Abstract
The coronavirus disease 2019 (COVID-19) pandemic caused by the novel severe acute respiratory syndrome coronavirus (SARS-CoV-2) has affected almost every country in the world resulting in severe morbidity, mortality and economic hardship, altering the landscape of healthcare forever. Its devastating and most frequent thoracic and cardiac manifestations have been well reported since the start of the pandemic. Its extra-thoracic manifestations are myriad and understanding them is critical in diagnosis and disease management. The role of radiology is growing in the second wave and second year of the pandemic as the multiorgan manifestations of COVID-19 continue to unfold. Musculoskeletal, neurologic and vascular disease processes account for a significant number of COVID-19 complications and understanding their frequency, clinical sequelae and imaging manifestations is vital in guiding management and improving overall survival. The authors aim to provide a comprehensive overview of the pathophysiology of the virus along with a detailed and systematic imaging review of the extra-thoracic manifestation of COVID-19. In Part I, abdominal manifestations of COVID-19 in adults and multisystem inflammatory syndrome in children will be reviewed. In Part II, manifestations of COVID-19 in the musculoskeletal, central nervous and vascular systems will be reviewed.
Keywords: COVID-19, Neuroimaging, Lower extremity CTA, Musculoskeletal
1. Introduction
COVID-19 is primarily a respiratory infection caused by the virus SARS-CoV-2 whose roots began in November of 2019 in Wuhan, China.1 Pneumonia is its most common manifestation with cases complicated by acute respiratory distress syndrome (ARDS) and pulmonary embolism. Cardiac manifestations are less common but include myocardial injury, arrhythmia, arterial and venous thromboembolism, myocarditis, cardiomyopathy, cardiogenic shock and cardiac arrest.2 However, COVID-19 is an unpredictable infection that can take many additional paths in the human body that are extra-thoracic. The abdominal manifestations are vast including hepatobiliary, genitourinary and gastrointestinal disease. Additionally, there are numerous extra-abdominal processes that can surface in the nervous, musculoskeletal or vascular systems. This article highlights the manifestations of COVID-19 in the musculoskeletal, the central nervous system and the central and peripheral vascular systems. Prevailing theories of the pathophysiology of SARS-CoV-2, the frequency of its involvement in various organ systems, and the imaging manifestations of COVID -19 in these systems will be discussed. The information presented is based on a review of current literature on COVID-19 and the cases discussed are from our own academic medical center in a hot zone in the Bronx, New York
2. Musculoskeletal imaging of COVID
Musculoskeletal symptoms are relatively common both at the onset and throughout the disease course of COVID-19, and include myalgia (16-19% of patients), back pain (10% of patients), arthralgia and fatigue (26-36% of patients) associated with a muscular component.3., 4., 5. 19% of patients present with myalgia as the initial symptom of COVID-19 and proximal muscle weakness may be a symptom of COVID-19-associated myositis.4., 6. The presence of musculoskeletal symptoms is not surprising as angiotensin-converting enzyme 2 (ACE2), the major SARS-CoV-2 entry receptor has been found not only in the lung, bowel, endothelium of small vessels, and smooth muscle, but also in skeletal muscle and synovial tissue.4., 5. Uncommon musculoskeletal manifestations of COVID-19 include myositis and peripheral neuropathy. In contrast, due to the frequent use of anticoagulation and prolonged hospital stays, intramuscular hematomas and ischiosacral decubiti are more commonly seen.
2.1. Myositis
Myositis, a relatively uncommon feature of COVID-19, presents with proximal muscle weakness and myalgia and may precede respiratory symptoms.6 Reflexes may be diminished on physical exam.7 Serum creatine kinase levels are elevated and antibody tests such as anti-SSA, anti-SAE 1 may also be positive.6., 7. Mehan et al. reported a series of 7 cases out of 9 COVID-19 patients who underwent spine MRI for back pain, lower extremity weakness or lower extremity paresthesia and had imaging evidence of paraspinal myositis characterized by intramuscular edema and/or enhancement.8 MRI is the most sensitive imaging modality to detect myositis. In the acute setting the muscle demonstrates T2 hyperintense signal consistent with edema and diffuse hyperenhancement post contrast. In the chronic setting, muscle atrophy is characterized by decreased bulk, varying degrees of fatty infiltration, and persistent edema (Fig. 1 ).7 MRI is critical not only in confirming myositis but also in guiding potential muscle biopsy.
Fig. 1.
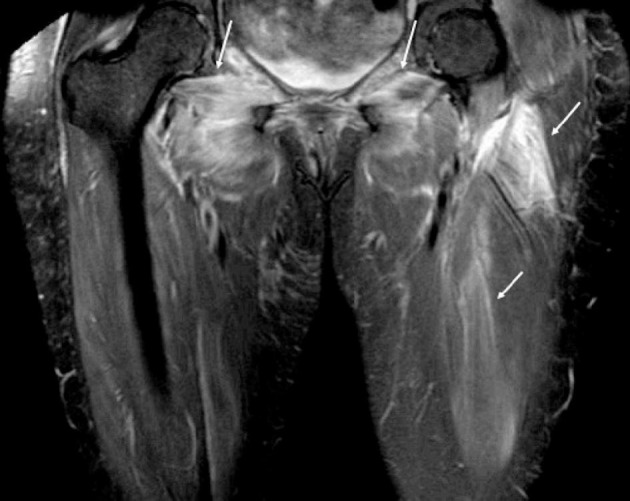
57-year-old woman hospitalized for COVID-19 pneumonia developed severe proximal thigh weakness with a markedly elevated creatine phosphokinase level in the 10,000 s. (a) Coronal fat-suppressed T2-weighted (SPIR) image from a bilateral thigh MRI shows bilateral streaky high intramuscular signal (arrows) in both adductor and gluteal muscles and in the left thigh muscles indicating nonspecific myositis. Subsequent thigh biopsy confirmed nonspecific myositis and the patient was treated successfully with prednisone.
Rhabdomyolysis has also been reported as a late complication of COVID-19, which may quickly become fatal.2., 9. It is clinically suspected when some combination of myalgia, fatigue, and tea-colored urine due to myoglobinuria are present. Serum creatine kinase is markedly elevated. Potential serious complications include compartment syndrome and acute kidney injury. Aggressive intravenous hydration is vital to prevent prerenal azotemia.10 The involved muscle or muscle groups may be subtly enlarged on imaging. CT scan reveals heterogeneous hypoattenuation of the involved muscle that may be associated with peripheral hyperenhancement. MRI demonstrates two patterns of rhabdomyolysis. In type 1, the involved muscle has homogeneous high signal on T2-weighted images with homogeneous hyperenhancement, seen in early stages of myonecrosis before liquefaction. In type 2, the involved muscle has heterogeneous high signal on T2-weighted images with peripheral hyperenhancement, seen in more advanced cases.2 Advanced myonecrosis may also appear as liquefactive zones of very high signal on T2-weighted images with rim hyperenhancement or internal stippled foci of preserved enhancement along with rim hyperenhancement (the “stipple sign”).2., 11.
2.2. Peripheral neuropathy
A variety of peripheral nerve abnormalities can occur with COVID-19, including postinfectious inflammatory neuropathy and traumatic neuropathy due to the use of proning in patient management. On imaging, the affected nerve may be enlarged, with alteration of its normal fascicular architecture. On ultrasound the affected nerve will be hypoechoic and MRI demonstrates high signal on T2-weighted images. The denervated muscles demonstrate geographic or diffuse edema in the acute setting which may progress to geographic or diffuse atrophy over time (Fig. 2 ).12
Fig. 2.
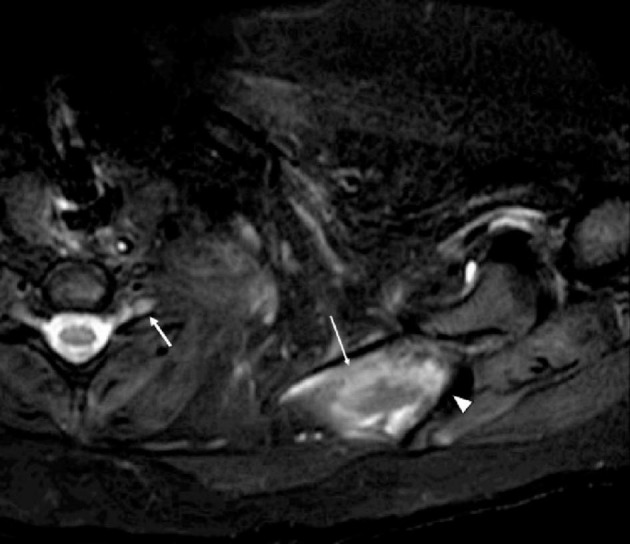
30-year-old man hospitalized for COVID-19 pneumonia and treated with intermittent proning, developed left upper extremity weakness. EMG showed diffuse upper-plexus-predominant plexopathy. Axial fat-suppressed T2-weighted (SPAIR) image shows asymmetric thickening and increased signal abnormality of the left C5-T1 nerve roots, brachial plexus trunks, divisions, and cords (arrow), which could reflect postinfectious inflammatory plexopathy or traumatic plexopathy secondary to proning. There is also intramuscular high signal (long thin arrow) in the supraspinatus muscles suspicious for denervation edema. Scapular spine is denoted with an arrowhead. Corresponding axial T1-weighted image fails to show any abnormality (not shown).
2.3. Intramuscular hematoma
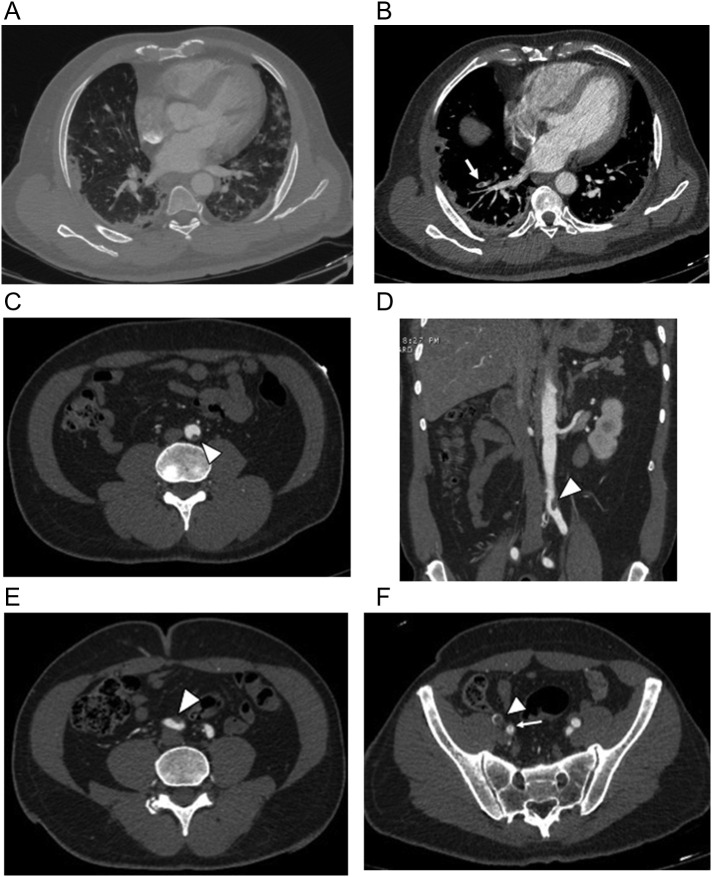
Due the frequent use of anticoagulation in prevention and treatment of COVID-19 thromboembolic disease, intramuscular hematomas are common. Hematomas can present as palpable lumps, often associated with pain, ecchymosis, and induration. They sometimes cause neurologic symptoms from mass effect on adjacent neurovascular structures.12 On CT, acute intramuscular hematomas manifest with muscular enlargement and are hyperdense on non-contrast exam (Fig. 3 ). The hematocrit sign (cellular-fluid level) is common in hematomas and is considered a highly sensitive and specific sign of coagulopathic hemorrhage characterized by dependent hyperdense cellular content and nondependent hypodense fluid content.13 MRI signal characteristics vary based on the age of the hematoma. Over time, hematomas may develop a distinct rim on all imaging modalities and may also develop septations and calcifications.
Fig. 3.
Two patients with hematomas as a result of anticoagulation. (a) 92-year-old woman with COVID-19 developed a palpable abdominal wall mass after receiving anticoagulation for deep vein thrombosis. Axial contrast- enhanced CT image shows an expansile right rectus abdominis mass (arrow) with mixed attenuation consistent with hematoma. (b) 53-year-old woman with COVID pneumonia and ARDS with an acute drop in hematocrit following anticoagulation to prevent venous thromboembolism. She required blood transfusions. Axial unenhanced CT image shows a large right sided-retroperitoneal hematoma (arrow) with bilateral psoas hematomas (long thin arrow) all demonstrating hematocrit levels; incidental note is made of an enlarged fibroid uterus (asterisk). She was later readmitted with pulmonary emboli, and lower extremity cellulitis and bilateral common femoral vein DVT despite anticoagulation.
2.4. Decubiti
Sacral and ischial decubiti are also recognized in COVID-19 patients with prolonged hospital stays (Fig. 4 ). Pressure ulcers are known to occur because of constant external force on dependent skin areas, typically seen in critically ill or immobile patients. COVID-19 patients who are intubated are at higher risk as they are often difficult to turn and the risk of infectious exposure to clinical staff may reduce attempts to move the patient. Diarrhea has been considered a contributing factor to sacral decubiti in ICU patients and is a common symptom of COVID-19.14 Sacral “ulcers,” distinct from sacral decubiti, are also reported in COVID-19 patients and are associated with immobility, prolonged bed rest, incontinence, poor nutrition, diabetes and vascular disease. They are reported in critically ill COVID-19 patients with multi-organ disease and present clinically with a distinct cutaneous appearance including purpuric lesions, violaceous induration, livedoid plaques and black eschars. Their etiology includes a combination of systemic coagulopathy, cutaneous ischemia and pressure-induced injury.15, 16. The role of wound care specialists in managing these patients becomes vital to help prevent, monitor and treat these entities, especially since these ulcers can be a portal of entry for secondary infection and sepsis.16 There is no literature describing any unique imaging features that distinguish sacral decubiti from sacral “ulcers” in COVID-19 patients.
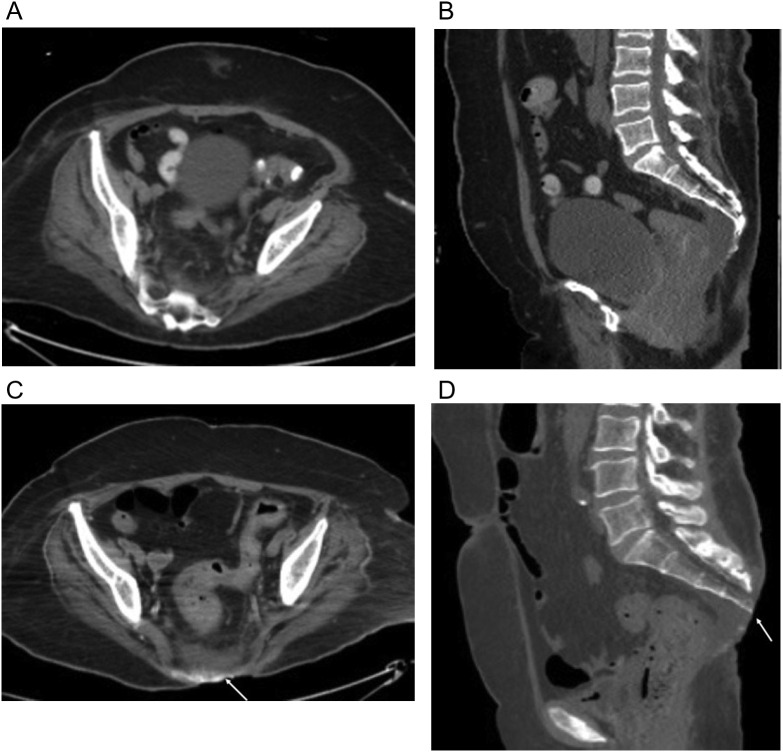
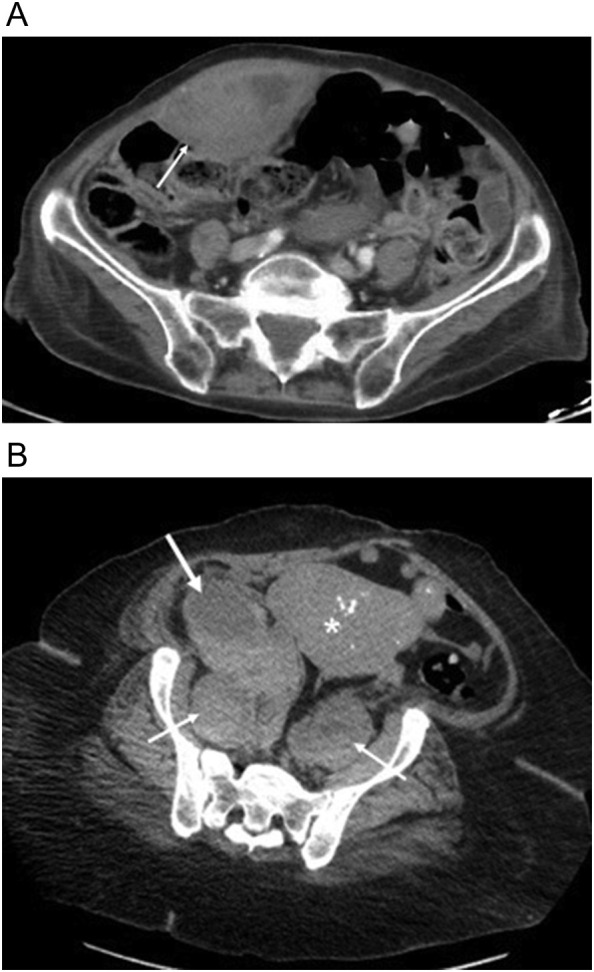
Fig. 4.
66-year-old-woman underwent a three-month hospitalization for COVID-19 pneumonia complicated by ARDS leading to tracheostomy, pseudomembranous colitis and E coli bacteremia ultimately died from septic complications. (a, b) Axial and sagittal unenhanced axial CT scan image from the first week of her first admission shows no evidence of sacral decubitus. (c, d) Axial and sagittal unenhanced images from CT scan 3 months later shows a large sacral decubitus (arrow) eroding the distal sacrum and coccyx. Note presacral edema. Dermatology consult determined a clean typical pressure ulcer with no signs of infection.
3. Neuroimaging of COVID-19
Among neurological syndromes associated with SARS-CoV-2 infection, the most common are anosmia, encephalopathy and stroke.17 Other neurological symptoms include encephalitis, meningitis and peripheral neuropathy. While SARS-CoV-2 particle and RNA can be detected in olfactory neurons and in anatomically connected regions of the brain, the evidence to date suggests that the predominant mechanism leading to neurological disease is due to inflammatory response and prothrombotic state, rather than direct neuron infection.18., 19. Studies from post-mortem series using electron microscopy indicate infection of vascular endothelial cells rather than neurons suggesting a role of the endothelial bed and a hematogenous route as the most likely pathway for SARS-CoV-2 into the central nervous system. Expression of the ACE2 receptor by the vascular endothelium further supports this mechanism.20 Subsequent disruption of the blood brain barrier can result in the virus gaining access to the central nervous system. The relative contributions of varying pathogenic mechanisms including inflammatory response, prothrombotic state, and direct viral invasion of neurons and cerebrovascular endothelium that lead to neurologic complications still remain unclear.21
The most common indication for neuroimaging in COVID-19 patients is “altered mental status” or “encephalopathy.” Most patients will have no abnormality on neuroimaging. However, among those with positive imaging findings, the most common diagnoses are infarct and hemorrhage, seen in 1.1% of hospitalized patients in one New York City study.22 In severely ill patients with longer hospitalization time, leukoencephalopathy and microhemorrhages can be seen. In rare cases, severe complications including large multifocal hemorrhage, cerebral edema, and anoxic brain injury can occur. Additional imaging patterns include venous thrombosis, cytotoxic lesions of the corpus callosum, olfactory bulb involvement, Guillain Barré syndrome and cranial nerve enhancement.
Radiologists should be aware of lung findings of COVID-19 incidentally detected on CTA of the Neck or CT and MRI of the spine as these may be the first recognized manifestations of infection and should be communicated with the referring physician23., 24., 25. (Fig. 5 ). A recent study has correlated the severity of lung findings on CT with the likelihood of acute neuroimaging findings.26 Patients with a neurological syndrome at presentation show a modest but significantly higher risk of mortality independent of overall COVID-19 disease severity.27 In the following sections, neuroimaging findings of COVID-19 will be reviewed. Standard head CT and MRI brain protocols can be utilized, as they are sensitive in the detection of neurological complications of COVID-19.
Fig. 5.
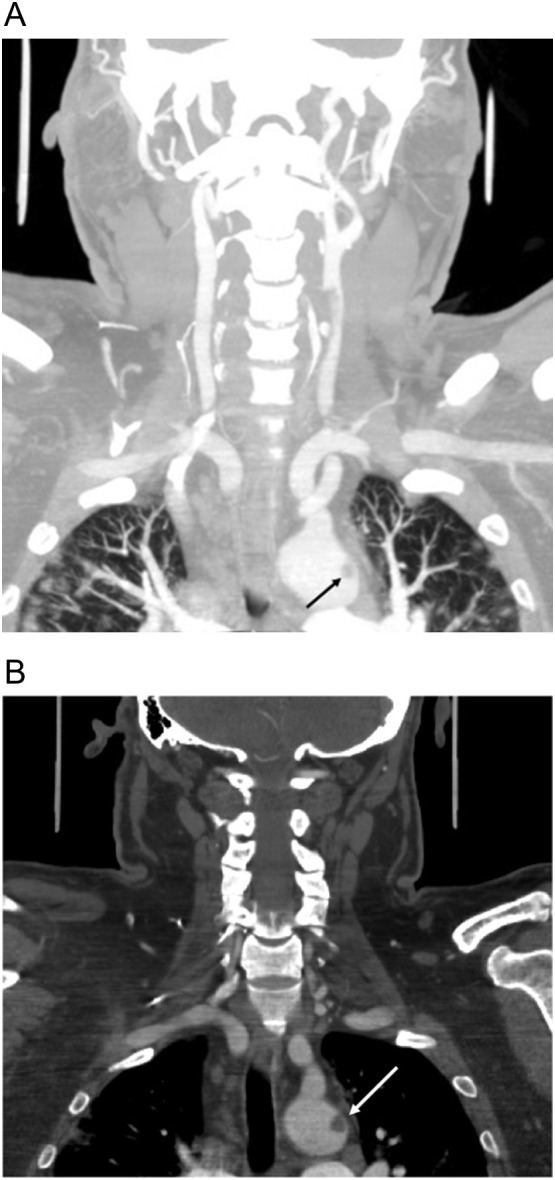
56-year-old man diagnosed with COVID-19 11 days prior to admission presented with mixed aphasia and right hemiparesis. (a) Coronal CTA neck image with lung windows shows subtle bilateral upper lobe peripheral ground glass opacities compatible with COVID-19 pneumonia, unsuspected at the time of imaging. Note a floating thrombus present within the aortic arch (black arrow), better seen on coronal image (b) with soft tissue windows (white arrow).
3.1. Acute infarct
The overall the incidence of infarct in hospitalized COVID-19 is low, reported at around 1% in large cohort studies.28., 29. However, in hospitalized patients with positive neuroimaging, infarct is the most common finding and is a strong prognostic marker for morbidity and mortality.22., 30. Most patients with infarction present with encephalopathy instead of focal neurological deficits.28 COVID-19 is a strong independent risk factor for development of stroke in hospitalized patients. Compared to patients without COVID-19, infarcts in patients with COVID-19 occurred in a younger population, with greater stroke severity, and greater morbidity and mortality.31 Prophylactic dose anticoagulation therapy is suggested by the American Society of Hematology in critically ill and severely ill patients with COVID-19.32
The cause of infarct is likely multifactorial, related to hypercoagulability and endotheliopathy. Supporting this theory are reports of unusually large intraluminal thrombi in the carotid arteries without significant underlying plaque [Fig. 6 ].33 Additionally, several case series have described large vessel occlusion (LVO) infarcts as the initial presenting symptom of COVID-19 infection with unexpected features including a younger than typical patient population34 or large clot burden with involvement of multiple vascular territories [Fig. 7, Fig. 8 ]. Infarcts on CT are characterized by loss of gray-white differentiation in a vascular distribution. Large vessel occlusion can be identified on CTA or MRA as an abrupt cut-off in a proximal intracranial vessel. These findings can be confirmed on MRI with restricted diffusion on diffusion-weighted imaging (DWI). Multifocal small infarcts can also be seen in external and internal border zone distributions (Fig. 9 ).
Fig. 6.
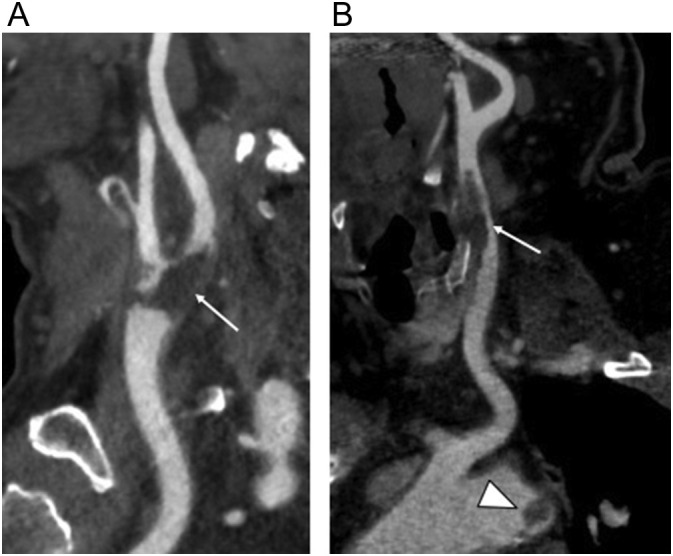
Two COVID-19 patients with carotid thrombi. (a) 76-year-old man with no respiratory symptoms who presented with right upper extremity weakness. Curved planar reformatted neck CTA image shows low attenuation arterial filling defect consistent with adherent thrombus in the carotid bifurcation (arrow). (b) 56-year-old man who presented with mixed aphasia and right hemiparesis with thrombus in the common carotid artery (arrow) and nodular floating thrombus in the aortic arch (arrowhead).
Fig. 7.
83-year-old man with 18-day history of COVID-19 who developed new left sided weakness after weaning sedation following extubation. (a) MIP image from CTA of the head shows abrupt cutoff of the mid M1 segment of the right middle cerebral artery (arrow) compatible with large vessel occlusion. (b) Corresponding non-contrast axial head CT image shows well-demarcated infarct in the right middle cerebral artery territory (arrow).
Fig. 8.
77-year-old woman hospitalized for COVID-19 pneumonia who developed dense left hemiparesis on her fourth hospital day. (a) Non-contrast axial head CT image shows multifocal large vessel territory infarcts (arrow) involving the right middle cerebral artery and left posterior cerebral artery territories. (b) More caudad image shows additional smaller infarcts (arrow) in the occipital lobes and left cerebellar hemisphere.
Fig. 9.
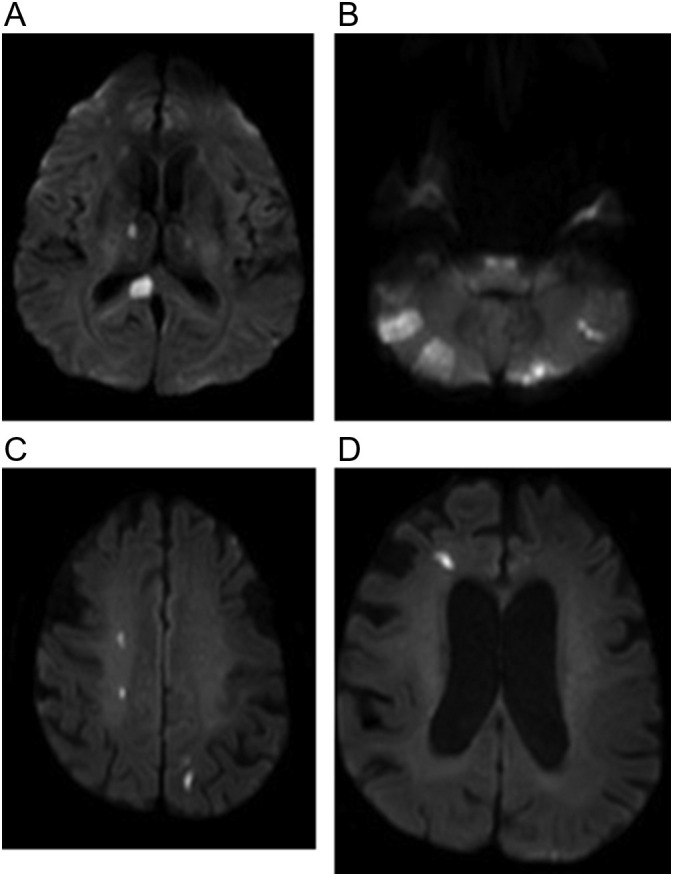
Two patients with COVID-19 who developed multifocal infarcts. (a, b) 62-year-old woman with no significant past medical history who presented with altered mental status. Axial diffusion weighted images show multiple foci of restricted diffusion in the posterior circulation. (c, d) 62-year-old man diagnosed with COVID-19 2 days earlier who developed headache and altered mental status. Axial diffusion weighted images showing multiple foci of restricted diffusion in border zones.
3.2. Parenchymal hemorrhage
Hemorrhage is another possible complication in severely ill COVID-19 patients and is the second most common finding after ischemic infarct. In a reported cohort of 33 patients with hemorrhage, the majority of cases were detected during their hospital stay (median hospital day 17) with only a minority presenting at admission. The majority of cases demonstrated microhemorrhage, while five developed large hemorrhages complicated by mass effect and herniation with a mortality rate of 100%.35 In this cohort, a majority of patients were on anti-coagulation for elevated D-Dimer, which is a possible risk factor. Other possible causes include a combination of critical care therapy, extracorporeal membrane oxygenation (ECMO), and consumptive coagulopathy.36., 37. Large parenchymal hematomas and multifocal hemorrhage can be seen, sometimes with rapid change (Fig. 10, Fig. 11 ). The rate of multifocal hemorrhage in COVID-19 patients is greater than that of the general population, reported to be 5 times the general population's rate.38 Multifocal hemorrhage can be associated with more extensive parenchymal injury with complicated posterior reversible encephalopathy syndrome (PRES) and leukoencephalitis-like appearances, which will be discussed later. Microhemorrhage detectable on MRI but not on CT is more typically seen in the subacute to chronic phase of severely ill patients in combination with white matter changes and will be discussed in the next section.
Fig. 10.
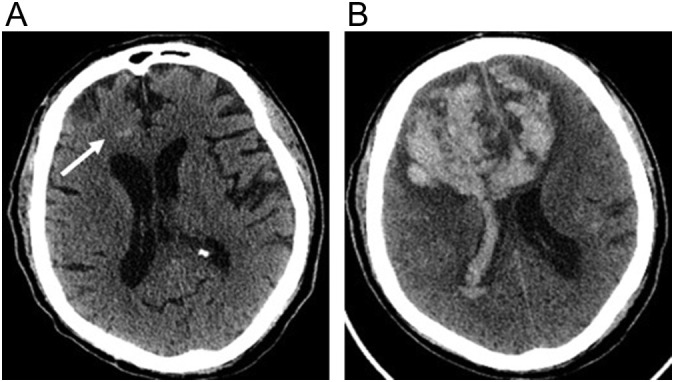
71-year-old male with COVID-19 on therapeutic anticoagulation because of elevated inflammatory markers who developed confusion. (a) Initial axial non-contrast CT image shows a small focus of hemorrhage (arrow) in the right frontal lobe. (b) Axial non-contrast CT image performed 48 h later shows marked enlargement of hemorrhage with extension across the corpus callosum and into the lateral ventricles.
Fig. 11.
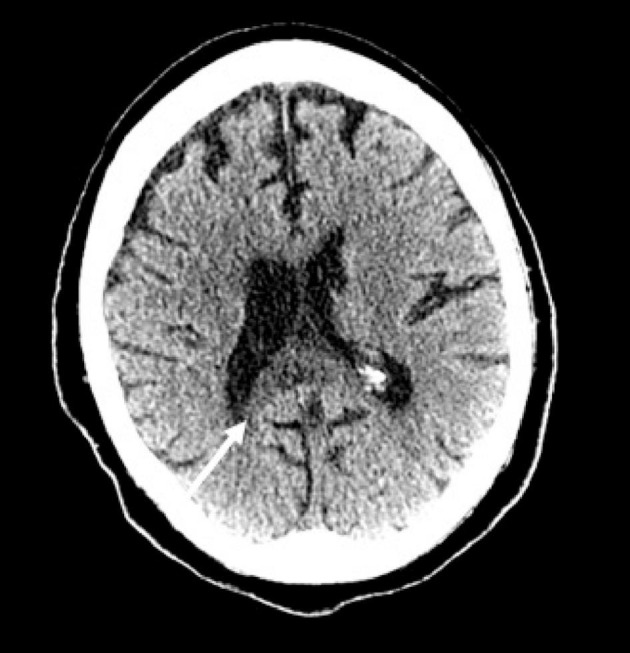
85-year-old woman with COVID-19 respiratory failure and acute kidney injury requiring hemodialysis that developed encephalopathy off sedation. (a) Axial non-contrast CT image shows hypoattenuation and mild expansion of the splenium of the corpus callosum (arrow).
Hemorrhage is detected on CT as a hyperdense collection. It can be characterized on MRI depending on the stage of blood. Susceptibility weighted imaging is sensitive for the T2 shortening effect of certain stages of blood. It can readily detect foci of microhemorrhage not seen on other sequences, and is more sensitive than gradient echo sequences in the detection of microhemorrhage.
3.3. White matter changes and hemorrhage
White matter changes with microhemorrhages are a less common imaging finding in COVID-19 patients. The incidence of these findings will vary based on image utilization and severity of cases in the hospital. In a study of 7146 COVID-19 patients, leukoencephalopathy was detected in 7 and positively correlated with clinical risks including obesity, acute renal failure, mild hypernatremia, and anemia.39 In most cases, leukoencephalopathy is found in patients with severe illness, long hospitalizations, and a clinical history of encephalopathy.40., 41., 42, 43., 44. The etiology of these findings is difficult to determine given the complex multisystem dysfunction and polypharmacy in the patients, however, vascular, demyelinating, or hypoxia related injury have been proposed.39., 45. Similar findings have been reported in high-altitude cerebral edema,46 leading some to hypothesize that a similar pathophysiology is responsible. A more severe appearance of white matter injury with macrohemorrhages can be seen in rare cases. Similar findings have been reported with other coronavirus infections such as Middle Eastern respiratory syndrome (MERS).47
On CT, the findings can be subtle with hypodensity and mild expansion in the corpus callosum (Fig. 11). On MR imaging, T2 FLAIR can show signal abnormality in the corpus callosum, periventricular white matter, and subcortical white matter and can be associated with restricted diffusion (Fig. 12 ). Microhemorrhages can be an associated finding in the corpus callosum or along the gray-white junction, and are much better delineated on susceptibility weighted imaging (SWI) (Fig. 13 ). In some instances, this can appear like PRES or acute hemorrhagic leukoencephalitis (Fig. 14 ).
Fig. 12.
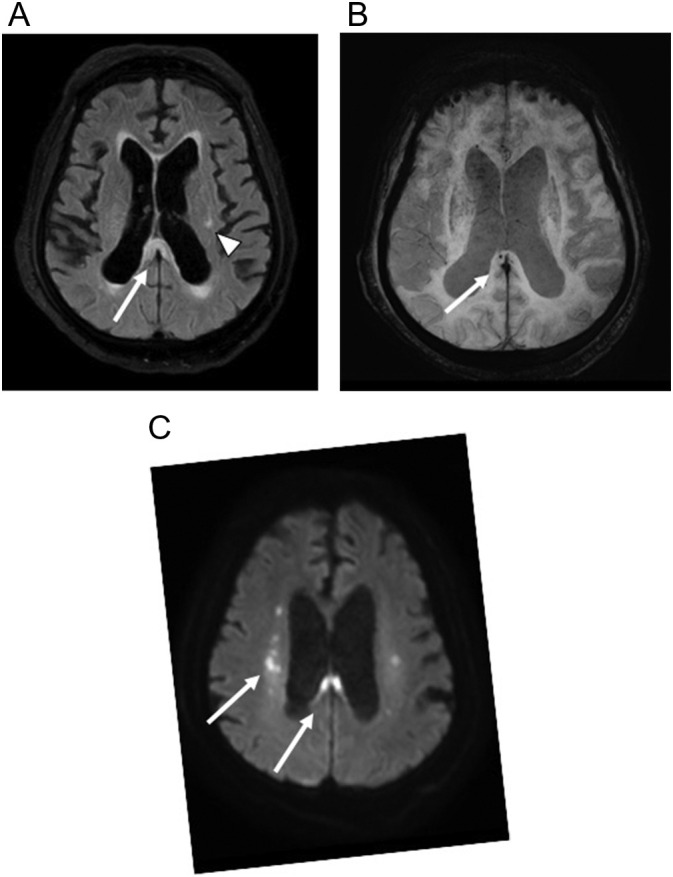
69-year-old man with COVID-19 pneumonia complicated by ARDS, pseudomonas and MRSA pneumonia, e. faecalis bacteremia, cytomegalovirus viremia, and acute kidney injury requiring dialysis who developed encephalopathy. (a) Axial T2-weighted (FLAIR) image demonstrates high signal in the splenium of the corpus callosum (arrow) and nonspecific white matter changes in the corona radiata (arrowhead). (b) Axial susceptibility-weighted (SWI) image shows punctate focus of microhemorrhage associated with corpus callosum signal abnormality. (c) Axial diffusion-weighted (DWI) image shows restricted diffusion within the splenium of the corpus callosum and corona radiata.
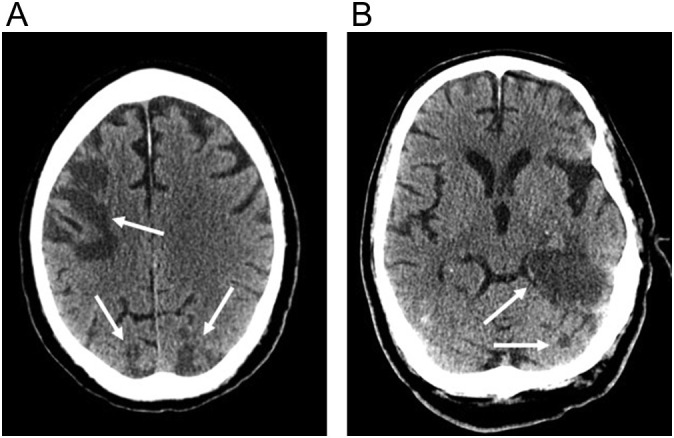
Fig. 13.
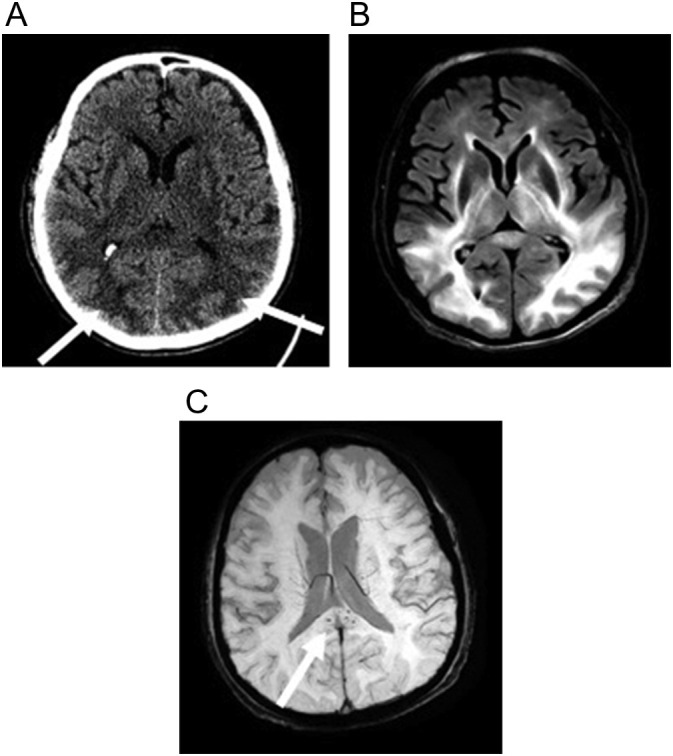
80-year-old man with COVID-19 respiratory failure requiring mechanical ventilation who developed a seizure on hospitalization day 41. (a) Non-contrast axial CT image shows ill-defined hypodensity in the subcortical white matter of the occipital lobes (arrow) and subtle hypodensity in the splenium of the corpus callosum. (b) Axial T2-weighted (FLAIR) image better shows extent of confluent white matter signal abnormality which involves the subcortical and periventricular white matter of the temporal and occipital lobes, surrounding the basal ganglia, and abnormality within the thalami and corpus callosum. (c) Axial susceptibility-weighted (SWI) image shows foci of microhemorrhage (arrow) within the splenium of the corpus callosum.
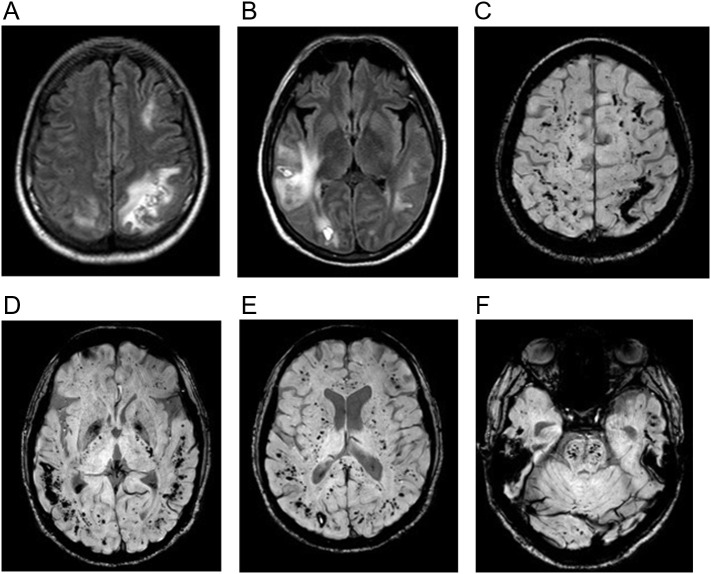
Fig. 14.
40-year-old man with no past medical history who presented with hypoxemia from COVID-19. He developed respiratory failure requiring mechanical ventilation and septic shock with multi-organ failure. On hospital day 22 he developed disorientation after extubation with his MRI revealing separate regions of subcortical leukoencephalopathy with associated hematomas and microhemorrhages. (a, b) Axial T2-weighted (FLAIR) images show large but separate regions of subcortical white matter signal abnormality in the frontal, parietal, and temporal lobes. (c-f) Multiple axial susceptibility-weighted (SWI) images show larger foci of hemorrhage associated with the white matter abnormality on T2-weighted (FLAIR) image, as well as more extensive microhemorrhage at the gray white junction and within the brainstem.
3.4. Venous sinus thrombosis
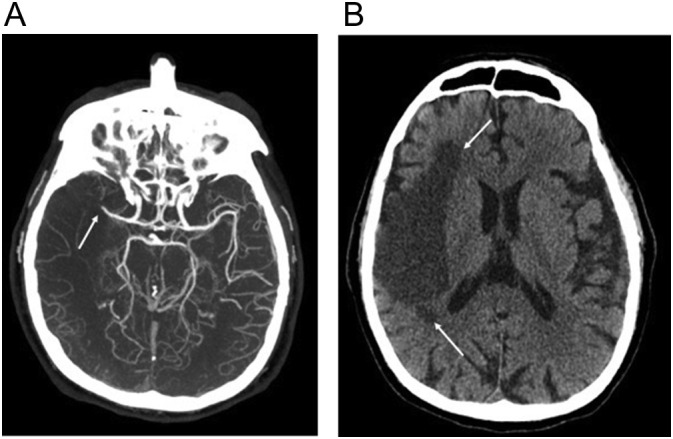
Venous sinus thrombosis is uncommon in COVID-19 but has been reported.48., 49, 50., 51., 52 In a recent review of the literature, a total of 33 COVID-19 patients with cerebral venous sinus thrombosis were reported. Headache was the most common symptom seen in 48.6%, with seizure, decreased consciousness, or focal neurological deficits present in over a quarter of the patients.52 Contrary to cerebral venous sinus thrombosis in the general population, reported cases have had a slight male majority and an older population. Significant elevation of inflammatory markers in these patients suggests a possible systemic prothrombotic state leading to cerebral venous sinus thrombosis. Non-contrast CT of the head can show increased density and size of the dural venous sinuses or central venous sinuses and can be confirmed with CT or MRI with contrast (Fig. 15 ).
Fig. 15.
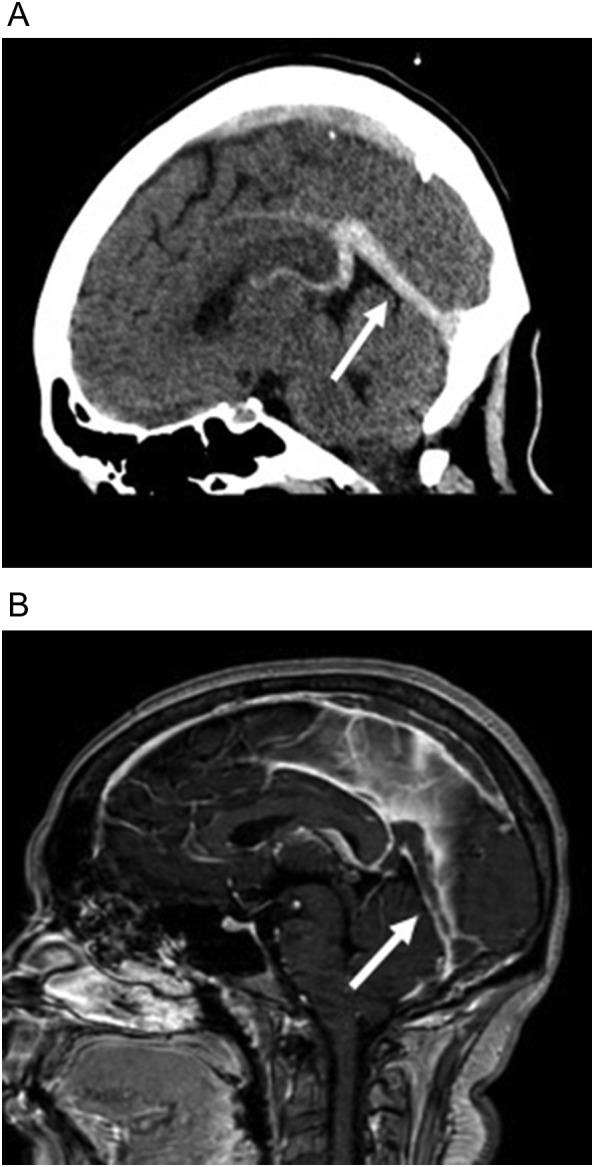
32-year-old obese woman with COVID-19 who presented with headache and no respiratory symptoms. (a) Sagittal non-contrast head CT image shows increased density in the vein of Galen, straight sinus (arrow), and lesser extent the superior sagittal sinus. (b) Sagittal T1 contrast-enhanced image shows absent enhancement within the vein of Galen, straight sinus (arrow), and superior sagittal sinus compatible with central venous and dural venous sinus thrombosis.
3.5. Anoxic injury
Anoxic brain injury can occur in severely ill COVID-19 patients although reported very infrequently (1 out of 126 patients in a descriptive literature review).53 Limited data is available to evaluate the cause in these patients given the few case reports, however, in severely ill patients the etiology is likely multifactorial including cardiovascular shock and cardiopulmonary arrest (Fig. 16 ). Anoxic injury is demonstrated on CT by diffuse loss of gray white differentiation progressing to cerebral edema with sulcal effacement.
Fig. 16.
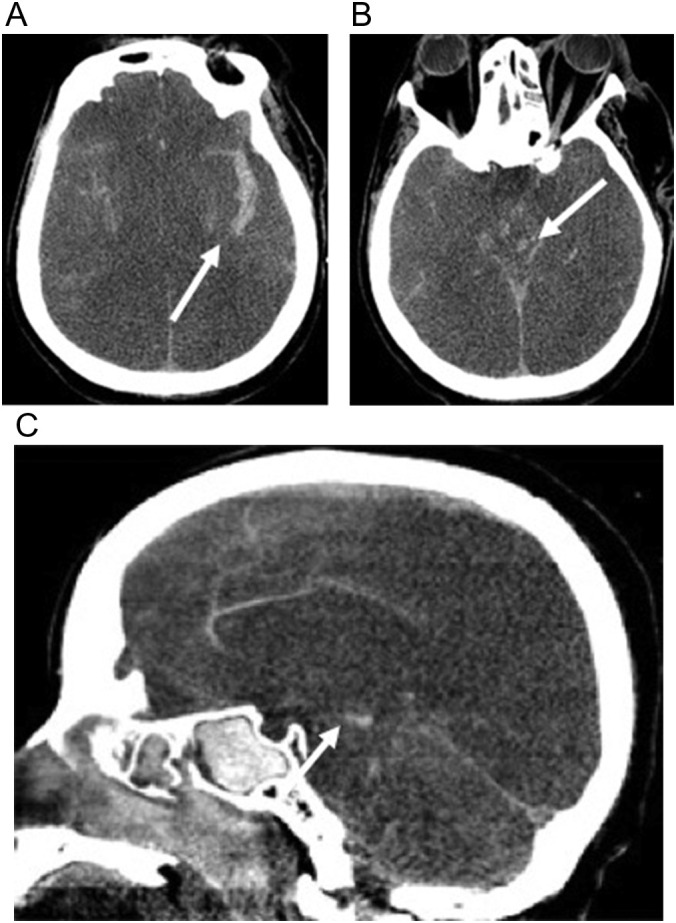
59-year-old man with COVID-19 respiratory failure requiring a prolonged intensive care unit stay of 18 days complicated by acute kidney injury requiring hemodialysis and Klebsiella and Pseudomonas bacteremia. He had a poor mental status when he was weaned off sedation due to anoxic brain injury and herniation. (a, b) Axial non-contrast CT images show diffuse loss of gray white differentiation and effacement of cerebral sulci compatible with global anoxic injury. There is (a) subarachnoid hemorrhage (arrow) and (b) brainstem hemorrhage (arrow). (c) Sagittal non-contrast head CT image demonstrates loss of gray white differentiation, cerebral edema, and resultant transtentorial herniation with foci of duret hemorrhage (arrow) in the brainstem.
3.6. Meningitis/encephalitis
Diagnosis of meningitis or encephalitis is based on a combination of clinical, laboratory (RT-PCR positive for SARS-CoV-2 in the cerebrospinal fluid), and radiological findings.54 Imaging findings include cortical FLAIR hyperintensities, cortical restricted diffusion, or leptomeningeal enhancement.55
3.7. Cranial and spinal nerve neuropathy
Acute immune-mediated polyneuropathies such as Guillain-Barré syndrome (GBS) and its subtypes like Miller Fischer syndrome (acute onset of external ophthalmoplegia, ataxia, and areflexia) and polyneuritis cranialis (isolated multiple cranial neuropathy) are rare complications of COVID-19.56., 57., 58., 59. Imaging findings include cranial nerve or cauda equina enhancement.
3.8. Olfactory bulb involvement
Anosmia and dysgeusia have been reported as common early symptoms in patients with COVID-19, occurring in greater than 80% of patients in one European series.60 Imaging findings are not as commonly observed, however. Increased T2 signal in the olfactory bulbs and tracts with or without contrast enhancement has been reported in only 19% of patients in one series.61
Task based functional MRI in a patient with persistent olfactory and gustatory symptoms after COVID-19 infection demonstrated lack of BOLD signal in orbitofrontal cortex (secondary and tertiary olfactory and gustatory area) suggesting central olfactory pathway impairment may be involved in the underlying etiology of the persistence symptoms.62 However, olfactory bulb atrophy after COVID-19 induced anosmia has been described in patients with prolonged postinfectious anosmia, suggesting persistent olfactory bulb injury plays a role as well.63
3.9. Optic neuritis
Orbital manifestations of COVID-19 such as optic neuritis have been reported.64 In a multicenter cohort of 129 patients presenting with severe COVID-19, 7% patients had one or several FLAIR-WI hyperintense nodules of the posterior pole of the globe. The clinical significance or the etiology of these nodules is currently unknown.65
3.10. Orbit
Orbital manifestations of COVID-19 such as optic neuritis have been reported.64 In a multicenter cohort of 129 patients presenting with severe COVID-19, 7% of patients had one or several FLAIR hyperintense nodules of the posterior pole of the globe. The clinical significance and etiology of these nodules are currently unknown.65
4. Imaging of COVID-19 in the aorta and peripheral vascular system
SARS-CoV-2 can directly infect the vascular system because ACE-2 receptors are located on endothelial cells lining our vessels and organ vascular beds. Resultant endotheliitis may precipitate an immune reaction with widespread endothelial dysfunction associated with apoptosis. This leads to a complex pathway of vasoconstriction with subsequent organ ischemia, inflammation and a procoagulant state.66 A patient's hypoxic status and the presence of ARDS further contribute to the progression of a proinflammatory thromboembolic state.67 The pathogenesis of COVID-19 coagulopathy is multifactorial and not fully understood. It remains unclear whether COVID-19 follows the same path of spiraling events seen in sepsis-induced coagulopathy and thrombotic microangiopathy typical of non-COVID critically ill patients.3
COVID-19 coagulopathy can manifest with varying venous or arterial thromboembolic events including deep venous thrombosis (DVT), pulmonary embolus (PE), limb ischemia, stroke and myocardial infarction.3 It is associated with a higher risk of death. Based on a meta-analysis of 425 studies evaluating thromboembolism (TE) in COVID-19 till June 2020, the pooled odds of mortality were 74% higher among patients who develop TE compared to those who did not. A high TE rate in COVID-19 was also determined with an overall TE rate of 21%.68 It has also been suggested that TE occurs more often in COVID-19 patients than in other critically ill non-COVID patients. In a retrospective study comparing 81 COVID-19 patients to 81 non-COVID patients, 11% of COVID-19 patients had TE (5 had arterial abdominal or lower extremity TE, 4 had splenic or renal infarcts, 1 had portal vein thrombosis and one had renal vein thrombosis) and only one non-COVID patient had known portal vein thrombosis from known HCC.69 Finally, elevated serums markers are seen that reflect COVID-19’s unique coagulopathy. Thrombocytopenia and elevation of fibrin D-dimer and fibrinogen levels are commonly seen and are associated with poor outcomes.70 Additional immune-mediated inflammatory markers due to cytokine storm may also be elevated including CRP, interleukins, ESR, and ferritin.71
4.1. Deep venous thrombosis (DVT)
Based on a meta-analysis of 20 studies including 1988 COVID-19 patients, the weighted mean prevalence was 31.3% for venous thromboembolism (VTE), 19.8% for DVT and 18.9% for PE.72 The risk of DVT is increased not just in ICU patients but also in non-ICU hospitalized patients and affects both the upper and lower extremity. Upper extremity DVT, however, is much rarer than lower extremity DVT in COVID-19 with sparse reports only of spontaneous upper extremity DVT in the literature.73., 74., 75. The risk of general VTE, including DVT, PE and venous thrombosis of other sites, rises with prolonged hospitalization where cumulative incidence rates rose to 59% on hospitalization day 21 of ICU patients.76 The incidence of DVT in COVID-19 patients with PE appears lower than in non-COVID patients suggesting that an in situ thromboinflammatory insult unique to SARS-CoV-2 plays a role in triggering PE in COVID-19 patients rather than migration of peripheral venous thrombi. Based on a meta-analysis of 27 studies of patients with COVID-19, DVT was present in only 42.4% of patients with PE, lower than the usual prevalence (60%) of DVT in non-COVID patients.77 In a prospective study of 26 hospitalized COVID-19 patients with PE who were screened for lower extremity DVT with ultrasound, only 2 patients (7.7%) had DVT.78
Venous duplex US with compression US technique remains the first-line imaging tool for diagnosis of suspected lower and upper extremity DVT and has the advantage of bedside portable performance.79 Thrombi typically appear as occlusive or non-occlusive echogenic venous filling defects on gray-scale images that may distend the affected vein and may arise denovo or at sites of central line placement3 (Fig. 17 ). Contrast-enhanced CT with venous timing can show a sharply defined occlusive or non-occlusive venous filling defect or an asymmetrically non-enhanced venous segment. It has the advantage of detecting IVC and intrapelvic DVT not optimally detected on lower extremity Duplex US (Fig. 18 ).
Fig. 17.
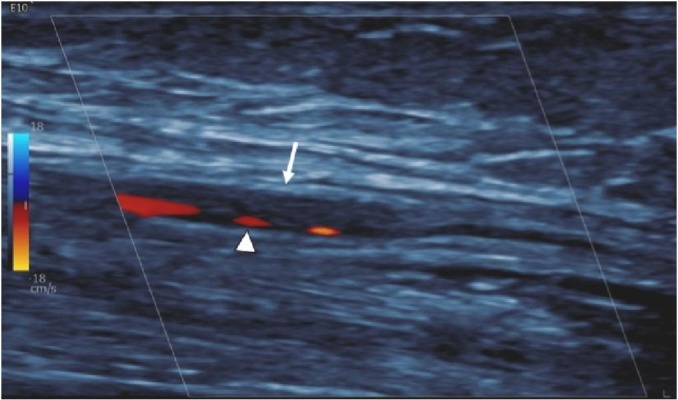
73-year-old woman with HTN, cardiac history and COVID pneumonia whose course was complicated by sepsis and duodenal ulcer perforation requiring surgical repair. She developed a cool right hand after right radial arterial line placement. Longitudinal Doppler Ultrasound image shows an elongated non-occlusive echogenic clot (arrow) with preserved peripheral flow (arrowhead) in the radial artery. The line was removed and hand ischemia improved with conservative measures.
Fig. 18.
75-year-old man with HCV cirrhosis and COVID pneumonia presented to the hospital with pleuritic chest pain and hypoxia 10 days after viral illness onset. (a) Axial chest CTA image shows multifocal PE (arrows) and bilateral pneumonia. (b) Axial abdominal contrast-enhanced CT image show a well-defined non-occlusive low attenuation clot in the IVC above the bifurcation (arrow) that extends into the left common iliac vein (arrowhead). (c) More caudal axial contrast-enhanced CT image shows non-occlusive left external iliac vein clot (arrowhead). (d) Further caudal axial contrast-enhanced CT image shows left common femoral vein clot (arrowhead). (e) Transverse gray-scale D Ultrasound image shows non-occlusive echogenic filling defect in the right popliteal vein (arrow). (f) Longitudinal color Doppler image shows preserved flow (arrowheads) surrounding the clot.
4.2. Aortic and peripheral arterial thromboembolism
Arterial thromboembolic events are much less common than venous thromboembolic events in COVID-19 and are potentially more deadly when their recognition is delayed. Although the true incidence of arterial thromboemboli of the aorta and extremities in COVID-19 patients is not known, there are growing reports of it in the literature.80, 81., 82., 83., 84., 85., 86., 87., 88. A systematic review of 27 studies reporting arterial thromboembolic events among COVID-19 patients till June 2020 included cardiac, aortic, mesenteric, central nervous system and limb ischemia and revealed a pooled incidence of arterial thromboembolic events of 4.4%. The most common site of disease was the limb arteries (39%), with the remaining distribution including cerebral arteries (24%), great vessels (aorta, common iliac, common carotid and brachiocephalic trunk, 19%), coronary arteries (9%) and superior mesenteric artery (8%).89 Many of the patients who suffer arterial thromboembolic events lack predisposing peripheral arterial disease81., 88. and thromboprophylaxis does not always prevent arterial thromboembolic ischemia.89 Atrial fibrillation, although it may be concomitant in COVID-19, is not as common a predisposing condition of arterial TE in COVID-19 as it is in non-COVID patients.90., 91.
Aortic involvement in COVID-19 can present as floating thrombi or segments of partial or complete occlusion. Involvement of the aorta tends to occur in patients with more severe infection and evidence of cytokine storm with elevated serum inflammatory markers.87 Both aortic floating thrombi (AFT) and abdominal aortic occlusion (AAO) are rare and life-threatening conditions in the general population. AFT are pedunculated mural thrombi that have the potential to break off causing visceral and peripheral embolism and can present silently or with chest or abdominal pain. In non-COVID patients they are associated with abnormal coagulation, abnormal aortic morphology and aortic stents.92 In COVID-19 patients they have been reported in the arch, descending aorta and ascending aorta in patients over 60 with elevated serum inflammatory markers.86 Abdominal aortic occlusion (AAO) can cause sudden onset of lower extremity pain, paralysis, and pallor and can be complicated by mesenteric, renal or spinal cord ischemia. Isolated events of AAO in COVID-19 patients have been described one to four weeks after clinical onset of infection, in patients with positive lupus anticoagulant presumed a viral-induced antiphospholipid syndrome and with elevated inflammatory serum markers.87 Finally, aortic TE in COVID-19 patients can be multifocal accompanied by additional PE and IVC clot.84
Aortoiliac and lower extremity arterial TE is another uncommon manifestation of COVID-19 with upper extremity arterial TE less frequently reported. Both occlusive and non-occlusive arterial TE have been reported. Cases reported are in hospitalized COVID-19 patients who are most often male, have elevated serum inflammatory markers and often develop arterial TE in a delayed fashion after viral illness onset, typically up to 2 weeks later.80, 81., 82., 85., 88., 93. In the largest series of sixteen COVID-19 hospitalized patients who underwent CT angiography (CTA) of the abdominal aorta and lower extremities for suspected ischemia, all sixteen patients had at least one arterial thrombus compared with only 69% of controls. Among COVID-19 patients arterial TE were more often proximal than in controls and clot burden was larger than in controls. Death of limb amputation was more common in COVID-19 patients but COVID-19 patients who presented with isolated leg ischemia instead of pulmonary or systemic symptoms were more likely to avoid amputation or death.80
When arterial TE is suspected in a COVID-19 patient, CTA of the chest, abdomen and pelvis is the first-line test and should include CTA of the extremities if peripheral ischemia is suspected. Arterial thrombi are readily detected as hypodense occlusive or non-occlusive filling defects with potential abrupt cutoff of contrast flow and with variable distal reconstitution via collaterals (Fig. 19 ). It is advised to carefully inspect all vessels and organs for thrombi as multifocal disease can occur, including organ ischemia.3 Floating thrombi are typically pedunculated aortic filling defects (cylindrical, striped or spindle-shaped) of varying lengths whose proximal segment is attached to the aortic wall and whose distal segment is free-floating either perpendicular to or along the direction of blood flow92 (Fig. 20 ). Doppler Ultrasound is also valuable in diagnosis of arterial TE as it can be performed portably in hospitalized COVID-19 patients.3 A thorough radiologic search for arterial TE is critical and communication of findings to clinical staff vital so that appropriate aggressive anticoagulation measures and/or endovascular or vascular surgery management expedited.
Fig. 19.
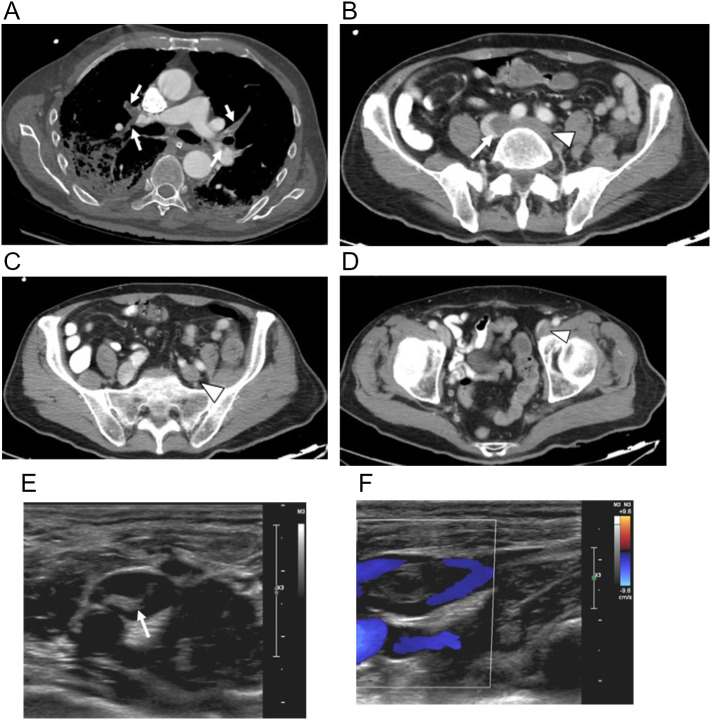
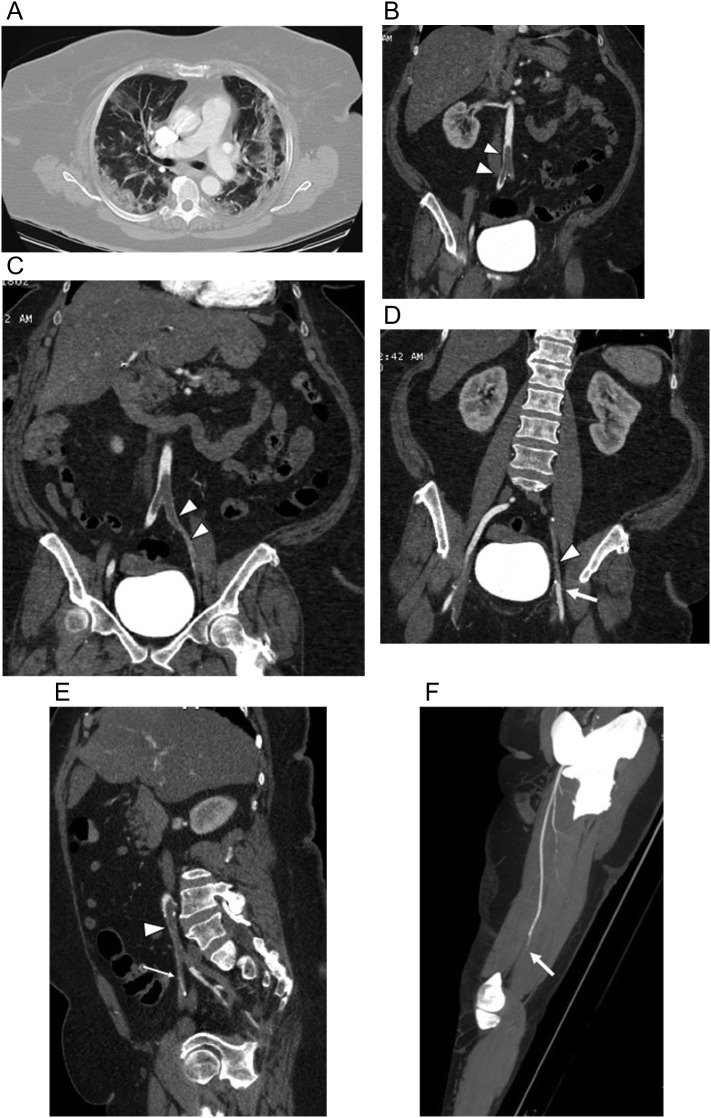
63-year-old woman with history of obesity, hypertension and breast cancer presented to the emergency room with 2 days of a painful and weak left leg and absent pulses. She had not respiratory complaints but was hypoxic. (a) Axial chest CTA image with lung windows on day of admission shows bilateral peripheral mixed ground glass and small consolidative infiltrates with no pulmonary emboli. Coronal multiplanar reformatted images from CTA of the abdomen, pelvis and lower extremities shows (b) low attenuation infrarenal abdominal aortic clot extending into the proximal right common iliac artery (arrowheads), (c) extending into the left common iliac and external iliac arteries (arrowheads), and (d) extending into the left external iliac artery (arrowhead) with reconstitution of the left femoral artery (arrow). (e) Sagittal multiplanar reformatted image shows the long segmental nature of the clot extending from the left common iliac artery (arrowhead) into the left external iliac artery (thin arrow) and focally into the proximal left internal iliac artery (thick arrow). (f) Oblique sagittal MIP image of the left thigh shows abrupt occlusion (arrow) of the distal left superficial femoral artery with no distal flow. The patient required left above knee amputation.
Fig. 20.
60-year-old man with type II DM presented to the emergency room with 3-day history of a cold and painful right lower extremity and asymptomatic COVID pneumonia. Axial CT image from CT angiogram (CTA) of the abdomen, pelvis and lower extremities showed (a) peripheral bibasilar interstitial pneumonia on lung windows and (b) segmental embolus (arrow) in the lateral right lower lobe pulmonary artery segmental branch on soft tissue windows. (c) Axial CT image of the aorta shows a peripheral round non-occlusive clot (arrowhead) in the distal infrarenal aorta that appears “floating” on (d) coronal 3D reformatted image of the aorta. (e) Axial CT image below the aortic bifurcation shows a second non-occlusive clot in the right common iliac artery (arrowhead). (f) Axial CT image below the right iliac bifurcation show a nearly occlusive clot of the right external iliac artery (arrowhead) and a punctate non-occlusive clot of the right internal iliac artery (thin arrow). (g) Sagittal MIP image of the mid right leg shows abrupt occlusion of the proximal right popliteal artery (arrow) with no distal flow. The patient was treated successfully with open right leg thrombectomy.
5. Conclusion
The SARS-CoV-2 virus is an intimidating agent because of its easy and aggressive spread all over the world and its ability to penetrate so many organ systems in the human body. There is a growing role of radiology in the second wave of the pandemic in detection and monitoring of complications of COVID-19. Therefore, it is critical for radiologists to be familiar with the broad spectrum of imaging manifestations of COVID-19, to better understand the pathophysiology of SARS-CoV-2, and to be knowledgeable about how frequently various organ systems may be affected. Increased vigilance about the complications of COVID-19 is necessary to ensure prompt diagnosis and further aid patient management.
References
- 1.Shereen M.A., Khan S., Kazmi A., Bashir N., Siddique R. COVID-19 infection: origin, transmission, and characteristics of human coronaviruses. J Adv Res. Mar 16 2020;24:91–98. doi: 10.1016/j.jare.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Revzin M.V., Raza S., Srivastava N.C., Warshawsky R., D'Agostino C., Malhotra A., Bader A.S., Patel R.D., Chen K., Kyriakakos C., Pellerito J.S. Multisystem imaging manifestations of COVID-19, part 2: from cardiac complications to pediatric manifestations. Radiographics. Nov-Dec 2020;40(7):1866–1892. doi: 10.1148/rg.2020200195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abdullahi A., Candan S.A., Abba M.A., Bello A.H., Alshehri M.A., Afamefuna Victor E., Kundakci B. Neurological and musculoskeletal features of COVID-19: a systematic review and meta-analysis. Front Neurol. 2020;11:687. doi: 10.3389/fneur.2020.00687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ciaffi J., Meliconi R., Ruscitti P., Berardicurti O., Giacomelli R., Ursini F. Rheumatic manifestations of COVID-19: a systematic review and meta-analysis. BMC Rheumatol. 2020;4(1):65. doi: 10.1186/s41927-020-00165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cipollaro L., Giordano L., Padulo J., Oliva F., Maffulli N. Musculoskeletal symptoms in SARS-CoV-2 (COVID-19) patients. J Orthop Surg Res. 2020;15(1):178. doi: 10.1186/s13018-020-01702-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shah S., Danda D., Kavadichanda C., Das S., Adarsh M.B., Negi V.S. Autoimmune and rheumatic musculoskeletal diseases as a consequence of SARS-CoV-2 infection and its treatment. Rheumatol Int. 2020;40(10):1539–1554. doi: 10.1007/s00296-020-04639-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang H., Charmchi Z., Seidman R.J., Anziska Y., Velayudhan V., Perk J. COVID-19-associated myositis with severe proximal and bulbar weakness. Muscle Nerve. 2020;62(3):E57–E60. doi: 10.1002/mus.27003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mehan W.A., Yoon B.C., Lang M., Li M.D., Rincon S., Buch K. Paraspinal myositis in patients with COVID-19 infection. AJNR Am J Neuroradiol. 2020;41(10):1949–1952. doi: 10.3174/ajnr.A6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Husain R., Corcuera-Solano I., Dayan E., Jacobi A.H., Huang M. Rhabdomyolysis as a manifestation of a severe case of COVID-19: a case report. Radiol Case Rep. 2020;15(9):1633–1637. doi: 10.1016/j.radcr.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Torres P.A., Helmstetter J.A., Kaye A.M., Kaye A.D. Rhabdomyolysis: pathogenesis, diagnosis, and treatment. Ochsner J. 2015;15(1):58–69. [PMC free article] [PubMed] [Google Scholar]
- 11.Cunningham J., Sharma R., Kirzner A., et al. Acute myonecrosis on MRI: etiologies in an oncological cohort and assessment of interobserver variability. Skeletal Radiol. 2016;45(8):1069–1078. doi: 10.1007/s00256-016-2389-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fernandez C.E., Franz C.K., Ko J.H., Walter J.M., Koralnik I.J., Ahlawat S., Deshmukh S. Imaging review of peripheral nerve injuries in patients with COVID-19. Radiology. 2020;203116 doi: 10.1148/radiol.2020203116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Federle M.P., Pan K.T., Pealer K.M. CT criteria for differentiating abdominal hemorrhage: anticoagulation or aortic aneurysm rupture? AJR Am J Roentgenol. 2007 May;188(5):1324–1330. doi: 10.2214/AJR.05.1911. [DOI] [PubMed] [Google Scholar]
- 14.Tang J., Li B., Gong J., Li W., Yang J. Challenges in the management of critical ill COVID-19 patients with pressure ulcer. Int Wound J. 2020;17(5):1523–1524. doi: 10.1111/iwj.13399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mawhirt S.L., Frankel D., Diaz A.M. Cutaneous manifestations in adult patients with COVID-19 and dermatologic conditions related to the COVID-19 pandemic in health care workers. Curr Allergy Asthma Rep. Oct 12 2020;20(12):75. doi: 10.1007/s11882-020-00974-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Young S., Narang J., Kumar S. Large sacral/buttocks ulcerations in the setting of coagulopathy: a case series establishing the skin as a target organ of significant damage and potential morbidity in patients with severe COVID-19 [published online ahead of print, 2020 Aug 7] Int Wound J. 2020 doi: 10.1111/iwj.13457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ellul M.A., Benjamin L., Singh B., Lant S., Michael B.D., Easton A., Kneen R., Defres S., Sejvar J., Solomon T. Neurological associations of COVID-19. Lancet Neurol. Sep 2020;19(9):767–783. doi: 10.1016/S1474-4422(20)30221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meinhardt J., Radke J., Dittmayer C., Franz J., Thomas C., Mothes R., Laue M., Schneider J., Brünink S., Greuel S., Lehmann M., Hassan O., Aschman T., Schumann E., Chua R.L., Conrad C., Eils R., Stenzel W., Windgassen M., Rößler L., Goebel H.H., Gelderblom H.R., Martin H., Nitsche A., Schulz-Schaeffer W.J., Hakroush S., Winkler M.S., Tampe B., Scheibe F., Körtvélyessy P., Reinhold D., Siegmund B., Kühl A.A., Elezkurtaj S., Horst D., Oesterhelweg L., Tsokos M., Ingold-Heppner B., Stadelmann C., Drosten C., Corman V.M., Radbruch H., Heppner F.L. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat Neurosci. 2021 Feb;24(2):168–175. doi: 10.1038/s41593-020-00758-5. [DOI] [PubMed] [Google Scholar]
- 19.Solomon T. Neurological infection with SARS-CoV-2 - the story so far. Nat Rev Neurol. Feb 2021;17(2):65–66. doi: 10.1038/s41582-020-00453-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matschke J., Lütgehetmann M., Hagel C., Sperhake J.P., Schröder A.S., Edler C., Mushumba H., Fitzek A., Allweiss L., Dandri M., Dottermusch M., Heinemann A., Pfefferle S., Schwabenland M., Sumner Magruder D., Bonn S., Prinz M., Gerloff C., Püschel K., Krasemann S., Aepfelbacher M., Glatzel M. Neuropathology of patients with COVID-19 in Germany: a post-mortem case series. Lancet Neurol. Nov 2020;19(11):919–929. doi: 10.1016/S1474-4422(20)30308-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maury A., Lyoubi A., Peiffer-Smadja N., de Broucker T., Meppiel E. Neurological manifestations associated with SARS-CoV-2 and other coronaviruses: a narrative review for clinicians. Rev Neurol. 2020;177(1–2):51–64. doi: 10.1016/j.neurol.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jain R., Young M., Dogra S., Kennedy H., Nguyen V., Jones S., Bilaloglu S., Hochman K., Raz E., Galetta S., Horwtiz L. COVID-19 related neuroimaging findings: a signal of thromboembolic complications and a strong prognostic marker of poor patient outcome. J Neurol Sci. Jul 15 2020;414 doi: 10.1016/j.jns.2020.116923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jain R., Young M., Dogra S., Kennedy H., Nguyen V., Raz E. Surprise diagnosis of COVID-19 following neuroimaging evaluation for unrelated reasons during the pandemic in hot spots. AJNR Am J Neuroradiol. Jul 2020;41(7):1177–1178. doi: 10.3174/ajnr.A6608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Esenwa C., Lee J.A., Nisar T., Shmukler A., Goldman I., Zampolin R., Hsu K., Labovitz D., Altschul D., Haramati L.B. Utility of apical lung assessment on computed tomography angiography as a COVID-19 screen in acute stroke. Stroke. Dec 2020;51(12):3765–3769. doi: 10.1161/STROKEAHA.120.030959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hossain R., Lazarus M.S., Roudenko A., Dako F., Mehta V., Alis J., Zalta B., Lei B., Haramati L.B., White C.S. CT scans obtained for nonpulmonary indications: associated respiratory findings of COVID-19. Radiology. Sep 2020;296(3) doi: 10.1148/radiol.2020201743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahammedi A., Ramos A., Bargalló N., et al. Brain and lung imaging correlation in patients with COVID-19: could the severity of lung disease reflect the prevalence of acute abnormalities on neuroimaging? A global multicenter observational study. AJNR Am J Neuroradiol. Mar 11 2021 doi: 10.3174/ajnr.A7072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eskandar E.N., Altschul D.J., de la Garza Ramos R., et al. Neurologic syndromes predict higher in-hospital mortality in COVID-19. Neurology. Mar 16 2021;96(11) doi: 10.1212/WNL.0000000000011356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Katz J.M., Libman R.B., Wang J.J., Sanelli P., Filippi C.G., Gribko M., Pacia S.V., Kuzniecky R.I., Najjar S., Azhar S. Cerebrovascular complications of COVID-19. Stroke. Sep 2020;51(9) doi: 10.1161/STROKEAHA.120.031265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qureshi A.I., Baskett W.I., Huang W., Shyu D., Myers D., Raju M., Lobanova I., MFK Suri, Naqvi S.H., French B.R., Siddiq F., Gomez C.R., Shyu C.R. Acute ischemic stroke and COVID-19: an analysis of 27?676 patients. Stroke. Mar 2021;52(3):905–912. doi: 10.1161/STROKEAHA.120.031786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ntaios G., Michel P., Georgiopoulos G., Guo Y., Li W., Xiong J., Calleja P., Ostos F., González-Ortega G., Fuentes B., Alonso de Leciñana M., Díez-Tejedor E., García-Madrona S., Masjuan J., DeFelipe A., Turc G., Gonçalves B., Domigo V., Dan G.A., Vezeteu R., Christensen H., Christensen L.M., Meden P., Hajdarevic L., Rodriguez-Lopez A., Díaz-Otero F., García-Pastor A., Gil-Nuñez A., Maslias E., Strambo D., Werring D.J., Chandratheva A., Benjamin L., Simister R., Perry R., Beyrouti R., Jabbour P., Sweid A., Tjoumakaris S., Cuadrado-Godia E., Campello A.R., Roquer J., Moreira T., Mazya M.V., Bandini F., Matz K., Iversen H.K., González-Duarte A., Tiu C., Ferrari J., Vosko M.R., HJF Salzer, Lamprecht B., Dünser M.W., Cereda C.W., ÁBC Quintero, Korompoki E., Soriano-Navarro E., Soto-Ramírez L.E., Castañeda-Méndez P.F., Bay-Sansores D., Arauz A., Cano-Nigenda V., Kristoffersen E.S., Tiainen M., Strbian D., Putaala J., GYH Lip. Characteristics and outcomes in patients with COVID-19 and acute ischemic stroke: the global COVID-19 stroke registry. Stroke. Sep 2020;51(9) doi: 10.1161/STROKEAHA.120.031208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Srivastava P.K., Zhang S., Xian Y., Xu H., Rutan C., Alger H.M., Walchok J., Williams J., de Lemos J.A., Decker-Palmer M.R., Alhanti B., MSV Elkind, Messé S.R., Smith E.E., Schwamm L.H., Fonarow G.C. Acute ischemic stroke in patients with COVID-19: an analysis from get with the guidelines-stroke. Stroke. Mar 17 2021 doi: 10.1161/STROKEAHA.121.034301. [DOI] [PubMed] [Google Scholar]
- 32.Cuker A., Tseng E.K., Nieuwlaat R., Angchaisuksiri P., Blair C., Dane K., Davila J., MT DeSancho, Diuguid D., Griffin D.O., Kahn S.R., Klok F.A., Lee A.I., Neumann I., Pai A., Pai M., Righini M., Sanfilippo K.M., Siegal D., Skara M., Touri K., A Akl E., Bou Akl I., Boulos M., Brignardello-Petersen R., Charide R., Chan M., Dearness K., Darzi A.J., Kolb P., Colunga-Lozano L.E., Mansour R., Morgano G.P., Morsi R.Z., Noori A., Piggott T., Qiu Y., Roldan Y., Schünemann F., Stevens A., Solo K., Ventresca M., Wiercioch W., Mustafa R.A., Schünemann H.J. American Society of Hematology 2021 guidelines on the use of anticoagulation for thromboprophylaxis in patients with COVID-19. Blood Adv. Feb 9 2021;5(3):872–888. doi: 10.1182/bloodadvances.2020003763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Esenwa C., Cheng N.T., Lipsitz E., Hsu K., Zampolin R., Gersten A., Antoniello D., Soetanto A., Kirchoff K., Liberman A., Mabie P., Nisar T., Rahimian D., Brook A., Lee S.K., Haranhalli N., Altschul D., Labovitz D. COVID-19-associated carotid atherothrombosis and stroke. AJNR Am J Neuroradiol. Nov 2020;41(11):1993–1995. doi: 10.3174/ajnr.A6752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oxley T.J., Mocco J., Majidi S., Kellner C.P., Shoirah H., Singh I.P., De Leacy R.A., Shigematsu T., Ladner T.R., Yaeger K.A., Skliut M., Weinberger J., Dangayach N.S., Bederson J.B., Tuhrim S., Fifi J.T. Large-vessel stroke as a presenting feature of Covid-19 in the young. N Engl J Med. May 14 2020;382(20) doi: 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dogra S., Jain R., Cao M., Bilaloglu S., Zagzag D., Hochman S., Lewis A., Melmed K., Hochman K., Horwitz L., Galetta S., Berger J. Hemorrhagic stroke and anticoagulation in COVID-19. J Stroke Cerebrovasc Dis. Aug 2020;29(8) doi: 10.1016/j.jstrokecerebrovasdis.2020.104984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beyrouti R., Best J.G., Chandratheva A., Perry R.J., Werring D.J. Characteristics of intracerebral haemorrhage associated with COVID-19: a systematic review and pooled analysis of individual patient and aggregate data. J Neurol. Feb 5 2021:1–11. doi: 10.1007/s00415-021-10425-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Usman A.A., Han J., Acker A., Olia S.E., Bermudez C., Cucchiara B., Mikkelsen M.E., Wald J., Mackay E., Szeto W., Vernick W.J., Gutsche J.T. A case series of devastating intracranial hemorrhage during venovenous extracorporeal membrane oxygenation for COVID-19. J Cardiothorac Vasc Anesth. Nov 2020;34(11):3006–3012. doi: 10.1053/j.jvca.2020.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Melmed K.R., Cao M., Dogra S., Zhang R., Yaghi S., Lewis A., Jain R., Bilaloglu S., Chen J., Czeisler B.M., Raz E., Lord A., Berger J.S., Frontera J.A. Risk factors for intracerebral hemorrhage in patients with COVID-19. J Thromb Thrombolysis. Sep 24 2020:1–8. doi: 10.1007/s11239-020-02288-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rapalino O., Pourvaziri A., Maher M., Jaramillo-Cardoso A., Edlow B.L., Conklin J., Huang S., Westover B., Romero J.M., Halpern E., Gupta R., Pomerantz S., Schaefer P., Gonzalez R.G., Mukerji S.S., Lev M.H. Clinical, imaging, and lab correlates of severe COVID-19 leukoencephalopathy. AJNR Am J Neuroradiol. Jan 7 2021 doi: 10.3174/ajnr.A6966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Breit H., Jhaveri M., John S. Concomitant delayed posthypoxic leukoencephalopathy and critical illness microbleeds. Neurol Clin Pract. Oct 2018;8(5) doi: 10.1212/CPJ.0000000000000513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fanou E.M., Coutinho J.M., Shannon P., Kiehl T.R., Levi M.M., Wilcox M.E., Aviv R.I., Mandell D.M. Critical illness-associated cerebral microbleeds. Stroke. 2017 Apr;48(4):1085–1087. doi: 10.1161/STROKEAHA.116.016289. [DOI] [PubMed] [Google Scholar]
- 42.Radmanesh A., Derman A., Lui Y.W., et al. COVID-19-associated diffuse leukoencephalopathy and microhemorrhages. Radiology. Oct 2020;297(1) doi: 10.1148/radiol.2020202040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Agarwal S., Jain R., Dogra S., Krieger P., Lewis A., Nguyen V., Melmed K., Galetta S. Cerebral microbleeds and leukoencephalopathy in critically ill patients with COVID-19. Stroke. Sep 2020;51(9):2649–2655. doi: 10.1161/STROKEAHA.120.030940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sachs J.R., Gibbs K.W., Swor D.E., Sweeney A.P., Williams D.W., Burdette J.H., West T.G., Geer C.P. COVID-19-associated leukoencephalopathy. Radiology. Sep 2020;296(3) doi: 10.1148/radiol.2020201753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Agarwal S., Conway J., Nguyen V., Dogra S., Krieger P., Zagzag D., Lewis A., Melmed K., Galetta S., Jain R. Serial imaging of virus-associated necrotizing disseminated acute leukoencephalopathy (VANDAL) in COVID-19. AJNR Am J Neuroradiol. Jan 2021;42(2):279–284. doi: 10.3174/ajnr.A6898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hackett P.H., Yarnell P.R., Weiland D.A., Reynard K.B. Acute and evolving MRI of high-altitude cerebral edema: microbleeds, edema, and pathophysiology. AJNR Am J Neuroradiol. Mar 2019;40(3):464–469. doi: 10.3174/ajnr.A5897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arabi Y.M., Harthi A., Hussein J., Bouchama A., Johani S., Hajeer A.H., Saeed B.T., Wahbi A., Saedy A., Okaili R., Sadat M., Balkhy H., AlDabbagh T. Severe neurologic syndrome associated with Middle East respiratory syndrome corona virus (MERS-CoV) Infection. Aug 2015;43(4):495–501. doi: 10.1007/s15010-015-0720-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cavalcanti D.D., Raz E., Shapiro M., Dehkharghani S., Yaghi S., Lillemoe K., Nossek E., Torres J., Jain R., Riina H.A., Radmanesh A., Nelson P.K. Cerebral venous thrombosis associated with COVID-19. AJNR Am J Neuroradiol. Aug 2020;41(8):1370–1376. doi: 10.3174/ajnr.A6644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thompson A., Morgan C., Smith P., et al. Cerebral venous sinus thrombosis associated with COVID-19. Pract Neurol. Oct 8 2020 doi: 10.1136/practneurol-2020-002678. [DOI] [PubMed] [Google Scholar]
- 50.Hughes C., Nichols T., Pike M., Subbe C., Elghenzai S. Cerebral venous sinus thrombosis as a presentation of COVID-19. Eur J Case Rep Intern Med. 2020 Apr 29;7(5) doi: 10.12890/2020_001691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hemasian H., Ansari B. First case of Covid-19 presented with cerebral venous thrombosis: a rare and dreaded case. Rev Neurol (Paris) Jun 2020;176(6):521–523. doi: 10.1016/j.neurol.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Abdalkader M., Shaikh S.P., Siegler J.E., et al. Cerebral venous sinus thrombosis in COVID-19 patients: a multicenter study and review of literature. J Stroke Cerebrovasc Dis. Mar 4 2021;30(6) doi: 10.1016/j.jstrokecerebrovasdis.2021.105733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gulko E., Oleksk M.L., Gomes W., Ali S., Mehta H., Overby P., Al-Mufti F., Rozenshtein A. MRI brain findings in 126 patients with COVID-19: initial observations from a descriptive literature review. AJNR Am J Neuroradiol. Dec 2020;41(12):2199–2203. doi: 10.3174/ajnr.A6805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moonis G., Filippi C.G., CFE Kirsch, Mohan S., Stein E.G., Hirsch J.A., Mahajan A. The spectrum of neuroimaging findings on CT and MRI in adults with coronavirus disease (COVID-19) AJR Am J Roentgenol. Nov 25 2020 doi: 10.2214/AJR.20.24839. [DOI] [PubMed] [Google Scholar]
- 55.Kandemirli S.G., Dogan L., Sarikaya Z.T., Kara S., Akinci C., Kaya D., Kaya Y., Yildirim D., Tuzuner F., Yildirim M.S., Ozluk E., Gucyetmez B., Karaarslan E., Koyluoglu I., Demirel Kaya H.S., Mammadov O., Kisa Ozdemir I., Afsar N., Citci Yalcinkaya B., Rasimoglu S., Guduk D.E., Kedir Jima A., Ilksoz A., Ersoz V., Yonca Eren M., Celtik N., Arslan S., Korkmazer B., Dincer S.S., Gulek E., Dikmen I., Yazici M., Unsal S., Ljama T., Demirel I., Ayyildiz A., Kesimci I., Bolsoy Deveci S., Tutuncu M., Kizilkilic O., Telci L., Zengin R., Dincer A., Akinci I.O., Kocer N. Brain MRI findings in patients in the intensive care unit with COVID-19 infection. Radiology. Oct 2020;297(1) doi: 10.1148/radiol.2020201697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gutiérrez-Ortiz C., Méndez-Guerrero A., Rodrigo-Rey S., San Pedro-Murillo E., Bermejo-Guerrero L., Gordo-Mañas R., de Aragón-Gómez F., Benito-León J. Miller fisher syndrome and polyneuritis cranialis in COVID-19. Neurology. Aug 4 2020;95(5):e601–e605. doi: 10.1212/WNL.0000000000009619. [DOI] [PubMed] [Google Scholar]
- 57.Ray A. Miller fisher syndrome and COVID-19: is there a link? BMJ Case Rep. Aug 11 2020;13(8) doi: 10.1136/bcr-2020-236419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Scheidl E., Canseco D.D., Hadji-Naumov A., Bereznai B. Guillain-Barré syndrome during SARS-CoV-2 pandemic: a case report and review of recent literature. J Peripher Nerv Syst. Jun 2020;25(2):204–207. doi: 10.1111/jns.12382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Toscano G., Palmerini F., Ravaglia S., Ruiz L., Invernizzi P., Cuzzoni M.G., Franciotta D., Baldanti F., Daturi R., Postorino P., Cavallini A., Guillain-Barré Micieli G. Syndrome associated with SARS-CoV-2. N Engl J Med. Jun 25 2020;382(26):2574–2576. doi: 10.1056/NEJMc2009191. Epub 2020 Apr 17. PMID: 32302082; PMCID: PMC7182017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lechien J.R., Chiesa-Estomba C.M., De Siati D.R., Horoi M., Le Bon S.D., Rodriguez A., Dequanter D., Blecic S., El Afia F., Distinguin L., Chekkoury-Idrissi Y., Hans S., Delgado I.L., Calvo-Henriquez C., Lavigne P., Falanga C., Barillari M.R., Cammaroto G., Khalife M., Leich P., Souchay C., Rossi C., Journe F., Hsieh J., Edjlali M., Carlier R., Ris L., Lovato A., De Filippis C., Coppee F., Fakhry N., Ayad T., Saussez S. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. Aug 2020;277(8):2251–2261. doi: 10.1007/s00405-020-05965-1. Epub 2020 Apr 6. PMID: 32253535; PMCID: PMC7134551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Klironomos S., Tzortzakakis A., Kits A., et al. Nervous system involvement in coronavirus disease 2019: results from a retrospective consecutive neuroimaging cohort. Radiology. Dec 2020;297(3) doi: 10.1148/radiol.2020202791. Epub 2020 Jul 30. PMID: 32729812; PMCID: PMC7393954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ismail I.I., Gad K.A. Absent blood oxygen level-dependent functional magnetic resonance imaging activation of the orbitofrontal cortex in a patient with persistent Cacosmia and Cacogeusia after COVID-19 infection. JAMA Neurol. Jan 22 2021 doi: 10.1001/jamaneurol.2021.0009. Epub ahead of print. PMID: 33480976. [DOI] [PubMed] [Google Scholar]
- 63.Chiu A., Fischbein N., Wintermark M., Zaharchuk G., Yun P.T., Zeineh M. COVID-19-induced anosmia associated with olfactory bulb atrophy. Neuroradiology. Jan 2021; ;63(1):147–148. doi: 10.1007/s00234-020-02554-1. Epub 2020 Sep 15. PMID: 32930820; PMCID: PMC7490479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Benito-Pascual B., Gegúndez J.A., Díaz-Valle D., Arriola-Villalobos P., Carreño E., Culebras E., Rodríguez-Avial I., Benitez-Del-Castillo J.M. Panuveitis and optic neuritis as a possible initial presentation of the novel coronavirus disease 2019 (COVID-19) Ocul Immunol Inflamm. Aug 17 2020;28(6):922–925. doi: 10.1080/09273948.2020.1792512. [DOI] [PubMed] [Google Scholar]
- 65.SFNR’s COVID Study Group. Lecler A., Cotton F., Lersy F., Kremer S., Héran F. Ocular MRI findings in patients with severe COVID-19: a retrospective multicenter observational study. Radiology. Feb 16 2021 doi: 10.1148/radiol.2021204394. Epub ahead of print. PMID: 33591889; PMCID: PMC7887777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Varga Z., Flammer A.J., Steiger P., et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Frantzeskaki F., Armaganidis A., Orfanos S.E. Immuno- thrombosis in acute respiratory distress syndrome: cross talks between in ammation and coagulation. Respiration. 2017;93(3):212–225. doi: 10.1159/000453002. [DOI] [PubMed] [Google Scholar]
- 68.Malas M.B., Naazie I.N., Elsayed N., Mathlouthi A., Marmor R., Clary B. Thromboembolism risk of COVID-19 is high and associated with a higher risk of mortality: a systematic review and meta-analysis. EClinicalMedicine. Dec 2020;29 doi: 10.1016/j.eclinm.2020.100639. Epub 2020 Nov 20. PMID: 33251499; PMCID: PMC7679115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dane B., Smereka P., Wain R., Kim D., Katz D.S. Hypercoagulability in patients with coronavirus disease (COVID-19): identification of arterial and venous thromboembolism in the abdomen, pelvis, and lower extremities. AJR. Jan 2021;216(1):104–105. doi: 10.2214/AJR.20.23617. Epub 2020 Sep 22. PMID: 32603220. [DOI] [PubMed] [Google Scholar]
- 70.McBane R.D., 2nd, Torres Roldan V.D., Niven A.S., et al. Anticoagulation in COVID-19: a systematic review, meta-analysis, and rapid guidance from Mayo Clinic. Mayo Clin Proc. 2020;95(11):2467–2486. doi: 10.1016/j.mayocp.2020.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Long X., Zhang Z., Zou W., Ling J., Li D., Jing L., Yu S., Zou X., Bian Y., Wu W., Li S., Fang M. Coagulopathy of patients with COVID-19 is associated with infectious and inflammatory markers. Risk Manag Healthc Policy. 2020;13:1965–1975. doi: 10.2147/RMHP.S268238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Di Minno A., Ambrosino P., Calcaterra I., MND Di Minno. COVID-19 and venous throm- boembolism: a meta-analysis of literature studies [published online ahead of print, 2020 Sep 3] Semin Thromb Hemost. 2020 doi: 10.1055/s-0040-1715456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ramalingam S., Arora H., Gunasekaran K., et al. Isolated radial vein thrombosis: upper extremity deep vein thrombosis in a patient with COVID-19 infection. Cureus. 2021;13(1) doi: 10.7759/cureus.12856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bozzani A., Arici V., Franciscone M.M. Severe acute respiratory syndrome coronavirus 2 infection and the upper limb deep vein thrombosis risk. Ann Vasc Surg. Jul 2020;66:11–13. doi: 10.1016/j.avsg.2020.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Marone E.M., Rinaldi L.F. Upsurge of deep venous thrombosis in patients affected by COVID-19: preliminary data and possible explanations. J Vasc Surg Venous Lymphat Disord. 2020;8(4):694–695. doi: 10.1016/j.jvsv.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Middeldorp S., Coppens M., van Haaps T.F., et al. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020;18(8):1995–2002. doi: 10.1111/jth.14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Suh Y.J., Hong H., Ohana M. Pulmonary embolism and deep vein thrombosis in COVID-19: a systematic review and meta-analysis. Radiology. 2020;00:1–11. doi: 10.1148/radiol20202035557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Franco-Moreno A., Herrera-Morueco M., Mestre-Gómez B. Thrombosis research group. Incidence of deep venous thrombosis in patients with COVID-19 and pulmonary embolism: compression ultrasound COVID study. J Ultrasound Med. Oct 5 2020 doi: 10.1002/jum.15524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Karande G.Y., Hedgire S.S., Sanchez Y., et al. Advanced imaging in acute and chronic deep vein thrombosis. Cardiovasc Diagn Ther. 2016;6(6):493–507. doi: 10.21037/cdt.2016.12.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Goldman I.A., Ye K., Scheinfeld M.H. Lower-extremity arterial thrombosis associated with COVID-19 is characterized by greater thrombus burden and increased rate of amputation and death. Radiology. Nov 2020;297(2) doi: 10.1148/radiol.2020202348. E263-E269. Epub 2020 Jul 16. PMID: 32673190; PMCID: PMC7370378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schweblin C., Hachulla A.L., Roffi M., Glauser F. Delayed manifestation of COVID-19 presenting as lower extremity multilevel arterial thrombosis: a case report. Eur Heart J. 2020:1–4. doi: 10.1093/ehjcr/ytaa371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kaur P., Qaqa F., Ramahi A. Acute upper limb ischemia in a patient with COVID-19 [published online ahead of print, 2020 May 13] Hematol Oncol Stem Cell Ther. 2020 doi: 10.1016/j.hemonc.2020.05.001. [S1658-3876(20)30096-0] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Roncati L., Manenti A., Manco G., Farinetti A. Abdominal aortic thrombosis complicating COVID-19 pneumonia. Ann Vasc Surg. 2020;67:8–9. doi: 10.1016/j.avsg.2020.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gandotra P., Supariwala A., Selim S., Garra G., Gruberg L. Aortic arch thrombus and pulmonary embolism in a COVID-19 patient. J Emerg Med. Aug 4 2020 doi: 10.1016/j.jemermed.2020.08.009. S0736-4679(20)30851-9. Epub ahead of print. PMID: 32917441; PMCID: PMC7402365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Woehl B., Lawson B., Jambert L., et al. 4 cases of aortic thrombosis in patients with COVID-19. J Am Coll Cardiol Case Rep. 2020;2(9):1397–1401. doi: 10.1016/j.jaccas.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.de Carranza M., Salazar D.E., Troya J. Aortic thrombus in patients with severe COVID-19: review of three cases [published online ahead of print, 2020 Jul 9] J Thromb Thrombolysis. 2020:1–6. doi: 10.1007/s11239-020-02219-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Baeza C., Gonzalez A., Torres P., et al. Acute aortic thrombosis in COVID-10. J Vasc Surg Cases Innov Tech. 2020;6(3):483–486. doi: 10.1016/j.jvscit.2020.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mestres G., Puigmacià R., Blanco C., Yugueros X., Esturrica M., Riambau V. Risk of peripheral arterial thrombosis in COVID-19. J Vasc Surg. 2020;72(2):756–757. doi: 10.1016/j.jvs.2020.04.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cheruiyot I., Kipkorir V., Ngure B., Misiani M., Munguti J., Ogeng'o J. Arterial thrombosis in coronavirus disease 2019 patients: a rapid systematic review. Ann Vasc Surg. 2021;70:273–281. doi: 10.1016/j.avsg.2020.08.087. Epub 2020 Aug 28. PMID: 32866574; PMCID: PMC7453204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kashi M., Jacquin A., Dakhil B., Zaimi R., Mahé E., Tella E., Bagan P. Severe arterial thrombosis associated with Covid-19 infection. Thromb Res. Aug 2020;192:75–77. doi: 10.1016/j.thromres.2020.05.025. Epub 2020 May 16. PMID: 32425264; PMCID: PMC7229939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lameijer J.R.C., van Houte J., van Berckel M.M.G., et al. Severe arterial thromboembolism in patients with Covid-19. J Crit Care. 2020;60:106–110. doi: 10.1016/j.jcrc.2020.08.002. doi: 10.1016/j.jcrc.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yang S., Yu J., Zeng W., Yang L., Teng L., Cui Y., Shi H. Aortic floating thrombus detected by computed tomography angiography incidentally: five cases and a literature review. J Thorac Cardiovasc Surg. 2017;153(4):791–803. doi: 10.1016/j.jtcvs.2016.12.015. [DOI] [PubMed] [Google Scholar]
- 93.Vulliamy P., Jacob S., Davenport R.A. Acute aorto-iliac and mesenteric arterial thromboses as presenting features of COVID-19. Br J Haematol. 2020;189:1053–1054. doi: 10.1111/bjh.16760. [DOI] [PMC free article] [PubMed] [Google Scholar]