Introduction
Despite a common CD4+ T-cell precursor, helper T (Th) 1 and 2 lymphocytes affect different immunologic responses, including cellular and humoral pathways, respectively. Th1 cells stimulate the activation of type 1 macrophages, IgG/immunoglobulin M production, and cytokine release (eg, interferon gamma [IFN-γ], interleukin 2) to respond to intracellular pathogens. In contrast, Th2 cells target extracellular pathogens, leading to increases in immunoglobulin E and proliferation of eosinophils, basophils, B lymphocytes, type 2 macrophages, and mast cells through the actions of interleukin 4 (IL-4), interleukin 5, interleukin 13 (IL-13), and interleukin 31.1 Th17 cells are important for host defense against extracellular bacteria and fungi, as well as injury, and are thought to be key drivers of autoimmune diseases. Th17 cells produce interleukin 17A, interleukin 17F, interleukin 22, and C-C motif chemokine ligand 20 and are characterized by neutrophil and macrophage recruitment. The Th2 cytokine, IL-4, has been shown to inhibit Th17 development.2 Dupilumab (Dupixent, Regeneron Pharmaceuticals and Sanofi Genzyme), a fully human monoclonal (IgG4) antibody, inhibits the signaling of IL-4/13 by blocking the shared receptor subunit, IL-4 receptor α. Dupilumab is an effective treatment for a number of Th2-driven conditions, including atopic dermatitis (AD), asthma, and chronic rhinosinusitis with nasal polyposis. Multiple sclerosis (MS) is an inflammatory disease of the central nervous system, which is characterized by multifocal demyelination and neuroaxonal degeneration, where the immune system is skewed toward Th1/Th17 axes.3 Interestingly, epidemiologic studies have suggested that a history of allergic diseases may confer some protection from MS.4 We report a temporal association of relapsing-remitting MS flareup and dupilumab treatment for AD. We provide a potential mechanism by which dupilumab leads to Th2 suppression with secondary Th1/17 dysregulation.
Case report
A 34-year-old woman with a history of AD presented to the neuroimmunology clinic for 12-months of intermittent falls related to tripping on her left foot. Four months prior to presentation, she began treatment with dupilumab for AD in the context of inadequately controlled disease with topical betamethasone 0.05% ointment daily (as needed) and diphenhydramine 25 mg 4 times daily (as needed). Two months prior to presentation, she developed paresthesias in her legs and perineum bilaterally, with a reduced sensation of bowel and urinary urgency. On presentation, neurologic examination was significant for mild left hip flexor weakness, left patellar hyperreflexia, absent right plantar, and mildly reduced vibration at the right great toe, concerning for thoracic myelopathy. Magnetic resonance imaging of the cervical and thoracic spine showed nonenhancing short-segment spinal cord lesions at C6, T8, and T10. Magnetic resonance imaging scans of the brain revealed multiple contrast-enhancing and nonenhancing white matter lesions in the periventricular and juxtacortical/cortical zones and the corpus callosum, consistent with active MS (Fig 1, A-C). Cerebrospinal fluid demonstrated mild lymphocytic pleocytosis, 11 oligoclonal bands, and an elevated IgG index of 0.93. Serum autoimmune workup was otherwise negative. Based on her clinical evaluation, imaging, and cerebrospinal fluid findings, she was diagnosed with relapsing-remitting MS. It was postulated that dupilumab, through its anti–IL-4 mechanism of action, may have triggered the second and/or worse flareup of MS. Dupilumab was stopped, and she was started on ocrelizumab (an anti-CD20 biologic) as MS disease-modifying therapy. The patient has since been stable on ocrelizumab with no residual, recurrent, or new neurologic symptoms.
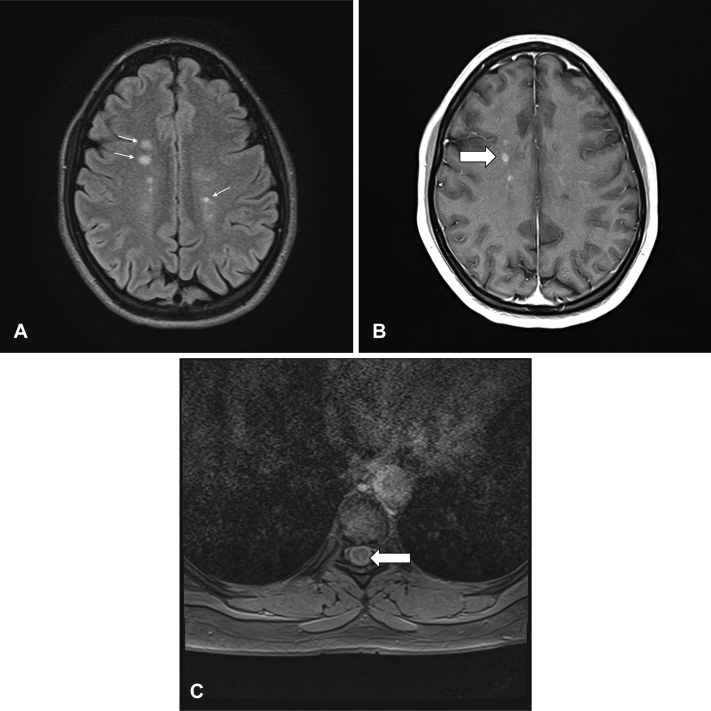
Fig 1.
Magnetic resonance imaging (MRI) scans of the brain and thoracic spine. A, Axial T2 brain MRI scan showed multiple hyperintense demyelinating lesions typical of multiple sclerosis. B, Axial T1 brain MRI scan with contrast demonstrated several enhancing multiple sclerosis lesions (arrow). C, Axial T2 spine MRI scan with dorsal thoracic cord MS lesion (arrow).
Discussion
AD is characterized by cutaneous and systemic type 2 inflammation with the recruitment of Th2 cells, eosinophils, and dendritic cells to skin lesions. Although the canonical Th2 cytokines (IL-4, interleukin 5, and IL-13) are the predominant Th pathway in AD skin, there is some evidence for a more minor role of T22, Th17, and Th1 cells with the production of interleukin 22, interleukin 17A, and IFN-γ, respectively. IL-4 and IL-13 promote Th2 survival, immunoglobulin E class switching, eosinophil recruitment to tissue, enhanced pruritus, and alterations in keratinocyte differentiation.5 These characteristic findings are found in the majority of patients with AD with moderate-to-severe disease and are largely reversed by dupilumab.6,7 No cases of exacerbated or newly diagnosed MS associated with dupilumab administration have been reported.
Research on MS pathogenesis suggests an inflammatory state involving the disruption of the blood-brain barrier and central nervous system trafficking of peripherally activated Th1, Th17, and CD8+ effector T cells. These cells secrete IFN-γ, interleukin 17, interleukin 21/22, tumor necrosis factor-α, and cytotoxic granules that act on the central nervous system microglia, astrocytes, and macrophages, resulting in the release of reactive oxygen species and axonal demyelination. Under normal conditions, this cascade is inhibited by the peripheral nervous system FoxP3+ regulatory T and natural killer cells, regulatory CD8+ cells, and regulatory B cells. In relapsing-remitting MS, regulatory B cells serve as antigen-presenting cells for 1 or more epitopes that lead to highly activated pathogenic T cells without antiinflammatory counter regulation.2 We posit that dupilumab's inhibition of IL-4 downstream actions may have altered our patient's cytokine milieu, shifting the balance of Th profiles in favor of Th1/17 pathways (ie, interleukin 22, interleukin 17, and IFN-γ), which is the T cell phenotype thought to drive MS pathogenesis. Research has also illustrated that IL-4 downregulates the Th1/17 axis by reducing the production of interleukin 23 from dendritic cells.8 Accordingly, dupilumab-IL-4 receptor α blockage may have inhibited this regulatory step, thus promoting the MS-pathogenic B cell-T cell interface and inducing a recurrence or exacerbation of MS-type symptoms in this patient.
A role for IL-4 inhibition of Th17 cells is suggested by studies on IL-4 receptor (IL4R) polymorphisms. IL-4 has been shown to block the Th17 differentiation in vitro, and this effect is diminished in T cells from individuals with a hypofunctional IL4R mutation.9 Moreover, hypofunctional IL4R alleles observed in patients with rheumatoid arthritis are associated with increased serum interleukin 17 levels, increased peripheral blood Th17 cells, poor clinical response to methotrexate, and increased bone erosions. Thus, there should be concerns that in genetically predisposed individuals, blockade of IL-4 receptors may initiate or induce flareups in diseases where Th17 cells play a pathogenic role. A review of rare side effects reported with dupilumab treatment lends support to this immunopathologic mechanism. For example, psoriasis has been reported in dupilumab-treated patients with AD with pathophysiology attributed to a shift of Th2 to Th17/interleukin 23 bioactivity, the latter axis a known inducer of this cutaneous disease.10 Until further data are known, we recommend that caution be used when prescribing dupilumab in people with MS.
Conflicts of interest
Dr Beck is a consultant for Abbvie, Allakos, Astra-Zeneca, Benevolent AIBio, DermTech, Incyte, Janssen, LEO Pharma, Lilly, Novartis, Pfizer, Principia Biopharma, Rapt Therapeutics, Regeneron, Sanofi/Genzyme, Sanofi-Aventis, and Stealth Biotherapeutics and an investigator for Abbvie, Astra-Zeneca, Kiniksa, LEO Pharma, Pfizer, and Regeneron and Sanofi. Drs Laageide, Verhave, Samkoff, and Looney have no conflicts of interest to declare.
Footnotes
Funding sources: None.
IRB approval status: Not applicable.
References
- 1.Cosmi L., Maggi L., Santarlasci V., Liotta F., Annunziato F. T helper cells plasticity in inflammation. Cytometry A. 2014;85(1):36–42. doi: 10.1002/cyto.a.22348. [DOI] [PubMed] [Google Scholar]
- 2.Romagnani S. Human Th17 cells. Arthritis Res Ther. 2008;10(2):206. doi: 10.1186/ar2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Villoslada P., Zamvil S.S. The immune signatures of multiple sclerosis: lessons from twin studies. Proc Natl Acad Sci U S A. 2020;117(39):24013–24015. doi: 10.1073/pnas.2016711117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pedotti R., Farinotti M., Falcone C. Allergy and multiple sclerosis: a population-based case-control study. Mult Scler. 2009;15(8):899–906. doi: 10.1177/1352458509106211. [DOI] [PubMed] [Google Scholar]
- 5.Blakely K., Gooderham M., Papp K. Dupilumab, a monoclonal antibody for atopic dermatitis: a review of current literature. Skin Ther Lett. 2016;21(2):1–5. [PubMed] [Google Scholar]
- 6.Beck L.A., Thaçi D., Hamilton J.D. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N Engl J Med. 2014;371(2):130–139. doi: 10.1056/NEJMoa1314768. [DOI] [PubMed] [Google Scholar]
- 7.Callewaert C., Nakatsuji T., Knight R. IL-4Rα blockade by dupilumab decreases Staphylococcus aureus colonization and increases microbial diversity in atopic dermatitis. J Invest Dermatol. 2020;140(1):191–202.e7. doi: 10.1016/j.jid.2019.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guenova E., Skabytska Y., Hoetzenecker W. IL-4 abrogates T(H)17 cell-mediated inflammation by selective silencing of IL-23 in antigen-presenting cells. Proc Natl Acad Sci U S A. 2015;112(7):2163–2168. doi: 10.1073/pnas.1416922112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wallis S.K., Cooney L.A., Endres J.L. A polymorphism in the interleukin-4 receptor affects the ability of interleukin-4 to regulate Th17 cells: a possible immunoregulatory mechanism for genetic control of the severity of rheumatoid arthritis. Arthritis Res Ther. 2011;13(1):R15. doi: 10.1186/ar3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jaulent L., Staumont-Sallé D., Tauber M. De novo psoriasis in atopic dermatitis patients treated with dupilumab: a retrospective cohort. J Eur Acad Dermatol Venereol. 2021;35(4):e296–e297. doi: 10.1111/jdv.17050. [DOI] [PubMed] [Google Scholar]