Abstract
Background
Copy number variation (CNVs) is a key factor in breast cancer development. This study determined prognostic molecular characteristics to predict breast cancer through performing a comprehensive analysis of copy number and gene expression data.
Methods
Breast cancer expression profiles, CNV and complete information from The Cancer Genome Atlas (TCGA) dataset were collected. Gene Expression Omnibus (GEO) chip data sets (GSE20685 and GSE31448) containing breast cancer samples were used as external validation sets. Univariate survival COX analysis, multivariate survival COX analysis, least absolute shrinkage and selection operator (LASSO), Chi square, Kaplan-Meier (KM) survival curve and receiver operating characteristic (ROC) analysis were applied to build a gene signature model and assess its performance.
Results
A total of 649 CNV related-differentially expressed gene obtained from TCGA-breast cancer dataset were related to several cancer pathways and functions. A prognostic gene sets with 9 genes were developed to stratify patients into high-risk and low-risk groups, and its prognostic performance was verified in two independent patient cohorts (n = 327, 246). The result uncovered that 9-gene signature could independently predict breast cancer prognosis. Lower mutation of PIK3CA and higher mutation of TP53 and CDH1 were found in samples with high-risk score compared with samples with low-risk score. Patients in the high-risk group showed higher immune score, malignant clinical features than those in the low-risk group. The 9-gene signature developed in this study achieved a higher AUC.
Conclusion
The current research established a 5-CNV gene signature to evaluate prognosis of breast cancer patients, which may innovate clinical application of prognostic assessment.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13048-021-00823-y.
Keywords: Copy number variation, Breast cancer, Gene signature, TCGA, Prognosis, Bioinformatics
Introduction
Copy number variations (CNVs), which are DNA fragments with varied copy number from 1 kb to several Mb in the human genome, include DNA fragment deletions, insertions, duplications, and compound multipoint variants [1]. CNVs are often present in various types of tumors, and are currently considered as a key factor in genetic variation of tumors [2–5]. CNVs at multiple sites in the genome can cause heterogeneity of the genome and molecular phenotype, leading to the occurrence and development of complex diseases including cancers [2, 6, 7]. Ding et al. reported the diversity of genomes of patients with primary breast cancer that are manifested as frequent gene rearrangements and copy number changes [8]. Shlien et al. used gene chips to compare 770 normal genomes, and found that 49 oncogenes were surrounded by CNV [9]. Stolz et al. demonstrated that about 50% of lung cancer patients show cell cycle-checkpoint kinase 2 gene (CHEK2) inactivation [10].
According to data released by the American Cancer Society in 2018, breast cancer is the most common malignancy among women worldwide and the second leading cause of cancer-related death to women with high [11]. In recent decades, the incidence of breast cancer in China is increasing and is showing a younger trend, noticeably, breast cancer has become a malignant tumor with the highest incidence among Chinese women [12, 13]. The causes of breast cancer are highly complex [14]. In recent decades, great progress has been made in the diagnosis, surgery, chemotherapy and molecular therapy of breast cancer, but the prognosis of breast cancer is still unsatisfactory due to its high heterogeneity and complexity. Therefore, the biological molecular mechanism of breast cancer development should be further studied and explored.
In this study, we examined the correlation between CNV-associated gene expression profiles and clinical outcomes in 1069 breast cancer patients recorded in the Cancer Genome Atlas (TCGA). CNV-associated genes were used to develop a prognostic model for the prediction of overall survival (OS) of breast cancer patients. The results of this study may provide a strategy targeting autophagy for predicting and monitoring the prognosis of breast cancer patients.
Material and methods
Microarray data profile
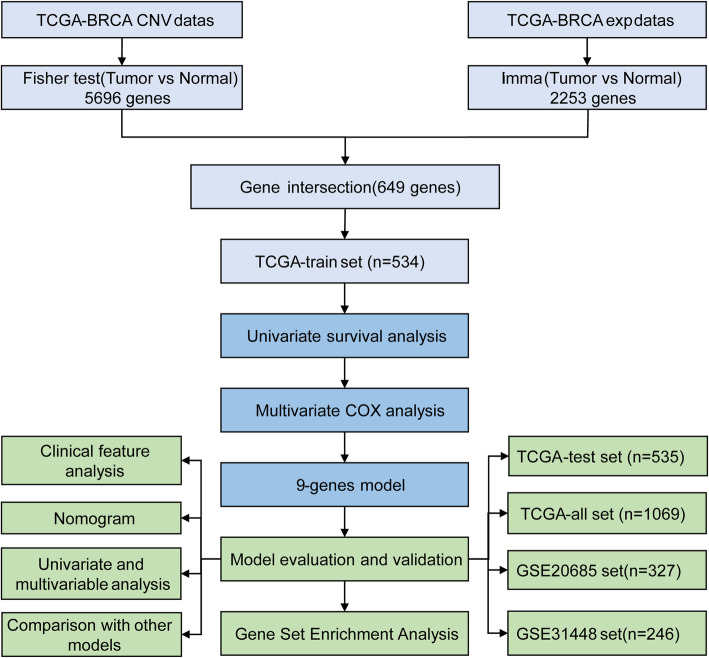
The study design is shown in Fig. 1. Gene expression profile and CNV dataset TCGA [15] with complete follow-up information were obtained on June 30, 2020, 1069 tumor samples with integral clinical information were obtained and randomly classified into the training cohort (n = 534), the testing cohort (n = 536). The two groups were similar in age distribution, sex, follow-up time, and proportion of death. After clustering the gene expression profiles of the two data sets, the number of samples of dichotomy was similar.
Fig. 1.
Work flow chart
The GSE20685 [16] and GSE31448 [17] chip data sets with survival time of 327 and 246 samples were downloaded from Gene Expression Omnibus (GEO) (http://www.ncbi.nlm.nih.gov/geo/) on June 30, 2020. The clinical information of the three data groups is shown in Table 1.
Table 1.
Sample clinical information for three data sets
| Clinical Features | TCGA-BRCA | GSE20685 | GSE31448 |
|---|---|---|---|
| Event | |||
| 0 | 921 | 244 | 167 |
| 1 | 148 | 83 | 79 |
| T Stage | |||
| T1 | 279 | 101 | |
| T2 | 617 | 188 | |
| T3 | 132 | 26 | |
| T4 | 38 | 12 | |
| TX | 3 | 0 | |
| N Stage | |||
| N0 | 502 | 137 | |
| N1 | 357 | 87 | |
| N2 | 120 | 63 | |
| N3 | 73 | 40 | |
| NX | 17 | 0 | |
| M Stage | |||
| M0 | 884 | 319 | |
| M1 | 22 | 8 | |
| MX | 163 | 0 | |
| Stage | |||
| I | 181 | ||
| II | 606 | ||
| III | 240 | ||
| IV | 20 | ||
| X | 22 | ||
| ER Status | |||
| Negative | 235 | ||
| Positive | 786 | ||
| Unknown | 48 | ||
| PR Status | |||
| Negative | 335 | ||
| Positive | 683 | ||
| Unknown | 51 | ||
| Her2 Status | |||
| Negative | 549 | ||
| Positive | 159 | ||
| Unknown | 361 | ||
| Age | |||
| ≤ 60 | 598 | 282 | |
| > 60 | 471 | 45 | |
| Subtype | |||
| Basal | 187 | 72 | |
| Her2 | 77 | 22 | |
| LumA | 552 | 85 | |
| LumB | 202 | 42 | |
| Normal | 39 | 25 | |
| Unknown | 12 | 0 | |
Identification tumor-specific CNV and differentially expressed genes (DEGs)
The chromosome segments in the CNV segment file were matched to genes using bedTools [18], and only the mean value of CNV cells with absolute value greater than 0.2 were kept for further analysis. The difference of CNV identification between tumor samples and normal samples was determined by chi-square test (FDR < 0.05).
The DEGs between tumor and normal samples were calculated using the Limma package [19], and the threshold filter was FDR < 0.01 and |log2FC| > 1.
After drawing the Wayne map of the differentially expressed CNV and the DEGs, 649 common genes were found.
Functional enrichment
Gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) were used to analyzed correlation biological functions and pathways of DEG using WebGestaltR (v0.4.2) [20] in R package.
Identification of prognostic CNV-related genes
Univariate Cox regression, least absolute shrinkage and selection operator (LASSO) regression and multivariate Cox regression analyses were employed to explore the performance of CNV-related genes in predicting OS of breast cancer. Genes were determined as potential prognostic genes when p value was < 0.05 in Univariate Cox regression analysis. LASSO-penalized and multivariate analysis were next performed for further screening. Hazard ratios (HRs) and regression coefficient were calculated for each gene, and 9 CNV-related genes were ultimately included.
Construction of prognostic gene signature
The risk-score model for prognosis prediction of breast cancer patients was the combination of each optimal prognostic CNV-related gene expression level multiplying relative regression coefficient weight calculated from the multivariate model according to the following formula:
All patients in the training cohort were classified into low- and high-risk groups according to the median of risk scores. The Kaplan–Meier survival curves of both groups were plotted, and the receiver operating characteristic (ROC) curve for OS prediction was used to assess the specificity and sensitivity of the model.
Validation of gene signature
Risk score of the patients in TCGA testing cohort, entire TCGA cohort, GSE20685 and GSE31448 dataset were calculated, and patients were assigned into the high-risk and low-risk group with the cut-off value calculated from the training cohort. The Kaplan–Meier survival curves of both groups were plotted, and the ROC curve for OS prediction was used to assess the specificity and sensitivity of the model.
Analysis of clinical feature, mutation gene and immune score
Analysis of RiskScore in clinical feature including T, N, M, Stage, Age were analyzed. Mutation annotation format (MAF) files were processed and visualized by R package maftools [21]. StromalScore, ImmuneScore and ESTIMATEScore were analyzed using ESTIMATE [22] in package.
Comparison with published models
By referring to the literature, we selected three prognostic risk models (10-gene signature (Huang) [23], 4-gene signature (Qi) [24], 19-gene signature (Su) [25] and 6-gene signature (Wang) [26]) for comparison with our 9-gene model, and evaluated them by KM curve, receiver operating characteristic (ROC) curve.
Results
Genes with CNV and expression differences were screened
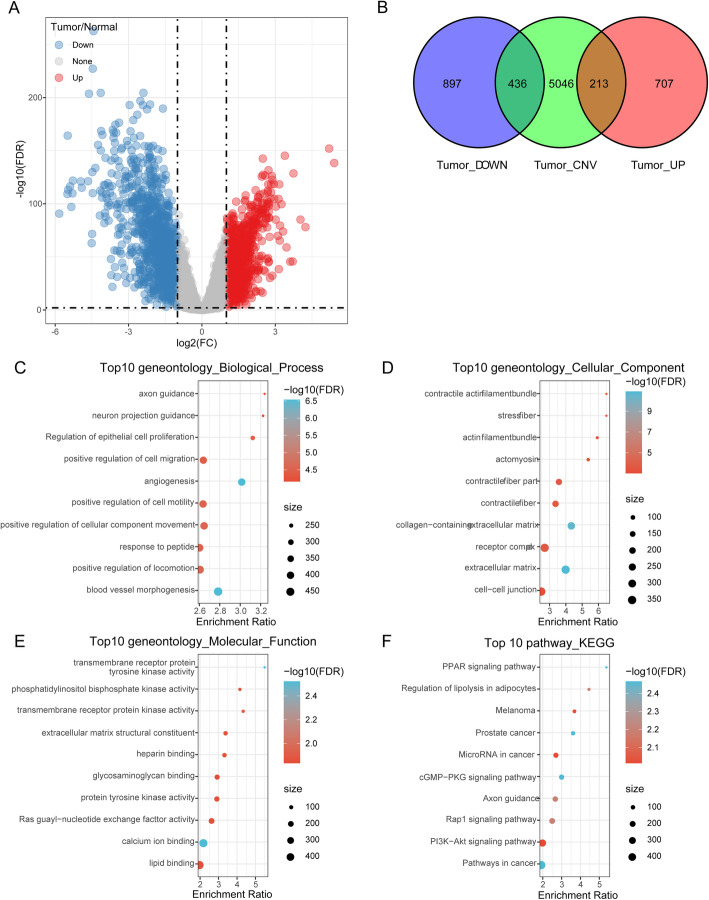
Bedtools was used to detect CNV genes related to breast cancer progression, here we screened 5696 significant differential CNV gene between breast cancer sample and normal sample. Under the condition of FDR < 0.01 and |log2FC| > 1, 920 up-regulation genes and 1333 down-regulation genes were obtained between breast cancer sample and normal sample (Fig. 2A) using Limma package. Venn diagram analysis showed that there were 649 genes with CNV and expression differences (Fig. 2B). KEGG and GO analyses conducted to explore the potential mechanism of these DEGs revealed that DEGs were mainly enriched in positive regulation of cell motility in biological process, cell-cell junction in cellular component and lipid binding in molecular function (Fig. 2C-E). Moreover, KEGG analysis demonstrated that those genes mainly were involved in PPAR signaling pathway, prostate cancer, Rap1 signaling pathway, PI3K-Akt signaling pathway and other pathways in cancer (Fig. 2F).
Fig. 2.
Genes with CNV and expression differences were screened. a: Volcano map of differentially expressed genes between Tumor and Normal. b: Venn diagram of specific CNV and differentially expressed genes. c: BP annotation map of differentially expressed genes. d: CC annotation map of differentially expressed genes. e: MF annotation map of differentially expressed genes. f: KEGG annotation map of differentially expressed genes
Establishment of CNV related genes prognostic model
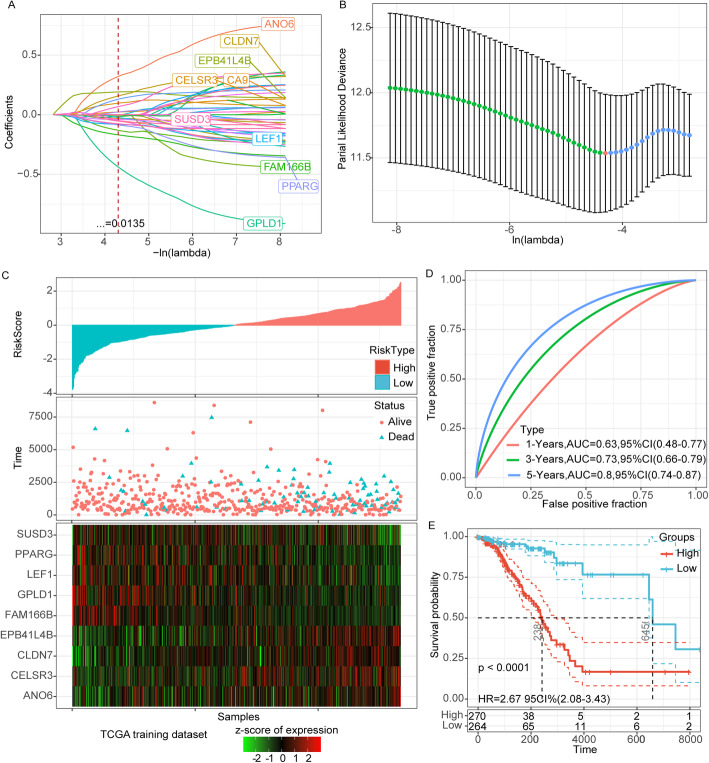
Base on TCGA training dataset, above 649 genes were subjected to univariate Cox survival analysis, and screened 39 DEG. A prognostic signature was developed to predict breast cancer patients’ overall survival. Based on the expression profile of the TCGA training dataset, LASSO Cox regression and multivariate Cox regression analyses were performed (Fig. 3A, B). A prognostic model was constructed based on ANO6, CELSR3, CLDN7, EPB41L4B, FAM166B, GPLD1, LEF1, PPARG and SUSD3. The risk score of breast cancer prognosis was determined with the following formula: RiskScore = 0.629*ANO6 + 0.147*CELSR3 + 0.381*CLDN7+ 0.273*EPB41L4B-0.357*FAM166B -0.843*GPLD1–0.202*LEF1–0.202*PPARG -0.127*SUSD3. KM survival analysis showed that apart from CLDN7 and GPLD1, other genes could accurately divide samples into higher and lower-risk group (Figure S1).
Fig. 3.
Establishment of CNV related genes prognostic model. a: The change trajectory of each independent variable, the horizontal axis represents the log value of the independent variable lambda, and the vertical axis represents the coefficient of the independent variable. b: The confidence interval under each lambda. c: RiskScore distribution, survival time and survival status and 9-gene expression in the TCGA training set. d: ROC curve and AUC of 9-gene signature in the TCGA training set. e: KM survival curve of 9-gene signature in the TCGA training set
The median level of the risk score was used to classify the breast cancer patients in TCGA training dataset into low- and high-risk groups. For the risk score and survival status calculated by the prognostic model and the heatmap of 9 genes, see Fig. 3C. Time-dependent ROC analysis demonstrated that AUC for 1-, 3-, 5-year survival was 0.63, 0.73, 0.8, respectively (Fig. 3D). KM survival analysis showed that the survival rate of the patients in the low-risk group was significantly higher than that in the high-risk group (p < 0.0001) (Fig. 3E).
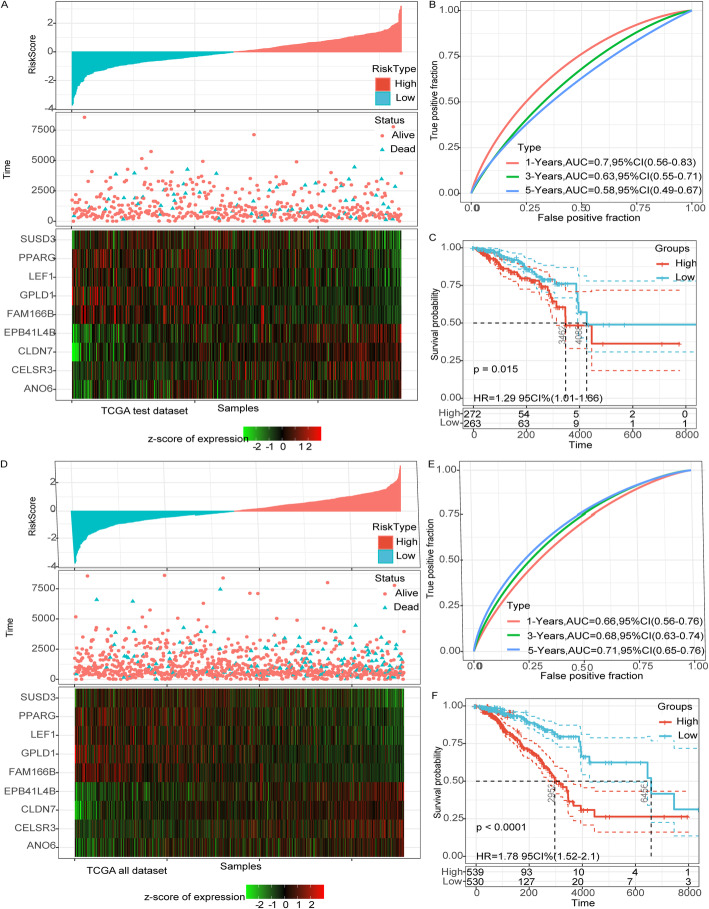
Validation of the risk score in TCGA test set and all TCGA dataset
In order to verify the robustness of the model, the same coefficient to the training set was used, and the model was applied to the TCGA validation dataset and entire dataset. The risk score of each sample was determined according to the expression level of the sample, and the RiskScore distribution and sample survival status was drew (Fig. 4A, D). Time-dependent ROC analysis demonstrated that AUC for 1-, 3-, 5-year survival was 0.7, 0.63, 0.58, respectively in TCGA test dataset, and 0.66, 0.69 and 0.71 respectively in all TCGA dataset (Fig. 4B, E). KM survival analysis showed that the survival rate of the patients in the low-risk group was significantly higher than that in the high-risk group in both TCGA test dataset (p = 0.015) and all TCGA dataset (p < 0.0001) (Fig. 4C, F).
Fig. 4.
Validation of the risk score in TCGA test set and all TCGA dataset. a: RiskScore distribution, survival time and survival status and 9-gene expression in the TCGA test set. b: ROC curve and AUC of 9-gene signature in the TCGA test set. c: KM survival curve of 9-gene signature in the TCGA test set. d: RiskScore distribution, survival time and survival status and 9-gene expression in the TCGA all data sets. e: ROC curve and AUC of 9-gene signature in the TCGA all data sets. f: KM survival curve of 9-gene signature in the TCGA all data sets
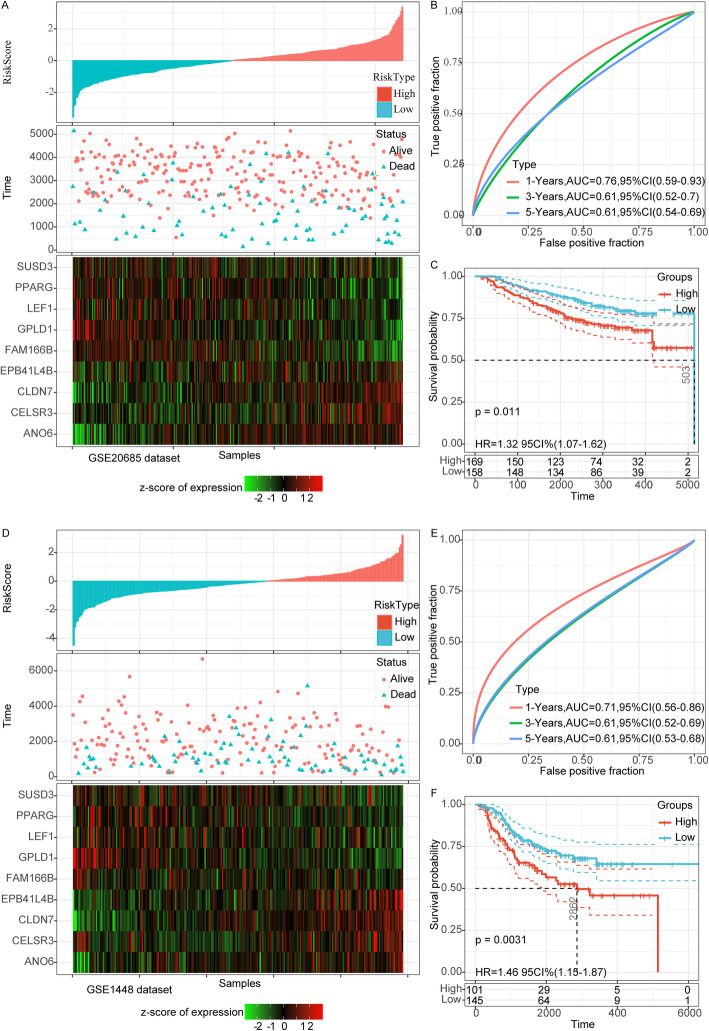
Validation of the risk score in GSE20685 and GSE31448
To determine cross-platform applicability, we applied the model to the GSE20685 and GSE31448 datasets with the same coefficients as the training set to calculate the risk score of each sample according to the expression of the model gene, and drew the RiskScore distribution (Fig. 5A, D). Time-dependent ROC analysis demonstrated that AUC for 1-, 3-, 5-year survival was 0.78, 0.61 and 0.61, respectively in GSE20685 dataset, and 0.71, 0.61 and 0.61 in GSE31448 dataset (Fig. 5B, E). KM survival analysis showed that the survival rate of the patients in the low-risk group was significantly higher than that in the high-risk group in both GSE20685 dataset (p = 0.011) and GSE31448dataset (p = 0.0031) (Fig. 5C, F).
Fig. 5.
Validation of the risk score in GSE20685 and GSE31448. a: RiskScore distribution, survival time and survival status and 9-gene expression in the GSE20685 data set. b: ROC curve and AUC of 9-gene signature in the GSE20685 data set. c: KM survival curve of 9-gene signature in the GSE20685 data set. d: RiskScore distribution, survival time and survival status and 9-gene expression in the GSE31448 data set. e: ROC curve and AUC of 9-gene signature in the GSE31448 data set. f: KM survival curve of 9-gene signature in the GSE31448 data set
Comparison of clinical characteristics between high and low risk groups
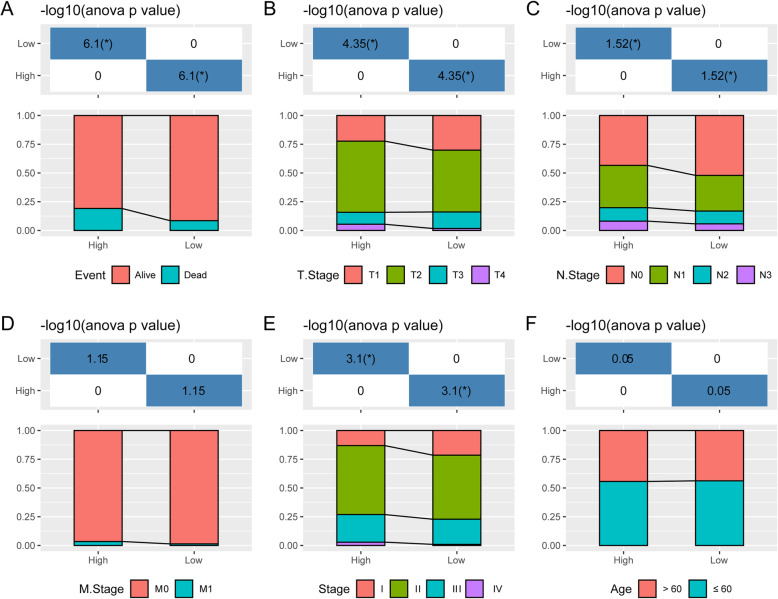
In the TCGA dataset, the distribution of clinical features in the high- and low- risk subgroups were compared. Results showed that there were more samples with a high-risk clinical features in high-risk group, such as T2, T3, and T4, higher degree of differentiation of N1 and N2 and N3, Stage II, III and IV (Fig. 6).
Fig. 6.
Comparison of clinical characteristics between high and low risk groups. a: Distribution of Alive and Dead sample between high and low risk groups in TCGA dataset. b: Distribution of T stage sample between high and low risk groups in TCGA dataset. c: Distribution of N stage sample between high and low risk groups in TCGA dataset. d: Distribution of M stage sample between high and low risk groups in TCGA dataset. e: Distribution of Stage stage sample between high and low risk groups in TCGA dataset. f: Distribution of Age sample between high and low risk groups in TCGA dataset
Comparison of molecular mutation and immune score between high- and low-risk groups
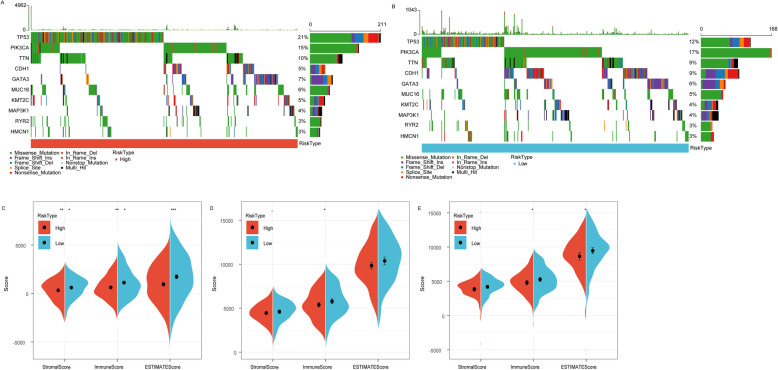
In the TCGA dataset, we compared the distribution of mutation frequencies across high- and low-risk groups, and found that TP53 mutation frequencies were higher, and CDH1 and PIK3CA mutation frequencies were lower in the high-risk group (Fig. 7A-B).
Fig. 7.
Comparison of molecular mutation and immune score between high and low risk groups. a: Distribution of molecular mutations in high risk groups in the TCGA dataset. b: Distribution of molecular mutations in low risk groups in the TCGA dataset. c: Comparison of immune scores between high and low risk groups in TCGA dataset. d: Comparison of immune scores between high and low risk groups in GSE20685 dataset. e: Comparison of immune scores between high and low risk groups in GSE31448 dataset
To examine the relationship of immune scores between high- and low-risk groups of the TCGA dataset, GSE20685 and GSE31448 datasets, the R software package ESTIMATE was used to assess StromalScore, ImmuneScore, ESTIMATEScore. The results showed that the three immune scores were higher in the low-risk group than those in the high-risk group (Fig. 7C-E).
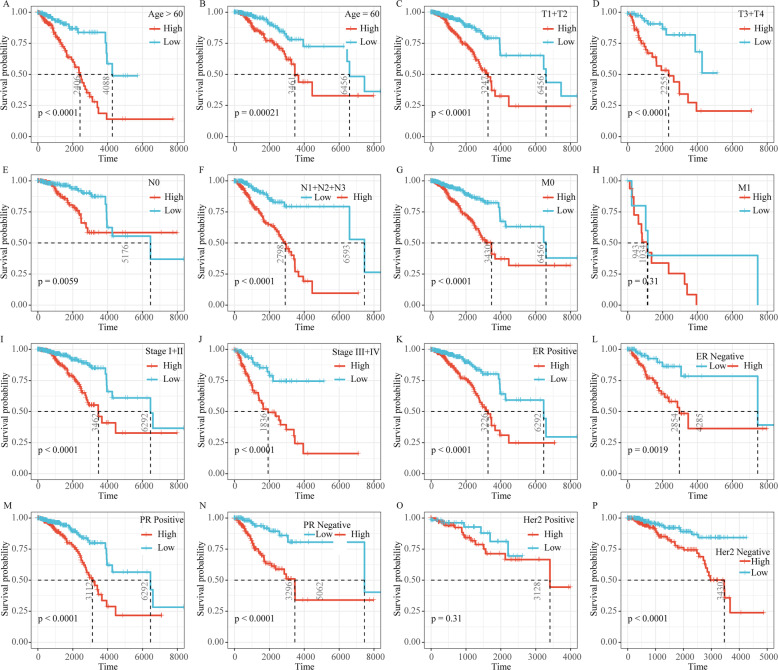
Analysis of clinical characteristics in RiskScore
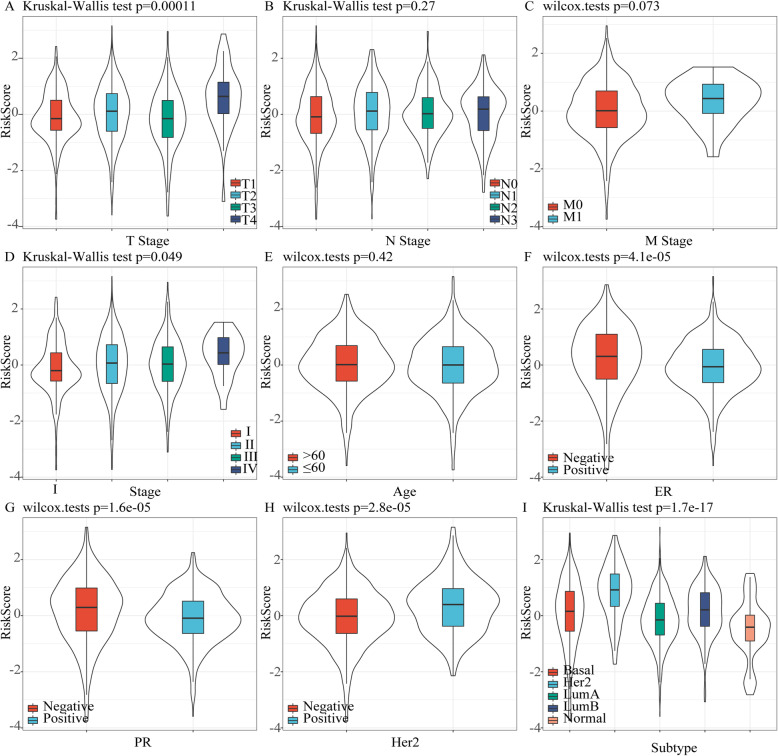
RiskScore analysis in clinical features showed that 9-gene signature could significantly distinguish high- and low-risk groups by age, T Stage, N Stage, M0 Stage, Stage, ER status, PR status and HER2 status in TCGA dataset (Fig. 8), but M1 Stage and Her2 positive could not effectively distinguish high and low risk groups. This further indicated that our model still had a strong predictive ability in different clinical signs.
Fig. 8.
The performance of the risk model on clinical features of the TCGA data set
By comparing the distribution of RiskScore between groups of clinical features, we found that there were significant differences between groups of T Stage, Stage, ER status, PR status, HER2 status and molecular subtypes (p < 0.05) (Fig. 9).
Fig. 9.
The distribution of RiskScore in different clinical characteristics and molecular subtypes in TCGA dataset
Independence of RiskScore
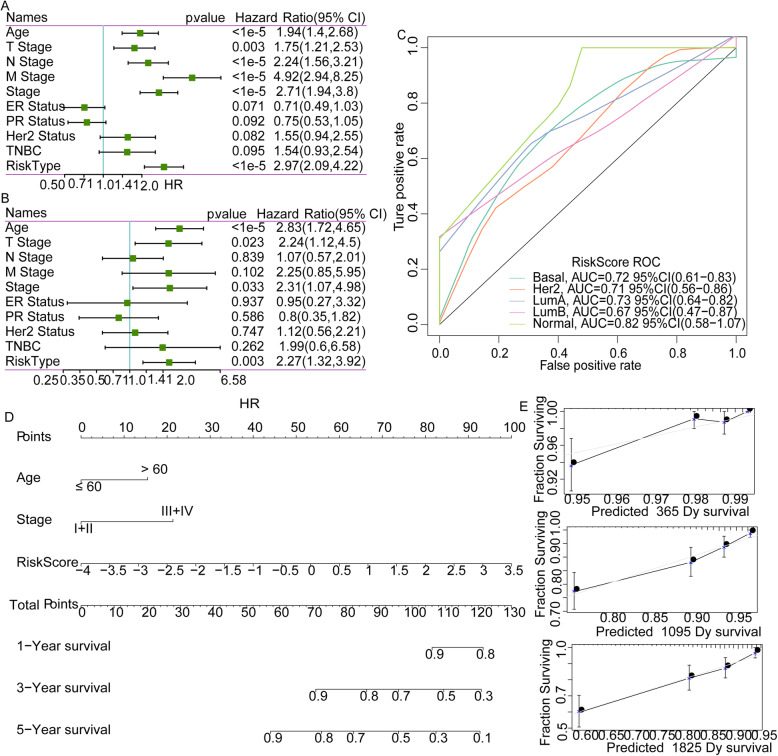
To assess whether the model was an independent predictor of breast cancer, univariate and multivariate analyses were performed on clinical factors and RiskScore. The results showed that showed independent prognostic power of Age, T Stage, Stage and RiskScore (Fig. 10A, B). We used clinical features Age, Stage, and RiskScore together to build a nomogram model using TCGA dataset. The results demonstrated that the RiskScore feature had the greatest influence on the survival prediction, indicating that the risk model based on the 9 genes can better predict patients’ prognosis (Fig. 10C). In addition, we also visualized the prediction performance of the nomogram data for 1-, 3- and 5-year survival (Fig. 10D), and the data proved that the nomogram had a strong prediction performance.
Fig. 10.
Independence of RiskScore. a: Univariate Cox survival analysis of clinical characteristics and RiskScore. b: Multivariate Cox survival analysis of clinical characteristics and RiskScore. c: Nomogram constructed by RiskScore and Clinical characteristics. d: Corrected plot of survival rates in nomogram
Advances of the model
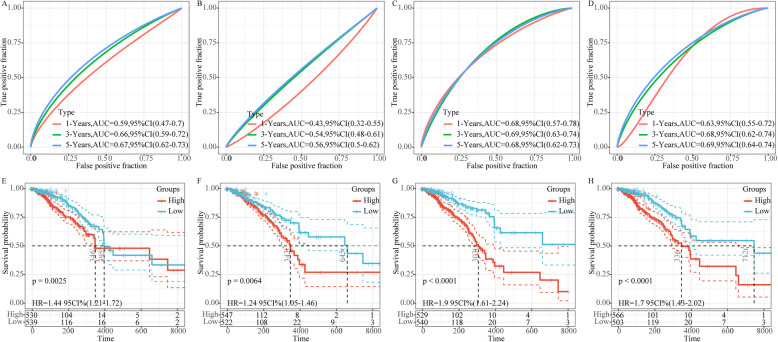
By consulting the literature, we further selected four prognostic-related risk models (a 10-gene signature (Huang), a 4-gene signature (Qi), a 19-gene signature (Su) and a 6-gene signature (Wang)) for comparison with our 9-gene model. In order to promote the comparability of the models, we calculated the risk scores of each BRCA sample in TCGA using the same method based on the corresponding genes in the four models, and divided the samples into the high-risk group and the low-risk group. The ROC curves of the four models showed that except for the 1-, 3-, and 5-year AUC of the 19-gene signature (Su) model, which are close to our model, the AUC of other three model were all lower than our model (Fig. 11A-D). KM curves indicated that the BRCA prognosis in the high- and low-risk group samples were different (log rank p < 0.05) (Fig. 11E-H).
Fig. 11.
Superiority of the model. a, e: ROC and KM curves of 10-gene signature (Huang) risk model; b, F: ROC and KM curves of 4-gene signature (Qi). c, G: ROC and KM curves of 19-gene signature (Su). d, H:ROC and KM curves of 6-gene signature (Wang)
Discussion
A total of 5696 CNV-related genes and 2253 DEGs were acquired from TCGA-BRCA dataset. After the intersection, 649 CNV-associated DEGs were determined and subjected to univariate survival analysis, multivariate COX analysis and LASSO regression analysis to construct a prognostic model. Finally, 9 CNV-related prognostic genes (ANO6, CELSR3, CLDN7, EPB41L4B, FAM166B, GPLD1, LEF1, PPARG and SUSD3) model was developed. After a comprehensive analysis of the clinical information, we found that these 9 genes were associated with multiple clinical features of breast cancer.
After reviewing the existing literature, in addition to tumor-associated mutations, researchers have also focused on other variant subtypes such as copy number variation [27]. Several pathological CNVs, such as CNV of BRCA1, MTUS1 and hTERT, have been identified in the initiation and progression of breast cancer subtypes, suggesting a specific contribution of CNVs to breast cancer [6, 28]. The CNV signature has the potential to be an effective biomarker for differentiating different tumors. However, considering that CNVs are widely distributed in tumor genomes, traditional experimental methods based on gene microarrays and real-time PCR to identify specific CNV patterns for specific tumor subtypes are often inefficient and time-consuming. In this aspect, tumor-specific CNVs could be used as a new tool to identify specific breast cancer-associated CNVs based on whole-genome sequencing data. Thus, copy number correlation studies may open a new direction to breast cancer treatment and prognosis. Several copy number-related prognostic indicators have been proposed. The CNV map of the MammaPrint™ gene or Oncotype DX® gene could predict the prognosis of patients with breast cancer [29, 30]. This study identified prognostic genes associated with CNV based on the whole genome sequence of breast cancer from the TCGA dataset, which may be provided new diagnostic indicators.
By reviewing the existing literature, we found that these 9 genes were more or less associated with tumor development. ANO6 has a higher expression in gliomas, and inhibition ANO6 suppresses the proliferation and invasion of gliomas cells [31]. The significance of ANO6 has also been found in bleeding disorders [32] and bone dysplasia [33]. CELSR3 mRNA expression is upregulated in hepatocellular carcinoma and indicates poor prognosis [34, 35]. Claudin-7 (CLDN7) is aberrantly expressed in some types of cancers including gastric cancer [36], human clear cell renal cell carcinoma [37] and colorectal cancer [38]. EPB41L4B is upregulated in prostate adenocarcinoma [39]. Knockout and suppression therapies designed for LEF1 have been shown to be effective in reducing tumor growth, migration, and invasion of CLL, CRC, glioblastoma multiforme (GBM), and renal cell carcinoma (RCC) [40]. PPARG promotes the differentiation of bladder epithelial cells and regulates the expression of mitochondrial genes [41]. A study has shown that a lack of SUSD3 expression in breast cancer tissues may be an important predictor of non-response to aromatase inhibitors [42]. However, FAM166B and GPLD1 have not been thoroughly studied in tumors.
Somatic mutation analysis of samples from the high- and low-risk groups indicated that differences in mutated genes may account for the genetic differences in breast cancer patients. The mutation of TP53 and TTN was higher, and PIK3R1 was lower in the high-risk group than in the low-risk group. Interestingly, these three genes have been shown to have some tumor suppressive effects in previous studies [43–45].
The advance of this study lies in the discovery that copy number variation is associated with the mechanism of breast cancer, which opens a new direction for breast cancer treatment. Also, we identified hub genes closely associated with breast cancer survival. Most of these genes have been shown to affect tumor progression and have the potential to be used in targeted therapies. However, most of the genes have not been well studied in relation to breast cancer.
This study found that copy number variants are associated with breast cancer and screened hub genes on copy number variants, which may become new targets for breast cancer treatment.
Supplementary Information
Acknowledgements
None.
Authors’ contributions
Conception and design of the research: DYZ; Acquisition of data: NL; Analysis and interpretation of data and Statistical analysis: KY; Drafting the manuscript: NL; Revision of manuscript for important intellectual content: DYZ and ZL; All authors read and approved the manuscript.
Funding
None.
Availability of data and materials
The analyzed data sets generated during the study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflicts of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Wei Hu and Mingyue Li contributed equally to this work.
Contributor Information
Jing Li, Email: lj13864152125@126.com.
Shusheng Qiu, Email: qss88055@163.com.
Liang Li, Email: liliangzb@sina.com.
References
- 1.Henrichsen CN, Chaignat E, Reymond A. Copy number variants, diseases and gene expression. Hum Mol Genet. 2009;18(R1):R1–R8. doi: 10.1093/hmg/ddp011. [DOI] [PubMed] [Google Scholar]
- 2.Shlien A, Malkin D. Copy number variations and cancer. Genome Med. 2009;1(6):62. doi: 10.1186/gm62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pollack JR, Sørlie T, Perou CM, Rees CA, Jeffrey SS, Lonning PE, et al. Microarray analysis reveals a major direct role of DNA copy number alteration in the transcriptional program of human breast tumors. Proc Natl Acad Sci U S A. 2002;99(20):12963–12968. doi: 10.1073/pnas.162471999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Freire P, Vilela M, Deus H, Kim YW, Koul D, Colman H, Aldape KD, Bogler O, Yung WKA, Coombes K, Mills GB, Vasconcelos AT, Almeida JS. Exploratory analysis of the copy number alterations in glioblastoma multiforme. PLoS One. 2008;3(12):e4076. doi: 10.1371/journal.pone.0004076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gorringe KL, George J, Anglesio MS, Ramakrishna M, Etemadmoghadam D, Cowin P, et al. Copy number analysis identifies novel interactions between genomic loci in ovarian cancer. PloS one. 2010;5(9):e11408. 10.1371/journal.pone.0011408. [DOI] [PMC free article] [PubMed]
- 6.Frank B, Bermejo JL, Hemminki K, Sutter C, Wappenschmidt B, Meindl A, Kiechle-Bahat M, Bugert P, Schmutzler RK, Bartram CR, Burwinkel B. Copy number variant in the candidate tumor suppressor gene MTUS1 and familial breast cancer risk. Carcinogenesis. 2007;28(7):1442–1445. doi: 10.1093/carcin/bgm033. [DOI] [PubMed] [Google Scholar]
- 7.Savinainen KJ, Saramäki OR, Linja MJ, Bratt O, Tammela TL, Isola JJ, et al. Expression and gene copy number analysis of ERBB2 oncogene in prostate cancer. Am J Pathol. 2002;160(1):339–345. doi: 10.1016/S0002-9440(10)64377-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ding L, Ellis MJ, Li S, Larson DE, Chen K, Wallis JW, Harris CC, McLellan MD, Fulton RS, Fulton LL, Abbott RM, Hoog J, Dooling DJ, Koboldt DC, Schmidt H, Kalicki J, Zhang Q, Chen L, Lin L, Wendl MC, McMichael JF, Magrini VJ, Cook L, McGrath SD, Vickery TL, Appelbaum E, DeSchryver K, Davies S, Guintoli T, Lin L, Crowder R, Tao Y, Snider JE, Smith SM, Dukes AF, Sanderson GE, Pohl CS, Delehaunty KD, Fronick CC, Pape KA, Reed JS, Robinson JS, Hodges JS, Schierding W, Dees ND, Shen D, Locke DP, Wiechert ME, Eldred JM, Peck JB, Oberkfell BJ, Lolofie JT, du F, Hawkins AE, O’Laughlin MD, Bernard KE, Cunningham M, Elliott G, Mason MD, Thompson Jr DM, Ivanovich JL, Goodfellow PJ, Perou CM, Weinstock GM, Aft R, Watson M, Ley TJ, Wilson RK, Mardis ER. Genome remodelling in a basal-like breast cancer metastasis and xenograft. Nature. 2010;464(7291):999–1005. doi: 10.1038/nature08989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shlien A, Malkin D. Copy number variations and cancer susceptibility. Curr Opin Oncol. 2010;22(1):55–63. doi: 10.1097/CCO.0b013e328333dca4. [DOI] [PubMed] [Google Scholar]
- 10.Stolz A, Ertych N, Bastians H. Loss of the tumour-suppressor genes CHK2 and BRCA1 results in chromosomal instability. Biochem Soc Trans. 2010;38(6):1704–1708. doi: 10.1042/BST0381704. [DOI] [PubMed] [Google Scholar]
- 11.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 12.Jiang X, Tang H, Chen T. Epidemiology of gynecologic cancers in China. J Gynecol Oncol. 2018;29(1):e7. doi: 10.3802/jgo.2018.29.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wen D, Wen X, Yang Y, Chen Y, Wei L, He Y, Shan B. Urban rural disparity in female breast cancer incidence rate in China and the increasing trend in parallel with socioeconomic development and urbanization in a rural setting. Thoracic Cancer. 2018;9(2):262–272. doi: 10.1111/1759-7714.12575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lawson JS, Günzburg WH, Whitaker NJ. Viruses and human breast cancer. Future Microbiol. 2006;1(1):33–51. doi: 10.2217/17460913.1.1.33. [DOI] [PubMed] [Google Scholar]
- 15.The TCGA Legacy Cell. 2018;173(2):281–2. doi: 10.1016/j.cell.2018.03.049. [DOI] [PubMed] [Google Scholar]
- 16.Kao KJ, Chang KM, Hsu HC, Huang AT. Correlation of microarray-based breast cancer molecular subtypes and clinical outcomes: implications for treatment optimization. BMC Cancer. 2011;11(1):143. doi: 10.1186/1471-2407-11-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sabatier R, Finetti P, Adelaide J, Guille A, Borg JP, Chaffanet M, Lane L, Birnbaum D, Bertucci F. Down-regulation of ECRG4, a candidate tumor suppressor gene, in human breast cancer. PLoS One. 2011;6(11):e27656. doi: 10.1371/journal.pone.0027656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quinlan AR, Hall IM. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics (Oxford, England). 2010;26(6):841–2. [DOI] [PMC free article] [PubMed]
- 19.Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43(7):e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang J, Vasaikar S, Shi Z, Greer M, Zhang B. WebGestalt 2017: a more comprehensive, powerful, flexible and interactive gene set enrichment analysis toolkit. Nucleic Acids Res. 2017;45(W1):W130–W1w7. doi: 10.1093/nar/gkx356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mayakonda A, Lin DC, Assenov Y, Plass C, Koeffler HP. Maftools: efficient and comprehensive analysis of somatic variants in cancer. Genome Res. 2018;28(11):1747–1756. doi: 10.1101/gr.239244.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chakraborty H, Hossain A. R package to estimate intracluster correlation coefficient with confidence interval for binary data. Comput Methods Prog Biomed. 2018;155:85–92. doi: 10.1016/j.cmpb.2017.10.023. [DOI] [PubMed] [Google Scholar]
- 23.Huang H, Chen Q, Sun W, Lu M, Yu Y, Zheng Z, Li P. Expression signature of ten genes predicts the survival of patients with estrogen receptor positive-breast cancer that were treated with tamoxifen. Oncol Lett. 2018;16(1):573–579. doi: 10.3892/ol.2018.8663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qi L, Yao Y, Zhang T, Feng F, Zhou C, Xu X, Sun C. A four-mRNA model to improve the prediction of breast cancer prognosis. Gene. 2019;721:144100. doi: 10.1016/j.gene.2019.144100. [DOI] [PubMed] [Google Scholar]
- 25.Su J, Miao LF, Ye XH, Cui MS, He XF. Development of prognostic signature and nomogram for patients with breast cancer. Medicine (Baltimore) 2019;98(11):e14617. doi: 10.1097/MD.0000000000014617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang F, Tang C, Gao X, Xu J. Identification of a six-gene signature associated with tumor mutation burden for predicting prognosis in patients with invasive breast carcinoma. Ann Transl Med. 2020;8(7):453. doi: 10.21037/atm.2020.04.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Azim HA, Jr, Nguyen B, Brohée S, Zoppoli G, Sotiriou C. Genomic aberrations in young and elderly breast cancer patients. BMC Med. 2015;13(1):266. doi: 10.1186/s12916-015-0504-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Silva FC, Lisboa BC, Figueiredo MC, Torrezan GT, Santos EM, Krepischi AC, et al. Hereditary breast and ovarian cancer: assessment of point mutations and copy number variations in Brazilian patients. BMC Medical Genetics. 2014;15(1):55. doi: 10.1186/1471-2350-15-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fatima A, Tariq F, Malik MFA, Qasim M, Haq F. Copy number profiling of MammaPrint™ genes reveals association with the prognosis of breast Cancer patients. J Breast Cancer. 2017;20(3):246–253. doi: 10.4048/jbc.2017.20.3.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahmed W, Malik MFA, Saeed M, Haq F. Copy number profiling of Oncotype DX genes reveals association with survival of breast cancer patients. Mol Biol Rep. 2018;45(6):2185–2192. doi: 10.1007/s11033-018-4379-1. [DOI] [PubMed] [Google Scholar]
- 31.Xuan ZB, Wang YJ, Xie J. ANO6 promotes cell proliferation and invasion in glioma through regulating the ERK signaling pathway. OncoTargets and therapy. 2019;12:6721–6731. doi: 10.2147/OTT.S211725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kmit A, van Kruchten R, Ousingsawat J, Mattheij NJ, Senden-Gijsbers B, Heemskerk JW, et al. Calcium-activated and apoptotic phospholipid scrambling induced by Ano6 can occur independently of Ano6 ion currents. Cell Death Dis. 2013;4(4):e611. doi: 10.1038/cddis.2013.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ehlen HW, Chinenkova M, Moser M, Munter HM, Krause Y, Gross S, et al. Inactivation of anoctamin-6/Tmem16f, a regulator of phosphatidylserine scrambling in osteoblasts, leads to decreased mineral deposition in skeletal tissues. J Bone Mineral Res. 2013;28(2):246–259. doi: 10.1002/jbmr.1751. [DOI] [PubMed] [Google Scholar]
- 34.Gu X, Li H, Sha L, Mao Y, Shi C, Zhao W. CELSR3 mRNA expression is increased in hepatocellular carcinoma and indicates poor prognosis. PeerJ. 2019;7:e7816. doi: 10.7717/peerj.7816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ouyang X, Wang Z, Yao L, Zhang G. Elevated CELSR3 expression is associated with hepatocarcinogenesis and poor prognosis. Oncol Lett. 2020;20(2):1083–1092. doi: 10.3892/ol.2020.11671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu Z, Shi J, Song Y, Zhao J, Sun J, Chen X, et al. Claudin-7 (CLDN7) is overexpressed in gastric cancer and promotes gastric cancer cell proliferation, invasion and maintains mesenchymal state. Neoplasma. 2018;65(3):349–359. doi: 10.4149/neo_2018_170320N200. [DOI] [PubMed] [Google Scholar]
- 37.Li Y, Gong Y, Ning X, Peng D, Liu L, He S, Gong K, Zhang C, Li X, Zhou L. Downregulation of CLDN7 due to promoter hypermethylation is associated with human clear cell renal cell carcinoma progression and poor prognosis. J Exp Clin Cancer Res. 2018;37(1):276. doi: 10.1186/s13046-018-0924-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tang W, Dou T, Zhong M, Wu Z. Dysregulation of Claudin family genes in colorectal cancer in a Chinese population. BioFactors (Oxford, England). 2011;37(1):65–73. [DOI] [PubMed]
- 39.Schulz WA, Ingenwerth M, Djuidje CE, Hader C, Rahnenführer J, Engers R. Changes in cortical cytoskeletal and extracellular matrix gene expression in prostate cancer are related to oncogenic ERG deregulation. BMC Cancer. 2010;10(1):505. doi: 10.1186/1471-2407-10-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Santiago L, Daniels G, Wang D, Deng FM, Lee P. Wnt signaling pathway protein LEF1 in cancer, as a biomarker for prognosis and a target for treatment. Am J Cancer Res. 2017;7(6):1389–1406. [PMC free article] [PubMed] [Google Scholar]
- 41.Liu C, Tate T, Batourina E, Truschel ST, Potter S, Adam M, Xiang T, Picard M, Reiley M, Schneider K, Tamargo M, Lu C, Chen X, He J, Kim H, Mendelsohn CL. Pparg promotes differentiation and regulates mitochondrial gene expression in bladder epithelial cells. Nat Commun. 2019;10(1):4589. doi: 10.1038/s41467-019-12332-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu Z, Jiang E, Wang X, Shi Y, Shangguan AJ, Zhang L, Li J. Sushi domain-containing protein 3: a potential target for breast Cancer. Cell Biochem Biophys. 2015;72(2):321–324. doi: 10.1007/s12013-014-0480-9. [DOI] [PubMed] [Google Scholar]
- 43.Cheng X, Yin H, Fu J, Chen C, An J, Guan J, Duan R, Li H, Shen H. Aggregate analysis based on TCGA: TTN missense mutation correlates with favorable prognosis in lung squamous cell carcinoma. J Cancer Res Clin Oncol. 2019;145(4):1027–1035. doi: 10.1007/s00432-019-02861-y. [DOI] [PubMed] [Google Scholar]
- 44.Chen L, Yang L, Yao L, Kuang XY, Zuo WJ, Li S, Qiao F, Liu YR, Cao ZG, Zhou SL, Zhou XY, Yang WT, Shi JX, Huang W, Hu X, Shao ZM. Characterization of PIK3CA and PIK3R1 somatic mutations in Chinese breast cancer patients. Nat Commun. 2018;9(1):1357. doi: 10.1038/s41467-018-03867-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Silwal-Pandit L, Langerød A, Børresen-Dale AL. TP53 Mutations in Breast and Ovarian Cancer. Cold Spring Harbor Perspectives Med 2017;7(1). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The analyzed data sets generated during the study are available from the corresponding author on reasonable request.