Sage E, et al. Inhaled nitric oxide dependency at the end of double-lung transplantation: a boosted propensity score cohort analysis. Transpl Int. 2019;32(3):244–256. doi: 10.1111/tri.13381. [DOI] [PubMed] [Google Scholar]
- 9.Kleyweg RP, van der Meché FG, Schmitz PI. Interobserver agreement in the assessment of muscle strength and functional abilities in Guillain-Barré syndrome. Muscle Nerve. 1991;14(11):1103–1109. doi: 10.1002/mus.880141111. [DOI] [PubMed] [Google Scholar]
- 10.Roux A, Sage E, Cerf C, Le Guen M, Picard C, Hamid AM, et al. Évolution et progrès en transplantation pulmonaire: étude de la cohorte de 600 premiers patients transplantés pulmonaires à l'hôpital Foch. Revue des Maladies Respiratoires. SPLF; 2019;36(2):142–154. doi: 10.1016/j.rmr.2018.02.014. [DOI] [PubMed] [Google Scholar]
- 11.Bharat A, Querrey M, Markov NS, Kim S, Kurihara C, Garza-Castillon R, et al. Lung transplantation for patients with severe COVID-19. Sci Transl Med. 2020;12(574) doi: 10.1126/scitranslmed.abe4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Combes A, Hajage D, Capellier G, Demoule A, Lavoué S, Guervilly C, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med. 2018;378(21):1965–1975. doi: 10.1056/NEJMoa1800385. [DOI] [PubMed] [Google Scholar]
- 13.Jurmann MJ, Schaefers HJ, Demertzis S, Haverich A, Wahlers T, Borst HG. Emergency lung transplantation after extracorporeal membrane oxygenation. ASAIO Journal. 1993;39(3):M448–M452. doi: 10.1097/00002480-199307000-00059. [DOI] [PubMed] [Google Scholar]
- 14.Brichon PY, Barnoud D, Pison C, Perez I, Guignier M. Double lung transplantation for adult respiratory distress syndrome after recombinant interleukin 2. Chest. 1993;104(2):609–610. doi: 10.1378/chest.104.2.609. [DOI] [PubMed] [Google Scholar]
- 15.Licker M, Schweizer A, Hohn L, Morel DR, Spiliopoulos A. Single lung transplantation for adult respiratory distress syndrome after paraquat poisoning. Thorax. 1998;53(7):620–621. doi: 10.1136/thx.53.7.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iacono A, Groves S, Garcia J, Griffith B. Lung transplantation following 107 days of extracorporeal membrane oxygenation. Eur J Cardiothorac Surg. 2010;37(4):969–971. doi: 10.1016/j.ejcts.2009.09.039. [DOI] [PubMed] [Google Scholar]
- 17.Salam S, Kotloff R, Garcha P, Krishnan S, Joshi D, Grady P, et al. Lung transplantation after 125 days on ECMO for severe refractory hypoxemia with no prior lung disease. ASAIO Journal. 2017;63(5):e66–e68. doi: 10.1097/MAT.0000000000000450. [DOI] [PubMed] [Google Scholar]
- 18.Harano T, Ryan JP, Chan EG, Noda K, Morrell MR, Luketich JD, et al. Lung transplantation for the treatment of irreversible acute respiratory distress syndrome. Clin Transplant. 2020:e14182. doi: 10.1111/ctr.14182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmidt M, Hajage D, Lebreton G, Monsel A, Voiriot G, Levy D, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome associated with COVID-19: a retrospective cohort study. Lancet Respir. 2020;8(11):1121–1131. doi: 10.1016/S2213-2600(20)30328-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barbaro RP, MacLaren G, Boonstra PS, Iwashyna TJ, Slutsky AS, Fan E, et al. Extracorporeal membrane oxygenation support in COVID-19: an international cohort study of the extracorporeal life support organization registry. The Lancet. 2020;396(10257):1071–1078. doi: 10.1016/S0140-6736(20)32008-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wadowski BJ, Bacchetta M, Kon ZN. Beware the Deus Ex machina of COVID-19. Ann Thorac Surg. 2020;110(6):1787–1788. doi: 10.1016/j.athoracsur.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cypel M, Keshavjee S. When to consider lung transplantation for COVID-19. Lancet Respir. 2020;8(10):944–946. doi: 10.1016/S2213-2600(20)30393-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Jonghe B, Bastuji-Garin S, Sharshar T, Outin H, Brochard L. Does ICU-acquired paresis lengthen weaning from mechanical ventilation? Intensive Care Med. 2004;30(6):1117–1121. doi: 10.1007/s00134-004-2174-z. [DOI] [PubMed] [Google Scholar]
- 24.Orens JB, Estenne M, Arcasoy S, Conte JV, Corris P, Egan JJ, et al. International guidelines for the selection of lung transplant candidates: 2006 update–a consensus report from the pulmonary scientific council of the international society for heart and lung transplantation. Vol. 25. J Heart Lung Transplant. 2006:745–755. doi: 10.1016/j.healun.2006.03.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
None