Abstract
For many years, conventional plastics are manufactured and used for packaging applications in different sectors. As the food industries are increasing, the demand for packaging material is also increasing. Plastics have transformed the food industry to higher levels; however, conventional petroleum-based plastics are non-degradable which has created severe ecological problems to the environment like a threat to aquatic life and degrading air quality. Biodegradable polymers or biopolymers emerged as an alternative approach for many industrial applications to control the risk caused by non-biodegradable plastic. According to the type of starting material, they have been categorized as polymers extracted from biomass, synthesized from monomers, and produced from microorganisms. The quality of biopolymers depends on the physical, mechanical, thermal, and barrier properties. The present review highlights the characteristics of various biopolymers and their blends, comparison of properties between non-biodegradable and biopolymers, the market potential for food packaging applications. The review also emphasizes different commercial forms like films, trays, bags, coatings, and foamed products for application as modified atmosphere packaging, active packaging, and edible packaging. Different issues affecting market growth like harmful products formed during production and consumer perception have also been discussed. Information on biopolymers is widely scattered over many sources, this article aims to provide an overview of biodegradable polymer packages for food applications.
Keywords: Biodegradable polymers, Packaging application, Properties, Packaging forms, Degradation
Graphical abstract
Highlights
-
•
Biopolymers emerged as an alternative approach for many industrial applications to control the risk by non-biodegradable plastic.
-
•
The present review highlights the characteristics of various biopolymers their blends, and the market potential for food packaging applications.
-
•
Different commercial forms like films, trays, bags, coatings and foamed products for food application are also discussed.
-
•
Issues affecting the market growth like harmful products formed during production and consumer perception have also been discussed.
-
•
This article aims to provide an overview on biodegradable polymer packages for food applications.
1. Introduction
In today's life, polymers form an integral part of day-to-day life due to their extensive desirable properties and ease in production. The worldwide production of plastics (Thermoplastics, Thermosets, Elastomers, Adhesives, Coatings and Sealants, and PP-Fibers) was approximately 348 million tonnes in 2017 and it reached 359 million tonnes in 2018. The major contributors include Asia (51%), China (30%), Europe (17%), Middle East & Africa (7%) and others (Europe & EPRO 2019). India is one of the leading nations for the production and use of plastic. In 2018–2019, Polyethylene (PE) was the most used plastic in India, utilized in the form of films and sheets with over 15 million tonnes of overall plastic production and is expected to increase 24 million tonnes by 2020 (Aryan et al., 2019).
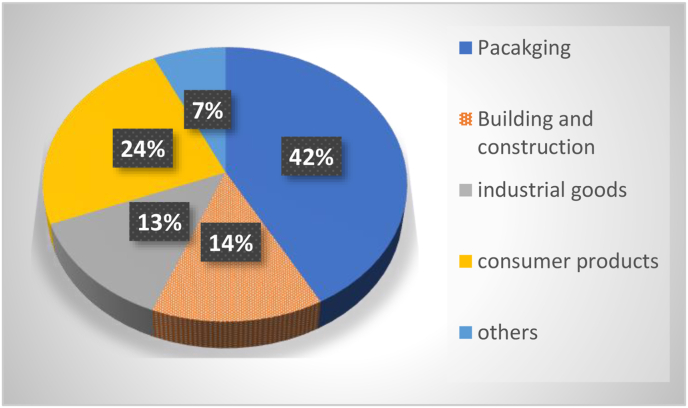
Approximately 95–99% of plastic material is manufactured from non-renewable sources (synthetic plastics) by petrochemical industries (Mangaraj et al., 2019). Synthetic plastic products have widely used in fields of medical appliances, packaging, building materials, and packaging, etc. 43% of the synthetic polymers produced annually in India is utilized by the packaging sector (FICCI, 2014). Fig. 1 represents different sectors of plastic utilization in India (Banerjee et al., 2014).
Fig. 1.
Plastics as packaging material in India (Banerjee et al., 2014).
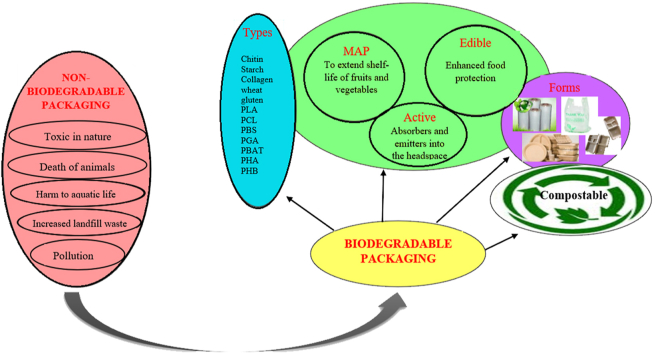
However synthetic plastic cannot undergo physical, chemical, and biological degradation and finally leads to an increase in waste (Vert et al., 2002). Waste creates numerous severe environmental and health-related problems. They accumulate on the streets and roads, choking drain that results in overflowing (Foolmaun and Ramjeeawon, 2012). A large amount of plastic waste is dumped into the ocean and rivers which harms aquatic life. Incineration leads to the release of harmful gases (carbon dioxide, carbon monoxide, chlorine, 1,3-butadiene, furans, amines, dioxin, etc.) that degrades the air quality and increases the threat of global warming and possess several health concerns (Smith, 2005). The increase in the difficulties for disposing of waste and the harmful effects on the environment and public health caused by the non-degradability of many synthetic polymers have increased concerns all over the world to find an alternative material that is environment friendly. (Luckachan and Pillai, 2011). Biodegradable polymers emerged as an alternative approach for many industrial applications to control the risk caused by non-biodegradable plastic.
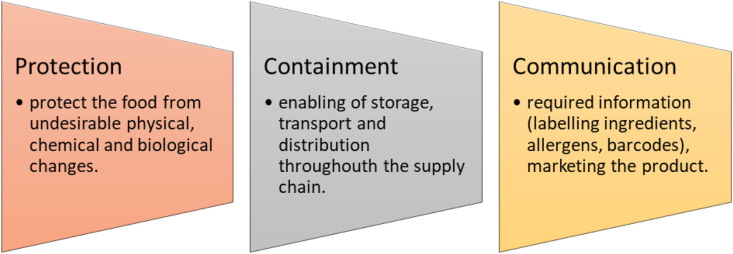
According to the American Society for Testing and Materials (ASTM), biodegradable plastic is ‘‘a plastic that degrades because of the action of naturally occurring microorganisms such as bacteria, fungi, and algae” (ASTM, 2004). They are produced from renewable sources and have similar properties (tensile strength, thermal properties, elongation at break, water vapor transmission rate and oxygen transmission rate) to conventional plastics like PET (polyethylene terephthalate), PP (polypropylene), PE (polyethylene), etc. (Kirwan and Strawbridge, 2003). Water, carbon dioxide, inorganic compounds, or biomass are the major products formed by the decomposition of biodegradable plastics. There is no accumulation of waste which is beneficial for the environment (Song et al., 2009). The main application of biodegradable plastics is in food packaging and agricultural sectors. In the food industry packaging performs different functions which are illustrated in Fig. 2.
Fig. 2.
Functioning of packaging.
The packaging is an integral part of the production, storage, distribution, preservation, and other unit operations (Ivankovic et al., 2017). In recent years, bioplastics are used as an alternative approach instead of conventional plastics for many applications. Bioplastic is a plastic of bio-based origin or biodegradable characteristic of a plastic. According to European standard EN 1675 bio-based is defined as “derived from biomass” (Van den Oever et al., 2017). Production of bioplastics requires 65% less energy than conventional plastics and also contributes to less production of greenhouse gases (Ahvenainen, 2003; Halley, 2002).
This paper aims to provide critical information on biopolymers as their role in packaging material which is a key innovation that can help in reducing the environmental impact of plastic pollution.
1.1. Production statistics
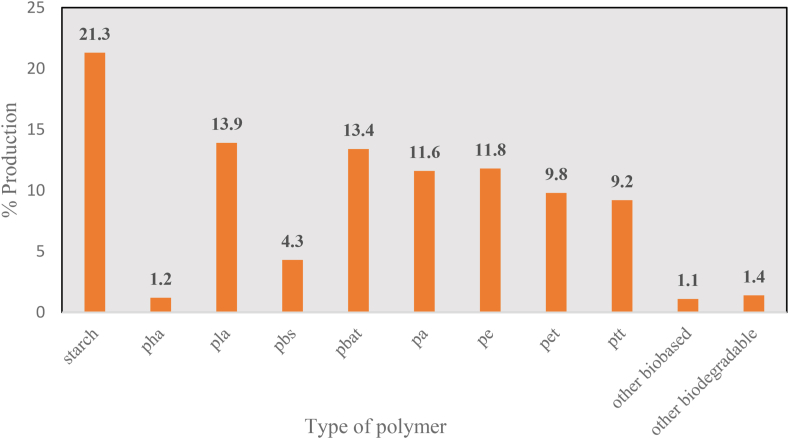
In 2019 the market for biodegradable plastic packaging was valued at USD 4.65 billion and by the end of 2025, it is expected to grow at a CAGR of 17.04% reaching a market value of up to 12.06 million. This increase is due to increasing environmental concerns and various government initiatives to reduce plastic waste. The global production of bioplastic in 2019 was 2.11 million tonnes and it is expected that by the end of 2024 the production will increase up to 2.43 million tonnes. Non-biodegradable, bio-based plastics which consist of PE (polyethylene), PET (polyethylene terephthalate), and PA (polyamides), altogether make up for over 44% of the global bioplastics production. Biodegradable plastics include PLA, PHA, starch blends, PBS, PBAT, and others constitute over 55.5% of the global bioplastics production (Market, 2020). In bioplastics production, the major contributions are seen by Asia (45%), Europe (25%), North America (18%), and South America (12%).
The production statistics of different biodegradable and biobased, non-biodegradable plastics have been represented in Fig. 3.
Fig. 3.
Production statistics.
Bioplastics are used in different sectors like packaging, consumer electronics, automotive, building/construction, agriculture/horticulture, coatings, rigid packaging, flexible packaging, and various other sectors. The packaging is the largest field of application more than 53% (1.14 million tonnes) of the total bioplastics produced in 2019. Biodegradable food packaging was the first successfully commercialized bioplastic product that is certified as industrially compostable. Since then, there is a tremendous increase in demand for bioplastics as food packaging. Flexible packaging mainly uses biodegradable polymers and rigid packaging mainly contributes to non-biodegradable packaging. Biodegradable polymers are also used for modified atmospheric storage for different fruits and vegetables (Mangaraj et al., 2018).
2. Classification
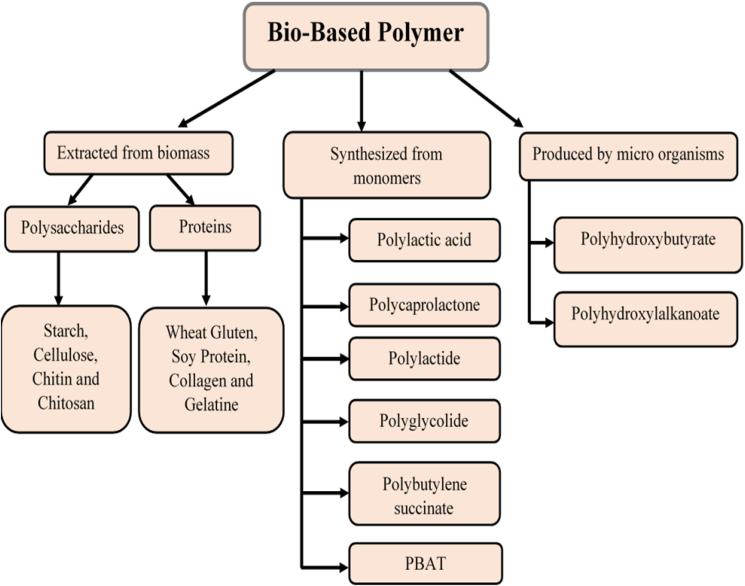
Bio-based polymers or bio-polymer based packaging materials can be classified into three main groups depending on their origin and method of production (Fig. 4).
Fig. 4.
Classification of polymers (Khalil et al., 2018).
2.1. Polymers extracted/isolated directly from biomass or natural material
2.1.1. Chitin and chitosan
Chitin is a linear copolymer with a β-1,4 linkage between N-acetylglucosamine and N-glucosamine. The monomers are randomly arranged through the polymer depending on the processing method. It is abundantly available and considered as amino cellulose. chitin is mainly present in shells of insects, crabs, shrimps, etc (Tokura and Tomura, 2007). Another source for chitin is from fungi cultivation where protein content ranges from 10 to 15%. The solubility of chitin is very low, so it is usually blended for packaging application. Chitinase degrades chitin (Teng et al., 2001).
Partial N-deacetylation of chitin forms chitosan that is insoluble in water and soluble in very few acidic solutions and has a compact crystalline structure and strong hydrogen bonding (Park et al., 2001). Chitosan is degraded by chitosanase or lysozymes. Their insolubility in most of the solvents limits the applications of chitin and chitosan. N-carboxymethyl chitosan or N-carboxyethyl chitosan is the modified chitosan prepared for application in different industries.
Both are applied to produce various biodegradable films for packaging, and the largest they use as an edible coating to prolong the shelf-life of fresh fruits and vegetables (Zhao and Mc Daniel, 2005). Chitin and chitosan have good antimicrobial properties to a variety of fungi, yeasts, and bacteria found in food.
2.1.2. Starch and cellulose
Starch is composed of amylopectin (poly-α-1,4-D-glucopyranoside and α-1,6-D-glucopyranoside) and amylose (poly-α-1,4-D-glucopyranoside). It is abundantly available and extracted from wheat, rice, potatoes, and corn. As the source changes, the content of amylose and amylopectin changes. The elongation and strength increase as the amylose content increases (Ratnayake et al., 2001). Starch can either be mixed with various resins as a filler to form blends because at a temp of 150–250 °C the linkage breaks and granules disintegrate (Angellier et al., 2006). Starch is mostly used as thermoplastic starch (TPS). TPS is highly sensitive to humidity and the thermal properties changes with the content of water. TPS or plasticized starch acts as an alternative for synthetic polymers. Recent research development in complete biodegradable “green” composites called bio-composites in which biodegradable polymers are blended with natural fibers that are also biodegradable. Biodegradation of TPS is done through hydrolysis of the acetal linkage. Amylases break α-1,4 linkage and glucosidases cleave α-1,6 linkage (Netravali and Chabba, 2003).
In food packaging applications corn-starch is used as TPS. Examples of some of the commercially available starch and their blends include Ecofram™ from National starch, solanyl™ from Rodenburg biopolymers, Biocool™ from Novamont, Bioplast™ from Biotec, and Plantic™ from Plantic Technologies.
Cellulose is a linear polymer formed from repeating units of cellobiose. It is crystalline and insoluble in organic solvents. Due to its insolubility and low fluency, it is transformed in different forms for their application. This transformation is achieved by various degrees of substitution. As the substitution degree increases the mechanical properties and degradation rate decreases. cellulose acetate (CA) is one of the important derivatives of cellulose with tensile strength like polypropylene. High glass transition temperature (Tg) limits the application of CA in thermal processing. Commercially available CA films include Bioceta™ developed by the company Mazzucchelli and EnviroPlastic Z™ developed by the company Planet Polymer. Biodegradation of cellulose is done by bacteria and fungi with enzyme oxidation specifically by peroxidases secreted by fungi (Klemm et al., 2002). Some of the commercially available cellulose-based polymers include Tenite™ from Eastman, Fasal™ from IFA, and Natureflex™ from UCB.
2.1.3. Collagen and gelatine
Collagen is a connective tissue protein composed of various polypeptides, which includes hydroxyproline, proline, glycine, and lysine. The glycine content is responsible for the flexibility of collagen. (Gelse et al., 2003). They are incorporated into cellulose and PVA films. Cellulose blended film is brittle and weak. A higher molecular weight polypeptide formed by chemical degradation of collagen is gelatine. It has excellent film forming abilities and consist of 19 amino acids. Molecular weight distribution, amino acid composition and type of plasticizer used greatly influence the barrier and mechanical property of the film (Gomez et al., 2009). Limited thermal stability during processing is an important factor which limit its applications. To improve or modify the mechanical and barrier properties of the film various additives are added to achieve excellent films for food packaging. 4% Gelatine film with 2.5% corn oil and 5% olive oil is used for packaging sausages (Ramos et al., 2016). Degradation of gelatine is caused by the enzyme protease.
2.1.4. Wheat gluten and soy protein
Wheat gluten is of low cost and a readily available by-product of the fabrication of starch. Their degradation speed is highest as compared to other polymers with no harmful by-products. It is an excellent film-forming agent but is brittle without a plasticizer. Soy protein concentrate does not have water-soluble carbohydrates. It has a protein concentration of 70%. Textured soy protein (TSP) is made by giving some texture to soy protein concentrate. TSP films do not have a good barrier and mechanical properties due to the hydrophilic nature of the protein. Films from isolated soy protein are sensitive to moisture. The addition of 25% of stearic acid improves the thermal and tensile properties and reduces moisture sensitivity (Lodha and Nteravali, 2005). Soy protein film incorporated with glycerol, gellan gum, or K-carrageenan is for the production of biodegradable soybean-based packaging containers (trays) (Mohareb and Mittal, 2007).
2.2. Polymers produced by classical chemical synthesis from bio-monomers
2.2.1. Polylactic acid (PLA)
PLA is a type of aliphatic polyester obtained by ring opening polymerization of lactide monomer. The lactic acid monomers are usually obtained from the fermentation of renewable materials like corn, sugar, and other feedstocks, etc. It is recyclable, compostable, and degrades within a short life span having a high molecular weight and has high transparency (Singla and Mehta, 2012). By changing the monomeric ratio, the properties of PLA can be changed from crystalline to amorphous. The glass transition temperature of PLA that is commercially available includes 63–63.8 °C (Briassoulis, 2004). The initial crystallinity and the monomer content change the rate of degradation of the complete polymer where the lowest degradation is showed by the highest monomer content due to high crystalline nature (Kale et al., 2006). Different companies commercialize PLA with different commercial names, for example, LLC (Blair, NB) the Natureworks™ PLA produced by Natureworks™, Galacid™ by Galacid, Lacty™ by Shimadzu, and Eco plastic™ by Toyota. PLA is currently used for food packaging of short-shelf products LLC (Blair, NB). BASF's bioplastic is a high-quality, completely compostable polymer. It consists of the biodegradable BASF polymer ecoflex® and polylactic acid (PLA). In food packaging applications it is used for short shelf-life products and for forming pads and trays for serving food (Siracusa et al., 2008). Table 1 characterizes different blends that have been formed by PLA.
Table 1.
Different blends of biopolymers.
| Plastic | Blend | Remarks | Application | Characterization technique | Reference |
|---|---|---|---|---|---|
| PLA | PLA/TPS | Adding TPS, properties of PLA was increased which ultimately increased shelf-life of the packaging material. Trays were immersed in bee-wax to improve the permeability of the material. |
Trays production | SEM, XRD, TGA | Reis et al. (2018) |
| PLA/PHB | Addition of PHB into PLA had enhanced barrier properties due to which the storage capability increased. OLA as plasticizer and carvacrol was incorporated in the film as active agent for anti-microbial packaging. |
Packaging film | SEM, XRD | Burgos et al. (2017) | |
| PLA/PBA | Addition of PBA with 1000 g/mol as plasticizer improved PLA properties. Degree of crystallinity increased with increasing PBA content. |
SEM, DSC, TGA, dynamic mechanical analysis. | Liu et al. (2017) | ||
| PLA/starch blend | Starch was used as a nucleating agent and glycerol as a plasticizer. With increase in starch content spherulites size decreased. ratio of 100/40 of PLA and starch gelatinized with water/glycerol had greatest superiority of mechanical properties. |
Food packaging | FTIR, DSC | Park (2001) | |
| PLA/corn starch | Maleic anhydride acted as a good compatibilizer, while maleated thermoplastic starch was not very effective for PLA/starch blend systems. The degradation rate of the blended film was higher. |
Packaging film | FTIR, SEM, DSC | Jang et al. (2007) | |
| PLA/PU | PLAPU polymers were synthesized through PLA diol with hexamethylene diisocynate, with chain extension by PCL diol. PLA: PCL of the ratio 1:3 had greater elongation at break at 1053% and the barrier properties were also enhanced. |
Packaging film | MR, FTIR, DSC | Akter et al., 2014 | |
| PLA/PBSA | Triphenyl phosphide was used a compatibilizer. Properties like tensile strength, impact strength and elongation at break increased. Improved phase adhesion made the blend more tough. |
Biodegradable active film for food packaging | FESEM | Ojijo et al. (2013) | |
| PHA | PHA/Zein | Zein fibers incorporation in PHA increased the oxygen and barrier properties. The mechanical properties were not significantly affected, and transparency was also decreased as the zein content increased. |
Packaging film | SEM, TGA | Fabra et al. (2014) |
| PHA/PA | By addition of PA the stiffness and toughness of the film increased. The complex viscosity and elastic share modulus of the blend increased with increasing PHA content. |
DMA, DSC, TGA, SEM | Yang et al. (2015) | ||
| PCL | PCL/PLA | The stiffness of the blend decreased with increase in PCL. The blend exhibited good toughness balance and the impact strength of the material increased with increased elongation at break. |
Medical equipment, packaging material, and dairy fields | SEM, XRD | Urquijo et al. (2015) |
| PCL/TPS/PLA | citric acid, maleic anhydride and methylene diphenyl diisocyanate (MDI citric acid, maleic anhydride and methylene diphenyl diisocyanate (MDI citric acid, maleic anhydride, and methylene diphenyl diisocynate were used as compatibilizing agent. The thermal stability was not affected but it induced crystallinity in the blend. Melt viscosity of the blend increased. |
Biodegradable film | DSC, TGA, FTIR, SEM | Carmona et al., 2015 | |
| PCL/PBS | Due to the difference between the melt viscosities of PCL and PBS a non-uniform immiscible film was formed. Carbon nanotubes addition increased the thermal, mechanical, and electrical properties. |
Biodegradable resin | SEM, PLOM | Gumede et al. (2018) | |
| PHB | PHB/PBAT | PHB/PBAT blend with addition of chlorinated agent such as chlorine bleach increased storage time of packaging material. The film also exhibited antimicrobial activity against E. ColiO157:H7, and Staphylococcus aureus. |
food packaging and medically related material | SEM, FTIR, TGA | Lin et al. (2018) |
| PHBV/PLA | PHBV content of 20–35% in PLA was found to be most suitable because of high compatibility and increased barrier properties. | Packaging application | DSC | Jost (2018) | |
| PHB/Chitosan | Trifluoroacetic acid was a co-solvent and the content of carbon, nitrogen and hydrogen was decreased in the blend. The ratio of 50:50 was found to be most thermal stable. |
Biomedical application | CHNS Analyzer, SEM, TGA | Karbasi et al. (2016) | |
| (PHB-HV)/maize starch | With increasing starch content young's modulus, strain to break, strength and puncture force decreased. There was lack of interfacial adhesion between the polymers. |
Packaging | FTIR, XRD, DSC, optical microscopy | Reis et al., 2018 | |
| Starch. | Corn-starch/Chitosan | Films made from the blend of corn starch and chitosan had good optical and morphological properties. The blend was sensitive to pH variations. |
Film production | TIR, DSC, Thermal degradation. | Silva-Pereira et al. (2015) |
| Rice starch/chitosan | Blend showed enhanced water vapor permeability, tensile strength, colour and a decrease in elongation strength and film solubility. Molecular miscibility was seen between the two polymers. |
Biodegradable film | FTIR, XRD, gravimetric Modified Cup method | Bourtoom and Chinnan (2008) | |
| Starch/PHB | Tensile strength was maximum for the ratio of 0.7:0.3 PHB: Starch. The thermal stability was increased by 30 °C |
Packaging film | DSC, TGA | Godbole et al. (2003) | |
| HPS/PE | The carbonyl index of blend increased but mechanical strength decreased with the increase in starch content. The degradation of the film increased. |
– | SEM, XRD | Kim (2003) | |
| Rice starch/four | Glycerol/sorbitol was used as a stabilizer. The blend with ratio of 2:8 showed highest Tensile strength. Films with sorbitol were less permeable to water and film made with glycerol had high permeability. |
SEM, FTIR | Dias et al. (2010) | ||
| Starch/PVA | Citric acid was used as plasticizer and glutaraldehyde as the cross-linker which increased tensile strength and degree of swelling of the film. Results showed that film can be an exceptional material for food packaging. |
Biodegradable plastic | FTIR, SEM, TGA. | Priya et al. (2014) | |
| Chitin/chitosan | Chitin/PHB | The thermal transition temperature was same as that of neat PHB. The blend showed high biodegradability because the crystallinity of the PHB was lowered. |
Biodegradable packaging | WAXD, DMTA | Ikejima and Inoue (2000) |
| Chitosan/Cellulose | Trifluoroacetic acid was used as a co-solvent. A reduction in water vapor permeability was seen. The blend demonstrated effective antimicrobial capability against Escherichia coli and Staphylococcus aureus. |
Wound dressing application | DMTA | Wu et al. (2004) | |
| PBS | PBS/CAB | The polymers were miscible at 0–30 % wt. of PBS. Due to the plasticizing effect of PBS the young's modulus of the blend decreased. By immersing in acetone porous film was obtained. |
– | XRD, DSC, Viscoelastic Analyzer | Tatsushima et al. (2005) |
| PBS/PLA | In presence of lysine triisocyanate (LTI) the impact strength of the blended film increased Results showed that LTI could be a good processing agent which increases the compatibility of PLA/PBS blend. |
Packaging application | MFR, SEC, LSCM, Charpy impact test | Harada et al. (2007) | |
| PBS/CA | The hydrophilicity of the cellulose acetate membrane improved upto 50% by addition of PBS. The thermal stability and degradation in compost was increased. |
– | SEM, TGA, biodegradability test | Ghaffarian et al. (2013) | |
| PBS/starch | The melting temperature decreased with addition of untreated and gelatinized starch. The tensile strength increased when untreated starch was replaced with gelatinized starch. |
Packaging film | Tension-meter, softness measurement. | Park et al. (2001) |
PLA: Polylactic acid, TPS: Thermoplastic starch, PHB: Polyhydroxy-butyrate, PBA: Polybutylene acrylate, PU: Polyurethane, OLA: Oligomeric lactic acid, PCL: Polycaprolactone, PBSA: Polybutylene succinate-co-butylene adipate, PA: Polyamide, PHA: Polyhydroxyalkonate, PBS: Polybutylene succinate, PBAT: Polybutylene adipate terephthalate, PHBV: Poly-3-hydroxybutyrate-co-3-hydroxyvalerate, PEO: Polyethylene oxide, HPS: Hydroxypropyl Starch, PE: Polyethylene, PVA: Polyvinyl alcohol, HPC: Hydroxypropyl cellulose, CAB: Cellulose acetate butyrate, CA: Cellulose acetate.
2.2.2. Polycaprolactone (PCL)
PCL is a semi-crystalline, completely biodegradable, easy to process, and cheap fossil-based polymer. It is soluble in many organic and inorganic solvents and has a glass transition temperature (Tg) of −60 °C which increases its application as a compatibilizer in formulations of polyurethane (Vroman and Tighzert, 2009). The addition of PCL into hydrophilic chitosan polymer increases the overall hydrophobicity of the blend. Low Water vapor transmission rate (WVTR) values are observed in the blend when compared to pure films. Due to this property food stored in such films have a longer shelf-life (Sarasam et al., 2006). It is commercially found under the trade names of Tone® from Union Carbide, Celgreen® from Daicel, CAPA® from Solvay.
2.2.3. Polybutylene succinate (PBS)
Polybutylene succinates belong to the polyalkenedicarboxylate family and are obtained by polycondensation of glycols such as 1,4- butanediol and ethylene glycol with aliphatic dicarboxylic acids, like adipic and succinic acid. It is a white crystalline polymer, with good processibility having a Tg of −45 to −10 °C and a melting point of 90–120 °C with 330% elongation at break. PBS has mechanical properties approximately like Polyethylene (PE) and polypropylene (PP) (Wang et al., 2007). They were first invented in 1990 under the trade name Bionolle (Showa Denko) in Japan. Since then many different copolymers have been prepared like polybutylene succinate-co-adipate (PBSA) obtained from incorporating adipic acid at a specific concentration. The molecular weight of the polymer can be increased by adding a small amount of coupling agents. Different industries commercialize different PBS by changing the monomeric units for example Skygreen® by SK Chemicals in Korea. The nature of diols and diacids used for condensation influences the properties and degradation rate of these polymers (Wang et al., 2007).
2.2.4. Polylactide aliphatic copolymer (CPLA)
CPLA is formed by a mixture of lactide which is a renewable resource and dicarboxylic acid which is an aliphatic polyester. It has properties like PP and PS which depend on % of polyester present in the mixture. It is stable up to a temperature of 200 °C. During combustion the amount of CO2 is very low as compared to that generated combustion of PE and PP. Any toxic substance is not produced during the incineration of CPLA. It starts to decompose after 2 weeks when mixed with food. In a natural environment, degradation takes 5–6 months, with 12 months to decompose completely (Siracusa et al., 2008). One commercialized film produced in Japan by Dainippon Ink Chemicals under the tradename of CPLA™.
2.2.5. Polyglycolide (PGA)
Polyglycolide or polyglycolic acid prepared by glycolic acid polycondensation. It is one of the simplest aliphatic polyesters with a glass transition temperature (Tg) of 35–40 °C and melting point (Tm) of approximately 220–250 °C. It is insoluble in water due to high crystallinity of 40–55% and soluble in most fluorinated solvents which can be used to form high molecular weight polymer films. The polymer is completely reabsorbed by the organism within 5–6 months (Tiberiu, 2011). Commercialized PGA includes Kurudex™ developed by Kureha Chemical Industries a high molecular weight PGA film for food packaging application. A low molecular weight film is also formed by The Chemours Company.
2.2.6. Polybutylene adipate-co-terephthalate (PBAT)
PBAT is a linear aromatic co-polyester obtained from the condensation of 1,4-butanediol with a mixture of terephthalic acid and adipic acid. At a terephthalic acid concentration of more than 35% mol, it exhibits excellent properties. As the content increases above 55% the biodegradation rate of PBAT decreases. PBAT is flexible and soft like PCL so it is used in the production of films, filaments, bottles, and molded products. PBAT can be blended with cellulose, starch, and other biodegradable polymers. For improving the hydrophobicity, mechanical, and thermal properties PBAT is blended with cellulose. The addition of PBAT in PHBV decreases the degree of crystallinity (Javadi et al., 2010). It is commercialized under the tradename of Ecoflex™ developed by BASF, Origo-Bi™ developed by Novamont, Easter Bio™ developed by Eastman Chemical.
2.2.7. Polyvinyl-alcohol (PVA)
PVA is a semicrystalline polymer comprising mainly amorphous phases with only a small amount of crystallinity and consists of 1, 3-diol units or 1, 2-diol units, depending on the hydrolysis degree of poly (vinyl-acetate). The properties of PVA generally depend on its molecular weight and degree of hydrolysis with the molecular weight of PVA generally ranging between 20,000–400,000 and based on the length of vinyl acetate used to produce PVA – the degree of hydrolysis is typically in the range of 80–99% (Abdullah et al., 2017).
2.2.8. Polypropylene carbonate (PPC)
PPC is the most common aliphatic polycarbonate which is produced from CO2 and propylene carbonate by copolymerization. PPC films have advantages such as better tensile and barrier (O2 and H2O vapor) properties compared to the PBAT, LDPE, and PE/starch blend. The tear resistance of the PPC films is lower than the PBAT but remains better than LDPE and PE/starch blends. However, the amorphous PPC has several limitations including poor thermal stability, high shrinkage, insufficient mechanical properties, low glass transition temperature (25–45 °C), and variability in the performance of the polymer depending on the type of catalyst used to prepare the PPC (Muthuraj et al., 2018).
2.3. Polymers obtained from natural or genetically modified organisms
2.3.1. Polyhydroxylalkanoates (PHAs)
PHA represents natural polyesters produced by bacterial fermentation of sugar, glucose, or vegetable oil feedstock. It is one of the most recent and widely used biodegradable polymers for food packaging applications. Bacteria accumulate PHAs as a reserve material intracellularly at a concentration of 30–80% dry weight under limited N2 and abundant C (Mercan et al., 2002). Its Tm ranges from 40–180 °C depending on monomers used for synthesis. Depending on the nutrient source for carbon and the organism, PHA may be manufactured from rigid brittle to a rubber-like polymer. (Zivkovic, 2009). A good barrier property film was formed when PHA is blended with zein. An increase of 39–48% and 27–35% was seen in the water vapor permeation coefficient and oxygen permeation coefficient respectively. A change in blend morphology was also observed due to the incorporation of zein (Fabra et al., 2014). PHAs are completely biodegradable. Biodegradation occurs through the esterase activity of linkage breaking of the monomer from the chain ends. The most common PHA is the PHB (polyhydroxybutyrate), formed by the polymerization of 3-hydroxybutyrate. PHB is known for its excellent UV-resistivity and high optical properties with Tm of 180 °C and Tg of 55 °C. PHB has a crystallinity of more than 50%. It is well known that PHB is unstable after melting temperature of 180 °C and when kept at temperature even below at 10 °C below melting point it can undergo molecular weight reduction which limits its processibility (Savenkova et al., 2000). To increase the processibility condition different strategies such as copolymerization with other alkanoates, addition of biodegradable polymer or blending with second polymer. Different routes yield different structural polymers.
PHB undergoes degradation by various bacteria, fungi, and algae in different environmental conditions. The hydrolytic degradation forms 3-hydroxy butyric acid, at a low rate. The copolymer polyhydroxybutyrate-valerate (PHBV) is synthesized by adding propionic acid to the feedstock. It is tougher and less stiff, so it is used as a packaging material. It degrades within 5–6 weeks in a microbiologically active environment, ending with CO2 and H2O in aerobic conditions. The degradation is faster, with the production of methane in anaerobic conditions (Kim et al., 2000). PHB and PHBV are commercialized under different trade names: Biopol™ from Mosanto, Nodax™ from Procter & Gamble and Kaneka corporation, Eamat™ from Tianan, and Biomer-P™ from Biomer. The properties of some blends of PHA and PHB are summarized in Table 1.
3. Properties
Properties such as tensile strength, water vapor transmission rate, oxygen transmission rate, elongation at break, and melting temperature of different biodegradable and non-biodegradable polymers have been given in Table 2.
Table 2.
Properties of thermoplastic and Biodegradable polymers.
| THERMOPLASTIC POLYMERS | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Class/type | polymer | Characteristic | Tensile strength (MPa) | WVTR at (38 °C), 90% RH (g/m2/day) |
OTR (25 °C), 0% RH (cc/m2/day) |
Elongation at break (%) | Melting point temperature (°C) | Reference | ||
| Polyolefin | LDPE | Tough, translucent material having excellent chemical resistance but sensitive to hydrocarbons, oils, and greases. Used to make food bags, films etc. |
9.93 | 16–23 | 7000–8500 | 349.0 | 109 | Jordan et al. (2016); Shebani et al. (2018) | ||
| LLDPE | molecular weight distribution is much narrower than LDPE. Greater resistance to chemical and cracking. |
15–17 | 4.8–7.9 | 2795–3500 | 745 | 134 | Manikanth & vardharaju (2012) | |||
| HDPE | Due to linear nature, the impact and tear strength are lower and tensile and bursting strength are much higher compared to LDPE. | 27.93 | 4.7–7.8 | 2300–3100 | 213.1 | 115–135 | Shebani et al. (2018); Contreras et al. (2018) | |||
| Polypropylene | Cast PP | Serve as both a plastic as well as a fiber. Very good resistance to chemical and grease. Due to brittleness at below freezing its application in food packaging is limited. |
40–50 | 9.3–11.0 | 2300–3100 | 100 | 160 | Khalifa (2016); Tetsuya et al. (2005) | ||
| OPP | BOPP has very high clarity. Improved moisture barrier property. Considered to be an alternative to cellophane, aluminium foil. |
31.2–48.2 | 3.9–6.2 | 1200–2500 | 46–60 | 170 | Khalifa (2016); Tetsuya et al. (2005) | |||
| Vinyl chloride | PVC | Flexible, light, excellent organoleptic properties which do not interact with the food. PVC bottles are made for storage of juices and oils. | 46–52 | 150–200 | 8–150 | 25.9 | 75–105 | Rostam et al. (2016); Sarfraz et al. (2012) | ||
| PVDC | Used as a shrink film because of improved tensile strength, flexibility, and impact strength. | 25–100 | 2–5 | 0.1–1 | 50–100 | 160–170 | Rostam et al. (2016) | |||
| Polyesters | PET | Used as a packaging material due to high tensile strength, excellent chemical resistance, and stability over a wide temperature range. Widely used for water and carbonated drink bottling. |
61.67 | 32 | 130 | 60–85 | 240–275 | Lianhua and Qiang (2017); Mirjalili et al. (2013) | ||
| PEN | Usually mixed with PET which is used to make bottle that are more heat resistance. | 91.47 | 10–40 | 0.03–0.05 | 5.26 | 270 | Bedia et al. (2001); Zhong et al. (2010) | |||
| PC | The film of PC is used for boil-in-bag packs and retort pouches due to its stability at high temperature | 58–65 | 10–30 | 9500–10 000 | 97–110 | 250–280 | Hassan and Jwu (2005); Diawara et al., 2020; Lagaron et al. (2004) | |||
| Polystyrene | OPS | poor barrier to water vapor and good barrier with high refractive index. Used for making disposable trays and containers. |
22 | 109–155 | 4350–6200 | 11–20 | 74–110 | Meenakshi et al. (2002) | ||
| HIPS | Excellent material for thermoforming. The tubes of HIPS are used in packaging of food material. | 27 | 79–340 | 320–400 | 36.7 | 90 | Soundararajan & Palanivelu 2014 | |||
| Vinyl Acetate | EVA | As the crystallinity decreases the oil and gas permeability decreases. Used to make multi-layered film. Due to low barrier properties its application is limited. |
3.8 | 70 | 10 000 | 550 | 65–90 | Soheilmoghaddam et al., 2017; Najarzadehet al., 2014; DuPont Teijin Films (2001) | ||
| EVOH |
Offers superior barrier to gases, odour, fragrances. It's application in food packaging increases food flavour retention. |
60 |
22–124 |
0.08–1.9 |
250 |
156–195 |
Khalifa (2016); Huang et al. (2004) |
|||
| BIODEGRADABLE POLYMERS | ||||||||||
|
Polymer |
Characteristic |
Tensile strength (MPa) |
WVTR (38 °C), 90% RH (g/m2/day) |
OTR (25 °C), 0% RH (cc/m2/day) |
Elongation at break (%) |
Melting temperature (°C) |
Reference |
|||
| Starch | Used as TPS which has sensitivity to humidity. In food packaging starch is mixed with other polymers like PVC to improve the efficiency of the film. | 4.8–8.5 | 7.78–9.0 | 12.11 | 35–100 | 160 | Ivankovic et al. (2017); Ghasemlou et al. (2013) | |||
| Cellulose | CA derivation is most common derivative of cellulose used in packaging due to high strength and great barrier properties. | 13–59 | 4.59–9.0 | 390 | 4.10–10.0 | 256 | Meenakshi et al. (2002) | |||
| Chitin/chitosan | Insoluble in most solvents which limits their application in the food industry. | 38.2–77.3 | 0.535–1.3 | 11–50 | 17–76 | 290 | Ivankovic et al. (2017) | |||
| Wheat gluten | Due to low tensile strength different additives are fillers are added so that they can be used for food coating application. They are usually brittle in nature. |
2.6 | 4.45–5.19 | 5.53–6.23 | – | – | Harry & lea 2000 | |||
| Collagen/Gelatine | The thermal and mechanical properties of the film made by gelatine is not stable which limits the application in food packaging | 17.46 | 290.64 | – | 20.28 | 150–160 | Wang et al. (2017) | |||
| PLA | High transparency increases its potential in the bottling industry. Mainly used for making containers for food storage. |
44 | 27–50 | 0.303–0.40 | 30.7 | 175 | Zhouyang Duan, 2013; Farah (2016); Messin et al. (2020) | |||
| PCL | Good chemical resistance to oils, greases. Usually blended with starch or chitosan to improve its barrier properties. |
16 | 20–25 | 700–800 | 250–300 | 58–60 | Jost (2018); Rosa et al. (2004) | |||
| PGA | Mainly used as copolymer-PGLA because PGA alone is insoluble in most organic solvents and brittle in nature has limited its alone application. | 13 | 10 | 1 | 40 | 220–230 | Samantaray et al. (2020); Murcia et al. (2020) | |||
| PBS | It has great compatibility with fibers so for food application films are made using a blend with different fibers like jute, cellulose etc | 40 | 2200–2300 | 1.72–2.00 | 150 | 90–120 | Messin et al. (2020) | |||
| PHB | Stiff and brittle, known for its UV resistivity property. Its mechanical and physical property are same as that of isostatic PP |
25 | 1.16 | 183 | 5 | 180 | Ivankovic et al. (2017) | |||
| CPLA | Products form CPLA can withstand high temperature without deforming. After crystallization it turns white in colour. |
59 | – | – | 7 | 165 | Farah (2016) | |||
LDPE: Low density polyethylene, LLDPE: Linear Low-density polyethylene, HDPE: High density polyethylene, PP: Polypropylene, OPP: Oriented Polypropylene, BOPP: Biaxially Oriented Polypropylene, PVC: Polyvinyl Chloride, PVDC: Polyvinylidene Chloride, PET: Polyethylene Terephthalate, PEN: Polyethylene naphthalate, PC: Polycarbonate, OPS: Oriented Polystyrene, HIPS: High Impact Polystyrene, EVA: Ethylene-vinyl acetate, EVOH; Ethylene vinyl alcohol, TPS: Thermoplastic starch, CA: Cellulose Acetate, PLA: Polylactic acid, PCL: Polycaprolactone, PGA: Polyglycolide, PBS: Polybutylene succinate, PHB: Polyhydroxy-butyrate, CPLA: Polylactide aliphatic copolymer. PGLA: Polyglycolide-co-lactide, UV: Ultraviolet.
3.1. Tensile strength
The maximum amount of stress that a material can withstand before its failure is the tensile strength of that material. It is one of the most common mechanical methods to determine the strength of any material (Westmoreland Mechanical Testing and Research 2020). The mechanical properties are important for the protection of food packaging. The tensile strength depends upon the type of polymer, processing condition, additives, chemical modification, and blends. The tensile strength of material changes with processing and storage (Briassoulis and Giannoulis, 2018). Additions of NPs (nanoparticles) in the material like PLA for bio-nano composites formation increases the mechanical properties (Lee, 2016). On comparing the tensile strength from Table 2 a variation can be observed in the order of PEN > PET > PVDC > PC > EVOH > PVC > PP > OPP > HDPE > HIPS > OPS > LLDPE > LDPE > EVA. The maximum and minimum strength was seen with PET and EVA respectively. In case of biodegradable polymers, CPLA > PLA > PBS > chitin > PHB > collagen > PCL > PGA > cellulose > starch > wheat gluten PLA have the maximum strength while as starch is having the lowest. The range of tensile strength depends on the type of additives used while forming the film. Many biodegradable polymers have the same tensile strength as compared to thermoplastic like CPLA, PLA, PHB having approximate values of PET, PVC, OPS respectively which suggests that these biodegradable polymers can be used as a great alternative.
3.2. Water vapor transmission rate
The amount of water vapor that passes per unit area and time of packaging material is called the water vapor transmission rate (WVTR) [kg mm−2 s−1] (Auras et al., 2006). Food products are susceptible to moisture as the moisture increases the shelf-life of the food product decreases. In some cases, the WVTR is an important factor while selecting a packaging material because some of the food products they need a certain range of moisture level like dairy products, meat, seafood, these require moisture inside their package (Flair Flexible Packaging Corporation 2020). WVTR is most measured at (38 °C), 90% RH. The WVTR in thermoplastic follows the order of PVC > OPS > HIPS > EVA > EVOH > PET > LDPE > PC > PEN > PP > HDPE > LLDPE > OPP > PVDC and in case of biodegradable polymers, the order followed is like; PBS > Collagen > PLA > PCL > PGA > starch > cellulose > Wheat gluten > PHB > chitin as given in Table 2. Biodegradable plastics have less water permeability than thermoplastic polymers so they can be used for the storage of dry products. PBS water vapor retention is very poor, so its alone application is minimum. PBS when blended with PLA in the ratio of 20:80 forms a blend with good water vapor retention (Bhatia et al., 2012).
3.3. Oxygen transmission rate
The oxygen permeability coefficients (OPC) show the amount of oxygen that can pass through the material per unit area and time under pressure [kg mm−2 s−1 Pa−1]. As a result of low OPC the oxidation process is inhibited which increases shelf-life of the product (Oliveira et al., 2004). The OTR is usually measured at 25 °C, 0% RH and OTR of different polymers have been given in Table 2. The OTR in thermoplastic polymers follows the order of PC > EVA > LDPE > OPS > LLDPE > PP > HDPE > OPP > HIPS > PET > PVC > PVDC > EVOH > PEN and in case of biodegradable polymers PCL > cellulose > PHB > chitin > starch > wheat gluten > PBS > PGA > PLA. The OTR in biodegradable polymers is very less i.e., they allow only a certain amount of oxygen to permeate. In some cases, blends are made by mixing biodegradable polymers to enhance the barrier properties. 20:80 chitosan:starch films showed a reduction in OTR as compared to native chitosan films the hydrophobicity of the film was also increased due to incorporation of chitosan (Akter et al., 2014).
3.4. Elongation at break
Elongation at break can be defined as the ratio of changed length to initial length. It measures to what extent the material can stretch or elongate without breaking. These values are an indication of how ductile a polymer is so that different shapes can be formed. (Plastic Materials 2020). It is measured in a percentage and the greater the value stronger is the polymer. Most of the thermoplastic has a high elongation percentage such as LLDPE, LDPE, EVA, and HIPS. Some other polymers have relatively low value include OPS, PEN, and PVC. Biodegradable polymers usually have low % elongation except PCL having 250–300% can be compared to HDPE because they have approximately the same values. In starch chitosan, composite films changing the ratio of chitosan to starch increases the elongation at break (Sun et al., 2019).
3.5. Melting point
The temperature at which material starts changing its structure or the point where a phase change is observed is called melting temperature (Tm). It is a thermodynamic property of the polymer to know the maximum temperature it can hold before deforming. Mostly melting temperatures are seen in different ranges. PC and PET having the highest Tm of 240–280 °C and 245–270 °C and are often recommended for the formation of bottles (Raj, 2005). In the case of biodegradable polymers, chitin has a Tm of 290–300 °C which is equal to PET or PC. But chitin alone cannot be molded in different shapes, so it is usually blended with other materials.
The Tm in biodegradable polymers follows the order of chitin > cellulose > PGA > PHB > PLA > CPLA > starch > Collagen > PBS > PCL and in case of thermoplastic polymers PC > PET > PEN > EVOH > OPP > PP > PVDC > LLDPE > HDPE > LDPE > HIPS > PVC > OPS > EVA. Properties of different thermoplastics and biodegradable plastics have been listed in Table 2.
3.6. Thermal stability
Thermal properties are relevant to the potential use of polymeric materials in many consumer-oriented applications. A detailed understanding of the thermal degradation of polymers is important in the design of materials with improved properties (Begum et al., 2020). Thermal stability of polymer is defined as the ability of the polymeric material to resist the action of heat and to maintain its properties, such as strength, toughness, or elasticity at given temperature. The thermal stability of polymers is usually determined by thermogravimetric analysis (TGA). Thermal stability of polymer depends on its chemical structure, degree of crystallinity, and molecular weight. Aromatic structures in the polymer backbone and cross-linking processes improve the thermal stability of polymers. On the other hand, double bonds or oxygen-containing structures in the main chain make polymers less resistant to high temperatures (Król-Morkisz and Pielichowska 2019).
4. Applications
4.1. Modified atmosphere packaging (MAP)
MAP is defined as ‘the packaging of a perishable product in an atmosphere which has been modified so that its composition is other than that of air’. It is one of the widely used techniques in packaging and preservation of agricultural products mainly fruits and vegetables by changing by gaseous composition in the headspace of the package (Coles et al., 2003). The final gaseous composition depends on a series of factors such as the weight of the product packed, the storage temperature, the commodity respiration rate, the cultivar, and the ripening stage (Briano et al., 2015).
MAP has been shown to lower respiration rates and delay the ripening of fruits by altering the O2 and CO2 concentration. It can also prevent water loss and fruit shriveling by maintaining a high humidity environment of 90–95% relative humidity (Giacalone et al., 2013). Biodegradable packaging acts as an alternative to polyethylene terephthalate and high- and low-density polyethylene that is being developed to package fresh agricultural produce (Peelman et al., 2013). The shelf life of strawberries cv. Camarosa was improved by including an oxygen absorber in bio-based packages. A biodegradable laminate was found to be suitable as a MAP material in the inert temperature range for fresh products, such as shredded lettuce and cabbage, head lettuce, cut broccoli, whole broccoli, tomatoes, sweet corn, and blueberries. The modified atmosphere extends the shelf life of berries, and the sealed container protects them from exposure to disease and other environmental contaminants (Briano et al., 2015). Xing et al. in 2010 evaluated the effect of chitosan coating containing anti-browning agents and modified atmosphere packaging (MAP) on the browning and shelf life of fresh-cut lotus root stored at 4 °C for 10 days and concluded that Both edible coating and MAP treatment cause changes in atmospheric composition and respiration rate of lotus root slices. This combined treatment could be used to control the browning and improve the storage life of this fresh-cut vegetable. PLA is a common biodegradable packaging material used for pork and other meat products. Muller et al., 2017 used trays made from PLA resin with sealing top films made from a layer combination of cellulose and PLA for packing pork meat.
Some of the examples of modified atmosphere packaging on fruits and vegetables have been listed in Table 3.
Table 3.
Application of Biodegradable polymers.
| Type of Polymer | Food product | Form of packaging | Application | Reference |
|---|---|---|---|---|
| Chitosan | mango | film | Mangoes kept in carton boxes covered with chitosan film and stored at 27 °C at 65% RH. The level of O2 and CO2 were decreased 5% and 3% respectively. The shelf-life of mangoes increased with no growth of fungus and flavour and colour of the mangoes were also maintained. |
Srinivasa et al. (2002) |
| Wheat gluten | refrigerated strawberry | coating and film | A bilayer coating of wheat gluten along with lipid was applied to refrigerated strawberries and the results indicated firmness retention, reduction of weight loss. The visual quality was maintained during the storage time and the strawberry coated with only gluten film was acceptable for consumption. |
Tanada-Palmu et al. (2005) |
| OPLA | fresh-cut tropical fruits | trays | OPLA compared with PET and OPS for storage of Mangoes, Melons, and Pineapples stored at 10 °C. The equilibrium modified atmosphere of O2 and CO2 were 19% and 3% for OPLA. A shelf-life stability of 6–8 days were seen which was same as fruits packed with PET with no visible fungal growth and surface slime. | Chonhenchob et al. (2007) |
| Chitosan | pet food | coating | Chitosan coated paper was compared with fluorinated resins for the fat-barrier property of the coating. in acidic conditions, a stabilization of the fatty acid emulsion was exhibited by the chitosan, which was due to its property to bind with anionic lipid molecules. The Ca+2 adsorption of the coating was also minor. |
Ham-Pichavant et al. (2005) |
| Gelatine | sea bass | film | Gelatine film incorporated with LEO 25% (w/w) was used for wrapping sea bass at a storage temp of 4 °C for 12 days and the microbiological, chemical, and physical changes were observed. The antimicrobial and antioxidative properties were enhanced. A retarded growth of psychrophilic bacteria, lactic acid bacteria, H₂S-producing bacteria and Enterobacteriaceae were recorded. |
Ahmad et al. (2012) |
| PLA | blueberry | container | Highbush blueberries were packed in non-ventilated PLA containers and stored at 10 °C for 18 days and at 23 °C for 9 days. Vented clamshell containers were used as control. Reduced fungal growth was seen in PLA containers as compared to clamshell containers. The fruit shelf-life was enhanced in PLA containers. |
Almenar et al. (2008) |
| Zein | broccoli | film | Freshly cut broccoli was packed in jars and covered with zein film. The film was plasticized with oleic acid and jar was stored for 6 days at 5 °C. There was no significant difference in firmness and colour of broccoli. Due to anoxic condition developed in packages there was no off-odour developed during refrigerated condition. |
Rakotonirainy et al. (2001) |
| PLA | melon | container | Freshly cut melon was packed in PLA and PET stored for 10 days at 4 °C and 10 °C. No difference was observed in colour, pH, firmness, TA or sensory evaluation of the packages at 4 °C, but differences in colour between the melon were found after 7 days of storage at 10 °C. Due to high WVTR and OTR, the PLA containers maintained the quality of fresh-cut melon better than the PET at 10 °C during 10 days of storage. |
Zhou et al. (2016) |
| PCL/ALG | broccoli | film | Bioactive film developed from the blend of PCL and ALG were compared with MC films to see the growth of Escherichia coli, Salmonella typhimurium and Listeria monocytogenes on fresh broccoli stored at 4 °C for 12 days. The initial concentration of organism used was 5 logs CFU/g sample. The PCL/ALG film showed better efficiency than MC film to control the growth of microorganisms at 4 °C |
Takala et al. (2013) |
| Chitosan | vegetable | film and bag | chitosan films were produced using 0.5 g chitosan in 100 ml aqueous solution with incorporation of 0.5–2 g banana flour for packaging of fresh-cut vegetables. The composite bags acted as antimicrobial agent by serving as good barrier and were effective to protect asparagus, Chinese cabbage, and baby corn against Staphylococcus aureus activity. The shelf-life of the vegetables stored in composite bags were higher. |
Pitak and Rakshit (2011) |
| Master-Bi® (starch) | tomatoes | bag | Organic tomatoes stored in LDPE bags and Master-Bi bags at 75–85% RH at 11 °C for 15 days and 22 days were compared for weight loss, colour, moisture content, firmness and flavour. The quality of tomatoes stored in biodegradable bags was same as that of tomatoes stored in LDPE bags. No significant changes were observed in firmness, colour and flavour. A slight reduction in weight was seen in biodegradable bags. |
Kantola and helen, 2001 |
| Chitosan-Gelatine | rainbow trout fillets | coating and film | Chitosan-gelatine coated rainbow trout (Oncorhynchus mykiss) fillets stored for 16 days at (4 ± 1 °C) were analysed for chemical, microbiological characteristics and also to examine the rancidity development. The bacterial contamination was reduced, and no significant difference was seen between the coating and film for reduction of bacterial pollution. The effect of coating against lipid oxidation was more than films. |
Nowzari et al. (2013) |
| Chitosan | pork sausages | active film | Green tea extract was incorporated in chitosan film and was used for the packaging of pork sausages stored at 4 °C. The chitosan film incorporated with extract when compared to control film showed higher inhibition of microbial growth and low count of yeast, LAB and molds which was due to the polyphenolic activity of the extract. |
Siripatrawan and Noipha (2012) |
| Starch | sunflower oil | active film | Wine grape pomace was encapsulated as in films made from cassava starch. Micro encapsuled film with gum arabic showed higher antioxidant activity as compared to micro encapsuled film produced with maltodextrin. Due to improvement in antioxidant activity the oxidative stability of oil increased leading to increased shelf-life. |
Stoll et al. (2016) |
| PLA | ready-to-eat salads | film | PLA film incorporated with Allium spp. extract were used for packaging ready-to-eat salads in a controlled atmosphere. films containing 5% and 6.5% of extract showed antimicrobial activity mainly for fresh lettuce. A decreased enterobacterial growth was also seen in the films with different extract concentration. Film with 6.5% extract was found to be most effective up to 5 days of storage against aerobic bacteria and 7 days of storage against molds. |
Llana-Ruiz-Cabello et al. (2015) |
| Chitosan | bread | film | Chitosan film was incorporated with grapefruit seed extract for improving anti-bacterial and antiviral properties. The result indicated that the shelf-life of the bread samples were two times longer because the film effectively blocked ultraviolet radiation and slowed the degradation. |
National University of Singapore (2016) |
| TPS/PBAT | pasta | active film | Active packaging film was produced by blown extrusion using TPS and PBAT. Potassium sorbate was used as an antimicrobial agent. The film controlled the growth of microorganisms and increasing the shelf-life of pasta. Film with 4.5% antimicrobial agent was found optimum for microbial growth control. |
Andrade-Molina et al., 2013 |
LEO- lemongrass essential oil, ALG-alginate, MC- methylcellulose, RH- Relative humidity, OPLA- Oriented Polylactic acid, PLA-Polylactic acid, WVTR- Water vapor transmission rate, OTR- Oxygen transmission rate, TA- Titratable acidity, PCL-Polycaprolactone, CFU-coliform forming unit, LDPE-Low density polyethylene, LAB- Lactic acid bacteria, TPS- Thermoplastic starch, PBAT- Polybutylene adipate terephthalate.
4.2. Edible packaging
Edible packaging is an excellent alternative for food applications because of its ability to protect foods with their barrier and mechanical properties, control-release active ingredients, and enhance sensory characteristics. They are an integral part of the food and are eaten along with the food product. Edible packaging mainly consists in form of films, sheets, coatings, and pouches (Janjarasskul and Krochta, 2010). Films and coatings are obtained from the same formulation, films are applied as solid sheets whereas coatings are applied as a liquid product (Galus and Kadzinska, 2015).
Edible packaging is mainly made from proteins, polysaccharides, and lipids. Chitosan and gelatine/collagen are the two widely used components. The gelatine coatings reduce O2, moisture, and does not allow migration of oil. Sausage casing made of collagen is the most successful edible protein film commercially available. Films wrapped over thawed and refrigerated beef steak reduces exudation without affecting colour or lipid oxidation. Collagen-based films are used for processed meats to increase juiciness, to reduce shrink loss, and to absorb fluid exudates for a variety of cooked meat products. The polysaccharide-based film found an application for extending the storage life of fruits and vegetables due to their good gas barrier properties and excellent adherence to the surfaces of cut fruits and vegetables. However, they are not good moisture resistant due to their hydrophilic nature (Falguera et al., 2011). For many years the Japanese meat industry is using polysaccharide-based films and coatings commercially. During processing, the coatings get dissolved and integrate into the meat which improves texture, decreases moisture loss, and produces higher yields (Cutter, 2006).
Cellulose derivative is mostly incorporated in all the commercial edible coatings due to their property to exhibit thermo-gelation i.e., forms gels when heated and comes back to original consistency when cooled (Shit and Shah, 2014). Some of the edible coatings incorporated with cellulose derivates have been given in Table 3. The food preservation efficiency increases when edible packaging is combined with non-edible packaging, the latter used for protection against the environment and bacterial contamination. Some of the examples of chitosan and gelatine as edible coating and film have been listed in Table 4.
Table 4.
Edible chitosan and gelatine coatings.
| Brand name | Company | Country |
|---|---|---|
| Semper-fresh | AgriCoat Industries Ltd | Berkshire, UK |
| Nature-Seal | Ecoscience Product System Division | Orlando, FL |
| Natural Shine 9000 | Pace International | USA |
| Pro-long | Courtaulds Group | London |
4.3. Active packaging
Active packaging is “deliberately incorporate components that would release or absorb substances into or from the packaged food or the environment surrounding the food”. It is an innovative technique to maintain the shelf-life and safety of the food product. The active packaging system includes Absorbers (scavenging system) which removes CO2, O2, moisture, odour, or ethylene, and emitters (releasing system) which incorporates different compounds like anti-microbial, antioxidant, flavors, etc into the headspace (Yildirim et al., 2018). The activity of certain active substances when directly incorporated into food may be inhibited or reduced due to the interaction between active substances and the food components in the bulk food system. Thus, the controlled release of active components in Active packaging is more effective for the bulk food system.
Antimicrobial packaging is a widely used application of active packaging. Trays by baking cassava bagasse with polyvinyl alcohol with the incorporation of clove or oregano essential oils were made to study the antimicrobial property. Two methods were used surface coating and direct incorporation; the surface coating method showed the highest antimicrobial activity against gram-positive, gram-negative bacteria, yeast, and molds (Debiagi et al., 2014). One of the current active packaging systems is the incorporation of zinc oxide and oregano essential oil in a very thin “bio-paper” made by PHBV. The paper has antimicrobial activity against Staphylococcus aureus and Escherichia coli (Barrett 2020).
Mixed cellulose/PP pillow packages can be used to extend the shelf life of iceberg lettuce if emitting sachets with eugenol, carvacrol, or trans-anethole are put inside. The sachets slowly release the natural antimicrobial agent and help preserve the food. An easy, low-cost method to confer antibacterial activity (Wieczynska et al., 2018).
Some of the examples of Active packaging system has been listed in Table 3.
5. Forms of biodegradable packaging
5.1. Films
Films are the widely used form of bio-packaging in every sector. Biodegradable films were originally designed for the replacement of PE film. They have better properties than non-degradable plastihave cs. Important characteristics of a good packaging film include:
-
•
Allowing controlled respiration.
-
•
Good barrier properties.
-
•
To maintain structural integrity
-
•
To prevent or reduce microbial spoilage.
A study of oxygen permeability and carbon dioxide of the biodegradable film as a form of packaging for tomatoes was carried out, results showed that films with the optimum permeability allowed proper respiration of the fruit, due to which the microbial contamination was prevented, and the quality of the fruit was maintained. (Muratore et al., 2005).
Blown films have been used as bags and other packaging applications. PLA was used as a base for blown film grading with excellent transparency and mechanical properties. As the degree of crystallinity changes the sealability property changes. Due to slow crystallization, low melting strength a single biodegradable polymer cannot be used for blown film formation. The co-extrusion process is used for the lamination of polyesters. For example, thermoplastic starch (TPS) is film blown in the coextrusion process while coating with polymers like PHA and PHB. Paragon™ developed by Avebe is used in the packaging of cheese (Tuil et al., 2000; Weber et al., 2002).
5.2. Containers
Thermoformed containers or trays can be used for the packaging of vegetables, salads, and fruits because a controlled atmosphere is required to maintain the quality of such food products. First, the polymer undergoes melt extrusion to form sheets and from sheets to a temp above Tg and Tm to form into a specific shape (Pawar and Purwar, 2013). Most of the trays made from biodegradable polymers are brittle and resistant to moisture. There is no change in the structural properties of the tray during freezing. Trays made from oriented PLA were used for the storage of mangoes, melons, and other tropical fruits. The shelf-life of the fruits packed was the same as that of fruits packed in PET trays (Chonhenchob et al., 2007).
5.3. Foamed product
For loose fill-application, starch-based foams are used. Different techniques used for the formation of foamed products include loose-fill molding, foam extrusion, expandable bead molding, and extrusion transfer molding (Tuil et al., 2000). Numerous foamed products like trays, clamshell, etc, based on starch can be used for food packaging but direct food contact coatings are required. On PLA and starch coatings are preferred of paraffin and other polymers. Adhesion between the foamed product and coating is very important. Novamont developed in the USA is a starch-based foam used in many packaging applications (Crow, 2020). Green Cell foam™ developed by the Landaal Packaging system is a sustainable alternative for PP foams. Under moist soil environment, it degraded completely in 4 weeks (Sustainable Packaging 2018).
5.4. Bags
The largest application of biodegradable bags is in the food industry because their raw material composition makes them flexible, strong, resistant to breakage, moisture, and temperature change. The biodegradable bags can be used for the storage and packaging of food products. The use of these bags in different industries requires the addition of additives (Ivankovic et al., 2017). The bags are completely environment friendly. Once their function of packaging is completed, they are decomposed to carbon dioxide, water, and other products within several weeks. The biodegradable bags are a great alternative to polyethylene bags (Nampoothiri et al., 2010).
5.5. Gels
Biodegradable gels include hydrogels, and it is most used to prevent microbial contamination. The development of complex hydrogels is an alternative for bio-based polymer production (Farris et al., 2009). Lettuce, when impregnating with hydrogel no positive effects were observed on maintaining the content of pectic substances and quality but when impregnated in the fruits of Solanum muricatum, the gel showed a positive effect on maintaining the beta-carotene (Schreiner et al., 2003). The combination of hydrogels of various polymeric materials decreases the shelf-life of certain fruits mainly caused by migration of water from the surrounding (Garcia and Barrett, 2002).
6. Biodegradation
Biodegradation can be defined as the conversion of polymer into carbon dioxide, water, or methane and biomass due to the action of microorganisms. During the biodegradation of polymeric materials, there are different steps which includes (Lucas et al., 2008).
-
•
Biodeterioration- The biodegradable material is converted into tiny fractions by the combined action of microbial organisms present in the soil and other abiotic factors.
-
•
Depolymerisation- Microorganisms release different catalytic agents mainly enzymes that cleave the molecule and form oligomers, dimers, and monomers.
-
•
Recognition- Some fragmented oligomers, dimers, and monomers are recognized by receptors of microbes, they pass the plasma membrane of the microbial cell. The unrecognized fragment is left in the extracellular surrounding.
-
•
Assimilation- Molecules enter the cytoplasm, integrate with the metabolism to produce numerous primary and secondary metabolites with biomass and energy.
-
•
Mineralization- Some metabolites like organic acids and aldehydes are secreted by microbial cells and they reach the extracellular surrounding. CO2, CH4, H2O, and other salts are also released in the environment.
The reaction occurring during the biodegradable polymer degradation is shown below.
| Biodegradable polymers → CO2 + H2O + Humus |
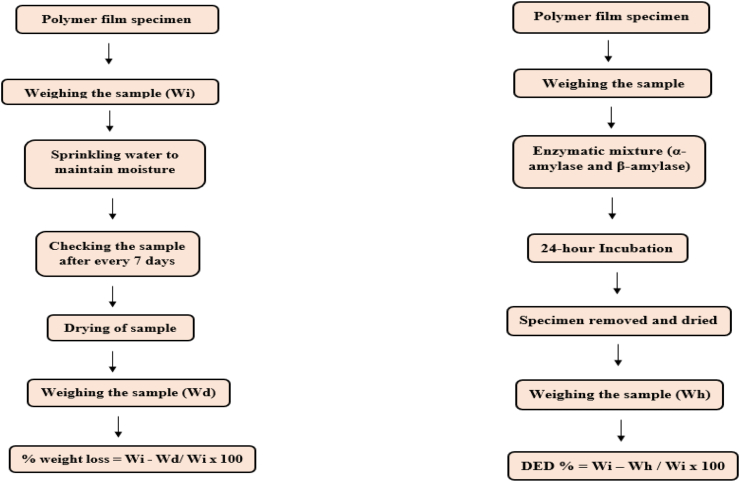
Biodiversity and the presence of microorganisms responsible for polymer-degradation vary depending on the environment, soil, sea, and compost, etc. The colonization of the exposed surface after the microorganism adherence on the polymer surface is the major mechanism involved in degradation (Barone and Arikan, 2007). Various factors that control the rate of biodegradation include the nature of enzymes, type of enzyme, location of the enzyme (extra, intracellular), type of substrate, and environmental conditions like soil, pH, light, temperature, oxygen, moisture, etc. The biodegradability of a polymer can be assessed mainly by soil burial test and enzymatic test. The flow charts of these methods are shown in Fig. 5 (Mangaraj et al., 2019).
Fig. 5.
Soil burial test and enzymatic test (Mangaraj et al., 2019).
7. Composting
Composting can be defined as the accelerated degradation of heterogeneous organic matter by a mixed microbial population in a moist, warm, aerobic environment under controlled conditions. It is an ancient method to convert organic matter into fertile humus. Biodegradation of such organic matter will produce compost as the major product along with water and carbon dioxide. The carbon-dioxide produced is already a part of the biological carbon cycle, so it does not contribute to greenhouse gases (Song et al., 2009). The soil is benefited by compositing as it helps to retain soil moisture, increases the microbiological activity, enriching the soil with nutrients, and also makes the soil more breathable (Mondini et al., 2004).
PLA decomposes to CO2, water, and biomass under controlled composting conditions in less than 90 days. The decomposition takes place in large plants where the temperature reaches up to 140 °C (Runjic, 2007). PHA is degradable under a normal biological environment by the enzyme PHA depolymerase secreted by 55% of penicillium yeast. Degradation under a controlled atmosphere can be completed within 45 days (Zivkovic, 2009). Biodegradation and composting products are a friendly alternative to protect the environment to preserve fossil fuels and reduce CO2 emissions.
The International Standards Organization (ISO) has developed ISO 17088, ‘Specification for Compostable Plastics’ at an international level which is in harmony with these European and US norms. The requirements of these standards for complete biodegradation under composting conditions are given as (Song et al., 2009).
-
1.
Conversion of polymer material in form of granule, film or powder to CO2, water and biomass through microbial activity.
-
2.
Approximately 90% of the carbon in the polymer conversion to CO2.
-
3.
The rate of biodegradation similar to degradation of natural materials like leaves, paper, grass and food scraps.
-
4.
The composting cycle or degradation time should be not more than 180 days and in case of radiolabelled polymer not more than 365 days.
8. Toxicity
During the formation of biodegradable plastics or bioplastics, different compounds like additives, antioxidants, stabilizers, chain initiators, cross-linking agents, nucleating agents, catalysts, etc. are added to improve the properties. Many of these compounds are not covalently bond to the polymer matrix, there is the possibility of a process called chemical migration which exposes humans to these compounds (Zimmermanna et al., 2020). The bioplastic evaluation of toxicity to increase environmental performance is usually carried at the production stage or the degradability stage. The release of chemicals by the material during the usage is often disregarded (Ernstoff et al., 2019). As the application of bioplastic increases the incorporation of chemicals will also increase.
Different assay methods are used to check the in-vitro toxicity such as Baseline toxicity, Oxidative stress response, and Endocrine activity. Some of the cellulose-based, starch-based. Bio-PE, Bio-PET, PBAT, and PHA samples inhibited bioluminescence of Aliivibrio fischeri which is an indicator for baseline toxicity, mostly with high potency (low EC20) (Zimmermanna et al., 2020). Cell culture tests proved that Bionolle produced by Showa Highpolymer made from polybutylene succinate has no toxic effects on cells. After degradation of PLA and nanocomposites (organoclays Cloisite 20A and Cloisite 30B) a decreasing mitotic index and increasing chromosomal abnormalities were reported which is possible due to the aneugenic action of the products formed after degradation; tested by bioassay method using Allium cepaas test organism for assessment of ecotoxicity (Souza et al., 2013). The phytotoxic effect of bioplastics showed be minimal for their application of agricultural and horticultural food products. Mater-bi a commercial bag made from vegetable starch was found to be a potential threat like other conventional plastics if let in the natural environment as it affected the plant radicle formation which was confirmed by phytotoxicity standard tests performed using seeds of Lepidium sativum L. It also showed a significant effect to change the characteristics of water (Balestri et al., 2019). Studies on the toxicity of PHB and PBAT leachates showed decreased survival of Daphnia magna already 48 h of exposure (Gottermann et al., 2015). 37 non-volatile chemicals were found in films and pellets made from a PLA/Bio-PE blend. Cyclic oligomers like adipic acid, phthalic acid, and butanediol were found in the highest concentration (Aznar et al., 2019).
Nanoparticles (NPs) are used to improve the properties of polymer for food packaging application but very few studies are reported for Nanotoxicology and nanoecotoxicology of different biodegradable polymers. These particles can be exposed as oral ingestions, inhalation, and contact. Oral ingestion is due to the chemical migration of NPs from polymer to food products (Maisanaba et al., 2015). The molecular weight of smaller nanoparticles is faster absorbed and readily distributed throughout the body damaging the cells. Studies on mice demonstrated that carbon nanotubes caused asbestos-like, length-dependent, toxic behavior when injected into the animal peritoneal cavity (Poland et al., 2008).
Toxicity is not the only parameter to study the sustainability of bioplastics other factors like land-use, greenhouse emissions, societal impacts must also be considered. Research on using safe chemical alternatives must be carried out based on scientific principles like the Tiered Protocol for Endocrine Disruption (TiPED) (Muncke et al., 2020). Despite all these toxicological effects most of the biodegradable polymers and bioplastics have found application in food industries as their production technology is changed with hazard identification and characterization performed before its large-scale commercial use.
9. Consumer perception on biodegradable packaging
Consumers play a very crucial role in the success of environmentally friendly food packaging through their decisions of buying the product. The decision depends on many trade-off attributes like design, colour, and shape like for example colour influences the taste of some products (Loose and Szolnoki, 2012). There are various barriers to not purchasing a sustainable product such as higher process, perceived lower quality, and lack of availability (Ketelsen et al., 2020). One of the main factors for the non-development market growth is the lack of recognition. Consumers identify these sustainable packaging through logos, labels, etc. Many people do not know about the biobased packaging and thinks of it as a marketing trick (Sijtsema et al., 2016). According to some studies, attributes like price and product quality were more important than green packaging. Consumers are only willing to pay only a small premium price.
To improve the market of environment-friendly packaging several ideas can be considered such as the packaging must have a third-party label certification to build trust in consumers. Food companies involved in environmentally friendly packaging should have an effective label on their product packages and must provide clear information on any benefits. Without good communication, packaging could not pay off for both the companies in the food industry and the environment (Ertz et al., 2017).
10. Conclusion
Biodegradable polymers help in reducing the environmental impact of plastic production and processing. As biodegradable polymers are made from renewable feedstocks, agricultural waste, there is a great opportunity for research work in harnessing this economic opportunity. Biodegradable polymers at present only replace about 1% of the plastics. Several factors like policy and legislative changes, as well as world demand for food and energy resources, influences the development of biodegradable packaging. The use of bio-based polymers is increasing for the packaging of food and other applications at a great speed. In food packaging, the biodegradable packaging can be used for modified atmosphere packaging, active packaging system, and edible packaging for different high-quality food products to enhance their shelf-life. However, before adopting any packaging for food proper studies on the interaction between food components and biopolymers during processing and storage need to be carried out. Future studies need to be focused on the use of nanotechnology and sensors which can help in communicating the information to the consumers. Biodegradable polymers can help in overall environmental sustainability.
CRediT authorship contribution statement
Salman Shaikh: Writing – original draft, drafting the manuscript, Approval of the version of the manuscript to be published. Mudasir Yaqoob: Conceptualization, Conception and design of study, Writing – original draft, Approval of the version of the manuscript to be published. Poonam Aggarwal: revising the manuscript critically for important intellectual content, Approval of the version of the manuscript to be published.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Salman Shaikh, Email: salman888909@gmail.com.
Mudasir Yaqoob, Email: Mudasiryaquob@gmail.com.
Poonam Aggarwal, Email: Sachdev_poonam@pau.edu.
References
- Abdullah Z.W., Dong Y., Davies I.J., Barbhuiya S. PVA, PVA blends, and their nanocomposites for biodegradable packaging application. Polym. Plast. Technol. Eng. 2017;56(12):1307–1344. [Google Scholar]
- Ahmad M., Benjakul S., Sumpavapol P., Nirmal N.P. Quality changes of sea bass slices wrapped with gelatin film incorporated with lemongrass essential oil. Int. J. Food Microbiol. 2012;155(3):171–178. doi: 10.1016/j.ijfoodmicro.2012.01.027. [DOI] [PubMed] [Google Scholar]
- Ahvenainen R. Wood head Publishing, Limited; Cambridge: 2003. Novel Food Packaging Techniques. [Google Scholar]
- Akter N., Khan R.A., Tuhin M.O., Haque M.E., Nurnabi M., Parvin F., Islam R. Thermomechanical, barrier, and morphological properties of chitosan-reinforced starch-based biodegradable composite films. J. Thermoplast. Compos. Mater. 2014;27(7):933–948. [Google Scholar]
- Almenar E., Samsudin H., Auras R., Harte B., Rubino M. Postharvest shelf life extension of blueberries using a biodegradable package. Food Chem. 2008;110(1):120–127. doi: 10.1016/j.foodchem.2008.01.066. [DOI] [PubMed] [Google Scholar]
- Angellier H., Molina-Boisseau S., Dole P., Dufresne A. Thermoplastic starch− waxy maize starch nanocrystals nanocomposites. Biomacromolecules. 2006;7(2):531–539. doi: 10.1021/bm050797s. [DOI] [PubMed] [Google Scholar]
- Aryan Y., Yadav P., Samadder S.R. Life Cycle Assessment of the existing and proposed plastic waste management options in India: a case study. J. Clean. Prod. 2019;211:1268–1283. [Google Scholar]
- Astm D. J ASTM Int; West Conshohocken, PA: 2004. -04 Standard Specification for Compostable Plastics; p. 6400. [Google Scholar]
- Auras R., Singh S.P., Singh J. Performance evaluation of PLA against existing PET and PS containers. J. Test. Eval. 2006;34(6):530–536. [Google Scholar]
- Aznar M., Ubeda S., Dreolin N., Nerín C. Determination of non-volatile components of a biodegradable food packaging material based on polyester and polylactic acid (PLA) and its migration to food simulants. J. Chromatogr. A. 2019;1583:1–8. doi: 10.1016/j.chroma.2018.10.055. [DOI] [PubMed] [Google Scholar]
- Balestri E., Menicagli V., Ligorini V., Fulignati S., Galletti A.M.R., Lardicci C. Phytotoxicity assessment of conventional and biodegradable plastic bags using seed germination test. Ecol. Indicat. 2019;102:569–580. [Google Scholar]
- Banerjee T., Srivastava R.K., Hung Y.T. Handbook of Environment and Waste Management: Land and Groundwater Pollution Control. 2014. Chapter 17: plastics waste management in India: an integrated solid waste management approach; pp. 1029–1060. [Google Scholar]
- Barone J.R., Arikan O. Composting and biodegradation of thermally processed feather keratin polymer. Polym. Degrad. Stabil. 2007;92(5):859–867. [Google Scholar]
- Barrett A. Biodegradable food packaging extending food shelf life. 2020. https://bioplasticsnews.com/2020/06/12/ypack-biodegradable-food-packaging-shelf-life/ Retrieved from. Accessed on 14th Oct 2020.
- Bedia E.L., Murakami S., Kitade T., Kohjiya S. Structural development and mechanical properties of polyethylene naphthalate/polyethylene terephthalate blends during uniaxial drawing. Polymer. 2001;42(17):7299–7305. [Google Scholar]
- Begum S.A., Rane A.V., Kanny K. Compatibilization of Polymer Blends. Elsevier; 2020. Applications of compatibilized polymer blends in automobile industry; pp. 563–593. [Google Scholar]
- Bhatia A., Gupta R.K., Bhattacharya S.N., Choi H.J. Analysis of gas permeability characteristics of poly (lactic acid)/poly (butylene succinate) nanocomposites. J. Nanomater. 2012;2012 [Google Scholar]
- Bourtoom T., Chinnan M.S. Preparation and properties of rice starch–chitosan blend biodegradable film. LWT-Food science and Technology. 2008;41(9):1633–1641. [Google Scholar]
- Briano R., Giuggioli N.R., Girgenti V., Peano C. Biodegradable and compostable film and modified atmosphere packaging in postharvest supply chain of raspberry fruits (cv. G randeur) J. Food Process. Preserv. 2015;39(6):2061–2073. [Google Scholar]
- Briassoulis D. An overview on the mechanical behaviour of biodegradable agricultural films. J. Polym. Environ. 2004;12(2):65–81. [Google Scholar]
- Briassoulis D., Giannoulis A. Evaluation of the functionality of bio-based food packaging films. Polym. Test. 2018;69:39–51. [Google Scholar]
- Burgos N., Armentano I., Fortunati E., Dominici F., Luzi F., Fiori S. Functional properties of plasticized bio-based poly (lactic acid) _poly (hydroxybutyrate)(PLA_PHB) films for active food packaging. Food Bioprocess Technol. 2017;10(4):770–780. [Google Scholar]
- Carmona V.B., Corrêa A.C., Marconcini J.M., Mattoso L.H.C. Properties of a biodegradable ternary blend of thermoplastic starch (TPS), poly (ε-caprolactone)(PCL) and poly (lactic acid)(PLA) J. Polym. Environ. 2015;23(1):83–89. [Google Scholar]
- Chonhenchob V., Chantarasomboon Y., Singh S.P. Quality changes of treated fresh‐cut tropical fruits in rigid modified atmosphere packaging containers. Packag. Technol. Sci.: Int. J. 2007;20(1):27–37. [Google Scholar]
- Coles R., McDowell D., Kirwan M.J., editors. vol. 5. CRC press; 2003. (Food Packaging Technology). [Google Scholar]
- Contreras I.N., Bader J., DuRant P., Grafman L. An analysis of recycling high density polyethylene with limited resources. International Journal for Service Learning in Engineering, Humanitarian Engineering and Social Entrepreneurship. 2018;13(2):45–68. [Google Scholar]
- Crow, Patrick . 2020. Novamont Buys Eastman’s ‘Eastar Bio’ Technology | ICIS. ICIS Explore.http://www.icis.com/resources/news/2004/09/08/611549/novamont-buyseastman-s-eastar-biotechnology [Google Scholar]
- Cutter C.N. Opportunities for bio-based packaging technologies to improve the quality and safety of fresh and further processed muscle foods. Meat Sci. 2006;74(1):131–142. doi: 10.1016/j.meatsci.2006.04.023. [DOI] [PubMed] [Google Scholar]
- de Camargo Andrade-Molina T.P., Shirai M.A., Grossmann M.V.E., Yamashita F. Active biodegradable packaging for fresh pasta. LWT-Food Science and Technology. 2013;54(1):25–29. [Google Scholar]
- Debiagi F., Kobayashi R.K., Nakazato G., Panagio L.A., Mali S. Biodegradable active packaging based on cassava bagasse, polyvinyl alcohol and essential oils. Ind. Crop. Prod. 2014;52:664–670. [Google Scholar]
- Dias A.B., Müller C.M., Larotonda F.D., Laurindo J.B. Biodegradable films based on rice starch and rice flour. J. Cereal. Sci. 2010;51(2):213–219. [Google Scholar]
- Diawara B., Fatyeyeva K., Chappey C., Colasse L., Ortiz J., Marais S. Evolution of mechanical and barrier properties of thermally aged polycarbonate films. J. Membr. Sci. 2020;607:117630. [Google Scholar]
- Duan Z. Loughborough University; 2013. Water Vapour Permeability of Bio-Based Polymers. Doctoral dissertation. [Google Scholar]
- DuPont Teijin Films . 2001. Oxygen and Water Vapour Barrier Properties of Flexible Packaging Films. [Google Scholar]
- Ernstoff A., Niero M., Muncke J., Trier X., Rosenbaum R.K., Hauschild M., Fantke P. Challenges of including human exposure to chemicals in food packaging as a new exposure pathway in life cycle impact assessment. Int. J. Life Cycle Assess. 2019;24(3):543–552. [Google Scholar]
- Ertz M., François J., Durif F. How consumers react to environmental information: an experimental study. J. Int. Consum. Market. 2017;29(3):162–178. [Google Scholar]
- Europe P., EPRO Plastics - the facts 2019. 2019. https://www.plasticseurope.org/en/resources/market-data
- Fabra M.J., Lopez-Rubio A., Lagaron J.M. Nanostructured interlayers of zein to improve the barrier properties of high barrier polyhydroxyalkanoates and other polyesters. J. Food Eng. 2014;127:1–9. [Google Scholar]
- Falguera V., Quintero J.P., Jiménez A., Muñoz J.A., Ibarz A. Edible films and coatings: structures, active functions and trends in their use. Trends Food Sci. Technol. 2011;22(6):292–303. [Google Scholar]
- Farah S., Anderson D.G., Langer R. Physical and mechanical properties of PLA, and their functions in widespread applications—a comprehensive review. Adv. Drug Deliv. Rev. 2016;107:367–392. doi: 10.1016/j.addr.2016.06.012. [DOI] [PubMed] [Google Scholar]
- Farris S., Schaich K.M., Liu L., Piergiovanni L., Yam K.L. Development of polyion-complex hydrogels as an alternative approach for the production of bio-based polymers for food packaging applications: a review. Trends Food Sci. Technol. 2009;20(8):316–332. [Google Scholar]
- Ficci . Potential of Plastics Industry in Northern India with Special Focus on Plastic Culture and Food Processing. 2014. [Google Scholar]
- Flair Flexible Packaging Corporation 2020. http://www.flairpackaging.com/home
- Foolmaun R.K., Ramjeeawon T. Disposal of post-consumer polyethylene terephthalate (PET) bottles: comparison of five disposal alternatives in the small island state of Mauritius using a life cycle assessment tool. Environ. Technol. 2012;33(5):563–572. doi: 10.1080/09593330.2011.586055. [DOI] [PubMed] [Google Scholar]
- Galus S., Kadzinska J. Food applications of emulsion-based edible films and coatings. Trends Food Sci. Technol. 2015;45(2):273–283. [Google Scholar]
- Garcia E., Barrett D.M. Preservative treatments for fresh-cut fruits and vegetables. Fresh-cut fruits and vegetables. 2002:267–304. [Google Scholar]
- Gelse K., Pöschl E., Aigner T. Collagens—structure, function, and biosynthesis. Adv. Drug Deliv. Rev. 2003;55(12):1531–1546. doi: 10.1016/j.addr.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Ghaffarian V., Mousavi S.M., Bahreini M., Afifi M. Preparation and characterization of biodegradable blend membranes of PBS/CA. J. Polym. Environ. 2013;21(4):1150–1157. [Google Scholar]
- Ghasemlou M., Aliheidari N., Fahmi R., Shojaee-Aliabadi S., Keshavarz B., Cran M.J., Khaksar R. Physical, mechanical and barrier properties of corn starch films incorporated with plant essential oils. Carbohydr. Polym. 2013;98(1):1117–1126. doi: 10.1016/j.carbpol.2013.07.026. [DOI] [PubMed] [Google Scholar]
- Giacalone G., Chiabrando V. Modified atmosphere packaging of sweet cherries with biodegradable films. International Food Research Journal. 2013;20(3) [Google Scholar]
- Godbole S., Gote S., Latkar M., Chakrabarti T. Preparation and characterization of biodegradable poly-3-hydroxybutyrate–starch blend films. Bioresour. Technol. 2003;86(1):33–37. doi: 10.1016/s0960-8524(02)00110-4. [DOI] [PubMed] [Google Scholar]
- Gomez-Guillen M.C., Pérez-Mateos M., Gomez-Estaca J., Lopez-Caballero E., Gimenez B., Montero P. Fish gelatin: a renewable material for developing active biodegradable films. Trends Food Sci. Technol. 2009;20(1):3–16. [Google Scholar]
- Gottermann S., Bonten C., Kloeppel A., Kaiser S., Brümme F. Marine littering - effects and degradation. Conference paper “24. Stuttgarter Kunststoffkolloquium. 2015 [Google Scholar]
- Gumede T.P., Luyt A.S., Muller A.J. 2018. Review on PCL, PBS, and PCL/PBS Blends Containing Carbon Nanotubes. [Google Scholar]
- Halley P. Biodegradable packaging for the food industry. Packag Bottling Int. 2002;4(4):56–57. [Google Scholar]
- Ham-Pichavant F., Sebe G., Pardon P., Coma V. Fat resistance properties of chitosan-based paper packaging for food applications. Carbohydr. Polym. 2005;61(3):259–265. [Google Scholar]
- Harada M., Ohya T., Iida K., Hayashi H., Hirano K., Fukuda H. Increased impact strength of biodegradable poly (lactic acid)/poly (butylene succinate) blend composites by using isocyanate as a reactive processing agent. J. Appl. Polym. Sci. 2007;106(3):1813–1820. [Google Scholar]
- Hassan A., Jwu W.Y. Polymer Symposium. Kebangsaan Ke-V; Malaysia: 2005. Mechanical properties of high impact ABS/PC blends–effect of blend ratio. [Google Scholar]
- Huang C.H., Wu J.S., Huang C.C., Lin L.S. Morphological, thermal, barrier and mechanical properties of LDPE/EVOH blends in extruded blown films. J. Polym. Res. 2004;11(1):75–83. [Google Scholar]
- Ikejima T., Inoue Y. Crystallization behavior and environmental biodegradability of the blend films of poly (3-hydroxybutyric acid) with chitin and chitosan. Carbohydr. Polym. 2000;41(4):351–356. [Google Scholar]
- Ivonkovic A., Zeljko K., Talic S., Lasic M. Biodegradable packaging in the food industry. Journal of Food Safety and Food Quality. 2017;68:26–38. [Google Scholar]
- Jang W.Y., Shin B.Y., Lee T.J., Narayan R. Thermal properties and morphology of biodegradable PLA/starch compatibilized blends. J. Ind. Eng. Chem. 2007;13(3):457–464. [Google Scholar]
- Janjarasskul T., Krochta J.M. Edible packaging materials. Annual review of food science and technology. 2010;1:415–448. doi: 10.1146/annurev.food.080708.100836. [DOI] [PubMed] [Google Scholar]
- Javadi A., Kramschuster A.J., Pilla S., Lee J., Gong S., Turng L.S. Processing and characterization of microcellular PHBV/PBAT blends. Polym. Eng. Sci. 2010;50(7):1440. [Google Scholar]
- Jordan J.L., Casem D.T., Bradley J.M. Mechanical properties of low-density polyethylene. J. dynamic behavior mater. 2016;2:411–420. [Google Scholar]
- Jost V. Packaging related properties of commercially available biopolymers–An overview of the status quo. Express Polym. Lett. 2018;12(5):429–435. [Google Scholar]
- Kale G., Auras R., Singh S.P. Degradation of commercial biodegradable packages under real composting and ambient exposure conditions. J. Polym. Environ. 2006;14(3):317–334. [Google Scholar]
- Kantola M., helen H. Quality changes in organic tomatoes packaged in biodegradable plastic films. J. Food Qual. 2001;24(2):167–176. [Google Scholar]
- Karbasi S., Khorasani S.N., Ebrahimi S., Khalili S., Fekrat F., Sadeghi D. Preparation and characterization of poly (hydroxy butyrate)/chitosan blend scaffolds for tissue engineering applications. Adv. Biomed. Res. 2016;5 doi: 10.4103/2277-9175.188490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketelsen M., Janssen M., Hamm U. Consumers' response to environmentally-friendly food packaging-A systematic review. J. Clean. Prod. 2020;254:120123. [Google Scholar]
- Khalifa Y.I.M. Effect of the printing remedies and lamination techniques on barrier properties “WVTR and OTR Value” for Polypropylene Film. EC Nutrition. 2016;5(2):1089–1099. [Google Scholar]
- Khalil H.A., Banerjee A., Saurabh C.K., Tye Y.Y., Suriani A.B., Mohamed A. Biodegradable films for fruits and vegetables packaging application: preparation and properties. Food Engineering Reviews. 2018;10(3):139–153. [Google Scholar]
- Kim M. Evaluation of degradability of hydroxypropylated potato starch/polyethylene blend films. Carbohydr. Polym. 2003;54(2):173–181. [Google Scholar]
- Kim M.N., Lee A.R., Yoon J.S., Chin I.J. Biodegradation of poly (3-hydroxybutyrate), Sky-Green® and Mater-Bi® by fungi isolated from soils. Eur. Polym. J. 2000;36(8):1677–1685. [Google Scholar]
- Kirwan M.J., Strawbridge J.M. Plastics in food packaging. In: Coles R., Macdowell D., Kirwan M.J., editors. Food Packaging Technology. Blackwell Publishing; Oxford: 2003. pp. 174–240. [Google Scholar]
- Klemm D., Shmauder H.P., Heinze T. Cellulose. In: Vandamme E.J., De Baets S., Steinbüchel A., editors. Polysaccharides II. vol. 6. Wiley-VCH; Weinheim, Germany: 2002. pp. 275–319. (Biopolymers). [Google Scholar]
- Król-Morkisz K., Pielichowska K. Thermal decomposition of polymer nanocomposites with functionalized nanoparticles. InPolymer Composites with Functionalized Nanoparticles. 2019 Jan 1:405–435. (Elsevier) [Google Scholar]
- Lagaron J.M., Catalá R., Gavara R. Structural characteristics defining high barrier properties in polymeric materials. Mater. Sci. Technol. 2004;20(1):1–7. [Google Scholar]
- Lee D.S. Carbon dioxide absorbers for food packaging applications. Trends Food Sci. Technol. 2016;57:146–155. [Google Scholar]
- Lianhua Y., Qiang C. IOP Conf. Ser. Mater. Sci. Eng. 2017;274 doi: 10.1088/1757-899X/279/1/012021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X., Fan X., Li R., Li Z., Ren T., Ren X., Huang T.S. Preparation and characterization of PHB/PBAT–based biodegradable antibacterial hydrophobic nanofibrous membranes. Polym. Adv. Technol. 2018;29(1):481–489. [Google Scholar]
- Liu Z., Lei Y., Hu Z., Kong W., Zhou C., Lei J. Preparation, characterization and properties of poly (lactic acid)/poly (1, 4-butylene adipate) blends for biodegradable packaging materials. Macromol. Res. 2017;25(5):439–445. [Google Scholar]
- Llana-Ruiz-Cabello M., Pichardo S., Baños A., Núñez C., Bermúdez J.M., Guillamón E. Characterisation and evaluation of PLA films containing an extract of Allium spp. to be used in the packaging of ready-to-eat salads under controlled atmospheres. LWT-Food Science and Technology. 2015;64(2):1354–1361. [Google Scholar]
- Lodha P., Netravali A.N. Thermal and mechanical properties of environment-friendly ‘green’plastics from stearic acid modified-soy protein isolate. Ind. Crop. Prod. 2005;21(1):49–64. [Google Scholar]
- Loose S.M., Szolnoki G. Market price differentials for food packaging characteristics. Food Qual. Prefer. 2012;25(2):171–182. [Google Scholar]
- Lucas N., Bienaime C., Belloy C., Queneudec M., Silvestre F., Nava-Saucedo J.E. Polymer biodegradation: mechanisms and estimation techniques–A review. Chemosphere. 2008;73(4):429–442. doi: 10.1016/j.chemosphere.2008.06.064. [DOI] [PubMed] [Google Scholar]
- Luckachan G.E., Pillai C.K.S. Biodegradable polymers-a review on recent trends and emerging perspectives. J. Polym. Environ. 2011;19(3):637–676. [Google Scholar]
- Maisanaba S., Prieto A.I., Pichardo S., Jordá-Beneyto M., Aucejo S., Jos A. Cytotoxicity and mutagenicity assessment of organomodified clays potentially used in food packaging. Toxicol. Vitro. 2015;29(6):1222–1230. doi: 10.1016/j.tiv.2015.03.010. [DOI] [PubMed] [Google Scholar]
- Mangaraj S., Yadav A., Bal L.M., Dash S.K., Mahanti N.K. Application of biodegradable polymers in food packaging industry: a comprehensive review. Journal of Packaging Technology and Research. 2019;3(1):77–96. [Google Scholar]
- Manikantan M.R., Varadharaju N. 2012. Preparation and Properties of Linear Low Density Polyethylene Based Nanocomposite Films for Food Packaging. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Market 2020, April 16. https://www.european-bioplastics.org/market/ Retrieved from.
- Meenakshi P., Noorjahan S.E., Rajini R., Venkateswarlu U., Rose C., Sastry T.P. Mechanical and microstructure studies on the modification of CA film by blending with PS. Bull. Mater. Sci. 2002;25(1):25–29. [Google Scholar]
- Mercan N., Aslim B., Yuksekdag Z.N., Beyatli Y. Production of poly-b-hydroxybutyrate (PHB) by some Rhizobium bacteria. Turkish J. Biol. 2002;26(4):215–219. [Google Scholar]
- Messin T., Marais S., Follain N., Guinault A., Gaucher V., Delpouve N., Sollogoub C. Biodegradable PLA/PBS multinanolayer membrane with enhanced barrier performances. J. Membr. Sci. 2020;598:117777. [Google Scholar]
- Mirjalili F., Moradian S., Ameri F. Enhancing the dyeability of polypropylene fibers by melt blending with polyethylene terephthalate. Sci. World J. 2013;2013 doi: 10.1155/2013/468542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohareb E., Mittal G.S. Formulation and process conditions for biodegradable/edible soy‐based packaging trays. Packag. Technol. Sci.: Int. J. 2007;20(1):1–15. [Google Scholar]
- Mondini C., Fornasier F., Sinicco T. Enzymatic activity as a parameter for the characterization of the composting process. Soil Biol. Biochem. 2004;36(10):1587–1594. [Google Scholar]
- Muller J., González-Martínez C., Chiralt A. Combination of poly (lactic) acid and starch for biodegradable food packaging. Materials. 2017 Aug;10(8):952. doi: 10.3390/ma10080952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muncke J., Andersson A.M., Backhaus T., Boucher J.M., Almroth B.C., Castillo A.C. Impacts of food contact chemicals on human health: a consensus statement. Environ. Health. 2020;19(1):1–12. doi: 10.1186/s12940-020-0572-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muratore G., Nobile D., Buonocore G.G., Lanza C.M., Asmundo N. The influence of using biodegradable packaging films on the quality decay kinetic of plum tomato (PomodorinoDatterino®) J. Food Eng. 2005;67(4):393–399. [Google Scholar]
- Murcia Valderrama M.A., van Putten R.J., Gruter G.J.M. PLGA barrier materials from CO2. The influence of lactide Co-monomer on glycolic acid polyesters. ACS applied polymer materials. 2020;2(7):2706–2718. doi: 10.1021/acsapm.0c00315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthuraj R., Misra M., Mohanty A.K. Biodegradable compatibilized polymer blends for packaging applications: a literature review. J. Appl. Polym. Sci. 2018;135(24):45726. [Google Scholar]
- Najarzadeh Z., Tabasi R.Y., Ajji A. Sealability and seal characteristics of PE/EVA and PLA/PCL blends. Int. Polym. Process. 2014;29(1):95–100. [Google Scholar]
- Nampoothiri K.M., Nair N.R., John R.P. An overview of the recent developments in polylactide (PLA) research. Bioresour. Technol. 2010;101(22):8493–8501. doi: 10.1016/j.biortech.2010.05.092. [DOI] [PubMed] [Google Scholar]
- National University of Singapore Eco-friendly food packaging material created by NUS researchers doubles shelf-life of food products. 2016. https://news.nus.edu.sg/press-releases/10049-all-naturalfood-packaging-material Available from:
- Netravali A.N., Chabba S. Composites get greener. Mat. Today Off. 2003;6:22–29. [Google Scholar]
- Nowzari F., Shabanpour B., Ojagh S.M. Comparison of chitosan–gelatin composite and bilayer coating and film effect on the quality of refrigerated rainbow trout. Food Chem. 2013;141(3):1667–1672. doi: 10.1016/j.foodchem.2013.03.022. [DOI] [PubMed] [Google Scholar]
- Ojijo V., Sinha Ray S., Sadiku R. Toughening of biodegradable polylactide/poly (butylene succinate-co-adipate) blends via in situ reactive compatibilization. ACS Appl. Mater. Interfaces. 2013;5(10):4266–4276. doi: 10.1021/am400482f. [DOI] [PubMed] [Google Scholar]
- Oliveira N.S., Oliveira J., Gomes T., Ferreira A., Dorgan J., Marrucho I.M. Gas sorption in poly (lactic acid) and packaging materials. Fluid Phase Equil. 2004;222:317–324. [Google Scholar]
- Park S.Y., Lee B.I., Jung S.T., Park J.H. Biopolymer composite films based on carrageenan and chitosan. Mater. Res. Bull. 2001;36:511–519. [Google Scholar]
- Pawar P.A., Purwar A.H. Biodegradable polymers in food packaging. American Journal of Engineering Research. 2013;2(5):151–164. [Google Scholar]
- Peelman N., Ragaert P., De Meulenaer B., Adons D., Peeters R., Cardon L. Application of bioplastics for food packaging. Trends Food Sci. Technol. 2013;32(2):128–141. [Google Scholar]
- Pitak N., Rakshit S.K. Physical and antimicrobial properties of banana flour/chitosan biodegradable and self-sealing films used for preserving fresh-cut vegetables. LWT-Food science and technology. 2011;44(10):2310–2315. [Google Scholar]
- Plastic Materials Free online database for plastic industry. 2020. https://omnexus.specialchem.com
- Poland C.A., Duffin R., Kinloch I., Maynard A., Wallace W.A., Seaton A. Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nat. Nanotechnol. 2008;3(7):423. doi: 10.1038/nnano.2008.111. [DOI] [PubMed] [Google Scholar]
- Priya B., Gupta V.K., Pathania D., Singha A.S. Synthesis, characterization and antibacterial activity of biodegradable starch/PVA composite films reinforced with cellulosic fibre. Carbohydr. Polym. 2014;109:171–179. doi: 10.1016/j.carbpol.2014.03.044. [DOI] [PubMed] [Google Scholar]
- Raj B. 2005. Plastics and Their Role in Food Packaging. [Google Scholar]
- Rakotonirainy A.M., Wang Q., Padua G.W. Evaluation of zein films as modified atmosphere packaging for fresh broccoli. J. Food Sci. 2001;66(8):1108–1111. [Google Scholar]
- Ramos M., Valdes A., Beltran A., Garrigós M.C. Gelatin-based films and coatings for food packaging applications. Coatings. 2016;6(4):41. [Google Scholar]
- Ratnayake W.S., Hoover R., Shahidi F., Perera C., Jane J. Composition, molecular structure, and physicochemical properties of starches from four field pea (Pisum sativum L.) cultivars. Food Chem. 2001;74(2):189–202. [Google Scholar]
- Reis M.O., Olivato J.B., Bilck A.P., Zanela J., Grossmann M.V.E., Yamashita F. Biodegradable trays of thermoplastic starch/poly (lactic acid) coated with beeswax. Ind. Crop. Prod. 2018;112:481–487. [Google Scholar]
- Rosa D.S., Guedes C.G., Pedroso A.G. Gelatinized and nongelatinized corn starch/poly (epsilon-caprolactone) blends: characterization by rheological, mechanical and morphological properties. Polímeros. 2004;14(3):181–186. [Google Scholar]
- Rostam S., Ali A.K., AbdalMuhammad F.H. Experimental investigation of mechanical properties of PVC polymer under different heating and cooling conditions. J. Eng. 2016;2016 [Google Scholar]
- Rujnic Sokele M. Myths and realities bioplastic. Environ. Protect. 2007;28:178–181. (in Croatian) [Google Scholar]
- Samantaray P.K., Little A., Haddleton D., McNally T., Tan B., Sun Z. Poly (glycolic acid) (PGA): a versatile building block expanding high performance and sustainable bioplastic applications. Green Chem. 2020;22(13):4055–4081. [Google Scholar]
- Sarasam A.R., Krishnaswamy R.K., Madihally S.V. Blending chitosan with polycaprolactone: effects on physicochemical and antibacterial properties. Biomacromolecules. 2006;7(4):1131–1138. doi: 10.1021/bm050935d. [DOI] [PubMed] [Google Scholar]
- Sarfraz A., Warsi M.F., Sarwar M.I., Ishaq M. Improvement in tensile properties of PVC–montmorillonite nanocomposites through controlled uniaxial stretching. Bull. Mater. Sci. 2012;35(4):539–544. [Google Scholar]
- Savenkova L., Gercberga Z., Nikolaeva V., Dzene A., Bibers I., Kalnin M. Mechanical properties and biodegradation characteristics of PHB-based films. Process Biochem. 2000;35(6):573–579. [Google Scholar]
- Schreiner M., Huyskens-Keil S., Krumbein A., Prono-Widayat H., Lüdders P. Effect of film packaging and surface coating on primary and secondary plant compounds in fruit and vegetable products. J. Food Eng. 2003;56(2–3):237–240. [Google Scholar]
- Shebani A., Klash A., Elhabishi R., Abdsalam S., Elbreki H., Elhrari W. The influence of LDPE content on the mechanical properties of HDPE/LDPE blends. Res. Dev. Mater. Sci. 2018;7(5):791–797. [Google Scholar]
- Shi S.C., Shah P.M. Edible polymers: challenges and opportunities. Journal of Polymers. 2014;2014 [Google Scholar]
- Sijtsema S.J., Onwezen M.C., Reinders M.J., Dagevos H., Partanen A., Meeusen M. Consumer perception of bio-based products—an exploratory study in 5 European countries. NJAS - Wageningen J. Life Sci. 2016;77:61–69. [Google Scholar]
- Silva-Pereira M.C., Teixeira J.A., Pereira-Júnior V.A., Stefani R. Chitosan/corn starch blend films with extract from Brassica oleraceae (red cabbage) as a visual indicator of fish deterioration. LWT-Food Science and Technology. 2015;61(1):258–262. (r) [Google Scholar]
- Singla R., Mehta R. Preparation and characterization of polylactic acid-based biodegradable blends processed under microwave radiation. Polym. Plast. Technol. Eng. 2012;51(10):1014–1017. [Google Scholar]
- Siracusa V., Rocculi P., Romani S., Dalla Rosa M. Biodegradable polymers for food packaging: a review. Trends Food Sci. Technol. 2008;19(12):634–643. [Google Scholar]
- Siripatrawan U., Noipha S. Active film from chitosan incorporating green tea extract for shelf life extension of pork sausages. Food Hydrocolloids. 2012;27(1):102–108. [Google Scholar]
- Smith R., editor. Biodegradable Polymers for Industrial Applications. CRC Press; 2005. [Google Scholar]
- Soheilmoghaddam M., Adelnia H., Bidsorkhi H.C., Sharifzadeh G., Wahit M.U., Akos N.I., Yussuf A.A. Development of ethylene‐vinyl acetate composites reinforced with graphene platelets. Macromol. Mater. Eng. 2017;302(2):1600260. [Google Scholar]
- Song J.H., Murphy R.J., Narayan R., Davies G.B.H. Biodegradable and compostable alternatives to conventional plastics. Phil. Trans. Biol. Sci. 2009;364(1526):2127–2139. doi: 10.1098/rstb.2008.0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soundararajan, S., & Palanivelu, K. Studies on Mechanical, Thermal, Electrical Properties and Accelerated UV Weathering of PP with HIPS Blends.
- Souza P.M.S., Corroqué N.A., Morales A.R., Marin-Morales M.A., Mei L.H.I. PLA and organoclays nanocomposites: degradation process and evaluation of ecotoxicity using Allium cepa as test organism. J. Polym. Environ. 2013;21(4):1052–1063. [Google Scholar]
- Srinivasa P., Baskaran R., Ramesh M., Prashanth K.H., Tharanathan R. Storage studies of mango packed using biodegradable chitosan film. Eur. Food Res. Technol. 2002;215(6):504–508. [Google Scholar]
- Stoll L., Costa T.M.H., Jablonski A., Flôres S.H., de Oliveira Rios A. Microencapsulation of anthocyanins with different wall materials and its application in active biodegradable films. Food Bioprocess Technol. 2016;9(1):172–181. [Google Scholar]
- Sun K., Li F., Li J., Li J., Zhang C., Chen S. Optimisation of compatibility for improving elongation at break of chitosan/starch films. RSC Adv. 2019;9(42):24451–24459. doi: 10.1039/c9ra04053f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sustainable Packaging Green packaging: point of purchase displays: biodegradable foam products. 2018. https://www.landaal.com/sustainable-packaging-environmentally-focused-packaging/biodegradable-foam-products/ Retrieved from. Accessed on 14th Oct 2020.
- Takala P.N., Vu K.D., Salmieri S., Khan R.A., Lacroix M. Antibacterial effect of biodegradable active packaging on the growth of Escherichia coli, Salmonella typhimurium and Listeria monocytogenes in fresh broccoli stored at 4 C. LWT-Food Science and Technology. 2013;53(2):499–506. [Google Scholar]
- Tanada-Palmu P.S., Grosso C.R. Effect of edible wheat gluten-based films and coatings on refrigerated strawberry (Fragaria ananassa) quality. Postharvest Biol. Technol. 2005;36(2):199–208. [Google Scholar]
- Tatsushima T., Ogata N., Nakane K., Ogihara T. Structure and physical properties of cellulose acetate butyrate/poly (butylene succinate) blend. J. Appl. Polym. Sci. 2005;96(2):400–406. [Google Scholar]
- Teng W.L., Khor E., Tan T.K., Lim L.Y., Tan S.C. Concurrent production of chitin from shrimp shells and fungi. Carbohydr. Res. 2001;332(3):305–316. doi: 10.1016/s0008-6215(01)00084-2. [DOI] [PubMed] [Google Scholar]
- Tetsuya T., Ishiaku U.S., Mizoguchi M., Hamada H. The effect of heat sealing temperature on the properties of OPP/CPP heat seal. I. Mechanical properties. J. Appl. Polym. Sci. 2005;97(3):753–760. [Google Scholar]
- Tiberiu N. Concepts in biological analysis of resorbable materials in oro-maxillo facial surgery. Oro-Maxillo-Fac Implantol (Romania) 2011;2(1):33–38. [Google Scholar]
- Tokura S., Tamura H. Chitin and chitosan. Compr. Glycosci. 2007;2:449–475. [Google Scholar]
- Tuil R.V., Schennink G., Beukelaer H.D., Heemst J.V., Jaeger R. The Food Biopack Conference. KVL; Copenhagen (Denmark: 2000. Converting biobased polymers into food packagings; pp. 27–29. Aug 2000. [Google Scholar]
- Urquijo J., Guerrica‐Echevarría G., Eguiazábal J.I. Melt processed PLA/PCL blends: effect of processing method on phase structure, morphology, and mechanical properties. J. Appl. Polym. Sci. 2015;132(41) [Google Scholar]
- Van den Oever M., Molenveld K., van der Zee M., Bos H. Bio-based and biodegradable plastics: facts and figures: focus on food packaging in The Netherlands (No. 1722) Wageningen Food & Biobased Research. 2017 [Google Scholar]
- Vert M., Santos I.D., Ponsart S., Alauzet N., Morgat J.L., Coudane J., Garreau H. Degradable polymers in a living environment: where do you end up? Polym. Int. 2002;51(10):840–844. [Google Scholar]
- Vroman I., Tighzert L. Biodegradable polymers. Materials. 2009;2(2):307–344. [Google Scholar]
- Wang X., Zhou J., Li L. Multiple melting behavior of poly (butylene succinate) Eur. Polym. J. 2007;43(8):3163–3170. [Google Scholar]
- Wang Z., Hu S., Wang H. Scale-up preparation and characterization of collagen/sodium alginate blend films. J. Food Qual. 2017;2017 [Google Scholar]
- Weber C.J., Haugaard V., Festersen R., Bertelsen G. Production and applications of biobased packaging materials for the food industry. Food Addit. Contam. 2002;19(S1):172–177. doi: 10.1080/02652030110087483. [DOI] [PubMed] [Google Scholar]
- Westmoreland Mechanical Testing and Research 2020. https://www.wmtr.com/ Inc.
- Wieczynska J., Cavoski I. Antimicrobial, antioxidant and sensory features of eugenol, carvacrol and trans-anethole in active packaging for organic ready-to-eat iceberg lettuce. Food Chem. 2018;259:251–260. doi: 10.1016/j.foodchem.2018.03.137. [DOI] [PubMed] [Google Scholar]
- Wu Y.B., Yu S.H., Mi F.L., Wu C.W., Shyu S.S., Peng C.K., Chao A.C. Preparation and characterization on mechanical and antibacterial properties of chitsoan/cellulose blends. Carbohydr. Polym. 2004;57(4):435–440. [Google Scholar]
- Xing Y., Li X., Xu Q., Jiang Y., Yun J., Li W. Effects of chitosan-based coating and modified atmosphere packaging (MAP) on browning and shelf life of fresh-cut lotus root (Nelumbo nucifera Gaerth) Innovat. Food Sci. Emerg. Technol. 2010;11(4):684–689. [Google Scholar]
- Yang S., Madbouly S.A., Schrader J.A., Grewell D., Kessler M.R., Graves W.R. Processing and characterization of bio‐based poly (hydroxyalkanoate)/poly (amide) blends: improved flexibility and impact resistance of PHA‐based plastics. J. Appl. Polym. Sci. 2015;132(27) [Google Scholar]
- Yildirim S., Röcker B., Pettersen M.K., Nilsen‐Nygaard J., Ayhan Z., Rutkaite R. Active packaging applications for food. Compr. Rev. Food Sci. Food Saf. 2018;17(1):165–199. doi: 10.1111/1541-4337.12322. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Mc Daniel M. Elsevier Ltd.; San Diego, California: 2005. Sensory Quality of Foods Associated with Edible Film and Coating Systems and Shelf-Life Extension, Innovations in Food Packaging; pp. 434–453. [Google Scholar]
- Zhong J., Tang H., Chen Y., Liu X. The preparation, mechanical and dielectric properties of PEN/HBCuPc hybrid films. J. Mater. Sci. Mater. Electron. 2010;21(12):1244–1248. [Google Scholar]
- Zhou H., Kawamura S., Koseki S., Kimura T. Comparative quality changes of fresh-cut melon in bio-based and petroleum-based plastic containers during storage. Environ. Control Biol. 2016;54(2):93–99. [Google Scholar]
- Zimmermanna L., Dombrowski A., Volker C., Wagner M. Are bioplastics and plant-based materials safer than conventional plastics? In vitro toxicity and chemical composition. Environ. Int. 2020;145:106066. doi: 10.1016/j.envint.2020.106066. [DOI] [PubMed] [Google Scholar]
- Zivkovic N. Polyhydroxy alkanoates, green chemistry (seminar) Food Technology Zagreb. 2009 (in Croatian) [Google Scholar]