Abstract
Objective:
To study the reprometabolic syndrome in normal weight, eumenorrheic women by infusing a combination of insulin and lipid. Women with obesity have been shown to have reduced gonadotropins and impaired LH and FSH response to gonadotropin releasing hormone (GnRH).
Design:
Randomized crossover.
Setting:
Academic medical center.
Participants:
15 women, median age 32 [IQR 26, 36] and BMI 21.9 [20.2, 22.9] were recruited
Interventions:
Early follicular phase, 6-hour infusions of insulin (20-40mg/mU/m2/min) and lipid (Intralipid)—insulin/lipid infusion; or saline infusion (controls). The first 4 hours of each study assessed endogenous gonadotropins; at 4hrs, a 75 ng/kg GnRH bolus was administered and sampling continued until 6hrs.
Main Outcome Measures:
Linear mixed model analysis was used to determine differences between insulin/lipid versus saline on endogenous LH pulse amplitude (primary outcome), mean FSH, and area under the curve (AUC) response to GnRH (secondary outcomes).
Results:
12 women completed both intended studies and an additional 3 women completed only one of the two studies. LH pulse amplitude, mean FSH, and both AUC responses to GnRH were reduced by insulin/lipid, mean FSH (P=0.03) and AUC for LH (P=0.05) were at or near statistical significance. LH response to GnRH was significantly reduced (P=0.02) when one participant with very high LH and AMH levels was excluded.
Conclusions:
Acute infusion of insulin/lipid to eumenorrheic, normal weight women recapitulated the reprometabolic syndrome of obesity. These findings imply that specific circulating factors in obese women contribute to their subfertility and thus may be amenable to discovery and treatment. (Clinicaltrials.gov #NCT02653092)
Keywords: Gonadotropins, LH, FSH, free fatty acids, hyperinsulinemia, GnRH
INTRODUCTION
Obesity exerts a number of detrimental effects on reproduction at many levels of the hypothalamic-pituitary-ovarian-uterine axis. In addition to delaying time to conception(1), obesity is associated with decreased pituitary and ovarian hormone production(2, 3), There is some evidence that obesity impairs the process of implantation(4, 5), although not all studies are in agreement on this outcome(6). The relationship of obesity to fertility is important to elucidate, because simple measures such as behavioral weight loss are not only extremely difficult to achieve, but have not been shown to be beneficial in terms of improving live birth rates (7, 8); in fact, both of the cited studies have had findings that verged on harm for the weight loss intervention; in one, the time to pregnancy was almost significantly prolonged in women pursuing weight loss(7); in the other, pregnancy losses trended higher in the behavioral weight loss group(8). Therefore, a deeper understanding of the mechanisms behind obesity related infertility is needed to derive targeted therapies that aim directly at the factors causing the fertility impairment.
We have previously demonstrated decreased LH pulse amplitude, dramatically reduced luteal progesterone metabolite excretion(9) and reduced responsiveness to exogenous gonadotropin releasing hormone (GnRH)(10) in women with obesity. We have also reported that exposure of normal weight women and men to excess nonesterified fatty acids (NEFA) and insulin, in an effort to reproduce the circulating metabolic milieu of obesity, resulted in decreased gonadotropin secretion(11). In this report, both hyperinsulinemia and NEFA excess were required to suppress gonadotropins, as either condition alone was insufficient. However, minute-to-minute characteristics of gonadotropin secretion were not well characterized, as serum was only sampled every two hours in the former study.
In order to elucidate more precisely how the circulating metabolic environment of obesity exerts its negative impact on the hypothalamic pituitary part of the reproductive axis, we examined detailed gonadotropin secretion patterns and responsiveness to GnRH in a sample of regularly cycling, normal weight women who underwent a saline infusion control study and, in a separate cycle, an insulin/lipid infusion. Assignment to saline or to insulin/lipid infusion was made in random order.
MATERIALS AND METHODS
This study was registered on clinicaltrials.gov (NCT02653092) and approved by the Colorado Multiple Institutional Review Board (COMIRB). All participants provided informed consent for the procedures described below.
Patient Population and Protocol.
Fifteen healthy, eumenorrheic (menses every 25-35 days) women were recruited through University campus-wide advertising. Participants were required to be aged 18-38 and of normal BMI (18-25 kg/m2), free of medications or chronic diseases that would interact with reproductive hormones or insulin metabolism, and without use of hormonal contraceptives for at least 3 months. Participants were screened for normal prolactin, thyroid stimulating hormone (TSH), and hemoglobin A1c (HgbA1c), and because of the frequent blood sampling tests were required to have a hemoglobin >11 gm/dl. Women with fasting triglycerides >300mg/dl at screening were excluded for safety reasons.
Participants were planned to undergo two 6-hour frequent blood sampling studies: one control study with infusion of saline and heparin (to maintain patency of the IV sampling line) and a second study in which lipid + insulin (also with heparin, see below for further details) were infused in a random-allocation, crossover study design. The study research coordinator (KK) recruited participants and the PI and research coordinator (KK, NS) determined eligibility. The study statistician (AF) provided all randomization allocations. All frequent blood sampling studies were performed in the early follicular phase of the menstrual cycle (cycle days 2-5 after the onset of menses) to standardize gonadotropin secretory patterns as much as could be practical. The order of the interventions (saline infusion versus insulin/lipid infusion) was assigned randomly. Participants were admitted after an overnight fast to the University of Colorado’s inpatient Clinical and Translational Research Center to accommodate a start time of 0800 hours. Participants did not have any oral intake during the infusion. Two intravenous lines were placed, one in each arm, one for frequent blood sampling every 10 minutes and the other for infusions. All women received heparin along with either saline or insulin and lipid. For the control study, saline and heparin (starting dose was 100units/ml at 24 units/kg/hr for heparin, with downward safety adjustments made for a partial thromboplastin time [PTT]>120 seconds at the 180 minute time point) were infused through the intravenous line in the arm opposite the one used for the blood sampling. For the insulin/lipid infusion, insulin, free fatty acids, and heparin were infused as follows. Insulin (Humulin, Eli Lilly and Company) was infused at a rate of 20-40 mU/m2/min. Initial studies (N=3) used the 40 mU/m2/min infusion rate, but this was adjusted to 20 mU/m2/min after the first set of studies because the sample was overall very insulin sensitive, and the volume of fluid administered to maintain euglycemia ranged from 733-1252 mls at the 40 mU/m2/min insulin infusion rate, leading to concern for hemodilution in the insulin/lipid studies compared to the control saline/heparin studies. Dextrose 10% was infused at a variable rate as needed to maintain blood glucose levels within the normal range, and a 20% lipid emulsion (Intralipid, Baxter Healthcare Corporation, Deerfield, IL, consisting of 20% soybean oil, 1.2% egg yolk phospholipids, and 2.25% glycerin—main fatty acid components: linoleic [44-64%], oleic [19-30%], palmitic [7-14%] linolenic [4-11%] and stearic [1.4-5.5%]) was co-infused with heparin (as described above) at a rate of 45 ml/hr. Heparin co-infusion was performed to release lipoprotein lipase into the circulation and assure that the lipid infusion induced elevation of both triglycerides and fatty acids. Blood glucose was checked at the bedside every 5 minutes using a Nova Biomedical Stat Strip Glucometer, and the glucose infusion rate was adjusted accordingly to maintain euglycemia. All blood sampling studies involving insulin infusion were attended directly by one of the authors (IES).
Blood was withdrawn every 10 minutes for 360 minutes (6 hours). For the first 4 hours, endogenous LH pulsatility and FSH secretion were assessed. At 4 hours (240 minutes), an intravenous bolus of GnRH (IND 118478), 75 ng/kg, previously found to be a physiologic dose of GnRH in women(12), was administered as a single IV bolus and q10’ blood sampling was continued for the next two hours until 360 minutes, at which time all IV lines were removed and the participants were discharged after being observed for any signs of hypoglycemia or persistently elevated PTT.
Each participant was intended to complete both studies (saline and insulin/lipid infusion) in random order. After the first study was completed, participants were followed with the intent to complete the second study within no more than 12 intervening menstrual cycles; all follow-up study visits were actually completed within 7 months as the maximum interval. The first study was completed on 5/15/2016 and the final study was completed on 10/17/2018.
Measurements.
Blood samples from the q10’ sampling (3cc) studies were stored overnight at 4° C and centrifuged the following morning to separate serum. Serum was stored at −80° C until thawed for assay. A 20ul aliquot from each time point was withdrawn from the serum samples before freezing to create a pool for the entire study.
LH and FSH were measured using a competitive chemiluminescent immunoassay (Advia Centaur XP; Siemens Healthcare Diagnostics). Inter- and intra-assay coefficients of variation (cvs) were 4.8 and 3.4% for LH, respectively, and 6.6 and 5.0% for FSH, respectively. AMH was measured using the Ansh pico ELISA (Ansh Labs, Webster, TX), which has a sensitivity of 0.0015 ng/ml and inter- and intra-assay cvs of 3.7-8.1% and 2.5-5.5%, respectively. Estradiol was measured using a commercial immunoassay (Siemens Centaur enhanced estradiol assay, Tarrytown, NY) which has a functional sensitivity of 19.0 pg/ml and inter- and intra-assays cvs of 10.6 and 7.5%, respectively. Median follicular phase ranges for this estradiol assay are 51.8 pg/ml with a range of 19.5-144.2(13).
Sample Size, Data Analysis and Statistics.
Our published comparative studies have indicated a 50% reduction in LH pulse amplitude in obese women compared to their normal weight counterparts(9). Based on the large percent change and effect size of previous studies ranging from 0.8-1.24 IU/L with SDs of 0.6-.088 IU/L, respectively (9, 11), a sample size of 10 women was required to demonstrate short term changes in LH amplitude (primary outcome) with 80% power at an α=0.05 in a 2-sided paired test. Hours 0-4 of each sampling period, which represented endogenous LH and FSH secretion, were initially analyzed separately from hours 2-4 (after steady state had been reached with the insulin infusion); however, since the results for 2-4 hours were similar to 0-4 hours, only the four hour results are shown. Mean FSH and the frequency and amplitude of LH pulses were assessed using a modified Santen and Bardin method, as previously described(9, 14). Mean LH pulse amplitude over the initial 4 hours of the frequent sampling study was the primary endpoint. Secondary end points were: 1. LH and 2. FSH response to GnRH (area under the curve and nadir-to-peak amplitude), 3. mean LH and 4. FSH over the initial 4 hours of the frequent sampling study. As analysis of FSH data did not reveal any discrete pulsations (as expected), only the response to GnRH and mean levels could be compared between the two study conditions for FSH.
Although a paired design was intended, not all participants completed both a control (saline) and a lipid (insulin/lipid) infusion study. In order to include as much data as possible, linear mixed models were used to incorporate the data from women who only completed 1 of the 2 intended study visits. The outcomes of mean LH pulse amplitude, mean FSH for 0-4 hours, and AUC of LH and FSH, were log-transformed; mean LH pulse frequency per hour was not log-transformed. The models included visit (saline or insulin/lipid) as the covariate and a random intercept per subject. While tests were performed using log-transformations for the specified variables, estimated means and standard errors (SEs) are presented in the raw scale. In case of outliers, all analyses were repeated excluding 1 subject at a time to evaluate the potential effect of outliers on the results. Baseline characteristics of the sample (age, weight, BMI, HBA1C, TSH, prolactin, and AMH) were examined using descriptive statistics, medians and interquartile ranges. Similarly to above, linear mixed models were used to assess if there were differences between the control and lipid visits for glucose, triglycerides, NEFA, or insulin. Triglycerides, insulin, and NEFA were log-transformed for modeling and presented as estimated means and SEs in the raw scale, while glucose did not need log-transformation. SAS 9.4 was used to perform analyses with a significance level of 0.05.
RESULTS
Participant description and study parameters.
Characteristics of the study participants are provided in Table 1. The 15 women who completed at least one of the intended pair of infusions had a median age of 32 years and a median BMI of 21.9 kg/m2. Ovarian reserve was variable, with AMH as low as 0.44 ng/ml and as high as 9.74 ng/ml in one participant. Median hemoglobin A1c was 5%. Thirteen of the 15 participants self-identified as White, one was Asian and one was Hispanic. Of the 12 women who completed both studies, 6 were done in consecutive cycles, 10 completed both infusions within 3 months, and 2 completed infusions within 7 months. There were no changes in body weight between the two studies. All studies were completed within the 2-5 day follicular phase window, and day of the cycle did not differ in any systematic way between the two infusions.
Table 1.
Baseline characteristics of the study sample for all participants who provided data from at least one study (N=15). Median and interquartile range (IQR: 25th and 75th percentiles) are provided for all parameters.
| Characteristic | Median (IQR) |
|---|---|
| AGE | 32.00 (26.00-36.00) |
| WEIGHT | 57.40 (52.70-66.00) |
| BMI | 21.90 (20.15-22.92) |
| HbA1C* | 5.00 (4.80-5.40) |
| TSH | 1.48 (1.28-2.06) |
| PROLACTIN | 9.20 (6.40-10.80) |
| AMH** | 4.10 (0.98-5.78) |
To convert AMH to SI units (pmol/L), multiply by 7.14. HbA1c=hemoglobin A1c; TSH=thyroid stimulating hormone; AMH=antimullerian hormone; BMI=body mass index
HbA1c was missing for one woman
AMH was missing for one woman
Euglycemia was maintained throughout the insulin/lipid infusion and average glucose did not differ between the two visits (Table 2). Due to the fasting state, NEFA elevations during the saline visits versus infusion-mediated NEFA elevation accompanied by insulin-mediated suppression of lipolysis during the insulin/lipid infusion visits resulted in similar NEFA levels in both experimental conditions (Table 2). Insulin/lipid infusion did result in a higher average NEFA level, but the elevation did not reach statistical significance, despite the co-infusion of heparin. Triglycerides and insulin were significantly higher at steady state during the insulin/lipid infusion than during the saline infusion (Table 2).
Table 2.
Effects of insulin/lipid infusion versus saline infusion on steady state metabolic parameters (estimated means and standard errors (SEs) for t=2-6 hours) from linear mixed models.
| Outcome | Control (N=15) |
Insulin/Lipid (N=12) |
P |
|---|---|---|---|
| NEFA (uEg/L) | 768.54 (1.08) | 906.68 (1.08) | 0.10 |
| Glucose (mg/dL) | 83.91 (1.36) | 85.89 (1.50) | 0.25 |
| Insulin (uIU/mL) | 1.81 (1.11) | 24.04 (1.12) | <0.01 |
| Triglycerides (mg/dL) | 47.47 (1.12) | 103.13 (1.12) | <0.01 |
To convert triglycerides to SI units (mmol/L), multiply by 0.0113
Gonadotropin Outcomes (Table 3).
Table 3.
Effects of Insulin/Lipid Infusion on Primary and Secondary Outcomes of Gonadotropin Secretion
All participants who completed at least 1 infusion study are included. Data shown are model estimated means and standard errors (SEs). AUC is shown as logged values without units. Estradiol is taken from a pooled sample comprised of an aliquot for each time point of the study from 0-360 minutes.
| Saline Infusion | Insulin/Lipid Infusion | ||||
|---|---|---|---|---|---|
| Outcome | N | Mean (SE) | N | Mean (SE) | P |
| LH pulse amplitude Hours 0-4 (IU/L)* | 15 | 2.33 (1.21) | 12 | 1.49 (1.23) | 0.14 |
| LH Pulses/hr | 15 | 0.73 (0.09) | 12 | 0.77 (0.10) | 0.72 |
| LH AUC* | 14 | 567.63 (1.22) | 12 | 355.12 (1.23) | 0.05 |
| FSH Mean (IU/L) Hours 0-4* | 15 | 9.39 (1.13) | 12 | 6.14 (1.14) | 0.03 |
| FSH AUC* | 14 | 242.46 (1.23) | 12 | 158.99 (1.25) | 0.12 |
| Estradiol (pg/ml) | 15 | 66 (7) | 12 | 75 (20) | 0.24 |
Estimated means and standard errors (SEs) are presented in the raw scale, but tests were performed using log-transformations. The N of 14 in the saline infusion excluded 1 woman who did not receive GnRH (therefore no AUC could be calculated). To convert estradiol to SI units, divide by .272
Primary and secondary outcomes are shown in this table and the Figure. Estradiol levels did not differ between the saline and insulin/lipid infusion studies.
LH secretory dynamics with saline vs insulin/lipid infusion.
Findings are provided in Table 3; graphical data are shown in Figure 1 for the aggregate data and a representative individual. Model estimated mean LH pulse amplitude in the saline infusion study was 2.33 (SE: 1.21) IU/L, and 1.49 (SE: 1.23) IU/L in the insulin/lipid infusion (P=0.14; Table 3). LH pulse frequency did not significantly differ between the two conditions (P=0.72).
Figure 1.
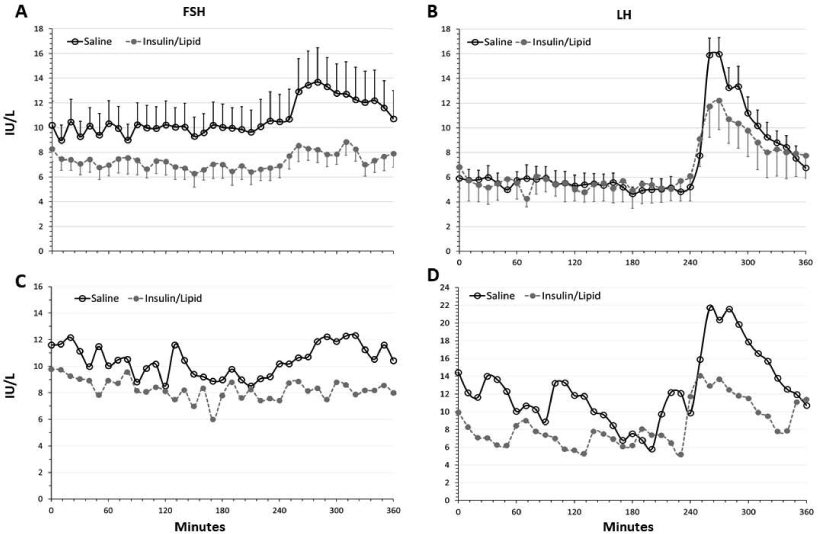
Mean LH (A) and FSH (B) with insulin/lipid infusion (closed circles) vs saline (control) infusion (open circles). Error bars indicate SEM. Bottom panels show FSH (C) and LH (D) in a representative participant who underwent saline control (open circles) and insulin/lipid infusion (closed circles). Women received heparin concurrent with both infusions (see text). A 75 ng/kg bolus of GnRH administered intravenously at 240 minutes.
Response to GnRH.
The estimated mean LH area under the curve was lower in the insulin/lipid infusion compared to saline (355.12 (SE: 1.23) vs 567.63 (SE: 1.22), respectively), but the difference was of borderline statistical significance (p=0.05; Table 3 and Figure 1). FSH AUC was lower in the insulin/lipid infusion compared to saline, with estimated means 158.99 (SE: 1.25) versus 242.46 (SE: 1.23) but was not statistically different between the two conditions (Table 3).
Mean LH and FSH response to saline vs insulin/lipid infusion.
Mean LH did not differ between the saline and insulin/lipid infusions (data not shown); however, mean FSH was 9.39 (SE: 1.13) IU/L during the saline infusion and decreased significantly to 6.14 (SE: 1.14) IU/L, P=0.03 (Table 3).
Sensitivity analysis.
One participant was noted to have very elevated LH levels and an AMH of 9.37 ng/ml., despite having regular menstrual cycles and meeting all other inclusion criteria. When this participant with high LH and AMH was excluded, LH pulse amplitude during the 4 hours of baseline LH secretion was marginally decreased by insulin/lipid infusion (P=0.06), as was LH AUC (p=0.02).
Adverse events.
There were no adverse events attributable to study procedures. One participant developed abdominal pain and a severe headache during a control (saline + heparin) infusion; examination by one of the study physicians (IES) and laboratory testing were negative. A subsequent examination and pelvic ultrasound performed by one of the study physicians (NS) did not indicate any pathology. This participant did not return for a second insulin + lipid infusion study. The amount of heparin infused was reduced per protocol when mid-study PTTs were found to exceed 120 seconds. Participants were observed in the clinical research unit until the PTT declined to <120second prior to discharge after obtaining a post-study level > 180 seconds in the first participant to complete the insulin + lipid + heparin infusion. No one developed bleeding complications.
DISCUSSION
Herein we demonstrate that inducing the conditions consistent with obesity (physiologically elevated insulin, triglycerides, and NEFA(15, 16)) in a sample of normal weight, regularly cycling women is capable of reproducing several of the deficits we have previously observed in association with obesity. These data strongly imply that hyperinsulinemia and hyperlipidemia regulate gonadotropin output in women and alterations in their concentration are capable of inducing short term deficits in gonadotropin secretion. These findings add to a growing body of literature implicating excess caloric intake with reduced reproductive capacity in both men and women.
We and others have shown that gonadotropin secretion is reduced in women with obesity(2, 3, 9, 17, 18) and that this is associated with reduced production of both estradiol and progesterone(3, 9) and their urinary metabolites(2). Moreover, pituitary response to exogenous GnRH is reduced in women with obesity(10) in the absence of any evidence of other deficits in pituitary hormone production. Lipotoxicity has been suggested as a mechanism by which gonadotropin secretion may be impaired, however the concentrations of NEFA needed to demonstrate induction of an unfolded protein response (UPR) and endoplasmic reticulum (ER) stress in mouse gonadotrophs(19) may be in excess of what is achieved in humans. The possibility that lipid infusion induces an acute inflammatory response also exists, however preliminary findings from our lab indicate that a host of pro-inflammatory cytokines and adipokines were unaltered by the insulin and lipid infusions carried out in this study(20). Careful examination of other pituitary hormones such as TSH and prolactin, and the downstream hormone produced by ACTH (cortisol) did not indicate evidence of acute, global pituitary deficits induced by the infusion protocol we utilized (21). Thus, the findings we report herein appear to be specific to gonadotrophs.
The ability to induce an acute deficit in FSH secretion using a short-term paradigm of insulin and lipid infusion suggests that the pathophysiology of obesity may have a reversible dietary component. Prior work by our group demonstrated a negative effect of NEFA and insulin on both LH and FSH secretion, in both women and men (11). The current study was undertaken to expand further upon those findings and to clarify whether the gonadotropin decrement we observed after insulin/lipid infusions was related to decreased pituitary sensitivity to GnRH by providing a GnRH bolus at the end of each study to specifically address pituitary response. Prior work by our group has demonstrated that in women with obesity, pituitary response to a weight-based dose of exogenous GnRH is blunted(10). Taken together, our data support these associations in that we observed some blunting of both unstimulated LH and FSH, and a blunted response to the same weight-based dose of GnRH in the women undergoing insulin/lipid infusion. However, results from the current study are consistently statistically significant only for FSH secretion, although the directionality of all of our findings point towards reduction of both LH and FSH output in association with the insulin/lipid infusion.
Exclusion of one participant with very high and non-reproducible LH levels markedly reduced the variation in the data and a clearer difference emerged for LH. Despite our use of prior data to derive an acceptable sample size, we note that the current sample of participants demonstrated a standard deviation for LH pulse amplitude, our primary outcome, that was approximately twice that of prior studies (1.6 IU/L vs 0.88 and 0.6). This increased variation likely caused us to be underpowered to detect a difference with our primary outcome. Indeed, when the one participant with high LH was excluded, the LH pulse amplitude was significantly different between the two infusions in the expected direction, suggesting that study power was marginal.
The rapid onset of the effect we observed is also remarkable, as we did not detect any difference in the first 2 hours of infusion versus the second 2 hours with respect to FSH and LH suppression. This finding indicates that the reduced gonadotropin output is not dependent upon achievement of a steady state of glucose and insulin.
The finding that FSH was more consistently suppressed by insulin/lipid infusion may be related to the fact that FSH synthesis and action are influenced not only by positive regulation via GnRH secretion, as is the case for LH, but also by negative regulation via inhibins and antimullerian hormone (AMH)(22, 23). The interplay of these regulatory factors in the acute experimental model presented herein may differ from the processes that occur in human overfeeding leading to obesity and the reprometabolic syndrome. However, they may provide a framework of inference from which further studies may proceed.
AMH is reduced in women with obesity(24), along with inhibin B(25) and early follicular phase estradiol(3). Taken together, these findings would be expected to result in increased and not decreased circulating FSH in the reprometabolic syndrome as a result of loss of all 3 known sources of negative feedback inhibition. The inappropriate suppression of FSH, and likely LH, in the face of this reduction in negative feedback implies strongly that the hypothalamic-pituitary axis is fundamentally dysregulated in the reprometabolic syndrome. AMH has been recently demonstrated to activate murine GnRH neurons in vitro and to increase GnRH-induced LH secretion(26). Thus, the relative lack of AMH and suppression of inhibin B in obesity may be a reflection of altered ovarian feedback that may be a primary consequence of obesity.
These data have clinical relevance because there have been virtually no successful inroads into the worldwide epidemic of obesity, and women are disproportionately affected. As more and more women acquire obesity, there is also increased risks to offspring and to future generations. In mice, epigenetic modifications in oocytes and (non-epigenetic) alterations in oocyte mitochondria function and number create long-lasting energy imbalances in the offspring that favor perpetuation of the obese phenotype(27). Although these rodent models are relatively extreme in their phenotype, involving the induction of frank diabetes along with ingestion of a very high fat diet, the implications for human reproduction are profound. Findings in humans indicate that obesity-related metabolic alterations predispose the offspring of obese mothers to obesity, type 2 diabetes and cardiometabolic disease risks(28, 29). Despite these known consequences of obesity, there are no current behavioral interventions that have demonstrated successful induction of fertility and reduced pregnancy complications(7, 8). While surgical weight loss appears to improve the reproductive phenotype(30), this modality has not been demonstrated to improve fertility. Although maternal risks of gestational diabetes and large for gestational age babies are reduced after weight loss surgery, the procedures carries their own set of perinatal(31) and maternal(32) risk, including increased maternal mortality. Moreover, surgical weight loss is not readily available to most reproductive aged women of high body mass due to access issues.
This study had several strengths. The detailed analysis of LH and FSH secretory characteristics and the ability to isolate the pituitary response by administering a bolus of GnRH are key strengths of the experimental paradigm. Our ability to study the same participant in a crossover design with good matching of estradiol levels in each of the two conditions studies is also a strength, as it rules out any independent contribution of estradiol feedback on gonadotropins that might introduce variability into the results. The utilization of a clinical research center to rigorously control the infusion of lipid and insulin, and to maintain euglycemia within a narrow range provides assurance that our findings are not due to excessive fluctuations in glucose. The fact that we doubled triglyceride concentration(33) and achieved a 25% increase in circulating free fatty acids(15, 16) and induced a 24 uIU/ml increase in circulating insulin levels (to just at or above the upper limit of normal) during the lipid + insulin infusions support the experimental model, as this magnitude of the changes induced is consistent with obesity and was not supraphysiological(15, 16, 33). Overall, the study population is also relatively homogenous in terms of metabolic characteristics. Weaknesses of the study include difficulty in pairing every single participant, as some declined participation after completing one of the two intended study treatments, and inherent variability in the timing within the menstrual cycle of the frequent sampling study. It is possible that some of our findings are due to the natural progression of change in LH pulsatility as a woman traverses the early to the mid follicular phase of her menstrual cycle, a time when LH pulses typically decrease in amplitude(34). However, we were not able to discern any systematic bias in our data in that women with lower pulse amplitudes were not more likely to be sampled later in their follicular phase. Our sample of regularly cycling women included a wide range of ovarian reserve as assessed by AMH, and this led to increased variability in the data. In particular, one participant with a very high AMH had elevated LH levels and therefore we examined our results with and without her data included. Our inclusion criteria did not include transvaginal ultrasound, and a formal assessment for hirsutism or hyperandrogenism was not performed. It is possible this participant would have met the Rotterdam or AEPCOS Society criteria for PCOS(35). Finally, there were several technical issues that required making minor alterations in the protocol which may have introduced variability into our findings and therefore biased our findings towards the null. The amount of insulin infused, originally set at 40 units/m2/min, resulted in large volumes of fluid being delivered to our participants over the course of the 6 hour study, and led to concerns about selective hemodilution that could have biased results. We decreased the amount of insulin, and consequently glucose, infused and examined our findings specifically to rule out hemodilution effects (21); as our participants were very healthy and midreproductive aged, they did not appear to have any evidence of fluid overload or significant hemodilution from the infusion volumes. Although heparin is not known to have a consistent effect on gonadotropin secretion(36, 37), data are scarce and it is possible the mid-study adjustment in the heparin infusion to avoid excessive elevation of the PTT (see Adverse Events) altered the outcomes. However, inspection of the data did not support any effect on gonadotropins in association with changes in the amount of heparin infused. Additionally, while there are known detrimental effects of hypoglycemia on gonadotropin secretion, these are presumably mediated via kisspeptin(38), and would not be expected to affect GnRH-induced LH secretion(39). In this study, we examined blood glucose every 5 minutes to scrupulously avoid hypoglycemia. Acute infusion of dextrose is also unlikely to have contributed to our findings, as there are not known effects on LH secretion of energy consumption during frequent blood sampling studies.
In summary, we have demonstrated that acute infusion of lipid and insulin to normal weight women is capable of partially reproducing reproductive hormonal features of the reprometabolic syndrome. The ability to demonstrate clear cut reductions in FSH secretion in a short term model of obesity-related reproductive dysfunction provides further evidence that the metabolic milieu of obesity is an important contributor to the reduced reproductive efficiency observed in women with obesity. The data support prior findings of suppression of LH and response to GnRH in association with the obese state. The ability to induce reprometabolic syndrome rapidly in our model also implies that it may be possible to identify and ameliorate the circulating factors responsible for the syndrome, thereby helping women with obesity to conceive with greater efficiency and hopefully mitigate adverse consequences to their pregnancy and to their offspring.
Acknowledgments
Funding: R01 HD0178314 (to NS) and UL1 TR002535 (Colorado Clinical and Translational Sciences Institute, to RJ Sokol)
Footnotes
Disclosures: The authors report no relevant disclosures regarding this manuscript
This clinical research study is registered at ClinicalTrials.gov (NCT02653092)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Gesink Law DC, Maclehose RF, Longnecker MP. Obesity and time to pregnancy. Hum Reprod. 2007;22(2):414–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Santoro N, Lasley B, McConnell D, Allsworth J, Crawford S, Gold EB, et al. Body size and ethnicity are associated with menstrual cycle alterations in women in the early menopausal transition: The Study of Women's Health across the Nation (SWAN) Daily Hormone Study. J Clin Endocrinol Metab. 2004;89(6):2622–31. doi: 10.1210/jc.2003-031578. [DOI] [PubMed] [Google Scholar]
- 3.Randolph JF Jr., Sowers M, Gold EB, Mohr BA, Luborsky J, Santoro N, et al. Reproductive hormones in the early menopausal transition: relationship to ethnicity, body size, and menopausal status. J Clin Endocrinol Metab. 2003;88(4):1516–22. [DOI] [PubMed] [Google Scholar]
- 4.Brewer CJ, Balen AH. The adverse effects of obesity on conception and implantation. Reproduction. 2010;140(3):347–64. doi: 10.1530/REP-09-0568. [DOI] [PubMed] [Google Scholar]
- 5.Gonzalez-Bulnes A, Pallares P, Ovilo C. Ovulation, implantation and placentation in females with obesity and metabolic disorders: life in the balance. Endocr Metab Immune Disord Drug Targets. 2011;11(4):285–301. doi: 10.2174/187153011797881193. [DOI] [PubMed] [Google Scholar]
- 6.Styne-Gross A, Elkind-Hirsch K, Scott RT Jr. Obesity does not impact implantation rates or pregnancy outcome in women attempting conception through oocyte donation. Fertil Steril. 2005;83(6):1629–34. doi: 10.1016/j.fertnstert.2005.01.099. [DOI] [PubMed] [Google Scholar]
- 7.Mutsaerts MA, van Oers AM, Groen H, Burggraaff JM, Kuchenbecker WK, Perquin DA, et al. Randomized Trial of a Lifestyle Program in Obese Infertile Women. N Engl J Med. 2016;374(20):1942–53. doi: 10.1056/NEJMoa1505297. [DOI] [PubMed] [Google Scholar]
- 8.Legro RS. FIT-PLESE. 36th Annual Meeting of the European Society for Humen Reproduction and Embryology (ESHRE) Virtual Meeting 2020. [Google Scholar]
- 9.Jain A, Polotsky AJ, Rochester D, Berga SL, Loucks T, Zeitlian G, et al. Pulsatile luteinizing hormone amplitude and progesterone metabolite excretion are reduced in obese women. J Clin Endocrinol Metab. 2007;92(7):2468–73. [DOI] [PubMed] [Google Scholar]
- 10.Al-Safi ZA, Liu H, Carlson NE, Chosich J, Lesh J, Robledo C, et al. Estradiol Priming Improves Gonadotrope Sensitivity and Pro-Inflammatory Cytokines in Obese Women. J Clin Endocrinol Metab. 2015;100(11):4372–81. doi: 10.1210/jc.2015-1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chosich J, Bradford AP, Allshouse AA, Reusch JE, Santoro N, Schauer IE. Acute recapitulation of the hyperinsulinemia and hyperlipidemia characteristic of metabolic syndrome suppresses gonadotropins. Obesity (Silver Spring). 2017;25(3):553–60. doi: 10.1002/oby.21754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin K, Santoro N, Hall J, Filicori M, Wierman M, Crowley WF Jr. Clinical review 15: Management of ovulatory disorders with pulsatile gonadotropin-releasing hormone. J Clin Endocrinol Metab. 1990;71(5):1081A–G. doi: 10.1210/jcem-71-5-1081. [DOI] [PubMed] [Google Scholar]
- 13.Christenson R, St. Onge S, McLaughlin L, Canfield W, Levy HR. The ADVIA Centaur Enhanced Estradiol Assay: Performance and Standardization 2012. Available from: https://cdn0.scrvt.com/39b415fb07de4d9656c7b516d8e2d907/1800000000212224/b4a47427c4b4/advia_centaur_enhanced_estradiol_assay-00212224_1800000000212224.pdf.
- 14.Santen RJ, Bardin CW. Episodic luteinizing hormone secretion in man. Pulse analysis, clinical interpretation, physiologic mechanisms. J Clin Invest. 1973;52(10):2617–28. Epub 1973/10/01. doi: 10.1172/JCI107454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller MR, Pereira RI, Langefeld CD, Lorenzo C, Rotter JI, Chen YD, et al. Levels of free fatty acids (FFA) are associated with insulin resistance but do not explain the relationship between adiposity and insulin resistance in Hispanic Americans: the IRAS Family Study. J Clin Endocrinol Metab. 2012;97(9):3285–91. Epub 2012/07/05. doi: 10.1210/jc.2012-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arner P, Ryden M. Fatty Acids, Obesity and Insulin Resistance. Obes Facts. 2015;8(2):147–55. doi: 10.1159/000381224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sherman BM, Korenman SG. Measurement of serum LH, FSH, estradiol and progesterone in disorders of the human menstrual cycle: the inadequate luteal phase. J Clin Endocrinol Metab. 1974;39(1):145–9. [DOI] [PubMed] [Google Scholar]
- 18.Grenman S, Ronnemaa T, Irjala K, Kaihola HL, Gronroos M. Sex steroid, gonadotropin, cortisol, and prolactin levels in healthy, massively obese women: correlation with abdominal fat cell size and effect of weight reduction. J Clin Endocrinol Metab. 1986;63(6):1257–61. [DOI] [PubMed] [Google Scholar]
- 19.Li S, Mbong EF, John DT, Terasaka T, Li D, Lawson MA. Induction of Stress Signaling In Vitro and Suppression of Gonadotropin Secretion by Free Fatty Acids in Female Mouse Gonadotropes. Endocrinology. 2018;159(2):1074–87. doi: 10.1210/en.2017-00638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tannous A, Bradford AP, Kuhn K, Fought A, Schauer IE, Santoro NF. A Randomised Trial Examining Inflammatory Signaling in Acutely Induced Hyperinsulinemia and Hyperlipidemia in Normal Weight Women-The Reprometabolic Syndrome. PLOS One. 2020; in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McDonald R, Bradford AP, Kuhn K, Santoro N. Cell type sepcific effects of hyperlipidemia and hyperinsulinemia, characteristic of reprometabolic syndrome, on pituitary function. American Society for Reproductive Medicine 2019 Annual Meeting; Philadelphia, PA2019. [Google Scholar]
- 22.Bernard DJ, Fortin J, Wang Y, Lamba P. Mechanisms of FSH synthesis: what we know, what we don't, and why you should care. Fertil Steril. 2010;93(8):2465–85. doi: 10.1016/j.fertnstert.2010.03.034. [DOI] [PubMed] [Google Scholar]
- 23.Sacchi S, D'Ippolito G, Sena P, Marsella T, Tagliasacchi D, Maggi E, et al. The anti-Mullerian hormone (AMH) acts as a gatekeeper of ovarian steroidogenesis inhibiting the granulosa cell response to both FSH and LH. J Assist Reprod Genet. 2016;33(1):95–100. doi: 10.1007/s10815-015-0615-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steiner AZ, Stanczyk FZ, Patel S, Edelman A. Antimullerian hormone and obesity: insights in oral contraceptive users. Contraception. 2010;81(3):245–8. doi: 10.1016/j.contraception.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Pergola G, Maldera S, Tartagni M, Pannacciulli N, Loverro G, Giorgino R. Inhibitory effect of obesity on gonadotropin, estradiol, and inhibin B levels in fertile women. Obesity (Silver Spring). 2006;14(11):1954–60. [DOI] [PubMed] [Google Scholar]
- 26.Cimino I, Casoni F, Liu X, Messina A, Parkash J, Jamin SP, et al. Novel role for anti-Mullerian hormone in the regulation of GnRH neuron excitability and hormone secretion. Nat Commun. 2016;7:10055. doi: 10.1038/ncomms10055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oestreich AK, Moley KH. Developmental and Transmittable Origins of Obesity-Associated Health Disorders. Trends Genet. 2017;33(6):399–407. doi: 10.1016/j.tig.2017.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Drake AJ, Reynolds RM. Impact of maternal obesity on offspring obesity and cardiometabolic disease risk. Reproduction. 2010;140(3):387–98. doi: 10.1530/REP-10-0077. [DOI] [PubMed] [Google Scholar]
- 29.Glastras SJ, Chen H, Pollock CA, Saad S. Maternal obesity increases the risk of metabolic disease and impacts renal health in offspring. Biosci Rep. 2018;38(2). doi: 10.1042/BSR20180050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rochester D, Jain A, Polotsky AJ, Polotsky H, Gibbs K, Isaac B, et al. Partial recovery of luteal function after bariatric surgery in obese women. Fertil Steril. 2009;92(4):1410–5. doi: 10.1016/j.fertnstert.2008.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Akhter Z, Rankin J, Ceulemans D, Ngongalah L, Ackroyd R, Devlieger R, et al. Pregnancy after bariatric surgery and adverse perinatal outcomes: A systematic review and meta-analysis. PLoS Med. 2019;16(8):e1002866. doi: 10.1371/journal.pmed.1002866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johansson K, Stephansson O, Neovius M. Outcomes of pregnancy after bariatric surgery. N Engl J Med. 2015;372(23):2267. doi: 10.1056/NEJMc1503863. [DOI] [PubMed] [Google Scholar]
- 33.Szczygielska A, Widomska S, Jaraszkiewicz M, Knera P, Muc K. Blood lipids profile in obese or overweight patients. Ann Univ Mariae Curie Sklodowska Med. 2003;58(2):343–9. [PubMed] [Google Scholar]
- 34.Filicori M, Santoro N, Merriam GR, Crowley WF Jr. Characterization of the physiological pattern of episodic gonadotropin secretion throughout the human menstrual cycle. J Clin Endocrinol Metab. 1986;62(6):1136–44. doi: 10.1210/jcem-62-6-1136. [DOI] [PubMed] [Google Scholar]
- 35.Williams T, Mortada R, Porter S. Diagnosis and Treatment of Polycystic Ovary Syndrome. Am Fam Physician. 2016;94(2):106–13. [PubMed] [Google Scholar]
- 36.Goodfriend TL, Pedersen TL, Grekin RJ, Hammock BD, Ball DL, Vollmer A. Heparin, lipoproteins, and oxygenated fatty acids in blood: a cautionary note. Prostaglandins Leukot Essent Fatty Acids. 2007;77(5-6):363–6. doi: 10.1016/j.plefa.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mai K, Bobbert T, Reinecke F, Andres J, Maser-Gluth C, Wudy SA, et al. Intravenous lipid and heparin infusion-induced elevation in free fatty acids and triglycerides modifies circulating androgen levels in women: a randomized, controlled trial. J Clin Endocrinol Metab. 2008;93(10):3900–6. doi: 10.1210/jc.2008-0714. [DOI] [PubMed] [Google Scholar]
- 38.McCosh RB, Kreisman MJ, Tian K, Ho BS, Thackray VG, Breen KM. Insulin-induced hypoglycaemia suppresses pulsatile luteinising hormone secretion and arcuate Kiss1 cell activation in female mice. J Neuroendocrinol. 2019;31(12):e12813. doi: 10.1111/jne.12813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lujan ME, Krzemien AA, Van Vugt DA. Hypoglycemia does not affect gonadotroph responsiveness to gonadotropin-releasing hormone in rhesus monkeys. Endocrine. 2003;21(2):109–14. doi: 10.1385/ENDO:21:2:109. [DOI] [PubMed] [Google Scholar]


