Abstract
Fetal bovine serum (FBS) is a widely used growth supplement in the in vitro culturing of animal and human cells, tissues and organs, notably due to the occurrence of abundant micro- and macronutrients, along with growth factors. Over the years, increasing demand, high price, batch-to-batch variability in quality and composition, increasing ethical concerns lead to the search for an alternative to FBS. Several approaches have been suggested and employed in the past, but none is implemented as widely as FBS, and each supplement has its own disadvantages. In this review, we described the importance of FBS in cell culture, discussed the issues associated with FBS use and presented the efforts made in the recent past to reduce or replace FBS. The potential of four different alternative sources to FBS, namely, bovine ocular fluid, sericin protein, human platelet lysate and earthworm heat inactivated coelomic fluid was evaluated. In the end, we present the conceptual perspective using the Human Platelet Lysate (HPL) and earthworm Heat Inactivated Coelomic Fluid (HI-CF) combination to alternate FBS and its context in scientific and economic impacts.
Keywords: Fetal bovine serum, Animal cell culture, Human platelet lysate, Coelomic fluid, Earthworm, Alternative to FBS
Fetal bovine serum, Animal cell culture, Human platelet lysate, Coelomic fluid, Earthworm, Alternative to FBS
1. Introduction
Since the establishment of the cell culture technique, the technique itself becomes a vital and significant tool in biological research (modelling disease, stem cell and cancer biology), reproductive technology (IVF- In vitro fertilization), biotechnology and pharmaceutical production units (antibodies, regenerative medicine, vaccine and other therapeutic production) [1, 2]. Importantly, the experiment that can only be performed in the living system have been replaced with cell culture as of now.
In this cell culture, the media and its supplements play an important role. Notably, the major supplement of basal media is animal-derived serum, which serves as a source for hormones, growth factors, amino acids, proteins, vitamins, inorganic salts, trace elements, carbohydrates, lipids, etc for cellular metabolisms and growth of cells [3]. In the 1950s, when FBS was introduced in cell culture, it was thought that FBS would only stimulate the cell growth [4]. Later it was identified that FBS has essential components required for cell attachment, proliferation and maintenance, such as serum albumin, fetuin [5], hormones, vitamins, trace elements, growth factor, etc., [6, 7]. Notably, compared to sera obtained from other sources, such as horse and calf serum, FBS is richer in growth factors and contains low level of γ-globulin (a cell growth inhibitor) [3, 8]. The serum contains approximately 1800 proteins [9, 10] and it also has more than 4000 metabolites [11]. In addition to that, the serum helps to improve the pH buffering capacity of the medium and reduce the physical damage caused by pipette manipulation and stirring [12].
Due to the above-mentioned advantages, FBS has already become a universal supplement. Therefore, not only FBS production increased but its demand also increased simultaneously. Meantime, some serious debate was formed over the usage of FBS, scientifically as well as ethically [13, 14]. Specifically, increasing demand for FBS leads to several issues and it is listed in the Table 1. Indeed, in vitro culturing technique majorly contributed to our understanding of cell biology to various drug discoveries over several decades. Although FBS is from the meat industry, blood is obtained from the foetuses if the slaughtering cattle are pregnant. Annually, there are more than 2 million bovine foetuses used worldwide to produce approximately 800,000 L of FBS [15]. It is not only criticised with the ethical concern but also in other ways of questioning the value of in vitro techniques. Nonetheless, we cannot compromise with such techniques because of their significant role in biological research and pharmaceutical production. But we can try to replace FBS with a suitable alternative. Therefore, moving towards finding a suitable alternative will be the only key to resolving the problem.
Table 1.
Issues associated with FBS and its classification.
| S. No | In market | Scientific | Moral |
|---|---|---|---|
| 1 | Unpredictable shortage in the supply of FBS [13, 95]. | Undefined composition of FBS [96]. | The worldwide increasing number of bovine fetus used and killed only for collecting FBS [97]. |
| 2 | Increasing the price of FBS (it was increased above 300% in the last few years as of 2016) [98]. | It can be contaminated with prion proteins [99], endotoxins [100], different kinds of microbes, immunoglobulin and viruses [101]. | Fetal distress during blood collection to prepare FBS leads to high anger among animal welfare and ethical association around the world [102]. |
| 3 | It creates a business opportunity in the loosely regulated market that leads to the exploitation of FBS [8, 97]. | The variation between the brands [103] seasonal (winter and spring) and geographical batch-to-batch variation in the quality (variation in composition, possible contamination specific to season and geographic) of FBS questioning the experimental reproducibility [96]. | - |
| 4 | - | It could be interfering with phenotypic cell stability and influence the experimental outcome [104, 105]. | - |
| 5 | - | The presence of non-human N-glycolylneuraminic acid (Neu5Gc) in the animal serum may promote chronic inflammation and influence tumor progression and vascular inflammation [106], which should have been excluding the usage of FBS in human embryonic stem cell (ES) for cellular therapy [107], yet, it is widely used [14]. | - |
2. Historical aspects and essential components of basal media
In cell culture technology, the culture media is an important factor for cell survival, cell growth and proliferation. However, the development of stable media was formulated by a series of research. Initially, the composition of salt solution was formulated which included only inorganic salt, occasionally glucose was added as a nutrient. For the first time, animal tissue has been cultured in vitro, briefly the heart of the frog was kept in the balanced salt solution and the pumping of the heart was observed. Balanced salt solution was developed by Sydney Ringer in 1882 and named as ringer's solution. Following the ringer's solution, several balanced salt solutions were developed namely, Locke's solution, Tyrode's solution, The Kerbs ringer bicarbonate solution, Gey's solution, Earle's solution and Hank's solution [12]. In the period of 1907–1912, researchers tried culturing of cells with lymph fluid for several weeks [16] and blood plasma [17] for several months. Subsequently, adding embryonic extract through blood plasma dramatically boost the cellular proliferation [18, 19]. Even then, it was not rightly helpful to maintain and proliferate primary cell cultures until the FBS came into the picture. Nevertheless, later it came to know that salts, amino acids, vitamins, hormones and glucose are the important component for cellular metabolism [20, 21]. Based on that knowledge, chemically defined media 199, EM media, DMEM media were developed with essential and non-essential amino acids to culture various types of cells under in vitro condition [22]. However, the inclusion of FBS in cell culture provides various advantages, yet the overall circumstances surrounded around FBS leads to various outrages and its further precedence to searching for a suitable alternative.
3. Efforts made to reduce or replace FBS
Worldwide researchers make efforts to reduce and replace the FBS from different sources like biological fluid or components, by-products of industry and combination of different chemicals. There are 260 unique cell types that can be cultured by using the serum-free media and media formulated from the literature that is given and regularly updated in the https://fcs-free.org/fcs-database [23]. However, there is no single alternate or chemically defined media or even the formulation of a group of components that are capable of replacing FBS to culture all possible cell types.
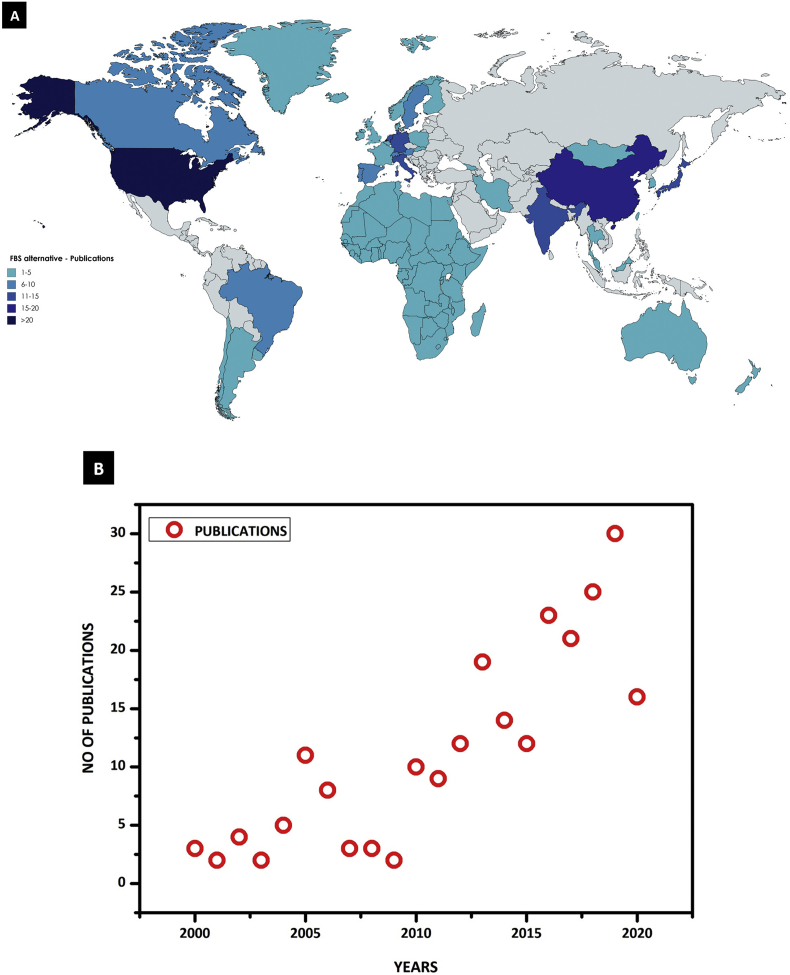
Surprisingly, global studies on the alternative to FBS are rather limited. Therefore, which is important to show the statistic on an alternative that will alert the scientific community for the importance of the need for a sustainable alternative. Totally there are 246 articles were published from 1979 to till date and the majority of the articles published by the USA (41), China (16), Italy (15) and followed by many other countries (Key word: FBS alternative in cell culture) which are shown in the Figure 1A. However, most of the work carried out in the last two decades was shown in the Figure 1B, which does not include any serum-free chemically defined media. Noticeably, according to the PubMed database (Key word: Serum free chemically defined media in cell culture) around 603 publications were published with the usage of serum-free chemically defined media between the years of 1961–2020. This data shows that a lot of research has to be done on developing a sustainable alternative for FBS, since it has a commercial value.
Figure 1.
(A) World-wide Scenario publications on serum alternatives: 1–5 = Africa (01), Ireland (01), Greece (01), Norway (01), Iceland (1), Argentina (01), Mongolia (01), Uruguay (1), Chile (02), Poland (02), Finland (02) and Slovenia (2), New Zealand (2), Australia (03), Thailand (3), Belgium (03), Taiwan (3), South Korea (04), Malaysia (4), Georgia (4), UK (05), Denmark (05), Iran (05) and France (5) = 62; 6–10 = Switzerland (6), Sweden (06), Spain (06) and Portugal (06), Brazil (09), Austria (09) and Canada (10) = 52; 11–15 = India (11), Germany (12), Netherland (12), Japan (14) and Italy (15) = 64; 16–20 = China (16) = 16 and >20 = US (41) = 41. (B) List of publications on alternatives to FBS in cell culture between 2000-2020: The number of publications was gradually increased from the year 2000–2015 and the maximum number of the article published in the recent years from 2016 to 2020. Since the total number of papers so far published in this particular period is 233.
Nevertheless, in the last six decades researchers have been trying to alternate FBS with different sources include hormones [24], pituitary extracts, chick embryo extracts, bovine milk fractions, bovine colostrum or platelet lysates [14], bovine ocular fluid [25], fish serum [26], plant origin [27] and sericin protein [28], other protein fractions from plant extracts, called vegetal serum [29] and earthworm coelomic fluid [30]. However, the serum-free chemically defined media is the good one for clinical and pharmaceutical productions since it is specific to every cell type [31]. The clinical and pharmaceutical products also carried out in the specific cell line needs; therefore, they can be used based on chemically defined media suitable for them. The advantage of chemically defined media is, its components and their concentrations are well defined. Also it is completely free from serum and animal-derived or a human-derived products including albumin [32]. But different cells need different requirements for cell survival, growth and differentiation. Therefore, the expensive chemically defined media will not be a feasible option for all other research purposes. Hence, a universal serum-free cell culture medium may not be achievable. But here, we explain some of the possible sources which can have the potential to alternate FBS as a combination or the other way and we gave our perspective on how to use these sources to culture most of the cells and thereby reduce the usage of FBS.
3.1. Bovine ocular fluid
The ocular fluid is the meat industry product and the fluid is collected from the eye of bovine within 6 h after the slaughtering. The combination of defibrinated 35% of sheep's plasma and 1.5% human serum albumin and mainly 63.5 % of bovine ocular fluid known as serum replacement (SR-2.05), required 8–9% for the culture of chicken embryonal fibroblasts. Similarly, Human Bone Marrow Fibroblasts (BMF182), Vero cells (Vervet Monkey Kidney) and WISH (Wistar Institute Human Amniotic Cells) grow much better and quicker in bovine ocular fluid when compared to FBS [27]. Similar to FBS, ocular fluid also contains fibronectin and hypoxanthine, insulin-like growth factor [33], vascular endothelial growth factor [34], S-100 (21-kDa acidic-Ca-binding protein) [35], which will help for cell attachment and proliferation.
In addition, reports also suggest that instead of FBS, buffalo ocular fluid can be used for the cryopreservation of mouse embryonic stem cells, and some primary cells such as mouse embryonic fibroblast cells, human peripheral blood mononuclear cells, and mouse bone marrow cells [36]. Moreover, it is also feasible to use instead of FBS for immature rodent and buffalo testis germplasm cryopreservation [37]. However, whether the ocular fluid-based supplement works for versatile cells, needs to be checked. Notably, even if the ocular fluid-based supplement may help to culture versatile cell types, yet here the concern, it cannot meet the required volume due to its limited quantities [38].
3.2. Sericin protein
Sericin is a glue protein of silk. This hydrolysed sericin derived from the raw silk during the degumming process from silkmoth Bombyx mori cocoon [39]. The sericin is discarded during filature production, but it can be developed as a by-product. This sericin protein boosts the cell attachment [40, 41] of human skin fibroblast [42] and growth of mouse fibroblasts [43] due to its mitogenic capacity. Researchers suggested that amino acids cysteine and methionine can help to promotes cellular proliferation as well as collagen production [44, 45] and enhances cellular proliferation [39, 46]. For example, the silk sericine extracted by heat with variable amino-acid content, The Thai native silk strains, Chul 1/1 (bivoltine, white shell) has the most methionine and cysteine content compare to other species and it promote the cellular growth and the collagen synthesis in the mouse fibroblast cell line (L929) and the sericin silk protein also not toxic to the same cell line [47]. The wound healing property of the methionine and cysteine also reported in the rat model [48]. Equally, it was reported that several human cell lines such as human marrow stromal cells (hMSCs), endothelial cells, T-lymphocytes, and hybridomas [49, 50]. HepG2, HeLa, murine hybridoma 2E3-O, human embryonal kidney 293 cells [39] and insect cell [47] were cultured using the sericin supplements. Moreover, the effect of sericin in cellular morphology, survival, proliferation capacity, cell cycle and transcriptomic analysis revealed that there is no significant difference between FBS and sericin in the Chinese hamster ovary (CHO) cells, African green monkey kidney (MARC-145) cells and HeLa cell lines, respectively [28]. Additionally, it has been reported that sericin can be used for cryopreservation of mammalian cells [51] and murine spermatogonial stem cells [52].
3.3. Human platelet lysate (HPL)
Blood platelets or thrombocytes having strong mitogens include platelet-derived growth factor, fibroblast growth factor, insulin-like growth factor, epidermal growth factor and various other growth factors like vascular endothelial growth factor, connective tissue growth factor [53], chemokines [54], cytokines and array of α-granule factors. During the clotting process, physiological activation of platelet releases these factors which help to promote cell stemness, migration [55], attachment, growth and proliferation in vitro [56]. It was well demonstrated that HPL support suspension as well as anchorage-dependent cells [57]. The large-scale, cost-effective, standardized manufacturing and the preparation of HPL are properly reviewed [53] and mainly donor thrombocytes from blood banks are easily accessible. The HPL helps to avoid the risks of xeno-immunization. Therefore, it is mainly used in the clinical setting for culturing the bone marrow mesenchymal stem cells [58], regenerative medicine [59] and immune cell therapies (Chimeric antigen receptor T cells) [60]. Compared to FBS, HPL is a better supplement to culture the human adipose tissue-derived stem cells which have a higher proliferation rate without compromising its genomic stability and differentiation capacity [61]. Interestingly, it can be used to culture primary human macrophages and human mesenchymal stem cells [62]. A recent study supports HPL supplement as a potential alternative for FBS to culture Vero and Hep-2 cells [63]. Like FBS, the composition and concentration of growth factors present in the human platelet lysate may vary, depending on the activating stimulus, preparation procedure and most importantly collected from different donors [55].
3.4. Earthworm heat inactivated coelomic fluid (HI-CF)
The celomic fluid (CF) circulates throughout earthworm body segments to distribute the nutrient and also act as a carrier for immune cells. Generally, in mammals or other higher animals’ immune cells are present in the blood. Though earthworm has the circulatory system, immune cells like various types of leukocytes are present in the coelomic fluid even some of that reflects vertebrate leukocytes [64]. Remarkably, CF contains plasma and a watery matrix which is similar to serum [65]. Interestingly, earthworm CF have several metabolites including amino acids, peptides, growth hormones, etc., which is important for cell growth and proliferation [66]. Besides, it has been reported the presence of sodium, potassium, calcium and chloride in earthworm CF [67]. Moreover, coelomic fluid of earthworms, Eudrilus eugeniae [68], Eisenia spp., [69] and Lumbricus spp. [70], are rich in riboflavin, which is essential for cellular proliferation [71, 72], promotes the neural cell proliferation [73] and neural stem cell differentiation in cell culture, respectively [74]. Additionally, CF is used as a supplement in plant tissue culture [75]. Importantly, the CF has the mitogenic effect and it has been demonstrated in the suspension cells of mouse and human lymphocytes [76]. The possible mitogenic factor is CMF (coelomic 5itogenic factor) which is one of the semi-purified fraction of CF and the size is 60-kDa [77]. These indications suggest that CF shall be an alternative for FBS in cell culture. But in the case of the adherent cell, the presence of fibrinolytic enzymes in CF does not allow the cells to attach and grow [78]. Nonetheless, the crude CF has a toxic effect on cells and interestingly our group [30] recently encounter and evaluated the feasibility of using 10% heat-inactivated coelomic fluid (HI-CF) from the earthworm, P. excavatus, for culturing mouse primary fibroblast cells and HeLa cell lines without FBS. The cells supplemented with HI-CF were grown well and show a similar growth rate and better cellular viability when compared to FBS. Notably, there is no cellular and molecular morphological changes were observed, while using HI-CF as an alternative supplement for FBS in cell culture experiments. Therefore, the HI-CF supplement could be a suitable alternative for FBS to culture adherent and suspension cells. However, HI-CF lacks the cell adherent factors like fibronectin, hence for initial cell attachment, it needs cell adherent factors to be become an efficient alternative to FBS.
4. Future perspectives
In this review, we have discussed four potential supplement sources and each source directly helps to culture different types of cells but unlikely to be adopted widely as a alternative for FBS. Searching an alternative for FBS in higher animals won't satisfy our expectations in terms of ethical issues associated with harvesting serum or other potential fluid. As an alternative, we can use industrial by-product like ocular fluid, sericin protein and fish serum, or immensely available invertebrate fluids, etc. In case of ocular fluid, which is a by-product from meat industry contains all the necessary factors for cellular attachment and growth but the availability is very limited and as a whole it cannot satisfy the demand and required quantity. Similarly, the fish serum is also one among the good abounded sources from the fish exporting industry around the world, but the mixture of various species, geographical and seasonal variation, and the different types of processing ultimately leads to variation in the undefined composition and the quantity. So, it leads to questioning in the reproducibility of the cell culture experiments. In the case of HPL, it has the mitotic factors and other necessary factors for cellular growth and many different cells were cultured [79, 80]. But it is mainly needed for clinical and health care purposes. Therefore, the scientific community cannot compromise the healthcare needs by occupying the HPL in such a volume as required.
Whatever biological fluid, researchers intended to use, which will definitely show variation in the composition of the micro and macronutrients similar to FBS. Though different types of serum-free chemically defined media are formulated for culturing different types of cells, but they are good for clinical therapies and pharmaceutical production, since they are cells specific and not cost-effective. Therefore, we cannot completely replace the FBS with chemically defined media or supplements like sericin and many others as well.
Indeed, to solve this issue, the supplement source should be vast and it should not violate the ethical concerns and the scientific reproducibility. In the meantime, if the source creates economic development, it will be much appreciated. In this context, earthworm P. excavates coelomic fluid can be a better option. Earthworm, P. excavatus is a tropical worm normally found in India, which has a vast reproductive capacity and it is mainly used in the vermicomposting industry around the world. The collection of the coelomic fluid is very simple and it won't cause any mortality in earthworms which is the most important things to consider and there are no ethical issues associated with earthworms. As well as, coelomic fluid can be collected from the earthworms using non-invasive methods and the processing of HI-CF was discussed clearly in our previous article [30]. Since the preparation is very simple so that it will not show much variability in the composition. Notably, we can use the same worm for the next round of coelomic fluid collection after 15–20 days. As already discussed, HI-CF has a similar effect on cellular growth as like FBS. Nevertheless, one of the positive sides of the FBS is the presence of lower levels of immunoglobulin but remarkably there is no presence of immunoglobulin in the CF, because earthworms are lower invertebrates. Moreover, many reports suggested that earthworm CF has antimicrobial activity [81, 82] and an author suggested that CF of P. excavatus has broad-spectrum antibacterial property and this may help to eliminate the use of antibiotics in the animal cell culture medium [83]. However, the HI-CF activity against microbes needs to be studied. If so, it also helps to improve the culture process by reducing the contamination. However, earthworm HI-CF have many advantages, but it lacks the attachment factors such as fibronectin. Though the suggested four sources are promoting cellular growth in cell culture, we are considering HI-CF as a source for alternative but not suggesting to use directly as an alternative since individual sources having few limitations on their own. Hence, we believe it can be a potential source to produce or develop an alternative one way or the other.
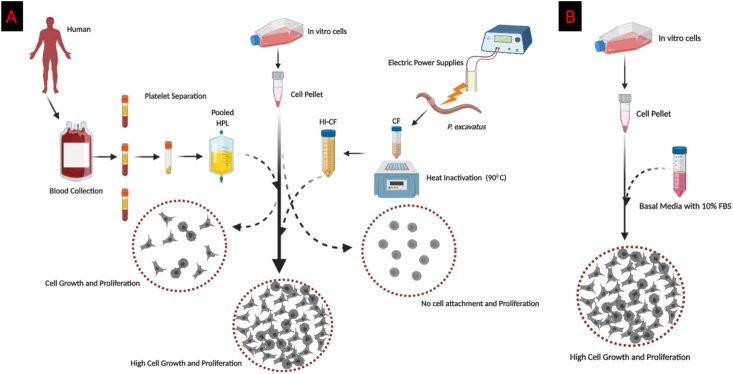
Here we have a perspective that the addition of a factor or a source is required for HI-CF in cell attachment, which could be a potential alternative to FBS. Though the addition of commercially available fibronectin with HI-CF in the cell culture may perhaps help to attach the cells but may not support all cell types to attach in the culture plate and growth of the cells. Notably these commercially available fibronectins also from bovine plasma and another way by biotechnological manufacturing (cloning). Therefore, the addition of commercially available factors cannot rectify our concerns on animals at the same time it will not be a feasible economic option too. Even coating the cell culture plate with materials like collagen, poly-D-lysine, peptides, etc failed to mimic FBS in cell culture. Therefore, the cell attaching material or addition of any single molecule with HI-CF shall not mimic the FBS like state. Based on these arguments, we would like to suggest that the combination of two sources, for example HPL and HI-CF can be a good alternative option. Consider the fact that all required factors for cellular growth available in the HPL and HI-CF but HI-CF is missing only the attachment factor for the cells to attach and grow. Even HPL alone enough for the cell attachment and growth, however, we cannot totally depend on HPL but we can use it in minimal. Since the minimum quantity of HPL is enough to support the attachment of the cell, the HI-CF can takeover further cellular growth and proliferation (Figure 2A) to achieve the standard cellular growth like it in FBS (Figure 2B). We discussed above the presence of all organic and amino acid, electrolyte and vitamins and several other molecules which are present in the CF represent the serum-like state. Though the availability of the HPL is 2×1011/unit in Europe and 3×1011/unit in USA [53], we require HPL in this HPL-(HI-CF) combination could be very minimal perhaps 5–10%. To minimise the use of HPL, the percentage of the HPL in the combination needs to be standardised. For the availability of HI-CF, earthworm P. excavatus not only found in India but also distributed in tropical Asia [84] and African countries [85], since it is commercially produced and highly used for the vermicomposting purpose in tropical and subtropical regions. It is also becoming highly popular in North America too. Time taken to develop from cocoon followed by maturation of worm and producing new cocoon is only 28 days [85]. Therefore, the animal numbers can meet the vast demand when it is needed as a commercial product for the production of HI- CF. Importantly, it will not be a biohazard for the worker when it comes to commercial production since it is widely used in the vermicomposting industry.
Figure 2.
Graphical representation of the vision of alternatives to FBS: With the minimum amount of HPL, cells can attach well and it slightly helps for proliferation. Whereas in the case of HI-CF, cells are not able to attach to the surface therefore there is no proliferation take place. But if we can combine HPL- (HI-CF) with the ratio 1:9 or 2:8 and 10% supplemented it in Basal media which will be enough for the cells to attach and proliferate (A) which will be similar to FBS media (B). Detailed methodology for the preparation of HPL discussed by Burnouf, T et al 2016 and HI-CF [30].
The donor platelets have a shelf-life of five days for the therapeutic purpose and it can retain all the growth factors for three weeks [86]. Even the outdated donor platelets is not showing many losses in its number and the Platelet-derived growth factor concentrations are also well documented [55]. Importantly, certain criteria should be considered before using pooled HPL that include the quality, composition, endotoxin levels and viral and bacterial safety of the HPL product [57]. The mycoplasma contamination can be eliminated by 0.2-micron filtration [87] and by following the mycoplasma elimination protocol. Statistically pooled HPL increases the possibility of risk of viral contamination like human immunodeficiency virus, hepatitis B and C, syphilis, human T-lymphotropic virus and cytomegalovirus [88], However, there are several viral inactivation methods are there to tackle this like heat treatment, nanofiltration, etc., [89, 90]. Yet it is the one of the major concerns on HPL [91] and a recent review discusses about the regulatory requirements, good manufacturing practice of HPL and recommended to release quality control criteria for HPL, including negative test results for pathogen screening, bacterial contamination, mycoplasma and endotoxin [92]. Over all the combination overcomes the things which we discussed about the FBS in Table 1.
In addition, HPL- (HI-CF-90°c) is in (pathogenic and toxin) inactivated form slightly better than heat inactivated FBS, so the presence of endotoxin and toxic virion contamination will be eliminated. Significantly, a combination of these (HPL and HI-CF) two sources has the components that can able to mimic the functional similarity with FBS in cell culture (Table 2) which may perhaps help to provide a better elevation for the replacement or reduction of FBS at least. Similarly, the HI-CF also support the growth of the immortal and diploid cells as discussed previously. Therefore, HPL- (HI-CF) combination will support the growth of all cells in cell culture. Considering this possibility, if further studies are conducted with a combination of two biological fluids like HPL and HI-CF or combination of HI-CF with other possible biological sources, the development of a good alternate may be possible to grow all types of mammalian and other animal cells.
Table 2.
Comparison of HPL and CF's possible components and its functional similarity with FBS.
| S.No | Components | Fetal Bovine Serum (FBS) [7] | Human Platelet Lysate (HPL) | Earthworm Coelomic Fluid (CF) |
|---|---|---|---|---|
| 1. | Serum proteins | Albumin Globulins (Example – Immunoglobulin- IgG) α1-Antitrypsin (Protease Inhibitor) α2-Macroglobulin (Protease Inhibitor) |
Immunoglobulins (IgG), bilirubin, thyroxine, cholesterol, progesterone, testosterone or fibrinogen [103]. | Coelomic cytolytic factor (CCF-1) [108], Fetidins [109],, Extracellular globin-2 and 4 [110]. |
| 2. | Transport proteins | Transferrin, Transcortin, α1-Lipoprotein, β1-Lipoprotein | - | Lysenin-related protein-1 and 2, Gelsolin-like protein- 1 [110]. |
| 3. | Attachment and Spreading Factors | Fibronectin, Laminin Serum Spreading Factor | P-selectin, Von willebrand Factor, Vitronectin, Fibronectin, Fibrinogen, Integrin αllbβ3, Integrin αVβ3 [53]. | - |
| 4. | Enzymes | Lactate Dehydrogenase, Alkaline Phosphatase, γ-Glutamyl Transferase, Alanine Aminotransferase (ALT/GPT), Aspartate Aminotransferase (AST/GOT) | Alkaline phosphatase, lactate dehydrogenase, Aspartate transaminase, Alanine transaminase [111]. | Chloragosomes, phenoloxidase, peroxidase [112], serine protease [113]. |
| 5. | Hormones | Insulin, Glucagon, Corticosteroids, Vasopressin, Thyroid Hormones, Parathyroid Hormone, Growth Hormone, Pituitary Glandotropic Factors, Prostaglandins. | - | Trace elements of plant hormone, auxin, indole-3-acetyl-L-valine [114]. |
| 6. | Growth Factors and Cytokines | Epidermal Growth Factor (EGF), Fibroblast Growth Factor (FGF), Nerve Growth Factor (NGF), Endothelial Cell Growth Factor (ECGF), Platelet-derived Growth Factor (PDGF), Insulin-like Growth Factors (IGFs), Interleukins, Interferons, Transforming Growth Factors (TGFs) | Platelet-derived growth factor, fibroblast growth factor, insulin-like growth, epidermal growth factor and various other growth factors like vascular endothelial growth factor, connective tissue growth factor, Interleukin-8, transforming growth factor-beta (TGF-β) [53]. | Epidermal Growth Factor (EGF), Fibroblast Growth Factor (FGF) [115], Actin-2 [110]. |
| 7. | Fatty Acids and Lipids | Free and Protein-bound Fatty Acids, Triglycerides, Phospholipids, Cholesterol Ethanolamine, Phosphatidylethanolamine | Triglycerides, Cholesterol, High-Density Lipoprotein, Low-Density Lipoprotein [111]. | lysenin, poly (ethyleneglycol) cholesteryl ether (fPEG-Chol) [116]. |
| 8. | Vitamins | Retinol/Retinoic Acid (Vitamin A), Vitamin B-Group: Thiamine Riboflavin Pyridoxine/Pyridoxalphosphate Cobalamin Folic Acid Niacinamide/Nicotinic Acid Panthotenic Acid Biotin Ascorbic Acid (Vitamin C) α-Tocopherol (Vitamin E) | Vitamin D3 [117]. | Vitamin B2 [118]. |
| 9. | Trace Elements | Selenium, Iron, Zinc, Copper, cobalt, chromium, iodine, Fluorine, manga- nese, molybdenum, Vanadium, Nickel and Sn | Sodium, potassium, magnesium, iron, Calcium [109]. | Sodium, potassium, calcium and chloride [67], acetate, fumarate, malonate, malate, formate, succinate [119]. |
| 10. | Carbo-hydrates | Glucose, Galactose, Fructose, Mannose, Ribose, Glycolytic Metabolites | Glucose [53]. | Inositol, glucose, galactose, and N-acetyl-glucosamine [110]. |
| 11. | Nonprotein Nitrogen | Urea, Purines/Pyrimidines, Polyamines, Creatinine, Amino Acids | Urea [109]. | Amino acids like alanine, lysine, etc [66], Adenosine, nicotinamide [119]. |
Certainly, the positive consequence of HPL- (HI-CF) will create a socio-economic impact. Since the vermicomposting industry is the main source for this production, it will rapidly boost the vermicomposting industry directly. Therefore, it will encourage farmers to use biofertilizer instead of chemical fertilizer and make sure the healthy agriculture products. This perspective provides a sustainable alternative to FBS and also potentially influences the sustainable biodiversity conservation, ecology, agriculture and human health. As on the 2016 report, the FBS market was $11,310.9 million in 2015 but it is expected to reach $18,630.7 million by 2020 [93]. In this highly growing vast market, alternative products should consider the 3R concept as a much-needed thing. This is not only to solve the scientific and moral issues on FBS but also to provide the above-mentioned advantages. If we can able to replace the use of FBS at least in basic research in academic and research institution to culture immortal cell lines, diploid cell lines and primary cells, the need for FBS will be reduced by half. In this context, a combination of HPL- (HI-CF) can be a good alternative. Meanwhile, there are 53 of 66 studies obviously utilizes the FBS in the manufacturing of stem cells for human clinical trials, even the presence of Neu5Gc in xenogenic FBS [94]. Though the xenogenic may not influence the clinical output, still we are unable to recommend HPL- (HI-CF) xenogenic mixture for translational research, clinical purpose, pharmaceutical and industrial production, until the clear composition and clinical research relevant to the HI-CF is documented.
5. Conclusion
Until now FBS is an immeasurable supplement for the cell culture experiments. However continuous use of FBS for the long term is not a good way to take forward cell culture. Since FBS is the universal supplement, we are in the position to replace it with a possible way. Though researchers around the world are trying to replace it with different sources like chemically defined media, proteins and growth factors, however, it is unable to mimic the same that FBS does in cell culture. Hence, the alternate has hidden only in the biological or animal fluid. Also, the source may be available in higher animals, but it cannot satisfy our expectations and goals. Subsequently, the extensively available and reproducible animal fluid from the invertebrate could be a potential candidate to alternate FBS. Though earthworm HI-CF has the factors to helps cellular growth, yet the combination of HPL and HI-CF can have the potential to attach, proliferation and the growth of the cells in vitro. The preparation method won't cause any mortality in earthworms and can use the same worm until its life span. Simple preparation and the huge source availability allow us to produce the HPL-(HI-CF) cost-effectively. It will reduce the use of FBS at least in the basic research and create an economic and ecological impact too. Unfortunately, FBS can be replaced only by animal fluids to culture most of the cells in vitro. Therefore, we suggest that HPL-(HI-CF) can be a meaningful option to alternate FBS. However, research needs to be done to validate this expected outcome.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This work was supported by the BIRAC- INDIA (Ref. No. BT/AIR0846/PACE 18/19), DST-SERB-INDIA (Ref. No. ECR/2016/000956), and a Young Scientist Fellowship (No. 12014/78/2020-HR/E-Office:8047109) from DHR/ICMR -New Delhi, INDIA.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Contributor Information
Jackson Durairaj Selvan Christyraj, Email: jacksondurairaj@sathyabama.ac.in.
Johnson Retnaraj Samuel Selvan Christyraj, Email: johnnbt@sathyabama.ac.in.
References
- 1.Leist M., Bremer S., Brundin P., Hescheler J., Kirkeby A., Krause K.H., Pörzgen P., Pucéat M., Schmidt M., Schrattenholz A., Zak N.B., Hentze H. The biological and ethical basis of the use of human embryonic stem cells for in vitro test systems or cell therapy. ALTEX. 2008;25:163–190. [PubMed] [Google Scholar]
- 2.Park D.-H., Eve D.J. Regenerative medicine: advances in new methods and technologies. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2009;15:RA233–R251. [PubMed] [Google Scholar]
- 3.Yao T., Asayama Y. Human preimplantation embryo culture media: past, present, and future. J. Mamm. Ova Res. 2016;33:17–34. [Google Scholar]
- 4.Puck T.T., Cieciura Steven J. Genetics of somatic mammalian cells∗ iii. Long-term cultivation of euploidcells from human and animal subjects. J. Exp. Med. 1958;108:945–956. doi: 10.1084/jem.108.6.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fisher Harold W., Puck Theodore T. Molecular growth requirements of single mammalian cells: the action of fetuin in cell attachment to glass. Proc. Natl. Acad. Sci. U. S. A. 1958;44:4–10. doi: 10.1073/pnas.44.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maurer H.R. Towards chemically-defined, serum-free media for mammalian cell culture. Anim. Cell Cult. 1986:13–31. https://ci.nii.ac.jp/naid/10027323360/en/ [Google Scholar]
- 7.Brunner D., Frank Jürgen, Appl Helmut, Schöffl Harald. Serum-free cell culture: the serum-free media interactive online database. ALTEX-Alt. Anim. Exp. 2010;27:53–62. doi: 10.14573/altex.2010.1.53. [DOI] [PubMed] [Google Scholar]
- 8.Gstraunthaler G., Lindl T., Van Der Valk J. A plea to reduce or replace fetal bovine serum in cell culture media. Cytotechnology. 2013;65:791–793. doi: 10.1007/s10616-013-9633-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson N.L., Polanski M., Pieper R., Gatlin T., Tirumalai R.S., Conrads T.P., Veenstra T.D., Adkins J.N., Pounds J.G., Fagan R., Lobley A. The human plasma proteome. Mol. Cell. Proteomics. 2004;3:311–326. doi: 10.1074/mcp.M300127-MCP200. [DOI] [PubMed] [Google Scholar]
- 10.Anderson N.L., Anderson N.G. The human plasma proteome: history, character, and diagnostic prospects. Mol. Cell. Proteomics. 2002;1:845–867. doi: 10.1074/mcp.r200007-mcp200. [DOI] [PubMed] [Google Scholar]
- 11.Psychogios N., Hau D.D., Peng J., Guo A.C., Mandal R., Bouatra S., Sinelnikov I., Krishnamurthy R., Eisner R., Gautam B., Young N., Xia J., Knox C., Dong E., Huang P., Hollander Z., Pedersen T.L., Smith S.R., Bamforth F., Greiner R., McManus B., Newman J.W., Goodfriend T., Wishart D.S. The human serum metabolome. PloS One. 2011;6 doi: 10.1371/journal.pone.0016957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yao T., Asayama Y. Animal-cell culture media: history, characteristics, and current issues. Reprod. Med. Biol. 2017;16:99–117. doi: 10.1002/rmb2.12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Der Valk J., Mellor D., Brands R., Fischer R., Gruber F., Gstraunthaler G., Hellebrekers L., Hyllner J., Jonker F.H., Prieto P., Thalen M., Baumans V. The humane collection of fetal bovine serum and possibilities for serum-free cell and tissue culture. Toxicol. Vitro. 2004;18:1–12. doi: 10.1016/j.tiv.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 14.van der Valk J., Brunner D., De Smet K., Fex Svenningsen Å., Honegger P., Knudsen L.E., Lindl T., Noraberg J., Price A., Scarino M.L., Gstraunthaler G. Optimization of chemically defined cell culture media - replacing fetal bovine serum in mammalian in vitro methods. Toxicol. Vitro. 2010;24:1053–1063. doi: 10.1016/j.tiv.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 15.Brindley D.A., Davie N.L., Culme-Seymour E.J., Mason C., Smith D.W., Rowley J.A. Peak serum: implications of serum supply for cell therapy manufacturing. Regen. Med. 2012;7:7–13. doi: 10.2217/rme.11.112. [DOI] [PubMed] [Google Scholar]
- 16.Harrison R.G., Greenman M.J., Mall F.P., Jackson C.M. Observations of the living developing nerve fiber. Anat. Rec. 1907;1:116–128. [Google Scholar]
- 17.Burrows M.T. The cultivation of tissues of the chick-embryo outside the body. J. Am. Med. Assoc. 1910;55:2057–2058. [Google Scholar]
- 18.Carrel A. Artificial activation of the growth in vitro of connective tissue. J. Exp. Med. 1913 Jan 1;17(1):14–19. doi: 10.1084/jem.17.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ebeling A.H. A ten year old strain of fibroblasts. J. Exp. Med. 1922;35:755–759. doi: 10.1084/jem.35.6.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eagle H. Nutrition needs of mammalian cells in tissue culture. Science. 1955 Sep 16;122(3168):501–504. doi: 10.1126/science.122.3168.501. [DOI] [PubMed] [Google Scholar]
- 21.Waymouth C. Growth, Nutr. Metab. Cells Cult. Elsevier; 1972. Construction of tissue culture media; pp. 11–47. [Google Scholar]
- 22.Ryan J.A. 2008. Introduction to Animal Cell Culture. Corning.http://catalog2.corning.com/lifesciences/media/pdf/intro_animal_cell_culture.pdf [Internet]. Available form: [Accessed: 2016-05-01] [Google Scholar]
- 23.3Rs database program of the 3rs-centre utrecht life sciences. https://fcs-free.org/fcs-%20database
- 24.Hayashi I., Sato G.H. Replacement of serum by hormones permits growth of cells in a defined medium. Nature. 1976;259:132–134. doi: 10.1038/259132a0. [DOI] [PubMed] [Google Scholar]
- 25.Filipic B., Shehata M., Toth S., Schwarzmeier J., Koren S. Novel serum replacement based on bovine ocular fluid: a useful tool for cultivation of different animal cells in vitro. ALTEX Altern. Zu Tierexperimenten. 2002;19:15–20. [PubMed] [Google Scholar]
- 26.Rathore G., Sood N., Swaminathan R. Primary cell culture from fish gills and kidney using fish serum. Indian J. Exp. Biol. 2001;39:936–938. [PubMed] [Google Scholar]
- 27.Pazos P., Boveri M., Gennari A., Casado J., Fernandez F., Prieto P. Culturing cells without serum: lessons learnt using molecules of plant origin. ALTEX. 2004;21:67–72. [PubMed] [Google Scholar]
- 28.Liu L., Wang J., Duan S., Chen L., Xiang H., Dong Y., Wang W. Systematic evaluation of sericin protein as a substitute for fetal bovine serum in cell culture. Sci. Rep. 2016;6:1–10. doi: 10.1038/srep31516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hartung A., Pazos T., Gennari P. Use of medium supplemented with molecules of plant origin to culture epithelial cells. ALTEX-Alt. Anim. Exp. 2003;20:175–176. [Google Scholar]
- 30.Chellathurai Vasantha N., Rajagopalan K., Selvan Christyraj J.D., Subbiahanadar Chelladurai K., Ganesan M., Azhagesan A., Rajaian Pushpabai R., Mohan M., Selvan Christyraj J.R.S. Heat-inactivated coelomic fluid of the earthworm Perionyx excavatus is a possible alternative source for fetal bovine serum in animal cell culture. Biotechnol. Prog. 2019;35:1–6. doi: 10.1002/btpr.2817. [DOI] [PubMed] [Google Scholar]
- 31.Freshney R.I. Cult. Anim. Cells. American Cancer Society; 2005. Serum-free media. [Google Scholar]
- 32.Broedel S.E., Papciak S.M. The case for serum-free media. Biopharm Int. 2003;1:56–58. [Google Scholar]
- 33.Webster L., Stanbury R.M., Chignell A.H., Limb G.A. Vitreous intercellular adhesion molecule 1 in uveitis complicated by retinal detachment. Br. J. Ophthalmol. 1998;82:438–443. doi: 10.1136/bjo.82.4.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller J.W., Adamis A.P., Shima D.T., D’Amore P.A., Moulton R.S., O’Reilly M.S., Folkman J., Dvorak H.F., Brown L.F., Berse B., Yeo T.K., Yeo K.T. Vascular endothelial growth factor/vascular permeability factor is temporally and spatially correlated with ocular angiogenesis in a primate model. Am. J. Pathol. 1994;145:574–584. [PMC free article] [PubMed] [Google Scholar]
- 35.Camiña J.P., Casabiell X.A., Pérez F.R., Lage M., Casanueva F.F. Isolation of a bioactive Ca2+-mobilizing complex lipid from bovine vitreous body. Biochem. Biophys. Res. Commun. 1998;244:696–700. doi: 10.1006/bbrc.1998.8320. [DOI] [PubMed] [Google Scholar]
- 36.Varma V.P., Devi L., Venna N.K., Murthy C.L.N., Idris M.M., Goel S. Ocular fluid as a replacement for serum in cell cryopreservation media. PloS One. 2015;10:1–17. doi: 10.1371/journal.pone.0131291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pothana L., Devi L., Venna N.K., Pentakota N., Varma V.P., Jose J., Goel S. Replacement of serum with ocular fluid for cryopreservation of immature testes. Cryobiology. 2016;73:356–366. doi: 10.1016/j.cryobiol.2016.09.169. [DOI] [PubMed] [Google Scholar]
- 38.Paul J. Walter de Gruyter GmbH & Co KG; 2019. Zell-und Gewebekulturen. [Google Scholar]
- 39.Terada S., Nishimura T., Sasaki M., Yamada H., Miki M. Sericin, a protein derived from silkworms, accelerates the proliferation of several mammalian cell lines including a hybridoma. Cytotechnology. 2002;40:3–12. doi: 10.1023/A:1023993400608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martínez-Mora C., Mrowiec A., García-Vizcaíno E.M., Alcaraz A., Cenis J.L., Nicolás F.J. Fibroin and sericin from Bombyx mori Silk stimulate cell migration through upregulation and phosphorylation of c-Jun. PloS One. 2012;7 doi: 10.1371/journal.pone.0042271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nayak S., Dey T., Naskar D., Kundu S.C. The promotion of osseointegration of titanium surfaces by coating with silk protein sericin. Biomaterials. 2013;34:2855–2864. doi: 10.1016/j.biomaterials.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 42.Tsubouchi K., Igarashi Y., Takasu Y., Yamada H. Sericin enhances attachment of cultured human skin fibroblasts. Biosci. Biotechnol. Biochem. 2005;69:403–405. doi: 10.1271/bbb.69.403. [DOI] [PubMed] [Google Scholar]
- 43.Minoura N., Aiba S.I., Gotoh Y., Tsukada M., Imai Y. Attachment and growth of cultured fibroblast cells on silk protein matrices. J. Biomed. Mater. Res. 1995;29:1215–1221. doi: 10.1002/jbm.820291008. [DOI] [PubMed] [Google Scholar]
- 44.Aramwit P., Kanokpanont S., De-Eknamkul W., Kamei K., Srichana T. The effect of sericin with variable amino-acid content from different silk strains on the production of collagen and nitric oxide. J. Biomater. Sci. Polym. Ed. 2009;20:1295–1306. doi: 10.1163/156856209X453006. [DOI] [PubMed] [Google Scholar]
- 45.Aramwit P., Kanokpanont S., Nakpheng T., Srichana T. The effect of sericin from various extraction methods on cell viability and collagen production. Int. J. Mol. Sci. 2010;11:2200–2211. doi: 10.3390/ijms11052200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cao T.T., Zhang Y.Q. Viability and proliferation of L929, tumour and hybridoma cells in the culture media containing sericin protein as a supplement or serum substitute. Appl. Microbiol. Biotechnol. 2015;99:7219–7228. doi: 10.1007/s00253-015-6576-3. [DOI] [PubMed] [Google Scholar]
- 47.Takahashi M., Tsujimoto K., Yamada H., Takagi H., Nakamori S. The silk protein, sericin, protects against cell death caused by acute serum deprivation in insect cell culture. Biotechnol. Lett. 2003;25:1805–1809. doi: 10.1023/a:1026284620236. [DOI] [PubMed] [Google Scholar]
- 48.Williamson M.B., Fromm H.J. Effect of cystine and methionine on healing of experimental wounds. Proc. Soc. Exp. Biol. Med. 1952;80:623–626. doi: 10.3181/00379727-80-19712. [DOI] [PubMed] [Google Scholar]
- 49.Verdanova M., Pytlik R., Kalbacova M.H. Evaluation of sericin as a fetal bovine serum-replacing cryoprotectant during freezing of human mesenchymal stromal cells and human osteoblast-like cells. Biopreserv. Biobank. 2014;12:99–105. doi: 10.1089/bio.2013.0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vepari C., Kaplan D.L. Silk as a biomaterial. Prog. Polym. Sci. 2007;32:991–1007. doi: 10.1016/j.progpolymsci.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ikeda K., Oumi Y., Ogawa A., Sasaki M., Yamada H., Terada S. Cells and culture. Cells Cult. 2010:10–13. [Google Scholar]
- 52.Ha S.J., Kim B.G., Lee Y.A., Kim Y.H., Kim B.J., Jung S.E., Pang M.G., Ryu B.Y. Effect of antioxidants and apoptosis inhibitors on cryopreservation of murine germ cells enriched for spermatogonial stem cells. PloS One. 2016;11:1–16. doi: 10.1371/journal.pone.0161372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Burnouf T., Strunk D., Koh M.B.C., Schallmoser K. Human platelet lysate: replacing fetal bovine serum as a gold standard for human cell propagation? Biomaterials. 2016;76:371–387. doi: 10.1016/j.biomaterials.2015.10.065. [DOI] [PubMed] [Google Scholar]
- 54.Semple J.W., Italiano J.E., Freedman J. Platelets and the immune continuum. Nat. Rev. Immunol. 2011;11:264–274. doi: 10.1038/nri2956. [DOI] [PubMed] [Google Scholar]
- 55.Rauch C., Feifel E., Amann E.-M., Spötl H.P., Schennach H., Pfaller W., Gstraunthaler G. Alternatives to the use of fetal bovine serum: human platelet lysates as a serum substitute in cell culture media. ALTEX-Altern. Anim. Exp. 2011;28:305–316. doi: 10.14573/altex.2011.4.305. [DOI] [PubMed] [Google Scholar]
- 56.van der Valk J., Bieback K., Buta C., Cochrane B., Dirks W.G., Fu J., Hickman J.J., Hohensee C., Kolar R., Liebsch M., Pistollato F., Schulz M., Thieme D., Weber T., Wiest J., Winkler S., Gstraunthaler G. Fetal bovine serum (FBS): past - present - future. ALTEX. 2018;35:99–118. doi: 10.14573/altex.1705101. [DOI] [PubMed] [Google Scholar]
- 57.Johansson L., Klinth J., Holmqvist O., Ohlson S. Platelet lysate: a replacement for fetal bovine serum in animal cell culture? Cytotechnology. 2003;42:67–74. doi: 10.1023/B:CYTO.0000009820.72920.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Astori G., Amati E., Bambi F., Bernardi M., Chieregato K., Schäfer R., Sella S., Rodeghiero F. Platelet lysate as a substitute for animal serum for the ex-vivo expansion of mesenchymal stem/stromal cells: present and future. Stem Cell Res. Ther. 2016;7:1–8. doi: 10.1186/s13287-016-0352-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Radtke S., Giebel B., Wagner W., Horn P.A. Platelet lysates and their role in cell therapy. ISBT Sci. Ser. 2014;9:193–197. [Google Scholar]
- 60.Canestrari E., Steidinger H.R., McSwain B., Charlebois S.J., Dann C.T. Human platelet lysate media supplement supports lentiviral transduction and expansion of human T lymphocytes while maintaining memory phenotype. J. Immunol. Res. 2019;2019 doi: 10.1155/2019/3616120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Trojahn Kølle S.F., Oliveri R.S., Glovinski P.V., Kirchhoff M., Mathiasen A.B., Elberg J.J., Andersen P.S., Drzewiecki K.T., Fischer-Nielsen A. Pooled human platelet lysate versus fetal bovine serum-investigating the proliferation rate, chromosome stability and angiogenic potential of human adipose tissue-derived stem cells intended for clinical use. Cytotherapy. 2013;15:1086–1097. doi: 10.1016/j.jcyt.2013.01.217. [DOI] [PubMed] [Google Scholar]
- 62.Tylek T., Schilling T., Schlegelmilch K., Ries M., Rudert M., Jakob F., Groll J. Platelet lysate outperforms FCS and human serum for co-culture of primary human macrophages and hMSCs. Sci. Rep. 2019;9:1–11. doi: 10.1038/s41598-019-40190-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mohamed H.E., Asker M.E., Kotb N.S., El Habab A.M. Human platelet lysate efficiency, stability, and optimal heparin concentration required in culture of mammalian cells. Blood Res. 2020;55:35–43. doi: 10.5045/br.2020.55.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vetvicka V. 1994. Immunology of Annelids. [Google Scholar]
- 65.Patil S.R., Biradar P.M. Earthworms coelomic fluid: extraction and importance. Int. J. Adv. Sci. Res. 2017;2:1–4. [Google Scholar]
- 66.Griffith C.M., Williams P.B., Tinoco L.W., Dinges M.M., Wang Y., Larive C.K. 1H NMR metabolic profiling of earthworm (Eisenia fetida) coelomic fluid, coelomocytes, and tissue: identification of a new metabolite - malylglutamate. J. Proteome Res. 2017;16:3407–3418. doi: 10.1021/acs.jproteome.7b00439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kamemoto F.I., Spalding A.E., Keister S.M. Ionic balance in blood and coelomic fluid of earthworms. Biol. Bull. 1962 Apr;122(2):228–231. [Google Scholar]
- 68.Subramanian E.R., Sudalaimani D.K., Selvan Christyraj J.R.S., Ramamoorthy K., Gopi Daisy N., Selvan Christyraj J.D., Renganathan K., Krishnan S., Sivasubramaniam S. Studies on organogenesis during regeneration in the earthworm, Eudrilus eugeniae, in support of symbiotic association with Bacillus endophyticus. Turkish J. Biol. 2017;41:113–126. [Google Scholar]
- 69.Plytycz B., Bigaj J., Falniowski A., Morgan A.J. Unexpected results and open questions from experiments on regeneration in lumbricid worms. Invertebr. Surviv. J. 2016;13:315–325. [Google Scholar]
- 70.Płytycz B., Homa J., Kozioł B., Rózanowska M., Morgan A.J. Riboflavin content in autofluorescent earthworm coelomocytes is species-specific. Folia Histochem. Cytobiol. 2006;44:275–280. [PubMed] [Google Scholar]
- 71.Morgan J.F., Morton H.J., Parker R.C. Nutrition of animal cells in tissue culture. I. Initial studies on a synthetic medium. Proc. Soc. Exp. Biol. Med. 1950 Jan;73(1):1–8. doi: 10.3181/00379727-73-17557. [DOI] [PubMed] [Google Scholar]
- 72.Nakano E., Mushtaq S., Heath P.R., Lee E.S., Bury J.P., Riley S.A., Powers H.J., Corfe B.M. Riboflavin depletion impairs cell proliferation in adult human duodenum: identification of potential effectors. Dig. Dis. Sci. 2011;56:1007–1019. doi: 10.1007/s10620-010-1374-3. [DOI] [PubMed] [Google Scholar]
- 73.Danielyan K.E. Subcomponents of vitamine B complex regulate the growth and development of human brain derived cells. Am. J. Biomed. Res. 2013;1:28–34. [Google Scholar]
- 74.Hirano K., Namihira M. FAD influx enhances neuronal differentiation of human neural stem cells by facilitating nuclear localization of LSD1. FEBS Open Bio. 2017;7:1932–1942. doi: 10.1002/2211-5463.12331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kashyap S., Kapoor N., Kale R.D. Coscinium fenestratum: callus and suspension cell culture of the endangered medicinal plant using vermicompost extract and coelomic fluid as plant tissue culture media. Am. J. Plant Sci. 2016;7(6):899. [Google Scholar]
- 76.Wojdani A., Stein E.A., Alfred L.J., Cooper E.L. Mitogenic effect of earthworm (Lumbricus terrestris)Coelomic fluid on mouse and human lymphocytes. Immunobiology. 1984;166:157–167. doi: 10.1016/S0171-2985(84)80034-0. [DOI] [PubMed] [Google Scholar]
- 77.Hanušová R., Bilej M., Brys L., De-Baetselier P., Beschin A. Identification of a coelomic mitogenic factor in Eisenia foetida earthworm. Immunol. Lett. 1999;65:203–211. doi: 10.1016/s0165-2478(98)00111-4. [DOI] [PubMed] [Google Scholar]
- 78.Zhao J., Qi S.P., Wu J., Li L., He R.Q. Earthworm fibrinolytic enzyme. Stud. Nat. Prod. Chem. 2005;30:825–847. [Google Scholar]
- 79.Fazzina R., Iudicone P., Mariotti A., Fioravanti D., Procoli A., Cicchetti E., Scambia G., Bonanno G., Pierelli L. Culture of human cell lines by a pathogen-inactivated human platelet lysate. Cytotechnology. 2016;68:1185–1195. doi: 10.1007/s10616-015-9878-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bieback K., Fernandez-Muñoz B., Pati S., Schäfer R. Gaps in the knowledge of human platelet lysate as a cell culture supplement for cell therapy: a joint publication from the AABB and the International Society for Cell & Gene Therapy. Cytotherapy. 2019;21:911–924. doi: 10.1016/j.jcyt.2019.06.006. [DOI] [PubMed] [Google Scholar]
- 81.Sethulakshmi K., Lakshmi R. Antibacterial activities of coelomic fluid of local earthworms against disease causing microorganisms. Asian J. Biol. 2018;5:1–7. [Google Scholar]
- 82.Rajesh C., Rajamanikkam K., Nadana G., Vadivu R. Coelomic fluid of earthworm, Eudrilus eugeniae, inhibits the growth of fungal hyphae, in vitro. Int. J. Eng. Adv. Technol. 2019;9:792–796. [Google Scholar]
- 83.John S.A., Packialakshmi N., Sundhararajan A. Investigation of cellular activity in the coelomic fluid of the earthworm (Perionyx excavatus Perrier) Asian J. Microbiol. Biotechnol. Environ. Sci. 2009;11:335–338. [Google Scholar]
- 84.Gates G.E. Burmese earthworms: an introduction to the systematics and biology of megadrile oligochaetes with special reference to Southeast Asia. Trans. Am. Philos. Soc. 1972;62:1–326. [Google Scholar]
- 85.Reinecke A.J., Viljoen S.A., Saayman R.J. The suitability of Eudrilus eugeniae, Perionyx excavatus and Eisenia fetida (Oligochaeta) for vermicomposting in southern Africa in terms of their temperature requirements. Soil Biol. Biochem. 1992;24:1295–1307. [Google Scholar]
- 86.Chan R.K., Liu P., Lew D.-H., Ibrahim S.I., Srey R., Valeri C.R., Hechtman H.B., Orgill D.P. Expired liquid preserved platelet releasates retain proliferative Activity1. J. Surg. Res. 2005;126:55–58. doi: 10.1016/j.jss.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 87.Folmsbee M., Howard G., McAlister M. Nutritional effects of culture media on mycoplasma cell size and removal by filtration. Biologicals. 2010;38:214–217. doi: 10.1016/j.biologicals.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 88.Organization W.H. Guidelines on viral inactivation and removal procedures intended to assure the viral safety of human blood plasma products. WHO Tech. Rep. Ser. 2004;924:150–224. [Google Scholar]
- 89.Klein H.G. Pathogen inactivation technology: cleansing the blood supply. J. Intern. Med. 2005;257:224–237. doi: 10.1111/j.1365-2796.2005.01451.x. [DOI] [PubMed] [Google Scholar]
- 90.Shih D.T., Chen J., Chen W., Kuo Y., Su C., Burnouf T. Expansion of adipose tissue mesenchymal stromal progenitors in serum-free medium supplemented with virally inactivated allogeneic human platelet lysate. Transfusion. 2011;51:770–778. doi: 10.1111/j.1537-2995.2010.02915.x. [DOI] [PubMed] [Google Scholar]
- 91.Hemeda H., Giebel B., Wagner W. Evaluation of human platelet lysate versus fetal bovine serum for culture of mesenchymal stromal cells. Cytotherapy. 2014;16:170–180. doi: 10.1016/j.jcyt.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 92.Oeller M., Laner-plamberger S., Krisch L., Rohde E., Strunk D., Schallmoser K. Human platelet lysate for good manufacturing practice-compliant cell production. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms22105178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Reports Reportsn. 2016. Cell Culture Market by Equipment (Bioreactor, Culture Vessels (Multiwell Plates, Petri Dish)), Consumables (FBS, ABS, Media, Reagents), Application (Therapeutics, Stem Cell), End Users (Pharmaceutical and Biotechnology, Research)- Forecast to 2020.http://www.reportsnreports.com/market-research/biotechnology/ Available at: [Google Scholar]
- 94.Karnieli O., Friedner O.M., Allickson J.G., Zhang N., Jung S., Fiorentini D., Abraham E., Eaker S.S., Yong T.K., Chan A., Griffiths S., When A.k., Oh S., Karnieli O. A consensus introduction to serum replacements and serum-free media for cellular therapies. Cytotherapy. 2017;19:155–169. doi: 10.1016/j.jcyt.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 95.Gstraunthaler G. Alternatives to the use of fetal bovine serum: serum-free cell culture. ALTEX Altern. Zu Tierexperimenten. 2003;20:275–281. [PubMed] [Google Scholar]
- 96.Baker M. Reproducibility: respect your cells! Nature. 2016;537:433–435. doi: 10.1038/537433a. [DOI] [PubMed] [Google Scholar]
- 97.Gstraunthaler G., Lindl T., Van Der Valk J. A severe case of fraudulent blending of fetal bovine serum strengthens the case for serum-free cell and tissue culture applications. ATLA Alt. Lab. Anim. 2014;42:207–209. doi: 10.1177/026119291404200308. [DOI] [PubMed] [Google Scholar]
- 98.RMBIO . 2016. Fetal Bovine Serum: Supply and Demand for US FBS.https://www.rmbio.com/fetal-bovine-serum-supply-and-demand-for-us-fbs [Google Scholar]
- 99.Chou M.L., Bailey A., Avory T., Tanimoto J., Burnouf T. Removal of transmissible spongiform encephalopathy prion from large volumes of cell culture media supplemented with fetal bovine serum by using hollow fiber anion-exchange membrane chromatography. PloS One. 2015;10:1–15. doi: 10.1371/journal.pone.0122300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kirikae T., Tamura H., Hashizume M., Kirikae F., Uemura Y., Tanaka S., Yokochi T., Nakano M. Endotoxin contamination in fetal bovine serum and its influence on tumor necrosis factor production by macrophage-like cells J774. 1 cultured in the presence of the serum. Int. J. Immunopharmacol. 1997 May 1;19(5):255–262. doi: 10.1016/s0192-0561(97)00066-0. [DOI] [PubMed] [Google Scholar]
- 101.Hawkes P.W. Fetal bovine serum: geographic origin and regulatory relevance of viral contamination. Bioresour. Bioprocess. 2015;2 [Google Scholar]
- 102.Jochems C.E.A., Van der Valk J.B.F., Stafleu F.R., Baumans V. The use of fetal bovine serum: ethical or scientific problem? ATLA Alt. Lab. Anim. 2002;30:219–227. doi: 10.1177/026119290203000208. [DOI] [PubMed] [Google Scholar]
- 103.Clavey V., Copin C., Mariotte M.C., Baugé E., Chinetti G., Fruchart J., Fruchart J.C., Dallongeville J., Staels B. Cell culture conditions determine apolipoprotein CIII secretion and regulation by fibrates in human hepatoma hepG2 cells. Cell. Physiol. Biochem. 1999;9:139–149. doi: 10.1159/000016311. [DOI] [PubMed] [Google Scholar]
- 104.Groothuis F.A., Heringa M.B., Nicol B., Hermens J.L.M., Blaauboer B.J., Kramer N.I. Dose metric considerations in in vitro assays to improve quantitative in vitro-in vivo dose extrapolations. Toxicology. 2015;332:30–40. doi: 10.1016/j.tox.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 105.Kramer N.I., Hermens J.L.M., Schirmer K. The influence of modes of action and physicochemical properties of chemicals on the correlation between in vitro and acute fish toxicity data. Toxicol. Vitro. 2009;23:1372–1379. doi: 10.1016/j.tiv.2009.07.029. [DOI] [PubMed] [Google Scholar]
- 106.Taylor R.E., Gregg C.J., Padler-Karavani V., Ghaderi D., Yu H., Huang S., Sorensen R.U., Chen X., Inostroza J., Nizet V., Varki A. Novel mechanism for the generation of human xeno-autoantibodies against the nonhuman sialic acid N-glycolylneuraminic acid. J. Exp. Med. 2010;207:1637–1646. doi: 10.1084/jem.20100575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Martin M.J., Muotri A., Gage F., Varki A. Human embryonic stem cells express an immunogenic nonhuman sialic acid. Nat. Med. 2005;11:228–232. doi: 10.1038/nm1181. [DOI] [PubMed] [Google Scholar]
- 108.Bilej M., Brys L., Beschin A., Lucas R., Vercauteren E., Hanušová R., De Baetselier P. Identification of a cytolytic protein in the coelomic fluid of Eisenia foetida earthworms. Immunol. Lett. 1995;45:123–128. doi: 10.1016/0165-2478(94)00248-p. [DOI] [PubMed] [Google Scholar]
- 109.Milochau A., Lassègues M., Valembois P. Purification, characterization and activities of two hemolytic and antibacterial proteins from coelomic fluid of the annelid Eisenia fetida andrei. Biochim. Biophys. Acta Protein Struct. Mol. Enzymol. 1997;1337:123–132. doi: 10.1016/s0167-4838(96)00160-4. [DOI] [PubMed] [Google Scholar]
- 110.Fiołka M.J., Czaplewska P., Macur K., Buchwald T., Kutkowska J., Paduch R., Kaczyński Z., Wydrych J., Urbanik-Sypniewska T. Anti-Candida albicans effect of the protein-carbohydrate fraction obtained from the coelomic fluid of earthworm Dendrobaena veneta. PloS One. 2019;14:1–30. doi: 10.1371/journal.pone.0212869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shanskii Y.D., Sergeeva N.S., Sviridova I.K., Kirakozov M.S., Kirsanova V.A., Akhmedova S.A., Antokhin A.I., Chissov V.I. Human platelet lysate as a promising growth-stimulating additive for culturing of stem cells and other cell types. Bull. Exp. Biol. Med. 2013;156:146–151. doi: 10.1007/s10517-013-2298-7. [DOI] [PubMed] [Google Scholar]
- 112.Valembois P., Seymour J., Roch P. Evidence and cellular localization of an oxidative activity in the coelomic fluid of the earthworm Eisenia fetida andrei. J. Invertebr. Pathol. 1991;57:177–183. [Google Scholar]
- 113.Ueda M., Noda K., Nakazawa M., Miyatake K., Ohki S., Sakaguchi M., Inouye K. A novel anti-plant viral protein from coelomic fluid of the earthworm Eisenia foetida: purification, characterization and its identification as a serine protease. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2008;151:381–385. doi: 10.1016/j.cbpb.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 114.Nadana G.R.V., Rajesh C., Kavitha A., Sivakumar P., Sridevi G., Palanichelvam K. Induction of growth and defense mechanism in rice plants towards fungal pathogen by eco-friendly coelomic fluid of earthworm. Environ. Technol. Innov. 2020;19:101011. [Google Scholar]
- 115.Grdiša M., Popović M., Hrženjak T. Stimulation of growth factor synthesis in skin wounds using tissue extract (G-90) from the earthworm Eissenia foetida. Cell Biochem. Funct. 2004;22:373–378. doi: 10.1002/cbf.1121. [DOI] [PubMed] [Google Scholar]
- 116.Ishitsuka R., Sato S.B., Kobayashi T. Imaging lipid rafts. J. Biochem. 2005;137:249–254. doi: 10.1093/jb/mvi041. [DOI] [PubMed] [Google Scholar]
- 117.Lohmann M., Walenda G., Hemeda H., Joussen S., Drescher W., Jockenhoevel S., Hutschenreuter G., Zenke M., Wagner W. Donor age of human platelet lysate affects proliferation and differentiation of mesenchymal stem cells. PloS One. 2012;7:14–16. doi: 10.1371/journal.pone.0037839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Koziol B., Markowicz M., Kruk J., Plytycz B. Riboflavin as a source of autofluorescence in Eisenia fetida coelomocytes. Photochem. Photobiol. 2006;82:570. doi: 10.1562/2005-11-23-RA-738. [DOI] [PubMed] [Google Scholar]
- 119.Bundy J.G., Osborn D., Weeks J.M., Lindon J.C., Nicholson J.K. An NMR-based metabonomic approach to the investigation of coelomic fluid biochemistry in earthworms under toxic stress. FEBS Lett. 2001;500:31–35. doi: 10.1016/s0014-5793(01)02582-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.