Abstract
Case report describing a patient infected with COVID-19 after initiation of Cladribine, with a favorable disease course and positive seroconversion.
Keywords: COVID-19, Cladribine, Multiple sclerosis, Seroconversion, Neuroimmunology
1. Introduction
Multiple sclerosis (MS) is a chronic immune-mediated disease of the central nervous system that usually requires long-term immunotherapy. In recent years, the number of FDA-approved disease-modifying therapies (DMTs) are gradually increasing, but effective treatment options for secondary progressive MS (SPMS) are limited and, in comparison to relapsing-remitting MS (RRMS), ineffective in preventing disease progression.(Correale et al., 2017) Emerging therapies provide possible alternative for patients with progressive disease (Macaron and Ontaneda, 2019). Due to the immunosuppressive mechanisms of several of the DMTs, previous studies have shown a higher prevalence of infections among treated MS patients, associated with higher rates of hospitalization and mortality compared to the general population.(Montgomery et al., 2013; Steelman, 2015) The patient described in this case report received Cladribine therapy, a DMT approved for use in patients with relapsing forms of MS, to include RRMS and SPMS, (Rocha Cabrero and Morrison, 2020) with frequently observed lymphopenia in patients undergoing treatment.(Cook et al., 2019) The patient was infected with COVID-19 1 month following initiation of Cladribine treatment.
During the emergence of the COVID-19 pandemic, there were significant health concerns for patients with MS. Initially, the main concern was whether immunosuppressive therapy, potentially exposing patients to an increased risk for acquiring viral infection would result in unfavorable outcomes and questioning the possible discontinuance of highly immunosuppressive therapies during an active infection.(Giovannoni et al., 2020; Louapre et al., 2020; Sormani et al., 2021) Now, with wide-spread administration of the vaccines for COVID-19, concerns have been raised related to the possibility of an inadequate immune response to vaccination in patients on certain DMTs.(Zheng et al., 2020) Due to these concerns, managing MS during the COVID-19 pandemic presents a major clinical dilemma. In this case report we describe a patient with MS who received oral Cladribine therapy, developed an active COVID-19 infection, and had a favorable outcome with an initial positive test to COVID-19 IgG titers.
2. Case report
A 54-year-old male patient diagnosed with SPMS in 2010, with initial relapsing MS diagnosis in 2001. He presented with a single episode of optic neuritis in 1989 without further inquiry, until diagnosis was established due to urinary incontinence in 2001 along with a supporting radiologically demyelinating findings on brain and spinal MRI (Polman et al., 2011). At this time intramuscular interferon beta-1a was initiated as preventative therapy.
Interferon beta-1a therapy was ineffective with recurrent relapses and gradual disability progression over the course of 10 years. Therefore, interferon beta-1a therapy was discontinued in 2011, with EDSS evaluated at 5.5. Other DMTs were Natalizumab, initiated in 2012 and discontinued in 2014 due to gradual deterioration under treatment; replaced with Fingolimod from 2014 and discontinued in September 2019 due to paroxysmal atrial fibrillation.(Rolf et al., 2014) Additional medical conditions included dry eye syndrome and medications included Dalfampridine and Metoprolol for Atrial Fibrillation rate control, without necessitating anticoagulative therapy. His last available Complete Blood-Count (CBC), from January 2020 showed normal white blood cell count of 6.6 K/uL (normal 4–10), and mild lymphopenia of 0.8 K/uL (normal 1.1–3). Brain MRI revealed no significant lesion dynamic since 2014–2021 (Fig. 1 ). Despite several referrals, no spinal MRI has been performed since 2011. Due to ongoing clinical deterioration therapy with Cladribine was recommended even though no relapse or MRI activity was documented. (See Fig. 2.)
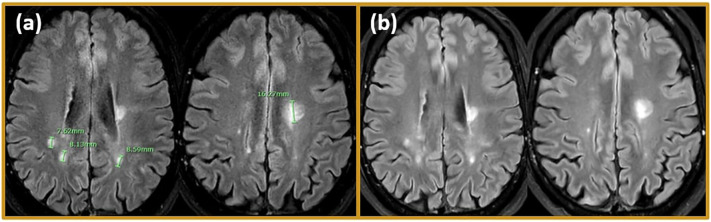
Fig. 1.
MRI findings. Representative MRI scans are shown. (a) Follow up MRI on December 26, 2018, 3 T imaging: T2 Flair showed periventricular hyperintense lesions bilaterally, and a hyperintense signal surrounding white matter around the posterior horns of the lateral ventricles. Hyperintense lesions were seen on T2WI in the left cerebellum. No lesion enhancement after gadolinium administration, overall MRI is stable compared previous MRI on September 28, 2017. (b) 2 months after initiating Cladribine treatment follow up MRI on April 16, 2020. 3 T imaging was stable, without new gadolinium enhancing lesions on T1, and FLAIR showed no dynamic in existing lesions or new formed lesions.
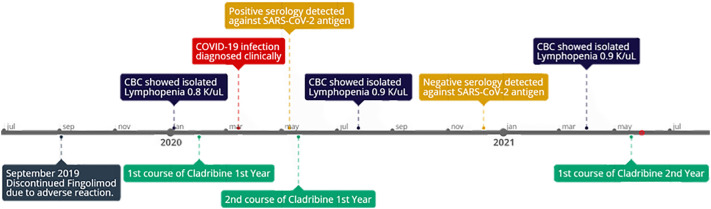
Fig. 2.
Timeline, following patient course from initial administration of Cladribine to COVID-19 infection. Seroconversion to IgG positive against COVID-19 was under 2 months, secondary seroconversion was detected >250 days post initial COVID-19 infection.
In February 2020, the first course of treatment with Cladribine tablets was initiated. The patient received 1st month, 1st year course of 70 mg over a 5-day period, per accepted treatment protocol. Prior to administration, neurological examination showed no cognitive impairment, language and speech conserved, no evidence of cranial nerve, and no facial motor or sensory impairment. Slight bilateral cupping in pronator drift was detected. Upper body muscle tone and strength were normal. Lower limbs were able to contract and provide some resistance, with bilateral asymmetrical hyperreflexia. Light touch, pinprick, proprioception, and vibration were intact. Rapid alternating movements and fine finger movements were intact with bilateral dysmetria. Patient had spastic gait, was unable to stand without 2-person support and wheelchair dependent due to unstable gait. In addition, he was diagnosed with neurogenic bladder. Accumulative EDSS was 7. MRI acquired prior to starting Cladribine, showed a moderate lesion burden and no gadolinium-enhancing lesion (Fig. 1). After the first treatment with Cladribine, the patient did not experience clinical or MRI worsening.
In March 2020, the patient exhibited respiratory symptoms, shortness of breath, fever, and loss of smell. He did not get tested for COVID-19 and the diagnosis was based on clinical symptoms. His mild symptoms ceased completely after a course of 21 days.
50 days after COVID-19 symptom onset, and 92 days after the first treatment course of Cladribine, serology for COVID-19 IgG was found positive (Abbot, Architect SARS-CoV-2 IgG (specificity 99.6%, sensitivity 100% >21 days post symptom onset (Manalac et al., 2020)). The second course of treatment with Cladribine was delayed an additional 21 days beyond the protocol spacing between the first and second course (51 days total between the first and second dose) and administered in May 2020. Follow-up CBC 1.5 months after the second Cladribine dose revealed normal white blood cell count of 6 K/uL and persisting lymphopenia 0.9 K/uL (similar to previous results). Nine months after COVID-19 diagnosis, on December 2020, he tested negative for COVID19 IgG serology (Abbot, Architect SARS-CoV-2 IgG).
In April 2021 the patient still exhibited mild lymphopenia 0.9 K/uL. In May 2021 the patient received 1st month, and a month later the 2nd year course of Cladribine, is planned. The course consisted of 70 mg over a 5-day period, per accepted treatment protocol (Fig. 2).
3. Discussion
We present here a patient with a progressive MS treated with Cladribine tablets and suffered a mild COVID-19 infection. Following the disease, he tested positive to COVID-19 IgG. The patient had lymphopenia that might be due to previous immunosuppressive medication (Nagy et al., 2020) that persist with no major change following the first course of Cladribine, yet only experienced a mild COVID-19 disease course and was able to develop a COVID-19 specific antibody response. He did not require any respiratory support throughout the COVID-19 infection, although he did exhibit mild respiratory symptoms. Despite non-selective lymphopenia in the first 6 months after dosing,(Cook et al., 2019) recently emerging data suggests that patients with MS treated with Cladribine tablets and who acquire COVID-19 are not at more risk of a severe outcome. There are multiple records of patients who experienced a mild disease course as described per our patient (De Angelis et al., 2020; Dersch et al., 2020; Jack et al., 2020). Also, reports of positive IgG serology proximal to infection were recorded (Celius, 2020). In some cases, patients infected with COVID-19 were advised to delay the second week of oral Cladribine if the infection occurred between the second and first weeks of treatment (Year 1 or Year 2), similar to the delay in the second week treatment for our patient, renewed after convalescence (Jack et al., 2020). The patient we describe showed initially adequate humoral mediated response, with post-infectious COVID-19 IgG recorded positive after 50 days from symptom onset. Later on, 9 months following COVID-19 infection, the serology was negative. In healthy individuals infected with COVID-19, the detection of antibodies within 20 days from infection was reported in several studies.(Figueiredo-Campos et al., 2020; Hartley et al., 2020; Maine et al., 2020) Hartley et al.,(Hartley et al., 2020) described that neutralizing antibody titers were highest in patients sampled approximately 20 days post-symptom onset and also showed a decline in specific IgG levels over time. Decline in IgG serum antibodies has also been previously described in recent studies.(Figueiredo-Campos et al., 2020; Hartley et al., 2020; Maine et al., 2020) The same group described that the decline in serum antibodies, as per the later obtained negative serological test for IgG in our patient, 273 days post-COVID-19 symptom-onset, does not ultimately indicate lack of immune response and memory. While specific IgG level in serum declined with time, post-symptom onset, COVID-19 -specific B and T memory cell may persist in the circulation (Hartley et al., 2020). In a recent interesting work, Schwarzkopf et al., focused on a subgroup of individuals with PCR-confirmed COVID-19 infection who did not react positive to an S1-specific COVID-19 IgG ELISA test. This subgroup was 17% of the entire cohort. Studying T cell responses, they showed that after a median of 2 months post-symptom onset, 79% of negative serology participants had detectable T-cell immunity, against the S1 antigen (Schwarzkopf et al., 2021).
The patient in the current report had rapid immune response and later decay of serum antibodies, and while previous studies have shown decay of antibodies over time, no current studies present follow up past 250 days (Hartley et al., 2020). Unfortunately, we have no serology data between 30 and 273 days post COVID-19 infection.
Despite initial humeral response, immune memory may be affected in patients treated with DMT's. IgG titers may not be the preferred method of confirming long-term immunity in DMT-treated patients, as seen in other subgroups of convalescent patients. Since the patient described in this case report was treated with Cladribine tablets, an immune reconstitution therapy, known to cause depletion of memory B cells, this could explain the later decay of antibodies.(Ceronie et al., 2018) This however is a premature conclusion since we lack data on longitudinal serology tests even in healthy untreated individuals.
4. Conclusion
In the current case report, we describe a positive serology test following COVID-19 infection in a Cladribine tablets-treated patient. The fact that the patient, even when lymphopenic, had a positive serology response is reassuring that an adequate immune response can be initiated toward COVID-19 in patients treated with Cladribine. In order to reassure MS patients, studies testing longitudinal serology and T cells responses to COVID-19 infection are needed both in MS patients and in healthy controls.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgements
None.
References
- Celius E.G. Normal antibody response after COVID-19 during treatment with cladribine. Mult. Scler. Relat. Disord. 2020 Nov;46:102476. doi: 10.1016/j.msard.2020.102476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceronie B., Jacobs B.M., Baker D., Dubuisson N., Mao Z., Ammoscato F., et al. Cladribine treatment of multiple sclerosis is associated with depletion of memory B cells. J. Neurol. 2018 May;265(5):1199–1209. doi: 10.1007/s00415-018-8830-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook S., Leist T., Comi G., Montalban X., Giovannoni G., Nolting A., et al. Safety of cladribine tablets in the treatment of patients with multiple sclerosis: an integrated analysis. Mult. Scler. Relat. Disord. 2019 Apr;29:157–167. doi: 10.1016/j.msard.2018.11.021. [DOI] [PubMed] [Google Scholar]
- Correale J., Gaitán M.I., Ysrraelit M.C., Fiol M.P. Progressive multiple sclerosis: from pathogenic mechanisms to treatment. Brain. 2017 Mar 1;140(3):527–546. doi: 10.1093/brain/aww258. [DOI] [PubMed] [Google Scholar]
- De Angelis M., Petracca M., Lanzillo R., Brescia Morra V., Moccia M. Mild or no COVID-19 symptoms in cladribine-treated multiple sclerosis: two cases and implications for clinical practice. Mult. Scler. Relat. Disord. 2020 Oct;45:102452. doi: 10.1016/j.msard.2020.102452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dersch R., Wehrum T., Fähndrich S., Engelhardt M., Rauer S., Berger B. COVID-19 pneumonia in a multiple sclerosis patient with severe lymphopenia due to recent cladribine treatment. Mult. Scler. 2020 Aug 7;26(10):1264–1266. doi: 10.1177/1352458520943783. [DOI] [PubMed] [Google Scholar]
- Figueiredo-Campos P., Blankenhaus B., Mota C., Gomes A., Serrano M., Ariotti S., et al. Seroprevalence of anti-SARS-CoV-2 antibodies in COVID-19 patients and healthy volunteers up to 6 months post disease onset. Eur. J. Immunol. 2020 Dec;50(12):2025–2040. doi: 10.1002/eji.202048970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannoni G., Hawkes C., Lechner-Scott J., Levy M., Waubant E., Gold J. The COVID-19 pandemic and the use of MS disease-modifying therapies. Mult. Scler. Relat. Disord. 2020 Mar 27;39:102073. doi: 10.1016/j.msard.2020.102073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley G.E., Edwards E.S.J., Aui P.M., Varese N., Stojanovic S., McMahon J., et al. Rapid generation of durable B cell memory to SARS-CoV-2 spike and nucleocapsid proteins in COVID-19 and convalescence. Sci. Immunol. 2020 Dec;22:5(54). doi: 10.1126/sciimmunol.abf8891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack D., Nolting A., Galazka A. Favorable outcomes after COVID-19 infection in multiple sclerosis patients treated with cladribine tablets. Mult. Scler. Relat. Disord. 2020 Aug 27;46:102469. doi: 10.1016/j.msard.2020.102469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louapre C., Collongues N., Stankoff B., Giannesini C., Papeix C., Bensa C., et al. Clinical characteristics and outcomes in patients with coronavirus disease 2019 and multiple sclerosis. JAMA Neurol. 2020 Sep 1;77(9):1079–1088. doi: 10.1001/jamaneurol.2020.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macaron G., Ontaneda D. Diagnosis and management of progressive multiple sclerosis. Biomedicines. 2019 Jul;29:7(3). doi: 10.3390/biomedicines7030056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maine G.N., Lao K.M., Krishnan S.M., Afolayan-Oloye O., Fatemi S., Kumar S., et al. Longitudinal characterization of the IgM and IgG humoral response in symptomatic COVID-19 patients using the Abbott architect. J. Clin. Virol. 2020 Oct 27;133:104663. doi: 10.1016/j.jcv.2020.104663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manalac J., Yee J., Calayag K., Nguyen L., Patel P.M., Zhou D., et al. Evaluation of Abbott anti-SARS-CoV-2 CMIA IgG and Euroimmun ELISA IgG/IgA assays in a clinical lab. Clin. Chim. Acta. 2020 Nov;510:687–690. doi: 10.1016/j.cca.2020.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery S., Hillert J., Bahmanyar S. Hospital admission due to infections in multiple sclerosis patients. Eur. J. Neurol. 2013 Aug;20(8):1153–1160. doi: 10.1111/ene.12130. [DOI] [PubMed] [Google Scholar]
- Nagy S., Kuhle J., Derfuss T. Lymphocyte recovery after fingolimod discontinuation in patients with MS. Neurol Neuroimmunol Neuroinflamm. 2020 Aug;14:7(6). doi: 10.1212/NXI.0000000000000874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polman C.H., Reingold S.C., Banwell B., Clanet M., Cohen J.A., Filippi M., et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann. Neurol. 2011 Feb;69(2):292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha Cabrero F., Morrison E.H. StatPearls Publishing; Treasure Island (FL): 2020. Cladribine. StatPearls. [PubMed] [Google Scholar]
- Rolf L., Muris A.-H., Damoiseaux J., van Daele M., Hupperts R. Paroxysmal atrial fibrillation after initiation of fingolimod for multiple sclerosis treatment. Neurology. 2014 Mar 18;82(11):1008–1009. doi: 10.1212/WNL.0000000000000218. [DOI] [PubMed] [Google Scholar]
- Schwarzkopf S., Krawczyk A., Knop D., Klump H., Heinold A., Heinemann F.M., et al. Cellular immunity in COVID-19 convalescents with PCR-confirmed infection but with undetectable SARS-CoV-2–specific IgG. Emerg. Infect. Dis. 2021 Jan;27(1):122–129. doi: 10.3201/2701.203772. [DOI] [PubMed] [Google Scholar]
- Sormani M.P., De Rossi N., Schiavetti I., Carmisciano L., Cordioli C., Moiola L., et al. Disease modifying therapies and Covid-19 severity in multiple sclerosis. Ann. Neurol. 2021 Jan;21 doi: 10.1002/ana.26028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steelman A.J. Infection as an environmental trigger of multiple sclerosis disease exacerbation. Front. Immunol. 2015 Oct 19;6:520. doi: 10.3389/fimmu.2015.00520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng C., Kar I., Chen C.K., Sau C., Woodson S., Serra A., et al. Multiple sclerosis disease-modifying therapy and the COVID-19 pandemic: implications on the risk of infection and future vaccination. CNS Drugs. 2020;34(9):879–896. doi: 10.1007/s40263-020-00756-y. [DOI] [PMC free article] [PubMed] [Google Scholar]