Abstract
Soils play a key role in meeting the UN Sustainable Development Goals (SDGs). In this study, we review the contribution of soils to the regulation of air quality, which is one of ‘Nature's Contributions to People’ identified by the Intergovernmental-Policy Platform on Biodiversity and Ecosystem Services (IPBES). This is particularly relevant for SDG3 (health and well-being) and 11 (sustainable cities and well-being) but also impacts other SDGs. Soils can act as both a source and a sink of air pollutants (and their precursors). In addition, soils support plant growth which plays a major role in regulating air quality. The scale of the soil impacts on air quality range from global (e.g. greenhouse gas fluxes, stratospheric ozone depletion) to local (e.g. odours, particulates, pathogen transport). Harmful emissions from soil can be increased or decreased by anthropogenic activity, while climate change is likely to modify future emissions patterns, both directly and in response to human mitigation and adaption actions. Although soils are not the only source of these pollutants, it is worthwhile managing them to reduce erosion and nutrient losses to maintain soil health so we may continue to benefit from the contributions to good quality of life they provide.
This article is part of the theme issue ‘The role of soils in delivering Nature's Contributions to People’.
Keywords: dust, air pollution, human health, ecosystem services, Nature's Contribution to People, Sustainable Development Goals
1. Introduction
Human society relies on the wise use of natural resources. In 2015, the UN adopted the Sustainable Development Goals (SDGs), to provide a guideline for all governments on what should be implemented to achieve sustainability for nature and people. Soil has a key role to play in reaching these goals as it contributes to people's well-being through what has been formalized as ‘ecosystem services’ [1], and more recently broadened to the term ‘Nature's Contributions to People’ or NCP [2]. In this paper, we will look specifically at soil and its role with respect to the NCP ‘regulation of air quality’. This NCP is defined by the Intergovernmental Platform on Biodiversity and Ecosystem Services (IPBES) as ‘the regulation (by impediment or facilitation) by ecosystems, of CO2/O2 balance, O3, sulphur oxide, nitrogen oxides (NO and NO2, collectively NOx), volatile organic compounds (VOC), particulates, aerosols, allergens. It concerns the filtration, fixation, degradation or storage of pollutants that directly affect human health or infrastructure’. Air quality regulation is essential for achieving SDG2 (zero hunger), SDG3 (good health and well-being), SDG11 (sustainable cities and communities), SDG13 (climate action) (see [3]) and SDG15 (life on land) [4]).
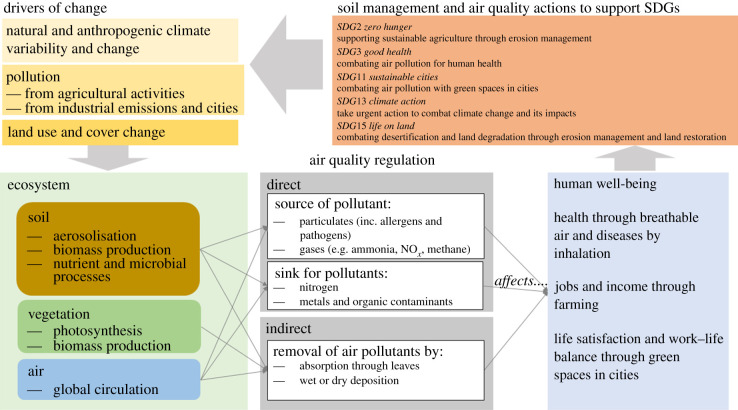
Soil interacts with air in both positive and negative ways for people's well-being (figure 1). It is critical for plant growth, which supports life on Earth through photosynthesis, and vegetation is increasingly used to improve air quality in urban and agricultural areas (e.g. [5]). Soil can also be a sink for airborne pollutants and has a direct regulatory function on gaseous atmospheric constituents through soil microbes responsible for nutrient cycling and releasing gases. However, soil can negatively affect air quality through being a source of particulates and gaseous pollutants. Good air quality is fundamental for human health but is negatively affected by particulate air pollution. Human health effects are mediated by the size, mineralogy and composition (both chemical and biological) of the dust particles. Atmospheric dust also influences the global climate through effects on radiative balance and cloud formation.
Figure 1.
Contribution of soil to air quality regulation. Examples of well-being topics are extracted from the OECD better life index (http://www.oecdbetterlifeindex.org/). In this paper, we explore the relationships between soil and air quality and discuss them in relation to the SDGs. (Online version in colour.)
Sand and dust storms are perhaps the most obvious way in which soil directly impacts air quality, with dust emissions arising either from natural phenomena or from land management activities. Dust emissions can also be experienced at a generally smaller scale on agricultural lands, and other activities such as construction or quarrying can also facilitate the release of dust.
2. How soils affect air quality
(a) . Direct source of pollutants or precursors to pollutants
(i) . Particulates
Soil particles, commonly referred to as dust, arise from the entrainment of soil particles into the air. This may occur through the direct entrainment by wind of small soil particles (less than 20 µm) that undergo long-range transport. Larger particles can be entrained through fragmentation of larger soil aggregates via saltation [6,7]. Direct emission of soil particles to air can arise from anthropogenic activities such as mining, quarrying and agriculture. Dust emissions are also indirectly influenced by anthropogenic activities and changes in soil structure, and hence the erodibility of the soils (e.g. [8,9]). Gaseous emissions from soil may also lead to particulate formation (see the next section).
The composition of the soil particles depends on mineralogy and land-use activities, with land use influencing both chemical contaminant and biotic contaminant load on the soil. The size and composition of dust particles influence both the biological (see §3a,b) and climate responses (see §3d).
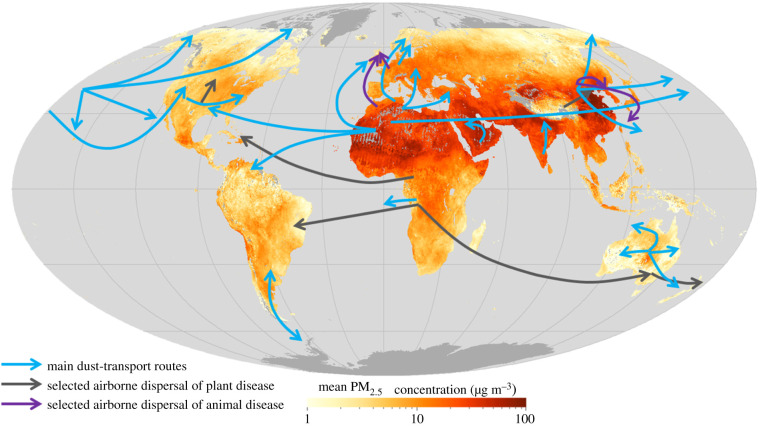
Dust storms can transport soilborne minerals and pathogens long distances (figure 2). Most dust comes from natural sources, with the majority coming from the Northern Hemisphere ‘Dust Belt’, that extends from the west coast of North Africa and the Middle East to central and South Asia. The relative contribution of these dust sources influenced by human action is highly uncertain (10–50%) [11] but likely to be around 25% [12]. Anthropogenic sources are dominated by agricultural activities, particularly around ephemeral water bodies [13]. Unsustainable agricultural and grazing activities and deforestation are the greatest causes, especially in southern Sahel, along the Mediterranean coast, North America and Argentina. Other climatic parameters influencing wind erosion and generation of dust include wind speed, wind direction, precipitation, evaporation and air temperature [12,14].
Figure 2.
Global transport of dust and selected airborne diseases. Adapted from Gonzalez-Martin et al. [10] and NASA (https://eoimages.gsfc.nasa.gov/images/imagerecords/86000/86075/sedac_gis_2010_2012_lrg.jpg). (Online version in colour.)
Trends in dust storms are variable across the globe, but simulations suggest global annual dust emissions have increased by 25–50% over the last century due to a combination of land use and climate changes [12,15]. Recent trends have shown that dust storm frequency has decreased in some areas (Iran, China, Mauritania), which was attributed to increases in precipitation [16].
(ii) . Gases
There are several gas fluxes from soils resulting from microbial, chemical and physical processes. These include carbon dioxide (CO2), methane (CH4), nitrous oxide (N2O), NOx and ammonia (NH3). Soils can also produce smaller amounts of volatile organic compounds (VOCs), hydrogen sulfide (H2S) and sulfur dioxide (SO2). While these have little influence on air quality at a global scale, they can impact air quality locally. CO2, CH4 and N2O are problematic due to their role as greenhouse gases, although N2O is also a major stratospheric ozone depleter [17]. By contrast, NH3 and NOx can lead to the formation of fine particulate matter, which is a risk to human health, as well as spreading nitrogen to environments where it could be damaging [18]. NOx and CH4 can also act as precursors to the production of tropospheric ozone [19], which can lead to respiratory problems and plant damage.
Ammonia and NOx. Bouwman et al. [20] estimated a global ammonia emissions inventory for 1990 of 54 Tg N yr–1. About 17% is from the application of synthetic fertilizers and almost 40% from the excreta of domestic animals, although some of this will be from manure management systems rather than from soils. For near-surface tropospheric NOx, soils are responsible for about 12% (5.5 Tg yr–1) of total emissions [19].
Globally, the fraction of urea-N lost as NH3 from applied urea on agricultural land ranges from 0.9 to 64.0%, with a mean of 18% [21].
Microbial processes in the soil can produce N2O and NO. Nitrification converts to nitrate (), reducing the potential for further NH3 losses; however, N2O and NO are produced as by-products. In addition, nitrate is more mobile than ammonium in the soil and presents a greater risk for water contamination. Nitrate is also the precursor to denitrification, a sequence of microbial reactions that can eventually convert nitrate to unreactive N2. However, the environmentally undesirable gases N2O and NO are produced as steps in this process and can be lost from the soil before denitrification is complete. The addition of nitrate fertilizers also increases denitrification. The rate and extent of these microbial processes are influenced by soil properties (e.g. pH, texture and soil organic carbon) and climate conditions (e.g. temperature and rainfall patterns), but can also be influenced by land-use change or intensification that results in soil degradation (e.g. soil organic matter loss, compaction, erosion, soil sealing, etc.)
Volatile organic carbons and odorous gases (SO2 and H2S). Soil organisms can produce a wide variety of VOCs, particularly under anaerobic conditions. The production of VOCs can have ecological significance, e.g. signalling for synchronous activities such as sporulation in fungi [22], and can be detectable at local levels, e.g. boreal forests [23]. Emissions from plants (see §4b) and anthropogenic sources rather than direct emissions from soil are generally considered to be a greater concern for air quality itself.
The odorous gases hydrogen sulfide (H2S) and sulfur dioxide (SO2) can be produced in some soils. High concentrations of H2S can be fatal to humans and animals and at lower concentrations, it has an unpleasant odour. Globally, soils1 account for less than 15% of the total H2S source [24]. Soils are not usually considered a major source of SO2. However, Macdonald et al. [25] estimated up to 3 Tg yr–1 S could be emitted from oxidation of sulfide-containing acid sulfate soils. This would make soils a source of SO2 of a similar magnitude to the 8 Tg yr–1 produced by volcanoes [26].
(b) . Direct sink for air pollutants
Gases and particulates may be removed from the atmosphere by wet (via precipitation) or dry deposition and consequently interact with either plants or soil. Pollutants that enter the soil can be subject to physical (e.g. adsorption, leaching), biological (e.g. plant uptake, nitrification) or chemical (e.g. oxidation/reduction) processes. These can result in the deposited material being bound to the soil, taken up by plants, re-emitted to the atmosphere or transported to waterways. The incorporation of nutrients from atmospheric deposition can be a source of nutrients, but it can also have negative impacts such as acidification of soils, eutrophication and other ecological effects [27].
Globally, it has been estimated that around 43 Tg nitrogen [28] and 220 Tg sulfur [29] are deposited annually to terrestrial ecosystems. The average wet S deposition rates range from 19 kg S ha–1 yr–1 in Asia down to 2 kg S ha–1 yr–1 in Oceania [29]. Holland et al. [28] found that global N deposition rates followed the pattern: Northern Hemisphere temperate regions > tropical regions > Southern Hemisphere temperate regions.
(c) . Support for plant growth
Soils perform a critical function influencing air quality by supporting the growth of plants, which in turn produce oxygen (O2). The process of producing oxygen (photosynthesis) removes CO2 from the atmosphere and sequesters carbon, assisting in climate regulation.
Beyond this fundamental role, there is perhaps limited recognition of the role soil plays in influencing air quality through supporting plant growth—despite the increasing recognition and use of plants to improve air quality in both rural and urban environments (e.g. [5] and references therein).
Vegetation can directly improve air quality through intercepting atmospheric particles and absorbing gaseous pollutants. The extent to which this occurs depends both on the individual species (including features such as leaf surface texture, size and configuration, and tree size) and on the planting configuration used. Various tree configurations can alter wind profiles or create local inversions to trap pollutants so that the localized removal of pollutants is enhanced and/or the exposure of people to pollutants is reduced (e.g. pedestrians alongside roadways) ([5] and references therein). However, trees can also contribute to air pollution through the emission of VOCs and pollen release.
In rural environments, plants are primarily used to reduce the potentially erosive effects of wind and dispersion of gases (e.g. around livestock pens, [30]) when planted as shelterbelts upwind of a field or facility, and when planted downwind to intercept and/or filter emitted particles and gases or to increase dispersion of emitted contaminants to minimize downwind effects [30,31].
In urban environments, multiple benefits in addition to air quality benefits are recognized as arising from urban vegetation. These are discussed in more detail in §3c.
3. Impacts of soil-induced air quality changes
Soils can affect air quality in many ways. The impacts of these changes can have positive or negative consequences for humans and other life forms. In this section, we discuss these impacts in relation to the UN SDGs [1].
(a) . Impacts on human health (SDG3)
The inhalation of particulate matter can negatively affect human health. Soils contribute to the atmospheric particulate load both directly as dust and indirectly via the reaction of gaseous compounds (e.g. VOCs, ammonia, NOx) (§2). Further, in the presence of NOx, VOCs can react in the atmosphere to form ozone, a noxious gas that can have acute and chronic impacts on human respiratory and cardiovascular system and can also cause damage to plants.
Particle size influences health effects, with the commonly described size fraction of PM10 (particles with a diameter of less than 10 µm) entering the lungs, and PM2.5 particles (particles with a diameter of less than 2.5 µm) able to penetrate deep lung tissue. Particles larger than 10 µm are generally trapped in the mucous membranes of the nose and throat and can be ingested, still resulting in exposure to adsorbed contaminants. Soil-derived particulates are generally larger than PM2.5.
The composition of particles also influences health responses. For example, diseases such as mesothelioma and silicosis are influenced by the mineralogical composition of the soil particles, with fibrous minerals such as naturally occurring asbestos and erionite giving rise to mesothelioma [32] and silica crystals giving rise to silicosis [33]. Soil particles may contain contaminants such as metals or organic compounds. Exposure via inhalation is typically lower than direct ingestion of contaminated soil but where contamination is widespread and wind erosion occurs, inhalation exposure may be greater.
Dust storms present an extreme example of the range of health effects arising from dust exposure and have been well documented in several countries, including developed countries [33,34]. Rublee et al. [35], for example, found that North American dust storms are associated with increases in the same day and lagged demand for critical care services at nearby hospitals. Respiratory ailments are among the most widely noted consequences of dust exposure, but other effects on human health range from cardiovascular ailments, conjunctivitis, dermatological disorders, and even injury and death related to transport accidents from dust [34,36]
Health effects may also arise from biotic material carried on soil particles such as bacteria, pollen spores, fungi and viruses. For example, endotoxins or lipopolysaccharides, the major components of the outer membrane of Gram-negative bacteria, are commonly used as indicators of the inflammatory potential of particulates [37]. Microbes causing infectious diseases such as influenza A, pulmonary coccidioidomycosis, bacterial pneumonia and meningococcal meningitis may also be present. More recently, PM2.5 particles and NO2 have been suggested as important factors triggering the spread and lethality of COVID-19 [38].
(b) . Zero hunger (SDG2)
SDG2 refers to food security, improved nutrition and the promotion of sustainable agriculture. Air pollution and food production are both interlinked: while agriculture contributes to air pollution via ammonia and other nitrogen compounds (see §2a(ii)) or increased wind erosion through tillage, air pollution can also negatively impact on food production and food security [39]. The impacts are either direct, affecting plant growth through the obstruction of photosynthesis and animal health, or indirect, affecting the effectiveness of agricultural inputs and thus crop yields. For instance, when soil microbes digest the ammonia in the atmosphere, they can make the soil more acidic, which can then reduce soil microbial community diversity [40]. Particles with heavy metals that are deposited onto topsoil through sedimentation, impact or interception can also affect plant growth and seed germination, ultimately reducing crop outputs [41,42]. However, this effect is minor compared with reduced productivity associated with the loss of the soil resource through wind erosion [36].
Airborne dust particles can also act as a vector for the transport of plant and animal pathogens (figure 2), which can then affect food production. Species within the genus Puccinia are responsible for much of the worldwide economic loss due to crop damage [43]; though it is known to spread aerially, it has not been identified in dust samples. A number of other plant and animal pathogens have been detected in dust (e.g. [10]).
By promoting soil conservation management practices to improve air quality, farmers can boost not only clean air but also soil health.
(c) . Sustainable cities (SDG11)
Urban vegetation is recognized to provide a plethora of benefits in addition to improving air quality, including ambient cooling and microclimate regulation (which can result in additional air quality gains through reducing local energy consumption and related emissions), storm water attenuation, improved mental and physical health, enhanced biodiversity, and climate change mitigation and adaptation (e.g. [44,45]). The extent to which these benefits are realized depends on the distribution and amount of green space across the urban area, as well as the individual species planted. Tools such as i-Tree, developed by the USDA, exist to support planning, management and advocacy for urban forests (e.g. [46,47]). Potentially negative impacts on air quality effects arising from biogenic VOC and pollen emissions can also be managed through judicious selection of species planted (e.g. [48]).
(d) . Climate action (SDG13)
A detailed discussion of the soil impacts on climate change are covered in Lal et al. [3]. However, in the context of soil impacts on air quality, it is worth commenting that global dust emissions are estimated to be the largest source of tropospheric aerosols and can have profound impacts on Earth's biosphere. Specifically, these particles impact climate by scattering and absorbing radiation (with clay particles being the most significant [6]), serve as nuclei for cloud formation and influence optical cloud properties [49]. Further climate effects arise from deposition of dust aerosols in the ocean, which provides limiting micronutrients such as iron influencing productivity and carbon sequestration of ocean ecosystems and affecting atmospheric concentrations of greenhouse gases [6].
(e) . Life on land and in water (SDG14 and 15)
Particulate and gaseous soil emissions (as well as other natural and anthropogenic emissions) can impact life on land and water both directly and indirectly. Some of these are discussed above in relation to food security and impacts on agricultural crops and animal health, and these same impacts are relevant to non-agricultural systems. Differing sensitivities to emissions that result in acidification, nutrient addition or pollutants can directly alter soil biodiversity and biomass/activity of soil organisms (e.g. [40,50,51]). Changes to aboveground plant communities resulting from emissions also have an indirect effect on soil biota through the feedback between the two [52]. These alterations to biodiversity and function then impact a host of other ecosystem processes and services (e.g. [53,54]). Effects on ocean ecosystems (as described in the preceding section) can, for instance, arise from the deposition of dust delivering limiting micronutrients that affect productivity.
4. Managing soils to improve air quality
The effectiveness of actions taken to manage soil to improve air quality depends on the nature and significance of the impact, and the efficacy of those actions. Specifically, management approaches to mitigation will have more impact on air quality in regions where the contribution of soil to the atmospheric load of a given contaminant is high. However, many of these management options have significant co-benefits, such as maintaining soil health or improving human well-being.
(a) . Erosion management
Strategies to control erosion in agricultural systems have largely been aimed at reducing soil exposure to wind, reducing wind speed or reducing soil movement [12] (table 1). The basic recommendation to reduce soil exposure to wind is by protecting the soil with live or dead vegetation and by limiting the time when the soil is bare. Vegetative barriers can act through filtration and/or interception of airborne particles and gases but also provide a root structure to keep the soil in place. Wind barriers can disrupt the erosive flow of wind over unprotected surfaces by slowing the airflow pattern over the land surface and reducing wind speed by 50–80% [12,31]. Other ways to stabilize soil movement involve conditioning the soil with water to control dust. Water management and water harvesting techniques can mitigate the suspension of soil particles in the air while helping other co-benefits such as reduced soil evaporation and soil moisture for better productivity [55].
Table 1.
Measures to control wind erosion in cropland, rangelands and natural ecosystems (from [12]).
| cropland | rangeland and natural ecosystems |
|---|---|
| reduce area and periods with little or no soil cover (e.g. adjustment of time of planting, reduced tillage) | manage vegetation in rangelands (e.g. reduce burning, avoid overgrazing, over-exploitation) |
| increase soil resistance to wind erosion (e.g. input of organic residue) | protect vegetation in natural steppe and desert areas (e.g. retain diverse vegetation cover) |
| reduce wind speed within and between fields | fix sand dunes (e.g. planting dead fences, grass, shrubs) |
| reduce soil movement (e.g. hedgerows, tree planting, tillage practices) |
Natural erosion in rangelands (natural grasslands or shrublands grazed by domestic livestock) vary as a function of climate, topography, vegetation composition and soil properties [56]. Preventive measures in rangelands, therefore, focus on limiting anthropogenic land degradation by avoiding overgrazing, burning or over-utilization in semi-arid and arid regions [49].
In natural ecosystems, protection measures also aim to reduce disturbance by retaining vegetation, reducing fire risk and minimizing disturbance of natural vegetation patchiness that could lead to desertification [57] (table 1).
(b) . Fertilizer management
Almost half the nitrogen received by the world's crops comes from synthetics fertilizers [58], but excessive N fertilizer use also results in reactive N entering the environment in harmful forms. This includes the gases NH3, NOx and N2O, and the water contaminant [59]. Fertilizer N inputs, therefore, need to be carefully managed to reduce N losses to the environment by matching N inputs to plant growth.
The form of the N fertilizer applied also affects the susceptibility to gaseous losses. Ammonium- and urea- (which hydrolyses to ammonium) based fertilizers are susceptible to NH3 losses. Urea-based fertilizers tend to have the highest volatilization losses due to the increase in pH following urea hydrolysis [60]. By contrast, while nitrate-based fertilizers do not produce NH3 (unless also containing ammonium), the nitrate is susceptible to leaching and denitrification to produce NOx and N2O.
Slow-release fertilizers avoid having large amounts of surplus N in the soil at any time. They can reduce NH3 losses by 40–78% compared with their conventional counterparts [61]. Organic fertilizers are similar to slow-release fertilizers in that time is required for the organic N to be mineralized to a plant-available form.
Another strategy is the use of urease inhibitors and nitrification inhibitors that slow the rates of urea hydrolysis and nitrification, respectively [61]. Slower urea hydrolysis reduces the amount of NH3 losses, while slower nitrification reduces the rate of leaching losses as well as N2O and NOx emissions. However, nitrification inhibitors can produce higher rates of ammonia emissions, particularly in soils with high pH and low cation exchange capacity [21,62]. One solution is to use both urease and nitrification inhibitors, which can reduce both NH3 and N2O [61].
The method of fertilizer application can also affect the gaseous losses. Subsurface application can decrease the loss of NH3. Yan et al. [63] found that on average, in upland crops and rice paddies in Asia, 23.5% of fertilizer N was lost as NH3 for top-dressed application but only 11.5% for incorporation of urea. Irrigating shortly after fertilizer application can also facilitate the transport of nitrogen down the soil profile where it is less vulnerable to gaseous loss. An average reduction in ammonia losses of 35% using irrigation compared with rain-fed or minimal irrigation was found by Pan et al. [21].
Other soil amendments that can reduce NH3 losses include compounds with a high ammonium-binding capacity (e.g. zeolite) or acidifying effect (e.g. humic or fulvic acid) [64,65].
The retention of crop residues on the soil surface is a common anti-erosion practice. However, a potential drawback is that it may prevent applied fertilizer from reaching the mineral soil, leading to increased NH3 losses [66].
(c) . Urban environments
Dust emissions from soils in urban environments are primarily restricted to localized activities such as construction, including land development and are often tightly managed as part of regulatory environmental management requirements. Thus, the main influence of soil on air quality in urban environments arises from their role in supporting plant growth, particularly tree growth. But underpinning any plant growth is soil. Urban soils are often poor quality, with low carbon and nutrient contents, compacted, and possibly contain contaminants. Poor soil quality is considered to be among the most significant limiting factors for optimal tree survival and growth in urban environments (e.g. [67]). As such, improving soil quality can be of considerable value to enhance plant growth. Several approaches can be used, although the addition of organic material, e.g. composts, mulches, is the most widespread and can be an ongoing strategy (e.g. [68]). During land development, other strategies such as retaining topsoil for use in vegetated areas and minimizing compaction of these soils will be helpful.
(d) . Soil-based technology
Biofiltration (removing gaseous contaminants through a bed containing soil) is a comparatively cheap method for removing odorous or toxic gases from polluted gas streams [69]. Examples of gases that may be removed using soil filters include hydrogen sulfide (H2S), ammonia (NH3) and hydrocarbons such as methane (CH4), carbon monoxide (CO) and ethylene (C2H2). The optimum performance of a biofilter requires that the bed be sufficiently porous to enable gas flow and that the soil moisture content and pH also be maintained at a sufficient level to sustain microbial activity [70,71]. The inoculation of soil with specific microbial species and/or conditioning soil with prior exposure to contaminant gases has also been shown to increase removal rates [72,73].
In addition to microbial processes, some chemical and physical processes in soils can remove pollutants. For example, iron-rich soils can be used to chemically remove H2S from the gas stream by the formation of iron sulfide [74,75].
Soils also play an important role in the reduction of pollution from landfills. Soil and clay are common materials used to cover landfills to reduce both the emission of gaseous pollutants from the waste and the infiltration of water into the landfill [76,77]. Soils from landfills can have large and highly active methanotroph (methane-oxidizing microbes) populations, which can eliminate 10–100% of the CH4 from the landfill gas [77,79].
(e) . Adapting to climate change
Climate change is likely to alter the contribution of soils to air quality not only through changes in temperature and rainfall patterns but also through changes in management practices to mitigate climate change impacts. However, it is difficult to gauge the level of change, with atmospheric dust also exerting an influence on climate through creating a dust–climate feedback from changes in the radiative balance due to atmospheric dust, and the sensitivity of the global dust cycle to climate with the potential to shape the climate of the major dust source regions such as Northern Africa and the Sahel [80]. Future dust activity and gaseous emissions are likely to depend on two main factors: land use in the source region and climate. For dust, both the climate in the dust region and the large-scale circulation that affects long-distance dust transport are important. Handmer et al. [81] noted low confidence in projecting future dust storm changes, due to difficulties in projecting future land use. However, dust storm activity is likely to increase where already dry regions will be prone to increased drought [12,82,83]. Climate change effects may not only increase dust emissions, but also reactivate areas prone to wind erosion [12]. Increased demand for water resources may also contribute to further desiccation of ephemeral water bodies and increased risks of dust storms. Reverse effects are also possible, with areas with increased precipitation projected in eastern Africa and east Asia.
NH3 emission and deposition are highly climate sensitive [84]. Future warming could potentially increase NH3 losses from agriculture. Shen et al. [85] predicted that under an intensely warming scenario, NH3 losses from agriculture in the USA could increase by 80% by 2100, but this could be mitigated by changing management practices.
5. Conclusion and outlook
Soils are an integral part of Earth's ecosystem and interact with the atmosphere both directly and by supporting the growth of plants. Plant photosynthesis is a major source of O2 required by most animal life. Soils also play an important role in the cycling of nitrogen, sulfur and carbon between the biosphere and the atmosphere.
Soil dust emissions, from largely natural sources, are estimated to be the largest source of tropospheric aerosols, giving rise to multiple impacts such as influencing the global radiative balance and cloud formation. However, human intervention to mitigate these emissions is challenging, given the vast areas from which the bulk of these emissions arise.
While agricultural soils are a source of food and income for people, they need to be carefully managed to avoid harmful emissions of dust, NH3 and greenhouse gases to the atmosphere, given these can lead to problems with human and animal health, and environmental degradation.
Soils (and the microbes and plants they support) can, however, be used to improve air quality at a local scale. Examples of this include the use of urban trees to reduce air pollution in cities and soil-based biofilters that can remove contaminants from pollution sources.
Climate change is likely to change the interactions between soil and air quality in a complex manner. Soil processes will be directly affected by changes in temperature and rainfall patterns, but also by changes in plant growth and management practices.
Careful management of soils is essential to ensure we continue to receive the many benefits they provide us.
Acknowledgements
We would like to thank Paul Mudge for his contribution to early discussions on the content of this paper, Robyn Simcock and Sandra Lavorel for comments on an early draft and Anne Austin for editing services.
Endnote
Salt marshes and estuaries, tropical forests (both soil and plant sources), soils (other than tropical forest) and wetlands. Note all sources were broken down by soil/plant.
Data accessibility
This article has no additional data.
Authors' contributions
All authors contributed to the conception and design of the manuscript and drafting and revising the paper. All authors gave final approval for publication and agree to be held accountable for the work performed therein.
Competing interests
We declare we have no competing interests.
Funding
Funding was provided by the Ministry of Business, Innovation and Employment (MBIE) strategic science investment fund.
References
- 1.Keesstra SD, et al. 2016The significance of soils and soil science towards realization of the United Nations Sustainable Development Goals. SOIL 2, 111-128. ( 10.5194/soil-2-111-2016) [DOI] [Google Scholar]
- 2.IPBES. 2019Summary for policymakers of the global assessment report on biodiversity and ecosystem services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services (eds S Díaz et al.). Bonn, Germany: IPBES Secretariat.
- 3.Lal R, Monger C, Nave L, Smith P. 2021The role of soils in regulation of climate. Phil. Trans. R. Soc. B 376, 20210084. ( 10.1098/rstb.2021.0084) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson CB, Seixas CS, Barbosa O, Fennessy MS, Díaz-José J, Herrera FB. 2019Determining nature's contributions to achieve the sustainable development goals. Sustain. Sci. 14, 543-547. ( 10.1007/s11625-018-0643-5) [DOI] [Google Scholar]
- 5.Barwise Y, Kumar P. 2020Designing vegetation barriers for urban air pollution abatement: a practical review for appropriate plant species selection. npj Clim. Atmos. Sci. 3, 12. ( 10.1038/s41612-020-0115-3) [DOI] [Google Scholar]
- 6.Kok JF. 2011A scaling theory for the size distribution of emitted dust aerosols suggests climate models underestimate the size of the global dust cycle. Proc. Natl Acad. Sci. USA 108, 1016-1021. ( 10.1073/pnas.1014798108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katra I. 2020Soil erosion by wind and dust emission in semi-arid soils due to agricultural activities. Agronomy 10, 89. ( 10.3390/agronomy10010089) [DOI] [Google Scholar]
- 8.Tagar AA, Adamowski J, Memon MS, Do MC, Mashori AS, Soomro AS, Bhayo WA. 2020Soil fragmentation and aggregate stability as affected by conventional tillage implements and relations with fractal dimensions. Soil Tillage Res. 197, 104494. ( 10.1016/j.still.2019.104494) [DOI] [Google Scholar]
- 9.Katra I, Laor S, Swet N, Kushmaro A, Ben-Dov E. 2017Shifting cyanobacterial diversity in response to agricultural soils associated with dust emission. Land Deg. Dev. 28, 878-886. ( 10.1002/ldr.2644) [DOI] [Google Scholar]
- 10.Gonzalez-Martin C, Teigell-Perez N, Valladares B, Griffin D. 2014The global dispersion of pathogenic microorganisms by dust storms and its relevance to agriculture. Adv. Agron. 127, 1-41. ( 10.1016/B978-0-12-800131-8.00001-7) [DOI] [Google Scholar]
- 11.Querol X, et al. 2019Monitoring the impact of desert dust outbreaks for air quality for health studies. Environ. Int. 130, 104867. ( 10.1016/j.envint.2019.05.061) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.UNEP, WMO, UNCCD. 2016Global assessment of sand and dust storms. Nairobi, Kenya: United Nations Environment Programme. [Google Scholar]
- 13.Ginoux P, Prospero JM, Gill TE, Hsu NC, Zhao M. 2012Global-scale attribution of anthropogenic and natural dust sources and their emission rates based on MODIS Deep Blue aerosol products. Rev. Geophys. 50, 1-35. ( 10.1029/2012RG000388) [DOI] [Google Scholar]
- 14.Pelletier JD. 2006Sensitivity of playa windblown-dust emissions to climatic and anthropogenic change. J. Arid Environ. 66, 62-75. ( 10.1016/j.jaridenv.2005.10.010) [DOI] [Google Scholar]
- 15.Stanelle T, Bey I, Raddatz T, Reick C, Tegen I. 2014Anthropogenically induced changes in twentieth century mineral dust burden and the associated impact on radiative forcing. J. Geophys. Res. Atmos. 119, 526-546. ( 10.1002/2014JD022062) [DOI] [Google Scholar]
- 16.Middleton N. 2019Variability and trends in dust storm frequency on decadal timescales: climatic drivers and human impacts. Geosciences 9, 261. ( 10.3390/geosciences9060261) [DOI] [Google Scholar]
- 17.Ravishankara AR, Daniel JS, Portman RW. 2009Nitrous oxide (N2O): the dominant ozone-depleting substance emitted in the 21st century. Science 326, 123-125. ( 10.1126/science.1176985) [DOI] [PubMed] [Google Scholar]
- 18.Galloway JN, Aber JD, Erisman JW, Seitzinger SP, Howarth RW, Cowling EB, Cosby BJ. 2003The nitrogen cascade. BioScience 53, 341-356. ( 10.1641/0006-3568(2003)053[0341:TNC]2.0.CO;2) [DOI] [Google Scholar]
- 19.Bradshaw J, Davis D, Grodzinsky G, Smyth S, Newell R, Sandholm S, Liu S. 2000Observed distributions of nitrogen oxides in the remote free troposphere from the NASA global troposphere experiment programs. Rev. Geophys. 38, 61-116. ( 10.1029/1999RG900015) [DOI] [Google Scholar]
- 20.Bouwman AF, Lee DS, Asman WAH, Dentener FJ, van der Hoek KW, Olivier JGJ. 1997A global high-resolution emission inventory for ammonia. Global Cycles 11, 561-587. ( 10.1029/97GB02266) [DOI] [Google Scholar]
- 21.Pan B, Lam SK, Mosier A, Luo Y, Chen D. 2016Ammonia volatilization from synthetic fertilizers and its mitigation strategies: a global synthesis. Agric. Ecosyst. Environ. 232, 283-289. ( 10.1016/j.agee.2016.08.019) [DOI] [Google Scholar]
- 22.Insam H, Seewald MSA. 2010Volatile organic compounds (VOCs) in soils. Biol. Fertil. Soils 46, 199-213. ( 10.1007/s00374-010-0442-3) [DOI] [Google Scholar]
- 23.Mäki M, Aaltonen H, Heinonsalo J, Hellén H, Pumpanen J, Bäck J. 2019Boreal forest soil is a significant and diverse source of volatile organic compounds. Plant Soil 441, 89-110. ( 10.1007/s11104-019-04092-z) [DOI] [Google Scholar]
- 24.Watts SF. 2000The mass budgets of carbonyl sulfide, dimethyl sulfide, carbon disulfide and hydrogen sulfide. Atmos. Environ. 34, 761-779. ( 10.1016/S1352-2310(99)00342-8) [DOI] [Google Scholar]
- 25.Macdonald BCT, Denmead OT, White I, Melville MD. 2004Natural sulfur dioxide emissions from sulfuric soils. Atmos. Environ. 38, 1473-1480. ( 10.1016/j.atmosenv.2003.12.005) [DOI] [Google Scholar]
- 26.Berglen TF, Berntsen TK, Isaksen ISA, Sundet JK. 2004A global model of the coupled sulfur/oxidant chemistry in the troposphere: the sulfur cycle. J Geophys. Res. 109, 19310. ( 10.1029/2003JD003948) [DOI] [Google Scholar]
- 27.Gao W, Yang H, Kou L, Li S. 2015Effects of nitrogen deposition and fertilization on N transformations in forest soils: a review. J. Soils Sediments 15, 863-879. ( 10.1007/s11368-015-1064-z) [DOI] [Google Scholar]
- 28.Holland EA, Dentener FJ, Braswell BH, Sulzman JM. 1999Contemporary and pre-industrial global reactive nitrogen budgets. Biogeochemistry 46, 7-43. [Google Scholar]
- 29.Gao Y, Ma M, Yang T, Chen W, Yang T. 2018Global atmospheric sulfur deposition and associated impaction on nitrogen cycling ecosystems. J Clean. Prod. 195, 1-9. ( 10.1016/j.jclepro.2018.05.166) [DOI] [Google Scholar]
- 30.United States Department of Agriculture (USDA). 2017Agricultural air quality conservation measures: reference guide for poultry and livestock production systems. See https://www.epa.gov/sites/production/files/2017-01/documents/web_placeholder.pdf.
- 31.United States Department of Agriculture (USDA). 2012Agricultural air quality conservation measures: reference guide for cropping systems and general land management. See https://www.epa.gov/sites/production/files/2016-06/documents/agaqconsmeasures.pdf.
- 32.Brook M, Black PM, Salmond J, Dirks KN, Berry T-A, Steinhorn G. 2020Erionite in Auckland bedrock and malignant mesothelioma: an emerging public and occupational health hazard? NZ Med. J. Viewpoint 133, 1518. [PubMed] [Google Scholar]
- 33.Schweitzer MD, Calzadilla AS, Salamo O, Sharifi A, Kumar N, Holt G, Campos M, Mirsaeidi M. 2018Lung health in era of climate change and dust storms. Environ. Res. 163, 36-42. ( 10.1016/j.envres.2018.02.001) [DOI] [PubMed] [Google Scholar]
- 34.Goudie AS. 2014Desert dust and human health disorders. Environ. Int. 63, 101-113. ( 10.1016/j.envint.2013.10.011) [DOI] [PubMed] [Google Scholar]
- 35.Rublee CS, Sorensen CJ, Lemery J, Wade TJ, Sams EA, Hilborn ED, Crooks JL. 2020Associations between dust storms and intensive care unit admissions in the United States, 2000–2015. Geohealth 4, e2020GH000260. ( 10.1029/2020GH000260) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Middleton N, Kang U. 2017Sand and dust storms: impact mitigation. Sustainability 9, 1053. ( 10.3390/su9061053) [DOI] [Google Scholar]
- 37.Rolphe C, et al. 2018Sources of airborne endotoxins in ambient air and exposure of nearby communities—a review. Atmosphere 9, 375. ( 10.3390/atmos9100375) [DOI] [Google Scholar]
- 38.Copat C, Cristaldi A, Fiore M, Grasso A, Zuccarello P, Signorelli SS, Conti GO, Ferrante M. 2020The role of air pollution (PM and NO2) in COVID-19 spread and lethality: a systematic review. Environ. Res. 191, 110-129. ( 10.1016/j.envres.2020.110129) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun F, Dai Y, Yu X. 2017Air pollution, food production and food security: a review from the perspective of food system. J. Integr. Agric. 16, 2945-2962. ( 10.1016/S2095-3119(17)61814-8) [DOI] [Google Scholar]
- 40.Fierer N, Jackson RB. 2006The diversity and biogeography of soil bacterial communities. Proc. Natl Acad. Sci. USA 103, 626-631. ( 10.1073/pnas.0507535103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peralta JR, Gardea-Torresdey JL, Tiemann KJ, Gomez E, Arteaga S, Rascon E, Parsons JG. 2001Uptake and effects of five heavy metals on seed germination and plant growth in alfalfa (Medicago sativa L.). Bull. Environ. Contam. Toxicol. 66, 727-734. ( 10.1007/s001280069) [DOI] [PubMed] [Google Scholar]
- 42.Pandey N, Sharma CP. 2002Effect of heavy metals Co2+, Ni2+ and Cd2+ on growth and metabolism of cabbage. Plant Sci. 163, 753-758. ( 10.1016/S0168-9452(02)00210-8) [DOI] [Google Scholar]
- 43.Strange RN, Scott PR. 2005Plant disease: a threat to global food security. Annu. Rev. Phytopathol. 4, 83-116. ( 10.1146/annurev.phyto.43.113004.133839) [DOI] [PubMed] [Google Scholar]
- 44.Jim CY, Chen W. 2009Ecosystem services and valuation of urban forests in China. Cities 26, 187-194. ( 10.1016/j.cities.2009.03.003) [DOI] [Google Scholar]
- 45.Oldfield EE, et al. 2015Growing the urban forest: tree performance in response to biotic and abiotic land management. Restor. Ecol. 23, 707-718. ( 10.1111/rec.12230) [DOI] [Google Scholar]
- 46.Nowak DS, Maco S, Brinkley M. 2018i-Tree: global tools to assess tree benefits and risks to improve forest management. Arboricult. Consult. 51, 10. [Google Scholar]
- 47.Badach J, Dymnicka M, Baranowski A. 2020Urban vegetation in air quality management: a review and policy framework. Sustainability 12, 1258. ( 10.3390/su12031258) [DOI] [Google Scholar]
- 48.Simpson J, McPherson EG. 2011The tree BVOC index. Environ. Pollut. 159, 2088-2093. ( 10.1016/j.envpol.2011.02.034) [DOI] [PubMed] [Google Scholar]
- 49.Ravi S, et al. 2011Aeolian processes and the biosphere. Rev. Geophys. 49, RG3001. ( 10.1029/2010RG000328) [DOI] [Google Scholar]
- 50.Elser JJ, et al. 2000Biological stoichiometry from genes to ecosystems. Ecol. Lett. 3, 540-550. ( 10.1046/j.1461-0248.2000.00185.x) [DOI] [Google Scholar]
- 51.Giller KE, Witter E, McGrath SP. 1998Toxicity of heavy metals to microorganisms and microbial processes in agricultural soils: a review. Soil Biol. Biochem. 30, 1389-1414. ( 10.1016/S0038-0717(97)00270-8) [DOI] [Google Scholar]
- 52.Van der Putten WH, et al. 2013Plant–soil feedbacks: the past, the present and future challenges. J. Ecol. 101, 265-276. ( 10.1111/1365-2745.12054) [DOI] [Google Scholar]
- 53.Singh BK, et al. 2014Loss of microbial diversity in soils is coincident with reductions in some specialized functions. Environ. Microbiol. 16, 2408-2420. ( 10.1111/1462-2920.12353) [DOI] [PubMed] [Google Scholar]
- 54.Cardinale BJ, et al. 2012Biodiversity loss and its impact on humanity. Nature 486, 59-67. ( 10.1038/nature11148) [DOI] [PubMed] [Google Scholar]
- 55.Schwilch G, Liniger HP, Hurni H. 2014Sustainable land management (SLM) practices in drylands: how do they address desertification threats? Environ. Manage. 54, 983-1004. ( 10.1007/s00267-013-0071-3) [DOI] [PubMed] [Google Scholar]
- 56.Webb NP, Herrick JE, Duniway MC. 2014Ecological site-based assessments of wind and water erosion: informing accelerated soil erosion management in rangelands. Ecol. Appl. 24, 1405-1420. ( 10.1890/13-1175.1) [DOI] [PubMed] [Google Scholar]
- 57.Okin GS, Parsons AJ, Wainwright J, Herrick JE, Bestelmeyer BT, Peters DC, Fredrickson EL. 2009Do changes in connectivity explain desertification? BioScience 59, 237-244. ( 10.1525/bio.2009.59.3.8) [DOI] [Google Scholar]
- 58.Smil V. 1999Nitrogen in crop production: an account of global flows. Glob. Biogeochem. Cycles 13, 647-662. ( 10.1029/1999GB900015) [DOI] [Google Scholar]
- 59.Congreaves KA, van Eerd LL. 2015Nitrogen management and cycling in intensive horticulture systems. Nutr. Cycl. Agroecosyst. 102, 299-318. ( 10.1007/s10705-015-9704-7) [DOI] [Google Scholar]
- 60.Turner DA, Edis RE, Chen D, Freney JR, Denmead OT. 2012Ammonia volatilization from nitrogen fertilizers applied to cereals in two cropping areas of southern Australia. Nutri. Cycl. Agroecosyst. 93, 113-126. ( 10.1007/s10705-012-9504-2) [DOI] [Google Scholar]
- 61.Dimkpa CO, Fugice J, Singh U, Lewis TD. 2020Development of fertilizers for enhanced nitrogen use efficiency—trends and perspectives. Sci. Tot. Environ. 731, 139113. ( 10.1016/j.scitotenv.2020.139113) [DOI] [PubMed] [Google Scholar]
- 62.Kim DG, Saggar S, Roudier P. 2012The effect of nitrification inhibitors on soil ammonia emissions in nitrogen managed soils: a meta-analysis. Nutr. Cycl. Agroecosystems 93, 51-64. ( 10.1007/s10705-012-9498-9) [DOI] [Google Scholar]
- 63.Yan X, Akimoto H, Ohara T. 2003Estimation of nitrous oxide, nitric oxide and ammonia emissions from croplands in East, Southeast and South Asia. Glob. Change Biol. 9, 1080-1096. ( 10.1046/j.1365-2486.2003.00649.x) [DOI] [Google Scholar]
- 64.Ahmed O, Aminuddin H, Husni M. 2006Reducing ammonia loss from urea and improving soil-exchangeable ammonium retention through mixing triple superphosphate, humic acid and zeolite. Soil Use Manag. 22, 315-319. ( 10.1111/j.1475-2743.2006.00040.x) [DOI] [Google Scholar]
- 65.Rosliza S, Ahmed OH, Muhamad AB, Majid N, Jalloh MB. 2009Reducing ammonia loss from urea by mixing with humic and fulvic acids isolated from coal. Am. J. Environ. Sci. 5, 420-426. ( 10.3844/ajessp.2009.420.426) [DOI] [Google Scholar]
- 66.De Ruijter FJ, Huijsmans JFM, Rutgers B. 2010Ammonia volatilization from crop residues and frozen green manure crops. Atmos. Environ. 44, 3362-3368. ( 10.1016/j.atmosenv.2010.06.019) [DOI] [Google Scholar]
- 67.Layman RM, Day SD, Mitchellc DK, Chen Y, Harrisa JR, Daniels WL. 2016Below ground matters: urban soil rehabilitation increases tree canopy and speeds establishment. Urban For. Urban Green. 16, 25-35. ( 10.1016/j.ufug.2016.01.004) [DOI] [Google Scholar]
- 68.Saebo A, Ferrini F. 2006The use of compost in urban green areas—a review for practical application. Urban For. Urban Green. 4, 159-169. ( 10.1016/j.ufug.2006.01.003) [DOI] [Google Scholar]
- 69.Barbusinski K, Kalemba K, Kasperczyk D, Urbaniec K, Kozik V. 2017Biological methods for odor treatment: a review. J Clean. Prod. 152, 223-241. ( 10.1016/j.jclepro.2017.03.093) [DOI] [Google Scholar]
- 70.La H, Hettiaratchi JPA, Achari G, Dunfield PF. 2018Biofiltration of methane. Bioresour. Technol. 268, 759-772. ( 10.1016/j.biortech.2018.07.043) [DOI] [PubMed] [Google Scholar]
- 71.Vikrant K, Kailasa SK, Tsang DCW, Lee SS, Kumar P, Giri BS, Singh RS, Kim KH. 2018Biofiltration of hydrogen sulfide: trends and challenges. J. Clean. Prod. 187, 131-147. ( 10.1016/j.jclepro.2018.03.188) [DOI] [Google Scholar]
- 72.Frye RJ, Welsh D, Berry TM, Stevenson BA, McCallum T. 1992Removal of contaminant gases from air in closed systems by soil. Soil Biol. Biochem. 24, 607-612. ( 10.1016/0038-0717(92)90087-E) [DOI] [Google Scholar]
- 73.Elsgaard L. 1998Ethylene removal by a biofilter with immobilized bacteria. Appl. Environ. Microb. 64, 4168-4173. ( 10.1128/AEM.64.11.4168-4173.1998) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Skerman AG, Heubeck S, Batstone DJ, Tait S. 2017Low-cost filter media for removal of hydrogen sulphide from piggery biogas. Process Saf. Environ. Protect. 105, 117-126. ( 10.1016/j.psep.2016.11.001) [DOI] [Google Scholar]
- 75.Pham CH, Saggar S, Berben P, Palmada T, Ross C. 2019Removing hydrogen sulfide contamination in biogas produced from animal wastes. J. Environ. Qual. 48, 32-38. ( 10.2134/jeq2018.07.0271) [DOI] [PubMed] [Google Scholar]
- 76.Sadavisam BY, Reddy KR. 2014Landfill methane oxidation in soil and bio-based cover systems: a review. Rev. Environ. Sci. Biotech. 13, 79-107. ( 10.1007/s11157-013-9325-z) [DOI] [Google Scholar]
- 77.Ménard C, Ramirez AA, Nikiema J, Heitz M. 2012Biofiltration of methane and trace gases from landfills: a review. Environ. Rev. 20, 40-53. ( 10.1139/a11-022) [DOI] [Google Scholar]
- 78.Tate KR, Walcroft AS, Pratt C. 2012Varying atmospheric methane concentrations affect soil methane oxidation rates and methanotroph populations in pasture, an adjacent pine forest, and a landfill. Soil Biol. Biochem. 52, 75-81. ( 10.1016/j.soilbio.2012.04.011) [DOI] [Google Scholar]
- 79.Nikiema J, Brzezinsk R, Heitz M. 2007Elimination of methane generated from landfills by biofiltration: a review. Rev. Environ. Sci. Biotech. 6, 261-284. ( 10.1007/s11157-006-9114-z) [DOI] [Google Scholar]
- 80.Kok JF, Ward DS, Mahowald NM, Evan AT. 2018Global and regional importance of the direct dust–climate feedback. Nat. Commun. 9, 241. ( 10.1038/s41467-017-02620-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Handmer J, et al. 2012Changes in impacts of climate extremes: human systems and ecosystems. In Managing the risks of extreme events and disasters to advance climate change adaptation. A special report of working groups I and II of the Intergovernmental Panel on Climate Change (IPCC) (eds Field CD, et al.), pp. 231-290. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 82.Clifford HM, et al. 2019A 2000 year Saharan dust event proxy record from an ice core in the European alps. J. Geophys. Res. Atmos. 124, 12 882-12 900. ( 10.1029/2019JD030725) [DOI] [Google Scholar]
- 83.Achakulwisut P, et al. 2019Effects of increasing aridity on ambient dust and public health in the U.S. southwest under climate change. GeoHealth 3, 127-144. ( 10.1029/2019GH000187) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sutton MA, et al. 2013Towards a climate-dependent paradigm of ammonia emission and deposition. Phil. Trans. R. Soc. B 368, 20130166. ( 10.1098/rstb.2013.0166) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shen H, Chen Y, Hu Y, Ran L, Lam SK, Pavur GK, Zhou F, Pleim JE, Russell AG. 2020Intense warming will significantly increase cropland ammonia volatilization threatening food security and ecosystem health. One Earth 3, 126-134. ( 10.1016/j.oneear.2020.06.015) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.