Highlights
-
•
For patients with atrial fibrillation within 12 months after percutaneous coronary intervention:
-
•
Factor IIa inhibitor 110 mg bid plus a P2Y12 inhibitor is suitable for patients at high risk of bleeding.
-
•
Both Novel oral anticoagulants and vitamin K antagonists could be chose as antithrombotic strategy for patients with high risk of thrombosis.
-
•
Different kinds of antithrombotic strategy (including vitamin K antagonists) should be selected according to different risk factors of patients.
Abbreviations: OACs, Oral anticoagulants; AF, Atrial fibrillation; PCI, Percutaneous coronary intervention; RR, Risk ratios; 95% CI, 95% confidence intervals; TIMI, Thrombolysis In Myocardial Infarction; ISTH, International Society on Thrombosis and Hemostasis; VKA, Vitamin K antagonist; NOACs, Novel oral anticoagulants; IIa, Factor IIa; Xa, Factor Xa; DAT, Oral anticoagulants plus single antiplatelet therapy; TAT, Vitamin K antagonist plus a P2Y12 inhibitor and aspirin; SD, Standard deviation; MI, Myocardial infarction; SUCRA, Surface under the cumulative ranking curve
Keywords: Atrial fibrillation, Percutaneous coronary intervention, Antithrombotic therapy, Oral anticoagulant
Abstract
Background
The optimal antithrombotic strategy, especially regarding oral anticoagulants (OACs) for atrial fibrillation (AF) patients with bleeding and thrombosis risk after percutaneous coronary intervention (PCI), remains unknown. This study explored the optimal oral anticoagulants for AF patients after PCI using a meta-analysis.
Methods
Randomised controlled trials were identified from PubMed, Embase, and the Cochrane Library through December 2020. Risk ratios, 95% confidence intervals, and random-effects models were used to compare different antithrombotic strategies through network meta-analysis, and the combination of antithrombotic agents was ranked according to the surface under the cumulative ranking curve and rankograms. Interval plots were drawn to observe pairwise comparisons between the different strategies.
Results
Five studies of 11,532 patients were included. Factor IIa inhibitor 110 mg bid plus a P2Y12 inhibitor had the greatest advantage for reducing Thrombolysis In Myocardial Infarction (TIMI) major or minor bleeding; Factor Xa inhibitor plus a P2Y12 inhibitor had the greatest advantage for reducing International Society on Thrombosis and Hemostasis major bleeding. For patients at risk of stroke plus all-cause death, factor IIa inhibitor 150 mg bid plus a P2Y12 inhibitor should be prioritised, and for those at risk of myocardial infarction and stent thrombosis, vitamin K antagonists plus a P2Y12 inhibitor were preferred.
Conclusion
Factor IIa inhibitor 110 mg, factor IIa inhibitor 150 mg, factor Xa inhibitor and vitamin K antagonists should be selected in different situations.
1. Introduction
Atrial fibrillation (AF) is always associated with an increased risk of thromboembolic complications, including ischemic stroke and extracranial systemic embolic events [1], [2], [3], [4]. Among AF patients, approximately 20% to 40% also have coronary artery disease, and these patients most likely require percutaneous coronary intervention (PCI) to restore coronary blood flow [5]. In such cases, patients should take both oral anticoagulants (OACs) and antiplatelet therapy to decrease the risk of stent thrombosis and other thrombotic events [6], [7], [8].
Currently, the 2018 North American perspective [9] proposes that PCI for AF patients with high thrombosis and low bleeding risks should be replaced with dual antithrombotic therapy (OAC + single antiplatelet therapy; DAT) after 1 month of triple antithrombotic therapy (vitamin K antagonist [VKA] plus a P2Y12 inhibitor and aspirin; TAT). Moreover, DAT should be replaced with OAC alone 12 months after surgery, while patients with a high risk of bleeding and low thrombotic risk can directly undergo DAT for 6 months followed by OAC treatment (preferred novel OACs [NOACs] over VKA if no contraindication). The 2019 American Heart Association/American College of Cardiology/Heart Rhythm Society guidelines for the management of patients with atrial fibrillation [10] stress that if patients are in the peri-PCI period, they should use TAT, and if the patients have a high thrombotic risk and low bleeding risk, they can use TAT for 1 month. For DAT, i.e., OAC and a P2Y12 inhibitor, initiating therapy as soon as possible after discharge is the preferred strategy.
OACs include VKA and NOACs [11], [12]. NOACs are mainly divided into factor Xa and factor IIa inhibitors [13], [14], [15]. The former includes apixaban, rivaroxaban, and edoxaban, while the latter includes dabigatran. Although the advantages of DAT have been proven in many studies, due to the lack of direct comparisons between different OACs in dual antithrombotic strategies, it is still not possible to determine which OAC is suitable for AF patients after PCI.
Our study is dedicated to exploring the optimal OACs for AF patients with different risk factors for bleeding and thrombosis after PCI using network meta-analysis.
2. Methods
2.1. Protocol and registration
This meta-analysis was registered in the International Prospective Register of Systematic Reviews (PROSPERO; ID: CRD42020198662, https://www.crd.york.ac.uk/prospero/display_record.php?ID = CRD42020198662). We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines [16] for the systematic review and meta-analysis. Supplementary Table S1 shows the position of each PRISMA guideline indicator in our meta-analysis.
2.2. Search strategy and study selection
A number of main databases were searched systematically, including PubMed, Embase, and Cochrane Library, from the earliest possible search date through December 2020. For a more comprehensive search, ‘atrial fibrillation’, ‘percutaneous coronary intervention’, ‘stents’, ‘bare-metal stents’, ‘drug-eluting stents’, ‘oral anticoagulants’, ‘warfarin’, ‘apixaban’, ‘rivaroxaban’, ‘edoxaban’, ‘dabigatran’, ‘dual anti-platelet therapy’, ‘aspirin’, ‘clopidogrel’, ‘ticagrelor’, ‘prasugrel’, ‘purinergic P2Y receptor antagonists’, and ‘fibrinolytic agents’ were set as keywords, and we searched the entry terms of the keywords through MeSH in the NCBI. Detailed retrievals can be found at https://www.crd.york.ac.uk/PROSPEROFILES/198662_STRATEGY_20200714.pdf. The retrieval work was completed independently by two researchers (Shuo Wang and Ying Liu), and the literature was retrieved again before data extraction to ensure the accuracy of our retrieval results as far as possible. To ensure the authority of the search, only English randomised controlled trials were included in our meta-analysis. After discussion, we decided to include patients taking NOAC + a P2Y12 inhibitor + aspirin in our study to ensure the comprehensiveness of the analysis. In Supplementary Table S2, we show the inclusion and exclusion criteria for the studies and patients.
2.3. Quality assessment and data extraction
Two researchers (Shuo Wang and Ying Liu) independently evaluated the included studies and extracted the data. We evaluated the included studies using the Newcastle Ottawa Scale [17] for quality assessment (http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp).
The following data were extracted from the included literature as the baseline characteristics of the patients: (1) author or name of the trial, (2) publication year, (3) age of the patients (mean [standard deviation {SD}]), (4) antithrombotic drug strategy (composition of antithrombotic drug strategy), (5) the number of people with different drug strategies (n), (6) proportion of men and women (%), (7) follow-up time (months) (8) stent type (e.g., drug-eluting stent, bare metal stent, or drug-eluting stent plus bare metal stent; n [%]), (9) median time in therapeutic range (international normalised ratio 2·0–3·0) (%) (mean [standard deviation {SD}]), (10) HAS-BLED score (mean [SD]), (11) CHA2DS2-VASc score (mean [SD]), (12) creatinine clearance (mL/min; n [%]), (13) type of index event (n [%]), (14) diabetes mellitus (n [%]), (15) hypertension (n [%]), (16) prior myocardial infarction (MI; n [%]), (17) prior stroke (n [%]), and (18) use of P2Y12 inhibitors (clopidogrel, ticagrelor, and prasugrel; n [%]).
To obtain more comprehensive raw data, e-mails were sent to the authors of the included literature. The definitions of ISTH major bleeding and TIMI major or minor bleeding events are shown in Supplementary Table S3.
2.4. Network meta-analysis and statistical analyses
Stata MP 14 software [18] was used for the network meta-analysis to detect the best combination of DAT. Risk ratio (RR) and 95% confidence interval (CI) were chosen as the effect measures. Six different antithrombotic strategies were selected to investigate the best strategy for most AF patients (TAT, Xa inhibitor + P2Y12 inhibitor, Xa inhibitor + P2Y12 inhibitor + aspirin, VKA + P2Y12 inhibitor, IIa inhibitor 110 mg bid + P2Y12 inhibitor, and IIa inhibitor 150 mg bid + P2Y12 inhibitor). We set TIMI major or minor bleeding and ISTH major bleeding as safety outcomes; MI, stent thrombosis, and stroke plus all-cause death as efficacy outcomes to evaluate the therapeutic effects.
Before using the consistency model in the network meta-analysis, we used an inconsistency model to check for global inconsistency; if the P-value of the inconsistency model exceeded 0.05, the inconsistency model was considered insignificant, and then the consistency model was used for further analysis. The node-splitting method [19] was also used to identify local inconsistencies. If the P-value of the inconsistency model was less than 0.05, investigators would explore the inconsistencies in the results caused by each study by removing single studies. When the source of the inconsistencies was found, group members would consider removing this study from the results of the subsequent analysis. If there was no source of inconsistency, follow-up analysis would still be conducted; however, these results were considered to be potentially unreliable.
After the consistency model processing, the data were used to create a network map. The combinations of antithrombotic agents were ranked according to the surface under the cumulative ranking curve (SUCRA) [19], [20] and rankograms. Finally, interval plots were drawn to observe pairwise comparisons between the different strategies.
2.5. Risk of bias analysis
The Cochrane Collaboration’s tool in Review Manager version 5.3 [21] was used to do risk of bias analysis. Investigators created risk-of-bias graphs and a risk-of-bias summary in which green represented a low risk of bias, yellow an unclear risk of bias, and red a high risk of bias. The work was performed by two researchers (Shuo Wang and Ying Liu), and the results were checked by all the researchers.
3. Results
3.1. Study selection and characteristics
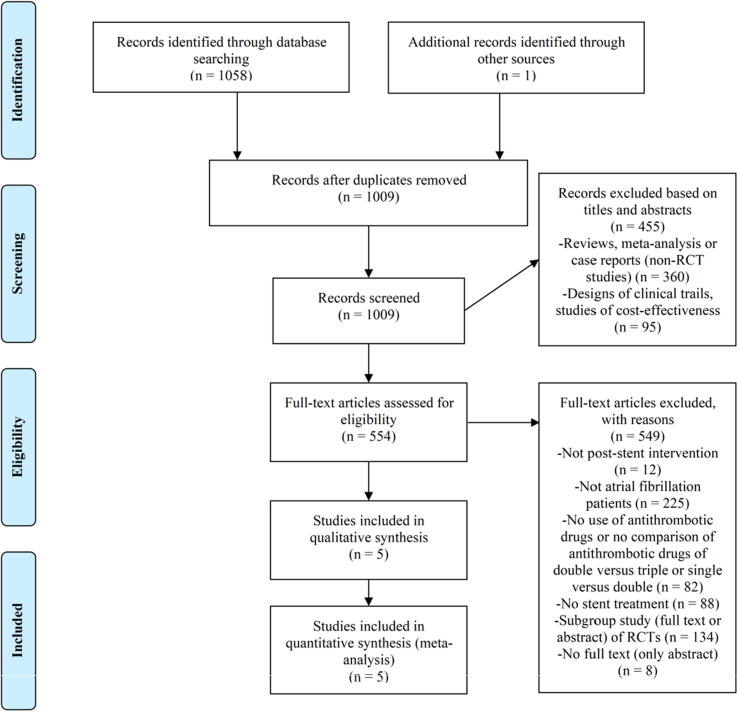
As shown in Fig. 1, 1059 articles were retrieved from the databases, of which five were eventually included in our study (AUGUSTUS [22], ENTRUST-AF PCI [23], PIONEER AF-PCI [24], RE-DUAL PCI [25], and WOEST [26]). After evaluation using the Newcastle Ottawa Scale, all the included studies had a score of ≥ 7 (Table 2), that is, the studies were of high quality. The five articles contained a total of 11,532 patients (Table 1). Among the studies, VKA + clopidogrel compared with traditional TAT were investigated in the WOEST study. In the AUGUSTUS, ENTRUST-AF PCI, and PIONEER AF-PCI studies, patients took a P2Y12 inhibitor and X factor inhibitors (apixaban, edoxaban, and rivaroxaban) as DAT, while the RE-DUAL PCI study defined 110 mg and 150 mg bid factor IIa inhibitor + P2Y12 inhibitors as DAT. Importantly, in the WOEST study, not all patients had AF (only 69%). Most studies comparing DAT with TAT had a follow-up period of approximately 12 months (AUGUSTUS: 6 months, RE-DUAL PCI: 14 months on average). Other baseline data are shown in Supplementary Table S4.
Fig. 1.
Literature screening procedure and exclusion criteria for meta-analysis. RCT, randomized controlled trial.
Table 2.
Quality evaluation of the included studies using Newcastle Ottawa scale
| Authors | Selection | Comparability | Outcome | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Representativeness of the exposed cohort | Selection of the nonexposed cohort | Ascertainment of exposure | Demonstration that outcome of interest was not present at start of | Studies controlling the most important factors | Studies control- ling the other main factors | Assessment of outcome | Was follow-up long enough for outcomes to | Adequacy of cohorts | ||
| AUGUSTUS | * | * | * | * | * | * | * | * | 8 | |
| ENTRUST-AF PCI | * | * | * | * | * | * | * | * | * | 9 |
| PIONEER AF-PCI | * | * | * | * | * | * | * | * | * | 9 |
| RE-DUAL PCI | * | * | * | * | * | * | * | * | * | 9 |
| WOEST | * | * | * | * | * | * | * | * | * | 9 |
Table 1.
Characteristics of studies included in the meta-analysis
| AUGUSTUS | ENTRUST AF-PCI | PIONEER AF-PCI | RE-DUAL PCI | WOEST | |
|---|---|---|---|---|---|
| First author/ Publication year | Renato D. Lopes /2019 | Pascal Vranckx /2019 | C. Michael Gibson/2016 | Christopher P. Cannon/2017 | Willem J M Dewilde/2013 |
| Antithrombotic drug strategy (composition of antithrombotic drug strategy) | Apixaban + P2Y12 inhibitor and Aspirin/ Apixaban + P2Y12 inhibitor and Placebo/ VKA + P2Y12 inhibitor and Aspirin/ VKA + P2Y12 inhibitor and Placebo Or : Apixaban/VKA /Aspirin / Aspirin-Matched Placebo | Edoxaban + a P2Y12 inhibitor for 12 months/ a VKA in combination with a P2Y12 inhibitor and aspirin for 1–12 months | Low-dose rivaroxaban + a P2Y12 inhibitor for 12 months/ very-low-dose rivaroxaban + DAPT for 1, 6, or 12 months/ a dose-adjusted VKA (once daily) + DAPT for 1, 6, or 12 months | Dual therapy with dabigatran (110 mg twice daily) + a P2Y12 inhibitor (clopidogrel or ticagrelor)/ Triple therapy with warfarin plus a P2Y12 inhibitor (clopidogrel or ticagrelor) and aspirin (for 1 to 3 months)/ Dual therapy with dabigatran (150 mg twice daily) + a P2Y12 inhibitor (clopidogrel or ticagrelor)/ Corresponding triple therapy with warfarin with dual therapy with dabigatran (150 mg twice daily) group | OAC + clopidogrel alone (double therapy)/ OAC + clopidogrel + aspirin (triple therapy) |
| The number of people with different drug strategies (n = ) | 1153/1153/1154/1154 Apixaban/ VKA/ Aspirin/ Aspirin-Matched Placebo (2306/2308/2307/2307) | 751/755 | 709/709/706 | 981/981/763/764 | 279/284 |
| Follow-up time (month) | 6 (additional visit at month 7) | 12 | 12 | Median: 14 | 12 |
| Creatinine clearance (ml/min) [mean (SD)] (experimental group/ control group) | NR | 71.8(53.7–91.1)/71.7(54.0–90.9) | 78.3 ± 31.3/77.5 ± 31.8/80.7 ± 30.0 | 76.3 ± 28.9/75.4 ± 29.1/83.7 ± 31.0/81.3 ± 29.6 | NR |
| Type of index event [n(%)] | ACS and PCI:873(38.0)/841(36.6)/844(36.8)/870(37.8) Medically managed ACS: 547(23.8)/550(23.9)547(23.9)/550(23.9) Elective PCI: 877(38.2)/907(39.5) 902(39.3)/882(38.3) | ACS: 388(52)/389(52) Stable coronary artery disease: 363(48)/366(48) | Non-ST elevation myocardial infarction: 130(18.5)/129(18.3)/123(17.8) ST elevation myocardial infarction: 86(12.3)/97(13.8)/74(10.7) Unstable angina : 145(20.7)/148(21.1)/164(23.7) | Stable angina or positive stress test: 433(44.1)/429(43.7)/320(41.9)/339(44.4) ACS: 509(51.9)/475(48.4)/391(51.2)/369(48.3) Staged procedure: 156(15.9)/168(17.1)/138(18.1)/134(17.5) Other: 43(4.4)/62(6.3)/65(8.5)/50(6.5) | ACS: 69(25)/86(30) |
| Diabetes mellitus [n(%)] (experimental group/ control group) | 842(36.5)/836(36.2)/842(36.5)/836(36.2) | 259(34)/258(34) | 204(28.8)/199(28.1)/221(31.3) | 362(36.9)/371(37.9)/260 (34.1)/303(39.7) | 68(24)/72(25) |
| Hypertension [n(%)] (experimental group/ control group) | 2042(88.6)/2031(88.0)/2031(88.0)/2042(88.5) | 674(90)/687(91) | 520(73.3)/519(73.2)/532(75.4) | NR | 193(69)/193(68) |
| Prior myocardial infarction [n(%)] (experimental group/ control group) | NR | 188(25)/177(23) | 140(19.8)/180(25.4)/157(22.2) | 237(24.2)/268(27.3)/194(25.4)/211(27.6) | 96(34)/100(35) |
| Prior stroke [n(%)] (experimental group/ control group) | NR | 97(13)/92(12) | NR | 74(7.5)/100(10.2)/52(6.8)/77(10.1) | 49(18)/50(18) |
NR: not reported. OAC: oral anticoagulant. VKA: vitamin K antagonist. DAPT: dual antiplatelet therapy. ACS: acute coronary syndrome
3.2. Exploration of the optimal OAC within 12 months of PCI according to safety outcomes
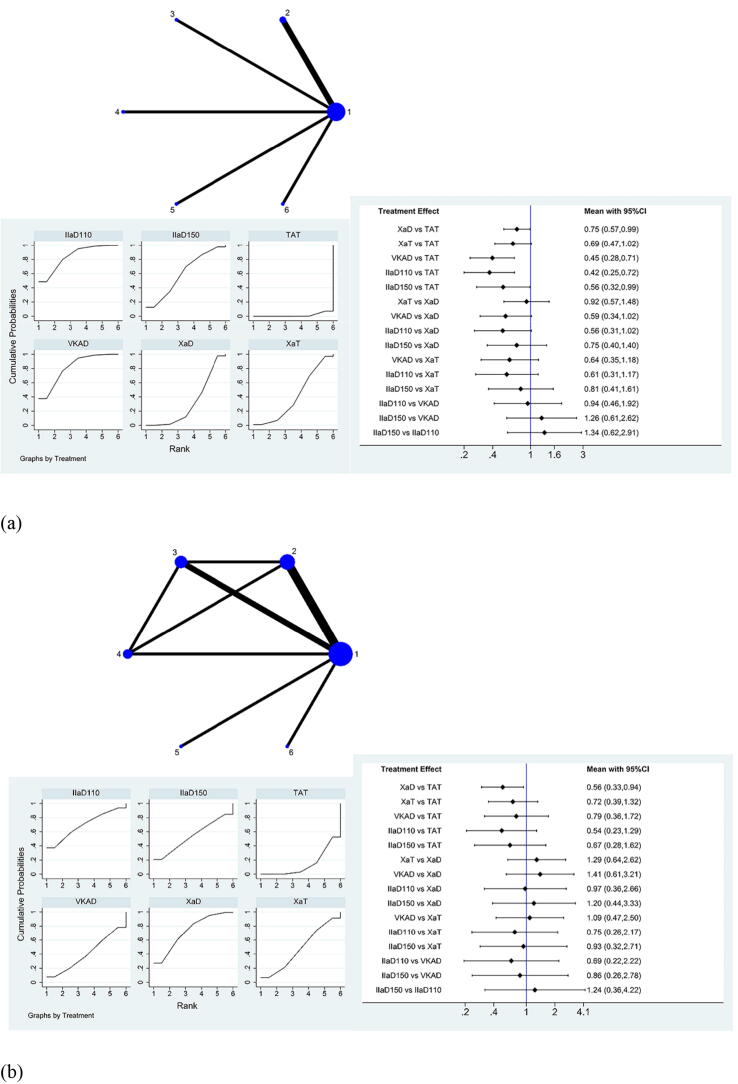
TIMI major and minor bleeding events can be seen in the network map in Fig. 2a. After further analysis through SUCRA and interval plots (Fig. 2a), compared to TAT, Xa inhibitor + P2Y12 inhibitor (RR: 0.75, 95% CI: 0.57–0.99), VKA + P2Y12 inhibitor (RR: 0.45, 95% CI: 0.28–0.71), IIa inhibitor 110 mg bid + P2Y12 inhibitor (RR: 0.42, 95% CI: 0.25–0.72), and IIa inhibitor 150 mg bid + P2Y12 inhibitor (RR:0.56, 95% CI: 0.32–0.99) showed a significant reduction in TIMI major and minor bleeding events. Importantly, of all the combinations of antithrombotic strategies, IIa inhibitor 110 mg bid + P2Y12 inhibitor showed the greatest advantage in reducing the occurrence of these events, while VKA + P2Y12 inhibitor ranked second, IIa inhibitor 150 mg bid + P2Y12 inhibitor ranked third, Xa inhibitor + P2Y12 inhibitor ranked fifth. (Supplementary Table S5).
Fig. 2.
Network meta-analysis of (a) TIMI major and minor bleeding events and (b) ISTH major bleeding events for the therapeutic effect evaluation of antithrombotic drugs within 12 months after PCI. TIMI, Thrombolysis In Myocardial Infarction; ISTH, International Society on Thrombosis and Hemostasis; PCI, percutaneous coronary intervention; CI, confidence interval; 1 or TAT, warfarin + P2Y12 inhibitor + aspirin; 2 or XaD, Xa inhibitor + P2Y12 inhibitor; 3 or XaT, Xa inhibitor + P2Y12 inhibitor + aspirin; 4 or VKAD, VKA + P2Y12 inhibitor; 5 or IIaD110, IIa inhibitor 110 mg bid + P2Y12 inhibitor; 6 or IIaD150, IIa inhibitor 150 mg bid + P2Y12 inhibitor.
Similarly, in the comparison of ISTH major bleeding events, compared to TAT, Xa inhibitor + P2Y12 inhibitor (RR: 0.56, 95% CI: 0.33–0.94) significantly reduced the occurrence of ISTH major bleeding events. The interval plot shows the comparison between different drug groups (Fig. 2b). The specific RRs and 95% CIs in the comparison are shown in Supplementary Table S6. Of all the combinations of antithrombotic strategies, Xa inhibitor + P2Y12 inhibitor was slightly safer than IIa inhibitor 110 mg bid + P2Y12 inhibitor and had the highest SUCRA ranking, IIa inhibitor 150 mg bid + P2Y12 inhibitor ranked third, while VKA + P2Y12 inhibitor ranked fifth.
There was no source of inconsistency in TIMI major and minor bleeding events, and the inconsistency in ISTH major bleeding events was only reflected in the local inconsistency of traditional TAT and Xa inhibitor + P2Y12 inhibitor + aspirin, Xa inhibitor + P2Y12 inhibitor and VKA + P2Y12 inhibitor, and Xa inhibitor + P2Y12 inhibitor + aspirin and VKA + P2Y12 inhibitor, indicating that the major local inconsistency of the results was derived from the group containing factor Xa.
In summary, IIa inhibitor 110 mg bid + P2Y12 inhibitor and Xa inhibitor + P2Y12 inhibitor have the greatest advantage for reducing TIMI major and minor bleeding and ISTH major bleeding, respectively.
3.3. Exploration of the optimal OAC within 12 months of PCI according to efficacy outcomes
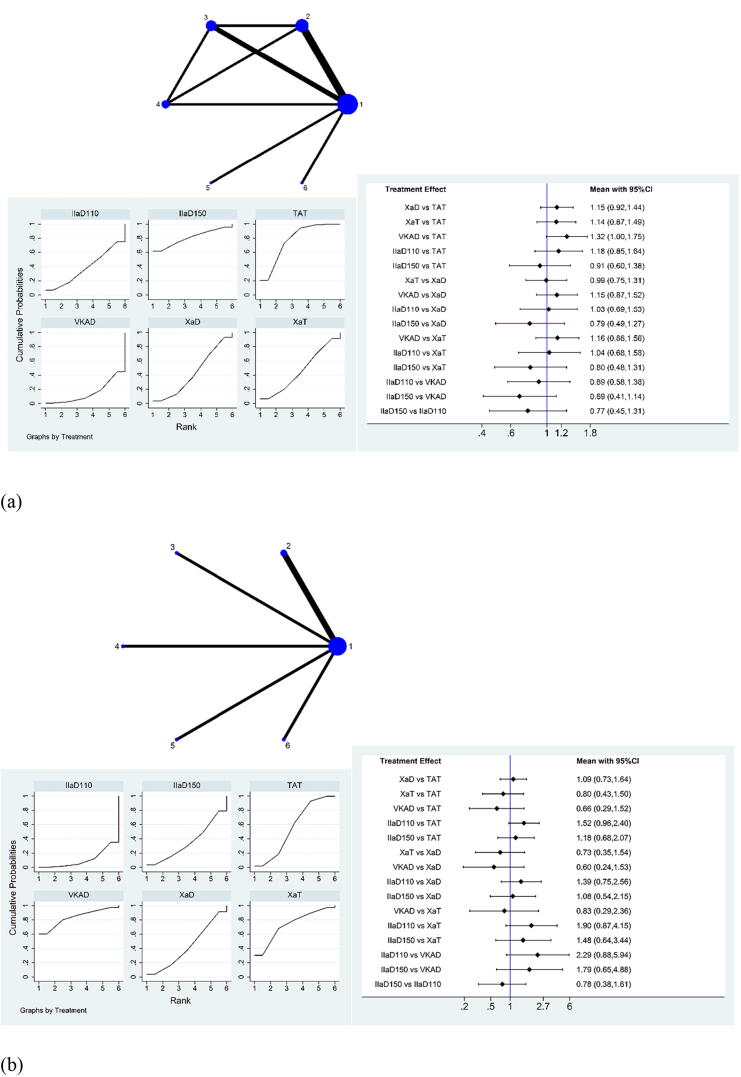
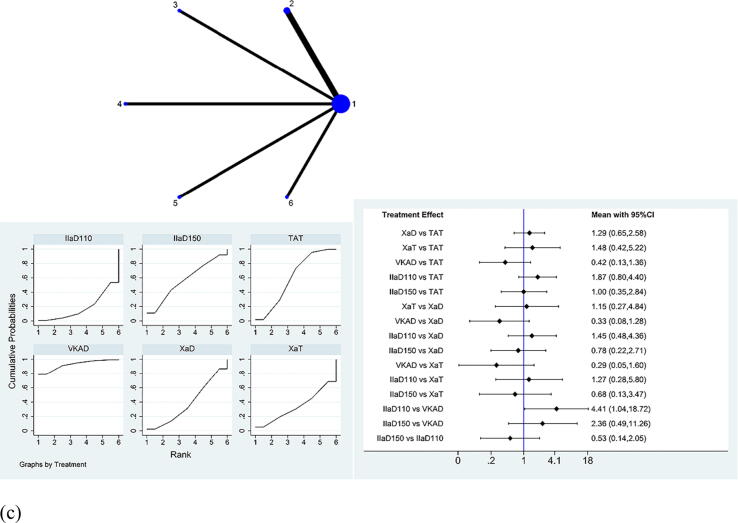
For stroke plus all-cause death, among all the groups, IIa inhibitor 150 mg bid + P2Y12 inhibitor was slightly more effective than traditional TAT, which is reflected in the highest SUCRA ranking (Fig. 3a). SUCRA ranking revealed that Xa inhibitor + P2Y12 inhibitor ranked fourth, IIa inhibitor 110 mg bid + P2Y12 inhibitor ranked fifth, whereas VKA + P2Y12 inhibitor ranked the lowest (Supplementary Table S7). The comparison of the efficacy of different drugs is demonstrated in the interval plot.
Fig. 3.
Network meta-analysis of (a) stroke plus all-cause death events, (b) acute myocardial infarction events, and (c) stent thrombosis events for the therapeutic effect evaluation of antithrombotic drugs within 12 months after PCI. PCI, percutaneous coronary intervention; CI, confidence interval; 1 or TAT, warfarin + P2Y12 inhibitor + aspirin; 2 or XaD, Xa inhibitor + P2Y12 inhibitor; 3 or XaT, Xa inhibitor + P2Y12 inhibitor + aspirin; 4 or VKAD, VKA + P2Y12 inhibitor; 5 or IIaD110, IIa inhibitor 110 mg bid + P2Y12 inhibitor; 6 or IIaD150, IIa inhibitor 150 mg bid + P2Y12 inhibitor.
For acute MI events, among all the groups, VKA + P2Y12 inhibitor ranked the highest, Xa inhibitor + P2Y12 inhibitor ranked fourth, and IIa inhibitor 150 mg bid + P2Y12 inhibitor ranked fifth and IIa inhibitor 110 mg bid + P2Y12 inhibitor ranked last (Supplementary Table S8). The detailed RRs and 95% CIs of the comparison are shown in Fig. 3b.
For stent thrombosis events, VKA + P2Y12 inhibitor ranked first and showed a significant advantage compared to IIa inhibitor 110 mg bid + P2Y12 inhibitor, (RR: 0.23, 95% CI: 0.05–0.96). IIa inhibitor 150 mg bid + P2Y12 inhibitor ranked third and Xa inhibitor + P2Y12 inhibitor ranked fourth, whereas IIa inhibitor 110 mg bid + P2Y12 inhibitor ranked last. (Fig. 3c and Supplementary Table S9).
There was no source of inconsistency in acute MI events and stent thrombosis events. In stroke plus all-cause death results, there was global inconsistency. Through the analysis of each included study, we found that the inconsistency originated from the WOEST study. Not all patients in the WOEST study had AF, and the sample size of the included patients was also small. After a group discussion, we decided to remove WOEST from the stroke plus all-cause death analysis. Because it did not cause obvious heterogeneity or inconsistency in the other results, we retained the WOEST study in the other analyses.
In summary, for stroke plus all-cause death events, doctors can select IIa inhibitor 150 mg bid + P2Y12 inhibitor as DAT; for acute MI or stent thrombosis, VKA + P2Y12 inhibitor can be selected as the best DAT combination.
3.4. Risk of bias analysis
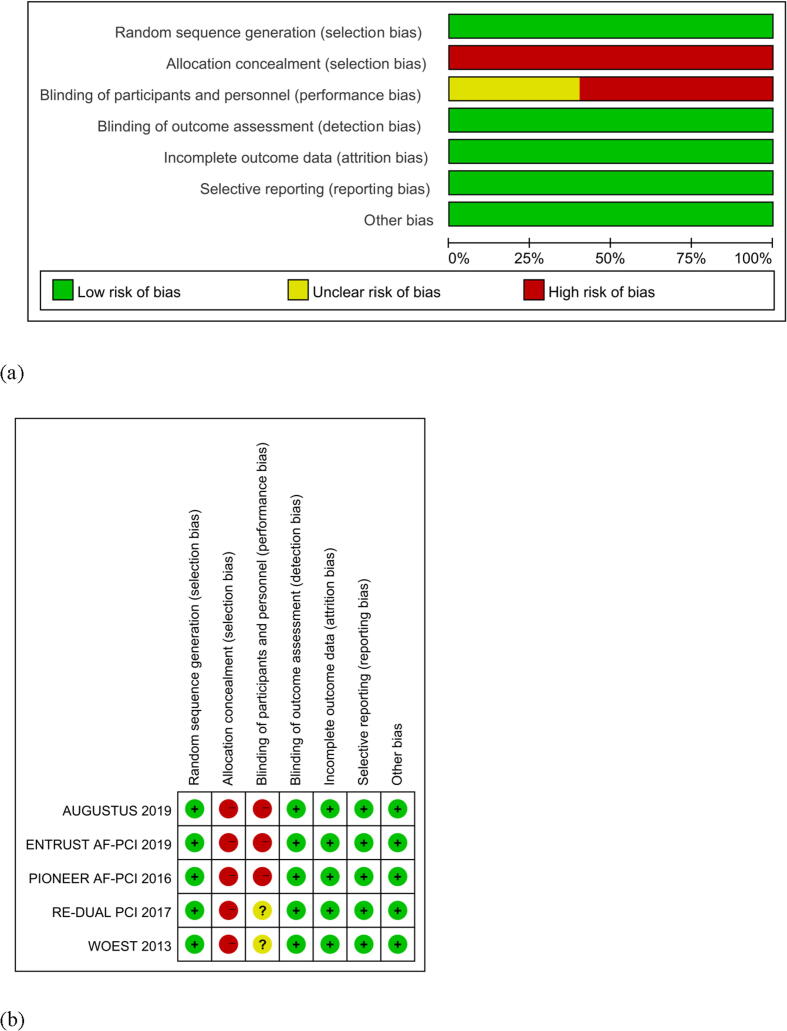
Since the included studies were all open-label studies, there was a risk of selection bias. Some articles were not blinded or did not describe the blinding of participants and personnel; thus, there was a risk of performance bias; the risk of other biases was low (Fig. 4a, b).
Fig. 4.
(a) Risk-of-bias graph (b) Risk-of-bias summary of the included studies with the help of Cochrane Collaboration's tool.
4. Discussion
Our research led to the following conclusions. For reducing TIMI major or minor bleeding events, factor IIa inhibitor 110 mg bid + P2Y12 inhibitor had the greatest advantage. For reducing ISTH major bleeding events, Xa inhibitor + P2Y12 inhibitor was best. For stroke plus all-cause death, factor IIa inhibitor 150 mg bid + P2Y12 inhibitor should be prioritised, and for MI and stent thrombosis, VKA + P2Y12 inhibitor was most beneficial.
NOACs are increasingly recognised as the drug of choice for the prevention of stroke in AF patients [27]. Both factor IIa inhibitors [28] and factor Xa inhibitors [29] were non-inferior to warfarin in reducing the incidence of stroke. Moreover, there is evidence that rivaroxaban can reduce the rate of MI in patients with AF compared to VKAs, and low-dose rivaroxaban can reduce mortality and atherothrombotic events in patients with coronary artery disease [30]. However, do the benefits of NOACs apply to AF patients who have undergone PCI?
In AF patients who have undergone PCI, the predominance of DAT has been demonstrated compared with TAT [22], [23], [24], [25], [26], especially after the publication of the AUGUSTUS study in which it was revealed that the apixaban-containing group had reduced bleeding events compared to the VKA-containing group, providing an intuitive comparison between NOAC and VKA. Regardless, it was still unclear which class of NOACs has more advantages in terms of efficacy and safety. Therefore, we performed a network meta-analysis of the results.
For our research, firstly, the literature included in this study was analyzed. The average CHA2DS2-VASC score of patients included in each study was mostly between 3 and 4, but some patients' score was 2–3 or 4–5. Similarly, most of the patients in various studies had a HAS-BLED median score between 2 and 3, but some patients had a score close to 4. Partial differences in thrombosis and bleeding profiles may cause additional heterogeneity and affect the results. However, due to the small span of CHA2DS2-VASC and HAS-BLED score differences, and the mean values of the two scores in each study were similar, the results of our meta-analysis based on the data from these studies were relatively reliable. In addition, VKA rather than NOAC was used in the WOEST study. The fact that patients in the PIONEER AF-PCI study received rivaroxaban in the very low-dose rivaroxaban + DAPT group, rather than the NOAC dose for stroke prevention used in other trials, may also have influenced the results. However, since our meta-analysis was based on different antithrombotic strategies, we need to fully analyze its advantages and disadvantages to achieve the purpose of comprehensive analysis, so we made a cross-sectional comparison of all drug groups included in the literature.
Next, we analyze our results. Bleeding events are a common safety outcome when evaluating antithrombotic therapy [31], [32], [33]. Thus, to avoid bleeding events, which antithrombotic strategy should we choose? We found that for TIMI major and minor bleeding events, the advantage of factor IIa 110 mg bid + P2Y12 inhibitor compared with factor Xa inhibitor + P2Y12 inhibitor approached statistical significance (RR: 0.56, 95% CI: 0.31–1.02), and the same result was shown for the SUCRA score (84.5 vs. 31.6). For ISTH major bleeding events, the SUCRA score of factor Xa inhibitor + P2Y12 inhibitor was only slightly higher than that of factor IIa 110 mg bid + P2Y12 inhibitor (73.3 vs. 69.5). Therefore, we believed that factor IIa 110 mg bid + P2Y12 inhibitor was more advantageous in reducing bleeding events.
Regarding efficacy outcomes, although many studies have shown that NOAC dual therapy can significantly reduce bleeding events compared with traditional TAT, there may be no difference in the occurrence of stent thrombosis events and MI events [34], [35]. The same results were observed in our meta-analysis: although there was no statistical difference between each DAT group, VKA + P2Y12 inhibitor had the highest SUCRA score. This result was mainly due to the WOEST study: four patients (1.4%) in the VKA dual therapy group had stent thrombosis, and nine (3.2%) had MI. The sample size of the WOEST study was the smallest among the five studies. However, due to its low cost and wider treatment range [36], [37], we can still consider using VKAs in patients with low bleeding risk and a relatively high financial burden, even if NOACs have obvious advantages.
Moreover, our results revealed that factor IIa (150 mg bid + P2Y12 inhibitor) had the greatest benefit for reducing stroke and all-cause death. However, in the included studies, we were only able to obtain stroke and cardiovascular death in the PIONEER AF-PCI study. Therefore, it may have influenced the overall data of the factor Xa group. However, although the difference between factor IIa 150 mg bid + P2Y12 inhibitor and factor Xa + P2Y12 inhibitor was not statistically significant (RR: 0.79, 95% CI: 0.49–1.27), the SUCRA score of factor IIa 150 mg bid + P2Y12 inhibitor was greater than that of factor Xa + P2Y12 inhibitor (80.8 vs. 43.1); thus, we can consider that factor Xa + P2Y12 inhibitor had little influence on the result, and the result remains valuable.
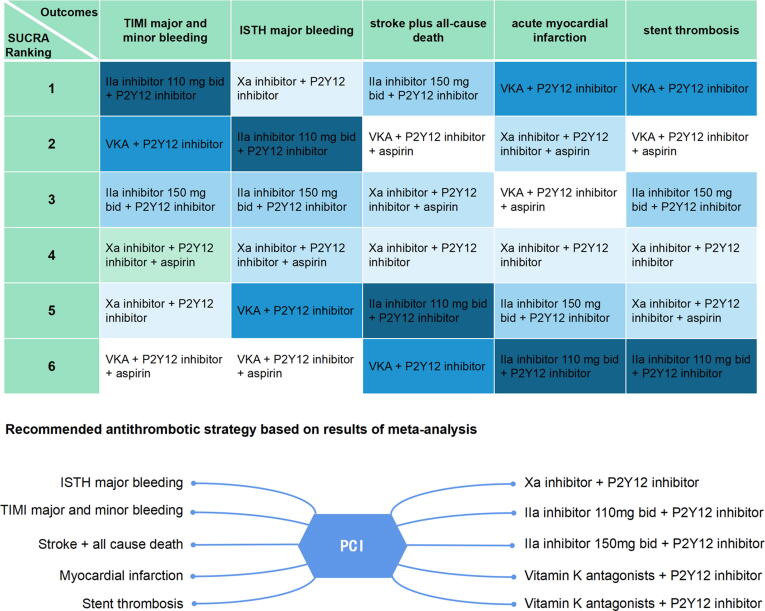
Finally, we created a representative figure to summarise our findings (Fig. 5).
Fig. 5.
Summary of results of meta-analysis. ISTH, International Society on Thrombosis and Hemostasis; TIMI, Thrombolysis In Myocardial Infarction; PCI, percutaneous coronary intervention; bid, bis in die; NOAC, novel oral anticoagulant.
5. Limitations
Our study has some limitations. First, because of the literature itself, not all of the patients included in the literature had AF. Second, as for the baseline data of patients in the included literatures, there were certain differences in the bleeding profiles and thrombosis profiles of patients in the 4 NOAC antithrombotic therapy studies. This might have some influence on our results. Besides, some of the results may have potential risks. For example, the source of inconsistency could not be identified in some of the results of the network meta-analysis or the inconsistency was high. Finally, due to incomplete raw data, it was not possible to analyse the specific factor Xa to develop a more comprehensive treatment plan for AF patients. Clinical studies that directly compare different antithrombotic strategies are needed to facilitate more optimised treatments for AF patients.
6. Conclusions
In conclusion, factor IIa inhibitor 110 mg bid + P2Y12 inhibitor had the greatest advantage in reducing TIMI major or minor bleeding and may have the greatest advantage in reducing bleeding events; Xa inhibitor + P2Y12 inhibitor had the greatest advantage in reducing ISTH major bleeding. For stroke plus all-cause death, factor IIa inhibitor 150 mg bid + P2Y12 inhibitor should be prioritised, and for MI and stent thrombosis, VKAs + P2Y12 inhibitors should be preferred.
Funding
This work was supported by the State Key Project of National Natural Science of China (81530052).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2021.100850.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Freedman B., Potpara T.S., Lip G.Y.H. Stroke prevention in atrial fibrillation. Lancet (London, England) 2016;388(10046):806–817. doi: 10.1016/S0140-6736(16)31257-0. [DOI] [PubMed] [Google Scholar]
- 2.Go A.S., Reynolds K., Yang J., Gupta N., Lenane J., Sung S.H., Harrison T.N., Liu T.I., Solomon M.D. Association of Burden of Atrial Fibrillation With Risk of Ischemic Stroke in Adults With Paroxysmal Atrial Fibrillation: The KP-RHYTHM Study. JAMA Cardiol. 2018;3(7):601. doi: 10.1001/jamacardio.2018.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lip G, Freedman B, De Caterina R, Potpara TS. Stroke prevention in atrial fibrillation: Past, present and future. Comparing the guidelines and practical decision-making. Thrombosis and haemostasis 2017; 117: 1230-1239. [DOI] [PubMed]
- 4.Michniewicz E., Mlodawska E., Lopatowska P., Tomaszuk-Kazberuk A., Malyszko J. Patients with atrial fibrillation and coronary artery disease - Double trouble. Adv. Med. Sci. 2018;63(1):30–35. doi: 10.1016/j.advms.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 5.Capodanno D., Huber K., Mehran R., Lip G.Y.H., Faxon D.P., Granger C.B. Management of Antithrombotic Therapy in Atrial Fibrillation Patients Undergoing PCI: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019;74:83–99. doi: 10.1016/j.jacc.2019.05.016. [DOI] [PubMed] [Google Scholar]
- 6.Andrade J.G., Deyell M.W., Wong G.C., Macle L. Antithrombotic Therapy for Atrial Fibrillation and Coronary Disease Demystified. Can. J. Cardiol. 2018;34(11):1426–1436. doi: 10.1016/j.cjca.2018.08.028. [DOI] [PubMed] [Google Scholar]
- 7.Camm A.J., Simantirakis E., Goette A., Lip G.Y.H., Vardas P., Calvert M., Chlouverakis G., Diener H.-C., Kirchhof P. Atrial high-rate episodes and stroke prevention. Europace. 2017;19(2):169–179. doi: 10.1093/europace/euw279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hijazi M., Aljohani S., Alqahtani F., Chaker Z., Al Hajji M., Al Hallak A., Alkhouli M. Perception of the Risk of Stroke and the Risks and Benefits of Oral Anticoagulation for Stroke Prevention in Patients With Atrial Fibrillation: A Cross-Sectional Study. Mayo Clin. Proc. 2019;94(6):1015–1023. doi: 10.1016/j.mayocp.2018.08.043. [DOI] [PubMed] [Google Scholar]
- 9.Angiolillo D.J., Goodman S.G., Bhatt D.L., Eikelboom J.W., Price M.J., Moliterno D.J., Cannon C.P., Tanguay J.-F., Granger C.B., Mauri L., Holmes D.R., Gibson C.M., Faxon D.P. Antithrombotic Therapy in Patients With Atrial Fibrillation Treated With Oral Anticoagulation Undergoing Percutaneous Coronary Intervention: A North American Perspective-2018 Update. Circulation. 2018;138(5):527–536. doi: 10.1161/CIRCULATIONAHA.118.034722. [DOI] [PubMed] [Google Scholar]
- 10.January C.T., Wann L.S., Calkins H., Chen L.Y., Cigarroa J.E., Cleveland J.C., Jr 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration With the Society of Thoracic Surgeons. Circulation. 2019;140:e125–e151. doi: 10.1161/CIR.0000000000000665. [DOI] [PubMed] [Google Scholar]
- 11.Forslund T., Wettermark B., Andersen M., Hjemdahl P. Stroke and bleeding with non-vitamin K antagonist oral anticoagulant or warfarin treatment in patients with non-valvular atrial fibrillation: a population-based cohort study. Europace. 2018;20(3):420–428. doi: 10.1093/europace/euw416. [DOI] [PubMed] [Google Scholar]
- 12.O'Brien E.C., Kim S., Thomas L., Fonarow G.C., Kowey P.R., Mahaffey K.W., Gersh B.J., Piccini J.P., Peterson E.D. Clinical Characteristics, Oral Anticoagulation Patterns, and Outcomes of Medicaid Patients With Atrial Fibrillation: Insights From the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF I) Registry. J. Am. Heart Ass. 2016;5(5) doi: 10.1161/JAHA.115.002721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Polzin A., Dannenberg L., Wolff G., Helten C., Achilles A., Hohlfeld T., Zeus T., Kelm M., Massberg S., Petzold T. Non-vitamin K oral anticoagulants (NOAC) and the risk of myocardial infarction: Differences between factor IIa and factor Xa inhibition? Pharmacol. Ther. 2019;195:1–4. doi: 10.1016/j.pharmthera.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 14.Brown K.S., Zahir H., Grosso M.A., Lanz H.J., Mercuri M.F., Levy J.H. Nonvitamin K antagonist oral anticoagulant activity: challenges in measurement and reversal. Critical Care (London, England) 2016;20:273. doi: 10.1186/s13054-016-1422-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Favaloro E.J., Pasalic L., Curnow J., Lippi G. Laboratory Monitoring or Measurement of Direct Oral Anticoagulants (DOACs): Advantages, Limitations and Future Challenges. Curr. Drug Metab. 2017;18:598–608. doi: 10.2174/1389200218666170417124035. [DOI] [PubMed] [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS medicine 2009; 6: e1000097. [DOI] [PMC free article] [PubMed]
- 17.Lo C.K., Mertz D., Loeb M. Newcastle-Ottawa Scale: comparing reviewers' to authors' assessments. BMC Med. Res. Method. 2014;14:45. doi: 10.1186/1471-2288-14-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shim S., Yoon B.-H., Shin I.-S., Bae J.-M. Network meta-analysis: application and practice using Stata. Epidem. & Health. 2017;39:e2017047. doi: 10.4178/epih.e2017047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh S., Fumery M., Sandborn W.J., Murad M.H. Systematic review and network meta-analysis: first- and second-line biologic therapies for moderate-severe Crohn's disease. Aliment. Pharmacol. Ther. 2018;48(4):394–409. doi: 10.1111/apt.14852. [DOI] [PubMed] [Google Scholar]
- 20.Tenforde MW, Shapiro AE, Rouse B, Jarvis JN, Li T, Eshun-Wilson I, et al. Treatment for HIV-associated cryptococcal meningitis. The Cochrane database of systematic reviews 2018; 7: Cd005647. [DOI] [PMC free article] [PubMed]
- 21.Zeng X., Zhang Y., Kwong J.S.W., Zhang C., Li S., Sun F., Niu Y., Du L. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. J. Evid. -Based Med. 2015;8(1):2–10. doi: 10.1111/jebm.12141. [DOI] [PubMed] [Google Scholar]
- 22.Lopes R.D., Heizer G., Aronson R., Vora A.N., Massaro T., Mehran R., Goodman S.G., Windecker S., Darius H., Li J., Averkov O., Bahit M.C., Berwanger O., Budaj A., Hijazi Z., Parkhomenko A., Sinnaeve P., Storey R.F., Thiele H., Vinereanu D., Granger C.B., Alexander J.H. Antithrombotic Therapy after Acute Coronary Syndrome or PCI in Atrial Fibrillation. New England J. Med. 2019;380(16):1509–1524. doi: 10.1056/NEJMoa1817083. [DOI] [PubMed] [Google Scholar]
- 23.Vranckx P., Valgimigli M., Eckardt L., Tijssen J., Lewalter T., Gargiulo G., Batushkin V., Campo G., Lysak Z., Vakaliuk I., Milewski K., Laeis P., Reimitz P.-E., Smolnik R., Zierhut W., Goette A. Edoxaban-based versus vitamin K antagonist-based antithrombotic regimen after successful coronary stenting in patients with atrial fibrillation (ENTRUST-AF PCI): a randomised, open-label, phase 3b trial. Lancet (London, England) 2019;394(10206):1335–1343. doi: 10.1016/S0140-6736(19)31872-0. [DOI] [PubMed] [Google Scholar]
- 24.Gibson C.M., Mehran R., Bode C., Halperin J., Verheugt F.W., Wildgoose P., Birmingham M., Ianus J., Burton P., van Eickels M., Korjian S., Daaboul Y., Lip G.Y.H., Cohen M., Husted S., Peterson E.D., Fox K.A. Prevention of Bleeding in Patients with Atrial Fibrillation Undergoing PCI. New England J. Med. 2016;375(25):2423–2434. doi: 10.1056/NEJMoa1611594. [DOI] [PubMed] [Google Scholar]
- 25.Cannon C.P., Bhatt D.L., Oldgren J., Lip G.Y.H., Ellis S.G., Kimura T., Maeng M., Merkely B., Zeymer U., Gropper S., Nordaby M., Kleine E., Harper R., Manassie J., Januzzi J.L., ten Berg J.M., Steg P.G., Hohnloser S.H. Dual Antithrombotic Therapy with Dabigatran after PCI in Atrial Fibrillation. New England J. Med. 2017;377(16):1513–1524. doi: 10.1056/NEJMoa1708454. [DOI] [PubMed] [Google Scholar]
- 26.Dewilde W.JM., Oirbans T., Verheugt F.WA., Kelder J.C., De Smet B.JGL., Herrman J.-P., Adriaenssens T., Vrolix M., Heestermans A.ACM., Vis M.M., Tijsen J.GP., van 't Hof A.W., ten Berg J.M. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open-label, randomised, controlled trial. Lancet (London, England) 2013;381(9872):1107–1115. doi: 10.1016/S0140-6736(12)62177-1. [DOI] [PubMed] [Google Scholar]
- 27.Valgimigli M., Bueno H., Byrne R.A., Collet J.-P., Costa F., Jeppsson A., Jüni P., Kastrati A., Kolh P., Mauri L., Montalescot G., Neumann F.-J., Petricevic M., Roffi M., Steg P.G., Windecker S., Zamorano J.L., Levine G.N., Badimon L., Vranckx P., Agewall S., Andreotti F., Antman E., Barbato E., Bassand J.-P., Bugiardini R., Cikirikcioglu M., Cuisset T., De Bonis M., Delgado V., Fitzsimons D., Gaemperli O., Galiè N., Gilard M., Hamm C.W., Ibanez B., Iung B., James S., Knuuti J., Landmesser U., Leclercq C., Lettino M., Lip G., Piepoli M.F., Pierard L., Schwerzmann M., Sechtem U., Simpson I.A., Uva M.S., Stabile E., Storey R.F., Tendera M., Van de Werf F., Verheugt F., Aboyans V., Windecker S., Aboyans V., Agewall S., Barbato E., Bueno H., Coca A., Collet J.-P., Coman I.M., Dean V., Delgado V., Fitzsimons D., Gaemperli O., Hindricks G., Iung B., Jüni P., Katus H.A., Knuuti J., Lancellotti P., Leclercq C., McDonagh T., Piepoli M.F., Ponikowski P., Richter D.J., Roffi M., Shlyakhto E., Simpson I.A., Zamorano J.L., Windecker S., Aboyans V., Agewall S., Barbato E., Bueno H., Coca A., Collet J.-P., Coman I.M., Dean V., Delgado V., Fitzsimons D., Gaemperli O., Hindricks G., Iung B., Jüni P., Katus H.A., Knuuti J., Lancellotti P., Leclercq C., McDonagh T., Piepoli M.F., Ponikowski P., Richter D.J., Roffi M., Shlyakhto E., Simpson I.A., Zamorano J.L., Roithinger F.X., Aliyev F., Stelmashok V., Desmet W., Postadzhiyan A., Georghiou G.P., Motovska Z., Grove E.L., Marandi T., Kiviniemi T., Kedev S., Gilard M., Massberg S., Alexopoulos D., Kiss R.G., Gudmundsdottir I.J., McFadden E.P., Lev E., De Luca L., Sugraliyev A., Haliti E., Mirrakhimov E., Latkovskis G., Petrauskiene B., Huijnen S., Magri C.J., Cherradi R., Ten Berg J.M., Eritsland J., Budaj A., Aguiar C.T., Duplyakov D., Zavatta M., Antonijevic N.M., Motovska Z., Fras Z., Montoliu A.T., Varenhorst C., Tsakiris D., Addad F., Aydogdu S., Parkhomenko A., Kinnaird T. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS) Eur. Heart J. 2018;39(3):213–260. doi: 10.1093/eurheartj/ehx419. [DOI] [PubMed] [Google Scholar]
- 28.Connolly S.J., Ezekowitz M.D., Yusuf S., Eikelboom J., Oldgren J., Parekh A., Pogue J., Reilly P.A., Themeles E., Varrone J., Wang S., Alings M., Xavier D., Zhu J., Diaz R., Lewis B.S., Darius H., Diener H.-C., Joyner C.D., Wallentin L. Dabigatran versus warfarin in patients with atrial fibrillation. New England J. Med. 2009;361(12):1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 29.Patel M.R., Mahaffey K.W., Garg J., Pan G., Singer D.E., Hacke W., Breithardt G., Halperin J.L., Hankey G.J., Piccini J.P., Becker R.C., Nessel C.C., Paolini J.F., Berkowitz S.D., Fox K.A.A., Califf R.M. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. New England J. Med. 2011;365(10):883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 30.Petzold T., Thienel M., Dannenberg L., Mourikis P., Helten C., Ayhan A., M’Pembele R., Achilles A., Trojovky K., Konsek D., Zhang Z., Regenauer R., Pircher J., Ehrlich A., Lüsebrink E., Nicolai L., Stocker T.J., Brandl R., Röschenthaler F., Strecker J., Saleh I., Spannagl M., Mayr C.H., Schiller H.B., Jung C., Gerdes N., Hoffmann T., Levkau B., Hohlfeld T., Zeus T., Schulz C., Kelm M., Polzin A. Rivaroxaban Reduces Arterial Thrombosis by Inhibition of FXa-Driven Platelet Activation via Protease Activated Receptor-1. Circ. Res. 2020;126(4):486–500. doi: 10.1161/CIRCRESAHA.119.315099. [DOI] [PubMed] [Google Scholar]
- 31.Kato E.T., Giugliano R.P., Ruff C.T., Koretsune Y., Yamashita T., Kiss R.G., Nordio F., Murphy S.A., Kimura T., Jin J., Lanz H., Mercuri M., Braunwald E., Antman E.M. Efficacy and Safety of Edoxaban in Elderly Patients With Atrial Fibrillation in the ENGAGE AF-TIMI 48 Trial. J. Am. Heart Ass. 2016;5(5) doi: 10.1161/JAHA.116.003432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Al-Shahi Salman R., Dennis M.S., Sandercock PAG, Sudlow CLM, Wardlaw J.M., Whiteley W.N., Murray G.D., Stephen J., Newby D.E., Sprigg N., Werring D.J., White P.M., Baigent C., Lasserson D., Sullivan F., Carrie J., Rojas J., Amoils S., Bamford J., Armitage J., Rinkel G., Lowe G., Emberson J., Innes K., Dinsmore L., Drever J., Williams C., Perry D., McGill C., Buchanan D., Walker A., Hutchison A., Matthews C., Fraser R., McGrath A., Deary A., Anderson R., Walker P., Hansen C., Parker R., Rodriguez A., Macleod M.R., Gattringer T., Palmer J., Sakka E., Adil-Smith J., Minks D., Mitra D., Bhatnagar P., du Plessis J., Joshi Y., Lerpiniere C., O'Brien R., Burgess S., Mead G., Paulton R., Doubal F., McCormick K., Hunter N., Taylor P., Parakramawansha R., Perry J., Blair G., MacRaild A., Parry-Jones A., Johnes M., Lee S., Shaw K.M., Burger I., Punter M., Ingham A., Perez J., Naing Z., Morell J., Marsden T., Hall A., Marshall S., Harrison L., Jarapa R., Wood E., O'Loughlin V., Cohen D., Davies S., Njoku K., Mpelembue M., Burgess L., Licenik R., Ngwako M., Nisar N., Niranchanan R., Roganova T., Bathula R., Devine J., David A., Oshodi A., Guo F., Owoyele E., Sukdeo V., Ballantine R., Abbdul-saheb M., Chamberlain A., Chandrakumar A., Poku P., Harkness K., Blank C., Richards E., Ali A., Kibutu F., Balitska O., Birchall K., Bayliss P., Doyle C., Stocks K., Majis A., Howe J.o., Kamara C., Barron L., Maatouk A., Lindert R., Dakin K., Redgrave J., Bhaskaran B., Salih I., Kelly D., Szabo S., Tomlin D., Bearne H., Buxton J., Fitzell P., Ayres G., Saulat A., Horan K., Garfield-Smith J., Bhakri H., Guyler P., Sinha D., Loganathan T., Siddiqui A., Siddiqui A., Coward L., Kunhunny S., Tysoe S., Orath Prabakaran R., Kelavkar S., Rashmi S., Ngo D., Ng K.X., Menon N., Shah S., Barber M., Esson D., Brodie F., Anjum T., Wani M., Krishnan M., Quinn L., Spencer J., Jones T., Thompson-Jones H., Dacey L., Chenna S., Storton S., Thomas S., Beaty T., Treadwell S., Davies C., Tucker S., Connor L., Slade P., Gainard G., Muddegowda G., Sanyal R., Remegoso A., Abano N., Causley C., Carpio R., Stevens S., Butler A., Varquez R., Denic H., Alipio F., Moores A., Barry A., Maguire H., Grocott J., Finney K., Lyjko S., Roffe C., Hiden J., Ferdinand P., Cvoro V., Ullah K., Chapman N., Couser M., Pound S., McCormick K., Mcauley S., Raghunathan S., Shelton F., Hedstrom A., Godfrey M., Havard D., Buck A., Krishnan K., Gilzeane N., Roffe J., Clarke J., Whittamore K., Sheikh S., Keshvara R., Jordan C., Jackson B., Wilkes G., Appleton J., Law Z., Matias O., Vasileiadis E., Mason C., Parry A., Landers G., Holden M., Aweid B., Rashed K., Balian L., Vickers C., Keeling E., Board S., Allison J., Buckley C., Williams-Yesson B., Board J., Pitt-Kerby T., Tanate A., Wood D., Kini M., Chadha D., Walstow D., Fong R., Luder R., Adesina T., Gallagher J., Bridger H., Murali E., Bhargava M., van Someren C., Harrington F., Mate A., James A., Courtauld G., Schofield C., Adie K., Lucas L., Bond K., Maund B., Ellis S., Mudd P., James M., Keenan S., Bowring A., Cageao J., Kingwell H., Roughan C., Hemsley A., Sword J., Strain D., Miller K., Goff A., Gupwell K., Thorpe K., Emsley H., Punekar S., McLoughlin A., Sultan S., Gregory B., Raj S., Doyle D., Muir K., Smith W., Welch A., Moreton F., Cheripelli B.K., El Tawil S., Kalladka D., Huang X., Day N., Ramachandran S., Crosbie C., Elliot J., Rudd T., Marks K., Bhalla A., Birns J., Kullane S., Weir N., Allen C., Pressly V., Crawford P., Battersby-Wood E., Blades A., Egerton S., Walters A., Evans S., Marigold J.R., Smith F., Howard G., Gartrell I., Smith S., Creeden R., Cox C., Boxall C., Hewitt J., Nott C., Sarah P., Whiteman J., Buckle S., Wallace R., Mardania R., Gray J., Triscott C., Nair A., Greig J., Rana P., Robinson M., Alam M.I., Wilson D., Watchurst C., Brezitski M., Crook L., Jones I., Banaras A., Patel K., Erande R., Hogan C., Hostettler I., Ashton A., Feerick S., Francia N., Oji N., Elliott E., Al-Mayhani T., Lerpiniere C., Fraser R., Dutta D., Brown P., Ward D., Davis F., Turfrey J., Hughes C., Collins K., Bakawala R., O'Connell S., Glass J., Broughton D., Tryambake D., Dixon L., Chapman K., Young A., Bergin A., Sigsworth A., Manoj A., Fletcher G., Lopez P., Cox P., Wilkinson M., Fitzsimmons P., Sharma N., Choulerton J., Button D., Dow L., Gbadamoshi L., Avis J., Madigan B., McCann S., Shaw L., Howcroft D., Lucas S., Stone A., Cluckie G., Lovelock C., Clarke B., Chopra N., Clarke N., Patel B., Kennedy K., Williams R., Blight A., O'Reilly J., Orefo C., Dayal N., Ghatala R., Adedoyin T., Watson F., Trippier S., Choy L., Moynihan B., Khan U., Jones V., Jeyaraj N., Kerin L., Thavanesan K., Tiwari D., Cox C., Ljubez A., Tucker L., Iqbal A., Bagnall C., Keltos M., Roberts J., Jupp B., Ovington C., Rogers E., David O., Bell J.o., Longland B., Hann G., Cooper M., Nasar M., Rajapakse A., Wynter I., Anwar I., Skinner H., Nozedar T., McArdle D., Kumar B., Crawford S., Annamalai A., Ramshaw A., Holmes C., Caine S., Osborn M., Dodd E., Murphy P., Devitt N., Baker P., Steele A., Guthrie L.B., Clarke S., Hassan A., Waugh D., Veraque E., Makawa L., Kambafwile M., Randall M., Papavasileiou V., Cullen C., Peters J., Thant H., Ingram T., Zoe M., Durairaj R., Harrison M., Stevenson S., Shackcloth D., Ewing J., Sutton V., McCarron M., McKee J., Doherty M., McVerry F., Blair C., MacLeod M., Irvine J., Gow H., Furnace J., Joyson A., Jagpal B., Ross S., Klaasen K., Nelson S., Clarke R., Crouch N., MacLennan B., Taylor V., Epstein D., Jones I., Shukla A., Krishnamurthy V., Nicholas P., Qureshi S., Webber A., Penge J., Ramadan H., Maguire S., Patterson C., Bellfield R., Hairsine B., Stewart K., Hooley M., Quinn O., Richard B., Moseley S., Nott C., Buckle S., Sarah P., Whiteman J., Edwards M., Lawson H., Wallace R., Triscott C., Tayler M., Pai Y., Dhakal M., Esisi B., Dima S., Smith G.M., Garside M., Naeem M., Baliga V., Rogers G., Brown E., Bruce D., Hayman R., Clayton S., Gamble E.d., Grue R., Charles B., Hague A., Blane S., Lambert C., Chaudhry A., Harrison T., Saastamoinen K., Hove D., Howaniec L., Grimwood G., Redjep O., Humphries F., Argandona L., Cuenoud L., Erumere E., Amlani S., Auld G., Salek-Haddadi A., Schulz U., Kennedy J., Ford G., Mathieson P., Reckless I., Teal R., Lenti G., Harston G., O'Brien E., Mcgee J., Mitchell J., Amis E., Handley D., Kelly S., Zachariah G., Francis J., Crisp S., Sesay J., Finlay S., Hayhoe H., Hannon N., Hughes T., Morse B., De Berker H., Tallantyre E., Osman A., White S., Schwarz S., Jelley B., Yadava R., Azhar K., Reddan J., Sangombe M., Stafford S., Fotherby K., Morgan D., Baig F., Jennings-Preece K., Butler D., Ahmad N., Willberry A., Stevens A., Rai B., Siddegowda P., Howard P., Saulat A., Hyatt L., Dobson T., Jarrett D., Ponnambath S., Tandy J., Harrington-Davies Y., Butler R., James C., Valentine S., Suttling A., Langhorne P., Kerr G., Wright F., Graham R., McAlpine C., Iqbal M.S., Humphreys L., Pasco K., Balazikova O., Nasim A., Peixoto C., Gallagher L., Shahmehri S., Ghosh S., Barrie E., Gilmour D., Henry M., Webb T., Cowie L., Rudenko H., McDonald S., Schumacher N., Walker S., Cosier T., Verrion A., Beranova E., Thomson A., Venter M., Kar A., Mashate S., Harvey K., Gardener L., Nguyen V., Halse O., Geraghty O., Hazel B., Wilding P., Tilley V., Esisi B., Cassidy T., McClelland B., Bokhari M., England T., Hedstrom A., Maddula M., Donnelly R., Findlay P., Macaden A., Shread I., Barr C., Mohd Nor A., Brown C., Persad N., Eglinton C., Weinling M., Hyams B., Shah A., Baker J., Byrne A., McGhee C., Smart A., Copeland C., Carpenter M., Walker M., Davey R., Needle A., Fathima R., Bateman G., Datta P., Stanners A., Jackson L., Ball J., Davis M., Atkinson N., Fawcett M., Thompson T., Guy H., Hogg V., Hays C., Woodward S., Haque M., Hakim E., Symonds S., Maanoosi M., Herman J., Black T., Miriam S., Clarke C., Anthony A., Tribbeck M., Cronin J., Mead D., Fennelly R., McIlmoyle J., Dickinson C., Jeffs C., Anwar S., Howard J., Jones K., Dhar S., Clay C., Siddiq M., Ivatts S., Baird Y., Sally M., Amey I., Newton S., Clayton-Evans L., Chadbourn I., Rayessa R., Naylor C., Rodgers A., Wilson L., Wilson S., Clarkson E., Davies R., Owings P., Sangster G., Gott V., Little V., Weir P., Cherian S., Jose D., Moroney H., Downham S., Dodd A., Vettimootal Johnson V., Codd L., Robinson N., Ahmed A., Albazzaz M.o., Johnson S., Denniss C., Cunningham M., Zahoor T., Webster T., Leason S., Haider S., Chatterjee K., Nallasivan A., Perkins C., Seagrave S., Jenkins C., Price F., Hughes C., Mercer L., Hussain M., Brown S., Harvey M., Homan J., Khan M., Whiting R., Foote L., Hunt N., Durman H., Brotherton L., Foot J., Pawley C., Foster E., Whitcher A., Metcalf K., Jagger J., McDonald S., Waterfield K., Sutton P., Shinh N., Anversha A., Ravenhill G., Greenwood R., Saada J., Wiltshire A., Perfitt R., Andole S., Gadapa N., Dunne K., Krommyda M., Burssens E., King S., Plewa C., Smyth N., Wilson J., Giallombardo E., Eglinton C., Sykes L., Kumar P., Barker J., Huggett I., Dunn L., Culmsee C., Thomas P., Myint M., O'Brien R., Brew H., Majmudar N., O'Connell J., Bunea G., Fox C., Gulliver D., Smith A., Mokoena B., Sattar N., Krishnamurthy R., Osborne E., Wilson D., Wroath B., Dynan K., Power M., Thompson S., Adell V., Orugun E., Poultney U., Glover R., Crowther H., Thornthwaite S., Wiggam I., Wallace A., Kerr E., Fulton A., Hunter A., Tauro S., Cuddy S., Mangion D., Hardwick A., Markova S., Lawrence T., Constantin C., Fletcher J.o., Thomas I., Pettitt K., Sekaran L., Tate M., Bharaj K., Simon R., Justin F., Sethuraman S., Phiri D., Mohammed N., Chauhan M., Elfandi K., Khan U., Stafford S., Reddan J., Eveson D., Mistri A., Manning L., Khan S., Patel C., Moqsith M., Sattar S., Lam M.Y., Musarrat K., Stephens C., Kalathil L., Miller R., Salehin M., Gautam N., Bailey D., Amor K., Meir J., Nicolson A., Imam J., Wood L., White J., Sajid M., Ghaly G., Ball M., Gascoyne R., Proeschel H., Sharpe S., Horton S., Beaves E., Jones S., Yip B., Bell M., MacLiver L., MacInnes B., Esson D., Sims D., Hurley J., Willmot M., Sutton C., Littleton E., Maiden S., Jones R., Cunningham J., Green C., Bates M., Shekhar R., Waterfield K., Gilham E., Ahmed I., Crown R., Fuller T., Goorah N., Bell A., Kelly C., Singh A., Walford J., Tomlinson B., Patel F., Duberley S., Kane I., Rajkumar C., Gaylard J., Breeds J., Gainsborough N., Pitt-Ford A., Barbon E., Latter L., Thompson P., Hervey S., Krishnamoorthy S., Vassallo J., Walter D., Cochrane H., Srinivasan M., Campbell R., Donaldson D., Motherwell N., Hurford F., Mukherjee I., Kenton A., Nyabadza S., Martin I., Hunt B., Hassan H., O'Toole S., Dallol B., Putterill J., Jha R., Gallifent R., Kakar P., Pusalkar A., Chan K., Dangri P., Beadle H., Cook A., Crabtree K., Subramonian S., Owusu-Agyei P., Temple N., Butterworth-Cowin N., Ragab S., Knops K., Jinks E., Dickson C., Gleave L., Dube J., Leggett J., Garcia T., Ispoglou S., Evans R., Ankolekar S., Hayes A., Ni H., Rahman B., Milligan J., Graham C., Jose J., Keegan B., Doherty M., Kelly J., Blair C., Dewar R., White J., Thomas K. Effects of antiplatelet therapy after stroke due to intracerebral haemorrhage (RESTART): a randomised, open-label trial. Lancet (London, England) 2019;393(10191):2613–2623. doi: 10.1016/S0140-6736(19)30840-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Vries T.I., Eikelboom J.W., Bosch J., Westerink J., Dorresteijn J.A.N., Alings M., Dyal L., Berkowitz S.D., van der Graaf Y., Fox K.A.A., Visseren F.L.J. Estimating individual lifetime benefit and bleeding risk of adding rivaroxaban to aspirin for patients with stable cardiovascular disease: results from the COMPASS trial. Eur. Heart J. 2019;40(46):3771–3778a. doi: 10.1093/eurheartj/ehz404. [DOI] [PubMed] [Google Scholar]
- 34.Schäfer A, Flierl U, Bauersachs J. Anti-thrombotic strategies in patients with atrial fibrillation undergoing PCI. Clinical research in cardiology 2020 Jul 21. [DOI] [PMC free article] [PubMed]
- 35.Russo V., Rago A., Proietti R., Attena E., Rainone C., Crisci M., Papa A.A., Calabrò P., D’Onofrio A., Golino P., Nigro G. Safety and Efficacy of Triple Antithrombotic Therapy with Dabigatran versus Vitamin K Antagonist in Atrial Fibrillation Patients: A Pilot Study. Biomed. Res. Int. 2019;2019:1–6. doi: 10.1155/2019/5473240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Al Said S, Alabed S, Kaier K, Tan AR, Bode C, Meerpohl JJ, et al. Non-vitamin K antagonist oral anticoagulants (NOACs) post-percutaneous coronary intervention: a network meta-analysis. The Cochrane database of systematic reviews 2019; 12: Cd013252. [DOI] [PMC free article] [PubMed]
- 37.Cîmpan P.L., Chira R.I., Mocan M., Anton F.P., Farcaş A.D. Oral Anticoagulant Therapy-When Art Meets Science. J. Clin. Med. 2019 Oct 21;8(10):1747. doi: 10.3390/jcm8101747. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.