Abstract
Progression of severe traumatic brain injury (TBI) is associated with worsening cerebral inflammation, but it is unknown how a concomitant bone fracture (FX) affects this progression. Enoxaparin (ENX), a low molecular weight heparin often used for venous thromboembolic prophylaxis, decreases penumbral leukocyte (LEU) mobilization in isolated TBI and improves neurological recovery. We investigated if TBI accompanied by an FX worsens LEU-mediated cerebral inflammation and if ENX alters this process. CD1 male mice underwent controlled cortical impact (CCI) or sham craniotomy with or without an open tibial FX, and received either ENX (1 mg/kg, three times/day) or saline for 2 days following injury. Randomization defined four groups (Sham, CCI, CCI+FX, CCI+FX+ENX, n = 10/group). Two days after CCI, neurological recovery was assessed with the Garcia Neurological Test (GNT); intravital microscopy (LEU rolling and adhesion, microvascular leakage) and blood hemoglobin levels were also evaluated. Penumbral cerebral neutrophil sequestration (Ly-6G immunohistochemistry [IHC]) were evaluated post-mortem. In vivo LEU rolling was greater in CCI+FX (45.2 ± 4.8 LEUs/100 μm/min) than in CCI alone (26.5 ± 3.1, p = 0.007), and was suppressed by ENX (23.2 ± 5.5, p = 0.003 vs. CCI + FX). Neurovascular permeability was higher in CCI+FX (71.1 ± 2.9%) than CCI alone (42.5 ± 2.3, p < 0.001). GNT scores were lower in CCI+FX (15.2 ± 0.2) than in CCI alone (16.3 ± 0.3, p < 0.001). Hemoglobin was lowest in the CCI+FX+ENX group, lower than in Sham or CCI. IHC demonstrated greatest polymorphonuclear neutrophil (PMN) invasion in CCI+FX in uninjured cerebral territories. A concomitant long bone FX worsens TBI-induced cerebral LEU mobilization, microvascular leakage, and cerebral edema, and impairs neurological recovery at 48 h. ENX suppresses this progression but may increase bleeding.
Keywords: : ENX, intravital microscopy, multiple trauma, TBI, tibial FX
Introduction
Traumatic Brain injury (TBI) is a principal source of mortality and disability worldwide.1 In the United States alone, it results in ∼50,000 deaths, and 80,000–90,000 cases of long-term disability per year.2 TBI, defined as the acute neurodegeneration that occurs to the brain parenchyma following the application of an external force, is typically classified into two phases.3,4 The “primary injury” encompasses the cerebral tissue damage sustained as a result of impact kinetic energy transfer. After impact, subsequent neuroinflammation results in “secondary brain injury.” The intensity and persistence of secondary brain injury is variable and may lead to cerebral edema, intracranial hypertension, and lethal complications such as cerebral herniation. Immediately after brain injury, a number of early interventions can be employed, such as surgical evacuation of the hematoma. As a result, alleviating the progression of secondary brain injury may contribute considerably to subsequent neurological recovery.5
TBI is often accompanied by multiple other injuries, including those of solid and hollow viscera as well as those of the axial and peripheral skeleton. A variety of mediator-based interactions between potent cellular triggers such as pathogen-associated molecular pattern molecules (PAMPs) and damage-associated molecular pattern molecules (DAMPs) have been articulated to exist in the setting of these injuries, which lead to increases in inflammatory cytokines via receptor of advanced glycation end-product (RAGE), and toll-like receptor (TLR)- based mechanisms,6–9 which may be produced locally and may influence brain injury progression. For example, a recent study showed that a concomitant long bone FX exacerbates secondary brain injury following TBI, and results in worsened behavioral testing and brain injury on magnetic resonance imaging (MRI) weeks after injury.10–12
Although multiple trauma is much more common than isolated TBI, few combined injury models have been studied, and cumulative and synergistic effects specific to adding one given organ injury over TBI, remain sparse. In particular, the important role of leukocyte (LEU)- mediated tissue destruction that commonly follows multiple trauma may have an effect on evolving brain tissue injury. Nonetheless, extensive pre-clinical and clinical data suggest not only that the immune privileged cerebral tissue after TBI is now open to systemic inflammatory modulation, but also that other concomitant non-cerebral injuries can result in significant systemic immune alterations that directly affect cerebral injury progression and healing. Shultz and his Melbourne colleagues have elegantly studied and described how cytokines (interleukin [IL]-1β, tumor necrosis factor [TNF]-α, IL-6) reactive oxygen species and growth factors released locally by fractured bone and circulating systemically are directly involved in modulating exacerbation and healing of TBI.13 The authors have further called for more multi-trauma investigations to better characterize TBI alterations in these settings, noting that most of the contradictory clinical studies are retrospective, with little control of baseline characteristics.14 They further point out that existing pre-clinical brain injury and FX studies within a more controlled experimental setting have clearly demonstrated alterations in the systemic cytokine milieu, proposing that increases in systemic inflammation may not simply be additive of the separate injuries but rather synergistic, as the resultant levels are well beyond the sum of the individual responses. Yet despite these data, we were unable to find a study that has evaluated how a simultaneously fractured bone affects live LEU trafficking to injured brain tissue, in particular, the in-vivo interactions between LEUs and endothelial cells (ECs) at the blood–brain barrier (BBB).
Enoxaparin (ENX) is a low molecular weight heparin (LMWH) routinely used clinically to prevent venous thromboembolic (VTE) complications. Although heparins are primarily known for their anticoagulant effect, more recently they have been shown to effectively reduce LEU-mediated tissue inflammation and swelling15,16 while improving cognitive recovery after isolated TBI.17,18 However, no study to date has investigated the effect of heparinoids on LEU-mediated inflammation balanced with risk of bleeding in a multi-trauma TBI and bone FX model.
Considering the foregoing, we hypothesized that a concomitant long bone FX worsens the progression of cerebral microvascular inflammation, and, consequently, intensifies secondary brain injury after TBI. We further hypothesized that ENX can ameliorate these effects, but at the cost of greater bleeding risk.
Methods
Experimental design and study groups
All experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Pennsylvania, and adhered to the policies and guidelines set forth in public health service (PHS) care and use of laboratory animals. Adult male CD1 mice (weighing 25–30 g) (Charles River Laboratories, Wilmington, MA) were housed for 5–7 days in standard facilities before experiments, with access to water and food ad libitum. Forty animals were randomized into four groups: sham craniotomy and vehicle (0.9% normal saline) without controlled cortical impact (CCI) or tibial FX (Sham, n = 10); TBI and vehicle (CCI, n = 10); TBI and tibial FX + vehicle (CCI+FX, n = 10); TBI and tibial fracture and ENX (CCI+FX+ENX, n = 10). ENX (1 mg/kg, Winthrop, Sanofiaventis, NJ) or an equal volume of 0.9% normal saline (1 mL/kg, Baxter Deerfield, IL) were injected into all animals subcutaneously at 2, 8, 14, 23, and 32 h after CCI (Fig. 1).
FIG. 1.
Experimental design and procedures. (A) Timeline of the experimental protocol. (B) Schematic representation of localization of both craniotomies. (C) A fluoroscopic image of tibial fracture with internal fixation 2 days following injury. The white arrow indicates the tibial fracture site. The fracture was isolated to the tibia (not the fibula), and an intermedullary pin was used for fixation.
TBI model: CCI
To simulate severe TBI, we adopted the validated CCI model for mice, which is widely used in experimental TBI reports.19,20 At time 0, mice were anesthetized with intraperitoneal ketamine, xylazine, and acepromazine (KXA) (100, 10, 1 mg/kg, respectively) and placed prone in a stereotactic device. After scalp incision, a left-sided 3 mm craniotomy was created with a trephine, centered between the sagittal, bregma, and lambda sutures (Fig.1B). The exposed left parietotemporal cortex, covered with dura mater, was then directly injured using a CCI device (AMS 201; AmScien Instruments, Richmond, VA). The velocity, area, and depth of impact remained constant for all animals, consistent with severe TBI (impact velocity of 6 m/sec, 3 mm diameter impactor tip, and cortical deformation of 1.0 mm).19 Sham animals underwent craniotomy but not CCI.
Long bone injury: Open tibial diaphyseal FX with intramedullary fixation
To simulate the most common concomitant injury with TBI, we adopted a validated long-bone FX model. Immediately after CCI and prior to anesthetic reversal, a right-sided open midshaft tibial FX was created and immediately repaired with intramedullary fixation under aseptic conditions.21 Briefly, the mouse was placed in a lateral decubitus position, and a skin incision (2 mm) was made on the proximal end of the leg anteriorly. A small hole was made on the articular tibial plateau, extending into the medullary cavity, using a 26 G needle. The skin incision was distally extended by 5 mm to expose the tibia, and a transverse osteotomy was performed across the proximal diaphysis with Iris scissors. The tibial FX was then stabilized with an intramedullary rod (inner trocar of a 22 G spinal needle) inserted through the tibial plateau hole, along the bone marrow cavity to the distal tibial shaft. The protruding pin extending beyond the cortex at the site of insertion was trimmed (Fig.1C). Sham and CCI animals only underwent skin incision without FX or fixation.
All animals received buprenorphine (0.1 mg/kg) for analgesia, every 6 h for 48 h after surgery, regardless of group assignment.
Live intravital microscopy (IVM)
IVM of the cerebral microcirculation was conducted.20,22 Forty-eight hours after CCI, animals were anesthetized and the right jugular vein was cannulated for administration of fluorescent dyes. Animals were placed in a stereotactic frame, and a second circular craniotomy (2.5 × 2.5 mm) was created adjacent to the first craniotomy using a handheld dental drill (Henry Schein, Melville, NY) (Fig.1B) and covered with a 5 mm glass cover-slip. Animals were then transferred to an intravital microscope (ECLIPSE FN1; Nikon Instruments, Melville, NY) and received a 50 μL IV bolus of 0.3% rhodamine 6G (Sigma-Aldrich, St. Louis, MO) to fluorescently label circulating LEUs. Non-branching venules, 25–50 μm in diameter, were randomly chosen, and 1 min footage of the pial microcirculation was recorded under a 590 nm epi-illumination emission filter using a digital camera (QuantEM; Photometrics, Tucson, AR).
Intravenous bovine fluorescein isothiocyanate (FITC)-labeled albumin (Sigma-Aldrich; 100 mg/kg) was then administered, and the same pial regions were visualized for determination of fluorescent albumin (FITC) leakage, a surrogate for microvascular BBB leakage. A 10 sec video was recorded under the 488 nm fluorescent filter.
Pial LEU/endothelial interactions and FITC albumin leakage (n = 40)
LEU rolling and adhesion were evaluated using digital analysis software (NIS-elements; Nikon Instruments, Melville, NY). LEU rolling (mean number of labeled cells crossing a 100 μm venular segment; LEUs/100 μm/min) and LEU adhesion (labeled cells stationary for at least 30 sec during recording period; LEUs/100 μm) were counted by a blinded observer unaware of treatment groups (Fig. 2A). Microvascular permeability was then determined using the same software, where FITC-albumin leakage was determined as the light intensity (grays) measured in three distinct regions within the vessel (venular intensity [Iv]) and outside the vessel wall (perivenular intensity [Ip]). The ratio of mean Ip to Iv for 10 sec was calculated to determine the mean permeability index for the given vessel [(Ip/Iv) × 100 %] (Fig. 3A).
FIG. 2.
In vivo leukocyte/endothelial cell interactions. (A) Representative image of the pial microcirculation. White arrows indicate Rhodamine-labeled leukocytes (LEUs). (B) LEU rolling on endothelium 48 h after controlled cortical impact (CCI.) The increased number of rolling LEUs in CCI was significantly enhanced with a concomitant long bone fracture (FX), and enoxaparin (ENX) reduced this significantly. (C) Forty-eight hour LEU adhesion to endothelium showed that CCI+FX increased the number of adherent LEUs compared with Sham procedure. *p < 0.01 versus Sham; **p < 0.05 versus Sham; #p < 0.01 versus CCI+FX.
FIG. 3.
In vivo microvascular permeability. (A) Representative image of a post-capillary pial venule after fluorescein isothiocyanate (FITC)-albumin injection. Light intensity (grays) was measured in three separate locations outside the vessel (perivenular intensity [Ip]) and within the vessel (venular intensity [Iv]), where blood–brain barrier (BBB) permeability was calculated as mean Ip/mean Iv. (B) The proportion of FITC-albumin leakage 48 h after controlled cortical impact (CCI) was greatest in CCI+fracture (FX) and was reduced significantly by enoxaparin (ENX) administration. *p < 0.01 versus Sham; **p < 0.05 versus Sham; #p < 0.01 versus CCI+FX.
Body weight loss, neurological recovery (n = 40)
Animal body weights were measured before as well as 24 and 48 h after CCI with weight loss extent expressed as a ratio [(W0h – W24h or 48h)/W0h × 100 %].
Neurological function was evaluated at 24 and 48 h after CCI or sham craniotomy using the Garcia Neurological Test (GNT),23,24 which scores motor, sensory, reflex, and balance ability to a maximum sum of 18 points.
Bleeding assessment (n = 40)
All animals underwent left common carotid artery cannulation before euthanasia to measure mean arterial blood pressure. Immediately after euthanasia, blood was obtained via cardiac puncture to measure hemoglobin levels using an i-STAT blood analyzer (Abbott Laboratories, Abbott Park, IL).
Following euthanasia, bleeding assessments were conducted via autopsy to determine the extent of hemorrhage in different sites. Classification (no or mild [< 2 mm diameter] [1 point], moderate [2–5mm diameter] [2 points] or extensive [> 5 mm diameter] [3 points] hematoma) of each surgical site (tibia fracture, craniotomy, neck area) was conducted via gross inspection by an investigator unaware of treatment groups, and is presented as mean score per group.
Brain and lung water content (n = 20)
After euthanasia, and prior to autopsy, whole brains and left lungs were excised for wet-to-dry ratio determination from five mice in each group. Brains were divided along the midline into hemispheres. Samples were immediately weighed (wet weight [WW]) and again after 72 h of drying at 70°C (dry weight [DW]) in an incubator. Tissue water content in each sample was calculated using standard wet-to-dry ratios [(WW – DW)/WW × 100 %].
Immunohistochemistry (IHC) for neutrophils in brain (n = 20)
In five animals from each group (n = 20), excised brains were post-fixed in 10% neutral buffered formalin (NBF) (Sigma Aldrich) after transcardial perfusion with phosphate buffered solution (PBS) and 10% NBF. Brains were dissected into 2 mm thick coronal blocks and processed to paraffin using standard techniques; 8 μm thick sections were prepared for staining procedures.
Following deparaffinization and rehydration, tissue sections were immersed in aqueous hydrogen peroxide (15 min) to quench endogenous peroxidase activity. Antigen retrieval was performed in a microwave pressure cooker with immersion in preheated Tris ethylenediaminetetraacetic acid (EDTA) buffer. Subsequent blocking was achieved using 1% normal goat serum (Vector Labs, Burlingame, CA) in Optimax buffer (BioGenex, San Ramon, CA) for 30 min. A rat monoclonal primary antibody specific for the protein Ly-6G (1: 15K, BD Bio sciences, San Jose, CA)25 was applied for 20 h at 4°C.
Incubation with the primary antibodies was performed for 20 h at 4°C. After rinsing, sections were incubated with the appropriate biotinylated secondary antibody for 30 min (Vector Labs, Burlingame, CA), followed by avidin biotin complex as per the manufacturer's instructions (Vectastain Universal Elite kit, Vector Labs, Burlingame, CA). Finally, visualization was achieved using via 3,3’-diaminobenzidine (DAB), as per manufacturer's instructions (Vector Labs, Burlingame, CA). Counterstaining with hematoxylin was performed.
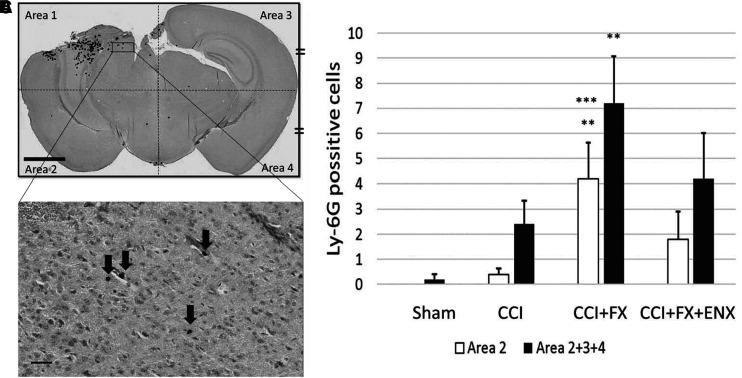
Slides were digitally scanned and visualized at 20 × magnification in Image Scope software (Leica Biosystems, Wetzlar, Germany). The captured cross-sectional brain image was divided into four equal quadrants (superior ipsilateral hemisphere [Area 1], inferior ipsilateral hemisphere [Area 2], superior contralateral hemisphere [Area 3] and inferior contralateral hemisphere [Area4]) (Fig. 4A). The number of polymorphonuclear neutrophils (PMNs), as identified by Ly-6G staining, was counted for each of the four designated areas.
FIG. 4.
Immunohistochemical analysis for polymorphonuclear neutrophil (PMN) sequestration in coronal brain sections 48 h after controlled cortical impact (CCI). (A) Representative image (20 × magnification, Scale bar = 2 mm) demonstrating Ly-6G staining for PMNs in four equal quadrants. (B) Ly-6G-positive cells are indicated by black arrows in inset detail at 200 × magnification (Scale bar = 30 μm). The spherical Ly-6G-positive cells with a 5–20 μm diameter were counted as PMNs. (C) In the non-contused quadrants (Areas 2 + 3 + 4), CCI+fracture (FX) resulted in greater PMN sequestration than Sham. **p < 0.05 versus Sham; ***p < 0.05 versus CCI.
Statistical analysis
All data are presented as mean ± SEM. Statistical analyses were performed using SPSS (version19, SPSS, Chicago, IL). Differences among multiple groups were compared using analysis of variance followed by Tukey's post-hoc comparisons. P < 0.05 was considered statistically significant.
Results
In vivo LEU–endothelial interactions
Forty-eight hours after CCI, LEUs rolling and adhering to the endothelium were more numerous in the CCI+FX group and least visualized in the Sham group (Fig. 2B). LEUs rolling in CCI+FX (45.2 ± 4.8 LEUs/100 μm/min) were also greater in number than in CCI alone (26.5 ± 3.1 LEUs/100 μm/min, p = 0.007), and were significantly suppressed by ENX administration (23.2 ± 5.5 LEUs/100 μm/min, p = 0.003), declining almost to isolated CCI levels. There were no significant differences between LEU rolling in the CCI and the CCI+FX+ENX groups. LEU adhesion was similar among all injured animals. (Fig. 2C).
In vivo microvascular permeability
The greatest proportion of FITC-albumin leakage was in the CCI+FX group and the lowest proportion was in the Sham group (Fig. 3B). Transendothelial albumin leakage in the CCI+FX group (71.1 ± 2.9%) was also greater than in the CCI alone group (42.5 ± 2.3, p < 0.001). Again, ENX treatment (46.6 ± 3.2, p < 0.001 vs. CCI+FX) almost restored microvascular permeability levels to that of CCI alone. Differences between the CCI and CCI+FX+ENX groups were not significant.
Body weight loss, neurological recovery
There were no significant differences in body weight loss ratio among all groups 24 h after CCI (Fig. 5A); 48 h after CCI, both the CCI+FX group (10.1 ± 1.6%) and the CCI+FX+ENX group (11.5 ± 1.6%) demonstrated significantly greater weight loss ratios than the Sham group (4.4 ± 1.2% vs. CCI+FX, p = 0.018; vs. CCI+FX+ENX, p = 0.007). Only one animal died in the entire study, shortly after induction of CCI.
FIG. 5.
Animal outcomes. (A) No significant differences were shown in body weight loss ratios 24 h after controlled cortical impact (CCI). CCI+fracture (FX) and CCI+FX+ enoxaparin (ENX) demonstrated significantly greater weight loss than Sham. (B) Neurological recovery evaluated with the Garcia Neurological Test (GNT). Sham animals demonstrated perfect scores 48 h after craniotomy. The CCI+FX group demonstrated significantly lower GNT scores compared with the other three groups, at both 24 and 48 h after CCI. Treatment with ENX significantly improved GNT scores to levels nearing that of CCI alone. (C) In the ipsilateral hemisphere, the CCI+FX group showed significantly higher water content ratio than the Sham and CCI groups. There were no significant differences in the contralateral hemisphere. (D) Lung water content was similar in all groups. *p < 0.01 versus Sham; **p < 0.05 versus Sham; #p < 0.01 versus CCI+FX; ##p < 0.05 versus CCI+FX.
Assessing neurological recovery by GNT scores, CCI+FX animals (12.8 ± 0.1) demonstrated significantly slower neurological recovery than CCI counterparts (14.1 ± 0.4, p = 0.007) at 24 h and at 48 h (15.2 ± 0.2 vs. 16.3 ± 0.3, p < 0.001) (Fig. 5B).
ENX administration resulted in score improvements at both time intervals (CCI+FX vs. CCI+FX+ENX; 24 h, 12.8 ± 0.1 vs. 13.9 ± 0.3, p = 0.029; 48 h, 15.2 ± 0.2 vs. 16.8 ± 0.2, p < 0.001).
Sham animals demonstrated the best neurological recovery among all groups, with perfect scores 48 h after CCI.
Brain and lung water content
CCI+FX animals (83.0 ± 1.1%) showed the highest water content ratio in the injured cerebral hemisphere at 48 h following CCI (Fig. 5C). This was significantly greater than both the Sham group (73.4 ± 1.5%, p = 0.001) and the CCI group (76.6 ± 1.2%, p = 0.02). No significant differences were found between the CCI+FX group and CCI+FX+ENX group.
In the uninjured contralateral cerebral hemisphere and in the lungs, there were no significant differences among any of the groups (Fig. 5D).
Bleeding assessment
At 48 h after CCI, the CCI+FX+ENX group demonstrated the lowest mean hemoglobin level (10.0 ± 0.5 g/dL), which was significantly lower than that found in the Sham (12.7 ± 0.6 g/dL, p = 0.026) and CCI (12.6 ± 0.5 g/dL, p = 0.034) groups (Fig. 6A). No differences were found in mean arterial blood pressures among any groups (Sham: 74 ± 7.2 mm Hg, CCI: 74.6 ± 6.5 mm Hg, CCI+FX: 70.4 ± 5.0 mm Hg, CCI+FX+ENX: 69.6 ± 8.6 mm Hg, p > 0.05 for all comparisons).
FIG. 6.
Bleeding assessments 48 h after controlled cortical impact (CCI). (A) Treatment with enoxaparin (ENX) resulted in significantly lower blood hemoglobin levels than those in the Sham or CCI groups. (B) Post-mortem autopsy bleeding assessments were conducted in the tibial fracture, injured brain, and neck surgery sites. In the tibial fracture area, larger hematomas were observed with ENX treatment, but this was not statistically significant. **p < 0.05 versus Sham; ***p < 0.05 versus CCI.
Autopsy bleeding assessments were conducted for the right hindlimb at the site of the open tibial fracture, the craniotomy site, and the neck wound created for vascular access. Although no significant differences were found, hemorrhage scores showed a tendency to larger hematomas in the tibial fracture area in CCI+FX+ENX animals (Fig. 6C). There were no bleeding extent differences in the craniotomy and neck sites.
IHC and quantification for neutrophils in brain
In coronal brain sections LY-6G positive cells (PMNs) were counted by a blinded observer in four equal areas (Fig. 4A, B). There were no significant differences among groups in the neutrophil density of the injury quadrant. However, in the inferior contralateral hemisphere (Area 2), CCI+FX animals (4.2 ± 1.4/cells) demonstrated a significantly higher number of PMNs than the Sham (0/cells, p = 0.023) or CCI groups (0.4 ± 0.2/cells, p = 0.043) (Fig. 4C). The sum total of PMNs found in non-contused quadrants showed the same significant differences with CCI+FX having the highest PMN sequestration.
Discussion
In the current study, we demonstrate how the addition of a commonly encountered open long bone injury to a standard severe TBI model resulted in worsening penumbral inflammation through greater local in vivo influx of LEUs interacting with endothelium and resulting in greater cerebral tissue PMN accumulation. The addition of an FX was also associated with increased microvascular leakage and resultant cerebral edema 48 h after brain injury. Finally, the addition of a fractured bone further demonstrated an additional impediment to neurological recovery including greater body weight loss, and diminished neurological scores on objective assessments. The administration of ENX generally counteracted the untoward effects of the added bone FX on brain inflammation, but resulted in a tendency to increased bleeding.
Maintenance of homeostasis at the BBB is crucial for preventing progression of secondary brain injury following TBI. However, when brain injury is accompanied by additional remote injuries, systemic circulation of locally released pro-inflammatory factors from those remote sites may degrade BBB integrity and impair healing.26 Our model validates that cerebral edema follows a disrupted BBB after remote injury and that this is ameliorated by a novel anti-inflammatory agent. Mechanistically, in the acute period after tissue injury, LEUs support a localized immune inflammatory response through degranulation and release of oxygen radicals and proteinases that injure adjacent ECs.27 This, in turn, promotes further LEU sequestration, attracting and activating more circulating LEUs that seek first to marginalize intravascularly and roll on the EC surface through interactions between surface selectins and EC counter-ligands.28,29 This is then followed by firm LEU adherence to ECs, driven by PMN surface CD11b binding to intercellular adhesion molecule 1 (ICAM-1) and vascular cellular adhesion molecule 1 (VCAM-1) on the EC surface.30 LEU adherence weakens the stability of the vascular endothelium, thereby enhancing microvascular permeability as a result of reactive oxygen species generation, and elaboration of inflammatory cytokines and other cytotoxic substances.31 Neutrophils and cytotoxic proteins then leak out of the intravascular space resulting in tissue inflammation and injury, which further worsens BBB disruption and loss of integrity32 This influx of inflammatory proteins into the brain parenchyma promotes ongoing secondary brain injury via activation of local resident astrocytes and microglia.33
How the concomitant release of inflammatory byproducts from remote sites of injury may influence the course of brain injury remains elusive. This is problematic, as severe TBI most commonly occurs with kinetic energy transmission at multiple sites, not just the head. In a previous rodent study evaluating closed brain injury, the addition of a bone fracture resulted in greater brain edema and Evans blue dye extravasation compared with isolated TBI at 24 h.10 The authors further demonstrated that dually injured animals also fared worse on behavioral testing (Y-maze) and demonstrated increased brain MRI inflammatory findings up to 30 days after injury. Similarly, in our experiments, brain edema was also acutely increased 2 days after injury if a bone FX was present. However, we further demonstrated in vivo that this observation was underpinned by enhanced penumbral microvascular macromolecular leakage indicating greater permeability of the assessed pial vessels.
These leaky vessels were the same ones in which CCI+FX animals revealed greater live LEU mobilization than those with isolated CCI. In addition, ex vivo cerebral Ly-6G IHC demonstrated significantly greater cerebral neutrophil accumulation with the addition of an FX to TBI, but only in uninjured brain territories. This result raises the possibility that a bone FX may particularly direct PMN trafficking toward normal, uninjured brain tissue following TBI: an unexpected finding. However, in the pericontusional area, the addition of an FX did not increase PMN mobilization, which is in contradiction to our in vivo results visualizing the pericontusional brain. This may have been observed because histological results are static and may not directly represent live occurrences minutes or seconds before animal death. Others using a weight drop TBI and closed tibial fracture model have also demonstrated how brain neutrophil accumulation is greatly increased when polytrauma (bone FX) accompanies TBI and how this is associated with significantly more brain water.10,34
It would have been interesting to conduct IVM of the contralateral uninjured brain hemisphere as well. One could reasonably hypothesize that even greater differences in neutrophil influx would have been encountered. Irrespectively, taken together, these results support the mechanism of LEU-mediated BBB permeability degradation resulting in cerebral edema after TBI. Although this has been demonstrated previously in isolated brain injury,17,18 our study adds novel evidence that this might also be the mechanism by which a concomitant bone FX appears to worsen ultimate neurological and behavioral function days and weeks after brain injury. Indeed, in a different murine study evaluating CCI with or without a concurrent open tibial fracture, investigators reported that the dually injured animals sustained blunted neurological recovery by Neurological Severity Scoring (NSS) and diminished sensorimotor abilities with greater somatosensory neglect in the initial days after injury.7,10
The concurrently injured bone–brain axis and how the presence of one injury affects the local progression of the other injury is further highlighted when evaluating bone remodeling and repair in the setting of a concomitant brain injury. In a murine study evaluating CCI with or without a concomitant femoral osteotomy repaired with an external fixator, bone callus formation (as evaluated by micro computed tomography [CT]) demonstrated significantly greater callus volume formation in the bone FX animals if they sustained a concurrent brain injury as early as the second postoperative week.35 The same authors further demonstrated accelerated mineral density, rates of gap bridging, and greater bone torsional strength in the healing fractured bone of animals with a concurrent brain injury.36 Another group of investigators evaluating a similar combined rodent injury model but utilizing a tibial fracture also noted greater bone volume calluses and increased amounts of trabecular bone in animals with a concurrent TBI.37 Therefore, it is evident by these studies, that locally released factors involved in TBI circulate systemically and profoundly affect healing at the site of a concomitant bone injury importantly impacting FX repair and remodeling.
In multiple previous animal TBI studies, heparinoids (including unfractionated heparin [UFH] and ENX) administered after injury, reduced progression of secondary brain injury.18,38–40 In particular, heparinoids blunt upregulation of surface LEU selectins (L-, E-selectin)41 and their counter ligands on the surface of ECs (ICAM-1 and VCAM-1), effectively reducing their interactions in the microcirculation.42,43 This, in turn appears to minimize LEU-mediated BBB injury and leakiness of microvessels.18,44 As we and others have shown, ENX may be acting through high-mobility group protein (HMGB)-1 or IL-1 blockade reducing neutrophil trafficking to the area of injury.34,45 Similar to our findings in isolated brain injury,17 ENX in a dual TBI/fracture model also resulted in a reduction of in vivo LEU interactions with endothelium and diminished microvascular leakage, along with subsequently improved neurological recovery. The additional effect of bone injury on secondary brain inflammation was again partly reduced by ENX, findings that complement findings of previous studies and further support an anti-inflammatory effect of ENX in a multi-injury model through inhibition of neurovascular LEU/EC interactions. Nevertheless, a major concern in administering heparinoids after injury is the risk of hemorrhage.46 As highlighted in our surgical field bleeding observations, ENX exacerbated bleeding observed in dual injury animals at 48 h. In parallel, we found ENX administration to result in the greatest degree of weight loss and apparent partial cancellation of beneficial effects, reducing brain edema potentially through superimposed worsening of bleeding.
The current study is small and, therefore, has important limitations. First, we only evaluated acute, 2 day outcomes following TBI in a dual injury setting; long-term outcomes and recovery are unknown. We chose a 48 h study end-point because previous reports have shown that the number of circulating neutrophils in the cerebral vasculature peaks between 36 and 72 h following TBI.47 Second, we used a therapeutic dose of ENX, much higher than clinical prophylactic doses used in humans. We selected this dose because in our past experiments this dose was shown to reduce secondary brain injury.17 Also, in multiple other studies, this dose has been shown to be most effective at reducing tissue edema while causing the least bleeding.40,48 In common clinical settings, one might be hesitant to administer such high-dose ENX to multiply injured patients, particularly after TBI. As such, it would be important to assess whether a parallel prophylactic dose would achieve similar neurological improvements without the risk of undesired hemorrhage. Third, the clinical neurological scale used, The GNT, was not created with the intent of assessing rodents with a repaired tibial FX, and, as such, CCI+FX animals may have scored inadvertently lower, for non-neurological reasons such as local pain, swelling, or reduced function. Nonetheless, per IACUC requirements, the protocol required buprenorphine administration when animals were judged to have pain at or near the surgical site, and none received any analgesia in the FX group upon repeated evaluations in their cages for 48 h. Additionally we have since evaluated an FX-only group in a similar but separate study, and these animals were found to score identically to Sham counterparts (∼18 points). Fourth, we did not assess if other aspects of our murine model (limb or head skin incisions, skull craniectomy, repair of the tibia) were in themselves contributory to increased cerebral PMN trafficking and edema as they too, similar to the FX, could be contributing to the observed cerebral inflammation. Finally, we did not collect hemodynamic data, and it is entirely possible that a bone fracture worsened animal hemodynamic status, directly contributing to worsened cerebral inflammation and outcome. However, we utilized a standard open FX model that has previously shown no effects on hemodynamics.21
Conclusion
In summary, utilizing a dual injury TBI model, we demonstrated that a concomitant long bone FX worsens progression of brain injury through augmenting LEU-mediated neurovascular inflammation and cerebral edema. Administration of ENX suppressed LEU mobilization but only partially corrected brain edema and neurological recovery, resulting in greater evidence of bleeding. Additional animal studies will be required to further develop multi-injury TBI models, and to better understand the pathophysiological mechanisms leading brain injury to worsen in the setting of bone FX or other organ injuries, and to what extent LEU and endothelium are implicated in this process.
Acknowledgments
We relay our gratitude to Robin Armstrong for her technical and organizational assistance. We also thank Chao Xie, MD, Department of Orthopaedics and Rehabilitation, Center for Musculoskeletal Research, University of Rochester for his technical assistance with tibia bone fractures.
Author Disclosure Statement
No competing financial interests exist.
References
- 1. Rosenfeld J.V., Maas A.I., Bragge P., Morganti-Kossmann M.C., and Gruen R.L. (2012). Early management of severe traumatic brain injury. Lancet 380, 1088–1098 [DOI] [PubMed] [Google Scholar]
- 2. Thurman D.J., Alverson C., Dunn K.A., Guerrero J., and Sniezek J.E. (1999). Traumatic brain injury in the United States: A public health perspective. J. Head Trauma Rehabil. 14, 602–615 [DOI] [PubMed] [Google Scholar]
- 3. Blennow K., Hardy J., and Zetterberg H. (2012). The neuropathology and neurobiology of traumatic brain injury. Neuron 76, 886–899 [DOI] [PubMed] [Google Scholar]
- 4. Xiong Y., Mahmood A., and Chopp M. (2013). Animal models of traumatic brain injury. Nat. Rev. Neurosci. 14, 128–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pop V., and Badaut J. (2011). A neurovascular perspective for long-term changes after brain trauma. Transl. Stroke Res. 2, 533–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Degos V., Maze M., Vacas S., Hirsch J., Guo Y., Shen F., Jun K., van Rooijen N., Gressens P., Young W.L., and Su H. (2013). Bone fracture exacerbates murine ischemic cerebral injury. Anesthesiology 118, 1362–1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yang L., Guo Y., Wen D., Yang L., Chen Y., Zang G., and Fan Z. (2016). Bone Fracture enhances trauma brain injury. Scand. J. Immunol. 83, 26–32 [DOI] [PubMed] [Google Scholar]
- 8. Pisetsky D. (2011). Cell death in the pathogenesis of immune-mediated diseases: the role of HMGB1 and DAMP-PAMP complexes. Swiss Med. Wkly. 141, w13256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Andersson U., and Tracey K.J. (2011). HMGB1 is a therapeutic target for sterile inflammation and infection. Annu. Rev. Immunol. 29, 139–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shultz S.R., Sun M., Wright D.K., Brady R.D., Lui S., Beynon S., Schmidt S.F., Kaye A.H., Hamilton J.A., O'Brien T.J., Grills B.L., and McDonald S.J. (2015). Tibial fracture exacerbates traumatic brain injury outcomes and neuroinflammation in a novel mouse model of multitrauma. J. Cereb. Blood Flow Metab. 35, 1339–1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sims G.P., Rowe D.C., Rietdijk S.T., Herbst R., and Coyle A. J. (2010). HMGB1 and RAGE in inflammation and cancer. Annu. Rev. Immunol. 28, 367–388 [DOI] [PubMed] [Google Scholar]
- 12. Park J.S., Gamboni-Robertson F., He Q., Svetkauskaite D., Kim J.Y., Strassheim D., Sohn J.W., Yamada S., Maruyama I., Banerijee A., Ishizawa A., and Abraham E. (2006). High mobility group box 1 protein interacts with multiple Toll-like receptors. Am. J. Physiol. Cell Physiol. 290, C917–C924 [DOI] [PubMed] [Google Scholar]
- 13. Sun M, McDonald S.J., Brady R.D., O'Brien T.J., and Shultz S.R. (2018). The influence of immunological stressors on traumatic brain injury. Brain Behav. Immun. 69, 618–628 [DOI] [PubMed] [Google Scholar]
- 14. McDonald S.J., Sun M., Agoston D.V., and Shultz S.R. (2016). The effect of concomitant peripheral injury on traumatic brain injury pathobiology and outcome. J. Neuroinflammation 13, 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Borsig L. (2010). Antimetastatic activities of heparins and modified heparins. Experimental evidence. Thromb. Res. 125, S66–71 [DOI] [PubMed] [Google Scholar]
- 16. Rao S.V., Melloni C., Myles-DiMauro S., Broderick S., Kosinski A.S., Kleiman N.S., Dzavik V., Tanguay J.F., Chandna H., Gammon R., Rivera E., Alexander J.H., Fier I., Roach J., and Becker R.C. (2010). Evaluation of a new heparin agent in percutaneous coronary intervention. Results of the phase 2 evaluation of M118 IN pErcutaNeous Coronary intErvention (EMINENCE) Trial. Circulation 121, 1713–1721 [DOI] [PubMed] [Google Scholar]
- 17. Li S., Marks J.A., Eisenstadt R., Kumasaka K., Samadi R., Johnson V.E., Holena D.N., Allen S.R., Browne K., Smith D.H., and Pascual J.L. (2015). Enoxaparin ameliorates post-traumatic brain injury edema and neurologic recovery, reducing cerebral leukocyte endothelial interactions and vessel permeability in vivo. J. Trauma Acute Care Surg. 79, 78–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nagata K., Kumasaka K., Browne K.D., Li S., St-Pierre J., Cognetti J., Marks J., Johnson V.E., Smith D.H, and Pascual J.L. (2016). Unfractionated heparin after TBI reduces in vivo cerebrovascular inflammation, brain edema and accelerates cognitive recovery. J. Trauma Acute Care Surg. 81, 1088–1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Smith D.H., Soares H.D., Pierce J.S., Perlman K.G., Saatman K.E., Meaney D.F., Dixon C.E., and Mclntosh T.K. (1995). A model of parasagittal controlled cortical impact in the mouse: cognitive and histopathologic effects. J. Neurotrauma 12, 169–178 [DOI] [PubMed] [Google Scholar]
- 20. Pascual J.L., Murcy M.A., Li S., Gong W., Eisenstadt R., Kumasaka K., Sims C., Smith D.H., Browne K., Allen S., and Baren J. (2013). Neuroprotective effects of progesterone in traumatic brain injury: blunted in vivo neutrophil activation at the blood–brain barrier. Am. J. Surg. 206, 840–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yukata K., Xie C., Li T.F., Takahata M., Hoak D., Kondabolu S., Zhang X., Awad H.A., Schwartz E.M., Beck C.A., Jonasaon J.H., and O'Keefe R.J. (2014). Aging periosteal progenitor cells have reduced regenerative responsiveness to bone injury and to the anabolic actions of PTH 1-34 treatment. Bone 62, 79–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Marks J.A., Li S., Gong W., Sanati P., Eisenstadt R., Sims C., Smith D.H., Reilly P.M., and Pascual J.L. (2012). Similar effects of hypertonic saline and mannitol on the inflammation of blood–brain barrier microcirculation after brain injury ia a mouse model. J. Trauma Acute Care Surg. 73, 351–357 [DOI] [PubMed] [Google Scholar]
- 23. Garcia J.H., Wagner S., Liu K.F., and Hu X.J. (1995). Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats. Statistical validation. Stroke 26, 627–635 [DOI] [PubMed] [Google Scholar]
- 24. Manaenko A., Lekic T., Ma Q., Ostrowski R.P., Zhang J.H., and Tang J. (2011). Hydrogen inhalation is neuroprotective and improves functional outcomes in mice after intracerebral hemorrhage. Acta Neurochir. Suppl. 111, 179–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Daley J.M., Thomay A.A., Connolly M.D., Reichner J.S., and Albina J.E. (2008). Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J. Leukoc. Biol. 83, 64–70 [DOI] [PubMed] [Google Scholar]
- 26. Scholz M., Cinatl J., and Schadel-Hopfner M. (2007). Neutrophils and the blood-brain barrier dysfunction after trauma. Med. Res. Rev. 27, 401–416 [DOI] [PubMed] [Google Scholar]
- 27. DiStasi M.R., and Ley K. (2009). Opening the flood-gates: how neutrophil–endothelial interactions regulate permeability. Trends Immunol. 30, 547–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ley K., Laudanna C., Cybulsky M.I., Cybulsky M.I., and Nourshagh S. (2007). Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat. Rev. Immunol. 7, 678–689 [DOI] [PubMed] [Google Scholar]
- 29. Kubes P., and Ward P.A. (2000). Leukocyte recruitment and the acute inflammatory response. Brain Pathol. 10, 127–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pascual J.L., Khwaja K.A., Chaudhury P., and Christou N.V. (2003). Hypertonic saline and the microcirculation. J. Trauma 54, S133–140 [DOI] [PubMed] [Google Scholar]
- 31. Xiao W., Mindrinos M.N., Seok J., Cuschieri J., Cuenca A.G., Gao H., Hayden D.L., Hennessy L., Moore E.E., Minei J.P., Bankey P.E., Johnson J.L., Sperry J., Nathens A.B., Billiar T.R., West M.A., Brownstein B.H., Mason P.H., Baker H.V., Finnerty C.C., Jeschke M.G., López M.C., Klein M.B., Gamelli R.L., Gibran N.S., Arnoldo B., Xu W., Zhang Y., Calvano S.E., McDonald-Smith G.P., Schoenfeld D.A., Storey J.D., Cobb J.P., Warren H.S., Moldawer L.L., Herndon D.N., Lowry S.F., Maier R.V., Davis R.W., Tompkins R.G., and Inflammation and Host Response to Injury Large-Scale Collaborative Research Program. (2011). A genomic storm in critically injured humans. J. Exp. Med. 208, 2581–2590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Thal S.C., and Neuhaus W. (2014). The blood–brain barrier as a target in traumatic brain injury treatment. Arch. Med. Res. 45, 698–710 [DOI] [PubMed] [Google Scholar]
- 33. Lukaszewicz A.C., Soyer B., and Payen D. (2011) Water, water, everywhere: sodium and water balance and the injured brain. Curr. Opin. Anaesthesiol. 24, 138–143 [DOI] [PubMed] [Google Scholar]
- 34. Sun M., Brady R.D., Wright D.K., Kim H.A., Zhang S.R., Sobey C.G., Johnstone M.R., O'Brien T.J., Semple B.D., McDonald S.J., Shultz S.R. (2017). Treatment with an interleukin-1 receptor antagonist mitigates neuroinflammation and brain damage after polytrauma. Brain Behav. Immun. 66, 359–371 [DOI] [PubMed] [Google Scholar]
- 35. Tsitsilonis S., Seemann R., Misch M., Wichlas F., Hass N.P., Schmidt-Bleek K., Kleber C., and Schaser K.D. (2015). The effect of traumatic brain injury on bone healing: an experimental study in a novel in vivo animal model. Injury 46, 661–665 [DOI] [PubMed] [Google Scholar]
- 36. Locher R.J., Lunnemann T., Garbe A., Schaser K., Schmidt-Bleek K., Duda G., and Tsitsilonis S. (2015). Traumatic brain injury and bone healing: radiographic and biomechanical analyses of bone formation and stability in a combined murine trauma model. J. Musculoskelet. Neuronal. Interact. 15, 309–315 [PMC free article] [PubMed] [Google Scholar]
- 37. Brady R.D., Grills B.L., Church J.E., Walsh N.C., McDonald A.C., Agoston D.V., Sun M., O'Brien T.J., Shultz S.R., and McDonald S.J. (2016). Closed head experimental traumatic brain injury increases size and bone volume of callus in mice with concomitant tibial fracture. Sci. Rep. 6, article number 34491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sen O., Sonmez E., and Cekinmez M., (2011). Antithrombin III and enoxaparin treatment inhibit contusion-triggered cell death, inflammation, hemorrhage and apoptosis after severe traumatic brain injury in rats. Turk. Neurosurg. 21, 203–209 [DOI] [PubMed] [Google Scholar]
- 39. Stutzmann J.M., Mary V., Wahl F., Grosjean-Piot O., Uzan A., and Pratt J. (2002). Neuroprotective profile of enoxaparin, a low molecular weight heparin, in vivo models of cerebral ischemia or traumatic brain injury in rats: a review. CNS Drug Rev. 8, 1–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wahl F., Grosjean-Piot O., Bareyre F., Uzan A., and Stultzmann J.M. (2000). Enoxaparin reduces brain edema, cerebral lesions, and improves motor and cognitive impairments induced by a traumatic brain injury in rats. J. Neurotrauma 17, 1055–1065 [DOI] [PubMed] [Google Scholar]
- 41. Wang L., Brown J.R., Varki A., and Esko J.D. (2002). Heparin's anti-inflammatory effects require glucosamine 6-O-sulfation and are mediated by blockade of L-and P-selectins. J. Clin. Invest. 110, 127–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Manduteanu I., Voinea M., Capraru M., Dragomir E., and Simionescu M. (2002). A novel attribute of enoxaparin: inhibition of monocyte adhesion to endothelial cells by a mechanism involving cell adhesion molecules. Pharmacology 65, 32–37 [DOI] [PubMed] [Google Scholar]
- 43. Manduteanu I., Voinea M., Antohe F., Dragomir E., Capraru M., Radulescu L., and Simionescu M. (2003). Effect of enoxaparin on high glucose-induced activation of endothelial cells. Eur. J. Pharmacol. 477, 269–276 [DOI] [PubMed] [Google Scholar]
- 44. Weber J.R., Angstwurm K., Rosenkranz T., Lindauer U., Freyer D., Burger W., Brusch C., Einhaupl K.M., and Dirnagl U. (1997). Heparin inhibits leukocyte rolling in pial vessels and attenuates inflammatory changes in a rat model of experimental bacterial meningitis. J. Cereb. Blood Flow Metab. 17, 1221–1229 [DOI] [PubMed] [Google Scholar]
- 45. Li S., Marks J.A., Eisenstadt R., Kumasaka K., Samadi D., Johnson V.E., Holena D.N., Allen S.R., Browne K.D., Smith D.H., Pascual J.L. (2015). Enoxaparin ameliorates post-traumatic brain injury edema and neurologic recovery, reducing cerebral leukocyte endothelial interactions and vessel permeability in vivo. J. Trauma Acute Care Surg. 79, 78–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Glassner S., Srivastava K., Cofnas P., Cofinas P., Deegan B., Demaria P., Denis R., and Ginzburg E. (2013). Prevention of venous thrombotic events in brain injury: review of current practices. Rambam Maimonides Med. J. 4, e0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rhind S.G., Crnko N.T., Baker A.J., Morrison L.J., Shek P.N., Scarpelimi S., and Rizoli S.B. (2010). Prehospital resuscitation with hypertonic saline-dextran modulates inflammatory, coagulation and endothelial activation marker profiles in severe traumatic brain injured patients. J. Neuroinflammation. 18, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Župan, Ž., Pilipović K., Dangubić B., Frković V., Šustić A., and Župan G. (2011). Effects of enoxaparin in the rat hippocampus following traumatic brain injury. Neuropsychopharmacol. Biol. Psychiatry 35, 1846–1856 [DOI] [PubMed] [Google Scholar]