Abstract
Background
Studies of insulin-like growth factor 1 (IGF-1) as a novel therapy for the treatment of cardiovascular diseases have proven promising. However, elevated IGF-1 levels have also been associated with poor patient outcomes in heart failure with reduced ejection fraction. IGF-1 therapy has additionally been shown to not be beneficial in the percutaneous coronary intervention setting. Although IGF-1 activation of the PI3K/Akt and ERK1/2 pathways have been demonstrated as cardioprotective, other cellular mechanisms have not been fully investigated.
Methods
Neonatal rat cardiac myocytes (NCMs) and fibroblasts (NCFs) were isolated from 1 to 2-day old pups using enzymatic digestion. NCMs and NCFs were pre-treated with IGF binding protein 6, inhibitors for the PI3K/Akt Wortmannin, ERK1/2 U0126, Rho Associated Protein Kinase (ROCK) GSK576371, Apoptosis Signal-regulating Kinase-1 (ASK-1) G2261818A, and p38MAPK RWJ67657 pathways before stimulation with IGF-1 for 62 and 50 h, respectively. Cardiac myocyte hypertrophy and fibroblast collagen synthesis were determined by 3H-leucine and 3H-proline incorporation, respectively.
Results
IGF-1 dose-dependently stimulated NCM hypertrophy and NCF collagen synthesis.
Treatment with IGFBP6 and the kinase inhibitors, Wortmannin, U0126, GSK576371, G2261818A and RWJ67657 significantly inhibited IGF-1 stimulated NCM hypertrophy and NCF collagen synthesis.
Conclusion
This study is the first to demonstrate that IGF-1 treatment in NCMs and NCFs activates the ROCK, ASK-1 and p38MAPK pathways. Future research may be guided by consideration of the PI3K/Akt and ERK1/2 pathways potentially increasing collagen synthesis, and the utilisation of a biased agonist to reduce activation of the ROCK, ASK-1 and p38MAPK pathways to maximise cardioprotective benefit whilst mitigating risks.
Keywords: Insulin-like growth factor 1, Rho Associated Protein Kinase, Apoptosis Signal-regulating Kinase 1, p38 MAPK, Cardiac cellular remodelling
1. Introduction
Insulin-like growth factor 1 (IGF-1) as a novel therapy in the setting of cardiovascular disease (CVD) has become an increasing focus of cardiovascular research, with promising pre-clinical and clinical results. However, some studies have outlined potential limitations of IGF-1 therapy, indicating a potentially incomplete understanding of the underlying mechanisms of IGF-1 therapy and that further research is required before its translation into clinical practice.
Studies have suggested that IGF-1 may have broad applicability in the cardiovascular setting. Clinical trials have demonstrated correlations between lower IGF-1 levels and increased mortality [1], [2] the presence of CVD, worse self-reported perception of overall health [1], the presence of heart failure (HF) [2] and more severe New York Heart Association functional impairment [2]. Additional studies have shown IGF-1 treatment improved cardiac function in HF patients[3]. In the pre-clinical setting, IGF-1 treatment enhanced left ventricular remodelling and function following myocardial infarction [4] and decreased infarct size in the ischaemia–reperfusion context [5], [6].
However, other research suggests that IGF-1 therapy should be considered with caution. Some studies have illustrated that increased levels of IGF-1 have also been associated with elevated all-cause mortality and development of HF [7]. Associations between IGF-1 levels and decreased mortality and hospitalisation for HF with reduced ejection fraction (HFrEF) were not found in HF with preserved ejection fraction (HFpEF) [8]. HFpEF patients also had significantly higher IGF-1 levels than their HFrEF counterparts [8]. Chronic exposure to IGF-1 has also been linked to cancer development such as in colorectal carcinoma [9]. Additionally, in the post percutaneous coronary intervention setting, IGF-1 treatment was not beneficial for left ventricular function [10]. As such, IGF-1 therapy may not be applicable in all cardiovascular settings and may need to be tailored to specific patient groups.
Pathways known to be activated by IGF-1 include inhibiting cell apoptosis via the PI3K/Akt pathway [6], [11], and promotion of cell growth via the ERK1/2 pathway [11]. In the ischemia–reperfusion setting, IGF-1 activates the PI3K/Akt pathway reducing cardiac dysfunction and fibrosis [12].
Given the mixed success of clinical trials [10], association of mortality and HF with elevated IGF-1 levels [7], and possible non-cardiac deleterious impacts [9], future IGF-1 therapy must be carefully considered. In this context, our current study sought to further explore the mechanisms underlying the effects of IGF-1 on cardiac cellular remodelling.
2. Methods and materials
2.1. Materials
IGF binding protein 6 (IGFBP6) and mutant IGF binding protein 6 (mIGFBP6) were cloned, expressed and IGF binding activity verified as previously described [13], [14], [15]. Recombinant human IGF-1 was purchased from PeproTech (New Jersey, United States). Wortmannin, a PI3K inhibitor, and U0126, an ERK1/2 inhibitor, were purchased from Sigma-Aldrich. The Rho Associated Protein Kinase (ROCK) inhibitor GSK576371 (GSK) and Apoptosis Signal-regulating Kinase-1 (ASK-1) inhibitor G2261818A (G226) were gifts provided by GlaxoSmithKline. The p38MAPK inhibitor RWJ67657 (RWJ) was a gift from Johnson & Johnson.
2.2. Cardiac myocyte and fibroblast culture
Animal use was approved by AMREP Animal Ethics Committee (E/1653/2016/M). Neonatal rat cardiac myocytes (NCMs) and fibroblasts (NCFs) were isolated from 1 to 2-day old Sprague-Dawley rat pups using enzymatic digestion and maintained as previously reported [16]. After digestion with collagenase, NCMs and NCFs were separated by Percoll gradient. NCMs were maintained in serum-free Dulbecco’s Modified Eagle Medium DMEM supplemented with insulin, apo-transferrin, and potassium chloride. Bromodeoxyuridine was used for the initial three days to inhibit proliferation of fibroblasts in case of contamination. NCFs were cultured in high-glucose DMEM containing 1% antibiotic/antimycotic and 10% fetal bovine serum (Sigma) and used at passage 2.
2.3. Cardiac myocyte hypertrophy assay
NCM hypertrophy was assessed by 3H-leucine incorporation as previously detailed [16]. NCMs were pre-treated with or without the selective inhibitors for 2 h prior to the addition of IGF-1 and 1 μCi per well of 3H-leucine for a further incubation of 60 h. Cells were harvested with 10% trichloroacetic acid precipitation and the level of 3H-leucine incorporation quantified using a beta counter.
2.4. Cardiac fibroblasts collagen synthesis assay
Collagen synthesis of NCFs was measured by a 3H-proline incorporation assay [16]. After 48 h of serum starvation, NCFs were pre-treated for 2 h with or without selective inhibitors prior to stimulation with IGF-1 and the addition of 3H-proline (1 μCi per well). Cells were incubated for 48 h before harvesting, and the levels of 3H-proline incorporation quantified as described previously [16].
2.5. Statistical analysis
The data were analysed using non-parametric ANOVA (Kruskal-Wallis test) for comparison among multiple groups. Due to the low number of replicates, data were assumed to not follow a Gaussian distribution and the analysis was performed with a non-parametric t-test (Mann-Whitney test) to compare the difference between two groups. All data were presented as median and IQR. GraphPad Prism Version 9.1.2 (GraphPad Software Inc., USA) was used to perform all of the statistical analyses.
3. Results
3.1. IGF-1 stimulates NCM hypertrophy and NCF collagen synthesis
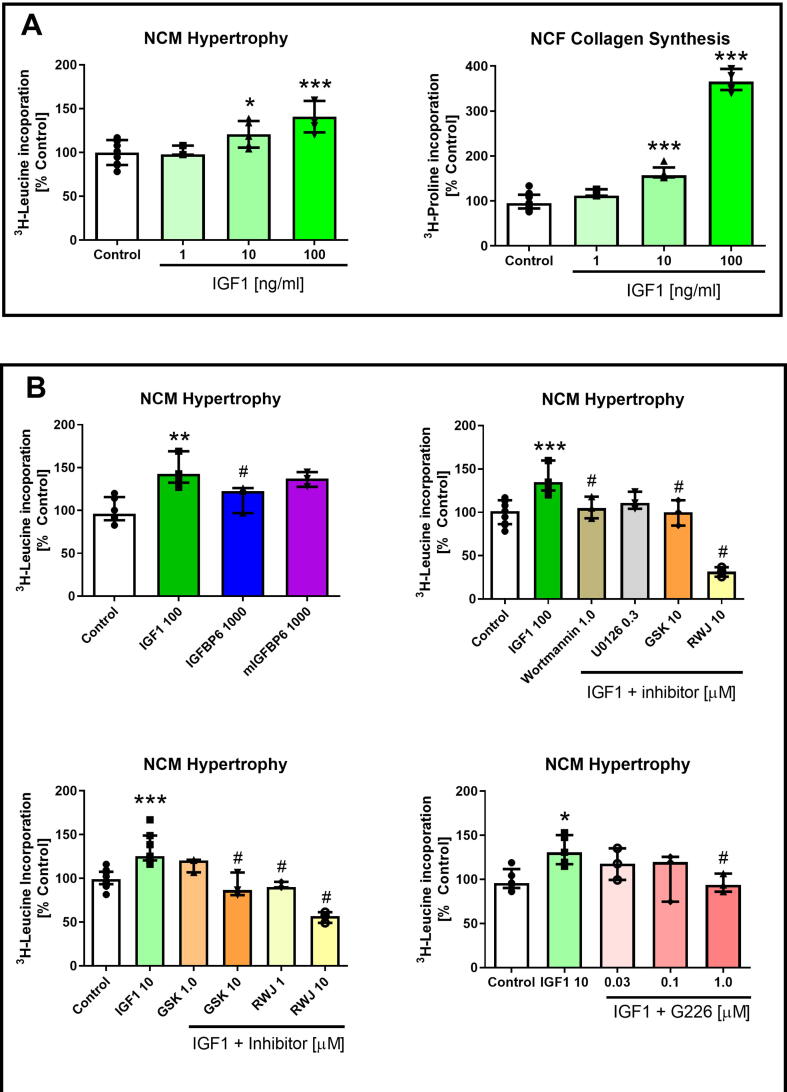
As illustrated in Fig. 1A, IGF-1 dose-dependently stimulated NCM hypertrophy and NCF collagen synthesis. In terms of NCM hypertrophy, IGF-1 concentrations of 10 ng/mL (p < 0.05) and 100 ng/mL (p < 0.001) were significantly greater than compared to the control. Likewise, NCF collagen synthesis was significantly elevated at 10 ng/mL and 100 ng/mL IGF-1 (both p < 0.0001 vs control).
Fig. 1.
A. IGF-1 dose-dependently stimulated NCM hypertrophy and NCF collagen synthesis. B. IGFBP6 (but not a non-IGF binding mIGFBP6), Wortmannin, U0126, GSK, G226 and RWJ treatment inhibited IGF-1-stimulated NCM hypertrophy. *p < 0.05 **p < 0.01, and ***p < 0.001 vs control, #p < 0.05 vs IGF1. All results are presented as median and IQR. All treatment groups were performed in triplicate and repeated three times (n = 3). Control and IGF-1 groups were performed in triplicates and also repeated 4 to 5 times (n = 4 or 5). GraphPad Prism Version 9.1.2 (GraphPad Software Inc., USA) was used to perform all of the statistical analyses.
3.2. Inhibition of several unique pathways attenuates IGF-1-stimulated NCM hypertrophy
As shown in Fig. 1B, IGFBP6 (1000 ng/ml) significantly reduced NCM hypertrophy stimulated by IGF-1 (p < 0.05 vs IGF-1) whilst mIGFBP6 (1000 ng/ml) had no effect. In addition, Wortmannin, U0126, GSK, G226 and RWJ significantly inhibited IGF-1 stimulated NCM hypertrophy (p < 0.01, 0.05, 0.01 and 0.001 vs IGF-1, respectively) in a dose dependent manner.
3.3. Inhibition of several unique pathways reduces IGF-1 induced NCF collagen synthesis
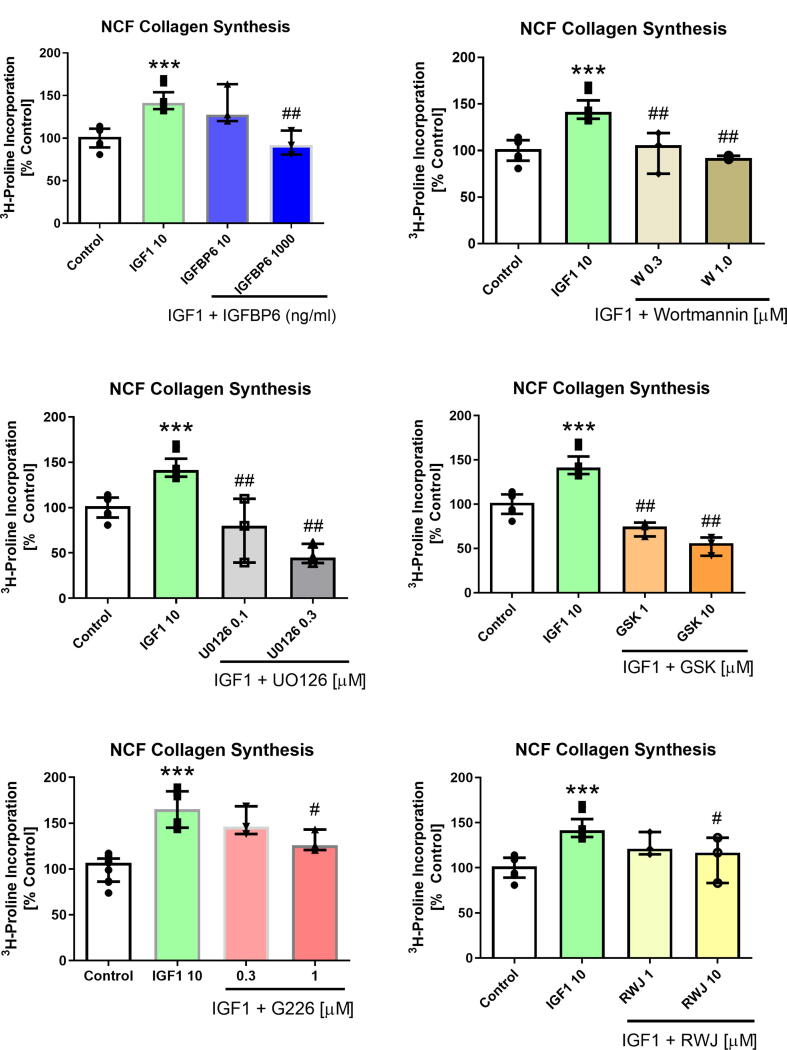
As depicted in Fig. 2, IGF-1 induced NCF collagen synthesis was significantly attenuated by IGFBP6. The inhibitors, Wortmannin, U0126, G226, RWJ and GSK also attenuated IGF-1-stimulated NCF collagen synthesis in a dose dependent manner.
Fig. 2.
IGFBP-6, Wortmannin, U0126, GSK, G226 and RWJ dose-dependently inhibited IGF-1-stimulated NCF collagen synthesis. ***p < 0.001 vs control, #p < 0.05 and ##p < 0.01 vs IGF1 (10 ng/ml). All results are presented as median and IQR. All treatment groups were performed in triplicate and repeated three times (n = 3). Control and IGF-1 groups were performed with triplicates and also repeated 4 to 5 times (n = 4, or 5). GraphPad Prism Version 9.1.2 (GraphPad Software Inc., USA) was used to perform all of the statistical analyses.
4. Discussion
4.1. IGF-1 induces hypertrophy and collagen synthesis via the PI3K/Akt and ERK1/2 pathways
Our study demonstrates that IGF-1 stimulates NCM hypertrophy and NCF collagen synthesis and that IGFBP6 reduces this hypertrophy and collagen synthesis, suggesting that IGF-1 is the direct cause of these deleterious effects. As such, developing an IGFBP-like compound may prevent IGF-1 induced hypertrophy and fibrosis. We have additionally shown that these IGF-1 driven effects are attenuated by inhibition of the PI3K/Akt and ERK1/2 pathways. Although an increase in hypertrophy via these pathways has been described in the literature [11], [12], the increase in fibrosis is an additional finding which may warrant further research into its underlying mechanisms and implications.
4.2. IGF-1 induced NCM hypertrophy and NCF collagen synthesis via the Rho-associated protein kinase, Apoptosis Signal-regulating Kinase-1 and p38MAPK pathways
Our study is the first to illustrate that the ROCK pathway is also activated by IGF-1. The ROCK pathway increases inflammation, reactive oxygen species production, and the development of cardiovascular disease [17]. Its activation leads to cardiac hypertrophy [17] and inhibition reduces fibrosis and dysfunctional remodelling [18]. Using the ASK-1 inhibitor, G226, our study demonstrates that IGF-1 additionally activates the ASK-1 pathway. ASK-1 in the cardiac setting leads to increased cardiomyocyte size and cardiac hypertrophy [19]. p38MAPK is a key convergent point of inflammation and our results highlight that IGF-1 also activates this pathway in cardiac myocytes and fibroblasts. Inhibition through the p38MAPK inhibitor, RWJ, led to a significant reduction in hypertrophy and fibrosis. These results are consistent with previously conducted studies which have shown that activating the p38MAPK pathway results in hypertrophy [11] and fibrosis [20].
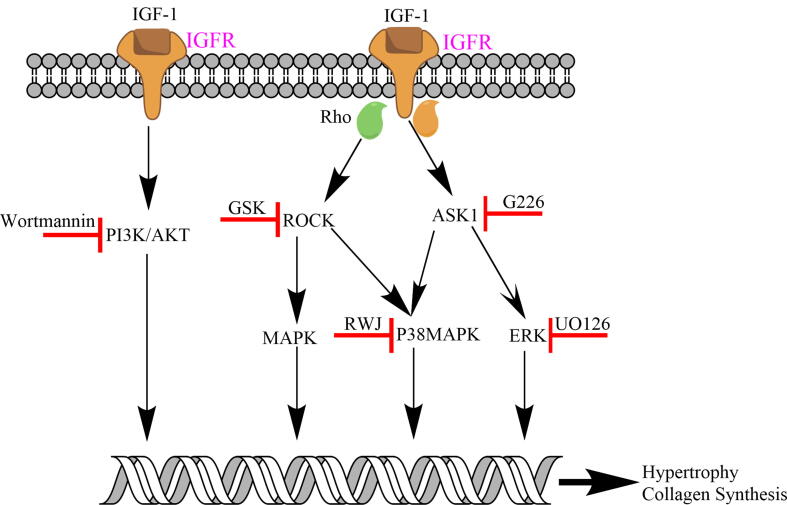
Our study utilising the ROCK inhibitor GSK, ASK-1 inhibitor G226 and p38MAPK inhibitor RWJ, demonstrated a significant reduction in IGF-1 induced NCM hypertrophy and NCF collagen synthesis. These pathways depicted in Fig. 3 at least in part contribute to IGF-1 induced NCM hypertrophy and NCF collagen synthesis leading to fibrosis.
Fig. 3.
Multiple pathways activated by IGF-1 in the setting of cardiac cells. PI3K/Akt, ROCK, ASK1, p38MAPK and ERK inhibition results in reduced hypertrophy and collagen synthesis.
The novel identification of IGF-1 as an activator of the ROCK, ASK-1 and p38MAPK pathways may assist in understanding the potential limitations including adverse effects of IGF-1 therapy in addition to correlations between elevated IGF-1 levels with mortality and heart failure. IGF-1 activation of these pathways may offset the cardioprotective effects of the PI3K/Akt and ERK1/2 pathways. As such, future therapies may need to avoid these deleterious effects by specifically inhibiting these pathways or through biased agonism preferentially activating cardioprotective pathways. Potential links between ROCK, ASK-1 and p38MAPK pathways suggest the possibility that IGF-1 therapies may not require the direct inhibition of all of these pathways but only that of p38MAPK, although future research into this hypothesis is required.
4.3. Study limitations
A limitation of our study is the use of a single inhibitor for each pathway analysed. A single inhibitor may have non-specific off-target effects which could be mitigated using multiple inhibitors individually. This remains an avenue for future research. Additionally, as cell-based assays, the direct applicability of our results in animal models and in the clinical setting requires further research to demonstrate significant benefit whilst avoiding adverse effects. The results of this study are preliminary. The use of adult cardiomyocytes and an appropriate in vivo model is a possible avenue of exploration for future research.
5. Conclusion
Our study has identified three additional pathways ROCK, ASK-1 and p38MAPK not previously known to be activated by IGF-1, which result in NCM hypertrophy and NCF collagen synthesis. Moreover, there may indeed be additional pathways not yet discovered which may likewise lead to deleterious effects of IGF-1 therapy. As such, our study provides the opportunity to guide future research. This may involve preferentially inhibiting some or all of these pathways by utilising a biased agonist to reduce activation of the ROCK, ASK-1 and p38MAPK pathways and carefully considering the activation of the PI3K/Akt and ERK1/2 pathways to balance cardioprotective benefits with potentially increased collagen synthesis in order to maximise cardioprotective benefit and minimise potential hypertrophy and fibrosis.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This research was supported by the National Health and Medical Research Council of Australia (Program Grant #1092642 and Project Grant #1087355).
References
- 1.Perkel D., Naghi J., Agarwal M., Morrissey R.P., Phan A., Willix R.D., Schwarz E.R. The potential effects of IGF-1 and GH on patients with chronic heart failure. J. Cardiovasc. Pharmacol. Ther. 2012;17(1):72–78. doi: 10.1177/1074248411402078. [DOI] [PubMed] [Google Scholar]
- 2.Watanabe S., Tamura T., Ono K., Horiuchi H., Kimura T., Kita T., Furukawa Y. Insulin-like growth factor axis (insulin-like growth factor-I/insulin-like growth factor-binding protein-3) as a prognostic predictor of heart failure: association with adiponectin. Eur. J. Heart Fail. 2010;12(11):1214–1222. doi: 10.1093/eurjhf/hfq166. [DOI] [PubMed] [Google Scholar]
- 3.Donath M., Sütsch G., Yan X. Acute cardiovascular effects of insulin-like growth factor I in patients with chronic heart failure. J. Clin. Endocrinol. Metabol. 1998;83:3177–3183. doi: 10.1210/jcem.83.9.5122. [DOI] [PubMed] [Google Scholar]
- 4.Cittadini A., Monti M.G., Petrillo V., Esposito G., Imparato G., Luciani A., Urciuolo F., Bobbio E., Natale C.F., Saccà L., Netti P.A. Complementary therapeutic effects of dual delivery of insulin-like growth factor-1 and vascular endothelial growth factor by gelatin microspheres in experimental heart failure. Eur. J. Heart Fail. 2011;13(12):1264–1274. doi: 10.1093/eurjhf/hfr143. [DOI] [PubMed] [Google Scholar]
- 5.Heinen A., Nederlof R., Panjwani P., Spychala A., Tschaidse T., Reffelt H., Boy J., Raupach A., Gödecke S., Petzsch P., Köhrer K., Grandoch M., Petz A., Fischer J.W., Alter C., Vasilevska J., Lang P., Gödecke A. IGF1 treatment improves cardiac remodeling after infarction by targeting myeloid cells. Mol. Ther. 2019;27(1):46–58. doi: 10.1016/j.ymthe.2018.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liao Y., Li H., Pi Y., Li Z., Jin S. Cardioprotective effect of IGF-1 against myocardial ischemia/reperfusion injury through activation of PI3K/Akt pathway in rats in vivo. J. Int. Med. Res. 2019;47(8):3886–3897. doi: 10.1177/0300060519857839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andreassen M., Raymond I., Kistorp C., Hildebrandt P., Faber J., Kristensen L.O. IGF1 as predictor of all cause mortality and cardiovascular disease in an elderly population. Eur. J. Endocrinol. 2009;160(1):25–31. doi: 10.1530/EJE-08-0452. [DOI] [PubMed] [Google Scholar]
- 8.Faxén U.L., Hage C., Benson L., Zabarovskaja S., Andreasson A., Donal E., Daubert J.-C., Linde C., Brismar K., Lund L.H. HFpEF and HFrEF Display Different Phenotypes as Assessed by IGF-1 and IGFBP-1. J. Card. Fail. 2017;23(4):293–303. doi: 10.1016/j.cardfail.2016.06.008. [DOI] [PubMed] [Google Scholar]
- 9.Wu Y., Yakar S., Zhao L., Hennighausen L., LeRoith D. Circulating Insulin-like Growth Factor-I Levels Regulate Colon Cancer Growth and Metastasis. Am. Associat. Cancer Res. 2002;62(4):1030–1035. [PubMed] [Google Scholar]
- 10.Caplice N.M., DeVoe M.C., Choi J., Dahly D., Murphy T., Spitzer E., Van Geuns R., Maher M.M., Tuite D., Kerins D.M., Ali M.T., Kalyar I., Fahy E.F., Khider W., Kelly P., Kearney P.P., Curtin R.J., O’Shea C., Vaughan C.J., Eustace J.A., McFadden E.P. Randomized placebo controlled trial evaluating the safety and efficacy of single low-dose intracoronary insulin-like growth factor following percutaneous coronary intervention in acute myocardial infarction (RESUS-AMI) Am. Heart J. 2018;200:110–117. doi: 10.1016/j.ahj.2018.03.018. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y. Mitogen-activated protein kinases in heart development and diseases. Circulation. 2007;116(12):1413–1423. doi: 10.1161/CIRCULATIONAHA.106.679589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khan R.S., Martinez M.D., Sy J.C., Pendergrass K.D., Che P.-L., Brown M.E., Cabigas E.B., Dasari M., Murthy N., Davis M.E. Targeting extracellular DNA to deliver IGF-1 to the injured heart. Sci. Rep. 2015;4(1) doi: 10.1038/srep04257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fu P., Thompson J.A., Bach L.A. Promotion of cancer cell migration: an insulin-like growth factor (IGF)-independent action of IGF-binding protein-6. J Biol. Chem. 2007;282(31):22298–22306. doi: 10.1074/jbc.M703066200. [DOI] [PubMed] [Google Scholar]
- 14.S.J. Headey, D.W. Keizer, S. Yao, et al. C-terminal domain of insulin-like growth factor (IGF) binding protein-6: structure and interaction with IGF-II, Mol. Endocrinol., 2004;18(11):2740–2750. [DOI] [PubMed]
- 15.Headey S.J., Leeding K.S., Norton R.S., Bach L.A. Contributions of the N- and C-terminal domains of IGF binding protein-6 to IGF binding. J. Mol. Endocrinol. 2004;33(2):377–386. doi: 10.1677/jme.1.01547. [DOI] [PubMed] [Google Scholar]
- 16.Lekawanvijit S., Adrahtas A., Kelly D.J., Kompa A.R., Wang B.H., Krum H. Does indoxyl sulfate, a uraemic toxin, have direct effects on cardiac fibroblasts and myocytes? Eur. Heart J. 2010;31(14):1771–1779. doi: 10.1093/eurheartj/ehp574. [DOI] [PubMed] [Google Scholar]
- 17.Shimokawa H., Sunamura S., Satoh K. RhoA/Rho-Kinase in the Cardiovascular System. Circ. Res. 2016;118(2):352–366. doi: 10.1161/CIRCRESAHA.115.306532. [DOI] [PubMed] [Google Scholar]
- 18.Yu B., Sladojevic N., Blair J.E., Liao J.K. Targeting Rho-associated coiled-coil forming protein kinase (ROCK) in cardiovascular fibrosis and stiffening. Expert. Opin. Ther. Targets. 2020;24(1):47–62. doi: 10.1080/14728222.2020.1712593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hirotani S.O.K., Nishida K., Higuchi Y.M.T., Nakayama H., Yamaguchi O., Mano T.M.Y., Ueno H., Tada M.H.M. Involvement of Nuclear Factor-B and Apoptosis Signal-Regulating Kinase 1 in G-Protein–Coupled Receptor Agonist-Induced Cardiomyocyte Hypertrophy. Circulation. 2002;105:509–515. doi: 10.1161/hc0402.102863. [DOI] [PubMed] [Google Scholar]
- 20.Liao P., Georgakopoulos D., Kovacs A., Zheng M., Lerner D., Pu H., Saffitz J., Chien K., Xiao R.-P., Kass D.A., Wang Y. The in vivo role of p38 MAP kinases in cardiac remodeling and restrictive cardiomyopathy. PNAS. 2001;98(21):12283–12288. doi: 10.1073/pnas.211086598. [DOI] [PMC free article] [PubMed] [Google Scholar]