Abstract
Soils play an important role in mediating chemical weathering reactions and carbon transfer from the land to the ocean. Proposals to increase the contribution of alkalinity to the oceans through ‘enhanced weathering’ as a means to help prevent climate change are gaining increasing attention. This would augment the existing connection between the biogeochemical function of soils and alkalinity levels in the ocean. The feasibility of enhanced weathering depends on the combined influence of what minerals are added to soils, the formation of secondary minerals in soils and the drainage regime, and the partial pressure of respired CO2 around the dissolving mineral. Increasing the alkalinity levels in the ocean through enhanced weathering could help to ameliorate the effects of ocean acidification in two ways. First, enhanced weathering would slightly elevate the pH of drainage waters, and the receiving coastal waters. The elevated pH would result in an increase in carbonate mineral saturation states, and a partial reversal in the effects of elevated CO2. Second, the increase in alkalinity would help to replenish the ocean's buffering capacity by maintaining the ‘Revelle Factor’, making the oceans more resilient to further CO2 emissions. However, there is limited research on the downstream and oceanic impacts of enhanced weathering on which to base deployment decisions.
This article is part of the theme issue ‘The role of soils in delivering Nature's Contributions to People’.
Keywords: ocean alkalinity, enhanced weathering, ocean acidification
1. Introduction
The Earth's climate is regulated by processes on the land and ocean. Soils play an important role in both spheres as a medium for organic carbon accumulation and turnover. Soils also facilitate mineral weathering, which removes CO2 from the atmosphere, converts it into bicarbonate ions, which contribute to the alkalinity of the ocean. This relationship between terrestrial and oceanic processes is an important feature in the natural carbon cycle [1], specifically as a feedback balancing volcanic degassing and other natural CO2 accumulation in the atmosphere. Weathering will also consume all anthropogenic CO2 emissions over 103–106 years [2,3].
The role of soils is particularly relevant to ‘enhanced weathering’ proposals that consider adding minerals to the land to help mitigate climate change [4,5]. Every year, the Earth's rivers naturally add around 500 million tonnes of dissolved calcium to the oceans [6]. This calcium originates from the weathering of carbonate or silicate minerals, which (along with other cations: Mg, Na and K) also consumes CO2 (e.g. equations (1.1) and (1.2)).
| 1.1 |
and
| 1.2 |
Equations (1.1) and (1.2) show the reaction of single minerals (calcite CaCO3 and wollastonite CaSiO3) with CO2, but typically a range of minerals in a rock weather to produce an array of dissolved species as well as new mineral phases (including clay minerals and iron oxides) but, as above, typically consume CO2 [4]. Approximately 0.25 billion tonnes (Gt) of carbon (1 GtC = 1 peta gram C) may be removed from the atmosphere by natural weathering of silicate minerals [7–9], and a similar amount from carbonate weathering [10]. On geological timescales, this removal is balanced with CO2 emissions from volcanic sources. Changes in this balance are fundamental in the climate system, and the temperature dependence of weathering rate provides a long-term negative feedback, stabilizing global climate [11].
Soils play an important role as a medium in which weathering reactions take place [12]. Mineral weathering is naturally accelerated in soils through physical (freeze–thaw, wetting–drying and anthropogenic activities [13]) and biochemical (CO2 respiration, and proton/organic molecule exudation from plant roots, microbes and fungal hyphae, [14,15]) processes. Being composed mainly of secondary minerals (minerals that form through environmental processes, see below, e.g. clays, carbonates and iron oxides/hydroxides), soils are also a product of weathering, which may occlude fresh primary minerals in underlying rock and reduce further mineral dissolution [16]. Soil is also a medium for the reverse reaction of equation (1.1), in which ‘pedogenic’ carbonates are formed. The quantification of the carbonate content of soil has typically been confined to arid environments where it is the largest carbon pool [17]. Estimates suggest that 695–748 GtC are stored globally as pedogenic carbonate, in which the calcium is derived primarily from remobilised lithogenic carbonate [18].
Here, the fundamental role that soils play in the terrestrial-oceanic inorganic carbon cycle are explored, and how, through the action of soils, enhanced weathering may help to ameliorate ocean acidification.
2. The role of soils in enhanced weathering
The application of crushed carbonate minerals to soils is a standard practice in agriculture (agricultural lime) to amend soil porewater pH. It is likely that in excess of 100 Mt of agricultural lime (CaCO3) are applied globally (e.g. 20–30 Mt in the US alone [19], although global figures are not readily available). By mimicking natural weathering, but using similar processes and supply chains for agricultural lime, some have suggested the intentional addition of silicate minerals to the land surface may help to prevent climate change [20–23], with the additional consequence of increasing ocean alkalinity [24]. Enhanced weathering may be part of a portfolio of approaches that intend to remove multiple GtCO2 yr−1 from the atmosphere by 2100 [25,26]. For instance, a recent study suggests that the application of crushed basalt to 35–59% of cropland area in 12 countries could be sufficient to remove 2 GtCO2 yr−1 by 2050 at a cost of $60–220 per tCO2 [5]. The technical challenges associated with enhanced weathering are dominated by the need to crush rock to a small particle size, such that the rate of mineral dissolution is sufficiently rapid that a large proportion of the mineral dissolves over only a few years. As such, the costs in Beerling et al. [5] account for emissions produced by the supply chain. Below we consider the properties of, and processes in, soils that may control the function of enhanced weathering. Rather than adding silicate minerals, it is theoretically possible to add carbonate minerals to the land surface (e.g. expanding the use of agricultural lime). However, as we discuss below, such a proposal may be considerably limited in the CO2 removed per unit of land.
The inorganic components of soil are conceptually divided into primary and secondary minerals. Primary minerals have not been significantly chemically altered since their crystallization from molten material [27]. They are mainly silicate minerals with varied bonding structure [28]. Other common primary minerals in soils include oxides/hydroxides of titanium/iron/manganese, carbonates, as well as non-crystalline inorganic materials such as volcanic glasses [29]. Primary minerals undergo various physical, chemical, biochemical and human-induced weathering in soils. One of the main weathering pathways is the reaction with natural aqueous solutions, such as rainwater, where carbonic acid forms by dissolution of atmospheric CO2. Carbonic acid reacts with the surfaces of primary minerals causing them to dissolve. On short timescales, weathering of carbonate minerals (e.g. equation (1.1)) results in less net sequestration of CO2 than weathering of silicate minerals (e.g. equation (1.2)), and that, over longer timescales (over hundreds of thousands to millions of years), weathering of carbonates results in no net CO2 sequestration due to eventual re-precipitation of carbonates in the ocean [4].
In soils, the CO2 partial pressure may be between 10 to 100 times greater than that of the atmosphere due to plant and microbial respiration, bringing it into the same range as power station flue gas [30,31]. This elevated partial pressure generates additional acidity, accelerating mineral weathering. Moreover, weathering in soils is enhanced by the release of organic acids from plant roots, e.g. malic and acetic acid [32,33], microorganisms, e.g. fulvic, humic, phenolic acids [34,35] and fungi, e.g. citric and oxalic acid [36,37]. In addition, organic compounds can form complexes with the cations in silicate minerals, facilitating breakdown as well as altering the formed products [38]. Furthermore, earthworms, lauded by Aristotle as ‘the intestines of the earth’, play a significant role in enhancing mineral degradation, via organic acids, digestive enzymes and gut microbes during ingestion as well as via burrow aeration and transport processes [39,40].
Since most weathering occurs via contact between primary minerals and aqueous solutions, mineral solubility is important. Generally, silicate minerals with less silica polymerization, e.g. olivine, dissolve at faster rates than minerals with greater silica polymerization, e.g. quartz [41,42] owing to the stronger Si–O bond compared to the M–O bond (where M = Na, Mg or Ca, etc). The dissolution of carbonate minerals (equation (1.1)) is orders of magnitude fasted than silicate minerals, and carbonate dissolution is congruent, meaning the molar ratios of the dissolved elements in solution are similar to that of the solid. However, most primary silicate minerals dissolve incongruently, which means their more soluble components are released preferentially [28]. For instance, when in contact with natural waters, minerals tend to release monovalent cations (e.g. Na+, K+), before divalent cations (Mg2+, Ca2+), before trivalent cations (Fe3+, Al3+), according to the correlation between the ease of hydrolysis and electrostatic valency of the species [43].
In soils, the dissolved products of primary silicate mineral weathering increase the availability of some limiting nutrients such as Si, K and P [44]. These can boost plant productivity and increase the size of the terrestrial carbon pool [45]. This process is critical in natural soil formation [46]. Some of the dissolved products, namely bicarbonate HCO3−, are transported by rivers to the oceans, increasing its total alkalinity, and counteracting ocean acidification (see below [47]). Furthermore, dissolved Si, P and Fe could stimulate biological productivity in oceans, removing additional CO2 from the atmosphere as organic carbon [48–50].
Alongside production of bioavailable dissolved products, incongruent dissolution of some primary minerals also produces solid residues, referred to as secondary minerals. For example, during weathering, primary mineral feldspars, MAlSi3O8, hydrolyse, releasing soluble cations M+ and H4SiO4, and leaving behind the solid secondary (clay) mineral kaolinite, Al2Si2O5(OH)4 (e.g. equation (2.1)).
| 2.1 |
Other common secondary minerals in soils include oxides, e.g. Fe2O3, hydroxides, e.g. Al(OH)3, carbonates, e.g. CaCO3, and phosphates, e.g. Ca5(PO4)3(F, Cl, OH).H2O. Secondary minerals may also precipitate directly from aqueous solution rather than by continuous modification of a primary mineral [51]. The compositions, structures and quantities of these secondary minerals together with organic molecules determine a soils' cation exchange capacity (CEC) and thus its ability to hold nutrients and buffer against acidification [52]. Although clays are more stable to weathering than the primary minerals from which they are derived, they too undergo weathering. In tropical soils, where temperature and precipitation are high, and where decaying organic matter is plentiful, clays undergo additional breakdown [28]. For example, kaolinite may hydrolyse, forming gibbsite (Al2O3.3H2O):
| 2.2 |
Field and laboratory studies [53–57] have shown that clay formation can significantly limit the extent and rate of primary mineral weathering and control elemental fluxes [53,58]. There are primarily three ways in which the precipitation of clays moderate dissolution rates of primary minerals: (i) via control of the saturation state of primary minerals in natural waters; (ii) forming passivating coatings on primary minerals restricting their reactive surface area; and (iii) reducing the hydraulic conductivity of the soil and/or creating preferential flow channels [57].
Another major factor in soil weathering is the presence of the transition metals Fe and Mn and their related redox processes [59]. In primary minerals, Fe and Mn mainly occur in their reduced form, i.e. Fe(II) and Mn(II). Their oxidation creates a charge imbalance which destabilizes the mineral lattice, enabling weathering [28]. In addition, the acidity created by oxidation in aqueous environments facilitates further mineral breakdown (equation (2.3)).
| 2.3 |
The global organic carbon content of soils is roughly three times more than that of atmospheric or terrestrial biomass [60] and a small perturbation to this pool can have a dramatic effect on atmospheric CO2 concentrations [61,62]. Secondary minerals play a very large role in the stabilization and retention of soil organic matter [63]. Secondary minerals form micro- and macro-aggregates with organic matter creating a physical barrier against attacking microbes [64–69]. Soil organic matter can also become stabilized by chemical or physicochemical binding with secondary minerals to form organomineral complexes [70,71]. Without these protections, organic carbon would decompose and mineralize, entering the atmosphere, and eventually result in acidification of the oceans [72].
As such, soil pore water chemistry is fundamental to enhanced weathering, while the ‘carrying capacity’ of rainwater, soil porewaters, and runoff may be constrained by secondary mineral formation. For instance, table 1 considers the metal cation concentration (Mg2+ or Ca2+) and dissolved inorganic carbon (DIC) of a solution in equilibrium with a range of primary and secondary minerals and 400 µatm of CO2 (approximately the partial pressure of CO2 in the atmosphere), and 50 000 µatm of CO2 (a typical partial pressure of CO2 in soil pore gases). The total alkalinity varies by over 8 orders of magnitude depending on what minerals are dissolving or precipitating, and the partial pressure of CO2. An effective enhanced weathering strategy may require spatial removal on the order of 10's tCO2 ha−1 yr−1 [5], which is thermodynamically possible for most silicate minerals at 50 000 µatm CO2, but only for a smaller selection of primary/secondary mineral pairs at 400 µatm CO2. Thus, the feasibility of enhanced weathering depends on the combined influence of dissolving primarily minerals, the formation of secondary minerals, and the partial pressure of CO2. These determine the maximum possible flux of basic cations to oceans via river transport and thus the transport of alkalinity to the ocean.
Table 1.
Resulting (a) total dissolved inorganic carbon and (b) total alkalinity from geochemical equilibrium between primary dissolving minerals (rows) and secondary precipitating minerals (columns). (c) The conversion of DIC to spatial flux assuming 500 mm rainfall. Calculated using PHREEQC [73] and the LLNL.dat database file (apart from gehlenite, which was calculated using minteq.dat file).
 |
Table 1 also highlights the limitations of using calcite, the mineral in agricultural lime, within enhanced weathering strategies. Here a spatial CO2 draw-down of 0.1–1 tCO2 ha−1 is 1–2 orders of magnitude smaller than what might be possible with silicate minerals. However, calcite may dissolve orders of magnitude faster than some silicate minerals, which may result in lower processing requirements and potentially cheaper removal costs. Its effectiveness as a CO2 removal technology may be constrained if the intention is large CO2 removal over a definite land area. However, it still may be possible to dissolve carbonate minerals within engineered systems where the produced alkaline solutions are added to the ocean [74].
Agricultural activities can substantially enhance mineral weathering and the flux of alkalinity to the oceans. For example, tillage exposes less-weathered minerals at depth and brings them to the surface where weathering rates are faster. Acidification resulting from application of fertilisers may also enhance mineral dissolution [75–77]. Nitrification of nitrogen-rich fertilisers can create nitric acid, HNO3, which reacts with minerals (equation (2.4)) at rates exceeding that of natural carbonic acid.
| 2.4 |
However, the role of nitrification in mediating weathering has previously been thought not to result in sequestration of atmospheric CO2, and in the case of carbonate weathering could promote CO2 emission [75,77–79]. Similarly, sulphur deposition (e.g. dissolved into rainwater), water acidification through oxidation of sulphur-bearing minerals (e.g. acid mine drainage), could promote weathering while resulting in the emission of CO2 [80].
Research on natural and enhanced weathering suggests that soils have an important influence on the generation of alkalinity which is ultimately transported to the oceans. This alkalinity influences the oceanic carbon cycle and the ability of the oceans to take up CO2.
3. The ocean carbon cycle and acidification
The ocean is the largest carbon pool at the Earth's surface containing approximately 40 000 GtC. This includes organic carbon contained within living biomass (3 GtC) and dissolved organic carbon (700 GtC). Molecules within the carbonate system, namely aqueous carbon dioxide (CO2(aq)), bicarbonate ions (HCO3−) and carbonate ions (CO32−) comprise the majority of oceanic carbon, of which approximately 920 GtC resides in surface waters and 37 200 GtC in the deep ocean [81]. Figure 1 presents a schematic of the oceanic inorganic ‘carbonate’ cycle, in which all 850 Gt of atmospheric C is cycled through DIC (CT in figure 1) within a decade. Marine autotrophic organisms consume DIC to produce biomass, but some calcifiers (e.g. corals, coccolithophores) also use this carbon to form mineral carbonate shells [83], which ultimately becomes particulate inorganic carbon (PIC). Note that unlike autotrophy, carbonate shell formation consumes ocean alkalinity and generates CO2/acid (reverse of equation (1.1). Much of the PIC is remineralized back into CO2, HCO3− and CO32− as it sinks into corrosive deeper waters (or through biological mediated weathering in the surface ocean) with only a minor amount (approx. 0.3 GtC yr–1) reaching the ocean floor and being permanently removed as sediment [6].
Figure 1.
The global ocean carbonate cycle. Adapted from Andersson and Sabine & Tanhua [6,82]. Arrows represent fluxes in Gt C per year (red arrows denote remineralization). CT represents the dissolved inorganic carbon pools in Gt C. PIC, particulate inorganic carbon.
The ease by which organisms create mineral carbonate shells is related to the product of the activity of the dissolved constituents (here Ca2+ and CO32−) normalized to mineral solubility (equation (3.1), [84,85]). The activity of calcium in seawater is relatively stable. However, CO32− ions are in dynamic equilibrium with CO2 in seawater, such that its activity is reduced by elevated aqueous CO2 (equation (3.2)).
| 3.1 |
and
| 3.2 |
The ocean has absorbed nearly 40% of anthropogenic CO2 emissions since the industrial revolution [86], and subsequently depressed the saturation state of the carbonate mineral aragonite (CaCO3) (referred to as ‘ocean acidification’ [84]). This process can be represented by equation (3.3), in which CO32− ions are consumed through reaction with CO2 to produce HCO3− (thus decreasing ). This places stress on marine calcifying organisms, some of which are sensitive to these changes [87,88] and additional acidification caused by contemporary and future emissions may have severe impacts on some ecosystems. Taylor et al. [89] suggest that an enhanced weathering scheme may be able to counteract the changes caused by saturation state through a globally deployed enhanced weathering scheme. However, the protection offered to calcifying organisms may be geographically limited to regions in which enhanced weathering is deployed.
| 3.3 |
Research over the last 20 years to understand the impact of ocean acidification [84] has produced variable results [90,91]. Species that can maintain calcium carbonate saturation levels in their internal calcifying sites may be less affected by changes in seawater pH. However, elevated CO2 will force calcifying organisms to expend a greater amount of energy in shell building, which could have the largest impact on sensitive organisms/ecosystems (e.g. some corals [92,93]), and some marine environments may dip below safe calcium carbonate saturation levels by mid-century [94].
By reacting away aqueous CO2 (equation (3.3)) additional CO2 can be removed from the atmosphere. This buffering capacity was formalised by Revelle & Suess [95], into what has subsequently been termed the ‘Revelle Factor’ (RF, equation (3.4)).
| 3.4 |
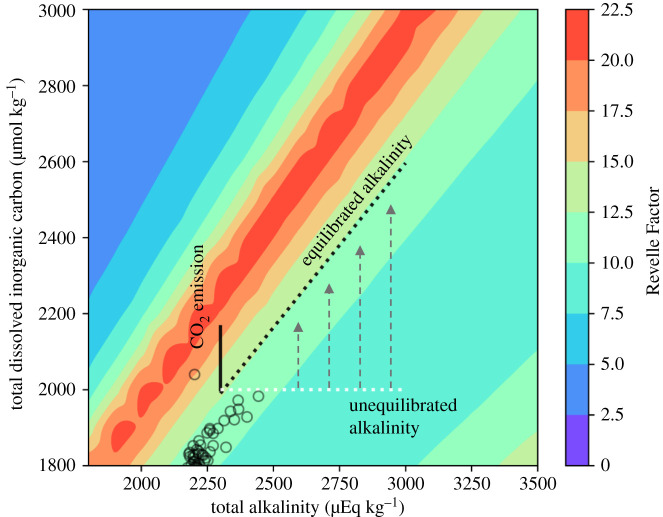
where the partial differentials denote that other state variables (e.g. total alkalinity) are held constant. RF describes the respective change of DIC with changes in atmospheric pCO2. A larger RF equates to a reduction in oceanic buffering capacity. Figure 2 shows a projection of RF under an RCP6.0 type emissions scenario, in which current oceanic values have already diverted from preindustrial and will continue to increase over the coming century, equating to a reduction in the buffering capacity by approximately 34% between 2000 and 2100 [98].
Figure 2.
The Revelle Factor (derived from Egleston et al. [96]) for values of total dissolved inorganic carbon and total alkalinity. The open circles show estimated values over the past 2 million years (derived from Hönisch [97]). The lines show stylised trajectories of an RCP6.0 magnitude emissions scenario (see Renforth & Henderson [24]) that is unabated (solid line) or wholly mitigated by enhanced weathering (dotted lines). (Online version in colour.)
Figure 2 also shows that it may be possible to maintain an RF value of the surface ocean if all anthropogenic CO2 emissions were mitigated by increasing ocean alkalinity (e.g. through mineral weathering). Initially, when alkalinity is increased it will not be equilibrated with atmospheric CO2 (figure 2, white line) and the RF would be reduced. Following the equilibration with CO2, the RF value would be maintained for a given emission. While mitigating all anthropogenic emissions by increasing ocean alkalinity is unlikely to be technically possible or desirable, this hypothetical exercise illustrates that enhanced weathering may also help to maintain the CO2 buffering capacity of the ocean.
As in the case of the re-precipitation of carbonate minerals via marine calcification, the ‘reverse weathering’ of silicate minerals in seawater can also occur. This involves the combination of dissolved metal anions, silicic acid, aluminium hydroxide and bicarbonate, to precipitate cation-poor clay minerals and generate CO2, which may be released to the atmosphere. Such reactions play an important role in controlling the global geochemical balance [99]. They remove alkalinity from the ocean and control the partitioning of CO2 in the ocean atmosphere system. For example, the formation of saponite, Ca0.15Na0.1Mg2.5Fe0.8Si3AlO10(OH)2 (equation (3.5)):
| 3.5 |
The source of Si may also be biogenic opal and the source of Al may be ‘degraded clays’ [100]. Reverse weathering reactions can also involve reactions with solids, e.g. FeOOH-rich coatings on substrate grains [100]. Reactions such as the one in equation (3.5) primarily occur in marine and deltaic environments. For example, in situ clay formation has been observed in the Amazon and Mississippi river deltas [53]. However, it has also been found to occur in the closed-basin lakes of Ethiopia [101]. The extent to which reverse weathering occurs at hydrothermal vents is subject to debate [102–104].
Michalopolous & Aller [53] determined that reverse weathering reactions in Amazon shelf sediments could consume as much as 10% of the continental riverine K+ flux. However, the true extent of reverse weathering is difficult to quantity due to the small quantities of clays formed in addition to interference from terrestrially derived clays [100]. Thus, the process is poorly understood, and its contribution remains uncertain [105]. A range of clay minerals are formed in marine environments, including greenalite, minnesotaite, palygorskite, montmorillonite, glauconite, berthierine, chamosite, clinochlore, sudoite, odenite and corrensite. Of these, only greenalite and minnesotaite are thought to be formed exclusively in marine environments and may be used to help distinguish between marine and terrestrial sources [105]. Better understanding of reverse weathering has been made possible using isotope tracking, particularly K [106], Li [107], and more recently Be [108].
Although formation of clays via reverse weathering is thermodynamically favoured, they may be spatially and kinetically constrained owing to a silica limitation [105]. Indeed, it is postulated that the late ecological rise of siliceous organisms and the resulting decline in silica-rich conditions inhibited the rate of reverse silicate mineral weathering, causing higher ocean alkalinity and lower atmospheric CO2 levels [105]. This silica limitation on reverse weathering has been observed in experiments in the Amazon delta [53]. On the other hand, supply of Al and/or Fe were kinetic limiters in clay formation in the Mississippi delta [109]. Reverse weathering reactions generate CO2 and consume seawater alkalinity. As such, the relative rates of these processes could affect the efficacy of using ocean alkalinity enhancement as an atmospheric CO2 management strategy and as a way of helping chemically counter ocean acidification [110]. For instance, saponite formation has been reported during olivine dissolution experiments in a laboratory shaker [111] and vermiculite and saponite were observed in flume weathering studies [112]. Formation of these clays reduces the efficiency of ocean alkalinity enhancement, e.g. coastal enhanced weathering of olivine which aims to sequester CO2 as bicarbonate in the ocean [113].
4. Conclusion
Sustainable Development Goal 14 aims to ‘conserve and sustainably use the oceans, sea and marine resources for sustainable development’, with a target to ‘minimize and address the impacts of ocean acidification, including through enhanced scientific cooperation at all levels'. The most effective approach to prevent impacts of ocean warming, acidification and sea level rise on SDG 14 is to stabilize if not reduce atmospheric CO2 concentrations by quickly moving to net-zero CO2 emissions. This requires both a redoubled effort to dramatically reduce CO2 emissions as well as employing methods to pro-actively remove billions of tonnes per year of atmospheric CO2.
In the context of this required CO2 removal, we have outlined the significant role soils already play in the land–ocean carbon cycle, and how safely accelerating chemical transformations here could contribute to CO2 removal efforts as well as help rebalance ocean chemistry. Reactions mediated in soils including respiration and organic acid exudation can accelerate weathering rates, while secondary mineral formation and elevated CO2 may limit the maximum alkalinity flux to the ocean. This alkalinity contributes to removing CO2 from the atmosphere for storage as DIC in the ocean.
Secondary mineral formation depends on the type of primary mineral dissolving as well as the local climate and biota present. CO2 sequestration potential of enhanced weathering in soils will need to be determined by in situ monitoring with special care to characterize the secondary minerals formed in order to accurately determine the total amount of carbon removed from that atmosphere.
The oceans remove approximately 25% of anthropogenic CO2 emissions, and some of this excess CO2 is neutralised through carbonate buffering (i.e. the ‘Revelle Factor’). The remaining CO2 contributes to a reduction in ocean pH, which will decline further if CO2 emissions continue to rise. This buffering capacity in the ocean has diminished as a consequence of reaction with CO2, and will continue to reduce with additional CO2 emissions, thus reducing the amount of CO2 that can be removed and neutralised. By increasing the alkalinity flux from land to the ocean, via enhanced weathering schemes, it may be possible to partially replenish the buffering capacity of the ocean and ameliorate some of the impacts of ocean acidification.
Data accessibility
This article has no additional data.
Authors' contributions
P.R. concept, drafting and figure creation, J.C. concept and drafting.
Competing interests
We declare we have no competing interests.
Funding
The authors acknowledge UKRI funding under the UK Greenhouse Gas Removal Programme (NE/P019943/1, NE/P019730/1), and EU funding under the H2020 ‘Fighting and adapting to climate change’ programme OceanNETs project 869357.
References
- 1.Berner RA, Kothavala Z. 2001Geocarb III: a revised model of atmospheric CO2 over Phanerozoic time. Am. J. Sci. 301, 182-204. ( 10.2475/ajs.301.2.182) [DOI] [Google Scholar]
- 2.Archer D, et al. 2009Atmospheric lifetime of fossil fuel carbon dioxide. Annu. Rev. Earth Planet. Sci. 37, 117-134. ( 10.1146/annurev.earth.031208.100206) [DOI] [Google Scholar]
- 3.Lord NS, Ridgwell A, Thorne MC, Lunt DJ. 2016An impulse response function for the ‘long tail’ of excess atmospheric CO2 in an Earth system model. Global Biogeochem. Cycles 30, 2-17. ( 10.1002/2014GB005074) [DOI] [Google Scholar]
- 4.Hartmann J, West AJ, Renforth P, Köhler P, La Rocha CLD, Wolf-Gladrow DA, Dürr HH, Scheffran J. 2013Enhanced chemical weathering as a geoengineering strategy to reduce atmospheric carbon dioxide, supply nutrients, and mitigate ocean acidification. Rev. Geophys. 51, 113-149. ( 10.1002/rog.20004) [DOI] [Google Scholar]
- 5.Beerling DJ, et al. 2020Potential for large-scale CO2 removal via enhanced rock weathering with croplands. Nature 583, 242-248. ( 10.1038/s41586-020-2448-9) [DOI] [PubMed] [Google Scholar]
- 6.Andersson AJ. 2013The oceanic CaCO3 cycle. Treatise Geochem. 8, 519-542. ( 10.1016/B978-0-08-095975-7.00619-7) [DOI] [Google Scholar]
- 7.Gaillardet J, Dupré B, Louvat P, Allègre CJ. 1999Global silicate weathering and CO2 consumption rates deduced from the chemistry of large rivers. Chem. Geol. 159, 3-30. ( 10.1016/S0009-2541(99)00031-5) [DOI] [Google Scholar]
- 8.Hartmann J, Jansen N, Dürr HH, Kempe S, Köhler P. 2009Global CO2-consumption by chemical weathering: what is the contribution of highly active weathering regions? Glob. Planet. Change 69, 185-194. ( 10.1016/j.gloplacha.2009.07.007) [DOI] [Google Scholar]
- 9.Suchet PA, Probst JL. 1995A global model for present-day atmospheric/soil CO2 consumption by chemical erosion of continental rocks (GEM-CO2). Tellus B 47, 273-280. ( 10.1034/j.1600-0889.47.issue1.23.x) [DOI] [Google Scholar]
- 10.Liu Z, Dreybrodt W, Liu H. 2011Atmospheric CO2 sink: silicate weathering or carbonate weathering? Appl. Geochem. 26, S292-S294. ( 10.1016/j.apgeochem.2011.03.085) [DOI] [Google Scholar]
- 11.Berner RA, Lasaga AC, Garrels RM. 1983The carbonate-silicate geothermal cycle and its effect on atmospheric carbon dioxide over the past 100 million years. Am. J. Sci. 283, 641-683. ( 10.2475/ajs.283.7.641) [DOI] [PubMed] [Google Scholar]
- 12.Granger DE, Riebe CS. 2007Cosmogenic nuclides in weathering and erosion. Treatise Geochem . 5 , 401–436. ( 10.1016/B978-0-08-095975-7.00514-3). [DOI] [Google Scholar]
- 13.Millot R, Gaillardet J, Dupré B, Allègre CJ. 2002The global control of silicate weathering rates and the coupling with physical erosion: new insights from rivers of the Canadian Shield. Earth Planet. Sci. Lett. 196, 83-98. ( 10.1016/S0012-821X(01)00599-4) [DOI] [Google Scholar]
- 14.Banwart SA, Nikolaidis NP, Zhu Y-G, Peacock CL, Sparks DL. 2019Soil functions: connecting earth's critical zone. Annu. Rev. Earth Planet. Sci. 47, 333-359. ( 10.1146/annurev-earth-063016-020544) [DOI] [Google Scholar]
- 15.Uroz S, Calvaruso C, Turpault M-P, Frey-Klett P. 2009Mineral weathering by bacteria: ecology, actors and mechanisms. Trends Microbiol. 17, 378-387. ( 10.1016/j.tim.2009.05.004) [DOI] [PubMed] [Google Scholar]
- 16.Dixon JL, von Blanckenburg F. 2012Soils as pacemakers and limiters of global silicate weathering. Comptes Rendus Geosci. 344, 597-609. ( 10.1016/j.crte.2012.10.012) [DOI] [Google Scholar]
- 17.Goudie AS. 1996Organic agency in calcrete development. J. Arid Environ. 32, 103-110. ( 10.1006/jare.1996.0010) [DOI] [Google Scholar]
- 18.Batjes NH. 2008ISRIC-WISE harmonized global soil profile dataset (ver. 3.1) . Report 2008/02. Wageningen, The Netherlands: World Soil Information. ( 10.13140/2.1.4306.7683) [DOI]
- 19.West TO, McBride AC. 2005The contribution of agricultural lime to carbon dioxide emissions in the United States: dissolution, transport, and net emissions. Agric. Ecosyst. Environ. 108, 145-154. ( 10.1016/j.agee.2005.01.002) [DOI] [Google Scholar]
- 20.Schuiling RD, Krijgsman P. 2006Enhanced weathering: an effective and cheap tool to sequester CO2. Clim. Change 74, 349-354. ( 10.1007/s10584-005-3485-y) [DOI] [Google Scholar]
- 21.Moosdorf N, Renforth P, Hartmann J. 2014Carbon dioxide efficiency of terrestrial enhanced weathering. Environ. Sci. Technol. 48, 4809-4816. ( 10.1021/es4052022) [DOI] [PubMed] [Google Scholar]
- 22.Renforth P. 2012The potential of enhanced weathering in the UK. Int. J. Greenh. Gas Control 10, 229-243. ( 10.1016/j.ijggc.2012.06.011) [DOI] [Google Scholar]
- 23.Beerling DJ, et al. 2018Farming with crops and rocks to address global climate, food and soil security. Nat. Plants 4, 392. ( 10.1038/s41477-018-0162-5) [DOI] [PubMed] [Google Scholar]
- 24.Renforth P, Henderson G. 2017Assessing ocean alkalinity for carbon sequestration. Rev. Geophys. 55, 636-674. ( 10.1002/2016RG000533) [DOI] [Google Scholar]
- 25.Minx JC, et al. 2018Negative emissions - Part 1: research landscape and synthesis. Environ. Res. Lett. 13, 063001 ( 10.1088/1748-9326/aabf9b) [DOI] [Google Scholar]
- 26.Smith P, et al. 2015Biophysical and economic limits to negative CO2 emissions. Nat. Clim. Change 6, 42-50. ( 10.1038/nclimate2870) [DOI] [Google Scholar]
- 27.Lapidus DF. 2003Geology (Collins Dictionary of). Glasgow, UK: HarperCollins.
- 28.Churchman GJ, Lowe DJ. 2012Alteration, formation, and occurrence of minerals in soils. See https://researchcommons.waikato.ac.nz/handle/10289/9024.
- 29.Nanzyo M, Kanno H. 2018Inorganic constituents in soil. Singapore: Springer. [Google Scholar]
- 30.Robbins CW. 1986Carbon dioxide partial pressure in lysimeter soils 1. Agron. J. 78, 151-158. ( 10.2134/agronj1986.00021962007800010031x) [DOI] [Google Scholar]
- 31.Karberg NJ, Pregitzer KS, King JS, Friend AL, Oecologia JRW. 2005Soil carbon dioxide partial pressure and dissolved inorganic carbonate chemistry under elevated carbon dioxide and ozone. Berlin, Germany: Springer. [DOI] [PubMed] [Google Scholar]
- 32.Manning DAC, Renforth P. 2013Passive sequestration of atmospheric CO2 through coupled plant-mineral reactions in urban soils. Environ. Sci. Technol. 47, 135-141. ( 10.1021/es301250j) [DOI] [PubMed] [Google Scholar]
- 33.Renforth P, Manning DAC. 2011Laboratory carbonation of artificial silicate gels enhanced by citrate: implications for engineered pedogenic carbonate formation. Int. J. Greenh. Gas Control 5, 1578-1586. ( 10.1016/j.ijggc.2011.09.001) [DOI] [Google Scholar]
- 34.Drever JI, Vance GF. 1994Role of soil organic acids in mineral weathering processes. In Organic acids in geological processes (eds ED Pittman, MD Lewan), pp. 138-161. Berlin, Germany: Springer. [Google Scholar]
- 35.Pettit RE. 2008 Organic matter, humus, humate, humic acid, fulvic acid and humin: their importance in soil fertility and plant health. harvestgrow.com.
- 36.Moira EK, Henderson RBD. 1963The release of metallic and silicate ions from minerals, rocks, and soils by fungal activity. Soil Sci. 14, 236-246. ( 10.1111/j.1365-2389.1963.tb00949.x) [DOI] [Google Scholar]
- 37.Jongmans AG, et al. 1997Rock-eating fungi. Nature 389, 682-683. ( 10.1038/39493) [DOI] [Google Scholar]
- 38.Velde P, Barré P. 2009Soils, plants and clay minerals: mineral and biologic interactions. Berlin, Germany: Springer.
- 39.Carpenter D, Hodson ME, Eggleton P, Kirk C. 2008The role of earthworm communities in soil mineral weathering: a field experiment. Mineralogical Mag. 72, 33-36. ( 10.1180/minmag.2008.072.1.33) [DOI] [Google Scholar]
- 40.Lian B, Jiang G-F, Liu D, Wang B, Jiang G. 2011Degradation of potassium rock by earthworms and responses of bacterial communities in its gut and surrounding substrates after being fed with mineral. PLoS ONE 6, e28803. ( 10.1371/journal.pone.0028803) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goldich SS. 1938A study in rock-weathering. J. Geol. 46, 17-58. ( 10.1086/624619) [DOI] [Google Scholar]
- 42.Franke WA, Teschner-Steinhardt R. 1994An experimental approach to the sequence of the stability of rock-forming minerals towards chemical weathering. CATENA 21 , 279–290. ( 10.1016/0341-8162(94)90018-3) [DOI] [Google Scholar]
- 43.Paton TR, Humphreys GS, Mitchell PB. 1995Soils: a new global view. New York, NY: Routledge.
- 44.Harley AD, Gilkes RJ. 2000Factors influencing the release of plant nutrient elements from silicate rock powders: a geochemical overview. Nutrient Cycl. Agroecosyst. 56, 11-36. ( 10.1023/A:1009859309453) [DOI] [Google Scholar]
- 45.Beerling DJ, et al. 2018Farming with crops and rocks to address global climate, food and soil security perspective. Nat. Plants 4, 138-147. ( 10.1038/s41477-018-0108-y) [DOI] [PubMed] [Google Scholar]
- 46.Jenny H. 1941Factors of soil formation: a system of quantitative pedology. New York, NY: McGraw-Hill. [Google Scholar]
- 47.Wolf-Gladrow DA, Zeebe RE, Klaas C, Körtzinger A, Dickson AG. 2007Total alkalinity: the explicit conservative expression and its application to biogeochemical processes. Mar. Chem . 106 , 287–300. ( 10.1016/j.marchem.2007.01.006) [DOI] [Google Scholar]
- 48.Buesseler KO, Science PWB. 2003Will ocean fertilization work? science.sciencemag.org.
- 49.Forrest H. 2015Did phosphorus derived from the weathering of large igneous provinces fertilize the Neoproterozoic ocean? Geochem. Geophys. Geosyst. 16, 1723-1738. ( 10.1002/2015GC005792) [DOI] [Google Scholar]
- 50.Cornelis JT, Delvaux B. 2016Soil processes drive the biological silicon feedback loop. Funct. Ecol. 30, 1298-1310. ( 10.1111/1365-2435.12704) [DOI] [Google Scholar]
- 51.Curtis CD. 1985Clay mineral precipitation and transformation during burial diagenesis. Phil. Trans. R. Soc. A 315, 19850031. ( 10.1098/rsta.1985.0031) [DOI] [Google Scholar]
- 52.Rayment GE, Higginson FR. 1992Australian laboratory handbook of soil and water chemical methods. Melbourne, Australia: Inkata Press. Available https://www.cabdirect.org/cabdirect/abstract/19921973446. [Google Scholar]
- 53.Michalopoulos P, Aller RC. 1995Rapid clay mineral formation in Amazon delta sediments: reverse weathering and oceanic elemental cycles. Science 270, 614-617. ( 10.1126/science.270.5236.614) [DOI] [Google Scholar]
- 54.Alekseyev VA, Medvedeva LS, Prisyagina NI, Meshalkin SS, Balabin AI. 1997Change in the dissolution rates of alkali feldspars as a result of secondary mineral precipitation and approach to equilibrium. Geochim. Cosmochim. Acta 61, 1125-1142. ( 10.1016/S0016-7037(96)00405-X) [DOI] [Google Scholar]
- 55.Stefánsson A, Gisiason SR. 2001Chemical weathering of basalts, southwest Iceland: effect of rock crystallinity and secondary minerals on chemical fluxes to the ocean. Am. J. Sci. 301, 513-556. ( 10.2475/ajs.301.6.513) [DOI] [Google Scholar]
- 56.Zhu C, Blum AE, Veblen DR. 2004Feldspar dissolution rates and clay precipitation in the Navajo aquifer at Black Mesa, Arizona, USA. In Proc. 11th Int. Symp. water–rock interaction (eds RB Wanty, RI Seal), pp. 895–899. Saratoga Springs, NY: A. A. Balkema. [Google Scholar]
- 57.Maher K, Steefel CI, White FA, Stonestrom DA. 2009The role of reaction affinity and secondary minerals in regulating chemical weathering rates at the Santa Cruz soil chronosequence, California. Geochim. Cosmochim. Acta 73, 2804-2831. ( 10.1016/j.gca.2009.01.030) [DOI] [Google Scholar]
- 58.Stefánsson A, Gíslason SR, Arnórsson S. 2001Dissolution of primary minerals in natural waters: II. Mineral saturation state. Chem. Geol. 172, 251-276. ( 10.1016/S0009-2541(00)00262-X) [DOI] [Google Scholar]
- 59.Georges M. 1970Geology of clays: weathering, sedimentology, geochemistry. Heidelberg, Germany: Springer. [Google Scholar]
- 60.Schmidt MWI, et al. 2011Persistence of soil organic matter as an ecosystem property. Nature 478, 49-56. ( 10.1038/nature10386) [DOI] [PubMed] [Google Scholar]
- 61.Smith P, et al. 2008Greenhouse gas mitigation in agriculture. Phil. Trans. R. Soc. B 363, 789-813. ( 10.1098/rstb.2007.2184) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Eglin T, et al. 2010Historical and future perspectives of global soil carbon response to climate and land-use changes. Tellus B 62, 700-718. ( 10.1111/j.1600-0889.2010.00499.x) [DOI] [Google Scholar]
- 63.Singh M, et al. 2018Stabilization of soil organic carbon as influenced by clay mineralogy. Adv. Agron. 148, 33-84. ( 10.1016/bs.agron.2017.11.001) [DOI] [Google Scholar]
- 64.Elliott ET. 1986Aggregate structure and carbon, nitrogen, and phosphorus in native and cultivated soils. Soil Sci. Soc. Am. J. 50, 627-633. ( 10.2136/sssaj1986.03615995005000030017x) [DOI] [Google Scholar]
- 65.Besnard E, Chenu C, Balesdent J, Puget P, Arrouays D. 1996Fate of particulate organic matter in soil aggregates during cultivation. Eur. J. Soil Sci. 47, 495-503. ( 10.1111/j.1365-2389.1996.tb01849.x) [DOI] [Google Scholar]
- 66.Skjemstad JO, Clarke P, Taylor JA, Research JMO-S. 1996The chemistry and nature of protected carbon in soil. Aust. J. Soil Res . 34 , 251–271. ( 10.1071/SR9960251) [DOI] [Google Scholar]
- 67.Six J, Paustian K, Elliott ET, Combrink C. 2000Soil structure and organic matter I. Distribution of aggregate-size classes and aggregate-associated carbon. Soil Sci. Soc. Am. J. 64, 681-689. ( 10.2136/sssaj2000.642681x) [DOI] [Google Scholar]
- 68.Bossuyt H, Six J, Hendrix PF. 2002Aggregate-protected carbon in no-tillage and conventional tillage agroecosystems using carbon-14 labeled plant residue. Soil Sci. Soc. Am. J. 66, 1965-1973. ( 10.2136/sssaj2002.1965) [DOI] [Google Scholar]
- 69.Yang C, Liu N, Zhang Y. 2017Effects of aggregates size and glucose addition on soil organic carbon mineralization and Q10 values under wide temperature change conditions. Eur. J. Soil Biol. 80, 77-84. ( 10.1016/j.ejsobi.2017.04.002) [DOI] [Google Scholar]
- 70.Von Lützow M, Kögel-Knabner I, Guggenberger G, Marschner B. 2006Stabilization of organic matter in temperate soils: mechanisms and their relevance under different soil conditions—a review. Eur. J. Soil Sci. 57, 426-445. ( 10.1111/j.1365-2389.2006.00809.x) [DOI] [Google Scholar]
- 71.Kleber M, Schwendenmann L, Veldkamp E, Rößner J, Jahn R. 2007Halloysite versus gibbsite: silicon cycling as a pedogenetic process in two lowland neotropical rain forest soils of La Selva, Costa Rica. Geoderma 138, 1-11. ( 10.1016/j.geoderma.2006.10.004) [DOI] [Google Scholar]
- 72.Paustian K, Six J, Elliott ET, Hunt HW. 2000Management options for reducing CO2 emissions from agricultural soils. Biogeochem. 48, 147-163. ( 10.1023/A:1006271331703) [DOI] [Google Scholar]
- 73.Parkhust DL, Appelo CAJ. 1999User's guide to PHREEQC (version 2): a computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculations . Water-Resources Investigations Report 99-4259. ( 10.3133/wri994259) [DOI]
- 74.Rau GH. 2011CO2 mitigation via capture and chemical conversion in seawater. Environ. Sci. Technol. 45, 1088-1092. ( 10.1021/es102671x) [DOI] [PubMed] [Google Scholar]
- 75.Perrin A-S, Probst A, Probst J-L. 2008Impact of nitrogenous fertilizers on carbonate dissolution in small agricultural catchments: implications for weathering CO2 uptake at regional and global scales. Geochim. Cosmochim. Acta 72, 3105-3123. ( 10.1016/j.gca.2008.04.011) [DOI] [Google Scholar]
- 76.Pierson-Wickmann A-C, Aquilina L, Martin C, Ruiz L, Molénat J, Jaffrézic A, Gascuel-Odoux C. 2009High chemical weathering rates in first-order granitic catchments induced by agricultural stress. Chem. Geol. 265, 369-380. ( 10.1016/j.chemgeo.2009.04.014) [DOI] [Google Scholar]
- 77.Semhi K, Amiotte Suchet P, Clauer N, Probst J-L. 2000Impact of nitrogen fertilizers on the natural weathering-erosion processes and fluvial transport in the Garonne basin. Appl. Geochem. 15, 865-878. ( 10.1016/S0883-2927(99)00076-1) [DOI] [Google Scholar]
- 78.Hartmann J, Kempe S. 2008What is the maximum potential for CO2 sequestration by ‘stimulated’ weathering on the global scale? Naturwissenschaften 95, 1159-1164. ( 10.1007/s00114-008-0434-4) [DOI] [PubMed] [Google Scholar]
- 79.Hamilton SK, Kurzman AL, Arango C, Jin L, Robertson GP. 2007Evidence for carbon sequestration by agricultural liming. Global Biogeochem. Cycles 21, 1-12. ( 10.1029/2006GB002738) [DOI] [Google Scholar]
- 80.Taylor LL, Driscoll CT, Groffman PM, Rau GH, Blum JD, Beerling DJ. 2020Increased carbon capture by a silicate-treated forested watershed affected by acid deposition. Biogeosci. Discuss. 2020, 1-29. ( 10.5194/bg-2020-288) [DOI] [Google Scholar]
- 81.Ciais P, et al. 2014Carbon and other biogeochemical cycles. In Climate change 2013: the physical science basis. Contribution of working group I to the fifth assessment report of the intergovernmental panel on climate change, pp. 465-570. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 82.Sabine CL, Tanhua T. 2010Estimation of anthropogenic CO2 inventories in the ocean. Annu. Rev. Mar. Sci. 2, 175-198. ( 10.1146/annurev-marine-120308-080947) [DOI] [PubMed] [Google Scholar]
- 83.Weiner S. 2003An overview of biomineralization processes and the problem of the vital effect. Rev. Mineral. Geochem. 54, 1-29. ( 10.2113/0540001) [DOI] [Google Scholar]
- 84.Doney SC, Fabry VJ, Feely RA, Kleypas JA. 2009Ocean acidification: the other CO2 problem. Annu. Rev. Mar. Sci. 1, 169-192. ( 10.1146/annurev.marine.010908.163834) [DOI] [PubMed] [Google Scholar]
- 85.Zeebe RE. 2012History of seawater carbonate chemistry, atmospheric CO2, and ocean acidification. Annu. Rev. Earth Planet. Sci. 40, 141-165. ( 10.1146/annurev-earth-042711-105521) [DOI] [Google Scholar]
- 86.Friedlingstein P, et al. 2019Global carbon budget 2019. Earth Syst. Sci. Data 11, 1783-1838. ( 10.5194/essd-11-1783-2019) [DOI] [Google Scholar]
- 87.Cyronak T, et al. 2018Taking the metabolic pulse of the world's coral reefs. PLoS ONE 13, e01872 ( 10.1371/journal.pone.0190872) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Langer G, Geisen M, Baumann KH, Kläs J, Riebesell U, Thoms S, Young JR. 2006Species-specific responses of calcifying algae to changing seawater carbonate chemistry. Geochem. Geophys. Geosyst. 7, 1-12. ( 10.1029/2005GC001227) [DOI] [Google Scholar]
- 89.Taylor LL, Quirk J, Thorley RMS, Kharecha PA, Hansen J, Ridgwell A, Lomas MR, Banwart SA, Beerling DJ. 2016Enhanced weathering strategies for stabilizing climate and averting ocean acidification. Nat. Clim. Change 6, 402-406. ( 10.1038/nclimate2882) [DOI] [Google Scholar]
- 90.Ries JB, Cohen AL, McCorkle DC. 2009Marine calcifiers exhibit mixed responses to CO2-induced ocean acidification. Geology 37, 1131-1134. ( 10.1130/G30210A.1) [DOI] [Google Scholar]
- 91.Bove CB, Umbanhowar J, Castillo KD. 2020Meta-analysis reveals reduced coral calcification under projected ocean warming but not under acidification across the Caribbean Sea. Front. Mar. Sci. 7, 127. ( 10.3389/fmars.2020.00127) [DOI] [Google Scholar]
- 92.Schoepf V, Jury CP, Toonen RJ, McCulloch MT. 2017Coral calcification mechanisms facilitate adaptive responses to ocean acidification. Proc. R. Soc. B 284, 20172117. ( 10.1098/rspb.2017.2117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.De'ath G., Lough JM, Fabricius KE. 2009Declining coral calcification on the great barrier reef. Science 323, 116-119. ( 10.1126/science.1165283) [DOI] [PubMed] [Google Scholar]
- 94.Ricke KL, Orr JC, Schneider K, Caldeira K. 2013Risks to coral reefs from ocean carbonate chemistry changes in recent earth system model projections. Environ. Res. Lett. 8, 34003. ( 10.1088/1748-9326/8/3/034003) [DOI] [Google Scholar]
- 95.Reveller R, Suess HE. 1957Carbon dioxide exchange between atmosphere and ocean and the question of an increase of atmospheric CO2 during the past decades. Tellus 9, 18-27. ( 10.1111/j.2153-3490.1957.tb01849.x) [DOI] [Google Scholar]
- 96.Egleston ES, Sabine CL, Morel FMM. 2010Revelle revisited: buffer factors that quantify the response of ocean chemistry to changes in DIC and alkalinity. Global Biogeochem. Cycles 24, 1-9. ( 10.1029/2008GB003407) [DOI] [Google Scholar]
- 97.Hönisch B, Hemming NG, Archer D, Siddall M, McManus JF. 2009Atmospheric carbon dioxide concentration across the mid-Pleistocene transition. Science 324, 1551-1554. ( 10.1126/science.1171477) [DOI] [PubMed] [Google Scholar]
- 98.Jiang L-Q, Carter BR, Feely RA, Lauvset SK, Olsen A. 2019Surface ocean pH and buffer capacity: past, present and future. Sci. Rep. 9, 18624. ( 10.1038/s41598-019-55039-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Garrels RM, Thompson ME. 1962A chemical model for sea water at 25 degrees C and one atmosphere total pressure. Am. J. Sci. 260, 57-66. ( 10.2475/ajs.260.1.57) [DOI] [Google Scholar]
- 100.Mackenzie FT, Kump LR. 1995Reverse weathering, clay mineral formation, and oceanic element cycles. Science 270, 586. ( 10.1126/science.270.5236.586) [DOI] [Google Scholar]
- 101.Von Damm KL, Edmond JM. 1984Reverse weathering in the closed-basin lakes of the Ethiopian Rift. Am. J. Sci. 284, 835-862. ( 10.2475/ajs.284.7.835) [DOI] [Google Scholar]
- 102.Rasmussen B, Buick R, Taylor WR. 1998Removal of oceanic REE by authigenic precipitation of phosphatic minerals. Earth Planet. Sci. Lett. 164, 135-149. ( 10.1016/S0012-821X(98)00199-X) [DOI] [Google Scholar]
- 103.Rahman S, Aller RC, Cochran JK. 2016Cosmogenic 32Si as a tracer of biogenic silica burial and diagenesis: major deltaic sinks in the silica cycle. Geophys. Res. Lett. 43, 7124-7132. ( 10.1002/2016GL069929) [DOI] [Google Scholar]
- 104.Morifuji N, Nakashima S. 2018Hydrothermal transformation of inorganic and biogenic silica as studied using in situ hydrothermal infrared microspectroscopy. Appl. Spectrosc. 72, 1487-1497. ( 10.1177/0003702818771817) [DOI] [PubMed] [Google Scholar]
- 105.Isson TT, Planavsky NJ. 2018Reverse weathering as a long-term stabilizer of marine pH and planetary climate. Nature 560, 471-475. ( 10.1038/s41586-018-0408-4) [DOI] [PubMed] [Google Scholar]
- 106.Santiago RDP, Morgan LE, Lloyd NS, Higgins JA. 2018Reverse weathering in marine sediments and the geochemical cycle of potassium in seawater: insights from the K isotopic composition (41K/39K) of deep-sea pore-fluids. Geochim. Cosmochim. Acta 236, 99-120. ( 10.1016/j.gca.2018.02.035) [DOI] [Google Scholar]
- 107.Misra S, Froelich PN. 2012Lithium isotope history of Cenozoic seawater: changes in silicate weathering and reverse weathering. Science 335, 818-823. ( 10.1126/science.1214697) [DOI] [PubMed] [Google Scholar]
- 108.Bernhardt A, Oelze M, Bouchez J, von Blanckenburg F, Mohtadi M, Christl M, Wittmann H. 202010Be/9Be ratios reveal marine authigenic clay formation. Geophys. Res. Lett. 47, e2019GL086061. ( 10.1029/2019GL086061) [DOI] [Google Scholar]
- 109.Presti M, Michalopoulos P. 2008Estimating the contribution of the authigenic mineral component to the long-term reactive silica accumulation on the western shelf of the Mississippi River delta. Cont. Shelf Res. 28, 823-838. ( 10.1016/j.csr.2007.12.015) [DOI] [Google Scholar]
- 110.Hangx SJT, Spiers CJ. 2009Coastal spreading of olivine to control atmospheric CO2 concentrations: a critical analysis of viability. Int. J. Greenh. Gas Control 3, 757-767. ( 10.1016/j.ijggc.2009.07.001) [DOI] [Google Scholar]
- 111.Oelkers EH, Declercq J, Saldi GD, Gislason SR, Schott J. 2018Olivine dissolution rates: a critical review. Chem. Geol. 500, 1-19. ( 10.1016/j.chemgeo.2018.10.008) [DOI] [Google Scholar]
- 112.Schuiling RD, de Boer PL. 2010Coastal spreading of olivine to control atmospheric CO2 concentrations: a critical analysis of viability. Comment: nature and laboratory models are different. Int. J. Greenh. Gas Control 4, 855-856. ( 10.1016/j.ijggc.2010.04.012) [DOI] [Google Scholar]
- 113.Montserrat F, Renforth P, Hartmann J, Leermakers M, Knops P, Meysman FJR. 2017Olivine dissolution in seawater: implications for CO2 sequestration through enhanced weathering in coastal environments. Environ. Sci. Technol. 51, 3960-3972. ( 10.1021/acs.est.6b05942) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.