Abstract
Massive vaccination against COVID-19 has become a global priority. Simultaneously, concerns regarding the safety of vaccines are growing. We describe two patients who developed sensory Guillain-Barre syndrome (GBS) shortly after the first dose of the ChAdOx1 vaccine. We also summarize 12 published cases of GBS after ChAdOx1 vaccination, highlighting their unique clinical and paraclinical features. We propose a possible association between the risk of GBS and the ChAdOx1 vaccine and recommend surveillance for GBS following vaccination. Population-based studies are needed to determine causality and whether specific subpopulations are susceptible.
Keywords: Guillain-Barre syndrome, SARS-CoV-2, COVID-19, ChAdOx1, Vaccines
Graphical abstract
1. Introduction
Massive vaccination against severe acute respiratory coronavirus-2 (SARS-CoV-2) has become a global priority. However, concerns regarding the safety of COVID-19 vaccines are growing. For example, a causal relationship between the ChAdOx1 vaccine and thrombotic immune thrombocytopenia was recently established (Cines and Bussel, 2021). Among the four major COVID-19 vaccines: ChAdOx1, BNT162b2 mRNA, mRNA-1273, and Ad26.COV2·S; ChAdOx1 is the most used in South Korea, administered to >60% of people who have received ≥1 dose of any COVID-19 vaccine (Agency KDCaP, n.d). We describe two patients who developed sensory Guillain-Barre syndrome (GBS) shortly after receiving the first dose of the ChAdOx1 vaccine. Further, we provide a summary of published post-ChAdOx1 vaccine-GBS cases, highlighting their unique features (Allen et al., 2021; Maramattom et al., 2021; Patel et al., 2021).
2. Case presentations
2.1. Case 1
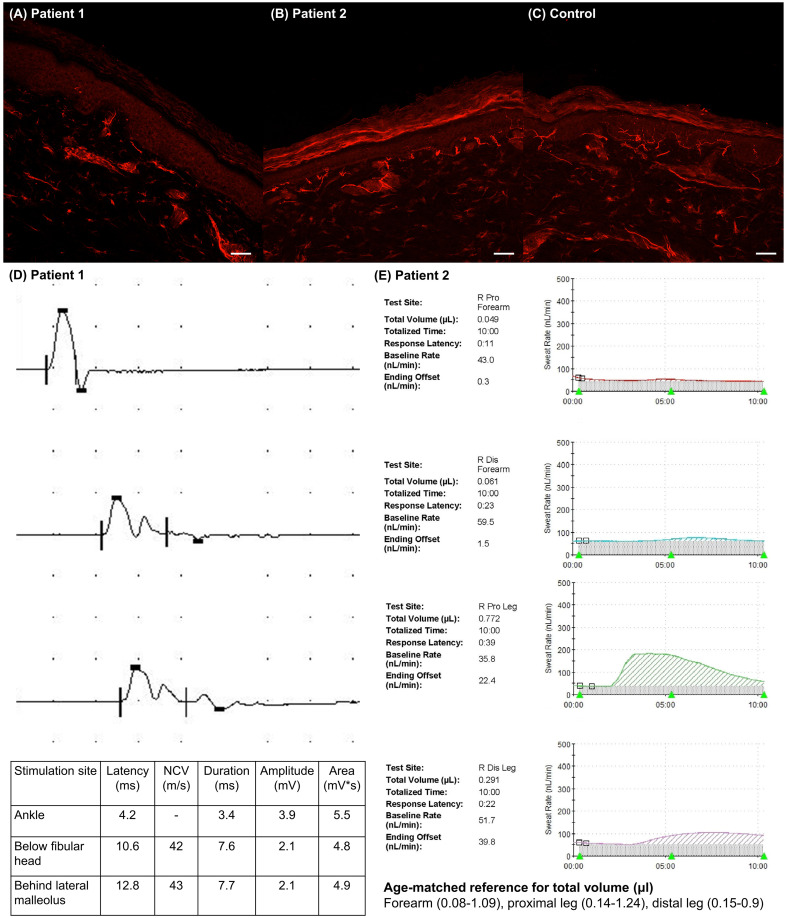
A 58-year-old man with unremarkable medical history and no recent infections experienced focal aching pain on his right toes three days after the first dose of the ChAdOx1 vaccine. Over the next week, he developed severe burning and tingling sensations on both feet, which modestly improved with gabapentin (900 mg per day). Neurological examination on the 15th day post-vaccination revealed mild hypoesthesia in vibration, temperature, and pain on both feet. The Modified Research Council (MRC) grades and deep tendon reflexes (DTR) were normal in all four limbs. A nerve conduction study (NCS) revealed decreased sensory nerve action potential amplitudes on both sural nerves, temporal dispersion on the left peroneal nerve, and absent peroneal motor responses on the right (Fig. 1D). A skin biopsy in the right distal leg (10 cm above the lateral malleolus) revealed an abnormal decrease in intraepidermal nerve fiber density (IENFD) (Fig. 1A). On magnetic resonance imaging (MRI), no abnormal signal changes, enhancement, or enlargement were noted in the spinal cord and nerve roots. Cerebrospinal fluid (CSF) analysis revealed albuminocytologic dissociation (2 white blood cells/μL and protein 70 mg/dL). IgG and IgM for GM1, GD1b, GQ1b as well as IgM for myelin-associated glycoprotein (MAG) tested negative. Nasopharyngeal swabs were negative for SARS-CoV-2 on the reverse transcription-polymerase chain reaction (RT-PCR) test.
Fig. 1.
Skin biopsy and electrophysiological findings of patient 1 (A, D) and patient 2 (B, E). (A) Intraepidermal nerve fiber density (IENFD), immunostained by protein gene product 9.5, was decreased in patient 1's distal leg (2.7/mm, age- and sex-matched cut-off 9.1) (Provitera et al., 2016). (B) Distal leg IENFD was also decreased in patient 2 (6.2/mm, age- and sex-matched cut-off 11.3). (C) Representative example of normal IENFD in healthy control. (D) Peroneal motor nerve conduction study (NCS) of patient 1 showed temporal dispersion, indicative of demyelination. (E) The quantitative sudomotor axon reflex test (QSART) in patient 2 was impaired in the forearm (abnormal) and distal leg (low normal) (reference value shown in the figure). Scale bars = 50 μm.
2.2. Case 2
A 37-year-old woman without any previous medical history and infections developed tingling sensations over both lower extremities four days after receiving her first dose of the ChAdOx1 vaccine. Afterwards, we developed painful cold sensations, and symptoms got worse over the next two weeks. Neurological examination on the 25th post-vaccination day revealed cold hypoesthesia in both legs, especially in the feet and posterior calf. The MRC grades and DTRs were normal in all four limbs. NCS, spine MRI, ultrasonography, and computed tomography angiography of the lower extremities were normal. However, a decrease in the distal leg IENFD demonstrated a small fiber neuropathy (Fig. 1B). Furthermore, the quantitative sudomotor axon reflex was abnormal in the right forearm and low normal in the right calf (Fig. 1E). The following laboratory tests were normal or negative: GM1, GD1b, GQ1b IgG/IgM, MAG IgM, anti-nuclear antibodies, anti-neutrophil cytoplasmic antibodies, complement levels, serum electrophoresis with immunofixation, hemoglobin A1c, folate, thiamine, thyroid function test, D-dimer, and HIV antibody. Her symptoms moderately improved with gabapentin, duloxetine, and tramadol. Nasopharyngeal swabs were negative for SARS-CoV-2 on the RT-PCR test.
3. Discussion
The two patients shared many clinical features: pure sensory manifestations, short-latency from vaccination to onset, progression duration, and no serum antibodies against gangliosides. Neuropathic pain symptoms partially responded to pharmacotherapy in both patients. Due to the absence of weakness or respiratory disturbances, intravenous immunoglobulin (IVIg) was not administered. Other possible causes, such as systemic autoimmune diseases or paraneoplastic syndromes, were not suspected based on the detailed laboratory investigations. Therefore, sensory GBS was considered the most probable diagnosis.
Classically, acute flaccid weakness is required to be diagnosed with GBS (Asbury and Cornblath, 1990; Fokke et al., 2014). However, patients can also present with atypical features or variant forms (Leonhard et al., 2019). Uncini and Yuki suggested an operative definition of sensory GBS, an acute monophasic polyneuropathy with exclusive sensory symptoms and signs that reach a nadir within six weeks without alternative cause (Uncini and Yuki, 2012). Although albuminocytologic dissociation or ganglioside antibodies strongly support the diagnosis, they are not prerequisites. Sensory GBS can be further categorized into three subtypes according to the involved fiber types and locations: acute sensory demyelinating polyneuropathy (demyelination on NCS); acute sensory large fiber axonopathy-ganglionopathy (GD1b antibodies); and acute sensory small fiber neuropathy-ganglionopathy (normal NCS but small fiber denervation in skin biopsy). Patient 1 would represent acute sensory demyelinating polyneuropathy, and Patient 2 acute small fiber neuropathy-ganglionopathy.
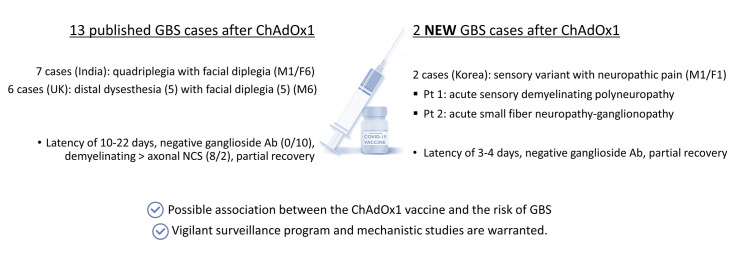
At the time of writing this writing, there are 13 published cases of GBS after the ChAdOx1 vaccine reported in the United Kingdom or India (Allen et al., 2021, Azam and Khalil, 2021, Maramattom et al., 2021, Patel et al., 2021). Table 1 summarizes all 15 patients, including the present report. All patients developed GBS after the first dose with a latency between 3 and 22 days. All but one showed rare phenotypes such as distal paresthesia with facial diplegia or quadriparesis with facial diplegia. There were marked differences in sex ratio and clinical patterns across countries, possibly attributed to genetic background. All tested patients revealed elevated CSF proteins, while three also showed lymphocytosis. Limb NCS revealed demyelinating features in eight patients, axonal in two, and was normal in four. Antibodies against gangliosides, frequently found in axonal GBS subtypes, were negative in every tested patient (0/10). Ten patients received IVIg and/or plasma exchange, mostly leading to a partial recovery.
Table 1.
Summary of 15 cases of post-ChAdOx1 vaccine GBS.
| Author (n) | Country | Sex/Age | Time to onset/nadir (days) | Clinical presentation | Limb NCS | CSF WBC (/μl) | CSF protein (mg/dl) | Ganglioside antibodies | Severity at nadir | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Marammatom et al. (7) | India | F/43 | 10/20 | Quadriparesis, facial diplegia | Demyelinating | 2 | 85 | NA | MRCSS 8 Respiratory failure |
IVIg, MV | Full recovery |
| F/67 | 14/16 | Quadriplegia, facial diplegia, abducens palsy, bulbar palsy, distal pinprick impairment | Axonal | 3 | 345 | Negative | MRCSS 4 Respiratory failure |
IVIG, PE, MV | Bed-bound | ||
| F/53 | 12/16 | Quadriplegia, facial diplegia, right facial and tongue numbness, lower limb pinprick and vibration impairment | Demyelinating | 3 | 120 | Negative | MRCSS 8 Respiratory failure |
IVIg, MV | |||
| F/68 | 14/18 | Quadriplegia, facial diplegia, bulbar palsy, limb numbness | Demyelinating | 4 | 75 | Negative | MRCSS 7 Respiratory failure |
IVIg, MV | |||
| M/70 | 11/NA | Quadriparesis, facial diplegia, limb numbness, bulbar palsy | Demyelinating | NA | NA | NA | MRCSS 24 Respiratory failure |
IVIg, MV | |||
| F/69 | 12/NA | Quadriplegia, facial diplegia, ophthalmoplegia, bulbar palsy, distal limb numbness | Demyelinating | NA | NA | NA | MRCSS 30 | IVIG, PE | |||
| F/69 | 13/NA | Quadriplegia, facial diplegia, bulbar palsy, distal limb numbness | Demyelinating | 2 | 83 | NA | MRCSS 2 Respiratory failure |
IVIG, MV | |||
| Allen et al. (4) | UK | M/54 | 12/16 | Distal dysesthesia, facial diplegia | Normal (limb) | 19 | 163 | Negative | MRCSS 60 | Oral steroid | NA |
| M/20 | 22/23 | Distal dysesthesia, facial diplegia | Normal (limb) | 14 | 123 | Negative | MRCSS 60 | Oral steroid | |||
| M/57 | 11/23 | Back pain, facial diplegia, distal dysesthesia | Normal (limb) | 8 | 247 | Negative | MRC 3–4 (UL), 4–5 (LL) | IVIg | |||
| M/55 | 22/31 | Lower limb paresthesia and numbness, facial diplegia | NA | 4 | 89 | Negative | MRCSS 60 | None | |||
| Patel et al. (1) | M/37 | 14/NA | Distal paresthesia, quadriparesis | Axonal | <1 | 177 | NA | MRCSS 44 | IVIg | Slow recovery | |
| Azam et al. (1) | M/67 | 15/NA | Quadriparesis with facial diplegia | Demyelinating | 0 | 390 | Negative | MRC NA (UL), 3 (LL) SIADH |
IVIg | Partial recovery | |
| Min et al. (2) | South Korea | M/58 | 3/12 | Distal dysesthesia (clinically pure sensory) | Demyelinating | 2 | 70 | Negative | MRCSS 60 Severe pain |
Neuropathic pain drugs | Partial response |
| F/37 | 4/16 | Normal | NA | NA | Negative |
Abbreviations: NCS, nerve conduction study; CSF, cerebrospinal fluid; F, female; M, male; NA, not available; WBC, white blood cell; MRCSS, medical research council sum score; MRC, medical research council; UL, upper limb; LL, lower limb; SIADH, syndrome of inappropriate antidiuretic hormone secretion; IVIG, intravenous immunoglobulin; MV, mechanical ventilation; PE, plasma exchange.
A causal relationship between COVID-19 and GBS is under active discussion since the first report on their co-occurrence in January 2020 (Dalakas, 2020; Fantini et al., 2020; Keddie et al., 2021; Palaiodimou et al., 2021; Zhao et al., 2020). A large-scale population-based study reported that the incidence of the whole GBS did not increase during the pandemic (Keddie et al., 2021). However, a recent meta-analysis based on 11 cohorts found an increased risk of the demyelinating subtype in COVID-19 patients compared to non-infected or historical counterparts (Palaiodimou et al., 2021).
Conversely, little is known about the relationship between COVID-19 vaccines and GBS. No GBS occurred in clinical trials of COVID-19 vaccines, except for one among 19,630 Ad26.COV2·S recipients (Baden et al., 2021; Heath et al., 2021; Polack et al., 2020; Sadoff et al., 2021; Voysey et al., 2021). Although rare, we propose a possible association between GBS and the ChAdOx1 vaccine. The frequent observation of rare variants and demyelinating subtypes, which increased in COVID-19 GBS, further supports our suspicion. However, the pathophysiological mechanisms underlying this vaccine-associated neuro-autoimmunity remain elusive; whether antibodies against the spike protein could cross-react with peripheral nerve constituents is controversial (Dalakas, 2020, Fantini et al., 2020, Keddie et al., 2021). As for DNA vaccines (ChAdOx1 and Ad26.COV2·S), adenovirus vectors or aberrant splice variants may be alternative sources of autoimmunity (Almuqrin et al., 2021). Further mechanistic research is needed to demonstrate the pathophysiology of post-COVID-19 vaccine-GBS and whether a particular vaccine is associated with the increased risk.
4. Conclusion
We describe two cases of sensory GBS after ChAdOx1 vaccinations and provide a literature review on 12 additional GBS cases following the ChAdOx1 vaccine, highlighting their unique clinical and paraclinical features. Vigilance of GBS following COVID-19 vaccinations is mandatory to determine a causal association. Moreover, it would be interesting to investigate the overall outcomes of the post-COVID-19 vaccine-GBS and if there are populations at an increased risk.
Funding
This work was supported by the National Research Foundation of Korea(NRF) grant funded by the Korean government (2017R1D1A1B03035105 and 2019M3C7A1031867).
Authors' contributions
YGM and J-YS conceived the study and wrote the main manuscript. WJ, SL, Y-EH and J-JB collected the data. WJ supported graphical presentation. J-YS and J-JS supervised the work. All authors contributed to the discussion of the results and reviewed the manuscript.
Ethical approval
This study was approved by the local institutional review board and all participants provided written informed consent.
Declaration of competing interest
There are no competing interests.
References
- Agency KDCaP Vaccination Information in South Korea. https://ncv.kdca.go.kr/ Available:
- Allen C.M., Ramsamy S., Tarr A.W., Tighe P.J., Irving W.L., Tanasescu R., et al. Guillain-Barre syndrome variant occurring after SARS-CoV-2 vaccination. Ann. Neurol. 2021;90:315–318. doi: 10.1002/ana.26144. [DOI] [PubMed] [Google Scholar]
- Almuqrin A., Davidson A.D., Williamson M.K., Lewis P.A., Heesom K.J., Morris S., et al. SARS-CoV-2 vaccine ChAdOx1 nCoV-19 infection of human cell lines reveals low levels of viral backbone gene transcription alongside very high levels of SARS-CoV-2 S glycoprotein gene transcription. Genom. Med. 2021;13:43. doi: 10.1186/s13073-021-00859-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asbury A.K., Cornblath D.R. Assessment of current diagnostic criteria for Guillain-Barré syndrome. Ann. Neurol. 1990;27(Suppl):S21–S24. doi: 10.1002/ana.410270707. [DOI] [PubMed] [Google Scholar]
- Azam S., Khalil A. Vol. 9. 2021. Taha AJAJoMCR. Guillain-Barré Syndrome in a 67-year-old Male Post COVID-19 Vaccination (Astra Zeneca) pp. 424–427. [Google Scholar]
- Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cines D.B., Bussel J.B. SARS-CoV-2 vaccine-induced immune thrombotic thrombocytopenia. N. Engl. J. Med. 2021;384:2254–2256. doi: 10.1056/NEJMe2106315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalakas M.C. Guillain-Barre syndrome: the first documented COVID-19-triggered autoimmune neurologic disease: more to come with myositis in the offing. Neurol Neuroimmunol Neuroinflamm. 2020;7 doi: 10.1212/NXI.0000000000000781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantini J., Di Scala C., Chahinian H., Yahi N. Structural and molecular modelling studies reveal a new mechanism of action of chloroquine and hydroxychloroquine against SARS-CoV-2 infection. Int. J. Antimicrob. Agents. 2020;55:105960. doi: 10.1016/j.ijantimicag.2020.105960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fokke C., van den Berg B., Drenthen J., Walgaard C., van Doorn P.A., Jacobs B.C. Diagnosis of Guillain-Barré syndrome and validation of Brighton criteria. Brain. 2014;137:33–43. doi: 10.1093/brain/awt285. [DOI] [PubMed] [Google Scholar]
- Heath P.T., Galiza E.P., Baxter D.N., Boffito M., Browne D., Burns F., et al. Safety and efficacy of NVX-CoV2373 Covid-19 vaccine. N. Engl. J. Med. 2021 doi: 10.1056/NEJMoa2107659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keddie S., Pakpoor J., Mousele C., Pipis M., Machado P.M., Foster M., et al. Epidemiological and cohort study finds no association between COVID-19 and Guillain-Barre syndrome. Brain. 2021;144:682–693. doi: 10.1093/brain/awaa433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonhard S.E., Mandarakas M.R., Gondim F.A.A., Bateman K., Ferreira M.L.B., Cornblath D.R., et al. Diagnosis and management of Guillain-Barre syndrome in ten steps. Nat. Rev. Neurol. 2019;15:671–683. doi: 10.1038/s41582-019-0250-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maramattom B.V., Krishnan P., Paul R., Padmanabhan S., Cherukudal Vishnu Nampoothiri S., Syed A.A., et al. Guillain-Barre syndrome following ChAdOx1-S/nCoV-19 vaccine. Ann. Neurol. 2021;90:312–314. doi: 10.1002/ana.26143. [DOI] [PubMed] [Google Scholar]
- Palaiodimou L., Stefanou M.I., Katsanos A.H., Fragkou P.C., Papadopoulou M., Moschovos C., et al. Prevalence, clinical characteristics and outcomes of Guillain-Barre syndrome spectrum associated with COVID-19: a systematic review and meta-analysis. Eur. J. Neurol. 2021;00:1–13. doi: 10.1111/ene.14860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S.U., Khurram R., Lakhani A., Quirk B. Guillain-Barre syndrome following the first dose of the chimpanzee adenovirus-vectored COVID-19 vaccine, ChAdOx1. BMJ Case Rep. 2021;14 doi: 10.1136/bcr-2021-242956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provitera V., Gibbons C.H., Wendelschafer-Crabb G., Donadio V., Vitale D.F., Stancanelli A., Caporaso G., et al. A multi-center, multinational age- and gender-adjusted normative dataset for immunofluorescent intraepidermal nerve fiber density at the distal leg. Eur. J. Neurol. 2016;23:333–338. doi: 10.1111/ene.12842. [DOI] [PubMed] [Google Scholar]
- Sadoff J., Gray G., Vandebosch A., Cárdenas V., Shukarev G., Grinsztejn B., et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N. Engl. J. Med. 2021;384:2187–2201. doi: 10.1056/NEJMoa2101544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uncini A., Yuki N. Sensory Guillain-Barre syndrome and related disorders: an attempt at systematization. Muscle Nerve. 2012;45:464–470. doi: 10.1002/mus.22298. [DOI] [PubMed] [Google Scholar]
- Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet (London, England) 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H., Shen D., Zhou H., Liu J., Chen S. Guillain-Barré syndrome associated with SARS-CoV-2 infection: causality or coincidence? Lancet Neurol. 2020;19:383–384. doi: 10.1016/S1474-4422(20)30109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]