Abstract
Background
COVID-19 is a pandemic disease that has no definite treatment or preventive medication until late 2020 when vaccines were developed. Vaccines are a very useful new tool against COVID-19 that stimulate the immune system after recognition of viruses or their parts. Complications post vaccination could happen and they depend on the types, vaccine mechanism of action as well as some other patients' factors.
Case report
We are reporting a 39 years old lady who is known as allergic to a strawberry and kiwi, otherwise medically free. She presented to the outpatient unit with a ten-days history of palms of the hands and soles of the feet itchiness that is associated with occasional redness after receiving the first dose of COVID-19 vaccination. There were no skin rashes or pruritis at any other sites. The patient was treated conservatively by antihistamine and the symptoms gradually resolved within five days.
Conclusion
Allergic reaction is one of the expected complications post any COVID-19 or non-COVID-19 vaccination. Although the type, distributions and severity of allergic reactions are variable from person to another, yet isolated itching to palms and soles could be a rare side effect post first dose of COVID-19 vaccination (Pfizer-BioNTech) which is worth reporting. This presentation could be a type of allergic reaction which may require holding further doses, debate is there for further case reporting, research or evaluation.
Learning points:
-
•
Isolated itching to palms and soles could be one of the side effects of COVID-19 vaccination (Pfizer-BioNTech) which is worth reporting.
Keywords: Itching, Allergy, COVID-19, Vaccination (BioNTech), Palms and soles
Introduction
COVID-19 is a coronavirus that first appeared in the Chinese city, Wuhan, at the end of December 2019 in the form of acute pneumonia and turned to be pandemic in March 2020 [1]. The presentation of the illness ranging from the common cold to severe acute respiratory distress syndrome (ARDS) or respiratory failure requires critical care admission and ventilatory support. However, several strategies and algorithms to treat the patient affected by COVID-19 were introduced during 2020, but none was considered as definitive treatment. Since then, the drug companies tried their best to come up with a suitable vaccine aiming to stop the spread of COVID-19. By the end of 2020, COVID-19 Vaccine was made to prevent Coronavirus Disease 2019 (COVID-19) that was caused by SARS-CoV-2. So far, there has been five different types of vaccine available to prevent spreading of COVID-19 infection, and each has different mechanisms of action and possible complications. Furthermore, some unpleasant side effects could be detected as in our case scenario, for that we are reporting one of the possible side effects of COVID-19 vaccination.
Case report
A 39 years old lady who is known to be allergic to a strawberry and kiwi, otherwise medically free. She presented to an outpatient unit with ten days history of both palms and soles itchiness that associated with on and off redness after receiving the first dose of COVID-19 vaccination (BioNTech) by few hours gradually increasing in severity to the limit that not allowing her to sleep, peaked after ten days from vaccination. There were no skin rashes or pruritis at any other sites. There was no fever, cough or sore throat. No new medication or herbs were used. Systemic review was unremarkable.
Physical examination
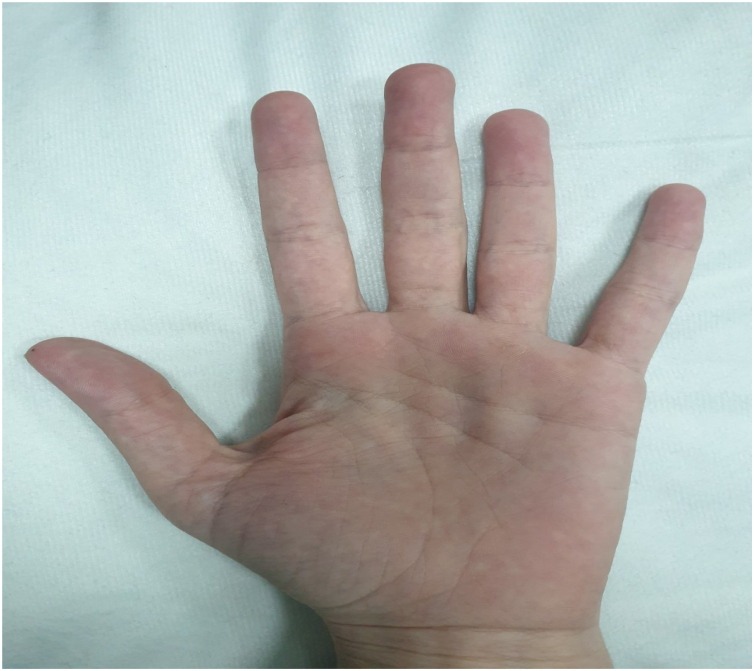
Well-built female, fully oriented and conscious, not in any distress. Her vital signs were stable and afebrile with a temperature of 37.2C. There were no palpable cervical, axillary or inguinal lymphadenopathies. Insignificant respiratory, cardiovascular and gastrointestinal examination. Musculoskeletal and skin examination were unremarkable apart from mild redness at the palms (Fig. 1 ).
Fig. 1.
Photo provided by the patient for the palm of the right hand showed mild redness.
Management and follow up
Given the history and in correlation with timing of vaccination, Suspicion of post vaccination reaction was made. Routine investigations including full blood count and biochemical blood results were unremarkable. Additional lab test such as IGE level was not available in our center. Patient was treated conservatively by antihistamine and the symptoms gradually resolved within five days. No hospital admission was required during her course of illness.
Discussion
COVID-19 vaccination, Pfizer (BioNTech) is a mRNA introducing vaccine that acts by triggering the human being's immune system and creating antibodies against SARS-COV2. As recommended by Centers for Disease Control and Prevention (CDC), this vaccine is administered in two doses within 6 weeks apart [2]. Initially, the vaccine was approved for adults above eighteen years old, nowadays age frame down to sixteen, even approved recently to twelve years of age, further evaluations to involve pediatric age are ongoing [3]. There are some side effects that have been reported with the Pfizer-BioNTech COVID-19 Vaccine including injection site swelling and arm pain, swollen lymph nodes, fever, loose bowel motion as well as nausea and vomiting, body ache and muscle pain, headache, allergic reactions either severe or non-severe allergic reactions such as rash and itching [3,4]. Severe allergic reactions occurred within fifteen minutes from vaccine injection and it is more reported in females and most of the reported cases have background of allergy [[5], [6], [7], [8]]. Moreover, 87% of the reactions and itchiness were at the site of injection and occurred in the first week of injection and most cases occurred with the second dose in comparison to the first one [9]. The incidence of severe allergic reaction due to the mRNA-1273 vaccine was inaccurate. In a report by CDC after reviewing the events of severe allergic reaction among 175 patients, 84% of the incidence were unconfirmed [7].
However, some various types of reaction could be there but not yet reported. For example, no report was found about delayed, isolated itchiness in both palms and soles post COVID-19 vaccination. Unfortunately, delayed types of reaction were infrequently reported, but the site of the reaction makes our case somehow unique. As a differential diagnosis, palms and hand itchiness can be happening in some situations such as in eczema, allergic reactions or reactions to medication, atopic dermatitis, nerve disorders, diabetes, psoriasis palmaris and other skin diseases such as lichen planus, porphyria cutanea tarda as well as infectious skin diseases such as tinea manus [10]. Since there was no previous medical illness in our case scenario, along with her presenting sign supported by laboratory markers which was against other differential, the diagnoses of reaction related to vaccination was made. Baden et al. reported a delayed site of injection reaction to the mRNA-1273 vaccine, which was in forms of tenderness, erythema and induration. Moreover, the median time of reaction in Baden et al. study was 8 days (4–11 days). Furthermore, 0.8% of such reactions occurred after the first dose of vaccination, on the other hand, 0.2% occurred post the second one [11]. A case series of 12 patients with localized delayed reaction were reported by Kimberly et al. and the biopsy from the induration or reaction site showed infiltration by T cells, eosinophils and lymphocytes. The author concluded that the delayed reaction or hypersensitivity reaction should not be a reason to omit further doses as the reaction was self-limited or controlled by conservative measures [12,13]. Some reported a challenge of vaccine dose-splitting in patients with high risk of allergy but this was not examined in mRNA-1273 vaccine [13,14]. Up to date, no reported cases with such a presentation of palms and soles itchiness post COVID-19 vaccination as in our case scenario. For that this report was written.
In conclusion, allergic reaction is one of the expected complications post any COVID-19 or non-COVID-19 vaccination. Although the type, distributions and severity of allergic reactions vary from person to person, yet, isolated itchiness in both palms and soles is a rare allergic reaction. Isolated itching to palms and soles could be an uncommon side effect post first dose of COVID -19 vaccination (Pfizer) which is worth reporting. This presentation could be a type of allergic reaction which may require holding further doses, debate is there for further case reporting, research or evaluation.
Author contributions
None to declare.
Financial disclosure or funding
not applicable.
Informed consent
Patient consented for publication and picture taken.
IRB & Ethic committee approval
Not required to get IRB in case report publication as per KFMMC-Dhahran ethical committee policy.
Conflict of interest
There are no conflicts of interest regarding the publication of this paper.
Acknowledgement
No acknowledgement.
References
- 1.Cucinotta D., Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed. 2020;91(March (1)):157–160. doi: 10.23750/abm.v91i1.9397. PMID: 32191675; PMCID: PMC7569573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.FDA. Fact sheet for healthcare providers administering vaccine (vaccination providers): Emergency Use Authorization (EUA) of the Pfizer-BioNTech COVID-19 vaccine to prevent coronavirus disease 2019 (COVID-19). Revised January 2021. Accessed January 17, 2021. https://www.fda.gov/media/144413/download.
- 3.FDA. Fact sheet for recipients and caregivers emergency use authorization (EUA) of the Pfizer-biontech COVID-19 vaccine to prevent coronavirus disease 2019 (COVID-19) in individuals 16 years of age and older. https://www.fda.gov/media/144414/download.
- 4.Shimabukuro T., Nair N. Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine. JAMA. 2021;325(8):780–781. doi: 10.1001/jama.2021.0600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McNeil M.M., DeStefano F. Vaccine-associated hypersensitivity. J Allergy Clin Immunol. 2018;141(2):463–472. doi: 10.1016/j.jaci.2017.12.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McNeil M.M., Weintraub E.S., Duffy J., Sukumaran L., Jacobsen S.J., Klein N.P. Risk of anaphylaxis after vaccination in children and adults. J Allergy Clin Immunol. 2016;137:868–878. doi: 10.1016/j.jaci.2015.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention COVID-19 Response Team Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine — United States, December 14–23, 2020. MMWR. 2021;70:46–51. doi: 10.15585/mmwr.mm7002e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.CDC COVID-19 Response Team; Food and Drug Administration Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine - United States, December 14-23, 2020. MMWR Morb Mortal Wkly Rep. 2021;70(2):46–51. doi: 10.15585/mmwr.mm7002e1. Published 2021 Jan 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Local reactions, systemic reactions, adverse events, and serious adverse events: Pfizer-BioNTech COVID-19 vaccine. https://www.cdc.gov/vaccines/covid-19/info-by_product/pfizer/reactogenicity.html.
- 10.Elke Weisshaar, Ursula Kallen, Melanie Weiß. “The itching hand “– important differential diagnoses and treatment. JDDG, First published: 26 November 2012 10.1111/j.1610-0387.2012.08002.x. [DOI] [PubMed]
- 11.Baden L.R., El Sahly H.M., Essink B. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMc2102131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blumenthal Kimberly G., Freeman Esther E. Delayed large local reactions to mRNA-1273 vaccine against SARS-CoV-2. N Engl J Med. 2021;384:1273–1277. doi: 10.1056/NEJMc2102131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kelso J.M., Greenhawt M.J., Li J.T. Adverse reactions to vaccines practice parameter 2012 update. J Allergy Clin Immunol. 2012;130:25–43. doi: 10.1016/j.jaci.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 14.Banerji A., Wickner P.G., Saff R. mRNA vaccines to prevent COVID-19 disease and reported allergic reactions: current evidence and suggested approach. J Allergy Clin Immunol Pract. 2021;9(4):1423–1437. doi: 10.1016/j.jaip.2020.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]