Abstract
Microscopies based on focused electron probes allow the cell biologist to image the 3D ultrastructure of eukaryotic cells and tissues extending over large volumes, thus providing new insight into the relationship between cellular architecture and function of organelles. Here we compare two such techniques: electron tomography in conjunction with axial bright-field scanning transmission electron microscopy (BF-STEM), and serial block face scanning electron microscopy (SBF-SEM). The advantages and limitations of each technique are illustrated by their application to determining the 3D ultrastructure of human blood platelets, by considering specimen geometry, specimen preparation, beam damage and image processing methods. Many features of the complex membranes composing the platelet organelles can be determined from both approaches, although STEM tomography offers a higher ~ 3 nm isotropic pixel size, compared with ~5 nm for SBF-SEM in the plane of the block face and ~30 nm in the perpendicular direction. In this regard, we demonstrate that STEM tomography is advantageous for visualizing the platelet canalicular system, which consists of an interconnected network of narrow (~50 to 100 nm) membranous cisternae. In contrast, SBF-SEM enables visualization of complete platelets, each of which extends ~2 μm in minimum dimension, whereas BF-STEM tomography can typically only visualize approximately half of the platelet volume due to a rapid non-linear loss of signal in specimens of thickness greater than ~1.5 μm. We also show that the limitations of each approach can be ameliorated by combining 3D and 2D measurements using a stereological approach.
Keywords: Scanning transmission electron microscopy (STEM), electron tomography, serial block face scanning electron microscopy (SBF-SEM), human blood platelets
Summary
SBF-SEM and BF-STEM tomography are complementary approaches for acquiring three-dimensional data, and each imaging technique has its advantages and disadvantages. When addressing a scientific question, it is important to consider the following: the size of the object imaged; the spatial resolution or level of detail that is needed; the efficiency and time required for imaging; and the sample preparation protocol which affects contrast and signal-to-noise ratio. For studying small details at the ultrastructural level, BF-STEM tomography provides superior nearly isotropic spatial resolution, whereas SBF-SEM offers a major advantage for imaging larger volumes of eukaryotic cells and tissues. Comparison between 3D structures of blood platelets obtained from the two approaches illustrates the trade-off between these considerations. Although a different specimen preparation protocol is required for each technique, the two methods are even more powerful when used in combination since they enable the imaging of large volumes, while providing a higher resolution to visualize similar but not identical structures of interest. Currently, the performance of SBF-SEM is limited by radiation damage, which reduces the depth information perpendicular to the block face due to shrinkage of the embedding material; and the maximum specimen thickness in axial BF STEM tomography is limited by the attenuation of electron probe by the stain that is needed to provide contrast. New approaches to 3D image segmentation based on deep learning techniques are likely to be available soon, which promise to extend greatly the usefulness of both methods.
1. Introduction
Three-dimensional imaging techniques are essential for visualizing the ultrastructure of cells and the intricate spatial organization of their organelles including the supramolecular assemblies from which the organelles are composed and the membranes that define their shapes. It is usually difficult to decipher cellular complexity by extrapolation of two-dimensional images. Indeed, it is only with the help of high-resolution 3D representations of cellular architecture obtainable by electron microscopy, and correlated with dynamic optical images of living cells, that we can hope to understand the structural basis of cellular function. Moreover, it is often desirable to obtain the 3D ultrastructure of entire eukaryotic cells since organelles can extend over relatively large volumes and display complicated membrane geometry and connectivity.
Cryo-electron tomography has proven to be a valuable technique for determining the high-resolution 3D ultrastructure of viruses and prokaryotic cells, as well as thin regions of eukaryotic cells that are vitrified by rapid freezing directly onto electron microscope grids (Grünewald et al., 2003; Khursigara et al., 2008; Liu et al., 2008; Beck and Baumeister, 2016). This technique can also be performed on cryosectioned specimens or on thin lamellae obtained from vitrified specimens by using a focused ion beam (FIB) (Marko et al., 2007; Rigort et al., 2013); and most notably Mahamid et al. (2017) have recently achieved a spatial resolution of ~3 nm in cryo electron tomography of 200-nm thick FIB-milled lamellae of HeLa cell nuclei. Although cryo-electron tomography provides the highest spatial resolution and the best preservation of native structures, it generally cannot provide the 3D ultrastructure of an entire eukaryotic cell because specimens must be prepared with a thickness of less than approximately 300 nm, which destroys the rest of the cell. Although the complementary techniques of freeze-fracture and freeze-etch can provide exquisite detail about the 3D structure of membranes and cytoskeletal proteins, they also do not generally reveal the overall morphology of eukaryotic cells (Carson, 2014). Cryo-tomography using absorption of soft x-rays generated at a synchrotron facility provides another valuable approach for imaging eukaryotic cells in a frozen hydrated state with a spatial resolution of approximately 35-50 nm (Do et al., 2015). Liquid phase scanning transmission electron microscopy (STEM) offers the possibility of imaging intact eukaryotic cells close to their native state, and is particularly useful for mapping the distribution of heavy atoms labels attached to individual protein assemblies in the plasma membrane or elsewhere in the cell (de Jonge et al., 2010).
In applications for which it is not essential to prepare samples in a frozen or liquid state, extended volumes of eukaryotic cells and tissues can be prepared by using freeze-substitution or conventional chemical fixation along with heavy atom staining and embedding in plastic. Although artifacts can occur, unlike in frozen preparations, staining and embedding coupled with high throughput 3D electron microscopy have the advantage of providing high-contrast 3D images of large sample volumes. Over the past ten years, various microscopies have been developed to image such specimens in 3D, and these techniques have been fine-tuned by improving sample preparation, electron optics, and detectors, as well as image acquisition and processing methods (Denk and Horstmann, 2004; Briggman and Denk, 2006; Yakushevska et al., 2007; Aoyama et al., 2008; Heymann et al., 2009; Hohmann-Marriott et al., 2009; Sousa et al., 2011; Mourik et al., 2015; Narayama and Submramaniam, 2015; Glancy et al., 2015; Pfeifer et al., 2015; Shomorony et al., 2015; Kremer et al., 2015; Wacker et al., 2015; Bouwer et al., 2016; Kittlemann et al., 2016). Much of the effort to develop these 3D imaging techniques has stemmed from the strong interest within the scientific community to map neuronal circuitry at the nanoscale in brain (Briggman et al., 2011; Helmstaedter et al., 2013; Holcomb et al., 2013; Hayworth et al., 2014; Maco et al., 2014; Perez et al., 2014).
Here we compare the capabilities of two 3D techniques: serial block face (SBF)-SEM (Denk and Horstmann, 2004; Briggman and Denk, 2006), and axial bright-field STEM tomography (Yakushevska et al., 2007; Aoyama et al, 2008; Hohmann-Marriott et al., 2009; Sousa et al., 2009; Sousa et al., 2011). Each of these approaches enables cell and structural biologists to visualize the arrangement of internal membrane-bound organelles, to the extent allowed by the specimen preparation protocols. There is in our experience, however, uncertainty within the scientific community about the relative capabilities of the SBF-SEM and STEM tomography for elucidating 3D cellular ultrastructure. We therefore examine systematically the relative strengths of the techniques in terms of specimen geometry, the incorporation of heavy atom stains, signal generation, spatial resolution, acquisition time, and image processing requirements. We illustrate the capabilities and limitations of the techniques with an application a eukaryotic cell that is sufficiently complex to contain several types of organelles and membrane geometries, yet one that is simple enough to have well-defined structures that have previously been characterized. Human blood platelets satisfy these conditions. Platelets are small anucleated cells, originating from megakaryocytes, which maintain homeostasis by circulating in the bloodstream and forming clots over damaged vasculature. In addition to mitochondria and fragments of endoplasmic reticulum, platelets contain three secretory organelles: lysosomes, alpha granules and dense granules. Upon activation, platelets form pseudopods and secrete molecular factors, such as Von Willebrand factor and beta thromboglobulin (King and Reed, 2002). A more comprehensive understanding of platelet ultrastructure and physiology under normal conditions could contribute to a better understanding of diseased states, since platelets are important in the pathology of several cardiovascular diseases, including atherosclerosis.
1.1. 3D Imaging Based on SEM
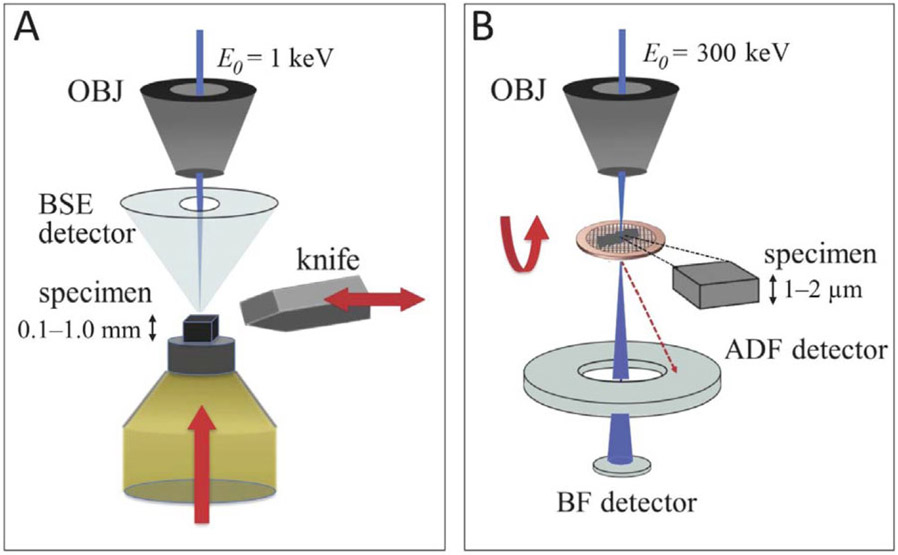
Serial block face SEM was originally conceived by Leighton (1981) and then developed into a practicable and valuable technique some twenty years later by Denk and Horstmann (2004). In the SBF-SEM, a low energy (~1 keV) electron probe a few nanometers in diameter originating from a field-emission gun is scanned across the trimmed surface of a heavy-atom stained, resin-embedded block of cells or tissue (Fig. 1A). An image of the block face is formed by collecting the signal from an annular backscattered electron (BSE) detector positioned just beneath the objective lens pole-piece. The BSE signal is sensitive to the heavy atoms that stain cellular structures within the specimen, which is mounted in a special specimen stage that includes an automated microtome. After an image is acquired the sample is raised by a set distance (25-200 nm) and the ultramicrotome slices off a thin section from the block, so that the diamond knife remains on the focal plane throughout the data collection. A new surface is imaged and the process repeats for a specified number of slices. In this way, SBF-SEM can provide large (103-106 μm3) volumes of ultrastructural data with a pixel size of 5–20 nm in the x, y plane. SBF-SEM has been applied to determine the ultrastructure of a variety of tissues and complex organ systems such as: neuronal circuitry in brain (Briggman and Denk, 2006; Holcomb et al, 2013); lung capillaries (West et al., 2010); neuronal circuitry in retina (Briggman et al., 2011; Helmstaedter et al., 2013); cochlear anatomy (Anttonen et al., 2012); pancreatic islets of Langerhans (Pfeifer et al., 2015; Shomorony et al., 2015; Zhu et al., 2017); and hepatic microarchitecture (Shami et al., 2016).
Figure 1.
(A) In SBF-SEM, a heavy-atom stained, resin-embedded biological specimen is first raster-scanned with a low energy electron beam (keV). A back scattered detector (BSD) is used to image the electrons back-scattered from a certain depth of the specimen. A diamond knife, mounted in situ, then slices off a thin layer and exposes a new surface to be imaged. The block is then raised a desired height, which dictates the cutting slice thickness and the pattern repeats. A series of consecutive images produces a volumetric dataset. (B) In STEM, 300 keV incident electrons have multiple ways of interacting with a thick (>1 μm) resin-embedded material: a red dotted line represents electrons that arrive at the bottom of the specimen with large displacement from the center, which land on the high angle-annular dark field (HAADF) detector; blue line represents the electrons along the central axis of the specimen which land on the on-axis bright field (BF) detector. When imaging thick specimens, the use of HAADF detector produces blurry unfocused images, while BF detector’s larger depth-of-field allows imaging of thick specimens without the loss of focus through the entire thickness of the plastic section. BF combined with tomography allows one to obtain three-dimensional structural information.
The resolution of SBF-SEM in the z-direction is limited by the minimum slice thickness of approximately 25 nm, although techniques have been developed to improve the z-resolution to less than the slice thickness, based on successively imaging the block face with probes of different primary energies (Boughorbel et al., 2012; He et al., 2017; de Goede et al., 2017). The spatial resolution in the x, y plane is limited by the total electron fluence which causes shrinkage of the plastic block; to cut uniform slices of this thickness, it is found that the fluence per image should not exceed approximately 20 e/nm2.
SEMs that incorporate a focused ion beam (FIB) milling capability, rather than the built-in microtome of the SBF-SEM offer an alternative approach for generating large 3D volumes of ultrastructural data (Heymann et al., 2006; de Winter et al., 2009; Hekking et al., 2009; Heymann et al., 2009; Villinger et al., 2012; Narayama and Submramaniam, 2015). FIB-SEMs provide an improved z-resolution relative to SFB-SEM because, in principle, arbitrarily thin layers of the specimen can be eroded by the ion beam. An important advantage of the FIB-SEM technique is that multiple 3D regions of the block can be analyzed sequentially because each 3D dataset only results in local destruction of the sample block (Weiner et al., 2016). With SBF-SEM, several different regions can be analyzed by scanning multiple areas after each successive cut of the block face, but these different regions have to be selected before the acquisition is started. However, it takes longer to remove a given volume of the specimen by FIB milling than by slicing it off the block with the diamond knife of the SBF-SEM ultramicrotome. Therefore, for very large volumes, the SBF-SEM offers an important advantage.
It has been reported that block face images revealing cellular ultrastructure can be obtained from frozen hydrated specimens in a cryo FIB-SEM by imaging with the secondary electron signal, but the contrast mechanism of secondary electrons emitted from the native frozen block face is influenced by subtle differences in surface potential, which can be difficult to understand quantitatively (Schertel et al., 2013). Cryo FIB-SEM has also been applied successfully to biomineralization studies for which there is high BSE contrast originating from the higher atomic number of the mineral phase (Vidavskya et al., 2016; Sviben et al., 2016)
Sample preparation for both SBF-SEM and FIB-SEM differs substantially from that used for standard TEM (Holcomb et al., 2013; Kremer et al., 2015; Kizilyaprak et al, 2015; He et al., 2016). To avoid the problem of electrical charging in the SEM while maintaining optimal spatial resolution, it is necessary to introduce sufficient heavy atom stain to make the specimen conductive. In addition, a high concentration of stain is required to provide sufficient backscattered electron signal to reveal cellular ultrastructure. Even when the maximum level of stain is introduced into the specimen, sometimes electrical charging cannot be avoided when the SBF-SEM is running in high vacuum mode. In that case, electrical charge build-up can be eliminated by introducing a controlled level of nitrogen gas into the SEM column (at a pressure of 5 to 30 Pa), although operating the SEM in variable pressure mode can result in poorer spatial resolution since the probe diameter is broadened and the landing energy is decreased due to the probe’s interaction with the gas. It has recently been demonstrated, however, that charge build-up in the sample can be mitigated without loss of spatial resolution by focal injection of nitrogen gas through a needle positioned close to the block-face during imaging (Deerinck et al., 2017).
1.2. 3D Imaging based on STEM Tomography
Axial bright-field STEM tomography provides an entirely different approach for imaging the 3D ultrastructure of fixed, stained and plastic-embedded cells. A schematic diagram of the experimental setup (Fig. 1B) shows a focused electron probe of energy 300 keV, which is incident on a thick (1 to 2 μm) plastic section. When a transmission electron microscope (TEM) is operated in STEM mode, there are no imaging lenses after the specimen, so that the chromatic aberration of the objective lens does not compromise the spatial resolution in the presence of strong multiple inelastic scattering (Yakushevska et al., 2007; Aoyama et al., 2008; Hyun et al., 2008; Hohmann-Marriott et al., 2009; Sousa et al., 2009; Sousa et al., 2011). However, parameters pertaining to the probe-forming lens must be optimized. In this regard, it is necessary to reduce the geometrical broadening of the electron probe by selecting a much smaller convergence semi-angle than the one typically used for STEM. Use of a small probe convergence semi-angle of ~1–2 mrad results in large depth-of-field extending several micrometers, i.e., throughout the entire specimen thickness, and broadening of the probe diameter to ~1–2 nm by diffraction is not a limiting factor for the attainable spatial resolution. It is preferable to collect scattered electrons that exit the specimen at angles close to the optical axis by using an axial bright-field (BF) detector, rather than an annular dark-field (ADF) detector, which is commonly used for STEM imaging (Fig. 1B). This is because the BF detector rejects a large fraction of electrons that have undergone multiple elastic scattering involving high scattering angles, and those electrons therefore have a broader lateral spatial distribution as they reach the lower surface of the specimen. It is important to point out that the BF detector does not reject multiple scattering events involving small angles, but those electrons have a narrower lateral spatial distribution as they travel through the specimen. In contrast, the ADF detector collects a large fraction of the high-angle scattering events, and this has been shown to result in degraded spatial resolution particularly in the lower half of micrometer-thick specimens (Sousa et al., 2009). Axial BF STEM tomographic reconstructions obtained from two orthogonal (dual-axis) tilt series display an isotropic pixel size of ~3 to 5 nm. The total electron fluence used to acquire these dual-axis STEM tomograms is about 104 incident electrons per nm2, which is about a factor of 500 higher than that required for SBF-SEM (Sousa et al., 2011). This difference in susceptibility of the specimen to damage is expected since scattering cross sections are roughly inversely proportional to the primary energy, i.e., ~1 keV for SBF-SEM and ~300 keV for BF-STEM tomography. Although the cross section for damage-induced mass loss is much greater at low primary electron energies, so is the cross section for generating the elastic BSE signal. Therefore, in terms of radiation damage, it is not immediately obvious which one of the two techniques has an advantage, but this is addressed in the discussion below.
For optimal staining of thick plastic-embedded samples for BF-STEM tomography, it is usually necessary to use a lower level of heavy atom staining relative to that used for preparation of conventional TEM specimens (He et al., 2016). This reduces attenuation of the transmitted electron probe due to elastic scattering in 1–2 μm-thick specimens, as they are tilted to 60° during acquisition of a tomographic tilt series, with an effective doubling in thickness. Thus, it is often advantageous to use only osmium tetroxide to stain membranes lightly, together with a low level of uranyl acetate, making the staining protocol for STEM tomography very different from that of SBF-SEM. In addition, en bloc staining is often required for STEM tomography, which can produce higher stain concentrations towards the surfaces than in the middle of the section. This unevenness in stain distribution is not present in samples prepared for SBF-SEM.
Axial BF-STEM tomography has been applied to determine the structure of diverse biological systems ranging from prokaryotes (Wolf et al., 2014; Hickey et al., 2017) to eukaryotic cells, including malaria-infected erythrocytes (Hohmann-Marriott et al., 2009), and blood platelets (Pokrovskaya et al., 2016). It has also been applied to complex tissues such as retina (Graydon et al., 2015), and cultured hippocampal neurons (Chen et al., 2015; Sweeney et al., 2017).
2. Materials and Methods
2.1. Specimen Preparation
Platelets were obtained from human donors following an IRB-approved protocol (Pokrovskaya et al., 2016). To suppress activation, the resting platelets were fixed with glutaraldehyde within five minutes of blood draw. After fixation, the samples were either stained and processed for SBF-SEM imaging (Holcomb et al., 2013) or were subjected to high pressure freezing and freeze substitution for BF-STEM tomography (Pokrovskaya et al., 2016).
For SBF-SEM, platelets were stained as previously described (Holcomb et al., 2013). In brief, a platelet pellet was fixed with 0.1 M cacodylate buffer containing 2.5% glutaraldehyde and 2 mM calcium chloride for 1 h in ice. The pellet was re-suspended and washed three times with cold 0.1 M sodium cacodylate buffer containing 2 mM calcium chloride and spun at 600 g for 5 minutes. Samples were fixed in reduced osmium containing 3% potassium ferrocyanide in 0.3 M cacodylate buffer with 4 mM calcium chloride with an equal volume of 4% aqueous tetroxide for 1 h in ice. Samples were then placed in a 0.22 μm-Millipore-filtered 1% thiocarbohydrazide (TCH) solution in double-distilled water for 20 minutes following 5 washes with double-distilled water at room temperature each for 3 minutes. Samples were fixed in 2% osmium tetroxide in double-distilled water for 30 minutes at room temperature following 5 washes with double-distilled water at room temperature each for 3 minutes. Samples were then placed in 1% uranyl acetate (aqueous) and left overnight at 4 °C. The next day, samples were washed five times with double-distilled water at room temperature each for 3 minutes and processed for en bloc Walton’s lead aspartate staining. Samples were placed in lead aspartate solution and were transferred to a 60°C oven for 30 minutes, following five washes with double-distilled water at room temperature each for 3 minutes. Samples were then dehydrated and processed for resin embedding.
For BF STEM tomography, plastic blocks of embedded platelets, prepared by fixation in glutaraldehyde and osmium tetroxide, were sectioned to a thickness of 1.5 μm. Sections were lightly post-stained with 0.2% uranyl acetate in ethanol for 2 h. Sections were deposited onto 200-mesh bare copper grids, which were carbon-coated and gold nanoparticles of diameter 20 nm were applied to both sides of the grid.
2.2. SBF-SEM
Blocks of embedded platelets were trimmed and mounted on aluminum pins such that the mounted side of each block had the heavily stained cellular material in contact with the pin to prevent charging; the pin was then sputter-coated with ~40 nm of gold. Image stacks were acquired using a Zeiss Sigma VP SEM (Carl Zeiss Microscopy, US), equipped with a Gatan 3View SBF system (Gatan Inc., US) operating in high vacuum mode at primary beam energy of 1.1 keV with a 1 μs per pixel dwell time. Each image stack contained 2000 x 2000 x 250 voxels with a voxel dimension of 6.8 nm (x-direction) × 6.8 nm (y-direction) × 30 nm (z-direction), giving the total acquired volume of ~1,400 μm3. Following alignment of the image stacks using Digital Micrograph software (Gatan Inc., US), the platelets were segmented manually in Amira (FEI, Visage Imaging). Using these segmentations, the models were surface-rendered and further quantitative analyses were performed.
2.3. Axial BF-STEM Tomography
Imaging was performed at 300 kV in a Tecnai TF30 (Thermo Fisher Scientific, Hillsboro, US) field-emission TEM, equipped with a Gatan BF/DF STEM detector (Gatan, Pleasanton, California, United States) using a probe semi-convergence angle of 1–2 mrad (Sousa et al., 2009; Sousa et al., 2011). Dual-axis tomographic tilt series were acquired by tiling to ±68° with a 2° angular increment on each orthogonal axis, with each image containing 2,048 × 2,048 pixels with a pixel size of 3.31 nm. The tomograms were reconstructed using weighted back projection (IMOD software, University of Colorado), binned by a factor of 2 in x, y and z, and then post-processed with an anisotropic diffusion filter. 3D models were created in Amira (Thermo Fisher Scientific, Hillsboro, US) via manual segmentation and surface rendering.
3. Results and Discussion
As summarized in Table 1, many different parameters affect the performance of SBF-SEM and axial BF-STEM tomography, and the optimal choice between these two approaches might not be obvious to the structural biologist. To compare their advantages and limitations, we find it helpful to consider separately the factors that relate to (1) specimen geometry, (2) specimen preparation, (3) electron scattering processes, and (4) image acquisition and processing methods used to extract 3D ultrastructure. The 3D ultrastructure of human blood platelets is used to illustrate the comparison between the two approaches. In addition, consideration of the parameters enables us to assess which types of technical improvement might enhance future performance. Our present aim is to demonstrate that the task of analyzing statistical variations between organelles is one that can be suitably addressed by both SBF-SEM and axial BF-STEM tomography. For a discussion of the biological findings that can be obtained from 3D electron microscopy of blood platelets, the reader is referred to Pokrovskaya et al., (2016) and Yadav et al., (2017).
Table 1.
Comparison of sample preparation, data acquisition, and data processing between STEM tomography and SBF-SEM
| Parameter╲Technique | Serial block face SEM | BF-STEM tomography |
|---|---|---|
| Cell / tissue volume | ~ 103 – 106 μm3 | ~ 101 – 102 μm3 |
| Specimen shape | solid block | thick sections cut to thickness of 1 – 2 μm |
| Acquisition mode | image stack | dual-axis tilt series (~ ±70°) with ~2° angular increment |
| Specimen preparation | conventional fixation or freeze substitution; heavy staining with Os, U, Pb; plastic embedding | conventional fixation or freeze-substitution; light staining with Os, U; plastic embedding |
| Incident electron energy | 1.0 – 1.5 keV | 200 – 300 keV |
| Minimum voxel size | 5 nm in x, y 25 nm in z | 3 – 5 nm approximately isotropic |
| Acquisition time | ~ 103 −105 s | ~ 102 −103 s |
| Processing time for reconstruction | — | ~ 103 – 104 s |
| Reconstruction method | — | weighted back-projection SIRT, compressed sensing |
| Processing time for segmentation | ~ 105 −106 s | ~ 104 −105 s |
| Electron fluence | < 20 electrons / nm2 | < 104 electrons / nm2 after initial pre-irradiation |
| Effect of electron fluence | shrinkage of block during acquisition of image stack; irregular cutting | shrinkage normal to section plane prior to acquisition of tilt series; shortened voxels along z -axis |
| Artifacts image processing | electrical charging of block | missing wedge or missing pyramid; reconstruction errors |
3.1. Specimen Geometry
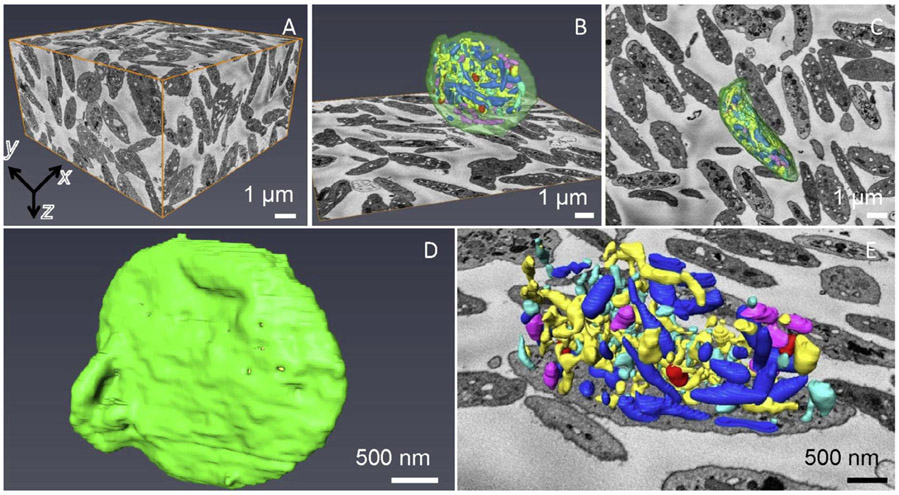
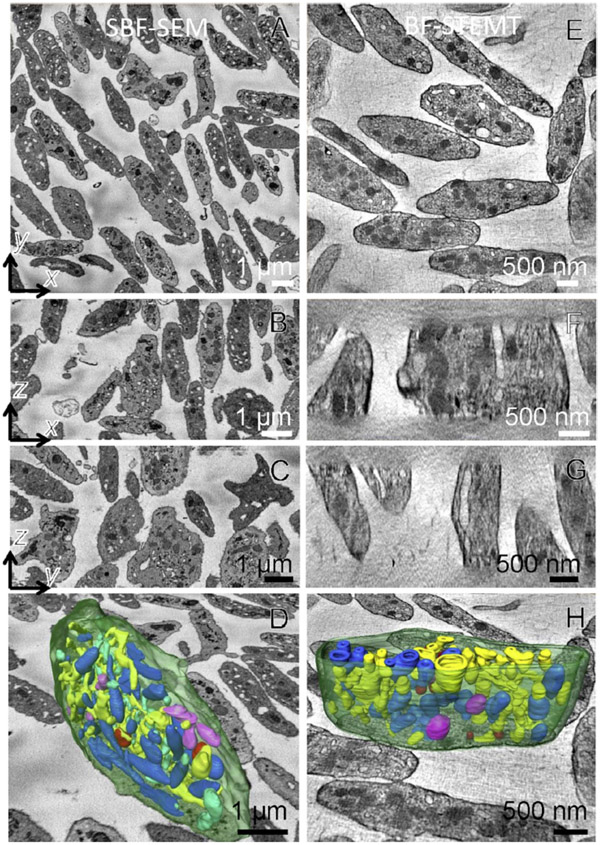
SBF-SEM enables acquisition of 3D ultrastructural data from embedded blocks ranging from volumes of 103 μm3 to volumes as large as 106 μm3, with a minimum useful voxel size of ~5 nm in the x, y plane and ~25 nm along the z-axis. The limitations of spatial resolution will be discussed below. In the present context, we are interested in assessing the capabilities of the technique for the minimum SBF-SEM voxel size, which we illustrate by considering block face data acquired from 250 slices at a cutting increment of 30 nm (i.e., a depth into the block of 7.5 μm), with a scanned area of 13.5 μm × 13.5 μm, corresponding to a total volume of 1,400 μm3. As illustrated in Fig. 2A, this volume includes some 100 complete platelets, in addition to many incomplete cells. By analyzing data volumes of this size, it is possible to assess the statistical variations among the volumes and shapes of entire platelets, as well as the distributions of volumes, shapes, and numbers of specific organelles within these cells, quantities that are of interest to platelet researchers. The capabilities of the technique are illustrated in Fig. 2B - 2E), which shows surface rendering after manual segmentation in one platelet, including (i) open canalicular system (yellow), (ii) closed canalicular system (cyan), (iii) mitochondria (purple), (iv) alpha granules (blue), (v) dense granules (red), (vi) dense cores (burgundy), and (vii) plasma membrane (green). Low magnification views of a segmented platelet are shown in Figs. 2B and 2C. The segmented outer plasma membrane in Fig. 2D indicates the overall shape of the platelet, whereas a magnified visualization of the organelles in Fig. 2E reveals the complexity of interconnected membranes inside the cell.
Figure 2.
SBF-SEM acquired 3D data of human resting platelets. (A) xy, yz and xz planes (orthoslices) define a volume of 7.5 μm in z, containing more than one hundred platelets. Each voxel is 6.8 nm x 6.8 nm x 30 nm. A complete platelet surface rendering in xz (B) and xy (C) views, together with an xy orthoslice, which shows organization of different membrane-bound organelles: open canalicular system (yellow), closed canalicular system (cyan), mitochondria (purple), alpha granules (blue), dense granules (red), dense cores (burgundy), plasma membrane (green). (D) Segmented outer plasma membrane of a complete platelet. (E) Magnified segmented platelet organelles without the membrane together with the xy orthoslice.
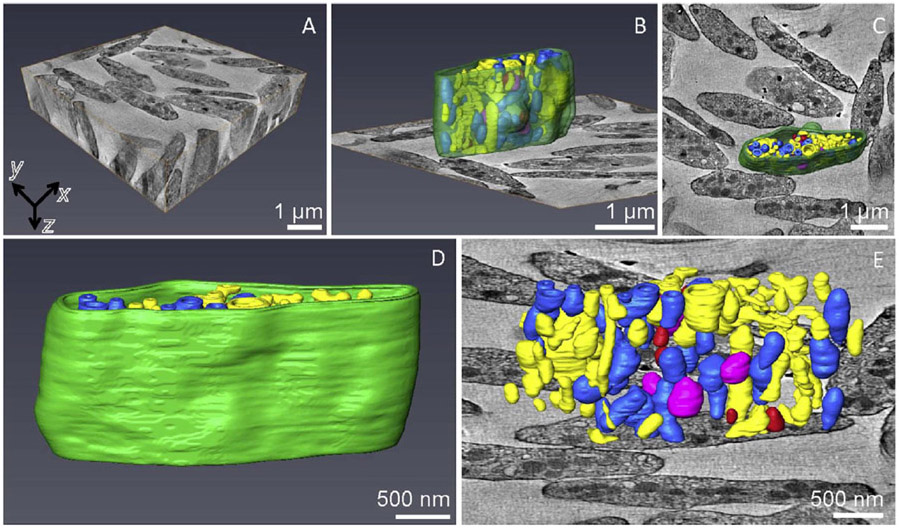
BF STEM tomography provides an improvement in spatial resolution with a minimum useful isotropic voxel size of ~3 nm in x, y, and z. The reconstructed volume in Fig. 3A contains resting platelets from a plastic section cut to a thickness of 1.5 μm, obtained by acquisition of dual-axis tilt series with a scanned area in the x, y plane of 6 μm × 6 μm. This volume is a factor of ~25 smaller than that imaged in the SBF-SEM dataset. Whereas no complete platelets are found in the BF-STEM tomographic reconstruction, many complete cells are found in the 3D volume obtained by SBF-SEM. This is explained by the minimum dimension of the platelets being comparable with the section thickness of 1.5 μm and, with random orientation of the cells, it is unlikely that a platelet would be exactly contained within the section thickness. We found several cells with 1/2 to 3/4 of their volumes contained within the section, but such a determination can only be made after the tomographic reconstruction. Surface rendering of the organelles within such a platelet (Figs. 3B - 3E) reveals the ultrastructure with a smaller voxel size and higher spatial resolution than is obtainable by SBF-SEM.
Figure 3.
BF STEM tomography of human resting platelets. (A) Full dataset from a 1.5 μm thick plastic section (in z), which contains roughly 10 partial platelets with isotropic voxels of dimension 6.6 nm in x, y, z) Surface rendering of one of the platelets in xz (B) and xy (C) views together with the xy orthoslice, which shows organization of different membrane-bound organelles. Color key is same as for Fig. 2. (D) Segmented outer plasma membrane of a partial platelet. (E) Magnified segmented platelet organelles without the membrane together with the xy orthoslice.
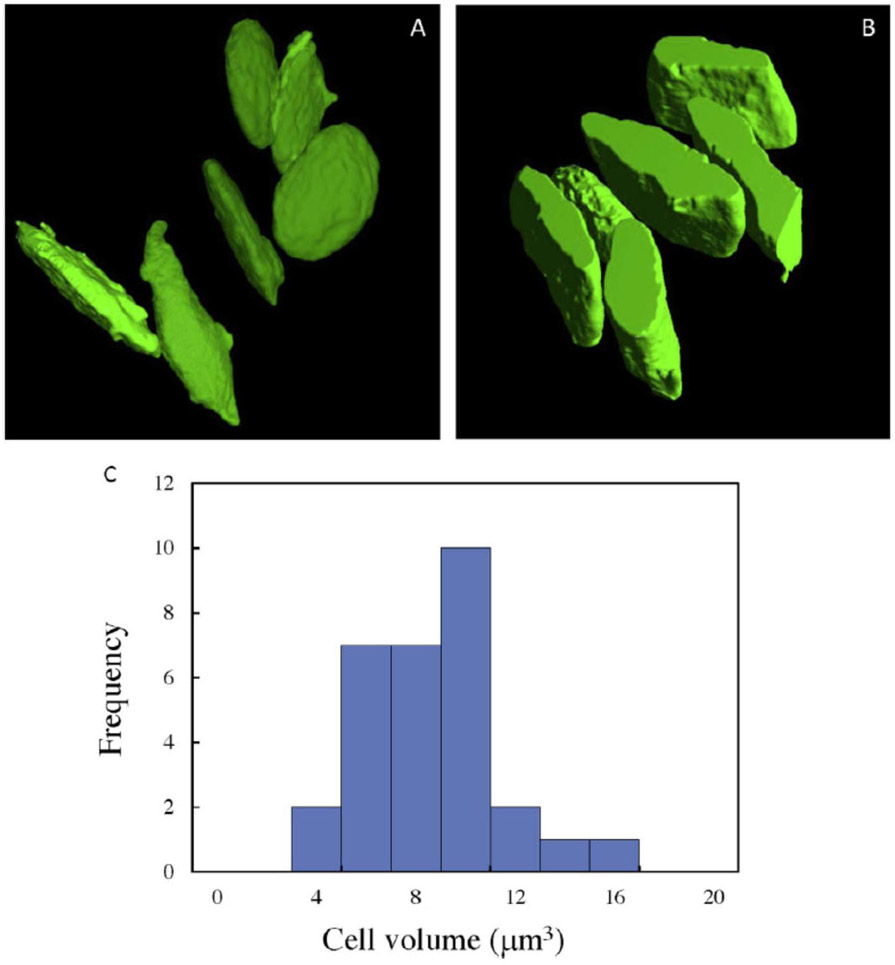
The characteristic platelet shape is evident from the volumes generated by SBF-SEM and STEM tomography in Fig. 4A and 4B, respectively, which show the surface rendered plasma membranes from six unactivated cells in each dataset. The distributions of platelet volumes from 30 cells obtained by SBF-SEM (Fig. 4C) gives a mean volume of 7.7 ± 2.6 μm3 (± std. dev.). Since each volume can be measured with high accuracy, the uncertainty in the mean volume from a large population of cells can be estimated in terms of the standard error of the mean, or ± 0.5 μm3. It is evident from the volume generated by STEM tomography in Fig. 4B that not one entire platelet is found, even in sections that are 1.5 μm in thickness. In fact, it is rare to find more than half of a platelet’s volume in the STEM tomography reconstructions. This result illustrates the importance of 3D block face techniques such as SBF-SEM for determining cell volume.
Figure 4.
Surface rendered plasma membrane of six unactivated platelets obtained by (A) SBF-SEM, and (B) STEM tomography reveals the characteristic platelet shape. In (B) only about half of the platelets are contained within the 1.5 μm-thickness of the plastic section. (C) Histogram of platelet volumes from 30 cells, obtained by SBF-SEM, with mean value of 7.7 μm3 ± 2.6 μm3 (± std. dev.).
Orthoslices through the 3D volumes in the x,y and x,z planes obtained by SBF-SEM (Figs. 5A - 5D) and by STEM tomography (Figs. 5E - 5H) show similar subcellular structure at low magnifications, and this enables most of the cellular organelles in the platelet to be segmented as shown in Figs. 5D and 5H for SBF-SEM and STEM tomography, respectively.
Figure 5.
Comparison of SBF-SEM and BF-STEM tomography techniques. Portion of the dataset is shown in two columns for SBF-SEM and BF-STEM tomography. xy, xz and yz planes for SBF-SEM (A-C) and BF-STEM tomography (E-G), respectively. The outlined region contains a surface rendered cell for SBF-SEM in (D) and BF-STEM tomography in (H).
3.2. Analysis of the platelet cananicular system
To illustrate the relative capabilities of SBF-SEM and STEM tomography for obtaining quantitative geometrical parameters of subcellular organelles, we consider a system of membrane compartments in the platelet, known as the canalicular system (CS). From a comparison of 2D x,y slices through volumes generated by the two techniques (Fig. 6), it is evident that both SBF-SEM (Fig. 6A) and STEM tomography (Fig. 6D) show similar structural detail, as expected since the x,y pixel size is approximately the same (~7 nm) in both datasets. Differences in contrast in the x,y images obtained by the two techniques can be attributed to differences in the specimen preparation protocols. In this regard, the incorporation of higher concentrations of uranium and lead in the SBF-SEM preparation diminishes the relative contrast of the membranes compared with the contrast observed in STEM tomography datasets.
Figure 6.
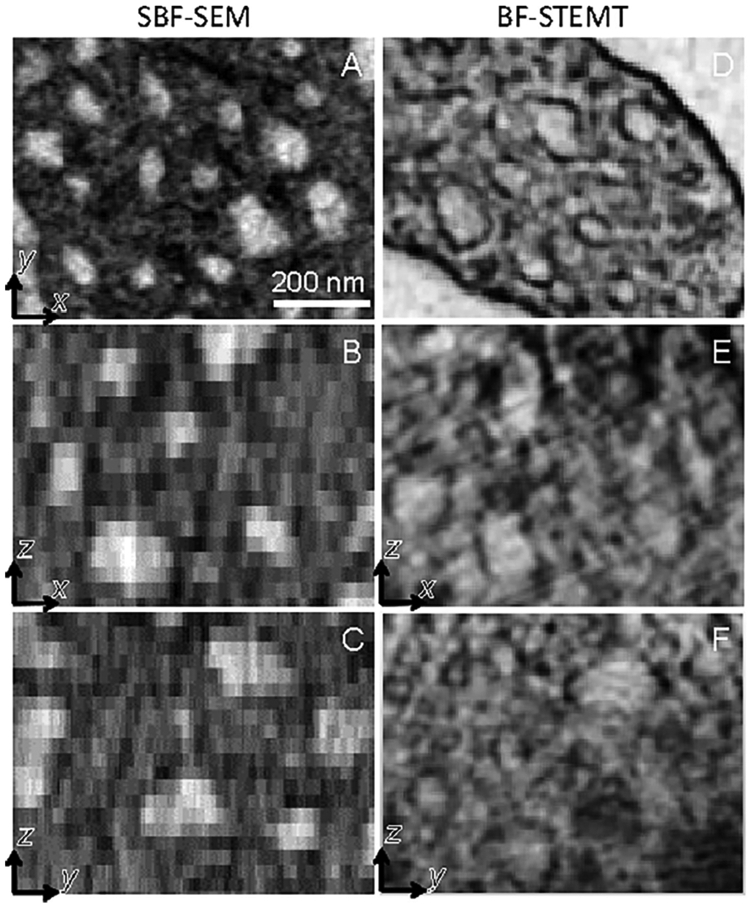
Slices at higher magnification through platelets in the xy, xz and yz planes containing membranes of the canalicular system obtained by SBF-SEM (A-C) and by BF-STEM tomography (D-F). Elongation of the voxels in the z-direction is evident in the SBF-SEM data.
Although the CS can be easily visualized in x,y slices of the SBF-SEM dataset, it is more difficult to visualize the CS in x,z slices because the 30-nm z-distance between successive cuts is commensurate with the dimension of the CS compartments (between 30 nm and 100 nm in dimension). This limitation is evident in Figs. 6B and 6C, where the CS is blurred along the z-direction resulting in loss of information and inability to segment all CS membranes throughout the 3D volume. Since the STEM tomography reconstructions have isotropic pixels, it is possible to visualize CS not only in the x,y slices but also in the x,z slices (Figs. 6D - 6F).
To circumvent the limitations of the extended voxel size along the z-direction, it is possible to extract quantitative values for the CS surface area SCS and volume VCS per unit volume of platelet from the SBF-SEM data by applying a stereological approach to the x,y slices in the 3D volume (Russ and Dehoff, 2000):
| (1) |
| (2) |
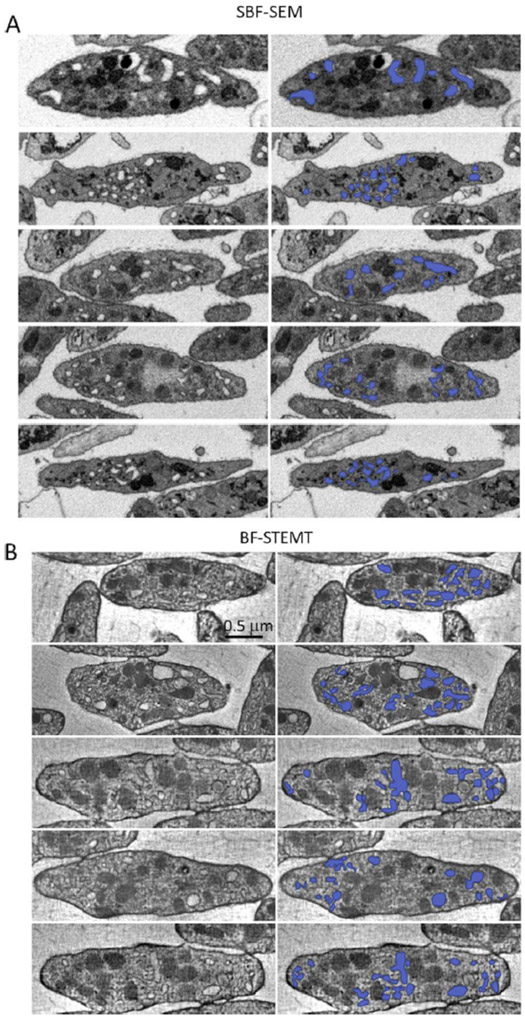
where the sums are over the line contours (LCS)nm and areas (ACS)nm of the n = 1…N regions of CS within a cross section m of a cell of area (Acell)m, and Vcell is the mean platelet volume averaged over the m = 1….M cross sections. Eqs. 1 and 2 are only valid if a sufficiently large number of cross-sectioned cells M are analyzed and the measurements averaged. Fig. 7A shows the segmentation of CS compartments for five representative cross sections through the SBF-SEM dataset and, for comparison, Fig. 7B shows the segmentation for five representative orthoslices through the STEM tomography dataset. The distributions of CS compartments (colored blue in Fig. 7) obtained with the two techniques appear similar.
Figure 7.
Segmentation of canalicular system membranes in five representative slices (A) through the SBF-SEM dataset, and (B) through the STEM tomography dataset. Images on left are shown without segmentation, and images on the right with segmentation in blue.
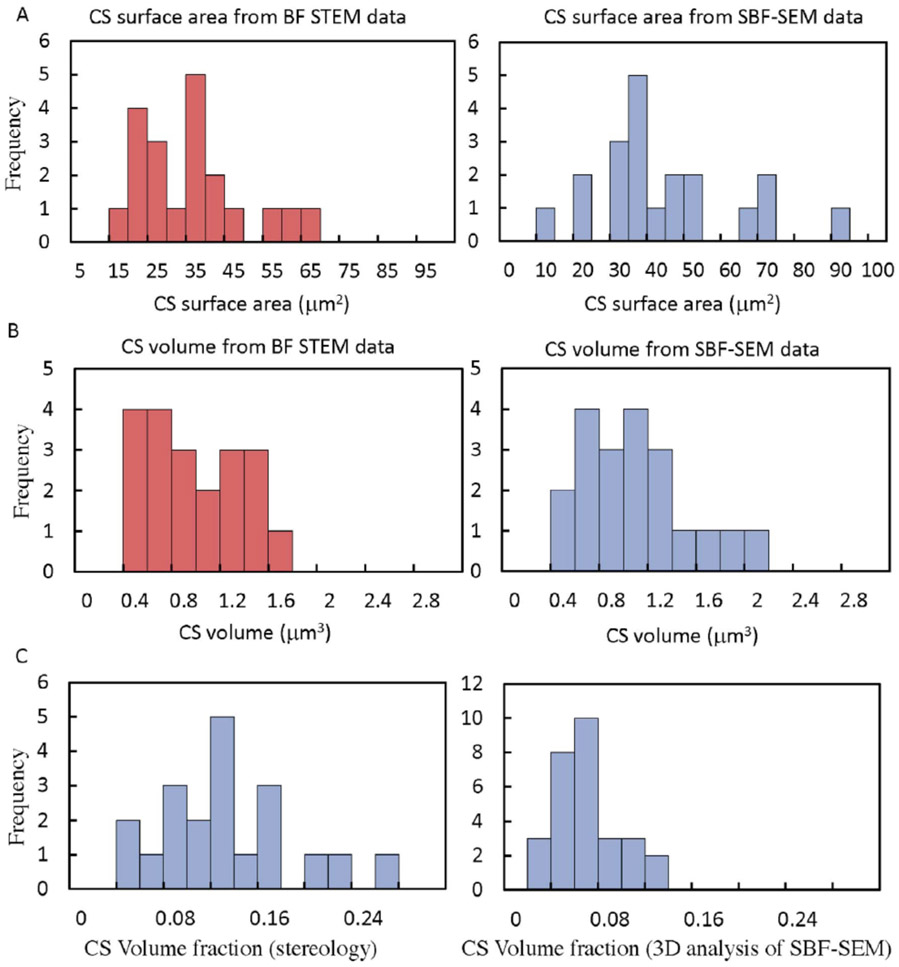
We analyzed 20 random cross sections though different cells in STEM tomography and SBF-SEM datasets, and have plotted the values of and , as histograms in Fig. 8. From Eq. 1, the stereological measurements give an average CS surface area per platelet of 31.1 μm2, ± 13.9 μm2 (± std. dev.) from STEM tomography (Fig. 8A, red plot), and an average CS surface area per platelet of 39.4 μm2 ± 19.4 μm2 (± std. dev.) from SBF-SEM (Fig. 8A, blue plot). From Eq. 2, the stereological measurements give an average CS volume per platelet of 0.79 μm3, ± 0.38 μm3 (± std. dev.) from STEM tomography (Fig. 8B, red plot), and an average CS volume per platelet of 0.89 μm3 ± 0.43 μm3 (± std. dev.) from SBF-SEM (Fig. 8B, blue plot). Stereological measurements from STEM tomography and from SBF-SEM are thus in agreement to within experimental error. The large standard deviation in the measurements can be attributed to variation in morphology in the random x-y orthoslices through the platelets. Since results are obtained from 20 orthoslices for each technique, the standard error of the mean (not shown) probably gives a more reliable estimate of the uncertainty in the measurements of CS surface area and volume per platelet.
Figure 8.
Histogram of stereological measurements on 20 random cross sections through platelets obtained from STEM tomography and SBF-SEM datasets: (A) shows use of stereological approach for calculating the CS surface area per platelet from STEM tomography data (red plot), and from SBF-SEM data (blue plot); (B) shows use of stereological approach for calculating the CS volume per platelet from STEM tomography data (red plot), and from SBF-SEM data (blue plot); (C) compares determination of the platelet CS volume fraction using stereological measurements on the SBF-SEM dataset (plot on left), and using 3D analysis of the segmented CS compartments in the same dataset (plot on right).
A comparison of 2D stereological measurements of the CS volume fraction obtained from the SBF-SEM dataset with direct 3D measurements from the same dataset in Fig. 8C, reveals that the stereological measurements (Fig. 8C, plot on left side) gives a mean volume fraction of 0.12 ± 0.06 (± std. dev.), which is more than a factor of two larger than the volume fraction of 0.05 ± 0.03 (± std. dev.) obtained from 3D measurements of the segmented volume (Fig. 8C, plot on right side). This failure of 3D analysis of the SBF-SEM data to provide quantitation of the CS volume is attributed to missing data due to elongated voxels in the z-direction, which makes it difficult to connect segmented regions in successive slices.
Although SBF-SEM provides accurate quantitative 3D structural information from membrane-bound organelles of dimension greater than ≳100 nm (e.g., Denk and Horstmann, 2004; Helmstaedter et al., 2013), the current work shows that care should be taken in analyzing systems of membranes with dimensions ≲ 100 nm, such as the CS in blood platelets, by using solely 3D segmentation methods. This is because of missing data in the z-direction, which can prevent accurate segmentation. However, it is possible to obtain quantitative information from smaller organelles like the platelet CS by combining stereological measurements on 2D orthoslices with full 3D segmentation of the cell volume. This approach resembles the one we used previously to analyze secretory granules in pancreatic islets of Langerhans (Shomorony et al., 2015).
3.3. Specimen Considerations
As described in the Materials and Methods, optimal staining protocols for SBF-SEM and BF-STEM tomography differ considerably. In SBF-SEM, the aim is two-fold: first, to generate at the block face as much signal as possible originating from backscattered electrons; and second, to incorporate as much metal stain as possible into the block to avoid electrical charging. As discussed earlier, to avoid electrical charge build-up in the specimen block and the need to operate the SEM in variable pressure mode, it is desirable to introduce as much heavy atom stain as possible while also avoiding stain artifacts that might disrupt the ultrastructure.
In BF-STEM tomography, the transmitted electrons provide projections of the ultrastructure at different tilt angles. Even though BF-STEM tomography is performed at primary beam energies of 200–300 keV (compared with ~1 keV for SBF-SEM), the technique requires a relatively low density of stain, so that information is retained in the projections through several micrometers of section thickness. The effect of stain concentration on the maximum section thickness in BF-STEM tomography is highly nonlinear since the transmitted beam is attenuated exponentially as a function stain concentration (He et al., 2016). This explains why BF-STEM tomography at a primary energy of 300 keV can only be applied to specimens of thickness 1–2 μm (or 2–4 μm effective thickness when tilted to 60°). Specimens prepared for SBF-SEM would be opaque in this thickness range.
3.4. Electron Scattering and Beam Damage
In the SBF-SEM, the low-energy (~1.0 to 1.5 keV) primary electron beam is scattered by heavy atoms of stain to provide a backscattered signal from atoms within 10 to 30 nm of the block face (Hennig and Denk, 2007; Bouwer et al., 2016). This distance is determined mainly by inelastic scattering processes which reduce the electron energies so that the electrons are unable to escape from the surface and contribute to the backscattered electron signal; this therefore defines the depth of each voxel in the z-direction. The minimum voxel dimension in the x, y plane is determined not only by lateral spreading of the probe by elastic scattering but also by radiation damage considerations.
It is found that radiation damage limits the maximum electron fluence that can be delivered to the block face in SBF-SEM and, for a primary beam energy of 1.5 keV, the maximum fluence is approximately 20 electrons/nm2 for Epon/Araldite or Durcupan embedded blocks (Kizilyaprak et al., 2015; Pfeifer et al., 2015; Shomorony et al., 2015). For fluences exceeding 20 electrons / nm2, shrinkage of the block causes the diamond knife to cut erratically, and results in discontinuities in the x-z and y-z planes in the generated 3D volumes.
In BF-STEM tomography, the specimen is pre-irradiated with a broad TEM beam at an electron fluence > 104 electrons/nm2, so that shrinkage of the block occurs before acquisition of the tomographic tilt series, which are acquired with a total fluence of approximately 104 electrons/nm2. It is found that shrinkage of the 1–2 μm thick sections tends to occur normal to the plane of the section (along the z-direction) and is typically 10–20% with negligible shrinkage in the x,y plane of the section in reasonable agreement with previous data (de Jonge et al., 2010; Aronova et al., 2010; Kizilyaprak et al., 2015). This results in some shortening of the voxels along the z-direction, which can be corrected if necessary after 3D reconstruction. The fractional shrinkage of the 1–2 μm thick sections is much smaller than that observed for thinner (<200 nm thick) sections, which can be as high as 50%, but the explanation for this difference in behavior is not clear.
3.5. Image Processing
In SBF-SEM, the stack of images acquired after each successive cut of the block directly represents the 3D volume, with the z-resolution determined by the slice thickness, so that it is only necessary to align the images, which can easily be accomplished by cross correlation without further processing. However, methods have been developed to obtain sub-slice thickness resolution by sequentially acquiring images at multiple primary beam energies from each block face. This sub-slice thickness resolution can be achieved because different primary energies penetrate different distances into the block; the resulting images acquired at different primary beam energies can be processed to improve the z-resolution by at least a factor of two lower than the slice thickness (He et al., 2017).
Image processing in BF-STEM is more complicated and involves tomographic reconstruction. In this regard, many laboratories including ours have used the IMOD software developed at the University of Colorado at Boulder (Kremer et al., 1996; Mastronarde, 1997). Reconstructions may be performed using weighted back-projection (WBP), simultaneous iterative reconstruction technique (SIRT), or regularization methods based on a compressed sensing (CS) approach [Saghi et al. 2016; Guay et al., 2016]. Tomographic reconstructions from a limited range of tilt angles can be susceptible to missing wedge or, in the case of dual-axis tilt series, missing pyramid artifacts. Such effects result in some loss of resolution along the z-direction in the 3D reconstructions.
The limiting factor for both techniques is the time required to segment the 3D volumes, rather than the acquisition time in SBF-SEM or the reconstruction time in BF-STEM tomography. Many laboratories, including ours, currently use manual segmentation techniques to trace contours of organelles in each slice of the 3D volume, and then employ software such as Amira to obtain surface rendered visualizations of the organelles. This can be enormously time-consuming, and can take several months to segment a single 3D dataset. Other laboratories make use of semi-automated software (Kreshuk et al., 2011; Kreshuk et al., 2014; Perez et al., 2014), which can be very helpful to reduce the time required for segmentation. Machine learning techniques based on convolutional neural networks now give automated annotation of cellular cryo electron tomograms (Chen et al., 2107). This approach offers a vast improvement in time required to segment 3D volumes, and is likely to transform the capabilities of SBF-SEM and other 3D electron microscopy techniques, including BF-STEM tomography (Guay et al., 2017).
Acknowledgments
This work was supported by the intramural research program at the National Institute of Biomedical Imaging and Bioengineering at the National Institutes of Health in Bethesda, Maryland. The research performed in the Storrie laboratory at the University of Arkansas for Medical Sciences in Little Rock, Arkansas was funded in part by NIH grant R01 HL119393.
References
- Anttonen T, Kirjavainen A, Belevich I, Laos M, Richardson WD, Jokitalo E, Brakebusch C, Pirvola U, 2012. Cdc42-dependent structural development of auditory supporting cells is required for wound healing at adulthood. Sci. Rep 2: 978. DOI: 10.1038/srep00978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyama K, Takagi T, Hirase A, Miyazawa A, 2008. STEM tomography for thick biological specimens. Ultramicroscopy 109, 70–80. [DOI] [PubMed] [Google Scholar]
- Aronova MA, Sousa AA, Zhang G, Leapman RD, 2010. Limitations of beam damage in electron spectroscopic tomography of embedded cells. J. Microsc 239(3), 223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck M, Baumeister W, 2016. Cryo-electron tomography: can it reveal the molecular sociology of cells in atomic detail? Trends Cell Biol. 26(11), 825–837. [DOI] [PubMed] [Google Scholar]
- Boughorbel F, Kooijman CS, Lich BH, Bosch EGT, 2012. SEM imaging method, US Patent 8232523.
- Bouwer JC, Deerinck TJ, Bushong E, Astakhov V, Ramachandra R, Peltier ST, Ellisman MH, 2016. Deceleration of probe beam by stage bias potential improves resolution of serial block-face scanning electron microscopic images. Adv. Struct. Chem. Imag 2(11), 1–13. DOI 10.1186/s40679-016-0025-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggman KL, Helmstaedter M, Denk W, 2011. Wiring specificity in the direction-selectivity circuit of the retina. Nature 471, 183–188. [DOI] [PubMed] [Google Scholar]
- Briggman KL, Denk W, 2006. Towards neural circuit reconstruction with volume electron microscopy techniques. Curr. Opin. Neurobiol 16, 562–570. [DOI] [PubMed] [Google Scholar]
- Carson JL, 2014. Fundamental technical elements of freeze-fracture/freeze-etch in biological electron microscopy. J. Vis. Exp 91, 51694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Dai W, Sun SY, Jonasch D, He CY, Schmid MF, Chiu W, Ludtke SJ, 2017. Convolutional neural networks for automated annotation of cellular cryo-electron tomograms. Nat. Methods 14(10), 983–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Levy JM, Hou A, Winters C, Azzam R, Sousa AA, Leapman RD, Nicoll RA, Reese TS, 2015. PSD-95 family MAGUKs are essential for anchoring AMPA and NMDA receptor complexes at the postsynaptic density. Proc. Natl. Acad. Sci. USA 112(50), E6983–E6992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deerinck TJ, Bushong EA, Thor A, Ellisman MH, 2010. NCMIR Methods for 3D EM: A new protocol for preparation of biological specimens for serial block face scanning electron microscopy. (National Center for Microscopy and Imaging Research). [Google Scholar]
- Deerinck TJ, Shone TM, Bushong EA, Ramachandra R, Peltier ST, Ellisman MH, 2017. High-performance serial block-face SEM of nonconductivebiological samples enabled by focal gas injection-based charge compensation. J. Microsc (in press) doi: 10.1111/jmi.12667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Goede M, Johlin E, Sciacca B, Boughorbel F, Garnett EC, 2017. 3D multi-energy deconvolution electron microscopy. Nanoscale 9, 684–689. [DOI] [PubMed] [Google Scholar]
- de Jonge N, Sougrat R, Northan BM, Pennycook SJ, 2010. Three-dimensional scanning transmission electron microscopy of biological specimens. Microsc. Microanal 16(1), 54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denk W, Horstmann H, 2004. Serial block-face scanning electron microscopy to reconstruct three-dimensional tissue nanostructure. PLoS Biol. 2(11), e329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do M, Isaacson SA, McDermott G, Le Gros MA, Larabell CA, 2015. Imaging and characterizing cells using tomography. Arch. Biochem. Biophys 581, 111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glancy B, Hartnell LM, Malide D, Yu Z-X, Combs CA, Connelly PS, Subramaniam S, Balaban RS, 2015. Mitochondrial reticulum for cellular energy distribution in muscle. Nature 523, 617–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graydon CW, Zhang J, Oesch NW, Sousa AA, Leapman RD, Diamond JS, 2015. Passive diffusion as a mechanism underlying ribbon synapse vesicle release and resupply. J. Neuroscience 34, 8948–8962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grünewald K, Desai P, Winkler DC, Heymann JB, Belnap DM, Baumeister W, Steven AC, 2003. Three-dimensional structure of herpes simplex virus from cryo-electron tomography. Science 302, 1396–1398. [DOI] [PubMed] [Google Scholar]
- Guay MD, Czaja W, Aronova MA, Leapman RD, 2016. Compressed sensing electron tomography for determining biological structure. Sci. Rep 6, 27614. doi: 10.1038/srep27614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guay MD, Zeyad E, Anderson A, Leapman RD, 2017. Exploring Deep Neural Network Architectures for Automated Electron Micrograph Segmentation. Biophys. J. Suppl (in press). [Google Scholar]
- Hanssen E, Knoechel C, Klonis N, Abu-Bakar N, Deed S, LeGros M, Larabell C, Tilley L 2011. Cryo transmission X-ray imaging of the malaria parasite, P. falciparum. J. Struct. Biol 173, 161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayworth KJ, Morgan JL, Schalek R, Berger DR, Hildebrand DGC, Lichtman JW, 2014. Imaging ATUM ultrathin section libraries with WaferMapper: a multi-scale approach to EM reconstruction of neural circuits. Front. Neural. Circuits 8, 68 (epub pp. 1–18). [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Q, Leapman RD, 2016. Choice of specimen thickness in axial bright-field STEM tomography of cells. Microsc. Microanal 22(suppl. 3), 1138–1139. [Google Scholar]
- He Q, Joy DC, Zhang G, Leapman RD, 2017. Sub-surface serial block face of biological structures at near isotropic spatial resolution. Biophys. J 108(2) Suppl. 1, 619a–620a. [Google Scholar]
- Hekking LHP, Lebbink MN, de Winter DAM, Schneijdenberg CTWM, Brand CM, Humbel BM, Verkleij AJ, Post JA, 2009. Focused ion beam-scanning electron microscope: exploring large volumes of atherosclerotic tissue. J. Microsc 235, 336–347. [DOI] [PubMed] [Google Scholar]
- Helmstaedter M, Briggman KL, Turaga SC, Jain V, Seung HS, Denk W, 2013. Connectomic reconstruction of the inner plexiform layer in the mouse retina. Nature 500, 168–174. [DOI] [PubMed] [Google Scholar]
- Hennig P, Denk W, 2007. Point-spread functions for backscattered imaging in the scanning electron microscope. J. Appl. Phys 102, 123101. [Google Scholar]
- Heymann JAW, Hayles M, Gestmann I, Giannuzzi LA, Lich B, Subramaniam S, 2006. Site-specific 3D imaging of cells and tissues with a dual beam microscope. J. Struct. Biol 155, 63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heymann JAW, Shi D, Kim S, Bliss D, Milne JLS, Subramaniam S, 2009. 3D imaging of mammalian cells with ion-abrasion scanning electron microscopy. J. Struct. Biol 166, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey WJ, Shetty AR, Massey RJ, Toso DB, Austin J, 2017. Three-dimensional bright-field scanning transmission electron microscopy elucidate novel nanostructure in microbial biofilms. J. Microsc 265(1), 3–10. [DOI] [PubMed] [Google Scholar]
- Hohmann-Marriott M, Sousa AA, Azari AA, Glushakova S, Zhang G, Zimmerberg J, Leapman RD, 2009. Nanoscale 3D cellular imaging by axial scanning transmission electron tomography. Nat. Methods 6(10), 729–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holcomb PS, Hoffpauir BK, Hoyson MC, Jackson DR, Deerinck TJ, Marrs GS, Dehoff M, Wu J, Ellisman MH, Spirou GA, 2013. Synaptic inputs compete during rapid formation of the Calyx of Held: a new model system for neural development. J. Neurosci 33(32), 12954–12969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyun JK, Ercius P, Muller DA, 2008. Beam spreading and spatial resolution in thick organic specimens. Ultramicroscopy 109, 1–7. [DOI] [PubMed] [Google Scholar]
- Khursigara CM, Wu X, Subramaniam S, 2008. Chemoreceptors in Caulobacter crescentus: trimers of receptor dimers in a partially ordered hexagonally packed array. J. Bacteriol 190(20), 6805–6810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King S, Reed G, 2002. Development of platelet secretory granules. Sem. Cell Devel. Biol 13(4), 293–302. [DOI] [PubMed] [Google Scholar]
- Kittlemann M, Hawes C, Hughes L, 2016. Serial block face scanning electron microscopy and the reconstruction of plant cell membrane systems. J. Microsc 263(2), 200–211. [DOI] [PubMed] [Google Scholar]
- Kizilyaprak C, Longo G, Daraspe J, Humbel BM, 2015. Investigation of resins suitable for the preparation of biological sample for 3-D electron microscopy. J. Struct. Biol 189, 135–146. [DOI] [PubMed] [Google Scholar]
- Kremer A, Lippens S, Bartunkova S, Asselbergh B, Blanpain C, Fendrych M, Goossens A, Holt M, Janssens S, Krols M, Larsimont J, McGuire C, Nowack M, Saelens X, Schertel A, Schepens B, Slezak M, Timmerman V, Theunis C, Van Brempt R, Visser Y, Guérin C, 2015. Developing 3D SEM in a broad biological context. J. Microsc 259(2), 80–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer JR, Mastronarde DN, McIntosh JR, 1996. Computer visualization of three-dimensional image data using IMOD. J. Struct. Biol 116, 71–76. [DOI] [PubMed] [Google Scholar]
- Kreshuk A, Straehle CN, Sommer C, Koethe U, Cantoni M, Knott G, Hamprecht FA, 2011. Automated detection and segmentation of synaptic contacts in nearly isotropic serial electron microscopy images. PLoS One 6(10), e24899. doi: 10.1371/journal.pone.0024899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreshuk A, Koethe U, Pax E, Bock DD, Hamprecht FA, 2014. Automated detection of synapses in serial section transmission electron microscopy image stacks. PLoS One 9(2), e87351. doi: 10.1371/journal.pone.0087351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leighton SB, 1981. SEM images of block faces, cut by a miniature microtome within the SEM—A technical note. Scan. Electron Microsc 2, 73–76. [PubMed] [Google Scholar]
- Liu J, Bartesaghi A, Borgnia MJ, Sapiro G, Subramaniam S, 2008. Molecular architecture of native HIV-1 gp 120 trimers. Nature 455(7209), 109–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maco B, Cantoni M, Holtmaat A, Kreshuk A, Hamprecht F, Knott G, 2014. Semiautomated correlative 3D electron microscopy of in vivo-imaged axons and dendrites. Nat. Prot 9, 1354–1366. [DOI] [PubMed] [Google Scholar]
- Mahamid J, Pfeffer S, Schaffer M, Villa E, Danev R, Cuellar JK, Förster F, Hyman A A.A., Plitzko JM, Baumeister W, 2017. Visualizing the molecular sociology at the HeLa cell nuclear periphery. Science 351(6276), 969–972. [DOI] [PubMed] [Google Scholar]
- Marko M, Hsieh C, Schalek R, Frank J, Mannella C, 2007. Focused-ion beam thinning of frozen-hydrated biological specimens for cryo-electron microscopy. Nat. Methods 4, 215–217. [DOI] [PubMed] [Google Scholar]
- Mastronarde DN, 1997. Dual-axis tomography: an approach with alignment methods that preserve resolution. J. Struct. Biol 120, 343–352. [DOI] [PubMed] [Google Scholar]
- Mourik MJ, Faas FGA, Zimmermann H, Eikenboom J, Koster AJ, 2015. Towards the imaging of Weibel-Palade body biogenesis by serial block face-scanning electron microscopy. J. Microsc 259(2), 97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan K, Subramaniam S, 2015. Focused ion beams in biology. Nat. Methods 12(11), 1021–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez AJ, Seyedhosseini M, Deerinck TJ, Bushong EA, Panda S, Tasdizen T, Ellisman MH, 2014. A workflow for the automatic segmentation of organelles in electron microscopy image stacks. Frontiers Neuroanat. 8, article 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer CR, Shomorony A, Aronova MA, Zhang G, Cai T, Xu H, Notkins AL, Leapman RD, 2015. Quantitative analysis of mouse pancreatic islet architecture by serial block-face SEM. J. Struct. Biol 189(1), 44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokrovskaya I, Aronova MA, Kamykowski J, Prince A, Hoyne J, Calco G, Kuo B, He Q, Leapman RD, Storrie B, 2016. STEM tomography reveals that the canalicular system and α-granules remain separate compartments during early secretion stages in blood platelets. J. Throm. Haem 14(3), 572–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigort A, Bäuerlein FJB, Villa E, Eibauer M, Laugks T, Baumeister W, Plitzko JM, 2012. Focused ion beam micromachining of eukaryotic cells for cryoelectron tomography. Proc. Natl. Acad. Sci. USA 109(12), 4449–4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russ JC, Dehoff RT (2000). Practical Stereology (2nd Ed.), publ. Springer Science + Business Media, New York, p. 257. ISBN 978–1-4613–5453-6. [Google Scholar]
- Saghi Z, Divitini G, Winter B, Leary R, Spiecker E, Ducati C, Midgley PA, 2016. Compressed sensing electron tomography of needle-shaped biological specimens – Potential for improved reconstruction fidelity with reduced dose. Ultramicroscopy 160, 230–238. [DOI] [PubMed] [Google Scholar]
- Schertel A, Snaidero N, Han H-M, Ruhwedel T, Laue M, Grabenbauer M, Möbius W, 2013. Cryo FIB-SEM: Volume imaging of cellular ultrastructure in native frozen specimens. J. Struct. Biol 184, 355–360. [DOI] [PubMed] [Google Scholar]
- Shami GJ, Cheng D, Huynh M, Vreuls C, Wisse E, Braet F, 2016. 3-D EM exploration of the hepatic microarchitecture – lessons learned from large-volume in situ serial sectioning. Sci. Rep 6, 36744. DOI: 10.1038/srep36744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shomorony A, Pfeifer C, Aronova M, Zhang G, Cai T, Xu H, Notkins A, Leapman RD, 2015. Combining quantitative 2D and 3D image analysis in the serial block face SEM: applications to secretory organelles of pancreatic islet cells. J. Microsc 259(2), 155–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa AA, Hohmann-Marriott MF, Zhang G, Leapman RD, 2009. Monte Carlo electron-trajectory simulations in bright-field and dark-field STEM: implications for tomography of thick biological sections. Ultramicroscopy 109, 213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa AA, Azari AA, Zhang G, Leapman RD, 2011. Dual-axis electron tomography of biological specimens: Extending the limits of specimen thickness with bright-field STEM imaging. J. Struct. Biol 174, 107–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sviben S, Gal A, Hood MA, Bertinetti L, Politi Y, Bennet M, Krishnamoorthy P, Schertel A, Wirth R, Sorrentino A, Pereiro E, Faivre D, Scheffel A, 2016. A vacuole-like compartment concentrates a disordered calcium phase in a key coccolithophorid alga. Nat. Commun 7 11228. DOI: 10.1038/ncomms11228∣www.nature.com/naturecommunications. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney AM, Fleming KE, McCauley JP, Rodriguez MF, Martin ET, Sousa AA, Leapman RD, Scimemi A, 2017. PAR1 activation induces rapid changes in glutamate uptake and astrocyte morphology. Sci. Rep 7, 43606. DOI: 10.1038/srep43606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidavskya N, Addadi S, Schertel A, Ben-Ezra D, Muki Shpigel M, Addadi L, Weiner S, 2017. Calcium transport into the cells of the sea urchin larva in relation to spicule formation. Proc. Natl. Acad. Sci. USA 113(45), 12637–12642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villinger C, Schauflinger M, Gregorius H, Kranz C, Höhn K, Nafeey S, Walther P, 2014. Three-dimensional imaging of adherent cells using FIB/SEM and STEM. In: Kuo John (ed.), Electron Microscopy: Methods and Protocols, Methods in Molecular Biology, vol. 1117, DOI 10.1007/978-1-62703-776-1_27, publ. Springer Science+Business Media, New York. [DOI] [PubMed] [Google Scholar]
- Wacker I, Chockley P, Bartels C, Spomer W, Hofmass A, Gengenbach U, Singh S, Thaler M, Grabher C, Schröder RR, 2015. Array tomography: characterizing FAC-sorted populations of zebrafish immune cells by their 3D ultrastructure. J. Microsc 259(2), 105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner A, Mellouk N, Lopez-Montero N, Chang Y-Y, Souque C, Schmitt C, Enninga J, 2016. Macropinosomes are key players in early Shigella invasion and vacuolar escape in epithelial cells. PLoS Pathog 12(5), e1005602. doi: 10.1371/journal.ppat.1005602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West JB, Fu X, Deerinck TJ, Mackey MR, Obayashi JT, Ellisman MH, 2010. Structure–function studies of blood and air capillaries in chicken lung using 3D electron microscopy. Respir. Physiol. Neurobiol 170, 202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf SG, Houben L, Elbaum M, 2014. Cryo-scanning transmission electron tomography of vitrified cells. Nat. Methods 11(4), 423–428. [DOI] [PubMed] [Google Scholar]
- Yadav S, Williamson JK, Aronova MA, Prince AA, Pokrovskaya ID, Leapman RD, Storrie B, 2017. Golgi proteins in circulating human platelets are distributed across non-stacked, scattered structures. Platelets 28(4), 400–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakushevska AE, Lebbink MN, Geerts WJC, Spek L, van Donselaar EG, Jansen KA, Humbel BM, Post JA, Verkleij AJ, Koster AJ, 2007. STEM tomography in cell biology. J. Struct. Biol 159, 381–391. [DOI] [PubMed] [Google Scholar]
- Zhu L, Almac J, Dadi PK, Hong H, Sakamoto W, Rossi M, Lee RJ, Vierra NC, Lu H, Cui Y, McMillin SM, Perry NA, Gurevich VV, Lee A, Kuo B, Leapman RD, Matschinsky FM, Doliba NM, Urs NM, Caron1 MG, Jacobson DA, Caicedo A, Wess J, 2017. Beta-arrestin-2 is an essential regulator of pancreatic beta-cell function under physiological and pathophysiological conditions. Nat. Commun 8, 14295. doi: 10.1038/ncomms14295. [DOI] [PMC free article] [PubMed] [Google Scholar]