Abstract
Aim:
This concept analysis aims to analyze the concept of sleep disturbance (SD) in the context of heart failure (HF) to guide the development of a clearly defined definition.
Background:
The term “sleep disturbance” has been used in the literature to describe sleep problems and sleep disorders among individuals with HF. Environmental, physical, psychological, behavioral, and developmental factors complicate the phenomenon of SD in HF.
Design:
Walker and Avant’s method was used for this concept analysis.
Data Source:
Published literature from 2000 to 2020 was identified from electronic health profession-related databases. The current definition and usages of SD were abstracted from empirical work and electronic databases.
Review Methods:
A focused review of abstracts and full text relating to SD in HF was performed. Studies featuring original data and peer-reviewed articles written in English were included to investigate the multifactorial contextual meaning of the concept.
Results:
SD in HF can be described as a condition in which individuals experience difficulty initiating and maintaining sleep, and difficulty continuing or resuming sleep due to frequent nocturnal arousals due to HF symptoms, sleep-disordered breathing, insomnia, and psychological burdens.
Conclusions:
To evaluate SD in HF, clinicians must examine the underlying causes to provide the contextual meaning of the concept. A clearly defined and distinguishable concept of SD in HF provides a possibility for accurate measurements of sleep quality, exploring interventions, and evaluating outcomes.
Keywords: advanced heart failure, cardiac failure, concept analysis, heart failure, sleep disturbance
1 |. INTRODUCTION
Heart failure (HF) is a chronic disabling irreversible condition affecting an estimated 6.0 million Americans. The number of people diagnosed with HF is projected to increase by 46% (more than 8 million) by 2030.1 Approximately 75% of individuals with HF experienced sleep disturbances (SDs),2–4 which are affected by demands of daily activities, the disease itself, and HF-related symptoms, such as nocturnal dyspnea, cough, and palpitation.5 Sleep-disordered breathing (SDB), comprised of obstructive sleep apnea (OSA), and central sleep apnea (CSA), contributes to respiratory SD,6 is highly prevalent (51%–71%) in HF and negatively impacts clinical outcomes.7,8 Hypoxia and partial neurological arousals resulting from SDB lead to disruption to the sleep architecture,9 fragmented sleep, sleep deprivation, and daytime sleepiness.10 Further, SDB contributes to poor sleep quality, accelerates the progress of HF,11 and worsens morbidity and mortality.12,13 Sleep-related daytime symptoms and poor sleep quality are associated with poor medication adherence,14–16 and inadequate self-care.17–19
The eight steps in Walker and Avant’s concept analysis framework include: select a concept, establish aims of the analysis, identify uses of the concept, determine defining attributes, identify a model case, identify borderline and illegitimate cases, identify antecedents and consequences, and define empirical referents.20 The purpose of this paper is to present a concept analysis of SD in the context of HF using Walker and Avant’s framework to guide evaluation and intervention development to address health-related issues associated with SD in individuals living with HF.
2 |. CONCEPT SELECTION AND AIM
Concept analysis is a process of deconstructing a term to assess the fundamental elements within the concept, allowing for the development of an in-depth understanding, a sound definition, and precise measurements of the concept.20 In the general population, SD was traditionally described as disorders of initiating and maintaining sleep, excessive somnolence and sleep-wake schedule, and dysfunctions associated with sleep, sleep stages, or partial arousals (parasomnias).21 The term “sleep disturbance” has been used in the literature to describe sleep problems and sleep disorders among individuals with HF, but it lacks consistency, specificity, and clarity in definition and causes. The concept of SD is chosen to provide a deeper understanding of the SD phenomenon in HF.
Insomnia, defined as repeated SD despite adequate time and opportunity for sleep, resulting in daytime distress or impairment,22 is characterized by three primary symptoms: difficulty falling asleep, difficulty staying asleep, and early morning awakening.23 Many of the essential characteristics of SD in HF in the current literature are identical with these commonly reported insomnia symptoms.24 While these terms may represent the complexity of SD, none of these terms provide the contextual multifactorial meaning of SD in individuals with HF. Environmental, physical, psychological, behavioral, and developmental factors complicate the phenomenon of SD.25 This analysis aims to provide a clearly defined and distinguishable concept of SD, offer operationalizable components of the concept, and opens possibilities for accurate measurements, exploring interventions, and evaluating outcomes.
To find the usage of SD in the context of HF in the scientific literature, a focused systematic search of scientific databases (CINAHL, PubMed, Medline, and PsyInfo) from 2000 to 2020 was conducted using the following key terms and Boolean operators (“AND,” “OR”): “sleep,” “sleep disturbance,” “disturbed sleep,” “disrupt sleep,” “sleep awake,” “insomnia,” “sleep disordered breathing,” “sleep-disordered breathing,” “sleep fragmentation,” “fragmented sleep” AND “heart failure” OR “cardiac failure”. Last, reference lists of the peer-reviewed articles were used to identify seminal works. Studies featuring original data and peer-reviewed articles written in English were included to investigate the contextual meaning of the concept.
3 |. USE OF THE CONCEPT
Prior studies and other published peer-reviewed literature use the terms “sleep disturbance,” “disturbed sleep,” and “disrupted sleep” interchangeably. “Sleep disruption,” “sleep dysfunction,” “fragmented sleep,” “sleep fragmentation,” and “sleep quality” are used to describe different aspects of sleep problems and disorders in both the general population and HF. Sleep fragmentation and fragmented sleep, characterized by frequent arousals, are two terms used interchangeably with SD in some literature.25 To analyze the concept of SD among individuals with HF, the defining attributes, antecedents, and consequences (Figure 1) were identified.
FIGURE 1.
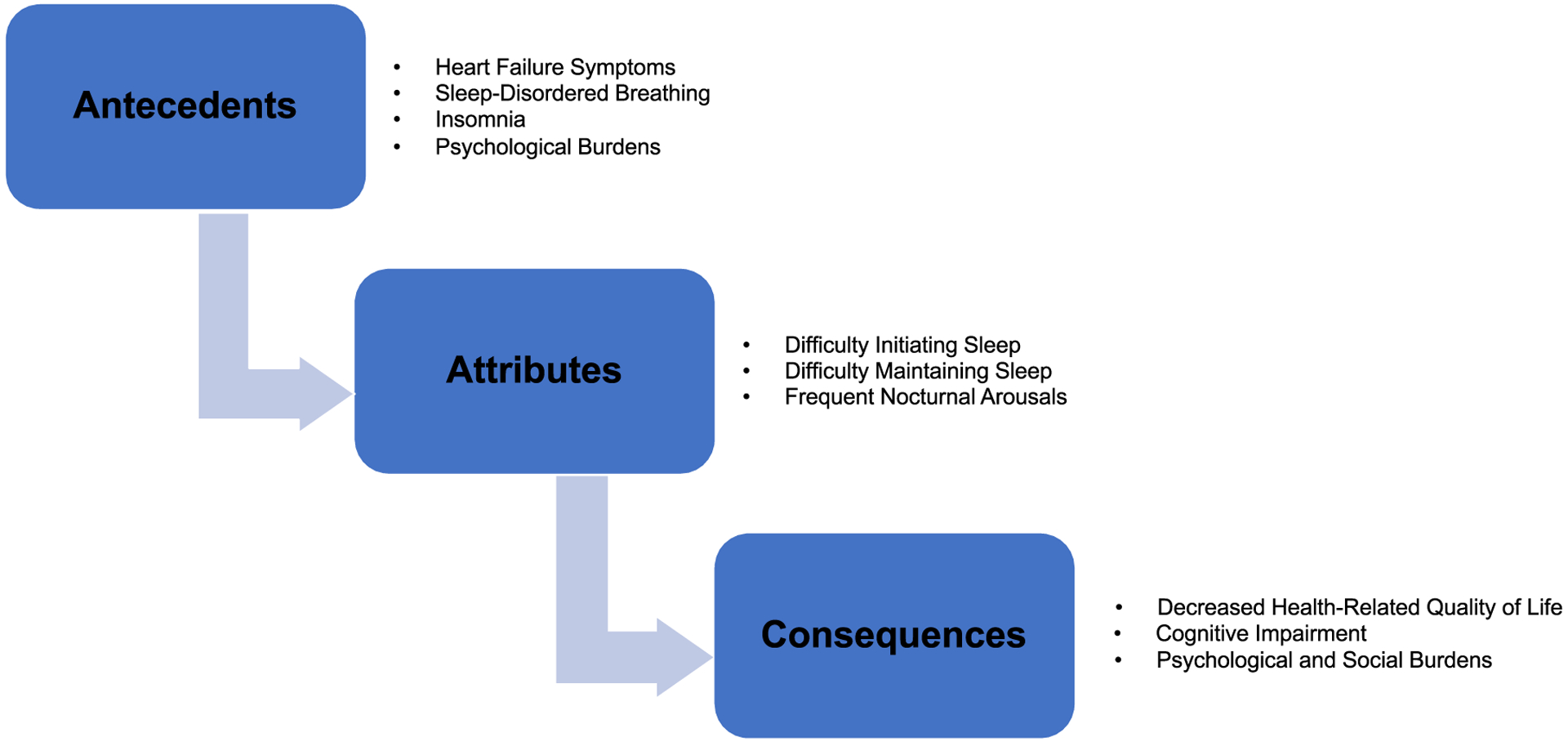
Antecedents, attributes, and consequence of sleep disturbance in heart failure
4 |. DEFINING ATTRIBUTES
Many forms of HF occur in individuals with advanced age1 and some changes in sleep are associated with normal aging,26 such as changes in sleep duration, fragmentation, and depth. In HF, individuals experience SDs, such as shorter sleep duration, frequent arousals, and altered stages of deep and rapid eye movement sleep.27 One significant physiologic factor contributing to SD in HF is SDB, a consequence of and a contributor to the severity of HF. The cause of SD in patients with HF can be physical, psychological, and situational.5 The defining attributes of SD include difficulty initiating sleep and difficulty maintaining sleep due to frequent nocturnal arousals.
4.1 |. Difficulty initiating sleep
Difficulty initiating sleep is one significant complaint in individuals with HF. In one study, 46.3% of male participants and 46.7% of female participants with HF reported moderate to major difficulty initiating sleep.27 In a separate study, over 50% of individuals with HF reported difficulty initiating sleep due to dyspnea and orthopnea.26 Further, hemodynamic deterioration during sleep and SDB may exacerbate respiratory and HF-related symptoms leading to difficulty in initiating sleep and compromised sleep quality.28,29 Anxiety and thoughts about the possibility of cardiac events (e.g., defibrillator shocks) occurring during sleep,26 activities in daily life, prognosis, and symptoms of the disease itself, induce difficulties for individuals with HF to initiate sleep.27
4.2 |. Difficulty maintaining sleep/frequent nocturnal arousals
Difficulty in maintaining sleep is a common component of SD, present in 20% of female participants and 23% of male participants with HF.27 Difficulty maintaining sleep is a result of frequent nocturnal arousals, commonly reported in HF26,27,30,30,31 related to nighttime cardiopulmonary symptoms (e.g., dyspnea and palpitations),5,32–34 pain,5,26,34,35 nocturnal polyuria.,27,32,35,36 and periodic limb movement.37 SDB, as in OSA and CSA with or without Cheyne-Stokes respiration, also contributes to nocturnal arousals.30,38
5 |. ANTECEDENTS
Antecedents are factors, events, or situations that must arise before the occurrence or manifestation of the concept of interest but are not the same as defining attributes.20,39 In HF, SD results from poorly controlled HF,40 SDB,38 and psychological burdens.5 The antecedents of SD can be measured objectively and subjectively, including HF symptoms, SDB, insomnia, and psychological burdens.
5.1 |. HF symptoms
Poorly controlled HF symptoms commonly disturb sleep, including nighttime cardiopulmonary symptoms (e.g., dyspnea and palpitations),5,32–34 pain,5,26,34,35 and nocturnal polyuria.27,32,35,36 Dyspnea during sleep is related to pulmonary congestion caused by postural changes related to alternation of bodily fluid distribution.33 Fluid volume overload leads to a greater volume of fluid entering the vascular space,41 elevated left atrial pressure, and subsequent pulmonary congestion, resulting in arousals from sleep due to a need to breathe.33 Pain is a common cause of SD in individuals with HF.24,26,27,34 Pain is associated with early morning awakening,26,34 self-reported difficulty initiating and/or maintaining sleep,27 nonrestorative sleep.24 Nocturnal polyuria (due to diuretics) causes frequent arousals leading to difficulty in maintaining restorative sleep.32,35,36
5.2 |. Sleep-disordered breathing
SDB (OSA and CSA) activates the sympathetic nervous system, which initiates a vicious cycle of disturbances in initiating sleep and maintaining sleep, with increased wakefulness and persistent thoughts about the next day.42 Individuals with HF may experience either OSA or CSA or mixed apnea.38 Nocturnal arousal is one common characteristic of SD among OSA and CSA. However, these two types of sleep apnea have differing underlying pathophysiologic mechanisms.26,27,30,30,43 Some literature indicates that CSA could contribute to the development of OSA due to pharyngeal narrowing or occlusion in healthy individuals.31 Arousals due to CSA can be measured objectively by polysomnography (PSG), and arousals can occur during hyperventilation or in the presence of congested lungs and a stiff respiratory system.44 In contrast, OSA is characterized by intermittent narrowing and collapse of the pharyngeal airway during sleep. The collapsed airway leads to increased respiratory effort, resulting in arousals that disrupt sleep continuity.
Cheyne-Stokes respiration is a common abnormal breathing pattern observed in individuals with HF and can cooccur with CSA.30 Cheyne-Stokes breathing pattern is characterized as periodic waxing and waning of the depth of respiration on the polysomnographic recording with regularly recurring periods of apnea.45 Cheyne–Stokes respiration leads to a shorter total duration of sleep, disturbed sleep structure with frequent arousals, and sleep stage changes.46
5.3 |. Insomnia
More than half of individuals with HF report at least one insomnia symptom,27,47 and insomnia is associated with worse New York Heart Association (NYHA) functional class,4,48 older age,49 and female gender.49 Individuals with insomnia present with difficulty initiating sleep resulting in an increased amount of time in bed to secure more opportunities to sleep. Moreover, spending more time in bed leads to increased wakefulness, fragmented sleep, and variability in sleep timing.22
5.4 |. Psychological burdens
Self-reported psychological factors contribute to SD in HF. Stressful life events, chronic illness, and stress significantly impair sleep due to the difficulty in initiating sleep.50 Stress induces the release of glucose, increased cardiovascular tone, and sharpened senses, all of which make initiating sleep a challenge.25 Activities and problems in individuals’ daily lives lead to thoughts and anxiety resulting in SD. Furthermore, the HF disease condition, and the perceived effects of symptom burden and negative effects of the disease (e.g., deterioration from the disease), disturb individuals’ sleep.5
6 |. CONSEQUENCES
As previously identified, many underlying factors contribute to SD in HF. It is also important to understand how SD leads to adverse health consequences, including decreased health-related quality of life (HRQOL), cognitive impairment, and emotional and social burdens.
6.1 |. Decreased health-related quality of life
HRQOL is used to describe areas of life that are more likely to be influenced by a health condition, including physiological, psychological, and sociological domains.51 Daytime sleepiness, a physiological symptom and a result of Cheyne–Stokes respiration cooccurring with CSA in HF,27 leads to social, economic, and medical impairment that negatively impacts the quality of life.43,52,53 SD and depressive symptoms have a bidirectional relationship, affecting the quality of life of individuals living with HF. Depressive symptoms mediate the effects of SD on HRQOL. Conversely, SD increases the risk for depressive symptoms,4,24 particularly in those with poor health perceptions, alcohol abuse, and economic burden associated with medical care and living alone.24
6.2 |. Cognitive impairment
SD adversely affects cognitive function, such as a loss of concentration.5 Cognitive impairment is common in HF, including decreased attention and concentration, memory loss, diminished psychomotor speed and perception, and decreased executive function.54 Overnight uninterrupted sleep plays an important role in consolidating spatial navigation memory. This process is lost when the rapid eye movement sleep stage is interrupted as a result of OSA.55 OSA not only shortens and fragments rapid eye movement sleep but also induces intermittent hypoxia,55 that negatively impacts cognition.56 Furthermore, OSA was found to be associated with neurocognitive impairment due to intermittent and sustained nocturnal cerebral tissue deoxygenation and a degree of cerebral dysfunction.57
6.3 |. Psychological and social burdens
SD negatively affects individuals with HF psychologically and socially. Some individuals reported feeling listless with decreased motivation and energy to do things; others reported that SD has led to a short temper, anger, and irritation.5 Fatigue resulting from SD induced a desire for seclusion to get more rest.5 Individuals experiencing SD are at a two- to threefold increased risk of developing depressive symptoms.4 Moreover, depressive symptoms exacerbate the effects of SD on HRQOL.58
7 |. MODEL CASE
Mr. Davidson is a 62-year-old veteran with advanced HF (NYHA functional Class IV). He is waiting to receive a Left Ventricular Assist Device (LVAD) due to ineligibility for a cardiac transplant. Mr. Davidson reports that his sleep quality is progressively getting worse. He sleeps in the same room as his spouse and wakes up five to six times a night due to shortness of breath. He says, “I am worried about my condition and how my life would change after getting the device. When I wake up in the middle of the night, it is hard for me to fall back to sleep, even if I try. I feel like I am in the cycle of not getting back to sleep and worrying about how I would feel in the morning without a goodnight’s sleep. I sleep only about 5 hours most nights. I feel sleepy during the day, and sometimes I doze off while watching the news in the morning. It is nice having my grandchildren visit, but I am often tired and unable to do much with them because I need some space to rest. I do not remember if I have taken my pills sometimes or if I have checked the mail. My wife is now helping me with these tasks. I might need to ask my doctor for some sleeping pills, but I am not sure if I can take more medications now since I am very sick.”
8 |. BORDERLINE CASE
Mrs. Shah is a 60-year-old LVAD recipient due to advanced HF and ineligibility for a cardiac transplant. It has been 6 months since she received her LVAD. Mrs. Shah has trouble falling asleep sometimes. However, she sleeps through the night. She has about 7 hours of sleep each night. Occasionally, Mrs. Shah feels a lack of energy and motivation, but she continues her volunteer work at the community center.
9 |. ILLEGITIMATE CASE
Mr. Smith is a 45-year-old engineer with advanced HF. He received an LVAD as a bridge to transplant. He reports that he sleeps better after the LVAD implant, even though he has to sleep with a new device. He sometimes has trouble falling asleep if he has coffee after lunch. Mr. Smith wakes up a couple of times at night because his cat tries to jump into bed. During the day, Mr. Smith is able to perform LVAD related tasks (e.g., dressing change and controller check) and manage his HF medications independently. Mr. Smith is able to go to the office to work 2 days a week and feels that having this new device has improved his quality of life.
10 |. EMPIRICAL REFERENTS
Empirical referents are what make the concept of SD measurable, including subjective and objective measurements. Subjective measures focus on self-reported SD. The Pittsburgh Sleep Quality Index (PSQI) is the most widely used instrument for SD. The PSQI measures subjective sleep quality and quantity over a 1-month interval. PSQI assesses seven domains of sleep quality.59 The Patient-Reported Outcomes Measurement Information System (PROMIS™) SD is another instrument for measuring SD. The PROMISE SD instrument assesses the qualitative aspect of sleep and wake function and gauges the severity of sleep-wake problems across a range of conditions.60
Objective measures of SD provide information on the mechanism of underlying causes of SD and compare self-reported sleep outcomes with objective data. PSG and actigraphy are two traditional objective measures of SD in both HF and the general population. PSG is a gold standard physiologic measure for sleep. PSG collects brain waves, the oxygen level in the blood, heart rate, breathing, and eye and leg movements.61 Actigraphy assesses sleep duration and continuity and is noninvasive. Actigraphy records arm movement used to estimate sleep parameters with specialized algorithms using computer software programs.62,63 A new device, the WatchPAT®, integrating advanced actigraphy to differentiate wake and sleep periods, Peripheral Arterial Tone (PAT) signal, heart rate, and oximetry, is able to calculate a true sleep time to prevent underestimation of respiratory events and arousals.64 The PAT signal amplitude is used to measure the pulsatile volume changes in the fingertip, reflecting the variations in sympathetic tones caused by respiratory disturbances during sleep.65 Analyzing the PAT signal amplitude, heart rate, and oxygen saturation simultaneously using the automatic algorithm detects breathing problems and respiratory-related arousals, uncovering the physiologic changes of sleep and quantifying SD.
11 |. POTENTIAL CONTRIBUTION TO NURSING SCIENCE
The clarification of SD in individuals with HF has implications for clinical nursing practice and research. SD affects a significant number of individuals with HF, and individuals experiencing SD are at risk for developing adverse health consequences. A clear understanding of SD characteristics in individuals with HF will allow nurses and clinicians to assess and determine the causes of poor sleep quality. In-depth knowledge of contributing factors will lead to the development, implementation, and evaluation of interventions to assist individuals with sleep self-management. Healthy sleep may enable better participation in self-care and long-term management of HF and augment HRQOL. Last, a succinct understanding of SD will help researchers differentiate SD from other related concepts.
12 |. FURTHER NEEDED DEVELOPMENT
Riegel and Weaver developed a conceptual framework of how sleep may contribute to self-care and outcomes in persons with HF in 2019.36 Since then, sleep-related daytime symptoms and poor sleep quality are found to be associated with poor medication adherence,14–16 and inadequate self-care.17–19 One important relationship of this framework, sleep and cognition, is understudied, particularly in individuals with advanced HF living with mechanical circulatory support devices, such as LVAD. Current literature indicates that cognition declines with increased severity of HF. However, the underlying causes of cognitive impairment are not well understood. Symptoms of SD, cognitive impairment, and psychological symptoms, such as anxiety and depression, adversely affect the overall HRQOL in individuals with HF. While HF-related symptoms may be improved by novel advanced HF therapy, the root causes of these symptoms are multifactorial, and relationships among these symptoms are multidirectional, likely complicated by individual comorbidities. Future studies can investigate the highly relevant physiologic linkages among these HF-related symptoms.
13 |. CONCLUSION
SD is a commonly used term when describing sleep problems and sleep disorders in both the general population and individuals with HF. To evaluate SD in individuals with HF, we must examine the underlying causes to provide the contextual multifactorial meaning of the concept. Defining SD as a condition in which individuals experience difficulty initiating and maintaining sleep, and difficulty continuing or resuming sleep due to frequent nocturnal arousals creates the possibility for accurate measurements of SD, exploring interventions, and evaluating outcomes.
ACKNOWLEDGMENTS
Special thanks to Cynthia Dougherty, PhD, ARNP, FAAN, for her exceptional support throughout the development of this manuscript. This work was supported, in part, by the National Institutes of Health, National Institute of Nursing Research, Omics and Symptom Science Training Program at the University of Washington (T32NR016913). The content is solely the responsibility of the author and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Virani SS, Alonso A, Aparicio HJ, et al. Heart disease and stroke statistics—2021 update: a report from the American Heart Association. Circulation. Published online 2021;143:e254–e743. 10.1161/CIR.0000000000000950 [DOI] [PubMed] [Google Scholar]
- 2.Redeker NS, Jeon S, Muench U, Campbell D, Walsleben J, Rapoport DM. Insomnia symptoms and daytime function in stable heart failure. Sleep. 2010;33(9):1210–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Redeker NS, Stein S. Characteristics of sleep in patients with stable heart failure versus a comparison group. Heart & Lung. 2006;35(4): 252–261. 10.1016/j.hrtlng.2005.10.007 [DOI] [PubMed] [Google Scholar]
- 4.Riegel B, Glaser D, Richards K, et al. Modifiable factors associated with sleep dysfunction in adults with heart failure. Eur J Cardiovasc Nurs. 2012;11(4):402–409. 10.1016/j.ejcnurse.2011.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Broström A, Strömberg A, Dahlström U, Fridlund B. Patients with congestive heart failure and their conceptions of their sleep situation. J Adv Nurs. 2001;34(4):520–529. 10.1046/j.1365-2648.2001.01781.x [DOI] [PubMed] [Google Scholar]
- 6.Jeon S, Redeker NS. Sleep disturbance, daytime symptoms, and functional performance in patients with stable heart failure: a mediation analysis. Nurs Res. 2016;65(4):259–267. 10.1097/NNR.0000000000000169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oldenburg O, Teerlink JR. Screening for sleep-disordered breathing in patients hospitalized for heart failure. JACC: Heart Failure. 2015;3(9):732–733. 10.1016/j.jchf.2015.07.006 [DOI] [PubMed] [Google Scholar]
- 8.Paulino A, Damy T, Margarit L, et al. Prevalence of sleep-disordered breathing in a 316-patient French cohort of stable congestive heart failure. Arch Cardiovasc Dis. 2009;102(3):169–175. 10.1016/j.acvd.2008.12.006 [DOI] [PubMed] [Google Scholar]
- 9.Khayat R, Small R, Rathman L, et al. Sleep disordered breathing in heart failure: identifying and treating an important but often unrecognized comorbidity in heart failure patients. J Card Fail. 2013; 19(6):431–444. 10.1016/j.cardfail.2013.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Redeker NS, Muench U, Zucker MJ, et al. Sleep disordered breathing, daytime symptoms, and functional performance in stable heart failure. Sleep. 2010;33(4):551–560. 10.1093/sleep/33.4.551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lanfranchi PA, Somers VK, Braghiroli A, Corra U, Eleuteri E, Giannuzzi P. Central sleep apnea in left ventricular dysfunction: prevalence and implications for arrhythmic risk. Circulation. 2003;107(5):727–732. 10.1161/01.CIR.0000049641.11675.EE [DOI] [PubMed] [Google Scholar]
- 12.Wang H, Parker JD, Newton GE, et al. Influence of obstructive sleep apnea on mortality in patients with heart failure. J Am Coll Cardiol. 2007;49(15):1625–1631. 10.1016/j.jacc.2006.12.046 [DOI] [PubMed] [Google Scholar]
- 13.Lanfranchi PA, Braghiroli A, Bosimini E, et al. Prognostic value of nocturnal Cheyne-Stokes respiration in chronic heart failure. Circulation. 1999;99(11):1435–1440. 10.1161/01.CIR.99.11.1435 [DOI] [PubMed] [Google Scholar]
- 14.Riegel B, Moelter ST, Ratcliffe SJ, et al. Excessive daytime sleepiness is associated with poor medication adherence in adults with heart failure. J Card Fail. 2011;17(4):340–348. 10.1016/j.cardfail.2010.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knafl GJ, Riegel B. What puts heart failure patients at risk for poor medication adherence? Patient Prefer Adherence. 2014;8:1007–1018. 10.2147/PPA.S64593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Riegel B, Lee CS, Ratcliffe SJ, et al. Predictors of objectively measured medication nonadherence in adults with heart failure. Circ Heart Fail. 2012;5(4):430–436. 10.1161/CIRCHEARTFAILURE.111.965152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kamrani A-AA, Foroughan M, Taraghi Z, et al. Self care behaviors among elderly with chronic heart failure and related factors. Pak J Biol Sci. 2014;17(11):1161–1169. 10.3923/pjbs.2014.1161.1169 [DOI] [PubMed] [Google Scholar]
- 18.Riegel B, Vaughan Dickson V, Goldberg LR, Deatrick JA. Factors associated with the development of expertise in heart failure self-care. Nurs Res. 2007;56(4):235–243. 10.1097/01.NNR.0000280615.75447.f7 [DOI] [PubMed] [Google Scholar]
- 19.Kessing D, Denollet J, Widdershoven J, Kupper N. Fatigue and self-care in patients with chronic heart failure. European Journal of Cardiovascular Nursing. 2016;15(5):337–344. 10.1177/1474515115575834 [DOI] [PubMed] [Google Scholar]
- 20.Walker LO, Avant KC. Concept analysis. In: Walker LO, Avant KC eds. Strategies for Theory Construction in Nursing. 5th ed. Boston, MA: Prentice Hall; 2011. [Google Scholar]
- 21.Cormier RE. Sleep disturbances. In: Walker HK, Hall WD, Hurst JW eds. Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd ed. Boston, MA: Butterworths; 1990. http://www.ncbi.nlm.nih.gov/books/NBK401/ [PubMed] [Google Scholar]
- 22.Suzuki M, Furihata R, Konno C, et al. Sleep disturbance is associated with not only shorter sleep duration, but also longer time in bed: a Japanese general population survey. Sleep Biol Rhythms. 2019;17(4): 407–415. 10.1007/s41105-019-00228-x [DOI] [Google Scholar]
- 23.St-Onge M-P, Grandner MA, Brown D, et al. Sleep duration and quality: impact on lifestyle behaviors and cardiometabolic health. Circulation. 2016;134(18):e367–e386. 10.1161/CIR.0000000000000444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johansson P, Riegel B, Svensson E, et al. The contribution of heart failure to sleep disturbances and depressive symptoms in older adults. J Geriatr Psychiatry Neurol. 2012;25(3):179–187. 10.1177/0891988712458366 [DOI] [PubMed] [Google Scholar]
- 25.Lee KA. Impaired sleep. In: Carrieri-Kohlman V, Lindsey AM, West CM eds. Pathophysiological Phenomena in Nursing: Human Responses to Illness. 3rd ed. Philadelphia, PA: Saunders; 2003. [Google Scholar]
- 26.Erickson VS, Westlake CA, Dracup KA, Woo MA, Hage A. Sleep disturbance symptoms in patients with heart failure. AACN Clin Issues. 2003;14(4):477–487. 10.1097/00044067-200311000-00009 [DOI] [PubMed] [Google Scholar]
- 27.Broström A, Strömberg A, Dahlström U, Fridlund B. Sleep difficulties, daytime sleepiness, and health-related quality of life in patients with chronic heart failure. J Cardiovasc Nurs. 2004;19(4):234–242. 10.1097/00005082-200407000-00003 [DOI] [PubMed] [Google Scholar]
- 28.Roux F, D’Ambrosio C, Mohsenin V. Sleep-related breathing disorders and cardiovascular disease. Am J Med. 2000;108(5):396–402. 10.1016/S0002-9343(00)00302-8 [DOI] [PubMed] [Google Scholar]
- 29.Bradley TD, Hall MJ, Ando S, Floras JS. Hemodynamic effects of simulated obstructive apneas in humans with and without heart failure. Chest. 2001;119(6):1827–1835. 10.1378/chest.119.6.1827 [DOI] [PubMed] [Google Scholar]
- 30.Hanly P, Zuberi-Khokhar N. Daytime sleepiness in patients with congestive heart failure and Cheyne-Stokes respiration. Chest. 1995;107(4):952–958. 10.1378/chest.107.4.952 [DOI] [PubMed] [Google Scholar]
- 31.Rowley JA, Badr MS. Central sleep apnea in patients with congestive heart failure. Sleep Med Clin. 2017;12(2):221–227. 10.1016/j.jsmc.2017.03.001 [DOI] [PubMed] [Google Scholar]
- 32.Casida JM, Brewer RJ, Smith C, Davis JE. An exploratory study of sleep quality, daytime function, and quality of life in patients with mechanical circulatory support. Int J Artif Organs. 2012;35(7): 531–537. 10.5301/ijao.5000109 [DOI] [PubMed] [Google Scholar]
- 33.Coats AJS. Monitoring for sleep-disordered breathing in heart failure. Eur Heart J Suppl. 2019;21(Suppl M):M36–M39. 10.1093/eurheartj/suz233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang T-J, Lee S-C, Tsay S-L, Tung H-H. Factors influencing heart failure patients’ sleep quality. J Adv Nurs. 2010;66(8):1730–1740. 10.1111/j.1365-2648.2010.05342.x [DOI] [PubMed] [Google Scholar]
- 35.Lee KS, Lennie TA, Heo S, Song EK, Moser DK. Prognostic importance of sleep quality in patients with heart failure. Am J Crit Care. 2016;25(6):516–525. 10.4037/ajcc2016219 [DOI] [PubMed] [Google Scholar]
- 36.Riegel B, Weaver TE. Poor sleep and impaired self-care: towards a comprehensive model linking sleep, cognition, and heart failure outcomes. Eur J Cardiovasc Nurs. 2009;8(5):337–344. 10.1016/j.ejcnurse.2009.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hanly PJ, Zuberi-Khokhar N. Periodic limb movements during sleep in patients with congestive heart failure. Chest. 1996;109(6): 1497–1502. 10.1378/chest.109.6.1497 [DOI] [PubMed] [Google Scholar]
- 38.Broström A, Johansson P. Sleep disturbances in patients with chronic heart failure and their holistic consequences—what different care actions can be implemented? Eur J Cardiovasc Nurs. 2005; 4(3):183–197. 10.1016/j.ejcnurse.2005.04.005 [DOI] [PubMed] [Google Scholar]
- 39.Fitzpatrick JJ, McCarthy G. Concept Analysis. In: Fitzpatrick JJ, McCarthy G eds. Nursing Concept Analysis: Applications to Research and Practice. New York, New York: Springer Publishing Company; 2016. [Google Scholar]
- 40.Jeon S, Redeker NS. Daytime symptoms mediate the effects of sleep disturbance on functional performance in stable heart failure. Nurs Res. 2016;65(4):259–267. 10.1097/NNR.0000000000000169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.White LH, Bradley TD. Role of nocturnal rostral fluid shift in the pathogenesis of obstructive and central sleep apnoea. J Physiol. 2013;591(Pt 5):1179–1193. 10.1113/jphysiol.2012.245159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Broström A, Strömberg A, Dahlström U, Fridlund B. Congestive heart failure, spouses’ support and the couple’s sleep situation: a critical incident technique analysis. J Clin Nurs. 2003;12(2):223–233. 10.1046/j.1365-2702.2003.00692.x [DOI] [PubMed] [Google Scholar]
- 43.Baldwin CM, Griffith KA, Nieto FJ, O’Connor GT, Walsleben JA, Redline S. The association of sleep-disordered breathing and sleep symptoms with quality of life in the sleep heart health study. Sleep. 2001;24(1):96–105. 10.1093/sleep/24.1.96 [DOI] [PubMed] [Google Scholar]
- 44.Javaheri S, McKane SW, Cameron N, Germany RE, Malhotra A. In patients with heart failure the burden of central sleep apnea increases in the late sleep hours. Sleep. 2018;42(1):zsy195. 10.1093/sleep/zsy195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dark DS, Pingleton SK, Kerby GR, et al. Breathing pattern abnormalities and arterial oxygen desaturation during sleep in the congestive heart failure syndrome: improvement following medical therapy. Chest. 1987;91(6):833–836. 10.1378/chest.91.6.833 [DOI] [PubMed] [Google Scholar]
- 46.Yamashiro Y, Kryger MH. Review: sleep in heart failure. Sleep. 1993; 16(6):513–523. 10.1093/sleep/16.6.513 [DOI] [PubMed] [Google Scholar]
- 47.Redeker NS. Sleep disturbance in people with heart failure: implications for self-care. J Cardiovasc Nurs. 2008;23(3):231–238. 10.1097/01.JCN.0000305094.20012.76 [DOI] [PubMed] [Google Scholar]
- 48.Príncipe-Rodríguez K, Strohl KP, Hadziefendic S, Piña IL. Sleep symptoms and clinical markers of illness in patients with heart failure. Sleep Breath. 2005;9(3):127–133. 10.1007/s11325-005-0023-0 [DOI] [PubMed] [Google Scholar]
- 49.Kanno Y, Yoshihisa A, Watanabe S, et al. Prognostic significance of insomnia in heart failure. Circ J. 2016;80(7):1571–1577. 10.1253/circj.CJ-16-0205 [DOI] [PubMed] [Google Scholar]
- 50.Kalmbach DA, Anderson JR, Drake CL. The impact of stress on sleep: pathogenic sleep reactivity as a vulnerability to insomnia and circadian disorders. J Sleep Res. 2018;27(6):e12710. 10.1111/jsr.12710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sandau KE, Lee CS, Faulkner KM, et al. Health-related quality of life in patients with a left ventricular assist device (QOLVAD) questionnaire: initial psychometrics of a new instrument. J Cardiovasc Nurs. 2020;36: 172–184. 10.1097/JCN.0000000000000774 [DOI] [PubMed] [Google Scholar]
- 52.Coman AC, Borzan C, Vesa CS, Todea DA. Obstructive sleep apnea syndrome and the quality of life. Clujul Med. 2016;89(3):390–395. 10.15386/cjmed-593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guilleminault C, Stoohs R, Clerk A, Cetel M, Maistros P. A cause of excessive daytime sleepiness: the upper airway resistance syndrome. Chest. 1993;104(3):781–787. 10.1378/chest.104.3.781 [DOI] [PubMed] [Google Scholar]
- 54.Pressler SJ. Cognitive functioning and chronic heart failure: a review of the literature (2002-July 2007). J Cardiovasc Nurs. 2008;23(3): 239–249. 10.1097/01.JCN.0000305096.09710.ec [DOI] [PubMed] [Google Scholar]
- 55.Varga AW, Kishi A, Mantua J, et al. Apnea-induced rapid eye movement sleep disruption impairs human spatial navigational memory. J Neurosci. 2014;34(44):14571–14577. 10.1523/JNEUROSCI.3220-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McMorris T, Hale BJ, Barwood M, Costello J, Corbett J. Effect of acute hypoxia on cognition: a systematic review and meta-regression analysis. Neurosci Biobehav Rev. 2017;74:225–232. 10.1016/j.neubiorev.2017.01.019 [DOI] [PubMed] [Google Scholar]
- 57.Schwarz EI, Furian M, Schlatzer C, Stradling JR, Kohler M, Bloch KE. Nocturnal cerebral hypoxia in obstructive sleep apnoea—a randomised controlled trial. Eur Respir J. 2018;51:1800032. 10.1183/13993003.00032-2018 [DOI] [PubMed] [Google Scholar]
- 58.Johansson P, Alehagen U, Svensson E, Svanborg E, Dahlström U, Broström A. Determinants of global perceived health in community-dwelling elderly screened for heart failure and sleep-disordered breathing. J Cardiovasc Nurs. 2010;25(5):E16–E26. 10.1097/JCN.0b013e3181d6de6f [DOI] [PubMed] [Google Scholar]
- 59.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. 10.1016/0165-1781(89)90047-4 [DOI] [PubMed] [Google Scholar]
- 60.Yu L, Buysse DJ, Germain A, et al. Development of short forms from the PROMIS sleep disturbance and sleep-related impairment item banks. Behav Sleep Med. 2011;10(1):6–24. 10.1080/15402002.2012.636266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rundo JV, Downey RP. Handbook of clinical neurology. Handb Clin Neurol. 2019;160:381–392. 10.1016/B978-0-444-64032-1.00025-4 [DOI] [PubMed] [Google Scholar]
- 62.Martin JL, Hakim AD. Wrist actigraphy. Chest. 2011;139(6): 1514–1527. 10.1378/chest.10-1872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Walia HK, Mehra R. Practical aspects of actigraphy and approaches in clinical and research domains. Handb Clin Neurol. 2019;160: 371–379. 10.1016/B978-0-444-64032-1.00024-2 [DOI] [PubMed] [Google Scholar]
- 64.Yalamanchali S, Farajian V, Hamilton C, Pott TR, Samuelson CG, Friedman M. Diagnosis of obstructive sleep apnea by peripheral arterial tonometry: meta-analysis. JAMA Otolaryngol Head Neck Surg. 2013;139(12):1343–1350. 10.1001/jamaoto.2013.5338 [DOI] [PubMed] [Google Scholar]
- 65.Herscovici S, Pe’er A, Papyan S, Lavie P. Detecting REM sleep from the finger: an automatic REM sleep algorithm based on peripheral arterial tone (PAT) and actigraphy. Physiol Meas. 2007;28(2): 129–140. 10.1088/0967-3334/28/2/002 [DOI] [PubMed] [Google Scholar]


