Abstract
Background
Several ABO blood groups have been associated with the likelihood of infection, severity, and/or outcome of COVID-19 in hospitalized cohorts, raising the hypothesis that anti-A isoagglutinins in non-A-group recipients could act as neutralizing antibodies against SARS-CoV-2.
Materials and methods
We run live virus neutralization tests using sera from 58 SARS-CoV-2 seronegative blood donors (27 O-group and 31 A-group) negatives for SARS-CoV-2 IgG to investigate what degree of neutralizing activity could be detected in their sera and eventual correlation with anti-A isoagglutinin titers.
Results
We could not find clinically relevant neutralizing activity in any blood group, regardless of anti-isoagglutinin titer,
Discussion
Our findings suggest that mechanisms other than neutralization explain the differences in outcomes from COVID19 seen in different ABO blood groups.
Keywords: COVID-19, SARS-CoV-2, Convalescent plasma, Neutralizing antibody, ABO blood group, Anti-A isoagglutinins
1. Introduction
The COVID-19 pandemic is totaling 190 million cases and 4 million deaths worldwide as of July 19, 2021. Specific therapeutics are still lacking, and mass vaccination campaigns are now tapering the pandemic in most countries. Several genetic protective factors have been hypothesized, and the ABO blood group locus is one of them: in preliminary small-scale series, non-A blood groups seemed to have increased risk for severity and mortality, while O-group appeared to confer relative protection [1].
A similar phenomenon was previously reported for SARS-CoV-1, where anti-A isoagglutinins were shown to neutralize viral infection [2]. Since the odds ratio between A- and non-A blood groups is not very high, several investigators have postulated that anti-A isoagglutinin titers could explain the variability among non-A patients. Of course, this would have implications for COVID-19 convalescent plasma (CCP) donor selection and efficacy [3].
Recently, Deleers et al reported significantly lower IgM anti-A+ anti-B agglutination scores in blood group O patients (76.93 vs 88.29, p-value = 0.034) and lower levels of anti-B (24.93 vs 30.40, p-value = 0.028) and anti-A antibodies (28.56 vs 36.50, p-value = 0.048) in blood group A and blood group B patients, respectively, compared to controls [4].
We hence investigated whether these relationships could be explained by the neutralizing activity of factors related to the ABO-blood group, and in particular to anti-A isoagglutinins.
2. Materials and methods
2.1. Sera
58 consecutive, SARS-CoV-2 IgG-negative blood donors (27 O-group or 31 A-group) were enrolled in the study. The two groups were matched for age and gender. Residual sera originally sampled for mandatory serological disease screening were used for the study. The research was conducted under institutional review board-approved protocols (CEAVNO, Pisa University Hospital). All the donors tested SARS-CoV-2 IgG negative on the Abbott Alinity® instrument and represented a nonconvalescent cohort.
A single residual convalescent serum sample from a SARS-CoV-2 RT-PCR-positive patient was chosen as a positive control. Expecting a very low neutralizing activity in any of the blood groups tested, to build a titration curve, we selected the convalescent serum with the lowest possible detectable neutralizing antibody (nAb) titer in the microneutralization (MN) assay, i.e., 1:10.
2.2. Anti-A1 IgG isoagglutinin titration
O-group nonconvalescent donors were titrated for anti-A IgG isoagglutinins using A1 references cells on the Neo Iris® analyzer (Immucor Inc, USA). The method employs 100 μl of donor serum, Capture-R Select® plates, Capture R Indicator® cells, and LISS. Sera which tested >1:128 in the low-titer method were retested in the high-titer method (which detects titers up to 1:4096).
2.3. SARS-CoV-2 microneutralization tests
We carried out live virus MN assay with limiting dilution method using SARS-CoV-2 isolate Human/ITA/PAVIA10734/2020 (belonging to clade G) that was propagated in Vero E6 cells cultivated in D-MEM supplemented with 10% heat-inactivated fetal bovine serum, L-glutamine, penicillin, and streptomycin. Viral strains and cell lines were provided by NeuCoV-NET. Viral preparations were titrated in Vero E6, checked for absence of Mycoplasma, aliquoted at 5 50% tissue culture infectious dose (TCID)50, and stored at −80 °C until use. To titrate serum nAbs, Vero E6 cells (12.000/well) were plated the day before on a 96-well plate in DMEM with 10% FBS. Inactivated serum samples were diluted 4-fold in duplicates from 1:10 to 1:640 in MEM, 2% FBS. 5 TCID50 SARS-CoV-2 preparation was added to the serum dilutions and incubated for 1 h at 37 °C. The virus-serum mixture was then added to the cells and incubated at 37 °C with 5% CO2 until the cytopathic effect became overtly manifested (three-four days). Culture supernatants were collected from each well for RNA extraction and subsequent reverse-transcription PCR (RTPCR). Cells were thenfixed with 1% paraformaldehyde and stained by Gram crystal violet to visualize cell destruction by spectrophotometry (optical density). nAbs titer was expressed as the endpoint serum dilution that inhibited cytopathic effect by 90% (MN90).
2.4. Polymerase chain reaction for SARS-CoV-2
Viral RNA was extracted from 200 μl of cell supernatant using the Nimbus platform (Hamilton, Reno, NV, USA), and then amplified by using the Allplex™ 2019-nCoV assay (Seegene, Seoul, South Korea) on the CFX96 instrument (Bio-Rad, Hercules, CA, USA), according to the manufacturer's instructions. The real-time Allplex™ 2019-nCoV assay simultaneously detects three genes of the SARS-CoV-2 genome (i.e. the nucleocapsid (N), envelope (E) and RNA-dependent RNA polymerase (RdRP) genes). The cycle threshold (Ct) value was recorded for each of the three genes, and a total mean Ct value was calculated and used as a surrogate measure for SARS CoV-2 load. Samples were considered positive when a PCR signal was detected at Ct <40 for any gene.
2.5. Statistical analysis
SPSS software version 23 (IBM, Chicago, IL, USA) and MedCalc statistical software version 18.2.1 (Ostend, Belgium) were used for statistical analysis. Differences between distributions were calculated by using the non-parametric Mann-Whitney U test. Correlations between variables were assessed using Spearman rho correlation coefficient and Student's t-test. All p values presented are based on two-tailed tests, and p < 0.05 was considered statistically significant.
3. Results
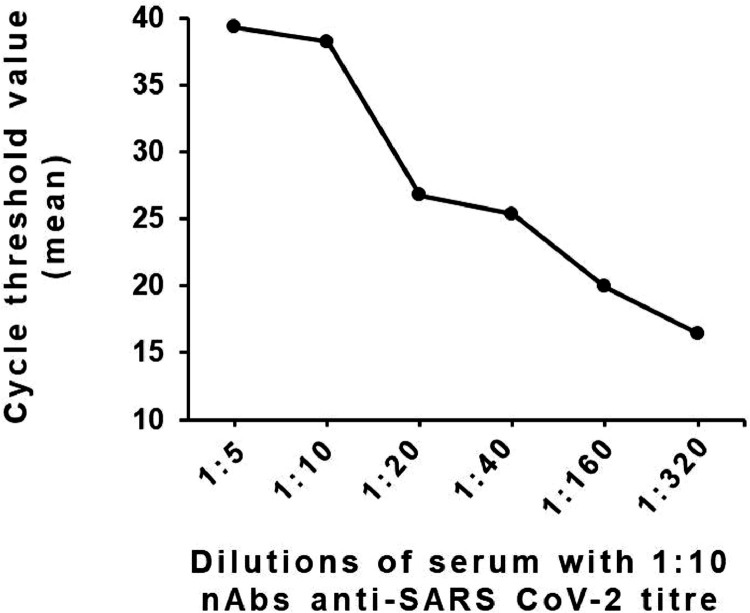
A reverse linear correlation was observed between nAbs titers in the serum of the convalescent donor and viral loads in supernatants (Fig. 1 ). The experiment was performed using the serum with nAb titer of 1:10 when measured by standard neutralization assay and serial dilution at 1:2 folds up to 1:320. The reverse correlation was used as the parameter for a more objective and faster proxy for neutralization activity.
Fig. 1.
Total mean cycle threshold values for SARS-CoV-2 PCR in supernatants of MN90 run using 5 TCID50 of SARS-CoV-2 and serial dilutions of convalescent serum with a nAbs titer of 1:10 when measured by standard neutralization assay. 1:10.
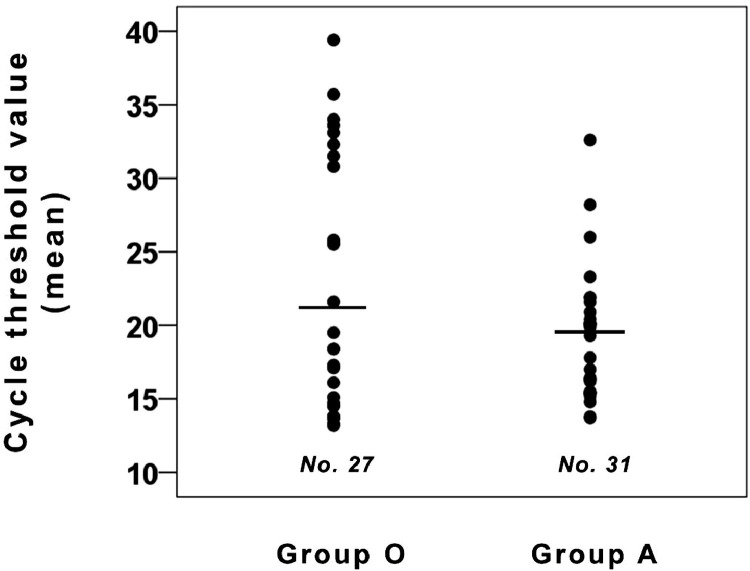
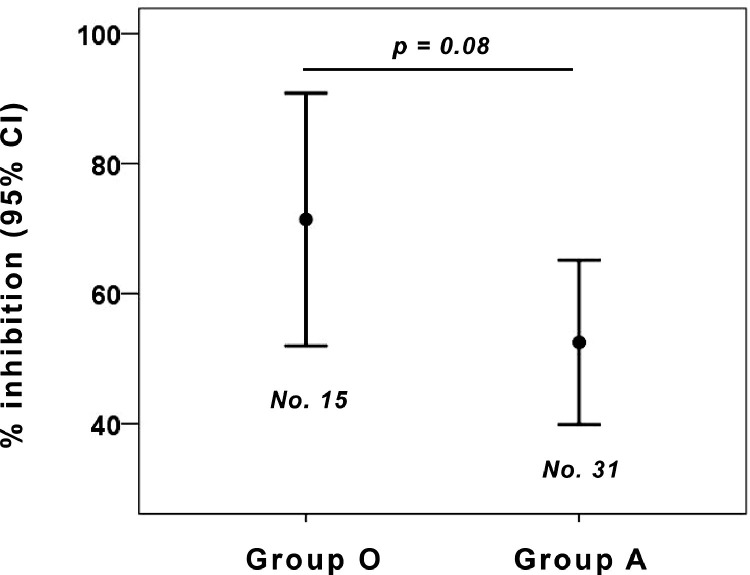
A non-significant difference in neutralizing activity, measured either as viral genome in supernatants of viral neutralization tests (Fig. 2 ) or as vitality of replication-competent cells (Fig. 3 ) was seen between the 2 ABO blood groups.
Fig. 2.
Neutralizing activity (expressed as total mean cycle threshold values in supernatants of viral neutralization tests) of nonconvalescent sera from O-group and A- group donors. The non-significant difference in neutralizing activity was seen between the 2 ABO blood groups.
Fig. 3.
Neutralizing activity (expressed as percentage of inhibition) of nonconvalescent sera from O-group and A-group donors. The non-significant difference in neutralizing activity was seen between the 2 ABO blood groups. The percent of inhibition at each well was calculated by the formula: 100 − [(X‐average of ʻno virusʼ wells)/(average of ʻvirus onlyʼ wells‐average of ʻno virusʼ wells)*100], where X is the read for each well.
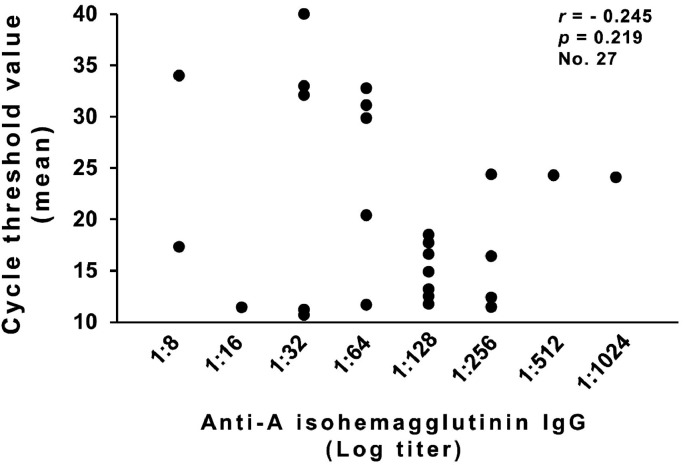
Among 27 O-group nonconvalescent donors, there was no statistically significant relationship between anti-A isoagglutinin titers and neutralizing activity (measured as viral genome in supernatants of viral neutralization tests) (Fig. 4 ).
Fig. 4.
Neutralizing activity (expressed as total mean cycle threshold values in supernatants of viral neutralization tests) in relationship to anti-A1 IgG isoagglutinin titer in 27 O-group nonconvalescent donors.
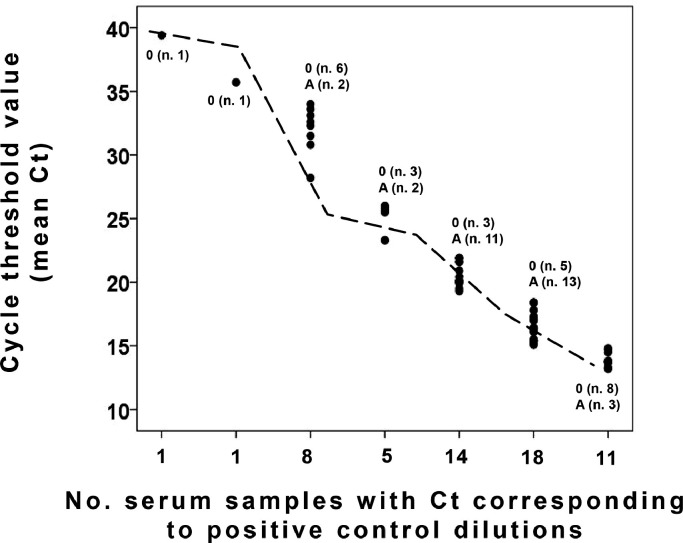
The nonconvalescent serum with the highest neutralizing activity had a titer which could be compared to 1:2 on the titration curve obtained from a convalescent serum (Fig. 5 ). Since such titer was achieved using a 50-fold lower viral inoculum than in routine viral neutralization tests (where the minimum protective titer is largely agreed to be ≥ 1:160 [5]), it is far from being clinically relevant for protection.
Fig. 5.
Distribution of the neutralizing activity (expressed as total mean cycle threshold values in supernatants of viral neutralization tests) of the nonconvalescent sera concerning the titration curve of a convalescent serum. The number of group 0 and A samples with Ct corresponding to positive control dilutions is reported.
4. Discussion
Recent large case series [6, 7] and meta-analyses [8, 9] are minimizing the protective role of the O-blood group in COVID19, denying early small-scale studies. No study to date has confirmed a relevant impact of morbidity or mortality [10], while protection from infection largely varies according to cohort selection (hospitalized vs. asymptomatic) [11]. In the unique epidemiological setting of 1,688 sailors on a French aircraft carrier, it was shown that the rate of infection among young adults is independent of ABO blood group [12].
We have demonstrated here that nonconvalescent sera contain negligible SARS-CoV-2 neutralizing activity, regardless of ABO blood group or anti-A isoagglutinin titer, which is clinically irrelevant.
Other factors, that have not been fully investigated in this study, could theoretically affect the relationship. Previous exposure to seasonal coronaviruses could be one of them: anyway we recently reported a minimal impact from such factor [13]. Given that the relationship between COVID19 and outcome has been confirmed in extremely large series, it is likely to represent an independent variable.
5. Conclusions
The differences in outcomes from COVID19 patients seen in different ABO blood groups are hence related to mechanisms downstream of viral entry. For instance, the O-blood group is associated with lower levels of von Willebrand factor (vWF) [14], and lower increases of vWF with ageing [15]. O antigen is inversely associated with thrombosis [16, 17] (particularly deep vein thrombosis), total cholesterol, low-density lipoprotein cholesterol, while the A1 antigen tends to have associations in reverse to O [18]. Since COVID19 is a mostly thrombophilic disorder [19], it is possible that ABO blood group conditions outcomes via factors not strictly related to viral replication but rather to thrombosis [20].
Our study needs to be consolidated by replicas in different cell lines that could express different ABO antigens, and on larger datasets including B-blood group patients.
Declaration of Competing Interest
We declare we have no conflict of interest related to this manuscript.
Acknowledgments
Acknowledgments
None.
Funding and resources
None.
Authorship contributions
Daniele Focosi conceived the study and wrote the first draft. Alfredo Rosellini performed viral neutralization tests. Pietro Giorgio Spezia and Lisa Macera performed PCR. Maria Lanza, Aldo Paolicchi, Fabio di Francesco, Andreina Baj, and Mauro Pistello critically revised the manuscript. Fabrizio Maggi analyzed the data, produced figures, and approved the final version.
References
- 1.Ellinghaus D, Degenhardt F, Bujanda L, Buti M, Albillos A, Invernizzi P, et al. Genomewide association study of severe Covid-19 with respiratory failure. N. Engl. J. Med. 2020;383:1522–1534. doi: 10.1056/NEJMoa2020283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guillon P, Clément M, Sébille V, Rivain J-G, Chou C-F, Ruvoën-Clouet N, et al. Inhibition of the interaction between the SARS-CoV Spike protein and its cellular receptor by anti-histo-blood group antibodies. Glycobiology. 2008;18:1085–1093. doi: 10.1093/glycob/cwn093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Focosi D. Anti-A Isohemagglutinin titers and SARS-CoV2 neutralization: implications for children and convalescent plasma selection. Br. J. Haematol. 2020;190:e148–ee50. doi: 10.1111/bjh.16932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deleers M, Breiman A, Daubie V, Maggetto C, Barreau I, Besse T, et al. Covid-19 and blood groups: ABO antibody levels may also matter. Int. J. Infect. Dis.: IJID: Off. Publ. Int. Soc. Infect. Dis. 2020 doi: 10.1016/j.ijid.2020.12.025. S1201-9712:32549-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Focosi D, Anderson AO, Tang JW, Tuccori M. Convalescent plasma therapy for COVID-19: state of the art. Clin. Microbiol. Rev. 2020;33:e00072. doi: 10.1128/CMR.00072-20. -20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gamal N, Villa E, Rolli M, Pecorari M, Mirabella G, Bertellini E, et al. Subjects with blood group O are not at lower risk to acquire SARS-CoV-2 infection. Vox Sang. 2020 doi: 10.1111/vox.13062. [DOI] [PubMed] [Google Scholar]
- 7.Levi JE, Telles PR, Scrivani H, Campana G. Lack of association between ABO blood groups and susceptibility to SARS-CoV-2 infection. Vox Sang. 2020 doi: 10.1111/vox.13015. [DOI] [PubMed] [Google Scholar]
- 8.Bhattacharjee S, Banerjee M, Pal R. ABO blood groups and severe outcomes in COVID-19: a meta-analysis. Postgrad. Med. J. 2020 doi: 10.1136/postgradmedj-2020-139248. [DOI] [PubMed] [Google Scholar]
- 9.Liu N, Zhang T, Ma L, Zhang H, Wang H, Wei W, et al. The impact of ABO blood group on COVID-19 infection risk and mortality: a systematic review and meta-analysis. Blood Rev. 2020 doi: 10.1016/j.blre.2020.100785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Solmaz İ, Araç S. ABO blood groups in COVID-19 patients; Cross-sectional study. Int. J. Clin. Pract. 2020:e13927. doi: 10.1111/ijcp.13927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Focosi D, Iorio MC, Lanza M. ABO blood group correlations with COVID-19: cohort choice makes a difference. Clin. Infect. Dis.: Off. Publ. Infect. Dis. Soc. Am. 2020 doi: 10.1093/cid/ciaa1495. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laurys B, Frédéric J, Olivier B, Fabien D. ABO blood groups are not associated with risk of acquiring the SARS-CoV-2 infection in young adults. Haematologica. 2020;105:2841–2843. doi: 10.3324/haematol.2020.265066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Focosi D, Genoni A, Lucenteforte E, Tillati S, Tamborini A, Spezia PG, et al. Previous humoral immunity to the endemic seasonal alphacoronaviruses NL63 and 229E is associated with worse clinical outcome in COVID-19 and suggests. Orig. Antigen. Sin. 2021;11:298. doi: 10.3390/life11040298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franchini M, Capra F, Targher G, Montagnana M, Lippi G. Relationship between ABO blood group and von Willebrand factor levels: from biology to clinical implications. Thromb. J. 2007;5:14. doi: 10.1186/1477-9560-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Biguzzi E, Castelli F, Lijfering WM, Cannegieter SC, Eikenboom J, Rosendaal FR, et al. Rise of levels of von Willebrand factor and factor VIII with age: role of genetic and acquired risk factors. Thromb. Res. 2021;197:172–178. doi: 10.1016/j.thromres.2020.11.016. [DOI] [PubMed] [Google Scholar]
- 16.Wang C, Le Ray I, Lee B, Wikman A, Reilly M. Association of blood group and red blood cell transfusion with the incidence of antepartum, peripartum and postpartum venous thromboembolism. Sci. Rep. 2019;9:13535. doi: 10.1038/s41598-019-49566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scheiner B, Northup PG, Gruber AB, Semmler G, Leitner G, Quehenberger P, et al. The impact of ABO blood type on the prevalence of portal vein thrombosis in patients with advanced chronic liver disease. Liver Int.: Off. J. Int. Assoc. Study Liver. 2020;40:1415–1426. doi: 10.1111/liv.14404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li S. Schooling CM. A phenome-wide association study of ABO blood groups. BMC Med. 2020;18:334. doi: 10.1186/s12916-020-01795-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calderon-Lopez MT, Garcia-Leon N, Gomez-Arevalillo S, Martin-Serrano P, Matilla-Garcia A. Coronavirus disease 2019 and coagulopathy: other prothrombotic coagulation factors. Blood Coagul. Fibrinolysis: Int. J. Haemost. Thromb. 2021;32:44–49. doi: 10.1097/MBC.0000000000000996. [DOI] [PubMed] [Google Scholar]
- 20.Zalba Marcos S, Antelo ML, Galbete A, Etayo M, Ongay E, García-Erce JA. Infection and thrombosis associated with COVID-19: possible role of the ABO blood group. Med. Clin. 2020;155:340–343. doi: 10.1016/j.medcle.2020.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]