Abstract
Background
Breakthrough infections after SARS-CoV-2 vaccination have been reported. Clinical outcomes in these persons are not widely known.
Methods
We evaluated all vaccinated persons with breakthrough infection ≥14 days after the second vaccine dose and unvaccinated controls matched on age, sex, nationality, and reason for testing between December 23, 2020 and March 28, 2021 in Qatar. Our primary outcome was severe disease defined as hospitalization, mechanical ventilation, or death.
Results
Among 456 persons cases of breakthrough infection and 456 unvaccinated matched controls with confirmed infection, median age was 45 years, 60.7% were males, and ≥1 comorbid condition was present in 61.2% of the vaccinated and 47.8% of the unvaccinated persons (P=0.009). Severe disease was recorded in 48 (10.5%) of the vaccinated and 121 (26.5%) of the unvaccinated group (P<0.001). Factors associated with severe disease included increasing age (HR vs. <40 years old: >40–60 years, HR 2.32; >60–70 years, HR 4.34; >70 years, HR 5.43); presence of symptoms at baseline (HR 2.42, 95%CI 1.44-4.07); and being unvaccinated (HR 2.84, 95%CI 1.80-4.47).
Conclusions
In persons with breakthrough SARS-CoV-2 infection, increasing age is associated with a higher risk of severe disease or death, while vaccination is associated with a lower risk. Presence of comorbidities was not associated with severe disease or death among persons with breakthrough infection.
Keywords: SARS-CoV-2, COVID-19, vaccination, breakthrough infection, Qatar
Several highly efficacious vaccines against the SARS-CoV-2 infection are now available. In the real-world settings, the Pfizer-BNT162b2 vaccine is approximately 90% effective in preventing SARS-CoV-2 infection and 94-100% effective in preventing severe or fatal disease.(Abu-Raddad et al., 2021; Dagan et al., 2021) Risk of reinfection after natural infection is very low, with a reported prevalence of approximately 0.18% in Qatar.(Abu-Raddad et al., 2020) More recently, breakthrough infections in fully vaccinated persons have been reported though little is known about the risk factors, clinical presentation and outcomes for breakthrough infections compared with demographically and clinically similar controls.(Butt et al., 2021; Hacisuleyman et al., 2021; Teran et al., 2021) Qatar was among the first nations to begin a national vaccination campaign, with the first inoculation on December 23, 2020. Since then, over half the population in Qatar has received at least one dose of the vaccine and over one-third have been fully vaccinated. Vaccines used in Qatar include the Pfizer-BNT162b2 and the Moderna-mRNA-1273 vaccines, both of which require two doses 21 and 28 days apart respectively for optimal coverage. After initial anecdotal reports of breakthrough infections among fully vaccinated persons, we sought to determine the risk factors and outcomes associated with breakthrough infections in the national COVID-19 database in Qatar. Our aim was not to determine vaccine efficacy or effectiveness, which has already been reported by numerous groups.
Methods
Study Population and Participants
Qatar has created a national database of COVID-19 infected persons, which includes demographics, reason and results of SARS-CoV-2 testing and vaccination related information. Among this national database, we identified all persons who had received both doses of the Pfizer-BNT162b2 vaccine between December 23, 2020 and March 28, 2021, and who had a documented positive SARS-CoV-2 PCR on a nasopharyngeal swab ≥14 days after the second dose of the vaccine. For each person thus identified with breakthrough infection, we identified a matched unvaccinated control with confirmed infection, with matching done on age, sex, nationality, and reason for testing from the national database. Demographic and clinical information was obtained through structured chart reviews of each case and control.
Definitions
Presence of SARS-CoV-2 infection was defined as a nasopharyngeal swab positive by RT-PCR. Comorbidities were retrieved from individual chart review and recorded as being present based on at least one confirmatory physician note. Severe disease was defined as hospitalization, admission to an intensive care or monitored setting, invasive or non-invasive mechanical ventilation, or death.
Statistical Analyses
Baseline characteristics of persons with SARS-CoV-2 infection with and without prior vaccination were compared using McNemar test for categorical and Mann–Whitney U test for continuous variables. We determined the proportion of persons with severe infection by various baseline characteristics. We used a Cox proportional hazards model to calculate the hazard ratios and 95% confidence intervals for factors associated with development of severe disease. Kaplan-Meier curves were constructed to determine the proportion of persons with event-free survival among the vaccinated and unvaccinated persons.
Results
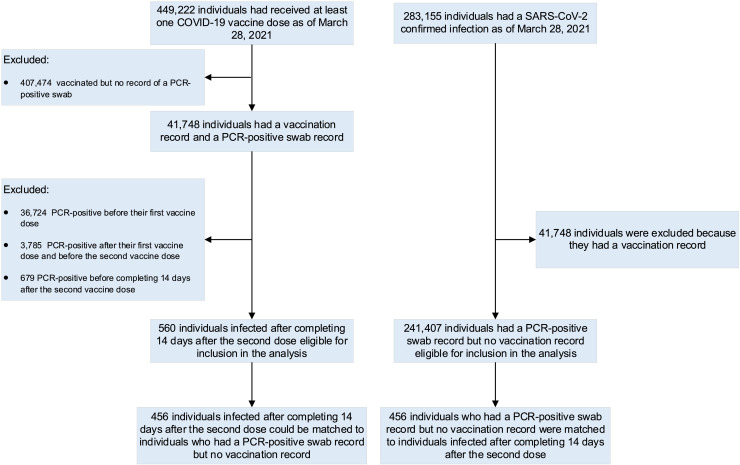
Between December 23, 2020 and March 28, 2021, 449,222 persons in Qatar had received at least one dose of the SARS-CoV-2 vaccine. During this time period, only the Pfizer-BNT162b2 vaccine was available in Qatar and 100% of the vaccinees received this vaccine. We excluded those persons who had any positive PCR test for SARS-CoV-2 before and up to 14 days after the second vaccine dose. Among those with at least 2 doses of the vaccine and no positive SARS-CoV-2 test up to 14 days after the second dose, we identified 456 persons who had a confirmed SARS-CoV-2 PCR positive test on a nasopharyngeal swab at least ≥14 days after the second dose of the vaccine. We also identified 456 unvaccinated matched controls with confirmed infection as per the matching criteria described above. ( Figure 1 ) The median age in both groups was 45 years, 60.7% were males, and 31.6% were Qatari nationals. ( Table 1 ) The most common reason for testing was clinical suspicion. At least one comorbid condition was present in 61.2% of the vaccinated and 47.8% of the unvaccinated persons (P=0.009) with hypertension and diabetes being the most common comorbidities in both groups. ( Table 1 )
Figure 1.
Construction of the study dataset.
Table 1.
Baseline characteristics of persons with documented SARS-CoV-2 infection after vaccination compared with infection in those who were not vaccinated.
| Vaccinated | Unvaccinated | P-value | |
|---|---|---|---|
| N=456 | N=456 | ||
| Median age (IQR) years | 45 (36, 59.8) | 45 (36, 59.8) | 1 |
| Male sex no. (%) | 60.7% | 60.7% | 1 |
| Qatari/ Non-Qatari | 31.6% | 31.6% | 1 |
| Median of average CT value (IQR) | 24 (19, 30) | 23 (18, 28) | 0.001 |
| Symptomatic at baseline | 52.6% | 55.3% | 0.02 |
| Reason for testing | <0.0001 | ||
| Clinical suspicion | 45.6% | 27.2% | |
| Surveillance | 23.9% | 21.9% | |
| Pre-travel requirement | 2.9% | 2.9% | |
| Contact tracing | 9.6% | 5.7% | |
| Individual request | 5.9% | 6.1% | |
| Port of entry testing | 6.1% | 6.1% | |
| Healthcare routine testing | 5.3% | 5.0% | |
| Other | 0.7% | 0.7% | |
| Missing | 0.0% | 24.3% | |
| Body mass index, kg/m2 | |||
| Median (IQR) | 29.4 (25.7, 33.1) | 29.6 (25.6, 33.4) | 0.7 |
| <=30 | 183 | 125 | 0.75 |
| >30 | 171 | 129 | |
| Missing | 102 | 202 | |
| Comorbidity count | <0.0001 | ||
| None | 38.8% | 52.2% | |
| 1-2 comorbidities | 32.7% | 30.9% | |
| 3 or more comorbidities | 28.5% | 16.9% | |
| Comorbidities | |||
| Diabetes | 25.4% | 23.7% | 0.74 |
| Hypertension | 30.7% | 25.0% | 0.088 |
| Coronary artery disease | 11.8% | 5.3% | 0.001 |
| Chronic kidney disease | 6.6% | 2.9% | 0.008 |
| Chronic lung disease | 6.6% | 5.0% | 0.49 |
| Anemia | 2.9% | 2.9% | 1 |
| Cancer diagnosis | 3.3% | 2.0% | 0.31 |
| Stroke | 0.4% | 0.7% | 1 |
| Immune diseases | 4.4% | 1.1% | 0.007 |
| Hyperlipidemia / Dyslipidemia | 16.9% | 10.5% | 0.009 |
| Hypothyroid | 6.4% | 5.7% | 0.78 |
| Chronic liver disease | 2.0% | 2.0% | 1 |
| Transplant | 1.8% | 0.9% | 0.16 |
| Vitamin D deficiency | 10.7% | 7.7% | 0.15 |
| Pregnancy | 0.2% | 1.3% | |
| Current smoker (if available) | 9.9% | 5.3% | 0.01 |
Severe disease was recorded in 48 persons (10.5%) among the vaccinated and 121 (26.5%) persons among the unvaccinated group (P<0.001). ( Table 2 ) The proportion of persons with severe disease or death was significantly higher among unvaccinated persons in nearly every subgroup (except age ≤40 years or >70 years where the number of persons in those groups was very small). Factors associated with severe disease or death included increasing age (hazards ratios and 95% CI compared with those <40 years old: >40 – 60 years, HR 2.32, 95% CI 1.15-4.66; >60 – 70 years, HR 4.34, 95% CI 2.10-9.10; >70 years, HR 5.43, 95% CI 2.20-13.53); presence of symptoms at baseline (HR 2.42, 95% CI 1.44-4.07); and being unvaccinated (HR 2.84, 95% CI 1.80-4.47). ( Table 3 ) Patient's sex, nationality, body mass index, comorbidity count, reason for testing, and cycle threshold value for PCR, an indicator of viral load, were not associated with severe disease or death.
Table 2.
Disease severity (severe disease or death) rate per 1,000 person days by subgroups among those who were vaccinated.
| Severe/ total Vaccinated N | (%) | Severe/ total Unvaccinated N | (%) | P-value* | |
|---|---|---|---|---|---|
| Total number | (48/456) | 10.5% | (121/456) | 26.5% | <0.0001 |
| By age | |||||
| <=40 | 9/173 | 5.2% | 16/173 | 9.2% | 0.15 |
| >40 – 60 | 13/185 | 7.0% | 51/185 | 27.6% | <0.0001 |
| >60 – 70 | 16/76 | 21.1% | 42/76 | 55.3% | <0.0001 |
| >70 | 10/22 | 45.5% | 12/22 | 54.5% | 0.55 |
| By nationality | |||||
| Qatari | 14/144 | 9.7% | 40/144 | 27.8% | <0.0001 |
| Non-Qatari | 34/312 | 10.9% | 81/312 | 26.0% | <0.0001 |
| By sex | |||||
| Male | 28/277 | 10.1% | 73/277 | 26.4% | <0.0001 |
| Female | 20/179 | 11.2% | 48/179 | 26.8% | <0.0001 |
| By BMI, kg/m2 | |||||
| <30 | 13/183 | 7.1% | 41/125 | 32.8% | <0.0001 |
| >=30 | 19/171 | 11.1% | 38/129 | 29.5% | <0.0001 |
| By co morbidities | |||||
| None | 5/177 | 2.8% | 37/238 | 15.5% | <0.0001 |
| 1-2 | 13/149 | 8.7% | 50/141 | 35.5% | <0.0001 |
| 3 or more | 30/130 | 23.1% | 34/77 | 44.2% | 0.002 |
Vaccinated vs. unvaccinated.
Table 3.
Factors associated with severe disease or death among persons with breakthrough infection and unvaccinated persons with infection.
| Hazards ratio (95% CI) | P-value | |
|---|---|---|
| Unvaccinated (vs. vaccinated) | 2.84 (1.80-4.47) | <0.0001 |
| Age (comparator: ≤40) | ||
| 40 – 60 | 2.32 (1.15-4.66) | 0.02 |
| >60 – 70 | 4.34 (2.10-9.10) | <0.0001 |
| >70 | 5.43 (2.20-13.53) | <0.0001 |
| Male sex | 1.11 (0.71-1.76) | 0.64 |
| Qatari Nationality (comparator: Non-Qatari) | 1.11 (0.69-1.73) | 0.69 |
| Body mass index >=30 kg/m2 (comparator: <30) | 1.02 (0.66-1.59) | 0.93 |
| Symptoms at baseline (comparator: no symptoms) | 2.42 (1.44-4.07) | 0.001 |
| Ct value (comparator >=30) | ||
| 25 – 30 | 1.87 (0.92-3.77) | 0.08 |
| 20 – 24.99 | 1.28 (0.62-2.64) | 0.5 |
| <20 | 1.60 (0.80-3.19) | 0.18 |
| Comorbidities (comparator: none) | ||
| 1-2 | 1.46 (0.80-2.66) | 0.22 |
| 3 or more | 1.70 (0.87-3.13) | 0.13 |
| Reason for testing (comparator: surveillance) | ||
| Clinical suspicion | 1.36(0.55-3.35) | 0.5 |
| Contact tracing | 1.64 (0.92-2.92) | 0.09 |
| Port of entry testing | 1.26 (0.61-2.62) | 0.54 |
| All others combined | 1.26 (0.74-2.33) | 0.43 |
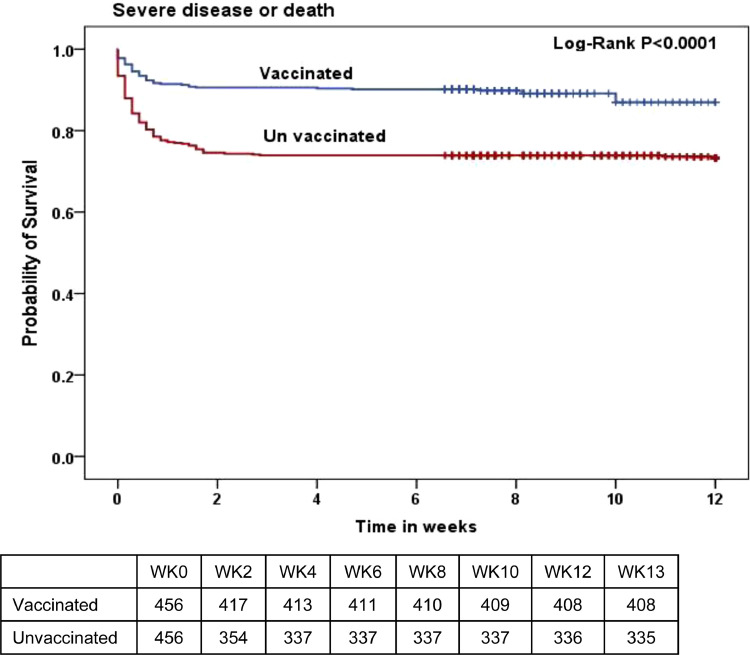
In Kaplan-Meier survival analysis, unvaccinated persons had a significantly lower probability of being free of severe disease or death over a 12 week follow up period (log-rank P-value <0.0001). ( Figure 2 ) The difference in event-free survival was evident within the first two weeks and then plateaued, which reflects the outcome definition (i.e. hospital or intensive care unit admission, mechanical ventilation, or death).
Figure 2.
Kaplan-Meier curves demonstrating the probability of remaining free of severe disease or death by vaccination status.
Discussion
We provide the rates and risk factors for severe outcomes in one of the largest cohorts of persons with breakthrough SARS-CoV-2 infection after full vaccination and infection among matched unvaccinated controls.
Fully vaccinated persons who developed a breakthrough infection were significantly less likely to experience severe disease or death compared with matched unvaccinated persons who developed infection. We found that the rate of severe disease or death among the unvaccinated persons was nearly 3-fold higher than their vaccinated counterparts. Most currently available vaccines consistently provide >90-100% primary protection against severe disease or death. Our data provides further assurances of the effectiveness of the vaccines even when the vaccine was not able to prevent infection. These results are important since no SARS-CoV-2 vaccine provides 100% protection against infection, and even with ∼95% efficacy observed with the mRNA vaccines, a significant number of breakthrough infections can be expected. Knowing that these vaccines provide a high level of protection against severe disease or death in case such breakthrough infection occurs, can boost the public's confidence in vaccines and improve vaccination rates.
Increasing age was strongly and independently associated with a higher risk of severe disease or death in persons with breakthrough infection. While this association has been well described in persons with primary infection,(Grasselli et al., 2020; Zhou et al., 2020) our results confirm that this association holds true in the population with breakthrough infection as well. It may be postulated that older persons have a higher burden of comorbidities that may predispose them to poorer clinical outcomes. However, we found an independent and “dose-dependent” association between age (higher age groups with progressively higher hazards) and severe disease or death. Poor immune response in older age groups and overall frailty may be potential explanations for a higher risk among older persons.
Burden of comorbidities was not associated with a higher risk of severe disease or death in our study. Comorbidities are well-recognized risk factors for poorer clinical outcomes in patients with SARS-CoV-2 infection.(Grasselli et al.; Wu et al.) On the other hand, symptomatic infection was associated with a higher risk. This is intuitive since those with symptoms are the ones who seek medical care and meet the definition of clinical outcomes in our study.
While there is no doubt that vaccination is among the most important and critical elements of the pandemic response, identifying factors associated with critical disease among those with breakthrough infection is of obvious clinical and policy significance. This knowledge can help the providers identify and prioritize the triage of those at highest risk and ensure appropriate monitoring. Policymakers can also determine the resources required to provide the most effective and efficient services based on the population characteristics.
The strengths of our study include a national population, appropriately matched control group, excellent data collection and follow up, and accurate characterization of the outcomes based on individual chart reviews. Limitations include relatively short follow up, and non-randomized protocols for treatment of SARS-CoV-2 infection. The latter is less relevant to our primary outcome of interest, since outcome for the majority (admission to the hospital) was determined prior to when any treatment effects would have been apparent. It is possible that patients received some treatment prior to admission to the hospital, but we would expect that to be relatively similar in the case and control groups.
In summary, increasing age is the most important factor determining poor clinical outcomes in persons with breakthrough SARS-CoV-2 infection, while vaccination is associated with a strong protective effect. Older persons who develop breakthrough infection, particularly those with any symptoms, should be quickly evaluated and monitored for severe outcomes.
Acknowledgement and disclaimer
The authors are grateful for the leadership and assistance provided by the Ministry of Public Health in Qatar, the System-Wide Incident Command and Control Center and the Business Intelligence Unit at Hamad Medical Corporation, and all the dedicated frontline healthcare workers who have selflessly served and provided care and comfort to all patients in Qatar. The views expressed in this article are those of the authors and do not necessarily represent official government views or policy of the State of Qatar or Hamad Medical Corporation.
Conflict of Interest
Dr. Butt has received investigator initiated grant support (to the institution, Veterans Health Foundation of Pittsburgh), which is unrelated to the current work. Other authors declare no conflict of interest.
Funding Source
There was no external funding source for this work.
Ethical Approval
The study was approved by the Institutional Review Board at Hamad Medical Corporation. A waiver of informed consent was granted due to the non-interventional nature of the study and public health relevance.
References
- Abu-Raddad L.J., Chemaitelly H., Butt A.A. Effectiveness of the BNT162b2 Covid-19 Vaccine against the B.1.1.7 and B.1.351 Variants. N Engl J Med. 2021;385:187–189. doi: 10.1056/NEJMc2104974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu-Raddad L.J., Chemaitelly H., Malek J.A., et al. Assessment of the risk of SARS-CoV-2 reinfection in an intense re-exposure setting. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt A.A., Khan T., Yan P., Shaikh O.S., Omer S.B., Mayr F. Rate and risk factors for breakthrough SARS-CoV-2 infection after vaccination. J Infect. 2021;83:237–279. doi: 10.1016/j.jinf.2021.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagan N., Barda N., Kepten E., et al. BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Mass Vaccination Setting. N Engl J Med. 2021;384:1412–1423. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasselli G., Zangrillo A., Zanella A., et al. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacisuleyman E., Hale C., Saito Y., et al. Vaccine Breakthrough Infections with SARS-CoV-2 Variants. N Engl J Med. 2021;384:2212–2218. doi: 10.1056/NEJMoa2105000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teran R.A., Walblay K.A., Shane E.L., et al. Postvaccination SARS-CoV-2 Infections Among Skilled Nursing Facility Residents and Staff Members — Chicago, Illinois, December 2020–March 2021. MMWR Morb Mortal Wkly Rep ePub. 2021 doi: 10.15585/mmwr.mm7017e1. 21 April 2021http://dx.doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C., Chen X., Cai Y., et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA internal medicine. 2020;180:934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]