Abstract
Introduction
Corona-virus Disease 2019 (COVID-19) has had a huge impact on the delivery of healthcare worldwide, particularly elective surgery. There is a lack of data regarding risk of postoperative COVID-19 infection in children undergoing elective surgery, and regarding the utility of pre-operative COVID-19 testing, and preoperative “cocooning” or restriction of movements. The purpose of this present study was to examine the safety of elective paediatric Otolaryngology surgery during the COVID-19 pandemic with respect to incidence of postoperative symptomatic COVID-19 infection or major respiratory complications.
Materials and methods
Prospective cohort study of paediatric patients undergoing elective Otolaryngology surgery between September and December 2020. Primary outcome measure was incidence of symptomatic COVID-19 or major respiratory complications within the 14 days after surgery. Parents of prospectively enrolled patients were contacted 14 days after surgery and enquiry made regarding development of postoperative symptoms, COVID-19 testing, or diagnosis of COVID-19.
Results
302 patients were recruited. 125 (41.4%) underwent preoperative COVID-19 RT-PCR testing. 66 (21.8%) restricted movements prior to surgery. The peak 14-day COVID-19 incidence during the study was 302.9 cases per 100,000 population. No COVID-19 infections or major respiratory complications were reported in the 14 day follow-up period.
Conclusion
The results of our study support the safety of elective paediatric Otolaryngology surgery during the pandemic, in the setting of community incidence not exceeding that observed during the study period.
Keywords: COVID, Pediatric otolaryngology, Patient outcomes, Perioperative care
1. Introduction
The corona disease 2019 (COVID-19) pandemic has had a major adverse impact on surgical services globally. Elective surgery has been particularly impacted. During the initial stages of the outbreak, much hospital capacity was diverted to the care of patients with COVID-19. However, even as the initial surge passed, concerns persisted regarding the safety of elective surgery, due to the risks of elective surgical patients becoming symptomatic with COVID-19 in the peri-operative period, with risks of severe respiratory complications, as well as risk of nosocomial outbreaks. At the same time, risks due to delay or cancellation of elective procedures have increased. To minimize the risks of elective surgery, new protocols have been introduced, encompassing additional protectives measures such as personal protective equipment, social distancing within healthcare facilities, and pre-operative testing of patients booked for elective surgery.
The considerations for elective paediatric surgery in the context of the pandemic are different than to the adult population. Firstly, the available data suggests that children are less susceptible to acquiring COVID-19 than adults [1], particularly those younger than 12–14 years [2]. In the USA, children <18 years account for approximately 10–12% of laboratory confirmed cases to the Centres for Disease Control and Prevention [3]. Secondly, children who do acquire COVID-19 are more likely to have a very mild disease course, with only 2.5–4% requiring hospitalization [4], and an estimated 26% of cases remaining asymptomatic [5]. However, severe disease can develop among children with COVID-19, with critical care requirement reported up to 33% among children requiring hospitalization [6]. Of note, while high case fatality ratios of 20–25% have been reported among adults who develop COVID-19 in the peri-operative period, there is minimal data on corresponding outcomes in children [7,8]. Finally, preoperative testing of children is more problematic than adults, due to greater difficulty obtaining nasopharyngeal swabs, which can be an unpleasant experience for small children and poor co-operation, which may reduce the accuracy of the results.
The purpose of the present study was to assess the safety of elective paediatric surgery during the COVID-19 pandemic with respect to perioperative COVID-19 infection, by determination of the risk of developing COVID-19 or respiratory complications, within 14 days of surgery, among paediatric patients undergoing elective Otolaryngology surgery in Ireland during the COVID-19 pandemic.
2. Materials and methods
2.1. Study design
Ethical approval for this study was granted by the National Research Ethics Committee on August 13th 2020 (20-NREC-COV-087). Study design was aided by the National Office of Clinical Audit and the Department of Surgical Affairs, Royal College of Surgeons in Ireland (RCSI). Statistical support was provided by the Data Science Centre, RCSI. Institutional approval was granted by all local sites prior to data collection.
A prospective cohort study was carried out in five hospital sites (Children's Health Ireland at Crumlin, Dublin and Children's Health Ireland at Temple Street, Dublin; University Hospital Galway; University Hospital Waterford; and the South Infirmary Victoria University Hospital, Cork). Two of the participating hospitals are dedicated children's hospitals and national paediatric referral centres for tertiary paediatric otolaryngology care. The other three hospitals were also adult hospitals. All served as regional otolaryngology referral centres, with children admitted to dedicated pediatric wards. 4 of the 5 hospitals were ‘COVID-receiving’ hospitals, and one (Cork) non-COVID receiving.
Inclusion criteria were all paediatric patients presenting for elective otolaryngology surgery between September 5 and December 18, 2020. Exclusion criteria were patients presenting for emergency surgery. Paediatric was defined as aged less than 16 years at the time of surgery. Parents were invited to participate in the study by a member of the surgical team at the time of hospital admission. Parents agreeable to participate were then formally contacted by the research coordinator in the days immediately after surgery, and informed consent for the study was given over the telephone.
Study data were collected and managed using a secure web-based software platform REDCap hosted at the RCSI [9]. Variables collected included patient demographics, diagnosis, details of surgery performed, whether patients had “cocooned” (self-isolated) prior to hospital admission (including a cessation of educational activities), pre-operative screening procedures, length of hospital stay, and post-operative cocooning.
Prior to hospital admission, standard pre-operative questionnaires, covering details of any respiratory symptoms, any contact with known case of COVID-19, or travel outside Ireland within the preceding 2 weeks, were filled by parents (Appendix A). In addition, in Crumlin and Cork, preoperative testing with reverse transcriptase-polymerase chain reaction (RTPCR, AllplexTM 2019 nCoV Assay, Seegene Inc.) on nasopharyngeal and oropharyngeal swabs, taken within 48 h of hospital admission, was performed. Only patients who completed the 14 day follow up are included in the results.
Primary outcome measure was the development of symptomatic COVID-19 or any respiratory symptoms in the 14 days after the date of surgery. Parents or guardians were contacted 14 days after the date of procedure and answered a telephone questionnaire on whether their child had developed any cardinal symptoms of COVID-19 or was tested for COVID-19 in the post-operative period and about their post-operative behaviour (Appendix B). Major respiratory complication was defined as any post-operative respiratory symptoms requiring readmission to hospital.
2.2. Relationship to community prevalence
Community incidence data for the entire population and for children was obtained from the Health Surveillance Protection Centre (HSPC) [10]. For the calculation of COVID-19 incidence in children, a cut-off of 18 years was used, correlating to data available from the HSPC. As the participating centres were located in 4 different locations in Ireland, and as all served as regional referral centres for surrounding counties, national incidence rather than incidence in local counties is given.
2.3. Statistical analysis
The study was reported according to STROBE and SAMPL guidelines [11,12]. Missing data were recorded where applicable. Descriptive statistics were performed including frequencies and percentages for categorical data and means (standard deviations, SD) for continuous data. Confidence intervals (95%) were constructed for incidence rates of positive COVID-19 using exact Poisson confidence intervals. Where zero frequencies were observed, only the upper confidence limit is reported. Statistical analysis was performed with Stata Release 16.
3. Results
3.1. Participants and procedures
During the study period in the respective institutions 742 paediatric otolaryngology procedures were carried out. 373 parents or guardians of patients were approached for inclusion from 5 institutions across the Republic of Ireland. Of these 8 did not consent to the study and 63 were uncontactable for follow-up despite 3 or more telephone calls attempts.
Therefore, 302 patients were enrolled from the five institutions. Demographics and procedure information is shown in Table 1 . The main operative procedures were tonsillectomy, adenoidectomy and examination of ears under general anaesthesia with grommet insertion. In total 158 (52.3%) of children had day case procedures.
Table 1.
Demographics of participants and procedures performed on participants included in the study.
| N | Total | 302 |
|---|---|---|
| Sex | Male | 158 |
| Female | 144 | |
| Age | Mean | 7.23 |
| Range | 0.25–17 | |
| Standard Deviation | 4.00 | |
| Centre | CHI at Crumlin | 15 |
| Galway University Hospital | 29 | |
| South Infirmary University Hospital | 99 | |
| CHI at Temple Street | 102 | |
| University Hospital Waterford | 57 | |
| Procedures | Tonsillectomy±Adenoidectomy ± Grommets | 156 |
| EUA Ears/Grommet insertion | 85 | |
| Microlaryngoscopy± bronchoscopy | 13 | |
| Minor Nasal Surgery | 11 | |
| Major Ear Surgery with drilling | 9 | |
| Adenoidectomy with grommets | 5 | |
| Tongue tie release | 6 | |
| Manipulation of Nasal Bones | 5 | |
| Other | 12 |
3.2. Pre-operative screening protocols
All children had preadmission questionnaire completed by parents. 125 patients underwent preoperative RT-PCR testing. 122 (98%) of these were carried out in accordance with local hospital policy; the remaining swabs were carried out because of recent travel history outside the country or the presence of symptoms. During the study period, one patient had a positive COVID-19 swab and elective surgery was postponed.
3.3. Patient behaviour
Pre-operatively 66 (21.8%) patients restricted their movements including stopping schooling and educational activities, in the 14 days prior to surgery. 127 (42%) patients cocooned once nasopharyngeal swabs were taken. This data was missing for 9 patients. In the post operative period 153 (50.6%) of children had restricted movements including stopping schooling and education activities for 14 days. This data was missing for 29 patients.
3.4. Patients’ follow up
No COVID-19 infections were detected among any of the 302 children who were followed up after 14 days. 16 children did undergo RT-PCR tests within the 14 days post-surgery. The indication for these swabs was readmission to hospital (8, 4 with post tonsillectomy bleed, and 4 having planned second surgical procedure), respiratory symptoms (5), and close contacts of known COVID-19 cases patients (3). All 16 tests were negative. 8 children had postoperative respiratory symptoms reported by their parents. Five of these underwent RT-PCR testing (all negative). Testing was not performed in the remaining 3 cases. In 2 cases medical attention was not sought, while in the remaining case testing was deemed unnecessary by the attending doctor.
3.5. Estimated risk of COVID-19 in patients undergoing elective surgery
Based on an observed frequency of zero from a sample of 302 patients, an upper 95% confidence limit of 1·2% is estimated for the risk of symptomatic COVID-19 infection in the postoperative period.
3.6. Community incidence
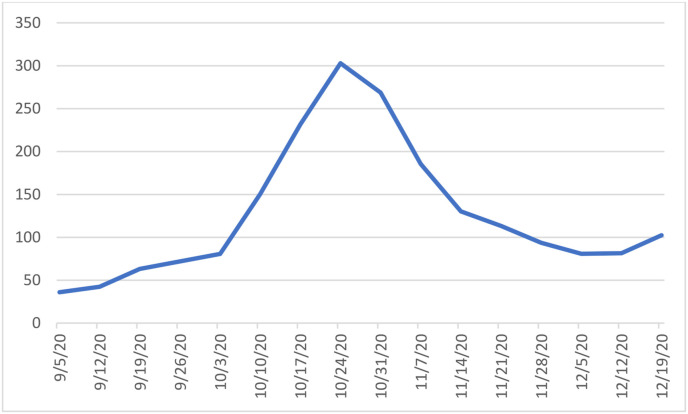
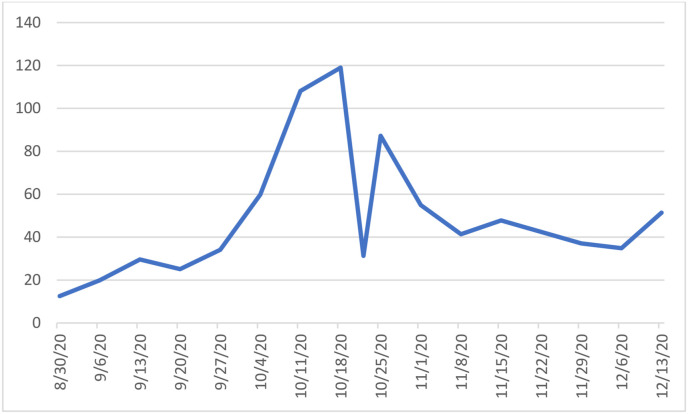
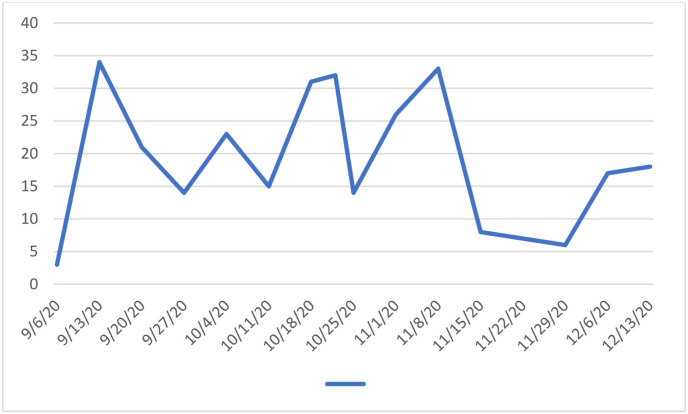
The 14-day incidence of confirmed COVID-19 cases for the Republic of Ireland per 100,000 population is shown in Fig. 1 . The 14-day incidence of confirmed cases for children per 100,000 is shown in Fig. 2 . The 14-day incidence reached a peak around the midpoint of the study from October 11th to 18th with peak 14-day incidence in the whole population of 302.9 and among children 118.9 per 100,000 respectively. Fig. 3 shows the temporal distribution of surgical cases performed on the study participants throughout the study period.
Fig. 1.
14 day Incidence rates of confirmed COVID-19 cases per 100,000 population in Ireland.
Fig. 2.
14 day Incidence rates of confirmed COVID-19 cases per 100,000 paediatric population in Ireland.
Fig. 3.
The number of cases carried out per week of the study.
4. Discussion
One of the major concerns regarding elective surgery during the COVID-19 pandemic is the possibility of patients having presymptomatic or asymptomatic COVID-19 at time of hospital admission, and consequently having an increased risk of adverse postoperative outcome, as well as presenting a risk of disease transmission within the hospital. Early data from the pandemic suggested a case fatality ratio of 20.5–24% among adult patients who developed COVID-19 in the perioperative period, and 51% developing postoperative pulmonary complications [7,8]. The risks of surgery on children with COVID-19 is unknown. Paediatric anaesthesia in the context of an ongoing respiratory viral infection has been shown to result in an increased incidence of peri-operative complications varying in severity from minor respiratory symptoms to death [13,14], yet little data has been published regarding the consequences of anaesthesia and surgery for children with SARS-CoV-2 infection specifically.
Preoperative COVID-19 testing has become widely adopted among adult patients as a means of reducing the risk of COVID-19 in hospitals. Current policies for testing of paediatric cases range from universal testing of all patients [15] to varying protocols depending on community transmission rates. In the present study, there was significant variation in testing protocols between the participating units. Among the 177 participants not undergoing preoperative testing, there were no cases of postoperative symptomatic COVID-19 infections. However, it is likely that this low incidence of post-operative infection is mainly related to the low community transmission rates in Ireland during the period of the study, rather than providing any conclusive evidence that preoperative COVID-19 testing of children is unnecessary.
The role of preoperative “cocooning” or restriction of movements is difficult to elucidate from the present study. Adherence to cocooning in children is reported to be poor and is not recommended as a routine practice for children undergoing elective surgery [16]. In the present study, we also observed low levels of patient cocooning, however, this did not appear to impact adversely on outcomes. It should be noted that for the entire study period, even though enhanced restrictions were put in place when incidence spiked in late October, normal educational activities for children continued throughout the study period.
The main difference between the present study and previous papers is that while the main outcome measure in other studies was the incidence of positive tests for COVID-19 among patients presenting for pre-operative testing, the purpose of the present paper was to examine the incidence of symptomatic COVID-19 or adverse postoperative outcome in the 14 days after surgery, with the main outcome measure established by systematic 14-day follow-up in all participants. Because of the variable incubation period of COVID-19, ranging up to 14 days, we believe this design gave us the optimal opportunity to detect any cases of symptomatic COVID-19 or respiratory complications that may have presented after hospital discharge. The only study to our knowledge to include formal 14-days follow up of patients was that by Sii et al. In a much smaller cohort of 66 patients undergoing elective surgery with negative preoperative test, the authors reported no case of COVID-19 at 14-day follow-up [17].
Among other measures used to minimize the risk of hospital transmission of COVID-19 among elective surgical patients during the COVID-19 pandemic are admission to hospital for the minimum period of time, social distancing within hospitals, use of masks and appropriate PPE by staff and adult patients, and use of aerosol precautions in cases were aerosol generating procedures (AGPs) are performed. In the present study, we report no cases of postoperative symptomatic COVID-19 or major respiratory complication among any of the 302 children enrolled in this study. Based on sample size we estimated the upper limit of the 95% confidence interval for risk of postoperative COVID-19 to be 1.2%. These findings would suggest that in the setting of community incidence comparable to that present in Ireland during the time period of the study, elective otolaryngological surgery during the COVID-19 pandemic is safe and associated with low risk of COVID-19 infection or complications.
4.1. Limitations
The major limitation of the current study is that due to the high incidence of asymptomatic infection in children, and the fact that three children with postoperative respiratory symptoms were not tested for COVID-19 in the postoperative period, it is possible that some cases of post-operative COVID-19 remained undetected. We also did not enquire about presence of gastrointestinal symptoms, which may be a symptom of COVID-19 in paediatric patients [18]. However, the absence of any major respiratory complications in the post-operative period would suggest that the risks of adverse postoperative outcome among elective Otolaryngology surgery among asymptomatic children during the pandemic is very low. An additional consideration is that our results should be interpreted in the setting of the 14-day incidence of COVID-19 in Ireland during the study period, and so may not be extrapolatable to periods of surge in disease incidence. In particular, our findings regarding lack of benefit of preoperative testing may not be applicable to periods of increased community transmission. A further weakness is the fact that only 41% of patients undergoing elective surgery at participating hospitals during the study period were enrolled in the study. Achieving high enrollment in this study was challenging, due to the need for prospective enrollment and consenting of all participants in 5 separate sites. In view of the significant logistics to carry out the study, we were very pleased with the enrollment rate. However, we cannot rule out selection bias which may have impacted results. Finally, most of the enrolment in this study occurred before the appearance of novel more transmissible variants of SARS-CoV-2 in Ireland, in particular the B.1.1.7 strain [19]. However, evidence to date does not suggest that children are any more susceptible to this new strain [20]. On the other hand, major advantages of the study include the systematic 14-day follow up of patients, which gave us the best opportunity to capture any cases of symptomatic COVID-19 or respiratory complications.
5. Conclusions
In the present study, we report no cases of postoperative symptomatic COVID-19 or major respiratory complication among any of the 302 children enrolled in this study. Based on sample size we estimated the upper limit of the 95% confidence interval for risk of postoperative COVID-19 to be 1.2%. These findings would suggest that in the setting of community incidence comparable to that present in Ireland during the time period of the study, which reached a peak of 302.9 for adults and 118.9 per 100,000 population, elective otolaryngological surgery during the COVID-19 pandemic is safe and associated with low risk of COVID-19 infection or complications.
Acknowledgements
We would like to thank the many surgeons and nurses who cooperated with this study. The authors wish to acknowledge Kathleen Bennett in the RCSI Data Science Centre (DSC) for the providing statistical advice and support and Kiernan Ryan, director of the Department of Surgical Affairs in the RCSI, Ireland, Brid Moran, Collette Tully, National Office of Clinical Audit, Ireland.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijporl.2021.110861.
Contributor Information
ICE Collaborative Group:
So Jeong Kang, Ryan O'Sullivan, Brian Kennedy, Conor Tiernan, Oisín ó Murchú, Agnieska Urbaniak, Colm Hannon, Peter O'Sullivan, Habib Khan, Andrew Dias, Darragh Coakley, Rania Mehanna, Stephen Hone, Stephen Garry, Coleen Heffernan, Eimear Phelan, Stephen Kieran, Seamus Boyle, Michael Fitzsimons, Orla Young, Mona Thornton, John Lang, Peter Gormley, Thavakumar Subramaniam, Moustafa Aly, Tahir Zaman, Khalid Majeed, Ola Fapohunda, Ross Byrne, Joanne Cregg, Jesvin Cheema, David Thornton, Oisin O'Domhaill, Martin Donnelly, David Smith, Liam Skinner, and Bangalore Mahesh
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.Zimmermann P., Curtis N. Coronavirus infections in children including COVID-19: an overview of the epidemiology, clinical features, diagnosis, treatment and prevention options in children. Pediatr. Infect. Dis. J. 2020;39:355–368. doi: 10.1097/INF.0000000000002660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Lusignan S., Dorward J., Correa A., Jones N., Akinyemi O., Amirthalingam G., Andrews N., Byford R., Dabrera G., Elliot A., Ellis J., Ferreira F., Lopez Bernal J., Okusi C., Ramsay M., Sherlock J., Smith G., Williams J., Howsam G., Zambon M., Joy M., Hobbs F.D.R. Risk factors for SARS-CoV-2 among patients in the oxford Royal College of general practitioners research and surveillance centre primary care network: a cross-sectional study. Lancet Infect. Dis. 2020;20:1034–1042. doi: 10.1016/S1473-3099(20)30371-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.CDC COVID Data Tracker https://covid.cdc.gov/covid-data-tracker/#cases_casesper100klast7days (n.d.)
- 4.Stokes E.K., Zambrano L.D., Anderson K.N., Marder E.P., Raz K.M., El Burai Felix S., Tie Y., Fullerton K.E. Coronavirus disease 2019 case surveillance — United States, january 22–may 30, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020;69:759–765. doi: 10.15585/mmwr.mm6924e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang F., Shi S., Zhu J., Shi J., Dai K., Chen X. Clinical characteristics and outcomes of cancer patients with COVID‐19. J. Med. Virol. 2020;92 doi: 10.1002/jmv.25972. jmv.25972. [DOI] [PubMed] [Google Scholar]
- 6.Kim L., Whitaker M., O'Halloran A., Kambhampati A., Chai S.J., Reingold A., Armistead I., Kawasaki B., Meek J., Yousey-Hindes K., Anderson E.J., Openo K.P., Weigel A., Ryan P., Monroe M.L., Fox K., Kim S., Lynfield R., Bye E., Shrum Davis S., Smelser C., Barney G., Spina N.L., Bennett N.M., Felsen C.B., Billing L.M., Shiltz J., Sutton M., West N., Talbot H.K., Schaffner W., Risk I., Price A., Brammer L., Fry A.M., Hall A.J., Langley G.E., Garg S., Coates A., Daily Kirley P., Libby T., Roland J., Alden N., Herlihy R., McLafferty S., Clogher P., Kayalioglu H., Maslar A., Misiorski A., Niccolai L., Olson D., Parisi C., Fawcett E., Gretzinger S., Lengacher K., Williams J., Blythe D., Brooks A., Park R., Wilson M., Como-Sabetti K., Danila R., Cline C., Angeles K., Eisenberg N., Flores K., Habrun C., Hancock E., Khanlian S., Novi M., Phipps E., Salazar-Sanchez Y., Dufort E., Muse A., Bushey S., Gaitan M., Kurtz R., Owusu-Dommey A., Snyder L., Michaelis K., Seeley K., Markus T., Chatelain R., George A., Hill M., McCullough L., Spencer M., Swain A., McCaffrey K., Holstein R., Meador S., Wortham J. Hospitalization rates and characteristics of children aged <18 Years hospitalized with laboratory-confirmed COVID-19 — COVID-NET, 14 states, march 1–july 25, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020;69:1081–1088. doi: 10.15585/mmwr.mm6932e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lei S., Jiang F., Su W., Chen C., Chen J., Mei W., Zhan L.Y., Jia Y., Zhang L., Liu D., Xia Z.Y., Xia Z. Clinical characteristics and outcomes of patients undergoing surgeries during the incubation period of COVID-19 infection. EClinicalMedicine. 2020;21 doi: 10.1016/j.eclinm.2020.100331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collaborative C. Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS-CoV-2 infection: an international cohort study. Lancet. 2020;396:27–38. doi: 10.1016/S0140-6736(20)31182-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harris P.A., Taylor R., Minor B.L., Elliott V., Fernandez M., O'Neal L., McLeod L., Delacqua G., Delacqua F., Kirby J., Duda S.N. The REDCap consortium: building an international community of software platform partners. J. Biomed. Inf. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Health Protection Surveillance Centre HPSC . HPSC; Dublin: 2019. Epidemiology of COVID 19 in Ireland – 14 Day Report.https://www.hpsc.ie/a-z/respiratory/coronavirus/novelcoronavirus/surveillance/covid-1914-dayepidemiologyreports/C No Title, (n.d.) [Google Scholar]
- 11.von Elm E., Altman D.G., Egger M., Pocock S.J., Gøtzsche P.C., Vandenbroucke J.P. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335:806–808. doi: 10.1136/bmj.39335.541782.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lang T.A., Altman D.G. Basic statistical reporting for articles published in biomedical journals: the “‘Statistical analyses and methods in the published literature’” or the SAMPL guidelines §, inspect-Lb.Org. (n.d.) 2014. [DOI] [PubMed]
- 13.Cohen M.M., Cameron C.B. Should you cancel the operation when a child has an upper respiratory tract infection? Anesth. Analg. 1991;72:282–288. doi: 10.1213/00000539-199103000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Tait A.R., Malviya S. Anesthesia for the child with an upper respiratory tract infection: still a dilemma? Anesth. Analg. 2005;100:59–65. doi: 10.1213/01.ANE.0000139653.53618.91. [DOI] [PubMed] [Google Scholar]
- 15.Ingram M.C.E., Mehl S., Rentea R.M., Lopez M.E., Raval M.V., Newton C., Berman L. Sharing strategies for safe delivery of surgical care for children in the COVID-19 Era. J. Pediatr. Surg. 2021;56:196–198. doi: 10.1016/j.jpedsurg.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Guidance for the Recovery of Elective Surgery in Children, (n.d).
- 17.Sii C.K.S., Lee J.A., Nah S.A. Early experience with universal preoperative and pre-procedural screening for COVID-19 in low-risk pediatric surgical patients requiring general anesthesia. Pediatr. Surg. Int. 2020;36:1407–1411. doi: 10.1007/s00383-020-04760-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Waterfield T., Watson C., Moore R., Ferris K., Tonry C., Watt A., McGinn C., Foster S., Evans J., Lyttle M.D., Ahmad S., Ladhani S., Corr M., McFetridge L., Mitchell H., Brown K., Amirthalingam G., Maney J.A., Christie S. Seroprevalence of SARS-CoV-2 antibodies in children: a prospective multicentre cohort study. Arch. Dis. Child. 2021;106:680–686. doi: 10.1136/archdischild-2020-320558. [DOI] [PubMed] [Google Scholar]
- 19.Mahase E. Covid-19: what have we learnt about the new variant in the UK? BMJ. 2020;371:m4944. doi: 10.1136/bmj.m4944. [DOI] [PubMed] [Google Scholar]
- 20.Davies N.G., Abbott S., Barnard R.C., Jarvis C.I., Kucharski A.J., Munday J.D., Pearson C.A.B., Russell T.W., Tully D.C., Washburne A.D., Wenseleers T., Gimma A., Waites W., Wong K.L.M., van Zandvoort K., Silverman J.D. CMMID COVID-19 working Group, COVID-19 Genomics UK (COG-UK) consortium, K. Diaz-ordaz, R. Keogh, R.M. Eggo, S. Funk, M. Jit, K.E. Atkins, W.J. Edmunds, estimated transmissibility and impact of SARS-CoV-2 lineage B.1.1.7 in England. Science. 2021 doi: 10.1126/science.abg3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.