Abstract
Background:
Tricuspid regurgitation imposes a volume overload on the right ventricle (RV) that can lead to progressive RV dilation and dysfunction. Overt RV dysfunction is associated with poor prognosis and increased operative risk. Abnormalities of myocardial strain may provide the earliest evidence of ventricular dysfunction. CMR feature-tracking techniques now allow assessment of strain from routine cine-images, without specialized pulse sequences. Whether abnormalities of RV strain measured using CMR feature-tracking have prognostic value in patients with tricuspid regurgitation is unknown.
Objectives:
To evaluate the prognostic value of CMR feature-tracking derived RV free wall longitudinal strain (RVFWLS) in a large multicenter population of patients with severe functional tricuspid regurgitation.
Methods:
Consecutive patients with severe functional tricuspid regurgitation undergoing CMR at four US medical centers were included in this study. Feature-tracking RVFWLS was calculated from 4 chamber cine-views. The primary endpoint was all-cause death. Cox proportional hazards regression modeling was used to examine the independent association between RVFWLS and death. The incremental prognostic value of RVFWLS was assessed in nested models.
Results:
Of the 544 patients in this study, 128 died during a median follow-up of 6 years. By Kaplan-Meier-analysis, patients with RVFWLS ≥median (-16%) had significantly reduced event free survival compared to those with RVFWLS <median (log-rank p<0.001). By Cox multivariable regression modeling, RVFWLS was associated with increased risk-of-death after adjustment for clinical and imaging risk factors including RV size and ejection fraction (HR=1.14 per %; p<0.001). Addition of RVFWLS in this model resulted in significant-improvement in the global-chi-square (31 to 78;p<0.001).
Conclusions:
CMR feature-tracking derived RVFWLS is an independent predictor of mortality in patients with severe functional tricuspid regurgitation, incremental to common clinical and imaging risk factors.
Keywords: tricuspid regurgitation, cardiac magnetic resonance imaging, prognosis, mortality, right ventricular function, global longitudinal strain, feature tracking
INTRODUCTION
Severe tricuspid regurgitation (TR) is associated with poor prognosis(1–5). However compared to mitral or aortic disease, the natural history and underlying pathophysiology of TR and its relationship to right ventricular (RV) function is less understood(6). There is also incomplete knowledge on the role and timing of tricuspid valve surgery and newer transcatheter repair approaches(6–8). Better understanding of underlying mechanisms and prognostic assessment of TR is essential for developing adequate and timely treatments. It is known that severe TR imposes a volume overload on the ventricle that can lead to progressive RV dilation and dysfunction(2,6,9). Overt RV dysfunction is associated with poor prognosis and increased operative risk(2,7,10). However, patients with severe TR are a heterogeneous population at different stages of right heart modeling(11).
Abnormalities of myocardial strain may provide the earliest evidence of right ventricular dysfunction(12). CMR feature-tracking techniques now allow assessment of strain from routine cine-images, without specialized pulse sequences(13). CMR also allows accurate assessment of RV function and size. Whether abnormalities of RV strain measured using CMR feature-tracking have prognostic value in patients with severe tricuspid regurgitation is unknown. We hypothesized that feature tracking derived RV longitudinal strain (RVFWLS) may provide prognostic information incremental to clinical and standard CMR derived parameters in this patient group.
The aim of this study was to evaluate the prognostic value of CMR feature-tracking derived RVFWLS in a large multicenter population of patients with severe functional tricuspid regurgitation.
METHODS
Study Design
Four geographically diverse medical centers in the United States participated in this retrospective, observational, multicenter study. The University of Illinois in Chicago served as the data-coordinating center using a cloud-based database (CloudCMR, www.cloudCMR.com) containing de-identified searchable data from consecutive patients with full DICOM datasets from the participating centers. Institutional review board approval was obtained at each center.
Study Population
In this retrospective study, consecutive patients with clinically reported severe tricuspid regurgitation who had undergone CMR with both cine and late gadolinium enhancement (LGE) imaging formed the study population of 544 patients. Severe TR was assessed by an experienced level 3 CMR reader with a qualitative multiparametric integrative approach based on a combination of right atrial enlargement, lack of leaflet coaptation, vena contracta>7mm, signal void area >50% of right atrium, RV enlargement, and tricuspid annular dilatation(14–17). Exclusion criteria included tricuspid valve prolapse, endocarditis, tumor, as well as pacemaker or congenital heart disease. Baseline demographics (age, gender, BMI, history of diabetes, history of hyperlipidemia, history of hypertension, history of smoking, history of pulmonary hypertension, cardiac medications) were obtained by local site investigators at the time of the clinical study. History of diabetes, history of hyperlipidemia, history of hypertension, history of smoking, and history of pulmonary hypertension were assessed based on documentation of the diagnosis in the electronic medical record at the time of the CMR exam.
CMR Acquisition
Images were acquired with phased-array receiver coils according to the routine scan protocol at each site using a variety of scanners from all three major vendors (Siemens, Philips and General Electric) at both 1.5 and 3 Tesla. A typical protocol included steady-state free-precession cine images acquired in multiple short-axis and three long-axis views (LV 4, 2, and 3-chamber) with short-axis views obtained every 1cm to cover the entire left ventricle(18). Typical temporal resolution of cine images was <45msec. LGE imaging was performed 10–15 minutes after Gadolinium contrast (0.15 mmol/kg) administration using a 2D segmented gradient echo inversion-recovery sequence in the same views used for cine-CMR. Inversion delay times were typically 280 to 360 ms.
CMR Analysis and RVFWLS Assessment
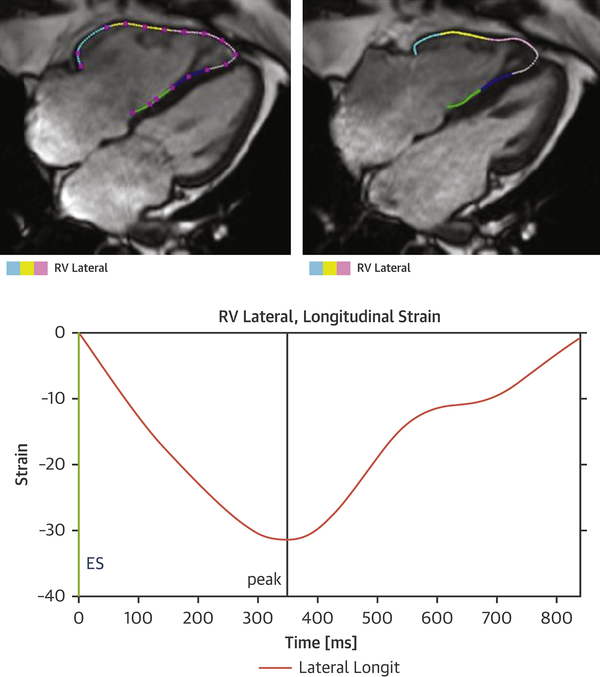
The study site investigators analyzed images on locally available workstations and were blinded to follow-up data. LV and RV volumes and ejection fractions as well as LV mass were determined by quantitative analysis according to Society of Cardiovascular Magnetic Resonance (SCMR) recommendations (18). Right atrial area was measured in the cine 4-chamber view, just before tricuspid valve opening. Tricuspid annular plane systolic excursion (TAPSE) was measured from cine CMR images. As previously described, the presence of LGE was determined by visual inspection on a 17-segment model per standardized SCMR recommendations (18–25). For feature tracking analysis, endocardial RV contours were manually traced (by a single physician who was blinded to patient information and outcomes) in the long-axis 4-chamber cine view to derive RVFWLS using Segment CMR software (Medviso AB, Lund, Sweden) (Figure 1). In 50 randomly selected patients, a blinded CMR physician measured RVFWLS for assessment of inter-observer variability
Figure 1. Measurement of RVFWLS using the Segment CMR package (Medviso AB, Lund, Sweden).
Endocardial right ventricular contours were manually traced in the 4 chamber cine view to derive RVFWLS.
Follow-up
Patients were followed for the primary outcome of all cause mortality using the United States Social Security Death Index. Time to event was calculated as the period between the CMR study and death. Patients who did not experience the primary outcome were censored at the time of assessment.
Statistical Analysis
Normally distributed data were expressed as mean ± SD. Differences in baseline characteristics were compared with the Student’s t-test for continuous variables and the chi-squared test for dichotomous variables. Inter-observer variability for RVFWLS was assessed in a random sample of 50 patients using the Bland-Altman method. Kaplan-Meier methods were used to evaluate the relationship between RVFWLS and time to the primary outcome of all cause mortality. A cubic spline model was fitted to the observed data to illustrate the association between RVFWLS as a continuous variable and outcome. We used Cox proportional hazards regression modeling to examine the association between RVFWLS and all cause mortality. All models were assessed for collinearity and proportional hazards assumption. For the multivariable models, established important clinical and imaging risk factors based on prior prognostic literature were considered as covariates: Age, Gender, Diabetes, Hypertension, Pulmonary Hypertension, Left sided valve disease, LVEF, LGE, TAPSE, RVEDV, RVEF (3,11,26). The incremental prognostic value of RVFWLS was assessed in nested-models. A p value of <0.05 was considered statistically significant. Analyses were performed using STATA (StataCorp, TX).
RESULTS
Patient Characteristics
Table 1 summarizes baseline patient characteristics stratified by RVFWLS above and below the median (-16%). The mean age of the study population was 60.6(±18.6) years. Forty-one percent of patients were male and 16.5% had diabetes mellitus. History of pulmonary hypertension was present in 14.3%, while 42% had moderate or more left sided valve disease. The mean RVEF was 37%. The mean LVEF was 52.4 ± 15.5% and LGE was present in 36.6% of patients. The mean TAPSE was 14mm. Scatterplots showing the correlations between 1)RVFWLS and RVEF, 2)RVFWLS and RVEDV, 3)RVFWLS and LVEF are shown in Supplementary Figures 1–3. Bland-Altman analysis of inter-observer variability for RVFWLS showed a bias of 0.19%. The 95% limits of agreement were -2% to 1.66% (Supplementary Figure 4).
Table 1. Baseline characteristics of study population stratified by RVFWLS above and below the median (−16%).
BMI=Body Mass Index, LGE=Late Gadolinium Enhancement, LVEDV=Left Ventricular End Diastolic Volume, LVEF=Left Ventricular Ejection Fraction, LVESV=Left Ventricular End Systolic Volume, RA=Right Atrial, RVEDV = Right Ventricular End Diastolic Volume, RVEF=Right Ventricular Ejection Fraction, RVESV = Right Ventricular End Systolic Volume, SD=standard deviation. TAPSE=Tricuspid Annular Plane Systolic Excursion.
| CHARACTERISTICS | Total | RVFWLS <median (−16%) | RVFWLS ≥median (−16%) | P Value |
|---|---|---|---|---|
| Age (±SD), years | 60.60 (±18.6) | 59.14 (±19.7) | 61.87 (±17.2) | 0.123 |
| Male % | 41.0 | 37.9 | 44.3 | 0.126 |
| BMI (±SD), kg/m2 | 27.3 (±5.9) | 27.4 (±6.0) | 27.1 (±5.7) | 0.592 |
| Diabetes % | 16.5 | 15.7 | 17.4 | 0.592 |
| Hyperlipidemia % | 35 6 | 32.6 | 39.0 | 0.161 |
| Hypertension % | 53.7 | 49.4 | 58.7 | 0.048 |
| Smoking % | 27.3 | 26.9 | 27.8 | 0.830 |
| Aspirin % | 42.4 | 39.5 | 45.7 | 0.191 |
| Statin % | 29.8 | 27.6 | 32.3 | 0.284 |
| ACE inhibitor % | 25.7 | 22.9 | 28.9 | 0.158 |
| Beta Blocker % | 36.0 | 32.3 | 40.4 | 0.083 |
| Pulmonary hypertension % | 14.3 | 12.5 | 16.3 | 0.208 |
| Left sided valve disease*% | 42.1 | 36.4 | 48.1 | 0.006 |
| Heart Rate (±SD), beats/min | 79.8 (±18.2) | 77.5 (±15.8) | 82.4 (±20.2) | 0.004 |
| Systolic BP (±SD), mm Hg | 122(±22) | 125(±23) | 120(±21) | 0.024 |
| Diastolic BP (±SD), mm Hg | 72(±14) | 72(±14) | 72(±14) | 0.950 |
| LVEDV (±SD), ml | 136(±65) | 126(±51) | 145(±75) | <0.001 |
| LVESV (±SD), ml | 69(±60) | 5 5 (±41) | 83(±72) | <0.001 |
| LVEF (±SD), % | 52.4 (±15.5) | 57.0 (±12.0) | 47.4 (±17.2) | <0.001 |
| LGE % | 36.6 | 25.4 | 48.5 | <0.001 |
| TAPSE (±SD), mm | 14 (±7) | 16 (±7) | 11 (±6) | <0.001 |
| Tricuspid Valve Annulus (±SD), mm | 41(±8) | 40(±8) | 42(±8) | 0.017 |
| RA area, cm2 | 37(±14) | 36(±14) | 37(±14) | 0.284 |
| RVEDV (±SD), ml | 213(±99) | 200(±96) | 228(±101) | 0.001 |
| RVESV (±SD), ml | 137(±80) | 115(±65) | 159(±88) | <0.001 |
| RVEF (±SD), % | 37.2 (±14.2) | 42.1 (±13.2) | 32.0 (±13.5) | <0.001 |
moderate or more left sided valve disease.
Primary Outcome
Of the 544 patients in this study, 128 died during a median follow-up of 6.1 years (interquartile range: 3.4–9.8 years).
Outcomes and RVFWLS
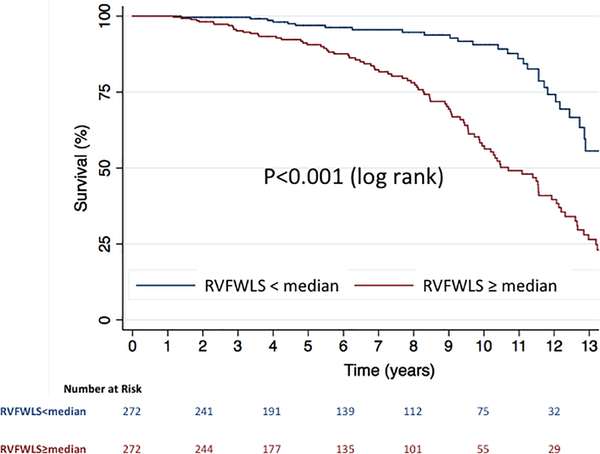
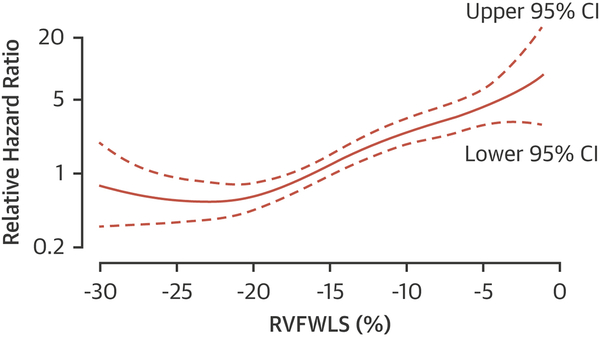
When stratified by the median value of RVFWLS (-16%), Kaplan-Meier analysis showed significantly increased risk of death in those with RVFWLS≥median (log-rank p<0.001) (Central Illustration). The continuous relationship between RVFWLS and the hazard of death is shown in the cubic spline in figure 2. The univariable associations between RVFWLS and death are shown in Supplementary Table 1.
Central Illustration. Kaplan-Meier survival curves in patients with severe functional tricuspid regurgitation, stratified by RVFWLS above and below the median value.
Kaplan-Meier analysis showed significantly increased risk of death in those with RVFWLS=median (log-rank p<0.001).
Figure 2. Relationship between RVFWLS and hazard of death (with 95% confidence intervals).
Hazard ratios are relative to those with median RVFWLS.
Multivariable Analysis and Incremental Prognostic Value
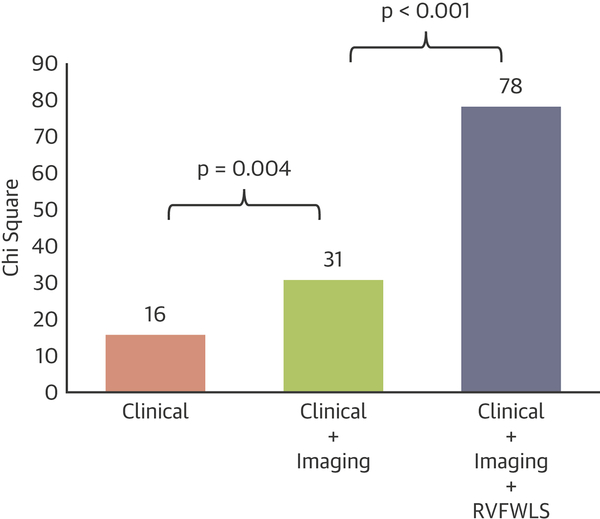
After multivariable adjustment for clinical and imaging risk factors (Age, Gender, Diabetes, Hypertension, Pulmonary Hypertension, Left sided valve disease, LVEF, LGE, TAPSE, RVEDV, RVEF), RVFWLS remained a significant independent predictor of death (HR=1.140; p<0.001) (Table 2 and Supplementary Table 2). In sequential nested Cox models, a model based on clinical variables alone (Age, Gender, Diabetes, Hypertension) was significantly improved by addition of imaging variables (Pulmonary Hypertension, Left sided valve disease, LVEF, LGE, TAPSE, RVEDV, RVEF), and further significantly improved by adding RVFWLS (Figure 3). Addition of RVFWLS into the model with clinical and imaging predictors resulted in a significant increase in model Chi square value (from 31 to 78; p<0.001).
Table 2. Multivariable model for death.
LGE=Late Gadolinium Enhancement, LVEF=Left Ventricular Ejection Fraction, RVEDV=Right Ventricular Diastolic Volume, RVEF=Right Ventricular Ejection Fraction, RVFWLS= Right Ventricular Fee Wall Global Longitudinal Strain, TAPSE=Tricuspid Annular Plane Systolic Excursion.
| VARIABLES | Multivariable Model for Death | |
|---|---|---|
| Hazard Ratio (95% CI) | P Value | |
| Age, per year | 1.024 (1.009–1.038) | 0.001 |
| Male | 1.109 (0.751–1.637) | 0.605 |
| Diabetes | 0.867 (0.555–1.353) | 0.529 |
| Hypertension | 1.210 (0.782–1.872) | 0.391 |
| Pulmonary hypertension | 1.392 (0.831–2.332) | 0.208 |
| Left sided valve disease | 0.912 (0.586–1.418) | 0.683 |
| LVEF, per % | 1.015 (1.000–1.031) | 0.052 |
| LGE | 1.052 (0.703–1.576) | 0.804 |
| TAPSE, per mm | 1.020 (0.984–1.057) | 0.275 |
| RVEDV, per ml | 1.001 (0.999–1.003) | 0.580 |
| RVEF, per % | 1.008 (0.990–1.026) | 0.405 |
| RVFWLS, per % | 1.140 (1.099–1.182) | <0.001 |
Figure 3. Sequential nested Cox models for death.
A model based on clinical variables alone (Age, Gender, Diabetes, Hypertension) was significantly improved by addition of imaging variables (Pulmonary Hypertension, Left sided valve disease, LVEF, LGE, TAPSE, RVEDV, RVEF), and further significantly improved by adding RVFWLS.
DISCUSSION
This study shows that CMR feature-tracking derived RVFWLS is an independent predictor of mortality in a multicenter population of patients with severe functional tricuspid regurgitation. We have demonstrated that RVFWLS provides prognostic information incremental to common clinical and CMR risk factors - including RV function and size as well as late gadolinium enhancement. These findings highlight the importance of long-axis RV function and suggest a role for feature tracking RVFWLS in identifying patients at highest risk of death.
Role of the RV in prognosis of TR
Prognosis in significant TR is influenced by RV adaptation and remodeling(2,11). The volume overload caused by regurgitation leads to eventual RV dilation and dysfunction(2,6). In addition, RV dilation and dysfunction leads to worsening TR resulting in a vicious cycle(2,6). However, this is a heterogenous process and has not been as well characterized and studied as for mitral regurgitation. Recently Dietz et al evaluated the relative prognostic value of RV dilation vs RV dysfunction (assessed by TAPSE) in patients with moderate and severe functional TR using echocardiography(11). They found that patients with worst RV function (measured by TAPSE) had worst survival regardless of presence of RV dilation. Most prior studies have been limited by the ability of 2D echocardiography to adequately image the RV. In the current study we used CMR as the gold standard technique for assessing RV volumes and ejection fraction.
Recently Prihadi and colleagues examined the prognostic value of RVFWLS in 896 patients from a single center with moderate or severe functional TR using speckle tracking echocardiography(26). They showed that RVFWLS was independently associated with all cause death after adjustment to clinical and echocardiographic parameters. However, as stated by the authors their study was limited by use of 2D echo measures of RV size and function. We have now shown that feature tracking RVFWLS provides independent and incremental prognostic information even when using CMR with accurate measurements of RV volumes and functions as well as tissue assessment with LGE. These findings suggest that RVFWLS is a fundamental and early marker of RV dysfunction in severe TR.
RV contraction and long axis function
The RV wall is composed of predominantly two layers. The superficial layer (approximately 25% of wall thickness) is formed primarily by circumferential fibers in a direction parallel to the atrioventricular groove that extend from one ventricle to another(27). The subendocardial RV layer is composed of longitudinally arranged fibers that pass through the apex toward the papillary muscles, tricuspid annulus, and RV outflow tract and are continuous with those of the septum. Shortening of the longitudinal fibers draws the tricuspid valve towards the apex and is an important component of normal RV function(12).
Possibly because of their subendocardial location, the longitudinal fibers maybe more sensitive to disturbance by various pathologies. At least in the left ventricle this appears to be related to greater compressive forces and higher oxygen consumption in the subendocardium(28,29). In this study we have shown that reduction of RV long axis function as detected by RVFWLS, is an independent predictor of mortality in patients with severe TR possibly because it is an early marker of subclinical pathological processes affecting the subendocardial longitudinal fibers.
Clinical Implications
This study demonstrates the incremental prognostic value of feature tracking derived RVFWLS over clinical and CMR variables in patients with severe TR. It highlights the value of evaluating longitudinal RV free wall deformation to provide a more complete assessment of RV function than that provided by RV volumes and EF– even when these are assessed by the gold standard technique of CMR volumetric measurements.
To our knowledge this is the first demonstration of the prognostic value of CMR in assessment of TR. Although echocardiography is the first line modality for evaluation of TR, our findings suggest a potential role for evaluation of severe TR using CMR and feature tracking RVFWLS. With the growing interest in transcatheter repair approaches for treatment of severe TR, early detection of RV dysfunction using feature tracking RVFWLS may potentially play a role in determining optimal timing of intervention. How exactly this information will affect clinical care requires further investigation and prospective studies are warranted to explore the role of feature tracking RVFWLS in clinical decision making. Ultimately, better identification of high-risk patients may allow closer follow-up and more directed therapies to be applied in a timely manner. Future studies will need to demonstrate that imaging driven patient management improves specific outcomes before such approaches can be advocated.
Limitations
Although this is a multicenter study, the patients in this paper may not be representative of all patients with severe functional TR in the community. In this retrospective study there is likely referral bias in undergoing the CMR exam for example resulting in exclusion of acutely sick patients, patients with large body size, severe renal impairment, severe claustrophobia or those with pacemakers and ICDs. At the time of scanning there was no validated widely used single measure for classification of TR severity by CMR or echocardiography. TR regurgitant volume quantification was not included in the analysis as the necessary phase contrast pulse sequences were not routinely performed at the time of clinical scanning in this retrospective study. Prospective studies using appropriate phase contrast sequences to incorporate tricuspid regurgitant fraction should be performed in the future and would be of great interest
Similar to echocardiography derived strain, there are algorithmic differences between various CMR feature tracking strain software programs, which may result in differing values (30). Thus, our findings would benefit from replication using other CMR feature tracking strain vendors. Certain limitations of 2D feature tracking related to tracking through plane motion and limited temporal resolution should be borne in mind. RV vertical long axis views were not used for RV strain assessment. This is because RV strain is typically measured in the 4-chamber view as recommended by expert consensus documents(31). Information about downstream cardiovascular procedures such as surgery, or revascularization was not available. However, this does not detract from the main findings of this study, that feature-tracking RVFWLS is a powerful predictor of death in these patients, independent of common clinical and imaging markers available at the time of CMR. Follow-up data was limited to the primary endpoint of all cause death and the cause of death was not known. However, many have argued that all–cause mortality is an extremely important and appropriate study endpoint because it is unbiased and clinically relevant, which is often not the case for other cardiac outcomes (32–34).
Conclusions
In this large multicenter study, CMR feature-tracking derived RVFWLS is an independent predictor of mortality in patients with severe functional tricuspid regurgitation. We have demonstrated that RVFWLS provides prognostic information incremental to common clinical and CMR risk factors - including RV function and size as well as late gadolinium enhancement.
Supplementary Material
CLINICAL PERSPECTIVES.
Competency in Medical Knowledge:
In this multicenter study, feature tracking RVFWLS measured during cine CMR is a significant independent predictor of mortality in patients with severe tricuspid regurgitation – incremental to common clinical and imaging risk factors
Translational Outlook:
How this information will affect clinical care requires further investigation and future studies are warranted to explore the role of CMR derived feature tracking RVFWLS in clinical decision making.
Abbreviations:
- CMR
Cardiac Magnetic Resonance
- EF
Ejection Fraction
- LGE
Late Gadolinium Enhancement
- RV
Right Ventricle
- RVFWLS
Right Ventricular Free Wall Longitudinal Strain
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Nath J, Foster E, Heidenreich PA. Impact of tricuspid regurgitation on long-term survival. J Am Coll Cardiol 2004;43:405–9. [DOI] [PubMed] [Google Scholar]
- 2.Prihadi EA, Delgado V, Leon MB, Enriquez-Sarano M, Topilsky Y, Bax JJ. Morphologic Types of Tricuspid Regurgitation: Characteristics and Prognostic Implications. JACC Cardiovascular imaging 2019;12:491–499. [DOI] [PubMed] [Google Scholar]
- 3.Wang N, Fulcher J, Abeysuriya N et al. Tricuspid regurgitation is associated with increased mortality independent of pulmonary pressures and right heart failure: a systematic review and meta-analysis. European heart journal 2019;40:476–484. [DOI] [PubMed] [Google Scholar]
- 4.Chorin E, Rozenbaum Z, Topilsky Y et al. Tricuspid regurgitation and long-term clinical outcomes. European heart journal cardiovascular Imaging 2020;21:157–165. [DOI] [PubMed] [Google Scholar]
- 5.Benfari G, Antoine C, Miller WL et al. Excess Mortality Associated With Functional Tricuspid Regurgitation Complicating Heart Failure With Reduced Ejection Fraction. Circulation 2019;140:196–206. [DOI] [PubMed] [Google Scholar]
- 6.Hahn RT, Waxman AB, Denti P, Delhaas T. Anatomic Relationship of the Complex Tricuspid Valve, Right Ventricle, and Pulmonary Vasculature: A Review. JAMA cardiology 2019;4:478–487. [DOI] [PubMed] [Google Scholar]
- 7.Taramasso M, Gavazzoni M, Pozzoli A et al. Tricuspid Regurgitation: Predicting the Need for Intervention, Procedural Success, and Recurrence of Disease. JACC Cardiovascular imaging 2019;12:605–621. [DOI] [PubMed] [Google Scholar]
- 8.Asmarats L, Puri R, Latib A, Navia JL, Rodes-Cabau J. Transcatheter Tricuspid Valve Interventions: Landscape, Challenges, and Future Directions. J Am Coll Cardiol 2018;71:2935–2956. [DOI] [PubMed] [Google Scholar]
- 9.Dreyfus GD, Martin RP, Chan KM, Dulguerov F, Alexandrescu C. Functional tricuspid regurgitation: a need to revise our understanding. J Am Coll Cardiol 2015;65:2331–6. [DOI] [PubMed] [Google Scholar]
- 10.Kammerlander AA, Marzluf BA, Graf A et al. Right ventricular dysfunction, but not tricuspid regurgitation, is associated with outcome late after left heart valve procedure. J Am Coll Cardiol 2014;64:2633–2642. [DOI] [PubMed] [Google Scholar]
- 11.Dietz MF, Prihadi EA, van der Bijl P et al. Prognostic Implications of Right Ventricular Remodeling and Function in Patients With Significant Secondary Tricuspid Regurgitation. Circulation 2019;140:836–845. [DOI] [PubMed] [Google Scholar]
- 12.Badano LP, Muraru D. Subclinical Right Ventricular Dysfunction by Strain Analysis: Refining the Targets of Echocardiographic Imaging in Systemic Sclerosis. Circ Cardiovasc Imaging 2016;9. [DOI] [PubMed] [Google Scholar]
- 13.Farzaneh-Far A, Romano S. Measuring longitudinal left ventricular function and strain using cardiovascular magnetic resonance imaging. European heart journal cardiovascular Imaging 2019;20:1259–1261. [DOI] [PubMed] [Google Scholar]
- 14.Myerson SG. Heart valve disease: investigation by cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2012;14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gulsin GS, Singh A, McCann GP Cardiovascular magnetic resonance in the evaluation of heart valve disease. BMC medical imaging 2017;17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reddy ST, Shah M, Doyle M et al. Evaluation of cardiac valvular regurgitant lesions by cardiac MRI sequences: comparison of a four-valve semi-quantitative versus quantitative approach. The Journal of heart valve disease 2013;22. [PubMed] [Google Scholar]
- 17.Hahn RT, Thomas JD, Khalique OK, Cavalcante JL, Praz F, Zoghbi WA. Imaging Assessment of Tricuspid Regurgitation Severity. JACC Cardiovascular imaging 2019;12:469–490. [DOI] [PubMed] [Google Scholar]
- 18.Schulz-Menger J, Bluemke DA, Bremerich J et al. Standardized image interpretation and post processing in cardiovascular magnetic resonance: Society for Cardiovascular Magnetic Resonance (SCMR) board of trustees task force on standardized post processing. Journal of cardiovascular magnetic resonance: official journal of the Society for Cardiovascular Magnetic Resonance 2013;15:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Romano S, Judd RM, Kim RJ et al. Association of Feature-Tracking Cardiac Magnetic Resonance Imaging Left Ventricular Global Longitudinal Strain With All-Cause Mortality in Patients With Reduced Left Ventricular Ejection Fraction. Circulation 2017;135:2313–2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Romano S, Judd RM, Kim RJ et al. Feature-Tracking Global Longitudinal Strain Predicts Death in a Multicenter Population of Patients With Ischemic and Nonischemic Dilated Cardiomyopathy Incremental to Ejection Fraction and Late Gadolinium Enhancement. JACC Cardiovascular imaging 2018;11:1419–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Romano S, Romer B, Evans K et al. Prognostic Implications of Blunted Feature-Tracking Global Longitudinal Strain During Vasodilator Cardiovascular Magnetic Resonance Stress Imaging. JACC Cardiovascular imaging 2020;13:58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Romano S, Judd RM, Kim RJ et al. Prognostic Implications of Mitral Annular Plane Systolic Excursion in Patients with Hypertension and a Clinical Indication for Cardiac Magnetic Resonance Imaging: A Multicenter Study. JACC Cardiovascular imaging 2019;12:1769–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Romano S, Judd RM, Kim RJ et al. Feature-Tracking Global Longitudinal Strain Predicts Mortality in Patients With Preserved Ejection Fraction: A Multicenter Study. JACC Cardiovascular imaging 2020;13:940–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dandekar VK, Bauml MA, Ertel AW, Dickens C, Gonzalez RC, Farzaneh-Far A. Assessment of global myocardial perfusion reserve using cardiovascular magnetic resonance of coronary sinus flow at 3 Tesla. Journal of cardiovascular magnetic resonance: official journal of the Society for Cardiovascular Magnetic Resonance 2014;16:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Indorkar R, Kwong RY, Romano S et al. Global Coronary Flow Reserve Measured During Stress Cardiac Magnetic Resonance Imaging Is an Independent Predictor of Adverse Cardiovascular Events. JACC Cardiovascular imaging 2019;12:1686–1695. [DOI] [PubMed] [Google Scholar]
- 26.Prihadi EA, van der Bijl P, Dietz M et al. Prognostic Implications of Right Ventricular Free Wall Longitudinal Strain in Patients With Significant Functional Tricuspid Regurgitation. Circ Cardiovasc Imaging 2019;12:e008666. [DOI] [PubMed] [Google Scholar]
- 27.Sanz J, Sanchez-Quintana D, Bossone E, Bogaard HJ, Naeije R. Anatomy, Function, and Dysfunction of the Right Ventricle: JACC State-of-the-Art Review. J Am Coll Cardiol 2019;73:1463–1482. [DOI] [PubMed] [Google Scholar]
- 28.Henein MY, Gibson DG. Long axis function in disease. Heart 1999;81:229–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chilian WM. Microvascular pressures and resistances in the left ventricular subepicardium and subendocardium. Circulation research 1991;69:561–70. [DOI] [PubMed] [Google Scholar]
- 30.Almutairi HM, Boubertakh R, Miquel ME, Petersen SE. Myocardial deformation assessment using cardiovascular magnetic resonance-feature tracking technique. The British journal of radiology 2017;90:20170072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Badano LP, Muraru D, Parati G, Haugaa K, Voigt JU. How to do right ventricular strain. European heart journal cardiovascular Imaging 2020;21. [DOI] [PubMed] [Google Scholar]
- 32.Stanton T, Leano R, Marwick TH. Prediction of all-cause mortality from global longitudinal speckle strain: comparison with ejection fraction and wall motion scoring. Circ Cardiovasc Imaging 2009;2:356–64. [DOI] [PubMed] [Google Scholar]
- 33.Klem I, Shah DJ, White RD et al. Prognostic value of routine cardiac magnetic resonance assessment of left ventricular ejection fraction and myocardial damage: an international, multicenter study. Circ Cardiovasc Imaging 2011;4:610–9. [DOI] [PubMed] [Google Scholar]
- 34.Lauer MS, Blackstone EH, Young JB, Topol EJ. Cause of death in clinical research: time for a reassessment? J Am Coll Cardiol 1999;34:618–20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.