Abstract
Purpose
Standard planning target volume (PTV) margins for lung stereotactic ablative radiation therapy (SABR) are 5 mm. High-dose-rate volumetric modulated arc therapy delivered using flattening filter-free (FFF) beams with modern immobilization systems may allow for PTV margin reduction. This study assesses whether PTV margins can be reduced from 5 to 3 mm.
Methods
Target intrafractional motions derived from pretreatment and posttreatment cone beam computed tomography (CBCT) scans for 33 patients receiving lung SABR treated with 10XFFF energy and 5-mm PTV margins from 2016 to 2019 were used to calculate the required PTV margin. Deformable registration of the planning CT scan and internal gross tumor volume (IGTV) contour to posttreatment CBCT scans for 36 consecutive patients with 4 fraction schedules was completed to capture volume changes and intrafractional movement. Plans were replanned with 3-mm margins and recalculated on each deformed CT scan to assess deformed IGTV (d-IGTV) coverage and organ-at-risk doses.
Results
Margin analysis showed PTV margins may be reduced to 3 mm. The mean d-IGTV coverage (percentage of the d-IGTV receiving ≥100% of the prescription dose [V100%] and the minimum dose covering 99.9% of the d-IGTV volume [D99.9%]) over 4 fractions for each patient was >95% with both margins. With 5-mm PTV margins, all 144 fractions had a d-IGTV V100% of >95% and a D99.9% >95%. With 3-mm PTV margins, the d-IGTV V100% was >95% in 99.3% of fractions (143 of 144) and the D99.9% was >95% in 98.6% of fractions (142 of 144). With 3-mm PTV margins, significant reductions in body V50%, body V80%, the volume of the lung receiving ≥20 Gy, and the mean lung dose and chest wall dose to 0.035 cm3 and 30 cm3 were observed (all P < .001). Using theoretical models, the normal tissue complication probability for radiation pneumonitis decreased by a mean of 0.8% (range, 0.1%-2.7%), and the mean 2-year tumor control probability was 96.1% and 95.2% with 5-mm and 3-mm PTV margins, respectively.
Conclusion
With modern treatment and immobilization techniques in lung SABR, 3-mm PTV margins maintain acceptable IGTV coverage, modestly reduce toxicity to organs at risk, and maintain a calculated 2-year local control rate of >95%.
Introduction
Stereotactic ablative radiation therapy (SABR) is an effective treatment for early-stage non-small cell lung cancer (NSCLC) with excellent local control rates (>90% at 2 years).1, 2, 3 It is the treatment of choice for patients with stage I medically inoperable NSCLC.4,5 Compared with conventional radiation therapy, SABR uses increased precision with image guidance, higher conformity with steeper dose gradients, and a larger dose per fraction in fewer fractions to deliver higher biologically effective doses to tumors while minimizing the dose to nearby normal tissue.6 Specialized planning, immobilization, and dose-delivery techniques are required to achieve this precision given that a geographic miss would have considerable implications.7, 8, 9 Components of this include 4-dimensional computed tomography (4DCT), used to create an internal gross tumor volume (IGTV)10, 11, 12 that accounts for tumor motion during respiration, as well as linear accelerator–mounted cone beam computed tomography (CBCT), used to make precise adjustments to patient set-up before treatment delivery.9
Using these strategies allows for smaller planning target volume (PTV) margins than those of conventional radiation therapy.9,13 The PTV margins are required to account for intrafractional shifts, random error, and systematic error encountered during treatment.14 Since the development of SABR, 5- to 10-mm PTV margins have been used by many institutions and continue to be a common standard.1,4,13,15 However, there have been advancements in image guidance, delivery techniques, and immobilization devices since the inception of SABR. Modern volumetric modulated arc therapy (VMAT) using flattening-filter-free (FFF) beams allows for much higher dose rates than intensity modulated radiation therapy (IMRT) with flattened beams.16 Higher dose rates result in reduced treatment times, potentially leading to decreased intrafractional motion.9,17 These factors may allow for reductions in PTV margin sizes while maintaining efficacy. This study had 2 purposes. The first was to evaluate the current PTV margin size and assess whether this can be reduced. The second was to analyze the dosimetry of 5-mm PTV margins compared with the calculated 3-mm PTV margins.
Methods
Posttreatment CBCT intrafractional shift data from 33 patients with early-stage NSCLC treated with 4- or 8-fractionation lung SABR from 2016 to 2019 were analyzed to determine a PTV margin with a probability of IGTV coverage of ≥95% of the prescription dose in 90% and 99% of patients. The mean displacements of the IGTV in longitudinal, vertical, and lateral directions were analyzed in a total of 173 posttreatment CBCT scans. The PTV setup margin (MPTV) for lung SABR was then calculated using the van Herk formula as follows:
| (1) |
where α is 2.5 when 90% of patients receive a minimum of 95% of the dose to the IGTV and 3.36 when 99% of patients receive a minimum of 95% of the dose to the IGTV, Σ is the standard deviation (SD) of the systematic error calculated using the mean displacements of intrafractional shifts of each patient, σ is the SD of the random error calculated using the SD of the shifts of each patient, β is 0.84 for an approximate prescription isodose level of 80% for a minimum of 95% of the PTV, and σp is 0.64 cm to accommodate for lung penumbra.14,15,18
To validate 3-mm PTV margins, 36 consecutive patients with early-stage NSCLC treated with lung SABR using 48 Gy in 4 fractions, 10XFFF energy (maximum dose rate, 2400 MU/min), and 5-mm PTV margins from 2016 to 2019 were included. For tumors superior to the carina, immobilization was accomplished with a long thermoplastic shell, no abdominal compression, and shoulder retractors (Combifix, CIVCO Radiotherapy, Orange City, Iowa). Immobilization for tumors below the carina used a vacuum mold and either arms up with a pneumatic belt for abdominal compression or arms down and a bridge and respiratory plate for abdominal compression (Body Pro-Lok, CIVCO Radiotherapy). Plans were created on the mean intensity projection data set from all 10 phases of a full-lung 4DCT cine scan, which was reviewed and approved by a physicist before planning. All patients were treated with coplanar VMAT with 2 or 3 partial (200°) arcs, with pretreatment and posttreatment CBCT scans.
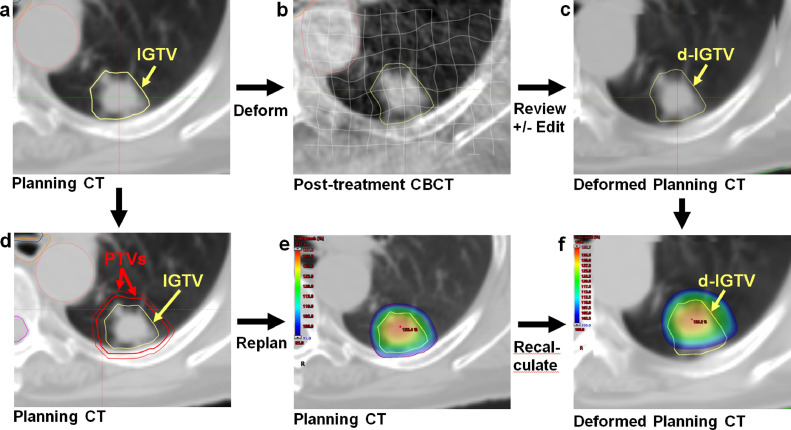
A graphical overview of our margin analysis workflow is shown in Figure 1. Deformable registration of the original planning CT (mean intensity projection from 4DCT) and IGTV contour to the posttreatment CBCT scan was completed for each fraction using the SmartAdapt, version 13, deformable registration algorithm (Varian Medical Systems, Palo Alto, California). This simultaneously captures the intrafraction translation and rotation of the IGTV and any volume changes. The volume of interest for deformation was set to enclose the original PTV plus a 1- to 2-cm margin to encompass tissues surrounding the PTV but exclude (and therefore preserve the integrity of) the external body contour. The CBCT scan and initial IGTV contour were used as references to review each deformed IGTV (d-IGTV) to ensure a high-quality registration and minimize deformation errors. Each d-IGTV contour was independently reviewed by a clinician and medical physicist.
Fig. 1.
A stepwise overview to margin analysis using deformable registration. The planning computed tomography (CT) internal gross tumor volume (IGTV) contour (A) for each case was deformed onto the posttreatment cone beam CT for every fraction (B). The deformed IGTVs (d-IGTVs) were reviewed and edited if necessary to ensure consistency (C). The initial planning CT planning target volume (PTV) expansion (A) was changed from 5 mm to 3 mm (D), and the case was replanned (E). The 5-mm and 3-mm PTV plans were both recalculated on the deformed planning CT scans (F). Coverage of the d-IGTV contour was analyzed for every fraction (F).
All plans were retrospectively replanned with 3-mm PTV margins using identical optimization and calculation parameters (Varian Eclipse Progressive Resolution Optimizer and Anisotropic Analytical Algorithm, version 11, with dose grid resolution of 2.5 mm) and normalized to match the coverage of the original plan (percentage of the PTV receiving ≥100% of the prescription dose [V100%] = 95%). Dose was recalculated on each deformed CT to assess d-IGTV coverage for both 5-mm and 3-mm PTV plans. The percentage of the d-IGTV receiving ≥100% of the prescription dose (V100%) and the minimum dose covering 99.9% of the d-IGTV volume (D99.9%) were used to assess d-IGTV coverage.
To study the difference in normal tissue treated, several metrics were compared between the 5-mm and 3-mm PTV plans calculated on the original (nondeformed) CT sets. The volume of the body receiving ≥50% and ≥80% of the prescription dose (V50% and V80%) was determined. For analysis of organs at risk (OARs), the volume of the lung receiving ≥20 Gy (V20Gy) and the mean lung dose (MLD) were calculated, and for those patients with any overlap of the 5-mm PTV with the chest wall (22 patients), the dose to 0.035 cm3 (D0.035cc) and 30 cm3 (D30cc) of chest wall (including ribs) was determined.
To analyze normal-tissue complication probability (NTCP) for radiation pneumonitis, the MLD was converted to MLD(3Gy) using the following equation calculated by Borst et al (derived from the linear quadratic model):
| (2) |
where α/β = 3 and the slope of the line of best fit (regression coefficient of 0.92) for SABR treatments of 12 Gy per fraction was 1.8.19 The NTCP for radiation pneumonitis was then calculated using the following equation:
| (3) |
where , TD50 = 20.8 Gy (the dose for a 50% NTCP), and m = 0.45 (steepness parameter in the Lyman model).19,20
Tumor control probability (TCP), used to predict 2-year local control, was determined by using the mean d-IGTV D99.9% from each patient's 5-mm and 3-mm plan to calculate the size-adjusted biological equivalent dose (sBED):
| (4) |
where BED10 is the biologically effective SABR dose using mean d-IGTV D99.9% and an α/β of 10 Gy, c is a constant (10 Gy/cm), and L is the tumor diameter (calculated assuming a spherical IGTV, ie, ), then by using the following formula validated by Ohri et al:
| (5) |
where TCD50 and k are parameters that define the shape of the TCP curve.21 Size-adjusted BED was used owing to the approximate linear reduction in effective dose with increasing tumor diameter, consistent with reported local control rates decreasing with tumor size.21
This study was approved by the University of British Columbia - BC Cancer Research Ethics Board (REB number H19-02195).
Results
Analysis of intrafractional shifts from 173 posttreatment CBCT scans from 33 patients using Equation 1 showed a PTV margin requirement of 2.3 mm to achieve ≥95% of the prescription dose delivered to the IGTV in 90% of patients (2.21 mm anterior-posterior, 1.60 mm superior-inferior, and 1.05 mm left-right). For the same coverage in 99% of patients, a PTV margin of 3.0 mm was found sufficient (2.95 mm anterior-posterior, 2.14 mm superior-inferior, and 1.40 mm left-right).
Dosimetric analysis included 144 fractions from 36 consecutively treated patients receiving lung SABR. The median time from the initial CBCT scan to the posttreatment CBCT scan was 7.48 min (interquartile range, 6.72-8.84 min; mean, 7.9 ± 1.9 min). The mean IGTV volume was 8.97 cm3 (range, 0.17-52.2 cm3). With 5-mm PTV margins, all 144 fractions had a d-IGTV V100% of >95% (Table 1) and a D99.9% of >95% (Fig 2). With 3-mm PTV margins, the d-IGTV V100% was >95% in 99.3% of fractions (143 of 144). Only 3 of 144 fractions had a d-IGTV V100% of <99% (98.9%, 97.9%, and 93.8%, respectively). With 3-mm PTV margins, the d-IGTV D99.9% was >95% in 98.6% of fractions (142 of 144). The mean d-IGTV coverage (V100% and D99.9%) over all 4 fractions for each patient was >95% for all patients with both margins. Although Wilcoxon signed rank tests revealed statistical differences in both the d-IGTV V100% and the D99.9% between the PTV margins (P < .05), for 3-mm PTV margins, both metrics achieved clinically acceptable coverage in >98% of all fractions and 100% of patients (averaged over 4 fractions), thus surpassing generally accepted criteria for PTV margin size.14
Table 1.
d-IGTV V100% by PTV margin size*
| PTV margin |
||||
|---|---|---|---|---|
| 5 mm |
3 mm |
|||
| d-IGTV V100%, % | Per fraction | Average over all fractions | Per fraction | Average over all fractions |
| 99.5-100 | 143 | 36 | 139 | 34 |
| 99.0-99.4 | 1 | 0 | 2 | 1 |
| 98.0-98.9 | 0 | 0 | 1 | 1 |
| 97.0-97.9 | 0 | 0 | 1 | 0 |
| 94.0-96.9 | 0 | 0 | 0 | 0 |
| 93.0-93.9 | 0 | 0 | 1 | 0 |
| < 93.0 | 0 | 0 | 0 | 0 |
Abbreviations: d-IGTV = deformed internal gross tumor volume, PTV = planning target volume; V100% = percent of the d-IGTV receiving at least 100% of the prescription dose.
Acceptable d-IGTV coverage was defined as V100% ≥ 95%.
Fig. 2.
The minimum percentage of the prescription dose covering 99.9% of the deformed internal gross tumor volume (d-IGTV) for each fraction with 5 mm versus 3 mm planning target volume margins. Acceptable coverage was defined as the minimum dose covering 99.9% of the d-IGTV volume ≥95% of the prescribed dose.
Body V50% and V80% data by PTV margin size are shown in Figure E1 and Table 2. For each patient's treatment course, the decrease in body V50% and V80% with 3-mm PTV margins was calculated. The mean volume reduction for the study population was 28 cm3 and 12 cm3, with a mean relative reduction of 28% and 31%, respectively. Using paired t tests, significant reductions in total lung V20Gy and MLD over patients’ treatment courses with 3-mm PTV margins were also observed. The mean absolute reduction in lung V20Gy was 0.7% (range, 0.2%-1.4%), whereas the mean relative reduction was 25% (range, 14%-42%). The mean MLD over patients’ treatment courses was reduced by an absolute 0.2 to 0.7 Gy (mean relative reduction, 12%-25%). The NTCP for MLD(3Gy), calculated using Equations 2 and 3 (with a TD50 of 20.8 Gy and m of 0.45), showed a 0.8% mean decrease in radiation pneumonitis risk with 3-mm PTV margins (range, 0.1%-2.7%) (Fig 3). For the subset of patients with overlap of the chest wall and the 5-mm margin PTV, use of a 3-mm PTV margin significantly reduced both the median D0.035cc and median D30cc of the chest wall (Table 2).
Table 2.
Organ-at-risk parameters by PTV margin size
| PTV margin |
|||
|---|---|---|---|
| 5 mm |
3 mm |
||
| Parameter | Median (range) | Median (range) | P value |
| Body V50%, cm3 | 93 (31-348) | 65 (20-268) | <.0001 |
| Body V80%, cm3 | 33 (11-147) | 24 (7-117) | <.0001 |
| Lung V20Gy, % | 2.5 (0.5-9.3) | 1.9 (0.3-7.9) | <.0001 |
| MLD, Gy | 2.5 (0.7-5.9) | 2.1 (0.5-5.2) | <.0001 |
| NTCPMLD(3Gy), % | 4.2 (1.8-13.8) | 3.5 (1.7-11.1) | <.0001 |
| Chest wallD0.035cc, Gy* | 51.4 (46.2-54.4) | 51.0 (38.4-52.6) | <.001 |
| Chest wallD30cc, Gy* | 24.9 (15.7-35.5) | 21.8 (13.0-32.1) | <.0001 |
Abbreviations: V50% and V80% = volume of the body receiving at least 50% or 80% of the prescription dose; D0.035cc = dose to 0.035 cm3 of the chest wall (including ribs); D30cc = dose to 30 cm3 of the chest wall (including ribs); V20Gy = percentage of lung receiving at least 20 Gy; MLD = mean lung dose; NTCPMLD(3Gy) = normal tissue complication probability for radiation pneumonitis, calculated using the mean lung dose in 2-Gy equivalents with an α/β of 3; PTV = planning target volume.
Only the 22 patients for whom the 5-mm margin PTV overlapped with the chest wall were included.
Fig. 3.
Normal-tissue complication probability reduction for radiation pneumonitis when planning target volume margins were reduced from 5-mm to 3-mm compared with the internal gross tumor volume. MLD(3Gy) is the mean lung dose in 2 Gy equivalents calculated using an α/β ratio of 3 Gy. The dotted line represents the linear best fit line, with R2 representing the regression coefficient.
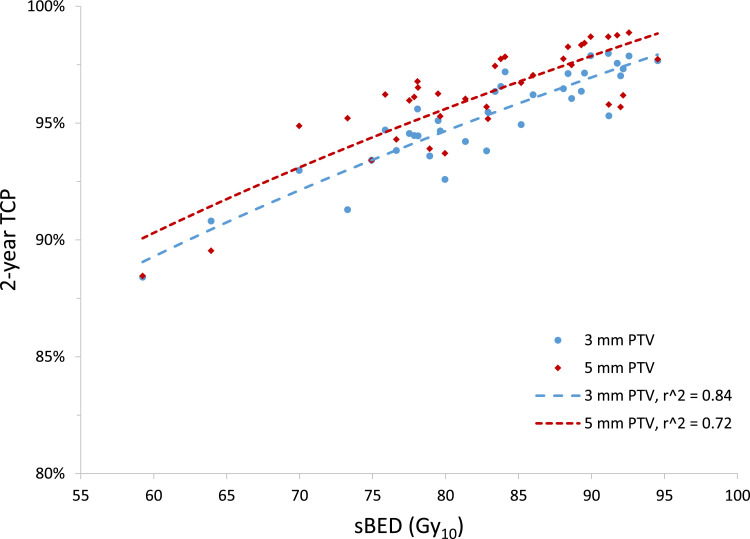
Using our average d-IGTV D99.9% values with Equations 4 and 5, the mean TCP with 5-mm PTV margins was 96.1%, with a mean sBED of 104.5 Gy (range 63.1-138.7 Gy) (Fig 4). For 3-mm PTV margins, the mean TCP was 95.2%, with a mean sBED of 95.5 Gy (range, 63.0-120.4 Gy). We estimated the uncertainty in these TCP calculations to be comparable to the standard deviations of the mean D99.9% values we obtained for each margin size: 7% and 5% for 5-mm and 3-mm PTV margins, respectively.
Fig. 4.
Two-year tumor-control probability, calculated from the mean deformed internal gross tumor volume (d-IGTV) dose covering 99.9% of the d-IGTV that was achieved with 5-mm and 3-mm planning target volume (PTV) margins versus the prescribed size-adjusted biological equivalent dose (sBED). The sBED is defined as the biological equivalent dose minus 10 times the tumor diameter in cm (using the linear quadratic model with an α/β ratio of 10 Gy). Power trendlines for each PTV margin are represented by dotted lines, with r2 representing the regression coefficient.
Discussion
Innovations in image guidance, planning, immobilization, and delivery techniques have allowed SABR to become an excellent treatment option in early-stage lung cancer. Success relies on adequate target coverage and avoidance of geometric misses. PTV margins of 5 mm are generally accepted as the current standard for lung SABR and are being used in modern trial design.4 To our knowledge, this study is the first to validate PTV margin reduction to 3 mm. The most significant difference compared with prior studies was the reduction in treatment time, which can primarily be attributed to the use of VMAT and FFF beams.13,16 Wierzbicki et al calculated that a 5-mm PTV margin covered ≥95% of the target volume ≥95% of the time; however, their mean treatment time was 17.8 minutes.13 Grills et al showed that 5-mm PTV margins were required to adequately account for intrafractional drift observed with treatment times (not reported) required by 6X noncoplanar IMRT.8 Purdie et al showed that mean intrafractional movement was significantly decreased when the interval between localization and repeat CBCT scans was shorter, with a mean time between localization and repeat CBCT scan of 34 minutes.9 However, a subsequent study did not reproduce this result, with a mean time of 25.9 minutes.15 Vloet et al investigated 6X noncoplanar VMAT versus 6X noncoplanar IMRT for potential margin reduction and reported a mean treatment time of 22 ± 6 minutes for the VMAT cohort.17 They recommended a 3-mm PTV margin only if a midtreatment CBCT scan and repositioning could be performed.17 Although the current evidence for correlating intrafraction motion and treatment time is still inconclusive, we hypothesize that our significantly shorter mean treatment time of 7.9 minutes allows for acceptable IGTV coverage with 3-mm PTV margins (V100% > 95% in 99.3% of fractions) without the requirement for repeat imaging and repositioning, which would increase overall treatment time.
The original van Herk formalism assumes an infinite fraction number and a nondeformed target and therefore is applicable for conventional nonhypofractionated schedules.14 The robustness of the formalism has been experimentally validated for scenarios with a large fraction number and nondeformed target.22 The modified formalism we used for our lung SABR PTV margin calculation (Eq 1) has been validated for lung SABR fractionation.15,18 However, for very small fraction numbers, the formalism may underestimate the required margin.23 Furthermore, no current margin calculation formalisms account for deformation or growth of the target lesion. As such, our use of deformable registration to 144 posttreatment CBCT scans provides a robust validation of 3-mm PTV margins while accounting for small fraction numbers and growth or deformation of the target lesion.
Smaller PTV margins may not be suitable for every patient. For single-fraction treatment, geographic accuracy has higher stakes.7 Using fractionated schedules allows for modest forgiveness in this stringency.7 The mean d-IGTV coverage with 3-mm PTV margins (V100% and D99.9%) was >95% for all patients over 4 fractions; however, 1 fraction for 1 patient did not meet both of these targets. This patient had a challenging body habitus that required plan modifications including an additional arc. When determining which patients can be planned with 3-mm PTV margins, consideration should be taken as to whether any alterations to the standard planning protocol were required, because these variables may intrinsically affect setup, immobilization, or tumor movement. Mitigation strategies could include increased CBCT milliampere-seconds (mAs) to improve image quality for patients with a challenging body habitus, and/or performing a midtreatment CBCT scan for repeat positioning if the treatment duration is expected to exceed standard times owing to planning modifications. Other parameters such as IGTV volume and tumor location did not appear to influence d-IGTV coverage and should not be regarded as contraindications to 3-mm PTV margins in fractionated SABR regimens.
Lung SABR has been associated with serious toxicity, such as radiation pneumonitis, bronchial stricture, esophageal fistula, or pulmonary hemorrhage.24, 25, 26, 27, 28 Smaller PTV volumes have been associated with reduced risk of radiation pneumonitis.29 A correlation between lung V20Gy and radiation pneumonitis risk in lung SABR has been identified in multiple studies.29,30 Barriger et al observed a 4.3% risk of grade 2 to 4 radiation pneumonitis when lung V20Gy was ≤4% versus 16.4% when V20Gy was >4%.30 Matsuo et al found a 15% risk of symptomatic radiation pneumonitis when V20Gy was <5.8% and a 42.9% risk when V20Gy was ≥5.8%.29 In this study's patient population, with the original 5-mm PTV margins, the median lung V20Gy was 2.5%. Of 8 patients with V20Gy of >4% and 3 patients with V20Gy of ≥5.8%, 4 and 2 patients, respectively, met the corresponding cutoff when planned with 3-mm PTV margins. The 4 patients who did not meet the lung constraint of V20Gy of ≤4% with 3-mm PTV margins had larger IGTV volumes than the mean of 8.97 cm3 in the study, and 3 of these patients had the largest IGTV volumes in our study population (23.7, 37.9, and 52.2 cm3, respectively). The patients with IGTV volumes ranging from 9.96 to 17.7 cm3 were the patients who met the lung V20Gy constraint after the margin reduction. When the NTCP for radiation pneumonitis was analyzed, it showed that the larger the IGTV, the greater the radiation pneumonitis risk reduction when PTV margins were reduced to 3 mm (regression coefficient, 0.724). These data all indicate that with smaller PTV margins, lower toxicity rates can be expected for patients with large IGTV volumes. However, it must be noted that these NTCP predictions are subject to significant uncertainties resulting from contouring variability, daily patient setup, linear accelerator output, patient-specific factors, and the NTCP model itself. As such, it is unlikely that the 0.8% mean reduction in NTCP reported here will translate into clinically significant reductions in lung toxicity rates for most patients receiving lung SABR.
The chest wall is rarely a dose-limiting structure for lung SABR; however, rib fractures and chest wall pain are clinically relevant toxicities for patients with lesions in close proximity to, or invading, the chest wall.31,32 Chest wall toxicity can be mitigated by reducing the maximum dose and the dose to absolute volumes.31,32 In our subset analysis of 22 patients who had chest wall overlap with the original 5-mm-margin PTV, reducing PTV margins to 3 mm significantly reduced both the D0.035cc and D30cc to the chest wall. In our institution, PTV coverage is prioritized over chest wall dose. For the current study population with 5-mm PTV margins, 18 patients exceeded our constraint of a D0.035cc of <50 Gy, and 1 patient exceeded our constraint of a D30cc of <34 Gy.31,32 With 3-mm PTV margins, the number of patients exceeding D0.035cc and D30cc constraints was reduced to 14 and 0, respectively.
When using Ohri et al's TCP model with our achieved mean d-IGTV D99.9% values, we anticipate excellent 2-year local control rates with both margins (96.1% and 95.2% with 5-mm and 3-mm PTV margins, respectively).21 To use this model, we assumed tumor volumes to be spherical, which introduced a margin of error into this calculation. Furthermore, the SDs on the mean d-IGTV D99.9% values we obtained for each margin size were approximately 5% to 7%. We suspect the TCP difference between margins was therefore unlikely to be clinically significant owing to its small magnitude and the aforementioned sources of uncertainty; however, additional data and analysis would be required to support this suspicion. Conduits of actuarial 2-year local control in lung SABR have been published in multiple studies including one by Ricardi et al, who observed a 2-year local progression-free survival rate of 92.7% using 45 Gy in 3 fractions.33 Haasbeek et al observed a 2-year local control rate of 94.3% using multiple fractionation schemes (60 Gy in 3 fractions, 60 Gy in 5 fractions, or 60 Gy in 8 fractions).34 Timmerman et al observed a 3-year primary tumor control rate of 97.6% using 54 Gy in 3 fractions.1 Timmerman et al's higher observed local control rate may be in part secondary to the higher BED10 of their prescribed dose (151 Gy BED10 for 54 Gy in 3 fractions vs 106 Gy BED10 for 48 Gy in 4 fractions).35 When OAR constraints dictate use of less hypofractionated schedules, reduced PTV margins may allow for improved ability to meet OAR constraints and consequently allow for increased BED10 delivery to the tumor.36 This may in turn result in improved outcomes for these patients, as increased BED10 correlates with improved 2-year local control.21,35
The current study population was treated at a single institution, which allowed for control of certain variables such as treatment energy and delivery as well as immobilization types; however, this limited the sample size and may have selected for a less diverse population. In addition, not all centers complete posttreatment CBCT scans because these scans only provide retrospective information on patient setup and are not a standard of practice, regardless of margin size. Posttreatment CBCT scans were integral to this study, which further limited the sample size. In general, posttreatment CBCT scans are felt to be representative of the worst-case intrafractional motion, but we acknowledge that using them to assess target coverage also introduces uncertainty because, as in reality, tumor motion and actual treatment position are more dynamic.
Using deformable registration comes with additional limitations, some of which are innate to this software, such as inaccuracy owing to tissue homogeneity or mass variation.37 Because of our volume of data, each d-IGTV was independently reviewed by 1 clinician and 1 medical physicist with respect to the CBCT scan and the original IGTV, but residual deformation errors cannot be excluded from the study. Deformable registration was completed for the region around the PTV, as it was not possible to complete it for the entire body contour on each fraction owing to CBCT field-of-view limitations. This should be noted when interpreting this study's body V50% and V80% and OAR data, which represent the planned doses, not the actual doses received, accounting for setup and intrafraction motion.
Conclusion
By analyzing posttreatment CBCT scans for 36 patients receiving lung SABR, we have shown that with modern treatment delivery and immobilization techniques, 3-mm PTV margins maintain acceptable IGTV coverage. Margin reduction treats less normal tissue, modestly lowering the risk of toxicity such as radiation pneumonitis. We anticipate that a 2-year TCP of >95% would be maintained. Future research directions include analyzing the benefit of margin reduction on the fractionation required as well as the dose reduction for other lung SABR OARs. In addition, with the increasing use of SABR for oligometastases, the benefit from margin reduction for patients with multiple lesions is an interesting topic for future research.
Footnotes
Sources of support: This work had no specific funding.
Disclosure: Dr Olson reports grants from Varian Medical Systems outside the submitted work. No other disclosures were reported.
Supplementary material for this article can be found at https://doi.org/10.1016/j.adro.2021.100750
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.adro.2021.100750.
Appendix. Supplementary materials
References
- 1.Timmerman R, Paulus R, Galvin J. Stereotactic body radiation therapy for inoperable early stage lung cancer. J Am Med Assoc. 2010;303:1070–1076. doi: 10.1001/jama.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ricardi U, Badellino S, Filippi AR. Stereotactic radiotherapy for early stage non-small cell lung cancer. Radiat Oncol J. 2015;33:57–65. doi: 10.3857/roj.2015.33.2.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palma D, Visser O, Lagerwaard FJ, Belderbos J, Slotman BJ, Senan S. Impact of introducing stereotactic lung radiotherapy for elderly patients with stage I non-small-cell lung cancer: A population-based time-trend analysis. J Clin Oncol. 2010;28:5153–5159. doi: 10.1200/JCO.2010.30.0731. [DOI] [PubMed] [Google Scholar]
- 4.Swaminath A, Wierzbicki M, Parpia S. Canadian phase III randomized trial of stereotactic body radiotherapy versus conventionally hypofractionated radiotherapy for stage I, medically inoperable non–small-cell lung cancer—Rationale and protocol design for the Ontario Clinical Oncology Group (OCOG)-LUSTRE Trial. Clin Lung Cancer. 2017;18:250–254. doi: 10.1016/j.cllc.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 5.Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman JR, Bharat A. Non-Small Cell Lung Cancer, NCCN Clinical Practice Guidelines in Oncology, Version 3.2020. J Natl Compr Cancer Netw. 2020;3 doi: 10.6004/jnccn.2022.0025. [DOI] [PubMed] [Google Scholar]
- 6.Potters L, Kavanagh B, Galvin JM. American Society for Therapeutic Radiology and Oncology (ASTRO) and American College of Radiology (ACR) practice guideline for the performance of stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys. 2010;76:326–332. doi: 10.1016/j.ijrobp.2009.09.042. [DOI] [PubMed] [Google Scholar]
- 7.Bissonnette J-P, Purdie TG, Higgins JA, Li W, Bezjak A. Cone beam computed tomographic image guidance for lung cancer radiation therapy. Int J Radiat Oncol Biol Phys. 2009;73:927–934. doi: 10.1016/j.ijrobp.2008.08.059. [DOI] [PubMed] [Google Scholar]
- 8.Grills IS, Hugo G, Kestin LL. Image-guided radiotherapy via daily online cone-beam CT substantially reduces margin requirements for stereotactic lung radiotherapy. Int J Radiat Oncol Biol Phys. 2008;70:1045–1056. doi: 10.1016/j.ijrobp.2007.07.2352. [DOI] [PubMed] [Google Scholar]
- 9.Purdie TG, Bissonnette J-P, Franks K. Cone-beam computed tomography for on-line image guidance of lung stereotactic radiotherapy: Localization, verification, and intrafraction tumor position. Int J Radiat Oncol Biol Phys. 2007;68:243–252. doi: 10.1016/j.ijrobp.2006.12.022. [DOI] [PubMed] [Google Scholar]
- 10.Chang JY, Dong L, Liu H. Image guided radiation therapy for non-small cell lung cancer. J Thorac Oncol. 2008;3:177–186. doi: 10.1097/JTO.0b013e3181622bdd. [DOI] [PubMed] [Google Scholar]
- 11.Ezhil M, Vedam S, Balter P. Determination of patient-specific internal gross tumor volumes for lung cancer using four-dimensional computed tomography. Radiat Oncol. 2009;4:1–14. doi: 10.1186/1748-717X-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brandner ED, Chetty IJ, Giaddui TG. Motion management strategies and technical issues associated with stereotactic body radiotherapy of thoracic and upper abdominal tumors: A review from NRG Oncology. Med Phys. 2017;44:2595–2612. doi: 10.1002/mp.12227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wierzbicki M, Mathew L, Swaminath A. A method for optimizing planning target volume margins for patients receiving lung stereotactic body radiotherapy. Phys Med Biol. 2018;63 doi: 10.1088/1361-6560/aadf26. [DOI] [PubMed] [Google Scholar]
- 14.van Herk M, Remeijer P, Lebesque JV. The probability of correct target dosage: Dose-population histograms for deriving treatment margins in radiotherapy. Int J Radiat Oncol Biol Phys. 2000;47:1121–1135. doi: 10.1016/s0360-3016(00)00518-6. [DOI] [PubMed] [Google Scholar]
- 15.Li W, Purdie TG, Taremi M. Effect of immobilization and performance status on intrafraction motion for stereotactic lung radiotherapy: Analysis of 133 patients. Int J Radiat Oncol Biol Phys. 2011;81:1568–1575. doi: 10.1016/j.ijrobp.2010.09.035. [DOI] [PubMed] [Google Scholar]
- 16.Yan Y, Yadav P, Bassetti M. Dosimetric differences in flattened and flattening filter-free beam treatment plans. J Med Phys. 2016;41:92–99. doi: 10.4103/0971-6203.181636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vloet A, Li W, Giuliani M. Comparison of residual geometric errors obtained for lung SBRT under static beams and VMAT techniques: Implications for PTV margins. Phys Med. 2018;52:129–132. doi: 10.1016/j.ejmp.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 18.Sonke J-J, Rossi M, Wolthaus J, van Herk M, Damen E, Belderbos J. Frameless stereotactic body radiotherapy for lung cancer using four-dimensional cone beam CT guidance. Int J Radiat Oncol Biol Phys. 2009;74:567–574. doi: 10.1016/j.ijrobp.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 19.Borst GR, Ishikawa M, Nijkamp J. Radiation pneumonitis after hypofractionated radiotherapy: Evaluation of the LQ(L) model and different dose parameters. Int J Radiat Oncol Biol Phys. 2010;77:1596–1603. doi: 10.1016/j.ijrobp.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 20.Lyman JT. Complication probability as assessed from dose-volume histograms. Radiat Res Suppl. 1985;8:S13–S19. [PubMed] [Google Scholar]
- 21.Ohri N, Werner-Wasik M, Grills IS. Modeling local control after hypofractionated stereotactic body radiation therapy for stage I non-small cell lung cancer: A report from the Elekta Collaborative Lung Research Group. Int J Radiat Oncol Biol Phys. 2012;84:e379–e384. doi: 10.1016/j.ijrobp.2012.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ecclestone G, Bissonnette JP, Heath E. Experimental validation of the van Herk margin formula for lung radiation therapy. Med Phys. 2013;40 doi: 10.1118/1.4824927. [DOI] [PubMed] [Google Scholar]
- 23.Gordon JJ, Siebers JV. Convolution method and CTV-to-PTV margins for finite fractions and small systematic errors. Phys Med Biol. 2007;52:1967–1990. doi: 10.1088/0031-9155/52/7/013. [DOI] [PubMed] [Google Scholar]
- 24.Haseltine JM, Rimner A, Gelblum DY. Fatal complications after stereotactic body radiation therapy for central lung tumors abutting the proximal bronchial tree. Pract Radiat Oncol. 2016;6:e27–e33. doi: 10.1016/j.prro.2015.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stephans KL, Djemil T, Diaconu C. Esophageal dose tolerance to hypofractionated stereotactic body radiation therapy: Risk factors for late toxicity. Int J Radiat Oncol Biol Phys. 2014;90:197–202. doi: 10.1016/j.ijrobp.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 26.Timmerman R, McGarry R, Yiannoutsos C. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol. 2006;24:4833–4839. doi: 10.1200/JCO.2006.07.5937. [DOI] [PubMed] [Google Scholar]
- 27.Tekatli H, Haasbeek N, Dahele M. Outcomes of hypofractionated high-dose radiotherapy in poor-risk patients with “ultracentral” non-small cell lung cancer. J Thorac Oncol. 2016;11:1081–1089. doi: 10.1016/j.jtho.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 28.Thompson M, Rosenzweig KE. The evolving toxicity profile of SBRT for lung cancer. Transl Lung Cancer Res. 2018;8:48–57. doi: 10.21037/tlcr.2018.10.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsuo Y, Shibuya K, Nakamura M. Dose-volume metrics associated with radiation pneumonitis after stereotactic body radiation therapy for lung cancer. Int J Radiat Oncol Biol Phys. 2012;83:e545–e549. doi: 10.1016/j.ijrobp.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 30.Barriger RB, Forquer JA, Brabham JG. A dose-volume analysis of radiation pneumonitis in non-small cell lung cancer patients treated with stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys. 2012;82:457–462. doi: 10.1016/j.ijrobp.2010.08.056. [DOI] [PubMed] [Google Scholar]
- 31.Andolino DL, Forquer JA, Henderson MA. Chest wall toxicity after stereotactic body radiotherapy for malignant lesions of the lung and liver. Int J Radiat Oncol Biol Phys. 2011;80:692–697. doi: 10.1016/j.ijrobp.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 32.Dunlap NE, Cai J, Biedermann GB. Chest wall volume receiving >30 Gy predicts risk of severe pain and/or rib fracture after lung stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys. 2010;76:796–801. doi: 10.1016/j.ijrobp.2009.02.027. [DOI] [PubMed] [Google Scholar]
- 33.Ricardi U, Filippi AR, Guarneri A. Stereotactic body radiation therapy for early stage non-small cell lung cancer: Results of a prospective trial. Lung Cancer. 2010;68:72–77. doi: 10.1016/j.lungcan.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 34.Haasbeek CJA, Lagerwaard FJ, Antonisse ME, Slotman BJ, Senan S. Stage I nonsmall cell lung cancer in patients aged ≥75 years: Outcomes after stereotactic radiotherapy. Cancer. 2010;116:406–414. doi: 10.1002/cncr.24759. [DOI] [PubMed] [Google Scholar]
- 35.Park S, Urm S, Cho H. Analysis of biologically equivalent dose of stereotactic body radiotherapy for primary and metastatic lung tumors. Cancer Res Treat. 2014;46:403–410. doi: 10.4143/crt.2013.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ahmed N, Hasan S, Schumacher L, Colonias A, Wegner RE. Stereotactic body radiotherapy for central lung tumors: Finding the balance between safety and efficacy in the “no fly” zone. Thorac Cancer. 2018;9:1211–1214. doi: 10.1111/1759-7714.12764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oh S, Kim S. Deformable image registration in radiation therapy. Radiat Oncol J. 2017;35:101–111. doi: 10.3857/roj.2017.00325. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.