Abstract
Soil and soil biodiversity play critical roles in Nature's Contributions to People (NCP) # 10, defined as Nature's ability to regulate direct detrimental effects on humans, and on human-important plants and animals, through the control or regulation of particular organisms considered to be harmful. We provide an overview of pathogens in soil, focusing on human and crop pathogens, and discuss general strategies, and examples, of how soils' extraordinarily diverse microbial communities regulate soil-borne pathogens. We review the ecological principles underpinning the regulation of soil pathogens, as well as relationships between pathogen suppression and soil health. Mechanisms and specific examples are presented of how soil and soil biota are involved in regulating pathogens of humans and plants. We evaluate how specific agricultural management practices can either promote or interfere with soil's ability to regulate pathogens. Finally, we conclude with how integrating soil, plant, animal and human health through a ‘One Health’ framework could lead to more integrated, efficient and multifunctional strategies for regulating detrimental organisms and processes.
This article is part of the theme issue ‘The role of soils in delivering Nature's Contributions to People’.
Keywords: soils, microbiology, pathogens, humans, crops
1. Introduction
(a) . Overview
Soil's vast biodiversity is crucial in regulating the impacts of pathogens and pests on microorganisms, plants and animals, including humans [1,2]. The complexity of soils' diverse communities and micro-environments provide a myriad of mechanisms, and potential solutions, for regulating detrimental organisms. Many of these biological processes are indirect, not visible, involve the actions of complex consortia of organisms, and are intimately linked to their physical environment and its management. Nonetheless, over the past few decades, the growing availability of new tools, both molecular and imaging, has expanded our ability to investigate and elucidate these complex soil phenomena.
The Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services (IPBES) introduced the framework of Nature's Contributions to People (NCP) defined as ‘all the positive contributions, losses or detriments, that people obtain from nature’ to capture both beneficial and harmful effects of nature on people's quality of life [3–5]. NCP include both positive and negative contributions of all of living nature (e.g. diversity of organisms, ecosystems and processes) to people's quality of life [6]. Here, we specifically explore how soil contributes to NCP #10, defined as nature's ability to regulate direct detrimental effects on humans, and on human-important plants and animals, through the control or stimulation of particular organisms considered to be harmful [6].
Though NCP #10 encompasses the regulation of a variety of categories of detrimental organisms, including pests, predators, competitors, parasites and other potentially harmful organisms, we focus specifically on microbial pathogens, with an emphasis on humans and crops. Considering both biological and abiotic processes and properties, we first review the ecological principles underpinning regulation of pathogens, as well as relationships between pathogen suppression and soil health. We provide specific examples of ways in which soil can control the potential for pathogens to infect humans and crops. Agricultural management practices used to control pathogens are discussed with respect to how they may promote or interfere with soils' ability to regulate detrimental organisms. Though our focus here is on agroecosystems, many concepts and examples are relevant to less disturbed ecosystems.
(b) . How soil biota regulates detrimental organisms and processes
Soil hosts arguably the most diverse biological communities on Earth [7,8]. Soil's heterogeneity, created by complex arrangements of minerals and organic matter of different structure and composition, organized into aggregates of different sizes, provides a vast variety of ecological niches [1]. Many of soil's inhabitants are either directly or indirectly involved in regulating organisms that may have detrimental effects on humans. At the same time, soil is also a reservoir of a variety of bacterial, fungal and viral pathogens capable of causing diseases in plants, animals or humans [9–15].
Pathogens are common members of soil communities who may be transient inhabitants, consistently present for parts of their life cycle, or permanent fixtures within the community [16]. Whether or not potential pathogens have negative impacts on humans and other biota depends on if they successfully colonize and establish themselves in soil (if they are not resident species) and come in contact with a susceptible host. Some pathogens require transfer via vectors or carriers who live in the soil. Other important factors include whether pathogen populations reach high enough levels to cause infections (inoculum potential) and whether they are metabolically capable of causing infection [17–19].
The survival of any organism in soil, pathogenic or not, depends on its interactions with other members of their biological communities (biotic factors). In soil, a diversity of interspecific interactions—mutualism, antagonism (including parasitism and predation), competition and commensalism—determine the presence and impacts of soil organisms, including those who become pathogenic. Some relationships are indirect, with pathogen invasion, establishment and survival depending on relatively general changes in soil communities, such as an increased population density or biomass. For example, diffuse competition with a broad swathe of the microbial community could regulate pathogens in soils by limiting their access to resources [20,21]. However, in other cases, more direct antagonistic or competitive interactions can prove critical. Kinkel et al. [22], for example, present a coevolutionary framework for investigating and managing soil communities capable of suppressing plant diseases. Drawing on the importance of antagonistic coevolutionary relationships in disease suppression, they demonstrate the importance of direct interactions between pathogens and non-pathogenic indigenous soil microbes for promoting and sustaining disease suppression in soils [22].
The remarkable diversity of soil microbial communities is crucial in deterring pathogen establishment and growth. Soil communities with greater diversity may be more likely to include antagonists or competitors that are particularly effective at suppressing invasive pathogens (i.e. the ‘sampling effect’) [23–25]. They may also contain more competitors or antagonists that act in concert to suppress pathogens (i.e. niche complementarity). Strong relationships between diversity and invasibility are observed in many ecological systems [25]; for example, non-native plants are more likely to invade grasslands as native plant diversity is lost [26]. Similarly, reduction of bacterial or fungal diversity in soil, demonstrated experimentally, increases vulnerability to invasion by pathogens [20,27].
Another consequence of high levels of biodiversity is greater ecological resilience; that is, ‘the capacity of a system to absorb disturbance and reorganize while undergoing change so as to still retain essentially the same function, structure, identity, and feedbacks' [28,29]. Soil microbiomes have a high degree of functional redundancy whereby multiple actors play similar roles in the soil ecosystem [30]. The resilience of complex biological systems is defined not just by the number of different species that respond to a specific disturbance, but more importantly by response diversity, i.e. the range of responses to the disturbance [31,32]. Diverse soil communities also have the capacity to bounce back more quickly from perturbations (such as invasion of a pathogen) and not bounce as far in subsequent exposures [33]. However, repeated disturbances that reduce the diversity of soil microbial communities, as is common in large-scale, intensive agricultural management, can reduce the response diversity, and, therefore, reduce the resistance and resilience of agroecosystems, creating opportunities for invasion by non-native organisms [32,34]. Fortunately, targeted management practices that increase soil microbial diversity and biomass can strengthen direct and indirect antagonistic interactions and consequently help regulate deleterious organisms.
Changes in soil abiotic factors are interdependent and exert substantial impacts on soil function and biodiversity [35]. For example, disturbing soil aggregates, key components of soil structure, homogenize the soil environment, expose protected organic matter to degradation, reduce niche complexity and lead to biodiversity losses [36]. Intensive agricultural practices, land-use changes and global climate change are recognized as major threats that modify the soil environment and reduce soil biodiversity [7,37]. Even so, none of these factors can be considered in isolation to test their effects on biodiversity as they interact and influence one another. Better understanding of mechanisms underlying management practices that focus on soil environment can facilitate the conservation of soil biomass and biodiversity [7,38].
(c) . Relationship between the concept of soil health and the regulation of detrimental organisms
The concept of soil health has reemerged over the last few decades and grown in importance in sustainable agriculture and climate resilience [39]. Healthy soils are broadly defined as those being ‘capable of supporting the production of food and fiber, to a level and with a quality sufficient to meet human requirements, together with continued delivery of other ecosystem services that are essential for maintenance of the quality of life for humans and the conservation of biodiversity’ [40]. Much of the emphasis in soil health assessments has been placed on soil organic carbon, nutrient cycling, soil structure and water relations, and general microbial activities. Most existing conceptual frameworks or tools for soil health do not directly consider suppression of detrimental organisms to plants and humans [41], despite the fact that suppressiveness is often an additional benefit of practices targeting other soil health indicators.
Two decades ago, van Bruggen & Semenov [42] proposed considering incidences of plant and animal disease as symptoms of an ecosystem in poor health. They argued that the ability of the biological community to suppress or reduce populations of pathogens (e.g. ‘disease suppression’) is an important indicator of a stable and healthy soil ecosystem. Several years later, Janvier et al. [43] proposed including suppressiveness as an indicator of soil health. More recently, Lehmann et al. [44] proposed a ‘new generation’ of soil health indicators and recommended the inclusion of biological assessments of ability of soil to suppress disease. Thus, an emerging consensus is disease suppressiveness is an integral component of soil health. A challenge is the difficulty in assessing disease potential in soils and thus in identifying appropriate and feasible indicators, as well as methodologies for measuring them [11,43,45].
2. Role of soil and soil biota in regulation of human and animal pathogens
(a) . Pathogens of humans and other animals in soil
Soil is a reservoir for a variety of microorganisms—including bacteria, fungi, protozoa and viruses—that can be pathogenic to humans and animals. Human and animal pathogens in soil can be categorized into four broad groups that reflect their degree of residency in soil: permanent, periodic, transient and incidental [46]. Permanent pathogens are soil inhabitants which spend their entire life cycle in soil and sometimes become infectious for humans and animals. Examples include organisms like Clostridium botulinum or Clostridium tetani that produce neurotoxins when contaminated food is ingested or through contaminated wounds, respectively. Many zoonotic pathogens also either live in soil or their vectors live or spend part of their life cycle in soil [15]. Periodic pathogens are soil organisms which require the soil environment to complete part of their life cycle. For example, Bacillus anthracis, the causative agent for anthrax in humans or livestock, is often found in soils and can survive for long periods as endospores [47]. Transient organisms are those which naturally occur in soil, often due to their hosts being in contact with soil, but do not require soil to complete their life cycle. For example, the protozoan parasite Giardia lamblia can be introduced into soil via urine and faeces of rodents but does not need soil to survive. Finally, incidental pathogens may be introduced to soil via anthropogenic sources, human and animal activities, or other pathways but only survive in soils for relatively short periods. For example, pathogens enter soil via application of improperly treated raw manure, contaminated irrigation water or via runoff, especially when croplands are in close proximity to livestock grazing areas or feedlots [48–51]. Examples include enteric pathogens (e.g. Salmonella enterica subsp. enterica and shiga toxin-producing Escherichia coli (STEC)) who are introduced into soil via raw or untreated manure or sewage [52,53]. Pathogen residency in soil is important as it determines the potential mechanisms for suppression (figure 1). The impacts of soil microbial pathogens on vertebrates have been reviewed extensively elsewhere [54,55].
Figure 1.
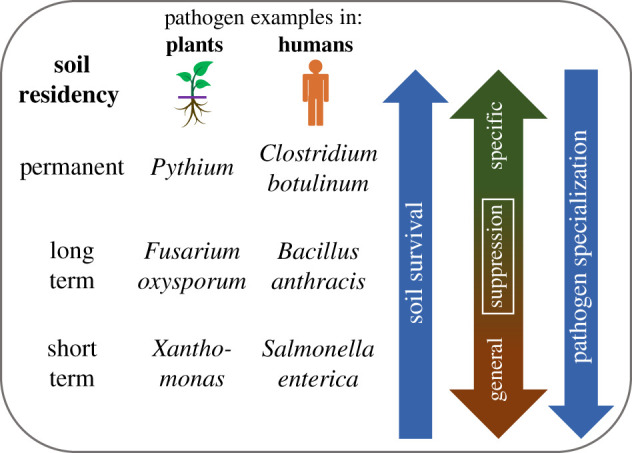
Pathogen ability to survive and grow in soil is typically inversely proportional to its specialization for a specific plant or animal (human) host. Long-term presence is necessary for pathogen–antagonist interactions that generate specific suppression; by contrast, pathogen presence is not required for antagonist–antagonist interactions that contribute to general suppression [41]. Permanent members of microbial communities are, therefore, likely controlled by specific suppression, while short-term survivors are likely regulated by general suppression. (Online version in colour.)
(b) . How soils regulate the establishment and suppression of human/animal pathogens
The soil environment and its indigenous communities strongly influence whether or not a pathogen can colonize and establish itself in soil, as well as whether it ultimately can infect a potential host [41,56,57]. Mechanisms can include diffuse competitive and antagonistic interactions between the pathogen and the entire microbial community, akin to ‘general suppression’ in the plant pathogen literature (see below). For example, larger and more diverse microbial communities, such as those associated with organically managed soils, may be more effective than communities under conventional management at suppressing pathogenic E. coli [58]. Similar trends have also been observed with Salmonella: soils with higher microbial diversity were more effective at decreasing Salmonella abundance and survival time [14]. Conversely, pathogen survival often increases dramatically when inoculated into soils that have been sterilized [59,60]. Introduced human enteric bacteria had lower rates of survival in microbially diverse soils [61], supporting the concept that invasion may be transient and limited [62]. Finally, it is important to note that soil microbes constitute only a part of the biological community in soils: arthropods, protists and other taxa can also play key roles in soil suppressiveness [58,63]. For example, dung beetles have been shown to reduce pathogenic E. coli on fresh produce farms by ingesting faeces contaminated with pathogenic E. coli and burying them, thereby further exposing the pathogens to competitors and antagonists within the soil microbial community [58].
Soil physical properties are also important in regulating establishment and survival of pathogens in soil. First, soil texture (i.e. the relative proportions of sand, silt and clay particles) helps determine the persistence and establishment of many pathogens, e.g. fine-textured clay soil may be more conducive to survival of pathogens [46,64]. Second, pH has also shown to be important for pathogen survival; for example, Listeria monocytogenes survived best at soil pH values greater than 7 [60]. Third, soil moisture can also determine the ability of a pathogen to persist and survive in soil. For example, one study found that pathogenic E. coli populations often peak following rainfall events [65] and another reported that rainfall and soil moisture were key factors influencing pathogenic E. coli survival [66]. Conversely, microbial growth decreases when soil becomes drier, as dry soils can impede microbial mobility, limit nutrient availability and slow nutrient diffusion through membranes [46]. Fourth, organic matter content, which often varies according to soil types and inputs, can also govern the survival of pathogens in soil. One study showed that Salmonella sp. survived longer in soils with higher organic matter content [51]; however, others show the opposite trend with higher organic matter supporting greater biologically induced suppression [67,68]. And fifth, concentrations of macro and micronutrients in soil influence pathogen survival; for example, the pool of exchangeable soil cations best explained survival of L. monocytogenes in different soils [60]. Critically, many factors listed above are strongly associated; for example, increasing organic matter in soils also improves moisture and nutrient retention, which can make the environment more conducive for pathogen proliferation.
Many human and animal pathogens are closely interrelated. Soil can play a role as an intermediary reservoir (e.g. pathogens from soil-applied animal manure infecting humans) or represent an integral part of pathogen life cycles [15]. Approximately 75% of new and emerging infectious diseases of humans are thought to originate from animals [69] and such diseases are defined as zoonotic diseases or zoonoses [70]. Some viruses find hosts in both humans and soil animals; for example, rodents living in soil burrows can be vectors for hantavirus [71]. How we manage agricultural soil can influence the prevalence of zoonotic diseases such as those caused by enteropathogenic bacteria such as Salmonella or pathogenic E. coli [72,73]. Potential sources and routes of faecal contamination of soil and produce include contaminated irrigation water, manure applications, proximity to livestock, wildlife or domesticated animal intrusion [48]. Moreover, husbandry and management practices (e.g. diet, health status, age, location) may affect the shedding of food-borne pathogens and increase persistence and risk of faecal contamination when animal manure is applied [48,74]. Manure application may also contribute to the propagation and dissemination of antibiotic residues, antibiotic-resistant bacteria and antibiotic-resistant resistance genes in the soil–water system and pose a public health issue [75]. The One Health approach to zoonotic disease transmission investigation involves multidisciplinary teams of biologists, ecologists, epidemiologists and physicians [76]. Growing evidence suggests that the prevention of livestock-associated zoonoses must start with developing animal health management routines and welfare programmes (such as vaccinations, prebiotic and probiotic feed additives) at the farm level and this will lead to improve overall animal and ecosystem health [77].
(c) . Soil health and human health
The capacity of soil biota to regulate organisms detrimental to human health can extend even beyond the soil itself and into the human body. First, the rich biodiversity of soils has been the source of most of the antibiotics and other antimicrobial agents currently used for human and animal health [16,78]. Waksman discovered streptomycin produced by soil actinomycetes using bacterial isolation techniques still employed by pharmaceutical industries today [79,80]. As antibiotic resistance rises among human pathogens, interest has renewed in identifying microorganisms that are capable of producing antimicrobial compounds in soil, building on our increasing understanding of soil community ecology and emergence of powerful new technologies (e.g. omics) [80,81].
Second, the relationship between human and soil microbiomes is a topic of rapidly growing interest for reasons of human health and nutrition [82,83]. Soils and humans have shared a long and intimate relationship [82,84]. Yet, humans have increasingly moved from rural to urban settings, leaving behind many opportunities for exposure to the soil microbiome. Recent evidence suggests that declines in human immunity and health may result from this disconnection from the natural environment. The ‘biodiversity hypothesis' states that ‘contact with natural environments enriches the human microbiome, promotes immune balance and protects from allergy and inflammatory disorders’ [85]. For example, early childhood exposure to environmental microorganisms appears to be associated with development of the body's immune system and certain positive health benefits [83,86]. Emerging research is suggesting that gut microbial diversities of healthy adults from rural communities throughout the world (e.g. Papua New Guinea, Malawi, Tanzania and Amazon) are higher than in urban populations in Italy and the USA [83]; however, soil's specific role in promoting these differences is yet to be isolated from contributions associated with diet and genetics.
Exploring relationships between the microbiomes of animals and surrounding soil is a new and evolving area of research. The composition of gut microbiomes of foraging baboons was better explained by the baboons' environment than by genetic factors, and in particular dependent on the soil's geologic history and exchangeable sodium [87]. Comparable research on human–soil relations is rare, speculative or does not yet exist but is underway with the expansion and ease of sequencing microbiomes in humans and of different environments [88,89]. A deeper understanding of the complex interrelationships and feedback between soil and humans will not only help reduce risks of soil-borne and food-borne disease but may also improve the general health of humans.
3. Role of soil and soil biota in regulation of plant pathogens
(a) . Pathogens of plants in soil
Plants face different challenges than do animals from the perspective of soil-borne diseases. Humans and most terrestrial animals are primarily in contact with the soil's surface and exposed to small amounts via ingestion, inhalation and dermal contact. Plants, on the other hand, are literally rooted in soils. To access the diffusely distributed nutrients and water in soil, their roots are in intimate contact with soil throughout their lifespans. A draw-back is that the plant has no ‘down time’ nor can it escape from the soil, and thus strategies are needed to deal with continuous assaults from soil pathogens. A benefit of this intimacy, however, is there is time (including evolutionary time) for the plant to develop collaborations with rhizosphere organisms to regulate impact of pathogens.
What are termed ‘disease suppressive soils'—soils whose indigenous microorganisms reduce establishment, persistence or impacts of pathogen—are excellent examples of soil's potential to regulate detrimental organisms [41]. Research differentiates between general and specific suppression of pathogens, yet recognizes there is a continuum from general to specific, ‘with the former underlying and potentially give rise to the latter over time’ [41,90] (figure 1).
For a disease to emerge in a crop requires a susceptible host plant, a pathogen that is virulent and an environment conducive to infection. Both abiotic and biotic processes in soil regulate the potential for and severity of a pathogen's impact on plants [43]. Soil-borne plant pathogens are taxonomically quite diverse and include bacteria, archaea, fungi, viruses and protozoa [41,57,91]. A number of classification schemes have been employed for plant pathogens, ranging from plan nutritional habit, plant physiology, parasitism to ecological perspectives (see Vega et al. [92] for an overview). Here, we focus on soil–pathogen rather than host–pathogen interaction, and, therefore, pathogen soil residency is a primary consideration (figure 1). Whether pathogens are (i) part of the soil microbial community, (ii) survive in soil for an extended period, or (iii) are transient entities that can only briefly exist outside of a suitable plant host determines the dynamics of their interaction with soil communities and abiotic factors and, therefore, determines the mechanisms by which soils may suppress them [41]. More extensive overviews of relationships between the plant rhizosphere, pathogens and beneficial organisms can be found elsewhere [93].
(b) . Mechanisms of suppressiveness of plant pathogens
What is termed ‘general suppressiveness' results from the activities of consortia or communities of microorganisms, via competition for resources or antagonistic activities, and is often effective against a variety of plant diseases [23,57,94,95]. Antagonistic interactions are influenced by the nutrient and energy supply available for growth in soil of both the pathogen and to its host [42]. Properties of general suppression are (i) not transferable from a suppressive soil to another soil, (ii) can be reduced or removed by sterilization of soil, (iii) often enhanced by inputs of organic amendments and tied to increases in abundance and activity/diversity of the microbial community [90,96]. Stimulation of indigenous microbial communities is thought to deplete limiting resources for pathogen growth and infection; suppression of Phytophthora root rot is an example [97]. Sometimes the impact is linked to major subgroups of the community. Specific amendments, like debris of wild rocket or rice bran, can enrich Streptomycetes spp. and help suppress the pathogen Fusarium oxysporum and potato scab diseases [98,99]. Secondary metabolites, both volatile and soluble, produced by many bacterial species in response to interspecies interactions and competition is also considered to play a role in general suppression [100].
Specific suppressiveness results from individual taxa of microorganisms and, unlike for general suppression, the benefit is often transferable from one soil to another (e.g. via inoculating with suppressive soil). Antibiosis is involved in specific suppression of take-all disease of cereals and often emerges after long periods of monocropping [57]. The specific suppression of the disease take-all, caused by Gaeumannomyces graminis, has been directly linked to antibiotic production by fluorescent Pseudomonas spp. [101,102]. An example of a parasitic antagonistic interaction is the suppression of the nematode Heterodera schachtii via the fungi Dactylella oviparasitica and F. oxysporum infecting nematode cysts and eggs [103]. Many plant growth-promoting rhizobacteria (PGPR) release antimicrobial or antifungal compounds that deter plant pathogens [23,57]. For example, fluorescent pseudomonads produce the antibiotic 2,4-DAPG which has been extensively studied as a protectant against soil-borne diseases [104,105].
Far from the view of plants as passive factories that transform sunlight into organic molecules, it is now recognized that plants actively respond to a complex assortment of physical and chemical environmental cues and precisely regulate their immediate soil environment. Recent advances in omics and visualization techniques have revealed complex and dynamic (i.e. responsive) relationships between plants and their rhizosphere microbial communities, and that plants recruit specific taxa and functional groups to help them uptake nutrients, promote growth, increase stress tolerance and avoid or fight off disease [106]. Responses include the recruitment of beneficial rhizosphere microbes [107]. When the plant cell membrane perceives a stressor, it releases a ‘cry for help’ [108] transmitted by downstream signalling networks which trigger an immune response [109] in the plant. For example, kinases are produced leading to accumulation of plant hormones like abscisic acid, salicylic acid (SA), jasmonic acid (JA) and ethylene. They stimulate changes in the plant root's exudates which target and recruit selective groups of microbes to colonize its rhizosphere. These recruitments essentially create a ‘suppressive soil memory’ [107,110] which protects successive plants of the same type growing in the same location from infection by those pathogens.
Elucidating who, and what mechanisms, are involved in the consortia of microbes conferring general soil suppressiveness is increasingly possible through coupling culture-dependent and culture-independent methods. In studies of soils suppressive of Rhizoctonia solani, Mendes et al. [95] could link higher relative abundance of key bacterial groups like Proteobacteria, Firmicutes and Actinobacteria, and genes coding for non-ribosomal peptide synthetases, to pathogen suppression. Similarly, in a study of soils that suppress Ralstonia solanacearum, specific rhizosphere taxa associated with whether or not plants developed disease symptoms included members of Pseudomonas and Bacillus, along with high abundance of genes encoding antimicrobial compounds [111]. This growing knowledge provides the foundation to design strategies to better select for beneficial native organisms, identify viable microbial inoculants or engineer plant rhizosphere microbiomes to reduce disease incidence in plants.
Abiotic properties of soil also play many roles in regulating plant disease, both directly and through their interactions with soil biota. Higher available nitrogen can increase disease susceptibility by favouring pathogen growth [112]; for example, increasing colonization by Pseudomonas syringae on winter wheat [113] and higher soil moisture content can increase impacts of pathogenic Ra. solanacearum on ginger [114]. These interactions are reviewed in greater detail elsewhere [115–117].
4. Effect of agricultural management practices on role of soil and soil biota in regulating detrimental organisms and biological processes
We have long known that environmental conditions, and consequently how we manipulate the environment to manage soil, profoundly affect soil biota and soil health [118]. Traditional and indigenous farming typically include practices of using organic inputs and diversified rotations, at least in part to reduce crop losses from detrimental organisms [119,120]. Many of these same practices are used today in organic farming, in some cases being re-integrated into conventional farming systems to both sustain and increase resiliency of agroecosystems.
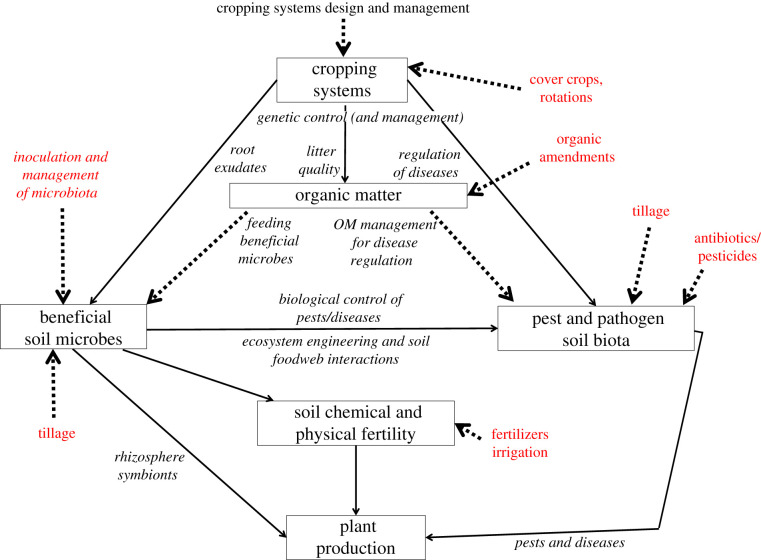
There are co-benefits and trade-offs associated with many management practices. Some implemented with the goal of improving fertility or soil structure also impact, either negatively or positively, soil's ability to regulate detrimental organisms [23]. Figure 2 depicts the major components of agricultural systems (boxes) and how biological processes mediate their interactions (arrows). Also shown are the impacts of different management practices on the biological underpinnings of the agroecosystem [2]. Whether intentional or not, farm managers choose practices that work with (or against) the ecological principles underpinning soil suppressiveness discussed earlier. Below, we briefly consider how selected management practices affect the ability of soil to regulate detrimental organisms and processes. More detailed reviews of this topic can be found in the following [11,118,121].
Figure 2.
The entry points for beneficial and pathogenic soil microbes, and impacts of cropping practices, on plant production. Modified from Brussaard et al. [2]. The boxes refer to major relevant components of the agroecosystem. The text in red and the dotted arrows refer to management practices. (Online version in colour.)
(a) . Organic amendments
Soil organic amendments are among the most frequently used practices to reduce deleterious impacts of soil pathogens. Amendments include composts made from a variety of sources—animal manure, food waste, green wastes—and non-composted materials such as green waste, biodigestates, biochar and biosolids from sewage treatment plants [122,123]. Organic amendments can have positive or negative effects on soil's ability to regulate detrimental organisms resulting from the introduction of nutrients or toxins, and beneficial organisms or pathogens.
Of all soil amendments, compost has received the most attention because it is widely available and less associated with negative impacts (e.g. risk of adding food-borne pathogens) than, for example, untreated animal manures. Compost increases soil micro- and macronutrients, alters physical properties like soil structure and increases microbial biomass, activity and diversity [43,96,124]. Mechanisms of disease suppression include competition among microbial populations, antagonism via antibiosis, hyperparasitism involving direct attacks on pathogens and systemic-induced resistance (SAR) of the plant [96]. The provision of a variety of carbon and nutrient inputs also modifies the interactions and equilibrium among members of the soil community and may help the plant in recruiting beneficial organisms [125,126].
Nonetheless, improperly processed composts, as well as untreated animal manures, can introduce pathogens to soil. For example, greater concentrations of Salmonella spp. were detected in soils amended with poultry-based organic amendments than synthetic fertilizer [51,127]. Factors determining the efficacy of compost inputs are type of manure, method of application, type of soil, storage, composting protocols, nutrient ratios, microbial diversity of the amended soils, as well as geographical and environmental factors [124]. Concerns that animal-based composts may contribute to soil pathogens in leafy greens and other vegetables have led to changes in compost processing and application to minimize pathogen risks [128]. Compost prepared following established guidelines [129,130] greatly reduces the potential for introducing pathogens.
Recent meta-analyses showed soil-borne fungal plant pathogen suppression in more than half of studies that used traditional organic amendments; however, in 20% of cases disease incidence increased [131,132]. Specific inputs can be designed based on understanding of soil microbial interactions. For example, adding biopolymers such as chitin and chitosan can stimulate the prevalence and activity of taxa that specifically break down cell walls of pathogenic fungi [133,134]. Organic inputs can also be combined with flooding of soils in anaerobic soil disinfestation (ASD) to select for anaerobic communities that generate pathogen-suppressing fermentation products [41].
Recently, biochar has received increasing attention for its potential benefits for soil health, water relations and disease suppression [135,136]. Biochar amendments have been shown to be effective in suppressing a wide range of pathogens including bacteria, fungi, virus and nematodes [136,137]. Some of the mechanisms involved may be similar to those of compost as discussed above; however, biochar's strong sorptive properties may also play a role. Biochar sorption and inactivation of pathogen-derived toxins and enzymes are potential mechanisms in disease suppression [138,139]. Broad generalizations about the efficacy of biochar in regulating deleterious organisms are difficult to make at this time however, given how dependent results are on biochar feedstock, synthesis conditions, application rate, as well as soil and crop properties [136,140].
(b) . Cover crops
Cover crops are typically included in rotations to improve soil parameters such as aggregation, water infiltration and water-holding capacity, and provide nitrogen if legumes are used. However, general suppressive activity can also result from using cover crops due to their stimulation of microbial biomass, diversity and activity [11,141]. Incorporated grasses (sorghum, millet, oats, rye), brassicas (rapeseed, mustard), legumes (alfalfa) and vines (kudzu) [142,143] as cover crops have been shown to decrease specific plant pathogen populations. Mixtures of cover crops, especially increasing plant functional group richness [144], may increase the resistance to pathogens in the following crop [144]; however, single cover crop species can also be effective [145]. There are instances, however, where cover crops increase enteric pathogen survival [146] so choosing the right cover crops is vitally important [147].
(c) . Diversifying crop rotations
Recognition of host–pathogen cycles have influenced development of diversified cropping systems because they reduce the build up of soil-borne plant pathogens that results when a crop is grown continuously [148,149]. A recent meta-analysis showed crop diversification can boost soil biodiversity and fertility while also regulating disease incidence [150]. Rotations regulate pathogen populations by disrupting the host–pathogen cycles [43,151]; altering the soil's physico-chemical traits, or biological communities [152] that make the environment less conducive to pathogen development or survival (general suppression); or through direct inhibition of pathogens via production of toxic chemicals or stimulating specific antagonists (specific suppression) [148]. The specific choice of crops in rotation is important to ensure planted crops are not compatible with pathogens selected for by the previous crop. Examples of successful rotations include red clover mitigating tuber disease in a potato [149] and using brassicas to combat Verticillium dahliae infestation in strawberry production [153]. The integration of animal and cropping systems is another strategy. For example, to reduce parasitic loads in cattle, grazing management using pasture diversification or rotation are recognized approaches to improve livestock husbandry [154,155].
(d) . Tillage
Reducing tillage of soils decreases erosion and loss of soil organic matter; however, its impacts on detrimental soil organisms are more complex and sometimes contradictory. Reduced or conservation tillage which leaves crop residues on the soil surface, or partially buried in soil, can create a competitive environment which facilitates competition and antagonistic interactions between microbes. This can result in disease suppression [156] and lead to niche differentiation and increased microbial diversity deeper in the soil profile [157]. In other cases, however, retention of crop residues can facilitate survival of pathogens by protecting them from microbial attack [158]. Some pathogens, even in the absence of their host plants, can survive as saprophytes or as spores until the host returns [159,160]. Conventional deep tillage may translocate the pathogens deeper into soil where the environment is less conducive to survival [161]. Other studies found no impact of tillage management on E. coli and Salmonella numbers [162,163].
(e) . Use of antibiotics and agrichemicals
Numerous agrichemicals including herbicides, insecticides and fungicides are routinely used in intensive, large-scale agricultural management, often with negative consequences for soil communities, particularly fungi and soil fauna [164–166]. Pesticides use has increased over the past decades [167]. Of what is applied to soil, only a small portion of the pesticide reaches the target organism [168] and the remainder stays in the soil where it may be biodegraded but may also expose soil communities [165]. Seed dressing with insecticides and fungicides can reduce activities of earthworms, mycorrhizal fungi and other microbial populations and processes [169]. Many processes governing nutrient cycling, e.g. nitrification, are sensitive to pesticides and other organic chemicals [164]. The available concentrations and bioavailability of pesticide on soil microbes depend on soil properties as well, but a common impact is the disruption of specific soil functions and reduced diversity [164,170]. Although the impacts of agrichemicals on soil communities have been well documented, there is little direct information about how these chemicals impact the soil's ability to regulate pathogens. However, in aquatic ecosystems, the use of herbicides and fungicides was associated with increases in populations of pathogens by reducing densities of protozoan predators and by altering competition with indigenous microbes [171], highlighting need for performing similar studies in soil.
Similarly, antibiotics have been widely used in the suppression or management of diseases in livestock [172] and some crops [173,174]. However, overuse of such antibiotics has increased potential threats to the soil microbial community and also elevated antibiotic resistance in the soil environment [175,176]. Impacts of high concentrations of antibiotics can, but not always, cause changes in enzyme activity and carbon use, reduction in microbial biomass and shifts in the community composition [175]. Low, sublethal concentrations of antibiotics are problematic because they exert a selective pressure for antibiotic resistance in microbial populations [177] and may promote persistence of certain human and other animal pathogens in soil [178,179]. Although much effort has gone into reducing antibiotic use in livestock production worldwide, the spread and dissemination of antimicrobial resistant bacteria and genes are still a global health concern [180,181]. Efforts to improve antimicrobial stewardship worldwide need to continue and expand to reduce impacts on human and environmental health [182].
5. Conclusion
There is broad consensus on the perilous state of the environment. In December 2020, the United Nations Secretary-General António Guterres called out humanity's suicidal ‘war on nature’ during his State of the Planet address at Columbia University [183]. The issues facing us are complex, multifaceted and profoundly interconnected, and will require similarly interdisciplinary, integrated approaches to solve.
Soil is an important part of the solution. With respect to impacts of deleterious organisms and processes, soil has tremendous capacity both to host human, animal and plant pathogens and also to suppress establishment and survival of these pathogens. Understanding the complex interactions and ecological phenomena that govern suppressiveness in a particular place and time will provide a foundation for new tools and indicators to predict potential outbreaks and develop preventative solutions [41,184]. Better knowledge of which tools to use, and when to stay out of the way of soil's native ability to regulate detrimental organisms, will come from ecological insights gained from studies of soil biodiversity [185].
Health is a concept that bridges across and links the microbiomes of humans, animals, plants, and soil and other living members of ecosystems [45,186]. Soil health has proven to be a powerful conceptual framework that helps integrate across multiple functions, processes and solutions at play [39,40] and could increasingly play a role in how we think about regulating deleterious organisms in soil. When one considers, however, the vast set of interactions among pathogens, vectors, hosts, antagonists, abiotic environment and other players [187–191], it is evident our perspective needs to be broader than soil to transcend the silos of specific disciplines and taxa. By focusing on the connections among human, other animal, plant, microbiome and environmental health, the evolving concept of One Health [45,186,192,193] brings together disciplinary areas that rarely interact, with the goal of transdisciplinary research and new solutions. For example, finding commonalities across plants and animals with regard to taxa and mechanisms involved in regulating detrimental organisms [193] could not only cross-fertilize approaches and conceptual models, but also identify root causes and find cross-cutting solutions [194,195]. A One Health perspective on soils' role in pathogen suppression will bring new ideas and synergism, and catalyse solution-based research, to help achieve the goals of NCP [6] and particularly of NCP #10, the regulation of detrimental effects on humans, human-important plants and animals.
Acknowledgements
This publication was also made possible by the USDA, National Institute of Food and Agriculture (NIFA) through Hatch Formula Funding CA 2122-H and multistate regional project W-2082.
Data accessibility
This article has no additional data.
Competing interests
We declare we have no competing interests.
Funding
Funding for this research was made possible by the Center for Produce Safety (grant no. 2019CPS03) and by the US Department of Agriculture's (USDA) Agricultural Marketing Service through grant no. USDA-AMS-TM-SCBG-G-18-003 as well as the USDA, Agricultural Research Service, National Program 108 Food Safety (animal and plant products).
Disclaimer
All opinions, findings, conclusions and recommendations expressed in this publication are those of the authors and do not necessarily reflect the view of The Center for Produce Safety, the National Institute of Food and Agriculture (NIFA) nor the United States Department of Agriculture (USDA).
References
- 1.Barrios E. 2007Soil biota, ecosystem services and land productivity. Ecol. Econ. 64, 269-285. ( 10.1016/j.ecolecon.2007.03.004) [DOI] [Google Scholar]
- 2.Brussaard L, de Ruiter PC, Brown GG. 2007Soil biodiversity for agricultural sustainability. Agric. Ecosyst. Environ. 121, 233-244. ( 10.1016/j.agee.2006.12.013) [DOI] [Google Scholar]
- 3.Díaz S, et al. 2015The IPBES conceptual framework—connecting nature and people. Curr. Opin. Environ. Sustain. 14, 1-16. ( 10.1016/j.cosust.2014.11.002) [DOI] [Google Scholar]
- 4.Díaz S, Demissew S, Joly C, Lonsdale WM, Larigauderie A. 2015A rosetta stone for nature's benefits to people. PLoS Biol. 13, e1002040. ( 10.1371/journal.pbio.1002040) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pascual U, et al. 2017Valuing nature's contributions to people: the IPBES approach. Curr. Opin. Environ. Sustain. 26–27, 7-16. ( 10.1016/j.cosust.2016.12.006) [DOI] [Google Scholar]
- 6.Díaz S, et al. 2018Assessing nature's contributions to people. Science 359, 270-272. ( 10.1126/science.aap8826) [DOI] [PubMed] [Google Scholar]
- 7.Nielsen UN, Wall DH, Six J. 2015Soil biodiversity and the environment. Annu. Rev. Environ. Resour. 40, 63-90. ( 10.1146/annurev-environ-102014-021257) [DOI] [Google Scholar]
- 8.Ranjard L, et al. 2013Turnover of soil bacterial diversity driven by wide-scale environmental heterogeneity. Nat. Commun. 4, 1434. ( 10.1038/ncomms2431) [DOI] [PubMed] [Google Scholar]
- 9.Baumgardner DJ. 2012Soil-related bacterial and fungal infections. J. Am. Board Fam. Med. 25, 734-744. ( 10.3122/jabfm.2012.05.110226) [DOI] [PubMed] [Google Scholar]
- 10.De Corato U. 2020Disease-suppressive compost enhances natural soil suppressiveness against soil-borne plant pathogens: a critical review. Rhizosphere 13, 100192. ( 10.1016/j.rhisph.2020.100192) [DOI] [Google Scholar]
- 11.Larkin RP. 2015Soil health paradigms and implications for disease management. Annu. Rev. Phytopathol. 53, 199-221. ( 10.1146/annurev-phyto-080614-120357) [DOI] [PubMed] [Google Scholar]
- 12.Paseka RE, et al. 2020Disease-mediated ecosystem services: pathogens, plants, and people. Trends Ecol. Evol. 35, 731-743. ( 10.1016/j.tree.2020.04.003) [DOI] [PubMed] [Google Scholar]
- 13.Pieterse CMJ, de Jonge R, Berendsen RL. 2016The soil-borne supremacy. Trends Plant Sci. 21, 171-173. ( 10.1016/j.tplants.2016.01.018) [DOI] [PubMed] [Google Scholar]
- 14.Schierstaedt J, Jechalke S, Nesme J, Neuhaus K, Sørensen SJ, Grosch R, Smalla K, Schikora A. 2020Salmonella persistence in soil depends on reciprocal interactions with indigenous microorganisms. Environ. Microbiol. 22, 2639-2652. ( 10.1111/1462-2920.14972) [DOI] [PubMed] [Google Scholar]
- 15.Steffan JJ, Derby JA, Brevik EC. 2020Soil pathogens that may potentially cause pandemics, including SARS coronaviruses. Curr. Opin. Environ. Sci. Health 17, 35-40. ( 10.1016/j.coesh.2020.08.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oliver MA, Gregory PJ. 2015Soil, food security and human health: a review. Eur. J. Soil Sci. 66, 257-276. ( 10.1111/ejss.12216) [DOI] [Google Scholar]
- 17.Boine B, Renner A-C, Zellner M, Nechwatal J. 2014Quantitative methods for assessment of the impact of different crops on the inoculum density of Rhizoctonia solani AG2-2IIIB in soil. Eur. J. Plant Pathol. 140, 745-756. ( 10.1007/s10658-014-0506-6) [DOI] [Google Scholar]
- 18.Garbelotto M, Schmidt D, Popenuck T. 2021Pathogenicity and infectivity of Phytophthora ramorum vary depending on host species, infected plant part, inoculum potential, pathogen genotype, and temperature. Plant Pathol. 70, 287-304. ( 10.1111/ppa.13297) [DOI] [Google Scholar]
- 19.Rees RW, Flood J, Hasan Y, Cooper RM. 2007Effects of inoculum potential, shading and soil temperature on root infection of oil palm seedlings by the basal stem rot pathogen Ganoderma boninense. Plant Pathol. 56, 862-870. ( 10.1111/j.1365-3059.2007.01621.x) [DOI] [Google Scholar]
- 20.van Elsas JD, Chiurazzi M, Mallon CA, Elhottovā D, Krištůfek V, Salles JF. 2012Microbial diversity determines the invasion of soil by a bacterial pathogen. Proc. Natl Acad. Sci. USA 109, 1159-1164. ( 10.1073/pnas.1109326109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mallon CA, van Elsas JD, Salles JF. 2015Microbial invasions: the process, patterns, and mechanisms. Trends Microbiol. 23, 719-729. ( 10.1016/j.tim.2015.07.013) [DOI] [PubMed] [Google Scholar]
- 22.Kinkel LL, Bakker MG, Schlatter DC. 2011A coevolutionary framework for managing disease-suppressive soils. Annu. Rev. Phytopathol. 49, 47-67. ( 10.1146/annurev-phyto-072910-095232) [DOI] [PubMed] [Google Scholar]
- 23.Garbeva P, van Veen JA, van Elsas JD. 2004Microbial diversity in soil: selection of microbial populations by plant and soil type and implications for disease suppressiveness. Annu. Rev. Phytopathol. 42, 243-270. ( 10.1146/annurev.phyto.42.012604.135455) [DOI] [PubMed] [Google Scholar]
- 24.Hooper DU, et al. 2005Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecol. Monogr. 75, 3-35. ( 10.1890/04-0922) [DOI] [Google Scholar]
- 25.Tilman D, Isbell F, Cowles JM. 2014Biodiversity and ecosystem functioning. Annu. Rev. Ecol. Evol. Syst. 45, 471-493. ( 10.1146/annurev-ecolsys-120213-091917) [DOI] [Google Scholar]
- 26.Zavaleta ES, Hulvey KB. 2004Realistic species losses disproportionately reduce grassland resistance to biological invaders. Science 306, 1175-1177. ( 10.1126/science.1102643) [DOI] [PubMed] [Google Scholar]
- 27.Bonanomi G, Capodilupo M, Incerti G, Gaglione SA, Scala F. 2014Fungal diversity increases soil fungistasis and resistance to microbial invasion by a non resident species. Biol. Control 72, 38-45. ( 10.1016/j.biocontrol.2014.02.005) [DOI] [Google Scholar]
- 28.Botton S, van Heusden M, Parsons JR, Smidt H, van Straalen N. 2006Resilience of microbial systems towards disturbances. Crit. Rev. Microbiol. 32, 101-112. ( 10.1080/10408410600709933) [DOI] [PubMed] [Google Scholar]
- 29.Holling CS. 1973Resilience and stability of ecological systems. Annu. Rev. Ecol. Syst. 4, 1-23. ( 10.1146/annurev.es.04.110173.000245) [DOI] [Google Scholar]
- 30.Escalas A, Hale L, Voordeckers JW, Yang Y, Firestone MK, Alvarez-Cohen L, Zhou J. 2019Microbial functional diversity: from concepts to applications. Ecol. Evol. 9, 12 000-12 016. ( 10.1002/ece3.5670) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elmqvist T, Folke C, Nyström M, Peterson G, Bengtsson J, Walker B, Norberg J. 2003Response diversity, ecosystem change, and resilience. Front. Ecol. Environ. 1, 488-494. ( 10.1890/1540-9295(2003)001[0488:RDECAR]2.0.CO;2) [DOI] [Google Scholar]
- 32.Ludwig M, Wilmes P, Schrader S. 2018Measuring soil sustainability via soil resilience. Sci. Total Environ. 626, 1484-1493. ( 10.1016/j.scitotenv.2017.10.043) [DOI] [PubMed] [Google Scholar]
- 33.Griffiths BS, Philippot L. 2013Insights into the resistance and resilience of the soil microbial community. FEMS Microbiol. Rev. 37, 112-129. ( 10.1111/j.1574-6976.2012.00343.x) [DOI] [PubMed] [Google Scholar]
- 34.Shade A, et al. 2012Fundamentals of microbial community resistance and resilience. Front. Microbiol. 3, 417. ( 10.3389/fmicb.2012.00417) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rillig MC, Ryo M, Lehmann A, Aguilar-Trigueros CA, Buchert S, Wulf A, Iwasaki A, Roy J, Yang G. 2019The role of multiple global change factors in driving soil functions and microbial biodiversity. Science 366, 886-890. ( 10.1126/science.aay2832) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rillig MC, Muller LA, Lehmann A. 2017Soil aggregates as massively concurrent evolutionary incubators. ISME J. 11, 1943-1948. ( 10.1038/ismej.2017.56) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tibbett M, Fraser TD, Duddigan S. 2020Identifying potential threats to soil biodiversity. PeerJ 8, e9271. ( 10.7717/peerj.9271) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thiele-Bruhn S, Bloem J, de Vries FT, Kalbitz K, Wagg C. 2012Linking soil biodiversity and agricultural soil management. Curr. Opin. Environ. Sustain. 4, 523-528. ( 10.1016/j.cosust.2012.06.004) [DOI] [Google Scholar]
- 39.Bünemann EK, et al. 2018Soil quality—a critical review. Soil Biol. Biochem. 120, 105-125. ( 10.1016/j.soilbio.2018.01.030) [DOI] [Google Scholar]
- 40.Kibblewhite MG, Ritz K, Swift MJ. 2008Soil health in agricultural systems. Phil. Trans. R. Soc. B 363, 685-701. ( 10.1098/rstb.2007.2178) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schlatter D, Kinkel L, Thomashow L, Weller D, Paulitz T. 2017Disease suppressive soils: new insights from the soil microbiome. Phytopathology 107, 1284-1297. ( 10.1094/PHYTO-03-17-0111-RVW) [DOI] [PubMed] [Google Scholar]
- 42.van Bruggen AHC, Semenov AM. 2000In search of biological indicators for soil health and disease suppression. Appl. Soil Ecol. 15, 13-24. ( 10.1016/S0929-1393(00)00068-8) [DOI] [Google Scholar]
- 43.Janvier C, Villeneuve F, Alabouvette C, Edel-Hermann V, Mateille T, Steinberg C. 2007Soil health through soil disease suppression: which strategy from descriptors to indicators? Soil Biol. Biochem. 39, 1-23. ( 10.1016/j.soilbio.2006.07.001) [DOI] [Google Scholar]
- 44.Lehmann J, Bossio DA, Kögel-Knabner I, Rillig MC. 2020The concept and future prospects of soil health. Nat. Rev. Earth Environ. 1, 544-553. ( 10.1038/s43017-020-0080-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Bruggen AHC, Goss EM, Havelaar A, van Diepeningen AD, Finckh MR, Morris JG. 2019One Health—cycling of diverse microbial communities as a connecting force for soil, plant, animal, human and ecosystem health. Sci. Total Environ. 664, 927-937. ( 10.1016/j.scitotenv.2019.02.091) [DOI] [PubMed] [Google Scholar]
- 46.Bultman MW, Fisher FS, Pappagianis D. 2013The ecology of soil-borne human pathogens. In Essentials of medical geology, revised edn (ed Selinus O), pp. 477-504. Dordrecht, The Netherlands: Springer. [Google Scholar]
- 47.Dragon DC, Rennie RP. 1995The ecology of anthrax spores: tough but not invincible. Can. Vet. J. 36, 295-301. [PMC free article] [PubMed] [Google Scholar]
- 48.Alegbeleye OO, Singleton I, Sant'Ana AS. 2018Sources and contamination routes of microbial pathogens to fresh produce during field cultivation: a review. Food Microbiol. 73, 177-208. ( 10.1016/j.fm.2018.01.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alegbeleye OO, Sant'Ana AS. 2020Manure-borne pathogens as an important source of water contamination: an update on the dynamics of pathogen survival/transport as well as practical risk mitigation strategies. Int. J. Hyg. Environ. Health 227, 113524. ( 10.1016/j.ijheh.2020.113524) [DOI] [PubMed] [Google Scholar]
- 50.Karp DS, Gennet S, Kilonzo C, Partyka M, Chaumont N, Atwill ER, Kremen C. 2015Comanaging fresh produce for nature conservation and food safety. Proc. Natl Acad. Sci. USA 112, 11 126-11 131. ( 10.1073/pnas.1508435112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shah MK, Bradshaw R, Nyarko E, Handy ET, East C, Millner PD, Bergholz TM, Sharma M. 2019Salmonella enterica in soils amended with heat-treated poultry pellets survived longer than bacteria in unamended soils and more readily transferred to and persisted on spinach. Appl. Environ. Microbiol. 85, e00334-19. ( 10.1128/AEM.00334-19) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fongaro G, García-González MC, Hernández M, Kunz A, Barardi CRM, Rodríguez-Lázaro D. 2017Different behavior of enteric bacteria and viruses in clay and sandy soils after biofertilization with swine digestate. Front. Microbiol. 8, 74. ( 10.3389/fmicb.2017.00074) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jechalke S, Schierstaedt J, Becker M, Flemer B, Grosch R, Smalla K, Schikora A. 2019Salmonella establishment in agricultural soil and colonization of crop plants depend on soil type and plant species. Front. Microbiol. 10, 967. ( 10.3389/fmicb.2019.00967) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hurst CJ. 2019Dirt and disease: the ecology of soil fungi and plant fungi that are infectious for vertebrates. In Understanding terrestrial microbial communities (ed. Hurst CJ), pp. 289-405. Cham, Switzerland: Springer International Publishing. [Google Scholar]
- 55.Tao L, Gowler CD, Ahmad A, Hunter MD, de Roode JC. 2015Disease ecology across soil boundaries: effects of below-ground fungi on above-ground host–parasite interactions. Proc. R. Soc. B 282, 20151993. ( 10.1098/rspb.2015.1993) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mazzola M. 2004Assessment and management of soil microbial community structure for disease suppression. Annu. Rev. Phytopathol. 42, 35-59. ( 10.1146/annurev.phyto.42.040803.140408) [DOI] [PubMed] [Google Scholar]
- 57.Weller DM, Raaijmakers JM, Gardener BBM, Thomashow LS. 2002Microbial populations responsible for specific soil suppressiveness to plant pathogens. Annu. Rev. Phytopathol. 40, 309-348. ( 10.1146/annurev.phyto.40.030402.110010) [DOI] [PubMed] [Google Scholar]
- 58.Jones MS, Fu Z, Reganold JP, Karp DS, Besser TE, Tylianakis JM, Snyder WE. 2019Organic farming promotes biotic resistance to foodborne human pathogens. J. Appl. Ecol. 56, 1117-1127. ( 10.1111/1365-2664.13365) [DOI] [Google Scholar]
- 59.Baker CA, Lee S, De J, Jeong KC, Schneider KR. 2020Survival of Escherichia coli O157 in autoclaved and natural sandy soil mesocosms. PLoS ONE 15, e0234562. ( 10.1371/journal.pone.0234562) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Locatelli A, Spor A, Jolivet C, Piveteau P, Hartmann A. 2013Biotic and abiotic soil properties influence survival of Listeria monocytogenes in soil. PLoS ONE 8, e75969. ( 10.1371/journal.pone.0075969) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mallon CA, Le Roux X, van Doorn GS, Dini-Andreote F, Poly F, Salles JF. 2018The impact of failure: unsuccessful bacterial invasions steer the soil microbial community away from the invader's niche. ISME J. 12, 728-741. ( 10.1038/s41396-017-0003-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hiddink GA, van Bruggen AHC, Termorshuizen AJ, Raaijmakers JM, Semenov AV. 2005Effect of organic management of soils on suppressiveness to Gaeumannomyces graminis var. tritici and its antagonist, Pseudomonas fluorescens. Eur. J. Plant Pathol. 113, 417-435. ( 10.1007/s10658-005-5402-7) [DOI] [Google Scholar]
- 63.Ongeng D, Geeraerd AH, Springael D, Ryckeboer J, Muyanja C, Mauriello G. 2015Fate of Escherichia coli O157:H7 and Salmonella enterica in the manure-amended soil-plant ecosystem of fresh vegetable crops: a review. Crit. Rev. Microbiol. 41, 273-294. ( 10.3109/1040841X.2013.829415) [DOI] [PubMed] [Google Scholar]
- 64.Obayomi O, Bernstein N, Edelstein M, Vonshak A, Ghazayarn L, Ben-Hur M, Tebbe CC, Gillor O. 2019Importance of soil texture to the fate of pathogens introduced by irrigation with treated wastewater. Sci. Total Environ. 653, 886-896. ( 10.1016/j.scitotenv.2018.10.378) [DOI] [PubMed] [Google Scholar]
- 65.Rochelle-Newall EJ, et al. 2016Effect of land use and hydrological processes on Escherichia coli concentrations in streams of tropical, humid headwater catchments. Sci. Rep. 6, 32974. ( 10.1038/srep32974) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pang H, Mokhtari A, Chen Y, Oryang D, Ingram DT, Sharma M, Millner PD, Doren JMV. 2020A predictive model for survival of Escherichia coli O157:H7 and generic E. coli in soil amended with untreated animal manure. Risk Anal. 40, 1367-1382. ( 10.1111/risa.13491) [DOI] [PubMed] [Google Scholar]
- 67.Dixon GR, Tilston EL. 2010Soil microbiology and sustainable crop production. Berlin, Germany: Springer. [Google Scholar]
- 68.Gossen BD, Kasinathan H, Deora A, Peng G, McDonald MR. 2016Effect of soil type, organic matter content, bulk density and saturation on clubroot severity and biofungicide efficacy. Plant Pathol. 65, 1238-1245. ( 10.1111/ppa.12510) [DOI] [Google Scholar]
- 69.Allen T, Murray KA, Zambrana-Torrelio C, Morse SS, Rondinini C, Di Marco M, Breit N, Olival KJ, Daszak P. 2017Global hotspots and correlates of emerging zoonotic diseases. Nat. Commun. 8, 1124. ( 10.1038/s41467-017-00923-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chomel BB. 2009Zoonoses. In Encyclopedia of microbiology, 3rd edn (ed. M Schaechter), pp. 820–829. London, UK: Academic Press. [Google Scholar]
- 71.Brevik EC, Slaughter L, Singh BR, Steffan JJ, Collier D, Barnhart P, Pereira P. 2020Soil and human health: current status and future needs. Air Soil Water Res. 13, 1178622120934441. ( 10.1177/1178622120934441) [DOI] [Google Scholar]
- 72.Gibbs EPJ. 2005Emerging zoonotic epidemics in the interconnected global community. Vet. Rec. 157, 673-679. ( 10.1136/vr.157.22.673) [DOI] [PubMed] [Google Scholar]
- 73.de Quadros Rodrigues R, Loiko MR, Minéia Daniel de Paula C, Hessel CT, Jacxsens L, Uyttendaele M, Bender RJ, Tondo EC. 2014Microbiological contamination linked to implementation of good agricultural practices in the production of organic lettuce in Southern Brazil. Food Control 42, 152-164. ( 10.1016/j.foodcont.2014.01.043) [DOI] [Google Scholar]
- 74.Pires AFA, Millner PD, Baron J, Jay-Russell MT. 2018Assessment of current practices of organic farmers regarding biological soil amendments of animal origin in a multi-regional US study. Food Prot. Trends 38, 347-362. [Google Scholar]
- 75.Checcucci A, Trevisi P, Luise D, Modesto M, Blasioli S, Braschi I, Mattarelli P. 2020Exploring the animal waste resistome: the spread of antimicrobial resistance genes through the use of livestock manure. Front. Microbiol. 11, 1416. ( 10.3389/fmicb.2020.01416) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dafale NA, Srivastava S, Purohit HJ. 2020Zoonosis: an emerging link to antibiotic resistance under ‘One Health Approach’. Indian J. Microbiol. 60, 139-152. ( 10.1007/s12088-020-00860-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cantas L, Suer K. 2014The important bacterial zoonoses in ‘One Health’ concept. Front. Public Health 2, 144. ( 10.3389/fpubh.2014.00144) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nesme J, Simonet P. 2015The soil resistome: a critical review on antibiotic resistance origins, ecology and dissemination potential in telluric bacteria. Environ. Microbiol. 17, 913-930. ( 10.1111/1462-2920.12631) [DOI] [PubMed] [Google Scholar]
- 79.Durand GA, Raoult D, Dubourg G. 2019Antibiotic discovery: history, methods and perspectives. Int. J. Antimicrob. Agents 53, 371-382. ( 10.1016/j.ijantimicag.2018.11.010) [DOI] [PubMed] [Google Scholar]
- 80.Lewis K. 2012Recover the lost art of drug discovery. Nature 485, 439-440. ( 10.1038/485439a) [DOI] [PubMed] [Google Scholar]
- 81.McArthur AG, Wright GD. 2015Bioinformatics of antimicrobial resistance in the age of molecular epidemiology. Curr. Opin. Microbiol. 27, 45-50. ( 10.1016/j.mib.2015.07.004) [DOI] [PubMed] [Google Scholar]
- 82.Brevik EC, Steffan JJ, Rodrigo-Comino J, Neubert D, Burgess LC, Cerdà A. 2019Connecting the public with soil to improve human health. Eur. J. Soil Sci. 70, 898-910. ( 10.1111/ejss.12764) [DOI] [Google Scholar]
- 83.Tasnim N, Abulizi N, Pither J, Hart MM, Gibson DL. 2017Linking the gut microbial ecosystem with the environment: does gut health depend on where we live? Front. Microbiol. 8, 1935. ( 10.3389/fmicb.2017.01935) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brevik EC, Burgess LC. 2012Soils and human health. Boca Raton, FL: CRC Press. [Google Scholar]
- 85.Haahtela T. 2019A biodiversity hypothesis. Allergy 74, 1445-1456. ( 10.1111/all.13763) [DOI] [PubMed] [Google Scholar]
- 86.Mosca A, Leclerc M, Hugot JP. 2016Gut microbiota diversity and human diseases: should we reintroduce key predators in our ecosystem? Front. Microbiol. 7, 455. ( 10.3389/fmicb.2016.00455) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Grieneisen LE, Charpentier MJE, Alberts SC, Blekhman R, Bradburd G, Tung J, Archie EA. 2019Genes, geology and germs: gut microbiota across a primate hybrid zone are explained by site soil properties, not host species. Proc. R. Soc. B 286, 20190431. ( 10.1098/rspb.2019.0431) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hanski I, et al. 2012Environmental biodiversity, human microbiota, and allergy are interrelated. Proc. Natl Acad. Sci. USA 109, 8334-8339. ( 10.1073/pnas.1205624109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.von Hertzen L, Hanski I, Haahtela T. 2011Natural immunity. EMBO Rep. 12, 1089-1093. ( 10.1038/embor.2011.195) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cook RJ. 2014Plant health management: pathogen suppressive soils. In Encyclopedia of agriculture and food systems (ed. Van Alfen NK), pp. 441-455. Oxford, UK: Academic Press. [Google Scholar]
- 91.Cook RJ, Baker KF. 1983The nature and practice of biological control of plant pathogens. St Paul, MN: American Phytopathological Society. [Google Scholar]
- 92.Vega D, Gally ME, Romero AM, Poggio SL. 2019Functional groups of plant pathogens in agroecosystems: a review. Eur. J. Plant Pathol. 153, 695-713. ( 10.1007/s10658-018-01616-8) [DOI] [Google Scholar]
- 93.Mendes R, Garbeva P, Raaijmakers JM. 2013The rhizosphere microbiome: significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiol. Rev. 37, 634-663. ( 10.1111/1574-6976.12028) [DOI] [PubMed] [Google Scholar]
- 94.Malajczuk N, Erwin D, Bartnicki-Garcia S, Tsao P. 1983Interactions between Phytophthora cinnamomi and Rhizobium isolates. Trans. Brit. Mycol. Soc. 82, 491-500. ( 10.1016/S0007-1536(84)80014-5) [DOI] [Google Scholar]
- 95.Mendes R, et al. 2011Deciphering the rhizosphere microbiome for disease-suppressive bacteria. Science 332, 1097-1100. ( 10.1126/science.1203980) [DOI] [PubMed] [Google Scholar]
- 96.Mehta CM, Palni U, Franke-Whittle IH, Sharma AK. 2014Compost: its role, mechanism and impact on reducing soil-borne plant diseases. Waste Manage. 34, 607-622. ( 10.1016/j.wasman.2013.11.012) [DOI] [PubMed] [Google Scholar]
- 97.Baker K, Cook RJ. 1974Biological control of plant pathogens. San Francisco, CA: WH Freeman and Company. [Google Scholar]
- 98.Klein E, Ofek M, Katan J, Minz D, Gamliel A. 2013Soil suppressiveness to fusarium disease: shifts in root microbiome associated with reduction of pathogen root colonization. Phytopathology 103, 23-33. ( 10.1094/PHYTO-12-11-0349) [DOI] [PubMed] [Google Scholar]
- 99.Tomihama T, Nishi Y, Mori K, Shirao T, Iida T, Uzuhashi S, Ohkuma M, Ikeda S. 2016Rice bran amendment suppresses potato common scab by increasing antagonistic bacterial community levels in the rhizosphere. Phytopathology 106, 719-728. ( 10.1094/PHYTO-12-15-0322-R) [DOI] [PubMed] [Google Scholar]
- 100.Olaf Tyc, Song C, Dickschat JS, Vos M, Garbeva P. 2017The ecological role of volatile and soluble secondary metabolites produced by soil bacteria. Trends Microbiol. 25, 280-292. ( 10.1016/j.tim.2016.12.002) [DOI] [PubMed] [Google Scholar]
- 101.Loper JE, et al. 2012Comparative genomics of plant-associated Pseudomonas spp.: insights into diversity and inheritance of traits involved in multitrophic interactions. PLoS Genet. 8, e1002784. ( 10.1371/journal.pgen.1002784) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Weller DM. 2015Take-all decline and beneficial pseudomonads. In Principles of plant-microbe interactions: microbes for sustainable agriculture (ed Lugtenberg B), pp. 363-370. Cham, Switzerland: Springer International Publishing. [Google Scholar]
- 103.Topalović O, Hussain M, Heuer H. 2020Plants and associated soil microbiota cooperatively suppress plant-parasitic nematodes. Front. Microbiol. 11, 313. ( 10.3389/fmicb.2020.00313) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Raaijmakers JM, Weller DM, Thomashow LS. 1997Frequency of antibiotic-producing Pseudomonas spp. in natural environments. Appl. Environ. Microbiol. 63, 881-887. ( 10.1128/aem.63.3.881-887.1997) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Raaijmakers JM, Weller DM. 1998Natural plant protection by 2,4-diacetylphloroglucinol-producing Pseudomonas spp. in take-all decline soils. Mol. Plant-Microbe Interact. 11, 144-152. ( 10.1094/MPMI.1998.11.2.144) [DOI] [Google Scholar]
- 106.Trivedi P, Leach JE, Tringe SG, Sa T, Singh BK. 2020Plant–microbiome interactions: from community assembly to plant health. Nat. Rev. Microbiol. 18, 607-621. ( 10.1038/s41579-020-0412-1) [DOI] [PubMed] [Google Scholar]
- 107.Kong HG, Song GC, Ryu C-M. 2019Inheritance of seed and rhizosphere microbial communities through plant–soil feedback and soil memory. Environ. Microbiol. Rep. 11, 479-486. ( 10.1111/1758-2229.12760) [DOI] [PubMed] [Google Scholar]
- 108.Bakker PAHM, Pieterse CMJ, de Jonge R, Berendsen RL. 2018The soil-borne legacy. Cell 172, 1178-1180. ( 10.1016/j.cell.2018.02.024) [DOI] [PubMed] [Google Scholar]
- 109.Pieterse CMJ, Zamioudis C, Berendsen RL, Weller DM, Van Wees SCM, Bakker PAHM. 2014Induced systemic resistance by beneficial microbes. Annu. Rev. Phytopathol. 52, 347-375. ( 10.1146/annurev-phyto-082712-102340) [DOI] [PubMed] [Google Scholar]
- 110.Raaijmakers JM, Mazzola M. 2016Soil immune responses. Science 352, 1392-1393. ( 10.1126/science.aaf3252) [DOI] [PubMed] [Google Scholar]
- 111.Wei Z, Gu Y, Friman V-P, Kowalchuk GA, Xu Y, Shen Q, Jousset A. 2019Initial soil microbiome composition and functioning predetermine future plant health. Sci. Adv. 5, eaaw0759. ( 10.1126/sciadv.aaw0759) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mur LAJ, Simpson C, Kumari A, Gupta AK, Gupta KJ. 2017Moving nitrogen to the centre of plant defence against pathogens. Ann. Bot. 119, 703-709. ( 10.1093/aob/mcw179) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Neumann S, Paveley ND, Beed FD, Sylvester-Bradley R. 2004Nitrogen per unit leaf area affects the upper asymptote of Puccinia striiformis f.sp. tritici epidemics in winter wheat. Plant Pathol. 53, 725-732. ( 10.1111/j.1365-3059.2004.01107.x) [DOI] [Google Scholar]
- 114.Jiang Y, Huang M, Zhang M, Lan J, Wang W, Tao X, Liu Y. 2018Transcriptome analysis provides novel insights into high-soil-moisture-elevated susceptibility to Ralstonia solanacearum infection in ginger (Zingiber officinale Roscoe cv. Southwest). Plant Physiol. Biochem. 132, 547-556. ( 10.1016/j.plaphy.2018.10.005) [DOI] [PubMed] [Google Scholar]
- 115.Dordas C. 2008Role of nutrients in controlling plant diseases in sustainable agriculture. A review. Agron. Sustain. Dev. 28, 33-46. ( 10.1051/agro:2007051) [DOI] [Google Scholar]
- 116.Gupta N, Debnath S, Sharma S, Sharma P, Purohit J. 2017Role of nutrients in controlling the plant diseases in sustainable agriculture. In Agriculturally important microbes for sustainable agriculture: volume 2: applications in crop production and protection (eds Meena VS, Mishra PK, Bisht JK, Pattanayak A), pp. 217-262. Singapore, Singapore: Springer. [Google Scholar]
- 117.Orr R, Nelson PN. 2018Impacts of soil abiotic attributes on Fusarium wilt, focusing on bananas. Appl. Soil Ecol. 132, 20-33. ( 10.1016/j.apsoil.2018.06.019) [DOI] [Google Scholar]
- 118.Norris CE, Congreves KA. 2018Alternative management practices improve soil health indices in intensive vegetable cropping systems: a review. Front. Environ. Sci. 6, 50. ( 10.3389/fenvs.2018.00050) [DOI] [Google Scholar]
- 119.Akullo D, Kanzikwera R, Birungi P, Alum W, Aliguma L, Barwogeza M. 2007Indigenous knowledge in agriculture: a case study of the challenges in sharing knowledge of past generations in a globalized context in Uganda. In World Library and Information Congress: 73rd IFLA General Conference and Council, Durban, South Africa, 19–23 August 2007, pp. 19-23. IFLA Library online. [Google Scholar]
- 120.Kumar A, Purohit AK. 2012The role of indigenous knowledge in biological control of plant pathogens: logistics of new research initiatives. In Plant defence: biological control (eds Mérillon JM, Ramawat KG), pp. 161-194. Dordrecht, The Netherlands: Springer. [Google Scholar]
- 121.Ghorbani R, Wilcockson S, Koocheki A, Leifert C. 2010Soil management for sustainable crop disease control: a review. In Organic farming, pest control and remediation of soil pollutants: organic farming, pest control and remediation of soil pollutants (ed. Lichtfouse E), pp. 177-201. Dordrecht, The Netherlands: Springer. [Google Scholar]
- 122.Ramos TM, Jay-Russell MT, Millner PD, Shade J, Misiewicz T, Sorge US, Hutchinson M, Lilley J, Pires AFA. 2019Assessment of biological soil amendments of animal origin use, research needs, and extension opportunities in organic production. Front. Sustain. Food Syst. 3, 73. ( 10.3389/fsufs.2019.00073) [DOI] [Google Scholar]
- 123.de Medeiros EV, Lima NT, de Sousa Lima JR, Pinto KMS, da Costa DP, Franco Junior CL, Souza RMS, Hammecker C. 2021Biochar as a strategy to manage plant diseases caused by pathogens inhabiting the soil: a critical review. Phytoparasitica 1, 1-14. ( 10.1007/s12600-021-00887-y) [DOI] [Google Scholar]
- 124.Sharma M, Reynnells R. 2018Importance of soil amendments: survival of bacterial pathogens in manure and compost used as organic fertilizers. Microbiol. Spectr. 4,159-175. ( 10.1128/9781555819644.ch9) [DOI] [PubMed] [Google Scholar]
- 125.Bonanomi G, Lorito M, Vinale F, Woo SL. 2018Organic amendments, beneficial microbes, and soil microbiota: toward a unified framework for disease suppression. Annu. Rev. Phytopathol. 56, 1-20. ( 10.1146/annurev-phyto-080615-100046) [DOI] [PubMed] [Google Scholar]
- 126.Inderbitzin P, Ward J, Barbella A, Solares N, Izyumin D, Burman P, Chellemi DO, Subbarao KV. 2017Soil microbiomes associated with verticillium wilt-suppressive broccoli and chitin amendments are enriched with potential biocontrol agents. Phytopathology 108, 31-43. ( 10.1094/PHYTO-07-17-0242-R) [DOI] [PubMed] [Google Scholar]
- 127.Gu G, et al. 2018Agricultural practices influence Salmonella contamination and survival in pre-harvest tomato production. Front. Microbiol. 9, 2451. ( 10.3389/fmicb.2018.02451) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Turner K, Moua CN, Hajmeer M, Barnes A, Needham M. 2019Overview of leafy greens-related food safety incidents with a California link: 1996 to 2016. J. Food Prot. 82, 405-414. ( 10.4315/0362-028X.JFP-18-316) [DOI] [PubMed] [Google Scholar]
- 129.USCC. 2002The test method for the examination of composting and compost (TMECC). Bethesda, MD: US Composting Council.
- 130.USDA-NOP. 2011National organic program handbook: guidance and instructions for accredited certifying agents and certified operations, https://www.ams.usda.gov/rules-regulations/organic/handbook.
- 131.Bonanomi G, Antignani V, Capodilupo M, Scala F. 2010Identifying the characteristics of organic soil amendments that suppress soilborne plant diseases. Soil Biol. Biochem. 42, 136-144. ( 10.1016/j.soilbio.2009.10.012) [DOI] [Google Scholar]
- 132.Rosskopf E, Di Gioia F, Hong JC, Pisani C, Kokalis-Burelle N. 2020Organic amendments for pathogen and nematode control. Annu. Rev. Phytopathol. 58, 277-311. ( 10.1146/annurev-phyto-080516-035608) [DOI] [PubMed] [Google Scholar]
- 133.Cretoiu MS, Korthals GW, Visser JHM, van Elsas JD. 2013Chitin amendment increases soil suppressiveness toward plant pathogens and modulates the actinobacterial and oxalobacteraceal communities in an experimental agricultural field. Appl. Environ. Microbiol. 79, 5291-5301. ( 10.1128/AEM.01361-13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Frąc M, Hannula SE, Bełka M, Jędryczka M. 2018Fungal biodiversity and their role in soil health. Front. Microbiol. 9, 707. ( 10.3389/fmicb.2018.00707) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Elad Y, Cytryn E, Harel YM, Lew B, Graber ER. 2011The Biochar effect: plant resistance to biotic stresses. Phytopathol. Mediterr. 50, 335-349. ( 10.14601/Phytopathol_Mediterr-9807) [DOI] [Google Scholar]
- 136.Kavitha B, Reddy PVL, Kim B, Lee SS, Pandey SK, Kim K-H. 2018Benefits and limitations of biochar amendment in agricultural soils: a review. J. Environ. Manage. 227, 146-154. ( 10.1016/j.jenvman.2018.08.082) [DOI] [PubMed] [Google Scholar]
- 137.Bonanomi G, Alioto D, Minutolo M, Marra R, Cesarano G, Vinale F. 2020Organic amendments modulate soil microbiota and reduce virus disease incidence in the TSWV-tomato pathosystem. Pathogens 9, 379. ( 10.3390/pathogens9050379) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bonanomi G, Ippolito F, Scala F. 2015A ‘black’ future for plant pathology? Biochar as a new soil amendment for controlling plant diseases. J. Plant Pathol. 97, 223-224. ( 10.4454/jpp.v97i2.3381) [DOI] [Google Scholar]
- 139.Jaiswal AK, Frenkel O, Tsechansky L, Elad Y, Graber ER. 2018Immobilization and deactivation of pathogenic enzymes and toxic metabolites by biochar: a possible mechanism involved in soilborne disease suppression. Soil Biol. Biochem. 121, 59-66. ( 10.1016/j.soilbio.2018.03.001) [DOI] [Google Scholar]
- 140.Frenkel O, Jaiswal AK, Elad Y, Lew B, Kammann C, Graber ER. 2017The effect of biochar on plant diseases: what should we learn while designing biochar substrates? J. Environ. Eng. Landsc. Manag. 25, 105-113. ( 10.3846/16486897.2017.1307202) [DOI] [Google Scholar]
- 141.Adetunji AT, Ncube B, Mulidzi R, Lewu FB. 2020Management impact and benefit of cover crops on soil quality: a review. Soil Tillage Res. 204, 104717. ( 10.1016/j.still.2020.104717) [DOI] [Google Scholar]
- 142.Larkin RP, Honeycutt CW, Olanya OM, Halloran JM, He Z. 2012Impacts of crop rotation and irrigation on soilborne diseases and soil microbial communities. In Sustainable potato production: global case studies (eds He Z, Larkin R, Honeycutt W), pp. 23-41. Dordrecht, The Netherlands: Springer. [Google Scholar]
- 143.Reddy PP. 2017Agro-ecological approaches to pest management for sustainable agriculture. Berlin, Germany: Springer. [Google Scholar]
- 144.Vukicevich E, Lowery T, Bowen P, Úrbez-Torres JR, Hart M. 2016Cover crops to increase soil microbial diversity and mitigate decline in perennial agriculture. A review . Agron. Sustain. Dev. 36, 48. ( 10.1007/s13593-016-0385-7) [DOI] [Google Scholar]
- 145.Richards A, Estaki M, Úrbez-Torres JR, Bowen P, Lowery T, Hart M. 2020Cover crop diversity as a tool to mitigate vine decline and reduce pathogens in vineyard soils. Diversity 12, 128. ( 10.3390/d12040128) [DOI] [Google Scholar]
- 146.Reed-Jones NL, Marine SC, Everts KL, Micallef SA. 2016Effects of cover crop species and season on population dynamics of Escherichia coli and Listeria innocua in soil. Appl. Environ. Microbiol. 82, 1767-1777. ( 10.1128/AEM.03712-15) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Schenck LA, Bakker MG, Moorman TB, Kaspar TC. 2019Effects of cover crop presence, cover crop species selection and fungicide seed treatment on corn seedling growth. Renew. Agric. Food Syst. 34, 93-102. ( 10.1017/S1742170517000345) [DOI] [Google Scholar]
- 148.Gaba S, et al. 2015Multiple cropping systems as drivers for providing multiple ecosystem services: from concepts to design. Agron. Sustain. Dev. 35, 607-623. ( 10.1007/s13593-014-0272-z) [DOI] [Google Scholar]
- 149.Peters RD, Sturz AV, Carter MR, Sanderson JB. 2003Developing disease-suppressive soils through crop rotation and tillage management practices. Soil Tillage Res. 72, 181-192. ( 10.1016/S0167-1987(03)00087-4) [DOI] [Google Scholar]
- 150.Tamburini G, Bommarco R, Wanger TC, Kremen C, van der Heijden MGA, Liebman M, Hallin S. 2020Agricultural diversification promotes multiple ecosystem services without compromising yield. Sci. Adv. 6, eaba1715. ( 10.1126/sciadv.aba1715) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bennett AJ, Bending GD, Chandler D, Hilton S, Mills P. 2012Meeting the demand for crop production: the challenge of yield decline in crops grown in short rotations. Biol. Rev. 87, 52-71. ( 10.1111/j.1469-185X.2011.00184.x) [DOI] [PubMed] [Google Scholar]
- 152.Peralta AL, Sun Y, McDaniel MD, Lennon JT. 2018Crop rotational diversity increases disease suppressive capacity of soil microbiomes. Ecosphere 9, e02235. ( 10.1002/ecs2.2235) [DOI] [Google Scholar]
- 153.Subbarao KV, Kabir Z, Martin FN, Koike ST. 2007Management of soilborne diseases in strawberry using vegetable rotations. Plant Dis. 91, 964-972. ( 10.1094/PDIS-91-8-0964) [DOI] [PubMed] [Google Scholar]
- 154.Grace C, Lynch MB, Sheridan H, Lott S, Fritch R, Boland TM. 2019Grazing multispecies swards improves ewe and lamb performance. Animal 13, 1721-1729. ( 10.1017/S1751731118003245) [DOI] [PubMed] [Google Scholar]
- 155.Prasad MSR, Sundaram SM, Gnanaraj PT, Bandeswaran C, Harikrishnan TJ, Sivakumar T, Azhahiannambi P. 2019Influence of intensive rearing, continuous and rotational grazing systems of management on parasitic load of lambs. Vet. World 12, 1188-1194. ( 10.14202/vetworld.2019.1188-1194) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kurle JE, Grau CR, Oplinger ES, Mengistu A. 2001Tillage, crop sequence, and cultivar effects on sclerotinia stem rot incidence and yield in soybean. Agron. J. 93, 973-982. ( 10.2134/agronj2001.935973x) [DOI] [Google Scholar]
- 157.Schmidt R, Gravuer K, Bossange AV, Mitchell J, Scow K. 2018Long-term use of cover crops and no-till shift soil microbial community life strategies in agricultural soil. PLoS ONE 13, e0192953. ( 10.1371/journal.pone.0192953) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bockus WW, Shroyer JP. 1998The impact of reduced tillage on soilborne plant pathogens. Annu. Rev. Phytopathol. 36, 485-500. ( 10.1146/annurev.phyto.36.1.485) [DOI] [PubMed] [Google Scholar]
- 159.Almeida ÁMR, Saraiva OF, Farias JRB, Gaudêncio CA, Torres E. 2001Survival of pathogens on soybean debris under no-tillage and conventional tillage systems. Pesqui. Agropecuária Bras. 36, 1231-1238. ( 10.1590/S0100-204X2001001000003) [DOI] [Google Scholar]
- 160.Wang H, Li X, Li X, Wang J, Li X, Guo Q, Yu Z, Yang T, Zhang H. 2020Long-term no-tillage and different residue amounts alter soil microbial community composition and increase the risk of maize root rot in northeast China. Soil Tillage Res. 196, 104452. ( 10.1016/j.still.2019.104452) [DOI] [Google Scholar]
- 161.Ntahimpera N, Dillard HR, Cobb AC, Seem RC. 1997Influence of tillage practices on anthracnose development and distribution in dry bean fields. Plant Dis. 81, 71-76. ( 10.1094/PDIS.1997.81.1.71) [DOI] [PubMed] [Google Scholar]
- 162.Endale DM, Schomberg HH, Jenkins MB, Franklin DH, Fisher DS. 2010Management implications of conservation tillage and poultry litter use for Southern Piedmont USA cropping systems. Nutr. Cycl. Agroecosyst. 88, 299-313. ( 10.1007/s10705-009-9318-z) [DOI] [Google Scholar]
- 163.Jenkins MB, Truman CC, Franklin DH, Potter TL, Bosch DD, Strickland TC, Nuti RC. 2014Fecal bacterial losses in runoff from conventional and no-till pearl millet fertilized with broiler litter. Agric. Water Manag. 134, 38-41. ( 10.1016/j.agwat.2013.11.013) [DOI] [Google Scholar]
- 164.Jacobsen CS, Hjelmsø MH. 2014Agricultural soils, pesticides and microbial diversity. Curr. Opin. Biotechnol. 27, 15-20. ( 10.1016/j.copbio.2013.09.003) [DOI] [PubMed] [Google Scholar]
- 165.Kalia A, Gosal SK. 2011Effect of pesticide application on soil microorganisms. Arch. Agron. Soil Sci. 57, 569-596. ( 10.1080/03650341003787582) [DOI] [Google Scholar]
- 166.Lo C-C. 2010Effect of pesticides on soil microbial community. J. Environ. Sci. Health Part B 45, 348-359. ( 10.1080/03601231003799804) [DOI] [PubMed] [Google Scholar]
- 167.Carvalho FP. 2017Pesticides, environment, and food safety. Food Energy Secur. 6, 48-60. ( 10.1002/fes3.108) [DOI] [Google Scholar]
- 168.Pimentel D, Burgess M. 2012Small amounts of pesticides reaching target insects. Environ. Dev. Sustain. 14, 1-2. ( 10.1007/s10668-011-9325-5) [DOI] [Google Scholar]
- 169.Van Hoesel W, et al. 2017Single and combined effects of pesticide seed dressings and herbicides on earthworms, soil microorganisms, and litter decomposition. Front. Plant Sci. 8, 215. ( 10.3389/fpls.2017.00215) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hussain S, Siddique T, Saleem M, Arshad M, Khalid A. 2009Impact of pesticides on soil microbial diversity, enzymes, and biochemical reactions. Adv. Agron. 102, 159-200. ( 10.1016/S0065-2113(09)01005-0) [DOI] [Google Scholar]
- 171.Staley ZR, Rohr JR, Senkbeil JK, Harwood VJ. 2014Agrochemicals indirectly increase survival of E. coli O157:H7 and indicator bacteria by reducing ecosystem services. Ecol. Appl. 24, 1945-1953. ( 10.1890/13-1242.1) [DOI] [PubMed] [Google Scholar]
- 172.Busch G, Kassas B, Palma MA, Risius A. 2020Perceptions of antibiotic use in livestock farming in Germany, Italy and the United States. Livest. Sci. 241, 104251. ( 10.1016/j.livsci.2020.104251) [DOI] [Google Scholar]
- 173.Leigh Archer UA. 2020Antibiotics in crop production. See https://edis.ifas.ufl.edu/hs1366 (accessed on 30 November 2020).
- 174.Stockwell V, Duffy B. 2012Use of antibiotics in plant agriculture. Rev. Sci. Tech. 31,199-210. ( 10.20506/rst.31.1.2104) [DOI] [PubMed] [Google Scholar]
- 175.Cycoń M, Mrozik A, Piotrowska-Seget Z. 2019Antibiotics in the soil environment—degradation and their impact on microbial activity and diversity. Front. Microbiol. 10, 338. ( 10.3389/fmicb.2019.00338) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Xie W-Y, Shen Q, Zhao FJ. 2018Antibiotics and antibiotic resistance from animal manures to soil: a review. Eur. J. Soil Sci. 69, 181-195. ( 10.1111/ejss.12494) [DOI] [Google Scholar]
- 177.Grenni P, Ancona V, Barra Caracciolo A. 2018Ecological effects of antibiotics on natural ecosystems: a review. Microchem. J. 136, 25-39. ( 10.1016/j.microc.2017.02.006) [DOI] [Google Scholar]
- 178.Jechalke S, Heuer H, Siemens J, Amelung W, Smalla K. 2014Fate and effects of veterinary antibiotics in soil. Trends Microbiol. 22, 536-545. ( 10.1016/j.tim.2014.05.005) [DOI] [PubMed] [Google Scholar]
- 179.Venglovsky J, Sasakova N, Placha I. 2009Pathogens and antibiotic residues in animal manures and hygienic and ecological risks related to subsequent land application. Bioresour. Technol. 100, 5386-5391. ( 10.1016/j.biortech.2009.03.068) [DOI] [PubMed] [Google Scholar]
- 180.Magouras I, Carmo LP, Stärk KDC, Schüpbach-Regula G. 2017Antimicrobial usage and -resistance in livestock: where should we focus? Front. Vet. Sci. 4, 148. ( 10.3389/fvets.2017.00148) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Thakur S, Gray GC. 2019The mandate for a global ‘One Health’ approach to antimicrobial resistance surveillance. Am. J. Trop. Med. Hyg. 100, 227-228. ( 10.4269/ajtmh.18-0973) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Gozdzielewska L, King C, Flowers P, Mellor D, Dunlop P, Price L. 2020Scoping review of approaches for improving antimicrobial stewardship in livestock farmers and veterinarians. Prev. Vet. Med. 180, 105025. ( 10.1016/j.prevetmed.2020.105025) [DOI] [PubMed] [Google Scholar]
- 183.Guterres A. 2020Secretary-General's address at Columbia University: ‘The State of the Planet’. U. N. Secr.-Gen. See https://www.un.org/sg/en/content/sg/speeches/2020-12-02/address-columbia-university-the-state-of-the-planet (accessed on 10 December 2020).
- 184.Garibaldi LA, Pérez-Méndez N, Garratt MPD, Gemmill-Herren B, Miguez FE, Dicks LV. 2019Policies for ecological intensification of crop production. Trends Ecol. Evol. 34, 282-286. ( 10.1016/j.tree.2019.01.003) [DOI] [PubMed] [Google Scholar]
- 185.Fierer N, Wood SA, Bueno de Mesquita CP, .2021How microbes can, and cannot, be used to assess soil health. Soil Biol. Biochem. 153, 108111. ( 10.1016/j.soilbio.2020.108111) [DOI] [Google Scholar]
- 186.Wall DH, Nielsen UN, Six J. 2015Soil biodiversity and human health. Nature 528, 69-76. ( 10.1038/nature15744) [DOI] [PubMed] [Google Scholar]
- 187.Gibbs EPJ. 2014The evolution of One Health: a decade of progress and challenges for the future. Vet. Rec. 174, 85-91. ( 10.1136/vr.g143) [DOI] [PubMed] [Google Scholar]
- 188.Keith AM, Schmidt O, McMahon BJ. 2016Soil stewardship as a nexus between Ecosystem Services and One Health. Ecosyst. Serv. 17, 40-42. ( 10.1016/j.ecoser.2015.11.008) [DOI] [Google Scholar]
- 189.Queenan K, Häsler B, Rushton J. 2016A One Health approach to antimicrobial resistance surveillance: is there a business case for it? Int. J. Antimicrob. Agents 48, 422-427. ( 10.1016/j.ijantimicag.2016.06.014) [DOI] [PubMed] [Google Scholar]
- 190.Rüegg SR, et al. 2018A systems approach to evaluate One Health initiatives. Front. Vet. Sci. 5, 23. ( 10.3389/fvets.2018.00023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Kock R, Haider N, Mboera LE, Zumla A. 2019A One-Health lens for anthrax. Lancet Planet. Health 3, e285-e286. ( 10.1016/S2542-5196(19)30111-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Berg G, Krause R, Mendes R. 2015Cross-kingdom similarities in microbiome ecology and biocontrol of pathogens. Front. Microbiol. 6, 1311. ( 10.3389/fmicb.2015.01311) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Mendes R, Raaijmakers JM. 2015Cross-kingdom similarities in microbiome functions. ISME J. 9, 1905-1907. ( 10.1038/ismej.2015.7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Adam E, et al. 2016Controlling the microbiome: microhabitat adjustments for successful biocontrol strategies in soil and human gut. Front. Microbiol. 7, 1079. ( 10.3389/fmicb.2016.01079) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Ramírez-Puebla ST, Servín-Garcidueñas LE, Jiménez-Marín B, Bolaños LM, Rosenblueth M, Martínez J, Rogel MA, Ormeño-Orrillo E, Martínez-Romero E. 2013Gut and root microbiota commonalities. Appl. Environ. Microbiol. 79, 2-9. ( 10.1128/AEM.02553-12) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.