Abstract
Background and purpose:
To determine rates of xerostomia after intensity-modulated radiotherapy (IMRT) or intensity-modulated proton therapy (IMPT) for oropharyngeal cancer (OPC) and identify dosimetric factors associated with xerostomia risk.
Materials and methods:
Patients with OPC who received IMRT (n = 429) or IMPT (n = 103) from January 2011 through June 2015 at a single institution were studied retrospectively. Every 3 months after treatment, each patient completed an eight-item self-reported xerostomia-specific questionnaire (XQ; summary XQ score, 0–100). An XQ score of 50 was selected as the demarcation value for moderate-severe (XQs ≥50) and no-mild (XQs <50) xerostomia. The mean doses and percent volumes of organs at risk receiving various doses (V5-V70) were extracted from the initial treatment plans. The dosimetric variables and xerostomia risk were compared using an independent-sample t-test or chi-square test.
Results:
The median follow-up time was 36.2 months. The proportions of patients with moderate-severe xerostomia were similar in the two treatment groups up to 18 months after treatment. However, moderate-severe xerostomia was less common in the IMPT group than in the IMRT group at 18–24 months (6% vs. 20%; p = 0.025) and 24–36 months (6% vs. 20%; p = 0.01). During the late xerostomia period (24–36 months), high dose/volume exposures (V25-V70) in the oral cavity were associated with high proportions of patients with moderate-severe xerostomia (all p < 0.05), but dosimetric variables regarding the salivary glands were not associated with late xerostomia.
Conclusion:
IMPT was associated with less late xerostomia than was IMRT in OPC patients. Oral cavity dosimetric variables were related to the occurrence of late xerostomia.
Keywords: Intensity-modulated radiotherapy, Intensity-modulated proton therapy, Xerostomia, Oropharyngeal cancer
Radiotherapy is one of the main treatment modalities for oropharyngeal cancer (OPC), especially human papillomavirus-associated OPC [1–4]. However, radiotherapy can cause severe xerostomia, which can dramatically and irreversibly impair quality of life [5]. Intensity-modulated radiotherapy (IMRT), which markedly reduces the incidence of xerostomia when compared with conventional radiotherapy, is now considered the standard radiotherapy technique for head and neck cancer [6, 7]. However, IMRT is inherently limited by the physical properties of photon beams and still inevitably results in xerostomia.
Protons have a physical advantages over photons in that they can deposit high energy at tumor targets but virtually no exit dose to normal tissues beyond the tumor. This physical advantage has led to great interest in the application of proton therapy for head and neck cancer because of the numerous critical normal structures in that region [8–13]. Treatment-planning comparisons have demonstrated that intensity-modulated proton therapy (IMPT) results in lower radiation doses to organs at risk (OARs) than does IMRT in head and neck cancer patients [14–16]. Recent studies of IMRT have shown that delivery of reduced radiation doses to the bilateral parotid glands, contralateral submandibular gland, and oral cavity is correlated with improvement of xerostomia [17, 18]. However, the literature contains few comparisons of xerostomia in OPC patients receiving IMRT and IMPT. Authors have reported improved xerostomia during the subacute phase with IMPT versus IMRT, but how this relates to long-term xerostomia is uncertain [10, 19]. To answer this question, we retrospectively reviewed patients with OPC to compare rates of xerostomia until 6 months after treatment and late xerostomia after IMRT versus IMPT and to identify potential associations between salivary gland or oral cavity dosimetric characteristics and subsequent occurrence of xerostomia in both treatment groups.
Materials and methods
Patient selection
Patients with OPC receiving photon-based IMRT or scanning-beam IMPT at MD Anderson from January 2011 to June 2015 were identified. Different radiotherapy techniques were selected based on the patients preference. Consecutive patients were included in the study if they met the following criteria: 1) a tissue diagnosis of squamous cell carcinoma originating in the oropharynx, 2) no prior radiotherapy, 3) no evidence of distant metastases, and 4) available xerostomia assessments after completion of radiotherapy. The final cohort used for this analysis was composed of 532 patients (429 who received IMRT and 103 who received IMPT) who fulfilled the eligibility criteria.
All patients’ data are from part of a large-scale Institutional Review Board approved programmatic prospective symptom survey. Study-specific informed consent was provided by all participants, who then completed the MD Anderson Symptom Inventory-Head and Neck module (MDASIHN). The patients treated with IMPT in this study also participated in two consecutive Institutional Review Board-approved prospective studies (ClinicalTrials.org identifiers: NCT00991094 and NCT01627093) [20, 21].
Radiotherapy
All patients underwent computed tomography-based treatment simulation and treatment delivery while supine and immobilized in a customized mold of the posterior head, neck, and shoulders. Delineation of the target volumes was described previously [22]. Briefly, OARs and treatment targets (gross tumor volume, clinical target volume [CTV], and planning target volume) were contoured. All contours in all cases were reviewed for quality assurance by a team of head and neck radiation oncology experts.
Doses were prescribed and referred to as either absolute absorbed photon dose or absorbed proton dose weighted according to a constant relative biological effectiveness of 1.1. For patients receiving concurrent chemotherapy, the prescribed dose delivered to the CTV1 was 70 Gy in 2.12-Gy fractions, that delivered to the CTV2 was 63 Gy in 1.9-Gy fractions, and that delivered to the CTV3 was 57 Gy in 1.7-Gy fractions. For patients receiving radiotherapy without chemotherapy, the prescribed dose delivered to the CTV1 was 66 Gy in 2.2-Gy fractions, that delivered to the CTV2 was 60 Gy in 2-Gy fractions, and that delivered to the CTV3 was 54 Gy in 1.8-Gy fractions. The specific dose constraints for the OARs are shown in Supplementary Table S1.
IMPT planning was performed with an Eclipse treatment-planning system (version 8.9; Varian Medical Systems, Palo Alto, CA, USA). Plan-specific quality assurance measurements were performed before treatment delivery [23]. IMPT was delivered with proton therapy equipment (Hitachi, Ltd., Tokyo, Japan, and Hitachi America, Ltd., Tarrytown, NY, USA). Planar X-rays were used for verifying the positioning of IMPT. Verification computed tomography scans were obtained at weeks 1 and 4 of therapy, and adaptive treatment replanning was considered if inadequate doses were delivered to the targets or OARs. The evaluation of adaptive replanning was as described previously [24]. Briefly, Deformable image registrations were performed between the 2 CT image sets, and the CTVs and major OARs were transferred to the verification CT images to generate the adaptive plan. The summation doses were obtained from the original and adaptive plans on the verification CT. IMRT planning was designed using a Pinnacle system (version 6.2b or later; Philips Medical Systems, Amsterdam, Netherlands). IMRT was delivered as 6-MV photons using linear accelerators (Varian Medical Systems, Palo Alto, CA, USA) with daily imaging guidance [25]. Cone-beam computed tomography and planar X-rays were used for verifying the positioning of IMRT. Adaptive treatment replanning was not performed for patients who underwent IMRT.
Chemotherapy
One hundred eighty-eight (35%) patients received neoadjuvant chemotherapy with platinum- and taxane-based regimens. Three hundred sixty-nine (69%) patients given concurrent chemoradiotherapy received platinum drugs with or without cetuximab.
Patient-reported xerostomia-specific questionnaire
All patients were asked to complete a validated xerostomia-specific questionnaire (XQ) about every 3 months after treatment for up to 36 months [26]. The XQ measures the severity of radiation-induced xerostomia and its effects on patients’ quality of life. The questionnaire consists of eight questions: four on aspects of mouth dryness while eating or chewing and four on mouth dryness in the absence of eating or chewing. The XQ was self-administered, and patients were asked to rate each symptom on a scale of 0–10, with higher scores indicating more severe dryness or discomfort due to dryness. Scores for each item were added, and the sum was transformed linearly to produce final summary scores ranging from 0 to 100. Scores of 0, 25, 50, 75, and 100 approximated reflected “no” “mild/slight,” “moderate,” “severe,” and “extreme,” respectively. Based on this, an XQ score of 50 was selected as the demarcation value for moderate-severe (XQ≥50) and no-mild (XQ<50) xerostomia [27].
Statistical analysis
Basic demographic variables, clinical disease stage, human papillomavirus status, and treatment-related information were compared in the patients given IMRT (n = 429) and IMPT (n = 103). Categorical variables were compared using a chi-square test or the Fisher exact test. The dosimetric parameters, percent volume of the salivary glands (parotid and submandibular) or oral cavity receiving doses of 5 to 70 Gy in 5-Gy intervals (V5-V70), and mean dose (Dmean) were extracted from the initial radiotherapy planning report. An independent t-test was used to compare the dosimetric parameters in the moderate-severe and no-mild xerostomia patients and in those receiving IMRT and IMPT. Multivariate analysis of the relationship of late xerostomia (moderate-severe xerostomia at 18–24 or 24–36 months) with all clinical co-factors was accomplished via binary logistic regression. The clinical variables included sex, age (≤60 vs >60 years), race, disease site, radiotherapy technology, use of induced or concurrent chemotherapy, primary tumor stage, and lymph node stage. Statistical analyses were performed using SPSS software (version 24; IBM, Armonk, NY), and p values less than 0.05 were considered statistically significant.
Results
The patient characteristics are shown in Table 1. We observed no significant differences between IMRT and IMPT group, regarding age, sex, race, tumor site, location, clinical stage, human papillomavirus status, or chemotherapy received.
Table 1.
Comparison of characteristics between IMRT and IMPT groups by Chi square test
| No. of Patients (%) | P Value | ||
|---|---|---|---|
| IMRT (n=429) | IMPT (n=103) | ||
| Age, years | |||
| Median(range) | 59 (32–84) | 60 (33–85) | |
| ≤60 | 247 (57.6) | 54 (52.4) | 0.38 |
| >60 | 182 (42.4) | 49 (47.6) | |
| Sex | |||
| Female | 61 (14.2) | 13 (12.6) | 0.75 |
| Male | 368 (85.8) | 90 (87.4) | |
| Race | |||
| White | 385 (89.7) | 95 (92.2) | 0.58 |
| Other | 44 (10.3) | 8 (7.8) | |
| Disease site | |||
| Base of tongue | 206 (48.0) | 53 (51.4) | 0.58 |
| Tonsil/other | 223 (52.0) | 50 (48.6) | |
| Tumor location | |||
| Left | 183 (42.7) | 55 (53.4) | 0.085 |
| Right | 239 (55.7) | 45 (43.7) | |
| Midline | 2 (0.5) | 1 (1.0) | |
| Bilateral | 5 (1.1) | 2 (1.9) | |
| T category | |||
| T1–2 | 292 (68.1) | 67 (65.0) | 0.56 |
| T3–4 | 137 (31.9) | 36 (35.0) | |
| N category | |||
| N0–1 | 76 (17.7) | 24 (22.9) | 0.21 |
| N2–3 | 353 (82.3) | 79 (77.1) | |
| HPV status | |||
| Positive | 295 (68.8) | 79 (76.7) | 0.16 |
| Negative | 56 (13.1) | 6 (5.8) | |
| Equivocal | 15 (3.5) | 2 (1.9) | |
| Not detected | 63 (14.6) | 16 (15.5) | |
| Induction CT | |||
| Yes | 158 (36.8) | 30 (29.1) | 0.17 |
| No | 271 (63.2) | 73 (70.9) | |
| Concurrent CT | |||
| Yes | 289 (67.4) | 79 (76.7) | 0.075 |
| No | 140 (32.6) | 24 (23.3) | |
Abbreviations: IMRT, intensity-modulated (photon) radiation therapy; IMPT, intensity-modulated proton therapy; HPV, human papillomavirus; CT, chemotherapy; T, tumor; N, lymph node.
The median follow-up time for all patients was 36.2 months (35.8 months for the IMRT group vs. 37.7 months for the IMPT group). The 532 study patients completed 1449 XQs during the follow-up period. The numbers of patients who completed the XQ at different times in both of the treatment groups are shown in Table 2.
Table 2.
The numbers and mean XQ scores of patients who filled out the questionnaire at each time point by treatment group.
| Number of patients | Mean XQ scores | SD | ||||||
|---|---|---|---|---|---|---|---|---|
| Time intervals | IMRT | IMPT | IMRT | IMPT | D-value | IMRT | IMPT | P value |
| 0–3 months | 266 | 80 | 39.7 | 38.0 | +1.7 | 24.8 | 22.1 | 0.58 |
| 3–6 months | 130 | 41 | 36.6 | 36.5 | +0.1 | 23.8 | 22.8 | 1.00 |
| 6–9 months | 130 | 54 | 36.5 | 37.8 | −1.3 | 22.6 | 20.0 | 0.72 |
| 9–12 months | 88 | 42 | 30.8 | 27.9 | +2.9 | 23.0 | 18.6 | 0.48 |
| 12–18 months | 142 | 47 | 28.7 | 24.0 | +4.7 | 22.4 | 18.6 | 0.20 |
| 18–24 months | 138 | 53 | 29.0 | 22.4 | +6.6 | 22.4 | 16.8 | 0.051 |
| 24–36 months | 172 | 65 | 27.3 | 22.7 | +4.6 | 23.5 | 17.4 | 0.15 |
Abbreviations: IMRT, intensity-modulated (photon) radiation therapy; IMPT, intensity-modulated proton therapy; XQ, xerostomia questionnaire; D-value, difference value of mean XQ scores between IMRT and IMPT (IMRT-IMPT); SD, Standard deviation.
The mean XQ scores for the IMRT and IMPT groups at seven intervals after treatment are also shown in Table 2. For the interval ≥ 6 months, the median value was analyzed for patients who had XQ scores at three time points. The scores were not markedly different between the two groups at any of the intervals, although the scores seemed to be lower in the IMPT group than in the IMRT group later in the follow-up period. This may be indicated that more patients in the IMRT group had higher XQ scores than those in the IMPT group after one year of radiotherapy.
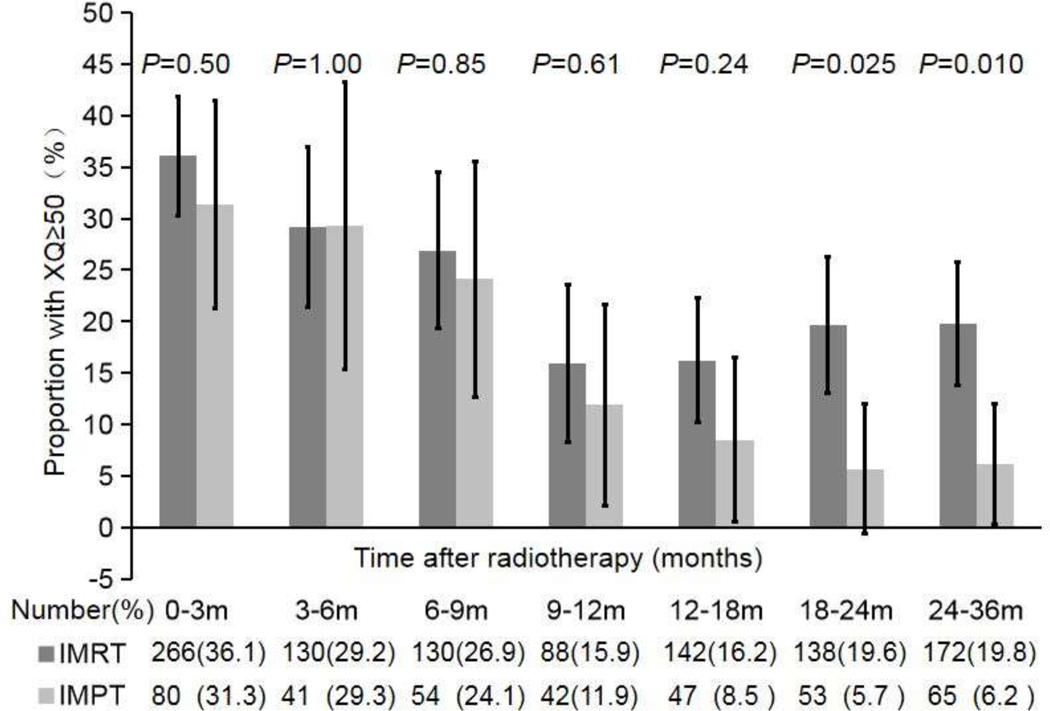
To further test this supposition, we compared the proportions of patients with moderate-severe xerostomia in the two treatment groups (Fig. 1). No difference in the proportions was evident up to 18 months after treatment. However, at 12–18 months, the proportions began to separate (16% for the IMRT group vs. 9% for the IMPT group; p = 0.24), and by 18–24 months, fewer patients in the IMPT group had moderate-severe xerostomia (3 of 53 [6%] patients vs. 27 of 138 [20%] patients in the IMRT group; p = 0.025), for a difference of 14%. At 24–36 months, this difference between the groups was maintained, with 4 of 65 (6%) patients in the IMPT group having moderate-severe xerostomia versus 34 of 172 (20%) patients in the IMRT group, for a difference of 14% (p = 0.01). Multivariate analysis demonstrated that IMPT was a significant factor reducing late moderate-severe xerostomia at 18–24 months (p = 0.017; 0.218 [0.062–0.765]) and 24–36 months (p = 0.017; 0.264 [0.088–0.789]) (Supplementary Table S2).
Fig. 1.
The proportions of patients with moderate-severe xerostomia (XQs ≥50) in both treatment groups (IMRT and IMPT) at the indicated times after treatment. The total numbers of patients in each group are shown under the graph. The p values are for comparison of the proportions of patients with moderate-severe xerostomia in the two groups (chi-square test). The error bars indicate 95% confidence intervals.
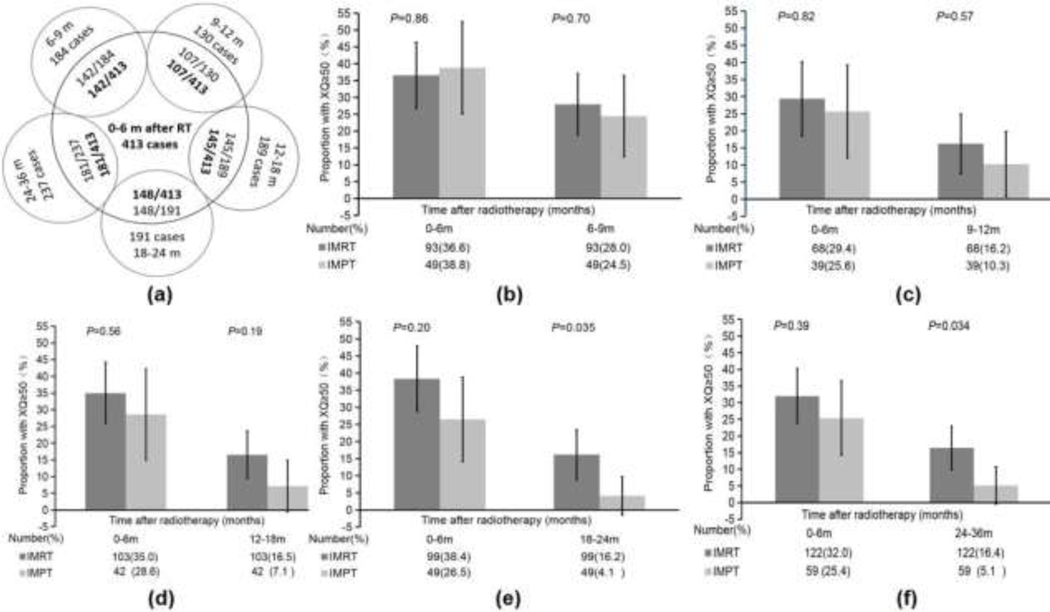
We also used the 413 patients with available XQ scores until 6 months after treatment as a reference and compared them with the patients who had XQ scores available at each interval thereafter. In total, 142 patients had XQ scores at 0–6 and 6–9 months, 107 had XQ scores at 0–6 and 9–12 months, 145 had XQ scores at 0–6 and 12–18 months, 148 had XQ scores at 0–6 and 18–24 months, and 181 had XQ scores at both 0–6 and 24–36 months (Fig. 2A). Among the 142 patients with XQ scores at 0–6 and 6–9 months, the proportions of those with moderate-severe xerostomia were not different in the IMPT and IMRT groups at 0–6 months (IMPT, 38% vs. IMRT, 37%; p = 0.86) or 6–9 months (IMPT, 25% vs. IMRT, 28%; p = 0.70) (Fig. 2B). Among the 107 patients with XQ scores at 0–6 and 9–12 months, the proportions of those with moderate-severe xerostomia were not different between the IMPT and IMRT groups at 0–6 months (IMPT, 26% vs. IMRT, 29%; p = 0.82) or 9–12 months (IMPT, 10% vs. IMRT, 16%; p = 0.57) (Fig. 2C). We found this similarity among the 145 patients with XQ scores at 0–6 and 12–18 months, as the proportions at 12–18 months were 7% in the IMPT group and 17% in the IMRT group (p = 0.19) (Fig. 2D). However, the IMPT group had a significantly smaller proportion of patients with moderate-severe xerostomia than did the IMRT group at 18–24 months after treatment (IMPT, 4% vs. IMRT, 16%; p = 0.035) (Fig. 2E) and at 24–36 months (IMPT, 5% vs. IMRT, 16%; p = 0.034) (Fig. 2F).
Fig. 2.
The numbers of patients with XQ scores at 0–6 months after treatment and at the indicated subsequent times. (A) Four hundred thirteen patients had XQ scores at 0–6 months, but only 142, 107, 145, 148, and 181 of those patients also had XQ scores at 6–9, 9–12, 12–18, 18–24, and 24–36 months, respectively. (B) The proportions of the 142 patients with moderate-severe xerostomia (XQs ≥50) in both treatment groups who had XQ scores at both 0–6 and 6–9 months. (C-F) The proportions of the (C) 107 patients with XQ scores at 0–6 and 9–12 months, (D) 145 patients with XQ scores at 0–6 and 12–18 months, (E) 148 patients with XQ scores at 0–6 and 18–24 months, and (F) 181 patients with XQ scores at 0–6 and 24–36 months in the IMRT and IMPT groups. Fewer patients in the IMPT group than in the IMRT group had moderate-severe xerostomia (XQs ≥50) at 18–24 months (p = 0.035) and 24–36 months (p = 0.034) after treatment.
We used the 181 patients who had XQ scores available at 0–6 and 24–36 months to investigate potential associations between the dose delivered to salivary glands or the oral cavity and xerostomia risk until 6 months after treatment and late xerostomia at those times. During the xerostomia period (0–6 months), higher Dmean, V5, V10, V15, V20, V25, V30, V35, V40, and V45 were all associated with higher proportions of patients with moderate-severe xerostomia (all p < 0.05), but we found no associations between dose/volume exposures to the ipsilateral parotid gland or oral cavity and the proportion of patients with moderate-severe xerostomia (Table 3). Similar to the parotid glands, dose/volume exposures to the contralateral submandibular rather than the ipsilateral submandibular gland were associated with high proportions of patients with moderate-severe xerostomia (Supplementary Table S3).
Table 3.
Dosimetric characteristics comparison for the ipsilateral glands, contralateral parotid glands and oral cavity by Moderate-Severe versus No-Mild xerostomia with the independent t test at 0–6 months and 24–36 months after treatment
| Ipsilateral Parotid | Contralateral Parotid | Oral Cavity | |||||||
|---|---|---|---|---|---|---|---|---|---|
| M-S X | N-M X | P value | M-S X | N-M X | P value | M-S X | N-M X | P value | |
| 0–6 months after treatment* | |||||||||
| Dmean,Gy | 37.9 | 37.2 | 0.69 | 23.4 | 18.5 | 0.001 | 44.3 | 42.8 | 0.48 |
| V5,% | 99.7 | 99.0 | 0.10 | 95.4 | 82.6 | 0.010 | 93.6 | 93.4 | 0.92 |
| V10,% | 87.5 | 85.2 | 0.27 | 70.6 | 54.4 | <0.001 | 90.4 | 89.7 | 0.81 |
| V15,% | 74.0 | 72.7 | 0.64 | 52.7 | 39.0 | <0.001 | 87.7 | 86.4 | 0.70 |
| V20,% | 65.0 | 65.0 | 0.99 | 42.1 | 31.2 | <0.001 | 84.4 | 82.2 | 0.56 |
| V25,% | 58.7 | 58.8 | 0.97 | 34.4 | 25.4 | <0.001 | 78.9 | 76.8 | 0.59 |
| V30,% | 53.8 | 53.7 | 0.96 | 28.5 | 20.7 | <0.001 | 71.8 | 70.5 | 0.73 |
| V35,% | 49.2 | 48.6 | 0.85 | 23.5 | 16.6 | <0.001 | 64.6 | 62.6 | 0.60 |
| V40,% | 44.7 | 43.7 | 0.74 | 18.9 | 13.1 | 0.010 | 56.8 | 54.3 | 0.48 |
| V45,% | 40.2 | 38.9 | 0.65 | 14.4 | 9.8 | 0.010 | 49.5 | 46.6 | 0.36 |
| V50,% | 35.5 | 33.8 | 0.58 | 10.3 | 7.00 | 0.050 | 43.2 | 40.1 | 0.30 |
| V55,% | 30.4 | 28.3 | 0.48 | 6.3 | 4.40 | 0.18 | 37.1 | 34.0 | 0.26 |
| V60,% | 24.4 | 22.1 | 0.44 | 3.4 | 2.30 | 0.32 | 30.9 | 27.8 | 0.22 |
| V65,% | 16.6 | 15.4 | 0.65 | 2.0 | 1.30 | 0.39 | 23.7 | 20.8 | 0.20 |
| V70,% | 8.4 | 7.9 | 0.82 | 0.9 | 0.60 | 0.57 | 13.5 | 11.1 | 0.25 |
| Late Period (24–36 months)** | |||||||||
| Dmean,Gy | 37.7 | 37.5 | 0.93 | 20.7 | 19.6 | 0.49 | 46.2 | 41.9 | 0.025 |
| V5,% | 99.9 | 99.1 | 0.23 | 90.2 | 85.9 | 0.51 | 97.3 | 92.9 | 0.13 |
| V10,% | 88.4 | 85.5 | 0.30 | 63.1 | 58.8 | 0.50 | 95.4 | 89.1 | 0.10 |
| V15,% | 74.0 | 73.0 | 0.80 | 44.1 | 43.0 | 0.84 | 93.7 | 85.7 | 0.080 |
| V20,% | 64.8 | 65.0 | 0.97 | 34.5 | 34.5 | 0.99 | 91.4 | 81.6 | 0.050 |
| V25,% | 58.6 | 58.8 | 0.94 | 27.9 | 28.2 | 0.94 | 86.8 | 76.1 | 0.040 |
| V30,% | 53.7 | 53.7 | 0.99 | 23.1 | 23.1 | 0.99 | 80.6 | 69.4 | 0.030 |
| V35,% | 49.3 | 48.7 | 0.90 | 19.3 | 18.6 | 0.82 | 72.6 | 61.8 | 0.030 |
| V40,% | 44.9 | 43.9 | 0.81 | 15.8 | 14.7 | 0.68 | 63.6 | 53.8 | 0.040 |
| V45,% | 40.6 | 39.1 | 0.72 | 12.6 | 11.0 | 0.54 | 55.6 | 46.3 | 0.030 |
| V50,% | 36.1 | 34.1 | 0.63 | 9.40 | 7.7 | 0.47 | 48.7 | 39.9 | 0.030 |
| V55,% | 31.4 | 28.6 | 0.51 | 6.3 | 4.8 | 0.45 | 42.0 | 33.9 | 0.030 |
| V60,% | 25.6 | 22.4 | 0.45 | 3.9 | 2.4 | 0.32 | 35.3 | 27.8 | 0.030 |
| V65,% | 16.5 | 15.7 | 0.83 | 2.2 | 1.4 | 0.44 | 27.9 | 20.7 | 0.030 |
| V70,% | 6.7 | 8.20 | 0.61 | 0.9 | 0.7 | 0.75 | 17.1 | 11.0 | 0.040 |
The bold values indicate significant statistical differences.
At 6 < months, n= 181 patients (127 patients with no-mild xerostomia and 54 patients [39 IMRT and 15 IMPT] with moderate-severe xerostomia);
At 24–36 months, n = 181 patients (158 patients with no-mild xerostomia and 23 patients [20 IMRT and 3 IMPT] with moderate-severe xerostomia).
Abbreviations: Dmean, mean dose; Vx, the percent volumes of organ at risk receiving x Gy; M-S X, Moderate-Severe Xerostomia; N-M X, No-Mild Xerostomia;
During the late xerostomia period (24–36 months), high dose/volume exposures (V25, V30, V35, V40, V45, V50, V55, V60, V65, and V70) in the oral cavity were associated with high proportions of patients with moderate-severe xerostomia (all p < 0.05), but we observed no associations between dose/volume exposures to either the parotid or submandibular glands and the proportion of patients with moderate-severe xerostomia (Table 3, Supplementary Table S3).
Finally, we compared dosimetric variables according to treatment (IMRT vs. IMPT) and found that IMPT delivered substantially lower V5, V35, V40, V45, V50, and V55 to the contralateral parotid gland; lower Dmean, V5, V10, V15, V20, V25, V30, V35, V40, V45, V50, V55, V60, and V65 to the oral cavity; and lower Dmean, V5, V30, V35, V40, V45, V50, V55, and V60 to the contralateral submandibular gland than did IMRT. However, IMPT delivered significantly higher Dmean, V5, V10, V15, V20, V25, V30, and V35 to the ipsilateral parotid gland than did IMRT (Table 4, Supplementary Table S4).
Table 4.
Dosimetric characteristics comparison for the ipsilateral parotid gland, contralateral parotid gland and oral cavity by treatment with the independent t test
| Ipsilateral Parotid | Contralateral Parotid | Oral Cavity | |||||||
|---|---|---|---|---|---|---|---|---|---|
| IMRT | IMPT | P value | IMRT | IMPT | P value | IMRT | IMPT | P value | |
| Dmean,Gy | 36.5 | 39.0 | 0.037 | 19.9 | 18.9 | 0.30 | 48.4 | 31.4 | <0.001 |
| V5,% | 99.6 | 98.4 | <0.001 | 91.5 | 76.6 | <0.001 | 100.0 | 80.3 | <0.001 |
| V10,% | 82.9 | 91.8 | <0.001 | 58.7 | 60.4 | 0.72 | 99.9 | 70.0 | <0.001 |
| V15,% | 67.9 | 83.3 | <0.001 | 41.6 | 46.2 | 0.21 | 99.2 | 62.0 | <0.001 |
| V20,% | 60.0 | 74.7 | <0.001 | 33.6 | 36.2 | 0.40 | 96.6 | 55.3 | <0.001 |
| V25,% | 54.7 | 66.9 | <0.001 | 28.2 | 28.1 | 0.98 | 91.4 | 49.6 | <0.001 |
| V30,% | 50.5 | 60.1 | <0.001 | 23.9 | 21.4 | 0.29 | 84.0 | 44.6 | <0.001 |
| V35,% | 46.6 | 53.1 | <0.001 | 20.2 | 15.7 | 0.040 | 74.7 | 40.2 | <0.001 |
| V40,% | 42.9 | 46.3 | 0.20 | 16.8 | 11.0 | <0.001 | 64.5 | 36.3 | <0.001 |
| V45,% | 39.2 | 39.4 | 0.90 | 13.3 | 7.2 | <0.001 | 54.9 | 32.7 | <0.001 |
| V50,% | 35.2 | 32.6 | 0.40 | 9.8 | 4.4 | <0.001 | 47.0 | 29.2 | <0.001 |
| V55,% | 30.6 | 25.7 | 0.10 | 6.2 | 2.6 | 0.010 | 39.7 | 25.4 | <0.001 |
| V60,% | 24.7 | 19.2 | 0.10 | 3.2 | 1.5 | 0.090 | 32.7 | 20.9 | <0.001 |
| V65,% | 17.6 | 12.2 | <0.001 | 1.9 | 0.8 | 0.16 | 24.7 | 15.5 | <0.001 |
| V70,% | 9.2 | 5.9 | 0.10 | 0.8 | 0.5 | 0.63 | 13.1 | 9.4 | 0.075 |
The bold values indicate significant statistical differences.
Abbreviations: Dmean, mean dose; Vx, the percent volumes of organ at risk receiving x Gy; IMRT, intensity-modulated (photon) radiation therapy; IMPT, intensity-modulated proton therapy.
Discussion
When we performed this analysis, only two published studies, both at the same institution, had reported xerostomia rates after proton therapy for OPC, but xerostomia was not the main endpoint of either study [10, 19]. In the present study, we evaluated a larger number of OPC patients than in those two studies to study xerostomia until 6 months after treatment and late xerostomia. To the best of our knowledge, this is the first report of IMPT for OPC leading to lower rates of late xerostomia (at 18–24 and 24–36 months) than did IMRT.
Researchers have used various strategies to reduce the risk of xerostomia after radiotherapy for head and neck cancer. Evaluating the merits of these strategies requires a reliable and reproducible way to score the severity of postradiotherapy xerostomia. Approaches used for this purpose include salivary gland imaging, salivary output measurement, observer-based toxicity scoring, and patient-reported scoring. Salivary gland images can be obtained via scintigraphy with 99MTc-pertechnetate, but the technical complexity of and requirement for dedicated nuclear medicine expertise in this approach preclude its use in routine clinical assessments. Measurements of salivary output are highly variable and lack a unified operational standard, leading to inconsistent results regarding the relationship between the salivary flow rate and quality of life [5, 28–31]. Observer-based toxicity scoring systems such as the Radiation Therapy Oncology Group and European Organisation for Research and Treatment of Cancer systems suffer from misinterpretation and omission errors and can underestimate patients’ subjective experience of xerostomia [28, 32, 33]. Indeed, because xerostomia is a subjective symptom, authors have proposed that assessment of xerostomia should be self-reported [34]. The University of Michigan specifically developed the XQ for this purpose [26]. Investigators have shown the XQ to have good psychometrics and to be reliable, valid, and reproducible in measuring patient-reported xerostomia [17, 33, 35–37]. We concur with these results and thus used the XQ to assess xerostomia in the present study.
We did not find that IMPT reduced the incidence of self-reported moderate-severe xerostomia until 6 months after treatment when compared with IMRT (IMPT, 31% vs. IMRT, 36% at 0–3 months; p = 0.5). However, Blanchard et al. [10] found that at 3 months, observers using the Radiation Therapy Oncology Group and European Organisation for Research and Treatment of Cancer scales found that grade ≥2 acute xerostomia was less common in the IMPT group (42% vs. 61% in the IMRT group; p = 0.009). This difference probably came from the use of the different assessment scales. As noted previously, the Radiation Therapy Oncology Group/European Organisation for Research and Treatment of Cancer grade often does not agree with patients’ reports [28, 33].
Of the 532 patients in the analysis, only 191 (36%) had XQ scores at 18–24 months after treatment, and 237 (45%) had them at 24–36 months after treatment. The missing XQ data may well have biased our results. For example, if more patients with no-mild xerostomia in the IMPT group had been lost to follow-up at 18–24 or 24–36 months, the proportion of patients with moderate-severe xerostomia would have been greater than the actual proportion, which would have skewed our results. To prevent this bias, we analyzed the proportions of the 148 patients with XQ scores at 0–6 and 18–24 months and the 181 with XQ scores at 0–6 and 24–36 months and found the same result that the IMPT group had a significantly smaller proportion of patients with moderate-severe xerostomia than did the IMRT group, leading us to conclude that IMPT may minimize the risk of late xerostomia.
Delivery of radiation doses to the parotid glands is well known to increase the risk of xerostomia [38–42]. Normal tissue complication probability models suggested that a low parotid gland dose, which can be achieved with IMPT, will further reduce the risk of xerostomia, especially for patients with tumors in the upper head and neck area [43–45]. However, very few authors have reported on associations between dosimetric variables and late xerostomia. Owosho et al. [46] showed that the Dmean delivered to the contralateral parotid gland (Dmeancontra) was significantly higher in patients with xerostomia than in those without xerostomia (p = 0.002) at 0–6 months after treatment. However, neither Dmeancontra nor the Dmean delivered to the ipsilateral parotid gland (Dmeanipsi) were associated with the risk of xerostomia at 12–24 months (Dmeancontra, p = 0.080; Dmeanipsi, p = 0.10). These results were similar to those in the present study. In our 181 patients with late xerostomia, we found that the radiation dose delivered to the contralateral parotid gland was related to xerostomia until 6 months after treatment, but we found no such associations between the dose delivered to the contralateral or ipsilateral parotid gland and late xerostomia.
Also, treatment-planning comparisons have shown that IMPT can reduce the dose delivered to the parotid glands relative to IMRT [47–52]. However, these studies are often criticized for comparing optimized IMPT plans generated by expert dosimetrists with IMRT plans generated purely for the purposes of comparison, often quickly and with less attention to detail [53]. To address this, Holliday et al. [53] quantified the doses delivered to critical structures in patients with OPC treated with IMPT and compared them with the doses in IMRT plans generated for the same patients and for a matched cohort of patients who actually received IMRT. They showed that IMPT did not lead to delivery of lower mean doses to the parotid glands than in either comparison group. Our results concur in that the parotid gland radiation dose/volume exposure from IMPT was no better than that from IMRT. Nevertheless, we still found fewer patients with moderate-severe late xerostomia in the IMPT group than in the IMRT group. Other researchers believe that radiation doses delivered to the parotid glands are irrelevant with regard to late xerostomia because the doses to other structures, such as the submandibular glands and oral cavity, are important contributors to xerostomia, as well. In that case, sparing the submandibular glands and oral cavity in addition to the parotid glands may reduce the incidence of xerostomia [54–56]. We found that IMPT produced less dose/volume exposure in the oral cavity than did IMRT and that this less exposure was associated with a lower proportion of patients with moderate-severe late xerostomia, which partially supported the viewpoint that IMPT for oropharyngeal cancer reduces rates of late xerostomia.
The replanning is currently adopted to account for interfractional anatomical changes for the patients. Those anatomical changes in HN cancers include shrinkage of primary tumors or nodal lesions, resolution of postoperative changes or edema, changes in nasal cavity filling, and weight loss [57]. In IMRT plans, interfractional geometric variations are handled by adding safety margins around the clinical target volume (CTV). The CTV is expanded to a larger planning target volume (PTV). If tumor has shrinkage during radiation therapy, both standard IMPT and IMRT techniques do not treat the shrinking targets. Previous work on IMRT technique indicates that target coverage is much more robust for PTV based IMPT planning for head and neck cancer [58]. The main advantage of adaptive planning for IMRT is to reduce margin so that normal tissues is better protected. Although we have protocols to treat shrinking targets through replanning for both IMPT and IMRT patients, we did not include those protocol patients in this work. However, due to the finite range of the proton beam, the proton dose distributions are more sensitive to density changes resulting in far more severe effects of uncertainties on the dose distribution during treatment. Although anatomical changes happen geometrically inside the PTV, PTV cannot account for the density change. As a result, it is even suggested that adaptive re-planning is mandatory for the proton patients [59, 60].
This study had some limitations, chief among them being the biases inherent in retrospective studies and the relatively small number of patients given IMPT. Another shortcoming was a lack of baseline (pretreatment) XQ scores, a significant factor affecting xerostomia risk, which precluded our assessment of changes in xerostomia risk relative to baseline values [61–63]. Finally, although we analyzed data on 532 OPC patients, the number of patients who completed XQs at each time point after treatment was suboptimal, likely because of inconsistencies in follow-up and low compliance with completing the questionnaires. Previously, all of the XQs used in our study were completed via telephone or mail. In the future, we will use online questionnaires to improve patient compliance and thus increase the integrity and validity of the data to verify the authenticity of the results.
We conclude that IMPT for OPC less often results in late xerostomia than does IMRT and that dosimetric variables regarding the oral cavity are related to late xerostomia. Further prospective studies with more detailed xerostomia survey data should be undertaken to clarify the influence of radiation technique (IMRT or IMPT) on the prevalence of late xerostomia.
Supplementary Material
Highlights.
IMPT resulted in less late xerostomia than IMRT for oropharyngeal cancer.
Oral cavity dosimetric variables were related to the occurrence of late xerostomia.
The dosimetric variables of parotids or submandibular were not associated with late xerostomia.
Key Points.
Intensity-modulated proton therapy (IMPT) resulted in less late xerostomia (18–36 months after treatment) than did intensity-modulated radiation therapy (IMRT) for oropharyngeal cancer.
Oral cavity dosimetric variables were related to the occurrence of late xerostomia.
Acknowledgements:
We would like to thank Donald R Norwood in Scientific Publications, Research Medical Library at The University of Texas MD Anderson Cancer Center for the editing assistance.
Funding sources: The University of Texas MD Anderson Cancer Center was supported in part by the National Cancer Institute Cancer Center Support Grant P30CA016672.
Footnotes
Conflict of interest statement: The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tân PF, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Clavel S, Nguyen DH, Fortin B, Després P, Khaouam N, Donath D, et al. Simultaneous integrated boost using intensity-modulated radiotherapy compared with conventional radiotherapy in patients treated with concurrent carboplatin and 5-fluorouracil for locally advanced oropharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2012;82:582–9. [DOI] [PubMed] [Google Scholar]
- [3].Lee NY, de Arruda FF, Puri DR, Wolden SL, Narayana A, Mechalakos J, et al. A comparison of intensity-modulated radiation therapy and concomitant boost radiotherapy in the setting of concurrent chemotherapy for locally advanced oropharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2006;66:966–74. [DOI] [PubMed] [Google Scholar]
- [4].Hübbers CU, Akgül B. HPV and cancer of the oral cavity. Virulence. 2015;6:244–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Eisbruch A, Rhodus N, Rosenthal D, Murphy B, Rasch C, Sonis S, et al. How should we measure and report radiotherapy-induced xerostomia? Semin Radiat Oncol. 2003;13:226–34. [DOI] [PubMed] [Google Scholar]
- [6].Nutting CM, Morden JP, Harrington KJ, Urbano TG, Bhide SA, Clark C, et al. Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): a phase 3 multicentre randomised controlled trial. Lancet Oncol. 2011;12:127–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Abel E, Silander E, Nyman J, Bove M, Johansson L, Björk-Eriksson T, et al. Impact on quality of life of IMRT versus 3-D conformal radiation therapy in head and neck cancer patients: A case control study. Adv Radiat Oncol. 2017;2:346–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ladra MM, Edgington SK, Mahajan A, Grosshans D, Szymonifka J, Khan F, et al. A dosimetric comparison of proton and intensity modulated radiation therapy in pediatric rhabdomyosarcoma patients enrolled on a prospective phase II proton study. Radiother Oncol. 2014;113:77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Leeman JE, Romesser PB, Zhou Y, McBride S, Riaz N, Sherman E, et al. Proton therapy for head and neck cancer: expanding the therapeutic window. Lancet Oncol. 2017;18:e254–e65. [DOI] [PubMed] [Google Scholar]
- [10].Blanchard P, Garden AS, Gunn GB, Rosenthal DI, Morrison WH, Hernandez M, et al. Intensity-modulated proton beam therapy (IMPT) versus intensity-modulated photon therapy (IMRT) for patients with oropharynx cancer - A case matched analysis. Radiother Oncol. 2016;120:48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].McDonald MW, Liu Y, Moore MG, Johnstone PA. Acute toxicity in comprehensive head and neck radiation for nasopharynx and paranasal sinus cancers: cohort comparison of 3D conformal proton therapy and intensity modulated radiation therapy. Radiat Oncol. 2016;11:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Romesser PB, Cahlon O, Scher E, Zhou Y, Berry SL, Rybkin A, et al. Proton beam radiation therapy results in significantly reduced toxicity compared with intensity-modulated radiation therapy for head and neck tumors that require ipsilateral radiation. Radiother Oncol. 2016;118:286–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sio TT, Lin HK, Shi Q, Gunn GB, Cleeland CS, Lee JJ, et al. Intensity Modulated Proton Therapy Versus Intensity Modulated Photon Radiation Therapy for Oropharyngeal Cancer: First Comparative Results of Patient-Reported Outcomes. Int J Radiat Oncol Biol Phys. 2016;95:1107–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kandula S, Zhu X, Garden AS, Gillin M, Rosenthal DI, Ang KK, et al. Spot-scanning beam proton therapy vs intensity-modulated radiation therapy for ipsilateral head and neck malignancies: a treatment planning comparison. Med Dosim. 2013;38:390–4. [DOI] [PubMed] [Google Scholar]
- [15].Stromberger C, Cozzi L, Budach V, Fogliata A, Ghadjar P, Wlodarczyk W, et al. Unilateral and bilateral neck SIB for head and neck cancer patients : Intensity-modulated proton therapy, tomotherapy, and RapidArc. Strahlenther Onkol. 2016;192:232–9. [DOI] [PubMed] [Google Scholar]
- [16].Mock U, Georg D, Bogner J, Auberger T, Pötter R. Treatment planning comparison of conventional, 3D conformal, and intensity-modulated photon (IMRT) and proton therapy for paranasal sinus carcinoma. Int J Radiat Oncol Biol Phys. 2004;58:147–54. [DOI] [PubMed] [Google Scholar]
- [17].Lin A, Kim HM, Terrell JE, Dawson LA, Ship JA, Eisbruch A. Quality of life after parotid-sparing IMRT for head-and-neck cancer: a prospective longitudinal study. Int J Radiat Oncol Biol Phys. 2003;57:61–70. [DOI] [PubMed] [Google Scholar]
- [18].Hawkins PG, Lee JY, Mao Y, Li P, Green M, Worden FP, et al. Sparing all salivary glands with IMRT for head and neck cancer: Longitudinal study of patient-reported xerostomia and head-and-neck quality of life. Radiother Oncol. 2018;126:68–74. [DOI] [PubMed] [Google Scholar]
- [19].Gunn GB, Blanchard P, Garden AS, Zhu XR, Fuller CD, Mohamed AS, et al. Clinical Outcomes and Patterns of Disease Recurrence After Intensity Modulated Proton Therapy for Oropharyngeal Squamous Carcinoma. Int J Radiat Oncol Biol Phys. 2016;95:360–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Data Collection for the Assessment of Acute and Late Normal Tissue in Patients Treated With Proton Therapy. Available online: https://clinicaltrials.gov/ct2/show/NCT00991094.
- [21].Medical Data Collection of Patients With Head and Neck Cancer Treated With Proton Therapy. Available online: https://clinicaltrials.gov/ct2/show/NCT01627093.
- [22].Frank SJ, Cox JD, Gillin M, Mohan R, Garden AS, Rosenthal DI, et al. Multifield optimization intensity modulated proton therapy for head and neck tumors: a translation to practice. Int J Radiat Oncol Biol Phys. 2014;89:846–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Zhu XR, Li Y, Mackin D, Li H, Poenisch F, Lee AK, et al. Towards effective and efficient patient-specific quality assurance for spot scanning proton therapy. Cancers (Basel). 2015;7:631–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wu RY, Liu AY, Sio TT, Blanchard P, Wages C, Amin MV, et al. Intensity-Modulated Proton Therapy Adaptive Planning for Patients with Oropharyngeal Cancer. Int J Part Ther. 2017;4:26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Garden AS, Dong L, Morrison WH, Stugis EM, Glisson BS, Frank SJ, et al. Patterns of disease recurrence following treatment of oropharyngeal cancer with intensity modulated radiation therapy. Int J Radiat Oncol Biol Phys. 2013;85:941–7. [DOI] [PubMed] [Google Scholar]
- [26].Eisbruch A, Kim HM, Terrell JE, Marsh LH, Dawson LA, Ship JA. Xerostomia and its predictors following parotid-sparing irradiation of head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2001;50:695–704. [DOI] [PubMed] [Google Scholar]
- [27].Vainshtein JM, Moon DH, Feng FY, Chepeha DB, Eisbruch A, Stenmark MH. Long-term quality of life after swallowing and salivary-sparing chemo-intensity modulated radiation therapy in survivors of human papillomavirus-related oropharyngeal cancer. Int J Radiat Oncol Biol Phys. 2015;91:925–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Deasy JO, Moiseenko V, Marks L, Chao KS, Nam J, Eisbruch A. Radiotherapy dose-volume effects on salivary gland function. Int J Radiat Oncol Biol Phys. 2010;76:S58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lin SC, Jen YM, Chang YC, Lin CC. Assessment of xerostomia and its impact on quality of life in head and neck cancer patients undergoing radiotherapy, and validation of the Taiwanese version of the xerostomia questionnaire. J Pain Symptom Manage. 2008;36:141–8. [DOI] [PubMed] [Google Scholar]
- [30].Braam PM, Roesink JM, Raaijmakers CP, Busschers WB, Terhaard CH. Quality of life and salivary output in patients with head-and-neck cancer five years after radiotherapy. Radiat Oncol. 2007;2:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Scrimger R, Kanji A, Parliament M, Warkentin H, Field C, Jha N, et al. Correlation between saliva production and quality of life measurements in head and neck cancer patients treated with intensity-modulated radiotherapy. Am J Clin Oncol. 2007;30:271–7. [DOI] [PubMed] [Google Scholar]
- [32].Trotti A, Colevas AD, Setser A, Basch E. Patient-reported outcomes and the evolution of adverse event reporting in oncology. J Clin Oncol. 2007;25:5121–7. [DOI] [PubMed] [Google Scholar]
- [33].Meirovitz A, Murdoch-Kinch CA, Schipper M, Pan C, Eisbruch A. Grading xerostomia by physicians or by patients after intensity-modulated radiotherapy of head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2006;66:445–53. [DOI] [PubMed] [Google Scholar]
- [34].Pellegrino F, Groff E, Bastiani L, Fattori B, Sotti G. Assessment of radiation-induced xerostomia: validation of the Italian version of the xerostomia questionnaire in head and neck cancer patients. Support Care Cancer. 2015;23:925–32. [DOI] [PubMed] [Google Scholar]
- [35].Jabbari S, Kim HM, Feng M, Lin A, Tsien C, Elshaikh M, et al. Matched case-control study of quality of life and xerostomia after intensity-modulated radiotherapy or standard radiotherapy for head-and-neck cancer: initial report. Int J Radiat Oncol Biol Phys. 2005;63:725–31. [DOI] [PubMed] [Google Scholar]
- [36].Pacholke HD, Amdur RJ, Morris CG, Li JG, Dempsey JF, Hinerman RW, et al. Late xerostomia after intensity-modulated radiation therapy versus conventional radiotherapy. Am J Clin Oncol. 2005;28:351–8. [DOI] [PubMed] [Google Scholar]
- [37].Kamal M, Rosenthal DI, Volpe S, Goepfert RP, Garden AS, Hutcheson KA, et al. Patient reported dry mouth: Instrument comparison and model performance for correlation with quality of life in head and neck cancer survivors. Radiother Oncol. 2018;126:75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Jellema AP, Doornaert P, Slotman BJ, Leemans CR, Langendijk JA. Does radiation dose to the salivary glands and oral cavity predict patient-rated xerostomia and sticky saliva in head and neck cancer patients treated with curative radiotherapy? Radiother Oncol. 2005;77:164–71. [DOI] [PubMed] [Google Scholar]
- [39].Eisbruch A, Ten Haken RK, Kim HM, Marsh LH, Ship JA. Dose, volume, and function relationships in parotid salivary glands following conformal and intensity-modulated irradiation of head and neck cancer. Int J Radiat Oncol Biol Phys. 1999;45:577–87. [DOI] [PubMed] [Google Scholar]
- [40].Roesink JM, Moerland MA, Battermann JJ, Hordijk GJ, Terhaard CH. Quantitative dose-volume response analysis of changes in parotid gland function after radiotherapy in the head-and-neck region. Int J Radiat Oncol Biol Phys. 2001;51:938–46. [DOI] [PubMed] [Google Scholar]
- [41].Blanco AI, Chao KS, El Naqa I, Franklin GE, Zakarian K, Vicic M, et al. Dose-volume modeling of salivary function in patients with head-and-neck cancer receiving radiotherapy. Int J Radiat Oncol Biol Phys. 2005;62:1055–69. [DOI] [PubMed] [Google Scholar]
- [42].Li Y, Taylor JM, Ten Haken RK, Eisbruch A. The impact of dose on parotid salivary recovery in head and neck cancer patients treated with radiation therapy. Int J Radiat Oncol Biol Phys. 2007;67:660–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].van der Laan HP, van de Water TA, van Herpt HE, Christianen ME, Bijl HP, Korevaar EW, et al. The potential of intensity-modulated proton radiotherapy to reduce swallowing dysfunction in the treatment of head and neck cancer: A planning comparative study. Acta Oncol. 2013;52:561–9. [DOI] [PubMed] [Google Scholar]
- [44].Johansson J, Blomquist E, Montelius A, Isacsson U, Glimelius B. Potential outcomes of modalities and techniques in radiotherapy for patients with hypopharyngeal carcinoma. Radiother Oncol. 2004;72:129–38. [DOI] [PubMed] [Google Scholar]
- [45].Jakobi A, Bandurska-Luque A, Stützer K, Haase R, Löck S, Wack LJ, et al. Identification of Patient Benefit From Proton Therapy for Advanced Head and Neck Cancer Patients Based on Individual and Subgroup Normal Tissue Complication Probability Analysis. Int J Radiat Oncol Biol Phys. 2015;92:1165–74. [DOI] [PubMed] [Google Scholar]
- [46].Owosho AA, Thor M, Oh JH, Riaz N, Tsai CJ, Rosenberg H, et al. The role of parotid gland irradiation in the development of severe hyposalivation (xerostomia) after intensity-modulated radiation therapy for head and neck cancer: Temporal patterns, risk factors, and testing the QUANTEC guidelines. J Craniomaxillofac Surg. 2017;45:595–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].van de Water TA, Lomax AJ, Bijl HP, de Jong ME, Schilstra C, Hug EB, et al. Potential benefits of scanned intensity-modulated proton therapy versus advanced photon therapy with regard to sparing of the salivary glands in oropharyngeal cancer. Int J Radiat Oncol Biol Phys. 2011;79:1216–24. [DOI] [PubMed] [Google Scholar]
- [48].Widesott L, Pierelli A, Fiorino C, Dell’oca I, Broggi S, Cattaneo GM, et al. Intensity-modulated proton therapy versus helical tomotherapy in nasopharynx cancer: planning comparison and NTCP evaluation. Int J Radiat Oncol Biol Phys. 2008;72:589–96. [DOI] [PubMed] [Google Scholar]
- [49].Taheri-Kadkhoda Z, Björk-Eriksson T, Nill S, Wilkens JJ, Oelfke U, Johansson KA, et al. Intensity-modulated radiotherapy of nasopharyngeal carcinoma: a comparative treatment planning study of photons and protons. Radiat Oncol. 2008;3:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Simone CB, 2nd, Ly D, Dan TD, Ondos J, Ning H, Belard A, et al. Comparison of intensity-modulated radiotherapy, adaptive radiotherapy, proton radiotherapy, and adaptive proton radiotherapy for treatment of locally advanced head and neck cancer. Radiother Oncol. 2011;101:376–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Lewis GD, Holliday EB, Kocak-Uzel E, Hernandez M, Garden AS, Rosenthal DI, et al. Intensity-modulated proton therapy for nasopharyngeal carcinoma: Decreased radiation dose to normal structures and encouraging clinical outcomes. Head Neck. 2016;38 Suppl 1:E1886–95. [DOI] [PubMed] [Google Scholar]
- [52].Steneker M, Lomax A, Schneider U. Intensity modulated photon and proton therapy for the treatment of head and neck tumors. Radiother Oncol. 2006;80:263–7. [DOI] [PubMed] [Google Scholar]
- [53].Holliday EB, Kocak-Uzel E, Feng L, Thaker NG, Blanchard P, Rosenthal DI, et al. Dosimetric advantages of intensity-modulated proton therapy for oropharyngeal cancer compared with intensity-modulated radiation: A case-matched control analysis. Med Dosim. 2016;41:189–94. [DOI] [PubMed] [Google Scholar]
- [54].Little M, Schipper M, Feng FY, Vineberg K, Cornwall C, Murdoch-Kinch CA, et al. Reducing xerostomia after chemo-IMRT for head-and-neck cancer: beyond sparing the parotid glands. Int J Radiat Oncol Biol Phys. 2012;83:1007–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Dijkema T, Raaijmakers CP, Braam PM, Roesink JM, Monninkhof EM, Terhaard CH. Xerostomia: a day and night difference. Radiother Oncol. 2012;104:219–23. [DOI] [PubMed] [Google Scholar]
- [56].Jellema AP, Slotman BJ, Doornaert P, Leemans CR, Langendijk JA. Impact of radiation-induced xerostomia on quality of life after primary radiotherapy among patients with head and neck cancer. Int J Radiat Oncol Biol Phys. 2007;69:751–60. [DOI] [PubMed] [Google Scholar]
- [57].Yang Z, Zhang X, Wang X, Zhu XR, Gunn B, Frank SJ, et al. Multiple-CT optimization: An adaptive optimization method to account for anatomical changes in intensity-modulated proton therapy for head and neck cancers. Radiother Oncol. 2020;142:124–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Müller BS, Duma MN, Kampfer S, Nill S, Oelfke U, Geinitz H, et al. Impact of interfractional changes in head and neck cancer patients on the delivered dose in intensity modulated radiotherapy with protons and photons. Phys Med. 2015;31:266–72. [DOI] [PubMed] [Google Scholar]
- [59].Kearney M, Coffey M, Leong A. A review of Image Guided Radiation Therapy in head and neck cancer from 2009–201 - Best Practice Recommendations for RTTs in the Clinic. Tech Innov Patient Support Radiat Oncol. 2020;14:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Hoffmann L, Alber M, Jensen MF, Holt MI, Møller DS. Adaptation is mandatory for intensity modulated proton therapy of advanced lung cancer to ensure target coverage. Radiother Oncol. 2017;122:400–5. [DOI] [PubMed] [Google Scholar]
- [61].Beetz I, Schilstra C, van der Schaaf A, van den Heuvel ER, Doornaert P, van Luijk P, et al. NTCP models for patient-rated xerostomia and sticky saliva after treatment with intensity modulated radiotherapy for head and neck cancer: the role of dosimetric and clinical factors. Radiother Oncol. 2012;105:101–6. [DOI] [PubMed] [Google Scholar]
- [62].Beetz I, Schilstra C, Visink A, van der Schaaf A, Bijl HP, van der Laan BF, et al. Role of minor salivary glands in developing patient-rated xerostomia and sticky saliva during day and night. Radiother Oncol. 2013;109:311–6. [DOI] [PubMed] [Google Scholar]
- [63].Beetz I, Schilstra C, van Luijk P, Christianen ME, Doornaert P, Bijl HP, et al. External validation of three dimensional conformal radiotherapy based NTCP models for patient-rated xerostomia and sticky saliva among patients treated with intensity modulated radiotherapy. Radiother Oncol. 2012;105:94–100. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.