Abstract
Ongoing environmental changes are affecting physical, chemical and biological soil components. Evidence of impacts of soil changes on pollinators' and seed dispersers' behaviour, fitness and density is scarce, but growing. Here, we reviewed information on such impacts and on a number of mechanisms that may explain its propagation, taking into account the full range of resources required by the large and diverse number of species of these two important functional groups. We show that while there is substantial evidence on the effects of soil nitrogen enrichment and changes in soil water content on the quality and quantity of floral and fruit resources, little is known on the effects of changes of other soil properties (e.g. soil pH, soil structure, other nutrients). Also, the few studies showing correlations between soil changes and pollinator and seed disperser foraging behaviour or fitness do not clearly identify the mechanisms that explain such correlation. Finally, most studies (including those with nitrogen and water) are local and limited to a small number of species, and it remains unclear how variable such effects are across time and geographical regions, and the strength of interactive effects between soil properties. Increasing research on this topic, taking into consideration how impacts propagate through species interaction networks, will provide essential information to predict impacts of ongoing environmental changes and help guide conservation plans that aim to minimize impacts on ecosystem functioning.
This article is part of the theme issue ‘The role of soils in delivering Nature's Contributions to People’.
Keywords: flower visitors, pollination, seed dispersal, frugivory, soil chemical and physical properties, soil microbiota
1. Introduction
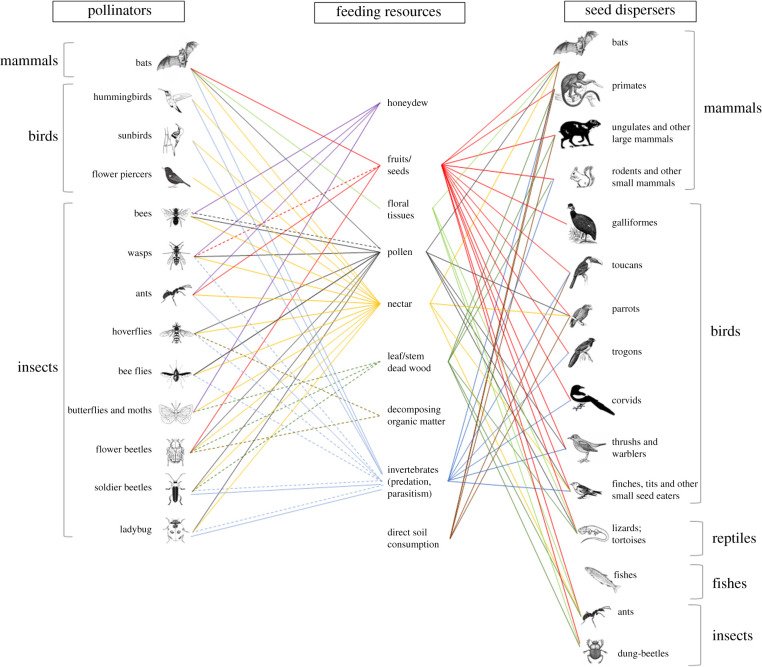
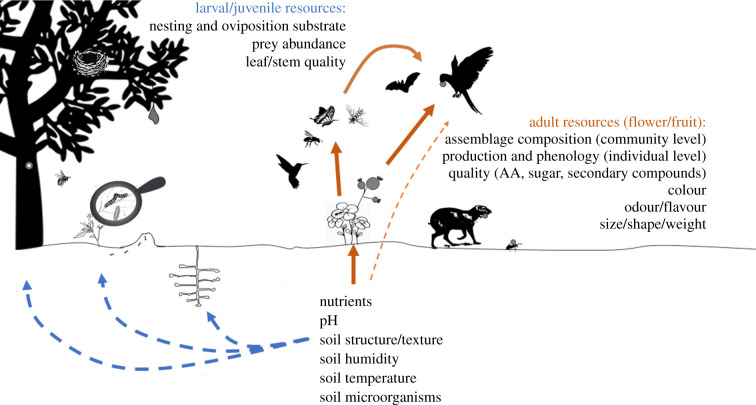
Pollination and seed dispersal are essential ecosystem functions upon which the reproduction of most plants depends [1,2] both being performed by a vast group of animals including a diverse set of invertebrates and vertebrates (figure 1). These functions make an important contribution to humankind, increasing production and quality of the vast majority of crops [3,4] and maintaining the populations of many plants [2] essential for several ecosystem services. Human activities have changed immensely chemical [5,6], physical [7,8] and biological [9] properties of the soil affecting plants at the species [10,11] and community level [12]. Yet, compared to other global changes (e.g. biological invasions, pollution, climate and land use changes [13,14]), little is known about how impacts of such changes in soil properties propagate through trophic levels and even less about the direct impacts on pollinators and seed dispersers [15,16]. In addition, the scarce existing information shows that impacts of soil changes vary in strength and direction depending on the species of the consumer evaluated. For example, some flower visitor species increase their visitation rates with nitrogen enrichment, while other species have their visitation rates maintained or even reduced [17,18]. Similarly, some vertebrate dispersers benefit from soil nutrient enrichment, while ant dispersers seem more frequent on infertile soils [16,19]. Diet preferences may partly explain such variability in species responses [20,21], which effects scale up to shape communities (e.g. increased dominance [18], reduced density and richness [21–23] and changes in overall interaction network patterns [24]). Understanding the mechanisms that mediate the propagation of soil changes to pollinators and seed dispersers is essential to improve our ability to predict impacts of ongoing environmental changes and define adequate conservation plans. Here, we explore potential mechanisms mediating impacts on pollinators and seed dispersers by summarizing the existing information on the impacts of changes of several soil properties on the great diversity of dietary (figure 1) and nesting resources of these important functional groups and how such changes can explain the reported effects on them (figure 2).
Figure 1.
Diversity of feeding resources used by pollinators, during larval or adult stages, that can be affected by soil properties. Dashed lines represent resources used during larval stages. References used for the construction of this figure are listed in the electronic supplementary material.
Figure 2.
Pathways through which changes in soil properties (chemical, physical and biotic) may affect pollinators and seed dispersers. Blue dashed arrows represent effects on nesting and dietary resources used mostly by immature stages, orange solid arrows represent effects on dietary resources collected by adult individuals and orange thin dashed line represents direct consumption of soil by adults.
2. Effects mediated by changes on abundance and diversity of floral and fruit resources
Soil abiotic and biotic characteristics are important regulators of plant physiology and affect the spatial abundance and diversity of flowers and fruits [25–28]. While the effects of increasing water availability on flower and fruit production tend to be generally positive, most plant species are better adapted to soils with low levels of nutrients, with nitrogen enrichment leading to local losses of plant diversity and promoting the expansion of invasive plants [25,26]. Similarly, soil phosphorous enrichment, which facilitates nitrogen uptake by plants [6], can lead to losses of plant richness [29]. Even in regions where plant richness is recovering (e.g. northwest Europe), increases of species are dominated by nitrophilous plants, benefitting only those pollinators that are able to make use of such species [21]. Other important macronutrients (phosphorus, potassium, calcium) may also affect flower availability, but are still poorly studied. On the other hand, whereas fertile soils have a higher percentage of fleshy-fruit species, infertile soils are more likely to have a community of plants that develop elaiosomes and arils (important rewards for many dispersers, including ants, birds and mammals [16,30,31]). Indeed, soils rich in potassium, calcium and phosphorus, are associated with vertebrate-attracting fleshy fruits, while in the nutrient-poor soil, seed dispersal communities may have a greater proportion of ants [19].
Effects of changes in soil microbiota on individual flower production can also vary among species. A reduction in the activity of soil microbiota may hence induce accentuated changes in plant community composition [32], the flower abundance of some species being positively affected by mycorrhizal fungi colonization ([33,34]), while others exhibit a negative or null response [33].
Phenology can also be affected by soil changes (e.g. nutrients [35], soil microclimate [36–38]), affecting the abundance of flowers and fruits through time, potentially creating temporal mismatches between plants and their pollinators and seed dispersers [39,40]. Some species have their flowering periods anticipated by increased nitrogen availability [41,42], while in others, flowering may be delayed or shortened [43,44]. Drought may delay the flowering period of certain plant species (e.g. [36–38]).
The changes in plant distribution, flower production and phenology described above may affect the availability of dietary resources with adequate nutritional content for pollinators and seed dispersers. Moreover, even when flower resource availability and assemblage composition are not affected, soil-driven changes in plant morphology, physiology and chemistry can have strong impacts on their consumers [45]. Below we describe in more detail effects of soil changes on such properties.
3. Effects mediated by changes on fruit and flower morphology
Alteration of soil nutrient levels [15,46], pH [47] and humidity [36,48] may change flower and fruit morphology. For example, under water restriction, certain plant species produce smaller flowers [36,48], while under lower soil pH, some species produce smaller inflorescences [47]. Nutrient increases have been related both to increases [46,49] and decreases [50] in fruit and flower size (e.g. corolla length, petal width), and soil changes may also affect flower and fruit colour [51,52]. Such changes can affect attractiveness to pollinators [53,54] and seed dispersers [16], or access of pollinators to floral resources [36,48]. Other morphological changes caused by soil changes (e.g. nutrient level, mycorrhizal fungi) may involve pollen sculpture [55] and size [33,56], which can affect pollen adherence to pollinator body and their performance as pollinators [57,58].
4. Effects mediated by changes on nutritional value of resources
Similar to other guilds of primary consumers, to satiate the dietary needs of pollinators and dispersers, the nutritional composition of plant resources consumed must match, at least partially, the requirements of those animals, which can greatly vary across species [21,59–62]. Nutrient enrichment may affect nectar [49,63,64] and pollen [56] production per flower, negative effects on nectar volume being more likely when nitrogen input is high [49,50]. Nectar and pollen quality [65–67] (i.e. amino acid (AA) content, sugar or secondary compounds levels) may also be affected by nutrient enrichment. For example, increasing nitrogen availability can increase nectar AAs and sugar content, some AAs being more affected than others [42,64,68–71], with climate mediating the strength of such effects [42]. Indeed, Gardener & Gillman [68] showed that glutamine and proline, key AAs in pollinator nectar selection, exhibit a large and significant increase under fertilizer treatment. Soil fertility (especially nitrogen and phosphorus) and irrigation may also affect the production of essential oils consumed by multiple flower visitor species [72–74]. For example, soil phosphorous increased the yield of essential oil in Achillea millefolium L. [75]. Yet, it is unclear if similar effects can be detected in non-volatile (fatty) floral oils, and if such effects depend on soil nutrients and water levels. Changes on mycorrhizal fungi root colonization can also affect the quality of nectar, affecting sugar content [33,34,76], flavonoids and other secondary compounds (e.g. [77–80]) found in floral resources.
Fruit quality may also be affected by soil nutrient enrichment [30,31]. An increase in potassium level may lead to more nutritious fruits [46], rich in sugars and AAs [30,81], and overall benefits for fleshy-fruited plants [19,82]. Increases in secondary compounds caused by nitrogen enrichment can be toxic when ingested [83,84], which many dispersers can counter by ingesting clay [83]. Soil ingestion may also help to compensate for fruit deficits in nitrogen and calcium [85,86]. In addition, soils with low nutrient levels tend to have plants with drier and harder fruits [30,31] and elaiosomes and arils are more common [19]. The production of edible fruits can also be limited by soil water stress, reducing fruit fleshiness and energetic content available to animals [87,88].
While many species of these two groups of animals have a generalized diet, resource quality changes mentioned above can have a strong influence on the foraging behaviour and fitness of pollinators [89,90] and seed dispersers [19,68,90–92], and potentially lead to local losses of diversity [93]. However, these effects may greatly vary among species. Low levels of nitrogen enrichment may be beneficial to some pollinator species (e.g. by reducing parasite loads [94,95]), but repel pollinators and negatively affect their physiology at high concentrations [96]. Finally, plants growing in heavy-metal-rich soils can accumulate metals into their nectar, shortening foraging time of pollinators and nectar robbers, leading to an overall positive effect on fitness of some plant species (e.g. [97]), possibly owing to increased cross-pollination.
5. Effects mediated by changes on floral and fruit scent
While vastly understudied, there is evidence that soil property changes can affect secondary compounds related to floral volatile compounds. Nitrogen enrichment can increase a phenylpropanoid floral volatile (eugenol) that attracts pollinators [17], while the number of floral volatiles and total fragrance emission can decrease with mycorrhizal fungi colonization rate [76]. Earthworm-mediated changes in soil properties can also affect the production of defence-related phytohormone jasmonic acid and of phenolic compounds [98]. Reduced soil humidity is also thought to limit the emission of olfactory cues used by dispersers to find seeds [59]. Given the importance of floral and fruit scent for detecting the presence of resources [60–62,84], any change in chemical composition of olfactory cues can affect pollinator and seed disperser foraging activity. Indeed, previous studies have suggested nitrogen-induced changes in floral volatiles increase pollinator visitation rates [17] and overall plant–pollinator communication [99].
6. Effects mediated by changes on dietary non-floral and non-fruit resources
Recognizing the diversity of resources used by consumers is essential to understand the mechanisms by which soil changes can affect them. While tight coevolutionary processes have resulted in highly specialized relationships between animals and plants [100], many pollinators feed on multiple species across multiple families [101,102], some including a variety of non-floral resources, especially during immature life stages (figure 1). Among seed dispersers, there is also variation in specialization levels, some being almost exclusive frugivorous species [1], while others include multiple alternative resources (figure 1). For pollinators that feed on leaves, decaying material, soil fungi and plant roots during immature stages (e.g. Lepidoptera and Syrphidae [103–107]), changes in leaf biomass, nutritious content and chemical clues caused by increased nutrient level or other soil changes affect the behaviour (e.g. oviposition, consumption patterns) and physiology of those insects [107–110]. Some species of pollinators also act as predators during larval stages, hence, depending on the populations of their prey. Such prey are frequently herbivores (e.g. aphids [110]) and, consequently are highly susceptible to plant chemical properties which are affected by soil properties [111]. Moreover, for pollinators highly specialized in their oviposition locations (e.g. on nitrophobous plant species [21,112]), any change in plant community composition will change oviposition opportunities. However, little is known on how changes in soil chemical and physical conditions affect larval stages of pollinators and seed dispersers and their role in ecosystem functioning.
7. Effects mediated by changes on nesting resources
Several pollinators and seed dispersers have a central place foraging pattern around a nesting location (e.g. bees, ants and vertebrates). For species constructing nests aboveground, nesting requirements are related to habitat structural complexity [28,113], which can be affected by soil properties (e.g. [114,115]). For those directly using soil as nesting substrate (e.g. the vast majority of non-parasitic bees [116,117] and several ant species) and for those that use soil to construct their cells above ground (e.g. Megachillidae [118]), the impacts of altered soil properties can be profound [119].
Although ground-nesting species can adapt to distinct soil types [120–122], some studies suggest soil texture is important. For example, many bee species tend to prefer sandy soils [122,123], deserts and dunes hosting a large diversity of bees, while clay or silt soils are less favourable. Slope [124] and soil compaction [125] also influence choice of nesting sites. While some bee communities prefer flat areas with little compaction [126], others require steep and sloping ground [127], or compacted soil in irregular surfaces [123,125]. Soil temperature and humidity can affect oviposition and the availability of nesting area for pollinators and seed dispersers (e.g. [128–131]). Central place foragers tend to minimize the difference between nest microclimate and optimal environmental foraging conditions [132], and exposed bare ground [126,127], litter cover [133], or direct sunlight and warmth [125] are documented to be preferred by some species.
Anthropogenic activities (e.g. agriculture, pasture or mining) can constrain nest availability [130,134] owing to intense soil disturbance and changes in the physical characteristics of soils and habitat structure [134]. Agricultural soils are known nesting sites of pollinators (wild bees) and seed dispersers (ants, birds) and, despite the limited knowledge, agricultural practices such as tillage or pesticide use may directly harm nest sites. Anecdotal evidence suggests that tillage can directly impact bee survival and delay emergence time [125,135]. For example, for squash bees, Peponapis pruinose, it has been reported that tilling can halve offspring survival owing to direct destruction of nests which, if males and females are at different depths, may also affect sex ratios [136]. However, tillage may also have positive effects on soil properties for ground-nesting by creating open bare ground, loosening compacted soils or changing the predator community [137]. In addition, in agricultural land, ground-nesting pollinators and seed dispersers can also be exposed to pesticides that accumulate in the soil (e.g. [138]). Substantial knowledge gaps remain around the toxicity and effects of neonicotinoids to arthropods, including ground-nesting bees. Further research on pollinator and seed disperser soil preferences is needed, and citizen science approaches integrating large-scale nesting occurrences documented by volunteers [139] with soil texture maps is a promising avenue to advance in this regard.
8. Effects of pollinators and seed dispersers on soil properties
Pollinators and seed dispersers are important in soil formation and maintenance. Soil nesting species impact soil characteristics owing to their excavation activities promoting vertical and horizontal mobilization of soil [116,140,141]. For example, a single bee species (Nomia meander) can move around 95 t yr−1 in the Touchet Valley of southeastern Washington [142]. Such activity may affect soil physical, chemical and hydrological profiles [141], as demonstrated in soils surrounding ants' nests [143]. This can be particularly relevant in arid and semi-arid ecosystems, where soil changes promoted by ants can generate islands of fertility [144]. Moreover, the ramified nests of communal nesters (i.e. bees nesting in aggregations) create an extensive network of tunnels that contribute to soil aeration. In addition, bees are known to bring in external soil into nests to complement its construction, contributing to soil mixing [121]. Overall, like earthworms, bees and ants contribute largely to soil aeration and rejuvenation.
9. Pathways for future research
Ongoing environmental changes are affecting soil in all its components. Evidence of impacts of soil changes on pollinators' and seed dispersers' behaviour, fitness and density is growing. While we have reviewed a number of mechanisms that may explain how such impacts propagate to these important groups of ecosystem service providers, most studies focus only on a subset of the pathways (e.g. effect of soil changes on resources, effects of resource changes on consumers). More experimental studies testing the full chain of effects from soil changes to both resources and consumers are needed. Moreover, it is likely that there is plenty of geographical variation in such impacts. Indeed, Zhong et al. [145] found differences in effects of nitrogen enrichment on soil respiration across biomes, with stronger negative effects in forests than in deserts. Global studies comparing strength and type of effects across climates and biomes are essential to better understand temporal and spatial variations of such effects. Moreover, much of the evidence on potential mechanisms presented here comes from a limited number of studies, and substantial knowledge gaps exist on the effects of most soil characteristics. Also, most studies focus on changes in nitrogen and water content, with more limited evidence on the effects of soil structure, pH, biota and other soil nutrients and pollutants. Experimental manipulation of other soil variables would help to disentangle interactive effects between multiple soil characteristics. Finally, multidisciplinary studies involving different guilds would help understand the indirect impacts of soil changes on pollinators and seed dispersers. For example, as pollinators have a strong influence on fruit abundance and quality, it is likely that any impact of soil changes on pollinators propagate to frugivores, affecting seed dispersal.
Data accessibility
This article has no additional data.
Authors' contributions
L.G.C. and I.B. conceived the initial structure of the manuscript. All authors contributed to the literature review and manuscript writing. L.G.C. coordinated and integrated all contributions.
Competing interests
We declare we have no competing interests.
Funding
L.G.C. and O.R. were funded by Fundação para Ciência e Tecnologia (FCT) and European Union via the programa operacional regional de Lisboa 2014/2020 (project no. EUCLIPO-028360). L.G.C. was also funded by the Brazilian National Council for Scientific and Technological Development (CNPq. Universal 421668/2018-0; PQ 305157/2018-3). S.T. was funded by Fundação para Ciência e Tecnologia (FCT: grant no. UID/BIA/04004/2020 and contract CEECIND/00135/2017). I.B. was funded by H2020 project SHOWCASE. C.F.T. was funded by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
References
- 1.Jordano P. 2000Fruits and frugivory. In Seeds: the ecology of regeneration in plant communities (ed. Fenner M), pp. 125-165. Wallingford, UK: CABI. [Google Scholar]
- 2.Ollerton J, Winfree R, Tarrant S. 2011How many flowering plants are pollinated by animals? Oikos 120, 321-326. ( 10.1111/j.1600-0706.2010.18644.x) [DOI] [Google Scholar]
- 3.Garibaldi LA, et al. 2013Wild pollinators enhance fruit set of crops regardless of honey bee abundance. Science 339, 1608-1611. ( 10.1126/science.1230200) [DOI] [PubMed] [Google Scholar]
- 4.Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services (IPBES) 2016The assessment report on pollinators, pollination and food production. ( 10.5281/ZENODO.3402857) [DOI] [PMC free article] [PubMed]
- 5.Fowler D, et al. 2013The global nitrogen cycle in the twenty-first century. Phil. Trans. R. Soc. B 368, 20130164. ( 10.1098/rstb.2013.0164) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smil V. 2000Phosphorus in the environment: natural flows and human interferences. Annu. Rev. Energy Environ. 25, 53-88. ( 10.1146/annurev.energy.25.1.53) [DOI] [Google Scholar]
- 7.Bai Z, et al. 2018Effects of agricultural management practices on soil quality: a review of long-term experiments for Europe and China. Agric. Ecosyst. Environ. 265, 1-7. ( 10.1016/j.agee.2018.05.028) [DOI] [Google Scholar]
- 8.Fageria NK. 2002Soil quality vs. environmentally-based agricultural management practices. Commun. Soil Sci. Plant Anal. 33, 2301-2329. ( 10.1081/CSS-120005764) [DOI] [Google Scholar]
- 9.Zogg GP, Zak DR, Ringelberg DB, White DC, MacDonald NW, Pregitzer KS. 1997Compositional and functional shifts in microbial communities due to soil warming. Soil Sci. Soc. Am. J. 61, 475-481. ( 10.2136/sssaj1997.03615995006100020015x) [DOI] [Google Scholar]
- 10.Hageman RH. 1980Effect of form of nitrogen on plant growth. In Nitrification inhibitors—potentials and limitations (eds Meisinger JJ, Randall GW, Vitosh ML), pp. 47-62. Maddison, WI: American Society of Agronomy and Soil Science Society of America. [Google Scholar]
- 11.Vitousek PM, Howarth RW. 1991Nitrogen limitation on land and in the sea: how can it occur? Biogeochemistry 13, 87-115. ( 10.1007/BF00002772) [DOI] [Google Scholar]
- 12.Tilman D, Lehman C. 2001Human-caused environmental change: impacts on plant diversity and evolution. Proc. Natl Acad. Sci. USA 98, 5433-5440. ( 10.1073/pnas.091093198) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Potts SG, Biesmeijer JC, Kremen C, Neumann P, Schweiger O, Kunin WE. 2010Global pollinator declines: trends, impacts and drivers. Trends Ecol. Evol. 25, 345-353. ( 10.1016/j.tree.2010.01.007) [DOI] [PubMed] [Google Scholar]
- 14.González-Varo JP, et al. 2013Combined effects of global change pressures on animal-mediated pollination. Trends Ecol. Evol. 28, 524-530. ( 10.1016/j.tree.2013.05.008) [DOI] [PubMed] [Google Scholar]
- 15.David TI, Storkey J, Stevens CJ. 2019Understanding how changing soil nitrogen affects plant–pollinator interactions. Arthropod. Plant. Interact. 13, 671-684. ( 10.1007/s11829-019-09714-y) [DOI] [Google Scholar]
- 16.Westoby M, French K, Hughes L, Rice B, Rodgerson L. 1991Why do more plant species use ants for dispersal on infertile compared with fertile soils? Aust. J. Ecol. 16, 445-455. ( 10.1111/j.1442-9993.1991.tb01074.x) [DOI] [Google Scholar]
- 17.Majetic CJ, Fetters AM, Beck OM, Stachnik EF, Beam KM. 2017Petunia floral trait plasticity in response to soil nitrogen content and subsequent impacts on insect visitation. Flora Morphol. Distrib. Funct. Ecol. Plants 232, 183-193. ( 10.1016/j.flora.2016.08.002) [DOI] [Google Scholar]
- 18.de Ramos DL, Bustamante MMC, Da Silva E, Silva FD, Carvalheiro LG. 2018Crop fertilization affects pollination service provision—common bean as a case study. PLoS ONE 13, 1-16. ( 10.1371/journal.pone.0204460) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Milewski AV. 1982The occurrence of seeds and fruits taken by ants versus birds in Mediterranean Australia and Southern Africa, in relation to the availability of soil potassium. J. Biogeogr. 9, 505-516. ( 10.2307/2844617) [DOI] [Google Scholar]
- 20.Pöyry J, Carvalheiro LG, Heikkinen RK, Kühn I, Kuussaari M, Schweiger O, Valtonen A, van Bodegom PM, Franzén M. 2017The effects of soil eutrophication propagate to higher trophic levels. Glob. Ecol. Biogeogr. 26, 18-30. ( 10.1111/geb.12521) [DOI] [Google Scholar]
- 21.Carvalheiro LG, et al. 2020Soil eutrophication shaped the composition of pollinator assemblages during the past century. Ecography 43, 209-221. ( 10.1111/ecog.04656) [DOI] [Google Scholar]
- 22.Öckinger E, Hammarstedt O, Nilsson SG, Smith HG. 2006The relationship between local extinctions of grassland butterflies and increased soil nitrogen levels. Biol. Conserv. 128, 564-573. ( 10.1016/j.biocon.2005.10.024) [DOI] [Google Scholar]
- 23.Van Dyck H, Van Strien AJ, Maes D, Van Swaay CAM. 2009Declines in common, widespread butterflies in a landscape under intense human use. Conserv. Biol. 23, 957-965. ( 10.1111/j.1523-1739.2009.01175.x) [DOI] [PubMed] [Google Scholar]
- 24.Burkle L, Irwin R. 2009The importance of interannual variation and bottom-up nitrogen enrichment for plant-pollinator networks. Oikos 118, 1816-1829. ( 10.1111/j.1600-0706.2009.17740.x) [DOI] [Google Scholar]
- 25.Tamis WLM, Van't Zelfde M, Van Der Meijden R, De Haes HAU. 2005Changes in vascular plant biodiversity in the Netherlands in the 20th century explained by their climatic and other environmental characteristics. Clim. Change 72, 37-56. ( 10.1007/s10584-005-5287-7) [DOI] [Google Scholar]
- 26.Bobbink R, et al. 2010Global assessment of nitrogen deposition effects on terrestrial plant diversity: a synthesis. Ecol. Appl. 20, 30-59. ( 10.1890/08-1140.1) [DOI] [PubMed] [Google Scholar]
- 27.McConkey KR, Prasad S, Corlett RT, Campos-Arceiz A, Brodie JF, Rogers H, Santamaria L. 2012Seed dispersal in changing landscapes. Biol. Conserv. 146, 1-13. ( 10.1016/j.biocon.2011.09.018) [DOI] [Google Scholar]
- 28.Plein M, Längsfeld L, Neuschulz EL, Schultheiß C, Ingmann L, Töpfer T, Böhning-Gaese K, Schleuning M. 2013Constant properties of plant–frugivore networks despite fluctuations in fruit and bird communities in space and time. Ecology 94, 1296-1306. ( 10.1890/12-1213.1) [DOI] [PubMed] [Google Scholar]
- 29.Wassen MJ, Schrader J, van Dijk J, Eppinga MB. 2021Phosphorus fertilization is eradicating the niche of northern Eurasia's threatened plant species. Nat. Ecol. Evol. 5, 67-73. ( 10.1038/s41559-020-01323-w) [DOI] [PubMed] [Google Scholar]
- 30.Hughes L, Westoby M, Johnson AD. 1993Nutrient costs of vertebrate- and ant-dispersed fruits. Funct. Ecol. 7, 54-62. ( 10.2307/2389867) [DOI] [Google Scholar]
- 31.Herrera CM. 1987Vertebrate-dispersed plants of the Iberian Peninsula: a study of fruit characteristics. Ecol. Monogr. 57, 305-331. ( 10.2307/2937089) [DOI] [Google Scholar]
- 32.Van Der Heijden MGA, Bardgett RD, Van Straalen NM. 2008The unseen majority: soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol. Lett. 11, 296-310. ( 10.1111/j.1461-0248.2007.01139.x) [DOI] [PubMed] [Google Scholar]
- 33.Barber NA, Soper Gorden NL. 2014How do belowground organisms influence plant-pollinator interactions? J. Plant Ecol. 8, 1-11. ( 10.1093/jpe/rtu012) [DOI] [Google Scholar]
- 34.Gange AC, Smith AK. 2005Arbuscular mycorrhizal fungi influence visitation rates of pollinating insects. Ecol. Entomol. 30, 600-606. ( 10.1111/j.0307-6946.2005.00732.x) [DOI] [Google Scholar]
- 35.Silvertown J, Poulton P, Johnston E, Edwards G, Heard M, Biss PM. 2006The park grass experiment 1856–2006: its contribution to ecology. J. Ecol. 94, 801-814. ( 10.1111/j.1365-2745.2006.01145.x) [DOI] [Google Scholar]
- 36.Caruso CM. 2006Plasticity of inflorescence traits in Lobelia siphilitica (Lobeliaceae) in response to soil water availability. Am. J. Bot. 93, 531-538. ( 10.3732/ajb.93.4.531) [DOI] [PubMed] [Google Scholar]
- 37.Desclaux D, Roumet P. 1996Impact of drought stress on the phenology of two soybean (Glycine max L. Merr) cultivars. F. Crop Res. 46, 61-70. ( 10.1016/0378-4290(95)00086-0) [DOI] [Google Scholar]
- 38.McMaster GS, White JW, Weiss A, Stephen Baenziger P, Wilhelm WW, Porter JR, Jamieson PD. 2015Simulating crop phenological responses to water deficits. In Response of crops to limited water (eds Ahuja L, Reddy V, Saseendran S, Yu Q), pp. 277-300. Hoboken, NJ: John Wiley & Sons, Ltd. [Google Scholar]
- 39.Schenk M, Krauss J, Holzschuh A. 2018Desynchronizations in bee–plant interactions cause severe fitness losses in solitary bees. J. Anim. Ecol. 87, 139-149. ( 10.1111/1365-2656.12694) [DOI] [PubMed] [Google Scholar]
- 40.Warren RJ, Bahn V, Bradford MA. 2011Temperature cues phenological synchrony in ant-mediated seed dispersal. Glob. Chang. Biol. 17, 2444-2454. ( 10.1111/j.1365-2486.2010.02386.x) [DOI] [Google Scholar]
- 41.Cleland EE, Chiariello NR, Loarie SR, Mooney HA, Field CB. 2006Diverse responses of phenology to global changes in a grassland ecosystem. Proc. Natl Acad. Sci. USA 103, 13 740-13 744. ( 10.1073/pnas.0600815103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hoover SER, Ladley JJ, Shchepetkina AA, Tisch M, Gieseg SP, Tylianakis JM. 2012Warming, CO2, and nitrogen deposition interactively affect a plant-pollinator mutualism. Ecol. Lett. 15, 227-234. (doi:10.1111/j.1461-0248.2011. 01729.x) [DOI] [PubMed] [Google Scholar]
- 43.Xia J, Wan S. 2013Independent effects of warming and nitrogen addition on plant phenology in the Inner Mongolian steppe. Ann. Bot. 111, 1207-1217. ( 10.1093/aob/mct079) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu Y, Miao R, Chen A, Miao Y, Liu Y, Wu X. 2017Effects of nitrogen addition and mowing on reproductive phenology of three early-flowering forb species in a Tibetan alpine meadow. Ecol. Eng. 99, 119-125. ( 10.1016/j.ecoleng.2016.11.033) [DOI] [Google Scholar]
- 45.Muñoz AA, Celedon-Neghme C, Cavieres LA, Arroyo MTK. 2005Bottom-up effects of nutrient availability on flower production, pollinator visitation, and seed output in a high-Andean shrub. Oecologia 143, 126-135. ( 10.1007/s00442-004-1780-3) [DOI] [PubMed] [Google Scholar]
- 46.Reuther W, Embleton TW, Jones WW. 1958Mineral nutrition of tree crops. Annu. Rev. Plant Physiol. 9, 175-206. ( 10.3168/ldhm.0849) [DOI] [Google Scholar]
- 47.Gentili R, Ambrosini R, Montagnani C, Caronni S, Citterio S. 2018Effect of soil pH on the growth, reproductive investment and pollen allergenicity of Ambrosia artemisiifolia L. Front. Plant Sci. 9, 1335. ( 10.3389/fpls.2018.01335) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Galen C. 2000High and dry: drought stress, sex-allocation trade-offs, and selection on flower size in the alpine wildflower Polemonium viscosum (Polemoniaceae). Am. Nat. 156, 72-83. ( 10.1086/303373) [DOI] [PubMed] [Google Scholar]
- 49.Burkle LA, Irwin RE. 2009The effects of nutrient addition on floral characters and pollination in two subalpine plants, Ipomopsis aggregata and Linum lewisii. Plant Ecol. 203, 83-98. ( 10.1007/s11258-008-9512-0) [DOI] [Google Scholar]
- 50.Burkle LA, Irwin RE. 2010Beyond biomass: measuring the effects of community-level nitrogen enrichment on floral traits, pollinator visitation and plant reproduction. J. Ecol. 98, 705-717. ( 10.1111/j.1365-2745.2010.01648.x) [DOI] [Google Scholar]
- 51.Miller R, Owens SJ, Rørslett B. 2011Plants and colour: flowers and pollination. Optics Laser Technol. 43, 282-294. ( 10.1016/i.optlastec.2008.12.018) [DOI] [Google Scholar]
- 52.Schaefer HM, Valido A, Jordano P. 2014Birds see the true colours of fruits to live off the fat of the land. Proc. R. Soc. B 281, 20132516. ( 10.1098/rspb.2013.2516) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kaczorowski RL, Seliger AR, Gaskett AC, Wigsten SK, Raguso RA. 2012Corolla shape vs. size in flower choice by a nocturnal hawkmoth pollinator. Funct. Ecol. 26, 577-587. ( 10.1111/j.1365-2435.2012.01982.x) [DOI] [Google Scholar]
- 54.Macukanovic-Jocic M, Stevanović ZD, Mladenović M, Jocić G. 2011Flower morphophysiology of selected Lamiaceae species in relation to pollinator attraction. J. Apic. Res. 50, 89-101. ( 10.3896/IBRA.1.50.2.01) [DOI] [Google Scholar]
- 55.Reshetova SA, Sumarokova IE. 2019Pollen of plants in urban areas as a bioindicator of industrial pollution (Transbaikalia). Geosfernye Issled. 4, 15-23. ( 10.17223/25421379/13/2) [DOI] [Google Scholar]
- 56.Lau T-C, Stephenson AG. 1993Effects of soil nitrogen on pollen production, pollen grain size, and pollen performance in Cucurbita pepo (Cucurbitaceae). Am. J. Bot. 80, 763-768. ( 10.2307/2445596) [DOI] [Google Scholar]
- 57.Hesse M. 2000Pollen wall stratification and pollination. Plant Syst. Evol. 222, 1-17. ( 10.1007/BF00984093) [DOI] [Google Scholar]
- 58.Tanaka N, Uehara K, Murata J. 2004Correlation between pollen morphology and pollination mechanisms in the Hydrocharitaceae. J. Plant Res. 117, 265-276. ( 10.1007/s10265-004-0155-5) [DOI] [PubMed] [Google Scholar]
- 59.Wall SBV. 2002Secondary dispersal of Jeffrey pine seeds by rodent scatter-hoarders: the roles of pilfering, recaching and a variable environment. In Seed dispersal and frugivory: ecology, evolution and conservation. Third International Symposium-Workshop on Frugivores and Seed Dispersal, São Pedro, BR. (eds Levey DJ, Silva WR, Galetti M), pp. 193-208. Wallingford, UK: CABI Publishing. [Google Scholar]
- 60.Dötterl S, Schäffler I. 2007Flower scent of floral oil-producing Lysimachia punctata as attractant for the oil-bee Macropis fulvipes. J. Chem. Ecol. 33, 441-445. ( 10.1007/s10886-006-9237-2) [DOI] [PubMed] [Google Scholar]
- 61.Wright GA, Schiestl FP. 2009The evolution of floral scent: the influence of olfactory learning by insect pollinators on the honest signalling of floral rewards. Funct. Ecol. 23, 841-851. ( 10.1111/j.1365-2435.2009.01627.x) [DOI] [Google Scholar]
- 62.Nevo O, Ayasse M. 2020Fruit scent: biochemistry, ecological function, and evolution. In Co-evolution of secondary metabolites (eds Mérillon JM, Ramawat K), pp. 403-425. Berlin, Germany: Springer Nature Switzerland. [Google Scholar]
- 63.Campbell DR, Halama KJ. 1993Resource and pollen limitations to lifetime seed production in a natural plant population. Ecology 74, 1043-1051. ( 10.2307/1940474) [DOI] [Google Scholar]
- 64.Gardener MC, Gillman MP. 2001The effects of soil fertilizer on amino acids in the floral nectar of corncockle, Agrostemma githago (Caryophyllaceae). Oikos 92, 101-106. ( 10.1034/j.1600-0706.2001.920112.x) [DOI] [Google Scholar]
- 65.Ceulemans T, Hulsmans E, Vanden Ende W, Honnay O. 2017Nutrient enrichment is associated with altered nectar and pollen chemical composition in Succisa pratensis Moench and increased larval mortality of its pollinator Bombus terrestris L. PLoS ONE 12, 1-15. ( 10.1371/journal.pone.0175160) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Audusseau H, Kolb G, Janz N. 2015Plant fertilization interacts with life history: variation in stoichiometry and performance in nettle-feeding butterflies. PLoS ONE 10, 1-15. ( 10.1371/journal.pone.0124616) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kurze S, Heinken T, Fartmann T. 2017Nitrogen enrichment of host plants has mostly beneficial effects on the life-history traits of nettle-feeding butterflies. Acta Oecol. 85, 157-164. ( 10.1016/j.actao.2017.11.005) [DOI] [Google Scholar]
- 68.Gardener MC, Gillman MP. 2002The taste of nectar—a neglected area of pollination ecology. Oikos 98, 552-557. ( 10.1034/j.1600-0706.2002.980322.x) [DOI] [Google Scholar]
- 69.Gijbels P, Van den Ende W, Honnay O. 2014Landscape scale variation in nectar amino acid and sugar composition in a Lepidoptera pollinated orchid species and its relation with fruit set. J. Ecol. 102, 136-144. ( 10.1111/1365-2745.12183) [DOI] [Google Scholar]
- 70.Gijbels P, Ceulemans T, Van den Ende W, Honnay O. 2015Experimental fertilization increases amino acid content in floral nectar, fruit set and degree of selfing in the orchid Gymnadenia conopsea. Oecologia 179, 785-795. ( 10.1007/s00442-015-3381-8) [DOI] [PubMed] [Google Scholar]
- 71.Ceulemans T, et al. 2017Phosphorus resource partitioning shapes phosphorus acquisition and plant species abundance in grasslands. Nat. Plants 3, 16224. ( 10.1038/nplants.2016.224) [DOI] [PubMed] [Google Scholar]
- 72.Machado IC. 2004Oil-collecting bees and related plants: a review of the studies in the last twenty years and case histories of plants occurring in NE Brazil. In Solitary bees, conservation, rearing and management for pollination. (eds BM Freitas, JOP Pereira), pp. 255–280. Fortaleza, Brazil: Universidade federal do Ceará. [Google Scholar]
- 73.Rasmussen C, Olesen J. 2000Oil flowers and oil-collecting bees. Det Nor. Videnskaps-akademi. I. Mat. Naturvidenskapelig Klasse, Skr. Ny Ser. 39, 23-31. [Google Scholar]
- 74.Buchmann SL. 1987The ecology of oil flowers and their bees. Annu. Rev. Ecol. Syst. 18, 343-369. ( 10.1146/annurev.es.18.110187.002015) [DOI] [Google Scholar]
- 75.Scheffer MC, Ronzelli JP, Koehler HS. 1993Influence of organic fertilization on the biomass, yield and composition of the essential oil of Achillea millefolium L. Acta Hortic. 331, 109-114. ( 10.17660/actahortic.1993.331.14) [DOI] [Google Scholar]
- 76.Becklin KM, Gamez G, Uelk B, Raguso RA, Galen C. 2011Soil fungal effects on floral signals, rewards, and aboveground interactions in an alpine pollination web. Am. J. Bot. 98, 1299-1308. ( 10.3732/ajb.1000450) [DOI] [PubMed] [Google Scholar]
- 77.Peipp H, Maier W, Schmidt J, Wray V, Strack D. 1997Arbuscular mycorrhizal fungus-induced changes in the accumulation of secondary compounds in barley roots. Phytochemistry 44, 581-587. ( 10.1016/S0031-9422(96)00561-4) [DOI] [Google Scholar]
- 78.Kapoor R, Anand G, Gupta P, Mandal S. 2017Insight into the mechanisms of enhanced production of valuable terpenoids by arbuscular mycorrhiza. Phytochem. Rev. 16, 677-692. ( 10.1007/s11101-016-9486-9) [DOI] [Google Scholar]
- 79.Araújo GC, Sousa NR, Castro PML. 2018The effect of fungal-bacterial interaction on the phenolic profile of Pinus pinea L. Plant Growth Regul. 86, 465-475. ( 10.1007/s10725-018-0445-x) [DOI] [Google Scholar]
- 80.Pedone-Bonfim MVL, da Silva DKA, da Silva-Batista AR, de Oliveira AP, da Silva Almeida JRG, Yano-Melo AM, Maia LC. 2018Mycorrhizal inoculation as an alternative for the sustainable production of Mimosa tenuiflora seedlings with improved growth and secondary compounds content. Fungal Biol. 122, 918-927. ( 10.1016/j.funbio.2018.05.009) [DOI] [PubMed] [Google Scholar]
- 81.Fischer RC, Richter A, Hadacek F, Mayer V. 2008Chemical differences between seeds and elaiosomes indicate an adaptation to nutritional needs of ants. Oecologia 155, 539-547. ( 10.1007/s00442-007-0931-8) [DOI] [PubMed] [Google Scholar]
- 82.Irion G. 1978Soil infertility in the Amazonian rain forest. Naturwissenschaften 65, 515-519. ( 10.1007/BF00439791) [DOI] [Google Scholar]
- 83.Cipollini ML, Levey DJ. 1997Secondary metabolites of fleshy vertebrate-dispersed fruits: adaptive hypotheses and implications for seed dispersal. Am. Nat. 150, 346-372. ( 10.1086/286069) [DOI] [PubMed] [Google Scholar]
- 84.Rodríguez A, Alquézar B, Peña L. 2013Fruit aromas in mature fleshy fruits as signals of readiness for predation and seed dispersal. New Phytol. 197, 36-48. ( 10.1111/j.1469-8137.2012.04382.x) [DOI] [PubMed] [Google Scholar]
- 85.Bravo A, Harms KE, Emmons LH. 2010Puddles created by geophagous mammals are potential mineral sources for frugivorous bats (Stenodermatinae) in the Peruvian Amazon. J. Trop. Ecol. 26, 173-184. ( 10.1017/S0266467409990472) [DOI] [Google Scholar]
- 86.Courts SE. 1998Dietary strategies of old world fruit bats (Megachiroptera, Pteropodidae): how do they obtain sufficient protein? Mamm. Rev. 28, 185-194. ( 10.1046/j.1365-2907.1998.00033.x) [DOI] [Google Scholar]
- 87.Bazzaz FA, Ackerly DD, Reekie EG. 2000Reproductive allocation in plants. In Seeds: the ecology of regeneration in plant communities (ed. Fenner M), pp. 1-29. Wallingford, UK: CABI Publishing. [Google Scholar]
- 88.Willson MF, Irvine AK, Walsh NG. 1989Vertebrate dispersal syndromes in some Australian and New Zealand plant communities, with geographic comparisons. Biotropica 21, 133-147. ( 10.2307/2388704) [DOI] [Google Scholar]
- 89.Alm J, Ohnmeiss TE, Lanza J, Vriesenga L. 1990Preference of cabbage white butterflies and honey bees for nectar that contains amino acids. Oecologia 84, 53-57. ( 10.1007/BF00665594) [DOI] [PubMed] [Google Scholar]
- 90.Nepi M. 2014Beyond nectar sweetness: the hidden ecological role of non-protein amino acids in nectar. J. Ecol. 102, 108-115. ( 10.1111/1365-2745.12170) [DOI] [Google Scholar]
- 91.Givnish TJ. 1999On the causes of gradients in tropical tree diversity. J. Ecol. 87, 193-210. ( 10.1046/j.1365-2745.1999.00333.x) [DOI] [Google Scholar]
- 92.Vanderplanck M, Vereecken NJ, Grumiau L, Esposito F, Lognay G, Wattiez R, Michez D. 2017The importance of pollen chemistry in evolutionary host shifts of bees. Sci. Rep. 7, 1-10. ( 10.1038/srep43058) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Elliott SE, Irwin RE, Adler LS, Williams NM. 2008The nectar alkaloid, gelsemine, does not affect offspring performance of a native solitary bee, Osmia lignaria (Megachilidae). Ecol. Entomol. 33, 298-304. ( 10.1111/j.1365-2311.2007.00974.x) [DOI] [Google Scholar]
- 94.Richardson LL, Adler LS, Leonard AS, Andicoechea J, Regan KH, Anthony WE, Manson JS, Irwin RE. 2015Secondary metabolites in floral nectar reduce parasite infections in bumblebees. Proc. R. Soc. B 282, 20142471. ( 10.1098/rspb.2014.2471) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Richardson LL, Bowers MD, Irwin RE. 2016Nectar chemistry mediates the behavior of parasitized bees: consequences for plant fitness. Ecology 97, 325-337. ( 10.1890/15-0263.1) [DOI] [PubMed] [Google Scholar]
- 96.Manson JS, Cook D, Gardner DR, Irwin RE. 2013Dose-dependent effects of nectar alkaloids in a montane plant-pollinator community. J. Ecol. 101, 1604-1612. ( 10.1111/1365-2745.12144) [DOI] [Google Scholar]
- 97.Xun E, Zhang Y, Zhao J, Guo J. 2018Heavy metals in nectar modify behaviors of pollinators and nectar robbers: consequences for plant fitness. Environ. Pollut. 242, 1166-1175. ( 10.1016/j.envpol.2018.07.128) [DOI] [PubMed] [Google Scholar]
- 98.Xiao Z, Jiang L, Chen X, Zhang Y, Defossez E, Hu F, Liu M, Rasmann S. 2019Earthworms suppress thrips attack on tomato plants by concomitantly modulating soil properties and plant chemistry. Soil Biol. Biochem. 130, 23-32. ( 10.1016/j.soilbio.2018.11.023) [DOI] [Google Scholar]
- 99.Majetic CJ, Raguso RA, Ashman TL. 2009Sources of floral scent variation. Plant Signal. Behav. 4, 129-131. ( 10.4161/psb.4.2.7628) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Anderson B, Johnson SD. 2008The geographical mosaic of coevolution in a plant-pollinator mutualism. Evolution 62, 220-225. ( 10.1111/j.1558-5646.2007.00275.x) [DOI] [PubMed] [Google Scholar]
- 101.Waser NM, Chittka L, Price MV, Williams NM, Ollerton J. 1996Generalization in pollination systems, and why it matters. Ecology 77, 1043-1060. ( 10.2307/2265575) [DOI] [Google Scholar]
- 102.Johnson SD, Steiner KE. 2000Generalization versus specialization in plant pollination systems. Trends Ecol. Evol. 15, 140-143. ( 10.1016/S0169-5347(99)01811-X) [DOI] [PubMed] [Google Scholar]
- 103.Ricarte A, Souba-Dols GJ, Hauser M, Marcos-García MÁ. 2017A review of the early stages and host plants of the genera Eumerus and Merodon (Diptera: Syrphidae), with new data on four species. PLoS ONE 12, e0189852. ( 10.1371/journal.pone.0189852) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Skevington JH, Dang PT. 2002Exploring the diversity of flies (Diptera). Biodiversity 3, 3-27. ( 10.1080/14888386.2002.9712613) [DOI] [Google Scholar]
- 105.Ssymank A, Kearns CA, Pape T, Thompson FC. 2008Pollinating flies (diptera): a major contribution to plant diversity and agricultural production. Biodiversity 9, 86-89. ( 10.1080/14888386.2008.9712892) [DOI] [Google Scholar]
- 106.Kristensen NP. 2003Band/Volume IV: Arthropoda: Insecta. Lepidoptera, moths and butterflies. Teilband/Part 36, vol. 2: morphology, physiology, and development. Berlin, Germany, Boston, MA: De Gruyter. ( 10.1515/9783110893724) [DOI] [Google Scholar]
- 107.Prudic KL, Oliver JC, Bowers MD. 2005Soil nutrient effects on oviposition preference, larval performance, and chemical defense of a specialist insect herbivore. Oecologia 143, 578-587. ( 10.1007/s00442-005-0008-5) [DOI] [PubMed] [Google Scholar]
- 108.Butler J, Garratt MPD, Leather SR. 2012Fertilisers and insect herbivores: a meta-analysis. Ann. Appl. Biol. 161, 223-233. ( 10.1111/j.1744-7348.2012.00567.x) [DOI] [Google Scholar]
- 109.Li F, Dudley TL, Chen B, Chang X, Liang L, Peng S. 2016Responses of tree and insect herbivores to elevated nitrogen inputs: a meta-analysis. Acta Oecol. 77, 160-167. ( 10.1016/j.actao.2016.10.008) [DOI] [Google Scholar]
- 110.Verheggen FJ, Arnaud L, Bartram S, Gohy M, Haubruge E. 2008Aphid and plant volatiles induce oviposition in an aphidophagous hoverfly. J. Chem. Ecol. 34, 301-307. ( 10.1007/s10886-008-9434-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Awmack CS, Leather SR. 2002Host plant quality and fecundity in herbivorous insects. Annu. Rev. Entomol. 47, 817-844. ( 10.1146/annurev.ento.47.091201.145300) [DOI] [PubMed] [Google Scholar]
- 112.WallisDeVries MF, van Swaay CAM. 2017A nitrogen index to track changes in butterfly species assemblages under nitrogen deposition. Biol. Conserv. 212, 448-453. ( 10.1016/j.biocon.2016.11.029) [DOI] [Google Scholar]
- 113.Breitbach N, Laube I, Steffan-Dewenter I, Böhning-Gaese K. 2010Bird diversity and seed dispersal along a human land-use gradient: high seed removal in structurally simple farmland. Oecologia 162, 965-976. ( 10.1007/s00442-009-1547-y) [DOI] [PubMed] [Google Scholar]
- 114.Roubik DW. 1989Ecology and natural history of tropical bees. Cambridge, UK: Cambridge University Press. [DOI] [PubMed] [Google Scholar]
- 115.Frankie GW, Newstrom L, Vinson SB, Barthell JF. 1993Nesting-habitat preferences of selected Centris bee species in Costa Rican dry forest. Biotropica 25, 322-333. ( 10.2307/2388790) [DOI] [Google Scholar]
- 116.Cane J, Neff J. 2011Predicted fates of ground-nesting bees in soil heated by wildfire: thermal tolerances of life stages and a survey of nesting depths. Biol. Conserv. 144, 2631-2636. ( 10.1016/j.biocon.2011.07.019) [DOI] [Google Scholar]
- 117.Kells AR, Goulson D. 2003Preferred nesting sites of bumblebee queens (Hymenoptera: Apidae) in agroecosystems in the UK. Biol. Conserv. 109, 165-174. ( 10.1016/S0006-3207(02)00131-3) [DOI] [Google Scholar]
- 118.Pinilla-Gallego MS, Crum J, Schaetzl R, Isaacs R. 2018Soil textures of nest partitions made by the mason bees Osmia lignaria and O. cornifrons (Hymenoptera: Megachilidae). Apidologie 49, 464-472. ( 10.1007/s13592-018-0574-2) [DOI] [Google Scholar]
- 119.Lybrand RA, Fedenko J, Tfaily M, Rao S. 2020Soil properties and biochemical composition of ground-dwelling bee nests in agricultural settings. Soil Sci. Soc. Am. J. 84, 1139-1152. ( 10.1002/saj2.20085) [DOI] [Google Scholar]
- 120.Orr MC, Griswold T, Pitts JP, Parker FD. 2016A new bee species that excavates sandstone nests. Curr. Biol. 26, R792-R793. ( 10.1016/j.cub.2016.08.001) [DOI] [PubMed] [Google Scholar]
- 121.Danforth BN, Minckley RL, Neff JL. 2019The solitary bees: biology, evolution, conservation. Princeton, NJ, Oxford, UK: Princeton University Press. [Google Scholar]
- 122.Cane J. 1991Soils of ground-nesting bees (Hymenoptera: Apoidea): texture, moisture, cell depth and climate. J. Kansas Entomol. Soc. 64, 406-413. [Google Scholar]
- 123.Tsiolis K. 2018Do bare soil landscapes encourage ground nesting bees? Masters thesis, Canterbury Christ Church University School of Human and Life Sciences, Canterbury, UK. [Google Scholar]
- 124.Burkle LA, Alarcón R. 2011The future of plant-pollinator diversity: understanding interaction networks across time, space, and global change. Am. J. Bot. 98, 528-538. ( 10.3732/ajb.1000391) [DOI] [PubMed] [Google Scholar]
- 125.Wuellner CT. 1999Nest site preference and success in a gregarious, ground-nesting bee Dieunomia triangulifera. Ecol. Entomol. 24, 471-479. ( 10.1046/j.1365-2311.1999.00215.x) [DOI] [Google Scholar]
- 126.Sardiñas HS, Kremen C. 2014Evaluating nesting microhabitat for ground-nesting bees using emergence traps. Basic Appl. Ecol. 15, 161-168. ( 10.1016/j.baae.2014.02.004) [DOI] [Google Scholar]
- 127.Potts SG, Vulliamy B, Roberts S, O'Toole C, Dafni A, Ne'eman G, Willmer P. 2005Role of nesting resources in organising diverse bee communities in a Mediterranean landscape. Ecol. Entomol. 30, 78-85. ( 10.1111/j.0307-6946.2005.00662.x) [DOI] [Google Scholar]
- 128.Hackwell GA. 1967The biology and behavior of the alkali bee Nomia Melanderi Cockerell (hymnoptera: apoidea). Doctoral dissertation, Oregon State University, Corvallis, OR, USA. [Google Scholar]
- 129.Kudo G, Cooper EJ. 2019When spring ephemerals fail to meet pollinators: mechanism of phenological mismatch and its impact on plant reproduction. Proc. R. Soc. B 286, 20190573. ( 10.1098/rspb.2019.0573) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Azcárate FM, Peco B. 2012Abandonment of grazing in a mediterranean grassland area: consequences for ant assemblages. Insect Conserv. Divers. 5, 279-288. ( 10.1111/j.1752-4598.2011.00165.x) [DOI] [Google Scholar]
- 131.Retana J, Cerdá X. 2000Patterns of diversity and composition of Mediterranean ground ant communities tracking spatial and temporal variability in the thermal environment. Oecologia 123, 436-444. ( 10.1007/s004420051031) [DOI] [PubMed] [Google Scholar]
- 132.Azcárate FM, Kovacs E, Peco B. 2007Microclimatic conditions regulate surface activity in harvester ants Messor barbarus. J. Insect Behav. 20, 315-329. ( 10.1007/s10905-007-9074-3) [DOI] [Google Scholar]
- 133.Grundel R, Jean RP, Frohnapple KJ, Glowacki GA, Scott PE, Pavlovic NB. 2010Floral and nesting resources, habitat structure, and fire influence bee distribution across an open-forest gradient. Ecol. Appl. 20, 1678-1692. ( 10.1890/08-1792.1) [DOI] [PubMed] [Google Scholar]
- 134.Azcárate FM, Peco B. 2003Spatial patterns of seed predation by harvester ants (Messor Forel) in Mediterranean grassland and scrubland. Insectes Soc. 50, 120-126. ( 10.1007/s00040-003-0635-y) [DOI] [Google Scholar]
- 135.Mathewson JA. 1968Nest construction and life history of the eastern cucurbit bee, Peponapis pruinosa (Hymenoptera: Apoidea). J. Kansas Entomol. Soc. 41, 255-261. [Google Scholar]
- 136.Ullmann KS, Meisner MH, Williams NM. 2016Impact of tillage on the crop pollinating, ground-nesting bee, Peponapis pruinosa in California. Agric. Ecosyst. Environ. 232, 240-246. ( 10.1016/j.agee.2016.08.002) [DOI] [Google Scholar]
- 137.Roger-Estrade J, Anger C, Bertrand M, Richard G. 2010Tillage and soil ecology: partners for sustainable agriculture. Soil Tillage Res. 111, 33-40. ( 10.1016/j.still.2010.08.010) [DOI] [Google Scholar]
- 138.Willis Chan DS, Prosser RS, Rodríguez-Gil JL, Raine NE. 2019Assessment of risk to hoary squash bees (Peponapis pruinosa) and other ground-nesting bees from systemic insecticides in agricultural soil. Sci. Rep. 9, 1-13. ( 10.1038/s41598-019-47805-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Maher S, Manco F, Ings TC. 2019Using citizen science to examine the nesting ecology of ground-nesting bees. Ecosphere 10, e02911. ( 10.1002/ecs2.2911) [DOI] [Google Scholar]
- 140.Houston TF. 1984Biological observations of bees in the genus Ctenocolletes. (Hymenoptera: Stenotritidae). Rec. West. Aust. Mus. 11, 153-172. [Google Scholar]
- 141.Zaitlin B, Hayashi M. 2012Interactions between soil biota and the effects on geomorphological features. Geomorphology 157–158, 142-152. ( 10.1016/j.geomorph.2011.07.029) [DOI] [Google Scholar]
- 142.Cane JH. 2003Annual displacement of soil in nest tumuli of alkali bees (Nomia melanderi) (Hymenoptera: Apiformes: Halictidae) across an agricultural landscape. J. Kansas Entomol. Soc. 76, 172-176. [Google Scholar]
- 143.Cammeraat LH, Willott SJ, Compton SG, Incoll LD. 2002The effects of ants' nests on the physical, chemical and hydrological properties of a rangeland soil in semi-arid Spain. Geoderma 105, 1-20. ( 10.1016/S0016-7061(01)00085-4) [DOI] [Google Scholar]
- 144.Mandel RD, Sorenson CJ. 1982The role of the western harvester ant (Pogonomyrmex occidentalis) in soil formation. Soil Sci. Soc. Am. J. 46, 785-788. ( 10.2136/sssaj1982.03615995004600040024x) [DOI] [Google Scholar]
- 145.Zhong Y, Yan W, Shangguan Z. 2016The effects of nitrogen enrichment on soil CO2 fluxes depending on temperature and soil properties. Glob. Ecol. Biogeogr. 25, 475-488. ( 10.1111/geb.12430) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.