Abstract
Background:
The Characterizing CFTR modulated changes in Sweat Chloride and their Association with Clinical Outcomes (CHEC-SC) study is a large epidemiologic study designed to determine the relationship between sweat chloride response and clinical outcomes in people with cystic fibrosis (CF) on commercially approved CFTR modulators. A challenge to study feasibility was capturing sweat chloride measurements before modulator initiation. We tested the hypothesis that historic sweat chloride approximated contemporary pre-modulator values to estimate CFTR modulator-induced changes, allowing a single-visit study design.
Methods:
GOAL and PROSPECT were multi-center prospective studies of individuals initiating ivacaftor or lumacaftor-ivacaftor. At enrollment, pre-modulator sweat chloride was measured and historic results recorded. Post-modulator sweat chloride was measured at 1, 3 and 6 months. For this analysis, differences between historic and pre-modulator sweat chloride were estimated. CFTR modulator-induced sweat chloride mean changes were compared using historic and pre-modulator sweat chloride.
Results:
Paired historic and pre-modulator sweat chloride (n=406 participants) revealed a non-significant mean change of −1.0 mmol/L (95% CI: −2.71, 0.66) over an average of 17.2 years. Calculating sweat response to ivacaftor or lumacaftor-ivacaftor using historic or pre-modulator values resulted in similar estimates of modulator response. Based on these results, the CHEC-SC study was designed with a single, post-modulator sweat chloride measurement.
Conclusions:
Historic sweat chloride values provide a reliable estimate of pre-modulator sweat chloride for people starting on modulator therapy. The CHEC-SC Study anticipates capturing approximately 5,000 sweat chloride values, providing an unprecedented understanding of sweat chloride across the CF population in the era of CFTR modulators.
Keywords: sweat chloride, sweat test, CFTR modulator, ivacaftor, lumacaftor-ivacaftor
I. Introduction
Cystic fibrosis (CF) is a life-shortening genetic disease arising from dysfunction or absence of an epithelial membrane anion channel, the CF transmembrane conductance regulator (CFTR)(1). In addition to pathologic consequences of CFTR protein dysfunction in the respiratory and gastrointestinal systems, reduced CFTR function in the sweat gland increases salt loss resulting in elevated sweat chloride concentrations(1–3). CFTR modulators increase CFTR protein presence and/or function at the apical surface membrane; four CFTR modulators are currently approved in the US: ivacaftor, lumacaftor/ivacaftor, tezacaftor/ivacaftor and elexacaftor/tezacaftor/ivacaftor (4–8). Modulator-associated increases in mutant CFTR protein function correlate with clinical response to these therapeutics.
Sweat chloride is a robust biomarker of CFTR function with strong correlations between diagnostic sweat chloride, genotype and phenotype, and between changes in sweat chloride and functional restoration of CFTR with modulator treatment (4, 9, 10). At a population level across clinical trials, average decreases in sweat chloride and lung function improvement strongly correlate (6). At the individual level, relationships between sweat chloride change and lung function improvement are less robust, possibly because clinical efficacy measures used for CF drug approvals (e.g. lung function improvement over months) may not be robust surrogates of CF lung disease progression (11–14). Ideally, modulator-associated sweat chloride values or associated changes in sweat chloride could be compared with corresponding rates of lung function decline and mortality risk over years to assess the viability of post-modulator sweat chloride as a predictor of long-term outcome.
Over 90% of individuals with CF in the US are followed in the CF Foundation Patient Registry (CFFPR) (15). As people with CF begin treatment with newly available CFTR modulators, an opportunity exists to study relationships between modulator-associated sweat chloride values, disease progression, and mortality using long-term clinical outcomes data in the CFFPR. However, the resources required to consent and enroll thousands of participants who might start modulator treatment and timing a pre-modulator sweat test proximate to modulator initiation are daunting.
We hypothesized that such a study could be realized with a single study visit among individuals followed in the CFFPR on CFTR modulator treatment, provided that their diagnostic sweat chloride concentration (most likely obtained early in infancy) is a reasonable estimate of their sweat chloride measurement taken immediately prior to modulator treatment initiation. In this report, we describe how CF diagnostic sweat chloride concentrations compare with those collected from subjects immediately prior to modulator treatment from two large naturalistic CFTR modulator studies (13, 14), and how this analysis informed the design of a large, pragmatic study of the effects of CFTR modulators on sweat chloride concentrations and disease progression, allowing a prospective link to be made with sweat chloride, a proxy for CFTR activity, and long term clinical outcome: the Characterizing CFTR modulated changes in Sweat Chloride and their Association with Clinical Outcomes (CHEC-SC) Study.
II. Methods
GOAL and PROSPECT were prospective, multi-center observational studies conducted across 46 centers within the CF Therapeutics Development Network (TDN) that sought to evaluate changes in biologic and clinical outcome measures among cohorts of CF patients prescribed ivacaftor or lumacaftor/ivacaftor, respectively. Details of these studies have been previously published (13, 14) and are available on clinicaltrials.gov (NCT01521338 and NCT02477319). Clinical, demographic, and sweat chloride concentration data at the time of enrollment into GOAL or PROSPECT, and just prior to CFTR modulator use, were collected for this study. Sweat testing at enrollment in the GOAL and PROSPECT studies were performed using the Macroduct® collection system and processed at the TDN Center for Sweat Analysis located at Children’s Hospital Colorado. Sweat testing was performed at 1, 3 and 6 months following CFTR modulator initiation. Standardized procedures for sweat induction, collection, shipment and chloride measurement were followed. Sweat was collected from left and right arm concurrently, and the average sweat chloride value of the two samples used for analysis. Corresponding historic sweat chloride values recorded at the time of CF diagnosis, with local site collection methods and laboratory analysis, were obtained as available from the US CFFPR and was linked to all GOAL and PROPSECT participants. This retrospective study was approved by the IRB at Seattle Children’s Hospital.
Statistical analysis: Differences between historic and pre-modulator study enrollment in GOAL or PROSPECT sweat chloride values were estimated with corresponding 95% confidence intervals (CIs) by mutation cohort and modulator therapy across studies and among study participants with both values available. Mutation specific cohorts for GOAL included: G551D/other (Cohort 1), R117H/other (Cohort 2), and Non-G551D gating mutation/other (Cohort 3), and for PROSPECT included F508del homozygous participants. The PROSPECT study also included an additional F508del heterozygous cohort who did not initiate CFTR modulators, but for whom enrollment sweat chloride data was collected. Corresponding estimates of the CFTR modulator-induced changes in sweat chloride (SC response) are provided with 95% CIs utilizing both historic and enrollment sweat chloride values as the pre-modulator baseline value.
III. Results
Utilization of Historic Sweat Chloride Values to Motivate a Streamlined CHEC-SC Study Design
Subject characteristics of the 406 individuals with CF (n=208 GOAL and 198 PROSPECT participants) included in the retrospective analysis are shown in Table 1 by genotype cohort.
Table 1.
Subject characteristics of those included in retrospective analyses comparing historic and enrollment sweat chloride values. Additional details of the GOAL and PROSPECT cohorts were previously published (13, 14).
| Total N= 406 individuals with paired samples | GOAL (Cohort 1: G551D/Other) (N=131) | GOAL (Cohort 2: R117H/other) (N=59) | GOAL (Cohort 3: Non-G551D Gating/other) (N=18) | PROSPECT (F508del homozygous) (N= 139) | PROSPECT (F508del heterozygous) (N= 59) |
|---|---|---|---|---|---|
|
| |||||
| Female, N (%) | 60 (45.8%) | 21 (35.6%) | 7 (38.9%) | 76 (54.7%) | 30 (50.8%) |
|
| |||||
| Age (years) at Enrollment: | |||||
| Mean (SD) | 20.9 (10.41) | 29.8 (19.97) | 21.3 (10.83) | 20.8 (10.24) | 26.3 (12.62) |
| Min, Max | 6.0,59.4 | 6.0,72.9 | 8.6,45.5 | 6.7,57.6 | 12.3,66.8 |
|
| |||||
| Age (years) at Historic SC: | |||||
| Mean (SD) | 4.7 (7.23) | 15.9 (19.82) | 7.1 (7.91) | 1.9 (4.80) | 6.9 (10.77) |
| Min, Max | 0.0,42.6 | 0.0,69.0 | 0.1,25.0 | 0.0,33.7 | 0.0,45.6 |
|
| |||||
| Time (years) from Historic SC to Enrollment: | |||||
| Mean (SD) | 16.2 (9.26) | 13.9 (12.37) | 14.2 (8.64) | 18.9 (9.28) | 19.4 (10.17) |
| Min,Max | 0.1,44.0 | 0.1,54.9 | 1.3,31.0 | 4.7,47.8 | 3.9,49.2 |
|
| |||||
| FEV1 % Predicted at Enrollment: | |||||
| Mean (SD) | 79.9 (24.78) | 85.8 (20.65) | 69.8 (23.03) | 82.8 (24.12) | 79.8 (25.13) |
| Min, Max | 22.9,138.2 | 31.7,118.9 | 22.2,113.8 | 20.6,132.9 | 34.5,138.8 |
Comparison of Historic and Enrollment Sweat Chloride Values
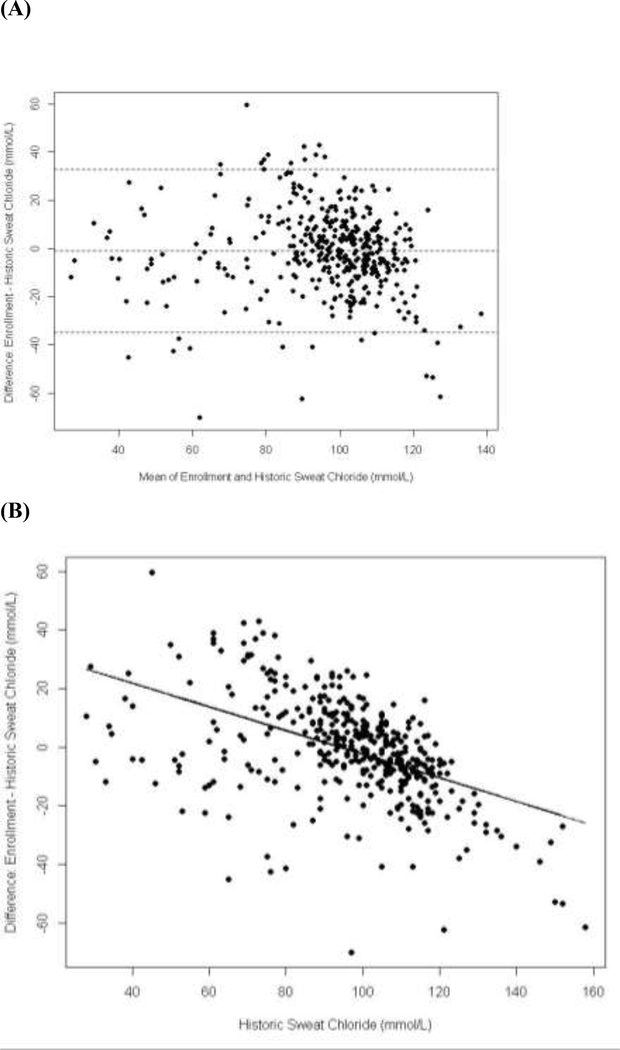
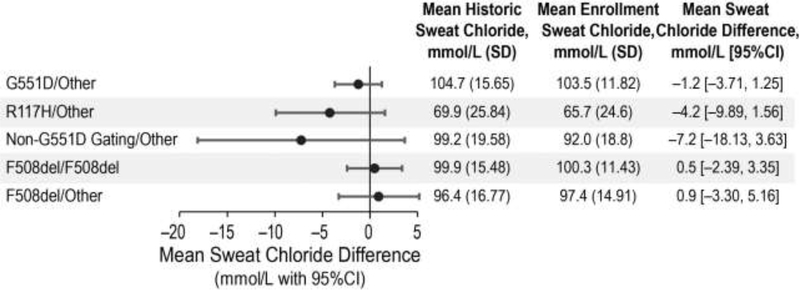
Figure 1 displays the differences between historic and enrollment sweat chloride populations across the population, with indication of a regression to the mean effect whereby higher historic values were associated with greater decreased change with respect to the enrollment value whereas the converse was also evident. For most of the study population, mean changes in sweat chloride values across the one to two decades between diagnosis and study enrollment prior to modulator initiation were not biologically or statistically significant (Figure 2). There were no significant systematic associations across cohorts in difference between historic and enrollment sweat chloride values and patient age or time between measurements. There was a minimal and non-significant mean change of −1.2 mmol/L in the G551D-CFTR cohort (95% CI −3.7,1.3) over an average 16.2 years, 0.5 mmol/L (95% CI −2.4,3.4) over an average 18.9 years in the homozygous F508del-CFTR cohort and 0.9 mmol/L (95% CI −3.3,5.2) over an average 19.4 years in the heterozygous F508del-CFTR cohort. Variability in historic and enrollment sweat chloride means were higher for the R117H-CFTR and non-G551D gating mutation cohorts due to small sample sizes and expected phenotypic diversity in the R117H-CFTR cohort related to variable poly T status. In these cohorts, historic sweat chloride values also trended higher than those at baseline, likely reflecting potential bias with the recording of the greatest sweat chloride values needed to confirm diagnosis in these milder mutation groups. Mean change in sweat chloride between diagnosis and study enrollment prior to modulator initiation for these cohorts was higher with wider confidence intervals but was not statistically significant [R117H-CFTR mean change −4.2 (95%CI −9.89, 1.56) and non-G551D-CFTR mean change −7.2 (95%CI 18.13,3.63), Figure 2]. Historic sweat chloride measurements, performed at local clinics and laboratories as opposed to a standardized collection technique and central lab, had higher variability across all mutation cohorts as compared to baseline sweat chloride performed in the context of a standardized research procedure (Figure 2).
Figure 1.
(A) Bland-Altman plot of the differences between historic and enrollment sweat chloride values. (B) Difference between historic and enrollment sweat chloride values vs. historic value with corresponding linear regression line.
Figure 2:
Mean difference between Historic and Pre-modulator Enrollment sweat chlorides in GOAL and PROSPECT Cohorts.
Comparison of Modulator-Associated Sweat Chloride Response using Historic versus Enrollment Values
Table 2 shows the impact on sweat chloride change of substituting historic sweat chloride values for enrollment sweat chloride values by mutation class. Substituting the historic values to calculate response resulted in minimal changes to estimates of modulator response. Further, Table 2 demonstrates the relative stability of the average sweat chloride response across time from 1 to 6 months post-modulator. These results indicate that for the purpose of estimating population, but not necessarily individual, changes in sweat chloride in response to CFTR modulators, the historic sweat chloride value serves as a robust alternative that can streamline data collection and improve feasibility for prospective, large population-based studies. Further, sweat chloride appears highly stable over time after initiation of modulator therapy, enabling efficient and time-independent sampling across a population of individuals with CF already on CFTR modulators (4). This is also consistent with results from multiple clinical efficacy trials of CFTR modulators (8, 16, 17).
Table 2.
Post-modulator changes in sweat chloride utilizing enrollment and historic sweat chloride values for pre-modulator baseline values.
| GOAL (Cohort 1: G551D/other) (N=131) | GOAL (Cohort 2: R117H/other) (N=59) | GOAL (Cohort 3: Non-G551D Gating/other) (N=18) | PROSPECT (F508del homozygous) (N= 91) | |
|---|---|---|---|---|
|
| ||||
| 1-month Post Modulator SC Change | ||||
|
| ||||
| N with SC post-modulator | 116 | 41 | 14 | 84 |
|
| ||||
| Change using SC (mmol/L) at Enrollment: | ||||
| Mean (SD) | −47.5 (20.1) | −20.4 (14.8) | −56.3 (17.8) | −18.1 (15.3) |
| 95% CI | (−51.2, −43.8) | (−25, −15.7) | (−66.6, −46.1) | (−21.5, −14.8) |
|
| ||||
| Change using Historic SC (mmol/L): | ||||
| Mean (SD) | −48.8 (23.7) | −26.6 (22.1) | −61 (22.2) | −19.2 (19.2) |
| 95% CI | (−53.2, −44.4) | (−33.5, −19.6) | (−73.9, −48.2) | (−23.3, −15) |
|
| ||||
| 3-month Post Modulator SC Change | ||||
|
| ||||
| N with SC post-modulator | 111 | 37 | 15 | 76 |
|
| ||||
| Change using SC (mmol/L) at Enrollment: | ||||
| Mean (SD) | −53.0 (21.1) | −23 (15.2) | −52.6 (24.1) | −16.8 (17.7) |
| 95% CI | (−57, −49.1) | (−28, −17.9) | (−65.9, −39.3) | (−20.8, −12.7) |
|
| ||||
| Change using Historic SC (mmol/L): | ||||
| Mean (SD) | −54.2 (24.2) | −29.2 (21.4) | −56.2 (28.5) | −18.5 (19.2) |
| 95% CI | (−58.8, −49.6) | (−36.3, −22) | (−72, −40.5) | (−22.9, −14.1) |
|
| ||||
| 6-month Post Modulator SC Change | ||||
|
| ||||
| N with SC post-modulator | 110 | 41 | 14 | 58 |
|
| ||||
| Change using SC (mmol/L) at Enrollment: | ||||
| Mean (SD) | −53.2 (22.6) | −25.6 (16.1) | −42.6 (38.2) | −16.4 (16.9) |
| 95% CI | (−57.5, −48.9) | (−30.7, −20.5) | (−64.7, −20.6) | (−20.8, −12) |
|
| ||||
| Change using Historic SC (mmol/L): | ||||
| Mean (SD) | −55.3 (26.2) | −30.7 (19.8) | −52.3 (38.5) | −17.2 (19.9) |
| 95% CI | (−60.2, −50.3) | (−37, −24.5) | (−74.5, −30) | (−22.5, −12) |
Use of Historic Sweat Tests in the CHEC-SC Study Design
CHEC-SC was designed as a multicenter, cross-sectional, cohort study to collect contemporary sweat chloride measurements from approximately 5000 CF patients receiving commercially approved CFTR modulator therapies. The primary objective of CHEC-SC is to comprehensively describe patterns of sweat chloride response to CFTR modulators and post-modulator sweat chloride levels across a representative cohort of CF patients receiving CFTR modulator therapy, and to characterize responses according to different CFTR modulators and across patient groups defined by genotype, age, weight, disease severity, and disease stage. Secondary objectives are to determine the relationship between sweat chloride values and long-term clinical outcomes including lung function, pulmonary exacerbations, airway microbiology and body mass index (BMI).
Data from our analysis of GOAL and PROSPECT historic and enrollment sweat values supported the concept that historic sweat chloride values, available in the CFFPR, provide a reliable estimate of baseline pre-modulator sweat chloride for people starting on modulator therapy. This finding negated the need for pre-modulator sweat chloride measurements for patients entering the CHEC-SC study and offered significant streamlining of the study design. In addition, as absolute sweat chloride values may be more important to determining phenotype and predicted long-term outcome than change in sweat chloride, measuring sweat chloride in those on modulator therapy has relevance even in the absence of diagnostic data or in more rare and mild genotype cohorts for which the historic sweat chloride may be more variable. Our study also provides estimates for a bias adjustment factor that can be considered in sensitivity analyses among the rare genotypes for which the historic sweat chloride may be downward biased on average.
CHEC-SC Study Design Overview
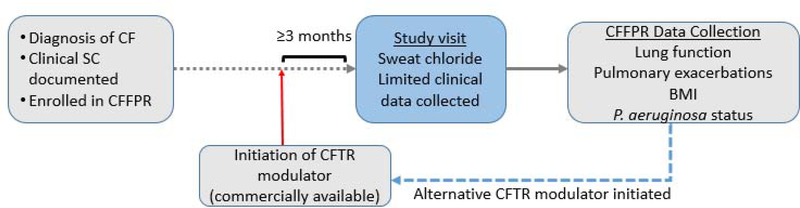
Eligible subjects who have been prescribed and taking a commercially approved CFTR modulator for at least 3 months will be enrolled for a single visit coinciding with a clinical care visit to collect sweat to be analyzed for sweat chloride at their local site laboratory. Limited clinical data obtained at this visit is augmented by retrospective and prospective data obtained from the CFFPR. Study subjects who have been prescribed and switch to an alternative commercially approved CFTR modulator will be approached to re-enroll in the study after being on the alternative modulator for at least 3 months so that a new sweat chloride value can be obtained (Figure 3).
Figure 3. CHEC-SC Study Design.
Individuals with CF are eligible if on a commercially available CFTR modulator for at least three months. A single study visit is completed with sweat chloride measurement and data collection. Data from the CFF Patient Registry is collected to determine long term outcomes. Patients may re-enroll if an alternative CFTR modulator is initiated.
Sample Size Rationale for the CHEC-SC Study
The current sample size is driven by the need to ensure sampling of a generalizable patient population across genotypes, age groups, gender, race and ethnicity, and disease stage. An additional key goal of the study will be to determine whether different sweat chloride response profiles to CFTR modulators are associated with differing long-term clinical outcomes, including rate of lung function decline. A total of 5000 sweat chloride collections across the CF population will enable cohorts of sufficient size to perform key statistical analyses requiring at least 500 unique participants per cohort. A recent study estimated the average rate of decline in forced expiratory volume over one second (FEV1) % predicted among 455 F508del homozygous patients receiving lumacaftor/ivacaftor to be −1.33 percentage points per year (SD=5.12) (18). For simplicity, we assume that we wish to compare two cohorts on CFTR modulators within our study identified with distinctively different sweat chloride response profiles associated with initiation of modulator therapy. To detect an average 0.9% annual difference in FEV1 decline among the group with greater sweat chloride response as compared to those with lesser sweat chloride response, nearly 500 patients per group will be needed to provide 80% power with a two-sided type 1 error of 0.05.
IV. Discussion
The advent of CFTR modulator therapy with increasing availability to individuals with CF marks a huge milestone in the treatment of CF. Sweat chloride values, the diagnostic goldstandard for CF since the late 1950’s, are poised to shift within the population with use of CFTR modulators. The CHEC-SC study reflects a large-scale study aimed at characterizing the changes in sweat chloride and how these changes reflect the population- both by explaining heterogeneity in response to therapy and the relation between response and clinical phenotype. The success of this study hinges on its ability to enroll large numbers of people representative of the CF population on CFTR modulator therapy across many sites. When initially conceived, collection of pre-modulator sweat chloride values at enrollment was perceived as a major barrier to a large epidemiologic study of sweat chloride changes, particularly given the rapid uptake of CFTR modulators following each new regulatory approval. Using retrospective data to determine the variability and change with age of sweat chloride values, we determined that diagnostic sweat chloride values, available for almost 90% of people with CF in the CFFPR(19), provides an adequate estimate of sweat chloride prior to treatment with CFTR modulators, thus negating the need for a pre-modulator sweat chloride measurement at enrollment. In populations with rare and phenotypically diverse CFTR-mutations, we found more variability in sweat chloride measurements and a bias towards higher historic sweat chloride values resulting in higher estimates of change, although the magnitude of the bias was small compared to the average treatment effect. Larger samples sizes as expected in the CHEC-SC study will also reduce some of the variability of sample means. In order to reduce this bias, future studies including CHEC-SC should consider including all historic sweat chloride measurements rather than selecting a single value to estimate changes.
The finding that historic sweat chloride measurements could substitute for pre-modulator enrollment measurements without substantially altering the estimate of sweat chloride change after modulator initiation made possible using CFFPR and available clinical trials data, allowed the development of a streamlined study design for the CHEC-SC study. Alternative study designs were considered for CHEC-SC including a longitudinal study enrolling fewer individuals with more frequent sweat chloride measurements and clinical data collection. Published data supports that sweat chloride values post-modulator initiation are highly stable within individuals, thus values obtained at one point in time can be reasonably extrapolated to represent the sweat chloride value of the individual as long as CFTR modulation therapy remains consistent (4) (8, 16, 17).. In addition, the CFFPR provides longitudinal data from before and after initiation of modulators. We acknowledge that observational data from the CFFPR may not be as robust as data collected prospectively during a clinical study. Thus, at study visits we are also collecting data including genotype, diagnostic sweat chloride values, dates of modulator treatments, and lung function at enrollment. Additional longitudinal data from CFFPR will add to our ability to measure associations with sweat chloride changes.
We found relatively high inter-individual variability in sweat chloride values for historic sweat chloride, consistent with prior reports, and at least in part due to differences in collection site and laboratories.(20, 21) Inter-individual variability was lower for sweat chloride measurements performed as part of GOAL and PROSPECT clinical studies which used standardized operating procedures, a consistent method of sweat collection, and a central laboratory. Variability was higher than previously reported in the F508del homozygous and G551D cohorts.(22, 23) Variability was higher in the R117H cohort congruent with differing phenotypes and CFTR activity with this mutation.(24, 25) We demonstrate however that in a population sized epidemiologic study such as CHEC, the larger variability with historic sweat chloride is mitigated by larger patient numbers, thus the effect sizes for the change in sweat chloride can still be estimated with adequate precision for interpretability. While we found little average difference between historic and enrollment sweat chloride values across cohorts, intraindividual differences were larger. Thus, these results should not be interpreted as negating the need for repeat sweat testing when clinically indicated. Previous studies have found coefficients of variation generally <10% for between and within-subject sweat chloride measurements; changes in sweat chloride of at least 10% within a population are likely to represent clinically meaningful differences in CFTR activity. (17, 23, 26, 27) Variability in sweat chloride values following ivacaftor treatment in the phase 2 clinical trials was also low particularly within-patient suggesting that changes following modulator therapy are stable.(4) Sweat chloride measurements from multiple clinical efficacy trials of CFTR modulators show similar stability with time (8, 16, 17). A strength of our study design is that the planned sample size will be sufficient to address any increased variance observed with historical SC collection methods.
Age has also been proposed to impact sweat chloride values, although we did not find this in our data. Collaco and colleagues examined changes with age in sweat chloride collected locally for observational studies including 2,678 sweat chloride values from 1,761 individuals.(21) Mean sweat chloride values increased slightly with age with mean sweat chloride of 99 mmol/L in F508del-CFTR homozygotes under age 1 year up to a mean of 106 mmol/L in those over 3 years of age. Our study relied on recording of historic sweat results from the CFFPR possibly resulting in bias (e.g. recording highest diagnostic values at the time of study enrollment) and included a wider range of ages at the time of historic sweat testing. The mean age at time of testing was also older than that reported by Collaco, thus age-related changes in sweat chloride may be limited to the first year or two of life.
Limitations of our data analysis include the reliance on historic results documented in the CFFPR and small cohorts. However, our intent was to determine the utility of relying on real world measurements rather than samples collected as part of a standardized clinical trial. By using historic values as our pre-modulator baseline values in CHEC-SC, we expect that variability will be higher but the large number of participants who can be enrolled with the stream-lined study design will help to overcome this limitation. In studies of CFTR modulators to date, the relationship between sweat chloride and clinical outcomes is unclear. Across populations, change in sweat chloride correlates well with improvement in lung function.(6) For example, larger mean changes in sweat chloride in response to ivacaftor in those with the G551D-CFTR mutation was associated with larger improvements in lung function compared to changes in sweat chloride and lung function in those homozygous for the F508del-CFTR mutation treated with lumacaftor/ivacaftor. However, in individuals, sweat chloride has relatively poor correlation to lung function response.(11, 12) In comparison to previous studies, CHEC-SC has the potential to enroll large numbers of participants on modulator therapy and to determine the relationship between sweat chloride values and long-term clinical outcomes by collecting longitudinal data through the CFFPR.
V. Conclusions
Using data from the CFFPR and well characterized clinical study cohorts, we found comparability between historic and pre-modulator baseline sweat chlorides when determining average sweat chloride response to modulation across a large population. This finding allows an efficient study design for CHEC-SC which is now poised to be the largest epidemiologic study of sweat chloride changes across the CF population in the era of CFTR modulators Results from CHEC-SC, combined with long-term data from the CFFPR, will allow us to determine the relationship between modulator-associated sweat chloride values and long term disease outcomes, a critical metric for continued advances in CFTR restoration.
Highlights.
Sweat chloride elevation is a key criterion to diagnose cystic fibrosis
CFTR modulators decrease sweat chloride and improve outcomes in many people with CF
CHEC-SC is a large study designed to measure sweat chloride response to modulators
Historic sweat chloride values estimate contemporary pre-modulator values without systematic bias
Thus, a single measurement is adequate to capture sweat changes in a large population
Acknowledgements:
The authors acknowledge the GOAL and PROSPECT clinical research study teams, participants and families and the Cystic Fibrosis Foundation Center for Sweat Analyses for sweat measurements obtained in GOAL and PROSPECT.
Funding support: Funding support: This work was supported by funding from the Cystic Fibrosis Foundation grants ZEMANI17Y5 (ETZ), ZEMANI17K0 (ETZ), and HAMBLE17K0 (NMH) and National Institutes of Health (NIH) grants P30 DK 089507 (NMH), UL1 TR002319 (NMH), R35 HL135816 (SMR), UL1TR003096 (SMR), P30DK072482 (SMR), UL1TR002548 (MWK), P01HL128192 (MWK).
Abbreviations:
- CFTR
Cystic fibrosis transmembrane conductance regulator
- GOAL
G551D Observational Study clinical study (NCT01521338)
- PROSPECT
A Two-Part Multicenter Prospective Longitudinal Study of CFTR-dependent Disease Profiling in Cystic Fibrosis clinical study (NCT02477319)
- CHEC-SC
Characterizing CFTR modulated changes in Sweat Chloride and their Association with Clinical Outcomes clinical study (NCT03350828)
- CFFPR
Cystic Fibrosis Foundation Patient Registry
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: ETZ, NMH, DRV, SMR, MWK: grants or consulting CFF; DRV consults for aMoon, Arrevus, Eloxx, Enbiotix, Felix, Ionis, Matinas, Merck, Polyphor, Respirion, Savara; SMR consults for Vertex; MWK consults for Anthera, AzurRx, Celtaxsys, Chiesi, Ionis, Kala, Laurent, Merck, Paranta, pH Pharma, Santhera, Vertex; JPC, KOD and MS: none.
References
- 1.Elborn JS. Cystic fibrosis. Lancet. 2016;388(10059):2519–31, 10.1016/S01406736(16)00576-6. [DOI] [PubMed] [Google Scholar]
- 2.Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. N Engl J Med. 2005;352(19): 1992–2001., 10.1056/NEJMra043184. [DOI] [PubMed] [Google Scholar]
- 3.Farrell PM, White TB, Ren CL, Hempstead SE, Accurso F, Derichs N, et al. Diagnosis of cystic fibrosis: Consensus guidelines from the Cystic Fibrosis Foundation. J Pediatr. 2017;181S:S4–S15 e1, 10.1016/j.jpeds.2016.09.064. [DOI] [PubMed] [Google Scholar]
- 4.Accurso FJ, Van Goor F, Zha J, Stone AJ, Dong Q, Ordonez CL, et al. Sweat chloride as a biomarker of CFTR activity: Proof of concept and ivacaftor clinical trial data. J Cyst Fibros. 2014;13(2): 139–47, 10.1016/j.jcf.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vermeulen F, Le Camus C, Davies JC, Bilton D, Milenkovic D, De Boeck K. Variability of sweat chloride concentration in subjects with cystic fibrosis and G551D mutations. J Cyst Fibros. 2017;16(1):36–40, 10.1016/j.jcf.2016.02.015. [DOI] [PubMed] [Google Scholar]
- 6.Fidler MC, Beusmans J, Panorchan P, Van Goor F. Correlation of sweat chloride and percent predicted FEV1 in cystic fibrosis patients treated with ivacaftor. J Cyst Fibros. 2017; 16(1):41–4. 10.1016/j.jcf.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Heijerman HGM EF; Downey DG; Van Braeckel E; Rowe SM; Tullis E et al. Efficacy and safety of the elexacaftor/tezacaftor/ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: a double-blind, randomised, phase 3 trial. Lancet. 2019;395(10238):1694, 10.1016/S0140-6736(19)32597-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Middleton PG, Mall MA, Drevinek P, Lands LC, McKone EF, Polineni D, et al. Elexacaftor-tezacaftor-ivacaftor for cystic fibrosis with a single Phe508del allele. N Engl J Med. 2019;381(19): 1809–19, 10.1056/NEJMoa1908639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muhlebach MS, Clancy JP, Heltshe SL, Ziady A, Kelley T, Accurso F, et al. Biomarkers for cystic fibrosis drug development. J Cyst Fibres. 2016;15(6):714–23, 10.1016/j.jcf.2016.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCague AF, Raraigh KS, Pellicore MJ, Davis-Marcisak EF, Evans TA, Han ST, et al. Correlating cystic fibrosis transmembrane conductance regulator function with clinical features to inform precision treatment of cystic fibrosis. Am J Respir Crit Care Med. 2019; 199(9): 1116–26., 10.1164/rccm.201901-0145OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Durmowicz AG, Witzmann KA, Rosebraugh CJ, Chowdhury BA. Change in sweat chloride as a clinical end point in cystic fibrosis clinical trials: the ivacaftor experience. Chest. 2013;143(1): 14–8, 10.1378/chest.l2-1430. [DOI] [PubMed] [Google Scholar]
- 12.Aalbers BE, Hofland RW, Bronsveld I, de Winter-de Groot KM, Arets HGM, de Kiviet AC, et al. Females with cystic fibrosis have a larger decrease in sweat chloride in response to lumacaftor/ivacaftor compared to males. J Cyst Fibres. 2020, 10.1016/j.jcf.2020.05.004. [DOI] [PubMed] [Google Scholar]
- 13.Sagel SD, Khan U, Heltshe SF, Clancy JP, Borowitz D, Gelfond D, et al. Clinical effectiveness of lumacaftor/ivacaftor in cystic fibrosis patients homozygous for F508del-CFTR. Ann Am Thorac Soc. 2020, 10.1513/AnnalsATS.202002-144OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rowe SM, Heltshe SF, Gonska T, Donaldson SH, Borowitz D, Gelfond D, et al. Clinical mechanism of the cystic fibrosis transmembrane conductance regulator potentiator ivacaftor in G55ID-mediated cystic fibrosis. Am J Respir Crit Care Med. 2014; 190(2): 175–84, 10.1164/rccm.201404-0703OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knapp EA, Fink AK, Goss CH, Sewall A, Ostrenga J, Dowd C, et al. The Cystic Fibrosis Foundation Patient Registry. Design and methods of a national observational disease registry. Ann AmThorac Soc. 2016; 13(7): 1173–9, 10.1513/AnnalsATS.201511-781OC. [DOI] [PubMed] [Google Scholar]
- 16.Ramsey BW, Davies J, McElvaney NG, Tullis E, Bell SC, Drevinek P, et al. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med. 2011;365(18): 1663–72, 10.1056/NEJMoa1105185 [doi],. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taylor-Cousar JL, Munck A, McKone EF, van der Ent CK, Moeller A, Simard C, et al. Tezacaftor-ivacaftor in patients with cystic fibrosis homozygous for Phe508del. N Engl J Med. 2017;377(21):2013–23, 10.1056/NEJMoa1709846. [DOI] [PubMed] [Google Scholar]
- 18.Konstan MW, McKone EF, Moss RB, Marigowda G, Tian S, Waltz D, et al. Assessment of safety and efficacy of long-term treatment with combination lumacaftor and ivacaftor therapy in patients with cystic fibrosis homozygous for the F508del-CFTR mutation (PROGRESS): a phase 3, extension study. The Lancet Respiratory Medicine. 2017;5(2): 107–18, 10.1016/s22132600(16)30427-1. [DOI] [PubMed] [Google Scholar]
- 19.Cystic Fibrosis Foundation Patient Registry. 2018 Annual Data Report. Bethesda, Maryland.: @2019 Cystic Fibrosis Foundation; 2019. [Google Scholar]
- 20.Traeger N, Shi Q, Dozor AJ. Relationship between sweat chloride, sodium, and age in clinically obtained samples. J Cyst Fibres. 2014;13(l):10–4, 10.1016/j.jcf.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 21.Collaco JM, Blackman SM, Raraigh KS, Corvol H, Rommens JM, Pace RG, et al. Sources of variation in sweat chloride measurements in cystic fibrosis. Am J Respir Crit Care Med. 2016; 194(11): 1375–82, 10.1164/rccm.201603-04590C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vermeulen F, Le Camus C, Davies JC, Bilton D, Milenkovic D, De Boeck K. Variability of sweat chloride concentration in subjects with cystic fibrosis and G551D mutations. J Cyst Fibros. 2016, 10.1016/j.jcf.2016.02.015. [DOI] [PubMed] [Google Scholar]
- 23.LeGrys VA, Moon TC, Laux J, Rock MJ, Accurso F. Analytical and biological variation in repeated sweat chloride concentrations in clinical trials for CFTR modulator therapy. J Cyst Fibros. 2017;17(l):43–9, 10.1016/j.jcf.2017.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Espel JC, Palac HL, Bharat A, Cullina J, Prickett M, Sala M, et al. The relationship between sweat chloride levels and mortality in cystic fibrosis varies by individual genotype. J Cyst Fibros. 2018; 17(l):34–42, 10.1016/j.jcf.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 25.Cirilli N, Raia V, Rocco I, De Gregorio F, Tosco A, Salvadori L, et al. Intra-individual biological variation in sweat chloride concentrations in CF, CFTR dysfunction, and healthy pediatric subjects. Pediatr Pulmonol. 2018;53(6):728–34, 10.1002/ppul.23992. [DOI] [PubMed] [Google Scholar]
- 26.Clancy JP, Rowe SM, Accurso FJ, Aitken ML, Amin RS, Ashlock MA, et al. Results of a phase Ha study of VX-809, an investigational CFTR corrector compound, in subjects with cystic fibrosis homozygous for the F508del-CFTR mutation. Thorax. 2012;67( 1): 12–8, 10.1136/thoraxjnl-2011-200393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rowe SM, McColley SA, Rietschel E, Li X, Bell SC, Konstan MW, et al. Lumacaftor/ivacaftor treatment of patients with cystic fibrosis heterozygous for F508del-CFTR. Ann AmThorac Soc. 2017;14(2):213–9, 10.1513/AnnalsATS.201609-6890C. [DOI] [PMC free article] [PubMed] [Google Scholar]