Abstract
The mRNA vaccines against COVID-19 infection have been effective in reducing the number of symptomatic cases worldwide. With widespread uptake, case series of vaccine-related myocarditis/pericarditis have been reported, particularly in adolescents and young adults. Men tend to be affected with greater frequency, and symptom onset is usually within 1 week after vaccination. Clinical course appears to be mild in most cases. On the basis of the available evidence, we highlight a clinical framework to guide providers on how to assess, investigate, diagnose, and report suspected and confirmed cases. In any patient with highly suggestive symptoms temporally related to COVID-19 mRNA vaccination, standardized workup includes serum troponin measurement and polymerase chain reaction testing for COVID-19 infection, routine additional lab work, and a 12-lead electrocardiogram. Echocardiography is recommended as the imaging modality of choice for patients with unexplained troponin elevation and/or pathologic electrocardiogram changes. Cardiovascular specialist consultation and hospitalization should be considered on the basis of the results of standard investigations. Treatment is largely supportive, and myocarditis/pericarditis that is diagnosed according to defined clinical criteria should be reported to public health authorities in every jurisdiction. Finally, we recommend COVID-19 vaccination in all individuals in accordance with the Health Canada and National Advisory Committee on Immunization guidelines. In patients with suspected myocarditis/pericarditis after the first dose of an mRNA vaccine, deferral of a second dose is recommended until additional reports become available.
Résumé
Les vaccins à ARNm contre la COVID-19 ont permis de réduire efficacement le nombre de cas symptomatiques de cette infection dans le monde entier. Par suite de l'usage généralisé du vaccin, une série de cas de myocardite ou de péricardite liées au vaccin a été signalée, en particulier chez les adolescents et les jeunes adultes. Le phénomène tend à toucher plus fréquemment les sujets de sexe masculin, et les symptômes apparaissent généralement au cours de la semaine suivant la vaccination. L’évolution clinique semble bénigne dans la très grande majorité des cas. À partir des données disponibles, nous dégageons un cadre de référence clinique auquel les fournisseurs pourront se reporter au moment d’évaluer, d'examiner, de diagnostiquer et de signaler les cas suspects et confirmés. Chez tout patient qui a des symptômes fortement évocateurs et présentant un lien temporel avec l'administration du vaccin à ARNm contre la COVID-19, le bilan diagnostique systématique comprend le dosage de la troponine sérique et le dépistage de la COVID-19 par PCR, d'autres analyses de laboratoire courantes et un électrocardiogramme (ECG) à 12 dérivations. L’échocardiographie est la technique d'imagerie recommandée en première intention chez les patients présentant une hausse inexpliquée du taux de troponine et/ou des modifications pathologiques du tracé de l'ECG. La consultation d'un spécialiste en soins cardiovasculaires et l'hospitalisation devraient être envisagées en fonction des résultats des examens standard. Le traitement est en grande partie axé sur les soins de soutien, et les cas de myocardite ou de péricardite diagnostiqués selon des critères cliniques définis devraient être signalés aux autorités de santé publique locales partout au pays. Enfin, nous recommandons la vaccination de chaque personne contre la COVID-19, conformément aux lignes directrices de Santé Canada et du Comité consultatif national de l'immunisation. En ce qui concerne les patients chez qui une myocardite ou une péricardite est soupçonnée après l'administration de la première dose d'un vaccin à ARNm, il est recommandé de reporter l'administration de la seconde dose jusqu’à ce que des données supplémentaires soient disponibles.
The mRNA vaccines against COVID-19 have shown unprecedented efficacy with respect to prevention of symptomatic infection and severe illness. With the development of COVID-19 variants of concern including variant B.1.617.2 for which 2 vaccinations are needed to confer immunity, continued public health initiatives promoting vaccine use has continued. Recently, an association with myocarditis and pericarditis has been reported to be related to mRNA vaccination.1 Historically, myocarditis/pericarditis has been reported after a smallpox live vaccine, with an incidence of 2.16-7.8 per 100,000 vaccines with reports occurring up to 30 days post vaccination. Most of these patients recover without any long-term sequelae. Other live viral vaccines (including measles-mumps-rubella, varicella, oral polio, or yellow fever vaccine) have a lower incidence of myocarditis/pericarditis (0.24 per 100,000 vaccines). The likelihood of an association between COVID-19 mRNA vaccines and myocarditis/pericarditis has understandably generated significant interest among health care providers, the scientific community, and the public. This issue is particularly germane during this phase of the pandemic with public health efforts increasingly focused on vaccination of adolescents and young adults, and because further expansion of vaccine efforts in children younger than 12 years of age is anticipated in the coming months.
The aim of this commentary is to provide a real-time pragmatic framework for cardiovascular care providers in Canada to address concerns of myocarditis/pericarditis potentially related to mRNA vaccination. The author group includes expertise in adult and pediatric cardiology, primary care, emergency medicine, policy, public health and vaccinology. Herein, we summarize the current state of knowledge regarding incidence and preliminary outcomes of mRNA vaccine-related myocarditis/pericarditis, address the evaluation, management, and reporting of suspected cases, and provide a suggested approach to informed decision-making with patients who receive the COVID-19 mRNA vaccination.
We recognize that this is a rapidly evolving area of great interest to multiple stakeholders, especially young patients and their families, and our understanding of the link between myocarditis/pericarditis and mRNA vaccines will likely be informed by new and emerging data. This commentary has been endorsed by the Canadian Cardiovascular Society's COVID-19 Rapid Response Team and by the Canadian Pediatric Cardiology Association.
Myocarditis/Pericarditis and COVID-19 mRNA Vaccines
Ongoing surveillance of COVID-19 mRNA vaccines has identified potential post-vaccination adverse events. Recently, data presented to the Advisory Committee on Immunization Practices by the Centers for Disease Control and Prevention reported on incidence of myocarditis/pericarditis after approximately 300,000,000 COVID-19 mRNA doses in the United States. Cases were much more common after the second dose, with a preponderance of men affected (approximately 5-10 times more frequent than in women). Similar to previous reports of myocarditis/pericarditis with other vaccines, one suspects that there is likely under-reporting of the true incidence on the basis of subclinical disease. Through June 5, 2021, an incidence of 12.6 cases per million second doses in the 12- to 39-year-old age group was observed in the 21 days after vaccination, with clustering within the first 6 days.1 Surveillance data in Canada (up to July 9, 2021) includes 163 cases of myocarditis/pericarditis, where cases have been seen after the first and second dose of vaccine, and between 5 hours and 92 days post exposure.2 These clinical findings are similar to multiple series reported worldwide. The references for these cases series are included in Supplemental Appendix S1. To date, myopericarditis has been reported to occur after both available mRNA vaccines (Moderna and Pfizer-BioNTech).
Case Definitions
Standardized case definitions are important to understand the true incidence of myocarditis and pericarditis after mRNA COVID-19 vaccination. This poses a number of challenges: the World Health Organization Global Advisory Committee on Vaccine Safety uses the Brighton Collaboration case definition for myocarditis, which discriminates 5 levels of certainty for the diagnosis. As a comparison, the Vaccine Adverse Events Reporting System in the United States draws from historic case definitions for myocarditis, categorizing events as suspected, probable, or confirmed—the latter specifically requires the presence of pathognomonic histological findings of myocardial inflammation to be present at biopsy or autopsy. In the Vaccine Adverse Events Reporting System, events may be self-reported and adjudication is challenging in the absence of medical records review and confirmatory diagnostic testing. Finally, and most relevant to the practitioner, myocarditis and pericarditis diagnostic criteria for the purpose of direct patient care might be different from those used by surveillance systems to classify a reported event with respect to certainty of diagnosis. In this regard, the Centers for Disease Control and Prevention working case definition for myocarditis, which categorizes cases as probable or confirmed, best aligns with our understanding of how myocarditis is currently worked up in Canada (a summary of the case definitions and references are shown in Supplemental Table S1).
Reporting
Adverse events after immunization should be reported to the Public Health authority, which in most provinces and territories is the local Medical Officer of Health (https://www.canada.ca/en/public-health/services/immunization/federal-provincial-territorial-contact-information-aefi-related-questions.html). This is important for detection of potential safety signals as well as future immunization recommendations for the individual recipient. Health care providers should report any serious or unusual events suspected to be associated with vaccination. Definitive proof of a causal association is not necessary, and for many events, cannot be obtained; however, events with a clear alternate explanation should not be reported. Myocarditis/pericarditis is not a listed adverse event on the current reporting forms in most jurisdictions because these are on the basis of the national form (https://www.canada.ca/content/dam/phac-aspc/documents/services/immunization/aefi-form-july23-2020-eng.pdf), but should be reported as “Other events—Other serious or unexpected events not listed on the form.”
All Canadian provinces and territories report into the Canadian Adverse Events Following Immunization Surveillance System, the national system for detection of serious and rare events that generates estimates of incidence (https://www.canada.ca/en/public-health/services/immunization/canadian-adverse-events-following-immunization-surveillance-system-caefiss.html). The Canadian Adverse Events Following Immunization Surveillance System has issued weekly summaries of reported adverse events with a focus on serious events and adverse events of special interest. This process also informs Health Canada, the vaccine regulatory authority, with responsibility for initial authorization of products for use and periodic updates to the product monograph including safety findings. Such updates have been made several times since initial emergency use authorizations of the COVID-19 vaccines, initially for the AstraZeneca/COVISHIELD vaccines for thrombosis with thrombocytopenia syndrome and capillary leak syndrome, and most recently for the mRNA vaccines with respect to myocarditis/pericarditis. Clinicians can remain current with such safety signals through subscribing to the MedEffect Canada system (https://www.canada.ca/en/health-canada/services/drugs-health-products/medeffect-canada.html).
Evaluation of Suspected Myocarditis/Pericarditis
Patients with suspected myocarditis or pericarditis after vaccination do not usually require extensive evaluation if symptoms are mild. Myocarditis/pericarditis should be suspected in patients who present with symptoms of chest pain, shortness of breath, palpitations, syncope, diaphoresis, or fatigue without obvious cause, which are also associated with evidence of myocardial injury by elevation in cardiac biomarkers (ie, a serum troponin level). Serum troponin level elevations in myocarditis are often significant (> 10 times the upper limit of normal) in patients with myocarditis. Electrocardiogram (ECG) changes are common and include ST elevation in multiple leads, nonspecific ST/T changes, and diffuse T-wave inversions. The differential diagnosis remains broad and includes infectious causes (viral, bacterial, fungal), including acute COVID-19 infection, or post-COVID-19 Multisystem Inflammatory Syndrome in Children (MIS-C)/Adults, drugs and toxins, systemic autoimmune disease, or specific etiologies such as sarcoidosis and giant cell myocarditis. Due diligence must be maintained to rule out other causes depending on the clinical presentation; myocardial ischemia due to coronary disease might also need to be considered in young persons with symptoms indicative of myocarditis/pericarditis.
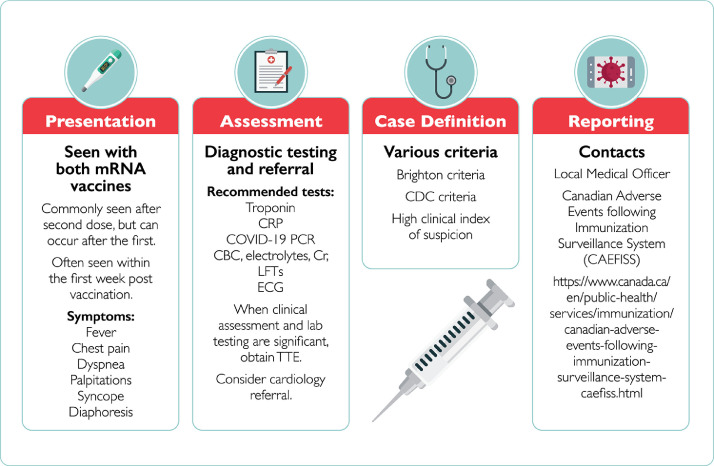
Suggested evaluation of a patient with high pretest probability of myocarditis/pericarditis should follow a rational clinical approach (Table 1 , Fig. 1 ). Careful history and physical examination are indicated for all patients with careful attention to timing of and type of antecedent vaccination. Most reports suggest that typical onset of myocarditis/pericarditis symptoms occurs within 5 days after COVID-19 mRNA vaccine exposure. Initial evaluation should include an ECG and laboratory tests: complete cell count, electrolytes, renal function, liver function tests, C-reactive protein and troponin, and COVID-19 polymerase chain reaction testing. Clinically significant myocarditis is unlikely in the setting of a normal ECG and cardiac biomarkers. Normal troponin level and a normal ECG on presentation does not exclude isolated pericarditis. Treatment should be started if there is a high index of suspicion. The need for more extensive cardiac imaging and additional testing will depend upon the results of the screening tests as listed. Echocardiography should be performed in cases compatible with myocarditis/pericarditis, especially in the setting of an elevated troponin level (in the absence of an alternative explanation). Cardiac magnetic resonance imaging may be considered where available and where an effect on management can reasonably be expected (ie, when echocardiography is normal with elevated troponin level, yet clinical suspicion remains high). Coronary artery imaging should be considered in appropriate cases to rule out myocardial infarction as a cause of biomarker/ECG abnormalities. An assessment flow diagram is presented in Supplemental Figure S1.
Table 1.
Evaluation of symptomatic patients with suspected myocarditis/pericarditis
| Investigation | Potential findings | Clinical indication |
|---|---|---|
| History and physical exam | Symptom onset temporally related to vaccination • Typical onset within first week post doseScreening history for alternative diagnosesHistory of previous COVID-19 infection or potential exposuresCommon symptoms suggestive of myocarditis/pericarditis:
|
All patients with symptoms potentially related to myocarditis/pericarditis temporally related to COVID-19 mRNA vaccine |
| ECG | ST elevation
|
All patients with symptoms potentially related to myocarditis/pericarditis temporally related to COVID-19 mRNA vaccine |
| Routine lab work | Cardiac biomarkers • Troponin elevation might be > 10 times ULN CRP • Elevated inflammatory markers are suggestive but not specific for myocarditis/pericarditis COVID-19 PCR testing • Positive test suggests acute or recent COVID-19 infection • Serology testing might be misleading in the setting of vaccination or previous exposure to COVID-19 CBC, LFTs, creatinine • Abnormalities might be nonspecific. Markers of end organ dysfunction assessment should consider the hemodynamic status of the patient |
All patients with symptoms potentially related to myocarditis/pericarditis temporally related to COVID-19 mRNA vaccination |
| Echocardiogram | Normal biventricular function does not rule out myocarditis/pericarditis Reduced LV/RV function might reflect more severe myocarditisPericardial effusion suggestive of pericarditis
|
Patients with moderate-high index of suspicion on the basis of clinical scenario, ECG, and troponin level elevation |
| Cardiac MRI | Findings might not be specific for vaccine-related myocarditis
|
Consider where available for symptomatic patients for whom diagnosis cannot be established with clinical scenario/ECG/labs/echocardiography |
Coronary artery assessment
|
Normal arteries suggestive of noncoronary cause for presentation Thrombotic occlusion, vasospasm, or dissection suggestive of primary coronary etiology |
Consider for patients with symptoms suggestive of ischemia with typical evolution of cardiac biomarkers and/or ECG changes suggestive of ischemia Need to consider pretest likelihood of coronary disease in all cases |
| Endomyocardial biopsy | Might be normal despite clinical picture consistent with myocarditis Changes consistent with myocarditis might be seen |
Rarely indicated
|
Included are the clinical indications for diagnostic testing and the findings that might be seen in a patient with myocarditis/pericarditis.
AV, atrioventricular; CBC, complete blood count; CRP, C-reactive protein; CT, computed tomography; ECG, electrocardiogram; LFTs, liver function tests; LGE, late gadolinium enhancement; LV, left ventricle; MRI, magnetic resonance imaging; mRNA, messenger ribonucleic acid; PCR, polymerase chain reaction; RV, right ventricle; ULN, upper limit of normal.
Figure 1.
A summary of the clinical considerations for diagnosis and reporting of a patient with COVID-19 mRNA vaccine myocarditis/pericarditis. https://wepik.com/. CBC, complete cell count; CDC, Centers for Disease Control and Prevention; Cr, creatinine; CRP, C-reactive protein; ECG, electrocardiogram; LFTs, liver function tests; mRNA, messenger ribonucleic acid; PCR, polymerase chain reaction; TTE, transthoracic echocardiogram.
Management of mRNA Vaccine-Related Myocarditis/Pericarditis
There is no clear evidence to support the use of anti-inflammatory therapy for all patients with myocarditis/pericarditis. Spontaneous resolution of symptoms is reportedly prevalent. Overall, the therapeutic approach for symptomatic patients might include pain management and nonsteroidal anti-inflammatory agents with or without colchicine (the latter suggested particularly when pericarditis is the predominant presentation). Use of intravenous immune globulins, corticosteroids, and biologic immune-modulating agents have been reported, and might be considered in severe cases.
For rare cases of hemodynamic instability requiring inotropic support, patients should be managed at tertiary care centres with the capacity for managing cardiogenic shock and critically ill cardiac patients. Supportive measures might include inotropic/vasoactive drugs, antithrombotic therapy, mechanical ventilation, or mechanical circulatory support including extracorporeal membrane oxygenation. For patients recovering from significant illness, follow-up should be coordinated in consultation with a cardiovascular specialist to provide guidance on further imaging/evaluation, treatment, and return to regular activities because strenuous exercise should be avoided until follow-up assessment.
Recommendations regarding further COVID-19 mRNA vaccination for those with confirmed myocarditis/pericarditis will evolve as evidence emerges. In the near term, it might be prudent to defer or delay the second or subsequent vaccine doses in accordance with the National Advisory Committee on Immunization (NACI) guidance.3
COVID-19-Related Cardiovascular Complications
Consideration of risks with COVID-19 mRNA vaccination have to be balanced against the risks of complications from COVID-19 infection. Proposed mechanisms of cardiac involvement in those infected with COVID-19 include sympathetic stimulation, proinflammatory effects, myocyte necrosis leading to myocarditis, and heightened risk of arrhythmia and left ventricular dysfunction. In addition, the hypercoagulable state associated with COVID-19 infection, along with direct vascular infection and concomitant cellular inflammation, contributes to higher risk of myocardial infarction.
Multiple studies have reported on the prevalence of cardiac complications in adults after COVID-19 infection, which include heart failure (23%-33.3%), myocardial injury/myocarditis (8%-27.8%), arrhythmia (16.7%), and thromboembolism (31%-40%). In those who develop myocarditis with elevated inflammatory biomarkers (leukocytosis, lymphopenia, d-dimer, C-reactive proteins, and pro-calcitonin) and elevated troponin levels, high mortality rates (51%-97%) have been described in several cases series.4
In contrast to adults, children have been largely spared COVID-19-related acute pulmonary infection and associated complications. However, children are vulnerable to a post exposure hyperinflammatory syndrome known as MIS-C. This syndrome can result in a severe clinical course requiring intensive care management. Cardiovascular complications from MIS-C include cardiac dysfunction (40.6%), shock (35.4%), pericardial effusion (23.9%), mitral regurgitation (25.5%), myocarditis (22.8%), heart failure (7.0%), and coronary artery dilatation or aneurysm (18.6%), with a mortality rate of 1%-2%.5 In light of current knowledge, it is reasonable to consider that despite the rarity of potential adverse outcomes after COVID-19 mRNA vaccine-related myocarditis/pericarditis in adults and children, the benefits from the vaccine in avoiding COVID-19 infection and its related complications outweigh the risk.
Summary and Guidance
On the basis of the current state of knowledge regarding the potential association between mRNA vaccination and myocarditis/pericarditis, “take away messages” for cardiovascular care providers can be articulated. These include:
-
•
The estimated incidence of myocarditis/pericarditis after COVID-19 mRNA vaccination across different age groups is likely to change with additional data; at present this is a rare event, with most reports suggesting incidence of 1 case per 10,000-100,000 vaccinations.
-
•
Most myocarditis/pericarditis cases have been reported after a second dose of COVID-19 mRNA vaccination, with greater incidence in men. Presentation is usually early (within the first week) after vaccination.
-
•
Most myocarditis/pericarditis cases reported have been mild and self-limited. Severe cases that present as more fulminant myocarditis with cardiogenic shock are exceedingly rare and likely merit more extensive investigation to exclude other causes.
-
•
The approach to investigation should be tailored to the clinical presentation. Suspected cases require a careful history and physical exam, ECG, routine laboratory work, and consideration for echocardiography. Patients with significant symptoms and routine testing supportive of a diagnosis of myocarditis/pericarditis should undergo additional cardiac investigations with specialized testing on the basis of availability and likelihood of an effect on clinical management.
-
•
Suspected cases should be reported to the Public Health Adverse Event Following Immunization Reporting System (https://www.canada.ca/en/public-health/services/immunization/reporting-adverse-events-following-immunization.html) by the health care provider of record.
-
•
No specific therapy can be recommended at present and care is largely supportive. A cardiac care provider should assess patients who require admission to hospital to provide guidance on monitoring, further management, and post-discharge follow-up instructions.
-
•
COVID-19 mRNA vaccination is recommended in all populations for which a benefit has been established, in accordance with Health Canada and NACI guidelines. On the basis of our current knowledge, the benefits of COVID-19 vaccination in all age groups far outweigh the potential risks of mRNA vaccination and that of possible COVID-19 infection in susceptible individuals.
-
•
For patients with myocarditis/pericarditis temporally associated with the first dose of COVID-19 mRNA vaccination, available evidence is currently insufficient to support any clinical practice recommendations. Questions remain as to whether these patients should avoid a second vaccination, have further delay between doses, or have a non-mRNA vaccine as a second dose.
-
•
As a precaution, and in accordance with NACI recommendations, patients with established myocarditis/pericarditis after the first mRNA vaccination should defer a second dose indefinitely pending additional data.
Conclusion
On the basis of our current state of knowledge, the association between myocarditis/pericarditis and COVID-19 mRNA vaccines in children and younger adults merits careful consideration for practitioners. Cases tend to be mild, do not usually require specific interventions, and potential risks of the vaccine are outweighed by the well defined risks of COVID-19 infection. Vaccination continues to be recommended in all eligible individuals. A thoughtful approach to evaluation, management, and reporting of suspected cases of myocarditis/pericarditis is indicated as a key public health measure.
Acknowledgments
Acknowledgements
The authors acknowledge members of the Canadian Cardiovascular Society's COVID-19 Rapid Response team in addition to Dr Kenny Wong (on behalf of the Canadian Pediatric Cardiology Association) for their review of an earlier version of this report.
Funding Sources
None.
Disclosures
The authors have no conflicts of interest to disclose.
Practical Clinical Practice Update
Footnotes
See page 1634 for disclosure information.
To access the supplementary material accompanying this article, visit the online version of the Canadian Journal of Cardiology at www.onlinecjc.ca and at doi:10.1016/j.cjca.2021.08.001.
Appendix. Supplementary materials
References
- 1.Shimabukuro T. COVID-19 Vaccine Safety Updates. Available at:https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-06/03-COVID-Shimabukuro-508.pdf. Accessed June 27, 2021.
- 2.Government of Canada. Reported side effects following COVID-19 vaccination in Canada. Available at: https://health-infobase.canada.ca/covid-19/vaccine-safety/. Accessed July 16, 2021.
- 3.Public Health Agency of Candada. Summary of National Advisory Committee on Immunization (NACI) Updates of July 2, 2021. Available at:https://www.canada.ca/content/dam/phac-aspc/documents/services/immunization/national-advisory-committee-on-immunization-naci/recommendations-use-covid-19-vaccines/summary-updates-july-2-2021-en.pdf. Accessed July 21, 2021.
- 4.Chang WT, Toh HS, Liao CT, Yu WL. Cardiac involvement of COVID-19: a comprehensive review. Am J Med Sci. 2021;361:14–22. doi: 10.1016/j.amjms.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Godfred-Cato S, Bryant B, Leung J, et al. COVID-19–associated multisystem inflammatory syndrome in children—United States, March-July 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1074–1080. doi: 10.15585/mmwr.mm6932e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.