Abstract
SARS-CoV-2 (Severe Acute Respiratory Syndrome Coronavirus 2) infection is a global medical challenge. Experience based medicines and therapies are being attempted and vaccines are being developed. SARS-CoV-2 exhibits varied patterns of infection and clinical presentations with varied disease outcomes. These attributes are strongly suggestive of some variables that differ among individuals and that affect the course of SARS-CoV-2 infection and symptoms of COVID-19 (Corona Virus Disease of 2019). Sex hormones vary with ageing, between the sexes, among individuals and populations. Sex hormones are known to play a role in immunity and infections. Progesterone is a critical host factor to promote faster recovery following Influenza A virus infection. Anti-inflammatory effects of progesterone are noted. In part 1 of the current study the regulatory role of progesterone for SARS-CoV-2 infection and COVID-19 is analyzed. The role of progesterone at different stages of the SARS CoV-2 infection is investigated with respect to two types of immunity status: immune regulation and immune dysregulation. Progesterone could have various alleviating impacts from SARS-CoV-2 entry till recovery: reversing of hypoxia, stabilizing of blood pressure, controlling thrombosis, balancing electrolytes, reducing the viral load, regulation of immune responses, damage repair, and clearance of debris among others. The present research adds to the available evidence by providing a comprehensive and thorough evaluation of the regulatory role of progesterone in SARS COV-2 infection, COVID-19 pathogenesis, and immune dysregulation. The available evidence has implications for upcoming studies about pathophysiology of COVID-19, as well as the roles of progesterone and other hormones in other infectious diseases.
Keywords: COVID-19, SARS-CoV-2, Pathogenesis, Progesterone, Immune regulation, Immune dysregulation, Cytokine storm
1. Introduction
Patients‘ responses to SARS-CoV-2 infection, the severity of symptoms, and disease outcomes are primarily determined by their immune responses to conquer the virus, and by their capability to regulate these immune responses appropriately. Recovery from COVID-19 is determined by the patients‘ ability to overcome the infection and to balance the elevated cell-mediated immunity by developing humoral immunity along with other regulatory mechanisms. Failing to do so engenders a state of immune imbalance, which could cause pathophysiological changes presenting as symptoms of sickness (Huntington and Gray, 2018). There is a strong interaction between sex hormones and the immune system. Immune cells have receptors for hormones, and circulating hormones can regulate the immune system through these receptors (Dosiou et al., 2008). It is rational to look for their possible role in COVID-19 pathogenesis and recovery.
The three major sex hormones are estrogen, progesterone, and testosterone. Estrogen has subtypes: estrone, estradiol, estriol, and estetrol (Baker, 2013). There is a predominance of different subtypes depending on age, reproductive status, and sex (Cooke et al., 2017). This variation makes it complicated to assess its role as a single common regulatory hormone parameter for the SARS-CoV-2 pandemic, which is affecting all age groups and sexes. Scientists have looked for testosterone‘s role in COVID-19 patients. Findings from these studies are debatable with two upcoming theories: one suggesting low levels of testosterone and the other indicating high levels of testosterone as aggravating the course of COVID-19 (Pozzilli and Lenzi, 2020). The role of progesterone in COVID-19 remains to be explored. Based on a detailed research study in female mice, Hall OJ et al from Johns Hopkins found that progesterone promotes faster recovery following Influenza A virus infection (Hall et al., 2016). Another study has concluded that progesterone is anti-inflammatory and has analgesic effects (Shilpa and Roby, 2019). Progesterone is a precursor to both estrogen and testosterone. The present research discovers if progesterone is a possible therapeutic regulator for COVID-19.
2. Methods
The present original theoretical research was carried out in the city of Mumbai, Maharashtra, India from April 1, 2020 to December 31, 2020.
COVID-19 was a novel and yet to be understood disease, when the present research was started. Physicians and health professionals involved in the care of COVID-19 patients were contacted through phone or electronic communications to gather their opinion about the most observed pathological changes and complications in SARS-CoV-2 infected patients. Opinions of Indian and international physicians were also gathered through live streaming interviews on electronic media. Among all the different pathogenesis, cytokine storm and immune-dysregulation was the major factor noted. Hence, based on the gathered information the study layout was prepared depicting two types, one being COVID-19 with progression towards recovery and the other, COVID-19 with immune dysregulation and severe disease.
Primary theoretical research was conducted to understand and explain each stage of the disease: beginning with virus entry, immune responses, pathogenesis, immune regulation, immune dysregulation, systemic injuries, repairs and debris overload, by keeping abreast with the upcoming published work in scientific journals.
To explore the main concept, which is if progesterone has a role in COVID-19, theoretical investigation based on formative research questions was carried out separately for each stage of disease in a stepwise manner. For example, how virus enters the body via priming its proteins using TMPRSS2 was an already established part of the research layout; to find if progesterone has a role to play, the following formative research questions were raised:
(1) Does progesterone block ACE2?
Finding: No.
(2) Does progesterone downregulate the TMPRSS2?
Finding: No.
(3) What are the factors that could affect any or both of the above-mentioned actions, which is blocking ACE2 and/or downregulating TMPRSS2?
Finding: Androgens do not downregulate but actually upregulate TMPRSS2.
(4) Does progesterone block the androgens?
Finding: Yes. Progesterone is anti-androgenic. This is a known fact but to establish this link was an outcome of stepwise research.
Such formative research questions are the methodological approach which guided the research throughout. For readers to have a ready context, the research findings are presented stage-wise starting with the hows and whys of SARS-CoV-2 infection and COVID-19 pathogenesis followed by the regulatory contributions of progesterone. All illustrations and tables in the manuscript are originally prepared.
3. Results And Discussion
Findings of the research are reported and discussed as per the stages of immune regulation and dysregulation; although within the body any or all of these processes could be in parallel or at random.
3.1. SARS-CoV-2 infection, immune regulation, and regulatory role of progesterone
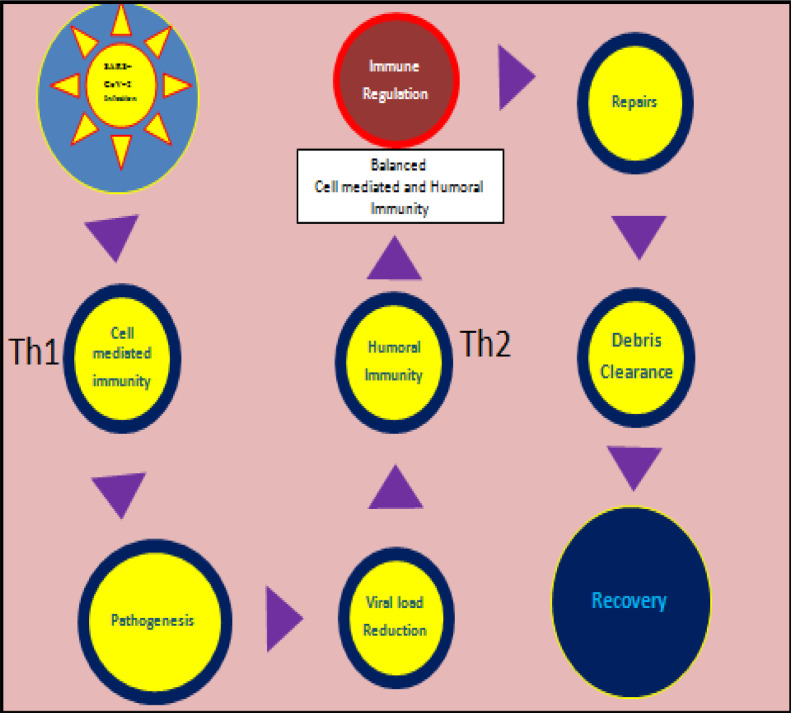
This section details research findings for COVID-19 from infection to recovery as per the layout of Fig. 1
Fig. 1.
Schematic illustration of immune regulation post immune attack for SARS-CoV-2 infection leading to recovery.
3.1.1. Stage 1A. SARS-CoV-2 infection
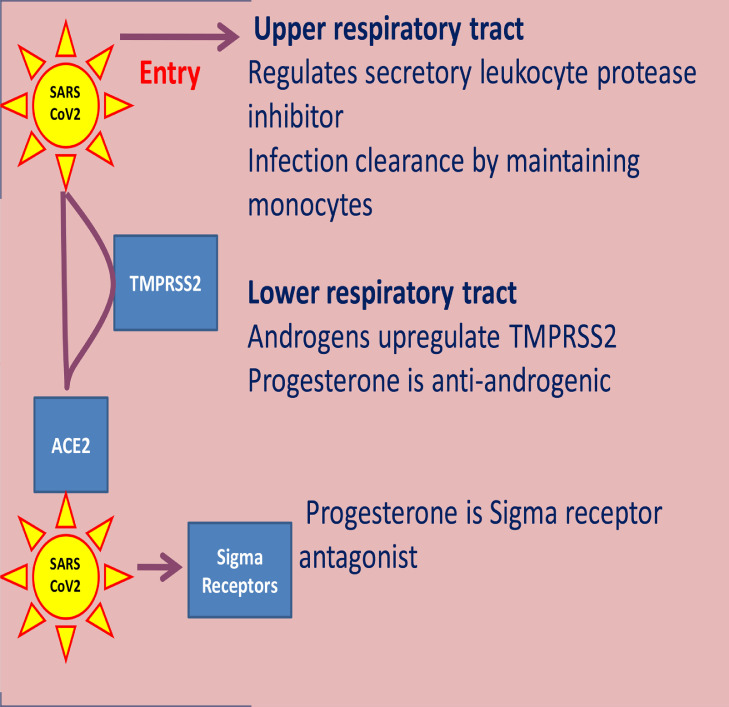
Progesterone could exhibit anti-viral activity at three stages of the SARS-CoV-2 infection: (i) upper respiratory tract, (ii) lower respiratory tract, and (iii) within the cells and neurons (Fig. 2 ).
Fig. 2.
SARS-CoV-2 infection and anti-viral activities of progesterone.
(i) Upper respiratory tract:
The major entry of SARS-CoV-2 is via the upper respiratory tract. Whether the virus can successfully enter and establish itself in the host cell or fails depends on the proteases and protease-inhibitors, which are secreted from the respiratory epithelium. More protease and less protease inhibitors is associated with increased susceptibility to respiratory viral infections and severity of symptoms (Meyer and Jaspers, 2015). Serine protease inhibitors (serpins) are the major protease inhibitors in the lung that protect cells from protease-mediated injury. They assist the immune system by limiting tissue damage and unwarranted cell death caused by infection-associated proteases (Askew and Silverman, 2008). They are the key regulators of numerous biological pathways that initiate inflammation, coagulation, angiogenesis, apoptosis, and complement activation; and are associated with cell survival, development, repairs, and host defense at the sites of infection and inflammation (Jakob et al., 2005, Gary et al., 2010). Progesterone increases secretory leukocyte protease inhibitor (SLPI), a serpin secreted by the human nasal epithelial cells and saliva. This is an important respiratory tract host defense protein which confers local protection against microbial, fungal, and viral infections (Sidharthan and Rao, 2011, Camper et al., 2016). Increase of SLPI by progesterone also contributes to the confinement of inflammation and tissue damage, thereby facilitating wound healing. It promotes infection clearance at mucosal sites by maintaining sufficient monocytes for infection and inflammation resolution (Doumas et al., 2005).
(ii) Lower respiratory tract:
Two main host factors are required for successful SARS-CoV-2 entry in the lung: angiotensin converting enzyme 2 (ACE2), and the transmembrane protease serine 2 (TMPRSS2). SARS-CoV-2 enters cells by binding its spike protein to ACE2. To accomplish this binding, the SARS-CoV-2 needs to prime its spike protein; which it does by using TMPRSS2 (Hoffmann et al., 2020). Androgens upregulate the TMPRSS2 and facilitate the SARS-CoV-2 to establish itself (Wambier et al., 2020). Progesterone is anti-androgenic and can block the upregulation of TMPRSS2 by androgens (DeLeo et al., 2016). Notably, progesterone and the serpin alpha-1 antitrypsin which inhibits the SARS-CoV-2 priming protease TMPRSS2 are higher in adult females than in adult males (Azouz et al., 2020, Lauretta et al., 2018, Sedrani et al., 1988). This is an important observation because SARS-CoV-2 infection and mortality due to COVID-19 are significantly more severe in males than in females (Jin et al., 2020).
(iii) Receptor binding:
Receptor binding is a major determinant of tissue tropism for SARS-CoV-2. Sigma receptors might play a role in the infectivity of SARS-CoV-2 (Gordon et al., 2020). These receptors are highly expressed in intracellular locations: the olfactory bulb, lung, liver, intestine, kidney, spleen, adrenal gland, central nervous system (brain and the periphery), and placenta. Sigma receptors are predominantly localized intracellularly in the endoplasmic reticulum (ER) (Hayashi and Su, 2005). When an agonist binds to the sigma receptor, it acts as an upstream accelerator of ER stress through modulation of a voltage-gated ion channel (Vela, 2020). Corona viruses exhibit identical effects on ER stress and may have a role in the agonist activation of the sigma receptors. Replication of the coronaviruses occurs in membranous compartments derived from the ER, which causes host cell stress and activates pathways to facilitate adaptation of the host cell machinery to viral needs. Progesterone is an antagonist of sigma receptors that blocks the receptor-mediated modulation of the voltage-gated ion channel (Johannessen et al., 2011). This way it blocks action of the agonist (Maurice and Su, 2009) on the sigma receptors and controls the ER stress. This is a disadvantage for the virus. Gordon et al (Gordon et al., 2020) have reported that progesterone could exert antiviral activity on SARS-CoV-2 through its sigma activity.
3.1.2. Stage 2A. Cell-mediated immunity
The SARS-CoV-2 virus being novel, humans who are coming across it for the very first time have no specific cellular or humoral immune memory for this virus. When this virus enters the body, cross-reacting or blocking antibodies, if any, from previous infections may provide initial barricade, but it is cell-mediated immunity which actually serves as the primary immune response and attack. SARS-CoV-2 being an intracellular infectious agent, an initial cell-mediated immune response is vital for its elimination (Stertz et al., 2007).
3.1.3. Stage 3A. Pathogenesis
The role of progesterone in COVID-19 pathogenesis is summarized in Table 1 .
Table 1.
Progesterone's regulatory actions on COVID-19 pathogenesis.
| Hypoxia | Airway smooth muscle relaxation, regulates microvascular leakage in airways, increases alveolar ventilation |
| Hypertension | Vasodialator, lowers blood pressure, regulates vascular tone. inhibits coronary hyperactivity |
| Low hemoglobin and iron overload | Increases hepcidin production |
| Thrombocytopenia | Progesterone metabolites regulate platelet aggregation and thrombosis |
| Electrolytes imbalance | Regulates potassium homeostasis and renal electrolyte balance |
3.1.3.1. Hypoxia:
A mega study on factors associated with hospitalization and critical illness conducted by Christopher M et al. on more than 4000 patients with COVID-19 in New York City revealed that though age and comorbidities are powerful predictors of hospitalization, it is on-admission oxygen impairment and markers of inflammation which are strongly associated with critical illness (Christopher et al., 2020). In COVID-19 patients, oxidative stress resulting from perpetuation of the cytokine storm, coagulopathy, cell hypoxia and inflammation are interconnected and co-exist (Cecchini and Cecchini, 2020).
Hypoxia induces glycolysis, thereby acidifies the tissue and perpetuates inflammation (McGarry et al., 2018). After analyzing a series of critically ill COVID-19, cases Bhatraju PK et al. found that COVID-19 patients requiring admission to intensive care units were hypoxemic with respiratory failure requiring mechanical ventilation, or hypotension requiring vasopressor treatment, or both. Mortality among these critically ill patients was high (Bhatraju et al., 2020).
The lung is a target organ for estradiol, progesterone, and prolactin (Ben-Harari et al., 1983). These hormones have a well-known role in the regulation of vascular tone. Progesterone and estrogen have also been associated with airway smooth muscle relaxation which reduces the contractile response of the bronchial smooth muscle (Zarazúa et al., 2016). Progesterone is an effective agent for improving ventilation. A decrease in progesterone level alters the contractility of smooth muscles and increases the hydration of airway mucosa because progesterone is important in the regulation of microvascular leakage in airways (Beynon et al., 1988). In humans, serum progesterone is positively associated with peak expiratory flow rate during the luteal phase of the menstrual cycle when the progesterone level is high (Mannan and Begum, 2012). Progesterone reduces alveolar pCO2 in adult men (Goodland et al., 1953). In patients with obesity-hypoventilation syndrome, progesterone therapy is reported to increase alveolar ventilation by an additional 29.3 per cent and total minute ventilation by 20.6 percent along with normalization of the arterial blood gas tensions and pH, correcting the respiratory acidosis. Further, abnormal ventilator carbon dioxide response curves get restored to normal by progesterone therapy (Lyons and Huang, 1968). Similarly, patients with the Pickwickian syndrome, characterized by obesity, hypoxemia, hypercapnia, who undergo progesterone therapy have highly significant rise in PaO2 and reduction in PaCO2 (Sutton et al., 1975). Progesterone is also found effective in lowering the PaCO2 in patients with emphysema and hypercapnia (Tyler, 1960). Before menopause, there is better oxygenation even at lower hemoglobin levels in women long residing at high altitude, and this is associated with higher levels of progesterone in the luteal phase of the cycle (León-Velarde et al., 2001).
3.1.3.2. Blood pressure and ACE2 (Angiotensin Converting Enzyme 2):
A pooled analysis of the current literature suggests that hypertension may be associated with an up to 2.5-fold higher risk of severe or fatal COVID-19, especially in older patients (Lippi et al., 2020). At the present time, it is not clear whether the upregulation of ACE2 in lung can be harmful or beneficial for patients with SARS-CoV-2 infection (Fernández-Fernández, 2020). Researchers have varied conclusions about it:
-
(1)
Patients with cardiac diseases, hypertension or diabetes, who are treated with ACE2-increasing drugs, are at higher risk for severe COVID-19 infection (Lei et al., 2020).
-
(2)
High ACE2 expression has a protective role against SARS-CoV2 fatality (Jiawei et al., 2020).
-
(3)
Overexpression of ACE2 reduces high BP on a long-term basis (Yamazato et al., 2007).
Major medical societies suggest continuing the angiotensin converting enzyme inhibitors versus angiotensin receptor blockers therapy in individuals who are already receiving treatment. Here it is important to mention that ACE2 does not activate angiotensin. Angiotensin is activated by the angiotensin converting enzyme (ACE), which is inhibited by the ACE inhibitors. However, for patients with a new diagnosis of hypertension who are at risk of COVID-19, an alternative anti-hypertensive agent could be preferred (Antwi-Amoabeng et al., 2020). Gonadal steroids have been implicated in the control of blood pressure and fluid homeostasis. Progesterone is a vasoactive hormone with regulatory effects on blood vessels and blood pressure (Barbagallo et al., 2001). Progesterone protects the cardiovascular system. It lowers blood pressure, inhibits coronary hyperactivity, and has powerful vasodilatory and natriuretic effects. Rapid non-genomic progesterone mechanisms are of physiological importance in regulating vascular tone (Thomas and Pang, 2013).
3.1.3.3. Low hemoglobin and iron overload:
Beyond the pulmonary immune-inflammation, COVID-19 is a disease marked by oxygen-deprived blood with dysregulated iron metabolism (Cavezzi et al., 2020). Both increase and depletion of body iron has harmful effects on health, as seen in iron deficiency anemia and iron overload-related organ damage. It is interesting to note that both iron deficiency and iron excess have been associated with an increased risk of thromboembolic events (Franchini et al., 2008). Proteins of the SARS-CoV-2 virus could coordinately attack the heme on the 1-beta chain of hemoglobin to dissociate the iron from the porphyrin. In turn, the viral surface glycoproteins could bind to the porphyrin. This kind of attack on the hemoglobin will reduce the amount of intact hemoglobin and cause acute iron overload. Plus, due to the severe stress caused by the SARS-CoV-2 infection and inability to exchange carbon dioxide and oxygen, the lung cells will suffer severe stress and inflammatory changes often resulting in ground-glass-like lung images (Wenzhong and Hualan, 2020).
Hepcidin is the master regulator of systemic iron homeostasis. Dysregulation of hepcidin leads to altered iron homeostasis and the development of pathological disorders including hemochromatosis, and iron loading and iron-restricted anemias. Increased iron levels in the plasma and iron storage in the cells stimulate production of hepcidin which reduces iron overload by blocking iron absorption and its further storage until the iron levels are normalized. Action of progesterone is mediated by its binding to progesterone receptor membrane component-1 which regulates hepcidin biosynthesis (Blanchette et al., 2016, Li et al., 2016).
3.1.3.4. Thrombocytopenia:
Generally, with viruses, evidence suggests that inflammation of immune and non-immune cells may lead to an imbalance of pro- and anticoagulant states during infection (Bassam et al., 2020). Thrombocytopenia is common in patients with COVID‐19 with progression of disease. SARS-CoV-2 infection may reduce platelet production; it may increase platelet consumption, and may also increase platelet destruction (Xu et al., 2020). Platelet count can discriminate between severe and non-severe SARS-CoV-2 infections. COVID-19 patients who do not survive have a significantly lower platelet count than survivors. A substantial decrease in platelet count may be an indicator of worsening illness. Low platelet count is associated with increased risk of severe disease and mortality in patients with COVID-19 (Yang et al., 2020). Human platelets possess receptors for progesterone metabolites: pregnanolone, isopregnanediol, and pregnanediol. These are the most effective stimulators of signal transduction in platelets. They rapidly stimulate calcium influx in human platelets by a src-dependent pathway. Platelets get activated when these progesterone metabolites bind to the platelet receptors. Thus, progesterone metabolites may regulate platelet aggregation and hence thrombosis in vivo (Blackmore, 2008).
3.1.3.5. Electrolytes imbalance:
Serum electrolyte derangements with hypokalaemia secondary to SARS-CoV-2 infection is common (Mabillard and Sayer, 2020). The correction of hypokalaemia is challenging because of continuous renal potassium loss resulting from the degradation of ACE2 (Dong et al., 2020). Progesterone inhibits aldosterone binding to the mineralocorticoid receptor. Elabidaet al have provided strong evidence for an important role of progesterone to regulate potassium homeostasis and renal electrolyte balance (Elabida et al., 2011).
3.1.3.6. Anosmia, hyposmia, and dysgeusia:
The angiotensin converting enzyme 2 receptor, to which SARS-CoV-2 binds for entry into cells, is found in brain vascular endothelium and smooth muscle (Hamming et al., 2004). SARS-CoV-2 replicates in neuronal cells in vitro (Chu et al., 2020). Possible entry routes to the nervous system include carriage across the blood-brain barrier following viremia, or through infected leukocytes or through the olfactory bulb (Ellul et al., 2020). SARS-CoV-2 virus infection in the olfactory bulb manifests as anosmia, hyposmia, and dysgeusia either solely or along with other features of COVID-19 (Lao et al., 2020).
Relationships between reproductive hormones and human olfactory function are complex and simple associations between circulating levels of gonadal hormones and measures of olfactory function are rarely present (Doty and Cameron, 2009). It will be interesting to find effects of exogenous progesterone on lost smell and appetite of COVID-19 patients, especially in context of a study by McNeil J et al which has found greater total odor scores, explicit wanting for high-fat foods and lipid intake, in women during the mid-luteal phase, which is the highest progesterone level phase during the menstrual cycle (McNeil et al., 2013, Carmina et al., 2014).
3.1.4. Stage 4A. Viral load reduction
Immune attack results in reduction of the viral load. Referring to stage 1, progesterone could have a role in SARS-CoV-2 viral load reduction. It is also reported to reduce the virus infection enhancing activity of estrone (Giron et al., 1971). It is relevant to mention here the effect of progesterone on other viruses. In a laboratory analysis, a progesterone concentration of 5μg/mL in tissue culture medium depressed the production of new orthopox viral particles nearly totally. This antiviral protective activity of progesterone against pathogenic actions of orthopox viruses was further confirmed in vivo by using rabbits for intradermal infections (Pfahler et al., 1987).
3.1.5. Stage 5A. Specific Humoral immunity to SARS-CoV-2
Specific IgM antibodies to SARS-CoV-2 are generated in COVID-19 patients after 1 week of symptom onset, and reach their peak level in 2-3 weeks; after which they start to decrease. Specific IgG antibodies to SARS-CoV-2 develop later than the specific IgM antibodies. The specific IgG antibodies to SARS-CoV-2 are maintained at a high level for about 2 months. The time for development of specific humoral immunity is in sync with the recovery time of COVID-19 patients. For patients with mild disease, recovery time is about two weeks, while patients with severe or critical disease recover within three to six weeks (WHO Director-General's opening remarks at the media briefing on COVID-19 2020). Further the anti SARS-CoV-2 antibody levels differ significantly among COVID-19 patients with different illness severities and outcomes (Hou et al., 2020).
3.1.6. Stage 6A. Immune regulation
Regulation of cell-mediated immune attack is necessary to limit inflammation and avoid systemic injuries. When the viral load starts to diminish, the cell-mediated immune attack needs to get restrained in response. Regulatory T cells (Beissert et al., 2006), dendritic cells (Lipscomb and Masten, 2002), and structural cells participate in immune regulation (Krausgruber et al., 2020). Vital to the regulation of an ongoing cell-mediated attack is its balancing by development of specific humoral immunity to combat the infection.
Kyurkchiev D et al designate progesterone as a regulator of regulators (Kyurkchiev et al., 2010). Progesterone regulates T cells, B cells, natural killer cells, dendritic cells, and mesenchymal cells. It has a stimulating effect on the differentiation of monocytes into dendritic cells (Ivanova et al., 2005). Progesterone is shown to impact cell-mediated and humoral-helper cells differentiation, cytokine production, and increased Treg differentiation (Moulton, 2018).
3.1.7. Stage 7A. Repairs & 3.8 Stage 8A. Debris clearance
The above two stages along with the role of progesterone are detailed later in the manuscript along with these identical stages for immune dysregulation. To state in brief, progesterone assists in repair of the damage and clearance of the debris that may have accumulated from the infection and immune attack, making way for recovery.
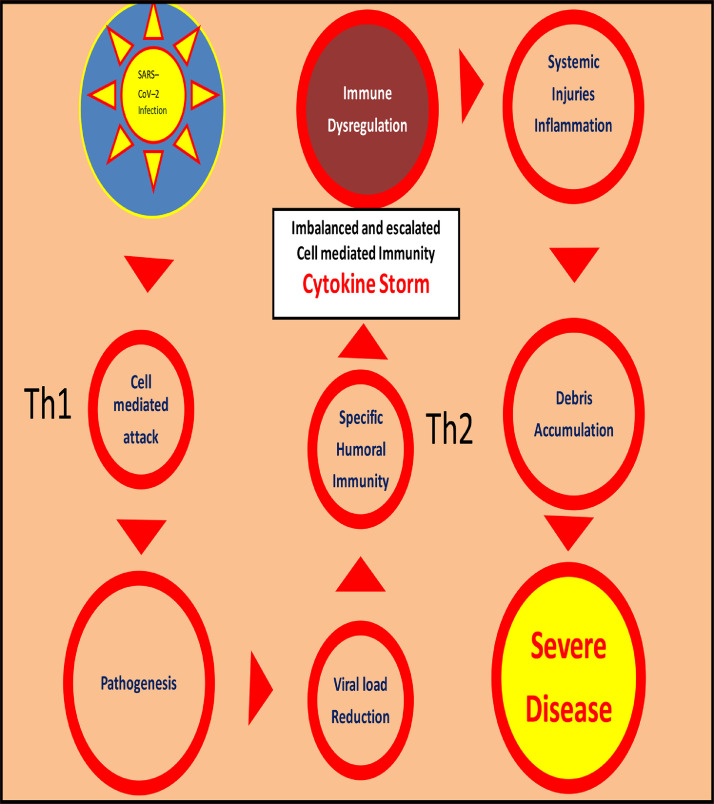
4. SARS-CoV-2 infection, immune dysregulation and regulatory role of progesterone
This section details research findings for COVID-19 from infection to severity as per the layout of Fig. 3. Immune dysregulation may indicate either suboptimum level or unavailability of progesterone or inability to meet the ’increased dysregulation importunity‘, indicating need for exogenous progesterone. The regulatory role of progesterone, as explained above, may fail partially or completely resulting in uncontrolled immune attack and pathogenesis with overproduction of proinflammatory cytokines (the cytokine storm), inflammation, systemic injuries, debris overload and severe illness.
Fig. 3.
Schematic illustration of immune dysregulation leading to severe COVID-19 disease.
4.1. Stages 1B to 5B
While the factors for increased dysregulation are detailed in part 2 of the manuscript, here the following sections highlight possible therapeutic regulatory actions of exogenous progesterone in COVID-19 patients from stage 6B to stage 8B of immune dysregulation (Fig. 3); the previous stages 1B to 5B being the same as the previously detailed stages 1A to 5A.
4.2. Stage 6B. Cytokine Storm and Inflammation
In many patients suffering from COVID-19 infection there is an aggressive hyperactive inflammatory response with release of large amounts of pro-inflammatory cytokines. Such an excessive production of cytokines is referred to as cytokine storm and is correlated directly with inflammation, lung injury, multi-organ failure, and poor prognosis of severe COVID-19 (Ragab et al., 2020). Steroid hormones are well-recognized regulators of the inflammatory response. The sex hormones have cell- and tissue-specific effects which can regulate inflammation. Progesterone is suggested as a corticosteroid-sparing, anti-inflammatory and anti-edema agent (Cheng et al., 2019). Progesterone signals through the progesterone receptor and to a lesser extent, through the glucocorticoid and mineralocorticoid receptors. Progesterone receptors are expressed on different immune cell types, including natural killer cells, macrophages, dendritic cells, and T cells, and thus progesterone could have broad anti-inflammatory effects on the immune system by decreasing leukocyte activation and production of pro-inflammatory mediators. Inhibition of nuclear factor kappa B is suggested to play a role in these effects of progesterone (Bereshchenko et al., 2018).
Progesterone can directly regulate cytokine levels and leukocyte trafficking in the progesterone receptor expressing tissues. In these tissues progesterone acts as an anti-inflammatory agent, with potential for the negative regulation of immune cell trafficking. Particularly, the proinflammatory cytokines, interleukins (IL), IL-6, IL-8; CXCL (C-X-C motif ligand), CXCL1, CXCL2, and CXCL3 are controlled by progesterone, both under physiological conditions and during pathological activation by lipopolysaccharide (Goddard et al., 2013). Comparisons of individuals with high and low c-reactive protein (CRP) levels revealed that those with high CRP had significantly lower progesterone (Clancy et al., 2013). In postmenopausal women, serum CRP levels tended to be reduced by progesterone treatment as compared to the pretreatment levels (Chaikittisilpa et al., 2003).
4.3. Stage 7B. Systemic injuries and repairs
The renin-angiotensin system (RAS) plays a main role in regulating blood pressure and maintaining fluid and electrolyte balance. RAS acts not only systemically, but also locally in a variety of tissues, including the lung. Maintenance of normal ACE2 levels in the lung is beneficial for the host to combat inflammatory lung disease (Hongpeng, 2016). Dysregulation of the RAS may play a central role in the pathophysiology of COVID-19 associated acute lung injury/acute respiratory distress syndrome (ALI/ARDS). RAS modulation may have a potential role in the treatment of patients with severe COVID-19 who are at risk for ALI/ARDS (Henry et al., 2020). It is important to note that angiotensin II has an inhibitory effect on progesterone and stimulatory effect on aldosterone (Johnson et al., 1997). Progesterone is a potent aldosterone antagonist (Oelkers, 2002). It is diuretic (Selye and Bassett, 1940), and it can reduce pericardial effusion. Its mineralocorticoid receptor antagonist property can have a therapeutic role in diseases of the cardiovascular system, primary aldosteronism, primary and resistant hypertension, heart failure, and chronic kidney disease (Guichard et al., 2013).
Occurrence of acute lung injury is a major determining factor of the severity of the SARS-CoV-2 infection. About 30% of ICU patients with COVID‐19 develop severe lung edema, dyspnea, hypoxemia, or acute respiratory distress syndrome (Liyang et al., 2020). Progesterone-based compounds modulate immune responses throughout the body, by binding to receptors located in immune cells including natural killer cells, macrophages, dendritic cells, and T cells, as well as in non-immune cells such as epithelial and endothelial cells. Progesterone-based compounds alter cellular signaling and activity to affect the outcome of infections at diverse mucosal sites (Hall and Klein, 2017). Progesterone-based treatments promote lung repair by increasing numbers of regulatory CD39+ Th17 cells in the lungs following clearance of influenza A viruses. Production of the epidermal growth factor amphiregulin is also increased following progesterone treatment. Amphiregulin promotes proliferation and repair of respiratory epithelial cells. Progesterone-based compounds reduce inflammation and expedite repair of lung tissue (Hall et al., 2016).
Neurological complications are common in patients suffering with severe COVID-19. Damage within the central nervous system or peripheral nervous system may be directly by the virus or by the body's immune attack on the SARS-CoV-2 infection. Some patients with COVID-19 may show nonspecific neurological symptoms, such as confusion and headache. A few patients with COVID-19 have more specific neurological manifestations, such as seizures or cerebrovascular problems. Neuroinvasion of SARS-CoV-2 may partially explain why some patients develop respiratory failure (Asadi-Pooya and Simani, 2020). Several hormones, including hypothalamic neuropeptides acting as neurotransmitters and neuromodulators in the central nervous system, are involved in the physiologic regulation of breathing and participate in adjustment of breathing in disease. In addition to central effects, some hormones also control breathing at peripheral chemoreceptors or have local effects on the lungs and airways (Tarja and Polo, 2002).
Progesterone is a neurosteroid that can be metabolized within all parts of the central nervous system. It is a neuromodulator with neuroprotective and neurogenic properties that can regulate neurotransmission and myelination (Schumacher et al., 2012, Schumacher et al., 2004). Progesterone has been shown to enhance brain mitochondrial energy metabolism, to decrease oxidative stress and reverse the decrease of mitochondrial respiration rate following brain injury. Actions of progesterone on mitochondrial function may participate in its neuroprotective properties (Gaignard et al., 2016). Low pulsatile doses of progesterone positively modulate neurotansmission (Ramirez and Dluzen, 1987). A study conducted on healthy adult males has concluded that Medroxyprogesterone acetate is capable of crossing the blood-brain barrier and potentially exerts its ventilatory stimulant effect by some central mechanism (Skatrud et al., 1978). Repeated low-dose post-ischemic progesterone treatment in animal studies is reported to reduce neurodegeneration induced by chronic cerebral hypoperfusion (Stnojlović et al., 2015). Dexamethasone is widely used in the management of brain edema. However, it can interact negatively with glioma therapy. Progesterone could serve as a viable alternative to dexamethasone in the management of brain edema (Cheng and Leung, 2015).
4.4. Stage 8B. Debris accumulation and clearance
Although essential for viral clearance, when excessive and extensive, the cytotoxic processes can compromise respiratory function through the loss of airway epithelium, disruption of the epithelial-endothelial barrier; and accumulation of apoptotic bodies and cellular debris in the airways. This can prolong severe inflammation and acute lung injury. As the body is recovering from an infection, it clears the virus litter—inactive debris of virus-infected cells—from the lungs. Neutrophils are the first cells recruited from the bloodstream to sites of infection. Their main function is phagocytosis. Neutrophils excel in eliminating infected cells and clearing post-immune attack debris. Neutrophils phagocytose microbial pathogens, virions, apoptotic bodies containing virus-infected dead cells, and debris (Newton et al., 2016). It has been shown that progesterone enhances chemotactic activity of neutrophils (Miyagi et al., 1992).
Apoptotic bodies and debris left by apoptotic neutrophils are then cleared by macrophages. Macrophages are involved in both innate and adaptive immune responses. As a preface to the immunity pattern concept introduced in part 2 of this manuscript, it would be interesting to mention here that depending on the types of cytokines that macrophages are exposed to, they are subjected to classical (Thelper cell 1) or alternative (Thelper cell 2) activation. In the first case, macrophages, particularly when activated by interferon gamma or by lipopolysaccharide, have the capacity to destroy the remaining microorganisms, intracellularly and extracellularly, in the inflammatory loci through the production of nitric oxide and other intermediates (Malysheva et al., 2006).
In the second case, after exposure to cytokines such as IL-4, IL-10, or IL-13, macrophages produce polyamines and proline, which induce proliferation, collagen production, and repairs (Classen et al., 2009). Progesterone has ability to selectively inhibit and augment the alternative macrophage activation (Menzies et al., 2011).
5. Conclusion
To conclude, progesterone has multifaceted functions. It could contribute by: antiviral activity on SARS-CoV-2, interference with the binding of SARS-CoV-2 to ACE2, recovery from pathogenesis, reduction in viral load, immune regulation, and hastened recovery from COVID-19. Progesterone could play a therapeutic regulatory role in the events of immune dysregulation and its related severe inflammatory morbidity in COVID-19 (Table 2 ). Part 2 of the present research evaluates the regulatory role of progesterone with respect to immunity patterns and severity of COVID-19. The present study indicates for future observational, clinical and pharmaceutical studies.
Table 2.
Progesterone: a therapeutic regulator for the immune dysregulation and systemic damages in COVID-19.
| COVID-19 | Progesterone |
|---|---|
| Cytokine storm | Regulates T cells, NK cells, dendritic cells, B cells and medicinal signaling cells. Regulates cytokine levels and leukocyte trafficking, reduces CRP levels. Is an anti-inflammatory and anti-edema agent. |
| Renin-angiotensin system | Aldosterone antagonist, diuretic, therapeutic for kidney functions. |
| Cardiovascular system | Reduces pericardial effusion, therapeutic for hypertension and heart failure. |
| Lung injuries | Increases amphiregulin production and lung repairs. |
| Nervous system | Neurosteroid and neuromodulator, with neuroprotective & neurogenic properties. |
| Debris accumulation | Enhances neutrophil chemotaxis and phagocytosis. |
Declaration of Competing Interest
None.
Acknowledgments
Acknowledgments
Author conveys sincere gratitude towards all her teachers. She thanks each of the authors for their referred work in the present manuscript. As this research is carried out during the SARS-CoV-2 pandemic lockdown period, author acknowledges her family for this work. She respectfully dedicates this manuscript to her parents Mrs. Hiraben Shah & Mr. Bhupatrai Shah, and brother Mr. Ajay Shah & SIL Mrs. Vijaya Shah for their blessings. Fond thanks to nieces Dr. Prathna and Aarshna for taking keen interest in the study and always wishing the best.
Author's Contributions
Conceptualization of the present research; reference work; published data collection; writing of the manuscript; validation of the concept; visualization and drawing of figures to explain the concepts; reviewing and editing the manuscript; and submission of the work to this journal. No medical writer or editor was involved in the creation of this manuscript.
Human And Animal Rights
No humans have participated in this research. No animals are used for this study.
Availability Of Data
There is no unreleased data for this study.
Funding
The present study had no funding source.
References
- Antwi-Amoabeng D., Beutler B.D., Moody A.E., Kanji Z., Gullapalli N., Rowan C.J. Management of hypertension in COVID-19. World J Cardiol. 2020;12(5):228–230. doi: 10.4330/wjc.v12.i5.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asadi-Pooya A.A., Simani L. Central nervous system manifestations of COVID-19: A systematic review. J Neurological Sciences. 2020:413. doi: 10.1016/j.jns.2020.116832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askew D., Silverman G. Intracellular and extracellular serpins modulate lung disease. J Perinatol. 2008;28:S127–S135. doi: 10.1038/jp.2008.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azouz N.P., Klingler A.M., Callahan V., Alpha 1 Antitrypsin is an Inhibitor of the SARS-CoV-2-Priming protease TMPRSS2. Preprint. bioRxiv. 2020 doi: 10.1101/2020.05.04.077826. [DOI] [PMC free article] [PubMed]
- Baker M.E. What are the physiological estrogens? Steroids. 2013;78(3):337–340. doi: 10.1016/j.steroids.2012.12.011. [DOI] [PubMed] [Google Scholar]
- Barbagallo M., Dominguez L.J., Licata G. Vascular effects of progesterone. Role of cellular calcium regulation. Hypertension. 2001;37:142–147. doi: 10.1161/01.hyp.37.1.142. [DOI] [PubMed] [Google Scholar]
- Bassam A., Saad I.M., Wael A.I.M. Anticoagulation in COVID-19. European Heart J Cardiovascular Pharmacotherapy. 2020;6(4):260–261. doi: 10.1093/ehjcvp/pvaa036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beissert S., Schwarz A., Schwarz T. Regulatory T cells. J Invest Dermatol. 2006;126(1):15–24. doi: 10.1038/sj.jid.5700004. [DOI] [PubMed] [Google Scholar]
- Ben-Harari R.R., Amit T., Youdim M.B. Binding of oestradiol, progesterone and prolactin in rat lung. J Endocrinol. 1983;97(2):301–310. doi: 10.1677/joe.0.0970301. [DOI] [PubMed] [Google Scholar]
- Bereshchenko O., Bruscoli S., Riccardi C. Glucocorticoids, sex hormones, and immunity. Front Immunol. 2018;9:1332. doi: 10.3389/fimmu.2018.0133220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beynon H.L., Garbett N.D., Barnes P.J. Severe premenstrual exacerbations of asthma: effect of intramuscular progesterone. Lancet. 1988;2:370–372. doi: 10.1016/s0140-6736(88)92837-1. [DOI] [PubMed] [Google Scholar]
- Bhatraju P.K., Ghassemieh B.J., Nichols M. Covid-19 in critically ill patients in the Seattle Region — case series. NEJM. 2020;382:2012–2022. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackmore P.F. Progesterone metabolites rapidly stimulate calcium influx in human platelets by a src-dependent pathway. Steroids. 2008;73(7):738–750. doi: 10.1016/j.steroids.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Blanchette N.L., Manz D.H., Torti F.M., Torti S.V. Modulation of hepcidin to treat iron deregulation: potential clinical applications. Expert Rev Hematol. 2016;9(2):169–186. doi: 10.1586/17474086.2016.1124757. 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camper N., Glasgow A.M., Osbourn M. A secretory leukocyte protease inhibitor variant with improved activity against lung infection. Mucosal Immunol. 2016;9(3):669–676. doi: 10.1038/mi.2015.90. [DOI] [PubMed] [Google Scholar]
- Carmina E., Stanczyk F.Z., Lobo R.A. Chapter 34 - Laboratory Assessment. Book: Yen & Jaffe's Reproductive Endocrinology. Imprint: Saunders. 2014 (7th Edition) Editors: Jerome Strauss and Robert Barbieri. 822-850. [Google Scholar]
- Cavezzi A., Troiani E., Corrao S. COVID-19: hemoglobin, iron, and hypoxia beyond inflammation. A narrative review. Clin Pract. 2020;10(2):1271. doi: 10.4081/cp.2020.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecchini R., Cecchini A.L. SARS-CoV-2 infection pathogenesis is related to oxidative stress as a response to aggression. Med Hypotheses. 2020;143 doi: 10.1016/j.mehy.2020.110102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaikittisilpa S., Chang L., Burry K., Mishra R.G., Hermsmeyer K., Stanczyk F.Z. Effects of low-dose transdermal progesterone on cardiovascular predictors: CRP, IL-6, and triglycerides. Fertility Sterility. 2003;79(2):p22. doi: 10.1016/S0015-0282(03)00130-4. [DOI] [Google Scholar]
- Cheng S.Y., Leung G.K.K. Can progesterone be a better alternative to dexamethasone for use in routine brain surgery? Neural Regen Res. 2015;10(9):1379–1380. doi: 10.4103/1673-5374.165221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y., Yeung W.L., Zhang P., Li N., Kiang M.Y., Leung G.K.K. Progesterone is more effective than Dexamethasone in prolonging overall survival and preserving neurologicfunction in experimental animals with orthotopic glioblastoma allografts. World Neurosurg. 2019;125:e497–e507. doi: 10.1016/j.wneu.2019.01.113. [DOI] [PubMed] [Google Scholar]
- Christopher M.P., Simon A.J., Jie Y. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369 doi: 10.1136/bmj.m1966. m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu H., Chan J.F.W., Yuen T.T.T. Comparative tropism, replication kinetics, and cell damage profiling of SARS-CoV-2 and SARS-CoV with implications for clinical manifestations, transmissibility, and laboratory studies of COVID-19: an observational study. Lancet Microbe. 2020;1:e14–e23. doi: 10.1016/S2666-5247(20)30004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy K.B.H., Klein L.D., Ziomkiewicz A., Nenko I., Jasienska G., Bribiescas R.G. Relationships between biomarkers of inflammation, ovarian steroids, and age at menarche in a rural polish sample. Am J Hum Biol. 2013;25:389–398. doi: 10.1002/ajhb.22386. [DOI] [PubMed] [Google Scholar]
- Classen A., loberas J., Celada A. Macrophage activation: Classical vs. alternative. In: Reiner N. (eds) Macrophages and Dendritic Cells. Methods in Molecular Biology™ (Methods and Protocols). Humana Press USA 2009;531.
- Cooke P.S., Nanjappa M.K., Ko C., Prins G.S., Hess R.A. Estrogens in male physiology. Physiol Rev. 2017;97(3):995–1043. doi: 10.1152/physrev.00018.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLeo V., Musacchia M.C., Cappelli V., Piomboni P., Morgante G. Hormonal contraceptives: pharmacology tailored to women‘s health. Human Reproduction Update. 2016;22(5):634–646. doi: 10.1093/humupd/dmw016. 16. [DOI] [PubMed] [Google Scholar]
- Dong C., Xiaokun L., Qifa S. Assessment of hypokalemia and clinical characteristics in patients with coronavirus disease 2019 in Wenzhou. China. JAMA Netw Open. 2020;3(6) doi: 10.1001/jamanetworkopen.2020.11122. e2011122 doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosiou C., Hamilton A.E., Pang Y., Overgaard M.T., Tulac S., Dong J. Expression of membrane progesterone receptors on human T lymphocytes and Jurkat cells and activation of G-proteins by progesterone. J Endocrinol. 2008;196(1):67–77. doi: 10.1677/JOE-07-0317. [DOI] [PubMed] [Google Scholar]
- Doty R.L., Cameron E.L. Sex differences and reproductive hormone influences on human odor perception. PhysiolBehav. 2009;97(2):213–228. doi: 10.1016/j.physbeh.2009.02.032. Erratum in: PhysiolBehav 2009;98(4):517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doumas S., Kolokotronis A., Stefanopoulos P. Anti-inflammatory and antimicrobial roles of secretory leukocyte protease inhibitor. Infect Immun. 2005;73(3):1271–1274. doi: 10.1128/IAI.73.3.1271-1274.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elabida B., Edwards A., Salhi A. Chronic potassium depletion increases adrenal progesterone production that is necessary for efficient renal retention of potassium. Kidney Int. 2011;80:256–262. doi: 10.1038/ki.2011.15. [DOI] [PubMed] [Google Scholar]
- Ellul M.A., Benjamin L., Singh B. Neurological associations of COVID-19. Lancet Neurology. 2020;19(9):767–783. doi: 10.1016/S1474-4422(20)30221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Fernández F.J. COVID-19, hypertension and angiotensin receptor-blocking drugs. J Hypertens. 2020;38(6):1191. doi: 10.1097/HJH.0000000000002468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchini M., Targher G., Montagnana M., Lippi G. Iron and thrombosis. Ann Hematol. 2008;87(3):167–173. doi: 10.1007/s00277-007-0416-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaignard P., Fréchou M., Schumacher M. Progesterone reduces brain mitochondrial dysfunction after transient focal ischemia in male and female mice. J Cereb Blood Flow Metab. 2016;36(3):562–568. doi: 10.1177/0271678X15610338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gary A.S., James C.W., Stephen P.B. Serpins flex their muscle I. Putting the clamps on proteolysis in diverse biological systems. J Biol Chem. 2010;285:24299–24305. doi: 10.1074/jbc.R110.112771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giron D.J., Schmidt J.P., Pindak F.F. Effect of progesterone and testosterone on interferon production and on the viral infection-enhancing activity of estrone and hydrocortisone. Infect Immun. 1971;4(5):537–540. doi: 10.1128/iai.4.5.537-540.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddard L.M., Ton A.N., Org T., Mikkola H.K., Iruela-Arispe M.L. Selective suppression of endothelial cytokine production by progesterone receptor. VasculPharmacol. 2013;59(1-2):36–43. doi: 10.1016/j.vph.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodland R.L., Reynolds J.G., McCoord A.B., Pommerenke W.T. Respiratory and electrolyte effects induced by estrogen and progesterone. FertilSteril. 1953;4:300–316. doi: 10.1016/s0015-0282(16)31327-9. [DOI] [PubMed] [Google Scholar]
- Gordon D.E., Jang G.M., Bouhaddou M. A SATS-CpV-2 protein interaction map reveals targets for drug repurposing. Nature. 2020;583(7816):459–468. doi: 10.1038/s41586-020-2286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guichard J.L., Clark D., Calhoun D.A., Ahmed M.I. Aldosterone receptor antagonists: current perspectives and therapies. Vascular Health Risk Management. 2013;9:321–323. doi: 10.2147/VHRM.S33759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall O.J., Klein S.L. Progesterone-based compounds affect immune responses and susceptibility to infections at diverse mucosal sites. Mucosal Immunol. 2017;10:1097–1107. doi: 10.1038/mi.2017.35. [DOI] [PubMed] [Google Scholar]
- Hall O.J., Limjunyawong N., Vermillion M.S., Robinson D.P., Wohlgemuth N., Pekosz A. Progesterone-based therapy protects against influenza by promoting lung repair and recovery in females. PLoSPathog. 2016;12(9) doi: 10.1371/journal.ppat.1005840. e1005840 doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamming I., Timens W., Bulthuis M.L., Lely A.T., Navis G., vanGoor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T., Su T. The sigma receptor: evolution of the concept in neuropsychopharmacology. CurrNeuropharmacol. 2005;3(4):267–280. doi: 10.2174/157015905774322516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry B.M., Vikse J., Lippi G. Rapid Response: COVID-19 induced Renin-Angiotensin System (RAS) imbalance may drive acute lung injury: the evidence and therapeutic options. Re: Response to the emerging novel coronavirus outbreak. BMJ. 2020;368 m406 DOI: 368/bmj.m406/rr-19. [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hongpeng J. Pulmonary angiotensin-converting enzyme 2 (ACE2) and inflammatory lung disease. Shock. 2016;46(3):239–248. doi: 10.1097/SHK.0000000000000633. [DOI] [PubMed] [Google Scholar]
- Hou H., Wang T., Zhang B. Detection of IgM and IgG antibodies in patients with coronavirus disease 2019. Clin Transl Immunology. 2020;9(5):e01136. doi: 10.1002/cti2.1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntington N.D., Gray D.H. Immune homeostasis in health and disease. Immunol Cell Biol. 2018;96:451–452. doi: 10.1111/imcb.12043. [DOI] [PubMed] [Google Scholar]
- Ivanova E., Kyurkchiev D., Altankova I., Dimitrov J., Binakova E., Kyurkchiev S. CD83 monocyte-derived dendritic cells are present in human decidua and progesterone induces their differentiation in vitro. Am J Reprod Immunol. 2005;53(4):199–205. doi: 10.1111/j.1600-0897.2005.00266.x. [DOI] [PubMed] [Google Scholar]
- Jakob R., Kasinath V., Alexandra L. Serpins the vasculature, and viral therapeutics. Front Biosci. 2005;11:1042–1056. doi: 10.2741/1862. [DOI] [PubMed] [Google Scholar]
- Jiawei C., Quanlong J., Xian X. Individual variation of the SARS-CoV-2 receptorACE2 gene expression and regulation. Aging Cell. 2020;19(7) doi: 10.1111/acel.13168. e13168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J.M., Bai P., He W. Gender differences in patients with COVID-19: Focus on severity and mortality. Front Public Health. 2020;8:152. doi: 10.3389/fpubh.2020.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannessen M., Fontanilla D., Mavlyutov T., Ruoho A.E., Jackson M.B. Antagonist action of progesterone at σ-receptors in the modulation of voltage-gated sodium channels. Am J Physiol Cell Physiol. 2011;300(2) doi: 10.1152/ajpcell.00383.2010. C328-37. Erratum in: Am J Physiol Cell Physiol. 2013 ;305(9):C997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M.C., Vega M., Vantman D., Troncoso J.L., Devoto L. Regulatory role of angiotensin II on progesterone production by cultured human granulosa cells. Expression of angiotensin II type-2 receptor. Molecular Human Reproduction. 1997;3(8):663–668. doi: 10.1093/molehr/3.8.663. [DOI] [PubMed] [Google Scholar]
- Krausgruber T., Fortelny N., Fife-Gernedl V. Structural cells are key regulators of organ-specific immune responses. Nature. 2020;583(7815):296–302. doi: 10.1038/s41586-020-2424-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyurkchiev D., Ivanova-Todorova E., Kyurkchiev S.D. New target cells of the immunomodulatory effects of progesterone. Reprod Biomed Online. 2010;21(3):304–311. doi: 10.1016/j.rbmo.2010.04.014. [DOI] [PubMed] [Google Scholar]
- Lao W.P., Imam S.A., Nguyen S.A. Anosmia, hyposmia, and dysgeusia as indicators for positive SARS-CoV-2 infection. World J Otorhinolaryngol Head Neck Surg. 2020 doi: 10.1016/j.wjorl.2020.04.001. doi: 10.1016/j.wjorl.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauretta R., Sansone M., Sansone A., Romanelli F., Appetecchia M. Gender in Endocrine Diseases: Role of Sex Gonadal Hormones. Int J Endocrinol. 2018 doi: 10.1155/2018/4847376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei F.a.n.g., Karakiulakis G., Michael R. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8(4) doi: 10.1016/S2213-2600(20)30116-8. e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- León-Velarde F., Rivera-Chira M., Tapia R., Huicho L., Monge-C C. Relationship of ovarian hormones to hypoxemia in women residents of 4,300 m. American J Physiology-Regulatory. Integrative Comparative Physiology. 2001;280(2):R488–R493. doi: 10.1152/ajpregu.2001.280.2.R488. [DOI] [PubMed] [Google Scholar]
- Li X., Rhee D.K., Malhotra R. Progesterone receptor membrane component-1 regulates hepcidin biosynthesis. J Clin Invest. 2016;126:389–401. doi: 10.1172/JCI83831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippi G., Wong J., Henry B.M. Hypertension in patients with coronavirus disease 2019 (COVID-19): a pooled analysis. Pol Arch Intern Med. 2020;130(4):304–309. doi: 10.20452/pamw.15272. [DOI] [PubMed] [Google Scholar]
- Lipscomb M.F., Masten B.J. Dendritic cells: immune regulators in health and disease. Physiol Rev. 2002;82(1):97–130. doi: 10.1152/physrev.00023.2001. [DOI] [PubMed] [Google Scholar]
- Liyang L., Qihong H., Diane C.W., David H.I., Xiangdong W. Acute lung injury in patients with COVID-19 infection. Clinical Translational Med. 2020;10(1) doi: 10.1002/ctm2.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons H.A., Huang C.T. Therapeutic use of progesterone in alveolar hypoventilation associated with obesity. American J Medicine. 1968;44(6):P881–P888. doi: 10.1016/0002-9343(68)90088-0. 17. [DOI] [PubMed] [Google Scholar]
- Mabillard H., Sayer J.A. Electrolyte disturbances in SARS-CoV-2 infection. Fl000Research. 2020;9:587. doi: 10.12688/f1000research.24441.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malysheva E.V., Kruglov S.V., Khomenko I.P., Bakhtina L.Y., Pshennikova M.G., Manukhina E.B., Malyshev I.Y. Role of extracellular and intracellular nitric oxide in the regulation of macrophage responses. Bull Exp Biol Med. 2006;141(4):404–406. doi: 10.1007/s10517-006-0183-3. [DOI] [PubMed] [Google Scholar]
- Mannan S., Begum N. Correlation of serum level of progesterone with peak expiratory flow rate (PEFR) in different phases of menstrual cycle. Anwer Khan Mod Med Coll J. 2012;3:6–9. [Google Scholar]
- Maurice T., Su T.P. The pharmacology of sigma-1 receptors. PharmacolTher. 2009;124(2):195–206. doi: 10.1016/j.pharmthera.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry T., Biniecka M., Veale D.J., Fearon U. Hypoxia, oxidative stress and inflammation. Free Radic Biol Med. 2018;125:15–24. doi: 10.1016/j.freeradbiomed.2018.03.042. [DOI] [PubMed] [Google Scholar]
- McNeil J., Cameron J.D., Finlayson G., Blundell J.E., Doucet É. Greater overall olfactory performance, explicit wanting for high fat foods and lipid intake during the mid-luteal phase of the menstrual cycle. PhysiolBehav. 2013 doi: 10.1016/j.physbeh.2013.02.008. 112-113:84-9.19. [DOI] [PubMed] [Google Scholar]
- Menzies F.M., Henriquez F.L., Alexander J., Roberts C.W. Selective inhibition and augmentation of alternative macrophage activation by progesterone. Immunology. 2011;134(3):281–291. doi: 10.1111/j.1365-2567.2011.03488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer M., Jaspers I. Respiratory protease/antiprotease balance determines susceptibility to viral infection and can be modified by nutritional antioxidants. Am J Physiol Lung Cell Mol Physiol. 2015;308(12):L1189–L1201. doi: 10.1152/ajplung.00028.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyagi M., Aoyama H., Morishita M., Iwamoto Y. Effects of sex hormones on chemotaxis of human peripheral polymorphonuclear leukocytes and monocytes. J Periodontol. 1992;63(1):28–32. doi: 10.1902/jop.1992.63.1.28. [DOI] [PubMed] [Google Scholar]
- Moulton V.R. Sex hormones in acquired immunity and autoimmune disease. FrontImmunol. 2018;9:2279. doi: 10.3389/fimmu.2018.02279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton A.H., Cardani A., Braciale T.J. The host immune response in respiratory virus infection: balancing virus clearance and immunopathology. Semin Immunopathol. 2016;38(4):471–482. doi: 10.1007/s00281-016-0558-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oelkers W. Antimineralocorticoid activity of a novel oral contraceptive containing drospirenone, a unique progestogen resembling natural progesterone. Eur J Contracept Reprod Health Care. 2002;7(3):19–43. [PubMed] [Google Scholar]
- Pfahler W.H., Reimann M., Munz E. Influence of progesterone on orthopox viruses in vitro and in vivo. ZentralblVeterinarmed B (J Veterinary Medicine. Series B) 1987;34(9):684–690. doi: 10.1111/j.1439-0450.1987.tb00449.x. [DOI] [PubMed] [Google Scholar]
- Pozzilli P., Lenzi A. Commentary: Testosterone, a key hormone in the context of COVID-19 pandemic. Metabolism. 2020;108 doi: 10.1016/j.metabol.2020.154252. doi: 10.1016/j.metabol.2020.154252 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragab D., Salah E.H., Taeimah M., Khattab R., Salem R. The COVID-19 cytokine storm; What we know so far. Front Immunol. 2020;11:1446. doi: 10.3389/fimmu.2020.01446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez V.D., Dluzen D. Is Progesterone a pre-hormone in the CNS? Steroid Biochem. 1987;27(1-3):589–598. doi: 10.1016/0022-4731(87)90358-x. [DOI] [PubMed] [Google Scholar]
- Schumacher M., Guennoun R., Robert F. Local synthesis and dual actions of progesterone in the nervous system: neuroprotection and myelination. Growth Horm IGF Res. 2004;14(A):S18–S33. doi: 10.1016/j.ghir.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Schumacher M., Hussain R., Gago N., Oudinet J.P., Mattern C., Ghoumari A.M. Progesterone synthesis in the nervous system: implications for myelination and myelin repair. Front Neurosci. 2012;6:10. doi: 10.3389/fnins.2012.0001021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedrani S.H., El-Hinnawi S.L., Warsy A.S. Establishment of a normal reference range for alpha 1-antitrypsin in Saudis using rate nephelometry. Am J Med Sci. 1988;296(1):22–26. doi: 10.1097/00000441-198807000-00005. [DOI] [PubMed] [Google Scholar]
- Selye H., Bassett L. Diuretic effect of progesterone. Proceedings Soc Exper Biol Med. 1940;44(2):502–504. [Google Scholar]
- Shilpa S., Roby R. Instant analgesic effect of sublingual progesterone dilution in fibromyalgia. Austin J Womens Health. 2019;6(1):1032. March 4, 2019. https://austinpublishinggroup.com/womens-health/fulltext/ajwh-v6-id1032.pdf. [Google Scholar]
- Sidharthan N.P., Rao A.J. Regulation of expression of secretory leukocyte protease inhibitor by progesterone in BeWo choriocarcinoma cells. J Steroids Hormon Sci. 2011;2:107. doi: 10.4172/2157-7536.1000107. [DOI] [Google Scholar]
- Skatrud J.B., Dempsey J.A., Kaiser D.G. Ventilatory response to Medroxyprogesterone Acetate in normal subjects: Time course and mechanism. J Appl Physiol Respir Environ ExercPhysiol. 1978;44(6):939–944. doi: 10.1152/jappl.1978.44.6.939. [DOI] [PubMed] [Google Scholar]
- Stertz S., Reichelt M., Spiegel M., Kuri T., Martínez-Sobrido L., García-Sastre A., Weber F., Kochs G. The intracellular sites of early replication and budding of SARS-coronavirus. Virology. 2007;361(2):304–315. doi: 10.1016/j.virol.2006.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stnojlović M., Guševac I., Grković I. Effects of chronic cerebral hypoperfusion and low-dose progesterone treatment on apoptotic processes, expression and subcellular localization of key elements within Akt and Erk signaling pathways in rat hippocampus. Neuroscience. 2015;311:308–321. doi: 10.1016/j.neuroscience.2015.10.040. [DOI] [PubMed] [Google Scholar]
- Sutton F.D., Jr, Zwillich C.W., Creagh C.E., Pierson D.J., Weil J.V. Progesterone for outpatient treatment of Pickwickian syndrome. Ann Intern Med. 1975;83(4):476–479. doi: 10.7326/0003-4819-83-4-476. [DOI] [PubMed] [Google Scholar]
- Tarja S., Polo O. Hormones and breathing. Chest. 2002;122(6):2165–2182. doi: 10.1378/chest.122.6.2165. [DOI] [PubMed] [Google Scholar]
- Thomas P., Pang Y. Protective actions of progesterone in the cardiovascular system: potential role of membrane progesterone receptors (mPRs) in mediating rapid effects. Steroids. 2013;78(6):583–588. doi: 10.1016/j.steroids.2013.01.003. [DOI] [PubMed] [Google Scholar]
- Tyler J.M. The effect of progesterone on the respiration of patients with emphysema and hypercapnia. J Clin Invest. 1960;39(1):34–41. doi: 10.1172/JCI104024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vela J.M. Repurposing Sigma-1 receptor ligands for COVID-19 therapy? Front Pharmacol. 2020;11 doi: 10.3389/fphar.2020.582310. 582310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wambier C.G., Goren A., Ossimetha A., Nau G., Qureshi A. Androgen driven COVID-19 pandemic theory. SSRN Electronic J. 2020 doi: 10.21.39/ssrn. [Google Scholar]
- Wenzhong L., Hualan L. COVID-19: Attacks the 1-Beta chain of hemoglobin and captures the porphyrin to inhibit human heme metabolism. Preprint. ChemRxiv. 2020 doi: 10.26434/chemrxiv.11938173.v7. [DOI] [Google Scholar]
- WHO Director-General's opening remarks at the media briefing on COVID-19. Published February 24, 2020, https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19—24-february-2020
- Xu P., Zhou Q., Xu J. Mechanism of thrombocytopenia in COVID-19 patients. Ann Hematol. 2020;99(6):1205–1208. doi: 10.1007/s00277-020-04019-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazato M., Yamazato Y., Sun C., Diez-Freire C., Raizada M.K. Overexpression of Angiotensin-Converting Enzyme 2 in the rostral ventrolateral medulla causes long-term decrease in blood pressure in the spontaneously hypertensive rats. Hypertension. 2007;49:926–931. doi: 10.1161/01.HYP.0000259942.38108.20. [DOI] [PubMed] [Google Scholar]
- Yang X., Yang Q., Wang Y. Thrombocytopenia and its association with mortality in patients with COVID-19. J ThrombHaemost. 2020;18(6):1469–1472. doi: 10.1111/jth.14848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarazúa A., González-Arenas A., Ramírez-Vélez G., Bazán-Perkins B., Guerra-Araiza C., Campos-Lara M.G. Sexual dimorphism in the regulation of estrogen, progesterone, and androgen receptors by sex steroids in the rat airway smooth muscle cells. Int J Endocrinol. 2016 doi: 10.1155/2016/8423192. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
There is no unreleased data for this study.